Abstract
Background
Integrase strand transfer inhibitors (InSTIs) decrease HIV plasma viral load faster than other antiretroviral classes. More rapid viral load decline has been associated with higher risk of immune reconstitution inflammatory syndrome (IRIS). There are conflicting reports on the association between InSTI and IRIS. We performed a systematic review and meta-analysis to compare the risk of IRIS among treatment-naïve HIV-positive patients starting InSTI versus non-InSTI regimens.
Methods
We searched PubMed, Scopus, Web of Science, Africa-Wide, and Cochrane databases from earliest available date to 26 November 2021, for randomised controlled trials (RCTs) having intervention arms with InSTI versus control arms without InSTI in patients initiating first-line antiretroviral therapy. The primary outcome was relative risk (RR) of IRIS, while the secondary outcome was RR of paradoxical tuberculosis-associated IRIS (TB-IRIS). Data were combined by random-effects meta-analysis according to the Mantel-Haenszel method. The protocol for this study is registered with PROSPERO, CRD42020213976.
Results
We included 14 RCTs comprising 8696 participants from six continents for the primary outcome of IRIS, and a subset of 674 participants (from three RCTs) for the secondary outcome of paradoxical TB-IRIS. Risk of IRIS was similar between InSTI and non-InSTI regimens (RR, 0.93, 95% confidence interval [CI], 0.75 – 1.14). There was a trend towards a lower risk of paradoxical TB-IRIS with InSTI versus efavirenz regimens that was not statistically significant (RR, 0.64, 95% CI, 0.34 – 1.19).
Conclusions
In this meta-analysis among treatment-naïve patients commencing first-line antiretroviral therapy, InSTI regimens were not associated with higher risk of IRIS.
Keywords: Antiretroviral therapy, HIV, integrase inhibitors, immune reconstitution inflammatory syndrome
Introduction
Dolutegravir, an integrase strand transfer inhibitor (InSTI), is replacing efavirenz as the preferred drug in first-line antiretroviral therapy (ART) in low- to middle-income countries (LMICs) due to its high genetic barrier to resistance, improved tolerability, and reduced cost as a generic fixed-dose combination with tenofovir and lamivudine 1-3.
InSTIs decrease plasma HIV viral load faster than other antiretroviral classes 4-6. All InSTIs are associated with similarly rapid HIV viral load decline 7. Rapid reduction in HIV viraemia does not appear to confer clinical benefit 5,8. However, rapid recovery of immune function during first-line ART may increase the risk of immune reconstitution inflammatory syndrome (IRIS), an immunopathological reaction characterised by paradoxical worsening of treated opportunistic infections or unmasking of subclinical infections. An association between more rapid HIV viral load decline and IRIS has been observed in certain cohort studies 9-11.
Tuberculosis is the commonest cause of morbidity and mortality in people living with HIV (PLWH) 12,13. Paradoxical tuberculosis-associated IRIS (TB-IRIS) can manifest shortly after ART initiation, occurring in 18% of patients receiving antituberculosis treatment according to a pooled estimate, with incidences ranging from 4 – 54% in individual studies 14. In sub-Saharan Africa, ART is often started when PLWH present for care with advanced immunosuppression (CD4 count below 100 cells/mm3) 15,16, which is an important risk factor for TB-IRIS 14,17. In populations with high prevalence of TB-IRIS risk factors (low CD4 counts and short interval between antituberculosis treatment and ART initiation), the incidence of TB-IRIS may exceed 50% 18,19. Paradoxical TB-IRIS is associated with significant morbidity, with 25% requiring hospitalisation 14.
InSTIs were associated with a two- to three-fold increased risk of IRIS in cohort studies, but only a few TB-IRIS events were reported 20-22. The most recent systematic review published on IRIS risk in randomised controlled trials (RCTs) 23 reported only 13 cases of IRIS; furthermore, the included RCTs excluded patients with Centers for Disease Control and Prevention (CDC) stage C disease at baseline, who have a higher risk of IRIS and IRIS-associated death 24. Since then, there have been several RCTs published on InSTI regimens that have enrolled patients with CDC stage C disease and HIV-associated tuberculosis. Therefore, we conducted a systematic review and meta-analysis of RCTs, comprising ART-naïve participants randomised to InSTI versus other antiretroviral classes, to provide robust evidence on the association of InSTI with IRIS and paradoxical TB-IRIS.
Study Population and Methods
Search strategy and selection criteria
The protocol for this study followed PRISMA guidelines and was approved by the Human Research Ethics Committee at the University of Cape Town (Ref 682/2020). We registered the study protocol on PROSPERO (CRD42020213976).
