Abstract
For many years, the high-affinity receptor for immunoglobulin E (IgE) FcεRI, which is expressed by mast cells and basophils, has been widely held to be the exemplar of cross linking (that is, aggregation-dependent) signaling receptors. We found, however, that FcεRI signaling could occur in the presence or absence of receptor crosslinking. Using both cell and cell-free systems, we showed that FcεRI signaling was stimulated by surface-associated monovalent ligands through the passive, size-dependent exclusion of the receptor-type tyrosine phosphatase CD45 from plasma membrane regions of FcεRI-ligand engagement. Similarly to the T cell receptor, FcεRI signaling was stimulated in a ligand-independent manner. These data suggest that a simple mechanism of CD45 exclusion–based receptor triggering could function together with crosslinking-based FcεRI signaling, broadening mast cell and basophil reactivity by enabling these cells to respond to both multivalent and surface-presented monovalent antigens. These findings also strengthen the case that a size-dependent, phosphatase exclusion–based receptor triggering mechanism might serve generally to facilitate signaling by noncatalytic immune receptors.
Introduction
The high-affinity immunoglobulin E (IgE) receptor, FcεRI, sensitizes mast cells and basophils to IgE-targeted antigens, thus, underpinning both anti-parasite and allergic responses (1). FcεRI comprises a tetrameric complex consisting of the IgE-binding chain, FcεRIα, associated with FcεRIβ and a covalent FcεRIγ homodimer (2). Binding of cognate antigen by FcεRI-bound IgE induces rapid phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic domains of FcεRIβ and FcεRIγ by the Src family kinase (SFK) Lyn (3), enabling recruitment and activation of spleen tyrosine kinase (Syk) through interactions of its Src homology 2 (SH2) domains with the phosphorylated ITAMs of FcεRIγ (4). Activated Syk then initiates a signaling cascade through phospholipases and calcium (Ca2+) mobilization that ultimately leads to the exocytosis of pro-inflammatory granules containing, for example, histamine (1).
For many years, IgE-initiated signaling was considered to be the quintessential example of aggregation-driven immunoreceptor triggering (5). Very early studies suggested that for IgE-mediated exocytosis, intermolecular crosslinking of cell-bound antibody was required for signaling (6). Accordingly, it was shown that multivalent antigens were generally capable of eliciting mast-cell responses, whereas univalent, monomeric ligands were not. It was also shown that crosslinking did not necessarily have to be antigen-mediated; bivalent anti-IgE antibody (7) or artificially aggregated IgE, or the Fc fragments of IgE, were all found to be active (8). It was subsequently proposed that dimers of IgE serve as a minimal signaling “unit” for mast cell degranulation (9), albeit weakly (10, 11). The current view is that for FcεRI to be triggered, the receptor must be crosslinked by multivalent antigens to achieve the necessary density and geometry required for receptor transphosphorylation by FcεRI-associated Lyn (3, 12). Alternatively, cross-linking–based aggregation might induce changes in the distribution of FcεRI within membrane domains that protect it from dephosphorylation (13, 14). Irrespective of the mechanism, constraining FcεRI reactivity to multivalent antigens would have the effect of severely restricting the breadth of mast cell and basophil responses.
Despite the enduring appeal of aggregation-based signaling, it was quickly realized that it created a paradox because antibody receptors were understood to be expressed at sufficiently high amounts, and enough of them are mobile, for dimers to form on a purely stochastic basis; however, spontaneous exocytosis is not observed (5). Further findings have produced new difficulties. The binding of free IgE to mast cells influences numerous cellular responses, including cytokine production, cell survival and differentiation, and FcεRI expression (15–17), implying that FcεRI is capable of sensing IgE in an aggregation-independent manner. These effects are, however, distinct from those of so-called ”highly cytokinergic” IgEs, which induce degranulation at very high concentrations, most likely through receptor crosslinking by surface-expressed autoantigens (18). In addition, FcεRI expressed in transfected fibroblasts is spontaneously phosphorylated (19), revealing that aggregation per se is not a strict requirement of receptor triggering. But most importantly, mast cell degranulation is robustly triggered by monovalent antigens attached to supported lipid bilayers (SLBs; 20, 21). Similar to a second immune receptor, that is, the B cell receptor (BCR) (22), which was also widely assumed to be triggered only by crosslinking, but is now known to be reactive to surface-presented monovalent ligands, it is now accepted that FcεRI can be triggered by both mono- and multivalent antigens.
Alongside these findings, it is becoming recognized that the properties of many common allergens are incompatible with, or at least seem unsuited to, aggregation-based FcεRI triggering in vivo. Firstly, many known allergens are monomeric (e.g. 23, 24–26). These could crosslink FcεRI in the presence of polyclonal IgE against non-overlapping epitopes, but this would only work within a narrow range of binding geometries according to studies of model antigens (11). On the other hand, most dimeric allergens that self-associate only do so transiently (27), with reported dissociation constant (Kd) values in the micro- to millimolar range (28, 29). Because exposure to common animal allergens is typically <1 to 300 ng/day (30, 31), which corresponds to ~0.02 to 8 pmol/day for the major cat allergen Fel d 1, and pollen allergens are encountered at 10- to 100-fold lower amounts (31, 32), these types of allergens will most likely be presented to mast cells and basophils as monomers, unless they accumulate in allergen-sensitive tissues.
Intrigued initially by differences in the functionality of surface-exposed and non-exposed mast cells, we explored the surface dependence of FcεRI signaling. We found that FcεRI was triggered by surfaces lacking FcεRI ligands, accounting for the characteristic behavior and morphology of basophilic cells in vitro. We then showed that signaling occurred in both the presence and absence of receptor aggregation and that it was robustly triggered by surface-associated monovalent ligands through the passive, size-dependent exclusion of the large receptor-type protein tyrosine phosphatase CD45 from regions of contact of FcεRI with its ligands. Whereas previous work suggested that CD45 exclusion and FcγR signaling are dependent on integrins forming an actin-tethered diffusional barrier to the phosphatase (33), we observed strong signaling by FcεRI in the absence of integrin activity, and spontaneous phosphatase exclusion from engaged receptors in both cells and cell-free systems. We propose that a phosphatase exclusion-based mechanism functions alongside aggregation-driven receptor triggering to broaden the reactivity of mast cells. In this way, we propose that the paradoxical features of FcεRI triggering can be reconciled.
Results
Mast cells sense the presence of surfaces
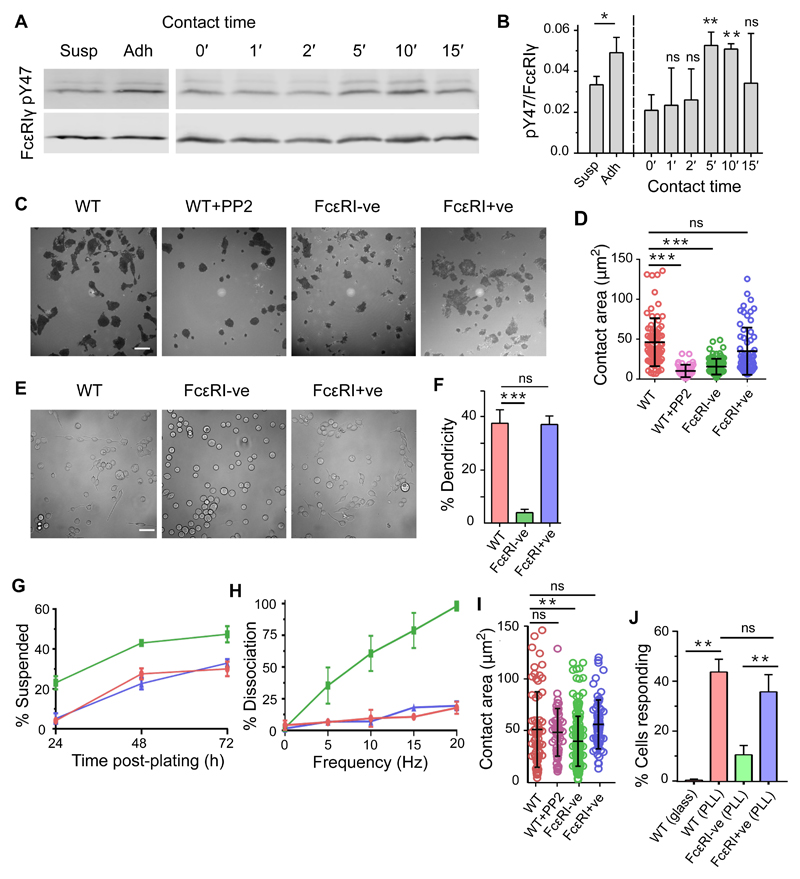
In the course of culturing rat basophilic leukemia (RBL) 2H3 cells, we observed small but statistically significant differences in FcεRI phosphorylation in cells kept in suspension by gentle end-over-end rotation versus those allowed to make contact with plastic surfaces (Fig. 1A, left, and Fig. 1B). FcεRI phosphorylation was detected on Western blots, using an anti-pY47-FcεRIγ phospho-specific antibody (34), as pairs of bands corresponding to short- and long-lived, mono- and dual-phosphorylated forms of FcεRIγ. We tested whether this was due to surface contact per se by quantifying phosphorylated FcεRIγ as the cells settled onto plastic surfaces in phosphate-buffered saline (PBS). The degree of phosphorylation was expressed as the ratio of dually-phosphorylated FcεRIγ intensity to total receptor intensity, normalized to the ratio of intensities measured in cells activated with anti-trinitrophenol (TNP) IgE and soluble bovine serum albumin-TNP (TNPBSA) as antigen. A small, time-dependent increase in FcεRIγ phosphorylation was observed as the cells settled, peaking at 5 to 10 min (Fig. 1A, right, and Fig. 1B). These results indicated that RBL-2H3 cells, as they are usually grown, are not ‘resting’ with respect to FcεRI phosphorylation direct from tissue culture.
Fig. 1. Mast cells sense the presence of surfaces.
(A) FcεRIγ phosphorylation detected on Western blots of whole-cell lysates of RBL-2H3 cells grown for 24 hours in suspension (Susp) or adherent (Adh) culture (left) or after contacting uncoated plastic for 0 to 15 min (right). The blots were incubated with anti-pY47 FcεRIγ (top) or anti-FcεRIγ (bottom) antibodies. (B) Anti-pY47 FcεRIγ signal relative to anti-FcεRIγ signal normalized against that for cell stimulate with anti-TNP IgE and TNPBSA. Statistical significance was calculated versus non-contacting cells (0 min). (C and D) Interference reflection microscopy (IRM) images of WT, PP2-treated WT, FcεRI-ve, and FcεRI+ve RBL-2H3 cells 30 min after contacting uncoated glass in growth medium (C). The size of the contact areas of cells under the indicated conditions were quantified (D). Each spot represents a single cell. (E and F) Differential interference contrast images of WT, FcεRI-ve, and FcεRI+ve RBL-2H3 cells cultured for 24 hours on uncoated glass revealing dendritic morphology (E). The percentage of the indicated cells with a dendritic morphology were quantified (F). (G) Percentage of RBL-2H3 cells that grew spontaneously in suspension under normal culturing conditions. (H) Percentage of cells that detach at different levels of mechanical agitation. For (G) and (H), WT cells are in red; FcεRI-ve cells are in green; FcεRI+ve cells are in blue.. (I) The contact area of RBL-2H3 cells 30 min after making contact with PLL-coated glass. Each spot represents a single cell. (J) The percentages of WT, FcεRI-ve, and FcεRI+ve RBL-2H3 cells that exhibited Ca2+ flux responses within 10 min of making contact with uncoated (glass) or PLL-coated glass. For all bar graphs, data are means ± SD of three independent experiments. Blots and images are representative of three independent experiments. Scale bars, 5 µm. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
Using interference reflection and contrast microscopy to visualize cell contact areas and morphologies after adherence to microscope cover glass, we examined in more detail the effects of cell culture-induced FcεRI signaling in wild-type (WT) RBL-2H3 cells and RBL-2H3 cells lacking FcεRIα, so-called FcεRI-ve cells, which were generated using CRISPR-Cas9 mutagenesis (35) (fig. S1A), under conditions that facilitated imaging. WT RBL-2H3 cells that attached to uncoated glass in fetal calf serum (FCS)-supplemented growth medium formed statistically significantly larger contacts after 30 min than did FcεRI-ve cells, whose contacts were similar to those of RBL-2H3 cells treated with the SFK inhibitor PP2 (Fig. 1, C and D). The contact areas formed by WT cells were not affected by the presence of FCS or by preloading with IgE (fig. S1B). Normal contact size was restored for FcεRI-ve cells by re-introduction of FcεRIα (‘FcεRI+ve’ cells; Fig. 1, C and D, and fig. S1A). Surface-grown WT RBL-2H3 cells exhibited more dendritic morphology (that is, they had protrusions extending away from the main cell body) than did FcεRI-ve cells (Fig. 1, E and F). Under normal culture conditions, RBL-2H3 cells grew predominantly as an adherent monolayer. However, substantially more FcεRI-ve cells grew in suspension compared to WT RBL-2H3 cells (Fig. 1G), and the surface-attached cells were more easily detached (Fig. 1H). FcεRI-ve RBL-2H3 cells were, however, as motile as WT cells (fig. S1C). Poly L-lysine (PLL)-coated surfaces induced RBL-2H3 cells to form large contacts in an FcεRI-independent manner (Fig. 1I), although FcεRI-ve cells formed statistically significantly, albeit slightly smaller, contacts than those of WT cells. Robust Ca2+ signaling was observed for WT and FcεRI+ve cells, but not FcεRI-ve cells, upon contact with PLL-coated glass (Fig. 1J), suggesting that this surface led to FcεRI triggering, similar to the case of T cells and the T cell receptor (TCR) (36). PLL also induced substantial FcεRIγ phosphorylation in RBL-2H3 cells and primary human basophils (fig. S1, D to F). Together, these data suggest that mast cells sense surfaces through FcεRI signaling, a consequence of which might be the characteristic, adherent nature of cultured RBL-2H3 cells. That PLL induced strong signaling was an initial indication that FcεRI signaling was not dependent on integrin mobilization.
FcεRI is triggered by immobilized ligands and sensitive to constitutive kinase and phosphatase activity
The capacity of FcεRI to be triggered without cognate ligands after contact with a surface was reminiscent of the TCR (37), and further undermined the aggregation-only theory of FcεRI signaling. To revisit its actual requirements, we used the classical approach to studying aggregation-based FcεRI signaling (12), that is, BSA-coupled hapten (TNPBSA) and anti-TNP IgE in solution and adsorbed onto glass. We also generated an artificial, monovalent FcεRI ligand mimic (His-Fcε), comprising the CH2 to CH4 domains of rat Fcε preceded by an N-terminal His12-tag, which we could present in an FcεRI-accessible orientation (fig. S2A) on Ni-coated glass surfaces (mimicking monovalent immobilized antigens) or SLBs (to mimic mobile, cell-presented monovalent ligands). We used Ca2+ signaling as a proxy for receptor triggering.
