Abstract
Hypothyroidism and hyperthyroidism are observationally associated with sex hormone concentrations and sexual dysfunction, but causality is unclear. We investigated whether TSH, fT4, hypo- and hyperthyroidism are causally associated with sex hormones and sexual function. We used publicly available summary statistics from genome-wide association studies on TSH and fT4 and hypo- and hyperthyroidism from the ThyroidOmics Consortium (N≤54,288). Outcomes from UK Biobank (women≤194,174/men≤167,020) and ReproGen (women≤252,514) were sex hormones (sex hormone binding globulin [SHBG], testosterone, estradiol, free androgen index [FAI]) and sexual function (ovulatory function in women: duration of menstrual period, age at menarche and menopause, reproductive lifespan, and erectile dysfunction in men). We performed two-sample Mendelian randomization (MR) analyses on summary level, and unweighted genetic risk score (GRS) analysis on individual level data. One SD increase in TSH was associated with a 1.332 nmol/L lower (95% CI: -0.717,-1.946;p=2x10-5) SHBG and a 0.103 nmol/l lower (-0.051,-0.154;p=9x10-5) testosterone in two-sample MR, supported by the GRS approach. Genetic predisposition to hypothyroidism was associated with decreased and genetic predisposition to hyperthyroidism with increased SHBG and testosterone in both approaches. The GRS for fT4 was associated with increased testosterone and estradiol in women only. The GRS for TSH and hypothyroidism were associated with increased and the GRS for hyperthyroidism with decreased FAI in men only. While genetically predicted thyroid function was associated with sex hormones, we found no association with sexual function.
Keywords: Mendelian randomization, thyroid function, SHBG, testosterone, reproductive lifespan, erectile dysfunction
Introduction
Normal thyroid function is important for normal sexual function. Thyroid hormones have direct effects on ovaries, testis and corpora cavernosa via specific nuclear receptors that regulate development and metabolism of these tissues (1–3). Additionally, thyroid hormones regulate bioavailability of sex hormones by increasing the concentrations of hepatic nuclear factor 4α, which in turn increases sex hormone binding globulin (SHBG) transcription and consequently SHBG concentrations (4). Since SHBG binds testosterone with higher affinity than estradiol, thyroid dysfunction may cause an imbalance in concentrations of bioavailable sex hormones and lead to ovulatory and erectile dysfunction. Inversely, estrogen therapy is known to increase thyroxin binding globulin concentrations (5), and consequently increase the need for thyroid hormone in hypothyroid women on estrogen therapy (6). Finally, an indirect effect on the gonads via an interaction between the hypothalamic-pituitary-thyroid axis and the hypothalamic-pituitary gonadal axis through prolactin has also been suggested (7).
Epidemiologically, both hypothyroidism and hyperthyroidism are associated with changes in concentrations of sex hormones (SHBG, testosterone and estradiol) in both sexes, ovulatory function (menstrual irregularities, menarche and menopause) in women, and erectile dysfunction in men (7–12).
Replacement therapy with thyroid hormones in patients with hypothyroidism causes SHBG, free testosterone, and prolactin to return to normal concentrations (3, 9), and sexual dysfunction to resolve (7, 11, 12). Taken together, this evidence supports the hypothesis that thyroid hormones may be causally associated with sex hormone concentrations and sexual function in the general population. However, this can only be determined by randomized controlled trials (RCTs). Since it is not feasible to perform RCTs in the general population (including euthyroid individuals), the Mendelian randomization (MR) approach can serve as a proxy to investigate if the observed associations between thyroid function and sex hormones and sexual function are causal.
The aim of this study was to examine whether thyrotropin (thyroid stimulating hormone [TSH]) and thyroxine (free tetraiodothyronine [fT4]) concentrations, hypothyroidism and hyperthyroidism are causally associated with sex hormone concentrations (SHBG, total testosterone, total estradiol, free androgen index [FAI]), ovulatory function in women (duration of menstrual cycle, age at menarche and menopause and reproductive lifespan) and erectile dysfunction in men. For this purpose, we employed the two-sample MR approach using publicly available summary level data from published genome wide association studies (GWAS) including the ThyroidOmics Consortium, UK Biobank and ReproGen. Additionally, we verified our findings using a genetic risk score (GRS) approach on individual level data in the UK Biobank.
Methods
In MR studies, randomization is by genetic variants, whereas it is by treatment and placebo in randomized controlled trials. According to Mendel’s first and second laws of inheritance, genetic variants are established at conception and inherited independently of potential confounders, thus circumventing reverse causation and confounding, and allowing causal inference (13).
For this study, we used genetic variants associated with lifelong thyroid function to examine the potential causal effect of these genetic variants on sex hormone concentrations and sexual function in population-based studies. As the effects of these genetic variants on thyroid function in pregnant women are unknown, we refrained from examining pregnancy related outcomes.
