Abstract
Background and Objectives
South Asians are known to have excess adiposity at a lower body mass index, with truncal fat accumulation. Whether this confers higher risk to develop severe COVID-19 is not known. This study evaluated body mass index, body fat mass and waist circumference as risk factors for COVID-19 severity and its progression, in South Asian adults.
Methods and Study Design
Details of COVID-19 patients (19-90 years) were obtained prospectively, along with weight, height, waist circumference and body fat mass assessed by bioelectrical impedance analysis. Binomial logistic and Poisson regression were performed to test associations between waist circumference, body fat mass and body mass index to evaluate the adjusted OR or relative risk for disease severity at admission and length of stay.
Results
After adjusting for age, sex, height and co-morbidities, body mass index >23 kg/m2 (adjusted OR 2.758, 95% CI 1.025, 7.427), waist circumference (adjusted OR 1.047, 95% CI 1.002, 1.093) and body fat mass (adjusted OR 1.111, 95% CI 1.013, 1.219) were associated with a significant risk for disease severity at admission, while only waist circumference (adjusted relative risk 1.004, 95% CI 1.001, 1.008), and body fat mass (adjusted relative risk 1.011, 95% CI 1.003, 1.018), were associated with a significantly longer length of stay.
Conclusions
Body mass index, at a lower cut-off of >23 kg/m2, is a significant risk factor for COVID-19 disease severity in the group of patients studied. The waist circumference and body fat mass are also good indicators for both severity at admission and length of stay.
Keywords: bioelectric impedance analysis, body mass index, body fat, central obesity, COVID-19, SARS-CoV-2, visceral adiposity, waist circumference
Introduction
Obesity and particularly central obesity as defined by body mass index (BMI) and waist circumference (WC), respectively, have consistently emerged as robust risk factors for COVID-19 severity at admission and outcome. For example, a recent UK study reported a dose-response increase in risk for COVID-19 severity at BMI >25 kg/m2, as well as a higher risk associated with central obesity.1 However, it is likely that the BMI-associated risk for severity may appear much earlier in South Asians, who are thought to have higher adiposity at lower BMI with greater truncal fat accumulation.2 It is then useful to additionally test the body fat mass (FM%) and WC (as a proxy for central or visceral adiposity) as direct risk factors. The present study evaluated the association between BMI, FM% and WC with COVID-19 severity at admission, and its progression as length of stay (LOS), in adult patients from a tertiary care hospital, with a BMI range that was similar to earlier reports.1,3
Methods
Admitted patients with COVID-19, aged between 19-90 years, were recruited into the study from a tertiary care hospital, after institutional ethics committee approval (IEC reference number 175/2021) and their written in formed consent. Pregnant women, patients admitted in intensive care (ICU), on inotropic support, dialysis, or who were unable to maintain posture during the height measurement, were excluded. Demographic details, clinical parameters (e.g., oxygen saturation, respiratory rate), inflammatory markers like D-dimer and C-reactive protein (CRP) and disease severity score at admission were obtained from patient records. Disease severity at admission was classified as mild, moderate and severe, based on published national guidelines.4 The hospital course of recruited participants included details on LOS, oxygen requirement, days on oxygen, ventilatory support, need for ICU support and mortality.
Body weight and height were recorded to the nearest 0.01 kg and the nearest 0.1 cm respectively. WC was measured at the mid-point between the lower edge of the rib cage and the iliac crest using a non-metal, non- stretchable measuring tape to the nearest 0.1 cm. Triplicate measurements by two investigators were averaged, with a CV <2%. Bioelectrical impedance was measured at 4 frequencies (BIA, Quadscan 4000, Bodystat Ltd, British Isles) with a standard quality protocol,5 to calculate FM% by equations provided by the manufacturer. Since regular electrode supply was disrupted due to the pandemic, preliminary measurements were made to evaluate whether electrocardiogram (ECG) electrodes could be used for this purpose. Simultaneous measurements by ECG and the Bodystat electrodes showed that the average difference between the measurements was 0.2 (95% CI, -0.1, 0.4) for FM%, with a good correlation (R2=0.98) between electrodes, and therefore, both types of electrodes were used.
