Abstract
Background and aims
Multiple observational studies and small-scale intervention studies suggest that high vitamin K intake is associated with improved markers for cardiovascular health. Circulating phylloquinone solely represents phylloquinone (vitamin K1) intake, while dephosphorylated uncarboxylated Matrix Gla Protein (dp-ucMGP) represents both phylloquinone and menaquinone (vitamin K2) intake. This study aims to investigate the causal relationship between genetically predicted vitamin K concentrations and the risk of CHD via a two-sample Mendelian Randomization approach.
Design
We used data from three studies: the European Prospective Investigation into Cancer and Nutrition (EPIC)-CVD case-cohort study, CARDIOGRAMplusC4D and the UK Biobank, resulting in 103,097 CHD cases. Genetically predicted vitamin K concentrations were measured using SNPs related to circulating phylloquinone and dp-ucMGP. We calculated a genetic risk score (GRS) including four SNPs (rs2108622, rs2192574, rs4645543 and rs6862071) related to circulating phylloquinone levels from a genome wide association study. Rs4236 was used as an instrumental variable for dp-ucMGP. Inverse-variance weighted (IVW) analysis was used to obtain Risk Ratios (RRs) for the causal relationship between phylloquinone and dp-ucMGP concentrations and CHD risk.
Results
Using the genetic score for circulating phylloquinone, we found that circulating phylloquinone was not causally related to CHD risk (RR 1.00 (95%-CI: 0.98;1.04)). Lower genetically predicted dp-ucMGP concentration was associated with a lower CHD risk with a RR of 0.96 (95%-CI: 0.93;0.99) for every 10 μg/L decrease in dp-ucMGP.
Conclusions
This study did not confirm a causal relationship between circulating phylloquinone and lower CHD risk. However, lower dp-ucMGP levels may be causally related with a decreased CHD risk. This inconsistent result may reflect the influence of menaquinones in the association with CHD.
Keywords: Vitamin K, Matrix Gla Protein, phylloquinone, coronary heart disease, Mendelian Randomization, Epidemiology
Introduction
Vitamin K occurs in two biologically active forms, phylloquinone and menaquinones. Phylloquinone is mainly derived from green leafy vegetables, while menaquinones mostly occur in animal products. Phylloquinone and menaquinones have the same 2-methylated-1,4 naphthoquinones ring structure, but differ in the length and saturation degree of the side chain (1). Circulating phylloquinone is a marker for vitamin K status but solely reflects phylloquinone intake or supplementation (2–4). Matrix Gla protein (MGP) is a vitamin K dependent protein since it requires vitamin K for activation (5). Inactive MGP, dephosphorylated uncarboxylated MGP (dp-ucMGP), serves as a circulating marker for vitamin K, where low dp-ucMGP levels represents long term high vitamin K intake (6). Both phylloquinone and menaquinone supplementation substantially reduce dp-ucMGP levels (7).
Observational studies for circulating phylloquinone show contradicting results: lower circulating phylloquinone was a risk factor for incident cardiovascular disease (CVD) risk (8), but studies focusing on coronary calcium showed contradicting results ranging from no to a positive association (9,10). Randomized placebo-controlled trials show that dietary phylloquinone supplementation reduces arterial elasticity (11), and progression of coronary calcium in adults treated for hypertension (4).
MGP is a strong inhibitor of arterial calcification (12) and dp-ucMGP is therefore suggested to reduce coronary heart disease (CHD) risk (13). A considerable number of observational studies showed that low dp-ucMGP levels, thus higher overall vitamin K intake, are associated with reduced coronary calcification levels and lower CVD risk (13). In line with observational studies, dietary menaquinone supplementation may be effective in reducing coronary calcification (6).
The current evidence points towards a beneficial role for vitamin K in prevention of cardiovascular disease risk. However, observational studies may be limited by reverse causation or confounding, while intervention studies are hampered by a short duration and focus on intermediate outcomes (14). Mendelian randomization (MR), where genetic variants are used as an instrumental variable, is an alternative design to minimize bias and infer causality (14). To date, two MR studies have been performed focusing on vitamin K concentrations and CHD risk (15,16). One MR study suggested that increased circulating phylloquinone is associated with an increased risk of CHD (15). No association was found between dp-ucMGP levels and CHD risk in a small-scale MR study (16). The MR study for phylloquinone was performed in a large dataset, but recently more genetic information in multiple cohort studies became available. Furthermore, the MR-study on dp-ucMGP was limited by a small-sample size.