For detailed description of the studies included, see Supplemental Methods. Briefly, we identified 60 SNPs and one indel (in 41 loci) associated with TSH, 31 SNPs (in 21 loci) associated with fT4, and 8 SNPs (in 8 loci) associated with (subclinical) hypo- and hyperthyroidism each (14). In an additional GWAS from 23andMe, we included data on overt hypothyroidism (15). Thyroid function was reflected by TSH and fT4 concentrations as exposures on a continuous scale and hypo-and hyperthyroidism as binary exposures. For binary exposures, we could only test the causal null hypothesis, because the causal estimates do not have a clear interpretation (16).
Outcomes were sex hormones (SHBG, total testosterone, total estradiol and FAI) and sexual function, defined as ovulatory function in women (duration of menstrual period, age at menarche and menopause, and reproductive lifespan) and erectile dysfunction in men. We extracted summary statistics for sex-stratified and sex-combined, as well as for crude and standardized (rank-based inverse normal transformed ["irn"]) continuous outcomes when available.
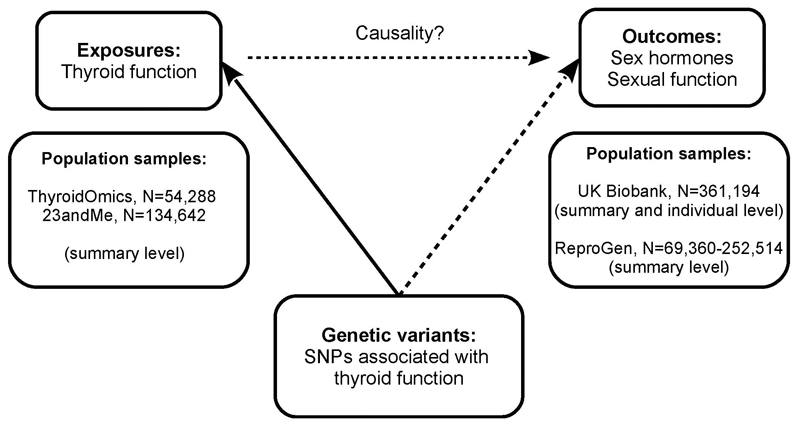
We used the UK Biobank to perform two-sample MR analyses on summary statistics data and GRS for thyroid function on individual level data, as thyroid function testing was not performed in the UK Biobank (Figure 1). The association with age at menarche and menopause were replicated by two-sample MR analyses in ReproGen (N=69,360-252,514 women).
Figure 1. Schematic diagram illustrating the study design.
Thyroid function was TSH and fT4 within reference range, and subclinical hypo- and hyperthyroidism from ThyroidOmics meta-genome-wide association study (GWAS) and overt hypothyroidism from 23andMe GWAS.
Within the UK Biobank, we used summary level as well as individual level data. Summary level data were from the rapid UK Biobank genome-wide association study provided by the Neale lab at: http://www.nealelab.is/uk-biobank.
Summary level data from ReproGen consortium were extracted from: https://www.reprogen.org/data_download.html.
SNP: single nucleotide polymorphism.
Statistical analysis
Analyses were performed using Stata 13.1 (StataCorp, Texas, USA, www.stata.com) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria, www.R-project.org). The effect allele was defined as the allele associated with increased level of TSH and fT4 levels, and increased risk of hypo- or hyperthyroidism (exposure). Thus, all β-coefficients for the SNP-exposure associations were positive. We then aligned the β-coefficients for the SNP-outcome association to the effect allele of the SNP-exposure association (data harmonization). Thus, the causal effect is estimated as change in outcome per one unit change of the respective exposure.
Strength of the genetic instruments was calculated as F= β2exposure/SE2exposure.
We performed sex-combined and sex-stratified analyses by using two-sample MR approach on summary level data, as well as GRS approach on individual level data.
We considered p<0.005 (0.05 divided by 9 outcomes: SHBG, testosterone, estradiol, FAI, duration of menstrual cycle, age at menarche, age at menopause, reproductive lifespan, erectile dysfunction) as the significance level threshold. However, as p-value mixes the estimated effect size with its’ estimated precision, and considering the large number of statistical test performed, we evaluated each result with a p<0.005 individually by considering the effect size (β), standard error, biological plausibility and removal of outlier SNPs (see MR-PRESSO below).
Two-sample MR
Two-sample MR analyses with summary data were performed by the freely available mrrobust Stata package (17). The main analysis was the inverse variance weighting (IVW) method, which requires that all SNPs are valid instrumental variables. The overall causal estimate was determined as inverse variance weighted (multiplicative) random effects meta-analysis (IVW-RE) applied across the individual causal estimates (Wald ratio estimates of the SNP-outcome and SNP-exposure association) and their standard errors (18). The IVW-RE takes into account the heterogeneity between the individual variants’ causal (ratio) estimates and provides the combined mean effect (19). We assessed the heterogeneity between the individual causal estimates with the I2 statistics, ranging from 0% to 100%, where high pleiotropy is a sign that one or more SNPs are likely to be pleiotropic (20–22). I2 is calculated as [100% x (Q-df)/Q], where Q is the Cochran’s Q statistics and df is degrees of freedom. While the Cochran’s Q statistics is sensitive to the number of variants included (calculated as the difference between the variants’ effect sizes and the combined effect size, which is then squared, weighted and summed), the I2 is not.
