Abstract
Objectives
This study aimed to standardize an “in house” immunoassay to detect anti-Toxocara IgG antibodies in human serum to estimate the seroprevalence of Toxocara infection, and to identify its potential risk factors in children living in poor areas of Salvador, a large northeastern Brazilian city.
Methods
Parents of 1309 children answered a questionnaire containing possible risk factor for acquisition of this infection. Blood was collected and the presence of anti-Toxocara IgG antibodies was detected by indirect ELISA using T. canis larval excretory–secretory antigens in sera previously absorbed with Ascaris lumbricoides antigens.
Results
Seroprevalence of Toxocara infection was 48.4%. Children's age, low maternal schooling, contact with dogs and cats, and household located in paved streets were shown to be risk factors for Toxocara infection.
Conclusions
The seroprevalence of Toxocara infection is high among children living in a poor urban setting of Brazil. The association of low maternal education with higher Toxocara infection supports studies showing that low socioeconomic status is a risk factor for the acquisition of this infection as a reflection of hygiene habits of the family. And both infected-dogs and cats may be involved in this parasite transmission in this children population.
Keywords: Toxocara infection, Seroprevalence, Children, Risk factors
1. Introduction
Visceral larva migrans (VLM) is a syndrome of human beings, transmitted mainly by accidental ingestion of embryonated eggs of Toxocara canis (dog round worms) or T. cati (cat round worm). In humans, the hatching larvae do not migrate to intestine as occur in the definite hosts, and remain migrating through the organs leading to several clinical pictures varying from asymptomatic to severe systemic forms such as prolonged fever, hepato-splenomegaly, meningoencephalitis and asthma-like symptoms (Despommier, 2003; Haralambidou et al., 2005; Saporito et al., 2008). Toxocara infection also causes a hypersensitivity reaction status, even in asymptomatic subject, leading to high eosinophilia, increase in total IgE and high susceptibility to asthma (Ferreira et al., 2007; Dattoli et al., 2011). Although this infection occurs worldwide, its prevalence is higher in non-affluent populations (Coelho et al., 2004; Espinoza et al., 2008), where its diagnosis is rarely performed, being considered a neglected disease. The toxocariasis serodiagnosis depends on the cultivation of T. canis larvae to produce excretory-secretory products used in ELISA as antigens. Currently, a few kits for serodiagnosis are commercial available, but is rarely used in Brazil due to their high cost. This infection is also prevalent in many developed countries and its global importance may be underestimated. In the United States of America, it is the most common helminthic infection, affecting millions of individuals (Hotez and Wilkins, 2009).
Stray and domiciliated dogs and cats from low income population play an important role in the transmission of Toxocara spp. providing environmental contamination, which perpetuates the spreading of the infection among the human populations (Regis et al., 2011). The contact with grounds contaminated with embryonated eggs is the most common Toxocara spp. transmission pathway, but contact with dogs and cats, presenting eggs in their fur, as well as the consumption of raw vegetables grown in contaminated gardens and raw or undercooked meat from paratenic hosts (Abougrain et al., 2009) have also been described as important ways of transmission of this zoonosis (Amaral et al., 2010). Studies in Brazil (Alcântara-Neves et al., 1999; Almeida et al., 2007; Tiyo et al., 2008) and worldwide (Mizgajska, 1997; Devera et al., 2008, Martin and Demonte, 2008) showed that soil of public areas such as plazas, parks, and beaches are important foci of Toxocara ssp. transmission and to frequent such places poses as an important risk factor to the human being to become infected. In addition, factors such as age, maternal education, low socioeconomic conditions, have also been related to this zoonosis (Wolfe and Wright, 2003). Most of these works however were carried out in small sample population of limited areas.
In this work, we aimed to standardize an "in house" immunoassay to detect anti-Toxocara IgG antibodies in human serum and determine the seroprevalence of Toxocara spp. infection in a large set of children living in poor areas of a large Brazilian city and to investigate the risk factors associated with the infection, helping to understand the epidemiology of Toxocara infection in this city and similar settings around the world.