Multivariate associations of severity at admission, hospital course parameters, and inflammatory markers were explored with WC, BMI and FM%, adjusting for age (>45y), sex, height (except in the BMI association), diabetes (DM) and hypertension (HTN). BMI was used as both, a continuous and categorical variable (with a cut-off of ≤23 and >23 kg/m2), based on the action point suggested for South Asians.6 However, WC and FM% were only analysed as continuous variables, as there were not adequate numbers available for applying separate sex-based cut-offs for either. For severity at admission, moderate and severe degree of disease were combined into a single category of ‘severe’. Binomial logistic regression was performed for severity at admission, considering ‘mild’ disease as reference. For the skewed distribution of serum CRP at admission, a log linear regression was used, and its slope reported. A Poisson regression model was used for LOS, because of a count response, with effect size in terms of relative risk (RR). An observed false positive rate of <5% was considered statistically significant. R statistical software, version 4.1.0 (R Core Team, 2021, Vienna, Austria), was used.
Results
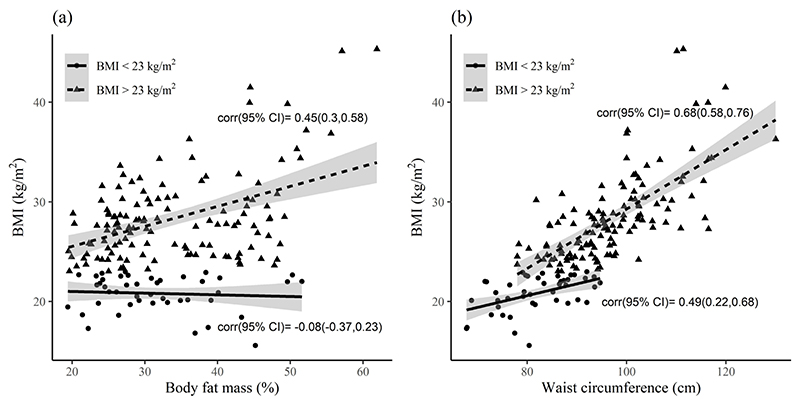
COVID-19 patients (n=172) admitted in a tertiary care hospital between 1st June 2021 to 15th June 2021, were studied. Their range of BMI was 16.8 to 45.3 kg/m2, and their demographic characteristics, anthropometry, BIA, oxygen saturation, inflammatory markers at admission and hospital course of the patients are summarised in Table 1. The sex ratio was skewed toward males (65%). The most common associated co-morbidities were DM (57%) and HTN (27%). About a third of the patients required either ICU care or step-down intensive therapy support during their hospital stay, while one fourth were discharged on home oxygen; 3 deaths occurred. WC was significantly and positively associated with BMI and was stronger (r=0.68, 95% CI 0.58-0.76) when BMI was >23 kg/m2, while FM% showed a significant positive correlation only with BMI >23 kg/m2 (Figure 1a and 1b).
Table 1. Demographics, anthropometry, and clinical parameters of patients.
| Parameter | Mean (SD) | Median (Q1, Q3) |
|---|---|---|
| At recruitment | ||
| Age, y | 51 (13) | 51(41, 60) |
| Anthropometry | ||
| Weight, kg | 70.3 (14.7) | 68.8 (60.6, 78.7) |
| Height, cm | 163 (8.7) | 164 (156, 169) |
| WC, cm | 93.1 (11.4) | 93.0 (86.1, 100) |
| BMI, kg/m2 | 26.4 (5.1) | 25.8 (22.9, 28.8) |
| FM, % | 33.4 (9.4) | 30.5 (25.9, 40.3) |
| At admission | ||
| SpO2, % | 87 (10) | 89 (82, 94) |
| CRP, mg/mL | 8.5 (7.9) | 6.2 (2.3, 11.9) |
| D-dimer, ng/mL | 725 (1238) | 384 (229, 626) |
| Hospital course | ||
| Length of hospital stay, days | 14.6 (9.0) | 12 (8, 20) |
| Days on oxygen | 12.8 (10.4) | 11 (5, 19) |
BMI: body mass index; CRP: C-reactive protein; FM: body fat mass; WC: waist circumference; SpO2: oxygen saturation.
Figure 1.
Association of FM% and WC with BMI below and above 23 kg/m2. Regression lines were fitted for BMI ≤23 (solid line) and >23 kg/m2 (dashed line) in relation to FM% and WC. Shaded portion is the 95% confidence band. (a) FM% in relation to BMI (b) WC in relation to BMI. BMI: body Mass Index; FM%: body fat mass; WC: waist circumference.
In multivariate linear regression, FM%, WC and categorical BMI >23 kg/m2 emerged as significant risk factors for disease severity at admission after adjusting for age, sex, height, DM and HTN (Table 2). BMI as a continuous variable was not associated with severity. For every unit increase in WC or FM%, the AOR of having ‘severe’ COVID-19 at admission was 5% or 11% higher, respectively. The AOR was nearly 3 times higher for severe COVID-19 at admission with BMI >23 kg/m2.
