Introduction
Asthma is a chronic, heterogeneous inflammatory condition of the lower airways characterized by reversible airway obstruction, and represents the culmination of distinct pathways that are associated with complex genetic backgrounds and environment exposure 1. Allergic disorders, including asthma, result mainly from an exacerbated Th2 immune response to antigens which are innocuous for most people 2. The prevalence of asthma and allergies has increased during the past decades, particularly in industrialized countries and, more recently, in developing countries 3, 4, 5, 6.
The temporal trend in the prevalence of allergic disease has been explained mainly by the “hygiene hypothesis”, originally proposed by Strachan, as a consequence of decreased exposure to pathogens (e.g., helminths and bacteria) in the environment during childhood 7.
The immune response against helminths is orchestrated by Th2 cytokines production, especially IL-4 and IL-5 that act on B cells to induce IgG and IgE class switching 8. To escape from host defense the helminths lead to develop robust immune regulatory mechanisms mediated by regulatory T (Treg) cells that act through the production of Transforming Growth Factor-Beta (TGF-β1) and IL-10 9–11.
TGF-β1 is a pleiotropic growth factor produced by various immune cells (epithelial cells, eosinophils, Th2 lymphocytes, macrophages and fibroblasts) that plays a key role in regulation of the immune response during intracellular infections and inflammatory events by inhibiting the differentiation of immune cells (Th1 lymphocytes, Th2 lymphocytes, cytotoxic T cells and B cells) as well as cytokine production (IFN-γ and IL-2) 12–14. Furthermore, TGF-β1 has been shown to be an important differentiation factor for regulatory T cells exerting powerful and diverse immunosuppressive effects.15
In genetic association studies, polymorphisms in genes encoding TGF-β1, have previously been associated with allergy and asthma phenotypes including rs4803455, rs1800470, rs1800469, rs2241712 16–20. The rs1800470 in the TGFB1 promoter and rs1800469 in codon 10 of exon 1 appear to influence TGF-β1 blood levels and gene expression 21, 22.
Despite the prominent role that TGF-β1 plays in helminthic infections, no association studies have been published examining the role of TGFB1 polymorphisms in risk of helminth infection and how it affects allergy.
Given the important regulatory role of TGF-β1 on inflammatory diseases and helminth infection, we sought to assess whether known polymorphisms in the TGFB1 gene are associated with asthma and allergic markers and whether they influence immunity to helminths.
Methods
Study population and design
The study population was selected from the city of Salvador in northeastern Brazil. The general study design has been extensively described elsewhere 9, 10, 23. Briefly, the study population included 1,335 unrelated children between 4 and 11 years old originally recruited in infancy by the program entitled Social Change, Asthma and Allergy in Latin America (SCAALA) for a prospective study that analyzed the effect of a citywide sanitation program on childhood morbidity 24.
Data were collected from children born between 1994 and 2001 who lived in sentinel neighborhoods in the city. In 2000, stool samples were collected to characterize intestinal helminth infection. Children were resurveyed in 2005 to determine asthma status and to obtain stool and blood samples. Written informed consent was obtained from parents or the legal guardian of participants as approved by the Brazilian National Ethical Committee (003-2005/CEP-ICS).
Asthma definition
As previously described 25, children were classified as having current wheeze by using a Portuguese-adapted phase II International Study of Asthma and Allergies in Childhood questionnaire (wheezing in the last 12 months)26 and were considered to have asthma if there was a history of wheezing in the previous 12 months and at least 1 of the following: (1) asthma diagnosis; (2) wheezing with exercise in the last 12 months; (3) 4 or more episodes of wheezing in the last 12 months; and (4) waking up at night because of wheezing in the last 12 months.
Specific serum IgE levels
Determination of specific IgE (sIgE) levels were performed for D. pteronyssinus, B. tropicalis, B. germanica, and P. americana using the ImmunoCAP assay (Phadia Diagnostics AB, Uppsala Sweden). Values equal or greater than 0.70 kU/L were considered positive. Allergy status was defined according to having a positive result for at least 1 sIgE to aeroallergens.
Skin prick tests
Skin prick tests (SPTs) were performed on the right forearm of participants using standardized extracts (ALK-Abelló, São Paulo, Brazil) of Dermatophagoides pteronyssinus, Blomia tropicalis, Blatella germanica, Periplaneta americana, cat and dog epithelia and a fungi mix (Aspergillus amstelodami, Aspergillus fumigatus, Aspergillus niger, Aspergillus terrus, Penicillium brevicompactum, Penicillium expansum, Penicillium notatum, Penicillium roqueforti, Cladosporium fulvum, and Cladosporium herbarum). Saline and 10mg/mL histamine solution were used as negative and positive controls, respectively. The reaction was read after 15 minutes. A reaction was considered positive if the wheal size was at least 3 mm greater than that elicited by the negative control.
