Abstract
Glucocorticoids such as cortisol are a class of steroid hormones that play an important role in co-ordinating the body's response to stress. Elevated cortisol levels and increased inflammation have frequently been reported in patients with depression. The currently accepted ‘glucocorticoid resistance’ model posits this increased inflammation as a consequence of reduced sensitivity to cortisol’s putative anti-inflammatory action. However, opposing evidence has accumulated that supports a more recent model, which instead proposes that cortisol possesses immune potentiating properties and may thus directly cause the increased inflammation seen in depression. Despite all of this, a clear explanation of the neuroendocrine mechanism that contributes to the development of depression is still lacking and thus requires further investigation in improved future studies.
Keywords: Hypothalamic-pituitary-adrenal axis, Inflammation, Major depressive disorder, Glucocorticoid resistance, Cortisol, Glucocorticoid receptor
Introduction
Increased inflammation and hypothalamic-pituitary-adrenal (HPA) axis alterations have been consistently associated with depression, or at least with a subgroup of individuals with depression [34]. It is widely known that the HPA axis is strictly implicated in inflammation. Its activation triggers the release of glucocorticoids, mainly cortisol in humans, which plays a crucial role in anti-inflammatory and immunosuppressive processes [3]. However, the clear relationships between the HPA axis and inflammation and depression and with one another have yet to be fully elucidated.
Initial findings regarding the neuroendocrine and biological correlates of major depressive disorder (MDD) consistently identified HPA axis hyperactivity and subsequent increased cortisol levels, which have also been observed in patients with treatment-resistant depression that is associated with greater severity. As a result, the ‘glucocorticoid resistance’ theory emerged in the late 90s, which proposes that the glucocorticoid receptor (GR) is less sensitive to cortisol and does not bind as effectively; thus, the regulation of the HPA axis through negative feedback inhibition becomes impaired, resulting in continued activation and production of the axis components [36,35,1]. This diminished sensitivity of the GR is considered to be due to reduced GR function and expression that has been reported in depression by a large number of experimental, biological and molecular studies.
During the last 10 years, evidence started to accumulate not only from animal studies showing that repeat social defeat (a form of chronic stress) can induce glucocorticoid-resistant monocytes, enhanced neuroinflammatory signalling and depressive-like behaviours in animal models [45] but also from human studies that noted the coexistence of reduced GR function/expression or HPA axis hyperactivity and elevated inflammation in depressed patients [34]. Therefore, scientists naturally inferred that this single molecular mechanism of glucocorticoid resistance is the common factor that is related to both HPA axis function and immune activation. That is to say, immune cells, which express GR [22], become less sensitive to cortisol’s ‘physiological anti-inflammatory action that may lead to the escape and hyperactivity of monocytes (and other immune cells), thus explaining the resulting increased inflammation that is observed. But despite all of this, the evidence is conflicting, as studies have reported findings that are in disagreement with the current or simplest version of the glucocorticoid resistance model.
Inflammation, HPA axis and glucocorticoids in MDD
Results from recent meta-analyses [32,31] and case–control studies [6,19,4] have confirmed the presence of increased inflammation in depression, particularly in treatment-resistant patients, through elevated levels of inflammatory markers like the C-reactive protein and higher immune cell counts compared with healthy controls, even in treatment-responsive patients. Most importantly, the largest study to have corroborated this finding utilised data on approximately 27,000 people with MDD from the UK Biobank and suggested the existence of a core biological association between depression and increased inflammation since this association remains significant after adjusting for clinical and psychosocial confounding factors [39]. Several human and animal studies have shown that this association is most likely due to stress system activation, leading to the release of pro-inflammatory cytokines and activated immune cells like monocytes, which occur along with HPA axis hyperactivity and increased cortisol levels [40,23,41]. Moreover, associations were found between specific inflammatory markers and different MDD symptoms [15,12,11], and individuals exposed to interferon-α, a pro-inflammatory trigger, displayed depressive-like symptoms [28,42]. Hence, clinical trials have investigated the putative therapeutic benefits of anti-inflammatory treatment in inflamed MDD/depressed patients with promising results [27,22].
