Abstract
Previous research found tobacco smoking and solid fuel use for cooking to increase the risk of chronic liver disease mortality, but previous cohort studies have not investigated their independent and joint associations with liver cancer incidence in contemporary China. The China Kadoorie Biobank (CKB) study recruited 0.5 million adults aged 30 to 79 years from 10 areas across China during 2004 to 2008. Participants reported detailed smoking and fuel use information at baseline. After an 11.1‐year median follow‐up via electronic record linkage, we recorded 2997 liver cancer cases. Overall, 29.4% participants were current smokers. Among those who cooked at least once per month, 48.8% always used solid fuels (ie, coal or wood) for cooking. Tobacco smoking and solid fuel use for cooking were independently associated with increased risks of liver cancer, with hazard ratios (95% confidence intervals [CIs]) of 1.28 (1.15‐1.42) and 1.25 (1.03‐1.52), respectively. The more cigarettes consumed each day, the earlier the age of starting smoking or the longer duration of solid fuels exposure, the higher the risk (P trend < .001, =.001, =.018, respectively). Compared with never smokers who had always used clean fuels (ie, gas or electricity), ever‐smokers who had always used solid fuels for cooking had a 67% (95% CIs: 1.29‐2.17) higher risk. Among Chinese adults, tobacco smoking and solid fuel use for cooking were independently associated with higher risk of liver cancer incidence. Stronger association was observed with higher number of daily cigarette consumption, the earlier age of starting smoking and longer duration of solid fuel use.
Keywords: liver cancer, prospective cohort study, solid fuel, tobacco smoking
What's new?
Tobacco smoking and cooking with solid fuels, such as coal or wood, increase the risk of death from chronic liver disease. Here, the authors investigated whether these factors might also drive liver cancer incidence. They recruited 500 000 adults from different regions of China and recorded their smoking and fuel use data at baseline. Over a median follow‐up of 11 years, they recorded nearly 3000 liver cancer cases. Smoking and cooking with solid fuels were independently associated with higher risk of liver cancer incidence, with longer duration of exposure associated with a larger increase in risk.

Abbreviations
- BMI
body mass index
- CKB
China Kadoorie Biobank
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IARC
International Agency for Research on Cancer
- MET‐h/d
metabolic equivalent hours per day
1. INTRODUCTION
Globally, liver cancer account for more than 810 000 deaths each year, 1 with 52% occurring in China alone, where liver cancer was the most commonly diagnosed cancer and the leading cause of cancer death in men under 60. 2 Alcohol abuse, hepatitis B and C virus (HBV and HCV) infections have been the major causes of liver cancer in China, estimated to account for 33%, 41% and 8% deaths, respectively. 1 However, new HBV and HCV infections have declined dramatically in China due to successful HBV vaccination, mandatory HCV screening and other precautionary measures against blood‐borne disease transmission, 3 , 4 so other modifiable risk factors are becoming increasingly relevant.
Notably, China has some of the world's large population of active smokers (>300 million) and solid fuel users (about 450 million). 5 , 6 Epidemiological studies have reported tobacco smoking as a risk factor for liver cancer, 7 but they were mostly case‐control studies or cohort studies conducted in Japan or other high‐income countries where the stages of smoking epidemic differed substantially from that in China or other low‐ and middle‐income countries. Previous cohort studies in China 8 , 9 , 10 , 11 , 12 , 13 , 14 were all carried out in the last century, lacking adjustment for key risk factors (eg, HBV infection, alcohol consumption) and were based on specific population. The International Agency for Research on Cancer (IARC) has classified solid fuels smoke as a Group 2A (probably carcinogenic to humans) and coal smoke as Group 1 (carcinogenic to humans) for lung cancer. 15 In contrast, there is no evidence on the impact of solid fuel smoke on liver cancer.
Previously, we have discovered positive associations of smoking and long‐term solid fuel use for cooking with risk of chronic liver diseases mortality among half a million adults in the China Kadoorie Biobank (CKB) study, 16 but this was constrained by the lack of data on specific liver diseases, especially on hospitalization for liver cancer. In this study, we intended to explore the association of tobacco smoking and solid fuel cooking on the risks of liver cancer incidence with longer follow‐up in the same population.
2. METHODS
2.1. Study population
Details of the CKB study have been described elsewhere. 17 , 18 Briefly, 512 715 participants aged 30 to 79 years were recruited from 10 areas across China, including five urban regions (Harbin, Qingdao, Suzhou, Liuzhou and Haikou) and five rural regions (Henan, Gansu, Sichuan, Zhejiang and Hunan), during 2004 to 2008. Participants with missing body mass index (BMI, n = 2), and those who reported potentially unreliable recall information (ie, the difference between the total number of years lived in the three most recent residences and baseline age >1, n = 2308) or those with a self‐reported prior diagnosis of any cancer at baseline (n = 2578) were excluded, leaving 507 837 participants for the primary analysis. For the cooking‐related analyses, we additionally excluded those who cooked irregularly (ie, less than once per month) at baseline (n = 129 845), reported using unspecified fuels at any recalled residences (n = 3463), switched from clean to solid fuels (n = 304) or switched between clean and solid fuels back and forth in the last three residences before baseline survey (n = 777), leaving 373 448 participants.
2.2. Assessment of exposure
Trained health workers administered a computer‐assisted questionnaire (with built‐in logic and error checks to avoid missing data and erroneous data entry) to collect detailed information about participants' current and past smoking habits and cooking behavior in the three most recent residences where they had lived for at least a year.
All participants provided their current and past (only among those who reported not smoking at baseline) smoking frequency (not smoking, occasionally, on most days, daily or almost every day). Among ever smokers, additional information on the age (years) of starting smoking on most days, the types of tobacco and quantity of daily (factory filter cigarettes, factory nonfilter cigarettes, hand‐rolled cigarettes, cigars) or monthly (hand‐rolled cigarettes, pipes or water pipes, liang per month, 1 liang equivalent to 50 g) consumption was collected. Equivalent number of daily cigarette consumption was calculated as (factory filter cigarettes + factory nonfilter cigarettes + 2 × cigars + 5/3 × hand‐rolled cigarettes + 5/3 × pipes or water pipes), by assuming a factory cigarette containing 1 g of tobacco and a cigar containing 2 g. 19 For ex‐smokers, to avoid reverse causality bias, those who had stopped smoking because of illness were grouped with current smokers as per previous studies. 20 Participants were categorized according to smoking status (never smoker, ex‐smoker and current smoker), number of cigarettes consumed per day (≤10, 11‐20 and >20) and the age of starting regular smoking (>25, 19‐25 and ≤18 years) among ever smokers.
