Abstract
Background
The tryptophan-kynurenine (KYN) pathway is linked to obesity-related systemic inflammation and metabolic health. The pathway generates multiple metabolites, with little available data on their relationships to early markers of increased metabolic disease risk in children. The aim of this study was to examine the association of multiple KYN pathway metabolites with metabolic risk markers in pre-pubertal Asian children.
Methods
Fasting plasma concentrations of KYN pathway metabolites were measured using liquid chromatography-tandem mass spectrometry in 8-year-old children (n=552) from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) prospective mother-offspring cohort study. Child’s weight and height were used to ascertain overweight and obesity using local body mass index (BMI)-for-age percentile charts. Body fat percentage was measured by quantitative magnetic resonance. Abdominal circumference, systolic and diastolic blood pressure, homeostatic model assessment for insulin resistance (HOMA-IR), triglyceride and HDL-cholesterol were used for the calculation of Metabolic syndrome scores (MetS). Serum triglyceride, BMI, gamma-glutamyltransferase (GGT), and abdominal circumference were used in the calculation of the Fatty liver index (FLI). Associations were examined using multivariable regression analyses.
Results
In overweight or obese children (n=93; 16.9% of the cohort), all KYN pathway metabolites were significantly increased, relative to normal weight children. KYN, kynurenic acid (KA), xanthurenic acid (XA), hydroxyanthranilic acid (HAA) and quinolinic acid (QA) all showed significant positive associations with body fat percentage (B(95% CI) = 0.32(0.22,0.42) for QA), HOMA-IR (B(95% CI) = 0.25(0.16,0.34) for QA), and systolic blood pressure (B(95% CI) = 0.14(0.06,0.22) for QA). All KYN metabolites except 3-hydroxykynurenine (HK) significantly correlated with MetS (B(95% CI) = 0.29(0.21,0.37) for QA), and FLI (B(95% CI) = 0.30(0.21,0.39) for QA).
Conclusions
Higher plasma concentrations of KYN pathway metabolites are associated with obesity and with increased risk for metabolic syndrome and fatty liver in pre-pubertal Asian children.
Keywords: GUSTO, obesity, metabolic risk, fatty liver index, kynurenine pathway, children
Introduction
Overweight and obesity in childhood is a major public health concern (1). Over the past four decades, the prevalence of children with obesity has risen more than 10-fold (2). In Singapore, the current estimates identify 12.7-15.9% of 6-12 year-old children being overweight or obese (3). Overweight children exhibit elevated prevalence of cardiovascular risk factors, such as elevated blood pressure, heightened inflammatory biomarkers and dyslipidaemia (2, 4). For these children, there is an increased probability of early and more severe chronic adult cardiovascular disease and type 2 diabetes (5, 6). Therefore, a major challenge in the prevention and mitigation of adulthood risk is the early identification of critical mechanisms of disease risk.
In studies during adulthood, the clustering of interconnected factors that increase the risk of cardiovascular complications in metabolic syndrome suggests systemic alterations in biochemical pathways involved in diverse biological actions. One key pathway is the kynurenine (KYN) pathway downstream from tryptophan (TRP) which participates in inter-organ communication and has been proposed as a metabolite pathway linking obesity, cardiovascular risk factors, inflammation and insulin resistance (7, 8). The KYN pathway is the major pathway (95%) of TRP metabolism and generates multiple metabolites (Figure 1), present across multiple tissues and in the circulation with diverse roles including inflammation, the immune response, metabolism, and neurotransmission (7, 9). The pathway commences with TRP conversion to KYN, catalysed by the rate-limiting enzymes indoleamine-2,3-dioxygenase-1 (IDO1) and in the liver by tryptophan-2,3-dioxygenase (TDO) (7, 9, 10). Increased KYN/TRP ratio, reflective of heightened IDO1 activity, has been reported in obese individuals (11–13), as well as in individuals with insulin resistance (14), type 2 diabetes (15) and coronary heart disease (16, 17). In younger people, an increased KYN/TRP ratio is associated with the presence of increased metabolic disease risk factors, including obesity (11) and insulin resistance (18).
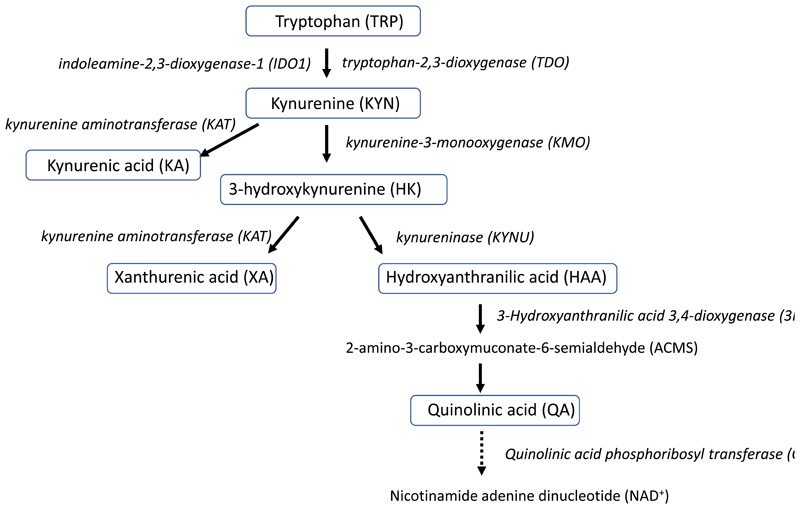
Figure 1. Simplified schematic of the KYN pathway.
The plasma KYN metabolites in this study are indicated by blue boxes. TRP is converted to KYN by indoleamine-2,3-dioxygenase-1 (IDO1) and in the liver by tryptophan-2,3-dioxygenase (TDO). KYN is then converted to HK by kynurenine-3-monooxygenase (KMO) or to KA by kynurenine aminotransferase (KAT). HK is converted to either HAA by kynureninase (KYNU) or to XA by kynurenine aminotransferase (KAT). HAA is converted to 2-amino-3-carboxymuconate-6-semialdehyde (ACMS) by 3-hydroxyanthranilic acid 3,4-dioxygenase (3HAO), which is then non-enzymatically converted to QA.
The KYN pathway extends to further downstream metabolites, which also demonstrate positive associations with metabolic disease; kynurenic acid (KA), 3-hydroxykynurenine (HK), 3-hydroxyanthranilic acid (HAA) and xanthurenic acid (XA) have been shown to be either individually or collectively elevated in obesity (12, 19), insulin resistance and type 2 diabetes (14, 20, 21) and cardiovascular disease risk (16, 17, 22). These associations are consistent with the demonstration of increased kynurenine monooxygenase (KMO) activity, which catalyses the conversion of KYN to HK (12). However, despite these metabolites representing the major pathway of TRP metabolism, there remains limited knowledge of the mechanisms of action, including the key regulatory tissues (7–9, 23), with few studies addressing relationships to metabolic disease risks in children.
There is consistent evidence demonstrating positive relationships between circulating concentrations of TRP, KYN and KYN/TRP ratio and aspects of metabolic risk. However the limited analyses in children have been in small cohorts (sample size <50) (18, 24), across an age range spanning pre- to post-puberty (11) and in a single ethnicity (25). There is also a lack of data in children examining relationships for downstream KYN metabolites, including HAA, XA and QA, for which there are emerging roles as contributors to insulin resistance and metabolic syndrome (20, 21, 23). Therefore, the aim of this study was to comprehensively examine relations of both individual metabolic parameters and composite risk scores of metabolic health, including fatty liver index, in a 8-year-old multi-ethnic Asian child cohort with circulatory concentrations of the KYN pathway metabolites (26), as well as the ratios of these metabolites which may indicate enzyme activities in the pathway.