2. Materials and methods
2.1. Study population
The present work is a transversal study, which evaluated whether the Toxocara spp. infection status assessed in 2005 was associated with exposures to potential risk factors for acquisition of the infection. It was conducted in the city of Salvador with nearly 2,800,000 inhabitants, mostly of mixed African descent, located in Northeast Brazil. Briefly, we studied 1309 children aged 4–11 years old, enrolled in a cohort recruited from 1997 to 2003 for evaluating the impact of a sanitation program on the incidence of childhood diarrhea, in different city areas, selected to represent the population without sanitation at that time (Strina et al., 2003). In 2005 these children were resurveyed and social (maternal schooling), demographic (age and gender) and environmental (presence of street pavement, presence of dog and/or cat at home, daycare attendance) data were collected. This children came from a typical urban poor population characterized by: absence of public sewage system in most of their household and 51.7% of the kids were from families having mensal income equal or less than 147 USD and only 3.3% had the family income equal or more than 500 USD. Informed consent was obtained from the children's parents or guardians. Ethical approval was granted by the Instituto de Saúde Coletiva at Universidade Federal da Bahia and the National Commission on Ethics in Research (CONEP), Brazil.
2.2. Blood collection
Blood collection was carried out in laboratory facilities established in each studied area. A blood sample of 5 mL was collected from each child and the sera were kept at −20 °C until use.
2.3. Obtaining excretory-secretory T. canis larval antigens
T. canis excretory/secretory larval antigens were obtained following the de Savigny (1975a) technique, modified by Alcântara-Neves et al. (2008).
2.4. Characterization of the excretory-secretory T. canis larval antigens by sodium duodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed according to Laemmli (1970) using a Mini-PROTEAN III Electrophoresis Cells (Bio-Rad Laboratories, Hercules, CA) and a 12% polyacrylamide gel in the presence of 10% sodium dodecyl sulphate (Merck & Co., Inc., White house Station, NJ, USA). Protein bands were stained with Coomassie Brilliant Blue R 250 (Sigma Chemical Co., San Louis, MO, USA). The relative molecular weights were calculated using prestained protein (Sigma Chemical Co., San Louis, MO, USA) of standard molecular weight according to the relative electrophoretical mobility (REM), using the following equation: REM = distance of the protein migration/distance of bromophenol blue migration.
2.5. Sera absorption with Ascaris lumbricoides antigens
In order to remove nonspecific antibodies that might cross react with excretory-secretory T. canis larval antigens each serum was pre-absorbed with adult A. lumbricoides somatic antigens in the presence of 3% polyetilenglycol (PEG 15,000; Sigma Chemical Co., San Louis, MO, USA) and 0.1% sodium azide diluted in PBS. After 30 min upon agitation at 4°C, the samples were centrifuged for 6082 × g during 10 min. Some samples were double absorbed with another incubation with the same conditions described above. Because 10.7% of the children were infected with Trichuris trichiura, a sample of the studied sera was also absorbed with antigens of this parasite and compared to the same sera absorbed with A. lumbricoides antigens alone or with both parasites. Since absorption with A. lumbricoides antigens alone or with both parasites provided comparable titers of anti-Toxocara spp. IgG, the remaining sera were absorbed with A. lumbricoides antigens only. Because this assay does not discriminate infection by T. canis or T. cati, we used the results of this assay as marker of past or present infection by both Toxocara species (Kennedy et al., 1987).
2.6. Immunoassay for detection of anti-Toxocara IgG antibodies
Anti-Toxocara IgG antibodies were detected in sera by indirect ELISA assay using excretory-secretory T. canis larval antigens according to de Savigny and Tizard (1975b). The only modification introduced was that the reaction was developed using an anti-human biotinylated IgG conjugate (BD Pharmingen, San Diego, CA, USA) instead of a peroxidase conjugate. It allowed a serum dilution of 1:1000 instead of dilution of 1:40–1:160 as reported in the literature (de Savigny and Tizard, 1975b; Nunes et al., 1999; Lynch et al., 1988). To determine the avidity of the antibodies binding to the antigen, the assay was performed in duplicate, and for each serum two wells were washed after the serum incubation with PBS-Tween 20, containing 6 M of urea (Urea P.A.–VETEC, Sao Paulo, Brazil). Toxocara-specific IgG avidity was calculated according to Dziemian et al. (2008) using the following formulae: relative avidity index (RAI) = O.D. in sera treated with urea/O.D. in sera non-treated with urea multiplied by 100. RAI up to 50 was considered as low avidity. The cut-off obtained (0.23) was calculated by the OD from the mean of the 14 negative controls (children without history of contact with dogs and cats) plus three standard deviations of this mean. Five previously assayed sera samples were used as positive controls.