However, in the 2000s, evidence opposing the glucocorticoid resistance model began to surface. For example, Munhoz and colleagues (2006) reported that, contrary to the anti-inflammatory effects of glucocorticoids via NF-κB inhibition, rats exposed to chronic unpredictable stress had elevated glucocorticoid levels, which resulted in increased NF-κB activation and pro-inflammatory gene expression induced by exposure to acute stress. Furthermore, pre-treatment with a GR antagonist weakened this effect, thus suggesting the putative immune potentiating properties of glucocorticoids. This pro-inflammatory action has also been observed in work conducted in our lab in human hippocampal progenitor cells that were treated with glucocorticoids prior to an inflammatory stimulus [13].
Moreover, investigating the biological outcomes of different types of stress in male mice failed to yield a consistent finding of HPA axis hyperactivity and increased inflammation, as researchers in our lab found that physical stress leads to hypercortisolaemia and reduced pro-inflammatory cytokine levels, while psychosocial stress leads to hypocortisolaemia and elevated pro-inflammatory cytokine levels [9]. Furthermore, again in our lab, Perrin and colleagues (2019) failed to identify a strong positive correlation between glucocorticoid resistance and inflammation in their meta-analysis of studies examining both cytokine levels in depressed patients and measures of glucocorticoid resistance, including plasma cortisol levels, dexamethasone (synthetic glucocorticoid) suppression test, GR expression and in vitro assays of GR function. Similarly [4], did not report a clear correlation between serum CRP inflammatory marker levels and glucocorticoid-related gene expression.
Therefore, this recent information has led the scientific community to postulate that the inflammation seen in depression may not solely be a consequence of glucocorticoid resistance and reduced GR signal but rather could be caused by the potential pro-inflammatory action of cortisol, whose levels are aberrantly increased due to HPA axis hyperactivity.
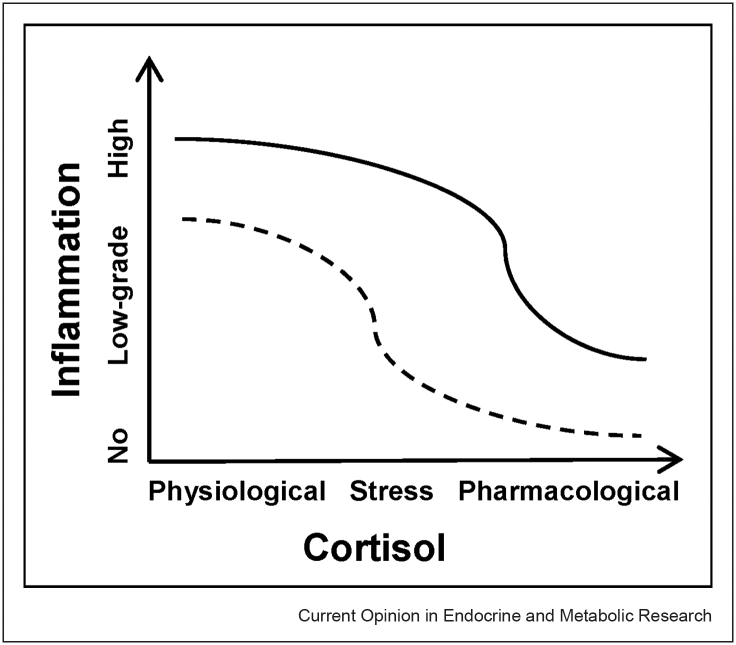
The two models
The ‘glucocorticoid resistance’ model was first proposed in the 1990s in the context of inflammatory diseases like asthma and inflammatory bowel disorders [16] and later became an established finding in psychiatry in the 2000s [41,36]. This phenomenon is the current consensus theory for HPA axis hyperactivity and the accompanied increased inflammation that are observed in depression. On the basis of this model, the physiological anti-inflammatory action of cortisol in humans increases as its concentration rises, which can range from physiological levels (during the day) to stress levels (those seen in depressed patients or induced experimentally) and to pharmacological levels (that is achieved with a large hydrocortisone or comparable synthetic glucocorticoid administration). A visual representation of this model is depicted in Figure 1 where despite the higher cortisol levels in depressed patients (straight line) compared to healthy controls (dotted line), their immune cells are resistant to cortisols aforementioned anti-inflammatory action; thus, inflammation is less inhibited in these individuals at different concentrations of cortisol than controls, and hence, resulting in the increased inflammation that is seen in depression. The scientific literature investigating this resistance to cortisol points to abnormalities involving the GR, which under normal conditions has a low affinity for cortisol, and therefore, requires high glucocorticoid concentrations to be fully activated [1]. Reviews and primary research have reported reduced function and/or expression of the GR in the immune cells of depressed patients, particularly those that are inflamed [36,1,35,20,4,5]. This is considered to be influenced by gene–environment interactions at FKBP5, a negative regulator of GR function, whereby early life adversity (even in utero) and FKBP5 risk alleles can lead to or exacerbate epigenetic alterations in this gene, thus increasing MDD risk [24,18,21] and potentially promoting NF-κB-driven peripheral inflammation [49]. Other evidence supporting this model includes the reports of glucocorticoid resistance, inflammation and depressive-like behaviours in the aforementioned repeated social defeat animal models of depression [45] and a study showing that administration of dexamethasone (a synthetic glucocorticoid and GR agonist) leads to reduced GR target gene expression in patients with depressive disorders and mouse models [2].