Sensitivity MR analyses included: weighted median, MR Egger (simulation extrapolation [SIMEX] adjusted) and MR-PRESSO (Mendelian Randomization Pleiotropy RESidual Sum and Outlier) regression analyses. The weighted median provides reliable estimates if at least 50% of the weight comes from valid genetic variants (23). MR Egger provides pleiotropy robust estimates in cases where even fewer than 50% of the genetic variants are valid (24). However, the trade-off is the large confidence intervals. Because MR Egger assumes no measurement error (NOME), the results can be biased when this assumption is violated. We quantified the NOME violation by I2GX (range: 0%-100%, decreases with increasing NOME violation), an adaptation of the I2 statistics from the field of meta-analysis (24). Furthermore, we adjusted for potential violations by using the bootstrapped SIMEX method (24). Finally, we examined horizontal pleiotropy by MR-PRESSO by MRPRESSO package in R (25). MR-PRESSO detects horizontal pleiotropy (by MR-PRESSO global test), corrects for horizontal pleiotropy via outlier removal, and tests whether there is a significant distortion in the causal estimates before and after outlier removal (by MR-PRESSO bias test). Outlier removal results in narrower confidence intervals.
Genetic risk score
To verify the findings from the two-sample MR approach based on summary statistics from an automated GWAS on numerous (>4,000) outcomes, we employed the GRS approach, a fixed-effect analysis, on individual level data. We generated an unweighted GRS using the different sets of genetic variants as described below. Thus, although the estimate sizes between the two-sample MR and the unweighted GRS approach are not directly comparable, the directions of the associations are. The GRS was standardized using the "scale" function in R, and the alleles were aligned to the increasing allele for each thyroid trait. Next, for these GRS, we performed linear regression for continuous (SHBG, testosterone, estradiol, FAI, duration of menstrual cycle, age at menarche and menopause, and reproductive lifespan) and logistic regression for erectile dysfunction, the only binary outcome. The analyses were adjusted for age, sex (for sex-combined only), 40 principal components, batch, BMI, and smoking, as all can affect thyroid hormone concentrations. Importantly, when we excluded BMI and smoking from the model, the results remained similar.
Stratification of TSH and fT4 variants
Autoimmune diseases in general have been associated with sex hormone concentrations (26). Therefore, we stratified TSH SNPs based on the presence or lack of their association with autoimmune thyroid disease (AITD) to investigate whether observed associations were caused directly by thyroid function or indirectly by autoimmunity, independent of thyroid hormone concentrations (Supplemental Table 1). AITD SNPs were defined as those associated with thyroperoxidase antibodies (TPOAb) (27), Hashimoto’s thyroiditis (28) and Graves’ disease (14).
Furthermore, because local tissue availability of thyroid hormones may be different from systemic plasma concentrations, we stratified fT4 SNPs based on their location within or outside DIO1 and DIO2 genes that regulate the bioavailability of active thyroid hormones. The stratifications should also decrease the heterogeneity within the sets of genetic variants.
Sets of genetic variants used in this study
We included a total of ten different sets of genetic variants: three for TSH (all variants and variants stratified by presence/absence of association with AITD), three for fT4 (all variants and variants stratified by their location within or outside DIO1 and DIO2 genes), two for hypothyroidism (subclinical from ThyroidOmics and overt from 23andMe GWAS) and two for hyperthyroidism (all variants and variants excluding FOXE1 rs925488, a pleiotropic SNP with questionable directionality, as the effect allele was associated (directly or indirectly through LD) with increased risk of both hypo- and hyperthyroidism) (Supplemental Figure 1).
Results
Summary measures for SHBG, total testosterone, total estradiol, FAI, duration of menstrual cycle, age at menarche and menopause, reproductive lifespan, erectile dysfunction, and GRS are shown in Supplemental Table 3.