2.7. Statistical analysis
Only children for whom complete data were available were included in the analysis. The following variables were studied as risk factors for acquisition of Toxocara spp. infection: whether the child attended nursery, maternal schooling, presence of dogs or cats at home, if the children houses were served by a paved road. We first performed a univariate analysis between each potential risk factor and outcome. Second, we built a multivariable model with standard logistic regression including only significant variables from the univariate analysis. Variables that remained statistically significant using a stepwise process remained in the final multivariate logistic regression model (Hosmer and Stanley, 2000). The association between outcome (seropositivity to Toxocara spp IgG antibodies) and risk factors was estimated with odds ratio and 95% confidence interval. Absortion test with A. lumbricoides antigens was analized by Dunn's test.
3. Results
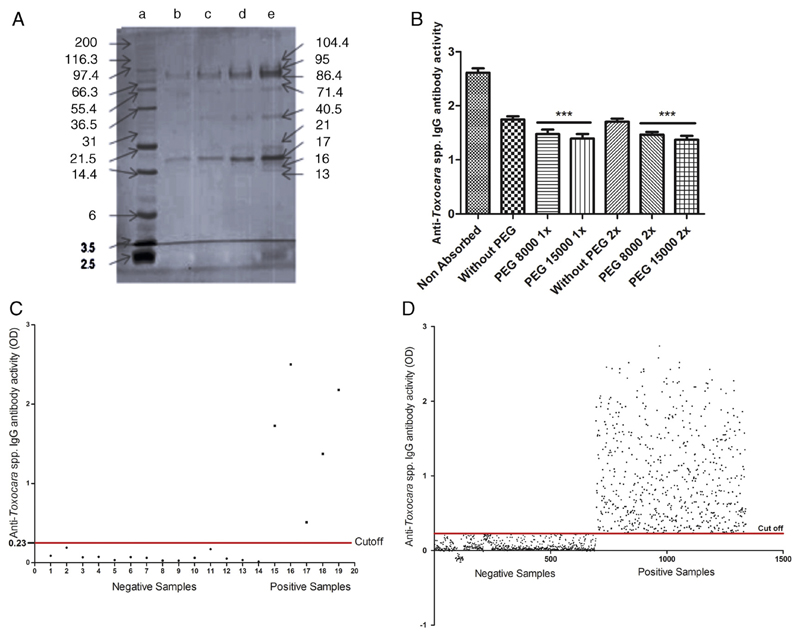
A total of 1309 children with complete data were used in the analysis. Fig. 1A shows the quality of the T. canis excretory/secretory larval antigens used as antigen to detect anti-Toxocara IgG in an indirect ELISA, determined by SDS-PAGE showing bands of 104, 95, 86, 71, 40, 21, 17, 16 and 13 kDa. Fig. 1B shows the results of the sera absorption with somatic antigens of adult stage of A. lumbricoides. Sera absorbed twice with PEG 15.000 had the best performance taking out the non-specific antibodies and decreasing optical density of the reaction up to 54%, Fig. 1C shows the determination of the assay cut-off. Fig. 1D shows the results of the determination of anti-Toxocara IgG antibodies in the whole population by the "in-house" indirect ELISA.
Fig. 1.
In house immunoassay to detect anti-T. canis IgG and results of the tested sera: (A) 12% polyacrilamide gel electrophoresis of T. canis excretory/secretory larval antigens for stained with blue Cooumasie (a) molecular weight markers; (b–d), antigen amount of 0.1, 0.2, 0.4 and 0.8 μg/well respectively); (B) results ofthe absorption of the children sera with somatic antigen of adult A. lumbricoides in the presence of polyetilenglycol as described in Section 2; (C) determination of the ELISA cut-off for anti-T.canis IgG as described in Section 2 and (D) dispersion of the anti-T.canis IgG in serum samples of the study population. Numbers in x-axis represent each sample.
Forty eight percent of the children were seropositive for anti-Toxocara IgG and only 2.8% of the 633 seropositive children had IgG of low avidity, indicating a recent infection (data not shown).
The following variables were positively associated with an increased prevalence of Toxocara canis infection at both univariated and multivariated analyses: to be ≥8 years old; living in house placed in paved streets; presence of a dog at home and presence of a cat at home. Day care attendance was positively associated with Toxocara infection only at univariated analysis and negative and statistically significant associations were found between Toxocara infection and mother with both, incomplete high school and complete high school or more, when compared with mothers with middle school or less, respectively (Table 1).