Methods
Study Population
Children were from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study, an Asian prospective mother-offspring cohort in Singapore (27). This birth cohort is a prospective observational study, which was registered on 1 July 2010 under the identifier NCT01174875. From June 2009 to September 2010, 1247 pregnant women were recruited at 11–14 weeks gestation from the two public maternity hospitals in Singapore, KK Women’s and Children’s Hospital (KKH) and National University Hospital (NUH). A total of 940 singleton children remained in the study at age 8 years, and 808 of these children attended the year 8 visit. This study included singleton children (n= 552) where KYN metabolite data was quantified at age 8 years. All participants provided assent and their parents provided informed written consent. The study was conducted according to the guidelines laid down in the Declaration of Helsinki. Ethical approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group and the Centralised Institutional Review Board of SingHealth.
Measurements
At age 8 years, weight (SECA803 weighing scale, Netherlands) and standing height were measured (SECA213 stadiometer, Netherlands). The body mass index (BMI)-for-age Z-scores of the children were determined using local BMI-for-age percentile charts for boys and girls (https://www.healthhub.sg/live-healthy/745/differencesbetweenchildandadultbmi, last accessed 10 August 2021). Children with BMI-for-age < -1 Z-scores were classified as underweight, while children with BMI-for-age > +1 Z-scores were classified as overweight and children with BMI-for-age > +2 Z-scores were classified as obese, as recommended by the World Health Organization (WHO) (28). Abdominal circumference was measured at the level of the iliac crest using an inelastic measuring tape. Peripheral systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured from the right upper arm while the child was in a sitting position (Dinamap CARESCAPE V100, GE Healthcare, Milwaukee, WI). All anthropometric measures were performed in duplicates and the average of the 2 measurements was used.
Whole body fat mass was determined by Quantitative Magnetic Resonance (QMR) (EchoMRI-Adolescent Humans Body Composition Analyzer, EchoMRI Corporation, Singapore) with a low magnetic field (0.007 Tesla), as previously described (29). Body fat percentage was calculated by dividing the total body fat in kg by the total body weight in kg multiplied by 100.
Laboratory analyses
Fasting blood samples were collected from the children at 8 years of age from a peripheral vein after an overnight fast of 8-10 hours. Glucose was measured in sodium fluoride plasma in the clinical laboratory using the hexokinase enzymatic method (Architect c8000 analyzer, Abbott and Beckman AU5800 analyzer, Beckman Coulter). Serum and EDTA plasma were obtained by centrifugation at 1600 g at 4°C for 10 minutes and stored at -80°C for later analyses. Insulin was measured in serum at the NUH clinical laboratory, accredited by the College of American Pathologists (30), using a sandwich immunoassay (Beckman DXL800 analyzer, Beckman Coulter). The homeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) was calculated by multiplying fasting insulin in mU/L by fasting glucose in mmol/mL and dividing by 22.5 (31).
Serum triglyceride, total cholesterol, HDL-cholesterol, gamma glutamyl transferase (GGT) and high sensitivity C-reactive protein (hsCRP) were measured in the NUH clinical laboratory using enzymatic colorimetric methods (Beckman AU5800 analyzer, Beckman Coulter). LDL-cholesterol was calculated using the Friedewald equation (32). As the distribution of hsCRP was skewed even after log-transformation, the following cut-offs were arbitrarily defined to categorize hsCRP levels in the children: (i) hsCRP below the detection limit <0.1 mg/L (n=98), (ii) hsCRP from 0.1 to 0.3 mg/L (n=211), (iii) hsCRP from 0.4 to 1.0 mg/L (n=130), and (iv) hsCRP >1.0 mg/L (n=93).
TRP, KYN, KA, HK, XA, HAA and QA were measured in fasting EDTA plasma by a targeted method based on liquid chromatography-tandem mass spectrometry (BEVITAL AS; www.bevital.no) as described previously (26). We calculated the KYN/TRP ratio*100, HK/KYN ratio, XA/HK ratio*100 and the HAA/HK ratio*100 as proxies for IDO1, KMO, kynurenine aminotransferase (KAT) and kynureninase (KYNU) activities, respectively (19). The between batch coefficients of variation (CV) of quality control samples between assays were 2.1% to 4.9%.
Calculation of the metabolic syndrome score (MetS) and fatty liver index (FLI)
We also examined these associations with the metabolic syndrome score (MetS), that combines the sex-specific risk factors of increased abdominal circumference, systolic and diastolic blood pressure, homeostasis model assessment of insulin resistance (HOMA-IR), serum triglyceride and decreased HDL-cholesterol (33). The MetS has been proposed as a tool for evaluating the need for intervention in clinical practice (33). Following a previously published study in children (33), MetS was calculated by adding the sex-standardized components: z_abdominal circumference + (z_SBP + z_DBP)/2 + z_HOMA-IR + (z_triglyceride + z_HDL-cholesterol)/2. Children with MetS ≥ 90th percentile were considered has having the metabolic syndrome (33).
Non-alcoholic fatty liver disease (NAFLD), characterized by fat accumulation in the liver, is the hepatic manifestation of metabolic syndrome, and is becoming a major health concern with an increasing prevalence in children and adolescents (34, 35). The prevalence increases with BMI, and NAFLD has been reported in 38% of obese adolescents (2). We further studied the relationship between KYN pathway metabolites and fatty liver index (FLI), an algorithm for prediction of fatty liver in the general population developed to identify individuals for intervention in clinical practice (36) based on the predictors BMI, abdominal circumference, serum triglycerides and gamma glutamyl transferase (GGT), which has been—used in children (37). FLI was calculated using published equation as (e0.953*loge(triglyceride) + 0.139*BMI + 0.718*loge(GGT) +0.053(abdominal circumference) – 15.745)/(1+ e0.953*loge(triglyceride) + 0.139*BMI + 0.718*loge(GGT) +0.053(abdominal circumference) – 15.745) *100 (36). A score of <30 was considered to rule out NAFLD (36).
Statistical analyses
Statistical analyses were performed using SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY). Independent samples t-test were used to compare mean values of normally distributed variables between girls and boys. Analysis of variance (ANOVA) (post-hoc test with Bonferroni correction) was used to compare mean values of normally distributed variables between Chinese, Malay and Indian ethnic groups. For variables that were not normally distributed, e.g. insulin and HOMA-IR, the variables were Log10 transformed before statistical comparison. Logistic regression analysis was used to compare mean values of normally distributed variables by increasing weight categories.
Multivariable regression analyses were performed with the KYN metabolites as the main exposures and metabolic health risk components or metabolic syndrome scores or fatty liver index as outcomes of interest. Both exposures and outcome variables were transformed into standardized scores so that the strengths of associations were comparable in the regression models. All regression models were adjusted for sex and ethnicity. Multivariable logistic regression analysis, with adjustment for sex and ethnicity, was used to determine the association between the standardized scores of the KYN metabolites and hsCRP categories. p-values <0.05 were considered significant.
Results
Characteristics of the study cohort
The characteristics of the study cohort is shown in Table 1. The mean age was 8 years ± 4.1 months, range 7.7-8.5 years. Among the 552 children, 51% were boys and 49% were girls. Most of the children were of Chinese ethnicity (55%), with a minority of Malay (27%) and Indian (18%) ethnicity. The mean BMI of the children was 16.5 kg/m2 (mean (SD) 16.7 (3.1) kg/m2 for boys and 16.4 (3.1) kg/m2 for girls). Indian children had higher BMI (17.0 (3.5) kg/m2 than Chinese (16.3 (2.7) kg/m2) and Malay (16.8 (3.5) kg/m2) children. Girls and children of Indian ethnicity had higher body fat percentage. Girls also had higher total cholesterol and LDL-cholesterol levels compared to boys. Indian children had lower HDL-cholesterol compared to Malay and Chinese children. Insulin resistance, as indicated by HOMA-IR, was highest in Indian children, as were MetS and FLI scores. Supplementary table 1 shows the comparison between the children who had KYN metabolites measured in their blood and those who did not consent for blood collection. There was no difference in BMI, abdominal circumference, fat percentage, systolic or diastolic blood pressure between those included in the study and those who were not.