Figure 1.
The glucocorticoid resistance model.
However, despite the existing findings in favour of this model, the previously mentioned meta-analysis only reported modest support for the association between glucocorticoid resistance and cytokine-mediated inflammation in depressed patients compared to controls [38]. In addition, the authors noted not only the limited number of included articles and study subjects for which information on both glucocorticoid resistance and inflammation was collected but also that most individual studies had utilised only a single method to quantify glucocorticoid resistance.
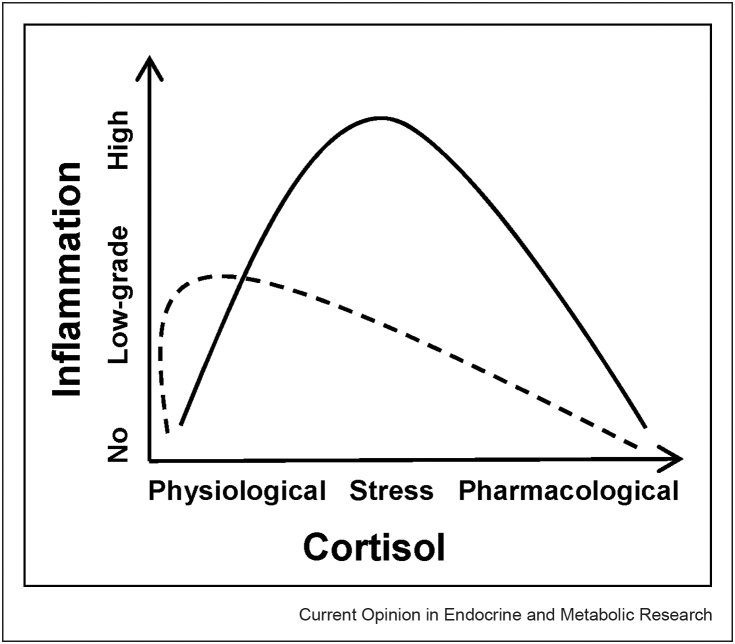
The accumulation of more recent evidence against the current model has resulted in a conceptual shift, providing an alternative explanation to the notions outlined in the first review published on the relevance of glucocorticoid resistance in depression [36]. This newer ‘pro-inflammatory cortisol’ model that has emerged proposes the idea that glucocorticoids may possess pro-inflammatory properties during stress, and so the high cortisol levels, typically observed in depressed patients, may be the cause of the elevated inflammation in MDD, instead of just a consequence of glucocorticoid resistance. This phenomenon is supported by findings from animal research demonstrating that increased concentrations of corticosterone (the primary glucocorticoid in rodents) induced by chronic unpredictable stress or achieved through glucocorticoid administration/manipulation resulted in enhanced inflammation in response to acute stress [25,26]. Furthermore [30], it is found that administration of metyrapone (a glucocorticoid synthesis inhibitor) and surgical removal of the adrenal glands (where glucocorticoids are synthesised) to inhibit corticosterone production, led to the prevention of neuroinflammatory signalling and inflammatory monocyte release into circulation when exposed to repeated social defeat stress, indicating that corticosterone increases inflammation in this model. In healthy human subjects, exposure to stress-associated concentrations of cortisol for 6 h via hydrocortisone (intravenous cortisol) administration elicited a pro-inflammatory response, including a significant increase in IL-6 cytokine levels, following an inflammatory stimulus [47,48]. Similar findings were reported by Ref. [13] in our lab, who investigated the effect of dexamethasone or cortisol treatment in human hippocampal progenitor cells prior to an inflammatory stimulus, which resulted in the increased expression of several innate immune genes. Moreover, these studies interestingly observed that this pro-inflammatory effect was maximal at intermediate (stress-relevant) cortisol levels but not at high (pharmacological) or low (physiological) concentrations, and when there was a delay or rest period of 24 h before the inflammatory stimulus. This model has not yet been tested in human patients with depression. As displayed in Figure 2, this alternative model postulates that cortisol possesses a pro-inflammatory action, and so the elevated stress levels of this hormone that are found in depressed patients (straight line) compared to healthy controls (dotted line) are in fact the reason for the increased inflammation that is present in these individuals, and not just merely resulting from glucocorticoid resistance.