Table 1. Logistic analyses of the association between seropositivity for anti-Toxocara IgG and the studied risk factors for acquisition of this infection in 1309 children.
| Variables | Anti-Toxocara IgG | ||
|---|---|---|---|
| Positivity n (%) | Bivariate OR (95% CI) | Multivariate OR (95% CI) | |
| Gender | |||
| Female | 289 (47.6) | 1 | - |
| Male | 344 (49.0) | 1.06 (0.85; 1.31) | |
| Age | |||
| ≤5 years | 152 (45.2) | 1 | 1 |
| 6–7 years | 245 (46.2) | 1.04 (0.79; 1.37) | 1.04 (0.79; 1.38) |
| ≥8 years | 236 (53.3) | 1.38 (1.04; 1.83) | 1.43 (1.07; 1.92) |
| Maternal schooling | |||
| Middle school or less | 177 (60.1) | 1 | |
| Incomplete high school | 322 (51.3) | 0.69 (0.52; 0.91) | 0.72 (0.54; 0.96) |
| Complete high school or more | 134 (34.5) | 0.35 (0.25; 0.47) | 0.36 (0.26; 0.50) |
| Daycare attendance | |||
| No | 514 (46.9) | 1.42 (1.05; 1.90) | - |
| Yes | 244 (52.6) | ||
| Street paving | |||
| No | 389 (46.0) | 1 | 1 |
| Yes | 244 (52.6) | 1.30 (1.04; 1.63) | 1.26 (1.00; 1.60) |
| Dog at home | |||
| No | 354 (44.7) | 1 | 1 |
| Yes | 279 (54.0) | 1.45 (1.16; 1.81 | 1.36 (1.07; 1.72) |
| Cat at home | |||
| 498 (46.2) | 1 | 1 | |
| 135 (58.2) | 1.62 (1.21;1.91) | 1.40 (1.03;1.91) | |
Boldface numbers are statistically significant.
4. Discussion
The diagnosis of toxocariasis is based on clinical findings associated with the demonstrations of IgG specific antibodies in serum. Many laboratories have developed "in house" assays for research purpose in order to determine the prevalence of this zoonosis (Aguiar-Santos et al., 2004). The T. canis excretory/secretory larval antigens obtained in our laboratory are similar in composition to antigens previously described, according to the size of the bands found in the SDS-PAGE (Rubinsky-Elefant et al., 2006; Iddawela et al., 2007; Roldán and Espinoza, 2009) and the standardization carried out in our laboratory provided an assay with a greater specificity when compared with the literature, since the serum dilution was 1:1000 instead of 1:200 (Nunes et al., 1997). Another point was the utilization of a biotin instead of a peroxidase-conjugate secondary antibody. Even using a cut-off determined by the mean of the negative controls (n = 14) plus 3 standard deviation we had a low cut off of 0.23 OD while some positive sera reached optical density values above the upper detection limit of the assay. Furthermore we absorbed the sera with A. lumbricoides antigens instead of A. suum that is customary used (Lynch et al., 1988, Nunes et al., 1997; Campos Junior et al., 2003; Roldán et al., 2006).Absorption of sera with antigens from other parasites is a practice that increases the specificity of the test, since many parasite species share similar antigens giving rise to cross-reactivity between these antibodies (Ishida et al., 2003). In our study population helminth infection are caused mainly by A. lumbricoides and Trichuris trichiura which occurs in 16.1% and 10.8% respectively. The absorption with Ascaris lumbricoides antigens decreased the reactivity of some sera up to 76.39% indicating a higher removal of specific antibodies to A. lumbricoides avoiding cross-reactions between this helminth and anti-T. canis antibodies and it also removed the cross-reactivity with T. trichiura. We have also absorbed some of the sera with Ancylostoma braziliensis antigens and there was no decrease of the anti-Toxocara IgG, showing that this hookworm does not share antigens reactive with anti-T. canis IgG (data not shown).
In our work we found a prevalence of IgG anti-Toxocara of 48.4%, this prevalence is similar to a previous work conducted by our group where we have reported 46.3% of Toxocara spp. sero-prevalence in blood donors in the same city of the present study (Dattoli et al., 2011). Others studies conducted in Latin America had reported smaller prevalences. In Argentina, Alonso et al. (2000) reported a positivity of 37.9% in children younger than 14 years and Radman et al. (2000) observed a prevalence of 39% infection. Espinoza et al. (2008) determined a seroprevalence of 32.4% in Peru and in Brazil, Chieffi et al. (2009) in a review, cited prevalence of T. canis varying from 3.72% to 40%.
Only 2.8% of the 633 seropositive children had IgG of low avidity. The avidity assay can distinguish between a chronic or past infection (high avidity) and a recent infection (low avidity). Because kids are exposed to Toxocara early in life it is very unusual to have recent infection by the age when these children were examined (4–11 years old).