The main search comprises individual searches using detailed medical subject heading (MeSH) terms for “HIV”, “AIDS”, “integrase inhibitor”, “dolutegravir”, “raltegravir”, “elvitegravir”, “bictegravir”, “cabotegravir”, and “RCT”, and related terms for relevant studies published in Medline (accessed via PubMed), Scopus, Web of Science, Africa-Wide, and Cochrane databases from earliest available date to 26 November 2021, without language restriction. The full search strategy is shown in Table 1, Supplemental Digital Content. We searched for grey literature, including the 2014-2021 proceedings from the Conference on Retroviruses and Opportunistic Infections (CROI) and the International AIDS Society Annual Conference. In addition, we searched the database www.clinicaltrials.gov and reviewed bibliographies of all included studies for potentially eligible studies. Search results were managed using the Rayyan platform 25. Two reviewers (YZ and PN) independently screened titles and abstracts in duplicate. YZ and PN evaluated full texts of potentially relevant articles independently and in duplicate using a standardised form. YZ and PN compiled and compared their own list of eligible studies. Any disagreement on eligibility was resolved through discussion and consultation with a third reviewer (GaM).
Eligibility criteria
Eligible studies were RCTs that enrolled adults (age ≥18 years) who were HIV-positive and initiating first-line ART, with intervention arms containing InSTI versus control arms with other antiretroviral classes. We included studies in which InSTI was used in combination with any other antiretrovirals (nucleoside reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, or protease inhibitor) and if the control arm did not have InSTI. RCTs were included if IRIS events were reported. We excluded observational studies due to potential bias in InSTI use, studies with a single-arm or cross-over design, studies with InSTI in all treatment arms, and studies evaluating the switch to InSTI regimens in virologically suppressed patients.
Data extraction and quality assessment
Two review authors (YZ and PN) collected data from text, tables, and figures of each eligible study onto a pre-designed standardised data extraction form (File 1, Supplemental Digital Content) independently, and in duplicate, and cross-checked results. Any disagreement in data extraction was resolved by discussion between the review authors. We extracted numbers of IRIS events reported by primary studies among participants initiating ART, who were at risk for IRIS. IRIS events reported as adverse events were also collected from the database www.clinicaltrials.gov and supplementary appendices of trial publications whenever available. Study authors were contacted in the case of missing data.
Studies were critically appraised to evaluate the risk of bias using the revised Cochrane risk of bias tool for randomised trials (RoB 2) 26. The effect of interest was the effect of assignment to the intervention at baseline (the “Intention-to-treat effect”). We assessed the risk of bias for outcomes reported in the included studies that we specified as outcomes for the current review (IRIS and paradoxical TB-IRIS). Bias was assessed in five distinct domains: bias arising from the randomisation process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; and bias in selection of the reported result. We used the proposed RoB 2 algorithms to report judgements for each domain and overall as low risk of bias, some concerns, or high risk of bias. Two review authors (YZ and AH) assessed the risk of bias for each outcome independently and in duplicate. Any disagreement in judgement was resolved by discussion between the review authors.
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to present the overall quality of evidence for the following outcomes: IRIS and paradoxical TB-IRIS 27. Two review authors (YZ and AH) independently rated the certainty of evidence for each outcome as high, moderate, low, or very low. We considered five criteria related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (indirectness). Any disagreement in judgement was resolved by discussion between the review authors. We used the GRADEpro GDT software to create a “Summary of findings” table. 28
Statistical analysis
We report proportions with IRIS events as point estimates with 95% confidence intervals (CIs). As none of the studies were specifically designed to assess IRIS as a primary outcome, our denominator for calculating proportions with IRIS events was defined as the total number of participants enrolled who initiated ART and were at risk of developing IRIS. Relative risks (RRs) for the comparison between InSTI and other antiretroviral classes were calculated for IRIS events and reported with corresponding 95% CIs. We conducted an overall meta-analysis of IRIS events across studies, as well as sub-group meta-analyses of IRIS events by individual InSTI drugs (dolutegravir, raltegravir, and elvitegravir). Meta-analyses were performed according to the Mantel-Haenszel method applying the random-effects models. Studies that enrolled participants with HIV-associated tuberculosis were combined in a sub-group meta-analysis to assess the secondary outcome of paradoxical TB-IRIS. We evaluated heterogeneity using the Cochrane’s Chi2 test (significant if P < 0.10) and the I2 statistic test (>50% indicative of substantial heterogeneity) 29.