Figure 2.
The pro-inflammatory cortisol model.
Epigenetics and cell/tissue type
The role of epigenetic mechanisms has been well investigated in relation to the ‘glucocorticoid resistance’ model, with several studies reporting the link between early life adversity/trauma, depression or stress-related conditions and the hypomethylation of the aforementioned FKBP5 gene or hypermethylation of the NR3C1 (GR) gene promoter, which leads to decreased GR activity or decreased GR mRNA and protein expression [24,10,43]. This reduced FKBP5 methylation was also associated with promoting inflammation [33,49], although more studies including this measure are required, as well as further research to clarify inconsistent findings [18,10]. Moreover, animal and cell culture studies found that histone deacetylation correlated with reduced transcription factor binding to the GR promoter and depressive behaviours [10], in addition to miRNA overexpression resulting in GR downregulation [14,44].
The contribution of epigenetics within the context of the ‘pro-inflammatory cortisol’ model has not yet been explored. However, it is known that the GR interacts with coactivators that possess histone acetyltransferase activity to regulate immune gene expression, and the suggested mechanisms for the immune-potentiating effects of glucocorticoids involve inducing the expression of genes with a pro-inflammatory function like TLR2, partly through GR interaction with NF-κB response elements [17,8,46].
It is also considered that glucocorticoids can exert contrasting actions depending on the cell or tissue type, with pro-inflammatory effects reportedly demonstrated in dendritic cells or the brain and anti-inflammatory effects in peripheral immune cells like neutrophils [17,46]. Although, both of these effects have been observed within the same cell type [7,46].
Future directions
In spite of this rationale, it is important to note that the interplay between the endocrine and immune systems is complex and paradoxical [38] since cortisol is not only implicated in increasing inflammation, but it can also bind the GR and repress the expression of genes encoding pro-inflammatory cytokines [1]. Moreover, this relationship is bidirectional as pro-inflammatory cytokines themselves can inhibit GR function [37] through activation of mitogen-activated protein kinases like p38 [35] and JNK (Zhang et al., 2020); thus, in turn, contributing to glucocorticoid resistance resulting in a feed-forward inflammatory cascade [41].
A potential explanation of cortisols biphasic effects on the immune system (that seem to be not only time- and dose-dependent but also reliant on the GR) may be that stress concentrations of glucocorticoids prime the innate immune system resulting in the existence of a certain level of intrinsic inflammation that leads to the exacerbating consequences stated above that are seen in depression [38,13,48]. Therefore, it can be speculated that the proposed models may occur simultaneously.
However, also considering the limitations of previous clinical studies, including small sample sizes, there is still a need for additional large mechanistic studies that specifically investigate cortisols GR-mediated effects on cytokine production in human depression subjects through utilising more comprehensive measures in order to disentangle this relationship.
Conflict of interest statement
Nothing declared.
Acknowledgments and disclosures
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Research funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre (BRC), London, United Kingdom. This work was also supported by the Wellcome Trust strategy award to the Neuroimmunology of Mood Disorders and Alzheimer's Disease (NIMA) Consortium (104025), which is also funded by Janssen, GlaxoSmithKline, Lundbeck and Pfizer.
Dr. Sforzini and Prof. Pariante have received research funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 853966–2, as part of the EU-PEARL project. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. Prof. Pariante has also received research funding from Johnson & Johnson as part of a program of research on depression and inflammation and speakers and consultation fees from Lundbeck and Boehringer Ingelheim.
