Abstract
Respiratory syncytial virus (RSV) disease is an important cause of morbidity and mortality in children and debilitated adults and remains one of the major global unmet challenges for vaccine development. Several immunological issues have delayed the development of vaccines, especially the poorly protective response to natural infection and the enhancement of disease following administration of formalin inactivated vaccines during trials conducted in the 1960s. Advances in knowledge of the immune system, of the virus and its antigenic properties combined with new vaccine technologies are now injecting new hope into the field and have given rise to many promising vaccine approaches. Some of these may be optimal for use in children, while others may be more appropriate for pregnant women or vulnerable older adults. With a multi-pronged approach to prevention, we propose that it may be possible to destabilise community circulation of RSV and thus to significantly lessen the impact of RSV disease.
Keywords: bronchiolitis, common colds, formalin inactivated vaccine, immune augmentation, respiratory syncytial virus
The burden of respiratory syncytial virus disease
Respiratory syncytial virus (RSV) is the leading cause of bronchiolitis in infants and a significant cause of morbidity and mortality in the elderly. It infects 65% of children in first year of life and continues to cause significant disease in children up to 5 years of age, affecting 34 million children each year of whom 3.4 million need admission to hospital and some 200,000 die; 99% of these deaths are in developing countries [1]. A recent prospective study of 56,560 children in Argentina showed that of 1293 children with respiratory infections, 61.6% were infected with RSV; of these, 13% had life-threatening disease [2]. The annual attributable mortality rate for RSV was 0.7 per 1000 infants, showing that life threatening and fatal RSV infections are common in the developing world.
In recent years, RSV has also been recognized as a significant problem in debilitated and elderly persons in whom infection may lead to cardiac failure and secondary bacterial pneumonia. Each year, 3-10% of adult colds are caused by RSV, and in the USA, 78% of the deaths due to RSV infection are in individuals over 65 years of age [3].
Despite measurable immune responses to primary and secondary infection, symptomatic RSV disease recurs throughout life [4]. Estimates of financial impact are largely limited to the direct costs of healthcare, but have been put at $680 million per year in the USA [5]. Although normally RSV causes only transient upper respiratory symptoms, severe RSV infection leads to lower respiratory tract illness such as pneumonia or bronchiolitis, resulting in cough, wheeze, respiratory obstruction and hypoxia [6]. The peak age of bronchiolitis is 2–4 months of age, perhaps reflecting the decline in protection afforded by maternal antibody, the maturation of the immune system and the physiological and anatomical features of the respiratory tract during this period of development [7,8]. Children reaching this susceptible age at the time of peak RSV circulation are most vulnerable. In addition, bronchiolitis appears to be causally related to wheeze in later life [9,10]. However, it remains unclear whether the delayed effects of early life infection are due to programming of a harmful adaptive response or due to other hitherto unrecognized factors.
The pathogenesis of RSV disease is diverse, but involves a dysregulation of immune responses in the airway with enhanced inflammation in the bronchial mucosa and surrounding tissue [4]. This is characterized by activation of innate immune responses and the appearance of neutrophils in the bronchial fluid [11–13]. The role of Th2 responses is not clear; in most cases, there is no lung eosinophilia (as there may be in some older children with asthma) [14]. Unlike asthma, viral bronchiolitis does not respond to steroids or bronchodilators [15,16]. In disease enhanced by prior vaccination with formalin-inactivated virus, the pathogenesis does appear to be Th2 related [17,18], and in some elderly persons with chronic lung disease (chronic bronchitis, emphysema and asthma), it is also possible that Th2 cytokines are involved [19].
Despite increased appreciation of the large global impact of RSV disease, there remain no specific antivirals and no licensed active vaccine. A number of significant hurdles remain; not least the many unanswered questions about the human immune response to RSV infection. RSV vaccine development is among the priorities for organizations such as the WHO and PATH as well as the pharmaceutical industry, which recognizes the unmet need. In addition, the Bill & Melinda Gates Foundation's current pneumonia strategy focuses on pneumococcus, influenza and RSV, and especially on maternal immunization to protect mothers and their newborn children [20]. Increasing investment therefore promises to accelerate both fundamental research and clinical development.
Antigenic properties of RSV proteins
RSV is an enveloped, nonsegmented negative-sense, single-stranded RNA virus belonging to the Paramyxoviridae family. Its genome is made up of 10 genes that encode 11 proteins. With the exception of the attachment glycoprotein (G), the viral proteins are very well conserved. The three surface glyco-proteins of RSV, fusion (F), small hydrophobic and G proteins, are the main targets for humoral immune responses. G is an attachment protein that mediates virus binding and is important for efficient replication in vivo [11,12]. It is produced in abundance and is not only cell/virus associated but also secreted, a form that may act as a decoy for antibody [21]. In addition, it bears a conserved CX3C fractalkine-like motif, which is thought to modulate inflammatory responses [11]. Recent in vitro studies have demonstrated the inhibitory effect of this chemokine analog on type I/III interferon production by epithelial cell lines and plasmacytoid dendritic cells with additional effects on TNF expression by plasmacytoid dendritic cells and IFN production by T cells [22]. This may occur via the inhibition of toll-like receptor (TLR) 3/4-dependent mechanisms and may contribute to impaired viral clearance [23]. Immunization of mice with RSV G protein-induced neutralizing antibodies that block G protein-CX3CR1 interactions with both RSV A and B strains [24], prophylactic treatment with which could reduce respiratory disease [25]. However, G protein is highly glycosylated and poorly conserved across RSV strains. Therefore, the majority of neutralizing antibodies against G protein are against hypervariable serine–threonine-rich domains that are heavily O-glycosylated, although induction of antibodies against the central conserved region of G could offer an advantage by reducing RSV-induced tissue damage.
In contrast, F protein is well conserved and, unlike G, is essential for infectivity, being responsible for fusion of the viral and cellular membranes [26,27]. It also has immunomodulatory functions and can activate TLR4. In human monocytes in vitro, the production of IL-6 in response to F protein is dependent on TLR4 signaling, and TLR4 knockout mice are unable to clear the virus [28]. Furthermore, analysis of single-nucleotide polymorphisms in TLR4 showed that mutations that contributed to reduced responsiveness to RSV in vitro were associated with increased susceptibility to RSV infection in high-risk infants and young children [29,30]. Thus, the immuno-genicity of the F protein coupled with its high degree of sequence conservation across all RSV strains has dictated its selection as the major vaccine antigen.
Recent advances in the understanding of the structure of F protein have invigorated RSV vaccine development. F protein is metastable and synthesized in a prefusion form that is stabilized on the virion. Proteolytic cleavage leads to a conformational change that causes the fusion of adjoining membranes [27]. Until recently, only the structure of the postfusion F protein was known [26]. A number of antigenic sites had been described including that recognized by palivizumab [26]. However, studies in which human sera were adsorbed on columns coated with postfusion F indicated that the greater proportion of virus neutralization was not due to antibodies against epitopes in this confirmation [31]. Recent studies have elucidated the structure of prefusion F and identified a novel antigenic site, termed site Ø [32]. Antibodies that recognize this epitope offer theoretical advantages by preferentially targeting F protein on infectious virions. However, analysis of site Ø suggests a higher degree of sequence variation surrounding it, implying that while antibodies recognizing this site may have greater antiviral efficacy, they might also encourage viral mutation in an attempt to evade immune pressure.