TSH and sex hormones
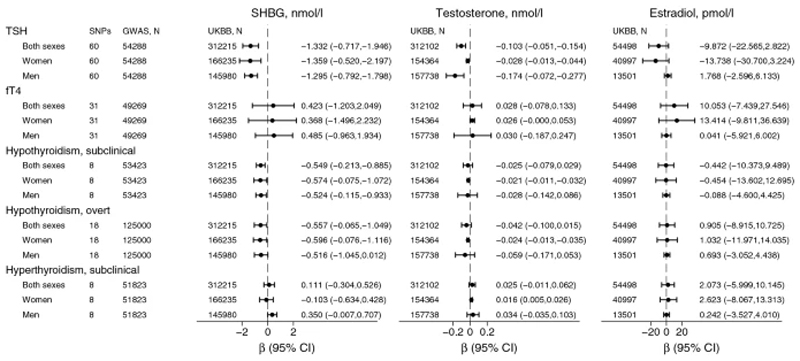
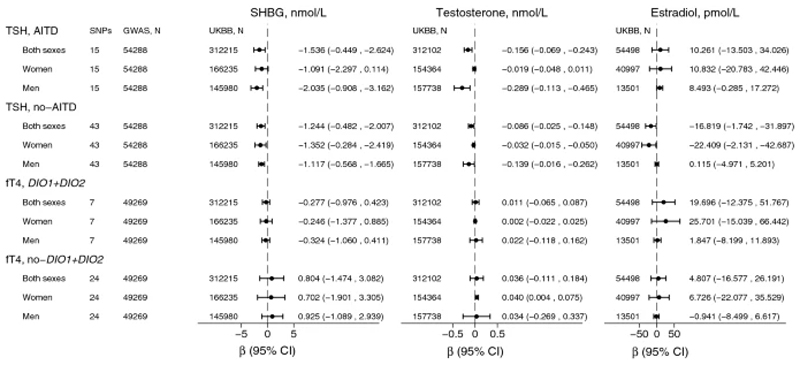
One SD increase in genetically predicted TSH was associated with 1.332 nmol/L lower SHBG (β=-1.332, 95% CI:-0.717,-1.946; p=2x10-5) and 0.103 nmol/L lower (β=-0.103, 95% CI:-0.051,-0.154; p=9x10-5) total testosterone concentrations (Figure 2, Supplemental Figure 2, Supplemental Table 4).The results were similar in sex-stratified analyses for raw and standardized SHBG, and for standardized testosterone (Supplemental Table 4, Figure 2, Supplemental Figure 2). However, the association with raw testosterone was substantially stronger in men than in women (Supplemental Table 4, Figure 3 and Supplemental Figure 2). The estimates were robust to inclusion of only no-AITD SNPs (Supplemental Tables 5-6, Figure 3, Supplemental Figure 2) and MR sensitivity analyses (Supplemental Tables 5-6).
Figure 2. Inverse variance weighted (IVW) random effects causal estimates for thyroid function on sex hormones.
The estimates were based on summary level data on 317,694 participants (170,101 women and 147,593 men) from the UK Biobank (UKBB).
SNP: single nucleotide polymorphism.
GWAS: genome-wide association study, refers to the latest ThyroidOmics GWAS for TSH (thyroid stimulating hormone) and fT4 (free tetraiodothyronine) and subclinical hypo- and hyperthyroidism, and additionally 23andMe GWAS for overt hypothyroidism.
SHBG: sex hormone binding globulin. Testosterone and estradiol refer to total, and not free concentrations.
CI: confidence intervals.
Figure 3. Mendelian randomization (MR) estimates for genetically increased TSH and fT4 concentrations on sex hormones.
Estimates are from inverse-variance weighted (IVW) random-effects method.
Analyses for TSH were stratified for association with autoimmune thyroid disease (AITD). Prior to stratification, SNPs in ABO and BCAS3 genes were excluded due to pleiotropy and low tissue specificity (Supplemental Figure 1). Analyses for fT4 were stratified by DIO1 and DIO2 genes.
SNP: single nucleotide polymorphism.
GWAS: genome-wide association study, refers to the latest ThyroidOmics GWAS.
SHBG: sex hormone binding globulin. Testosterone and estradiol refer to total and not free concentrations.
UKBB=UK Biobank.
CI: confidence intervals.
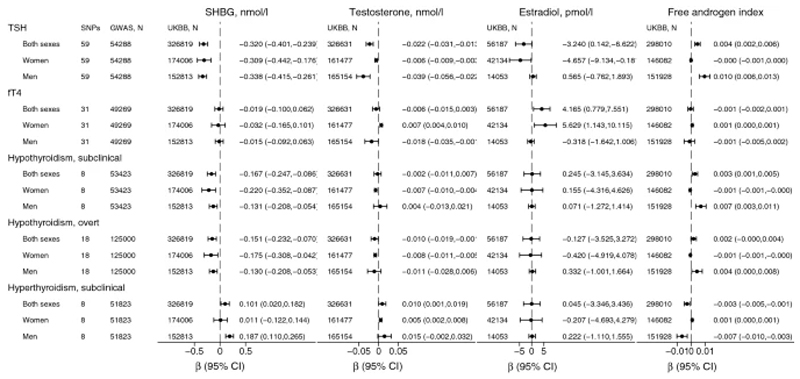
Overall, these findings were supported by the GRS approach (Supplemental Table 7, Figure 4). However, while the two-sample MR showed little evidence for association with estradiol in either sex, the GRS for TSH was associated with decreased estradiol in women, but not in men (Supplemental Table 7, Figure 4). Furthermore, the GRS for TSH was associated with increased FAI in men, but not in women (Supplemental Table 7, Figure 4).
Figure 4. Genetic risk score estimates for thyroid function on sex hormones.