Therefore, this study aims to investigate the causal relation between both circulating phylloquinone and dp-ucMGP and CHD using a two sample Mendelian randomization approach in the European Prospective Investigation into Cancer and Nutrition (EPIC)-CVD case cohort study, CARDIOGRAMplusC4D and the UK Biobank.
Subjects and Methods
Study population
For these analyses, we used data from three studies: EPIC-CVD, CARDIOGRAMplusC4D and the UK Biobank. Individual participant data was only available in EPIC-CVD. EPIC-CVD is a case-cohort study of cardiovascular disease nested within EPIC. EPIC consists of 366,521 women and 153,457 men, aged 35 to 70 years, recruited between 1991-1999, from 23 recruitment centres across 10 European countries. Participants were recruited in different ways in different countries and recruitment centres, as described previously (17). This case-cohort design includes a randomly selected subcohort of EPIC including 13,985 participants. The subcohort is supplemented with 9,296 participants who developed CHD during follow up. Due to the random selection of the subcohort from the baseline cohort, the subcohort also contains 531 participants that developed CHD during follow up. The EPIC study was approved by the ethical review boards of the International Agency for Research on Cancer and all recruitment centres. All participants gave informed consent prior to inclusion.
Summarized data was included from CARDIOGRAMplusC4D and the UK Biobank. CARDIOGRAMplusC4D is a meta-analysis of 48 studies involving 60,801 CAD cases and 123,504 controls (18). Data on coronary artery disease / myocardial infarction have been contributed by CARDIOGRAMplusC4D investigators and have been downloaded from www.CARDIOGRAMPLUSC4D.ORG. The UK Biobank (www.ukbiobank.ac.uk) recruited over 500,000 men and women (aged 37 to 73 years) between 2006 and 2010 (application number 29916) (19). The UK Biobank study was approved by the North West Multi-Centre Research Ethics Committee and all participants provided written informed consent to participate. Including CARDIOGRAMplusC4D and the UK Biobank increased our dataset to 103,097 CHD cases (Supplementary figure 1).
Genotyping
For EPIC-CVD, DNA was extracted from buffy coat from citrated blood plasma. EPIC-CVD participants were genotyped with the Human Core Exome array, Illumina 660 Quad array and Omni Exome Express array. Single nucleotide polymorphisms (SNPs) were removed with minor allele count <2 or Hardy Weinberg p-value <1e-6. Missing genotypes were imputed using the 1000 Genomes reference panel.
CARDIOGRAMplusC4D are genotyped using different assays, as described previously (18). Genotyping in the UK Biobank was performed using the Affymetrix UK BiLEVE Axiom array and the Affymetrix UK Biobank Axiom Array (20).
Circulating phylloquinone
We included four SNPs that were related to circulating phylloquinone (both plasma and serum) in a small genome wide association study (GWAS) (rs2108622, rs2192574, rs4645543 and rs6862071) (21).
Rs2108622 (chromosome 19) is a CYP4F2 variant, which functions as a phylloquinone oxidase (22). Previous studies showed that rs2108622 was associated with altered phylloquinone metabolism and warfarin dosage (23). Rs2192574 (chromosome 2) is a variant on the CTNNA2 locus, whereas a GWAS indicated an association between the CTNNA2 locus and bone mineral density (24). Vitamin K is suggested to increase bone mineral concentration, via activation of osteocalcin, another vitamin K dependent protein (25), which may explain the correlation between vitamin K and the CTNNA2 locus. Rs4645543 (chromosome 8; KCNK9) and rs6862071 (chromosome 5; CDO1) are SNPS on the potassium channel, and tumor suppressor gene respectively (26). The mechanism by which these genes influence phylloquinone concentration is unknown.
All SNPs passed the threshold of p<5×10−6, but not p<5×10−8 (21). No linkage disequilibrium (LD) at R2>0.9 was present among these four SNPs. Pleiotropic effects were investigated by searching the GWAS catalogue and Phenoscanner for the SNPs or their proxies (R2>0.8) (27,28), checking possible confounders with a threshold of p<5×10−6.
We calculated a genetic risk score (GRS) to take into account the differences in the effect of the individual SNP on circulating phylloquinone levels. The genetic variants are uncorrelated and therefore the estimates from a weighted regression analysis using summarized data are equal to a genetic risk score using individual level data (29). We refer to the analysis as a genetic risk score as this is likely to be more familiar to applied readers. The weights of the individual SNPs are shown in Table 1. The F-statistic for the GRS for circulating phylloquinone was 280.4.