For each eligible residence, we obtained information about participants' duration of living (in years) and their corresponding cooking frequency (no cooking facility, never/rarely, monthly, weekly and daily). For participants who cooked at least once per month, we further asked their primary cooking fuel (gas, coal, wood, electricity and other) in each residence. “Gas” refers to natural gas, coal gas and liquefied petroleum gas. The “other” category comprised all fuel types not specified above. We considered gas and electricity as clean fuels, whereas wood and coal as solid fuels. Although participants might have used multiple fuel types simultaneously, we only recorded the one used most frequently and for the longest duration in each residence. Based on the long‐term cooking fuel use in the last three residences, participants were categorized into three groups (eg, always clean fuels, solid to clean fuels and always solid fuels). Long‐term solid fuel users were also categorized according to the total duration of exposure (<15, 15‐29 and ≥30 years) and specific solid fuel types (always coal, always wood, mixed of coal and wood). For residences with cooking facilities, we further asked if all cooking stoves were equipped with a chimney or extractor (all stoves, not all stoves and none).
A composite exposure of smoking status (never‐ or eversmoker) and long‐term fuel use (always clean fuels, ever solid fuels) was derived to investigate the potential joint effects of both exposures, taking those who had never smoked and always used clean fuels for cooking as the reference group.
Within a few weeks of the baseline survey, about 3% (n = 15 720) of participants were randomly selected to a quality control survey. 18 The kappa coefficients between the baseline and quality control survey were 0.94 for smoking and 0.61 for cooking fuel, 21 indicating acceptable reliability.
2.3. Assessment of covariates
Through the same electronic questionnaire mentioned above, a variety of covariates were assessed at the baseline survey, including sociodemographic characteristics (eg, age, sex, marital status, education, household income and occupation), lifestyle and dietary habits (alcohol drinking, physical activity, frequency of fresh fruit, preserved vegetables, red meat, fish and grains consumption), living environment (environmental tobacco smoke, storing pesticides at home, having a refrigerator at home, whether heating in winter and the fuel types used), medical history (diabetes, hepatic cirrhosis and cancer) and family medical history of cancer. The total daily physical activity level was calculated by multiplying the metabolic equivalent of tasks (MET) value for a particular type of physical activity by hours spent on that activity per day and summing the MET hours per day (MET‐h/d) for all activities. 22
Body weight (kg) and standing height (m) were measured by uniformly trained staff using a standard protocol and calibrated instruments at baseline. BMI (kg/m2) was calculated by dividing weight in kilograms by the square of height in meters. For each participant, a 10‐mL nonfasting blood sample (with time of last meal recorded) was collected and tested for hepatitis B surface antigen (HBsAg) (ACON Biotech).
2.4. Follow‐up and outcome definition
Through record linkage to death and disease registries and a national health insurance system, participants were followed up from baseline to the date of any liver cancer (International Classification of Diseases, 10th Revision [ICD‐10]: C22) diagnosis, death (n = 48 480), loss to follow‐up (n = 5246) or 31 December 2017, whichever came first. Vital status and cause of death were ascertained through reviews of official residential records and death certificates submitted to the regional Center for Disease Control and Prevention. The national health insurance system was established in all study regions by 2009 and it documented detailed hospitalization information, including ICD‐10 codes, dates of diagnosis and procedures.
2.5. Statistical analysis
Linear regression and logistic regression were used to compare continuous and categorical baseline characteristics across baseline smoking status (never smoker, ex‐smoker and current smoker) and long‐term cooking fuel types (always clean fuels, solid to clean fuels and always solid fuels), respectively, adjusted for age, sex and study areas as appropriate.
Cox proportional hazard regression was used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the independent associations of tobacco smoking (taking never smoker as the common reference group) and cooking fuel use (taking those who had always used clean fuels as the common reference group) with liver cancer incidence. Potential confounders were selected based on prior knowledge of liver cancer risk factors and were adjusted for in a stepwise manner. In order to illustrate the impact of adjusting for different sets of key confounders, we presented three sets of models with increasing number of covariates. The basic models (model 1) were stratified by sex, baseline age groups (in 5‐year intervals) and study region and adjusted for education (no formal school, primary school, middle school, high school, college and above), household income (<10 000, 10 000‐19 999 and ≥20 000 CNY/year), occupation (manual, nonmanual and not working) and marital status (married, widowed, separated or divorced and never married). Model 2 further adjusted for alcohol consumption (never/occasional, ex‐regular, weekly but not daily, daily <15 g/day, 15‐29 g/day, 30‐59 g/day, ≥60 g/day), environmental tobacco smoke (never lived with smoker, lived with smoker for <20 years, lived with smoker for ≥20 years and exposure <20 hours per week [h/w], lived with smoker for ≥20 years and exposure ≥20 h/w), months of storing pesticides at home (continuous, month), long‐term heating fuel exposure (always used clean fuels, switched from solid to clean fuels, always used solid fuels, switched from clean to solid fuels, switched between clean and solid fuels back and forth, ever used unspecified fuels, not heating in winter), stoves with chimney/extractor (all stoves, not all stoves and none stoves), physical activity (continuous, MET‐h/d), BMI (<18.5, 18.5‐24.0, 24.0‐27.9 and ≥28.0 kg/m2 according to overweight/obesity definition of Chinese population 23 ), having a refrigerator at home (never, 1‐5, 6‐10, 11‐15 and >15 years), consumption frequency of fresh fruit, preserved vegetables, red meat, fish and grains at baseline (never/rarely, monthly, 1‐3 days per week [d/w], 4‐6 d/w, daily) and mutually adjusted for long‐term cooking fuel exposure (always used clean fuels, switched from solid to clean fuels, always used solid fuels, switched from clean to solid fuels, switched between clean and solid fuels back and forth, ever used unspecified fuels, cooked irregularly) and smoking habits (never/occasional, quit ≥5 years ago, quit <5 years ago, current <15 cigarettes per day, current 15‐24 cigarettes per day and current ≥25 cigarettes per day). Model 3 further included HBsAg status (negative, positive, unclear, missing [n = 8149]), family history of cancer (yes or no), medical history of hepatic cirrhosis (yes or no) and diabetes (yes or no) at baseline. Only results from the “fully adjusted” model 3 are considered final and are quoted in the main text.