Genetic risk score was based on individual level data on concentrations of SHBG in 326,819 participants (174,006 women and 152,813 men), testosterone in 326,631 (161,477 women and 165,154 men), estradiol in 56,187 (42,134 women and 14,053 men) and FAI in 298,010 participants (146,082 women and 151,928 men) from the UK Biobank (UKBB).
GWAS: genome-wide association study, refers to the latest ThyroidOmics GWAS for TSH (thyroid stimulating hormone) and fT4 (free tetraiodothyronine) and subclinical hypo- and hyperthyroidism, and additionally 23andMe GWAS for overt hypothyroidism.
SHBG: sex hormone binding globulin. Testosterone and estradiol refer to total, and not free concentrations. Free androgen index (FAI)=total testosterone/SHBG.
CI: confidence intervals.
fT4 and sex hormones
One SD increase in genetically predicted fT4 was not associated with concentrations of SHBG, testosterone or estradiol in any MR analyses (Figures 2 and 3, Supplemental Figures 2 and 3, Supplemental Tables 8-10).
GRS for fT4 (N=31) was not associated with SHBG, but was nominally associated with increased testosterone, estradiol and FAI, all in women only (and not in men, Supplemental Table 13, Figure 4). Stratification by fT4 DIO1 and DIO2 variants, diluted the association with testosterone for DIO1 and DIO2 variants (N=7), and estradiol for fT4 excluding DIO1 and DIO2 variants (N=24, Supplemental Table 11).
Hypothyroidism and sex hormones
Genetic predisposition to hypothyroidism was associated with decreased SHBG and total testosterone concentrations (p=0.001 for SHBG, p=0.361 for raw testosterone, and p=0.015 for standardized testosterone) in sex-combined, and similar in sex-stratified analyses (Supplemental Table 12, Figure 2, Supplemental Figure 2). However, for testosterone, the association was more evident in women than in men. In men, the results were inconsistent as weighted median and MR-Egger showed a possible causal association, but this was not supported by the main analysis (IVW-RE) or MR-PRESSO (Supplemental Table 12). Furthermore, the sensitivity analysis using SNPs associated with overt hypothyroidism showed no associations with testosterone concentrations in men (Supplemental Table 13).
Overall, these findings were supported by the GRS approach (Figure 4, Supplemental Table 14). Furthermore, the GRS for hypothyroidism was associated with decreased FAI in women and increased FAI in men (Supplemental Table 14, Figure 4).
Hyperthyroidism and sex hormones
Genetic predisposition to hyperthyroidism was associated with increased SHBG concentrations in men only, and particularly when the pleiotropic FOXE1 rs925488 was excluded from the analyses (Supplemental Tables 15-16, Figure 2). In addition, genetic predisposition to hyperthyroidism was associated with increased testosterone in women only (Supplemental Tables 15-16, Figure 2), and supported by the sensitivity analyses (Supplemental Tables 15-16).
Overall, these findings were supported by the GRS approach (Supplemental Table 17, Figure 4). However, while the two-sample MR showed no association with testosterone in men, the GRS (excluding FOXE1 rs925488) for subclinical hyperthyroidism was associated with increased testosterone in men (Supplemental Table 17). Furthermore, the GRS for subclinical hyperthyroidism was associated with decreased FAI in men (and possibly barely increased FAI in women, Supplemental Table 17, Figure 4).
Thyroid function and sexual function
Overall, genetically predicted thyroid function was not robustly associated with sexual function in neither the UK Biobank, nor ReproGen (Supplemental Tables 4-16).
However, TSH SNPs associated with AITD (N=15) were associated with decreased age at menopause in the IVW-RE analysis (β=-0.395, 95% CI: -0.650,-0.139, p=0.003) and the GRS approach (p=0.001), but not in other sensitivity analyses (Supplemental Tables 5 and 7).
The GRS for fT4 (all 31 variants) was associated with increased age at menopause (p=3x10-6). Stratification analyses abrogated the association for DIO1 and DIO2 variants (p=0.705, N=7), and augmented the association for all other fT4 excluding DIO1 and DIO2 variants (p=2x10-7, N=24, Supplemental Table 11).
Likewise, only the GRS for fT4 excluding DIO1 and DIO2 variants (N=24) was associated with increased reproductive lifespan (p=0.002, Supplemental Table 11). These associations were driven by rs4954192, rs55679545 and rs12907106 (Supplemental Table 10), and excluding these SNPs (from all fT4, as well as no-DIO1 and DIO2 fT4 variants) resulted in no apparent association with age at menopause or reproductive lifespan in both approaches (data not shown).
Discussion
This is the first study using genetic variation to investigate the causality between thyroid function and sex hormone concentrations and sexual function. We showed that genetically predicted thyroid function was associated with sex hormone concentrations, but not with sexual function.