Table 2. Risk factors for ‘severe’ COVID-19 at admission and length of hospital stay†.
| ‘Severe’ versus mild | LOS | CRP, mg/mL | |
|---|---|---|---|
| AOR (95% CI) | ARR (95% CI) | Slope (95% CI) | |
| WC, cm | 1.047 (1.002, 1.093)* | 1.004 (1.001, 1.008)* | 0.019 (0.000, 0.037)* |
| BMI, kg/m2 | 1.089 (0.982, 1.207) | 1.002 (0.994, 1.011) | 0.015 (-0.030, 0.059) |
| BMI > 23 kg/m2 | 2.758 (1.025, 7.427)* | 1.050 (0.960, 1.150) | 0.146 (-0.326, 0.618) |
| Body Fat Mass, % | 1.111 (1.013, 1.219)* | 1.011 (1.003, 1.018)** | 0.045 (0.006, 0.084)* |
AOR: adjusted odds ratio; ARR: adjusted relative risk; LOS: length of hospital stay; CRP: C-reactive protein; WC: waist circumference.
Binomial logistic regression was performed for COVID-19 severity at admission (‘severe’ versus mild); Log linear regression was used for CRP; Poisson regression model was used for LOS; adjusted by stepwise regression for age (>45 y), sex, height, diabetes (DM) and hypertension (HTN).
p<0.05
p<0.01.
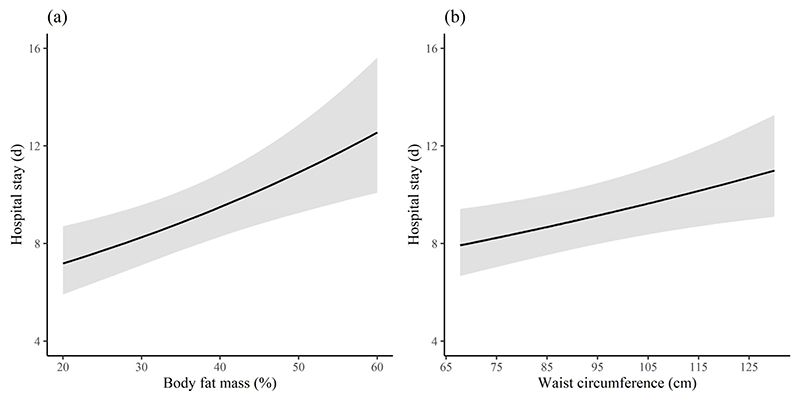
Every 10 cm increase in WC or 10 unit change in FM% had a risk of an extra 9-hour or ~1 day in LOS respectively (Figure 2a and 2b). However, BMI as either a continuous or categorical variable was not associated with LOS. Serum CRP was significantly and positively associated with WC and FM% but not with BMI (Table 2). There was no association of any other anthropometric measure with other characteristics such as days on oxygen, medications used, need for intensive care, other inflammatory markers or discharge outcome.
Figure 2.
Adjusted Poisson regression predicting LOS by FM% and WC. The Poisson regression model was adjusted for sex, age (>45 y), height, diabetes and hypertension. The solid line signifies the mean LOS in response to increasing FM% and WC. Shaded portion is the 95% confidence band. (a) LOS in relation to FM% (b) LOS in relation to WC. LOS: Length of hospital stay (days); FM%: body fat mass; WC: waist circumference.
Discussion
In the present study, the BMI, even at a relatively lower cut off of 23 kg/m2 showed a ~3 times higher odds of COVID-19 severity at admission, in adult patients from a tertiary care hospital. While a similar risk has been noted earlier in a UK group of patients, this magnitude of risk was noted at a much higher BMI range of 35-39.9 kg/m2.1 The finding points towards a higher risk for COVID-19 severity at a lower BMI but with a relatively higher fat accumulation, especially in the truncal region.
In keeping with the framework of excessive fat accumulation, particularly truncal fat, FM% and WC were also significant risk factors for COVID-19 severity at admission and LOS. The effect on LOS has implications for healthcare capacity, personnel utilization and costs, particularly in resource-poor settings. However, BMI did not emerge as a significant risk factor for disease severity at admission or LOS in continuous analyses probably because of the non-linear relation that existed between FM% and BMI, where the FM% variability was high in the BMI ≤23 kg/m2 category, with no correlation. However, there was a significant correlation between FM% and BMI in the >23 kg/m2 category (Figure 2a), with the attendant higher AOR for severity. In addition to this, WC significantly correlated across the BMI range, particularly for BMI >23 kg/m2 (Figure 2b), suggesting that fat mass accretion was probably centrally located.