The inner viral proteins are also well conserved and are major targets for cellular immunity. NS1 and NS2 are non-structural proteins that mediate inhibition of STAT2 expression and early IFN production [33]. Matrix (M) protein lines the inner surface of the viral envelope, having a central role in budding and formation of infectious virus [34]. Nucleoprotein (N), phosphoprotein (P) and polymerase (L) have roles in encapsidation, RNA replication and transcription, respectively [11]. It is of note that the minimal construct for an infectious virus-like particle (VLP) requires the M, F, N and P proteins [35]. We recently reviewed the role of cellular immunity in RSV, but comparatively little is known about the importance of human T cells in protection and immunopathology [4]. Further understanding of the targets of T cell immunity and the role of individual T cell subsets in viral clearance and B cell help will be necessary to direct the inclusion of RSV proteins or peptide epitopes in novel vaccine candidates.
Protective immunity against RSV
The majority of successful vaccines rely on replicating aspects of the protective immune response induced by natural infection without the pathological sequelae. However, since natural RSV infection does not induce complete immunity, simply imitating the response to infection is unlikely to be sufficient. There are two serogroups of human RSV (hRSV), termed A and B, in addition to multiple genotypes that can be distinguished by viral sequencing. Despite this, human sera and many monoclonal antibodies cross-react to both A and B serotypes, and immunity to RSV is only partially strain specific. In adult challenge studies, a single strain can reinfect with relative ease [36]. In addition, longitudinal studies show that reinfection can occur within even a single year, either with the same or a different serotype [37]. These findings suggest that RSV evades immunity in order to reinfect, but how it does this remains obscure.
It also remains unclear why some individuals suffer severe disease leading to respiratory failure, while others infected with the same virus show only minor or unapparent disease. Hypotheses include severe disease being driven by high viral load due to defective early control of viral replication. This might be compounded by RSV's ability to inhibit interferon (IFN) responses via early and abundant expression of viral NS proteins [38]. In addition, poor immune regulation may cause an overexuberant inflammatory response [4]. Thus, for a vaccine against RSV to be successful, it will need to induce immune responses beyond those stimulated by natural infection, but must also avoid the induction of harmful immunopathology.
Role of antibody in protection against RSV
Host responses to natural infection are only partially protective, but primary infection during infancy can be delayed by parenteral administration of palivizumab (Synagis), a humanized anti-RSV F protein monoclonal antibody. Palivizumab reduces hospitalization of at-risk infants by around 50% [39], suggesting that there are stable, serotype-independent neutralizing epitopes in the RSV F protein, and that antibody alone is capable of protecting against severe RSV disease [32].
A number of studies have investigated the role of serum antibody as a correlate of protection against RSV disease. Most have shown an association between low serum-neutralizing antibody titers and increased risk of RSV disease [40,41]. A number have also attempted to identify threshold values above which the likelihood of hospitalization with RSV falls significantly [42]. However, a lack of assay standardization makes it difficult to compare studies and populations. Furthermore, an upper threshold above which complete protection is seen has not been found. Therefore, in most RSV-experienced individuals, the variability in infection risk and disease severity is not fully determined by antibody levels. Thus, while the literature suggests that those with little or no neutralizing antibody are indeed at high risk of infection, even the highest levels of antibody induced by natural infection are insufficient to provide absolute protection. This is compounded by studies that indicate the short-lived nature of anti-RSV antibodies, which are only boosted temporarily on secondary infection and which drop rapidly with no sustained increment [43].
Palivizumab needs to be administered once a month during the RSV season in order to prevent disease and has no therapeutic efficacy in established infection [44]. In most countries, it is only given to children at greatest risk due to its high cost and the relatively low probability of preventing hospitalization in healthier individuals (since most children only suffer mild disease). Therefore, most current RSV vaccine strategies hope to replicate palivizumab's clinical efficacy through the induction of antibodies against F protein. Whether it will be possible to induce high-affinity neutralizing antibodies that persist at sufficiently high titer to provide long-term protection remains to be seen.
Cell-mediated immunity against RSV
The production of antibodies depends on T cell help [45]. Furthermore, if sterilizing immunity cannot be achieved by antibody, antigen-specific T cells may assist in the clearance of infected cells. In small animal models, depletion of CD4+ or CD8+ T cells impairs RSV clearance [4]. However, T cells have also been implicated in increased disease severity [46]. It therefore remains unclear whether induction of high frequencies of antigen-specific T cells by RSV vaccination is desirable. Recent studies in influenza have demonstrated the beneficial effect of pre-existing antigen-specific CD4+ and CD8+ T cells on subsequent disease severity, but no such evidence exists in RSV [45]. In infants hospitalized with bronchiolitis, CD8+ T cells dominate the adaptive response, which might suggest a role in pathogenesis [47]. However, children with defects in cell-mediated immunity suffer prolonged RSV infections, implying that induction of T cells may be beneficial as part of a balanced immune response [48]. Further analysis of T cell responses following infection is required to further delineate their role.
Impact of a failed vaccine
One of the key contributors to the slowing of RSV vaccine development was the failure in the 1960s of a formalin-inactivated vaccine candidate (FI-RSV). Formalin treatment is a common approach used for the inactivation of whole virus vaccines. It diminishes the replicative function, but the outer virion coat remains intact, which aids immune recognition. The method resulted in production of a safe and effective vaccine against poliovirus. Kim et al. developed a FI-RSV vaccine (Lot-100), which was tested in infants and children aged between 2 months and 9 years in the late 1960s [49]. FI-RSV vaccination was delivered intramuscularly in two or three doses separated by 1-3 months. Early results were promising; 43% of the vaccinees had a minimum fourfold increase in neutralizing antibodies and 91% had a minimum fourfold increase in complement fixing antibodies. However, upon natural RSV infection, 80% of the FI-RSV-vaccinated subjects were hospitalized, whereas only 5% of the parainfluenza-vaccinated control group required admission. Two children died and adverse effects were apparent in vaccinated infants, who were diagnosed with pneumonia, bronchiolitis, rhinitis and bronchitis.
The postmortem analysis of the lungs from the two fatal cases reported infiltration of mononuclear cells, neutrophils and eosinophils [49]. Further analysis of samples indicated that antibody–antigen complex deposition and complement activation were seen in the lung tissue. On more detailed investigation, serum antibodies directed against the surface proteins G and F in the sera of FI-RSV vaccinees were found to be poorly neutralizing, almost certainly contributing to decreased vaccine efficacy [50]. In addition, formalin inactivation was found to cause the formation of reactive carbonyl groups, which affected the quality of antibody responses [17].
