Abstract
Forest disturbance regimes are intensifying in many parts of the globe. In order to mitigate disturbance impacts a number of management responses have been proposed, yet their effectiveness in addressing changing disturbance regimes remains largely unknown. The strong positive relationship between forest age and the vulnerability to disturbances such as windthrows and bark beetle infestations suggests that a reduced rotation length can be a potent means for mitigating the impacts of natural disturbances. However, disturbance mitigation measures such as shortened rotation lengths (SRL) can also have undesired consequences on ecosystem services and biodiversity, which need to be considered in their application.
Here, we used the process-based landscape and disturbance model iLand to investigate the effects of SRL on the vulnerability of a 16,000 ha forest landscape in Central Europe to wind and bark beetle disturbances. We experimentally reduced the current rotation length (between 100 and 115 years) by up to −40% in 10% increments, and studied effects on disturbance dynamics under current and future climate conditions over a 200-year simulation period. Simultaneously, we quantified the collateral effects of SRL on forest carbon stocks and indicators of biodiversity. Shortening the rotation length by 40% decreased disturbances by 14%. This effect was strongly diminished under future climate change, reducing the mitigating effect of shortened rotation to < 6%. Collateral effects were severe in the initial decades after implementation: Reducing the rotation length by 40% caused a spike in harvested timber volume (+ 92%), decreased total forest carbon storage by 6% and reduced the number of large trees on the landscape by 20%. The long-term effects of SRL were less pronounced. At the same time, SRL caused an increase in tree species diversity. Shortening rotation length can reduce the impact of wind and bark beetle disturbances, but the overall efficiency of the measure is limited and decreases under climate change. Given the potential for undesired collateral effects we conclude that a reduction of the rotation length is no panacea for managing increasing disturbances, and should be applied in combination with other management measures reducing risks and fostering resilience.
Keywords: Forest disturbances, Climate change, Disturbance management, Wind and bark beetles, Central Europe
1. Introduction
Forest disturbances have increased in recent decades, and there is ample evidence that this trend will continue in the future due to ongoing climate change (Seidl et al., 2014b; Senf et al., 2018). Among the forest types affected by recent pulses of mortality, Europe's Norway spruce forests (Picea abies (L.) Karst) were impacted with a particular severity (Dobor et al., 2018; Marini et al., 2017; Mezei et al., 2017). The sensitivity of these forest types to large-scale dieback is on the scientific agenda since the 1980's (Hlásny and Sitková, 2010; Klimo et al., 2000), yet recent observation are particularly alarming and suggest that a tipping point has been reached (Hlásny et al., 2019; Lindenmayer et al., 2016). While the initial problems identified in the 1980 largely concerned areas where the species was cultivated at the edge of or even outside of its natural range, recent pulses of mortality occurred almost across the entire distributional range of the species in Central Europe (de Groot et al., 2019; Marini et al., 2017).
These developments have promoted the adoption of new policies which mainly strived to restore the natural tree species composition (Hlásny et al., 2017; Spiecker et al., 2004), aiming to increase the response diversity to changing environmental conditions and reduce the vulnerability to disturbances (Lindner et al. 2010). While the forest policy and management communities in Central Europe have largely focused on the question of which tree species and species mixtures could increase the robustness of forest stands to changing climate and disturbance regimes (e.g. Bolte et al. 2009), recent research indicates that also other management options provide considerable leverage. These, for instance, include intensifying thinnings to reduce drought risk (Elkin et al., 2015; Sohn et al., 2016), improving structural diversity and revegetation strategies to facilitate post-disturbance regeneration (Churchill et al., 2013; Lafond et al., 2014), and increasing landscape-scale diversity to contain the spread of disturbances (Honkaniemi et al., 2020; Seidl et al., 2018).
Another important means to influence the structure and functioning of managed forests is the timing of harvesting operations (Brown et al., 2018; Curzon et al., 2017; Russell and Jones, 2001). A central parameter in this regard, describing the time elapsed between final fellings in even-aged forest management, is rotation length (Roberge et al., 2016). In Central Europe, rotation lengths are typically determined based on past species- and site-specific growth performance to maximize profit (Faustmann, 1849; Newman, 2002). Forest planning under global change, however, requires broader consideration, accounting, for example, for changing tree growth performance (Blennow et al., 2010; Yousefpour et al., 2019), the need to mitigate climate change via increased carbon stocks (Ekholm, 2016; Liski et al., 2011), the requirement to increase biodiversity also in managed forests (Angelstam et al., 2018; Díaz-Rodríguez et al., 2012), and the uncertainty in future environmental conditions and societal demands (Daniel et al., 2017; Seidl and Lexer, 2013; Spittlehouse and Stewart, 2003).
The risk for many disturbances changes with stand age (Gardiner and Quine, 2000; Jactel et al., 2009; Roberge et al., 2016). Disturbances are thus another important factor to consider in determining forest rotation length (Meilby et al., 2001; Price, 1989). In the Norway spruce forests of Central Europe, the most important biotic disturbance agent is the European spruce bark beetle Ips typographus (L.). It typically prefers trees older than 60 years that have a diameter at breast height larger than 20–25 cm (although beetles may attack and reproduce also in smaller and younger trees at high population levels). A similar positive relationship between tree age and susceptibility exists for wind disturbance (Jactel et al., 2009). Taller trees receive higher wind loading (larger crown surface area) and turning moments (higher crowns), leading to an increased risk of windthrow and stem breakage. Moreover, considering the long disturbance return intervals and the stochastic nature of wind regimes, a longer rotation increases the chance that a severe windstorm will occur during the lifetime of a tree generation in a particular area (Jactel et al., 2009). Wind disturbance furthermore often serves as trigger for bark beetle outbreaks (Marini et al., 2017), an interaction that is expected to be further amplified under climate change (Seidl and Rammer, 2017). That the rotation length in Norway spruce forests exceeds 100 years in many regions of Europe (Lindner et al., 2000) thus results in large tracts of forests prone to both wind and bark beetle disturbances. Therefore, a shortening of the rotation length is increasingly discussed as a means to address intensifying disturbance regimes (Gardiner and Quine, 2000; Hlásny et al., 2017; Jactel et al., 2009; Kuboyama and Oka, 2000).
Reducing rotation length, however, can also have negative effects on the supply of ecosystem services (e.g., timber production, carbon storage) and the objectives of nature conservation (Felton et al., 2017; Roberge et al., 2016). Reducing rotation length can, for instance, result in a temporal surplus of timber to the market and reduce the supply of logs with larger dimensions (Lindner et al., 2000). Shorter rotation may also result in a loss of habitat features that are important for biodiversity conservation (Felton et al., 2017; Lange et al., 2014; Lassauce et al., 2013) and compromise supporting (water, soil nutrients) and cultural (aesthetics, cultural heritage) ecosystem services (Roberge et al., 2016; Weslien et al., 2009). The reduction of mature stands with high structural complexity can, for instance, affect habitat availability for some red-listed species (Bernes, 2011) and reduce the amount of dead wood, which accumulates with a higher intensity in older stands (Jonsson et al., 2006). The absence of old trees and the higher frequency of harvesting interventions can also affect forest aesthetic and recreation values negatively (e.g. Curtis, 1997). Reduced forest carbon stocks due to shorter rotation may counteract efforts to mitigate climate change through carbon storage in forest ecosystems (Ekholm, 2016; Kaipainen et al., 2004). Recent research also indicates that the maintenance of older forests on the landscape can help to sustain biodiversity and ecosystem services under climate change (Thom et al., 2019).
Here, we assessed how a reduced rotation length affects the future susceptibility of forests to wind and bark beetle disturbances. We subsequently evaluated a range of collateral effects of reduced rotation lengths, assessing their impact on indicators of forest carbon storage and biodiversity. We hypothesized that a reduced rotation length can buffer the expected increase in forest disturbances in the forests of Central Europe (Björkman et al., 2015; Jactel et al., 2009). However, we further expected that positive effects on disturbance risk are countered by negative effects of reduced rotation on indicators of forest carbon and biodiversity (Liski et al. 2011, Lassauce et al. 2013, Lundmark et al. 2018).
2. Methods and materials
2.1. Simulation model
To address the complex interdependencies between disturbance, rotation, and indicators of ecosystem services and biodiversity, we here used the forest landscape and disturbance model iLand (Seidl et al., 2012a). iLand is a process-based ecosystem model that simulates forest landscape dynamics within a hierarchical multi-scale framework (Mäkelä, 2003), i.e. the model treats different processes at different spatial and temporal scales. The main entity in the model is a tree, for which the demographic processes of growth, mortality, and regeneration are simulated. Processes at the stand and landscape scale constrain the dynamics of individual trees and thus allow for a robust scaling of tree-scale processes to large areas (Seidl et al., 2012a).
iLand integrates an agent-based model of forest management (Rammer and Seidl, 2015) in which general stand treatment programs (i.e., a sequence of management interventions carried out over the course of stand development) are dynamically adapted to the forest state emerging from the simulation. We here applied a single stand treatment program implemented by a sole agent across the entire study landscape. Stand treatment programs included planting after harvests or natural disturbances based on prescribed planting schemes, thinning operations, harvesting and post-disturbance salvaging. In addition to stand-level management the model also includes a landscape-level scheduling module that aims for equally distributed annual harvests (e.g., by re-scheduling other planned activities in response to salvage harvesting) and accounts for spatial contingencies.
iLand simulates forest disturbances in a spatially explicit manner (Seidl et al., 2014a; Seidl and Rammer, 2017). Wind disturbances are initiated by the wind speed of severe wind events provided as external input to the simulation. The model initiates wind disturbances in locations, where canopy rugosity changes abruptly, i.e., where vertical differences between the top heights of neighbouring grid cells exceed 10 m (e.g. Blennow and Sallnas, 2004). Next, wind speed at the canopy top height is calculated based on a vertical wind profile at the stand edge (Gardiner et al., 2000), and individual-tree turning coefficients (Hale et al., 2012) are calculated. The latter two information are used to calculate the critical wind speeds for uprooting and tree breakage based on the approach of Gardiner et al. (2000). If the soil is frozen, only the stem breakage is allowed to occur. The final evaluation of the impact of wind on forest is based on comparison of the prevailing wind speed to the critical windspeed; if the critical wind speed is exceeded, the tree is broken or uprooted. The disturbance impact is simulated iteratively, with forest structure (including the appearance of new edges) being updated after each iteration if breakage or windthrow was simulated.
