Abstract
Toll like receptor 3 (TLR3) belongs to a family of pattern recognition receptors that recognise molecules found on pathogens referred to as pathogen associated molecular patterns (PAMPs). Its involvement in innate immunity is well known but despite its presence in the central nervous system (CNS), our knowledge of its function is limited. Here, we have investigated whether TLR3 activation modulates synaptic activity in primary hippocampal cultures and induced pluripotent stem cell (iPSC)-derived neurons. Synaptically driven spontaneous action potential (AP) firing was significantly reduced by the TLR3 specific activator, poly I:C, in a concentration-dependent manner following both short (5 min) and long exposures (1h) in rat hippocampal cultures. Notably, the consequence of TLR3 activation on neuronal function was reproduced in iPSC-derived cortical neurons, with poly I:C (25μg/ml, 1h) significantly inhibiting sAP firing. We examined the mechanisms underlying these effects, with poly I:C significantly reducing peak sodium current, an effect dependent on the MyD88-independent TRIF dependent pathway. Furthermore, poly I:C (25μg/ml, 1h) resulted in a significant reduction in miniature excitatory postsynaptic potential (mEPSC) frequency and amplitude and significantly reduced surface AMPAR expression. These novel findings reveal that TLR3 activation inhibits neuronal excitability and synaptic activity through multiple mechanisms, with this being observed in both rat and human iPSC-derived neurons. These data might provide further insight into how TLR3 activation may contribute to neurodevelopmental disorders following maternal infection and in patients with increased susceptibility to herpes simplex encephalitis.
Keywords: TLR3, TRIF, poly I:C, neuron, iPSC-derived neuron, action potential, Na+ channel, mEPSCs
1. Introduction
Toll like receptors (TLRs) are pattern recognition receptors (PRR) that classically play a pivotal role in activating innate immunity in response to invading pathogens by sensing a diverse range of pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) (Gay et al., 2014; Achek et al., 2016). To date, 11 human and 13 mouse members of the TLR family have been identified with TLRs 1, 2, 4, 5, 6, 10 and 11 being expressed on the membrane surface for ligand recognition whereas TLRs 3, 7, 8 and 9 are primarily expressed intracellularly on endosomes where they are activated by pathogen nucleic acids. All TLRs, with the exception of TLR3 and under certain circumstances, TLR4, use the MyD88-dependent pathway resulting in the release of a diverse range of inflammatory cytokines (Gay et al., 2014; Achek et al., 2016). In contrast, TLR3, which is activated by double stranded RNA (dsRNA), acts through a MyD88-independent, TIR domain-containing adaptor protein inducing IFN-β (TRIF)-dependent pathway which when activated, results in the production of type one interferons (IFNs; Gay et al., 2014; Achek et al., 2016).
Within mammalian cells, TLRs are known to be expressed on a variety of cell types including numerous immune cell types (Gay et al., 2014; Achek et al., 2016). However, it is now known that TLRs are expressed in cells of the central nervous system (CNS) with expression initially proposed to be in astrocytes, microglia and oligodendrocytes but it is now established that neurons and neuronal progenitor cells also express TLRs (Okun et al., 2011; Barichello et al., 2015). TLRs have been proposed to play a role in CNS diseases including Alzheimer’s disease, Parkinson’s disease and multiple sclerosis (Lucas et al., 2006) implicating TLRs as therapeutic targets in these diseases (Gambuzza et al., 2014). In addition, TLRs have been implicated in neurodevelopment including modulating neural precursor cell proliferation and fate, adult neurogenesis and neurite outgrowth (Bsibi et al., 2010; Barichello et al., 2015, Vontell et al., 2015). Indeed, one TLR that has been implicated in both neuronal development and CNS disease is TLR3.
Within the CNS, TLR3 is known to be expressed on neurons, astrocytes, microglia and oligodendrocytes (Okun et al., 2011) with its activation shown to negatively regulate axonal growth, reduce neural precursor cell proliferation and contribute to the long term CNS effects associated with maternal viral infection including Zika virus-induced microcephaly (Cameron et al., 2007; Lathia et al., 2008; Reisinger et al., 2015; Faizan et al., 2016; Chen et al., 2017). In addition, TLR3 was also revealed to regulate spinal cord synaptic transmission and central sensitisation, thus highlighting its critical role in pruritus (Liu et al., 2012). Within the brain itself, TLR3 deficiency in mice leads to enhanced working memory, novel object recognition and contextual fear conditioning as well as increased hippocampal neurogenesis and GluA1 subunit expression (Okun et al., 2010). In contrast, direct activation of TLR3 via intracerebral infusion of a TLR3 ligand has been shown to result in impaired working, but not spatial, memory (Okun et al., 2010) and increased seizure susceptibility and epileptiform activity (Galic et al., 2009; Costello et al., 2013) with TLR3 also proposed to contribute to disease progression when investigated using a pilocarpine model of epilepsy (Gross et al., 2017).
Despite these recent findings, it remains to be elucidated what underlies the impairment in hippocampal memory retention and the exact mechanisms involved. In the present study we reveal that both short and long term exposure of rat primary hippocampal neurons to the TLR3 ligand, polyinosinic:polycytidylic acid (poly I:C), reversibly inhibits spontaneous action potential firing. These effects are mediated via the inhibition of Na+ channel function and a reduction in AMPA receptor expression and are dependent on the classical TLR3-TRIF pathway but are independent of astrocytic activation. Finally, consistent with its activation in rat cultures, TLR3 activation inhibits AP firing and Na+ channel function in human iPSC-derived cortical neurons.
2. Materials and Methods
All experimental procedures were performed in accordance with UK legislation including the Animals (Scientific Procedures) Act 1986 and with approval of the University of Strathclyde Animal Welfare and Ethical Review Body (AWERB). Ethical permission for the use of patient-derived iPSCs was obtained from the National Hospital for Neurology and Neurosurgery and the Institute of Neurology joint research ethics committee (study reference 09/H0716/64; see Sposito et al., 2015).
2.1. Rat primary hippocampal culture: Rat primary hippocampal culture
Primary rat hippocampal cultures were prepared as described previously (Gan et al., 2011) and maintained in a humidified incubator at 37°C/5% CO2 for up to 14 days in vitro (DIV) prior to functional studies. Cytosine β-D-arabinofuranoside (Ara-C, 10μM, Sigma, UK) was added after 5 DIV to prevent further glial cell proliferation. Primary mouse hippocampal cultures were obtained from TLR3-/- breeding colonies (Jackson Labs, stock number: 005217) with TLR3+/+ cultures obtained from MKP-2+/+ breeding colonies (Abdul Rahman et al., 2016). All experiments from both species were performed on cells taken from at least three separate cultures obtained from different animals.
2.2. Human astrocyte and neuron co-culture
Cortical neurons were differentiated from human iPSCs as previously described (Sposito et al., 2015). After 35 DIV, neurons were re-plated and co-cultured on top of human astrocytes (ScienCell cat# 1800, Caltag Medsystems, UK) on laminin and polyornithine coated 13mm glass coverslips and incubated in incubated in a culture medium as described previously (Verheyen et al., 2015). After plating, the media was supplemented with DAPT (10 μM, Sigma, UK) for 3 media changes in the first week. This media was then completely replaced and the co-cultures maintained in the supplemented Neurobasal-A:DMEM medium (Verheyen et al., 2015) and kept in a humidified incubator at 37°C/5% CO2 with half the media replaced twice weekly. All experiments were performed on cells taken from two separate differentiations of iPSCs into cortical neurons that were cultured for ≥80 DIV.