Supporting the concept that cellular immune responses contributed to the vaccine-enhanced disease, in vitro studies showed exaggerated proliferation of lymphocytes from peripheral blood mononuclear cells (PBMCs) of the FI-RSV vaccinees compared with a nonvaccinated control group following natural infection [51]. A number of animal models of FI-RSV vaccination have subsequently shown skewing of virus-specific CD4+ T cell responses toward a Th2 phenotype [17,52]. Th2 cells are the main producers of the cytokines IL-4 and IL-5, which typically support eosinophilia, IgE production and airway hyper-sensitivity [53]. It has been proposed that this Th2 bias might be due to the absence of the normal Th1 response elicited by viral infection with paucity of cytotoxic CD8+ T cell responses related to nonreplicating virus. In addition, it has recently been shown that enhanced disease is associated with a profound loss of regulatory T cells during viral challenge [54].
Thus, animal studies suggest that, via a number of dysregulated adaptive mechanisms, FI-RSV vaccination promoted harmful immunopathology while providing no immune protection. Although these studies must be interpreted carefully, caution surrounding the risks of any novel vaccine causing similar harm has compelled more systematic approaches that have rightly prioritized safety over speed of advancement.
Animal models of human RSV infection
The adverse effects of FI-RSV vaccines highlight the importance of preclinical safety testing of vaccine candidates. However, the use of animal models in hRSV has been problematic and no single animal model adequately replicates all aspects of hRSV disease. For example, young children are typically most severely affected by RSV infection and the presentation of disease changes with age. In neonates, nonspecific presentation with symptoms such as periodic breathing and apnea is common, whereas older infants are more likely to exhibit upper and lower respiratory tract signs such as nasal discharge, wheeze and labored breathing [55]. In elderly adults, viral lower respiratory tract infections with RSV are less well studied, but it is likely that additional factors, such as bacterial superinfection, contribute to increased mortality [56].
RSV disease is usually confined to the respiratory tract. Although the receptor(s) for virus entry is not fully elucidated, RSV preferentially infects respiratory epithelial cells; infection of other cell types is seen in vitro only at high multiplicities of infection [57]. Studies restricted to systemic immune responses, including measurements of serum antibody, may therefore not address the most relevant compartment. Analysis of respiratory samples from natural RSV infection or RSV-challenged adult volunteers has shown production of various cytokines and chemokines, including IL-6, TNF-α, IL-8, IP-10, RANTES and MIP-1, which diverge markedly from those detected in the blood [58,59]. The activity of these early inflammatory mediators shapes the innate and adaptive immune responses. Furthermore, in the bronchoalveolar lavage (BAL) of naturally infected infants, neutrophils have been reported to be the most abundant cells, followed by macrophages, eosinophils and T lymphocytes, the relative importance of which remains unclear [60,61]. An appropriate animal model should therefore allow detailed examination of mucosal immunity, despite the current lack of understanding of equivalent responses in humans.
An ideal animal model should allow determination of which antigens to use in a candidate vaccine, the dose and route of delivery as well as any adverse side effects to the host. It should also mimic the clinical and pathological characteristics of hRSV infection. Practically, it is not possible to find all these features in one model, and it is therefore essential to choose the most appropriate model in order to study the cells, mediators and responses of interest. The main animal models used today include primates, bovine calves, mice and cotton rats.
Chimpanzees
Chimpanzees have a historical place in hRSV studies as the virus was first recognized as an infectious agent in this species as chimpanzee coryza agent [62]. Although chimpanzees allow hRSV replication upon experimental challenge, they only experience mild symptoms, including nasal discharge, sneezing and coughing, typically seen in upper respiratory tract diseases [63]. To date, there are no reports indicating lower respiratory tract disease in hRSV-infected chimpanzees. In addition, the maintenance, logistics, ethical issues and the limitation of reagents in working with these animals make them a less popular choice. Nevertheless, the similarity in their genetics and anatomy to man can have advantages for vaccine testing.
Cattle
Although sharing some of the logistic difficulties studying primates, studies of RSV infection in calves have considerable advantages. Cattle are a natural host of bovine RSV (bRSV), which does not infect humans, but is a ubiquitous pathogen in commercial herds and a leading cause of respiratory infection [64]. The spectrum of diseases it causes overlaps with hRSV disease. Infected calves may be asymptomatic, but may alternatively show severe respiratory signs (tachypnea, hyperinflation and cyanosis) and die of respiratory failure [65]. Antonis et al. describe the association of age with the development of severe bRSV disease: neonatal (1-day-old) calves are clinically less affected than young (6-week-old) calves but have greater lung pathology with lung neutrophilia and macrophage recruitment and an increase in the levels of IFN-γ, TNF-α and IL-6 in BAL fluid [66]. A disadvantage of experimentation on cattle is the considerable cost of experimental studies with large animals and the restricted range of laboratory reagents.
Cotton rats
Cotton rats are appreciated as important models for studying infectious human disease and are now used as standard for the evaluation of new vaccines, antivirals and prophylactic drugs in RSV. They provided the majority of the preclinical data that led to the licensure of palivizumab, with pharmacokinetics having been established in the model [67]. In cotton rats, RSV replication occurs in the lower airways and virus can be detected in both the upper and lower respiratory tract, leading to moderate bronchiolitis or pneumonia [68]. Upon experimental hRSV challenge, there is an induction of early IFN, chemokine and TLR responses [69].
The effect of FI-RSV vaccination has been studied extensively in cotton rats. Vaccine-enhanced disease occurs with infiltration of neutrophils, macrophages and lymphocytes. This has proven useful for the elucidation of disease mechanisms, but Shaw et al. highlight the role of nonviral components in these studies. FI-RSV is often a crude virus preparation contaminated with cell debris. Although priming and challenge with RSV-containing preparations lead to enhanced respiratory disease, virus-free cell culture components also cause sensitization [70]. To circumvent this problem, most laboratories use purified RSV stocks [71]. It is vital to include appropriate controls in these studies and to appreciate the complexities of the models.
Although cotton rats are clearly useful in preclinical studies of hRSV vaccination, the immunological reagents and techniques that are available to those using cotton rats are more limited than those available for mouse studies. There are no fully inbred or transgenic cotton rats, limiting studies of precise immunological pathways.
Mice
Mice are the most commonly used animals for experimental studies of hRSV infection. There are numerous reasons for this including the availability of immunological reagents, a complete genome sequence and the availability of transgenics, congenics and knockouts. The mouse genome is similar to humans with 99% of mouse genes having a human homolog [72]. Similar to cotton rats, mice are semipermissive hosts for hRSV replication. Using varying doses of inoculum between 105 and 107 pfu, a mild infection, moderate-to-severe lung pathology and illness can be induced [55]. It is important to highlight that differing substrains and isolates of RSV and mice result in different effects. The most commonly used combination is BALB/c mice and the A2 strain of RSV; such mice exhibit moderate-to-severe respiratory tract disease with signs of illness including weight loss, hunching, reduced motile activity, hypoxia and increased breathing rate [55]. Mice infected with hRSV have an increase in early innate cytokines, such as TNF-α, IL-6, MIP-1α and RANTES, in the BAL [73], which parallels findings from human infant studies [74]. Mice have been especially useful in studying the detailed responses of the innate and cell-mediated immune responses to RSV infection and in elucidating the effects of immune enhancement by prior vaccination [12].