The process-based implementation of bark beetle disturbances considers bark beetle phenology and development, spatially explicit dispersal of beetles, colonization and tree defence, as well as temperature-related overwintering success (Seidl and Rammer, 2017). Large outbreaks are typically triggered by wind disturbance, but smaller outbreaks occur also independently based on a climate-sensitive background probability. Bark beetle development is simulated based on the beetle phenology model by Baier et al. (2007). The model tracks beetle cohorts rather than individuals, with a cohort being defined as the minimum number of beetles needed to successfully colonize a tree. Every brood tree disperses a number of beetle cohorts determined by the reproductive rate of the beetle (Wermelinger and Seifert, 1999). Attacking beetle cohorts need to first overcome the defence system of the tree, which is approximated by its dynamically simulated non-structural carbohydrate reserves. The tree can be attacked in multiple waves of beetle cohorts in one vegetation period if the climate allows for the development of multiple beetle generations per years.
The model was tested and evaluated across a range of ecosystems in Europe and North America in previous studies (Seidl et al., 2012b; Silva Pedro et al., 2015; Thom et al., 2017a). A detailed evaluation of simulated productivity, natural mortality and regeneration patterns for the landscape studied here was conducted by Dobor et al. (2018). All tests showed satisfactorily performance of the model in the current study region.
2.2. Study landscape
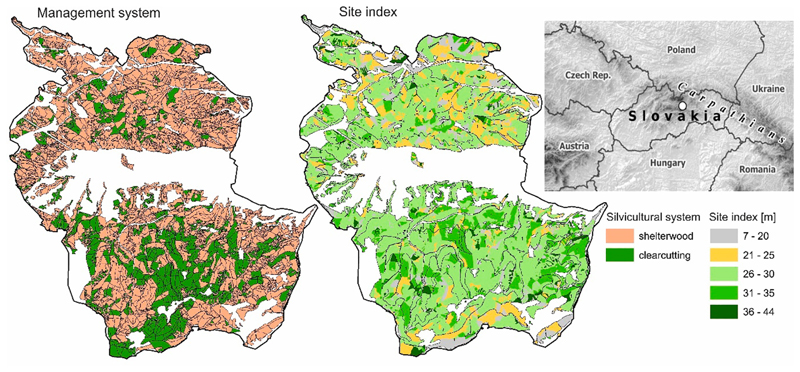
The study landscape Goat Backs Mts. is located in central-eastern Slovakia (Lon 20.088 – 20.275, Lat 48.920 – 49.061) and covers an area of 16,050 ha (Fig. 1). The forest cover is 70%, dominated by Norway spruce, which makes up 75% of the forested area. Other important tree species are European larch (Larix decidua Mill.), Scots pine (Pinus sylvestris L.), Silver fir (Abies alba Mill.), and European beech (Fagus sylvatica L.). The elevation range is 620–1550 m a.s.l. Air temperature during the growing season (April–September) ranges from 12 to 15 °C, and growing-season precipitation ranges from 380 to 510 mm. Cambisols and Podsols prevail, while Rendzinas occur on calcareous bedrock which dominates the highest reaches of the landscape.
Fig. 1.
Silvicultural systems applied in the study region (left). Clearcutting system is applied in pure stands of Norway spruce. Shelterwood system is applied in mixed stands. Site index map, with site index denoting the mean stand height at the age of 100 years (right). The insert shows the location of the study landscape in Central Europe (white circle).
The current silvicultural system is an even-aged management regime with a rotation length of approximately 100 years. The primary approach to tree regeneration in stands with fir and/or beech admixtures is a uniform shelterwood cut (Fig. 1). The shelterwood system contains 3–4 regeneration cuts applied over a period of approximately 30 years, followed by a final cut. In spruce monocultures, a small-scale clearcutting system is applied (cut-block size < 3 ha).
Wind and bark beetles (mainly Ips typographus) are the main agents of natural disturbance in the region. Over the last 20 years, the landscape has been subject to heavy windthrow and high severity bark beetle disturbance, affecting almost 40 percent of the study area (Dobor et al., 2018). Management responses to natural disturbances contain salvage logging of both wind and beetle-killed trees, and sanitation logging aimed to reduce the spread of bark beetles.
2.3. Vegetation, soil and climate data
We initialized the vegetation of the study landscape in iLand based on stand-level data from forest management plans (FMP) provided by the National Forest Centre of Slovakia. The data were collected in the field and contain attributes such as stand structure, species- and cohort-specific mean stand height and diameter, standing volume, site index, mean stand age and stand density.
Soil depth and plant available nitrogen – which are both important soil parameters for the simulation with iLand – were derived on a 100 × 100 m grid from the national forest soil database (National Forest Centre, Slovakia). Because soil depth was only available as categorical variable, depth information for each stand was sampled from a uniform distribution centred on the mean soil depth of each of the five depth categories. The soil database contained a relative nutrient content (0–1), which was used to estimate the plant-available N (kg m−2 year−1) based on iLand-internal model logic (Seidl et al., 2012a).
Two types of climate data were used to drive the simulations. A stationary reference climate series was created based on daily meteorological data from a nearby meteorological station (Poprad-Gánovce, Slovak Hydrometeorological Institute), and spatially expanded to the study landscape using MTClim in combination with topography information (Hungerford et al., 1989; see Dobor et al., 2018 for details). A reference climate series was generated by randomly sampling years with replacement from the period 1996–2016. Future climate was represented by seven GCM-RCM combinations driven by two Representative Concentration Pathway (RCP) scenarios (RCP4.5 and RCP8.5; Moss et al., 2010) (Appendix A). The data were developed in the framework of the CORDEX project (Giorgi et al., 2009). CO2 concentrations used to drive the forest simulations were defined by the two RCP scenarios, and reached 538 ppm and 936 ppm in 2100 under RCP4.5 and RCP8.5 runs, respectively. Climate data driving the simulations after 2100 were sampled with replacement from the period 2080–2100 for each RCM, while CO2 concentration was held at the level of 2100.
2.4. Simulation design
Prior to scenario simulations a 920-year spin-up run was performed to estimate the initial litter, dead wood and soil C pools, as well as to initialize stand structures (including individual tree positions) in a manner that is consistent with the internal logic of the model. The procedure used ('legacy spin-up') assimilates information on the current vegetation (here taken from FMP) in order to ensure that the resulting initial vegetation state for simulation is (i) consistent with the model-internal logic and (ii) represents the current structure and composition of the forest (see Thom et al. 2018 for details). Specifically, spin-up simulations are continuously compared to FMP reference data, and simulated management is dynamically adapted to ensure convergence between the simulation and the data at the level of individual stands. This approach accounts for the fact that the details of past land-use are often unknown, and ensures that the initial conditions of the simulation are in good correspondence with observations (e.g., with regard to structural legacies, Thom et al., 2018). The first 800 years of the spin-up included regular forest management operations but no natural disturbances. In a second 120-year spin-up phase, we allowed several low-intensity wind and bark beetle disturbances to ensure a smooth transition between the spin-up and scenario runs. The scenario simulations were run for 200 years, and each scenario was replicated 10 times to account for the stochasticity in the simulations.
The wind speed of simulated wind events was prescribed in the scenario simulations, with speed values sampled from a distribution parameterized based on past meteorological observations from the nearby meteorological station. In total, six wind events with the duration of 60 min were prescribed to occur during the 200-year simulation period. This series of wind events caused an average annual wind damage of 1.68–1.79 m3 ha−1 year−1 (range of the 10 replicate simulations) under the default rotation length and the reference climate. This level of wind damage corresponds well with the long-term range of 0.9–2.2 m3 ha−1 year−1 reported for Slovakia in the national forest disturbance statistics (Konôpka et al., 2016).
We note that while the occurrence of strong winds was prescribed, their impact on the forest ecosystem was simulated as an emergent property of the process-based wind model implemented in iLand (Seidl et al., 2014a). Also bark beetle disturbances were simulated dynamically, based on the iLand bark beetle module (Seidl and Rammer, 2017). A 60% salvaging intensity was applied in all simulations (see Dobor et al., 2020, 2019 for details on the implementation of salvage harvesting).
2.5. Rotation length experiment
The starting point for our analyses of rotation length effects was the default management currently implemented in the study region, as defined by national forestry legislation. The average rotation length (Udef) was 100 years for spruce stands, and 115 years for broadleaved species. The rotation age varies across the landscape, acknowledging a negative correlation of rotation length with site fertility. In addition to Udef, we simulated four alternative scenarios, reducing the rotation length of each stand by 10, 20, 30 and 40% (Ured10, Ured20 Ured30 and Ured40, respectively) relative to Udef. The rotation length was, however, not allowed to be < 60 years. All reduced rotation length and Udef simulations were run both under reference climate and climate change.
The transition phase to a shorter rotation length can – depending on the speed of the transition – generate different undesired effects (e.g., temporary increase in harvested timber, increased occurrence of cleared areas, rapid change in forest demography affecting biodiversity, etc.). To study realistic trajectories, we thus simulated a relatively long transition period of 30 years.