Parasitological analysis
Stool samples were collected twice and analyzed for A. lumbricoides and T. trichiura infection at each of the 2 sampling times, 2 weeks apart. Stool samples were analyzed by using the Hoffman technique 9 to determine the presence of helminths and the Kato-Katz technique 27 to determine parasitic load. All children with positive results were appropriately treated 23.
Occurrence of infections were defined as follows: (1) current infections: infections with A. lumbricoides, or T. trichiura detected in childhood (ie, survey conducted in 2005)and (2) coinfection: children infected with both A. lumbricoides and T. trichiura in 2005.
Total serum IgE levels and markers of infection: anti- A. lumbricoides IgE and IgG4 antibodies and anti- T. canis IgG
Total serum (tIgE) IgE levels were measured as previously described 9. Briefly, plate wells were coated with 4 mg/mL of an anti-human IgE antibody (BD PharMingen) overnight at 4°C, followed to blocking overnight at 4°C. Samples were diluted 1:10 in diluent solution and incubated overnight at 4°C. Plates were incubated with biotinylated anti-human IgE (Sigma), followed by streptavidin/peroxidase (BD PharMingen) and H2O2/orthophenylenediamine substrate (Merck) and read with a 480-nm filter.
Determination of worm-specific sIgE was performed for A. lumbricoides using the ImmunoCAP assay (Phadia Diagnostics AB, Uppsala, Sweden). Anti- A lumbricoides- sIgE levels equal or greater than 0.35 kU/L were considered positive.
Anti- A. lumbricoides IgG4 was detected by indirect ELISA as previously described 9. Briefly, plate wells were sensitized with 20 mg/mL of A. lumbricoides antigen. Sera were diluted 1:50 in diluent solution. Plates were incubated with biotinylated anti-human IgG4 (Sigma Chemical Co), followed by streptavidin/peroxidase (BD PharMingen) and H2O2/orthophenylenediamine substrate (Merck, White House Station, NJ) and read with a 480-nm filter. The assay cutoff for IgG4 for A. lumbricoides was determined as the mean plus an SD of negative controls (sera from children with 3 negative stool samples collected serially). Antibody levels of anti–A lumbricoides IgG4 more than the cutoff were defined as positive.
Anti- T. canis IgG antibodies were detected in sera by indirect ELISA assay using excretory-secretory T. canis larval antigens as previously described 28. The cut-off obtained (0.23) was calculated by the OD from the mean of the 14 negative controls (children without history of contact with dogs and/or cats) plus three standard deviations of this mean. Five previously assayed sera samples were used as positive controls.
Cell culture for IL-10 production
Venous blood was collected into heparinized tubes and cultured at a dilution of 1:4 in RPMI (Gibco, Auckland, New Zealand) containing 10 mmol/L glutamine (Sigma-Aldrich, St Louis, Mo) and 100 mg/mL gentamicin (Sigma-Aldrich). The cell cultures were started within 6 hours after the blood collection and were maintained in a humidified environment of 5% CO2 at 37°C for 24 hours for IL-10 detection in the absence or presence of pokeweed mitogen (PWM; Sigma-Aldrich, St. Louis, MO, USA) (2.5 μg/mL).
IL-10 measurement using ELISA
The IL-10 concentrations were measured in whole-blood culture supernatant by sandwich ELISA, according to the manufacturer’s instructions (BD PharMingen, San Diego, Calif). Cytokine concentrations were determined by means of interpolation of standard curves. The detection limits (low/high) were 31.25/500 pg/ml.
Genotyping
Four TGFB1 SNPs with prior associations with related phenotypes (rs4803455, rs1800470, rs1800469, rs2241712) were selected for genotyping18, 19.
DNA was extracted from peripheral blood samples by using commercial standard protocols (Gentra Puregene Blood Kit; Qiagen, Hilden, Germany). SNPs were typed by using the TaqMan probe–based, 59 nuclease assay minor groove binder chemistry 29 on the 7900HT Sequence Detection System (Applied Biosystems, Foster City, Calif). TaqMan-validated assays and master mix were manufactured by Applied Biosystems.
PCR was conducted in a 5mL volume by using a universal master mix and 4 predesigned and validated TaqMan assays for the SNPs (list of SNPs is shown in Table 1). The thermal cycling conditions were as follows: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds/60°C for 1 minute and an extension step of 60°C for 5 minutes. Nontemplate negative and genotyping-positive controls were included in each genotyping plate. Automatic calling was performed with a quality value of greater than 99%.