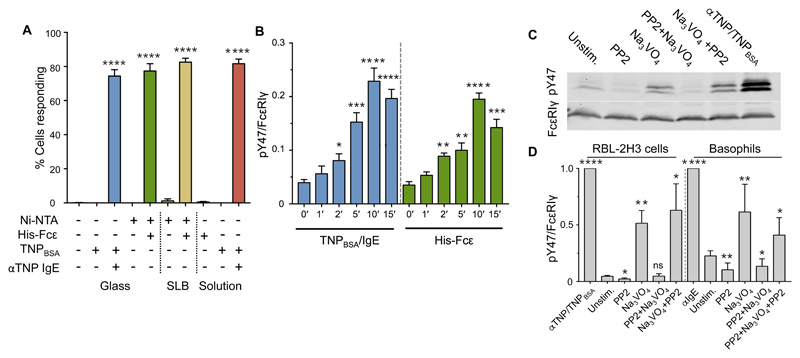
Previous work showed that soluble monovalent antigen does not trigger FcεRI signaling, whereas when it is attached in a mobile form to a SLB, the same antigen induces RBL-2H3 degranulation (20, 21). As expected, therefore, RBL-2H3 cells did not produce measurable Ca2+ responses to soluble His-Fcε or to Ni-coated glass that did not present His-Fcε (Fig. 2A). However, ~80% of cells produced detectable responses within 10 min of making contact with SLBs presenting His-Fcε (Fig. 2A), with an average delay between surface contact and triggering of 217 s. Equivalent fractions of the cell population produced Ca2+ responses within 10 min of contact with surfaces coated with TNPBSA-IgE or with His-Fcε, as did cells exposed to anti-TNP IgE followed by soluble TNPBSA (Fig. 2A). The consistency of the triggering fraction likely reflects the overall number of responsive cells. Nonetheless, phosphorylated FcεRIγ was somewhat more abundant in cells after contact with immobilized, cross-linked IgE than in cells exposed to immobilized monovalent ligand mimics (Fig. 2B). Soluble, cross-linked ligand produced the largest effect (normalized value of 1 in Fig. 2B), likely because it engaged more of the surface FcεRI than did any of the immobilized ligands. These observations indicate that, like the TCR, FcεRI can be triggered by receptor-clustering and surface-associated monovalent ligands.
Fig. 2. FcεRI responds to immobilized ligands and is sensitive to constitutive kinase and phosphatase activities.
(A) Percentages of RBL-2H3 cells that exhibited Ca2+ flux responses within 10 min of making contact with glass or SLBs functionalized with His-Fcε or TNPBSA-IgE versus uncoated surfaces or in response to soluble His-Fcε. Statistically significant differences refer to differences from zero. (B) Normalized anti-pY47 FcεRIγ signals relative to anti-FcεRIγ signals in whole-cell lysates from RBL-2H3 cells that made contact with the indicated surfaces. Statistically significant differences are in comparison to cells at time zero.. Data are from four independent experiments. (C) Western blotting analysis of FcεRIγ phosphorylation in RBL-2H3 cells after acute inhibition of kinase or phosphatase activity with PP2 or pervanadate (Na3VO4), respectively. (D) Quantitation of the effects measured in (C) in RBL-2H3 cells (left) and primary human basophils (right). Significances represent differences from unstimulated cells. Replicate primary basophil measurements were performed with blood from different donors. For all bar graphs, data are means ± SD of three independent experiments, unless stated otherwise. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
We sought to establish whether, also like the TCR (38–41), FcεRI is sensitive to the constitutive activities of kinases and phosphatases in situ. FcεRIγ phosphorylation was induced in RBL-2H3 cells and primary human basophils by the tyrosine phosphatase inhibitor, pervanadate (Fig. 2, C and D, and fig. S2B). However, receptor crosslinking resulted in greater amounts of phosphorylated FcεRIγ than were induced by pervanadate, implying that local kinase activity was enhanced by receptor clustering. Near-complete inhibition of FcεRI phosphorylation was observed if the SFK inhibitor PP2 was added 15 min before pervanadate, but not 15 min afterwards. These results indicate that in resting cells, and especially in resting primary basophils in which the spontaneous, PP2-inhibited abundance of phosphorylated FcεRIγ was relatively high (Fig. 2D and fig. S1, E and F), FcεRI is accessible to active SFKs, but its phosphorylation is constrained by constitutive tyrosine phosphatase activity. These observations are inconsistent with the notion that inactive FcεRIγ is in some way sequestered away from phosphatases (13, 14).
CD45 and FcεRI segregate after engagement by surface-associated IgE
The dependence of FcεRI triggering on the surface attachment of monovalent ligands and the sensitivity of the receptor to constitutively active kinases and phosphatases suggested that FcεRI might be triggered by local, size-dependent phosphatase exclusion. We (37) and others (33, 42) have reported that CD45 is excluded from contacts that leukocytes form with model surfaces. We previously proposed that ligand-induced receptor triggering arises in vivo if receptors, such as the TCR, are held in phosphatase-depleted contacts by ligands, favoring phosphorylation of the receptors [the “kinetic segregation” (KS) model; (43)].
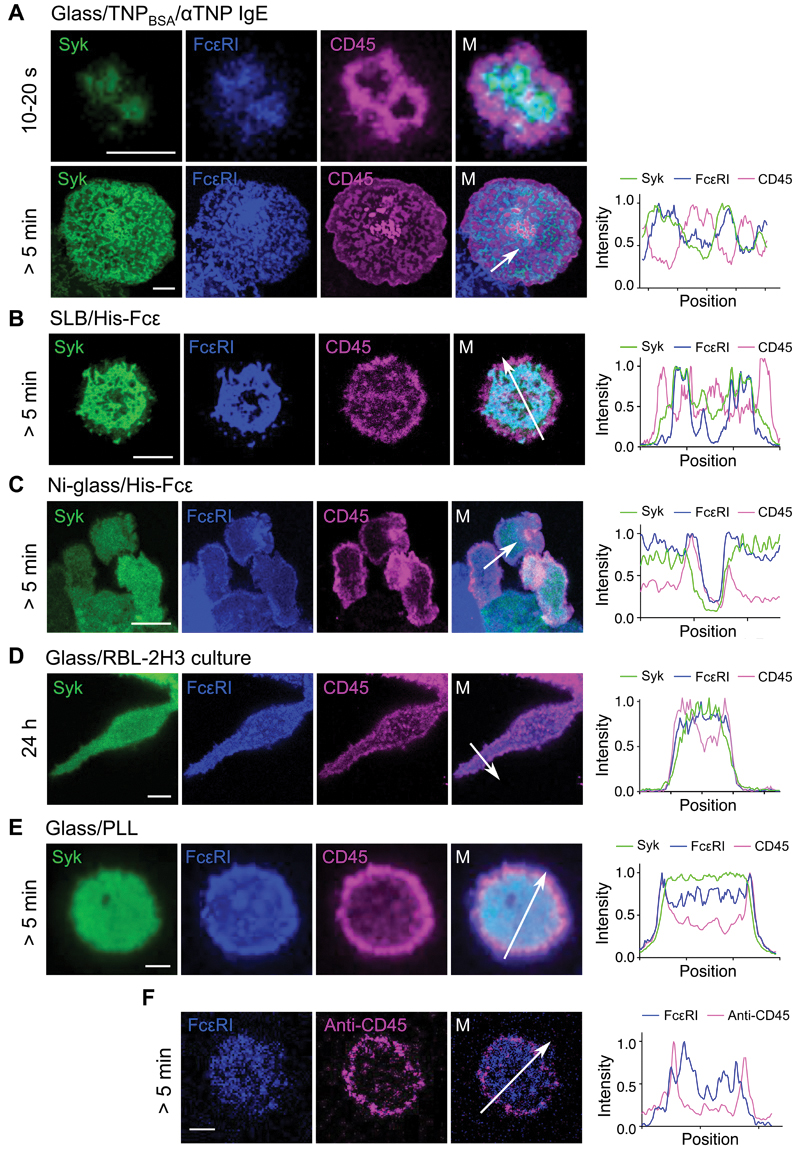
To test whether phosphatase exclusion accompanies the engagement of FcεRI by ligands, we studied the behavior of CD45, which is expressed by mast cells and regulates FcεRI activity through both inhibitory and activatory effects (44, 45). Basal surfaces of RBL-2H3 cells were imaged by confocal microscopy, detecting FcεRI, CD45R0 (the shortest CD45 isoform), and Syk, which were tagged with SNAP-tag, HaloTag, and Citrine, respectively. For all activating surfaces, that is, His-Fcε on Ni-glass or SLBs, and TNPBSA-IgE on glass, CD45 was excluded from areas containing FcεRI and Syk within 10 to 20 s of cell contact, forming exclusion zones that were 1 to 2 μm in diameter (Fig. 3, A to C; movie S1; additional example images are shown in fig. S3). Recruitment of Citrine-labelled Syk to regions of FcεRI accumulation (fig. S4A), similarly to the recruitment of ZAP70 to the TCR (37), was taken to be evidence of receptor triggering and phosphorylation: photo-bleaching analysis showing loss of the Citrine signal (fig. S4B), confirmed that Syk was localized at the membrane in FcεRI-, but not CD45-enriched areas. Cells interacting with immobile ligands (His-Fcε on Ni-glass and TNPBSA-IgE on glass) formed stably organized contacts that expanded as the cells spread (Fig. 3, A and C). For TNPBSA-IgE, this led to the formation of a complex network of mutually exclusive CD45- and FcεRI/Syk-enriched zones after 5 min (Fig. 3A, bottom). CD45 exclusion from FcεRI-enriched regions was also observed in cells preloaded with anti-TNP IgE before making contact with TNPBSA-coated glass (fig. S4C). For cells activated on His-Fcε–presenting SLBs (Fig. 3B), the initial contacts led to synapse formation within ~5 min, as reported previously (20), in which CD45 was excluded to the peripheral regions of the contact. CD45 was also excluded from the basal plane of cells cultured on coverslips in the absence of ligand (Fig. 3D), consistent with the greater abundance of phosphorylated FcεRIγ in resting RBL-2H3 cells grown in adherent versus suspension culture (Fig. 1, A and B). Similarly, CD45 and FcεRI were excluded from FcεRI-enriched regions when cells made contact with PLL-coated coverslips (Fig. 3E), but more so in the case of CD45, which may account for the signal-potentiating effects of PLL (Fig. 1J). Primary human basophils that made contact with coverslips coated with the common dust mite antigen Der f 1 exhibited exclusion of CD45 from FcεRI-enriched regions if they were first loaded with anti-Der f 1 hIgE (Fig. 3F), but not when either Der f 1 or anti-Der f 1 were absent (fig. S4, D and E).
Fig. 3. CD45 and FcεRI segregate following engagement of surface-associated IgE.
(A to E) Left: Confocal fluorescence images of Syk, FcεRI, and CD45 at the basal surfaces of RBL-2H3 cells that had made contact with (A) TNPBSA-IgE-coated glass, (B) a His-Fcε-functionalized SLB, (C) Ni-glass, (D) uncoated glass, or (E) PLL-coated glass. Timings indicate the period of contact with the surface. Right: Intensity line profiles correspond to the arrows in the merged views. (F) Exclusion of CD45 relative to FcεRI was observed for primary human basophils that were loaded with anti-Der f 1 IgE and then interacted with Der f 1–coated glass. Images are representative of observations made in at least three independent experiments. Replicates with primary basophils were performed with blood from different donors. Scale bars, 5 µm.
CD45 exclusion is passive
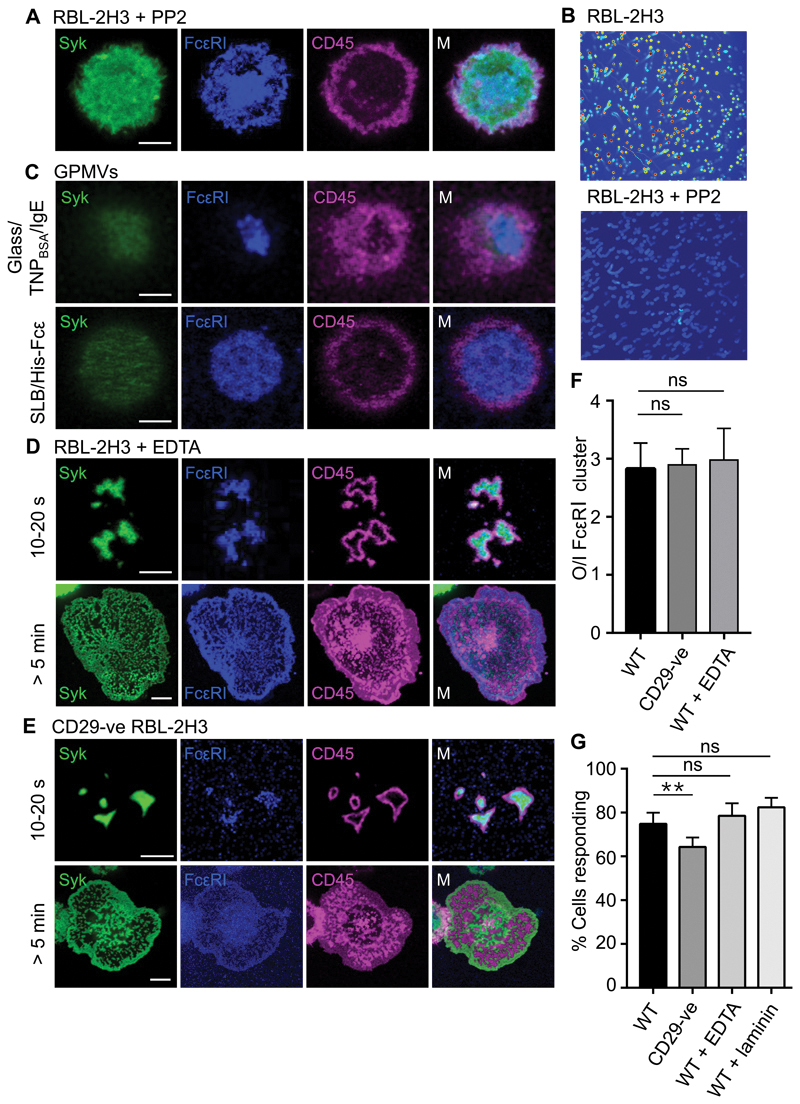
The observed segregation of CD45 and FcεRI could, in principle, be a result of signaling or effected by surface contact alone. On TNPBSA-IgE–coated glass, we observed the exclusion of CD45 from FcεRI-containing regions of contact in PP2-treated RBL-2H3 cells (Fig. 4, A and B, and fig. S5A), in which signaling was completely blocked (Fig. 4B). To confirm that CD45 exclusion was entirely passive, we examined the behavior of the tagged proteins in giant plasma membrane vesicles (GPMVs; 46)] derived from RBL-2H3 cells. GPMVs lack cytoskeletal structures and lose normal cellular energy homeostasis but retain the membrane lipid and protein compositions of the cells from which they are derived. GPMVs produced from RBL-2H3 cells presenting fluorescently tagged FcεRI, CD45, and Syk formed regions of contact upon settling on either TNPBSA-IgE-coated glass or SLBs presenting His-Fcε that were marked by the local exclusion of CD45R0 and accumulation of FcεRI (Fig. 4C and fig. S5B). These observations suggest that the exclusion of CD45 from contacts as they form is an entirely passive process.