We distinguished two broad groups of effects of shortened rotation length in our analysis, which we refer to as main effects (i.e., those primarily discussed by managers in the context of rotation length) and collateral effects (i.e., those that are not receiving broad attention in the management community, yet might also be important when making management decisions) (Table 1). Main effects include the modification of forest age, the amount of harvested wood, and the level of disturbance by wind and bark beetles. As collateral effects, we focused on two different groups of indicators, representing forest carbon stocks and biodiversity. In particular, we evaluated effects on total landscape carbon storage, tree size diversity, the abundance of large trees, tree species diversity and tree shade tolerance diversity. Tree species diversity was evaluated based on the Shannon entropy index (Shannon, 1948) using basal area shares. Shade tolerance diversity was evaluated based on the Rao's Quadratic Entropy (Ricotta and Szeidl, 2009) using shade tolerance ratings of the tree species occurring on the landscape. The shade tolerance ratings were based on Niinemets and Valladares (2006) with minor modifications for consistency with iLand framework (http://iland.boku.ac.at/species + parameter). We used shade tolerance ratings as they are a good proxy for the different successional roles of tree species in forest dynamics (e.g., early vs. late seral species), and thus capture an important component of functional diversity beyond the tree species richness and abundance. The Rao's Quadratic Entropy was calculated as
where dij is the dissimilarity between species i and j (here the absolute difference between species shade tolerance scores; note that dij is normalized between [0,1] by dividing it by the maximum difference between the species represented in the landscape), pi and pj are the relative proportions of species i and j. We used R package SYNCSA (Debastiani, 2020) for this analysis.
Table 1.
Indicators used to study the effect of a reduced rotation length on the forest ecosystems. All indicators were calculated as landscape-level averages, and changes are relative to the current rotation length (Udef).
| Type of effect | Indicator [% change] |
|---|---|
| Main effect | Mean forest age Regular timber volume harvested (excluding salvage logged timber) Growing stock affected by wind Growing stock affected by bark beetles |
| Collateral effect | Mean stock of the total landscape carbon Abundance of large trees (DBH above 60 cm) Tree size diversity index (H index) Shannon diversity of tree species Rao‘s Quadratic Entropy Index of tree species shade tolerance |
Tree size diversity (H) was evaluated based on the index presented by Staudhammer and LeMay (2001):
where NDBH and NH are the number of DBH and height classes present in the landscape, gi is the basal area (m2) of DBH or height class i, and G is the basal area of the landscape (m2). We used 5-cm classes for DBH and 2-m classes for height (Cordonnier et al. 2013) with a minimum values of 4 m height and 5 cm DBH.
Although there is ample evidence that structural complexity and the abundance of microhabitats (cavities, dead branches) increase with tree diameter, the thresholds for identifying valuable habitat trees differs widely between authors and ecosystems (e.g. Larrieu et al., 2012; Larrieu and Cabanettes, 2012). We here applied a 60 cm diameter threshold for identifying large trees, following a suggestion of Lachat and Butler (2007) for mixed forests.
For all indicators we evaluate the effect of shortened rotation length and the modulation of this effect by climate change. To do this, we compare simulations under different levels of shortened rotation against simulations under Udef. The compared pairs of simulations are always driven by the same climate scenario, i.e. reference climate and climate change projections.
For all collateral effects and the harvested volume, we separately analysed the short-term (average over the first 30 years of the simulation) and the long-term (average over the remaining 170 years of the study period) effects of shortening rotation lengths. For the disturbed growing stock, we analysed the 200-year averages only, because 30 years were too short to make robust assessments of the highly stochastic disturbance events. We report the median and 10 – 90% quantile range over the 14 simulated climate change scenarios and 10 replicates.
3. Results
3.1. Main effects of reduced rotation
3.1.1. Forest age
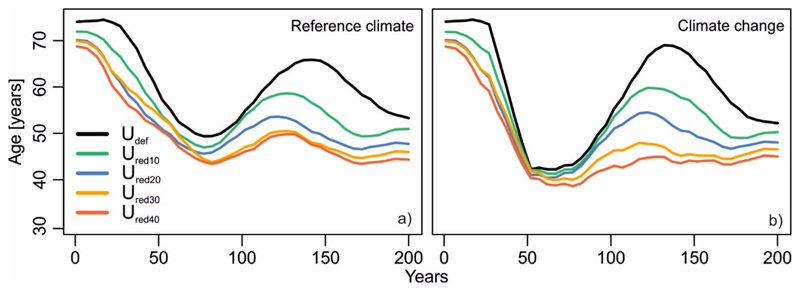
The average forest age during the 200-year simulation period was 61 years under Udef and reference climate (Table 2). Average forest age decreased by up to 18% (11 years) under the most extreme scenario Ured40. Because of the uneven distribution of current forest ages (resulting from past natural disturbances), forest age fluctuated regularly throughout the 200-year study period. The frequencies of this fluctuation roughly equalled the respective rotation age being simulated (Fig. 2ab). Climate change resulted in a higher amplitude of age variation over time, while conserving the general pattern also observed under reference climate.
Table 2.
The direct effects of reducing rotation length. Averages over the entire simulation period are shown. Each value shows the average of 10 simulation replicates. In case of climate change, also 14 climate scenarios are averaged. RC: reference climate, CC: climate change.
| Indicator | Absolute values | Differences from default rotation [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | Default rotation | Ured10 | Ured20 | Ured30 | Ured40 | ||||||
| RC | CC | RC | CC | RC | CC | RC | CC | RC | CC | ||
| Age | years | 60.6 | 58.3 | −8.0 | −7.3 | −13.7 | −12.7 | −15.7 | −16.7 | −18.1 | −20.1 |
| Harvested volume | m3 ha-1year−1 | 6.8 | 7.6 | 9.9 | 7.1 | 21.8 | 18.2 | 27.8 | 25.9 | 32.8 | 32.1 |
| Wind | m3 ha-1year−1 | 1.7 | 1.2 | −10.6 | −10.2 | −18.2 | −18.5 | −18.3 | −22.7 | −17.9 | −24.8 |
| Bark beetles | m3 ha-1year−1 | 2.1 | 4.5 | −5.2 | 0.1 | −10.6 | 0.1 | −10.6 | 0.1 | −11.5 | −0.7 |
| Totalkilled | m3 ha-1year−1 | 3.8 | 5.7 | −7.6 | −2.2 | −14.0 | −4.0 | −13.3 | −4.1 | −14.3 | −5.9 |
Fig. 2.
Temporal development of mean forest age on the study landscape under the default rotation age (Udef) and different levels of reduced rotation (Ured). A moving-window smoothing (k = 25) was applied on the age time series for presentation purpose. Each line shows the average of 10 replicated simulations. In case of climate change, also 14 climate scenarios were averaged.
3.1.2. Harvested volume
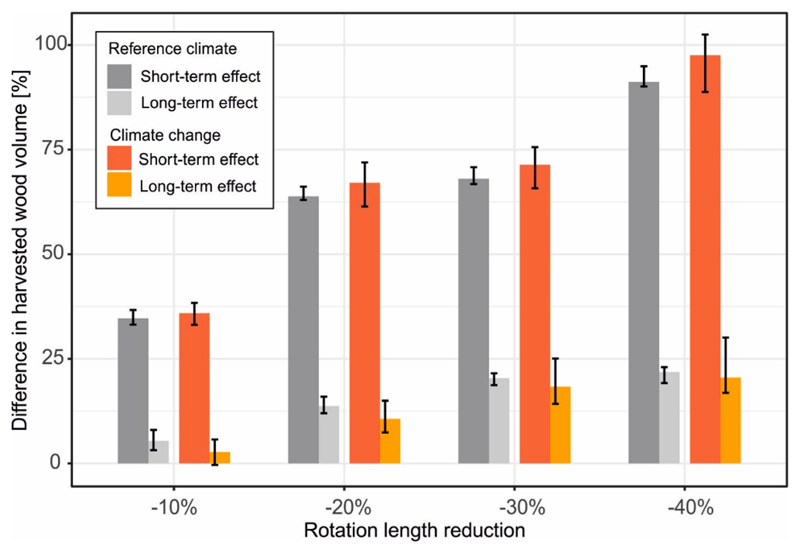
Reducing rotation length increased the amount of harvested wood volume (Table 2). In the short-term (i.e., the first 30 years of the simulation), this increase ranged between 35% and 92% of the harvest levels reached under Udef, depending on the intensity of reduction (Ured10 – Ured40) (Fig. 3). In the long-term (i.e. over the remaining 170 years of the simulation), the initially strong effect on harvested volume was reduced. Under Ured40, for instance, the long-term harvest level increase was 22% (i.e., a change from 6.7 to 8.1 m3ha-1year−1) (See Fig. 4).
Fig. 3.
Effect of reduced rotation length on the amount of harvested wood volume, expressed as percent difference relative to the default rotation. Short-term (average over the first 30 years of simulation) and long-term (average over the remaining 170 years of the study period) effects are shown. Columns show median values of replicated simulations and different climate change scenarios. Whiskers indicate 10 to 90% quantile range.
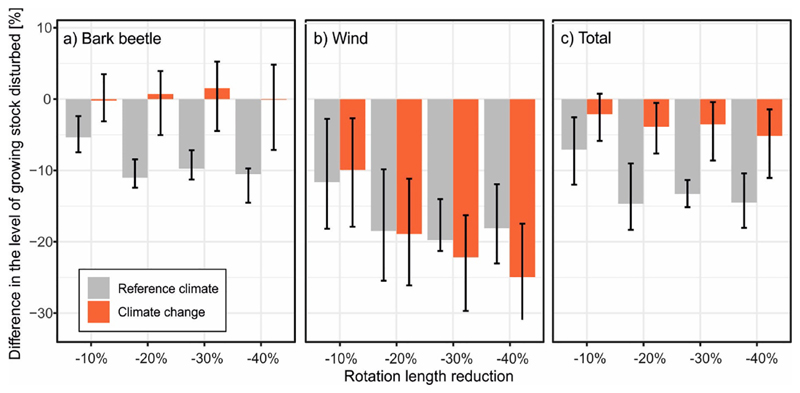
Fig. 4.