This review comes from a themed issue on Neuroendocrine Response to Stress
Edited by Megan Holmes and Ferenc Antoni
References
- 1.Anacker C., Zunszain P.A., Carvalho L.A., Pariante C.M. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arloth J., Bogdan R., Weber P., Frishman G., Menke A., Wagner K.V., Balsevich G., Schmidt M.V., Karbalai N., Czamara D., Altmann A., Trümbach D., Wurst W., Mehta D., Uhr M., Klengel T., Erhardt A., Carey C.E., Conley E.D., Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (PGC) … Genetic differences in the immediate transcriptome response to stress predict risk-related brain function and psychiatric disorders. Neuron. 2015;86:1189–1202. doi: 10.1016/j.neuron.2015.05.034. Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium PGC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellavance M.A., Rivest S. The HPA - immune Axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Ferrari C., Turner L., Mariani N., Enache D., Hastings C., Kose M., Lombardo G., McLaughlin A.P., Nettis M.A., Nikkheslat N., Sforzini L., Worrell C., Zajkowska Z., Cattane N., Lopizzo N., Mazzelli M., Pointon L., Cowen P.J., Cavanagh J.…Pariante C.M. Whole-blood expression of inflammasome- and glucocorticoid-related mRNAs correctly separates treatment-resistant depressed patients from drug-free and responsive patients in the BIODEP study. Transl Psychiatry. 2020;10:232. doi: 10.1038/s41398-020-00874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Presenting evidence against the ‘glucocorticoid resistance’ model as no clear correlations were identified between inflammatory biomarkers and GR expression mRNA in the whole blood of patients with MDD, but six inflammasome- and glucocorticoid-related mRNAs distinguished treatment-resistant from treatment-responsive individuals
- 5.Cattaneo A., Gennarelli M., Uher R., Breen G., Farmer A., Aitchison K.J., Craig I.W., Anacker C., Zunsztain P.A., McGuffin P., Pariante C.M. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology : Off Publ Am Coll Neuropsychopharmacol. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain S.R., Cavanagh J., de Boer P., Mondelli V., Jones D., Drevets W.C., Cowen P.J., Harrison N.A., Pointon L., Pariante C.M., Bullmore E.T. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatr : J Ment Sci. 2019;214:11–19. doi: 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Topete D., Cidlowski J.A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22:20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desmet S.J., De Bosscher K. Glucocorticoid receptors: finding the middle ground. J Clin Invest. 2017;127:1136–1145. doi: 10.1172/JCI88886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Preez A., Law T., Onorato D., Lim Y.M., Eiben P., Musaelyan K., Egeland M., Hye A., Zunszain P.A., Thuret S., Pariante C.M., Fernandes C. The type of stress matters: repeated injection and permanent social isolation stress in male mice have a differential effect on anxiety- and depressive-like behaviours, and associated biological alterations. Transl Psychiatry. 2020;10:325. doi: 10.1038/s41398-020-01000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Some novel insight into the neuroendocrine changes that can take place when exposed to different types of stress, but no results indicating both increased corticosterone reactivity and increased systemic inflammation in response to stress were reported, which is not in line with the ‘glucocorticoid resistance’ model
- 10.Farrell C., O'Keane V. Epigenetics and the glucocorticoid receptor: a review of the implications in depression. Psychiatr Res. 2016;242:349–356. doi: 10.1016/j.psychres.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Felger J.C., Haroon E., Patel T.A., Goldsmith D.R., Wommack E.C., Woolwine B.J., Le N.A., Feinberg R., Tansey M.G., Miller A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatr. 2020;25:1301–1311. doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried E.I., von Stockert S., Haslbeck J., Lamers F., Schoevers R.A., Penninx B. Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychol Med. 2020;50:2682–2690. doi: 10.1017/S0033291719002770. [DOI] [PubMed] [Google Scholar]
- Horowitz M.A., Cattaneo A., Cattane N., Lopizzo N., Tojo L., Bakunina N., Musaelyan K., Borsini A., Zunszain P.A., Pariante C.M. Glucocorticoids prime the inflammatory response of human hippocampal cells through up-regulation of inflammatory pathways. Brain Behav Immun. 2020;87:777–794. doi: 10.1016/j.bbi.2020.03.012. [DOI] [PubMed] [Google Scholar]; Further evidence of cortisol’s pro-inflammatory effect was demonstrated to rely on time, concentration and the GR in the human hippocampal progenitor cell model with significant relevance to depression