2.3. Electrophysiology
Cultures were perfused continuously with a HEPES-buffered saline (HBS) composed of (in mM): NaCl 140, KCl 5, MgCl2 2, HEPES 10, D-glucose 10 and CaCl2 2, pH 7.4 ± 0.02. Fire polished borosilicate glass microelectrodes (4-6 MΩ, Harvard Apparatus, UK) were filled with an internal solution containing (in mM): KMeSO3 130, KCl 20, HEPES 10, EGTA 0.5, MgATP 4 and GTP 0.3, pH 7.2. All experiments were performed at room temperature (21-23°C) using an Axopatch-200B amplifier (Molecular Devices, USA) connected to a personal computer interfaced with Digidata 1322A interface (Molecular Devices, USA) and captured using pClamp9.0 software (Molecular Devices, USA). In all experiments, neurons with an initial resting membrane potential greater than -55mV were rejected. Spontaneous action potential (sAP) firing, digitised at 5kHz, was recorded from neurons held at -65 mV using whole cell patch clamp in current clamp mode. For current–voltage relationships, cells were held at -70mV in voltage clamp mode with 10mV increments at 10s intervals. mEPSCs, digitized at 5 kHz, were recorded at -70 mV for 5 minute periods in voltage-clamp mode.. Data were analysed offline using pClamp9.2 software (Molecular Devices, USA) and MiniAnalysis software (Synaptosoft, USA).
2.4. Immunocytochemistry
To examine surface AMPA receptor labelling, staining was performed on rat hippocampal cultures (10-12 DIV) treated with vehicle or poly I:C (25μg /ml) for 1h, then immediately fixed with ice cold 4% paraformaldehyde for 10 minutes followed by blocking non-specific binding using a blocking buffer solution (5% FBS v/v and 1% BSA w/v in PBS, all Sigma, UK) for 30 minutes. The cultures were then incubated with a N-terminal GluA1antibody (1:100 in blocking buffer, kindly donated by Dr A. Irving, see Moult et al., 2010) at 4°C overnight followed by corresponding secondary antibodies (Alexa Fluor 488 anti-sheep, 1:200 dilution, Thermo Fisher, UK) in blocking solution for 60 min at room temperature. Image acquisition was performed taking random images with an Olympus BX51W1 microscope with a Q-imaging digital camera, with images acquired using WinFluor v3.4.4 imaging software (J. Dempster, University of Strathclyde, Glasgow, UK). GluA1 fluorescence intensity from vehicle and poly I:C treated cultures was measured using ImageJ software (NIH, USA) with 50 μm regions of interest added to 5 randomly selected neurites per coverslip. For neurites, n represents the number of neurites from at least three separate cultures, with all data expressed as mean ± S.E.M.
2.5. Neuronal Transfection
A plasmid containing a human TLR3 p.Ala795Pro mutant (A795P TLR3, Verstak et al., 2013) was subcloned into the mammalian expression vector, pcDNA3.1/Zeo (Thermo Fisher, UK) and verified by Sanger sequencing (GATC Biotech, Cologne, Germany). Plasmid DNA for mammalian cell transfections were prepared using EndoFree® Plasmids kits (Qiagen, UK). For hippocampal neuronal transfections, cultures (9-10 DIV) were placed in 4 well plates containing conditioned media (400μl). Transfection was achieved using Lipofectamine 2000 (Thermo Fisher, UK) according to the manufacturer’s instructions with either GFP alone (0.5μg/ml) or GFP (0.5μg/ml) and A795P TLR3 (0.5μg/ml) included. The transfection reagents (100μl) were added directly to the cultures for 3h, following which the transfection media removed and replaced by conditioned media. The cultures were maintained post-transfection in a humidified incubator at 37°C/5% CO2, with experiments performed 24-48h post transfection.
2.6. Cytokine quantification
Secreted cytokines were measured, in relation to standard curves, in rat primary hippocampal culture supernatants using the Rat IL-10 Quantikine ELISA Kit (R1000), Rat TNF-α Quantikine ELISA Kit (RTA00) and the Mouse/Rat CCL5/RANTES Quantikine ELISA Kit (MMR00) (all R&D Systems) according to manufacturer’s instructions.
2.7. Western blotting
Primary rat hippocampal cultures were treated with either vehicle or poly I:C (25μg/ml) for 1 hour, washed in ice-cold PBS and then lysed in lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 % Triton X-100, 0.05% deoxycholate, pH7.4, containing a phosphatase and protease inhibitor cocktail (Sigma Aldrich). Lysates were then centrifuged at 16,000 rpm for 10 minutes and the protein content of the supernatant estimated. Equal amounts of protein were heated to 95 °C in SDS sample buffer for 5 minutes, resolved by SDS-PAGE and transferred to nitrocellulose for immunoblotting analysis. Primary antibodies (caspase-3, Cell Signalling Cat# 9665S; total GluA1, Abcam Cat# 109450; GluA1 phospho S845, Abcam Cat# 76321, GluA1 phospho S831 Abcam Cat# 109464, Na+ Sigma Cat# S8809) were used at 1:1000 in PBS-T and secondary antibodies (IR dye; LICOR) at 1:10,000.
2.8. Statistics
All data are expressed as mean ± S.E.M. Data were compared by unpaired Student’s t-tests, one-way or two-way analysis of variance with Dunnett’s or Tukey’s comparison as appropriate, with differences considered significant when P < 0.05.