Stratified analyses by baseline characteristics, such as sex, region, BMI and physical activity, were performed to examine potential effect modifications. We conducted three sensitivity analyses separately: (a) excluding those who developed liver cancer during the first 2 years of follow‐up; (b) excluding those with family history of cancer; and (c) adjusting for detailed alcohol consumption (11 groups: never, occasional, ex‐regular, weekly but not daily, daily <15, 15‐29, 30‐44, 45‐59, 60‐74, 75‐89 and ≥90 g/day). All analyses were performed using Stata 15.0 (StataCorp, TX). The significance level was set at .05.
3. RESULTS
Among 507 837 participants with a mean (SD) age of 51.5 (10.7) years, 59.0% were female, 55.8% resided in rural areas, 29.4% were current smokers and 73.5% reported cooking regularly at baseline, of whom 48.8% had always used solid fuels for cooking. Current smokers and solid fuel users had less education and lower income, were less likely to be nonmanual workers, but more likely to be alcohol drinkers, to use solid fuels for heating and have poorer kitchen ventilation. Compared with those who had always used clean fuels for cooking, solid fuel users were more likely to be rural residents, current smokers and exposed to environmental tobacco smoke (Table 1).
TABLE 1.
Baseline characteristics of participants according to smoking status and long‐term cooking fuel use
| Smoking status | Cooking fuel | |||||
|---|---|---|---|---|---|---|
| Never | Ex‐smoker | Current smoker | Always clean | Solid to clean | Always solid | |
| N | 343 198 | 15 175 | 149 464 | 86 782 | 104 381 | 182 285 |
| Age (years) | 51.5 | 56.6 | 52.8 | 46.0 | 52.4 | 54.6 |
| Female (%) | 84.6 | 8.0 | 5.5 | 59.5 | 80.5 | 77.5 |
| Rural (%) | 52.7 | 50.7 | 63.2 | 12.2 | 18.2 | 91.1 |
| Married (%) | 91.1 | 91.8 | 88.7 | 88.5 | 89.7 | 89.1 |
| Middle school and above (%) | 51.3 | 48.2 | 44.7 | 62.7 | 52.6 | 38.3 |
| Household income >20 000 yuan/year (%) | 44.1 | 42.8 | 39.9 | 55.3 | 47.3 | 30.2 |
| Occupation (%) | ||||||
| Manual | 54.7 | 53.3 | 58.5 | 39.5 | 39.0 | 68.4 |
| Nonmanual | 14.0 | 12.9 | 11.1 | 17.6 | 14.9 | 5.8 |
| Not working | 31.3 | 33.8 | 30.4 | 42.9 | 46.0 | 25.8 |
| Passive smoker (%) | 42.6 | 44.4 | 48.0 | 44.8 | 48.1 | 51.3 |
| Current alcohol drinker (%) | 9.5 | 17.1 | 18.7 | 10.9 | 10.9 | 11.2 |
| Current smoker (%) | — | — | — | 17.9 | 18.9 | 20.1 |
| Ventilation with all stoves (%) | 45.3 | 43.8 | 42.6 | 65.3 | 60.3 | 26.8 |
| Heating fuel type (%) | ||||||
| Always clean | 8.9 | 8.5 | 8.0 | 18.5 | 7.1 | 4.5 |
| Always solid | 35.7 | 36.1 | 36.8 | 21.7 | 24.8 | 43.2 |
| Not heating | 43.0 | 42.2 | 42.2 | 43.3 | 43.6 | 45.7 |
| Storing pesticide (months) | 4.1 | 4.1 | 4.2 | 2.3 | 2.7 | 5.2 |
| Physical activity (MET‐h/d) | 21.0 | 20.9 | 21.4 | 18.6 | 19.7 | 22.5 |
| Overweight and obesity (%) | 45.9 | 51.2 | 38.2 | 47.6 | 51.4 | 40.6 |
| HBsAg positive (%) | 3.0 | 2.8 | 3.1 | 2.7 | 2.9 | 3.1 |
| History of diabetes (%) | 5.9 | 6.3 | 5.8 | 6.8 | 7.2 | 4.9 |
| History of hepatic cirrhosis (%) | 1.3 | 1.1 | 1.2 | 1.1 | 1.3 | 0.9 |
Note: Values are means or percentages of participants adjusted for age, sex and region, where appropriate.
Abbreviations: HBsAg, hepatitis B surface antigen; MET‐h/d, metabolic equivalent of tasks‐hours per day.
During a median follow‐up of 11.1 (interquartile range 10.2‐12.1) years, we documented 2997 incident liver cancer cases, of whom 24.6% were HBsAg positive compared to 2.9% in those who did not develop liver cancer. Compared with never smokers, current smokers had 28% (95% CIs: 1.15‐1.42) higher risk of liver cancer. Besides, the association followed a dose‐response trend with daily cigarette consumption and the starting age of regular smoking (P trend < .001 and P trend = .001, respectively, Table 2). Compared to never smokers, the HRs of liver cancer incidence increased from 1.18 (1.03‐1.36) among those who started smoking after 25 years old to 1.34 (1.17‐1.53) among those who started at 18 or earlier.
TABLE 2.