Fig. 4. CD45 exclusion is passive and independent of integrin activity.
(A) Confocal fluorescence images of Syk, FcεRI, and CD45 at the basal surface of an RBL-2H3 cells treated with PP2 after making contact with TNPBSA-IgE-coated glass (a line profile is shown in fig. S5). (B) Effect of PP2, as used in (A), on FcεRI triggering as measured by Ca2+ flux intensity. Images are 10-min maximum intensity projections for Ca2+ intensity scaled as a heat map; blue, low intensity; red, high intensity. (C) Confocal fluorescence images of Syk, FcεRI, and CD45 at the basal surface of RBL-2H3–derived GPMVs after making contact with TNPBSA-IgE-coated glass (upper) or a His-Fcε–functionalized SLB (lower). (D) Confocal fluorescence images of Syk, FcεRI, and CD45 at the basal surface of RBL-2H3 cells after making contact for the indicated times with TNPBSA-IgE-coated glass in the presence of EDTA to chelate divalent cations. (E) Confocal fluorescence images of Syk, FcεRI, and CD45 at the basal surface of CD29-ve RBL-2H3 cells after making contact for the indicated times with TNPBSA-IgE-coated glass. (F) Ratio of CD45 fluorescence intensity outside (O) versus inside (I) areas of enriched FcεRI intensity, for WT, CD29-ve, and EDTA-treated RBL-2H3 cells. Data are means ± SD of three independent experiments, with > 5 cells assessed per experiment. (G) Percentages of WT, CD29-ve, and EDTA-treated RBL-2H3 cells that produced Ca2+ flux responses within 10 min of making contact with glass coated with TNPBSA-IgE or TNPBSA-IgE and rat laminin peptide. Data are means ± SD of four independent experiments. Images are representative of observations made in at least three independent experiments. Scale bars, 5 µm. **P < 0.01; ns, not significant.
Integrins are not required for CD45 exclusion or FcεRI triggering
Previous work on FcγR signaling in macrophages suggested that integrin activation is needed to generate a diffusional barrier that excludes CD45 from the receptor, leading to signaling (33). We tested whether this applied to FcεRI signaling in mast cells using chemical and genetic interventions. We observed very efficient exclusion of CD45 from regions of contact of RBL-2H3 cells with TNPBSA-IgE–coated glass in the absence of free divalent cations, that is, after treatment with 1.5 mM EDTA treatment in the absence of Ca2+ or Mg2+, which abrogates the ligand binding activity of integrins [(47); Fig. 4D]. Similar results were obtained for primary basophils (fig. S6A). Similarly, CRISPR-mediated deletion of the expression of CD29 (fig. S6B), which is the most abundant β integrin subunit found in mast cells (48) and pairs with several of the most abundant α integrins present (49), had no effect on the exclusion of CD45 from contacts formed with TNPBSA-IgE–coated glass (Fig. 4E). The degree of exclusion within seconds of contact was comparable to that in WT cells (Fig. 4F and movie S2), with the cells progressing to also form the characteristic networks of mutually-excluding FcεRI and CD45 accumulation zones.
WT cells tested in divalent cation–free conditions and CD29-ve cells also produced robust Ca2+ responses. On TNPBSA-IgE-coated glass (Fig. 4G) and across a range of IgE concentrations (fig. S6C), the triggering fraction was reduced by only ~15% for CD29-ve cells (compared to WT) due to a slight increase in the lag time preceding triggering (fig. S6D). The RBL-2H3 cells tested in the divalent cation–free conditions exhibited a single Ca2+ flux of 40- to80-s duration rather than multiple fluxes of 10 to 15 s, and typically showed a reduced Ca2+ fluorescence peak (fig. S6E), likely due to the effects of Ca2+ chelation by EDTA on intracellular Ca2+ store replenishment. Neither EDTA nor CD29 deletion in RBL-2H3 cells impaired FcεRIγ phosphorylation (fig. S6F), and removal of divalent cations had no effect on FcεRIγ phosphorylation in primary basophils in response to Der f 1 (Fig. S6G).
Although integrins did not appear to contribute directly to CD45 exclusion or early signaling detected as Ca2+ fluxes, RBL-2H3 cells adhering to immobilized integrin ligands exhibit stronger degranulation and phospholipase activity than those on passivated surfaces (50, 51). FcεRI triggering by surface-bound antigen could conceivably be enhanced by integrin-mediated interactions that stabilize or increase the size of surface contacts, lowering the threshold for signaling. We tested whether RBL-2H3 cells were more sensitive to TNPBSA-IgE in the presence of a peptide derived from rat laminin, which promotes mast cell surface attachment (52) by binding to CD29 in complex with α integrin subunits, such as CD49f (53, 54). Ca2+ responses (Fig. 4G and fig. S6C) and the amount of phosphorylated FcεRIγ (fig. S6F) in cells that made contact with laminin peptide-coated surfaces were comparable to those observed in cells in the absence of laminin, suggesting that, at least for initial receptor signaling, there is little contribution from CD29.
Size-based CD45 exclusion drives FcεRI signaling
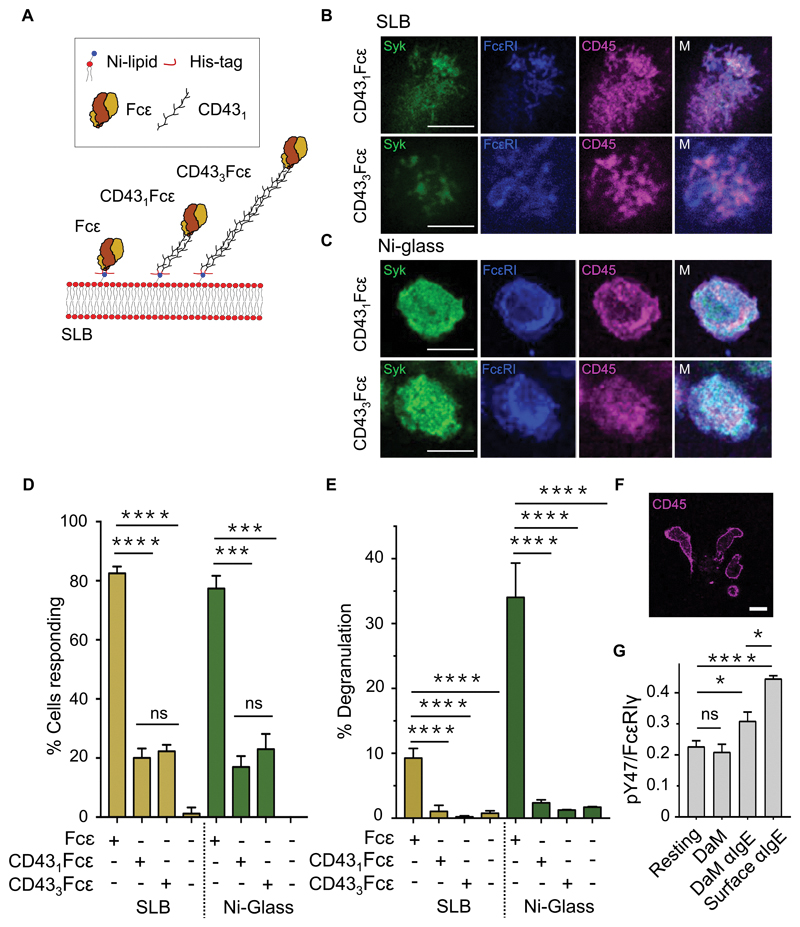
A likely cause of CD45 exclusion from regions of FcεRI engagement is the difference in size of IgE/receptor complexes and CD45. This is because the extracellular region of CD45R0 is 215Å (37) and the complex formed by the extracellular region of FcεRI and IgE measures ~100Å along an axis orthogonal to the plane of the plasma membrane (55). We tested this assertion by generating size-variant IgE ligand mimics composed of the short His-Fcε construct and incorporating either one or three copies of the human CD43 extracellular domain (amino acid residues 20 to 254) between the histidine tag and Fcε (generating CD431Fcε and CD433Fcε; Fig. 5A). Like His-Fcε, these proteins bound to FcεRI with affinities and specificities comparable to those of rat IgE (fig. S2, C and D). However, the relatively rigid CD43 domain(s) (56) were expected to force FcεRI to engage Fcε further from the SLB surface, reducing the effect of ligand engagement on size-dependent reorganization of kinases and phosphatases.
Fig. 5. CD45 exclusion is sensitive to the size of the surface-associated ligand.
(A) Schematic representation of CD43Fcε chimeras. (B and C) Confocal fluorescence images of Syk, FcεRI, and CD45 at the basal surface of an RBL-2H3 cell after making contact with an SLB (B) or Ni-glass (C) presenting extended CD431Fcε and CD433Fcε chimeras. (D) Percentages of RBL-2H3 cells producing Ca2+ fluxes within 10 min of making contact with an SLB or Ni-coated glass functionalized with extended CD431Fcε and CD433Fcε chimeras versus the same surfaces presenting His-Fcε. Data are means ± SD of four independent experiments. (E) Confocal fluorescence image of CD45 (that is, anti-CD45 Fab) at the basal surface of primary human basophils after making contact with anti-IgE–coated glass. (F) Normalized anti-pY47 FcεRIγ signals relative to anti-FcεRIγ signals obtained from Western blots of whole-cell lysates from primary human basophils after making contact anti-IgE conjugated directly or indirectly (through donkey anti-mouse; DaM) to glass. Data are means ± SD of three independent experiments. Resting denotes cells kept in suspension. (G) Percentage maximal degranulation of RBL-2H3 cells 30 min after making contact with an SLB or Ni-coated glass functionalized with extended CD431Fcε and CD433Fcε chimeras versus the same surfaces presenting His-Fcε. Data are means ± SD of four independent experiments. Images are representative of observations made in at least three independent experiments. Replicates with primary basophils were performed with blood from different donors. Scale bars, 5 µm. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
In contrast to the efficient exclusion observed on His-Fcε–coated surfaces (Fig. 3, B and C), CD45 exclusion was not observed in RBL-2H3 cells that made contact with surfaces presenting either mobile or immobile CD431Fcε or CD433Fcε (Fig. 5, B and C). Fewer RBL-2H3 cells produced Ca2+ calcium responses after contacting CD431Fcε- or CD433Fcε-functionalised surfaces compared to cells that interacted with His-Fcε (Fig. 5D); there was no measurable difference between the behaviors of cells that interacted with CD431Fcε or CD433Fcε. CD45 was excluded from contacts formed by freshly isolated (human IgE–bound) primary human basophils with glass coated with mouse anti-human IgE (Fig. 5E), and much less exclusion when the basophil-surface ”gap” was increased by coating the anti-human IgE indirectly onto the surface using donkey anti-mouse IgG (fig. S6H), despite the densities of anti-human IgE being comparable (fig. S6I). Moreover, primary basophils that interacted with a surface coated with anti-hIgE had an increased abundance of phosphorylated FcεRI than did cells that made contact with anti-hIgE/anti-mouse IgG–coated glass (Fig. 5F).
These observations suggest that FcεRI is triggered by CD45 exclusion, at least for surfaces presenting compact FcεRI ligands, that is, ~100 Å, the size of His-Fcε. Whereas we relied heavily on Ca2+ signaling as a reporter of FcεRI triggering per se, it seemed possible that the extent of signaling we observed due to CD45 exclusion comprised a background or sub-threshold response that was insufficient to trigger the important effector functions of mast cells. We therefore tested whether mast cell degranulation in response to surface-presented antigen is also dependent on phosphatase exclusion. We observed that mast cell degranulation was no higher than background levels for the CD431Fcε and CD433Fcε ligand mimics presented in either mobile or immobile forms (Fig. 5G). Immobile His-Fcε induced approximately three-fold greater degranulation than did mobile His-Fcε, perhaps due to the contact-stabilizing effects of glass-bound Ni2+ in the manner of PLL (36).