3. Results
3.1. TLR3 activation reduces spontaneous action potential frequency in rat primary hippocampal cultures
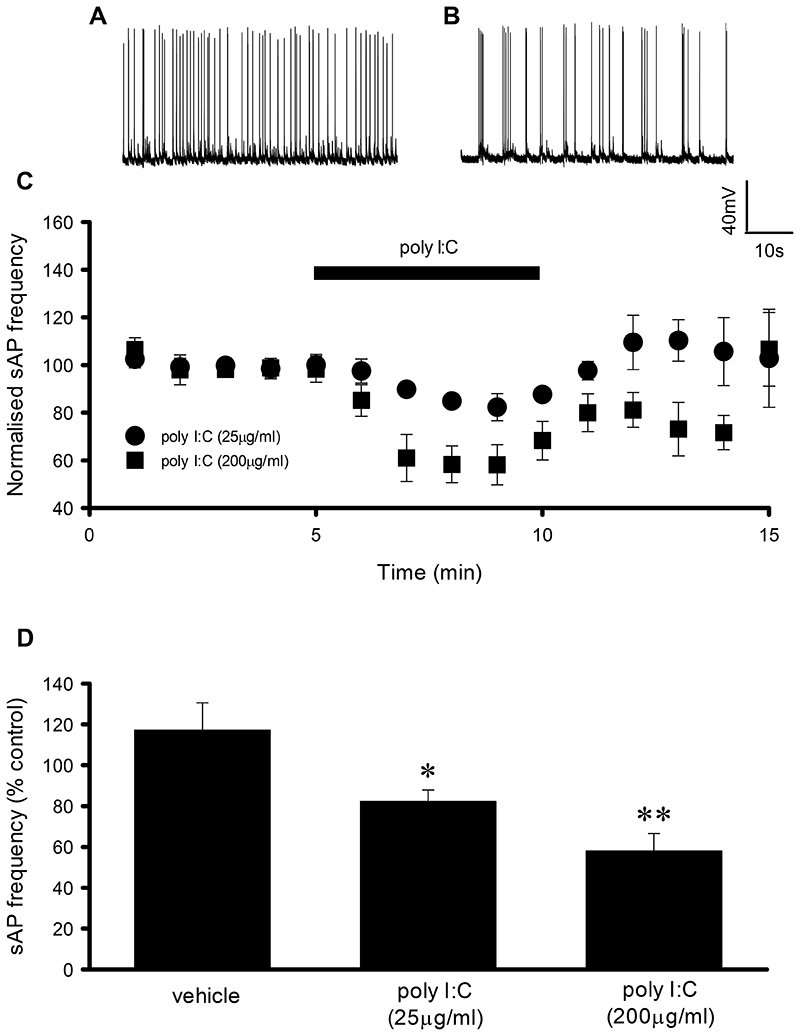
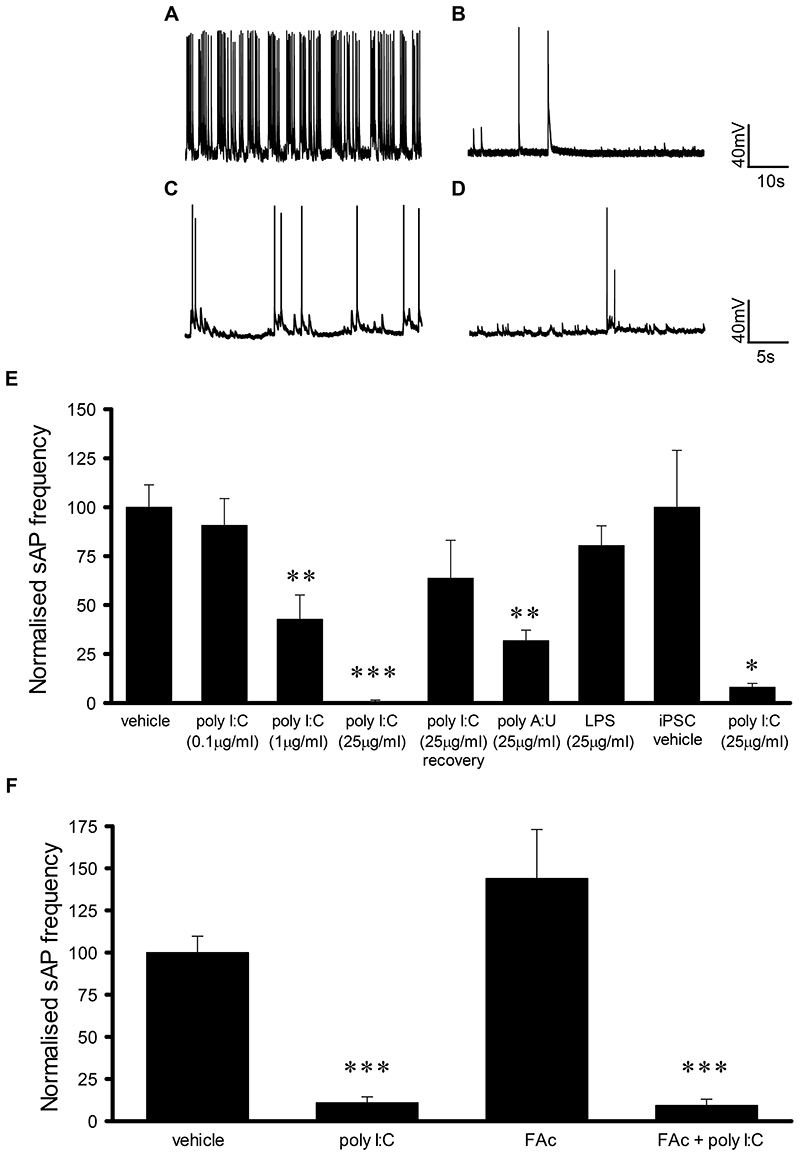
Application of the TLR3 agonist, poly I:C (5min) induced a reversible and concentration-dependent reduction in sAP frequency in cultured hippocampal neurons (25μg/ml, 82.3 ± 5.6% of control, n=6, p<0.05; 200μg/ml, 58.1 ± 8.4% of control, n=6, p<0.01, Fig. 1A-D). In addition, a reversible concentration-dependent reduction of sAP frequency was observed upon extended exposure (1h) to poly I:C (0.1μg/ml, 90.7 ± 13.7% of control, n=6; 1μg/ml, 42.7 ± 12.4% of control, n=6, p<0.01; 25μg/ml, 1.0 ± 0.5% of control, n=8, p<0.001,Fig. 2A-B & E). Furthermore another TLR3 agonist, poly A:U (25μg/ml, 1h) also resulted in a significant reduction in sAP frequency (31.8 ± 5.4% of control, n=5, p<0.01, Fig. 2E). In contrast, application of the TLR2/4 agonist, lipopolysaccharide (LPS, 25μg/ml, 1h) had no significant effect on sAP frequency (80.4 ± 10.1% of control, n=7, Fig. 2E). In addition, neither poly I:C (200μg/ml, 5 min, 0.4 ± 0.2 mV increase compared to control, n=7) nor poly I:C (200μg/ml, 1h, vehicle control 59 ± 1 mV (n=17), poly I:C 59 ± 1 mV (n=8)) altered the neuronal resting membrane potential. As TLR3 is expressed on both neuronal and non-neuronal cells, we also examined whether the effects of poly I:C were mediated directly via neuronally expressed TLR3 or indirectly via astrocytic TLR3. Hence, we inhibited astrocyte function using fluoroacetate (FAc, 10μM, 3h) which we have used to probe astrocyte function in previous studies (17). FAc had no significant effect on sAP frequency (144 ± 29% of control, n=5, Fig. 2F) whereas poly I:C (25μg/ml, 1h) still significantly reduced sAP frequency following FAc pre-treatment (9.3 ± 3.7% of control, n=5, p<0.001, Fig. 2F).
Fig. 1. Short term poly I:C application reduces spontaneous AP firing.
A + B) Representative traces of AP firing from neurons exposed to vehicle and poly I:C (200μg/ml) respectively. C) Time course revealing concentration-dependent and reversible effects of poly I:C application on spontaneous AP firing. D) Bar chart summarising the maximum effect of poly I:C on spontaneous AP firing, *P < 0.05, **P < 0.01. Data are mean ± S.E.M, with n≥6 for each data set taken from at least 3 different cultures.
Fig. 2. Chronic poly I:C application results in a concentration dependent but astrocyte independent reduction in AP firing in both rat and human neurons.