In this work and others from our group (Alcantara-Neves et al., 2011; Mendonça et al., 2012), maternal education was used as an indicator of socioeconomic status of the family since it is highly associated in this population. It also reflects the hygiene habits of the family which will be rely on greater chance of infection. The results of the seroprevalence of IgG anti-Toxocara found in this study were similar to those observed in other low-income populations, where prevalence of infection in children of mothers with fewer years of education was higher. Alderete et al. (2003) diagnosed a prevalence of 38.8% in children with a mean age of 9.4 years and determined that Toxocara infection was inversely proportional to family income.
Contact with dogs has been shown in several studies as an important risk factor for toxocariasis. A cross-sectional study estimated a frequency of 52% seropositivity for Toxocara spp. in 34 families in the Amazonas state in Brazil. Individuals who had contact with adult dogs at home, 60% were positive (chi2 = 14.317, p = 0.026), and who had contact with puppies at home, 66.6% were positive (chi2 = 22.149, p = 0.008), demonstrating the association between contact with dogs and the presence of anti-T.canis IgG (Damian et al., 2007). In Argentina, Chiodo et al. (2006) evaluated 100 individuals for IgG anti-Toxocara and 23% were positive, and all had contact with dog at home. Our results confirm these findings and the presence of dogs at home is a risk factor for Toxocara infection in this study population.
Several epidemiological studies indicate contamination of soil as a determinant in infection by Toxocara spp. In the present study was noted that paved street increased the chance of infection, which makes us hypothesizes that we could have higher egg concentrations in soil with paving then you would if you had no paving since in urban areas we would have less area for water to soak into the soil (unpaved areas) and concentrate soil contamination during rainfall and flooding. Another possibility it may occurs is that maybe cats which were also a risk factor in this study, may be polluting the environment with Toxocara eggs since they cannot bury their feces in paved streets leaving them exposed increasing the chance of infection. Few studies were conducted to estimate the infection of cats and their potential to cause VLM. Martínez-Barbabosa et al. (2003) determined a prevalence of 42.5% of T. cati infection in cats which makes one think that this parasite may be common and raises the importance of the development of a specie-specific ELISA for detection anti-Toxocara spp. IgG, useful for further studies about the epidemiology of VLM caused by both Toxocara species.
Some studies refer that soil contamination is not the only effective route in human toxocariasis and eggs of Toxocara spp. can be sprouted in fur and direct contact between humans and dogs and cats may be an alternative route of human infection (Wolfe and Wright, 2003). Aydenizöz-Ozkayhan et al. (2008) collected 51 fur samples and observed that 21.56% of the dogs had eggs in their fur. Roddie et al. (2008) examined 100 dogs for the presence of eggs in fur and found Toxocara spp. eggs in 67% of adult dogs and 95% of puppies. In the Netherlands, Overgaauw et al. (2009) found Toxocara eggs in 4.4% of dog fecal samples and in 12.2% of their fur samples. Moreover, many of the owners allowed their dogs to climb and sleep in their beds, and only 15% washed their hands after contact with your pet. Therefore, this close physical contact between pets and their owners possibly increase the risk of transmission of Toxocara spp. and point to the need for greater attention to the potential risk to which humans are exposed.
In conclusion this work shows that Toxocara infection is highly prevalent in the studied population and we postulate that it is closely related to social status and hygiene. The presence of the dog at home proved to be an important risk factor for this disease and is necessary to adopt sanitary measures more specific for resident dogs, since the control program of stray dogs is not the only way to control the disease. The association with presence of cats in house confirms previous work showing antigenic similarities between T. canis and T. cati ESLA and that anti-T. cati antibodies have influenced the outcome of the study. Although cats have a habit of burying their feces, paving the street may be influencing the increased exposure to cat feces as well as dogs feces since this variable was associated with increased risk of Toxocara infection. So, the importance of cat as a disseminator of this disease was suggested and deserves further attention in programs for this disease control.
Acknowledgements
We thank the WELLCOME TRUST for funding this work and CAPES, CNPQ and FAPESB for scholarships that supported the author and some of the co-authors.
Contributor Information
Lívia R. Mendonça, Email: mendoncalr@gmail.com.
Camila A. Figueiredo, Email: cavfigueiredo@gmail.com.
Renata Esquivel, Email: rme86@hotmail.com.
Rosemeire L. Fiaccone, Email: r_fiaccone@hotmail.com.
Lain Pontes-de-Carvalho, Email: lain@bahia.fiocruz.br.
Phillip Cooper, Email: pcooper@sgul.ac.uk.
Maurício L. Barreto, Email: mauricio@ufba.br.