Results
Study characteristics
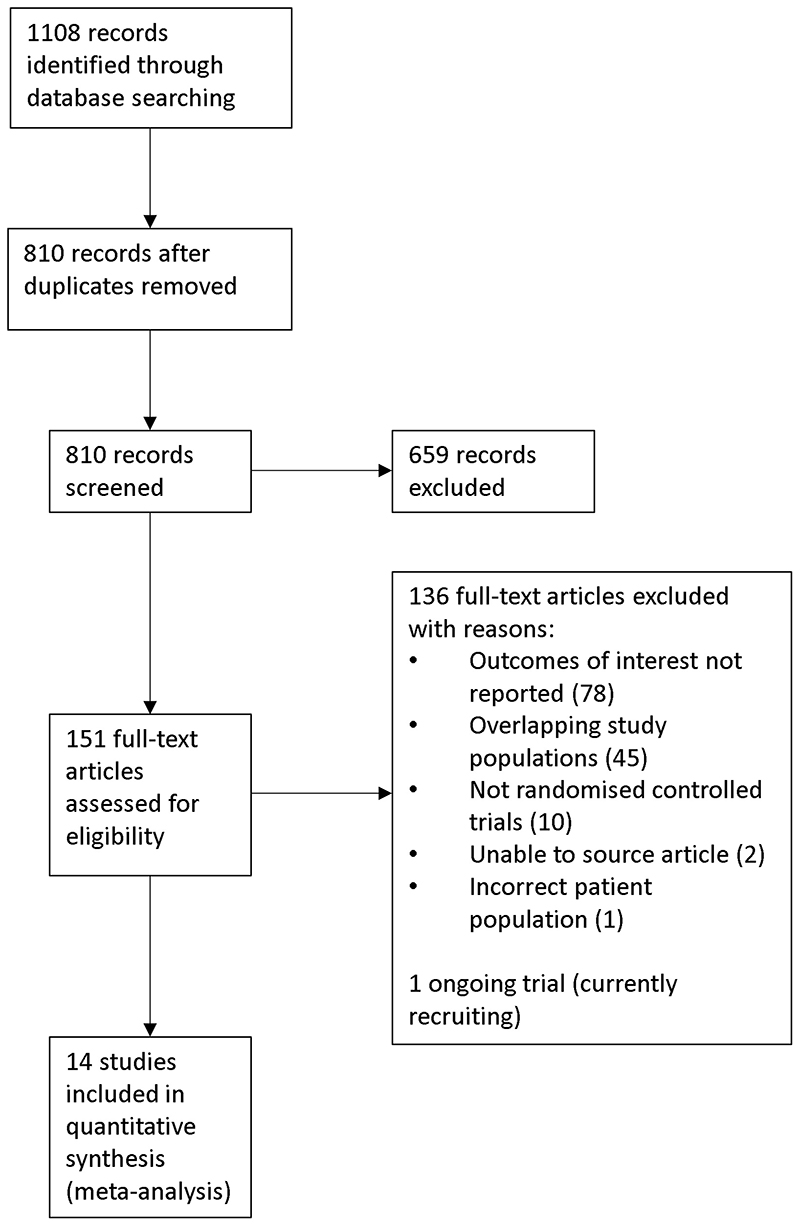
We identified 1108 records from five databases, 151 of which were eligible for full-text review. Fourteen RCTs were included in our meta-analysis (Figure 1).
Figure 1. PRISMA flow diagram.
The characteristics of the included studies are summarised in Table 2, Supplemental Digital Content. Most studies (n = 11) were multinational, and participants were recruited from Africa (n = 7), Europe (n = 6), North America (n = 5), South America (n = 5), Asia (n = 5), and Australia (n = 2). Two studies 5,30 enrolled children (aged ≥5 years) and/or adolescents (aged ≥13 years) in addition to adult participants. Both studies were included in our analysis as few participants were aged <18 years: 72/1805 (4.0%) participants were aged 5 – 17 years in REALITY 5 and 14/1053 (1.3%) aged 13 – 18 years in ADVANCE 30. Dolutegravir was the InSTI drug used in the intervention arms in seven studies 4,8,30-34, raltegravir in six studies 5,35-39, and elvitegravir in one study 40.
The primary outcome of included studies was proportion with virologic suppression 4,8,30,32-38,40, time to virologic or clinical failure 39, all-cause mortality 5, or median CD4 count increase at week 48 31. Seven trials defined IRIS as a secondary outcome 5,8,31,32,35,36,39, including four that specifically defined TB-IRIS as a secondary outcome 5,32,35,36. The case definition proposed by French 41 was used in the diagnosis of IRIS in two studies 5,8 and the International Network for the Study of HIV-associated IRIS (INSHI) case definition 42 in the diagnosis of paradoxical TB-IRIS in five studies 5,8,32,35,36. Nine studies did not specify the case definition used for IRIS adjudication 4,30,31,33,34,37-40. An independent endpoint review committee adjudicated IRIS events in six studies 5,8,30,32,35,36, three of which specified that adjudication was blinded to treatment arms 5,8,32.
Full safety data were available for only two studies as supplementary appendices to the main publications of the trials 4,33. The safety data reported by the other studies included serious adverse events (SAEs), adverse events leading to drug discontinuation, Grade 3 and 4 adverse events, and adverse events that occurred with a frequency threshold of 5%. Therefore, IRIS events classified as Grade 1 and 2 adverse events or occurred in <5% of participants and not reported by primary studies could not be assessed in this meta-analysis. The Late Presenter Treatment Optimisation (LAPTOP) study is ongoing and could not be included in this analysis (estimated date of completion in December 2021) 43.
Risk of bias in included studies
The RoB 2 judgements for all domains, and overall for the primary and secondary outcomes, are summarised in Table 3, Supplemental Digital Content. Six studies were evaluated as having some concerns for the overall risk of bias for the primary outcome of IRIS 8,30,35,36,38,40. Six studies were at overall high risk of bias for IRIS 4,31,33,34,37,39. The main reason for a study being assessed as having a high risk of bias was measurement of the outcome. Three studies contributing to the secondary outcome of paradoxical TB-IRIS were at overall low risk of bias 32 or had some concerns 35,36.