FcεRI triggering relies on the large extracellular domain of CD45
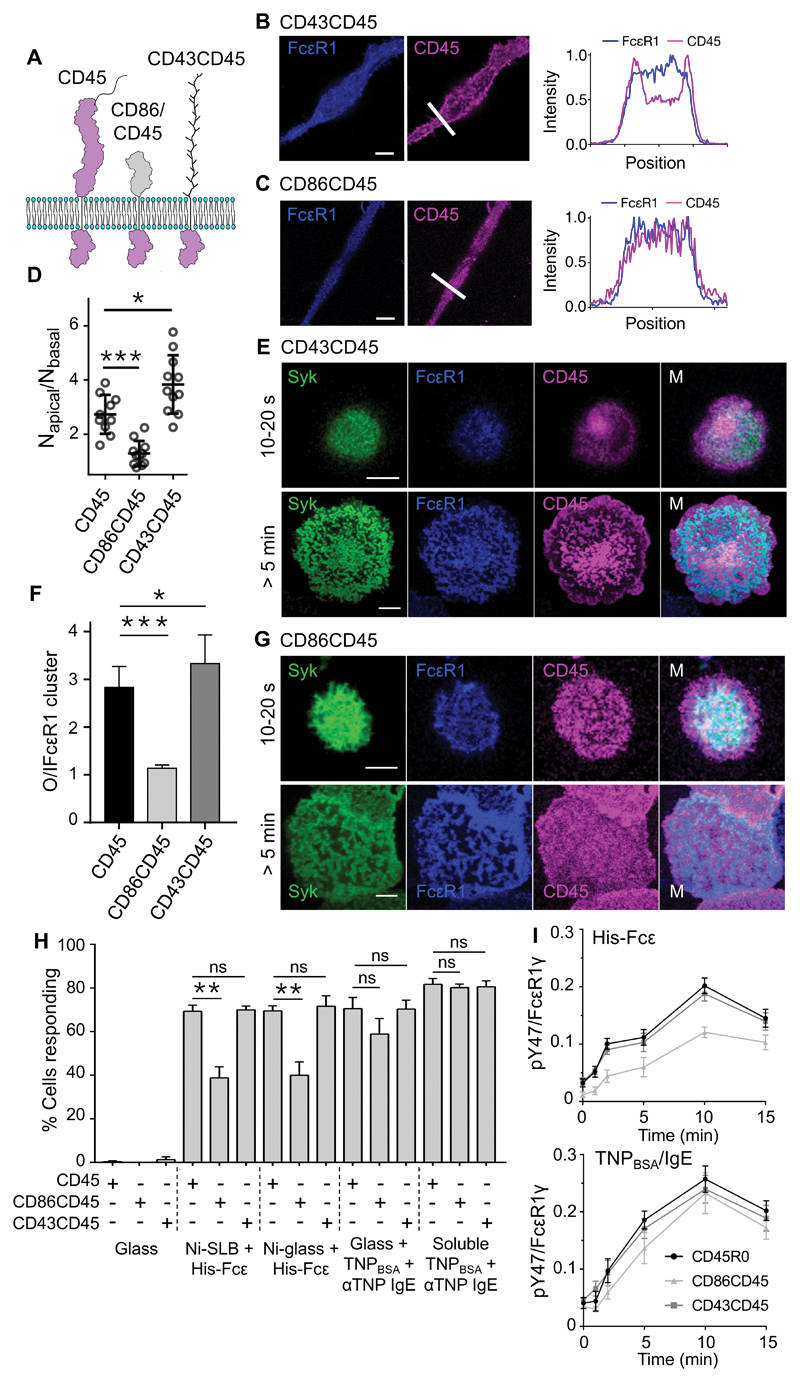
As a final test of the sensitivity of FcεRI to local, size-based exclusion of CD45, we tested whether signaling was dependent on the size of CD45 itself, as in the case of TCR-driven signaling (57, 58). We stably transfected RBL-2H3 cells with plasmids expressing chimeric forms of CD45 consisting of the transmembrane and intracellular regions of rat CD45 and the extracellular domains of either CD43 or CD86 (generating CD43CD45 and CD86CD45, respectively). CD43 has a mucin-like extracellular domain comparable in size to those of the longer CD45 isoforms [~400Å; (56)], whereas CD86 is much shorter [~75Å; (59); Fig. 6A]. After expression in RBL-2H3 cells, each form of CD45 was detected through a C-terminal HaloTag, together with FcεRI labeled with a SNAP-tag, and Syk tagged with Citrine. As observed for CD45R0 (Fig. 3D), CD43CD45 exhibited partial exclusion from FcεRI-enriched regions on the basal surface of cells cultured on glass for 24 hours, whereas CD86CD45 did not (Fig. 6, B and C). Measurement of the local concentration of CD45 molecules by fluorescence correlation spectroscopy indicated that there was 3- to 4-fold depletion of CD45R0 and CD43CD45 from the basal surface relative to the apical surface, whereas CD86CD45 was equally distributed at both sites (Fig. 6D).
Fig. 6. FcεRI triggering relies on the large extracellular domain of CD45.
(A) Schematic of WT and chimeric CD45 constructs, which are colored purple to reflect their color in the fluorescence images. (B and C) Left: FcεRI and CD43CD45 (B) or CD86CD45 (C) fluorescence at the basal surface of RBL-2H3 cells cultured for 24 hours on glass. Right: Line profiles of fluorescence intensity corresponding to the arrows in the images. (D) Local concentration, that is, the average number of molecules in the observation volume (measured by fluorescence correlation spectroscopy), for CD45 and CD43CD45 at the basal versus apical surfaces of RBL-2H3 cells compared to those for CD86CD45. Data are means ± SD of three independent experiments, with >3 cells analyzed per experiment. (E) Confocal fluorescence images of Syk, FcεRI, and CD43CD45 at the basal surface of an RBL-2H3 cell after making contact for the indicated times with TNPBSA-IgE-coated glass. (F) Fluorescence intensity for CD45, CD86CD45, and CD43CD45 outside (O) versus inside (I) areas of enriched FcεRI intensity. Data are means ± SD of three independent experiments, with >5 cells assessed per experiment. (G) Confocal fluorescence images of Syk, FcεRI, and CD86CD45 at the basal surface of an RBL-2H3 cell after making contact for the indicated times with TNPBSA-IgE-coated glass. (H) Percentages of RBL-2H3 cells expressing CD45, CD86CD45, or CD43CD45, that produced Ca2+ fluxes within 10 min of making contact with an SLB or Ni-coated glass functionalized with His-Fcε, TNPBSA-IgE-coated glass, or after being incubated with soluble anti-TNP and TNPBSA. Data are means ± SD of four independent experiments. (I) Normalized anti-pY47 FcεRIγ signals relative to anti-FcεRIγ signals for whole-cell lysates prepared from RBL-2H3 cells expressing CD45, CD86CD45, and CD43CD45 after making contact for the indicated times with Ni-coated glass functionalized with His-Fcε (top) or with TNPBSA-IgE-coated glass (bottom). Data are means ± SD of three independent experiments. All images are representative of observations made in at least three independent experiments. Scale bars, 5 µm. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
Truncation of its extracellular domain altered the distribution of CD45 on activating surfaces. CD43CD45, but not CD86CD45, was excluded from FcεRI-enriched regions on TNPBSA-IgE-coated glass, to an extent comparable to that of CD45R0 (Fig. 6, E to G, fig. S7, A and G, and movie S3). The ratio of CD45 fluorescence outside versus inside regions of FcεRI fluorescence was ~1 for CD86CD45 and > 1 for CD45R0 and CD43CD45 (Fig. 6F). Indeed, CD43CD45, which is larger than CD45R0, was slightly more excluded (Fig. 6F). Mast cells typically express CD45 isoforms larger than CD45R0 (60), and so native CD45 segregation may be more efficient than that we observed. Transfection of RBL-2H3 cells with vector expressing CD86CD45 reduced the number of cells producing Ca2+ responses within 10 min of making contact with either mobile or immobile His-Fcε by ~two-fold versus CD45R0- or CD43CD45-expressing cells (Fig. 6H). Although the fraction of responding cells was unaffected by CD86CD45 for cells that made contact with TNPBSA-IgE–coated glass (Fig. 6H), the triggering lag time was statistically significantly increased (fig. S7, C to E). CD86CD45-expressing cells also exhibited a reduced abundance of phosphorylated FcεRIγ upon making contact with His-Fcε, but not TNPBSA-IgE (Fig. 6I), and were less efficient at degranulation (fig. S7B). Expression of CD86CD45 but not CD43CD45 also reduced cell adhesion strength, suggestive of reduced basal FcεRI triggering (fig. S7F).
FcεRI triggering by unilamellar vesicles
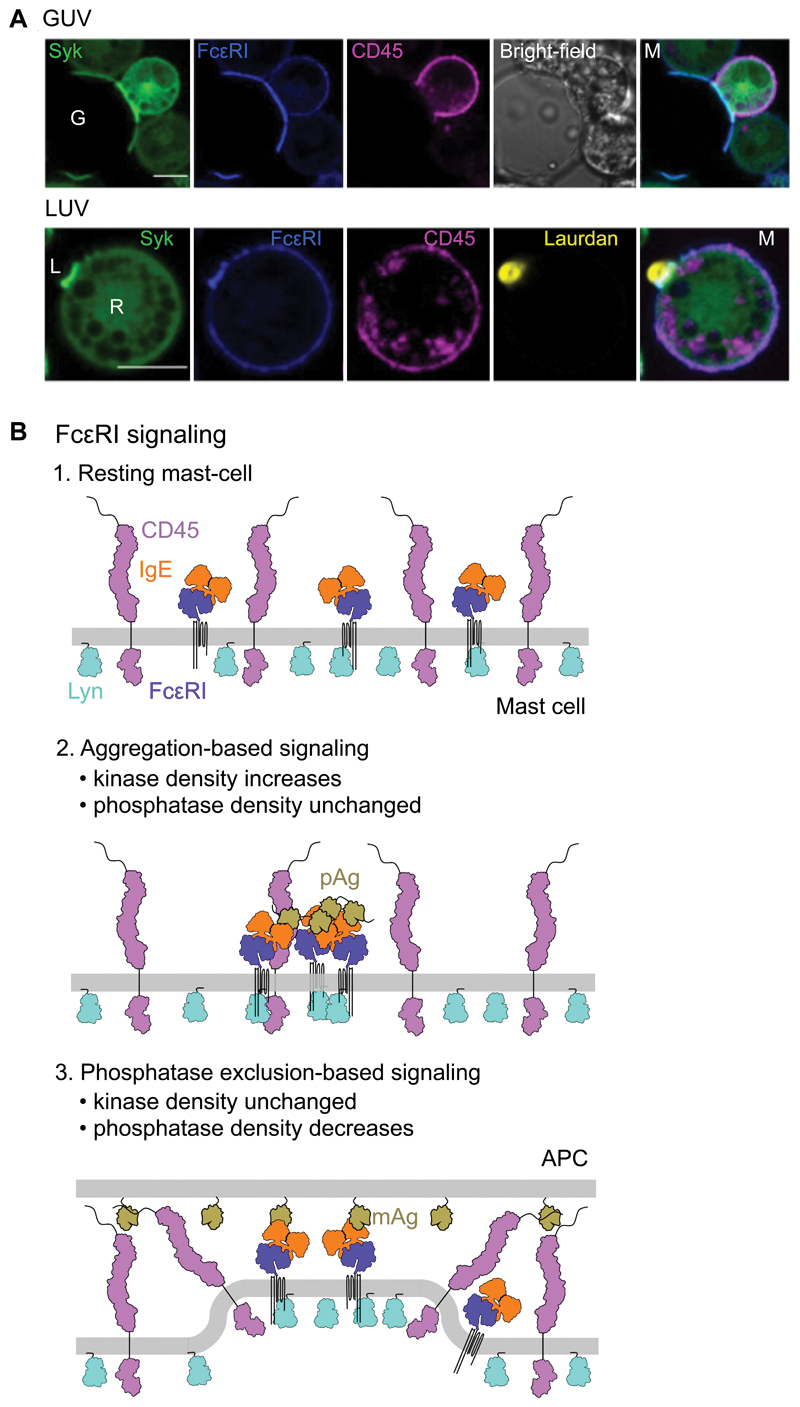
To confirm that signaling could in principle be driven by passive phosphatase exclusion alone, we tested whether signaling was initiated by unilamellar vesicles presenting FcεRI ligand mimics. Large and small unilamellar vesicles (LUVs and GUVs) enable ligand mimics to be presented under conditions more reflective of the membrane curvature and lower resistance to deformational forces of cells versus SLBs. GUVs are similar in size or smaller than whole cells but mimic cell-cell associations, whereas LUVs are typically smaller than 2 µm in diameter and so more closely replicate interactions with smaller structures, for example, fragments of opsonized cells or large allergens, such as pollen grains or microvilli. Both exclusion of CD45 and enrichment of FcεRI and Syk were observed at contacts between RBL-2H3 cells and either GUVs or LUVs loaded with His-Fcε (Fig. 7A). Exclusion of CD45 was once again impaired either by truncation of its extracellular domain or by extension of the His-Fcε ligand mimic (fig. S7H). In both cases, Syk recruitment was reduced accordingly, despite the accumulation of comparable amounts of FcεRI.
Fig. 7. FcεRI signaling can be triggered by unilamellar vesicles.
(A) Confocal fluorescence images of Syk, FcεRI, and CD45 at the equatorial plane of RBL-2H3 cells that interacted with GUVs (top) or laudan-stained LUVs (bottom) loaded with Fcε. G, GUV; L, LUV. Scale bars, are 5 µm. Images are representative of observations made in three independent experiments. (B) Schematic representation of the non-triggered state of the resting basophil surface (top) and after the triggering of FcεRI by polyvalent antigen (pAg; middle) or surface-associated monovalent antigen (mAg; bottom).
Discussion
For more than 40 years, FcεRI triggering was considered to be adequately explained by ligand-induced receptor aggregation; however, more recent findings have challenged this view. Principle among these is the observation that some of the best-studied allergens are monovalent (e.g. 23, 24) and that monovalent ligands provoke vigorous mast-cell activation when immobilized on model surfaces (20, 21). Here, we showed that, in the first instance, FcεRI phosphorylation was kept under tonic control by the local balance of kinase and phosphatase activities, which are affected by various factors in addition to receptor aggregation. In this context, the ability of surface-immobilized, monovalent ligands to induce signaling can be explained simply by the exclusion of large phosphatases from regions of FcεRI-ligand engagement. Supporting this view, we went on to show that (i) CD45 was excluded at sites of ligand engagement and Syk recruitment (that is, sites of receptor phosphorylation) after RBL-2H3 cells and primary basophil made contact with ligand-presenting surfaces; (ii) that this effect was size-dependent, that is, that CD45 exclusion and signaling were each abrogated either by increasing the size of the ligand or by reducing the size of the extracellular domain of CD45; (iii) that preventing CD45 exclusion from FcεRI-enriched regions inhibited triggering and degranulation in response to monovalent, surface-associated ligand; and (it) that the exclusion of CD45 was entirely passive, that is, it occurred both in the absence of signaling in intact cells and spontaneously in GPMVs devoid of any active metabolism or ATP. The strongest argument that signaling does not only depend on ligand-driven FcεRI aggregation is that we observed strong activation of RBL-2H3 cells and primary human basophils on surfaces coated with PLL, which appears only to be capable of immobilizing receptors and efficiently excluding CD45 from regions of contact (36).
We, and others, have shown that the TCR can be triggered by the passive, size-dependent exclusion of inhibitory phosphatases from sites of ligand engagement (37, 43, 61). Our findings here indicate that FcεRI can be triggered in an analogous manner. Mast cells form a degranulatory synapse upon encountering monovalent, surface-associated ligand (20), resulting in degranulation in the direction of FcεRI signaling (62), which enables a focused, targeted response to pathogens with surface-presented antigens. Given that basophils and mast cells form stable contacts with T cells and dendritic cells (63–65), phosphatase exclusion–dependent receptor triggering likely contributes to optimal basophil and mast cell responses in vivo. Our observation of low-level FcεRI triggering in cells that made contact with tissue-culture plastic may explain why increased cell adherence correlates with greater sensitivity to antigen (50, 51, 66), and why RBL-2H3 cells grown in suspension have altered protein expression (67) and pharmacological responses (68) compared to those of adherent cells. Signaling in the absence of FcεRI ligands may occur alongside, or even amplify, the effects of other factors that increase mast cell activity due to adhesion, including activation of focal adhesion kinase (69), integrins (51), or receptor tyrosine kinases (70). Nonetheless, such surface-dependent phosphorylation of FcεRI in the absence of ligands appears to be distinct from surface-driven, adhesion-based amplification of FcεRI-triggering dependent signaling. Our finding that FcεRI triggering is not exclusively dependent on the presence of ligands establishes clear ground between ligand-only theories of signaling and explanations that are not explicitly ligand-dependent, such as segregation-based theories.
