Understanding lymphocyte activation will require thorough characterization of the ‘resting’ state, and how it changes. Surfaces coated with the cationic homopolymer poly L-lysine (PLL) are widely used for total internal reflection fluorescence (TIRF) imaging of the resting organization of lymphocyte surface proteins (1–5), because PLL is assumed to be inert. Here we show that PLL initiates T-cell signaling and profoundly alters the behavior of membrane proteins such as the T-cell receptor (TCR). The emerging notion that receptors and signaling proteins cluster by default (1–5), based mostly upon studies of lymphocytes interacting with PLL-coated surfaces, therefore needs reconsideration.
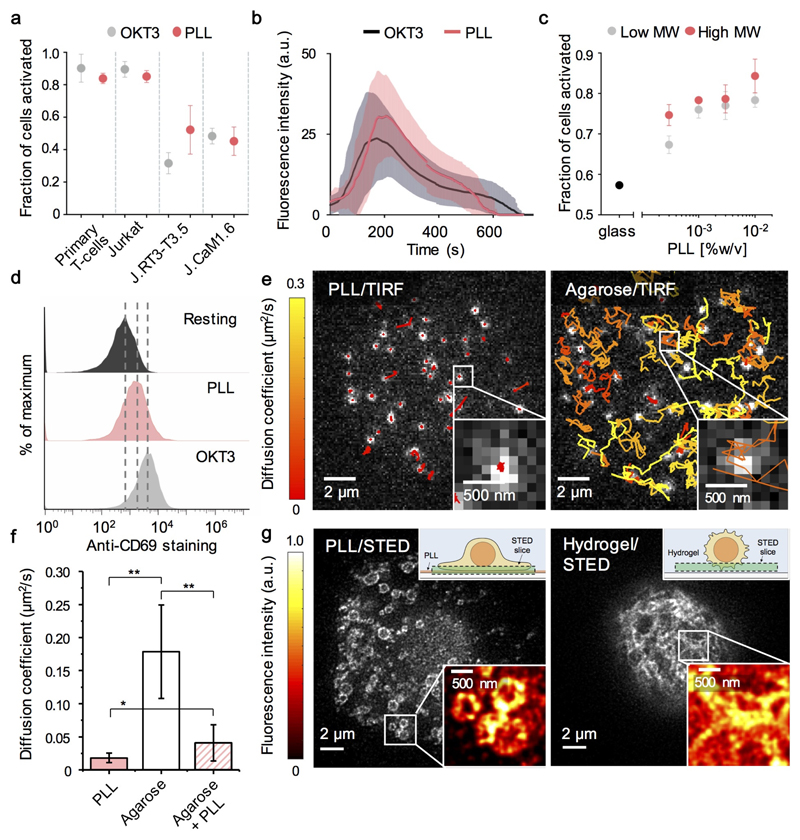
We examined whether PLL-coated surfaces are actually inert by testing their capacity to induce calcium signaling in primary CD4+ and Jurkat T-cells. Cells were labelled with Fluo-4AM and transferred to PLL- or OKT3- (anti-CD3 antibody) coated coverslips. Signaling occurred in ~80% of primary and Jurkat T-cells after 30s on PLL (total N=247 cells in n=3 experiments; Fig. 1a), and in 90% of cells after 30s on OKT3 (N=631, n=3). Similar results were obtained for Jurkats with the ratiometric reporter, Fura-2 (N=270, n=3; Supplementary Fig. 1a). The dynamics of Jurkat responses to each surface were also comparable (Fig. 1b). Signaling was largely insensitive to PLL coating-concentration, coating time and polymer size (Fig. 1c; Supplementary Fig. 1b). J.CaM1.6 and J.RT3-T3.5 cells exhibited diminished responses (Fig. 1a) that were restorable with Lck and TCRβ re-expression, respectively (Supplementary Fig. 1c), implying that PLL induces bona fide TCR signaling. PLL induced TCR-proximal signaling detected as ZAP70 phosphorylation, albeit weakly (Supplementary Fig. 1d), and TCR-distal CD69 upregulation (Fig. 1d). Although they were somewhat more refractory, calcium signaling was induced by PLL in murine primary CD4+ (C57BL/6) T-cells, and two of three T-cell hybridomas tested (e.g. Yae5b3k; Supplementary Fig. 1e). PLL also induced B-cell receptor-dependent signaling in a B-cell line (A20; Supplementary Fig. 1f).
Figure 1. PLL induces strong T-cell signaling and profoundly alters TCR diffusional behavior and organization.
(a) Fraction of primary (CD4+) and wild-type and mutant Jurkat T-cells eliciting calcium responses (detected with Fluo-4AM) upon contact with OKT3- (grey)- and PLL- (red) coated surfaces. Mean ± s.d. are shown for three experiments. (b) Fluo-4AM fluorescence intensity changes versus contact time for Jurkat T-cells on OKT3- (grey) or PLL- (red) coated surfaces (mean, solid lines; s.d., shaded area) were calculated for N=100 randomly chosen cells per condition. (c) Fraction of responding Jurkat T-cells as PLL coating-concentration is varied, for high (red, i.e. 150 kDa) and low (grey, i.e. 70 kDa) molecular weight (MW) PLL. Mean ± s.d. are shown for three experiments. (d) Surface expression of CD69 on cultured Jurkat T-cells (black), and after overnight incubation on PLL- (red) or OKT3- (grey) coated surfaces. Data are representative of three experiments. (e) Single-molecule, TIRF-based tracking of fluorescent Fab-labelled TCRs at basal surfaces of Jurkat T-cells contacting PLL- (left) or agarose- (right) coated surfaces. Tracks are coloured by diffusion coefficient (MSD analysis; colour bar). (f) TCR diffusion coefficients from single-molecule tracking for each cell calculated from ensemble average MSD curves; means ± s.d. for cell-to-cell variation are shown; *, p<0.001; **, p<1x10-10. (g) STED images of TMR-Halo labelled TCRs at basal surfaces of Jurkat T-cells contacting a PLL-coated glass surface (left) or suspended in hydrogel (right). Insets: diagrams of the experimental setup (upper right corners), and fluorescence intensity variation in the marked-up areas (lower right corners).