Table 1. Description of SNPs analyzed in this study, including allelic frequency and Hardy-Weinberg equilibrium data.
| SNP | Base pairs | Allele | MAF | HWE | functionGVS | Regulome DB Score |
|---|---|---|---|---|---|---|
| rs4803455 | 41855515 | A/C | 0.49 | 0.53 | intron | 7 |
| rs1800470 | 41858921 | C/T | 0.47 | 0.57 | missense | 4 |
| rs1800469 | 41860296 | T/C | 0.33 | 0.79 | near-gene-5 | 2b |
| rs2241712 | 41869756 | G/A | 0.28 | 1 | intron | 2b |
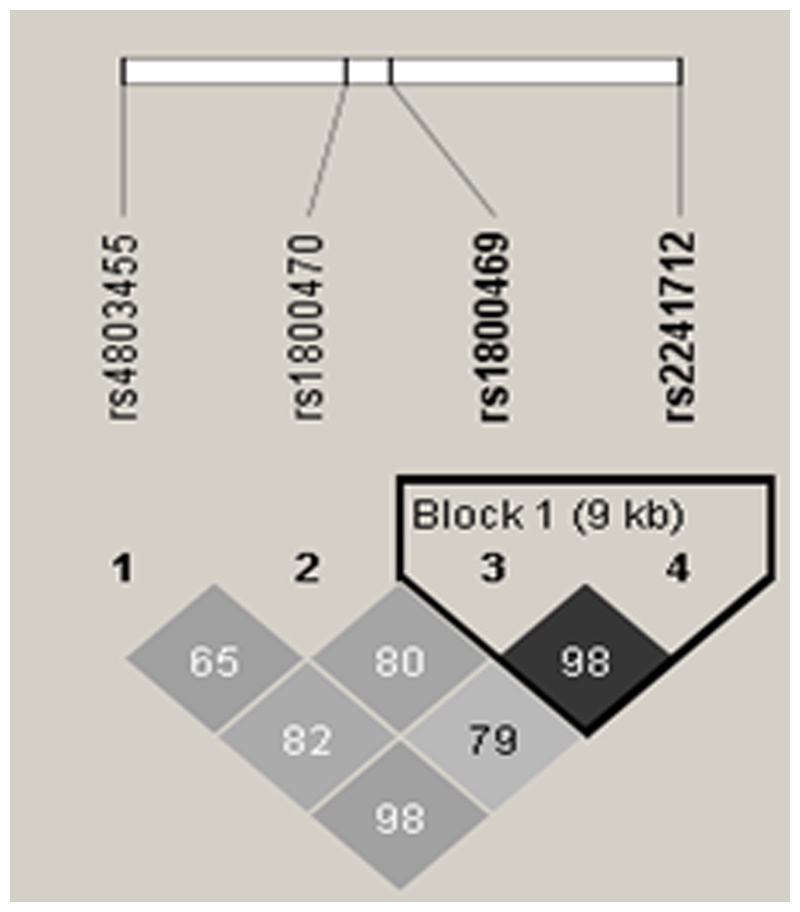
Ten percent of the samples were genotyped in duplicate with 100% reproducibility. All 4 SNPs were in Hardy-Weinberg equilibrium. Allele frequencies of the SNPs and SNP localization in the TGF-β1 gene (chromosome 19q13.1, position 41330531 to 41353933) are summarized in Table 1. Markers rs1800469 and rs2241712 are in strong linkage disequilibrium LD (eFigure 1) by Haploview 30.
Statistical analyses
Genotype and haplotype analyses were conducted for genetic associations using logistic regression to estimate odds ratio (ORs) and 95% CIs for the genetic risk factor (including sex, age and helminth infection as covariates). In the population of the current study, a negative association was previously described between T. trichiura (mainly) and A. lumbricoides infections and a SPT responses 31. Also, there is cross reactivity between the helminth-induced IgE and anti-IgE against aeroallergens. Thus, we have adjusted such models for helminth infections 31.
In addition, the first 2 principal components delineated through Eigenstrat on 269 ancestry informative markers (AIMs) were included in the model to address the potential effects of population stratification. Genotype analyses were performed using the additive, dominant and recessive models.
For continuous data (tIgE, anti- A. lumbricoides IgG4 and anti- A. lumbricoides IgE), analyses were conducted by using linear regression adjusted by sex, age, helminth infection and principal components 1 and 2. All genetic analyses were performed using PLINK 1.07 32, and Pairwise LD was created with Haploview 30. The differences were considered significant at P < 0.05. Additionally, the permutation procedures (Perm) were calculated to provide a computationally intensive approach to generating significance levels. This strategy has been used to control the false discovery rate solving the problem of multiple comparisons 33.
Results
4.1. Description of the study population
The Table 2 summarizes the clinical characteristics of the study population. We observed greater proportions of children with allergic and non-allergic asthma in the younger group (<5 years old); however, no difference according to sex was observed. Markers of allergy, specifically SPT reactivity (65.73%; p<0,001) and total IgE levels (1.71 kU/mL; p<0,05), were significantly higher in the allergic asthmatic group compared to non-asthmatic group. IgE anti-A. lumbricoides (77.53%; p<0,001) was great in allergic asthmatics subjects.