Primary outcome: Risk of IRIS
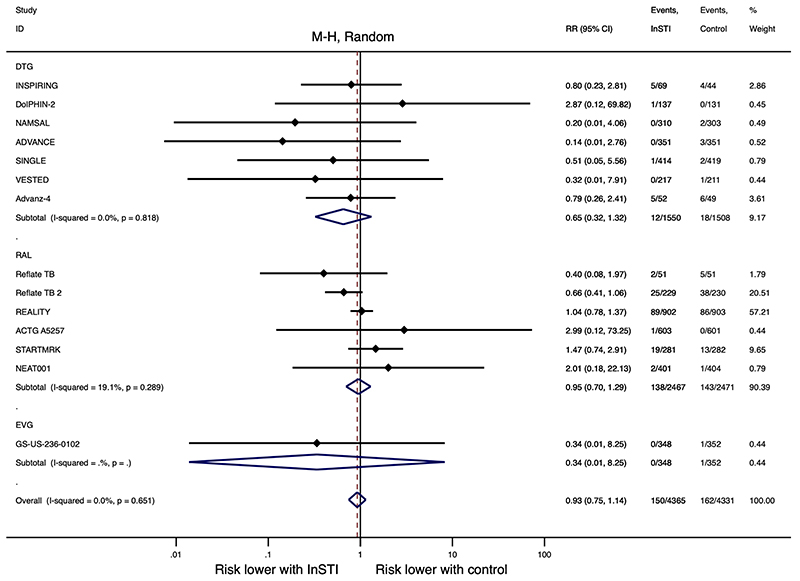
There is probably little to no difference in IRIS risk between InSTI and non-InSTI regimens (RR, 0.93, 95% CI, 0.75 – 1.14, I2 = 0.0%, 8696 participants, moderate-certainty evidence, Figure 2). Risk of IRIS was similar between individual member of InSTI class and non-InSTI regimens: dolutegravir (RR, 0.65, 95% CI, 0.32 – 1.32, I2 = 0.0%, 7 studies, 3058 participants), raltegravir (RR, 0.95, 95% CI, 0.70 – 1.29, I2 = 19.1%, 6 studies, 4938 participants), and elvitegravir (RR, 0.34, 95% CI, 0.01 – 8.25, 1 study, 700 participants). Heterogeneity among studies was low for the primary outcome (I2 = 0.0%, Pheterogeneity = 0.651). We downgraded the certainty of evidence one level for serious risk of bias. The evidence profile is presented in the “Summary of findings” table (Table 4, Supplemental Digital Content). IRIS events were reported for 1.94% (n = 4365, 95% CI, 0.33 – 4.57) participants on InSTI regimens versus 2.63% (n = 4331, 95% CI, 0.69 – 5.58) on non-InSTI regimens (Figure 3).
Figure 2. Meta-analysis of IRIS in randomized controlled trials of InSTI.
IRIS, immune reconstitution inflammatory syndrome; InSTI, integrase strand transfer inhibitor; DTG, dolutegravir; RAL, raltegravir; EVG, elvitegravir; M-H, Mantel-Haenszel; RR, risk ratio; CI, confidence interval.
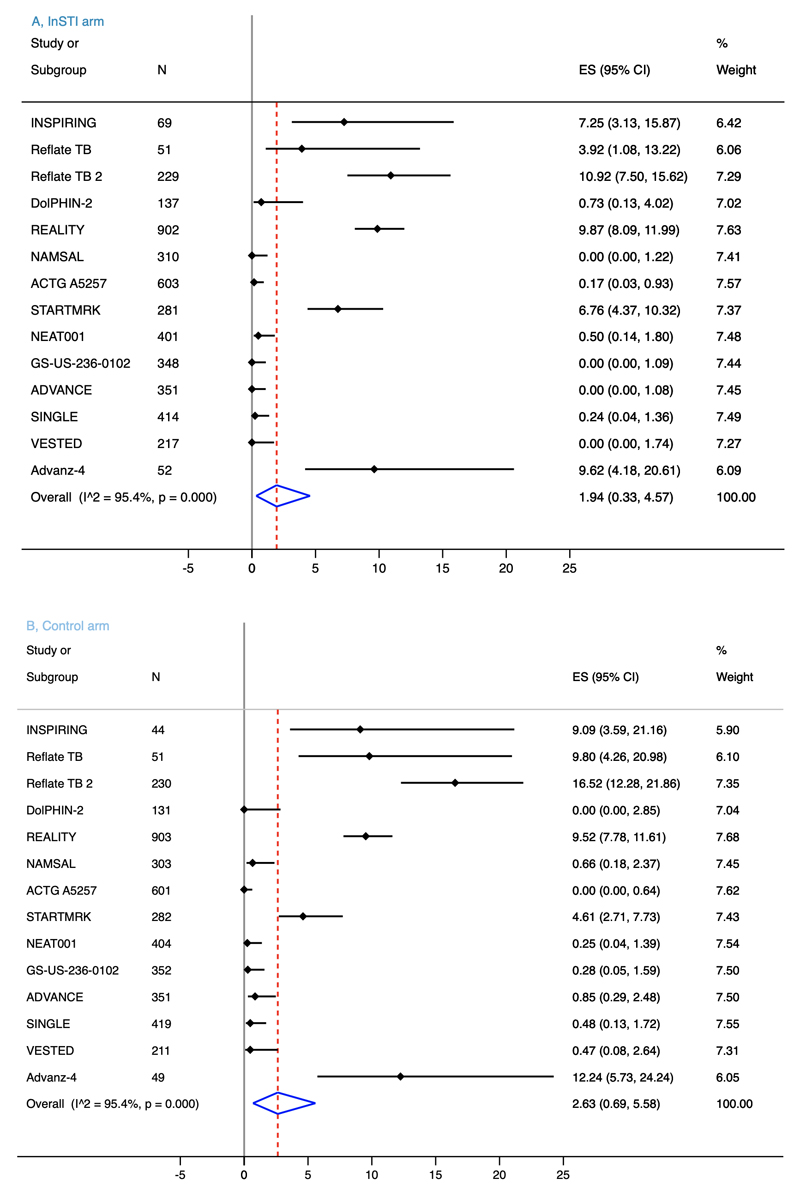
Figure 3. Forest plots of proportion of patients with IRIS on InSTI regimens (a) and non-InSTI regimens (b).