Experimental human infection of adult volunteers with RSV
Despite the practical advantages and the availability of genetically modified animals that allow study of precise mechanistic pathways, there are major drawbacks in only using animals in experimental studies of RSV infection. Mice are mostly inbred and clean, whereas humans are outbred and have experienced a range of past and present infections and other environmental influences. Humans are continuously exposed to a variety of pathogens living as they do in diverse environments and suffering multiple infections throughout life, leading to complex antigenic histories that program their immune systems in a variety of ways. For these reasons, the immunobiology of humans may be radically different from that of mice. For example, it is known that mice that are older than 15 months are more permissive to RSV replication than younger animals [75]. Although there is no record of primary RSV infection occurring in adults or in elderly persons, this does not appear to be the case in man; most severe RSV cases occur before the age of 2 years. In addition, severe disease in mice leads to weight loss, which is not a major feature of human infection.
Preclinical studies in animal models may not adequately predict vaccine responses in human beings. Numerous vaccine candidates that have demonstrated excellent immunogenicity in animals have failed in man, while a number of vaccine candidates whose development was stopped due to poor preclinical efficacy might possibly have been effective in humans. However, clinical efficacy trials of RSV vaccines have a number of logistical difficulties to overcome. These include decisions on which patient groups to vaccinate and how to determine the outcome when the RSV incidence may be relatively low, particularly in adult populations.
The experimental hRSV infection model may have an important role in this regard. Experimental infection of volunteers with RSV has an extensive history with substantial work in the field having been carried out at the MRC Common Cold Unit from 1946 to 1989. In these studies, adults are inoculated with a fixed dose of a well-characterized virus, quarantined and sampled longitudinally during the course of infection. Compared with natural infection studies, this offers the advantages of standardized study populations, defined as virus inoculum, the ability to examine immune correlates both before and after inoculation, definite diagnosis of infection and the richness of longitudinal data analysis [58]. For the purposes of vaccine development, this allows the determination of correlates of protection and can demonstrate efficacy of protection by vaccine candidates without reliance on the relatively unreliable event of natural RSV exposure. Although infection of healthy adults does not necessarily recapitulate at-risk infants or immunosuppressed individuals, it may directly inform attempts to vaccinate other groups of adults including pregnant women and the healthy elderly.
Who to vaccinate against RSV infection
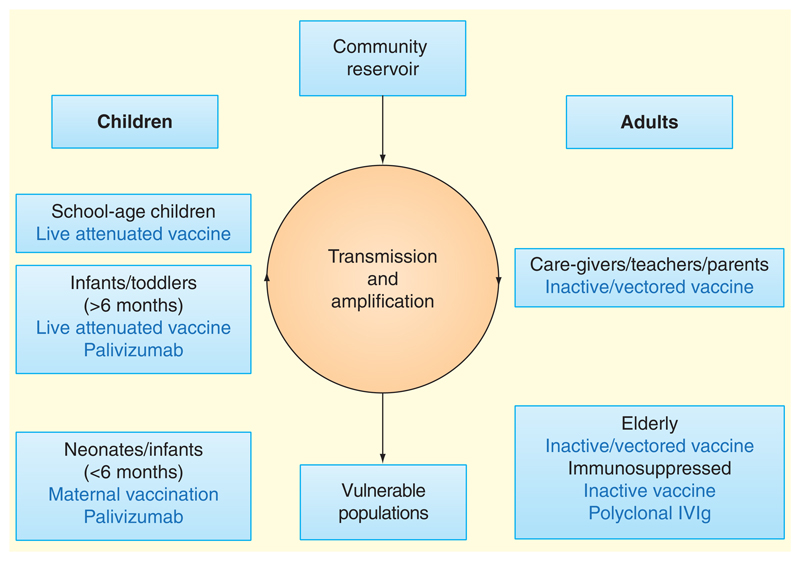
Although severe RSV primarily occurs in young children and the elderly, a variety of issues make these populations particularly difficult to target. Vaccine candidates are available that would be suitable to go into some of these populations (including live attenuated viruses in infants or young children and subunit vaccines for the elderly), and these may have both direct benefits to at-risk groups and interrupt transmission between susceptible groups, vectors and community reservoirs (Figure 1). However, high-risk populations are likely to have impaired immune responses, either due to immaturity, immunosuppression or immunosenescence. Vaccine safety is clearly an important issue, with the history and understanding of FI-RSV having caused extreme reluctance to vaccinate RSV-na'ive infants with an inactivated vaccine. In elderly adults, the efficacy trials that will be required for licensing may be impractical in view of the number of participants that would be required for an adequately powered study.
Figure 1. Targeting different populations with immune interventions against respiratory syncytial virus (RSV).
Multiple checkpoints may be targeted for the interruption of RSV infection. At-risk individuals may be protected directly through induction of protective immunity or indirectly via reduced circulation of infection in the community. Vaccination of healthy individuals aims to promote herd immunity and/or prevent transmission between school-aged children and adults with whom they are in contact. This would interrupt the amplification loop within households, hospitals and schools, reducing transmission to vulnerable populations (i.e., neonates, immunosuppressed individuals and the elderly). Vaccination of pregnant women during the last trimester aims to enhance maternofetal transfer of IgG to protective levels in term infants, but passive vaccination would probably still be required for preterm infants or immunosuppressed adults. Inactive or subunit RSV vaccines are most appropriate for RSV-experienced individuals in whom the risk of vaccine-enhanced disease is low. Live attenuated vaccines are likely to be safe in children, and the induction of mucosal immunity would have the added benefit of reducing virus shedding.
Recent emphasis has therefore fallen on maternal vaccination as a strategy for boosting protection in the neonate. IgG is actively transported across the placenta during the final weeks of pregnancy, and epidemiological studies have shown a negative correlation between the level of maternal neutralizing antibody and infectious disease incidence [76]. Recent studies examining maternal vaccination with trivalent inactivated influenza vaccine have suggested benefit to the mother and protection of infants up to 6 months of age. For these reasons, there has recently been renewed emphasis on the development of a maternally administered RSV vaccine in the hope of elevating the infant IgG level beyond the lower threshold of neutralizing antibody titer above which the risk of severe disease is reduced. A variety of vaccine strategies may be successful in this regard, with F protein subunit, VLPs and vectored vaccines all having shown success in the induction of neutralizing antibodies in preclinical models as well as some early Phases I and II trials [77–80]. Although this strategy would not protect premature infants, prolonging the window during which maternal antibody reduces the risk of infection before the immune system matures sufficiently for a normal anti-RSV response would be a major advance.
Current progress in vaccine development
Advances in our understanding of the structural biology of RSV F protein, estimates of global disease burden and the immune responses to infection outlined above, have led to widespread acceleration in the development of RSV vaccines, and these have recently been reviewed extensively elsewhere [81–83].