A-B) Representative traces of AP firing from rat hippocampal neurons and C-D) human iPSC-derived neurons exposed to vehicle, poly I:C (1μg/ml, 1h) and poly I:C (25μg/ml, 1h). E) Bar chart summarising the concentration dependent effect of poly I:C on spontaneous AP firing in both rat and human neurons. F) Bar chart summarising the lack of effect of the astrocyte inhibitor, FAc, on poly I:C-induced inhibition of spontaneous AP firing. *P < 0.05, **P < 0.01, ***P < 0.001. Data are mean ± S.E.M, with n≥5 for each data set taken from at least 3 different cultures for rat neurons and from at least 3 differentiations of iPSCs for human neurons.
Given the recent surge in interest in the use of human stem cell derived neurons as a potential means to overcome the known translational issues associated with CNS research, we also examined the consequence of TLR3 activation on sAP firing in human iPSC-derived cortical neurons co-cultured with human astrocytes. In agreement with our findings in rat primary cultures, sAP firing was significantly reduced following exposure to poly I:C (25 μg/ml, 1h), with sAP firing reduced to 8 ± 2% of vehicle-treated controls (control (n=6) v poly I:C treated (n=5), p<0.05, Fig. 2C, D & E).
3.2. TLR3 activation impairs Na+ channel function and reduces AMPA receptor surface expression
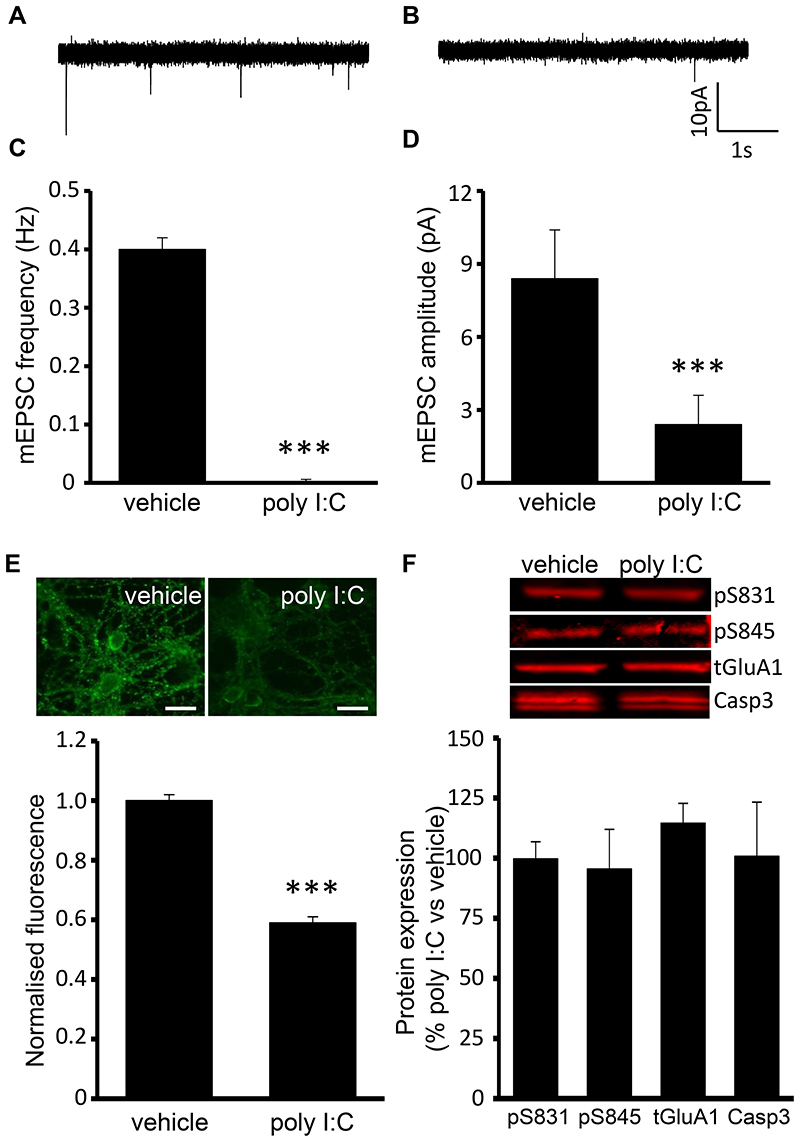
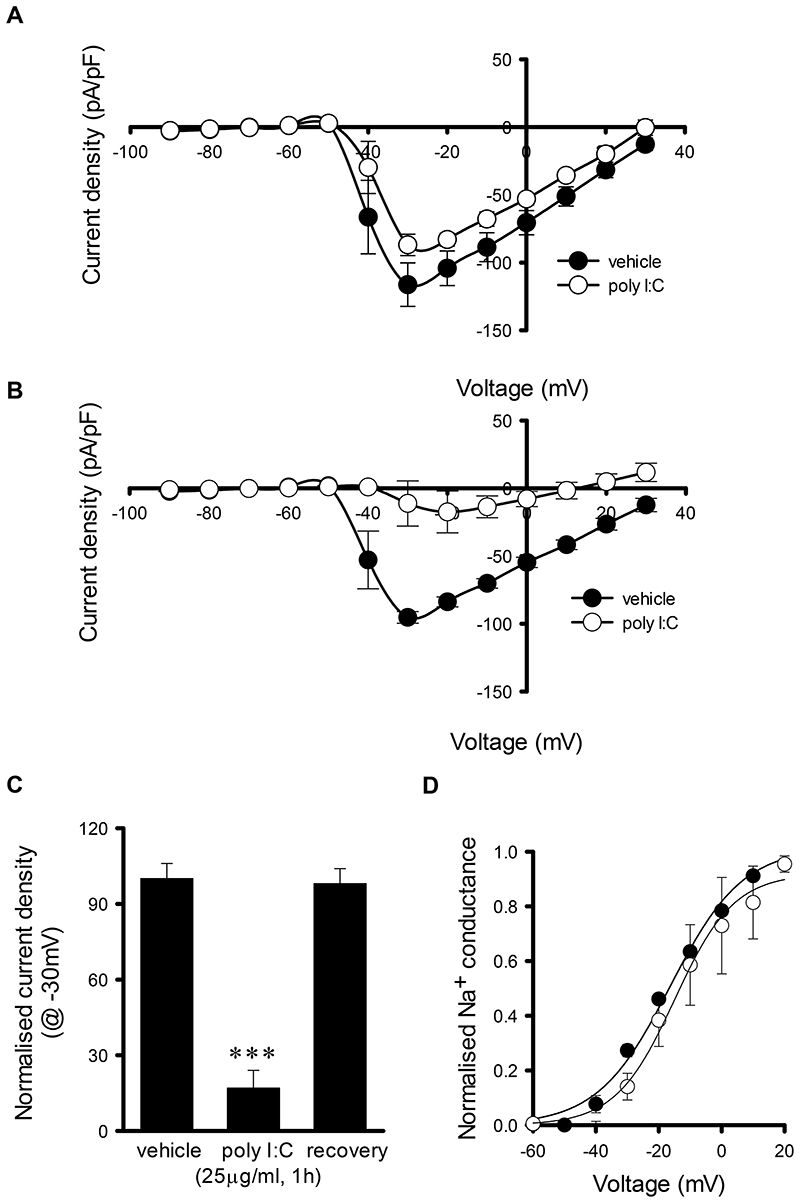
Having demonstrated that TLR3 activation suppresses sAP firing, we next investigated the possible mechanisms underlying this effect. Given that Na+ channels are critical for AP firing, we investigated whether modulation of Na+ channel function was a mechanism by which TLR3 activation inhibited sAP firing. Poly I:C application (200μg/ml, 5min) significantly reduced Na+ current density compared to vehicle treated controls (vehicle control (n=13) v poly I:C treated (n=7), F= 9.76, p=0.002) with peak Na+ current density being 75 ± 7% of control, (P<0.05, Fig. 3A). Furthermore, poly I:C (25μg/ml, 1h) reduced Na+ current density when compared to vehicle treated control cultures (vehicle control (n=21) v poly I:C treated (n=5), F= 123.89, p<0.001) with peak Na+ current density reduced to 17 ± 7% of control (p<0.001, Fig. 3B), which fully recovered upon washout with conditioned media (3h, 98 ± 6% of control, n=8, Fig. 3C). Poly I:C (25μg/ml, 1h) also resulted in a 10mV positive shifted in peak Na+ current density but analysis of Na+ channel activation revealed no significant difference between vehicle (n=21) and poly I:C-treated neurons (n=5, Fig. 3D). Moreover, poly I:C (25μg/ml, 1h) did not alter Na+ channel expression levels as these were 116 ± 38% of vehicle treated controls. As AP firing in primary hippocampal cultures is known to be synaptically driven, we examined whether TLR3 activation modulates miniature excitatory postsynaptic currents (mEPSCs) recorded in the presence of TTX (0.5μM). Poly I:C (200μg/ml, 5min) did not alter mEPSC frequency or amplitude whereas both the frequency (control, 0.4 ± 0.02 Hz, n=9; poly I:C treated, 0.001 ± 0.0005 Hz, n=7, p<0.05, Fig. 4A-C) and amplitude (control, 8.4 ± 2.0 pA, n=9; poly I:C treated, 2.4 ± 1.2 pA, n=7, p<0.05, Fig. 4A, B & D) of mEPSCs were reduced in poly I:C (25μg/ml, 1h) treated cultures. As TLR3 activation reduced the frequency and amplitude of mEPSCs and TLR3 has been reported to modulate AMPA receptor expression (13), we next investigated, using immunocytochemistry, the possibility that a reduction in AMPA receptor surface expression accounted for this observation. Exposure to poly I:C (25μg/ml, 1h) resulted in a significant reduction in AMPA receptor surface expression when compared to control cultures (58 ± 2% of control, p < 0.001, Fig. 4E) whereas, using western blotting techniques, no change in GluA1 S831 phosphorylation (99.7 ± 7.1% of control, n=3, Fig. 4F), GluA1 S845 phosphorylation (95.5 ± 16.5% of control, n=3, Fig. 4F), total GluA1 (114.5 ± 8.3% of control, n=3, Fig. 4F) or caspase 3 activation (100.8 ± 22.5% of control, n=3, Fig. 4F) was observed in poly I:C (25μg/ml, 1h) treated cultures when compared to vehicle treated controls.