References
- Abougrain AK, Nahaisi MH, Madi NS, Saied MM, Ghenghesh KS. Parasitological contamination in salad vegetables in Tripoli-Libya. Future Child. 2009;21:760–762. [Google Scholar]
- Aguiar-Santos AM, Andrade LD, Medeiros Z, Chieffi PP, Lescano SZ, Perez EP. Human Toxocariasis: frequency of anti-toxocara antibodies in children and adolescents from an outpatient clinic for lymphatic filariasis in Recife, Northeast Brazil. Rev Inst Med Trop. 2004;46:81–84. doi: 10.1590/s0036-46652004000200005. [DOI] [PubMed] [Google Scholar]
- Alcântara-Neves NM, Bavia E, Silvão Rm Carvalho E. Environmental contamination by Toxacara sp. eggs in public areas of Salvador, Bahia State, Brazil. Rev Soc Bras Med Trop. 1999;24:187–190. doi: 10.1590/s0037-86821989000400005. [DOI] [PubMed] [Google Scholar]
- Alcântara-Neves NM, dos Santos AB, Mendonça LR, Figueiredo CAV, Pontes-de-Carvalho L. An improved method to obtain antigen-excreting Toxocara canis larvae. Exp Parasitol. 2008;119:349–351. doi: 10.1016/j.exppara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Alcantara-Neves NM, Veiga RV, Dattoli VC, Fiaccone RL, Esquivel R, Cruz AA, Cooper PJ, Rodrigues LC, Barreto ML. The effect of single and multiple infections on atopy and wheezing in children. J Allergy Clin Immunol. 2011;129:359–367. e351–353. doi: 10.1016/j.jaci.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JMS, Jacob CMA, Pastorino AC, et al. Prevalence of Toxocara infection in schoolchildren for the Butanta, region, Sao Paulo, Brazil. Mem Inst Oswaldo Cruz. 2003;98:593–597. doi: 10.1590/s0074-02762003000500002. [DOI] [PubMed] [Google Scholar]
- Almeida ABPF, Sousa VRF, Dalcin L, Justino CHS. Contaminafao por fezes caninas das praças públicas de Cuiabá, Mato Grosso. Braz J Veter Res Anim Sci. 2007;44:132–136. [Google Scholar]
- Alonso JM, Bojanich MVL, Chamorro M, Gorodner JO. Toxocara sero-prevalence in children from a subtropical city in Argentina. Rev Inst Med Trop. 2000;42:235–237. doi: 10.1590/s0036-46652000000400010. [DOI] [PubMed] [Google Scholar]
- Amaral HLC, Rassier GL, Pepe MS, et al. Presence of Toxocara canis eggs on the hair of dogs: a risk factor for visceral larva migrans. Vet Parasitol. 2010;174:115–118. doi: 10.1016/j.vetpar.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Aydenizöz-Ozkayhan M, Yagci BB, Erat S. The investigation of Toxocara canis eggs in coats of different dog breeds as a potential transmission route in human toxocariasis. Vet Parasitol. 2008;152:94–100. doi: 10.1016/j.vetpar.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Campos Junior D, Elefant GR, Silva EOM, et al. Freqüência de Soropositividade para Antígenos de Toxocara canis em Crianças de Classes Sociais Diferentes. Rev Soc Bras Med Trop. 2003;36:509–513. [PubMed] [Google Scholar]
- Chieffi PP, Santos SV, Queiroz ML, Lescano SAZ. Human toxocariasis: contribution by Brazilian researchers. Rev Inst Med Trop. 2009;51:301–308. doi: 10.1590/s0036-46652009000600001. [DOI] [PubMed] [Google Scholar]
- Chiodo P, Basualdo J, Ciarmela L, Pezzani B, Apezteguía M, Minvielle M. Related factors to human toxocariasis in a rural community of Argentina. Mem Inst Oswaldo Cruz. 2006;101:397–400. doi: 10.1590/s0074-02762006000400009. [DOI] [PubMed] [Google Scholar]
- Coelho LMPS, Silva MV, Dini CY, Giacon Neto AA, Novo NF, Silveira EPR. Human toxocariasis: a seroepidemiological survey in schoolchildren of Sorocaba, Brazil. Mem Inst Oswaldo Cruz. 2004;99:553–557. [PubMed] [Google Scholar]
- Damian MM, Martins M, Sardinha JF, Souza LO, Chaves A, Tavares M. Freqüéncia de anticorpo anti-Toxocara canis em comunidade do Rio Uatumã, no Estado do Amazonas. Rev Soc Bras Med Trop. 2007;40:661–664. doi: 10.1590/s0037-86822007000600013. [DOI] [PubMed] [Google Scholar]