In this context, it is noteworthy that FcεRI seems to form the smallest possible complexes with its ligands. FcεRIα comprises a strikingly bent structure, such that the IgE-binding domain is located close to the proximal membrane (71). IgE also forms a curved structure and uses an FcεRIα-binding site located next to the hinge-region of the antibody, helping to minimize the distance between the mast cell membrane and the IgE Fab regions (55). These observations suggest that the small size of the complex formed by FcεRI with its ligands is important for its function, perhaps because it facilitates phosphatase exclusion. For a triggering system that relied on transphosphorylation, the most efficient structure for FcεRI would be one that minimizes its hydrodynamic diameter, enabling the closest approach of neighboring receptors. Simple modeling implies that this would be achieved by FcεRI extending directly from the membrane and binding IgE in such a way as to position the Fc orthogonal to the plane of the membrane. However, such a conformation would increase the membrane-Fab separation distance by 5 to 10 nm, likely preventing phosphatase exclusion. FcγRI adopts a similarly bent conformation and binds to the same region of IgG Fc (72), suggesting that for this receptor, the dimensions of the ligand-bound complex might also be pivotal. Together, the topological arguments appear to strengthen the case that an evolutionarily conserved, size-dependent, phosphatase exclusion–based mechanism might drive signaling by Fc receptors.
Studies indicating that FcγR signaling in macrophages also depends on CD45 exclusion are conflicting with regard to whether redistribution of the phosphatase is passive (73) or dependent on integrins first forming an actin-tethered diffusional barrier (33). We did not observe any integrin-dependence of FcεRI signaling. First, neither disrupting integrin activity with a chelating agent nor depletion of CD29, the major integrin β subunit expressed by RBL-2H3 cells, substantially affected FcεRI sensitivity to surface-bound ligand. Second, we observed passive CD45 exclusion and receptor triggering in cells interacting with SLBs that presented FcεRI ligand mimics and with both LUVs and GUVs. Finally, we observed CD45 exclusion in GPMVs which, lacking active cytoskeletons, are incapable of mobilizing any form of actin-tethered diffusional barrier. The chemical and genetic interventions we used have their caveats insofar as CD29 deletion does not globally impair integrin function, and removal of free divalent cations does not physically eliminate integrins from the cell. Nevertheless, the sum of our data argues against there being a strict requirement for integrin mobilization to secure phosphatase exclusion. The findings of Freeman et al. also raise the issue of what is it that “triggers the trigger”, that is, integrin activation, enabling FcγR signaling (33). At the very least, our results suggest that integrin-dependent phosphatase exclusion is not a universal feature of FcR triggering.
Although the case for phosphatase exclusion–based signaling appears to be strong, it needs also to be acknowledged that the role of CD45 in the activation of mast cells is complicated by the fact that it both dephosphorylates ITAMs in activated receptors and activates SFKs by reversing inhibitory phosphorylation at regulatory sites (74). Despite strenuous efforts to understand the role of CD45 in FcεRI signaling, the matter is still incompletely resolved. It has been reported that CD45 is not required for FcεRI phosphorylation or Syk activation in response to receptor aggregation (75), but it is unclear whether this reflects only partial abrogation of SFK activity in the CD45-deficient cells. Crosslinked FcεRI is also proposed to be sequestered into ordered membrane domains with higher kinase activity (14) and reduced phosphatase (including CD45) activity, leading to signaling (13), but other data suggest that rates of dephosphorylation are comparable for receptors in different membrane domains (76), perhaps because such domains are very short-lived (77). The role of CD45 is therefore very complex, and at present, for example, we cannot rule out the formal possibility that SFKs are activated (through the dephosphorylation of inhibitory tyrosines) in regions abundant in excluded CD45, and that these kinases then diffuse into CD45-depleted areas to promote ligand-dependent and -independent FcεRI signaling.
There is also no denying the very potent effects of soluble, multivalent ligands on FcεRI-mediated signaling. These types of ligands would not be expected to substantially affect phosphatase distribution per se (consistent with previous observations; 78), except insofar as crosslinking might lead to the formation of clusters of FcεRI-IgE-antigen that are too dense to allow passive CD45 entry, which could heighten the sensitivity of FcεRI to Lyn. However, even if this is not the case, the two mechanisms need not be mutually exclusive. Aggregation-driven FcεRI triggering likely occurs through local increases in kinase (i.e. Lyn) density (12) in a manner analogous to growth-factor induced signaling, which is simply the mirror-image of the local exclusion of inhibitory phosphatases. As we have suggested elsewhere (43), perhaps the common principle is that signaling is achieved no matter how the local balance in kinase and phosphatase activities is perturbed in favor of the kinases.
We propose that an overlap in the triggering mechanisms would enable them to act side-by-side, maximizing the versatility of FcεRI responses to antigens, depending only on their valency and mode of presentation (Fig. 7B). Some antigens are soluble, multimeric proteins that presumably cannot induce phosphatase exclusion but would be capable of FcεRI aggregation. Others may induce responses principally by excluding phosphatases and may be unable to crosslink FcεRI. Moreover, some antigens may use both mechanisms if they accumulate into aggregates of a sufficient size that they become an effective surface, for example, large aggregates of cytoskeletal proteins, such as the common helminth allergens tropomyosin and paramyosin (79). A capacity for phosphatase exclusion–based receptor triggering may increase the scope for polyclonal IgEs to induce robust responses, because some binding geometries unable to efficiently crosslink the receptor might instead exclude phosphatases in the manner of adhesion proteins, also increasing versatility.
Which triggering mechanism(s) could dominate in vivo is difficult to predict. An obvious requirement of the phosphatase exclusion–based mechanism is that IgE-targeted antigens must be surface-associated. Mast cells respond to surface-bound IgE by forming a secretory synapse in vitro (62), but it is unclear how commonly surface-associated IgE is presented in vivo. Very few protein families (~2%) comprise allergens (79, 80) and they have no shared structural folds (81). However, a shared feature of many common allergens is the ability to bind to cell membranes or components of the extracellular matrix. Lipid-binding activity has been observed for many allergens, including members of the largest allergen family, the prolamins (80), the major birch tree pollen antigen, Bet v 1 (82), and several of the most potent parasite allergens, for example, the fatty acid–binding nematode polyproteins (83, 84). Similarly, the principal cat allergen, Fel d 1, shares close homology with uteroglobin (85), which binds to components of the extracellular matrix (86) and cell membrane (87), whereas many common helminth allergens, for example, the secreted proteins of Ancylostoma (88, 89) and other venom and allergen-like proteins in a range of species (90, 91), belong to the cysteine-rich secretory protein family, at least some of which bind to surface ion channels (92). One sixth of all known allergens are enzymes with hydrolase activity, including lipases and proteases(80) that, together with the potential to increase antigen exposure through weakening of the epithelial barrier, could conceivably form bridging interactions between FcεRI/IgE complexes and physiological surfaces. Examples include dust mite protease allergens that target proteins expressed by human cells (93), and most of the common insect venom antigens, which bind to the extracellular matrix (hyaluronidases) or cell-surface components (phospholipases) (94). The prevalence of surface-binding allergens suggests that phosphatase exclusion–based signaling could be an important driver of mast cell and basophil responses. Furthermore, although FcεRI is primarily involved in degranulatory responses, it is also a driver of phagocytosis and antibody-dependent cellular cytotoxicity in monocytes (95), for which the IgE-targeted antigens are all surface-associated. The use of IgE in monoclonal antibody therapy has begun to show promise (96) and for this, as in the case of IgG (73), the size of target antigens may need to be a consideration given the likely requirement for phosphatase exclusion.
Our finding that FcεRI is triggered by passive phosphatase exclusion adds to the growing body of evidence that noncatalytic tyrosine-phosphorylated receptors (NTRs) might generally be triggered in this way. In other respects, signaling by this class of immune receptors, members of which are usually relatively compact, shares strikingly consistent features: ligand binding induces phosphorylation of the receptors’ immunoreceptor tyrosine-based motif(s) by SFKs, enabling recruitment of SH2 domain–containing tyrosine kinases (for activatory receptors) or tyrosine phosphatases (for inhibitory receptors), which propagate the signal (97). Although best confirmed in the case of the TCR (37, 43), various NTRs are sensitive to the formation and dimensions of close contacts, including C-type lectins (42), CD28 (98), and natural killer cell receptors (99, 100), consistent with a shared dependence on size-based, phosphatase exclusion–dependent receptor signaling. The capacity of FcεRI to also be triggered by receptor aggregation could be a refinement that enables it to respond to a uniquely broad array of ligands.
Materials and Methods
Cell isolation, culture, and handling
RBL-2H3 cells (ATCC CRL-2256) were cultured at 37°C, 5% CO2 in minimum essential medium eagle (MEM; Sigma-Aldrich) supplemented with 15% FCS, 4mM L-glutamine, and 1% penicillin-streptomycin-neomycin solution (Sigma-Aldrich). Twenty-four hours before imaging (or for experiments requiring suspension culture), cells were seeded at 1x106/ml and incubated in 50-ml culture tubes overnight on an end-over-end rotator (6 rpm) at 37°C. Primary human basophils were isolated from leukocyte cones provided by UK NHS Blood and Transplant. Nucleated cells were separated from erythrocytes with the HetaSep aggregation agent (StemCell Technologies) as per the manufacturer’s instructions. Basophils were isolated through negative selection with the EasySep Human Basophil Enrichment Kit (StemCell Technologies) and used immediately after isolation.
Cloning and stable expression of fluorescent constructs
Rat FceR1a was amplified from cDNA prepared from total RBL-2H3 cell RNA using the oligonucleotide primers 5’-TAGTAGACGCGTGCCACCATGGATACTGGAGGATCTGCCCGGCTG-3’ and 5’-CTACTAGGTACCGCCACTCCCACCTTTTTTTTTGCCTTTTCCAGTC-3’ and subcloned into the lentiviral pHR vector upstream of a SNAP-tag-encoding sequence. The resultant construct contained a flexible Gly-Ser-Gly sequence between FceR1a and SNAP-tag. Syk-Citrine was also generated in pHR by amplification of rat Syk from RBL-2H3 cDNA using the primers 5’-TAGTAGACGCGTGCCACCATGGCGGGCAATGCTGTGGACAATG-3’ and 5’-CTACTAGGATCCCCGTTAACCACGTCGTAGTAGTAATTG-3’ and subcloned upstream of the Citrine sequence. Rat CD45 was amplified from RBL-2H3 cDNA using the primers 5’-TAGTAGACGCGTGCCACCATGTATTTGTGGCTCAAACTTCTGGCC-3’ and 5’-CTACTAGGTACCCCTGAGCTCGGGGTTAGAGCTGGGC-3’, which was subcloned into pHR upstream of the HaloTag sequence. For the chimeric CD86CD45 and CD43CD45 forms, the transmembrane- and intracellular domain–encoding segments of rCD45 were amplified using the primers 5’-CCTCCCCCAGACCACGCACTGATTATATTCC-3’ or 5’-GAGAACTCACGAGGCGCACTGATTATATTCC-3’, respectively, to enable overlap with the extracellular domain–encoding segments of hCD86 and hCD43 amplified from cDNA prepared from RNA extracted from human THP-1 cells with the primers 5’-TAGTAGACGCGTGCCACCATGGATCCCCAGTGCACTATG-3’ and 5’-GGAATATAATCAGTGCGTGGTCTGGGGGAGG-3’ (for CD86) or 5’-TAGTAGACGCGTGCCACCATGGCCACGCTTCTCCTTCTCC-3’ and 5’-GGAATATAATCAGTGCGCCTCGTGAGTTCTC-3’ (for CD43). These fragments were used in chimeric PCR reactions to generate the full-length constructs, which were then inserted into pHR upstream of HaloTag. For all fluorescent constructs, human embryonic kidney (HEK) 293 cells were cotransfected with pHR vectors and with pMD.G and p8.91 with Genejuice (Novagen) as per the manufacturer’s instructions. Harvested lentivirus was incubated with RBL-2H3 cells in culture for 72 hours before expression of the relevant proteins was assessed by flow cytometry. A pure population of cells expressing all constructs was then derived by fluorescence-activated cell sorting.
Cloning and purification of His-rFcε fragments
The sequence encoding rat IgE heavy chain domains CH2-CH4 were amplified from cDNA prepared from total rat spleen RNA using the oligonucleotide primers 5’- TAGTAGGTTTAAACTCGGAGGCGCTCGACCTGTCAACATCACCAAGCC-3’ and 5’-CTACTAGAATTCCTACATGGAGGCCTGGGAGGGACGG-3’. For the short His-rFcε form, this was inserted into the pOPINEE12G vector immediately after a sequence encoding the tag N-HHHHHHSRAWRHPQFGGHHHHHH-C. The construct also encoded a heterologous secretory signal peptide (MGILPSPGMPALLSLVSLLSVLLMGCVAE) directly upstream of the His tag. For the CD431 and CD433 forms, the sequence encoding the extracellular domain of hCD43 after the native signal peptide (residues 20 to 254) was inserted either once or in three tandem repeats separated by restriction sites for KpnI, XhoI, and PmeI. The pOPINEE12G vectors were used to transfect Chinese hamster ovary K1 cells, which were then enriched through glutamine synthetase selection. Dilution-plated clones were assessed for expression of secreted protein by Western blotting analysis of their culture medium with anti-His5 and those clones with the best expression were moved to large culture. Cells were cultured in T175 tissue culture flaks in the presence of 2 mM sodium butyrate for 2 weeks before the cell culture medium was harvested and extraction of the His-tagged protein was performed with Ni-NTA agarose beads (Qiagen). Extracted protein was cleaned by fast protein liquid chromatography on a superose 6, 10/300 column (GE Healthcare) and ÄKTA pure system (GE Healthcare). Samples of the proteins were labeled using the Alexa Fluor 647 antibody labeling kit (ThermoFisher Scientific) as per the manufacturer’s instructions for use in flow cytometry experiments.