Table 1. Genetic variants associated with circulating phylloquinone and dp-ucMGP as reported in prior studies.
| SNP | Chromosome | Nearest gene | ß | p-value | |
|---|---|---|---|---|---|
| Phylloquinone(21) | Rs2108622 | 19 | CYP4F2 | 0.16 | 8.78 X 10-7 |
| Rs2192574 | 2 | CTNAA2 | 0.28 | 1.82 X 10-6 | |
| Rs4645543 | 8 | KCNK9 | 0.42 | 2.00 X 10-7 | |
| Rs6862071 | 5 | CDO1 | 0.94 | 2.29 X 10-5 | |
| Dp-ucMGP(16) | Rs4236 | 12 | MGP | -3.74 | 0.001# |
identified from candidate gene study
Dp-ucMGP
A candidate gene study identified three SNPs covering the MGP gene (chromosome 12) into the 3’ and 5’ flanking regions, that were associated with dp-ucMGP levels: rs2098435, rs2430692 and rs4236 (16). LD was present among these three SNPs (R2>0.75). Rs4236 showed the strongest association with dp-ucMGP (beta/SE), and was therefore included as the instrumental variable. Furthermore, pleiotropic effects were investigated by searching in the GWAS catalog and Phenoscanner, and no pleiotropic effects were identified (27,28). SNP rs4236 was complete for all participants with available genetic data. The F-statistic for rs4236 was 117.8.
CHD ascertainment
In EPIC-CVD, incident CHD cases were defined as first CHD event, both fatal and non-fatal as defined by ICD 10 codes: I20-I25. This includes myocardial infarction, acute ischaemic heart diseases, chronic ischaemic heart disease and angina pectoris. Several methods were used to ascertain CHD events including self-report, linkage to primary- and secondary-care registers and death registries (30). Case ascertainment in CARDIOGRAMplusC4D differed per study centre, as described previously (31). In the UK Biobank, endpoints were derived from linkage with hospital admissions data in England, Scotland and Wales and national death register data to identify the date of the first known CHD after the date of baseline assessment, identified by ICD 10 codes: I20-I25.
Statistical analyses
In EPIC-CVD individual participant data was analysed. Prentice-weighted Cox proportional hazards regression, with robust standard errors were used to account for the case-cohort design, was performed to assess the association between individual SNPs, the GRS and CHD risk. For each SNP and the GRS, we used the same model including age as the underlying time scale, with entry time defined as the participant’s age at recruitment and exit time as age at first fatal or non-fatal CHD event or censoring (whichever came first). This model was adjusted for sex, study centre, principal components and genetic array and analysed in strata of country. Hazard ratios from EPIC-CVD and the odds ratios from CARDIOGRAMplusC4d and UK Biobank were pooled to obtain risk ratios (RR) using random-effect meta-analysis. Then, the inverse-variance weighted (IVW) method was applied to assess the association between genetically predicted circulating phylloquinone, dp-ucMGP and CHD risk, were calculated using the formula reported by Burgess et al. (32).
To assess the robustness of the main result for the GRS for circulating phylloquinone, we performed a MR-Egger analysis using the ‘MendelianRandomization’ package in R (33). Circulating phylloquinone is carried predominantly in triglyceride rich lipoproteins and the GWAS also reported beta-coefficients adjusted for fasting triglycerides (21). Therefore, we performed sensitivity analyses in EPIC-CVD only; using these adjusted beta-coefficients and additionally adjusted the association between the GRS and CHD risk for triglycerides and hours of fasting. The study identifying the dp-ucMGP SNPs also adjusted for BMI, alcohol intake and smoking status (16). We performed sensitivity analyses including the same confounders. Missing values on triglycerides (6.6%), hours of fasting (32.4%) and alcohol intake (0.04%) were imputed using 10 imputations using the ‘mice’ package in R and combined according to Rubin’s Rules (34).
To explain the differences between CARDIOGRAMplusC4D and EPIC-CVD and UK Biobank for rs2108622 and rs4645543, we requested study-level results from CARDIOGRAMplusC4D and excluded studies with participants of non-European descent and small studies (n<1,000 cases) as sensitivity analyses. All analyses are performed using R Software version 3.1.1.
Results
Baseline characteristics of the EPIC-CVD participants are described in Supplementary table 1.