Table 2. Characteristics of the Social Changes Asthma and Allergy in Latin American population according to asthma status and variables included in this study.
| Nonasthmatic (n=962) |
No allergic Asthmatic (n=212) |
Allergic Asthmatic (n=178) |
P value | |
|---|---|---|---|---|
| Age | ||||
| ≤5 y | 290 (30.40%) | 108 (50.94%) | 75 (42.13%) | |
| 6-7 y | 351 (36.79%) | 67 (31.60%) | 59 (33.15%) | |
| ≥8 y | 313 (32.81%) | 37 (17.45%) | 44 (24.72%) | <0.0001 |
| Sex | ||||
| Male | 517 (53.74%) | 101 (47.64%) | 107 (60.11%) | |
| Female | 445 (46.26%) | 111 (52.36%) | 71 (39.89%) | 0.048 |
| Skin prink test response ≥ 1 allergen (>3mm) | 268 (27.86%) | 21 (9.91%) | 117 (65.73%) | <0.0001 |
| Skin prink test to B. Tropicalis (>3mm) | 192 (19.96%) | 10 (4.72%) | 90 (50.56%) | <0.0001 |
| Specific IgE for ≥ 1 allergen (> 0.70KU/L) | 331 (34.41%) | 0 (0.00%) | 178 (100.00%) | <0.0001 |
| Total IgE (KU/L) mean ± SD | 0.80 ± 5.46 | 0.28 ± 0.60 | 1.71 ± 4.59 | 0.0155 |
| T. canis current infection | 443 (46.05%) | 101 (47.64%) | 88 (49.44%) | 0.888 |
| T. trichiura current infection | 124 (12.89%) | 39 (18.40%) | 21 (11.80%) | 0.193 |
| T. trichiura chronic infection | 48 (4.99%) | 15 (7.08%) | 8 (4.49%) | 0.095 |
| A. lumbricoides chronic infection | 50 (5.20%) | 17 (8.02%) | 12 (6.74%) | 0.203 |
| IgG4 anti-Asc | 145 (15.07%) | 36 (16.98%) | 38 (21.35%) | 0.233 |
| IgE anti-Asc | 462 (48.02%) | 80 (37.74%) | 138 (77.53%) | <0.0001 |
| Coinfection (A. lumbricoides + T. trichiura) | 210 (21.83%) | 62 (29.25%) | 39 (21.91%) | 0.167 |
4.2. Association of TGFB1 SNPs with allergic asthma and markers of allergy
TGFB1 marker rs1800470 was negatively associated (OR 0.60; p <0.05) with allergic asthma in the recessive model (see Table 3). This marker was similarly negatively associated with specific IgE to at least one allergen tested (OR 0.52, p <0.05), with skin test to at least one allergen (OR 0.41; p <0.01) and skin test reactivity to Blomia tropicalis (OR 0.39; p <0.05), under the recessive model (see Table 4).
Table 3. Association between the TGF-β1 SNPs and allergic asthma by logistic regression adjusted for age, sex, helminth infections and principal components 1 and 2.
| Marker | Model | OR | 95% CI | P Value |
|---|---|---|---|---|
| Allergic asthma | ||||
| rs1800470 | Recessive | 0.60 | 0.37-0.95 | 0.030 |
Table 4. Association between TGFB1 SNPs and specific IgE and skin tests in the asthmatic subjects by logistic regression adjusted for age, sex, helminth infections and major components 1 and 2.
| Marker | Model | OR | 95% CI | p Value |
|---|---|---|---|---|
| Specific IgE for at least 1 aeroallergens (>0.70kU/L) | ||||
| rs1800470 | Recessive | 0.52 | (0.29-0.91) | 0.02171 |
| SPT response for at least 1 specific aeroallergens (≥3mm) | ||||
| rs1800470 | Recessive | 0.41 | (0.22 - 0.79) | 0.006929 |
| SPT response to B. tropicalis (≥3mm) | ||||
| rs1800470 | Recessive | 0.39 | (0.19-0.81) | 0.01183 |
| Specific IgE to B tropicalis | ||||
| rs1800470 | Recessive | 0.57 | (0.32-1) | 0.05 |
4.3. Association of TGFB1 SNPs and total IgE
No associations were observed between any of the TGFB1 markers and serum total IgE levels under any of the models tested (data not shown). However, the haplotypes TT (β -0.156; p<0.05), CTT (β -0.162; p< 0.05), TTG (β -0.211; p<0.01) and CTTG (β -0.197; p<0.01) were negatively associated with total IgE serum levels (see Table 5).
Table 5. Association between the TGF-β1 SNPs and total IgE in total case-control subjects by linear regression adjusted by age, sex, helminth infections and principal components 1 and 2.
| rs4803455 | rs1800470 | rs1800469 | rs2241712 | Freq | Beta | p Value |
|---|---|---|---|---|---|---|
| T | T | 0.0343 | -0.156 | 0.0137 | ||
| C | T | T | 0.0281 | -0.162 | 0.0243 | |
| T | T | G | 0.029 | -0.211 | 0.00284 | |
| C | T | T | G | 0.0271 | -0.197 | 0.00614 |
4.4. Association of TGFB1 SNPs and helminth infections
No association was found considering analysis between single genotype with helminth infections and markers of infection (data not shown). However, evaluating the association of possible haplotypes with helminth infections, significant associations were observed (see Table 6). Specially, haplotypes AC, ACC and ACCA showed a positive association with T. canis infection (OR 1.73, 2.09 and 2.07, respectively; p <0.001), T. trichiura current infection (OR 1.80, 1.80 and 1.85, respectively; p <0.01) and co-infection with T. trichiuras and A. lumbricoides (OR 1.61, 1.63, 1.67, respectively; p <0.01).