CRISPR/Cas9 knockout of FcεRIα
Genomic knockout of FceRIa from RBL-2H3 cells was performed with the lentiCRISPR v2.0 (Addgene plasmid #52961) system (101), which was a gift from Dr Feng Zhang (Massachusetts Institute of Technology). The guide sequence 5’-ATTACACAATTGAGTATCGT-3’ was ligated into the vector using BsmBI restriction sites. This sequence targets nucleotides 584 to 603 of rat FceR1a, corresponding to residues 196 to 201 of the translated protein, and has a fidelity score of 85 as determined by the CRISPR design tool (crispr.mit.edu). Lentiviral particles containing this vector sequence were generated by cotransfection of HEK 293T cells with lentiCRISPR v2.0 and with pMD.G and p8.91 using Genejuice (Novagen) as per the manufacturer’s instructions. Harvested lentivirus was incubated with RBL-2H3 cells in culture for 5 days before the cell surface expression of FcεRIα was assessed by flow cytometry analysis of cells stained with Alexa Fluor 647–conjugated mouse IgE. A pure FcεRI-ve population was then derived by fluorescence-activated cell sorting. To obtain an FcεRI-restored (FcεRI+ve) RBL-2H3 line, a CRISPR-resistant FcεRIα mutant was generated by site-directed mutagenesis of the target sequence to 5’-AcTAtACgATcGAaTAcCGg-3’ (mutations in lower case), which translates to the same protein sequence as that encoded by the WT gene. The mutant FceRIa gene was inserted into the lentiviral pHR vector and used to transduce FcεRI-ve RBL-2H3 cells as described earlier. As before, FcεRIα cell surface expression was assessed by flow cytometry and a pure FcεRI+ve RBL-2H3 population of cells was obtained by cell sorting.
CRISPR/Cas9 knockout of CD29
CD29-ve RBL-2H3 cells were generated in the same manner as that described for the FcεRI-ve RBL-2H3 cell line but using the guide sequence 5’-TCATCACATCGTGCAGAAGT-3’, which targets nucleotides 178 to 197 of rat Itgb1. This sequence has a fidelity score of 75 (crispr.mit.edu). A pure population of cells was sorted by staining with FITC-conjugated hamster anti-rat CD29 (Biolegend; 102205).
Flow cytometry
In all cases, cells were labeled with antibody/protein at 1 μg/ml in PBS + 0.05% sodium azide for 1 hour at 4°C. Cells were washed with three times with 4 ml of cold PBS-azide before resuspension in 400 μl of PBS-azide. Fluorescence was measured with a CyAn ADP flow cytometer (Beckman Coulter). To assess cell surface integrin expression, FITC-conjugated hamster anti-rat CD29 (Biolegend; 102205) and FITC-conjugated mouse anti-rat CD49d (Biolegend; 200103) were used. For general rFcεRI staining, mouse anti-TNP IgE conjugated to Alexa Fluor 647 was used. Primary human basophils were stained with anti-CD123 FITC (Biolegend; 306005) and anti-human IgE APC (Biolegend; 325507). To assess Fcε construct binding, RBL-2H3 cells were stained with the relevant Alexa Fluor 647–labeled protein, washed, and then incubated at 37°C in 4 ml of PBS-azide for various times after washing to enable dissociation of Fcε. Cells were then pelleted and resuspended in 400 μl of PBS-azide before analysis.
Preparation of activating surfaces
For all imaging experiments, glass bottom chambers (World Precision Instruments; FD3510) were used for coating, whereas Western blotting experiments were performed in coated plastic 24-well tissue culture plates. For TNPBSA-IgE–coating, surfaces were incubated with 200 µl of trinitrophenol-conjugated bovine serum albumin (TNPBSA; 50 µg/ml, Insight Biotechnology) in coating buffer [50 mM Na2CO3, 50mM NaHCO3, (pH 9.6)] overnight at 4°C. The chambers were washed three times with 200 µl of PBS before 200 µl of mouse anti-TNP IgE (5 µg/ml, unless otherwise specified; BD Biosciences; 554118) in PBS was added to the chamber and incubated at 4°C for 2 hours. Chambers were then washed three times with 200 µl of PBS and covered with 200 µl for PBS for experiments. For PLL coating, surfaces were incubated with 200 µl of 0.01% PLL for 30 min at room temperature and then washed three times with 200 µl of PBS before use. For laminin peptide coating, surfaces were coated with 200 µl of rat laminin peptide (150 µg/ml; Merck Millipore; SCR127) and TNPBSA in coating buffer (50 µg/ml) overnight at 4°C. Chambers were washed and coated with IgE as described earlier. For Der f 1 coating, surfaces were coated with 200 µl of purified Der f 1 (50 µg/ml; Indoor Biotechnologies; NA-DF1-2) in coating buffer overnight at 4°C, and washed three times with 200 µl PBS before use. Before they were stimulated on these surfaces, primary basophils were loaded with human anti-Der f 1 IgE (Absolute Antibody; 1 μg/ml; Ab00132-14.0) for 10 min then washed three times with PBS. For anti-IgE coating, surfaces were coated either with 200 µl of mouse anti-human IgE (50 µg/ml; Biolegend; 325507) or donkey anti-mouse IgG (50 µg/ml; Stratech; 715-001-003 JIR) in coating buffer overnight at 4°C. Surfaces were washed three times with 200 µl of PBS and then the donkey anti-mouse–coated surfaces were incubated with mouse anti-human IgE (5 µg/ml) for 2 hours at room temperature before washing three times with 200 µl of PBS. Coating efficiency was determined with anti-human IgE labeled with the Alexa Fluor 647 antibody-labeling kit (ThermoFisher Scientific) as per the manufacturer’s instructions, with fluorescence intensity at the coverslip surface measured with a Zeiss 780 LSM. SLBs were prepared as described previously (37). Briefly, glass slides were cleaned with piranha solution. Lipid mix (POPC:nickelated lipid, 96:4; 1 mg/ml) was deposited on the glass slides on a spin coater at 3000 rpm for 30 s and hydrated in HBS. His-Fcε (1 μg/ml) or equimolar equivalents of other Fcε chimeras were coated and washed as described previously (37). Ni-coated glass coverslips (Nanocs; CS-NTA-5) were coated with His-Fcε (1 μg/ml) or equimolar equivalents of other Fcε chimeras for 30 min before being washed three times with 500 μl of PBS. Coverslips were then blocked with 5% BSA for 30 min and washed three times with 500 μl of PBS.
Preparation of GPMVs
GPMVs were prepared as described previously (46). Briefly, RBL-2H3 cells were grown in 35-mm plastic cell culture petri dishes. Before GPMV preparation, cells were labeled with HaloTag and SNAP-tag probes and then washed twice with GPMV buffer [10 mM HEPES, 150 NaCl, 2 mM CaCl2, (pH 7.4)] and incubated for 1 hour in GPMV buffer containing 2 mM DTT and 25 mM PFA at 37 °C. GPMVs were then imaged on activating surfaces as described earlier.
Preparation of GUVs and LUVs
GUVs were prepared through an electro-formation method. Lipid mixture (POPC:nickelated lipid, 96:4 molar ratio; 1 mg/ml) was deposited on a platinum wire and dried. the wire was then dipped into a Teflon-coated chamber filled with 300 mM sucrose. GUV formation was triggered by applying a 10-Hz AC field for 1 hour, which was followed by the application of a 2-Hz AC field for 30 min. After GUV formation, 100 µl of the GUV suspension was incubated with His-tagged protein (1 µg/ml) for 30 min. To wash out any unbound protein, the GUV mixture was gently mixed with 1 ml of PBS and allowed to sediment for 30 min. The bottom 100-µl fraction was transferred to a new tube containing 1 ml of PBS. This process was repeated twice. GUVs were imaged in PBS as described earlier. To prepare LUVs, 50 µl of a lipid mix (POPC:nickelated lipid, 96:4 with 0.001 mol% Laurdan; 1 mg/ml) was dried in a glass vial under N2 flow. Next, 200 µl of HBS was added to the dried lipid film, which was then vortexed for 20 s. The suspension was then sonicated for 20 min and the LUVs were loaded in the same manner as described for GUVs.
Cell labeling
Cells were incubated with TMR-HaloTag ligand and SiR-SNAPtag ligand (each at a final concentration of 0.5 µM) at 37ºC for 30 min. Cells were then washed twice with fresh prewarmed medium supplemented with FCS by centrifugation (500g for 2 min) and were incubated in fresh full medium at 37ºC for 30 min to remove unbound fluorescent ligands. Finally, the cells were washed twice by centrifugation (500g for 2 min) and resuspended in imaging medium.
Confocal and IRM imaging
Confocal images were acquired using a Zeiss 780 LSM. Labeled cells were excited at 488 nm, 561 nm, and 633 nm for Citrine, the TMR-HaloTag ligand, and the SiR-SNAP-tag ligand, respectively. All channels were acquired with different tracks to eliminate crosstalk. A 40x W (NA 1.2) objective was used to acquire images. IRM images were obtained using the same instrument with a 488-nm laser line for excitation and 470 to 505 nm for detection in the transmission channel. Values for individual cell surface areas were determined from IRM images by measuring the number of pixels corresponding to thresholded images of each cell using the tracing tool in the ImageJ software. This was converted to μm2 by reference to pixel size.
Fluorescence correlation spectroscopy
FCS was measured using a 40x objective (NA 1.2) on a Zeiss 780 LSM. A diffraction-limited spot was focused on the basal or apical plane of the cells and three measurements for 10 s each were taken on each point. We measured a minimum of 10 cells for each condition. The autocorrelation functions were fitted with a 2D + Triplet model to obtain the transit diffusion time and the number of particles.
Ca2+ imaging
RBL-2H3 cells were labeled with 4 μM Fluo-4 for 30 min at room temperature in RPMI with no supplement. Cells were then washed in PBS and resuspended at ~1x106 cells/ml in PBS. A cell suspension (50 μl) was gently added to the 200 μl of solution on imaging coverslips and image collection was begun immediately. Cells were imaged with a 10x objective (NA=0.45) on a Zeiss spinning disk confocal microscope with 488 nm laser excitation for 600 frames at 1 frame/s and an exposure time of 800 ms. All imaging was performed inside a heated cabinet at 37°C and coverslips, cells, and solutions were allowed to equilibrate to 37°C before imaging. Ca2+ images were analyzed with CalQuo software (102), generating a triggering fraction value for each video and a triggering frame value for each triggered cell. For experiments with PP2-treated or divalent cation–free samples, cells were labeled with Fluo-4 as described earlier and resuspended in either PBS containing 10 μM PP2 or Mg2+/Ca2+-free PBS containing 1.5 mM EDTA. The solution used on the coverslip was the same as that used for cell treatment (that is, +PP2/EDTA). For experiments using soluble ligand, RBL-2H3 cells were plated onto glass coverslips at ~1x105/ml and cultured for 24 hours to obtain adherent cells. Cells were washed with PBS, labeled with Fluo-4, and washed as described earlier before being covered with 200 μl of PBS containing anti-TNP IgE (0.1 μg/ml) at 37°C for 10 min. Cells were washed three times with 200 μl of PBS and image acquisition was begun. At 20 frames (20 s), 20 μl of TNPBSA in PBS (1 μg/ml) was added to give a final concentration of 100 ng/ml.
Cell stimulation and Western blotting analysis
Cells were treated with either 10 μM PP2 or 10 μM pervanadate for 15 min at room temperature, or with PP2 for 15 min and then pervanadate for 15 min, or vice versa. Pervanadate was freshly prepared using 1 mM sodium orthovanadate and 0.3% hydrogen peroxide in 20 mM HEPES for 5 min before neutralization with bovine catalase. Stimulation of RBL-2H3 cells by soluble ligand was performed by incubation with anti-TNP IgE (1 μg/ml) for 15 min at room temperature, before washing and incubating with TNPBSA (100 ng/ml) for 15 min at room temperature. Primary basophils were stimulated in solution using mouse anti-human IgE (0.5 μg/ml). Cells stimulated on surfaces were allowed to settle at room temperature for various times before solublization. After stimulation, 0.5x106 cells in 100 μl of PBS were solublized for 20 min on ice by the addition of 10 μl of 10x RIPA buffer (Cell Signaling Technology) supplemented with 20 mM sodium orthovanadate, 30 mM EDTA-free protease inhibitors (Sigma-Aldrich; 4693132001), and 1% protease inhibitor cocktail (Sigma-Aldrich; P5726). Solublized samples were centrifuged at 14000g for 5 min to remove protein aggregates, diluted in 4x SDS protein loading buffer with 10% β-mercaptoethanol, and separated on mini-PROTEAN TGX polyacrylamide gels (BioRad). Proteins were transferred to nitrocellulose membrane, blocked with Odyssesy blocking buffer (Li-Cor) for 3 hours, and then incubated with rabbit anti-FcεRIγ (Merck-Millipore; 06727) and mouse phopho-specific anti-FcεRIγ antibodies (34), kindly provided by Dr Ryo Suzuki, Nagoya City University, Japan. Donkey anti-rabbit IRDye 680RD (Li-Cor) and donkey anti-mouse IRDye 800CW (Li-Cor) secondary antibodies were used to enable simultaneous imaging and quantification in both channels using a Li-Cor Odyessey CLx infrared imaging system. In all experiments, a soluble triggered sample was included for normalization. All data were normalized to this value using the following equation:
β-hexosaminidase release assay
RBL-2H3 cells (5 x 104) were allowed to settle onto activating or non-activating surfaces in a total volume of 100 μl of HEPES buffer (10 mM HEPES, 137 mM NaCl, 2.7 mM KCl, 0.4 mM Na2HPO4.7H2O, 5.6 mM glucose 1.3 mM MgSO4.7H2O, 0.04% BSA) for 30 min at 37°C without CO2. A sample of supernatant (75 μl) was removed and centrifuged at 11,000g for 1 min in a 1.5-ml tube to remove any cells. Of this, 50 μl was then transferred to a well of a transparent, flat-bottomed 96-well plate. Negative control wells containing only HEPES buffer were also plated. We then added 100 μl of p-nitrophenyl N-acetyl-β-D-glucosamide (3.5 mg/ml in 40 mM citric acid), 20 mM Na2HPO4.7H2O (pH 4.5) to each well and incubated the samples for 90 min at 37°C without CO2. The reaction was stopped after 90 min by the addition of 50 μl of 400 mM glycine (pH 10.7). Absorbance at 405 nm was measured using a SPECTROstar Nano platereader. Background was established relative to the wells containing HEPES buffer alone. The percentage degranulation was calculated by reference to the absorbance at 405 nm of RBL-2H3 cells solublized with 0.1% Triton X-100, which was defined as 100%.
Measurement of cell adhesion and dendricity
Cell adhesion was measured as a function of the mechanical force required to detach cells from a cultured plastic surface. RBL-2H3 cells were plated at 3x105 cells/well in a plastic 6-well plate in supplemented MEM and incubated for 24 hours. Medium containing any non-adherent cells was removed and the adherent cells were washed with 1 ml of PBS. Cells were then covered with 2 ml of PBS and placed onto a bench-top orbital shaker at varying speeds for 30 min at room temperature. After shaking, the number of detached cells in solution was counted using a hemocytometer. The remaining adherent cells were detached by incubation with trypsin and EDTA for 10 min and counted in the same manner. Spontaneous growth in suspension was determined by plating the RBL-2H3 cells at 3x105 cells/well in a plastic 6-well plate in supplemented MEM and incubating them for 24 to 72 hours before assessing the suspension and adherent fractions as described earlier. Cell dendricity was determined by collecting DiC images of RBL-2H3 cell populations cultured on glass coverslips for 24 hours after plating at 3x105 cells/ml. Cells were qualitatively classed as dendritic if they exhibited protruding dendrites from the main cell body, whereas those cells with roughly spherical morphologies were classed as non-dendritic.