Table 1. Characteristics of the study cohort by sex and ethnicity.
| Variable | All (N=552) |
Male (N=282) |
Female (N=270) |
P | Chinese (N=305) |
Malay (N=148) |
Indian (N=99) | P |
|---|---|---|---|---|---|---|---|---|
| Metabolic risk factors | ||||||||
| BMI (kg/m2) | 16.5 (3.1) | 16.7 (3.1) | 16.4 (3.1) | 0.209 | 16.3 (2.7) | 16.8 (3.5) | 17.0 (3.5) | 0.056 |
| Abdominal circumference (cm) | 58.6 (8.8) | 59.1 (8.9) | 58.1 (8.6) | 0.216 | 57.8 (7.9) | 58.4 (9.4) | 61.5 (10.0) | 0.002 |
| Body fat percentage (%) | 21.4 (8.1) | 19.4 (76) | 23.5 (8.1) | <0.001 | 20.8 (7.3) | 20.5 (8.4) | 24.8 (8.9) | 0.001 |
| Systolic blood pressure (mmHg) | 102.5 (8.7) | 103.6 (9.1) | 101.3 (8.1) | 0.002 | 102.4 (8.9) | 102.5 (8.6) | 102.7 (8.3) | 0.942 |
| Diastolic blood pressure (mmHg) | 60.4 (6.6) | 61.0 (6.7) | 59.9 (6.3) | 0.054 | 60.5 (6.8) | 60.1 (6.2) | 60.8 (6.4) | 0.596 |
| Fasting plasma glucose (mmol/L) | 4.6 (0.4) | 4.7 (0.4) | 4.6 (0.4) | <0.001 | 4.7 (0.4) | 4.5 (0.3) | 4.6 (0.3) | <0.001 |
| Fasting plasma insulin (mIU/L) | 5.3 (4.3) | 5.2 (4.2) | 5.5 (5.2) | 0.088 | 5.1 (3.6) | 5.1 (4.7) | 6.4 (5.8) | <0.001 |
| Homeostasis model assessment for insulin resistance (HOMA-IR) | 1.4 (1.6) | 1.3 (1.2) | 1.5 (1.9) | 0.299 | 1.4 (2.0) | 1.3 (0.9) | 1.7 (1.1) | 0.005 |
| High sensitivity C-reactive protein (hsCRP) (mg/L) | 0.4 (0.7) | 0.3 (0.7) | 0.4 (0.8) | 0.058 | 0.3 (0.5) | 0.5 (0.9) | 0.5 (1.0) | 0.004 |
| Triglyceride (mmol/L) | 0.8 (0.4) | 0.8 (0.4) | 0.9 (0.4) | 0.035 | 0.8 (0.4) | 0.8 (0.5) | 0.8 (0.3) | 0.591 |
| Total cholesterol (mmol/L) | 4.6 (0.8) | 4.5 (0.8) | 4.7 (0.8) | 0.004 | 4.7 (0.9) | 4.6 (0.7) | 4.5 (0.8) | 0.073 |
| HDL-cholesterol (mmol/L) | 1.5 (0.3) | 1.5 (0.3) | 1.5 (0.3) | 0.132 | 1.5 (0.3) | 1.5 (0.3) | 1.4 (0.3) | <0.001 |
| LDL-cholesterol (mmol/L) | 2.8 (0.7) | 2.7 (0.7) | 2.9 (0.7) | 0.001 | 2.8 (0.8 | 2.7 (0.6) | 2.7 (0.7) | 0.679 |
| Metabolic risk scores | ||||||||
| Metabolic syndrome score (MetS) | -0.3 (2.5) | -0.3 (2.7) | -0.2 (2.3) | 0.741 | -0.3 (2.5) | -0.6 (2.3) | 0.1 (2.5) | 0.08 |
| Fatty liver index (FLI) | 0.9 (1.6) | 0.9 (1.7) | 0.9 (1.6) | 0.728 | 0.8 (1.2) | 0.9 (1.6) | 1.3 (2.2) | 0.003 |
| Tryptophan-kynurenine pathway metabolite concentrations and ratios | ||||||||
| Tryptophan (TRP) (μmol/L) | 59.4 (9.9) | 59.2 (10.3) | 59.6 (9.5) | 0.576 | 60.7 (10.1) | 55.9 (8.5) | 60.7 (10.2) | <0.001 |
| Kynurenine (KYN) (μmol/L) | 1.5 (0.3) | 1.5 (0.3) | 1.5 (0.3) | 0.394 | 1.5 (0.3) | 1.5 (0.3) | 1.6 (0.3) | 0.017 |
| Kynurenic acid (KA) (nmol/L) | 47.6 (15.6) | 47.9 (16.9) | 47.3 (14.1) | 0.657 | 48.2 (16.2) | 46.1 (14.0) | 48.3 (15.9) | 0.381 |
| 3-hydroxykynurenine (HK) (nmol/L) | 50.0 (15.9) | 50.5 (14.4) | 49.5 (17.3) | 0.474 | 48.6 (14.3) | 51.1 (17.8) | 52.9 (17.2) | 0.042 |
| Xanthurenic acid (XA) (nmol/L) | 11.6 (5.7) | 11.8 (6.0) | 11.3 (5.3) | 0.251 | 12.5 (5.5) | 9.9 (5.8) | 11.0 (5.6) | <0.001 |
| Hydroxyanthranilic acid (HAA) (nmol/L) | 40.1 (12.2) | 41.4 (12.1) | 38.8 (12.2) | 0.011 | 42.2 (12.7) | 38.0 (10.1) | 36.9 (12.7) | <0.001 |
| Quinolinic acid (QA) (nmol/L) | 451.7 (111.2) | 461.8 (116.9) | 441.1 (104.1) | 0.028 | 452.0 (114.5) | 458.6 (102.0) | 440.6 (114.5) | 0.458 |
| KYN/TRP ratio *100 (indoleamine-2,3-dioxygenase-1 (IDO1) activity) | 2.6 (0.5) | 2.7 (0.6) | 2.6 (5.3) | 0.194 | 2.5 (0.5) | 2.8 (0.5) | 2.7 (0.6) | <0.001 |
| HK/KYN ratio (kynurenine monooxygenase (KMO) activity) | 32.8 (8.8) | 33.0 (8.1) | 32.7 (9.6) | 0.737 | 32.6 (8.5) | 33.0 (9.5) | 33.3 (8.9) | 0.763 |
| XA/HK ratio *100 (kynurenine aminotransferase (KAT) activity) | 24.0 (10.5) | 24.2 (10.7) | 23.8 (10.3) | 0.638 | 26.7 (10.5) | 19.7 (8.5) | 22.1 (11.1) | <0.001 |
| HAA/HK ratio *100 (kynureninase (KYNU) activity) | 84.9 (28.7) | 86.1 (28.0) | 83.6 (29.5) | 0.310 | 91.4 (30.0) | 78.9 (24.4) | 73.8 (25.6) | <0.001 |
Abbreviations: N: number of participants· Data shown are N (%) for categorical variables and mean (SD) for continuous variables except for insulin, homeostasis model assessment for insulin resistance (HOMA-IR), high sensitivity C-reactive protein (hsCRP), metabolic risk score (MRS) and fatty liver index (FLI) which are represented as median (interquartile range) as they are not normally distributed. Two sided P-values less than 0.05 considered significant·
Using the 2007 WHO classification of BMI-for-age Z-scores, 37 children (6.7%) were underweight, 76.4% had normal weight, 65 (11.8%) were overweight, and 28 (5.1%) were obese (Table 2). 45 boys (15.9%) and 48 girls (17.8%) were overweight or obese. Malay (20.3%) and Indian (25.3%) children were more likely to be overweight than Chinese (12.5%) children. Overweight children had higher blood pressure, fasting glucose, fasting insulin, HOMA-IR, hsCRP, triglyceride, MetS and FLI scores, but lower HDL-cholesterol. Using the Z-score ≥ 90th percentile definition of MetS, 0%, 1.6%, 35.3% and 76.9% of girls in the underweight, normal weight, overweight and obese categories respectively had MetS, while 0%, 1.9%, 27.6% and 86.7% of boys in the underweight, normal weight, overweight and obese categories had MetS.