Hazard ratios for liver cancer by smoking characteristics and long‐term cooking fuel use
| N (rates/100 000 PYs) | HRs (95% CIs) a | |||
|---|---|---|---|---|
| Model 1: basic adjustment | Model 2: basic + lifestyle factors | Model 3: basic + lifestyle + medical factors | ||
| Smoking status | ||||
| Never (common reference) | 1374 (36.6) | 1.00 | 1.00 | 1.00 |
| Ex‐smoker | 153 (95.8) | 1.15 (0.96‐1.39) | 1.12 (0.93‐1.35) | 1.13 (0.94‐1.36) |
| Current smoker | 1470 (92.8) | 1.33 (1.20‐1.48) | 1.26 (1.13‐1.40) | 1.28 (1.15‐1.42) |
| No. of cigarettes/day | ||||
| ≤10 | 532 (91.4) | 1.24 (1.10‐1.40) | 1.19 (1.05‐1.35) | 1.19 (1.05‐1.35) |
| 11‐20 | 761 (91.8) | 1.35 (1.20‐1.52) | 1.28 (1.14‐1.44) | 1.30 (1.15‐1.47) |
| >20 | 330 (99.4) | 1.38 (1.19‐1.59) | 1.27 (1.10‐1.48) | 1.32 (1.14‐1.54) |
| P trend | <.001 | <.001 | <.001 | |
| Age started smoking (years) | ||||
| >25 | 372 (98.0) | 1.20 (1.05‐1.37) | 1.16 (1.01‐1.33) | 1.18 (1.03‐1.36) |
| 19‐25 | 775 (90.2) | 1.31 (1.17‐1.47) | 1.24 (1.10‐1.40) | 1.26 (1.12‐1.42) |
| ≤18 | 476 (94.3) | 1.43 (1.25‐1.62) | 1.33 (1.16‐1.52) | 1.34 (1.17‐1.53) |
| P trend | <.001 | .003 | .001 | |
| Long‐term fuel type | ||||
| Always clean (common reference) | 363 (38.5) | 1.00 | 1.00 | 1.00 |
| Solid to clean | 513 (45.2) | 1.09 (0.94‐1.27) | 1.12 (0.96‐1.30) | 1.10 (0.94‐1.28) |
| Always solid | 1009 (50.7) | 1.29 (1.07‐1.55) | 1.23 (1.01‐1.49) | 1.25 (1.03‐1.52) |
| Types of solid fuels b | ||||
| Always coal | 356 (45.2) | 1.17 (0.91‐1.50) | 1.15 (0.89‐1.48) | 1.14 (0.88‐1.47) |
| Always wood | 480 (56.7) | 1.31 (1.08‐1.58) | 1.24 (1.01‐1.51) | 1.27 (1.04‐1.56) |
| Always solid (mixed) | 173 (48.7) | 1.39 (1.08‐1.79) | 1.33 (1.03‐1.72) | 1.34 (1.04‐1.73) |
| Duration exposed (years) | ||||
| 1‐14 | 322 (42.6) | 1.05 (0.89‐1.24) | 1.06 (0.90‐1.26) | 1.06 (0.89‐1.25) |
| 15‐29 | 498 (41.6) | 1.17 (1.00‐1.37) | 1.18 (1.00‐1.38) | 1.16 (0.99‐1.37) |
| ≥30 | 702 (60.0) | 1.23 (1.04‐1.45) | 1.22 (1.03‐1.44) | 1.21 (1.03‐1.44) |
| P trend | .008 | .018 | .018 | |
HRs were stratified by sex, baseline age groups and study regions, model 1 were adjusted for education, household income, occupation and marital status; model 2 were additionally adjusted for alcohol consumption, environmental tobacco smoke, months of storing pesticides at home, long‐term heating fuel exposure, stoves with chimney/extractor, physical activity, BMI, having a refrigerator at home, consumption frequency of fresh fruit, preserved vegetables, red meat, fish and grains at baseline and mutually adjusted for long‐term cooking fuel exposure and smoking habits; model 3 were additionally adjusted for hepatitis B test result, family history cancer, medical history of hepatic cirrhosis and diabetes.
Analysis was restricted to individuals who always solid fuels in the last 3 residences before the baseline survey (N = 182 285).
Compared to long‐term clean fuel users, long‐term solid fuel users had a higher risk of liver cancer (HRs: 1.25, 95% CIs: 1.03‐1.52). However, individuals who had switched from solid to clean fuels had no significant excess risk (1.10, 0.94‐1.28). Among those who had always used solid fuels for cooking, wood users and mixed solid fuel users had higher risks, with HRs of 1.27 (1.04‐1.56) and 1.34 (1.04‐1.73), respectively, whereas long‐term coal users had no significant excess risk (1.14, 0.88‐1.47). A dose‐response trend was observed between the duration of solid fuels exposure and liver cancer risks (P trend = .018, Table 2).
As shown in Figure 1, compared with never smokers who had always used clean fuels, ever smokers who had always used solid fuels had the highest risk of liver cancer (HRs: 1.67, 95% CIs: 1.31‐2.19). Both ever smokers who had used clean fuels and never smokers who had used solid fuels had smaller but significantly elevated risks, with HRs of 1.41 (1.11‐1.80) and 1.36 (1.09‐1.78), respectively.
FIGURE 1.