Statistical analysis
All statistical analysis was performed using the GraphPad Prism 7.0a software. Datasets were assessed for significant differences using an unpaired two-tailed t test. For comparisons of values from individual cells (cell contact area; time to triggering; FCS data), data from all cells were pooled before analysis. For all others, an individual value was determined for each independent experiment (for example, percentage cells triggered) and these were pooled for analysis.
Supplementary Material
Acknowledgments
We thank Dr Ryo Suzuki (Nagoya City University, Japan) for kindly providing the phopsho-specific FcεRIγ antibody, and Dr Feng Zhang (Massachusetts Institute of Technology) for supplying Addgene plasmid #52961.
Funding
This work was supported by the Wellcome Trust (098274/Z/12/Z to S.J.D., 100262/Z/12/Z to M.L.D., 104924/14/Z/14 to C.E., and 107375/Z/15/Z to J.H.F.) and the Kennedy Trust for Rheumatology. We further acknowledge financial support from the Wolfson Foundation, the Medical Research Council (MRC; grant MC_UU_12010/Unit Programmes G0902418 and MC_UU_12025), UK Research Councils (Grant MR/K01577X/1), and Oxford internal funds (EPA Cephalosporin Fund and John Fell Fund). We especially thank the Wolfson Imaging Centre as well as the Micron Advanced Imaging Unit (Welcome Trust Strategic Award 091911) for microscopy support. E.S. was supported by EMBO Long Term (ALTF 636-2013) and Marie Sklodowska-Curie Intra-European Fellowships (MEMBRANE DYNAMICS-627088). The Li-Cor Odyssey CLX imaging system was purchased with funding from the European Research Council (AdG 670930).
Footnotes
Author contributions: S.J.D., C.E., J.H.F., and E.S. conceptualized the project; S.J.D., C.E., and M.L.D. provided management and supervision; S.J.D., C.E., J.H.F., E.S., and M.L.D. devised the experimental approach; J.H.F., E.S., M.W., and H.B. performed the experiments; J.H.F. and E.S. analyzed the data; J.H.F., S.J.D., E.S., C.E., and M.L.D. wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
References and Notes
- 1.Rivera J, Olivera A. A current understanding of Fc epsilon RI-dependent mast cell activation. Current allergy and asthma reports. 2008;8:14–20. doi: 10.1007/s11882-008-0004-z. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 2.Blank U, Ra C, Miller L, White K, Metzger H, Kinet JP. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. published online EpubJan. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki R, Scheffel J, Rivera J. New insights on the signaling and function of the high-affinity receptor for IgE. Current topics in microbiology and immunology. 2015;388:63–90. doi: 10.1007/978-3-319-13725-4_4. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T, Suzuki R, Backlund PS, Yamashita Y, Yergey AL, Rivera J. Differential dephosphorylation of the FcR gamma immunoreceptor tyrosine-based activation motif tyrosines with dissimilar potential for activating Syk. Journal of Biological Chemistry. 2008;283:28584–28594. doi: 10.1074/jbc.M802679200. published online EpubOct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metzger H. The IgE-mast cell system as a paradigm for the study of antibody mechanisms. Immunol Rev. 1978;41:186–199. doi: 10.1111/j.1600-065x.1978.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 6.Metzger H. In: In Receptors and Recognition. Cuatrecasas P, Greaves MF, editors. Vol. 4. London: Chapman & Hall; 1977. The cellular receptor for IgE; p. 75. [Google Scholar]
- 7.Ishizaka K, Ishizaka T. Immune mechanisms of reversed type reaginic hypersensitivity. J Immunol. 1969;103:588. [PubMed] [Google Scholar]
- 8.Ishizaka K, Ishizaka T, Lee EH. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. 1970;7:687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- 9.Segal DM, Taurog JD, Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977;74:2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paar JM, Harris NT, Holowka D, Baird B. Bivalent Ligands with rigid double-stranded DNA spacers reveal structural constraints on signaling by Fc epsilon RI. Journal of Immunology. 2002;169:856–864. doi: 10.4049/jimmunol.169.2.856. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 11.Sil D, Lee JB, Luo D, Holowka D, Baird B. Trivalent ligands with rigid DNA spacers reveal structural requirements for IgE receptor signaling in RBL mast cells. Acs Chemical Biology. 2007;2:674–684. doi: 10.1021/cb7001472. published online EpubOct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vonakis BM, Chen HX, HaleemSmith H, Metzger H. The unique domain as the site on Lyn kinase for its constitutive association with the high affinity receptor for IgE. Journal of Biological Chemistry. 1997;272:24072–24080. doi: 10.1074/jbc.272.38.24072. published online EpubSep. [DOI] [PubMed] [Google Scholar]
- 13.Young RM, Zheng XM, Holowka D, Baird B. Reconstitution of regulated phosphorylation of Fc epsilon RI by a lipid raft-excluded protein-tyrosine phosphatase. Journal of Biological Chemistry. 2005;280:1230–1235. doi: 10.1074/jbc.M408339200. published online EpubJan. [DOI] [PubMed] [Google Scholar]
- 14.Young RM, Holowka D, Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. Journal of Biological Chemistry. 2003;278:20746–20752. doi: 10.1074/jbc.M211402200. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 15.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwakura JI, Otani IM, Kawakami T. Monomeric IgE and mast cell development, survival, and function. Mast Cell Biology: Contemporary and Emerging Topics. 2011;716:29–46. doi: 10.1007/978-1-4419-9533-9_3. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, Kinet JP, Galli SJ. IgE enhances mouse mast cell Fc epsilon RI expression in vitro and in vivo: Evidence for a novel amplification mechanism in IgE-dependent reactions. Journal of Experimental Medicine. 1997;185:663–672. doi: 10.1084/jem.185.4.663. published online EpubFeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwakura JI, Okayama Y, Furue M, Kabashima K, Shimada S, Ra C, Siraganian RP, Kawakami Y, Kawakami T. Most Highly Cytokinergic IgEs Have Polyreactivity to Autoantigens. Allergy Asthma & Immunology Research. 2012;4:332–340. doi: 10.4168/aair.2012.4.6.332. published online EpubNov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torigoe C, Metzger H. Spontaneous phosphorylation of the receptor with high affinity for IgE in transfected fibroblasts. Biochemistry. 2001;40:4016–4025. doi: 10.1021/bi0027534. published online EpubApr. [DOI] [PubMed] [Google Scholar]
- 20.Carroll-Portillo A, Spendier K, Pfeiffer J, Griffiths G, Li HT, Lidke KA, Oliver JM, Lidke DS, Thomas JL, Wilson BS, Timlin JA. Formation of a Mast Cell Synapse: Fc epsilon RI Membrane Dynamics upon Binding Mobile or Immobilized Ligands on Surfaces. Journal of Immunology. 2010;184:1328–1338. doi: 10.4049/jimmunol.0903071. published online EpubFeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balakrishnan K, Hsu FJ, Cooper AD, McConnell HM. Lipid Hapten Containing Membrane Targets Can Trigger Specific Immunoglobulin E-dependent Degranulation of Rat Basophil Leukemia Cells. Journal of Biological Chemistry. 1982;257:6427–6433. [PubMed] [Google Scholar]
- 22.Volkmann C, Brings N, Becker M, Hobeika E, Yang J, Reth M. Molecular requirements of the B-cell antigen receptor for sensing monovalent antigens. Embo J. 2016;35:2371–2381. doi: 10.15252/embj.201694177. published online EpubNov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chruszcz M, Chapman MD, Vailes LD, Stura EA, Saint-Remy JM, Minor W, Pomes A. Crystal Structures of Mite Allergens Der f 1 and Der p 1 Reveal Differences in Surface-Exposed Residues that May Influence Antibody Binding. Journal of Molecular Biology. 2009;386:520–530. doi: 10.1016/j.jmb.2008.12.049. published online EpubFeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouvinen J, Rautiainen J, Virtanen T, Zeiler T, Kauppinen J, Taivainen A, Mantyjarvi R. Probing the molecular basis of allergy - Three-dimensional structure of the bovine lipocalin allergen Bos d 2. Journal of Biological Chemistry. 1999;274:2337–2343. doi: 10.1074/jbc.274.4.2337. published online EpubJan 22. [DOI] [PubMed] [Google Scholar]
- 25.Shin DH, Lee JY, Hwang KY, Kim KK, Suh SW. High-resolution crystal structure of the non-specific lipid-transfer protein from maize seedlings. Structure. 1995;3:189–199. doi: 10.1016/s0969-2126(01)00149-6. published online EpubFeb 15. [DOI] [PubMed] [Google Scholar]
- 26.Yang XJ, Moffat K. Insights into specificity of cleavage and mechanism of cell entry from the crystal structure of the highly specific Aspergillus ribotoxin, restrictocin. Structure. 1996;4:837–852. doi: 10.1016/s0969-2126(96)00090-1. published online EpubJul 15. [DOI] [PubMed] [Google Scholar]
- 27.Rouvinen J, Janis J, Laukkanen L-M, Jylha S, Niemi M, Paivinen T, Makinen-Kiljunen S, Haahtela T, Soderlund H, Takkinen K. Transient Dimers of Allergens. Plos One. 2010;5 doi: 10.1371/journal.pone.0009037. published online EpubFeb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemi MH, Rytkonen-Nissinen M, Miettinen I, Janis J, Virtanen T, Rouvinen J. Dimerization of lipocalin allergens. Scientific Reports. 2015;5 doi: 10.1038/srep13841. Published online EpubSep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakurai K, Goto Y. Manipulating monomer-dimer equilibrium of bovine beta-lactoglobulin by amino acid substitution. Journal of Biological Chemistry. 2002;277:25735–25740. doi: 10.1074/jbc.M203659200. published online EpubJul 12. [DOI] [PubMed] [Google Scholar]
- 30.Antens CJM, Oldenwening M, Wolse A, Gehring U, Smit HA, Aalberse RC, Kerkhof M, Gerritsen J, de Jongste JC, Brunekreef B. Repeated measurements of mite and pet allergen levels in house dust over a time period of 8 years. Clinical and Experimental Allergy. 2006;36:1525–1531. doi: 10.1111/j.1365-2222.2006.02603.x. published online EpubDec. [DOI] [PubMed] [Google Scholar]
- 31.Custis NJ, Woodfolk JA, Vaughan JW, Platts-Mills TAE. Quantitative measurement of airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clinical and Experimental Allergy. 2003;33:986–991. doi: 10.1046/j.1365-2222.2003.01706.x. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 32.Traidl-Hoffmann C, Jakob T, Behrendt H. Determinants of allergenicity. Journal of Allergy and Clinical Immunology. 2009;123:558–566. doi: 10.1016/j.jaci.2008.12.003. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 33.Freeman SA, Goyette J, Furuya W, Woods EC, Bertozzi CR, Bergmeier W, Hinz B, van der Merwe PA, Das R, Grinstein S. Integrins Form an Expanding Diffusional Barrier that Coordinates Phagocytosis. Cell. 2016;164:128–140. doi: 10.1016/j.cell.2015.11.048. published online EpubJan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki R, Leach S, Dema B, Rivera J. Characterization of a Phospho-Specific Antibody to the Fc epsilon Receptor γ Chain, Reveals Differences in the Regulation of Syk and Akt Phosphorylation. Antibodies. 2013;2:321–337. [Google Scholar]
- 35.Wang HF, La Russa M, Qi LS. In: Annual Review of Biochemistry. Kornberg RD, editor. Vol. 85. Annual Reviews; Palo Alto: 2016. pp. 227–264. 85. [DOI] [PubMed] [Google Scholar]
- 36.Santos AM, Ponjavic A, Fritzsche M, Fernandes RA, de la Serna J Bernardino, Wilcock MJ, Schneider F, Urbancic I, McColl J, Anzilotti C, Ganzinger KA, et al. Capturing resting T-cells: perils of PLL. Naure Immunology. 2018;19:203–105. doi: 10.1038/s41590-018-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang VT, Fernandes RA, Ganzinger KA, Lee SF, Siebold C, McColl J, Jonsson P, Palayret M, Harlos K, Coles CH, Jones EY, et al. Initiation of T cell signaling by CD45 segregation at 'close contacts'. Nature Immunology. 2016;17:574. doi: 10.1038/ni.3392. +. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandes RA, Yu C, Carmo AM, Evans EJ, van der Merwe PA, Davis SJ. What Controls T Cell Receptor Phosphorylation? Cell. 2010;142:668–669. doi: 10.1016/j.cell.2010.08.018. published online EpubSep. [DOI] [PubMed] [Google Scholar]
- 39.Manz BN, Tan YX, Courtney AH, Rutaganira F, Palmer E, Shokat KM, Weiss A. Small molecule inhibition of Csk alters affinity recognition by T cells. Elife. 2015;4 doi: 10.7554/eLife.08088. published online EpubAug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustelin T, Coggeshall KM, Isakov N, Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990;247:1584–1587. doi: 10.1126/science.2138816. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 41.Secrist JP, Burns LA, Karnitz L, Koretzky GA, Abraham RT. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993;268:5886–5893. published online EpubMar. [PubMed] [Google Scholar]
- 42.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan ASH, Magee AS, Danielson ME, Weiss A, et al. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature. 2011;472:471–U541. doi: 10.1038/nature10071. published online EpubApr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nature Immunology. 2006;7:803–809. doi: 10.1038/ni1389. published online EpubAug. [DOI] [PubMed] [Google Scholar]
- 44.Grochowy G, Hermiston ML, Kuhny M, Weiss A, Huber M. Requirement for CD45 in fine-tuning mast cell responses mediated by different ligand-receptor systems. Cellular Signalling. 2009;21:1277–1286. doi: 10.1016/j.cellsig.2009.03.018. published online EpubAug. [DOI] [PubMed] [Google Scholar]
- 45.Murakami K, Sato S, Nagasawa S, Yamashita T. Regulation of mast cell signaling through high-affinity IgE receptor by CD45 protein tyrosine phosphatase. International Immunology. 2000;12:169–176. doi: 10.1093/intimm/12.2.169. published online EpubFeb. [DOI] [PubMed] [Google Scholar]