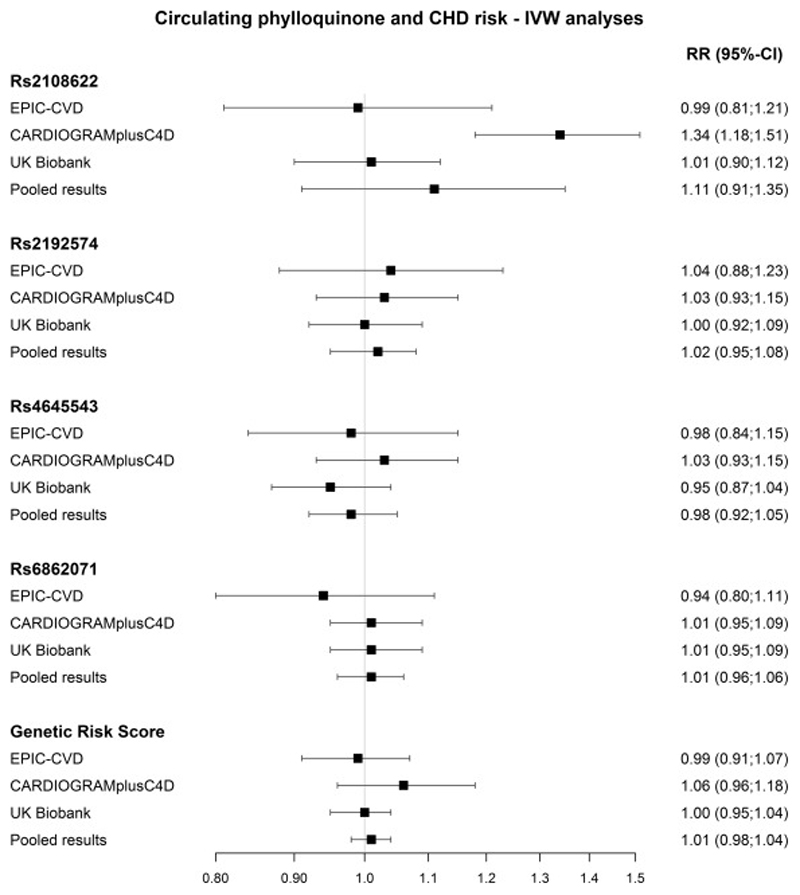
The IVW analyses showed no association between genetically-predicted concentrations of circulating phylloquinone risk and CHD risk in EPIC-CVD (HRGRS of 0.99 (95%-CI: 0.90;1.07) per ln-nmol/L increase in circulating phylloquinone) (Figure 1). Similar results were observed in CARDIOGRAMplusC4D (OR 1.06(95%-CI: 0.96-1.18)) and the UK Biobank (OR 1.00 (95%-CI: 0.95-1.04)). There was no heterogeneity between the three studies (I2=0.0) or genetic variants (I2=1.49, p=0.68). However, a causal relation was found between rs2108622 (CYP4F2) and CHD risk in CARDIOGRAMplusC4D (OR 1.34 (95%-CI: 1.18;1.51). To assess the difference in studies for rs2108622, sensitivity analysis was performed excluding small scale studies (n<1,000 cases) and participants of non-European descent from CARDIOGRAMplusC4D and this slightly decreased the RR (1.21 (95%-CI: 1.03;1.41)). The MR-Egger method indicated no significant pleiotropy (intercept=0.02, p=0.09), but the causal estimate slightly decreased to 0.95 (95%-CI: 0.88;1.02) per ln-nmol/L increase in circulating phylloquinone (Supplementary figure 2). EPIC-CVD data only, additional adjustment for triglycerides and hours of fasting did not change the results with a HR of 0.90 (95%-CI: 0.83;1.26) for the GRS (Supplementary table 2).
Figure 1.
The results of the Mendelian Randomization analyses for circulating phylloquinone in EPIC-CVD (n=9,296 cases), CARDIOGRAMplusC4D (n=60,801 cases) and UK Biobank (n=33,000 cases), and the pooled association (n=103,097 cases). The results are derived from the IVW analyses and presented as RR per ln-nmol/L increase in circulating phylloquinone.