Fig. 3. Short term and long term TLR3 activation significantly reduces Na+ current density.
A) I-V curve revealing short term poly I:C (25μg/ml, 5 min) inhibits Na+ current density. B) I-V curve revealing effects of long term poly I:C(25μg/ml, 1h) on Na+ current density. C) Bar chart summarising the effect of short term poly I:C on peak Na+ current. D) Activation curves are similar in the absence and presence of poly I:C (25μg/ml, 1h), *P < 0.05, ***P < 0.001. Data are mean ± S.E.M, with n≥5 for each data set taken from at least 3 different cultures.
Fig. 4. Long term TLR3 activation significantly reduces frequency and amplitude of mEPSCs and surface AMPAR expression.
A + B) Representative traces displaying mEPSCs in the absence and presence of poly I:C (25μg/ml, 1h). C + D) Bar charts revealing no effects of poly I:C (25μg/ml, 1h) on mEPSC frequency and amplitude respectively. E) Representative images and bar chart revealing the effects of poly I:C (25μg/ml, 1h) on AMPAR surface expression. F) Representative blots and bar chart revealing the effects of poly I:C (25μg/ml, 1h) on AMPAR and caspase 3 protein expression. ***P < 0.001. Data are mean ± S.E.M, with n≥7 for each data set taken from at least 3 different cultures. Scale bar = 20μm.
3.3. TLR3 inhibition of neuronal excitability requires the adaptor molecule TRIF
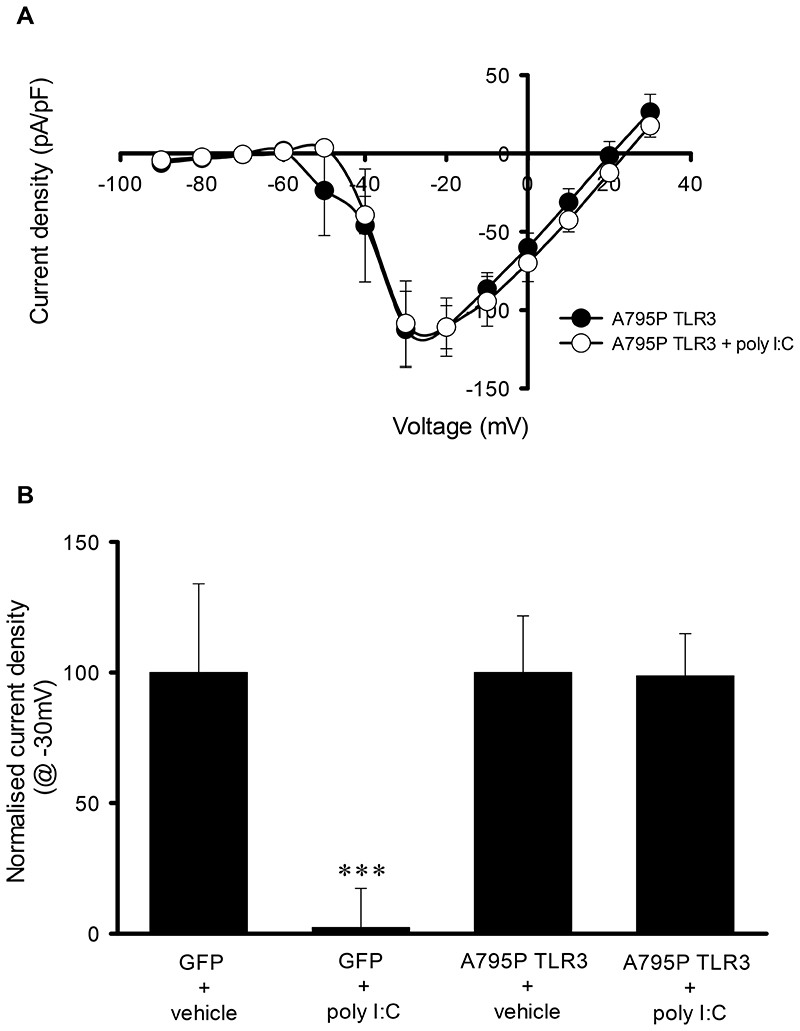
Having established that TLR3 activation reduces sAP firing via a reduction in neuronal excitability and synaptic activity, we next investigated whether these effects require the activation of the classical TLR3-TRIF pathway. Experiments were performed in an attempt to confirm that poly I:C mediates it’s action via TLR3 using hippocampal cultures from TLR3+/+ and TLR3-/- mice. However, poly I:C (25μg/ml, 1h) did not reduce sAP frequency in TLR3+/+ mouse cultures (101.8 ± 22.3% of vehicle treated controls (n=7) with sAP frequency also being unaffected in TLR3-/- cultures (95.5 ± 34.4% of vehicle treated controls, n=8). Hence to confirm the role of TLR3 in rat cultures, we transfected neurons with A795P TLR3, a TLR3 mutated receptor that switches TLR3 activation from the classical TRIF pathway to the MyD88-dependent pathway (Verstak et al., 2013). In agreement with our earlier observation, poly I:C (25μg/ml, 1h) significantly reduced the peak Na+ current density in GFP transfected cells (n=6, F=87.91, p<0.001) when compared to their vehicle-treated GFP controls (n=6) with peak Na+ current density reduced to 2 ± 15% of GFP control (n=6, p < 0.001, Fig. 5B). In contrast, poly I:C (25μg/ml) had no effect on the Na+ current density in neurons co-transfected with A795P TLR3 (A795P TLR3 control (n=7) v poly I:C treated (n=7), F= 0.26, p=0.61) with the peak Na+ current density being 99 ± 16% of control (Fig. 5A & B). With TLR3’s actions requiring the activation of the TRIF pathway, we used ELISA to examine whether cytokines classically produced via the TLR3-TRIF pathway in immune cells, were induced following exposure of hippocampal cultures to poly I:C (25μg/ml, 1h). Levels of RANTES were similar in conditioned media from both vehicle and poly I:C treated cultures (vehicle: 80 ± 9 pg/ml v poly I:C 81 ± 11 pg/ml, n=4 for both conditions) whereas levels of TNF-α and IL-10 were undetectable in both conditions.