- 46.Sezgin E, Kaiser HJ, Baumgart T, Schwille P, Simons K, Levental I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nature protocols. 2012;7:1042–1051. doi: 10.1038/nprot.2012.059. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 47.Zhang K, Chen JF. The regulation of integrin function by divalent cations. Celll Adhes Migr. 2012;6:20–29. doi: 10.4161/cam.18702. published online EpubJan-Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, Lassmann T, Carninci P, Hayashizaki Y, Zuberbier T, Forrest ARR, et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood. 2014;123:E58–E67. doi: 10.1182/blood-2013-02-483792. published online EpubApr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sperr WR, Agis H, Czerwenka K, Klepetko W, Kubista E, Boltznitulescu G, Lechner K, Valent P. Differential expression of cell surface integrins on human mast cells and human basophils. Annals of Hematology. 1992;65:10–16. doi: 10.1007/bf01715119. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 50.Apgar JR. Increased degranulation and phospholipase A(2), C, and D activity in RBL cells stimulated through Fc epsilon R1 is due to spreading and not simply adhesion. Journal of Cell Science. 1997;110:771–780. doi: 10.1242/jcs.110.6.771. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 51.Ra CS, Yasuda M, Yagita H, Okumura K. Fibronectin receptor integrins are involved in mast cell activation. Journal of Allergy and Clinical Immunology. 1994;94:625–628. doi: 10.1016/0091-6749(94)90139-2. published online EpubSep. [DOI] [PubMed] [Google Scholar]
- 52.Thompson HL, Burbelo PD, Seguireal B, Yamada Y, Metcalfe DD. Laminin promotes mast cell attachment. Journal of Immunology. 1989;143:2323–2327. published online EpubOct 1. [PubMed] [Google Scholar]
- 53.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microscopy Research and Technique. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::aid-jemt7>3.0.co;2-o. published online EpubNov 1. [DOI] [PubMed] [Google Scholar]
- 54.Vliagoftis H, Metcalfe DD. Characterization of adhesive interactions between mast cells and laminin isoforms: evidence of a principal role for alpha 6 integrin. Immunology. 1997;92:553–560. doi: 10.1046/j.1365-2567.1997.00368.x. published online EpubDec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holdom MD, Davies AM, Nettleship JE, Bagby SC, Dhaliwal B, Girardi E, Hunt J, Gould HJ, Beavil AJ, McDonnell JM, Owens RJ, et al. Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor Fc epsilon RI. Nature Structural & Molecular Biology. 2011;18:571.:U187. doi: 10.1038/nsmb.2044. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cyster JG, Shotton DM, Williams AF. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. Embo Journal. 1991;10:893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. published online EpubApr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cordoba S-P, Choudhuri K, Zhang H, Bridge M, Basat AB, Dustin ML, van der Merwe PA. The large ectodomains of CD45 and CD148 regulate their segregation from and inhibition of ligated T-cell receptor. Blood. 2013;121:4295–4302. doi: 10.1182/blood-2012-07-442251. published online EpubMay 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irles C, Symons A, Michel F, Bakker TR, van der Merwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nature Immunology. 2003;4:189–197. doi: 10.1038/ni877. published online EpubFeb. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz JCD, Zhang XW, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. published online EpubMar 29. [DOI] [PubMed] [Google Scholar]
- 60.Virts E, Barritt D, Siden E, Raschke WC. Murine mast cells and monocytes express distinctive sets of CD45 isoforms. Molecular Immunology. 1997;34:1191–1197. doi: 10.1016/s0161-5890(97)00142-9. published online EpubNov-Dec. [DOI] [PubMed] [Google Scholar]
- 61.James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. published online EpubJul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joulia R, Gaudenzio N, Rodrigues M, Lopez J, Blanchard N, Valitutti S, Espinosa E. Mast cells form antibody-dependent degranulatory synapse for dedicated secretion and defence. Nature Communications. 2015;6 doi: 10.1038/ncomms7174. published online EpubJan. [DOI] [PubMed] [Google Scholar]
- 63.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du YR, Zaph C, van Rooijen N, Comeau MR, et al. MHC class II-dependent basophil-CD4(+) T cell interactions promote T(H)2 cytokine-dependent immunity. Nature Immunology. 2009;10:697.:U645. doi: 10.1038/ni.1740. published online EpubJul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nature Immunology. 2009;10:713–U763. doi: 10.1038/ni.1738. published online EpubJul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4(+) T cells. Nature Immunology. 2009;10:706–U754. doi: 10.1038/ni.1737. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 66.Hamawy MM, Mergenhagen SE, Siraganian RP. Cell adherence to fibronectin and the aggregation of the high affinity immunoglobulin E receptor synergistically regulate tyrosine phosphorylation of 105-115-kDa proteins. Journal of Biological Chemistry. 1993;268:5227–5233. published online EpubMar. [PubMed] [Google Scholar]
- 67.Grodzki CGA, Pastor VDM, Sousa JF, Oliver C, Jamur MC. Differential expression of integrin subunits on adherent and nonadherent mast cells. Brazilian Journal of Medical and Biological Research. 2003;36:1101–1109. doi: 10.1590/s0100-879x2003000800017. published online EpubAug( [DOI] [PubMed] [Google Scholar]
- 68.Wolfe PC, Chang EY, Rivera J, Fewtrell C. Differential effects of the protein kinase C activator phorbol 12-myristate 13-acetate on calcium responses and secretion in adherent and suspended RBL-2H3 mucosal mast cells. Journal of Biological Chemistry. 1996;271:6658–6665. doi: 10.1074/jbc.271.12.6658. published online EpubMar ( [DOI] [PubMed] [Google Scholar]
- 69.Vial D, Oliver C, Jamur MC, Pastor VDM, Trindade ED, Berenstein E, Zhang J, Siraganian RP. Alterations in granule matrix and cell surface of focal adhesion kinase-deficient mast cells. J Immunol. 2003;171:6178–6186. doi: 10.4049/jimmunol.171.11.6178. published online EpubDec. [DOI] [PubMed] [Google Scholar]
- 70.Tan BL, Yazicioglu MN, Ingram D, McCarthy J, Borneo J, Williams DA, Kapur R. Genetic evidence for convergence of c-Kit- and alpha(4) integrin-mediated signals on class IAPI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood. 2003;101:4725–4732. doi: 10.1182/blood-2002-08-2521. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 71.Garman SC, Wurzburg BA, Tarchevskaya SS, Kinet JP, Jardetzky TS. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilon RI alpha. Nature. 2000;406:259–266. doi: 10.1038/35018500. published online EpubJul ( [DOI] [PubMed] [Google Scholar]
- 72.Kiyoshi M, Caaveiro MMJ, Kawai T, Tashiro S, Ide T, Asaoka Y, Hatayama K, Tsumoto K. Structural basis for binding of human IgG1 to its high-affinity human receptor Fc gamma RI. Nature Communications. 2015;6 doi: 10.1038/ncomms7866. published online EpubApr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakalar MH, Joffe AM, Schmid EM, Son S, Podolski M, Fletcher DA. Size-Dependent Segregation Controls Macrophage Phagocytosis of Antibody-Opsonized Targets. Cell. 2018;174:131–142.:e113. doi: 10.1016/j.cell.2018.05.059. published online EpubJun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Justement LB. The role of CD45 in signal transduction. Advances in Immunology, Vol 66. 1997;66:1–65. doi: 10.1016/s0065-2776(08)60595-7. Vol 66. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Siraganian RP. CD45 is essential for Fc epsilon RI signaling by ZAP70, but not Syk, in Syk-negative mast cells. J Immunol. 1999;163:2508–2516. published online EpubSep ( [PubMed] [Google Scholar]
- 76.Peirce M, Metzger H. Detergent-resistant microdomains offer no refuge for proteins phosphorylated by the IgE receptor. J Biol Chem. 2000;275:34976–34982. doi: 10.1074/jbc.M005819200. published online EpubNov. [DOI] [PubMed] [Google Scholar]
- 77.Barua D, Goldstein B. A Mechanistic Model of Early Fc epsilon RI Signaling: Lipid Rafts and the Question of Protection from Dephosphorylation. Plos One. 2012;7 doi: 10.1371/journal.pone.0051669. published online EpubDec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mao SY, Metzger H. Characterization of protein-tyrosine phosphatases that dephosphorylate the high affinity IgE receptor. J Biol Chem. 1997;272:14067–14073. doi: 10.1074/jbc.272.22.14067. published online EpubMay. [DOI] [PubMed] [Google Scholar]
- 79.Fitzsimmons CM, Fakone FH, Dunne DW. Helminth allergens, parasite-specific IgE, and its protective role in human immunity. Frontiers in Immunology. 2014;5 doi: 10.3389/fimmu.2014.00061. published online EpubFeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. Journal of Allergy and Clinical Immunology. 2008;121:847–852. doi: 10.1016/j.jaci.2008.01.025. published online EpubApr. [DOI] [PubMed] [Google Scholar]
- 81.Aalberse RC. Structural biology of allergens. Journal of Allergy and Clinical Immunology. 2000;106:228–238. doi: 10.1067/mai.2000.108434. published online EpubAug ( [DOI] [PubMed] [Google Scholar]
- 82.Mogensen JE, Ferreras M, Wimmer R, Petersen SV, Enghild JJ, Otzen DE. The major allergen from birch tree pollen, Bet v 1, binds and permeabilizes membranes. Biochemistry. 2007;46:3356–3365. doi: 10.1021/bi062058h. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy MW. The nematode polyprotein allergens/antigens. Parasitology Today. 2000;16:373–380. doi: 10.1016/s0169-4758(00)01743-9. published online EpubSep. [DOI] [PubMed] [Google Scholar]
- 84.Tweedie S, Paxton WA, Ingram L, Maizels RM, McReynolds LA, Selkirk ME. Brugia-pahangi and Brugia-malayi - a surface-associated glycoprotein (gp15-400) is composed of multiple tandemly repeated units and processed from a 400-kDa precursor. Experimental Parasitology. 1993;76:156–164. doi: 10.1006/expr.1993.1018. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 85.Morgenstern JP, Griffith IJ, Brauer AW, Rogers BL, Bond JF, Chapman MD, Kuo MC. Amino acid sequence of Fel dI, the major allergen of the domestic cat: protein sequence analysis and cDNA cloning. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:9690–9694. doi: 10.1073/pnas.88.21.9690. published online EpubNov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chowdhury B, Zhang ZJ, Mukherjee AB. Uteroglobin interacts with the heparin-binding site of fibronectin and prevents fibronectin-IgA complex formation found in IgA-nephropathy. Febs Letters. 2008;582:611–615. doi: 10.1016/j.febslet.2008.01.025. published online EpubMar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez KD, Nieto A. Binding of uteroglobin to microsomes and plasmatic membranes. Febs Letters. 1995;361:255–258. doi: 10.1016/0014-5793(95)00167-8. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 88.Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, Goud G, Bottazzi ME, Zhan B, Wang Y, Williamson A, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. Faseb Journal. 2005;19:1743. doi: 10.1096/fj.05-3936fje. +. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 89.Zhan B, Liu YY, Badamchian M, Williamson A, Feng JJ, Loukas A, Hawdon JM, Hotez PJ. Molecular characterisation of the Ancylostoma-secreted protein family from the adult stage of Ancylostoma caninum. International Journal for Parasitology. 2003;33:897–907. doi: 10.1016/s0020-7519(03)00111-5. published online EpubAug. [DOI] [PubMed] [Google Scholar]
- 90.Tawe W, Pearlman E, Unnasch TR, Lustigman S. Angiogenic activity of Onchocerca volvulus recombinant proteins similar to vespid venom antigen 5. Molecular and Biochemical Parasitology. 2000;109:91–99. doi: 10.1016/s0166-6851(00)00231-0. published online EpubJul. [DOI] [PubMed] [Google Scholar]
- 91.Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sanchez-Flores A, Brooks KL, Tracey A, Bobes RJ, Fragoso G, Sciutto E, Aslett M, et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63. doi: 10.1038/nature12031. published online EpubApr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamazaki Y, Koike H, Sugiyama Y, Motoyoshi K, Wada T, Hishinuma S, Mita M, Morita T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. European Journal of Biochemistry. 2002;269:2708–2715. doi: 10.1046/j.1432-1033.2002.02940.x. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 93.Schulz O, Laing P, Sewell HE, Shakib F. Der p I a major allergen of the house dust mite, proteolytically cleaves the low-affinity receptor for human IgE (CD23) European Journal of Immunology. 1995;25:3191–3194. doi: 10.1002/eji.1830251131. published online EpubNov. [DOI] [PubMed] [Google Scholar]
- 94.Ludman SW, Boyle RJ. Stinging insect allergy: current perspectives on venom immunotherapy. J Asthma Allergy. 2015;8:75–86. doi: 10.2147/JAA.S62288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Josephs DH, Bax HJ, Dodev T, Georgouli M, Nakamura M, Pellizzari G, Saul L, Karagiannis P, Cheung A, Herraiz C, Ilieva KM, et al. Anti-Folate Receptor-alpha IgE but not IgG Recruits Macrophages to Attack Tumors via TNFalpha/MCP-1 Signaling. Cancer Res. 2017;77:1127–1141. doi: 10.1158/0008-5472.CAN-16-1829. published online EpubMar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, Fear DJ, Thompson RG, East N, Burke F, Moore RJ, et al. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. published online EpubSep 1 ( [DOI] [PubMed] [Google Scholar]
- 97.Dushek O, Goyette J, van der Merwe PA. Non-catalytic tyrosine-phosphorylated receptors. Immunological Reviews. 2012;250:258–276. doi: 10.1111/imr.12008. published online EpubNov. [DOI] [PubMed] [Google Scholar]
- 98.Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert JCR, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse B, Hunig T, et al. Crystal structure of a soluble CD28-Fab complex. Nature Immunology. 2005;6:271–279. doi: 10.1038/ni1170. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 99.Brzostek J, Chai JG, Gebhardt F, Busch DH, Zhao R, van der Merwe PA, Gould KG. Ligand dimensions are important in controlling NK-cell responses. European Journal of Immunology. 2010;40:2050–2059. doi: 10.1002/eji.201040335. published online EpubJul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kohler K, Xiong SQ, Brzostek J, Mehrabi M, Eissmann P, Harrison A, Cordoba SP, Oddos S, Miloserdov V, Gould K, Burroughs NJ, et al. Matched Sizes of Activating and Inhibitory Receptor/Ligand Pairs Are Required for Optimal Signal Integration by Human Natural Killer Cells. Plos One. 2010;5 doi: 10.1371/journal.pone.0015374. published online EpubNov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nature Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. published online EpubAug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fritzsche M, Fernandes RA, Colin-York H, Santos AM, Lee SF, Lagerholm BC, Davis SJ, Eggeling C. CalQuo: automated, simultaneous single-cell and population-level quantification of global intracellular Ca2+ responses. Sci Rep. 2015;5:16487. doi: 10.1038/srep16487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.