Table 2. Characteristics of the study cohort by BMI categories.
| All | Underweight | Normal weight | Overweight | Obese | P | |
|---|---|---|---|---|---|---|
| N (%) | 552 | 37 (6.7%) | 422 (76.4%) | 65 (11.8%) | 28 (5.1%) | |
| Sex | ||||||
| Male | 282 (51.1%) | 18 (7.0%) | 219 (77.6%) | 30 (10.6%) | 15 (5.3%) | reference |
| Female | 270 (48.9%) | 19 (4.7%) | 203 (75.2%) | 35 (13.0%) | 13 (4.8%) | 0.825 |
| Ethnicity | ||||||
| Chinese | 305 (55.3%) | 21 (6.9%) | 246 (80.7%) | 28 (9.2%) | 10 (3.3%) | reference |
| Malay | 148 (26.8%) | 6 (4.1%) | 112 (75.7%) | 18 (12.2%) | 12 (8.1%) | 0.01 |
| Indian | 99 (17.9%) | 10 (10.1%) | 64 (64.6%) | 19 (19.2%) | 6 (6.1%) | 0.075 |
| Metabolic risk factors | ||||||
| BMI (kg/m2) | 16.5 (3.1) | 12.7 (0.6) | 15.7 (1.5) | 20.7 (1.1) | 25.3 (2.6) | <0.001 |
| Abdominal circumference (cm) | 58.6 (8.8) | 49.3 (2.1) | 56.1 (5.0) | 70.8 (4.5) | 80.5 (7.5) | <0.001 |
| Body fat percentage (%) | 21.4 (8.1) | 12.9 (2.7) | 19.2 (5.6) | 32.4 (4.5) | 36.7 (5.8) | <0.001 |
| Systolic blood pressure (mmHg) | 102.5 (8.7) | 99.6 (9.0) | 101.4 (8.2) | 114.1 (7.6) | 102.5 (8.7) | <0.001 |
| Diastolic blood pressure (mmHg) | 60.4 (6.6) | 58.6 (6.0) | 60.3 (6.7) | 60.6 (5.9) | 63.6 (6.1) | 0.026 |
| Fasting plasma glucose (mmol/L) | 4.6 (0.4) | 4.5 (0.5) | 4.6 (0.4) | 4.7 (0.3) | 4.7 (0.4) | <0.001 |
| Fasting plasma nsulin (mIU/L) | 6.7 (6.6) | 4.2 (2.9) | 5.4 (2.8) | 10.9 (7.7) | 18.4 (19.2) | <0.001 |
| Homeostasis model assessment for insulin resistance (HOMA-IR) | 1.4 (1.6) | 0.9 (0.6) | 1.1 (0.6) | 2.3 (1.8) | 4.0 (5.0) | <0.001 |
| High sensitivity C-reactive protein (hsCRP) (mg/L) | 1.2 (2.5) | 0.6 (1.0) | 0.8 (2.2) | 2.2 (3.1) | 3.6 (3.3) | <0.001 |
| Triglyceride (mmol/L) | 0.8 (0.4) | 0.8 (0.3) | 0.8 (0.4) | 1.0 (0.4) | 1.2 (0.7) | <0.001 |
| Total cholesterol (mmol/L) | 4.6 (0.8) | 4.7 (0.9) | 4.6 (0.8) | 4.6 (0.7) | 4.6 (0.8) | 0.994 |
| HDL-cholesterol (mmol/L) | 1.5 (0.3) | 1.6 (0.3) | 1.5 (0.3) | 1.3 (0.2) | 1.2 (0.2) | <0.001 |
| LDL-cholesterol (mmol/L) | 2.8 (0.7) | 2.8 (0.8) | 2.7 (0.7) | 2.9 (0.7) | 2.9 (0.8) | 0.128 |
| Metabolic risk scores | ||||||
| Metabolic syndrome score (MetS) | 0.0 (2.3) | -1.7 (1.3) | -0.6 (1.5) | 2.2 (1.8) | 5.2 (3.9) | <0.001 |
| Fatty liver index (FLI) | 2.7 (6.7) | 0.4 (0.2) | 1.0 (0.8) | 5.5 (2.9) | 22.1 (19.5) | <0.001 |
| Tryptophan-kynurenine pathway metabolite concentrations and ratios | ||||||
| Tryptophan (TRP) (µmol/L) | 59.4 (9.9) | 57.1 (11.4) | 59.3 (7.2) | 59.7 (9.9) | 64.4 (9.6) | 0.006 |
| Kynurenine (KYN) (µmol/L) | 1.5 (0.3) | 1.4 (0.3) | 1.5 (0.3) | 1.6 (0.3) | 1.8 (0.3) | <0.001 |
| Kynurenic acid (KA) (nmol/L) | 47.6 (15.6) | 45.5 (12.6) | 46.3 (14.4) | 53.3 (22.5) | 56.3 (15.6) | <0.001 |
| 3-hydroxykynurenine (HK) (nmol/L) | 50.0 (15.9) | 48.0 (15.4) | 49.0 (14.3) | 52.1 (15.2) | 62.3 (28.2) | <0.001 |
| Xanthurenic acid (XA) (nmol/L) | 11.6 (5.7) | 11.5 (7.0) | 11.1 (5.2) | 12.7 (6.4) | 15.4 (7.0) | <0.001 |
| Hydroxyanthranilic acid (HAA) (nmol/L) | 40.1 (12.2) | 34.6 (10.7) | 38.9 (11.1) | 45.2 (13.8) | 53.8 (14.1) | <0.001 |
| Quinolinic acid (QA) (nmol/L) | 451.6 (111.3) | 412.6 (133.3) | 439.7 (101.5) | 498.8 (119.4) | 571.3 (100.2) | <0.001 |
| KYN/TRP ratio *100 (indoleamine-2,3-dioxygenase-1 (IDO1) activity) | 2.6 (0.5) | 2.5 (0.6) | 2.6 (0.5) | 2.8 (0.5) | 2.8 (0.4) | 0.006 |
| HK/KYN ratio (kynurenine monooxygenase (KMO) activity) | 32.8 (8.8) | 34.6 (11.6) | 32.6 (8.1) | 32.1 (7.4) | 35.6 (15.4) | 0.750 |
| XA/HK ratio *100 (kynurenine aminotransferase (KAT) activity) | 24.0 (10.5) | 25.9 (15.1) | 23.5 (9.8) | 25.2 (11.5) | 26.3 (11.2) | 0.372 |
| HAA/HK ratio *100 (kynureninase (KYNU) activity) | 84.9 (28.7) | 78.5 (32.3) | 83.7 (27.5) | 90.9 (28.8) | 97.3 (37.9) | 0.001 |
Abbreviations: N: number of participants· Data shown are N (%) for categorical variables and mean (SD) for continuous variables. Two sided P-values less than 0.05 considered significant·
KYN metabolites
Boys had higher HAA and QA concentrations compared to girls (Table 1). Malay children had lower TRP concentrations compared to Chinese and Indian children, as well as lower XA concentrations compared to Chinese children. Indian children had higher KYN concentrations compared to Chinese children. Chinese children had higher HAA concentrations compared to Malay and Indian children. Chinese children also had lower KYN/TRP ratios but higher XA/HK and HAA/HK ratios compared to Malay and Indian children.