- Dattoli VCC, Freire SM, Mendonça LR, Santos PC, Meyer R, Alcântara-Neves NM. Toxocara canis infection is associated with eosinophilia and total IgE in blood donors from a large Brazilian centre. Trop Med Int Health. 2011;16:514–517. doi: 10.1111/j.1365-3156.2010.02719.x. [DOI] [PubMed] [Google Scholar]
- de Savigny D. In vitro maintenance of Toxocara canis larvae and a simple method forthe production of Toxocara ES antigen for the uses in serodiagnostic test for visceral larva migrans. J Parasitol. 1975a;61:781–782. [PubMed] [Google Scholar]
- de Savigny DH, Tizard IR. Serodiagnosis of Toxocara larva migrans visceral. Can J Public Health. 1975b;66:52–56. [Google Scholar]
- Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devera R, Blanco Y, Hernández H, Simoes D. Toxocara spp. and other helminths in squares and parks of Ciudad Bolivar, Bolivar State (Venezuela) Enferm Infecc Microbiol Clin. 2008;26:23–26. doi: 10.1157/13114391. [DOI] [PubMed] [Google Scholar]
- Dziemian E, Zarnowska H, Kolodziej-Sobociñska M, Machnicka B. Determination of the relative avidity of the specific IgG antibodies in human toxocariasis. Parasite Immunol. 2008;30:187–190. doi: 10.1111/j.1365-3024.2007.01010.x. [DOI] [PubMed] [Google Scholar]
- Espinoza YA, Huapaya PH, Roldán WH, Jiménez S, Arce Z, Lopez E. Clinical and serological evidence of Toxocara infection in school children from Morrope district, Lambayeque, Peru. Rev Inst Med Trop. 2008;50:101–105. doi: 10.1590/s0036-46652008000200007. [DOI] [PubMed] [Google Scholar]
- Ferreira MU, Rubinsky-Elefant G, Castro TG, et al. Bottle feeding and exposure to toxocara as risk factors for wheezing illness among under-five amazonian children: a population-based cross-sectional study. J Trop Pediatr. 2007;53:119–124. doi: 10.1093/tropej/fml083. [DOI] [PubMed] [Google Scholar]
- Haralambidou S, Vlachaki E, Ioannidou E, Milioni V, Haralambidis S, Klonizakis I. Pulmonary and myocardial manifestations due to Toxocara canis infection. Eur J Intern Med. 2005;16:601–602. doi: 10.1016/j.ejim.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Stanley L. Applied Logistic Regression. 2nd ed. John Wiley & Sons; New York: 2000. [Google Scholar]
- Hotez PJ, Wilkins PP. Toxocariasis: America's most common neglected infection of poverty and a helminthiasis of global importance? PLoS Neglected Trop Dis. 2009;3:1–4. doi: 10.1371/journal.pntd.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iddawela RD, Rajapakse Rpvj, Perera Nand, Agatsuma T. Characterization of a Toxocara canis species-specific excretory-secretory antigen (TcES-57) and development of a double sandwich ELISA for diagnosis of visceral larva migrans. Korean J Parasitol. 2007;45:19–26. doi: 10.3347/kjp.2007.45.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida MMI, Rubinsky-Elefant G, Ferreira AW, Hoshino-Shimizu S, Vaz AJ. Helminth antigens (Taenia solium, Taenia crassiceps, Toxocara canis, Schistosoma mansoni and Echinococcus granulosus) and cross-reactivities in human infections and immunized animals. Act Trop. 2003;89:73–84. doi: 10.1016/j.actatropica.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Kennedy MW, Maizels RM, Meghji M, Young L, Qureshi F, Smith HV. Species-specific carbohydrate epitopes on the secreted and surface antigens of Toxocara cati and Toxocara canis infective larvae. Parasite Immunol. 1987;9:407–420. doi: 10.1111/j.1365-3024.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly ofthe head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynch NR, Wilkes LK, Hodgen AN, Turner KJ. Specificity of Toxocara ELISA in tropical populations. Parasite Immunol. 1988;10:323–337. doi: 10.1111/j.1365-3024.1988.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Martin UO, Demonte MA. Urban Contamination with Zoonotic Parasites in the Central Region of Argentina. Medicina Buenos Aires. 2008;68:363–366. [PubMed] [Google Scholar]