Genetically increased TSH and genetic predisposition to hypothyroidism were associated with decreased concentrations of SHBG in both sexes. Other associations were sex-specific: genetic predisposition to hyperthyroidism was associated with increased SHBG in men and increased testosterone in women, and genetic predisposition to hypothyroidism was associated with decreased testosterone in women. While these findings were directionally consistent, the findings for fT4 differed between the two approaches: genetically predicted fT4 was not associated with sex hormones in the two-sample MR approach, but was associated with increased concentrations of testosterone and estradiol (in women only) in the GRS approach.
Our findings are in line with the epidemiological association between thyroid hormones and SHBG concentrations. Mechanistically, increased thyroid hormone concentrations increase hepatic metabolism and hepatocyte nuclear factor-4α concentrations (4). This stimulates the SHBG promoter to increase SHBG transcription, and consequently results in increased SHBG concentrations (4). SHBG inhibits testosterone and estradiol by binding to them and thereby decreasing their bioavailability. When fT4 increases (and TSH correspondingly decreases), SHBG concentration increases. Consequently, further down the pathway, the total testosterone and estradiol concentrations increase in order to maintain an appropriate bioavailability of these hormones. Thus, due to vertical pleiotropy, genetic variants associated with SHBG, should also be associated with testosterone and estradiol concentrations. Since testosterone binds to SHBG with stronger affinity than estradiol, this might explain why genetically predicted TSH was associated with testosterone in both sexes and both approaches, but only associated with estradiol in women in the GRS approach. This may illustrate the vertical pleiotropy, i.e. the observational association between thyroid function and estradiol could be secondary to the association with SHBG.
FAI is approximately the inverse of SHBG in women, who normally have very low testosterone concentrations. Thus, we could expect that hypothyroidism was associated with increased and hyperthyroidism with decreased FAI in women, i.e. the inverse of the association with SHBG. Surprisingly, the GRS approach showed the exact opposite: hypothyroidism was associated with decreased FAI in women and increased FAI in men, and vice versa for hyperthyroidism. Furthermore, our findings were directionally consistent and in opposite directions between the sexes, as the GRS for TSH was associated with increased FAI in men, and GRS for fT4 was associated with increased FAI in women. However, the usefulness of FAI is debated. FAI can be misleading when SHBG concentrations are low, and is mostly used to examine androgen status in women, but not in men, and in clinical studies (29).
Another surprising finding was the lack of association between genetically predicted fT4 and sex hormones using the two-sample MR approach. In contrast, using the GRS approach, we found that fT4 was associated with increased concentrations of testosterone (driven by no-DIO1 and DIO2 variants, N=24) and estradiol (driven by DIO1 and DIO2 variants, N=7) in women only, suggesting that fT4 SNPs may influence concentrations of testosterone and estradiol through other pathways than SHBG in women.
A possible explanation for the discrepant findings for TSH and fT4 with the two-sample MR approach could be that TSH is a much more sensitive biomarker than fT4 in detecting small changes in the set point of the hypothalamic–pituitary–thyroid axis, as suggested by previous studies (30–32). Additionally, the TSH and fT4 SNPs do not overlap. The fact that the fT4 SNPs have a much weaker association with TSH levels means that they do not play a central role in the hypothalamic–pituitary–thyroid axis, but rather determine fT4 concentrations in other ways, such as by peripheral thyroid hormone metabolism by DIO1, DIO2 and AADAT. Furthermore, the proportion of genetic variance explained by the genetic variants is higher for TSH than fT4 concentrations (9.4% versus 4.8%).
In accordance with this, we observed an association between genetic predisposition to hypothyroidism and decreased SHBG concentrations in both sexes and genetic predisposition to hyperthyroidism and increased SHBG concentrations in men. Interestingly, the associations between genetic predisposition to hypothyroidism and decreased testosterone and between genetic predisposition to hyperthyroidism and increased testosterone concentrations were more evident in women, than in men. This can possibly be explained by women having much higher SHBG and lower testosterone concentrations than men. Another possible explanation could be gene-by-sex interaction, since both hypo- and hyperthyroidism are more prevalent in women than in men. However, while the ThyroidOmics Consortium meta-GWAS investigated and rejected gene-by-sex interaction for TSH and fT4, it did not investigate this for hypo- and hyperthyroidism.
While we found no association between genetically predicted thyroid function and ovulatory function, a previous British case-control study of 178 women reported an association for Hashimoto’s thyroiditis (80% of cases were hypothyroid) with decreased age at menarche, increased age at menopause and increased reproductive lifespan (8). Indeed, the only associations we cannot completely exclude were in the opposite direction, that is, TSH AITD SNPs with decreased age at menopause, and fT4 (no-DIO1 and DIO2) SNPs with increased age at menopause. Furthermore, a Greek case-control study of 144 women found no association between subclinical hypothyroidism and duration of menstrual cycle, age at menopause and reproductive lifespan, but suggested that early menarche may be a risk factor for subclinical hypothyroidism (10).