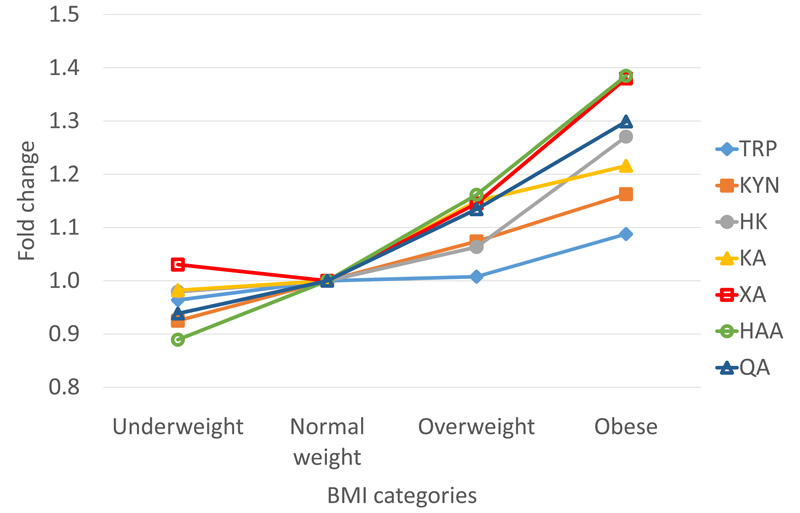
Overweight children had higher concentrations of all metabolites in the KYN pathway compared to normal weight and underweight children (Table 2). The KYN/TRP and HAA/HK ratios were also higher in overweight children. The HK/KYN and XA/HK ratios, however, did not increase with BMI. Figure 2 shows the fold change in metabolites of the KYN pathway in the underweight (< -1 SD), overweight (> +1 SD), and obese (> +2 SD) categories compared to the normal weight category (-1 SD to +1 SD). The increases in the concentrations of the distal downstream metabolites XA (1.38-fold in obese), HAA (1.38-fold in obese) and QA (1.30 fold in obese) were somewhat greater than metabolites more proximal to TRP, such as KYN (1.16 fold in obese), KA (1.22 fold in obese) and HK (1.27 fold in obese).
Figure 2. Fold change of KYN metabolites by BMI categories.
The body mass index (BMI)-for-age Z scores of the children were determined using local BMI-for-age percentile charts for boys and girls (https://www.healthhub.sg/livehealthy/745/differencesbetweenchildandadultbmi, last accessed 10 August 2021). Children with BMI-for-age < -1 Z-scores were classified as underweight, while children with BMI-for-age > +1 Z-scores were classified as overweight and children with BMI-for-age > +2 Z-scores were classified as obese. The fold increase in KYN metabolite was calculated by taking the average concentration for the children in the normal weight category as 1 and calculating the fold change by dividing the average concentration at the underweight, overweight or obese BMI categories by the average concentration at the normal weight category. TRP: solid diamond, KYN: solid square, KA: solid triangle, HK: solid circle, XA: open square, HAA: open circle, QA: open triangle.
Associations between KYN metabolites and metabolic risk factors
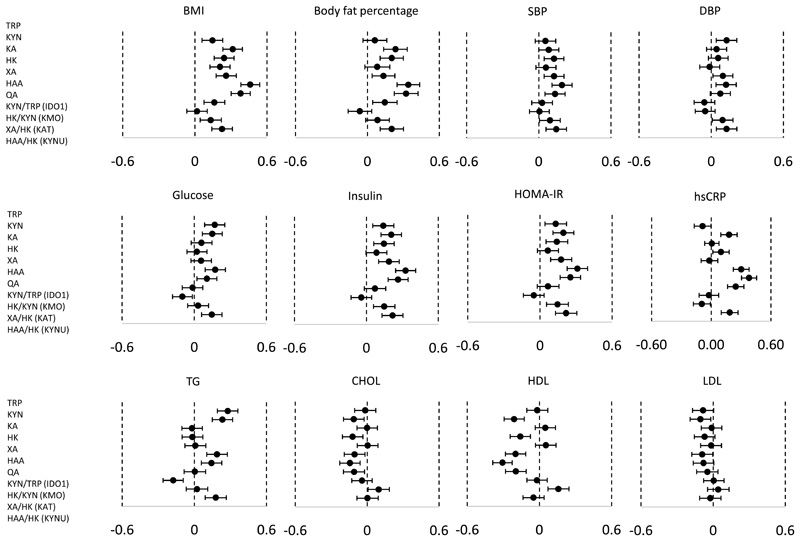
All analysed metabolites, as well as the KYN/TRP, XA/HK and HAA/HK ratios were associated positively with BMI in the children (Figure 3). The magnitudes of associations were highest for HAA and QA. For body fat percentage, all metabolites except TRP, HK, and the KYN/TRP and HAA/HK ratios showed positive associations. KA, XA, HAA and QA showed positive associations with systolic blood pressure, while TRP, XA and HAA showed positive associations with diastolic blood pressure. Although the activities of the upstream enzymes IDO1 (KYN/TRP) and KMO (HK/KYN) showed no associations with blood pressure, the downstream enzyme activities of KAT (XA/HK) and KYNU (HAA/HK) were positively associated with both systolic and diastolic blood pressure.
Figure 3. Association of KYN metabolites with metabolic risk factors.
X-axes show standardized scores of BMI, body fat percentage, systolic blood pressure (SBP), diastolic blood pressure (DBP), glucose, insulin, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), high sensitivity C-reactive protein (hsCRP), triglycerides (TG), total cholesterol (CHOL), high density lipoprotein-cholesterol (HDL) and low density lipoprotein-cholesterol (LDL). Forest plots show the differences (95% CI) in standardized score of metabolic risk factor of children at 8 years with change in each standardized score of cord KYN metabolite or ratio (Y-axis). Models were adjusted for sex and ethnicity. Total sample size (N) is not always 552 due to the missing values for outcomes.
TRP, KYN, HAA and QA were positively associated with fasting glucose concentrations. HK/KYN showed a negative association with fasting glucose, while HAA/HK showed a positive association with fasting glucose. TRP, KYN, KA, XA, HAA and QA were positively associated with fasting insulin concentrations and HOMA-IR. Although the activities of the upstream enzymes IDO1 (reflected by KYN/TRP) and KMO (reflected by HK/KYN) showed no association with blood pressure, the downstream enzymes activities of KAT (reflected by XA/HK) and KYNU (reflected by HAA/HK) appeared positively associated with fasting insulin and HOMA-IR. KYN, HK, XA, QA, KYN/TRP and HAA/HK ratios were all positively associated with hsCRP concentrations, while the XA/HK ratio showed an inverse association with hsCRP. TRP, KYN, HAA and QA were positively associated with triglyceride concentrations. The HK/KYN ratio was negatively associated and HAA/HK positively associated with triglyceride concentrations. KYN, HK, HAA and QA were inversely associated with total cholesterol and HDL-cholesterol concentrations. KYN/TRP ratio was negatively associated with HDL-cholesterol, while XA/HK ratio was positively associated with HDL-cholesterol.
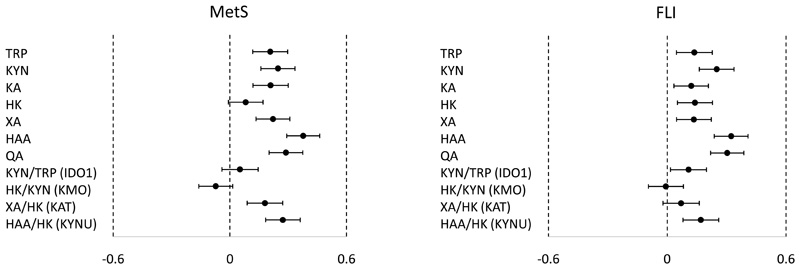
Plasma TRP, KYN, KA, XA, HAA and QA were positively associated with the MetS score, while TRP and all metabolites of the KYN pathway were positively associated with FLI score (Figure 4). Increases in one standardized score of HAA and QA were associated with 0.38 (0.29, 0.46) and 0.29 (0.20, 0.37) increases in MetS respectively, compared to increases of 0.21 (0.12, 0.30) and 0.25 (0.16, 0.34) in MetS with TRP and KYN. However, the KYN/TRP ratio showed no association with MetS score, while there was a positive association with FLI score. In contrast, the XA/HK ratio was positively associated with MetS score but not with FLI score. The HAA/HK ratio, indicative of KYNU activity, was positively associated with both MetS and FLI scores.
Figure 4. Association of KYN metabolites with metabolic syndrome score and fatty liver index.