The spectrum of vaccine candidates in development ranges from live attenuated viruses to vector-based and subunit vaccines in a wide variety of forms, and it is likely that no single vaccine will be appropriate for all age groups. With the elucidation of the prefusion F protein structure, the number of particle-based and subunit vaccines that aim to deliver antigen in this form is on the rise. In early human studies, these appear immunogenic and well tolerated [77]. These have the potential to induce incremental rises in serum neutralizing antibody titer greater than natural infection, but the longevity of these responses and their ability to stimulate appropriate T cell responses are yet to be investigated in detail. Furthermore, inactive vaccines are unlikely to be suitable for use in RSV-naïve children for whom safety concerns will exist and may not induce significant local immunity unless delivered intranasally. A number of vector-based vaccines do seek to induce responses beyond serum neutralizing antibody, and these offer the promise of inducing a more balanced immune response that may be preferred.
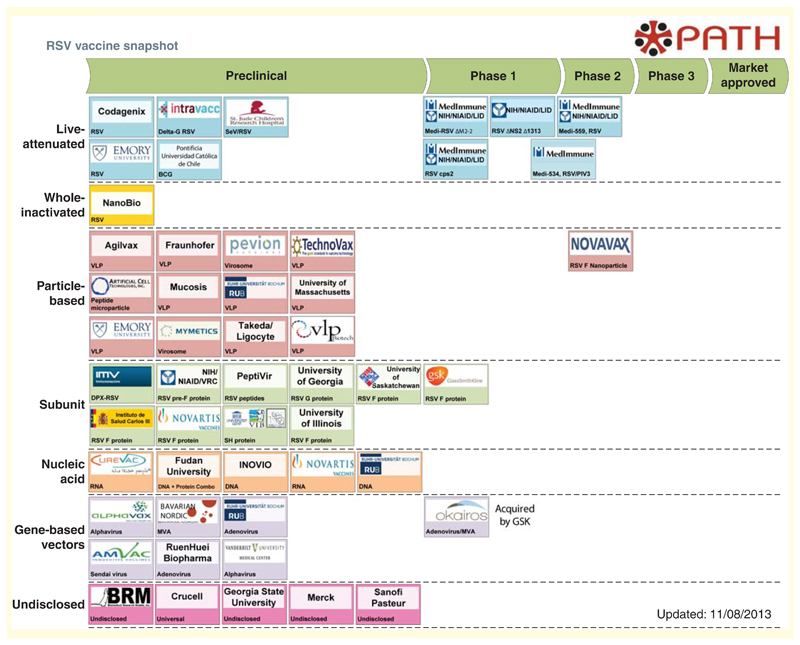
With this increase in knowledge and enhanced incentives to vaccine development, there are now many potential vaccines at various stages of testing. The current pipeline is summarized in Figure 2, which is reproduced with kind permission of PATH. This 'snapshot' of RSV vaccine development is regularly updated and available online [84].
Figure 2. Vaccines currently in development against human respiratory syncytial virus infection.
Many candidate vaccines are at preclinical stage. However, there is live attenuated, particle-based, subunit and gene-based vectors being tested at Phase I or II trials. The respiratory syncytial virus vaccine snapshot is reproduced with permission from PATH and can be found at [84]. Reproduced with permission from the PATH [101].
Live attenuated virus vaccines
Live attenuated vaccine candidates have historically dominated Phases I and II trials. RSV can be attenuated by multiple strategies including cold passage, mutagenesis and reverse genetics. An early cold passaged, temperature-sensitive RSV vaccine (cpts-248/404) with mutations in N, F and L genes, when delivered intramuscularly to seronegative infants, was safe but poorly immunogenic [85]. Encouragingly, this vaccine showed no significant side effects and reverse genetics was used to try to generate a more immunogenic strain. By introducing changes in the small hydrophobic gene, a further live attenuated RSV candidate (rA2cp248/404/1030ΔSH or Medi-559; MedImmune/National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA) was developed and tested in a Phase I clinical trials on healthy children and infants [86]. The majority of vaccinees made a serological response and/or developed viral shedding after inoculation, but an increase in the number of medical attendances led to further safety testing requirements. A Phase II trial to determine its ability to induce protective immunity upon natural exposure to RSV is underway [87,88]. Medi-559 has recently been improved for stability, resulting in a new formulation that is indistinguishable from MEDI-559 in terms of the replication and disease induction in seronegative chimpanzees. This may replace Medi-559 in subsequent clinical trials [89].
Although Medi-559 is the leading attenuated vaccine candidate, there are other promising strains in Phase I clinical trials. Medi-534 is a live, attenuated RSV/PIV3 chimeric virus vaccine candidate that has been tested in seronegative children between 6 and 24 months of age [80]. This vaccine candidate is thought to be safe, and administration of three doses over 4 months showed that the virus was infectious and immunogenic in the tested population [80]. A recent report examining local immune responses to a range of live attenuated viruses from Karron et al. showed increases in pro-, anti- and regulatory cytokines in nasal samples, suggesting that appropriate cytokine responses might contribute to additional protection [90].
Unfortunately, despite their good safety record and absence of vaccine-enhancement issues, poor immunogenicity, under-attenuation, genetic reversion and secondary transmissibility have dampened enthusiasm for live attenuated RSV vaccines [90]. Despite this, continuing advances in understanding of RSV disease and the technologies involved in virus generation may allow further development of optimally attenuated viruses for future study.
Particle-based vaccines
A number of VLP vaccines are in preclinical development. These multiprotein structures mimic the viral organization but lack the viral genome, providing noninfectious particles. The main advantage of VLPs is that the potency of the vaccine can be enhanced by exclusion of viral proteins with immunosuppressive function while presenting antigen in an optimum configuration. In addition, VLPs can bind pattern recognition receptors to trigger of innate immunity, which can promote B cell responses in the absence of adjuvants [91]. However, VLP manufacture may be difficult to optimize; despite the number of expression vectors that can be used to synthesize VLPs, the glycosylation and the correct folding of the proteins may be a limitation for their use in humans. Glycosylation cannot be achieved by bacteria and yeast is limited to high mannose glycoprotein modification that is inconsistent. Using mammalian culture systems is an option that favors the modification and assembly of the particles, but it is less controllable and more costly [92].
One leading VLP vaccine candidate against RSV is an F nanoparticle (Novavax, Inc., 9920 Belward Campus Drive, Rockville, MD 20850, USA). This particle is an insect cell-derived RSV F protein vaccine that is safe and protective in cotton rats [93]. As part of the Phase I clinical trials, it was administered intramuscularly to healthy adults [77]. The vaccine was found to be safe with no adverse effects, inducing a minimum sevenfold rise in anti-F IgG responses and achieving comparable to titers to those seen after administration of palivizumab.
Subunit vaccines
Immunization with purified proteins isolated from pathogens has a long and successful history. However, there are few subunit vaccines licensed for viral infections, and these are thought to assemble into VLPs [94]. Early clinical trials examined purified F protein (PFP) formulated with alum [95–98]. Gonzalez et al. evaluated the effects of immunization with the live attenuated virus cpts 248/404 followed by PFP-2 in adults in whom both vaccines were well tolerated. They induced a minimum fourfold rise in neutralizing antibody titers in both young and elderly participants [96]. Immunization of pregnant women with PFP-2 was also safe [95]. Following immunization, 95% of the recipients had a minimum fourfold increase in IgG levels. In addition, a modest increase in neutralizing antibody titers was observed in the babies at birth, 2 and 6 months after delivery. Anti-F IgA and IgG concentrations in breast milk were higher in mothers who received the PFP-2 vaccine [95].