Table 6. Association between haplotypes of TGF-β1 SNPs and helminthes Infections in total case-control subjects by additive logistic regression model adjusted for age, sex and principal components 1 and 2.
| AC | ACC | ACCA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Freq | OR | p Value | Freq | OR | p Value | Freq | OR | p Value | |
| Toxocara canis seroprevalence | 0.8 | 1.73 | 0.00078 | 0.08 | 2.09 | 0.00014 | 0.08 | 2.07 | 0.00017 |
| Tricuris trichiura current infection | 0.12 | 1.80 | 0.00172 | 0.09 | 1.80 | 0.0052 | 0.09 | 1.85 | 0.00349 |
| Tricuris trichiura chronic infection | 0.13 | 2.00 | 0.0124 | 0.11 | 2.00 | 0.0185 | 0.11 | 2.03 | 0.0159 |
| A. lumbricoides chronic infection | 0.13 | 1.69 | 0.0471 | 0.10 | 1.77 | 0.0435 | 0.11 | 1.85 | 0.0273 |
| Anti- A. lumbricoides IgE | 0.09 | 1.49 | 0.013 | 0.07 | 1.58 | 0.0134 | 0.07 | 1.61 | 0.0109 |
| Anti- A. lumbricoides IgG4 | 0.12 | 1.80 | 0.00081 | 0.09 | 1.92 | 0.00087 | 0.10 | 2.01 | 0.00033 |
| Coinfection (A. Lumbricoides + T. trichiura) | 0.11 | 1.61 | 0.00434 | 0.08 | 1.63 | 0.0104 | 0.08 | 1.67 | 0.00679 |
AC: rs4803455; rs1800470
ACC: rs4803455; rs1800470; rs1800469
ACCA: rs4803455; rs1800470; rs1800469; rs2241712
By linear regression, CC (β 1,67; p<0.001), CCC (β 1.48; p<0.05), CCA (β 1.57; p<0.001) and CCCA (β 1.4; p<0.05) haplotypes were positively associated with IgG4 anti-A. lumbricoides serum levels (eTable 1).
4.5. Association of TGFB1 SNPs with allergic asthma in helminth- infected or uninfected individuals
Marker rs1800470 was negatively associated (OR 0.31, p <0.001) with allergic asthma (recessive model) among the Toxocara canis infected subjects (see Table 7), but not among Toxocara canis uninfected individuals.
Table 7. Association between TGFB1 SNPs and allergic asthma in T. canis infected and uninfected subjects by logistic regression adjusted for age, sex, helminth infections and principal components 1 and 2.
| Marker | Model | OR | 95% CI | P value |
|---|---|---|---|---|
| Allergic asthma (Toxocara canis infected subjects) | ||||
| rs1800470 | Recessive | 0.31 | (0.14-0.72) | 0.005894 |
| Allergic asthma (Toxocara canis uninfected subjects) | ||||
| rs1800470 | Recessive | 0.84 | (0.44-1.59) | 0.595 |
There was no association with allergic asthma in the subgroup analysis for infected/non-infected individuals with A. lumbricoides or T. trichiura.
4.6. Association of TGFB1 SNPs with IL-10 levels
No association was found when testing associations between any single marker and pokeweed stimulated IL-10 levels (data no shown). However, evaluating the possible haplotypes with basal IL-10 production from PBMC without stimulus, we found a positive association with AC (β 9.78; p<0.05), ACC (β 21.7; p< 0.05) and ACCA (β 12.6; p<0,05) (see Table 8). These haplotypes were also associated with levels of IL-10 production under pokeweed stimulation: AC (β 46.8; p<0.001), ACC (β 51.1; p< 0.001) and ACCA (β 50.7; p<0,001) (see eTable 2).
Table 8. Association between TGFB1 haplotypes and IL-10 production under mitogen stimulation in total of cases-controls subjects by linear regression adjusted for age, sex, principal components 1 and 2 and helminth infections.
| rs4803455 | rs1800470 | rs1800469 | rs2241712 | Freq. | Beta | P Value |
|---|---|---|---|---|---|---|
| A | C | 0.0812 | 46.8 | 0.000413 | ||
| A | C | C | 0.0594 | 51.1 | 0.000575 | |
| A | C | C | A | 0.0592 | 50.7 | 0.000632 |
Discussion
Allergy is a complex disease in which environmental factors interact with multiple genetic variants modifying it susceptibility and severity. To elucidate the impact of the immune regulatory network on allergic disease and parasitic diseases, we investigated the role of common genetic polymorphisms in TGFB1, an important immune regulatory cytokine. We found that TGFB1 polymorphisms are negatively associated with allergic asthma and associated phenotypes and positively associated with helminth infections in a population of children living in Salvador, an urban, tropical environment for which extracellular parasitic disease is endemic. This observation may contribute to the better understanding of the importance of genetic variability on the modulation of allergic processes by helminth infections.