The WC is a simple method of measuring visceral adiposity and has been shown to be associated with COVID severity and death.1,7 A retrospective analysis of a UK cohort (n=489,769 adults) showed that with every 10 cm increase in WC (measured in 2006-2010) the unadjusted odds of COVID severity significantly increased by 35% (p<0.001).1 In comparison, the AOR was nearly two-fold higher in the present study, which emphasizes the risk associated with central fat accumulation in relatively thin people. In a retrospective analysis on 215 hospitalized patients with COVID-19, WC (≥102 cm for men and ≥82 cm for women) showed a significant association with chest X-ray derived severity scores, rather than the BMI, which is similar to the findings of the present study.8 Human visceral adipose tissue is implicated in severe COVID-19 pathogenesis because it is proinflammatory9 and has a higher gene expression for Angiotensin Converting Enzyme 2 (ACE2) receptor.10 Recent studies have demonstrated how SARS-CoV-2 can infect adipocytes in in vitro11 as well as in vivo settings and that macrophages and preadipocytes within the adipose tissue participate in replication of the virus and in inflammation.12 This is corroborated here with the significant association between FM% or WC with the inflammatory marker, serum CRP, at admission.
The strength of this study is the additional measurement of body composition and WC with BMI in hospitalised COVID patients. Drawbacks include study in a single hospital that is not generalizable, cross-sectional nature, with anthropometry and FM% measurements at different points of hospital stay. The confounding effect of different treatment protocols and premature discharge due to financial or social constraints are other limitations to data interpretation.
In conclusion, the risk of COVID-19 disease severity occurs at a much lower BMI cut-off in South Indians and could be considered for triaging at admission in this group of individuals. WC and FM% also show potential as good risk indicators that can be used for both severity at admission and LOS, and further studies on these lines are required to support the suggestion. The findings also highlight the need for public health interventions that focus on a better body composition and waist size reduction to buffer the impact of the pandemic.
Acknowledgements
We acknowledge the assistance of Ms. Shakila for data abstraction and entry from medical records.
Footnotes
Author Disclosures
The authors declare no conflict of interest. SGH and NS are Fellows of the Clinical Research Training Program of the Wellcome Trust-Dept of Biotechnology, India Alliance Clinical Research Centre grant, awarded to AVK
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Jr, Liang L. Association of obesity and its genetic predisposition with the risk of severe COVID-19: Analysis of population-based cohort data. Metabolism. 2020;112:154345. doi: 10.1016/j.metabol.2020.154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurpad AV, Varadharajan KS, Aeberli I. The thin-fat phenotype and global metabolic disease risk. Curr Opin Clin Nutr Metab Care. 2011;14:542–7. doi: 10.1097/MCO.0b013e32834b6e5e. [DOI] [PubMed] [Google Scholar]
- 3.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, Jebb SA. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9:350–9. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical guidance for management of adult COVID-19 patients. Ministry of Health and Family Welfare; [cited 2021/09/16]. Available from: https://www.icmr.gov.in/pdf/covid/techdoc/COVID_Management_Algorithm_17052021.pdf. [Google Scholar]
- 5.NIH technological assessment statement. Bioelectrical impedance analysis in body composition measurement. U.S.D.H.H.S; Bethesda, USA: 1994. pp. 1–35. [DOI] [Google Scholar]
- 6.World Health Organization. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 7.Peters SA, MacMahon S, Woodward M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK Biobank: comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes Metab. 2021;23:258–62. doi: 10.1111/dom.14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malavazos AE, Secchi F, Basilico S, Capitanio G, Boveri S, Milani V, et al. Abdominal obesity phenotype is associated with COVID-19 chest X-ray severity score better than BMI-based obesity. Eat Weight Disord. 2021;5:1–5. doi: 10.1007/s40519-021-01173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malavazos AE, Corsi Romanelli MM, Bandera F, Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity (Silver Spring) 2020;28:1178–9. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med. 2020;19:100283. doi: 10.1016/j.obmed.2020.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiterer M, Rajan M, Gómez-Banoy N, Lau JD, Gomez-Escobar LG, Gilani A, Alvarez-Mulett S, Sholle ET, Chandar V, Bram Y. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021;33:2174–88.:e5. doi: 10.2139/ssrn.3837640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Colón GJ, Ratnasiri K, Chen H, Jiang S, Zanley E, Rustagi A, et al. SARS-CoV-2 infects human adipose tissue and elicits an inflammatory response consistent with severe COVID-19. bioRxiv. 2021 doi: 10.1101/2021.10.24.465626. [DOI] [Google Scholar]