Effect of reduced rotation length on the level of disturbance by wind and bark beetles, expressed as the percent difference relative to the default rotation. Averages over the 200-year simulation period are shown. Columns show median values of replicated simulations and different climate change scenarios. Whiskers indicate 10 to 90% quantile range.
Climate change increased the overall amount of harvested wood volume from 6.8 to 7.6 m3ha-1year−1 (200-year average under Udef). The relative effects of reduced rotation on harvested volume did not change under climate change (Fig. 3, Table 2). The temporal evolution of harvested volumes was more erratic under climate change than under reference climate (Appendix C).
3.1.3. Wind and bark beetle disturbances
The level of natural disturbance decreased with shorter rotation lengths (Table 2). The average level of growing stock affected by wind and bark beetles under Udef was 3.8 m3 ha−1 year−1 under reference climate, and 5.7 m3 ha−1 year−1 under climate change (i.e. an increase by 50%) (Table 2). Climate change doubled bark beetle disturbances (from 2.2 to 4.5 m3 ha−1 year−1), while wind disturbance decreased from 1.7 to 1.2 m3 ha−1 year−1. Reducing the rotation length decreased the total disturbance by up to 14% under reference climate, but only by 6% under climate change (average change over simulations driven by all 14 climate scenarios). Reduced rotation buffered the impact of wind more effectively than the impact of bark beetles (-18% vs. −12% under reference climate, and −25% vs. −0.7% under climate change in the Ured40% scenario).
3.2. Collateral effects of reduced rotation length
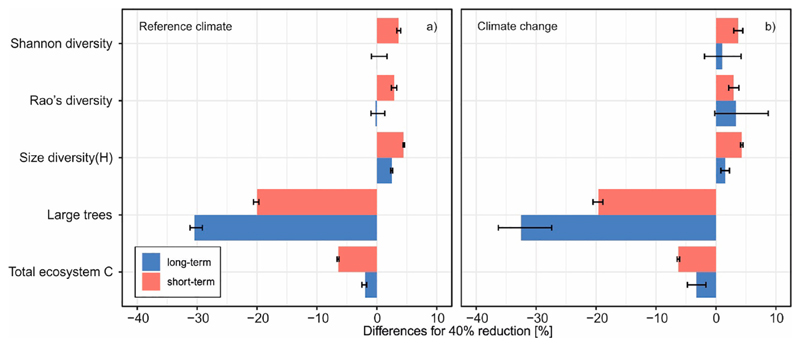
Reduced rotation length decreased the total amount of carbon stored in the landscape (Table 3). This effect was pronounced in the short-term (up to −7% under Ured40), while the long-term effect was −2% only. Climate change had a generally positive effect on forest carbon storage (Appendix C), but slightly amplified the negative effect of reduced rotation length (long-term effect of up to −3%) (Fig. 5, Table 3). However, the negative effects of reduced rotation length on carbon storage decreased over time, with Ured and Udef trajectories converging after 150 simulated years (Appendix B).
Table 3.
Indicator values for the collateral effects of reduced rotation length. Pairs of values indicate the short-term effects (upper value; average over the first 30 years of simulation) and the long-term effect (bottom value; average over the remaining 170 years). The values are averages over 10 replicate simulations. In case of climate change, also the 14 climate scenarios are averaged. RC: reference climate, CC: climate change.
| Indicator | Absolute values | Differences from default rotation [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | Default rotation | –10% | –20% | –30% | –40% | ||||||
| RC | CC | RC | CC | RC | CC | RC | CC | RC | CC | ||
| Landscape carbon | tC ha−1 | 420 | 423 | −2.9 | −2.8 | −5.1 | −5 | −3.9 | −3.8 | −6.5 | −6.3 |
| 421 | 453 | 0.1 | −0.5 | −0.8 | −1.4 | −1.4 | −2.4 | −2 | −3.3 | ||
| Large trees | no ha−1 | 33.8 | 33 | −10.2 | −10.2 | −17.5 | −17.2 | −14.2 | −13.9 | −20.1 | −19.7 |
| 27 | 26 | −11.2 | −11.4 | −20.8 | −19.5 | −26.2 | −27.3 | −30.2 | −32.1 | ||
| Structural diversity index (H) | – | 2.23 | 2.24 | 2.1 | 2.1 | 3.5 | 3.5 | 3.2 | 3.1 | 4.5 | 4.3 |
| 2.5 | 2.52 | 0.9 | 0.6 | 1.7 | 1 | 2.2 | 1.3 | 2.5 | 1.6 | ||
| Shannon diversity index | – | 0.25 | 0.25 | 0.9 | 0.9 | 1.9 | 2.0 | 1.9 | 1.9 | 3.6 | 3.7 |
| 0.04 | 0.13 | −3.2 | −0.2 | −2.8 | 0.3 | −2.1 | 0.3 | 0.2 | 1.0 | ||
| Rao‘s Quadratic Entropy Index | – | 0.35 | 0.36 | 0.3 | 0.3 | 1.1 | 1.1 | 1.2 | 1.3 | 2.9 | 2.9 |
| 0.44 | 0.52 | −3.0 | 0.3 | −2.9 | 1.5 | −2.2 | 2.4 | 0.0 | 3.8 | ||
Fig. 5.
Collateral effects of a 40% reduction in the current rotation length. Short-term (over the first 30 years of simulation) and long-term (over the remaining 170 years of the study period) effects are presented. Columns show median values of replicated simulations and different climate change scenarios. Whiskers indicate 10 to 90% quantile range.
Mean tree size diversity index H was 2.23 during the first 30 years of the simulation and 2.50 during the remaining 170 years. H increased in response to a reduced rotation length, with the increase being more pronounced in the short-term (up to 4.5%) than in the long-term (up to 2.5%). The effect of climate change on the rotation length response of H was negligible (Table 3). Reduced rotation decreased the number of large trees in the short-term by 20% under Ured40 (from 34 to 27 trees ha−1) in both the reference and climate change scenarios. The effect was more pronounced in the long-term, when the number of large trees decreased by 30 and 32% under reference climate and climate change, respectively (from 27 and 26 trees ha−1 to 19 and 18 trees ha−1).
Both tree species diversity and diversity in shade tolerance increased strongly during the simulation period as disturbances and harvesting opened up the canopy of the initial mature forest dominated by Norway spruce. In the long-term and under the reference climate, reduced rotation length had almost no effect on these two indicators. In the short-term, however, the two species diversity indicators increased slightly in response to the high harvesting intensity in the first decades of the simulation. Climate change increased Shannon and Rao's diversity in the long-term, while the short-term values remained similar to reference climate levels. Overall, the sensitivity of species and shade-tolerance diversity to reduced rotation was small.
4. Discussion
Addressing natural disturbances is an increasing concern for forest management in Europe, particularly due to a recent increase in disturbance activity and the propensity for further amplification of disturbance regimes under climate change (Seidl et al., 2014b; Senf et al., 2018). Yet, the efficiency of frequently discussed disturbance management measures remains insufficiently understood, and it is unclear whether past practices will retain their leverage also under future environmental conditions (e.g. Dobor et al. 2020). Furthermore, potential collateral effects of disturbance mitigation measures need to be considered, yet are rarely quantified comprehensively in studies focusing on disturbance management. We here show that a reduced rotation length can substantially reduce the impact of wind and bark beetle disturbances, but also highlight a decreasing efficiency of the measure under climate change. Furthermore, collateral impacts of reduced rotation lengths on forest biodiversity and carbon cycling indicators persisted.
4.1. Potential and limitations of reduced rotation lengths
4.1.1. Potential for reducing disturbances
We showed that shortened rotation lengths reduce the share of mature trees on the landscape and lower the risk of wind and bark beetle disturbances. Rotation reduction by 40% (i.e. the most severe variant studied here) reduced forest age by 18%, and decrease the total amount of disturbed growing stock by 14% under the reference climate. Climate change increased the amount of disturbed growing stock by 50% mainly due to increased bark beetle outbreaks, and reducing the rotation length was not able to fully compensate this increase. Overall, the relative effect of reduced rotation length on disturbances decreased with climate change from 14 to 6% under the Ured40. Our results thus suggest that expectations of lowered vulnerability of forest ecosystems to disturbances under climate change via reduced rotation lengths need to be reconsidered (e.g. Eidmann 1992, Björkman et al. 2015). This finding is in line with previous research indicating a decreasing efficiency of conventional disturbance management measures under climate change (Dobor et al., 2020, 2019). Overall, our study provides further evidence that controlling forest disturbances is increasingly challenging under climate change, underlining the growing relevance of alternative management approaches such as fostering resilience (i.e. strengthening the ecosystems ability to tolerate disturbances without changing substantially, and recover rapidly from them) (e.g. Seidl, 2014).
The fact that reduced rotation lengths decrease wind risk have been reported previously (Kuboyama and Oka, 2000; Moore and Quine, 2000). This effect mainly results from a reduced length that trees are exposed to extreme winds, and a smaller share of tall trees on the landscape, which are particularly predisposed to wind breakage or uprooting (Gardiner et al., 2000; Jactel et al., 2009). Quantitative evidence for positive effects of shortened rotation lengths remain, however, scarce for bark beetles (but see Taylor and Carroll, 2004; Whitehead et al., 2004 for Dendroctonous ponderosae). Nonetheless, research on the ecology (e.g. Wermelinger, 2004) and infestation patterns of Ips typographus clearly identified a preference of the beetle for older, larger diameter trees (Hlásny and Turčáni, 2013; Netherer et al., 2019; Netherer and Nopp-Mayr, 2005). In this regard an interesting finding of the current study is that reduced rotation lengths dampen wind disturbances more efficiently than bark beetle disturbances (-18% vs. −11% under Ured40%, relative to the default rotation). This suggests that a critically large number of suitable host trees remains on the landscape even under a 40% reduction in the rotation length. This difference was even greater under climate change (-25% vs. −0.7%), underscoring that under the elevated bark beetle population levels of the future, even isolated patches of potential host trees are increasingly at risk (Honkaniemi et al., 2020). It is important to note, however, that wind and bark beetle dynamics are intricately linked in Central European forests (Marini et al., 2017; Stadelmann et al., 2013), not least because they “compete” for the same resource, i.e. mature trees. For example, Dobor et al. (2019) found that the intense suppression of bark beetle disturbances increased the share of dense and mature forests on the landscape, which subsequently increased the susceptibility to wind disturbance. Conversely, an increased impact of bark beetles due to climate change may result in a concomitant decrease in wind impact, as observed here. We conclude by highlighting that the current study is the first to explore the dynamic relationships between multiple interacting disturbance agents and rotation length under climate change. Our analysis thus considerably improves the understanding of the potential future efficiency of important disturbance management measures.