Of the four TGFB1 SNPs evaluated in this study, the CC genotype of rs1800470 (T869C) showed a negative association with allergic asthma, serum sIgE to common allergens and skin test reactivity to allergens, including house dust mite B. tropicalis. However, several previous studies have described no association for rs1800470 with asthma34–36 and few studies show positive association20, 37. The discrepant results may be consequence of the LD with other variants within or near TGFB1, the ethnical differences among the studied populations and/or untested gene-by-gene or gene-by-environment interactions. Further work on this polymorphism is required to better understand their association with asthma.
The marker rs1800470 has also been reported to be associated with serum levels of the gene product, with the CC genotype associated with higher TGF-β1 concentration than other genotypes 38, 39. The association of TGF-β1 levels with allergy has been explored in several experimental studies. Intratracheal delivery of TGF-β1 suppressed allergen induced inflammation 11. In contrast, blocking transforming growth factor beta/Smad signaling in T cells enhances antigen-induced airway inflammation, airway reactivity and increased Th2 cytokine production 40. Moreover, reduced expression of TGF-β1 exacerbates pathology in an experimental asthma model related with increased eosinophilic inflammation and increased levels of specific IgE in serum 41.
The IgE is an important mediator involved in the allergic process as well as in the immune response against helminthes. IgE production is induced by Th2 cytokines, while immune regulatory cytokines (e.g. IL-10 and TGF-β1) down-regulate IgE levels 42. We identified four haplotypes in TGFB1 gene negatively associated with total IgE serum levels in this populationwhat characterizes the immunomodulatory property of TGF-β1. However, previous studies have found no association between TGFB1 SNPs and total IgE levels 43, 44.Due to its immune modulatory properties, TGF-β1 also leads to a failure in the immune response against helminths, resulting in increased susceptibility to infections. A study of children infected with helminths identified increased production of TGF-β1 in unstimulated peripheral blood leukocytes, being positively associated with burden of infection and negatively associated with immune reactivity, determined by IL-4 and IFN-γ production and cell proliferation in response to antigenic stimuli 45. In this study we evaluated the association between TGFB1 polymorphism and helminth infections. Although no association was found between TGFB1 genotypes and helminth infections, analysis of possible haplotypes as a mean of simultaneous SNPs occurring together, especially the haplotypes formed by the C allele of rs1800470 with the other SNPs, were positively associated with helminth infections, showing TGFB1 polymorphisms contribute to susceptibility to parasitic infections. This study was the first to describe the association of polymorphism in the TGFB1 gene and infection by T. canis, T. trichiura and A. lumbricoides. The results suggest that the genetic background may influence the susceptibility and resolution of the helminth infection. Thereby, the subject genetically predisposed when exposed to helminths will probably have a higher immunomodulatory response, characterized by high TGF and IL-10 production, an important mechanism of escape of the effector immune response against the helminths.
The relationship between helminth infection and TGF-β1 production seems to also influence the development of allergic diseases, through the modulation of the Th2 response 46, 31, 47. The interaction with the environment (e.g. infections) can represent important role on the manifestation of genetic susceptibility. In fact, we found that the CC genotype of rs1800470 is negatively associated with allergic asthma in T. canis infected individuals. However, this association was lost when only T. canis uninfected subjects were analyzed. Thus, we demonstrated that the T. canis infection contribute to modulate the immunologic response on allergies on genetically susceptible subjects. Thus, the intense immune regulatory role played by TGF-β1 induced in infected individuals may explain the protection against the development of immune-mediated diseases 7, 45, 48.
Our group previously demonstrated in the same population that children chronically infected with helminths produce higher levels of immune regulatory cytokine IL-10 9. Also in the SCAALA population, our group demonstrated that the relationship between allergies and IL-10 levels are determined not only by environmental factors, but also as a result of polymorphisms in the IL-10 gene that are positively associated with allergy and negatively associated with helminth infections 17. TGF-β1 is the primary regulator of the immune response acting as an important factor by inducing the differentiation and development of Foxp3+ regulatory T cells, and thus for the IL-10 production 49 50. For this reason we investigated whether polymorphisms in TGFB1 can impact the IL10 production. We found that the TGFB1 haplotypes were positively associated with spontaneous IL-10 production and IL-10 production stimulated by pokeweed mitogen. Such haplotypes were the same as those associated with helminth infections. Thus, not only polymorphisms in the IL10 gene but also in TGFB1 are involved in modulation of IL-10 levels which may contribute to susceptibility to infection and potentially modulation of allergy.
Individuals with genetic polymorphisms in TGFB1 have a lower risk of developing allergy and increased susceptibility to helminth infections. Additionally, we have shown that immune modulation of allergy is a complex response resulting not only from the environmental factors but also of the genetic polymorphisms, especially upon IL-10 production. Future works are needed to further elucidate the potential role of TGF-β1 on asthma and how it could be a strategy to control the disease.