More recently, a vaccine candidate containing a formulation of F, G and M subunits with or without alum has been trialed in elderly persons. All formulations were well tolerated, regardless of adjuvant. Interestingly, the nonadjuvanted formulation demonstrated most efficacy with 58% of the subjects demonstrating a minimum fourfold increase in the neutralizing antibody titers against RSV A [98].
Subunit F vaccines are well tolerated in seropositive individuals and induce neutralizing antibody, but in seronegative infants, there is a theoretical concern that immunization will induce vaccine-enhanced disease. Several groups are pursuing PFP subunit vaccines through Phase I clinical trials for the vaccination of adults, and particularly of pregnant women (Figure 2).
Vector-based vaccines
Viral vectors were initially developed for gene therapy, but their ability to induce innate and adaptive responses in mammalian hosts has also made them potential vaccine vehicles. Multiple vectors derived from viruses or bacteria exist for the delivery of genes-encoding proteins of interest. These vectors have the major advantage of mimicking natural infection in the host, and viral vectors allow transcription and translation via host cell machinery. This is not only important for processing and presentation of antigen to achieve priming of CD4+ and CD8+ T cells but also for the induction of relevant antibody responses. Moreover, vectors can be rendered replication incompetent and cannot evade immunity through selective gene expression, increasing their safety. Adenoviruses are a particularly attractive vector for vaccine delivery for many reasons: they can be grown to high titers in tissue culture, they can be inoculated systemically or at the mucosal surfaces and there are multiple serotypes available for use in humans [99]. Geraldine Taylor's group at the Pirbright Institute, in collaboration with Okairos (Basel, Switzerland), has recently presented work on a bRSV model where newborn calves without maternally derived RSV-specific antibodies were primed intranasally with a replication-defective chimpanzee Adenoviruses/RSV and boosted with Modified Vaccinia Ankara/RSV 8 weeks later. On Week 12 postpriming, the animals were challenged with bRSV and samples analysed on Week 13. Compared with control groups, prime/boosted animals had a significant rise in serum IgG, mucosal IgA and frequency of IFN-γ-producing CD4+ and CD8+ T cells, and calves were completely protected against bRSV infection. This strategy is now being evaluated in Phase I clinical trials [100].
Conclusion
Historically, RSV vaccine development has been hindered by two major stumbling blocks: limited immunogenicity and vaccine-enhanced disease. However, recent advances have increased likelihood that progress can be made in vaccination against RSV, which remains one of the greatest remaining challenges for vaccine development. This imperative is driving the research and development toward making rapid progress. We are of the opinion that the future for an RSV vaccine is now bright, and that the approaches outlined in this review have the potential to achieve a safe and effective vaccine.
Expert commentary
Of the major unmet challenges posed by infectious disease, prevention of RSV through vaccination is widely viewed as being among the most likely to be solved in the next decade, at least for certain target groups. Compared with tuberculosis, HIV or malaria, RSV infection would seem easier to prevent; however, attempts at developing an effective vaccine have been hampered by fundamental difficulties: poor immunogenicity, reactogenicity, fear of immune-mediated disease enhancement and antigen production.
The research agenda developed in academic laboratories has now been passed to small and large pharmaceutical companies with substantial records of success and well-developed vaccine divisions. Many lessons have been learned in other fields of vaccinology that can be applied to RSV vaccine research; with improved understanding of the key structural and antigenic requirements of RSV vaccination, we are optimistic that an RSV vaccine will be available within the next 10 years.
Five-year view
With the current level of increased activity in the field of RSV vaccine development, we anticipate that RSV vaccines may become available for use in one or more of the target groups. In addition to improved prophylaxis, we anticipate that it may be possible to interrupt RSV's community circulation by such interventions.
Key issues.
Improved understanding of protective and harmful immunity to respiratory syncytial virus.
Protein engineering of antigens that can induce broadly neutralizing antibody responses against respiratory syncytial virus.
Tailoring prevention to specific vulnerable or important groups (infants, toddlers, adult care givers, pregnant mothers and the elderly).
Financial & disclosure
The work is supported by the Wellcome Trust (programme grants 090382 and 25092, PO), the BBSRC (AG/PO) and the Medical Research Council UK (G0902266, CC). The authors acknowledge the NIHR support of the comprehensive Biomedical Research Centre (cBRC) at Imperial College NHS Healthcare Trust. P Openshaw grant co-funding from GlaxoSmithKline; no input in the content or writing of this manuscript. A Guvenel has also received grant cofunding from GlaxoSmithK-line.
Footnotes
competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Author contributions
All authors contributed to writing the manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Nair H, Verma VR, Theodoratou E, et al. An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children. BMC Public Health. 2011;11(Suppl 3):S30. doi: 10.1186/1471-2458-11-S3-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferolla FM, Hijano DR, Acosta PL, et al. Macronutrients during pregnancy and life-threatening respiratory syncytial virus infections in children. Am J Respir Crit Care Med. 2013;187(9):983–90. doi: 10.1164/rccm.201301-0016OC. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [ • In the USA, 78% of RSV-associated deaths occur among those aged 65 years or older, establishing that RSV is a major pathogen in older persons and is not just a disease of infancy ] [DOI] [PubMed] [Google Scholar]
- 4.Openshaw PJ, Chiu C. Protective and dysregulated T cell immunity in RSV infection. Curr Opin Virol. 2013;3(4):468–74. doi: 10.1016/j.coviro.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179(1):25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 6.Smyth RL, Openshaw PJM. Bronchiolitis. Lancet. 2006;368(9532):312–22. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 7.Parrott RH, Kim HW, Arrobio JO, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. II. Infection and disease with respect to age, immunologic status, race and sex. Am J Epidemiol. 1973;98(4):289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- 8.Ochola R, Sande C, Fegan G, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One. 2009;4(12):e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 10.Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368(19):1791–9. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 11.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162(1-2):80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Openshaw PJM, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18(3):541–55. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halfhide CP, Flanagan BF, Brearey SP, et al. Respiratory syncytial virus binds and undergoes transcription in neutrophils from the blood and airways of infants with severe bronchiolitis. J Infect Dis. 2011;204(3):451–8. doi: 10.1093/infdis/jir280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durant LR, Makris S, Voorburg CM, et al. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol. 2013;87(20):10946–54. doi: 10.1128/JVI.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cade A, Brownlee KG, Conway SP, et al. Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child. 2000;82(2):126–30. doi: 10.1136/adc.82.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartling L, Fernandes RM, Bialy L, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ. 2011;342:d1714. doi: 10.1136/bmj.d1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghaddam A, Olszewska W, Wang B, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12(8):905–7. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 18.Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J Immunol. 2007;179(8):5415–24. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- 19.Cherukuri A, Patton K, Gasser RA, et al. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20(2):239–47. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gates Foundation. Available from: www.gatesfoundation.org/What-We-Do/Global-Review .