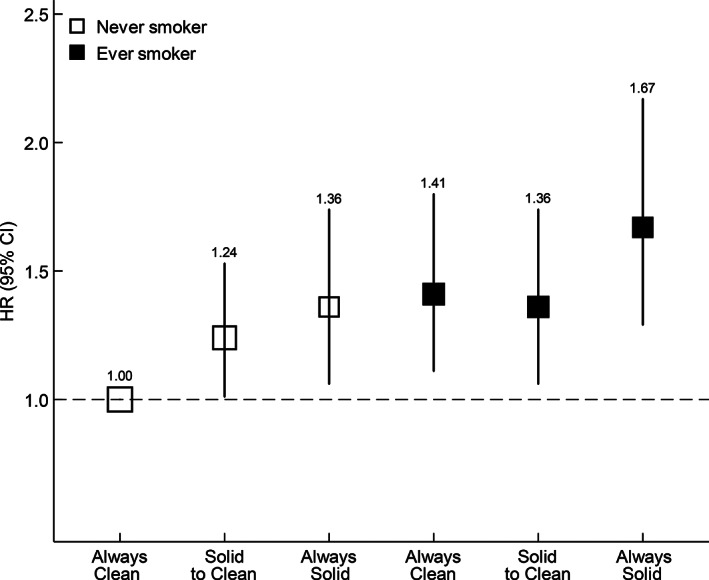
Associations of smoking status and long‐term cooking fuel exposure with liver cancer risk. HRs were stratified by sex, baseline age groups, study regions and adjusted for education, household income, occupation, marital status, alcohol consumption, environmental tobacco smoke, months of storing pesticides at home, long‐term heating fuel exposure, stoves with chimney/extractor, physical activity, BMI, having a refrigerator at home, consumption frequency of fresh fruit, preserved vegetables, red meat, fish and grains at baseline, hepatitis B test result, family history of cancer, medical history of hepatic cirrhosis and diabetes. The size of the box was inversely proportional to the variance of the logarithm of the category‐specific log risk and the vertical lines represent 95% CIs. The numbers above the vertical lines were point estimates for HRs. The analysis was restricted to individuals who had data on solid fuel use and smoking (N = 373 448)
In the subgroup analyses, the association with current smoking appeared greater in HBsAg seropositive participants (HRs: 1.35, 95% CIs: 1.09‐1.68 vs 1.26, 1.11‐1.43; P interaction = .042) and ever‐regular drinkers (1.42, 1.25‐1.62 vs 1.13, 0.94‐1.37; P interaction = 0.031) than their counterparts (Table 3). No significant interaction was found between the amount of daily cigarette consumption and liver cancer across all stratums (Table S1). When stratified by sex, solid fuel use remained significantly and positively associated with liver cancer risk in females (1.59, 1.14‐2.20), but not in males (1.13, 0.89‐1.45; P interaction < .001). The positive relationship between solid fuel types and liver cancer risks was much more pronounced among rural participants, with 51% (1.05‐2.19), 57% (1.11‐2.22) and 70% (1.17‐2.46) elevated risks for coal, wood and mix solid fuel users, respectively, but not among urban citizens (P interaction = .011, Table S2).
TABLE 3.
Hazard ratios for liver cancer associated with smoking status and long‐term cooking fuel use, stratified by baseline characteristics
| Smoking status | P interact | Cooking fuel type | P interact | |||||
|---|---|---|---|---|---|---|---|---|
| Never | Ex‐smoker | Current smoker | Always clean | Solid to clean | Always solid | |||
| Sex | .803 | <.001 | ||||||
| Male | 1.00 | 1.16 (0.95‐1.40) | 1.30 (1.15‐1.46) | 1.00 | 1.01 (0.83‐1.22) | 1.13 (0.89‐1.45) | ||
| Female | 1.00 | 0.82 (0.36‐1.86) | 1.20 (0.90‐1.60) | 1.00 | 1.34 (1.03‐1.75) | 1.59 (1.14‐2.20) | ||
| Region | .788 | .092 | ||||||
| Rural | 1.00 | 1.29 (0.99‐1.69) | 1.37 (1.18‐1.58) | 1.00 | 1.28 (0.86‐1.90) | 1.60 (1.12‐2.20) | ||
| Urban | 1.00 | 1.07 (0.83‐1.37) | 1.23 (1.05‐1.44) | 1.00 | 1.01 (0.87‐1.19) | 1.08 (0.82‐1.41) | ||
| HBsAg | .042 | .065 | ||||||
| Negative | 1.00 | 1.06 (0.85‐1.33) | 1.26 (1.11‐1.43) | 1.00 | 1.11 (0.92‐1.34) | 1.41 (1.12‐1.78) | ||
| Positive | 1.00 | 1.33 (0.91‐1.93) | 1.35 (1.09‐1.68) | 1.00 | 1.08 (0.81‐1.46) | 0.84 (0.56‐1.27) | ||
| Cirrhosis | .621 | .833 | ||||||
| No | 1.00 | 1.16 (0.95‐1.41) | 1.29 (1.15‐1.45) | 1.00 | 1.10 (0.94‐1.29) | 1.24 (1.01‐1.52) | ||
| Yes | 1.00 | 1.00 (0.56‐1.77) | 1.20 (0.87‐1.66) | 1.00 | 1.17 (0.70‐1.96) | 1.71 (0.88‐3.30) | ||
| Drinker | .031 | .209 | ||||||
| Never | 1.00 | 1.02 (0.70‐1.49) | 1.13 (0.94‐1.37) | 1.00 | 1.31 (1.02‐1.69) | 1.45 (1.08‐1.96) | ||
| Ever | 1.00 | 1.25 (1.01‐1.54) | 1.42 (1.25‐1.62) | 1.00 | 0.97 (0.80‐1.18) | 1.12 (0.86‐1.46) | ||
| BMI (kg/m2) | .546 | .117 | ||||||
| 18.5‐24.0 | 1.00 | 1.12 (0.85‐1.48) | 1.37 (1.18‐1.59) | 1.00 | 1.30 (1.03‐1.64) | 1.48 (1.13‐1.94) | ||
| ≥24 | 1.00 | 1.07 (0.83‐1.39) | 1.19 (1.01‐1.40) | 1.00 | 0.97 (0.79‐1.19) | 0.97 (0.71‐1.33) | ||
| Physical activity | .876 | .516 | ||||||
| Low | 1.00 | 1.15 (0.90‐1.47) | 1.35 (1.17‐1.57) | 1.00 | 1.12 (0.92‐1.37) | 1.38 (1.05‐1.81) | ||
| High | 1.00 | 1.11 (0.84‐1.46) | 1.21 (1.03‐1.41) | 1.00 | 1.04 (0.82‐1.32) | 1.11 (0.84‐1.47) | ||
Note: HRs were stratified by sex, baseline age groups, study regions and adjusted for education, household income, occupation, marital status, alcohol consumption, environmental tobacco smoke, months of storing pesticides at home, long‐term heating fuel exposure, stoves with chimney/extractor, physical activity, BMI, having a refrigerator at home, consumption frequency of fresh fruit, preserved vegetables, red meat, fish and grains at baseline, hepatitis B test result, family history of cancer, medical history of hepatic cirrhosis and diabetes, mutually adjusted for long‐term cooking fuel exposure and smoking habits.