Extended Data
eFigure 1.
Pairwise LD within Haploview by using the R` squared statistic for the TGF-β1 gene. Intensity of shading indicates the degree of confidence in the R` value.
eTable 1. Association between haplotypes of TGF-β1 SNPs and levels of IgG4 anti-Ascaris lumbricoides in total of cases-controls subjects by logistic regression adjusted for age, sex and principal components 1 and 2.
| rs4803455 | rs1800470 | rs1800469 | rs2241712 | Freq. | Beta | p Value | EMP1 |
|---|---|---|---|---|---|---|---|
| C | C | 0.178 | 1.67 | 9.3x10-5 | 0.0005999 | ||
| C | C | C | 0.116 | 1.48 | 0.0147 | 0.0188 | |
| C | C | A | 0.18 | 1.57 | 0.000405 | 0.0011 | |
| C | C | C | A | 0.12 | 1.4 | 0.0326 | 0.0011 |
EMP1, P value considering adaptive permutations
eTable 2. Association between TGFB1 haplotypes and IL-10 production under mitogen stimulation in total of cases-controls subjects by linear regression adjusted for age, sex, principal components 1 and 2 and helminth infections.
| rs4803455 | rs1800470 | rs1800469 | rs2241712 | Freq. | Beta | p Value | EMP1 |
|---|---|---|---|---|---|---|---|
| A | C | 0.08 | 46.8 | 0.000413 | 0.0003 | ||
| A | C | C | 0.06 | 51.1 | 0.000575 | 0.0069 | |
| A | C | C | A | 0.06 | 50.7 | 0.000632 | 9.999e-05 |
EMP1, P value considering adaptive permutations
References
- 1.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242(1):10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res. 2010;690(1–2):24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce N, Aït-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62(9):758–66. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper PJ, Rodrigues LC, Cruz AA, Barreto ML. Asthma in Latin America: a public heath challenge and research opportunity. Allergy. 2009;64(1):5–17. doi: 10.1111/j.1398-9995.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 6.Jenerowicz D, Silny W, Dańczak-Pazdrowska A, Polańska A, Osmola-Mańkowska A, Olek-Hrab K. Environmental factors and allergic diseases. Ann Agric Environ Med. 2012;19(3):475–81. [PubMed] [Google Scholar]
- 7.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7(12):975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueiredo CA, Barreto ML, Rodrigues LC, et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78(7):3160–7. doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueiredo CA, Alcantara-Neves NM, Amorim LD, et al. Evidence for a modulatory effect of IL-10 on both Th1 and Th2 cytokine production: the role of the environment. Clin Immunol. 2011;139(1):57–64. doi: 10.1016/j.clim.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joetham A, Takeda K, Takada K, et al. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol. 2007;178(3):1433–42. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 12.Yang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy. 2012;67(10):1193–202. doi: 10.1111/j.1398-9995.2012.02880.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura A, Wakabayashi Y, Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem. 2010;147(6):781–92. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 15.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25(3):455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Bottema RW, Kerkhof M, Reijmerink NE, et al. Gene-gene interaction in regulatory T-cell function in atopy and asthma development in childhood. J Allergy Clin Immunol. 2010;126(2):338–46. doi: 10.1016/j.jaci.2010.04.024. 346.e1-10. [DOI] [PubMed] [Google Scholar]
- 17.Figueiredo CA, Barreto ML, Alcantara-Neves NM, et al. Coassociations between IL10 polymorphisms, IL-10 production, helminth infection, and asthma/wheeze in an urban tropical population in Brazil. J Allergy Clin Immunol. 2013;131(6):1683–90. doi: 10.1016/j.jaci.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu H, Romieu I, Shi M, et al. Evaluation of candidate genes in a genome-wide association study of childhood asthma in Mexicans. J Allergy Clin Immunol. 2010;125(2):321–327.:e13. doi: 10.1016/j.jaci.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XX, Li FX, Wu YS, Wu D, Tan JY, Li M. Association of TGF-beta1, IL-4 and IL-13 gene polymerphisms with asthma in a Chinese population. Asian Pac J Allergy Immunol. 2011;29(3):273–7. [PubMed] [Google Scholar]
- 20.Li H, Romieu I, Wu H, et al. Genetic polymorphisms in transforming growth factor beta-1 (TGFB1) and childhood asthma and atopy. Hum Genet. 2007;121(5):529–38. doi: 10.1007/s00439-007-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grainger DJ, Heathcote K, Chiano M, et al. Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet. 1999;8(1):93–7. doi: 10.1093/hmg/8.1.93. [DOI] [PubMed] [Google Scholar]