Fig. 5. TLR3 mediated inhibition of Na+ current density is abolished in A795P TLR3 transfected neurons.
A) I-V curve revealing effects of poly I:C on Na+ current density observed in A795P TLR3 transfected neurons. B) Bar chart summarising the effect of poly I:C on peak Na+ current density in GFP- and A795P TLR3 transfected neurons. ***P < 0.001. Data are mean ± S.E.M, with n≥6 for each data set taken from at least 3 different cultures.
4. Discussion
In the present study, we show that TLR3 activation impairs neuronal excitability and synaptic activity in rat hippocampal neurons via the inhibition of Na+ channel function and reduced AMPA receptor expression. Furthermore, we provide evidence that the TLR3-mediated effects are mediated directly via neuronal TLR3 and the reduction in Na+ channel function relies on the activation of the MyD88-independent pathway. In addition, we reveal that TLR3 activation inhibits sAP firing in human iPSC-derived neurons.
4.1. TLR3 activation inhibits action potential firing
It is well established that TLRs play a key role in the activation of innate immunity by recognising both PAMPs and DAMPs with TLR3 proposed to play a crucial role in antiviral responses (Gay et al., 2014; Achek et al., 2016; Miller wet al., 2016). Indeed, this applies to CNS viral infections but evidence indicates a dichotomy for TLR3 in which it plays a protective and/or a detrimental role in CNS viral infections and pathogenesis (Perales-Linares and Navas-Martin, 2013). In addition to its well established role in innate immunity, TLR3 is expressed in neurons, astrocytes, microglia and oligodendrocytes within the CNS (Costello et al., 2013; Miller et al., 2016), but the consequence of its activation on neuronal excitability and synaptic activity remains unclear. Our data shows that TLR3 activation reduces action potential firing in primary rat hippocampal cultures, an effect that is independent of astrocytic activation. This reduction in neuronal activity may underlie the finding that TLR3 activation following direct infusion of poly I:C into the hippocampus results in impaired working memory (Okun et al., 2010). This study also revealed that TLR3-/- mice had improved hippocampal-dependent memory retention, with these findings indicating that TLR3 is an endogenous suppressor of hippocampal memory retention but whether endogenous activator(s) of TLR3 within the CNS exist remains to be elucidated (Okun et al., 2010). Our data supports this view that TLR3 activation modulates neuronal function, however our experiments using mouse hippocampal cultures are a caveat to this but given it is well documented that species differences occur with regard to TLR3 function (Zhang et al., 2013; Carty et al., 2014), further investigation into this is beyond the scope of this study. In contrast to the data presented here, it has previously been shown that systemic injection of poly I:C results in increased hippocampal network excitability (Costello et al., 2013), with this being dependent on IFNβ production from microglia and astrocytes. These differences may be a result of direct neuronal TLR3 activation with no role for glial cells in the present study whereas the increased excitability observed following systemic poly I:C injection results from an inflammatory response, which in part includes the activation of glial cells. However further investigations are required to examine these discrepancies. In addition, poly I:C has been shown to induce inward currents and elicit action potential firing in dorsal root ganglion cells (DRGs), in a TLR3-dependent manner (Liu et al., 2012). This action is proposed to underlie the increased neuronal excitability associated with pruritus and implies TLR3 as a potential target for anti-itch treatments. However, why the dichotomy exists between the effects of TLR3 activation on DRG and hippocampal neuronal excitability is as yet unknown.
4.2. TLR3 activation impairs AP firing in human iPSC-derived neurons
Having characterised the effects of TLR3 activation in rat hippocampal cultures, we then extended our investigation to determine whether similar effects are observed in human iPSC-derived cortical neurons. There has been intense recent speculation regarding the physiological credibility of iPSC-derived neurons but several recent studies have revealed electrical and synaptic maturity depending on the culture conditions (Livesey et al., 2016). The iPSC-derived neurons utilised in the present study exhibit spontaneous AP firing, thus we are confident that they are indeed physiologically viable for the present study. Hence, we show for the first time that exposure of human cortical neurons to poly I:C results in impaired AP firing similar to that seen in rat hippocampal cultures, with our data indicating that TLR3 is indeed present in human neurons in agreement with previous studies (Peltier et al., 2010; Vontell et al., 2013). Whilst these iPSC-derived neurons are electrically and synaptically mature when grown under the current conditions, they are still developmentally immature (Livesey et al., 2016). As there is intense interest in the relationship between maternal infection and neurodevelopmental disorders (Cordeiro et al., 2015) and with animal models of prenatal infection being used extensively (Meyer, 2014; Ibi and Yamada, 2015; Reisinger et al., 2015), our observation may offer another mechanism, in addition to the activation of inflammatory pathways, by which viral infections contribute to neurodevelopmental disorders.