Abbreviations: BMI, body mass index; HBsAg, hepatitis B surface antigen.
The associations of smoking and solid fuel use with liver cancer risks remained consistent with the main results after adjusting for 11 groups of alcohol consumption, excluding liver cancer cases within the first 2 years of follow‐up and those who had family history of cancer (Table S3).
4. DISCUSSION
In this large prospective study, we found that tobacco smoking and long‐term solid fuel use for cooking were both associated with higher risk of liver cancer, and these associations were stronger with higher daily cigarette consumption, earlier starting age of regular smoking and total duration of solid fuels exposure. HBV infection, alcohol drinking, sex and area of residence are potential effect modifiers.
The present study corroborates previous findings on smoking and liver cancer. 7 , 24 A meta‐analysis including 24 cohort studies published till 2016 found that regular smokers had 66% (95% CIs: 1.53‐1.80) higher risk of hepatocellular carcinoma (HCC). 7 Another meta‐analysis found similar results, with a pooled OR of 1.51 (95% CIs: 1.37‐1.67) for liver cancer associated with current smoking. 24 Both reviews reported higher risk of liver cancer associated with smoking than observed that discovered in the present study. Such difference could partly be explained by the study population. The studies enrolled in the meta‐analyses were mainly conducted in developed countries, where the smoking epidemic had reached its summit. We have further examined the dose‐response associations with the number of cigarettes consumed on the risk of liver cancer. Previous cohort studies conducted in China found similar results but with larger magnitude, with RRs of smoking >20 cigarettes per day ranging from 1.60 to 1.80. 14 , 25 However, one only enrolled males 14 while the other investigated liver cancer mortality in city factory workers. 25
For solid fuels combustion, although previous reports mainly focused on its association with cardiorespiratory diseases, 26 a recent study suggested that the liver might also be affected by air pollutants due to its role in detoxification. 27 A case‐control study including 314 HCC cases and 368 controls in China reported a 3.91‐fold HCC risk (95% CIs: 2.62‐5.83) among those exposed to indoor air pollution. 28 However, it lacked a detailed definition of indoor air pollution and only adjusted for education. In the present study, we observed 25% higher risk of liver cancer incidence among those who had always used solid fuels for cooking, and further identified apparently greater hazard in persistent wood users than coal users. A possible explanation might be that wood combustion produced much higher levels of air pollutants (especially particulate matter [PM], many of which are carcinogens such as polycyclic aromatic hydrocarbons [PAHs]) than coal. 29
The associations between tobacco smoking and liver cancer stratified by HBV infection status have been controversial. Similar to this study, a meta‐analysis of nine studies (five case‐control and four cohort studies) 30 and a recent study of 2011 liver cancer cases and 7933 controls in China 31 found that the association of smoking with liver cancer appeared stronger in HBV positive individuals. In contrast, some studies observed significant association only among HBV negative participants, 32 , 33 , 34 but all of them were case‐control studies with less than 200 cases. When stratified by alcohol drinking status, smoking remained significantly and positively associated with liver cancer risk among ever drinkers, but not among never drinkers. Previously two hospital‐based case‐control studies conducted in Greece and United States had found similar results, with 6‐ to 9‐fold higher risk of HCC among those exposed to both drinking and smoking compared with those exposed to neither. 35 , 36 The interaction is biologically plausible since the IARC had identified both tobacco and alcoholic beverages as Group 1 human carcinogens for liver cancer. 37 , 38
The association of solid fuel use with liver cancer incidence appeared greater in females than in males, possibly because females tend to cook much more regularly and intensely in China. 39 The positive association between solid fuel types and liver cancer risks remained significant among rural but not urban residents. This could be due to poorer ventilation and lower quality fuels and thus higher exposure to noxious pollutants in rural residents. 40
Solid fuels combustion could produce a large amount of air pollutants, especially PM and PAHs, which have been reported to induce the development and progression of liver cancer. 41 Our previous work found long‐term solid fuel use for cooking and smoking to be independently associated with a higher risk of death from chronic liver diseases. 16 Similarly, the positive relationship between solid fuels for cooking and incident liver cancer risk was much more pronounced among ever smokers than never smokers.
Our finding highlighted the double burden from tobacco smoking and solid fuels on liver cancer in China. These two risk factors are both more prevalent in less‐developed areas, and greater attention should be placed on smoking cessation and promotion of access to clean energy to reduce the burden caused by liver cancer, among other diseases. 42 , 43 Notably, no significant excess risk of liver cancer incidence was found among those who had switch from solid to clean fuels compared with long‐term clean fuel users, supporting the potential benefits of clean fuel transition. In addition, chronic carriers of HBV and alcohol drinkers, who are also more likely to smoke, 44 should be suggested to avoid smoking.
To the best of our knowledge, the present study was among the first prospective studies to investigate the independent and joint impact of smoking and solid fuel use for cooking on liver cancer incidence among Chinese adults. The strengths of this study included the large sample size, prospective cohort design and long‐term follow‐up via robust record linkage to hospitalization records. The comprehensive survey in CKB collected a range of risk factors for liver cancer, including hepatitis B infection status and history of hepatic cirrhosis, enabling adjustment for important confounders in our analyses. However, some limitations merit discussion. First, smoking habits and solid fuel use were self‐reported, which might entail recall and/or social desirability bias. Second, although we used a comprehensive questionnaire to measure both exposures, we lacked more detailed information for cooking fuel use, such as stove types, secondary fuel type used and intra‐day cooking frequency, which prevented us from getting more precise exposure classification. Although these data were unavailable, we adjusted for stove ventilation condition and excluded those who cooked irregularly at baseline to reduce noise and confounding. Third, we had no data on aflatoxins exposure, which is a strong risk factor for liver cancer. 45 Aflatoxins are usually produced from improper storage of food (corns, peanuts and spices) in warm and humid regions, and it is typically associated with lower socioeconomic status. 46 All analyses in this study were stratified by the 10 study areas and adjusted for a range of socioeconomic factors in order to reduce the confounding. Fourth, incident liver cancer cases were documented via linkage to death and disease registries and hospitalization records. Underdiagnosis might exist due to poor healthcare access or health awareness associated with lower socioeconomic status and/or rural residency, both of which were positively associated with higher solid fuel use in CKB, so the associations observed may be underestimated.