A key limitation of our analysis is the sole focus on wind and bark beetle disturbances, which are both positively related to older trees. A number of other important agents of tree mortality particularly affect young trees, and could thus be hypothesized to increase with decreasing rotation length. These include important pests such as the pine weevil Hylobius abietis (Leather et al., 1999), but also abiotic drivers such as increasing drought (Kolb et al., 2016). Furthermore, the edges created by shortened rotation length as well as mechanical damage from harvesting operations could further increase the susceptibility of trees to disturbance (Buras et al., 2018; Rönnberg, 2000; Woodcock et al., 2015). Climate change could further amplify these effects (Allen et al., 2010; Inward et al., 2012), which highlights the need to study the effects of reduced rotation length on tree mortality more comprehensively in the future.
4.1.2. Limitations arising from collateral effects
The choice of rotation length has manifold impacts on forest soils, carbon, biodiversity, non-wood forest products and timber production (Felton et al., 2017; Kaipainen et al., 2004; Roberge et al., 2016). Therefore, even though the decision to shorten the rotation length can be motivated by the prospect of reducing natural disturbances, its effects on other management objectives need to be considered. Mitigating climate change is increasingly recognized as an important objective for contemporary forestry (Canadell and Raupach, 2008; Luyssaert et al., 2018) and management should thus consider the effects of interventions on forest carbon stocks and sink strength (Pilli et al., 2016). We found that reduced rotation can be in conflict with this objective, particularly in the short term. This finding is in agreement with previous studies which reported mostly negative effect of shortened rotation lengths on forest carbon (Kaipainen et al., 2004; Liski et al., 2011; Lundmark et al., 2018). We note, however, that an initial decrease in landscape carbon storage can be offset by increased carbon storage in the wood products pool, and positive substitution effect are possible from increased timber use (Lamers et al., 2014). Future works should therefore consider the carbon cycle effects of management more broadly, accounting for impacts on the entire forestry sector (Lundmark et al., 2018; Seidl et al., 2008). Managing forest carbon under climate change also needs to consider an ameliorative effect of increased CO2, which is an important driver of the current global carbon sink (Bellassen and Luyssaert, 2014). In our simulations, this effect caused an overall increase in forest carbon storage under climate change, and compensated carbon loss due to reduced rotation lengths (Appendix C) (see also Dobor et al., 2018). However, experimental evidence of the persistence of a CO2 fertilization effect is limited (Lindner et al., 2014) and trade-offs with other climate change impacts on forest demography are likely (McDowell et al., 2020).
Growing interest in reducing forest rotation lengths has also raised concerns about potential effects on biodiversity, especially since big old trees and structurally complex overmatured forests provide important habitat for a wide range of species of conservation concern (Hilmers et al., 2018; Lassauce et al., 2013). This effect is important as production forests are increasingly recognized to also play an important role in the conservation of biodiversity Díaz-Rodríguez et al., 2012; Felton et al., 2017). Here we show that a 40% reduction in rotation length resulted in a 30% decrease in large trees. These effects could possibly be compensated by retention forestry practices (Gustafsson et al., 2012), promoting the retention of trees in harvested areas and mimicking natural disturbance regimes in harvesting patterns (Lassauce et al., 2013; North and Keeton, 2008). Another approach to compensate for potential negative effects of shortened rotation length in managed forests could be the increase of protected areas on the landscape (Felton et al. 2017). While the effects of a shortened rotation length negatively impacted the prevalence of large trees, tree species diversity as well as the diversity in different functional groups (light demanding vs. shade tolerant) increased. The increase in harvesting intensity resulting from a reduced rotation length thus accelerates the transformation of structurally and compositionally homogenous forests to more diverse conditions, similarly to the catalysing effect of natural disturbances (Thom et al., 2017b). This effect can be potentially beneficial from the perspective of climate change adaptation (Bouriaud et al., 2015), facilitating necessary transformations.
Despite the fact that this is – to our knowledge – the most comprehensive study on the multiple simultaneous effects of reduced rotation length to date, shortening the rotation length might also affect important indicators not considered here. Reduced rotation periods do, for instance, increase the exposure of soils to sun and rain, and could thus result in soil loss via erosion and elevated decomposition (Kreutzweiser et al., 2008). Furthermore, an increased frequency of harvests could reduce the protective effect of forest ecosystems against natural hazards such as debris flow events and floods (Sebald et al., 2019). A higher harvesting frequency and smaller tree dimensions could also have negative impacts on the recreational value of forest ecosystems (Curtis, 1997). This underscores the need for a broad and comprehensive assessment of potential collateral effects of forest management decisions before they are implemented.
4.2. Conclusions
Reduced rotation length is increasingly discussed as an important component of adapting managed forests to climate change (Bolte et al., 2009; Lindner et al., 2010), in particular addressing changing disturbance regimes (Gardiner and Quine, 2000; Jactel et al., 2009). In contrast to other adaptation measures with long lead times, such as changing the tree species composition (e.g. Keenan 2015), the effects of reduced rotation lengths can be obtained within a relatively short period of time. Such approaches are increasingly needed because proactive and anticipatory adaptation actions to climate change have been neglected in many regions of Europe (Sousa-Silva et al., 2018), and climate change is progressing at an accelerating rate.
Here we show that the reduction of forest rotation length is an important tool for managing natural disturbances in Central Europe. However, our analyses also clearly demonstrate that the efficiency of reduced rotation lengths to counter increasing disturbances is limited, and that the negative effects of climate change cannot be fully compensated by shortening the rotation length. Transitions towards shorter rotation lengths also generated a number of undesired effects on ecosystem services and biodiversity. We thus conclude that reducing rotation lengths needs to be applied with caution, evaluating its positive and negative implications in the context of the local conditions and objectives. While we here simulated simultaneous rotation length reductions across an entire landscape we note that spatially stratified approaches to risk management, i.e., considering landscape heterogeneity and the differential risk of stands within a landscape, hold high potential to maximize outcomes while minimizing undesired effects (Dobor et al., 2020; Seidl et al., 2018). Furthermore, reducing the rotation length is only one possible option to address increasing disturbances, and should be applied in concert with other disturbance management approaches such as fostering disturbance resilience (Hlásny et al., 2019; Seidl, 2014). We conclude that addressing changing climate and disturbances regimes remains a major challenge for forest management in Central Europe, and while shortened rotation lengths can make a potential contribution, they are no silver bullet solution.
Supplementary Material
Acknowledgements
This study was supported by the grant “EVA4.0”, No. CZ.02.1.01/0.0/0.0/16_019/0000803 financed by OP RDE. R. Seidl and W. Rammer acknowledge support from Austrian Science Fund FWF START grant no. Y895-B25.