- 22.Suthanthiran M, Li B, Song JO, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci U S A. 2000;97(7):3479–84. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barreto ML, Cunha SS, Alcântara-Neves N, et al. Risk factors and immunological pathways for asthma and other allergic diseases in children: background and methodology of a longitudinal study in a large urban center in Northeastern Brazil (Salvador-SCAALA study) BMC Pulm Med. 2006;6:15. doi: 10.1186/1471-2466-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreto ML, Genser B, Strina A, et al. Impact of a citywide sanitation program in Northeast Brazil on intestinal parasites infection in young children. Environ Health Perspect. 2010;118(11):1637–42. doi: 10.1289/ehp.1002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcantara-Neves NM, Veiga RV, Dattoli VC, et al. The effect of single and multiple infections on atopy and wheezing in children. J Allergy Clin Immunol. 2012;129(2):359–67. doi: 10.1016/j.jaci.2011.09.015. 367.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiland SK, Björkstén B, Brunekreef B, et al. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J. 2004;24(3):406–12. doi: 10.1183/09031936.04.00090303. [DOI] [PubMed] [Google Scholar]
- 27.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 28.Mendonça LR, Figueiredo CA, Esquivel R, et al. Seroprevalence and risk factors for Toxocara infection in children from an urban large setting in Northeast Brazil. Acta Trop. 2013;128(1):90–5. doi: 10.1016/j.actatropica.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ. Allelic discrimination using fluorogenic probes and the 5’ nuclease assay. Genet Anal. 1999;14(5–6):143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 30.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues LC, Newcombe PJ, Cunha SS, et al. Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy. 2008;38(11):1769–77. doi: 10.1111/j.1365-2222.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 32.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lage-Castellanos A, Martinez-Montes E, Hernandez-Cabrera JA, Galan L. False discovery rate and permutation test: an evaluation in ERP data analysis. Stat Med. 2010;29(1):63–74. doi: 10.1002/sim.3784. [DOI] [PubMed] [Google Scholar]
- 34.Heinzmann A, Bauer E, Ganter K, Kurz T, Deichmann KA. Polymorphisms of the TGF-beta1 gene are not associated with bronchial asthma in Caucasian children. Pediatr Allergy Immunol. 2005;16(4):310–4. doi: 10.1111/j.1399-3038.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Li Y, Zhang M, et al. Associations of genetic variants in ADAM33 and TGF-β1 genes with childhood asthma risk. Biomed Rep. 2014;2(4):533–538. doi: 10.3892/br.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wisniewski A, Obojski A, Pawlik A, et al. Polymorphism of the TGFB1 gene is not associated with bronchial allergic asthma in a Polish population. Hum Immunol. 2009;70(2):134–8. doi: 10.1016/j.humimm.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Mak JC, Leung HC, Ho SP, et al. Analysis of TGF-beta(1) gene polymorphisms in Hong Kong Chinese patients with asthma. J Allergy Clin Immunol. 2006;117(1):92–6. doi: 10.1016/j.jaci.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Salam MT, Gauderman WJ, McConnell R, Lin PC, Gilliland FD. Transforming growth factor-1 C-509T polymorphism, oxidant stress, and early-onset childhood asthma. Am J Respir Crit Care Med. 2007;176(12):1192–9. doi: 10.1164/rccm.200704-561OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunning AM, Ellis PD, McBride S, et al. A transforming growth factorbeta1 signal peptide variant increases secretion in vitro and is associated with increased incidence of invasive breast cancer. Cancer Res. 2003;63(10):2610–5. [PubMed] [Google Scholar]
- 40.Nakao A, Miike S, Hatano M, et al. Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192(2):151–8. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherf W, Burdach S, Hansen G. Reduced expression of transforming growth factor beta 1 exacerbates pathology in an experimental asthma model. Eur J Immunol. 2005;35(1):198–206. doi: 10.1002/eji.200425209. [DOI] [PubMed] [Google Scholar]
- 42.Alvaro M, Sancha J, Larramona H, et al. Allergen-specific immunotherapy: update on immunological mechanisms. Allergol Immunopathol (Madr) 2013;41(4):265–72. doi: 10.1016/j.aller.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Silverman ES, Palmer LJ, Subramaniam V, et al. Transforming growth factor-beta1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med. 2004;169(2):214–9. doi: 10.1164/rccm.200307-973OC. [DOI] [PubMed] [Google Scholar]
- 44.Acevedo N, Vergara C, Gusmão L, et al. The C-509T promoter polymorphism of the transforming growth factor beta-1 gene is associated with levels of total and specific IgE in a Colombian population. Int Arch Allergy Immunol. 2010;151(3):237–46. doi: 10.1159/000242361. [DOI] [PubMed] [Google Scholar]
- 45.Turner JD, Jackson JA, Faulkner H, et al. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J Infect Dis. 2008;197(8):1204–12. doi: 10.1086/586717. [DOI] [PubMed] [Google Scholar]
- 46.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9(1):29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10(1):3–12. doi: 10.1007/s11882-009-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreau E, Chauvin A. Immunity against helminths: interactions with the host and the intercurrent infections. J Biomed Biotechnol. 2010;2010:428593. doi: 10.1155/2010/428593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166(12):7282–9. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 50.Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]