- 21.Bukreyev A, Yang L, Fricke J, et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol. 2008;82(24):12191–204. doi: 10.1128/JVI.01604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chirkova T, Boyoglu-Barnum S, Gaston KA, et al. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol. 2013;87(24):13466–79. doi: 10.1128/JVI.01741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shingai M, Azuma M, Ebihara T, et al. Soluble G protein of respiratory syncytial virus inhibits Toll-like receptor 3/4-mediated IFN-beta induction. Int Immunol. 2008;20(9):1169–80. doi: 10.1093/intimm/dxn074. [DOI] [PubMed] [Google Scholar]
- 24.Choi Y, Mason CS, Jones LP, et al. Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C-CX3CR1 binding and cross-neutralize RSV A and B strains. Viral Immunol. 2012;25(3):193–203. doi: 10.1089/vim.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyoglu-Barnum S, Gaston KA, Todd SO, et al. A respiratory syncytial virus (RSV) anti-G protein F(ab')2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected BALB/c mice. J Virol. 2013;87(20):10955–67. doi: 10.1128/JVI.01164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85(15):7788–96. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Argüello MB, Martín D, Wharton SA, et al. Thermostability of the human respiratory syncytial virus fusion protein before and after activation: implications for the membrane-fusion mechanism. J Gen Virol. 2004;85(Pt 12):3677–87. doi: 10.1099/vir.0.80318-0. [DOI] [PubMed] [Google Scholar]
- 28.Haynes LM, Moore DD, Kurt-Jones EA, et al. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75(22):10730–7. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awomoyi AA, Rallabhandi P, Pollin TI, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179(5):3171–7. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 30.Tulic MK, Hurrelbrink RJ, Prêle CM, et al. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J Immunol. 2007;179(1):132–40. doi: 10.4049/jimmunol.179.1.132. [DOI] [PubMed] [Google Scholar]
- 31.Magro M, Andreu D, Gómez-Puertas P, et al. Neutralization of human respiratory syncytial virus infectivity by antibodies and low-molecular-weight compounds targeted against the fusion glycoprotein. J Virol. 2010;84(16):7970–82. doi: 10.1128/JVI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–17. doi: 10.1126/science.1234914. [ •• Excellent study reporting the prefusion structure of the F protein, showing a newly identified site of antigenicity, named antigenic site Ø. This potentially identifies a new target for passive prevention of RSV disease and suggests novel approaches to vaccine development. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo MS, Brazas RM, Holtzman MJ. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J Virol. 2005;79(14):9315–19. doi: 10.1128/JVI.79.14.9315-9319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez L, Cuesta I, Asenjo A, Villanueva N. Human respiratory syncytial virus matrix protein is an RNA-binding protein: binding properties, location and identity of the RNA contact residues. J Gen Virol. 2004;85(Pt 3):709–19. doi: 10.1099/vir.0.19707-0. [DOI] [PubMed] [Google Scholar]
- 35.Shaikh FY, Cox RG, Lifland AW, et al. A critical phenylalanine residue in the respiratory syncytial virus fusion protein cytoplasmic tail mediates assembly of internal viral proteins into viral filaments and particles. MBio. 2012;3:1. doi: 10.1128/mBio.00270-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–8. doi: 10.1542/peds.2013-0303. [ •• Large infant study of hospitalized children with acute RSV infection, showing that many children hospitalized with RSV infection have no identifiable risk factor ] [DOI] [PubMed] [Google Scholar]
- 37.Sande CJ, Mutunga MN, Okiro EA, et al. Kinetics of the neutralizing antibody response to respiratory syncytial virus infections in a birth cohort. J Med Virol. 2013;85(11):2020–5. doi: 10.1002/jmv.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling Z, Tran KC, Teng MN. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol. 2009;83(8):3734–42. doi: 10.1128/JVI.02434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7. [PubMed] [Google Scholar]
- 40.Kawasaki Y, Hosoya M, Katayose M, Suzuki H. Role of serum neutralizing antibody in reinfection of respiratory syncytial virus. Pediatr Int. 2004;46(2):126–9. doi: 10.1046/j.1442-200x.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 41.Shinoff JJ, O'Brien KL, Thumar B, et al. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J Infect Dis. 2008;198(7):1007–15. doi: 10.1086/591460. [DOI] [PubMed] [Google Scholar]
- 42.Piedra PA, Jewell AM, Cron SG, et al. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21(24):3479–82. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 43.Singleton R, Etchart N, Hou S, Hyland L. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J Virol. 2003;77(21):11303–11. doi: 10.1128/JVI.77.21.11303-11311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frogel MP, Stewart DL, Hoopes M, et al. A systematic review of compliance with palivizumab administration for RSV immunoprophylaxis. J Manag Care Pharm. 2010;16(1):46–58. doi: 10.18553/jmcp.2010.16.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tregoning JS, Wang BL, McDonald JU, et al. Neonatal antibody responses are attenuated by interferon-γ produced by NK and T cells during RSV infection. Proc Natl Acad Sci USA. 2013;110(14):5576–81. doi: 10.1073/pnas.1214247110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tregoning JS, Yamaguchi Y, Harker J, et al. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;82(8):4115–24. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heidema J, Lukens MV, van Maren WWC, et al. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol. 2007;179(12):8410–17. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
- 48.Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315(2):77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 49.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 50.Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26(8):1595–7. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HW, Leikin SL, Arrobio J, et al. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976;10(1):75–8. doi: 10.1203/00006450-197601000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Graham BS, Davis TH, Tang YW, Gruber WC. Immunoprophylaxis and immunotherapy of respiratory syncytial virus-infected mice with respiratory syncytial virus-specific immune serum. Pediatr Res. 1993;34(2):167–72. doi: 10.1203/00006450-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31(3):425–37. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loebbermann J, Durant L, Thornton H, et al. Defective immunoregulation in RSV vaccine-augmented viral lung disease restored by selective chemoattraction of regulatory T cells. Proc Natl Acad Sci USA. 2013;110(8):2987–92. doi: 10.1073/pnas.1217580110. [• In mice, there is a remarkable absence of regulatory T cells in those with vaccine-augmented disease. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301(2):L148–56. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee N, Lui GCY, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069–77. doi: 10.1093/cid/cit471. [DOI] [PubMed] [Google Scholar]
- 57.Domurat F, Roberts NJ, Walsh EE, Dagan R. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J Infect Dis. 1985;152(5):895–902. doi: 10.1093/infdis/152.5.895. [DOI] [PubMed] [Google Scholar]