Footnotes
CRediT authorship contribution statement
Sona Zimová: Data curation, Formal analysis. Laura Dobor: Methodology, Formal analysis, Writing - review & editing. Tomas Hlášy: Methodology, Supervision, Writing - original draft. Werner Rammer: Software, Writing - review & editing. Rupert Seidl: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, Mcdowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EHT, Gonzalez P, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 2010;259:660–684. doi: 10.1016/j.foreco.2009.09.001. [DOI] [Google Scholar]
- Angelstam P, Naumov V, Elbakidze M, Manton M, Priednieks J, Rendenieks Z. Wood production and biodiversity conservation are rival forestry objectives in Europe’ s Baltic Sea Region. Ecosphere. 2018;9:1–26. doi: 10.1002/ecs2.2119. [DOI] [PubMed] [Google Scholar]
- Baier P, Pennerstorfer J, Schopf A. PHENIPS-A comprehensive phenology model of Ips typographus (L.) (Col., Scolytinae) as a tool for hazard rating of bark beetle infestation. For Ecol Manage. 2007;249:171–186. doi: 10.1016/j.foreco.2007.05.020. [DOI] [Google Scholar]
- Bellassen V, Luyssaert S. Carbon sequestration: Managing forests in uncertain times. Nature. 2014;506:153–155. doi: 10.1038/506153a. [DOI] [PubMed] [Google Scholar]
- Bernes C. Biodiversity in Sweden. Swedish Environmental Protection Agency; Stockholm: 2011. [Google Scholar]
- Björkman C, Bylund H, Nilsson U, Nordlander G, Schroeder M. In: Climate Change and Insect Pests. Björkman C, Niemelä P, editors. Antony Rowe CPI Group (UK) Ltd; Preston, UK: 2015. Effects of New Forest Management on Insect Damage Risk in a Changing Climate; p. 291. [Google Scholar]
- Blennow K, Andersson M, Bergh J, Sallnäs O, Olofsson E. Potential climate change impacts on the probability of wind damage in a south Swedish forest. Clim Change. 2010;99:261–278. doi: 10.1007/s10584-009-9698-8. [DOI] [Google Scholar]
- Blennow K, Sallnäs O. WINDA - A system of models for assessing the probability of wind damage to forest stands within a landscape. Ecol Model. 2004;175:87–99. doi: 10.1016/j.ecolmodel.2003.10.009. [DOI] [Google Scholar]
- Bolte A, Ammer C, Löf M, Madsen P, Nabuurs G-J, Schall P, Spathelf P, Rock J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand J For Res. 2009;24:473–482. doi: 10.1080/02827580903418224. [DOI] [Google Scholar]
- Bouriaud L, Bouriaud O, Elkin C, Temperli C, Reyer C, Duduman G, Barnoaiea I, Nichiforel L, Zimmermann N, Bugmann H. Age-class disequilibrium as an opportunity for adaptive forest management in the Carpathian Mountains, Romania. Reg Environ Change. 2015;15:1557–1568. doi: 10.1007/s10113-014-0717-6. [DOI] [Google Scholar]
- Brown M, Canham C, Murphy L, Donovan T. Timber harvest as the pre-dominant disturbance regime in northeastern US forests: effects of harvest intensification. Ecosphere. 2018;9 doi: 10.1002/ecs2.2062. [DOI] [Google Scholar]
- Buras A, Schunk C, Zeiträg C, Herrmann C, Kaiser L, Lemme H, Straub C, Taeger S, Gösswein S, Klemmt HJ, Menzel A. Are Scots pine forest edges particularly prone to drought-induced mortality? Environ Res Lett. 2018;13:1–11. doi: 10.1088/1748-9326/aaa0b4. [DOI] [Google Scholar]
- Canadell JG, Raupach MR. Managing Forests for Climate Change Mitigation. Science. 2008;320:1456–1457. doi: 10.1126/science.1155458. [DOI] [PubMed] [Google Scholar]
- Cordonnier T, Berger F, Elkin C, Lamas T, Martinez M. Models and linker functions (indicators) for ecosystem services. Arange Deliverable D2.2, Project Report. FP7-289437. 2013
- Curtis RO. In: Creating a Forestry for the 21st Century. Kohm KA, Franklin JF, editors. Island Press; Washington DC: 1997. The role of extended rotations; pp. 165–170. [Google Scholar]
- Curzon MT, D’Amato AW, Fraver S, Palik BJ, Bottero A, Foster JR, Gleason KE. Forest Ecology and Management Harvesting influences functional identity and diversity over time in forests of the northeastern USA. For Ecol Manage. 2017;400:93–99. doi: 10.1016/j.foreco.2017.05.056. [DOI] [Google Scholar]
- Daniel CJ, Ter-Mikaelian MT, Wotton BM, Rayfield B, Fortin M-J. Incorporating uncertainty into forest management planning: Timber harvest, wildfire and climate change in the boreal forest. For Ecol Manage. 2017;400:542–554. doi: 10.1016/j.foreco.2017.06.039. [DOI] [Google Scholar]
- de Groot M, Diaci J, Ogris N. Forest management history is an important factor in bark beetle outbreaks : Lessons for the future. For Ecol Manage. 2019;433:467–474. doi: 10.1016/j.foreco.2018.11.025. [DOI] [Google Scholar]
- Debastiani VJ. Analysis of functional and phylogenetic patterns in meta-communities - package SYNCSA (1.3.4) 2020 [Google Scholar]
- Díaz-Rodríguez B, Blanco-García A, Gómez-Romero M, Lindig-Cisneros R. Filling the gap: Restoration of biodiversity for conservation in productive forest landscapes. Ecol Eng. 2012;40:88–94. doi: 10.1016/j.ecoleng.2011.12.017. [DOI] [Google Scholar]
- Dobor L, Hlásny T, Rammer W, Barka I, Trombik J, Pavlenda P, Šebeň V, Štěpánek P, Seidl R. Post-disturbance recovery of forest carbon in a temperate forest landscape under climate change. Agric For Meteorol. 2018;263:308–322. doi: 10.1016/j.agrformet.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobor L, Hlásny T, Rammer W, Zimová S, Barka I, Seidl R. Spatial configuration matters when removing windfelled trees to manage bark beetle disturbances in Central European forest landscapes. J Environ Manage. 2020;254:1–12. doi: 10.1016/j.jenvman.2019.109792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobor L, Hlásny T, Rammer W, Zimová S, Barka I, Seidl R. Is salvage logging an effective means to protect forests and their carbon stores from future disturbances? J Appl Ecol. 2019;57:67–76. [Google Scholar]
- Eidmann HH. Impact of bark beetles on forests and forestry in Sweden. J Appl Entomol. 1992;114:193–200. [Google Scholar]
- Ekholm T. Forest Policy and Economics Optimal forest rotation age under efficient climate change mitigation. Forest Policy and Economics. 2016;62:62–68. doi: 10.1016/j.forpol.2015.10.007. [DOI] [Google Scholar]
- Elkin C, Giuggiola A, Rigling A, Bugmann H. Short- and long-term efficacy of forest thinning to mitigate drought impacts in mountain forests in the European Alps. Ecol Appl. 2015;25:1083–1098. doi: 10.1890/14-0690.1. [DOI] [PubMed] [Google Scholar]
- Faustmann M. Berechnung des Wertes welchen Waldboden sowie noch nicht haubare Holzbestände für die Waldwirtschaft besitzen [Calculation of the value which forest land and immature stands possess for forestry] Allgemeine Forst- und Jagdzeitung. 1849;25:441–445. [Google Scholar]
- Felton A, Sonesson J, Nilsson U, Lämäs T, Lundmark T, Nordin A, Ranius T, Roberge J-M. Varying rotation lengths in northern production forests : Implications for habitats provided by retention and production trees. Ambio. 2017;46:324–334. doi: 10.1007/s13280-017-0909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner BA, Quine CP. Management of forests to reduce the risk of abiotic damage – a review with particular reference to the effects of strong winds. For Ecol Manage. 2000;135:261–277. [Google Scholar]
- Gardiner B, Peltola H, Kellomäki S. Comparison of two models for predicting the critical wind speeds required to damage coniferous trees. Ecol Model. 2000;129:1–23. doi: 10.1016/S0304-3800(00)00220-9. [DOI] [Google Scholar]
- Giorgi F, Jones C, Asrar GR. Addressing climate information needs at the regional level: the CORDEX framework. WMO Bulletin. 2009;58:175–183. [Google Scholar]
- Gustafsson L, Baker SC, Bauhus J, Beese WJ, Brodie A, Kouki J, Lindenmayer DB, Lõhmus A, Pastur GM, Messier C, Neyland M, et al. Retention Forestry to Maintain Multifunctional Forests : A World Perspective. Bioscience. 2012;62:633–645. doi: 10.1525/bio.2012.62.7.6. [DOI] [Google Scholar]
- Hale SE, Gardiner BA, Wellpott A, Nicoll BC, Achim A. Wind loading of trees: Influence of tree size and competition. Eur J Forest Res. 2012;131:203–217. doi: 10.1007/s10342-010-0448-2. [DOI] [Google Scholar]
- Hilmers T, Friess N, Bässler C, Heurich M, Brandl R, Pretzsch H, Seidl R, Müller J. Biodiversity along temperate forest succession. J Appl Ecol. 2018:1–11. doi: 10.1111/1365-2664.13238. [DOI] [Google Scholar]
- Hlásny T, Barka I, Roessiger J, Kulla L, Trombik J, Sarvašová Z, Bucha T, Kovalčík M, Čihák T. Conversion of Norway spruce forests in the face of climate change: a case study in Central Europe. Eur J Forest Res. 2017;136:1013–1028. doi: 10.1007/s10342-017-1028-5. [DOI] [Google Scholar]
- Hlásny T, Krokene P, Liebhold A, Montagné-Huck C, Müller J, Qin H, Raffa K, Schelhaas M-J, Seidl R, Svoboda M, Viiri H. Living with bark beetles: impacts, outlook and management options. From Science to Policy 8. European Forest Institute; 2019. [Google Scholar]
- Hlásny T, Sitková Z. Spruce forest decline in the Beskids. 1st ed. National forest centre - forest research institute Zvolen & Czech University of Life Sciences Prague & Forestry and Game Management Research Institute Jíloviště; Strnady: 2010. [Google Scholar]
- Hlásny T, Turčáni M. Persisting bark beetle outbreak indicates the unsustainability of secondary Norway spruce forests: Case study from Central Europe. Annals of Forest Science. 2013;70:481–491. doi: 10.1007/s13595-013-0279-7. [DOI] [Google Scholar]
- Honkaniemi J, Rammer W, Seidl R. Norway spruce at the trailing edge : the effect of landscape configuration and composition on climate resilience. Landscape Ecol. 2020 doi: 10.1007/s10980-019-00964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerford RD, Nemani RR, Running SW, Coughlan JC. MTCLIM: A mountain microclimate simulation model. USDA Forest Service Res. Paper 52. 1989