We found no association between genetically predicted thyroid function and erectile dysfunction in either approach. This is in disagreement with a previous report of an association between (overt) hyperthyroidism and erectile dysfunction (7). However, since we only examined the effect of normal and subclinical variation in thyroid function, our findings do not necessarily extend to more severe phenotypes. Thus, we cannot exclude that (untreated) overt hyperthyroidism is associated with erectile dysfunction. Indeed, proposed mechanisms linking thyroid function to erectile dysfunction include hyperthyroidism-associated anxiety and impairment of the nitric oxide-dependent relaxation of the corpora cavernosa (through thyroid hormone binding to its associated receptors in the genitourinary tract) (1, 12).
One major strength of this study is the employment of two different approaches: the two-sample MR on summary level and the GRS on individual level data, showing largely consistent results. Additionally, we included a sensitivity study for (well-treated) overt hypothyroidism, confirming our findings on subclinical hypothyroidism, as well as a replication study in ReproGen. Furthermore, the proportion of variance explained by the genetic variants for TSH and fT4 concentrations was high, suggesting high validity. Finally, we confirmed the observational association between thyroid function and sex hormones, further validating the genetic instruments used for thyroid function, and TSH in particular.
A potential limitation of our study is the restriction to individuals of European ancestry, meaning that our findings cannot automatically be extended to individuals of other ancestries. On the other hand, this also strengthened our study by decreasing the risk of population stratification. Another potential limitation is the lack of sex-stratified instrument-exposure summary statistics for hypo- and hyperthyroidism, which are more prevalent in women than in men.
In conclusion, using both summary and individual level data from the UK Biobank, we found evidence to support that thyroid function is causally associated with concentrations of sex hormones, but not with sexual function.
Supplementary Material
Acknowledgements
The two-sample MR approach is based on data freely available from the public domain. The authors would like to thank the ThyroidOmics consortium, 23andMe, UK Biobank, Neale lab, and ReproGen for sharing the data and making this project possible.
This research has been conducted using the UK Biobank Resource under Application Number 53723.
Funding
ADK is funded by an unrestricted grant by Novo Nordisk. AK is supported by the Exchange in Endocrinology Expertise (3E) program of the European Union of Medical Specialists (UEMS), Section and Board of Endocrinology. AP is funded by the NIHR Barts Biomedical Research Centre.
Declarations
Conflicts of interest
All the authors declare no conflicts of interest.
Ethics approval and consent: not applicable
Contributor Information
Alisa D. Kjaergaard, Steno Diabetes Center Aarhus, Aarhus University Hospital, Denmark
Eirini Marouli, Email: e.marouli@qmul.ac.uk, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Areti Papadopoulou, Email: areti.papadopoulou@qmul.ac.uk, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK, and National Institute of Health Research Barts Biomedical Research Centre, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Panos Deloukas, Email: p.deloukas@qmul.ac.uk, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK, and Princess Al-Jawhara Al-Brahim Centre of Excellence in Research of Hereditary Disorders (PACER-HD), King Abdulaziz University, Jeddah, Saudi Arabia.
Aleksander Kuś, Email: akus@wum.edu.pl, Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus Medical Center, Rotterdam, The Netherlands, and Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands, and Department of Internal Medicine and Endocrinology, Medical University of Warsaw, Warsaw, Poland.
Rosalie Sterenborg, Email: Rosalie.Sterenborg@radboudumc.nl, Radboud University Medical Center, Radboud Institute for Health Sciences, Department of Internal Medicine, Nijmegen, The Netherlands and Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus Medical Center, Rotterdam, The Netherlands, and Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands.
Alexander Teumer, Email: ateumer@uni-greifswald.de, Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, and DZHK (German Center for Cardiovascular Research), partner site Greifswald, Greifswald, Germany.
Stephen Burgess, Email: sb452@medschl.cam.ac.uk, MRC Biostatistics Unit, University of Cambridge, Cambridge, and Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge.
Bjørn O. Åsvold, Email: bjorn.o.asvold@ntnu.no, K.G. Jebsen Center for Genetic Epidemiology, Department of Public Health and Nursing, NTNU, Norwegian University of Science and Technology, and HUNT Research Center, Department of Public Health and Nursing, NTNU, Norwegian University of Science and Technology, and Department of Endocrinology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
Daniel I. Chasman, Email: DCHASMAN@research.bwh.harvard.edu, Harvard Medical School, Boston, Massachusetts, and Program in Medical and Population Genetics, The Broad Institute of MIT and Harvard, Cambridge, Massachusetts, and Division of Preventive Medicine, Brigham and Women's Hospital, Boston, Massachusetts, USA
Marco Medici, Email: Marco.Medici@radboudumc.nl, Department of Internal Medicine, Radboud University Medical Center, Nijmegen, The Netherlands, and Academic Center for Thyroid Diseases, Department of Internal Medicine, and Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands.
Christina Ellervik, Email: christina.ellervik@childrens.harvard.edu, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, DK-2200, Denmark, and Department of Pathology, Harvard Medical School, Boston, MA-02215, USA.
Data Availability
All data analyzed during this study are either publically available through http://www.nealelab.is/uk-biobank and https://www.reprogen.org/data_download.html, or available through application to the UK Biobank Resource.