Dp-ucMGP
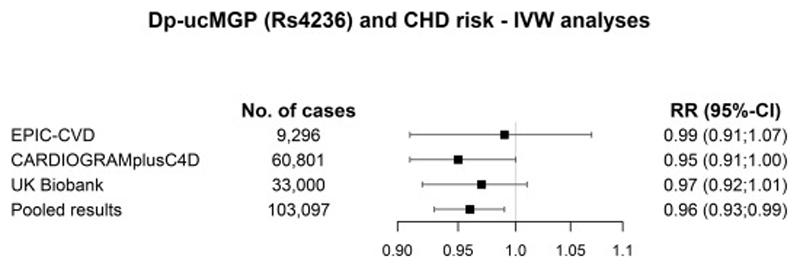
No causal relation was found between rs4236 and risk of CHD in EPIC-CVD with a HR of 0.99 (95%-CI: 0.91;1.07) (Figure 2). Adding CARDIOGRAMplusC4D and UK Biobank data resulted in a RR of 0.96 (95%-CI: 0.93;0.99) per 10 µg/L decrease in dp-ucMGP (p=0.02). Additional adjustment for smoking, drinking and alcohol intake, in EPIC-CVD only, did not change the results (HR 0.94 (95%-CI: 0.79;1.11)) (Supplementary table 2).
Figure 2.
The results of the Mendelian Randomization analyses for circulating dp-ucMGP (rs4236) in EPIC-CVD, CARDIOGRAMplusC4D and UK Biobank, and the pooled association (EPIC-CVD, CARDIOGRAMplusC4D and UK Biobank). The results are derived from the IVW analyses and presented as RR per every 10 μg/L decrease in dp-ucMGP.
Discussion
This study did not provide evidence for an association between genetically predicted phylloquinone concentrations and CHD risk. However, lower genetically predicted dp-ucMGP concentrations, representing long-term increased vitamin K intake, may be causally related with a decreased CHD risk.
Our results are in contrast with a previous MR-study showing that higher genetically predicted phylloquinone concentrations were associated with an increased CAD/MI risk in the CARDIOGRAM data (15). This well-conducted and reproducible study used CARDIOGRAM (2011) and CARDIOGRAMplusC4D (2013), while we used the CARDIOGRAMplusC4D 2015 release. The difference in results between the study from Schooling and our study were driven by two genetic variants: rs4645543 (KCNK9 gene) and rs2108622 (CYP4F2 gene). The results for rs4645543 differed in CARDIOGRAMplus4D 2015 release and the CARDIOGRAM 2011 release. The major differences between the releases are the studies including participants from non-European descent; however, excluding studies from non-European descent did not alter the results. Furthermore, restricting to the studies (n=12) in the CARDIOGRAMplusC4D 2015 release that were also in the CARDIOGRAM 2011 release, did not explain the discrepancies as well. Therefore, we cannot fully explain the discrepancy between the results of Schooling using earlier CARDIOGRAM releases and our study.
Rs2108622 (CYP4F2 gene) was positively associated with CHD risk in CARDIOGRAMplusC4D, like in all CARDIOGRAM releases, while no association was found in EPIC-CVD or in the UK Biobank. We excluded small-scale studies and studies from non-European descent in CARDIOGRAMplusC4D, and this slightly decreased the risk ratios, but we could not fully explain the discrepancy for rs2108622 in CARDIOGRAMplusC4D compared to EPIC-CVD or the UK Biobank. This genetic variant may be the most reliable marker for phylloquinone concentrations since the CYP4F2 gene functions as a phylloquinone oxidase, and could alter the phylloquinone metabolism (22). However, we did not detect a causal relation between this genetic variant and CHD risk.
In contrast with phylloquinone, decreased genetically predicted dp-ucMGP concentrations, reflecting increased vitamin K intake, were causally related with a decreased CHD risk. Liu et al. did not detect a causal relation between rs4236 and risk of CVD using an MR approach (16). However, the direction of the association is comparable to our study (HR 0.87 (95%-CI: 0.71;1.08)). The small sample size of this study (CVD events, n=87) may explain the non-significant finding (16). Indeed, our study only detected a causal relation in the pooled dataset of over 100,000 CHD cases.