X-axes show standardized scores of metabolic syndrome score (MetS) and fatty liver index (FLI). Forest plots show the differences (95% CI) in MetS (left) or standardized score of FLI (right) of children at 8 years with change in each standardized score of cord KYN metabolite or ratio (Y-axis). Models were adjusted for sex and ethnicity. Total sample size (N) is not always 552 due to the missing values for outcomes.
Discussion
Previous studies showed higher concentrations of TRP, KYN, KA and HAA, in men compared to women (38–40). In our study of pre-pubertal children, we observed no obvious sex differences in TRP and metabolites generated by the actions of IDO1. However, downstream of KMO, concentrations of HAA and QA were higher in boys than girls. The differences relative to previous studies in adulthood may be indicative of a sex-steroid regulation, as that estrogen is known to inhibit KAT (9), which converts KYN to KA and HK to XA, thus possibly shunting the KYN pathway to HAA and QA synthesis (Figure 1). It is unclear why pre-pubertal boys have higher circulating HAA and QA than pre-pubertal girls, or indeed what the metabolic impact may be.
Ethnic differences in KYN pathway metabolite concentrations have been described for Caucasian, African-American, Hispanic and Asian-American ethnic groups (40). In this study, we observed lower TRP concentrations in Malay children compared to Chinese and Indian children. This may be related to dietary differences or genetic differences in metabolic enzymes such as TDO. Indian children had the highest metabolic risk compared to Malay and Chinese children. They also had the highest KYN concentration. This supports the hypothesis that obesity and metabolic dysfunction activate IDO1 and KMO, driving the conversion of TRP to KYN and activating the KYN metabolic pathway. It is also possible that activation of the KYN metabolic pathway plays a role in the development of obesity and metabolic dysfunction through the regulation of energy metabolism (8). Further studies in larger cohorts will be necessary to examine these ethnic differences in depth.
In prepubertal Asian children, TRP and KYN metabolite concentrations were higher in overweight children and demonstrated positive associations with several metabolic risk factors. While previous studies have shown positive association between TRP, KYN and BMI in adults (11–13) and in children (18, 25), the current study provides evidence for extensive associations of the KYN pathway metabolites with BMI and metabolic risk factors in a mixed ethnicity cohort of Asian prepubertal children.
In this cohort, the prevalence of overweight (BMI-for-age >+1SD), obesity (BMI-for-age >+2SD) and severe obesity (BMI-for-age >+3SD) was 10.6%, 4.6% and 0.7% for boys and 13.0%, 3.3%, and 1.5% for girls respectively. These rates are relatively lower compared to those reported by a recent study on 6 to 9 year old children from 36 countries, where the overall prevalences of overweight, obesity and severe obesity in 8 year-olds were 28.7%, 12.5% and 3.5% for boys and 26.5%, 9.0%, 1.5% for girls (41). We found an average HOMA-IR of 1.4 in our cohort, with an average HOMA-IR of 2.3 in overweight children and 4.0 in obese children compared to 1.1 in normal weight and 0.9 in underweight children. In the IDEFICS cohort, HOMA-IR was 1.1 in girls and 1.0 in boys (42). In a study in Chinese children, a HOMA-IR cut-off for metabolic syndrome was 2.3 in all children, and 1.7 in prepubertal children (43). Therefore, despite the apparent lower rate of overweight and obesity compared to recent international comparisons, insulin resistance as determined by HOMA-IR for the overweight and obese children was similar, indicative of heightened metabolic risk in these children.
Continuous metabolic risk scores are useful clinically as they enable the tracking of cardiometabolic risk and earlier detection of people at risk (44). Multiple methods have been used for the generation of continuous metabolic risk scores in children (45, 46), with the sum or mean of age-standardized and sex-standardized components within the study population being one of the most common methods. The most commonly used score components are waist circumference (52%), triglycerides (87%), HDL-cholesterol (67%), glucose (43%) and systolic blood pressure (52%) (46). Despite the variety of methods, many of these continuous metabolic risk scores in children have been reported to have high validity (47–49) and correlate to: a) adverse lifestyle factors such as sedentary behavior, physical activity (18), poor handgrip strength and cardiorespiratory fitness (50), b) cross-sectional biomarkers of inflammation, endothelial damage and cardiovascular disease (51), and c) adulthood outcomes such as type 2 diabetes (52, 53), and cardiovascular disease (54). The MetS scores calculated for our cohort at the 90th percentile was 2.63 for girls and 2.85 for boys, compared to 4.75 for girls and 4.39 for boys in the IDEFICS study (33). As expected, there was a clear positive gradient of MetS score with BMI, with 82% of children in the obese category having metabolic syndrome, i.e. having a MetS score ≥ 90th percentile. However, even within the normal weight category, a small proportion (1.8%) of children were classified as having metabolic syndrome, while 18% of obese children did not have metabolic syndrome. Despite the predominant influence of BMI on metabolic health risk, there remains some unexplained variability of risk.
The FLI is often used to estimate NAFLD as magnetic resonance spectroscopy (MRS) is expensive and not readily available (37). It varies between 0 and 100; a threshold of <30 can be used to rule out NAFLD, while a threshold of >60 is used to rule in NAFLD (36, 37). In this cohort, 2 children had a FLI of >60 while another 4 children had FLI between 30 and 60. The 2 children with FLI >60 were in the obese weight category and had metabolic syndrome while out of the 4 children with FLI between 30 and 60, 2 were obese and 2 were overweight and all had metabolic syndrome. Ectopic fat accumulation may be a common mechanism underlying the association between BMI, metabolic syndrome and hepatic steatosis in these children (37).
Previous studies have focused on the KYN/TRP ratio (12, 13, 38); our data support this, but extend to metabolites mediated by further enzymatic steps. In the analysis of metabolites of the KYN pathway, it was those downstream of the enzymatic activity of KMO, including HAA and QA, that demonstrated the greatest increase in overweight and obese children, and the highest magnitude associations with metabolic risk. Further, the HAA/HK ratio, indicative of KYNU enzyme activity, associated positively with BMI, blood pressure, fasting glucose, insulin and HOMA-IR, as well as triglyceride, MetS and FLI. The possible function of these metabolites and their relationship to heightened metabolic risk is speculative. It is known that QA is the precursor of the redox cofactor nicotinamide adenine dinucleotide (NAD+), as well as functioning as an excitatory glutamate receptor agonist (9). Disordered NAD metabolism may be involved in metabolic dysfunction and changes in body composition (55, 56). HAA and QA are products of human microglia and monocytes upon activation by specific cytokines such as interferon gamma (57, 58), and QA is a pro-oxidant molecule (59). Furthermore, both HAA and QA have immunomodulatory functions, with a putative role in transition of immune Th1/Th2 balance towards Th2 (9). Further analysis is therefore required to elucidate whether these relationships are associative or if there is a mechanistic relationship in the onset and development of adverse metabolic health in those children with heightened adiposity.
Strengths of our study include a relatively large sample size and the inclusion of three Asian ethnic groups at a single age point, where information on KYN metabolite concentrations are limited in literature. We have systematically studied the association between downstream KYN metabolite concentrations and ratios indicative of the enzyme activities in the KYN metabolic pathway and multiple metabolic risk factors including risk scores. Another strength is the use of an accredited clinical laboratory (30) for analyses of clinical biomarkers related to metabolic risk such as lipids and insulin. In this study the entire sample set was analysed for TYP-KYN metabolites in a single laboratory (Bevital, AS) by a targeted LC-MS/MS method including authentic labelled internal standards for each analyte providing high analytical precision (60). The analyses were carried out in continuous sample batches, with small variability between each batch. Finally, standardized scores were used in the regression models so that the strength of associations were comparable.
This study has some limitations. Firstly, we did not examine the data on the dietary intakes of the children. As TRP cannot be synthesized by humans, the main source of TRP is from the diet (61). Diet would also influence BMI and metabolic risk factors. Also, we did not study the physical activity of the children. Physical activities of the children are likely to be a major contributor to the metabolic risk factors studied, and there is some evidence that fitness status influences KYN pathway metabolite concentrations (62). Further, the current study is an observational study, therefore it is not possible to establish possible causality or how these data are reflective of change in risk with time.