IRIS, immune reconstitution inflammatory syndrome; InSTI, integrase strand transfer inhibitor; ES, effect size; CI, confidence interval.
The Reflate TB 2 study, a multinational trial recruiting participants with HIV-associated tuberculosis, reported the highest incidence of IRIS: 11% (25/229) participants on raltegravir regimen versus 17% (38/230) on efavirenz regimen developed IRIS events 36. IRIS event rates were low (<1%) in seven studies 4,8,33,34,37,39,40. Five studies reported an incidence of IRIS between 1 and 10% 5,30,32,35,38. Proportions with IRIS reported in included studies are shown in Table 5, Supplemental Digital Content. We did not observe substantial changes to our overall estimate of IRIS risk between InSTI and non-InSTI regimens with a sub-group meta-analysis excluding studies that reported <5% IRIS events (RR, 0.93, 95% CI, 0.72 – 1.19, I2 = 9.1%, 6 studies, 3143 participants).
Eight IRIS events were reported as SAEs (2 on raltegravir regimen and 1 on efavirenz regimen in STARTMRK 44, 1 on dolutegravir regimen and 1 on efavirenz regimen in INSPIRING 32, 1 on raltegravir regimen in ACTG A5257 37, 1 on dolutegravir regimen in DolPHIN-2 8, and 1 on efavirenz regimen in GS-US-236-0102 40). One IRIS event led to discontinuation of ART regimen (raltegravir regimen of STARTMRK 44). In the REALITY study, 36/902 (4.0%) participants on raltegravir-intensified regimen died as result of IRIS, compared with 31/903 (3.4%) on standard ART regimen that did not contain raltegravir 5. Fatal IRIS occurred in 2/303 participants on efavirenz regimen in NAMSAL, manifesting as pulmonary tuberculosis and Kaposi sarcoma 33. In Reflate TB 2, TB-IRIS was the cause of death in two participants: 1/229 on raltegravir regimen and 1/230 on efavirenz regimen 36.
One study reported median time from ART initiation to IRIS occurrence as 3.4 (interquartile range, 2.0 – 6.3) weeks, with rates declining from the third week on ART 5. Four late-occurring IRIS events were reported in STARTMRK, with two cases on raltegravir regimen and two cases on efavirenz regimen developing after 48 weeks 38.
Secondary outcome: Risk of paradoxical TB-IRIS
Three studies (INSPIRING, Reflate TB, and Reflate TB 2) enrolled 674 participants at risk for paradoxical TB-IRIS (starting ART after a diagnosis of HIV-associated tuberculosis while on antituberculosis treatment) 32,35,36. The INSPIRING study compared a twice daily dolutegravir regimen to efavirenz regimen 32. Both Reflate TB studies compared raltegravir and efavirenz; Reflate TB included two raltegravir regimens using two different dosing strategies 35.
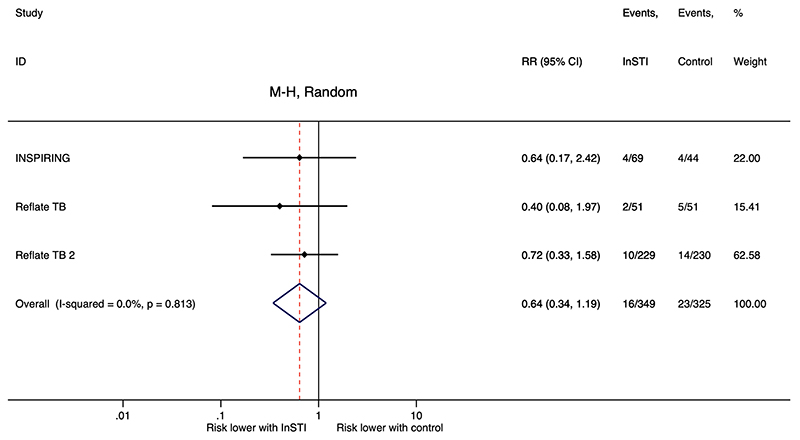
There may be a reduced risk of paradoxical TB-IRIS among participants initiating InSTI regimens than those initiating efavirenz regimens, but this was not statistically significant (RR, 0.64, 95% CI, 0.34 – 1.19, I2 = 0.0%, 674 participants, low-certainty evidence, Figure 4). Heterogeneity between the studies was low for this secondary outcome (I2 = 0.0%, Pheterogeneity = 0.813). We downgraded the certainty of evidence due to serious risk of bias and imprecision. The evidence profile is presented in the “Summary of findings” table (Table 4, Supplemental Digital Content). One SAE was attributed to paradoxical TB-IRIS (dolutegravir regimen in INSPIRING) 32. Two deaths due to paradoxical TB-IRIS were reported: 1 on efavirenz regimen and 1 on raltegravir regimen in Reflate TB 2 36.