Jurkats formed much larger contacts with PLL- versus OKT3-coated or uncoated glass (Movies S1-S3; Supplementary Fig. 1g). To test whether PLL perturbs receptor behavior, we used single-molecule tracking of fluorescently-labelled TCRs. On PLL-coated coverslips, TCR mobility was immediately reduced (diffusion coefficient D=0.018±0.01 μm2/s, versus D=0.06 μm2/s measured at the apical surface of a murine T-cell hybridoma (6); Fig. 1e left; Movie S4; Fig. 1f). To determine whether this required hard surfaces, we spin-coated agarose onto coverslips, producing a softer surface still suitable for TIRF imaging. Under these conditions, the TCR exhibited high mobility (D=0.18±0.07μm2/s; Fig. 1e right; Movie S4; Fig. 1f), which was reduced to near static levels when PLL was added to the surface (D=0.04±0.03 μm2/s; Movie S4; Fig. 1f). PLL therefore directly perturbed TCR dynamics, presumably via electrostatic polymer/receptor interactions.
But how might PLL initiate signaling, and how could such effects be avoided? CD45 is depleted at PLL-mediated contacts by ~50% (Supplementary Fig. 1h,i), which could favor signaling by increasing the local kinase/phosphatase ratio. Similar levels of phosphatase segregation suffice to initiate TCR phosphorylation and downstream signaling on SLBs (7). The immobilization of CD45 and TCRs in PLL-mediated contacts could potentiate signaling also by constraining receptor dephosphorylation. Overall, for imaging truly resting cells, surfaces might be best avoided altogether. ICAM-1 coated surfaces allow T cells to be captured without triggering strong calcium signaling (8), but integrin ‘out-to-in’ signaling will likely shift these cells away from the resting state nevertheless (9). Although TCR diffusion was apparently unimpeded on agarose surfaces, for imaging it was necessary to hold the cells in place using agarose pads, which induced signaling (79±10% responding cells, N=80, n=3). In >70% of experiments, however, signaling was undetectable when we suspended Jurkats in hydrogels straight from culture ((10); Supplementary Fig. 2a).
Super-resolution, stimulated emission depletion imaging revealed that TCR organization in Jurkats suspended in hydrogel and on PLL-coated surfaces was profoundly different (Fig. 1g). Intensity-based analysis revealed that the degree of TCR clustering was significantly higher for PLL-coated glass-contacting cells than for cells in hydrogel (p<0.001, n=5-14 images; Supplementary Fig. 2b). This suggests that the high levels of receptor and signaling-protein clustering reported previously for lymphocytes interacting with PLL-coated surfaces (1–5) is probably not reflective of resting-cell behavior. The extent to which the deformable surfaces that non-activated T-cells encounter in vivo affect signaling-protein organization, if at all, is a separate matter.
Supplementary Material
References
- 1.Lillemeier BF, et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman E, et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity. 2011;35:705–720. doi: 10.1016/j.immuni.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossy J, et al. Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nat Immunol. 2012;14:82–89. doi: 10.1038/ni.2488. [DOI] [PubMed] [Google Scholar]
- 4.Maity PC, et al. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci Signal. 2015;8:ra93. doi: 10.1126/scisignal.2005887. [DOI] [PubMed] [Google Scholar]
- 5.Pageon SV, et al. Superresolution microscopy reveals nanometer-scale reorganization of inhibitory natural killer cell receptors upon activation of NKG2D. Sci Signal. 2013;6:ra62. doi: 10.1126/scisignal.2003947. [DOI] [PubMed] [Google Scholar]
- 6.James JR, et al. Single-molecule level analysis of the subunit composition of the T cell receptor on live T cells. Proc Natl Acad Sci USA. 2007;104:17662–7. doi: 10.1073/pnas.0700411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang VT, et al. Initiation of T cell signaling by CD45 segregation at 'close contacts'. Nat Immunol. 2016;17:574–82. doi: 10.1038/ni.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaizuka Y, Douglass AD, Vardhana S, Dustin ML, Vale RD. The coreceptor CD2 uses plasma membrane microdomains to transduce signals in T cells. J Cell Biol. 2009;185:521–34. doi: 10.1083/jcb.200809136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans R, Lellouch AC, Svensson L, McDowall A, Hogg N. The integrin LFA-1 signals through ZAP-70 to regulate expression of high-affinity LFA-1 on T lymphocytes. Blood. 2011;117:3331–42. doi: 10.1182/blood-2010-06-289140. [DOI] [PubMed] [Google Scholar]
- 10.Sood N, et al. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23:758–80. doi: 10.3109/10717544.2014.940091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.