Code availability
We used standard statistical software (STATA and R) and packages to perform the analyses.
References
- 1.Carosa E, Lenzi A, Jannini EA. Thyroid hormone receptors and ligands, tissue distribution and sexual behavior. Mol Cell Endocrinol. 2018;467:49–59. doi: 10.1016/j.mce.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 2.La Vignera S, Vita R, Condorelli RA, et al. Impact of thyroid disease on testicular function. Endocrine. 2017;58(3):397–407. doi: 10.1007/s12020-017-1303-8. [DOI] [PubMed] [Google Scholar]
- 3.Silva JF, Ocarino NM, Serakides R. Thyroid hormones and female reproduction. Biol Reprod. 2018;99(5):907–21. doi: 10.1093/biolre/ioy115. [DOI] [PubMed] [Google Scholar]
- 4.Selva DM, Hammond GL. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4alpha. J Mol Endocrinol. 2009;43(1):19–27. doi: 10.1677/JME-09-0025. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Rafael Z, Struass JF, 3rd, Arendash-Durand B, Mastroianni L, Jr, Flickinger GL. Changes in thyroid function tests and sex hormone binding globulin associated with treatment by gonadotropin. Fertil Steril. 1987;48(2):318–20. doi: 10.1016/s0015-0282(16)59363-7. [DOI] [PubMed] [Google Scholar]
- 6.Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344(23):1743–9. doi: 10.1056/NEJM200106073442302. [DOI] [PubMed] [Google Scholar]
- 7.Gabrielson AT, Sartor RA, Hellstrom WJG. The Impact of Thyroid Disease on Sexual Dysfunction in Men and Women. Sex Med Rev. 2019;7(1):57–70. doi: 10.1016/j.sxmr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Phillips DI, Lazarus JH, Butland BK. The influence of pregnancy and reproductive span on the occurrence of autoimmune thyroiditis. Clin Endocrinol (Oxf) 1990;32(3):301–6. doi: 10.1111/j.1365-2265.1990.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 9.Krassas GE, Markou KB. The impact of thyroid diseases starting from birth on reproductive function. Hormones (Athens) 2019;18(4):365–81. doi: 10.1007/s42000-019-00156-y. [DOI] [PubMed] [Google Scholar]
- 10.Kotopouli M, Stratigou T, Antonakos G, Christodoulatos GS, Karampela I, Dalamaga M. Early menarche is independently associated with subclinical hypothyroidism: a cross-sectional study. Horm Mol Biol Clin Investig. 2019;38(1) doi: 10.1515/hmbci-2018-0079. [DOI] [PubMed] [Google Scholar]
- 11.Dittrich R, Beckmann MW, Oppelt PG, et al. Thyroid hormone receptors and reproduction. J Reprod Immunol. 2011;90(1):58–66. doi: 10.1016/j.jri.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Bates JN, Kohn TP, Pastuszak AW. Effect of Thyroid Hormone Derangements on Sexual Function in Men and Women. Sex Med Rev. 2020;8(2):217–30. doi: 10.1016/j.sxmr.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 14.Teumer A, Chaker L, Groeneweg S, et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun. 2018;9(1):4455. doi: 10.1038/s41467-018-06356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nature genetics. 2016;48(7):709–17. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33(10):947–52. doi: 10.1007/s10654-018-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiller W, Davies NM, Palmer TM. Software application profile: mrrobust—a tool for performing two-sample summary Mendelian randomization analyses. International Journal of Epidemiology. 2018;48(3):684–90. doi: 10.1093/ije/dyy195. [DOI] [Google Scholar]
- 18.Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45(6):1717–26. doi: 10.1093/ije/dyx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783–802. doi: 10.1002/sim.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–r208. doi: 10.1093/hmg/ddy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40. doi: 10.1002/sim.6522. [DOI] [PubMed] [Google Scholar]
- 23.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304–14. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–74. doi: 10.1093/ije/dyw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018;9:2279. doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medici M, Porcu E, Pistis G, et al. Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease. PLOS Genetics. 2014;10(2):e1004123. doi: 10.1371/journal.pgen.1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brcic L, Baric A, Gracan S, et al. Genome-wide association analysis suggests novel loci for Hashimoto's thyroiditis. J Endocrinol Invest. 2019;42(5):567–76. doi: 10.1007/s40618-018-0955-4. [DOI] [PubMed] [Google Scholar]
- 29.Keevil BG, Adaway J. Assessment of free testosterone concentration. J Steroid Biochem Mol Biol. 2019;190:207–11. doi: 10.1016/j.jsbmb.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160(6):985–91. doi: 10.1530/EJE-08-0953. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–54. doi: 10.1016/s0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Shan Z, Li C, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. 2014;24(11):1642–9. doi: 10.1089/thy.2014.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are either publically available through http://www.nealelab.is/uk-biobank and https://www.reprogen.org/data_download.html, or available through application to the UK Biobank Resource.
We used standard statistical software (STATA and R) and packages to perform the analyses.