- Martínez-Barbabosa I, Tsuji OV, Cabello RR, Cárdenas EMG, Chasin AO. The prevalence of Toxocara cati in domestic cats in Mexico City. Vet Parasitol. 2003;114:43–49. doi: 10.1016/s0304-4017(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Mendonça LR, Veiga Rv, Dattoli Vc, Figueiredo Ca, Fiaccone R, Santos J, Cruz Aa, Rodrigues Lc, Cooper Pj, Pontes-De-Carvalho Lc, Barreto Ml, et al. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban latin American. PLoS Negl Trop Dis. 2012;11:e1886. doi: 10.1371/journal.pntd.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizgajska H. The role of some environmental factors in the contamination ofsoil with Toxocara spp. and other geohelminth eggs. Parasitol Int. 1997;46:67–72. [Google Scholar]
- Nunes CM, Tundisi RN, Garcia JF, Heinemann MB, Ogassawara S, Richtzenhain LJ. Cross-Reactions Between Toxocara Canis And Ascaris Suum In The Diagnosis Of Visceral Larva Migrans By Western Blotting Technique. Rev Inst Med Trop. 1997;39:253–256. doi: 10.1590/s0036-46651997000500002. [DOI] [PubMed] [Google Scholar]
- Nunes CM, Tundisi RN, Heinemann MB, Ogassawara S, Richtzenhain LJ. Toxocariasis: serological diagnosis by indirect antibody competition Toxocariasis: serological diagnosis by indirect antibody competition ELISA. Rev Inst Med Trop. 1999;41(2):95–100. doi: 10.1590/s0036-46651999000200006. [DOI] [PubMed] [Google Scholar]
- Overgaauw PAM, Zutphen L, Hoek D, et al. Yaya FO, Roelfsema J, Pinelli E, Knapen F, Kortbeek LM. Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vet Parasitol. 2009;163:115–122. doi: 10.1016/j.vetpar.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Radman NE, Archelli SM, Fonrouge RD, Guardis MV, Linzitto OR. Human Toxocarosis. Its Seroprevalence in the City of La Plata. Mem Inst Oswaldo Cruz. 2000;95:281–285. doi: 10.1590/s0074-02762000000300001. [DOI] [PubMed] [Google Scholar]
- Regis SCS, Mendonça LR, Silva NS, Dattoli VCC, Alcântara-Neves NM, Barrouin-Melo SM. Seroprevalence and risk factorsfor canine toxocariaisis by detection of specific IgG as marker of infection in dogs from Salvador, Brazil. Acta Trop. 2011;120:46–51. doi: 10.1016/j.actatropica.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Roddie G, Stafford P, Holland C, Wolfe A. Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol. 2008;152:85–93. doi: 10.1016/j.vetpar.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Roldán W, Cornejo W, Espinoza Y. Evaluation of the dot enzyme-linked immunosorbent assay in comparison with standard ELISA forthe immunodiagnosis of human toxocariasis. Mem Inst Oswaldo Cruz. 2006;101:71–74. doi: 10.1590/s0074-02762006000100013. [DOI] [PubMed] [Google Scholar]
- Roldán WH, Espinoza YA. Evaluation of an enzyme-linked immunoelec-trotransfer blot test for the confirmatory serodiagnosis of human toxocariasis. Mem Inst Oswaldo Cruz. 2009;104:411–418. doi: 10.1590/s0074-02762009000300003. [DOI] [PubMed] [Google Scholar]
- Rubinsky-Elefant G, Shimizu SH, Sanchez MCA, Jacob CMA, Ferreira AW. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal. 2006;20:164–172. doi: 10.1002/jcla.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito L, Scarlata F, Colomba C, Infurnari L, Giordano S, Titone L. Human toxocariasis: a report of nine cases. Acta Paediatr. 2008;97:1301–1302. doi: 10.1111/j.1651-2227.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- Strina A, Cairncross S, Barreto ML, Larrea C, Prado MS. Childhood diarrhea and observed hygiene behavior in Salvador, Brazil. Am J Epidemiol. 2003;157:1032–1038. doi: 10.1093/aje/kwg075. [DOI] [PubMed] [Google Scholar]
- Tiyo R, Guedes TA, Falavigna DL, Falavigna-Guilherme AL. Seasonal contamination of public squares and lawns by parasites with zoonotic potential in southern Brazil. J Helminthol. 2008;82:1–6. doi: 10.1017/S0022149X07870829. [DOI] [PubMed] [Google Scholar]
- Wolfe A, Wright IP. Human toxocariasis and direct contact with dogs. Veter Rec. 2003;152:419–422. doi: 10.1136/vr.152.14.419. [DOI] [PubMed] [Google Scholar]