In conclusion, plasma metabolites of the TYP-KYN pathway positively associated with metabolic risk factors in young Asian children, suggesting possible activation of this pathway in childhood metabolic dysfunction. Our results suggest that examining metabolites of the KYN pathway in children may help predict the risk of obesity and metabolic syndrome and suggest new interventional approaches.
Supplementary Material
Acknowledgements
This study group includes: Airu Chia, Allan Sheppard, Amutha Chinnadurai, Anna Magdalena Fogel, Anne Eng Neo Goh, Anne Hin Yee Chu, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit Froukje Philipp Broekman, Bobby Kyungbeom Cheon, Boon Long Quah, Candida Vaz, Chai Kiat Chng, Cheryl Shufen Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Claudia Chi, Daniel Yam Thiam Goh, Dawn Xin Ping Koh, Desiree Y. Phua, Doris Ngiuk Lan Loh, E Shyong Tai, Elaine Kwang Hsia Tham, Elaine Phaik Ling Quah, Elizabeth Huiwen Tham, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Fabian Kok Peng Yap, Faidon Magkos, Falk Müller-Riemenschneider, George Seow Heong Yeo, Hannah Ee Juen Yong, Helen Yu Chen, Heng Hao Tan, Hong Pan, Hugo P S van Bever, Hui Min Tan, Iliana Magiati, Inez Bik Yun Wong, Ives Yubin Lim, Ivy Yee-Man Lau, Izzuddin Bin Mohd Aris, Jeannie Tay, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Jia Xu, Joanna Dawn Holbrook, Joanne Su-Yin Yoong, Joao Nuno Andrade Requicha Ferreira, Johan Gunnar Eriksson, Jonathan Tze Liang Choo, Jonathan Y. Bernard, Jonathan Yinhao Huang, Joshua J. Gooley, Jun Shi Lai, Karen Mei Ling Tan, Keith M. Godfrey, Kenneth Yung Chiang Kwek, Keri McCrickerd, Kok Hian Tan, Kothandaraman Narasimhan, Krishnamoorthy Naiduvaje, Kuan Jin Lee, Leher Singh, Li Chen, Lieng Hsi Ling, Lin Lin Su, Ling-Wei Chen, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael J. Meaney, Michelle Zhi Ling Kee, Min Gong, Mya Thway Tint, Navin Michael, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Peter David Gluckman, Pratibha Keshav Agarwal, Priti Mishra, Queenie Ling Jun Li, Rob Martinus van Dam, Salome A. Rebello, Sambasivam Sendhil Velan, Seang Mei Saw, See Ling Loy, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu, Sue-Anne Ee Shiow Toh, Suresh Anand Sadananthan, Swee Chye Quek, Varsha Gupta, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, Wen Lun Yuan, Yanan Zhu, Yap Seng Chong, Yin Bun Cheung, Yiong Huak Chan, Yung Seng Lee.
Funding
The study is supported by the National Research Foundation (NRF) under the Open Fund-Large Collaborative Grant (OF-LCG; MOH-000504) administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC) and the Agency for Science, Technology and Research (A*STAR). In RIE2025, GUSTO is supported by funding from the NRF’s Human Health and Potential (HHP) Domain, under the Human Potential Programme.
Footnotes
Author contributions
KMT was responsible for designing the study, analysing data, interpreting results, and writing the manuscript. MTT contributed to designing the study, extracting and analysing data, interpreting results, and writing the manuscript. KN contributed to analysing data. FY, KMG, YSL, KHT, PDG, YSC, and MFFC contributed to data collection. KMG, YSL, MFFC and JGE provided feedback on the manuscript. DCS was responsible for designing the study, interpreting results and writing the manuscript.
Competing interests
YSC, PDG and KMG are part of an academic consortium that has received research funding from companies selling nutritional products. KMG and DCS have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus+ Programme ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174, SP/F/21/150013). All other authors declare no financial relationships with any organizations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Organization. WH. Report of the Commission on Ending Childhood Obesity. Geneva, Switzerland: Ending Childhood Obesity Commission 2016; [Google Scholar]
- 2.Caprio S, Santoro N, Weiss R. Childhood obesity and the associated rise in cardiometabolic complications. Nature metabolism. 2020;2(3):223–32. doi: 10.1038/s42255-020-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foo LL, Vijaya K, Sloan RA, Ling A. Obesity prevention and management: Singapore's experience. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(Suppl 2):106–13. doi: 10.1111/obr.12092. [DOI] [PubMed] [Google Scholar]
- 4.Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nature reviews Cardiology. 2011;8(9):513–25. doi: 10.1038/nrcardio.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho TF. Cardiovascular risks associated with obesity in children and adolescents. Ann Acad Med Singap. 2009;38(1):48–9. [PubMed] [Google Scholar]
- 6.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119(4):628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 7.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349) doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 8.Dadvar S, Ferreira DMS, Cervenka I, Ruas JL. The weight of nutrients: kynurenine metabolites in obesity and exercise. J Intern Med. 2018;284(5):519–33. doi: 10.1111/joim.12830. [DOI] [PubMed] [Google Scholar]
- 9.Badawy AA. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modoux M, Rolhion N, Mani S, Sokol H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol Sci. 2021;42(1):60–73. doi: 10.1016/j.tips.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, Prassl R, et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 2014;22(1):195–201. doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- 12.Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring) 2015;23(10):2066–74. doi: 10.1002/oby.21199. [DOI] [PubMed] [Google Scholar]
- 13.Pertovaara M, Raitala A, Juonala M, Lehtimäki T, Huhtala H, Oja SS, et al. Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol. 2007;148(1):106–11. doi: 10.1111/j.1365-2249.2007.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu E, Papandreou C, Ruiz-Canela M, Guasch-Ferre M, Clish CB, Dennis C, et al. Association of Tryptophan Metabolites with Incident Type 2 Diabetes in the PREDIMED Trial: A Case-Cohort Study. Clin Chem. 2018;64(8):1211–20. doi: 10.1373/clinchem.2018.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebnord EW, Strand E, Midttun Ø, Svingen GFT, Christensen MHE, Ueland PM, et al. The kynurenine:tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia. 2017;60(9):1712–21. doi: 10.1007/s00125-017-4329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song P, Ramprasath T, Wang H, Zou MH. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci. 2017;74(16):2899–916. doi: 10.1007/s00018-017-2504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polyzos KA, Ketelhuth DF. The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie. 2015;35(2):128–36. doi: 10.5482/HAMO-14-10-0052. [DOI] [PubMed] [Google Scholar]
- 18.Barat P, Meiffred MC, Brossaud J, Fuchs D, Corcuff JB, Thibault H, et al. Inflammatory, endocrine and metabolic correlates of fatigue in obese children. Psychoneuroendocrinology. 2016;74:158–63. doi: 10.1016/j.psyneuen.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Groven N, Reitan SK, Fors EA, Guzey IC. Kynurenine metabolites and ratios differ between Chronic Fatigue Syndrome, Fibromyalgia, and healthy controls. Psychoneuroendocrinology. 2021;131:105287. doi: 10.1016/j.psyneuen.2021.105287. [DOI] [PubMed] [Google Scholar]