- 14.Jung S.H., Wang Y., Kim T., Tarr A., Reader B., Powell N., Sheridan J.F. Molecular mechanisms of repeated social defeat-induced glucocorticoid resistance: role of microRNA. Brain Behav Immun. 2015;44:195–206. doi: 10.1016/j.bbi.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappelmann N., Czamara D., Rost N., Moser S., Schmoll V., Trastulla L., Stochl J., Lucae S., CHARGE inflammation working group. Binder E.B., Khandaker G.M., Arloth J. Polygenic risk for immuno-metabolic markers and specific depressive symptoms: a multi-sample network analysis study. Brain Behav Immun. 2021;95:256–268. doi: 10.1016/j.bbi.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Lamberts S.W. The glucocorticoid insensitivity syndrome. Horm Res. 1996;45(Suppl 1):2–4. doi: 10.1159/000184815. [DOI] [PubMed] [Google Scholar]
- 17.Liberman A.C., Budziñski M.L., Sokn C., Gobbini R.P., Steininger A., Arzt E. Regulatory and mechanistic actions of glucocorticoids on T and inflammatory cells. Front Endocrinol. 2018;9:235. doi: 10.3389/fendo.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin E., Tsai S.J. Epigenetics and depression: an update. Psychiatr Invest. 2019;16:654–661. doi: 10.30773/pi.2019.07.17.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynall M.E., Turner L., Bhatti J., Cavanagh J., de Boer P., Mondelli V., Jones D., Drevets W.C., Cowen P., Harrison N.A., Pariante C.M., Pointon L., Clatworthy M.R., Bullmore E., Neuroimmunology of Mood Disorders and Alzheimer’s Disease (NIMA) Consortium Peripheral blood cell-stratified subgroups of inflamed depression. Biol Psychiatr. 2020;88:185–196. doi: 10.1016/j.biopsych.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Mariani N., Cattane N., Pariante C., Cattaneo A. Gene expression studies in Depression development and treatment: an overview of the underlying molecular mechanisms and biological processes to identify biomarkers. Transl Psychiatry. 2021;11:354. doi: 10.1038/s41398-021-01469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matosin N., Halldorsdottir T., Binder E.B. Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: the FKBP5 model. Biol Psychiatr. 2018;83:821–830. doi: 10.1016/j.biopsych.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Ménard C., Pfau M.L., Hodes G.E., Russo S.J. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology : Off Publ Am Coll Neuropsychopharmacol. 2017;42:62–80. doi: 10.1038/npp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mourtzi N., Sertedaki A., Charmandari E. Glucocorticoid signaling and epigenetic alterations in stress-related disorders. Int J Mol Sci. 2021;22:5964. doi: 10.3390/ijms22115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munhoz C.D., Lepsch L.B., Kawamoto E.M., Malta M.B., Lima L., Avellar M.C., Sapolsky R.M., Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci : Official J Soc Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munhoz C.D., Sorrells S.F., Caso J.R., Scavone C., Sapolsky R.M. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci : Official J Soc Neurosci. 2010;30:13690–13698. doi: 10.1523/JNEUROSCI.0303-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nettis M.A., Lombardo G., Hastings C., Zajkowska Z., Mariani N., Nikkheslat N., Worrell C., Enache D., McLaughlin A., Kose M., Sforzini L., Bogdanova A., Cleare A., Young A.H., Pariante C.M., Mondelli V. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology : Off Publ Am Coll Neuropsychopharmacol. 2021;46:939–948. doi: 10.1038/s41386-020-00948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nettis M.A., Veronese M., Nikkheslat N., Mariani N., Lombardo G., Sforzini L., Enache D., Harrison N.A., Turkheimer F.E., Mondelli V., Pariante C.M. PET imaging shows no changes in TSPO brain density after IFN-α immune challenge in healthy human volunteers. Transl Psychiatry. 2020;10:89. doi: 10.1038/s41398-020-0768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niraula A., Wang Y., Godbout J.P., Sheridan J.F. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J Neurosci : Off J Soc Neurosci. 2018;38:2328–2340. doi: 10.1523/JNEUROSCI.2568-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Pillinger T., Rodriguez I.M., Khandaker G.M., Pariante C.M., Howes O.D. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020;87:901–909. doi: 10.1016/j.bbi.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Largest meta-analysis to demonstrate reduced or unchanged variability in inflammatory markers in patients with depression compared to healthy controls, as well as increased mean levels of pro-inflammatory cytokines in depression