- Churchill DJ, Larson AJ, Dahlgreen MC, Franklin JF, Hessburg PF, Lutz JA. Restoring forest resilience : From reference spatial patterns to silvicultural prescriptions and monitoring. For Ecol Manage. 2013;291:442–457. doi: 10.1016/j.foreco.2012.11.007. [DOI] [Google Scholar]
- Inward DJG, Wainhouse D, Peace A. The effect of temperature on the development and life cycle regulation of the pine weevil Hylobius abietis and the potential impacts of climate change. Agric For Entomol. 2012;14:348–357. doi: 10.1111/j.1461-9563.2012.00575.x. [DOI] [Google Scholar]
- Jactel H, Nicoll BC, Branco M, Gonzalez-Olabarria JR, Grodzki W, Långström B, Moreira F, Netherer S, Orazio C, Piou D, Santos H, et al. The influences of forest stand management on biotic and abiotic risks of damage. Annals of Forest Science. 2009;66:1–18. doi: 10.1051/forest/2009054. [DOI] [Google Scholar]
- Jonsson M, Ranius T, Ekvall H, Bostedt G, Dahlberg A, Ehnström B, Nordén B, Stokland JN. Cost-effectiveness of silvicultural measures to increase substrate availability for red-listed wood-living organisms in Norway spruce forests. Biological Conservation I. 2006;27:443–462. doi: 10.1016/j.biocon.2005.09.004. [DOI] [Google Scholar]
- Kaipainen T, Liski J, Pussinen A, Karjalainen T. Managing carbon sinks by changing rotation length in European forests. Environ Sci Policy. 2004;7:205–219. doi: 10.1016/j.envsci.2004.03.001. [DOI] [Google Scholar]
- Keenan RJ. Climate change impacts and adaptation in forest management: a review. Annals of Forest Science. 2015;72:145–167. doi: 10.1007/s13595-014-0446-5. [DOI] [Google Scholar]
- Klimo E, Hager H, Kulhavý J. Spruce Monocultures in Central Europe – Problems and Prospects; Proceedings 33; Joensuu, Finland. 2000. p. 208. [Google Scholar]
- Kolb TE, Fettig CJ, Ayres MP, Bentz BJ, Hicke JA, Mathiasen R, Stewart JE, Weed AS. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For Ecol Manage. 2016;380:321–334. doi: 10.1016/j.foreco.2016.04.051. [DOI] [Google Scholar]
- Konôpka B, Zach P, Kulfan J. Wind – an important ecological factor and destructive agent in forests. Forestry Journal. 2016;62:123–130. doi: 10.1515/forj-2016-0013. [DOI] [Google Scholar]
- Kreutzweiser DP, Hazlett PW, Gunn JM. Logging impacts on the bio-geochemistry of boreal forest soils and nutrient export to aquatic systems : A review. Environmental Reviews. 2008;16:157–179. doi: 10.1139/A08-006. [DOI] [Google Scholar]
- Kuboyama H, Oka H. Climate Risks and Age-related Damage Probabilities – Effects on the Economically Optimal Rotation Length for Forest Stand Management in Japan. Silva Fennica. 2000;34:155–166. [Google Scholar]
- Lafond V, Lagarrigues G, Cordonnier T, Courbaud B. Uneven-aged management options to promote forest resilience for climate change adaptation : effects of group selection and harvesting intensity. Annals of Forest Science. 2014;71:173–186. doi: 10.1007/s13595-013-0291-y. [DOI] [Google Scholar]
- Lachat T, Butler R. Gestion des vieux arbres et du bois mort: Îlots de sénescence, arbres-habitat et métapopulations saproxyliques. Ecole polytechnique fédérale Lausanne WSL; Lausa: 2007. [Google Scholar]
- Lamers P, Junginger M, Dymond CC, Faaij A. Damaged forests provide an opportunity to mitigate climate change. GCB Bioenergy. 2014;6:44–60. doi: 10.1111/gcbb.12055. [DOI] [Google Scholar]
- Lange M, Türke M, Pašalić E, Boch S, Hessenmöller D, Müller J, Prati D, Socher SA, Fischer M, Weisser WW, Gossner MM. Forest Ecology and Management Effects of forest management on ground-dwelling beetles (Coleoptera; Carabidae, Staphylinidae) in Central Europe are mainly mediated by changes in forest structure. For Ecol Manage. 2014;329:166–176. doi: 10.1016/j.foreco.2014.06.012. [DOI] [Google Scholar]
- Larrieu L, Cabanettes A. Species, live status, and diameter are important tree features for diversity and abundance of tree microhabitats in subnatural montane beech–fir forests1This article is one of a selection of papers from the International Symposium on Dynamics and Ecologic. Can J For Res. 2012;42:1433–1455. doi: 10.1139/x2012-077. [DOI] [Google Scholar]
- Larrieu L, Cabanettes A, Delarue A. Impact of silviculture on dead wood and on the distribution and frequency of tree microhabitats in montane beech-fir forests of the Pyrenees. Eur J Forest Res. 2012;131:773–786. doi: 10.1007/s10342-011-0551-z. [DOI] [Google Scholar]
- Lassauce A, Larrieu L, Paillet Y, Lieutier F, Bouget C. The effects of forest age on saproxylic beetle biodiversity : implications of shortened and extended rotation lengths in a French oak high forest. Insect Conservation and Diversity. 2013;6:396–410. doi: 10.1111/j.1752-4598.2012.00214.x. [DOI] [Google Scholar]
- Leather SR, Day KR, Salisbury AN. The biology and ecology of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae): a problem of dispersal? Bull Entomol Res. 1999;89:3–16. doi: 10.1017/S0007485399000024. [DOI] [Google Scholar]
- Lindenmayer D, Messier C, Sato C. Avoiding ecosystem collapse in managed forest ecosystems. Front Ecol Environ. 2016;14:561–568. doi: 10.1002/fee.1434. [DOI] [Google Scholar]
- Lindner M, Fitzgerald JB, Zimmermann NE, Reyer C, Delzon S, van der Maaten E, Schelhaas MJ, Lasch P, Eggers J, van der Maaten-Theunissen M, Suckow F, et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J Environ Manage. 2014;146:69–83. doi: 10.1016/j.jenvman.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Lindner M, Lasch P, Erhard M. Alternative forest management strategies under climatic change – Prospects for gap model applications in risk analyses. Silva Fennica. 2000;34:101–111. doi: 10.14214/sf.634. [DOI] [Google Scholar]
- Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J, Seidl R, Delzon S, Corona P, Kolström M, Lexer MJ, et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manage. 2010;259:698–709. doi: 10.1016/j.foreco.2009.09.023. [DOI] [Google Scholar]
- Liski J, Pussinen A, Pingoud K, Mäkipää R, Karjalainen T. Which rotation length is favourable to carbon sequestration? Can J For Res. 2011;31:2004–2013. doi: 10.1139/x01-140. [DOI] [Google Scholar]
- Lundmark T, Poudel BC, Stål G, Nordin A, Sonesson J. Carbon balance in production forestry in relation to rotation length. Can J For Res. 2018;48:672–678. [Google Scholar]
- Luyssaert S, Marie G, Valade A, Chen Y, Djomo SN, Ryder J, Otto J, Naudts K, Lansø AS, Ghattas J, Mcgrath MJ. Trade-offs in using European forests to meet climate objectives. Nature. 2018;562:259–267. doi: 10.1038/s41586-018-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä A. Process-based modelling of tree and stand growth: towards a hierarchical treatment of multiscale processes. Can J For Res. 2003;33:398–409. doi: 10.1139/x02-130. [DOI] [Google Scholar]
- Marini L, Økland B, Jönsson AM, Bentz B, Carroll A, Forster B, Grégoire J, Hurling R, Nageleisen LM, Netherer S, Ravn HP, et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography. 2017;40:1426–1435. doi: 10.1111/ecog.02769. [DOI] [Google Scholar]
- McDowell NG, Allen CD, Anderson-Teixeira K, Aukema BH, Bond-Lamberty B, Chini L, Clark JS, Dietze M, Grossiord C, Hanbury-Brown A, Hurtt GC, et al. Pervasive shifts in forest dynamics in a changing world. Science. 2020;368 doi: 10.1126/science.aaz9463. [DOI] [PubMed] [Google Scholar]
- Meilby H, Strange N, Thorsen BJ. Optimal spatial harvest planning under risk of windthrow. For Ecol Manage. 2001;149:15–31. [Google Scholar]
- Mezei P, Jakuš R, Pennerstorfer J, Havašová M, Škvarenina J, Ferenčik J, Slivinský J, Bičárová S, Bilčík D, Blaženec M, Netherer S. Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—An infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agric For Meteorol. 2017;242:85–95. doi: 10.1016/j.agrformet.2017.04.004. [DOI] [Google Scholar]
- Moore J, Quine CP. A comparison of the relative risk of wind damage to planted forests in Border Forest Park, Great Britain, and the Central North Island, New Zealand. For Ecol Manage. 2000;135:345–353. [Google Scholar]
- Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, van Vuuren DP, Carter TR, Emori S, Kainuma M, Kram T, Meehl GA, et al. The next generation of scenarios for climate change research and assessment. Nature. 2010;463:747–756. doi: 10.1038/nature08823. [DOI] [PubMed] [Google Scholar]
- Netherer S, Nopp-Mayr U. Predisposition assessment systems (PAS) as supportive tools in forest management - Rating of site and stand-related hazards of bark beetle infestation in the High Tatra Mountains as an example for system application and verification. For Ecol Manage. 2005;207:99–107. doi: 10.1016/j.foreco.2004.10.020. [DOI] [Google Scholar]
- Netherer S, Panassiti B, Pennerstorfer J, Matthews B. Acute Drought Is an Important Driver of Bark Beetle Infestation in Austrian Norway Spruce Stands. Frontiers in Forests and Global Change. 2019;2 doi: 10.3389/ffgc.2019.00039. [DOI] [Google Scholar]
- Newman DH. Forestry' s golden rule and the development of the optimal forest rotation literature. Journal of Forest Economics. 2002;8:30602 [Google Scholar]
- Niinemets Ü, Valladares F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol Monogr. 2006;76:521–547. doi: 10.1890/0012-9615(2006)076[0521:TTSDAW]2.0.CO;2. [DOI] [Google Scholar]
- North MP, Keeton WS. In: Patterns and Processes in Forest Landscapes – Multiple Use and Sustainable Management. Lafortezza R, Chen J, Sanesi G, Crow TR, editors. Springer Verlag; The Netherlands: 2008. Emulating natural disturbance regimes: an emerging approach for sustainable forest management; pp. 341–372. [Google Scholar]