The differences between phylloquinone and dp-ucMGP could be explained by the different characteristics of both biomarkers to assess vitamin K status. Circulating phylloquinone is the primary circulating form of vitamin K and solely represents phylloquinone intake of the previous days (13). Inactive MGP, dp-ucMGP, is considered the best marker for vitamin K since dp-ucMGP reflects the bioavailability of both phylloquinone and menaquinone intake over multiple weeks to months. Menaquinones should be taken into account since menaquinones have a higher absorption and bioavailability than phylloquinone, and therefore may be more beneficial to human health (35). Moreover, dp-ucMGP is functionally associated with the mechanism explained the reduced CHD risk. Therefore, the association found for dp-ucMGP may be the most reliable marker to reflect vitamin K intake. However, candidate gene studies also suggested that rs4236 was associated with total MGP (36). Therefore, this SNP may also reflect the causal association between MGP itself and CHD rather than vitamin K.
To date, the literature regarding circulating phylloquinone and CHD risk is inconsistent. Observational studies focusing on circulating phylloquinone and differences in coronary artery calcium showed contradicting results ranging from no to a positive association (9,10), while intervention studies showed that phylloquinone supplementation reduces arterial elasticity and coronary calcification (4,11). It is possible that the protective effect can be reached with supplementary dosage and not with dietary intake or circulating levels of phylloquinone (9). The majority of the observational studies showed that low dp-ucMGP levels are associated with reduced cardiovascular disease (CVD) risk and presence of coronary calcification levels (13). Our MR-study is comparable to previous observational studies, since we were not able to detect an association between circulating phylloquinone and CHD risk. However, higher genetically predicted dp-ucMGP levels were associated with a reduced CHD risk, which could be explained by the influence of menaquinones.
A particular strength in two-sample MR study is the large sample size which can detect small effect sizes. Several issues may compromise the value of Mendelian randomization approach in assessing causality. First, genetic variants determining significant proportions of variance in the risk factor are needed. While the SNPs did not reach genome wide significance, the F-statistic did not show an indication of weak instrument bias. Additionally, the F-statistic for dp-ucMGP SNP showed no weak instrument bias as well. However, since this SNP is identified in a small scale candidate gene study, the effect size of the SNP may be overestimated (37). A GWA study on dp-ucMGP should be performed to verify the SNPs used in this MR-study. Circulating phylloquinone and dp-ucMGP are not available in EPIC-CVD, CARDIOGRAMplusC4D or UK Biobank, and therefore we were not able to assess the strength of our instrument in our study population. Furthermore, Mendelian Randomization studies assume that instruments are associated exclusively via the risk factor of interest, and the instrument is not associated with possible confounders. Therefore, we assessed possible pleiotropy using the MR-Egger, and we did not find an indication for pleiotropy in this study. However, our power to detect pleiotropy with a limited number of genetic instruments was low. Additionally, we cannot exclude the possibility of unmeasured pleiotropy for the genetic variants for phylloquinone, since the biological pathways by which the genetic variants influence phylloquinone concentrations is not clear yet. Furthermore, our instrument for dp-ucMGP has also been associated with levels of proteins encoded by other genes in the region (eg, ART) (38), which suggests potential alternative pathways via which any causal effect could be acting.
In conclusion, this study did not provide evidence for a causal association between circulating phylloquinone concentrations and CHD risk, but decreased dp-ucMGP concentrations, representing long-term increased vitamin K intake, may be causally associated with a decreased CHD risk. Whether this association reflects the influence of menaquinones in the association with CHD or MGP as a causal factor leading to CHD needs to be further investigated.
Supplementary Material
Acknowledgements
We thank all EPIC participants and staff for their contribution to the study. We thank staff from the EPIC-CVD and EPIC-InterAct Coordinating Centres for carrying out sample preparation and data-handling work, particularly Sarah Spackman (EPIC-CVD Data Manager) and Nicola Kerrison (EPIC-InterAct Data Manager). EPIC-CVD has been supported by the European Union Framework 7 (HEALTH-F2-2012-279233), the European Research Council (268834), the UK Medical Research Council (G0800270 and MR/L003120/1), the British Heart Foundation (SP/09/002 and RG/08/014 and RG13/13/30194), and the UK National Institute of Health Research. The establishment of the random subcohort was supported by the EU Sixth Framework Programme (FP6) (grant LSHM_CT_2006_037197 to the InterAct project) and the Medical Research Council Epidemiology Unit (grants MC_UU_12015/1 and MC_UU_12015/5). The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The funders played no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
Sources of support
SRZ and JWJB are supported by the Senior Dr. Dekker grant (2013T120) from The Dutch Heart Foundation. IS is supported by a personal Dr. Dekker Junior Postdoctoral grant (2015T19) from The Dutch Heart Foundation. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Abbreviations
- dp-ucMGP
dephosphorylated uncarboxylated Matrix Gla Protein
- CHD
coronary heart disease
- GRS
genetic risk score
- IVW
inverse variance weighted
Footnotes
Conflict of interest: SRZ and JWJB are supported by the Senior Dr. Dekker grant (2013T120) from The Dutch Heart Foundation. IS is supported by a personal Dr. Dekker Junior Postdoctoral grant (2015T19) from The Dutch Heart Foundation. The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’s contributions: SZ, JB, IS, YvdS and SB designed this research, SZ, JB, IS, YvdS and SB performed statistical analysis, SZ, JB, YvdS and SB wrote the paper, JB, EW, IS, YS, SB reviewed the manuscript. SZ, JB, YvdS and SB had primary responsibility for final content.