- 33.Palma-Gudiel H., Prather A.A., Lin J., Oxendine J.D., Guintivano J., Xia K., Rubinow D.R., Wolkowitz O., Epel E.S., Zannas A.S. HPA axis regulation and epigenetic programming of immune-related genes in chronically stressed and non-stressed mid-life women. Brain Behav Immun. 2021;92:49–56. doi: 10.1016/j.bbi.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pariante C.M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur Neuropsychopharmacol : J Eur Coll Neuropsychopharmacol. 2017;27:554–559. doi: 10.1016/j.euroneuro.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Pariante C.M., Miller A.H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatr. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 37.Pariante C.M., Pearce B.D., Pisell T.L., Sanchez C.I., Po C., Su C., Miller A.H. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- 38.Perrin A.J., Horowitz M.A., Roelofs J., Zunszain P.A., Pariante C.M. Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Front Psychiatr. 2019;10:423. doi: 10.3389/fpsyt.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitharouli M.C., Hagenaars S.P., Glanville K.P., Coleman J., Hotopf M., Lewis C.M., Pariante C.M. Elevated C-reactive protein in patients with depression, independent of genetic, health, and psychosocial factors: results from the UK Biobank. Am J Psychiatr. 2021;178:522–529. doi: 10.1176/appi.ajp.2020.20060947. [DOI] [PubMed] [Google Scholar]; Largest study to show that increased inflammation is a core biological feature of depression, and this association exists independently from psychosocial and clinical confounding factors
- 40.Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raison C.L., Miller A.H. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatr. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 42.Russell A., Hepgul N., Nikkheslat N., Borsini A., Zajkowska Z., Moll N., Forton D., Agarwal K., Chalder T., Mondelli V., Hotopf M., Cleare A., Murphy G., Foster G., Wong T., Schütze G.A., Schwarz M.J., Harrison N., Zunszain P.A., Pariante C.M. Persistent fatigue induced by interferon-alpha: a novel, inflammation-based, proxy model of chronic fatigue syndrome. Psychoneuroendocrinology. 2019;100:276–285. doi: 10.1016/j.psyneuen.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spies L.L., Verhoog N., Louw A. Acquired glucocorticoid resistance due to homologous glucocorticoid receptor downregulation: a modern look at an age-old problem. Cells. 2021;10:2529. doi: 10.3390/cells10102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vreugdenhil E., Verissimo C.S., Mariman R., Kamphorst J.T., Barbosa J.S., Zweers T., Champagne D.L., Schouten T., Meijer O.C., de Kloet E.R., Fitzsimons C.P. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–2228. doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- 45.Weber M.D., Godbout J.P., Sheridan J.F. Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology : Off Publ Am Coll Neuropsychopharmacol. 2017;42:46–61. doi: 10.1038/npp.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xavier A.M., Anunciato A.K., Rosenstock T.R., Glezer I. Gene expression control by glucocorticoid receptors during innate immune responses. Front Endocrinol. 2016;7:31. doi: 10.3389/fendo.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeager M.P., Pioli P.A., Collins J., Barr F., Metzler S., Sites B.D., Guyre P.M. Glucocorticoids enhance the in vivo migratory response of human monocytes. Brain Behav Immun. 2016;54:86–94. doi: 10.1016/j.bbi.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeager M.P., Pioli P.A., Guyre P.M. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose-response : Publ Int Hormesis Soc. 2011;9:332–347. doi: 10.2203/dose-response.10-013.Yeager. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zannas A.S., Jia M., Hafner K., Baumert J., Wiechmann T., Pape J.C., Arloth J., Ködel M., Martinelli S., Roitman M., Röh S., Haehle A., Emeny R.T., Iurato S., Carrillo-Roa T., Lahti J., Räikkönen K., Eriksson J.G., Drake A.J., Waldenberger M.…Binder E.B. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB-driven inflammation and cardiovascular risk. Proc Natl Acad Sci U S A. 2019;116:11370–11379. doi: 10.1073/pnas.1816847116. [DOI] [PMC free article] [PubMed] [Google Scholar]