- Pilli R, Grassi G, Kurz WA, Moris JV, Viñas RA. Modelling forest carbon stock changes as affected by harvest and natural disturbances. II. EU-level analysis. Carbon Balance and Management. 2016;11:1–19. doi: 10.1186/s13021-016-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. The Theory and Application of Forest Economics. Basil Blackwell; Oxford, UK: 1989. [Google Scholar]
- Rammer W, Seidl R. Coupling human and natural systems: Simulating adaptive management agents in dynamically changing forest landscapes. Global Environ Change. 2015;35:475–485. doi: 10.1016/j.gloenvcha.2015.10.003. [DOI] [Google Scholar]
- Ricotta C, Szeidl L. Diversity partitioning of Rao's quadratic entropy. Theor Popul Biol. 2009;76:299–302. doi: 10.1016/j.tpb.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Roberge J-M, Laudon H, Björkman C, Ranius T, Sandström C, Felton A, Sténs A, Nordin A, Granström A, Widemo F, Bergh J, et al. Socio-ecological implications of modifying rotation lengths in forestry. Ambio. 2016;45:109–123. doi: 10.1007/s13280-015-0747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg J. Logging Operation Damage to Roots of Clear-felled Picea abies and Subsequent Spore Infection by Heterobasidion annosum. Silva Fennica. 2000;34:29–36. [Google Scholar]
- Russell WH, Jones C. The effects of timber harvesting on the structure and composition of adjacent old-growth coast redwood forest, California, USA. Landscape Ecol. 2001;16:731–741. [Google Scholar]
- Sebald J, Senf C, Heiser M, Scheidl C, Pflugmacher D, Seidl R. The effects of forest cover and disturbance on torrential hazards : large-scale evidence from the Eastern Alps. Environ Res Lett. 2019;14:1–12. [Google Scholar]
- Seidl R. The Shape of Ecosystem Management to Come : Anticipating Risks and Fostering Resilience. Bioscience. 2014;64:1159–1169. doi: 10.1093/biosci/biu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Albrich K, Thom D, Rammer W. Harnessing landscape heterogeneity for managing future disturbance risks in forest ecosystems. J Environ Manage. 2018;209:46–56. doi: 10.1016/j.jenvman.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Lexer MJ. Forest management under climatic and social uncertainty : Trade-offs between reducing climate change impacts and fostering adaptive capacity. J Environ Manage. 2013;114:461–469. doi: 10.1016/j.jenvman.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Seidl R, Rammer W. Climate change amplifies the interactions between wind and bark beetle disturbances in forest landscapes. Landscape Ecol. 2017;32:1485–1498. doi: 10.1007/s10980-016-0396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Rammer W, Blennow K. Simulating wind disturbance impacts on forest landscapes: Tree-level heterogeneity matters. Environ Modell Software. 2014a;51:1–11. doi: 10.1016/j.envsoft.2013.09.018. [DOI] [Google Scholar]
- Seidl R, Rammer W, Jäger D, Lexer MJ. Impact of bark beetle (Ips typographus L.) disturbance on timber production and carbon sequestration in different management strategies under climate change. For Ecol Manage. 2008;256:209–220. doi: 10.1016/j.foreco.2008.04.002. [DOI] [Google Scholar]
- Seidl R, Rammer W, Scheller RM, Spies TA. An individual-based process model to simulate landscape-scale forest ecosystem dynamics. Ecol Model. 2012a;231:87–100. doi: 10.1016/j.ecolmodel.2012.02.015. [DOI] [Google Scholar]
- Seidl R, Schelhaas M-J, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nat Clim Change. 2014b;4:806. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Spies TA, Rammer W, Steel EA, Pabst RJ, Olsen K. Multi-scale Drivers of Spatial Variation in Old-Growth Forest Carbon Density Disentangled with Lidar and an Individual-Based Landscape Model. Ecosystems. 2012b;15:1321–1335. doi: 10.1007/s10021-012-9587-2. [DOI] [Google Scholar]
- Senf C, Pflugmacher D, Zhiqiang Y, Sebald J, Knorn J, Neumann M, Hostert P, Seidl R. Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat Commun. 2018;9:1–8. doi: 10.1038/s41467-018-07539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. A Mathematical Theory of Communication. Bell Syst Tech J. 1948;27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- Silva Pedro M, Rammer W, Seidl R. Tree species diversity mitigates disturbance impacts on the forest carbon cycle. Oecologia. 2015;177:619–630. doi: 10.1007/s00442-014-3150-0. [DOI] [PubMed] [Google Scholar]
- Sohn JA, Hartig F, Kohler M, Huss J, Bauhus J. Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecol Appl. 2016;26:2190–2205. doi: 10.1002/eap.1373. [DOI] [PubMed] [Google Scholar]
- Sousa-Silva R, Verbist B, Lomba Â, Valent P, Suškevičs M, Picard O, Hoogstra-Klein MA, Cosofret V-C, Bouriaud L, Ponette Q, Verheyen K, et al. Adapting forest management to climate change in Europe: Linking perceptions to adaptive responses. Forest Policy and Economics. 2018;90:22–30. doi: 10.1016/j.forpol.2018.01.004. [DOI] [Google Scholar]
- Spiecker H, Hansen J, Klimo E, Skovsgaard JP, Sterba H, vonTeuffel K. Norway spruce conversion: options and consequences. Brill; Leiden, Boston, Köln: 2004. [Google Scholar]
- Spittlehouse DL, Stewart RB. Adaptation to climate change in forest management. Journal of Ecosystems and Management Adaptation. 2003;4:1–11. doi: 10.1109/lsp.2009.2014096. [DOI] [Google Scholar]
- Stadelmann G, Bugmann H, Meier F, Wermelinger B, Bigler C. Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. For Ecol Manage. 2013;305:273–281. doi: 10.1016/j.foreco.2013.06.003. [DOI] [Google Scholar]
- Staudhammer CL, LeMay VM. Introduction and evaluation of possible indices of stand structural diversity. Can J For Res. 2001;31:1105–1115. doi: 10.1139/x01-033. [DOI] [Google Scholar]
- Taylor S, Carroll A. In: Shore TL, Brooks JE, Stone JE, editors. Disturbance, forest age, and mountain pine beetle outbreak dynamics in BC: a historical perspective; Mountain Pine Beetle Symposium: Challenges and Solutions; October 30–31, 2003; Kelowna, British Columbia, Canada. 2004. pp. 41–51. Report BC-X-399. [Google Scholar]
- Thom D, Golivets M, Edling L, Meigs G, Gourevitch J, Sonter L, Galford G, Keeton W. The climate sensitivity of carbon, timber, and species richness covaries with forest age in boreal-temperate North America. Glob Change Biol. 2019;25 doi: 10.1111/gcb.14656. [DOI] [PubMed] [Google Scholar]
- Thom D, Rammer W, Dirnböck T, Müller J, Kobler J, Katzensteiner K, Helm N, Seidl R. The impacts of climate change and disturbance on spatio-temporal trajectories of biodiversity in a temperate forest landscape. J Appl Ecol. 2017a;54:28–38. doi: 10.1111/1365-2664.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom D, Rammer W, Garstenauer R, Seidl R. Legacies of past land use have a stronger effect on forest carbon exchange than future climate change in a temperate forest landscape. Biogeosciences. 2018;15:5699–5713. [Google Scholar]
- Thom D, Rammer W, Seidl R. Disturbances catalyze the adaptation of forest ecosystems to changing climate conditions. Glob Change Biol. 2017b;23:269–282. doi: 10.1111/gcb.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermelinger B. Ecology and management of the spruce bark beetle Ips typographus – A review of recent research. For Ecol Manage. 2004;202:67–82. doi: 10.1016/j.foreco.2004.07.018. [DOI] [Google Scholar]
- Wermelinger B, Seifert M. Temperature dependent reproduction of the spruce bark beetle Ips typographus, and analysis of the potential population growth. Ecol Entomol. 1999;24:103–110. [Google Scholar]
- Weslien J, Finer L, Jónsson JÁ, Koivusalo H, Laurén A, Ranius T, Sigurdsson BD, Weslien J, Finér L, Jónsson JÁ, Koivusalo H, et al. Effects of increased forest productivity and warmer climates on carbon sequestration, run-off water quality and accumulation of dead wood in a boreal landscape : A modelling study. 2009:7581. doi: 10.1080/02827580903085171. [DOI] [Google Scholar]
- Whitehead RJ, Safranyik L, Russo G, Shore TL, Carroll AL. In: Shore TL, Brooks JE, Stone JE, editors. Silviculture to reduce landscape and stand susceptibility to the mountain pine beetle; Mountain Pine Beetle Symposium: Challenges and Solutions; October 30-31, 2003; Kelowna, British Columbia, Canada. 2004. pp. 233–244. Report BC-X-399. [Google Scholar]
- Woodcock P, Halme P, Edwards D. In: Routledge Handbook of Forest Ecology. Bergeron K, editor. Taylor & Francis; 2015. Ecological effects of logging and approaches to mitigating impacts; pp. 422–435. [Google Scholar]
- Yousefpour R, Nabel JEMS, Pongratz J. Simulating growth-based harvest adaptive to future climate change. Biogeosciences. 2019;16:241–254. doi: 10.5194/bg-16-241-2019. [DOI] [Google Scholar]
- Christensen OB, Drews M, Christensen JH, Dethloff K, Ketelsen K, Hebestadt I, Rinke A. The HIRHAM regional climate model version 5 (β), DMI Techical Report. Copenhagen: 2007. [Google Scholar]
- Strandberg G, Bärring L, Hansson U, Jansson C, Jones C, Kjellström E, Kolax M, Kupiainen M, Nikulin G, Samuelsson P, Ullerstig A, et al. CORDEX scenarios for Europe from the Rossby Centre regional climate model RCA4. SMHI Rep Meteorol Climatol. 2014;116:1–45. [Google Scholar]
- van Meijgaard E, van Ulft LH, van de Berg WJ, Bosveld FC, van den Hurk B, Lenderink G, Siebesma AP. Technical Report 302: The KNMI regional atmospheric climate model RACMO version 2.1. De Bilt: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.