- 58.DeVincenzo JP, Wilkinson T, Vaishnaw A, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182(10):1305–14. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oshansky CM, Barber JP, Crabtree J, Tripp RA. Respiratory syncytial virus F and G proteins induce interleukin 1alpha, CC, and CXC chemokine responses by normal human bronchoepithelial cells. J Infect Dis. 2010;201(8):1201–7. doi: 10.1086/651431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bem RA, Bos AP, Bots M, et al. Activation of the granzyme pathway in children with severe respiratory syncytial virus infection. Pediatr Res. 2008;63(6):650–5. doi: 10.1203/PDR.0b013e31816fdc32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNamara PS, Ritson P, Selby A, et al. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child. 2003;88(10):922–6. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blount RE, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92(3):544–9. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- 63.Whitehead SS, Bukreyev A, Teng MN, et al. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73(4):3438–42. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klem TB, Gulliksen SM, Lie K-I, et al. Bovine respiratory syncytial virus: infection dynamics within and between herds. Vet Rec. 2013;173(19):476. doi: 10.1136/vr.101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antonis AFG, Schrijver RS, Daus F, et al. Vaccine-induced immunopathology during bovine respiratory syncytial virus infection: exploring the parameters of pathogenesis. J Virol. 2003;77(22):12067–12073. doi: 10.1128/JVI.77.22.12067-12073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antonis AFG, de Jong MC, van der Poel WHM, et al. Age-dependent differences in the pathogenesis of bovine respiratory syncytial virus infections related to the development of natural immunocompetence. J Gen Virol. 2010;91(Pt 10):2497–506. doi: 10.1099/vir.0.020842-0. [DOI] [PubMed] [Google Scholar]
- 67.Prince GA, Hemming VG, Horswood RL, Chanock RM. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3(3):193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 68.Boukhvalova MS, Prince GA, Blanco JCG. The cotton rat model of respiratory viral infections. Biologicals. 2009;37(3):152–9. doi: 10.1016/j.biologicals.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boukhvalova MS, Sotomayor TB, Point RC, et al. Activation of interferon response through toll-like receptor 3 impacts viral pathogenesis and pulmonary toll-like receptor expression during respiratory syncytial virus and influenza infections in the cotton rat Sigmodon hispidus model. J Interferon Cytokine Res. 2010;30(4):229–42. doi: 10.1089/jir.2009.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw CA, Galarneau J-R, Bowenkamp KE, et al. The role of non-viral antigens in the cotton rat model of respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2013;31(2):306–12. doi: 10.1016/j.vaccine.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Prince GA, Jenson AB, Hemming VG, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986;57(3):721–8. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guénet JL. The mouse genome. Genome Res. 2005;15(12):1729–40. doi: 10.1101/gr.3728305. [DOI] [PubMed] [Google Scholar]
- 73.Jafri HS, Chavez-Bueno S, Mejias A, et al. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis. 2004;189(10):1856–65. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- 74.McNamara PS, Flanagan BF, Hart CA, Smyth RL. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2005;191(8):1225–32. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- 75.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26(2):153–62. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 76.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 77.Glenn GM, Smith G, Fries L, et al. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31(3):524–32. doi: 10.1016/j.vaccine.2012.11.009. [ •• An F protein nanoparticle vaccine given intramuscularly to volunteers was well tolerated and induced high levels of neutralizing anti-RSV antibodies. ] [DOI] [PubMed] [Google Scholar]
- 78.Nelson CL, Tang RS, Stillman EA. Genetic stability of RSV-F expression and the restricted growth phenotype of a live attenuated PIV3 vectored RSV vaccine candidate (MEDI-534) following restrictive growth in human lung cells. Vaccine. 2013;31(36):3756–62. doi: 10.1016/j.vaccine.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 79.Schickli JH, Kaur J, Tang RS. Nonclinical phenotypic and genotypic analyses of a Phase 1 pediatric respiratory syncytial virus vaccine candidate MEDI-559 (rA2cp248/ 404/1030DSH) at permissive and non-permissive temperatures. Virus Res. 2012;169(1):38–47. doi: 10.1016/j.virusres.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 80.Bernstein DI, Malkin E, Abughali N, et al. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr Infect Dis J. 2012;31(2):109–14. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 81.Jorquera PA, Oakley KE, Tripp RA. Advances in and the potential of vaccines for respiratory syncytial virus. Expert Rev Respir Med. 2013;7(4):411–27. doi: 10.1586/17476348.2013.814409. [DOI] [PubMed] [Google Scholar]
- 82.Anderson LJ. Respiratory syncytial virus vaccine development. Semin Immunol. 2013;25(2):160–71. doi: 10.1016/j.smim.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 83.Rudraraju R, Jones BG, Sealy R, et al. Respiratory syncytial virus: current progress in vaccine development. Viruses. 2013;5(2):577–94. doi: 10.3390/v5020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaccine Development Global Program. Available from: http://sites.path.org/vaccinedevelopment/respiratory-syncytial-virus-rsv/
- 85.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–42. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 86.Malkin E, Yogev R, Abughali N, et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-Seronegative children 5 to 24 months of age. PLoS One. 2013;8(10):e77104. doi: 10.1371/journal.pone.0077104. [ •• A significant immune response was seen in 59% of vaccine recipients without clinically significant adverse events in seronegative infants. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191(7):1093–104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 88.Clinical Trials.gov. 2013. Available from: www.clinicaltrials.gov.
- 89.Luongo C, Winter CC, Collins PL, Buchholz UJ. Increased genetic and phenotypic stability of a promising live-attenuated respiratory syncytial virus vaccine candidate by reverse genetics. J Virol. 2012;86(19):10792–804. doi: 10.1128/JVI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karron RA, Thumar B, Schappell E, et al. Attenuation of live respiratory syncytial virus vaccines is associated with reductions in levels of nasal cytokines. J Infect Dis. 2013;207(11):1773–9. doi: 10.1093/infdis/jit089. [ • Children given insufficiently attenuated live RSV vaccine intranasally show increased proinflammatory, anti-inflammatory, Th1, Th2 and regulatory cytokines, whereas those given fully attenuated vaccines do not. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bessa J, Schmitz N, Hinton HJ, et al. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur J Immunol. 2008;38(1):114–26. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- 92.Grgacic EVL, Anderson DA. Virus-like particles: passport to immune recognition. Methods. 2006;40(1):60–5. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith G, Raghunandan R, Wu Y, et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One. 2012;7(11):e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239(1):149–66. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21(24):3465–7. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez IM, Karron RA, Eichelberger M, et al. Evaluation of the live attenuated cpts 248/404 RSV vaccine in combination with a subunit RSV vaccine (PFP-2) in healthy young and older adults. Vaccine. 2000;18(17):1763–72. doi: 10.1016/s0264-410x(99)00527-7. [DOI] [PubMed] [Google Scholar]
- 97.Groothuis JR, King SJ, Hogerman DA, et al. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177(2):467–9. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- 98.Langley JM, Sales V, McGeer A, et al. A dose-ranging study of a subunit Respiratory Syncytial Virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults >or =65 years of age. Vaccine. 2009;27(42):5913–19. doi: 10.1016/j.vaccine.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 99.Tatsis N, Ertl HCJ. Adenoviruses as vaccine vectors. Mol Ther. 2004;10(4):616–29. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scarselli E. RSV Vaccines for the World Conference, Oral Presentation; 2013. [Google Scholar]
- 101.Path. [Last accessed on 20 December 2013]. Available from: www.path.org.