References
- 1.Beulens JWJ, Booth SL, van den Heuvel EGHM, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr. 2013;110:1357–68. doi: 10.1017/S0007114513001013. [DOI] [PubMed] [Google Scholar]
- 2.Booth SL, Tucker KL, McKeown NM, Davidson KW, Dallal GE, Sadowski JA. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J Nutr. 1997;127:587–92. doi: 10.1093/jn/127.4.587. [DOI] [PubMed] [Google Scholar]
- 3.Lamon-Fava S, Sadowski JA, Davidson KW, O’Brien ME, McNamara JR, Schaefer EJ. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am J Clin Nutr. 1998;67:1226–31. doi: 10.1093/ajcn/67.6.1226. [DOI] [PubMed] [Google Scholar]
- 4.Shea MK, O’Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–807. doi: 10.3945/ajcn.2008.27338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schurgers LJ, Cranenburg ECM, Vermeer C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thrombosis and Haemostasis. 2008:593–603. [PubMed] [Google Scholar]
- 6.Brandenburg VM, Schurgers LJ, Kaesler N, Püsche K, van Gorp RH, Leftheriotis G, Reinartz S, Koos R, Krüger T. Prevention of vasculopathy by vitamin K supplementation: Can we turn fiction into fact? Atherosclerosis. 2015:10–6. doi: 10.1016/j.atherosclerosis.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 7.Kyla Shea M, Booth SL. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients. 2016;8 doi: 10.3390/nu8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shea MK, Booth SL, Weiner DE, Brinkley TE, Kanaya AM, Murphy RA, Simonsick EM, Wassel CL, Vermeer C, Kritchevsky SB. Circulating Vitamin K Is Inversely Associated with Incident Cardiovascular Disease Risk among Those Treated for Hypertension in the Health, Aging, and Body Composition Study (Health ABC) J Nutr. 2017;147:888–95. doi: 10.3945/jn.117.249375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalmeijer GW, Van Der Schouw YT, Booth SL, De Jong PA, Beulens JWJ. Phylloquinone concentrations and the risk of vascular calcification in healthy women. Arterioscler Thromb Vasc Biol. 2014;34:1587–90. doi: 10.1161/ATVBAHA.114.303853. [DOI] [PubMed] [Google Scholar]
- 10.Shea MK, Booth SL, Miller ME, Burke GL, Chen H, Cushman M, Tracy RP, Kritchevsky SB. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: The Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;98:197–208. doi: 10.3945/ajcn.112.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braam LAJLM, Hoeks APG, Brouns F, Halmuyák K, Gerichhausen MJW, Vermeer C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: A follow-up study. Thromb Haemost. 2004;91:373–80. doi: 10.1160/TH03-07-0423. [DOI] [PubMed] [Google Scholar]
- 12.Luo GB, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 13.van Ballegooijen AJ, Beulens JW. The Role of Vitamin K Status in Cardiovascular Health: Evidence from Observational and Clinical Studies. Curr Nutr Rep. 2017;6:197–205. doi: 10.1007/s13668-017-0208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GD, Ebrahim S. Mendelian randomization: Prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 15.Schooling CM. Plasma levels of vitamin K and the risk of ischemic heart disease: a Mendelian randomization study. J Thromb Haemost. 2016;14:1211–5. doi: 10.1111/jth.13332. [DOI] [PubMed] [Google Scholar]
- 16.Liu YP, Gu YM, Thijs L, Knapen MHJ, Salvi E, Citterio L, Petit T, Delli Carpini S, Zhang Z, Jacobs L, et al. Inactive matrix gla protein is causally related to adverse health outcomes: A mendelian randomization study in a flemish population. Hypertension. 2015;65:463–70. doi: 10.1161/HYPERTENSIONAHA.114.04494. [DOI] [PubMed] [Google Scholar]
- 17.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondière UR, Hémon B, Casagrande C, Vignat J, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 18.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, CHopewell J, et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–30. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins R. What makes UK Biobank special? The Lancet. 2012:1173–4. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 20.UK Biobank. Protocol for a large-scale prospective epidemiological resource. 2006. Available from: www.ukbiobank.ac.uk/resources/