5. CONCLUSION
Tobacco smoking and solid fuel use for cooking were each independently associated with higher risk of liver cancer incidence. The strength of the association increased with the amount of daily cigarette consumption, earlier age of starting smoking and total duration of solid fuels exposure. Our findings reinforce the importance of smoking cessation and promotion of access to clean energy, especially in less‐developed areas.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Qiaorui Wen and Ka Hung Chan are joint first authors. Qiaorui Wen and Ka Hung Chan drafted the manuscript. Qiaorui Wen and Kexiang Shi analyzed the data. Yu Guo, Pei and Weijie Hu collected the data. Huaidong Du, Yiping Chen, Simon Gilbert and Daniel Avery were involved in data cleaning. Canqing Yu, Jun Lv and Ling Yang interpreted the results and contributed to the critical revision of the manuscript for important intellectual content. Zhengming Chen and Liming Li are the study guarantors. Liming Li, Zhengming, Canqing Yu and Junshi Chen designed the study. All authors have read and approved the final version of the manuscript. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
ETHICS STATEMENT
The CKB study was approved by the Ethical Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee at the University of Oxford (Oxford, UK). Written informed consent was obtained from all participants.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
The most important acknowledgement is to the participants in the study and the members of the survey teams in each of the 10 regional centers, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centers.
Wen Q, Chan KH, Shi K, et al. Tobacco smoking and solid fuels for cooking and risk of liver cancer: A prospective cohort study of 0.5 million Chinese adults. Int. J. Cancer. 2022;151(2):181‐190. doi: 10.1002/ijc.33977
Qiaorui Wen and Ka Hung Chan contributed equally to this study.
The members of steering committee and collaborative group are listed in the Supporting information.
Funding informationThis work was supported by National Natural Science Foundation of China (91843302, 81941018, 91846303). The CKB baseline survey and the first re‐survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long‐term follow‐up is supported by grants from the UK Wellcome Trust (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), grants (2016YFC0900500, 2016YFC0900501, 2016YFC0900504, 2016YFC1303904) from the National Key R&D Program of China, National Natural Science Foundation of China (81390540, 81390541, 81390544) and Chinese Ministry of Science and Technology (2011BAI09B01). Ka Hung Chan acknowledges support from the BHF Centre of Research Excellence, University of Oxford (RE/18/3/34214). The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report or the decision to submit the article for publication.
DATA AVAILABILITY STATEMENT
The dataset for this study is available at www.ckbiobank.org, as well as the access policy and procedures. Further information is available from the corresponding author upon request.
REFERENCES
- 1. Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(12):1683‐1691. doi: 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099‐2108. doi: 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen YS, Li L, Cui FQ, et al. A sero‐epidemiological study on hepatitis C in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32(9):888‐891. [PubMed] [Google Scholar]
- 5. Zhang J, Ou JX, Bai CX. Tobacco smoking in China: prevalence, disease burden, challenges and future strategies. Respirology. 2011;16(8):1165‐1172. doi: 10.1111/j.1440-1843.2011.02062.x [DOI] [PubMed] [Google Scholar]
- 6. Health Effect Institute . State of Global Air 2020. Boston, MA: Health Effects Institute; 2020. [Google Scholar]
- 7. Abdel‐Rahman O, Helbling D, Schöb O, et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10(4):245‐254. doi: 10.1111/jebm.12270 [DOI] [PubMed] [Google Scholar]
- 8. Tu JT, Gao RN, Zhang DH, Gu BC. Hepatitis B virus and primary liver cancer on Chongming Island, People's Republic of China. Natl Cancer Inst Monogr. 1985;69:213‐215. [PubMed] [Google Scholar]
- 9. Chen ZM, Xu Z, Collins R, Li WX, Peto R. Early health effects of the emerging tobacco epidemic in China. A 16‐year prospective study. JAMA. 1997;278(18):1500‐1504. doi: 10.1001/jama.278.18.1500 [DOI] [PubMed] [Google Scholar]
- 10. Lam TH, He Y, Li LS, Li LS, He SF, Liang BQ. Mortality attributable to cigarette smoking in China. JAMA. 1997;278(18):1505‐1508. [PubMed] [Google Scholar]
- 11. Sun Z, Lu P, Gail MH, et al. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology. 1999;30(2):379‐383. doi: 10.1002/hep.510300204 [DOI] [PubMed] [Google Scholar]
- 12. Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT. Eight‐year follow‐up of the 90,000‐person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev. 2002;11(4):369‐376. [PubMed] [Google Scholar]
- 13. Wang J, Gao YT, Wang XL, Liu EJ, Zhang YL, Yuan JM. Cigarette smoking and cancer mortality: a prospective cohort study in urban males in Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(10):837‐840. [PubMed] [Google Scholar]
- 14. Yu MW, Hsu FC, Sheen IS, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145(11):1039‐1047. doi: 10.1093/oxfordjournals.aje.a009060 [DOI] [PubMed] [Google Scholar]
- 15. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1‐538. [PMC free article] [PubMed] [Google Scholar]
- 16. Chan KH, Bennett DA, Kurmi OP, et al. Solid fuels for cooking and tobacco use and risk of major chronic liver disease mortality: a prospective cohort study of 0.5 million Chinese adults. Int J Epidemiol. 2020;49(1):45‐55. doi: 10.1093/ije/dyz216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Z, Lee L, Chen J, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC). Int J Epidemiol. 2005;34(6):1243‐1249. doi: 10.1093/ije/dyi174 [DOI] [PubMed] [Google Scholar]