Figure 4. Meta-analysis of paradoxical TB-IRIS in randomized controlled trials of InSTI.
TB-IRIS, tuberculosis-associated immune reconstitution inflammatory syndrome; InSTI, integrase strand transfer inhibitor; M-H, Mantel-Haenszel; RR, risk ratio, CI, confidence interval.
Discussion
In our meta-analysis of data from RCTs across six continents, we found no association between InSTI regimens and risk of IRIS. We found a trend towards a lower risk of paradoxical TB-IRIS with InSTI regimens compared with efavirenz regimens among patients with HIV-associated tuberculosis initiating ART, but precision was lacking for this secondary outcome. It is reassuring that initiating InSTI regimens did not increase the risk of IRIS in our systematic review that included trial participants across eight sub-Saharan African countries, therefore supporting the current move to first-line InSTI regimens in LMICs.
Our finding that InSTI regimens were not associated with risk of IRIS is contrary to the results from observational cohort studies. In the Dutch ATHENA cohort, 32% of patients starting InSTI and 18% starting non-InSTI regimens developed IRIS (odds ratio [OR] 2.17, 95% CI, 1.45 – 3.25) 20. Severe IRIS leading to hospitalisation occurred in 3% of patients on InSTI and 1.5% on non-InSTI regimens in a multicentre French cohort (RR 1.99, 95% CI, 1.09 – 3.47) 21. Psichogiou et al. reported InSTI use an independent risk factor for IRIS (OR 2.89, 95% CI, 1.26 – 6.64) 22. We found a trend towards a lower risk of paradoxical TB-IRIS with InSTI regimens compared with efavirenz regimens among patients with HIV-associated tuberculosis initiating ART. However, an increased risk of paradoxical TB-IRIS was reported in patients with newly diagnosed tuberculosis initiating InSTI regimen in a retrospective cohort study (OR 3.33, 95% CI, 1.01 – 11.1) 45. Bias and unmeasured confounders may have contributed to the observed association with IRIS in observational cohort studies. First, diagnoses of IRIS rely on the detection of clinical features that fulfil case definitions and are challenging due to the lack of specific diagnostic tests. An increasing awareness of a possible association between InSTI and IRIS and the nature of an unblinded study and retrospective chart review may lead to ascertainment bias. Second, patients presenting with specific opportunistic infections were more likely to receive InSTI regimens to avoid drug-drug interactions with concomitant medications in one study 20, resulting in patients most at risk for IRIS being preferentially channelled to InSTI regimens.
InSTI regimens result in more rapid HIV viral load declines 4-6. The hypothesis that IRIS would be more common with InSTI regimens was based on cohort studies reporting the rapid decline in HIV viral load after ART initiation an independent risk factor for paradoxical TB-IRIS 10,11. In a retrospective observational study, patients who developed IRIS had more marked decline in viral load within 90 days of starting ART (P < 0.001), and a viral load decrease of 2 log10 copies/mL at 90 days on ART was associated with a 3.7-fold increase in risk of developing IRIS (95% CI, 1.55 – 8.64; P = 0.003) 9. Similarly, in a study of immunologic predictors of TB-IRIS, a viral load decline ≥4 log10 copies/mL at week 12 was associated with TB-IRIS (OR 2.56, 95% CI, 1.00 – 6.59) 46. However, our review of RCTs found no excess of IRIS events observed on InSTI regimens, despite the substantially faster viral load decline with InSTI regimens as demonstrated with raltegravir-intensified regimen in the REALITY study 5. Our findings suggest that rapid viral load decline with ART initiation might not be an independent risk factor for IRIS. Further research into the immunological mechanisms underlying IRIS is needed to better predict the development of this condition.
Our study serves to update an earlier systematic review which excluded patients with CDC stage C disease and HIV-associated tuberculosis 23. Nevertheless, this review has important limitations. First, there was likely under-reporting of IRIS events as none of the RCTs assessed IRIS as a primary outcome and due to restrictions used by RCTs in the reporting of safety results. One study reported only fatal IRIS events 33 and three studies reported IRIS events that met criteria for an SAE 8,37,40. Two studies reported only Grade 3 and 4 paradoxical TB-IRIS events 35,36. Our pooled estimates of IRIS events were low compared with other meta-analyses 14,47 and likely reflected an under-ascertainment of IRIS events. However, under ascertainment was expected to be similar in participants randomised to InSTI and non-InSTI regimens. Our findings may thus reflect the more clinically significant and severe spectrum of IRIS events. Second, adjudication of IRIS event was not masked to treatment allocation in some studies and case definitions used to ascertain IRIS events differed across studies. Third, we did not have individual participant data to explore risk of IRIS in sub-groups such as patients with low CD4 counts.