- 21.Dashti HS, Shea MK, Smith CE, Tanaka T, Hruby A, Richardson K, Wang TJ, Nalls MA, Guo X, Liu Y, et al. Meta-analysis of genome-wide association studies for circulating phylloquinone concentrations. Am J Clin Nutr. 2014;100:1462–9. doi: 10.3945/ajcn.114.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edson KZ, Prasad B, Unadkat JD, Suhara Y, Okano T, Peter Guengerich F, Rettie AE. Cytochrome P450-dependent catabolism of vitamin K: ω-hydroxylation catalyzed by human CYP4F2 and CYP4F11. Biochemistry. 2013;52:8276–85. doi: 10.1021/bi401208m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 Is a Vitamin K1 Oxidase: An Explanation for Altered Warfarin Dose in Carriers of the V433M Variant. Mol Pharmacol. 2009;75:1337–46. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng HF, Duncan EL, Yerges-Armstrong LM, Eriksson J, Bergström U, Leo PJ, Leslie WD, Goltzman D, Blangero J, Hanley DA, et al. Meta-analysis of genome-wide studies identifies MEF2C SNPs associated with bone mineral density at forearm. J Med Genet. 2013;50:473–8. doi: 10.1136/jmedgenet-2012-101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch M, Schulze J, Hansen U, Ashwodt T, Keene DR, Brunken WJ, Burgeson RE, Bruckner P, Bruckner-Tuderman L. A novel marker of tissue junctions, collagen XXII. J Biol Chem. 2004;279:22514–21. doi: 10.1074/jbc.M400536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brait M, Ling S, Nagpal JK, Chang X, Park HL, Lee J, Okamura J, Yamashita K, Sidransky D, Kim MS. Cysteine dioxygenase 1 is a tumor suppressor gene silenced by promoter methylation in multiple human cancers. PLoS One. 2012;7:1–19. doi: 10.1371/journal.pone.0044951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, Junkins H, McMahon A, Milano A, Morales J, et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45:D896–901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, Paul DS, Freitag D, Burgess S, Danesh J, et al. PhenoScanner: A database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–9. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess S, Thompson SG. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251–60. doi: 10.1093/aje/kwu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danesh J, Saracci R, Berglund G, Feskens E, Overvad K, Panico S, Thompson S, Fournier A, Clavel-Chapelon F, Canonico M, et al. EPIC-Heart: The cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. Eur J Epidemiol. 2007;22:129–41. doi: 10.1007/s10654-006-9096-8. [DOI] [PubMed] [Google Scholar]
- 31.Preuss M, König IR, Thompson JR, Erdmann J, Absher D, Assimes TL, Blankenberg S, Boerwinkle E, Chen L, Cupples LA, et al. Design of the coronary ARtery disease genome-wide replication and meta-Analysis (CARDIoGRAM) study: A genome-wide association meta-analysis involving more than 22 000 cases and 60 000 controls. Circ Cardiovasc Genet. 2010;3:475–83. doi: 10.1161/CIRCGENETICS.109.899443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess S, Thompson SG. Mendelian randomization : methods for using genetic variants in causal estimation. 2015:142. [Google Scholar]
- 33.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–1739. doi: 10.1093/ije/dyx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45 [Google Scholar]
- 35.Beulens JWJ, Booth SL, van den Heuvel EGHM, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr. 2013;110:1357–68. doi: 10.1017/S0007114513001013. [DOI] [PubMed] [Google Scholar]
- 36.Cancela L, Hsieh CL, Francke U, Price PA. Molecular structure, chromosome assignment, and promoter organization of the human matrix Gla protein gene. J Biol Chem. 1990;265:15040–8. [PubMed] [Google Scholar]
- 37.Iles MM. Obtaining unbiased estimates of tagging SNP performance. Ann Hum Genet. 2006;70:254–61. doi: 10.1111/j.1529-8817.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 38.Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, Burgess S, Jiang T, Paige E, Surendran P, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558:73–9. doi: 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.