4.3. TLR3 modulation of action potential firing involves modulating Na+ channels and neurotransmitter release
Having established that TLR3 activation reduces spontaneous AP firing, we next sought to examine the mechanisms that underlie this. As spontaneous AP firing in primary hippocampal cultures is dependent on Na+ channel activation and is synaptically driven (Gan et al., 2011), we examined the consequence of TLR3 activation on Na+ channel function and mEPSCs. To the best of our knowledge, our data reveals for the first time that TLR3 activation reversibly reduces Na+ channel currents following both 5 minute and 1 hour exposure to poly I:C without any effect on resting membrane potential. In DRGs, TLR3 activation results in inward currents that are suggested to be mediated via coupling with ion channels (Liu et al., 2012), but which channels this involved was not investigated further. Since the first discovery of the role of Na+ channels in AP generation, it is accepted that inhibition of Na+ channel function results in impaired neuronal excitability with Na+ channel dysfunction associated with a number of channelopathies (Kruger and Isom, 2016). Hence the TLR3-mediated Na+ channel inhibition is likely to underlie, at least in part, the reduction in spontaneous AP firing observed following exposure to poly I:C. As mentioned earlier, whether endogenous CNS activators exist for TLR3 remains to be elucidated, but recently two Toll receptors were shown to be neurotrophin receptors in the Drosophila CNS (McIlroy et al., 2013). Whether this extends to TLRs in mammalian systems remains unknown, but it has been reported that neurotrophin 3 reduces sodium channels expression in DRGs (Wilson-Gerwing et al., 2008). However, our data indicates no change in sodium channel expression following exposure to TLR3 activation under our experimental conditions but it would be intriguing to determine whether the connection between neurotrophins and TLR3 holds true in rat hippocampal and human neurons. We also examined whether TLR3 activation modulated synaptic activity given that AP firing in primary hippocampal cultures is synaptically driven. Short term application of poly I:C had no effect on mEPSCs whereas a 1 hour exposure resulted in a significant decrease in both frequency and amplitude of mEPSCs. These data indicate that prolonged TLR3 activation mediates its effect on mEPSCs postsynaptically. Hence, we examined GluA1 surface expression in our cultures and reveal that prolonged TLR3 activation results in reduced expression. This reduced GluA1 surface expression is independent of S831 and S845 dephosphorylation, sites proposed to be involved in AMPA receptor trafficking (Henley and Wilkinson, 2016). Other phosphorylation sites are also proposed for AMPA receptor trafficking but receptor internalisation may also depend on activation of other signalling pathways including caspase-3 (Li et al., 2010). Given that TLRs are linked to caspase activation (Gay et al., 2014), we also examined whether poly I:C exposure activated caspase-3 in our rat hippocampal preparations. However no change is caspase-3 activity was observed which indicates an as yet undefined mechanism underlies the TLR3-mediated GluA1 internalisation. Our findings therefore indicate that reduced GluA1 surface expression underlies the effect on mEPSCs and combined with the effects on Na+ channel function, results in impaired spontaneous AP firing.
Given our findings that the effects of TLR3 activation on neuronal excitability and synaptic activity are independent of astrocytic activation, we next examined whether these neuron specific effects were mediated through the classical TLR3-MyD88-independent pathway. In experiments utilising mouse TLR3+/+ and TLR-/- hippocampal cultures, in contrast to the effects observed in rat hippocampal cultures, poly I:C was without effect on sAP firing, indicating species variation regarding the consequence of TLR3 activation on neuronal function. Given that TLR species variation is acknowledged (Zhang et al., 2013; Carty et al., 2014), we show that neuronal overexpression of A795P TLR3 abolished poly I:C-induced Na+ channel inhibition revealing that the TLR3-TRIF pathway is indeed required for the actions observed here. This mutant TLR3 has previously been utilised to highlight that TLR adaptor specificity is controlled by small regions of the Toll/IL-1R domain (Verstak et al., 2013). Indeed, the fact that A795P TLR3 switches to the MyD88-dependent pathway supports our findings that LPS has no effect on AP firing in our studies. Having confirmed that the effects of poly I:C require the TLR3-MyD88-independent pathway, our findings that poly I:C did not induce the production of cytokines classically produced by immune cels following TLR3 activation suggest that an alternative and as yet unknown TLR3-mediated pathway(s) is responsible for the effects observed in the present study. These data are also interesting given the known link between the TLR3-MyD88-independent pathway and herpes simplex encephalitis (HSE) as it is now well documented that a number of patients who have identified genetic deficiencies in this pathway have an increased susceptibility to HSE (Zhang et al., 2007; Guo et al., 2011; Andersen et al., 2015; Sironi et al., 2017), presumably as the virus is more neuroinvasive. In addition, given the role of trans-synaptic spread in viral propagation (Miller et al., 2016), one could speculate that an intact TLR3-MyD88-independent pathway is an alternative mechanism to the canonical immune response by which to control the spread of viral infections.
5. Conclusion
Taken together, our novel data reveals for the first time that activation of neuronally expressed TLR3 results in impaired neuronal function. This extends our understanding of the role of TLR3 in modulating CNS function both under physiological conditions, but potentially under pathophysiological conditions including those observed during maternal viral infections and during HSE.
Supplementary Material
Acknowledgements
Sincere thanks to Aimee Franssen and Shehla Hridi for providing assistance with TLR+/+ and TLR3-/- cultures.
Funding
This work was supported by a BBSRC studentship to L.R. and a National Centre for the Replacement, Refinement and Reduction of Animals in Research [grant No. 35580-259130] to S.W., M.Z. and T.J.B.
Footnotes
Author contributions: L.R. and T.J.B. designed research; L.R., L.H.C., J.A.W., G.R. and T.J.B. provided culture assistance and performed research; L.R., J.A.W., G.R. and T.J.B. analyzed data; all authors contributed to the writing of the paper.
Conflicts of interest: The authors declare no conflict of interest.