A further limitation was our lack of precision for the risk of paradoxical TB-IRIS. Heterogeneity between the studies was low for this secondary outcome, but the associated 95% CI was wide due to imprecision in meta-analysis of few studies. It is also plausible that TB-IRIS was uncommon because patients at highest risk for TB-IRIS were underrepresented in the RCTs included in our sub-group analysis. Low CD4 counts and short intervals between antituberculosis treatment and ART initiation are risk factors most consistently associated with TB-IRIS 14,48. Patients with advanced immunosuppression (CD4 count <50 cells/mm3) were excluded from INSPIRING and underrepresented in Reflate TB (20%) and Reflate TB 2 (33%) 32,35,36. Median time to starting ART was over four weeks in two studies (5.9 weeks in Reflate TB 35; 35 and 33.5 days on dolutegravir and efavirenz regimens, respectively, in INSPIRING 32).
Conclusions
We found no increased risk of IRIS with InSTI regimens in this meta-analysis of RCTs. We do, however, highlight the need for further studies, including RCTs with less restrictive eligibility criteria, to determine whether InSTI increase the risk and/or severity of paradoxical TB-IRIS in high-risk patients (CD4 counts <50 cells/mm3 and starting ART within four weeks of antituberculosis treatment). Notwithstanding the limitations of the study, findings from this meta-analysis provide additional evidence to support the routine use of InSTI in first-line ART regimens in LMICs.
Supplementary Material
Acknowledgments
For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Sources of Funding
This work was supported by the Wellcome Trust through core funding from the Wellcome Centre for Infectious Diseases Research in Africa (203135/Z/16/Z). GrM was supported by the Wellcome Trust (214321/Z/18/Z) and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (Grant No 64787). M.E.E. was supported by grant (NW17SFRN33630027) from the American Heart Association. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Kandel CE, Walmsley SL. Dolutegravir–a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug design, development and therapy. 2015;9:3547. doi: 10.2147/DDDT.S84850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhu V, Wong C, Jenkins S, et al. New ARVs could represent over USD 3 billion in cost savings through 2025; Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2016. [Google Scholar]
- 3.Dutta A, Barker C, Kallarakal A. The HIV Treatment Gap: Estimates of the Financial Resources Needed versus Available for Scale-Up of Antiretroviral Therapy in 97 Countries from 2015 to 2020. PLoS Med. 2015;12(11):e1001907. doi: 10.1371/journal.pmed.1001907. discussion e1001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
- 5.Kityo C, Szubert AJ, Siika A, et al. Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: A randomised controlled trial. PLoS Med. 2018;15(12):e1002706. doi: 10.1371/journal.pmed.1002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahangdale L, Cates J, Potter J, et al. Integrase inhibitors in late pregnancy and rapid HIV viral load reduction. American journal of obstetrics and gynecology. 2016;214(3):385. e381–385. e387. doi: 10.1016/j.ajog.2015.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927–935. doi: 10.1016/S1473-3099(13)70257-3. [DOI] [PubMed] [Google Scholar]
- 8.Kintu K, Malaba TR, Nakibuka J, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. 2020;7(5):e332–e339. doi: 10.1016/S2352-3018(20)30050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shelburne SA, Visnegarwala F, Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. Aids. 2005;19(4):399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 10.Olalla J, Pulido F, Rubio R, et al. Paradoxical responses in a cohort of HIV-1-infected patients with mycobacterial disease. The International Journal of Tuberculosis and Lung Disease. 2002;6(1):71–75. [PubMed] [Google Scholar]
- 11.Navas E, Martín-Dávila P, Moreno L, et al. Paradoxical reactions of tuberculosis in patients with the acquired immunodeficiency syndrome who are treated with highly active antiretroviral therapy. Archives of Internal Medicine. 2002;162(1):97–99. doi: 10.1001/archinte.162.1.97. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Global Tuberculosis Report 2018. WHO; Geneva, Switzerland: 2018. [Google Scholar]
- 13.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. Aids. 2001;15(2):143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 14.Namale PE, Abdullahi LH, Fine S, et al. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future microbiology. 2015;10(6):1077–1099. doi: 10.2217/fmb.15.9. [DOI] [PubMed] [Google Scholar]
- 15.The IeDEA and ART Cohort Collaborations. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-and high-income countries. Journal of acquired immune deficiency syndromes 1999. 2014;65(1):e8. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona S, Bor J, Nattey C, et al. Persistent high burden of advanced HIV disease among patients seeking care in South Africa’s national HIV program: data from a nationwide laboratory cohort. Clinical Infectious Diseases. 2018;66(suppl_2):S111–S117. doi: 10.1093/cid/ciy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet M, Baudin E, Jani IV, et al. Incidence of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome and impact on patient outcome. PLoS One. 2013;8(12):e84585. doi: 10.1371/journal.pone.0084585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker NF, Stek C, Wasserman S, et al. The tuberculosis-associated immune reconstitution inflammatory syndrome: recent advances in clinical and pathogenesis research. Current Opinion in HIV and AIDS. 2018;13(6):512. doi: 10.1097/COH.