- 18. Chen Z, Chen J, Collins R, et al. China Kadoorie biobank of 0.5 million people: survey methods, baseline characteristics and long‐term follow‐up. Int J Epidemiol. 2011;40(6):1652‐1666. doi: 10.1093/ije/dyr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan M, Lv J, Yu C, et al. Family history, tobacco smoking, and risk of ischemic stroke. J Stroke. 2019;21(2):175‐183. doi: 10.5853/jos.2018.03566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Bragg F, Yang L, et al. Smoking and smoking cessation in relation to risk of diabetes in Chinese men and women: a 9‐year prospective study of 0·5 million people. Lancet Public Health. 2018;3(4):e167‐e176. doi: 10.1016/s2468-2667(18)30026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Qin C, Lv J, et al. Solid fuel use and incident COPD in Chinese adults: findings from the China Kadoorie biobank. Environ Health Perspect. 2019;127(5):57008. doi: 10.1289/ehp2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du H, Bennett D, Li L, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank Study. Am J Clin Nutr. 2013;97(3):487‐496. doi: 10.3945/ajcn.112.046854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut‐off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83‐96. [PubMed] [Google Scholar]
- 24. Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta‐analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38(6):1497‐1511. doi: 10.1093/ije/dyp280 [DOI] [PubMed] [Google Scholar]
- 25. Liaw KM, Chen CJ. Mortality attributable to cigarette smoking in Taiwan: a 12‐year follow‐up study. Tob Control. 1998;7(2):141‐148. doi: 10.1136/tc.7.2.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hystad P, Duong M, Brauer M, et al. Health effects of household solid fuel use: findings from 11 countries within the prospective urban and rural epidemiology study. Environ Health Perspect. 2019;127(5):57003. doi: 10.1289/ehp3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng H, Eckel SP, Liu L, Lurmann FW, Cockburn MG, Gilliland FD. Particulate matter air pollution and liver cancer survival. Int J Cancer. 2017;141(4):744‐749. doi: 10.1002/ijc.30779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niu J, Lin Y, Guo Z, Niu M, Su C. The epidemiological investigation on the risk factors of hepatocellular carcinoma: a case‐control study in Southeast China. Medicine (Baltimore). 2016;95(6):e2758. doi: 10.1097/md.0000000000002758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Q, Qi J, Jiang J, et al. Significant reduction in air pollutant emissions from household cooking stoves by replacing raw solid fuels with their carbonized products. Sci Total Environ. 2019;650:653‐660. doi: 10.1016/j.scitotenv.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 30. Chuang SC, Lee YC, Hashibe M, Dai M, Zheng T, Boffetta P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1261‐1268. doi: 10.1158/1055-9965.Epi-09-1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Baecker A, Wu M, et al. Interaction between tobacco smoking and hepatitis B virus infection on the risk of liver cancer in a Chinese population. Int J Cancer. 2018;142(8):1560‐1567. doi: 10.1002/ijc.31181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lam KC, Yu MC, Leung JW, Henderson BE. Hepatitis B virus and cigarette smoking: risk factors for hepatocellular carcinoma in Hong Kong. Cancer Res. 1982;42(12):5246‐5248. [PubMed] [Google Scholar]
- 33. Trichopoulos D, Day NE, Kaklamani E, et al. Hepatitis B virus, tobacco smoking and ethanol consumption in the etiology of hepatocellular carcinoma. Int J Cancer. 1987;39(1):45‐49. doi: 10.1002/ijc.2910390109 [DOI] [PubMed] [Google Scholar]
- 34. Tanaka K, Hirohata T, Takeshita S. Blood transfusion, alcohol consumption, and cigarette smoking in causation of hepatocellular carcinoma: a case‐control study in Fukuoka, Japan. Jpn J Cancer Res. 1988;79(10):1075‐1082. doi: 10.1111/j.1349-7006.1988.tb01529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42(2):218‐224. doi: 10.1016/j.jhep.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 36. Kuper H, Tzonou A, Kaklamani E, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85(4):498‐502. [PubMed] [Google Scholar]
- 37. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:3‐1383. [PMC free article] [PubMed] [Google Scholar]
- 38. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1‐1438. [PMC free article] [PubMed] [Google Scholar]
- 39. Lin J, Ni S, Shi Q, et al. Environmental exposure to cooking oil fumes and fatty liver disease. Ann Palliat Med. 2020;9(6):3810‐3817. doi: 10.21037/apm-20-1730 [DOI] [PubMed] [Google Scholar]
- 40. Mestl HE, Aunan K, Seip HM, Wang S, Zhao Y, Zhang D. Urban and rural exposure to indoor air pollution from domestic biomass and coal burning across China. Sci Total Environ. 2007;377(1):12‐26. doi: 10.1016/j.scitotenv.2007.01.087 [DOI] [PubMed] [Google Scholar]
- 41. Kim JW, Park S, Lim CW, Lee K, Kim B. The role of air pollutants in initiating liver disease. Toxicol Res. 2014;30(2):65‐70. doi: 10.5487/tr.2014.30.2.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Astell‐Burt T, Zhang M, Feng X, et al. Geographical inequality in tobacco control in China: multilevel evidence from 98 058 participants. Nicotine Tob Res. 2018;20(6):755‐765. doi: 10.1093/ntr/ntx100 [DOI] [PubMed] [Google Scholar]
- 43. Smith KR, Frumkin H, Balakrishnan K, et al. Energy and human health. Annu Rev Public Health. 2013;34:159‐188. doi: 10.1146/annurev-publhealth-031912-114404 [DOI] [PubMed] [Google Scholar]
- 44. Lajtha A, Sershen H. Nicotine: alcohol reward interactions. Neurochem Res. 2010;35(8):1248‐1258. doi: 10.1007/s11064-010-0181-8 [DOI] [PubMed] [Google Scholar]
- 45. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr Eval Carcinog Risks Hum. 2002;82:1‐556. [PMC free article] [PubMed] [Google Scholar]
- 46. Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80(5):1106‐1122. doi: 10.1093/ajcn/80.5.1106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The dataset for this study is available at www.ckbiobank.org, as well as the access policy and procedures. Further information is available from the corresponding author upon request.