- 20.Oxenkrug GF. Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol Neurobiol. 2015;52(2):805–10. doi: 10.1007/s12035-015-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuoka K, Kato K, Takao T, Ogawa M, Ishii Y, Shimizu F, et al. Concentrations of various tryptophan metabolites are higher in patients with diabetes mellitus than in healthy aged male adults. Diabetol Int. 2017;8(1):69–75. doi: 10.1007/s13340-016-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JJ, Movassat J, Portha B. Emerging role for kynurenines in metabolic pathologies. Curr Opin Clin Nutr Metab Care. 2019;22(1):82–90. doi: 10.1097/MCO.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 23.Meyramov G, Korchin V, Kocheryzkina N. Diabetogenic activity of xanturenic acid determined by its chelating properties? Transplant Proc. 1998;30(6):2682–4. doi: 10.1016/s0041-1345(98)00788-x. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Kido J, Matsumoto S, Shimizu K, Nakamura K. Associations among amino acid, lipid, and glucose metabolic profiles in childhood obesity. BMC Pediatr. 2019;19(1):273. doi: 10.1186/s12887-019-1647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, et al. Global metabolomic profiling targeting childhood obesity in the Hispanic population. The American journal of clinical nutrition. 2015;102(2):256–67. doi: 10.3945/ajcn.115.111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23(9):1371–9. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 27.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 28.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LW, Tint MT, Fortier MV, Aris IM, Shek LP, Tan KH, et al. Body composition measurement in young children using quantitative magnetic resonance: a comparison with air displacement plethysmography. Pediatr Obes. 2018;13(6):365–73. doi: 10.1111/ijpo.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding C, Chan Z, Chooi YC, Choo J, Sadananthan SA, Michael N, et al. Association between Serum Vitamin D Metabolites and Metabolic Function in Healthy Asian Adults. Nutrients. 2020;12(12) doi: 10.3390/nu12123706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 33.Ahrens W, Moreno LA, Mårild S, Molnár D, Siani A, De Henauw S, et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. International journal of obesity (2005) 2014;38(Suppl 2):S4–14. doi: 10.1038/ijo.2014.130. [DOI] [PubMed] [Google Scholar]
- 34.Clemente MG, Mandato C, Poeta M, Vajro P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J Gastroenterol. 2016;22(36):8078–93. doi: 10.3748/wjg.v22.i36.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaram SS, Zeitler P, Nadeau K. The metabolic syndrome and nonalcoholic fatty liver disease in children. Curr Opin Pediatr. 2009;21(4):529–35. doi: 10.1097/MOP.0b013e32832cb16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tint MT, Michael N, Sadananthan SA, Huang JY, Khoo CM, Godfrey KM, et al. Brown adipose tissue, adiposity and metabolic profile in preschool children. The Journal of clinical endocrinology and metabolism. 2021 doi: 10.1210/clinem/dgab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theofylaktopoulou D, Midttun Ø, Ulvik A, Ueland PM, Tell GS, Vollset SE, et al. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol. 2013;173(1):121–30. doi: 10.1111/cei.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deac OM, Mills JL, Shane B, Midttun Ø, Ueland PM, Brosnan JT, et al. Tryptophan catabolism and vitamin B-6 status are affected by gender and lifestyle factors in healthy young adults. J Nutr. 2015;145(4):701–7. doi: 10.3945/jn.114.203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badawy AA, Dougherty DM. Assessment of the Human Kynurenine Pathway: Comparisons and Clinical Implications of Ethnic and Gender Differences in Plasma Tryptophan, Kynurenine Metabolites, and Enzyme Expressions at Baseline and After Acute Tryptophan Loading and Depletion. Int J Tryptophan Res. 2016;9:31–49. doi: 10.4137/IJTR.S38189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spinelli A, Buoncristiano M, Nardone P, Starc G, Hejgaard T, Júlíusson PB, et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative-COSI 2015-2017. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2021:e13214. doi: 10.1111/obr.13214. [DOI] [PubMed] [Google Scholar]
- 42.Peplies J, Börnhorst C, Günther K, Fraterman A, Russo P, Veidebaum T, et al. Longitudinal associations of lifestyle factors and weight status with insulin resistance (HOMA-IR) in preadolescent children: the large prospective cohort study IDEFICS. Int J Behav Nutr Phys Act. 2016;13(1):97. doi: 10.1186/s12966-016-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin J, Li M, Xu L, Wang Y, Cheng H, Zhao X, et al. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetology & metabolic syndrome. 2013;5(1):71. doi: 10.1186/1758-5996-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeBoer MD, Gurka MJ. Clinical utility of metabolic syndrome severity scores: considerations for practitioners. Diabetes, metabolic syndrome and obesity: targets and therapy. 2017;10:65–72. doi: 10.2147/DMSO.S101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovascular diabetology. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamel M, Smith BT, Wahi G, Carsley S, Birken CS, Anderson LN. Continuous cardiometabolic risk score definitions in early childhood: a scoping review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2018;19(12):1688–99. doi: 10.1111/obr.12748. [DOI] [PubMed] [Google Scholar]
- 47.Eisenmann JC, Laurson KR, DuBose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetology & metabolic syndrome. 2010;2:8. doi: 10.1186/1758-5996-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoshhali M, Heshmat R, Esmaeil Motlagh M, Ziaodini H, Hadian M, Aminaei T, et al. Comparing the validity of continuous metabolic syndrome risk scores for predicting pediatric metabolic syndrome: the CASPIAN-V study. Journal of pediatric endocrinology & metabolism: JPEM. 2019;32(4):383–9. doi: 10.1515/jpem-2018-0384. [DOI] [PubMed] [Google Scholar]
- 49.Heshmat R, Heidari M, Ejtahed HS, Motlagh ME, Mahdavi-Gorab A, Ziaodini H, et al. Validity of a continuous metabolic syndrome score as an index for modeling metabolic syndrome in children and adolescents: the CASPIAN-V study. Diabetology & metabolic syndrome. 2017;9:89. doi: 10.1186/s13098-017-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen DD, Gómez-Arbeláez D, Camacho PA, Pinzon S, Hormiga C, Trejos-Suarez J, et al. Low muscle strength is associated with metabolic risk factors in Colombian children: the ACFIES study. PloS one. 2014;9(4):e93150. doi: 10.1371/journal.pone.0093150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olza J, Aguilera CM, Gil-Campos M, Leis R, Bueno G, Valle M, et al. A Continuous Metabolic Syndrome Score Is Associated with Specific Biomarkers of Inflammation and CVD Risk in Prepubertal Children. Annals of nutrition & metabolism. 2015;66(2-3):72–9. doi: 10.1159/000369981. [DOI] [PubMed] [Google Scholar]
- 52.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58(12):2745–52. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnussen CG, Cheriyan S, Sabin MA, Juonala M, Koskinen J, Thomson R, et al. Continuous and Dichotomous Metabolic Syndrome Definitions in Youth Predict Adult Type 2 Diabetes and Carotid Artery Intima Media Thickness: The Cardiovascular Risk in Young Finns Study. The Journal of pediatrics. 2016;171:97–103.:e1-3. doi: 10.1016/j.jpeds.2015.10.093. [DOI] [PubMed] [Google Scholar]
- 54.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. Journal of the American College of Cardiology. 2015;66(6):755–7. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26(1):34. doi: 10.1186/s12929-019-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migliavacca E, Tay SKH, Patel HP, Sonntag T, Civiletto G, McFarlane C, et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nature communications. 2019;10(1):5808. doi: 10.1038/s41467-019-13694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. The Biochemical journal. 1996;320(Pt 2):595–7. doi: 10.1042/bj3200595. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Werner ER, Bitterlich G, Fuchs D, Hausen A, Reibnegger G, Szabo G, et al. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life sciences. 1987;41(3):273–80. doi: 10.1016/0024-3205(87)90149-4. [DOI] [PubMed] [Google Scholar]
- 59.Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. The FEBS journal. 2012;279(8):1356–65. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 60.Ulvik A, McCann A, Midttun Ø, Meyer K, Godfrey KM, Ueland PM. Quantifying Precision Loss in Targeted Metabolomics Based on Mass Spectrometry and Nonmatching Internal Standards. Analytical chemistry. 2021;93(21):7616–24. doi: 10.1021/acs.analchem.1c00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int J Tryptophan Res. 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valente-Silva P, Cervenka I, Ferreira DMS, Correia JC, Edman S, Horwath O, et al. Effects of Tryptophan Supplementation and Exercise on the Fate of Kynurenine Metabolites in Mice and Humans. Metabolites. 2021;11(8) doi: 10.3390/metabo11080508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.