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PloS one. 2013;8(5):e63541. doi: 10.1371/journal.pone.0063541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wijting IEA, Wit F, Rokx C, et al. Immune reconstitution inflammatory syndrome in HIV infected late presenters starting integrase inhibitor containing antiretroviral therapy. E Clinical Medicine. 2019;17:100210. doi: 10.1016/j.eclinm.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutertre M, Cuzin L, Demonchy E, et al. Initiation of Antiretroviral Therapy Containing Integrase Inhibitors Increases the Risk of IRIS Requiring Hospitalization. J Acquir Immune Defic Syndr. 2017;76(1):e23–e26. doi: 10.1097/QAI.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 22.Psichogiou M, Basoulis D, Tsikala-Vafea M, et al. Integrase Strand Transfer Inhibitors and the Emergence of Immune Reconstitution Inflammatory Syndrome (IRIS) Curr HIV Res. 2017;15(6):405–410. doi: 10.2174/1570162X15666171122155708. [DOI] [PubMed] [Google Scholar]
- 23.Hill AM, Mitchell N, Hughes S, et al. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS. 2018;13(2):102–111. doi: 10.1097/COH.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 24.Sereti I, Sheikh V, Shaffer D, et al. Prospective international study of incidence and predictors of immune reconstitution inflammatory syndrome and death in people living with human immunodeficiency virus and severe lymphopenia. Clinical Infectious Diseases. 2020;71(3):652–660. doi: 10.1093/cid/ciz877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Systematic reviews. 2016;5(1):1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 28.GRADEpro GDT. 2015. [Accessed 14 January 2022]. Computer program McMaster University (developed by Evidence Prime) GRADEpro GDT Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666–e676. doi: 10.1016/S2352-3018(20)30241-1. [DOI] [PubMed] [Google Scholar]
- 31.Miro JM, Torres F, Manzardo C. Dolutegravir vs darunavir/r-based ART in very advanced patients: 48-week results. Chicago. Science Spotlight abstract 412; Conference on Retroviruses and Opportunistic Infections 6–10 March; 2021. [Google Scholar]
- 32.Dooley KE, Kaplan R, Mwelase N, et al. Dolutegravir-based Antiretroviral Therapy for Patients Coinfected With Tuberculosis and Human Immunodeficiency Virus: A Multicenter, Noncomparative, Open-label, Randomized Trial. Clin Infect Dis. 2020;70(4):549–556. doi: 10.1093/cid/ciz256. [DOI] [PubMed] [Google Scholar]
- 33.Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, et al. Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N Engl J Med. 2019;381(9):816–826. doi: 10.1056/NEJMoa1904340. [DOI] [PubMed] [Google Scholar]
- 34.Lockman S, Brummel SS, Ziemba L, et al. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. The Lancet. 2021;397(10281):1276–1292. doi: 10.1016/S0140-6736(21)00314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grinsztejn B, De Castro N, Arnold V, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 Reflate TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis. 2014;14(6):459–467. doi: 10.1016/S1473-3099(14)70711-X. [DOI] [PubMed] [Google Scholar]
- 36.De Castro N, Marcy O, Chazallon C, et al. Standard dose raltegravir or efavirenz-based antiretroviral treatment for patients co-infected with HIV and tuberculosis (ANRS 12 300 Reflate TB 2): an open-label, non-inferiority, randomised, phase 3 trial. The Lancet Infectious Diseases. 2021 doi: 10.1016/S1473-3099(20)30869-0. [DOI] [PubMed] [Google Scholar]
- 37.Lennox JL, Landovitz RJ, Ribaudo HJ, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med. 2014;161(7):461–471. doi: 10.7326/M14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lennox JL, Dejesus E, Berger DS, et al. Raltegravir versus Efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39–48. doi: 10.1097/QAI.0b013e3181da1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raffi F, Babiker AG, Richert L, et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet. 2014;384(9958):1942–1951. doi: 10.1016/S0140-6736(14)61170-3. [DOI] [PubMed] [Google Scholar]
- 40.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: A randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. The Lancet. 2012;379(9835):2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 41.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. Aids. 2004;18(12):1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 42.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. The Lancet infectious diseases. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NEAT ID Foundation. The Late Presenter Treatment Optimisation Study (LAPTOP) [Accessed 26 November 2021]. https://clinicaltrials.gov/ct2/show/NCT03696160 .
- 44.Teppler H, Brown DD, Leavitt RY, et al. Long-term safety from the raltegravir clinical development program. Curr HIV Res. 2011;9(1):40–53. doi: 10.2174/157016211794582650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaillet A, Calin R, Flandre P, et al. Increased risk of IRIS-associated tuberculosis in HIV-infected patients receiving Integrase Inhibitors. Infectious Diseases Now. 2021;51(1):90–93. doi: 10.1016/j.medmal.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Tieu HV, Ananworanich J, Avihingsanon A, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25(11):1083–1089. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. The Lancet infectious diseases. 2010;10(4):251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uthman OA, Okwundu C, Gbenga K, et al. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: a systematic review and meta-analysis. Annals of internal medicine. 2015;163(1):32–39. doi: 10.7326/M14-2979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.