References
- Abdul Rahman NZ, Greenwood SM, Brett RR, Tossell K, Ungless MA, Plevin R, Bushell TJ. Mitogen-Activated Protein Kinase Phosphatase-2 Deletion Impairs Synaptic Plasticity and Hippocampal-Dependent Memory. J Neurosci. 2016;36:2348–54. doi: 10.1523/JNEUROSCI.3825-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achek A, Yesudhas D, Choi S. Toll-like receptors: promising therapeutic targets for inflammatory diseases. Arch Pharm Res. 2016;39:1032–49. doi: 10.1007/s12272-016-0806-9. [DOI] [PubMed] [Google Scholar]
- Andersen LL, Mørk N, Reinert LS, Kofod-Olsen E, Narita R, Jørgensen SE, Skipper KA, Höning K, Gad HH, Østergaard L, Ørntoft TF, et al. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J Exp Med. 2015;212:1371–9. doi: 10.1084/jem.20142274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barichello T, Generoso JS, Goularte JA, Collodel A, Pitcher MR, Simões LR, Quevedo J, Dal-Pizzol F. Does Infection-Induced Immune Activation Contribute to Dementia? Aging Dis. 2015;6:342–8. doi: 10.14336/AD.2015.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Bajramovic JJ, Vogt MH, van Duijvenvoorden E, Baghat A, Persoon-Deen C, Tielen F, Verbeek R, Huitinga I, Ryffel B, Kros A, et al. The microtubule regulator stathmin is an endogenous protein agonist for TLR3. J Immunol. 2010;184:6929–37. doi: 10.4049/jimmunol.0902419. [DOI] [PubMed] [Google Scholar]
- Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, Flavell R, Strittmatter SM, Volpe J, Sidman R, Vartanian T. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27:13033–41. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M, Reinert L, Paludan SR, Bowie AG. Innate antiviral signalling in the central nervous system. Trends Immunol. 2014;35:79–87. doi: 10.1016/j.it.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Chen CY, Liu HY, Hsueh YP. TLR3 downregulates expression of schizophrenia gene Disc1 via MYD88 to control neuronal morphology. EMBO Rep. 2017;18:169–183. doi: 10.15252/embr.201642586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro CN, Tsimis M, Burd I. Infections and Brain Development. Obstet, Gynecol, Surv. 2015;70:644–55. doi: 10.1097/OGX.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DA, Lynch MA. Toll-like receptor 3 activation modulates hippocampal network excitability via glial production of interferon-β. Hippocampus. 2013;23:696–707. doi: 10.1002/hipo.22129. [DOI] [PubMed] [Google Scholar]
- Faizan MI, Abdullah M, Ali S, Naqvi IH, Ahmed A, Parveen S. Zika Virus-Induced Microcephaly and Its Possible Molecular Mechanism. Intervirology. 2016;59:152–158. doi: 10.1159/000452950. [DOI] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Henderson AK, Tsutsui S, Pittman QJ. Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol Dis. 2009;36:343–51. doi: 10.1016/j.nbd.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambuzza ME, Sofo V, Salmeri FM, Soraci L, Marino S, Bramanti P. Toll-like receptors in Alzheimer’s disease: a therapeutic perspective. CNS Neurol Disord Drug Targets. 2014;13:1542–58. doi: 10.2174/1871527313666140806124850. [DOI] [PubMed] [Google Scholar]
- Gan J, Greenwood SM, Cobb SR, Bushell TJ. Indirect modulation of neuronal excitability and synaptic transmission in the hippocampus by activation of protease-activated receptor-2. Br J Pharmacol. 2011;163:984–94. doi: 10.1111/j.1476-5381.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14:546–58. doi: 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- Gross A, Benninger F, Madar R, Illouz T, Griffioen K, Steiner I, Offen D, Okun E. Toll-like receptor 3 deficiency decreases epileptogenesis in a pilocarpine model of SE-induced epilepsy in mice. Epilepsia. 2017;58:586–596. doi: 10.1111/epi.13688. [DOI] [PubMed] [Google Scholar]
- Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011;208:2083–98. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016;17:337–50. doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- Ibi D, Yamada K. Therapeutic Targets for Neurodevelopmental Disorders Emerging from Animal Models with Perinatal Immune Activation. Int J Mol Sci. 2015;16:28218–29. doi: 10.3390/ijms161226092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger LC, Isom LL. Voltage-Gated Na+ Channels: Not Just for Conduction. Cold Spring Harb Perspect Biol. 2016;8:a029264. doi: 10.1101/cshperspect.a029264. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Okun E, Tang SC, Griffioen K, Cheng A, Mughal MR, Laryea G, Selvaraj PK, ffrench-Constant C, Magnus T, Arumugam TV, et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci. 2008;28:13978–84. doi: 10.1523/JNEUROSCI.2140-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, Cho K, Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–71. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lü N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey MR, Magnani D, Hardingham GE, Chandran S, Wyllie DJ. Functional properties of in vitro excitatory cortical neurons derived from human pluripotent stem cells. J Physiol. 2016;594:6573–6582. doi: 10.1113/JP270660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy G, Foldi I, Aurikko J, Wentzell JS, Lim MA, Fenton JC, Gay NJ, Hidalgo A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat Neuro. 2013;16:1248–1256. doi: 10.1038/nn.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75:307–15. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Miller KD, Schnell MJ, Rall GF. Keeping it in check: chronic viral infection and antiviral immunity in the brain. Nat Rev Neurosci. 2016;17:766–776. doi: 10.1038/nrn.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Cross A, Santos SD, Carvalho AL, Lindsay Y, Connolly CN, Irving AJ, Leslie NR, Harvey J. Leptin regulates AMPA receptor trafficking via PTEN inhibition. J Neurosci. 2010;30:4088–101. doi: 10.1523/JNEUROSCI.3614-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, Pit MA, Cheng A, Mughal MR, Wan R, Ashery U, Mattson MP. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2010;107:15625–30. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–81. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier DC, Simms A, Farmer JR, Miller DJ. Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling. J Immunol. 2010;184:7010–21. doi: 10.4049/jimmunol.0904133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales-Linares R, Navas-Martin S. Toll-like receptor 3 in viral pathogenesis: friend or foe? Immunology. 2013;140:153–67. doi: 10.1111/imm.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger S, Khan D, Kong E, Berger A, Pollak A. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther. 2015;149:213–26. doi: 10.1016/j.pharmthera.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Sironi M, Peri AM, Cagliani R, Forni D, Riva S, Biasin M, Clerici M, Gori A. TLR3 mutations in adult patients with Herpes Simplex Virus and Varicella-Zoster Virus Encephalitis. J Infect Dis. 2017;215:1430–1434. doi: 10.1093/infdis/jix166. [DOI] [PubMed] [Google Scholar]
- Sposito T, Preza E, Mahoney CJ, Setó-Salvia N, Ryan NS, Morris HR, Arber C, Devine MJ, Houlden H, Warner TT, Bushell TJ, et al. Developmental regulation of tau splicing is disrupted in stem cell-derived neurons from frontotemporal dementia patients with the 10 + 16 splice-site mutation in MAPT. Hum Mol Genet. 2015;24:5260–9. doi: 10.1093/hmg/ddv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen A, Diels A, Dijkmans J, Oyelami T, Meneghello G, Mertens L, Versweyveld S, Borgers M, Buist A, Peeters P, Cik M. Using Human iPSC-Derived Neurons to Model Tau Aggregation. PLoS One. 2015;10:e0146127. doi: 10.1371/journal.pone.0146127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstak B, Arnot CJ, Gay NJ. An alanine-to-proline mutation in the BB-loop of TLR3 Toll/IL-1R domain switches signalling adaptor specificity from TRIF to MyD88. J Immunol. 2013;191:6101–9. doi: 10.4049/jimmunol.1300849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontell R, Supramaniam V, Thornton C, Wyatt-Ashmead J, Mallard C, Gressens P, Rutherford M, Hagberg H. Toll-like receptor 3 expression in glia and neurons alters in response to white matter injury in preterm infants. Dev Neurosci. 2013;35:130–9. doi: 10.1159/000346158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontell R, Supramaniam V, Wyatt-Ashmead J, Gressens P, Rutherford M, Hagberg H, Thornton C. Cellular mechanisms of toll-like receptor-3 activation in the thalamus are associated with white matter injury in the developing brain. J Neuropathol Exp Neurol. 2015;74:273–85. doi: 10.1097/NEN.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Gerwing TD, Stucky CL, McComb GW, Verge VM. Neurotrophin-3 significantly reduces sodium channel expression linked to neuropathic pain states. Exp Neurol. 2008;213:303–14. doi: 10.1016/j.expneurol.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–7. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Abel L, Casanova JL. Mendelian predisposition to herpes simplex encephalitis. Handb Clin Neurol. 2013;112:1091–7. doi: 10.1016/B978-0-444-52910-7.00027-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.