Abstract
The innate immune system is implicated in Parkinson’s disease (PD), but peripheral in-vivo clinical evidence of the components and driving mechanisms involved and their relationship with clinical heterogeneity and progression to dementia remain poorly explored.
We examined changes in peripheral innate immune-related markers in PD cases (n=41) stratified according to risk of developing early dementia. ‘Higher Risk’(HR) (n=23) and ‘Lower Risk’ (LR) (n=18) groups were defined according to neuropsychological predictors and MAPT H1/H2 genotype, and compared to age, gender and genotype-matched controls. Monocyte subsets and expression of key surface markers were measured using flow cytometry. Serum markers including alpha-synuclein, inflammasome-related caspase-1 and bacterial translocation-related endotoxin were measured using quantitative immuno-based assays. Specific markers were further investigated using monocyte assays and validated in plasma samples from a larger incident PD cohort (n=95).
We found that classical monocyte frequency was elevated in PD cases compared to controls, driven predominantly by the HR group, in whom Toll-Like Receptor (TLR)4+ monocytes and monocyte Triggering Receptor Expressed on Myeloid cells-2 (TREM2) expression were also increased. Monocyte Human Leukocyte Antigen (HLA)-DR expression correlated with clinical variables, with lower levels associated with worse cognitive/motor performance. Notably, monocyte changes were accompanied by elevated serum bacterial endotoxin, again predominantly in the HR group.
Serum alpha-synuclein and inflammasome-related caspase-1 were decreased in PD cases compared to controls regardless of group, with decreased monocyte alpha-synuclein secretion in HR cases. Further, alpha-synuclein and caspase-1 correlated positively in serum and monocyte lysates, and in plasma from the larger cohort, though no associations were seen with baseline or 36-month longitudinal clinical data.
Principal Components Analysis of all monocyte and significant serum markers indicated 3 major components. Component 1 (alpha-synuclein, caspase-1, TLR2+ monocytes) differentiated PD cases and controls in both groups, while Component 2 (endotoxin, monocyte TREM2, alpha-synuclein) did so predominantly in the HR group. Component 3 (classical monocytes, alpha-synuclein) also differentiated cases and controls overall in both groups.
These findings demonstrate that systemic innate immune changes are present in PD and are greatest in those at higher risk of rapid progression to dementia. Markers associated with PD per-se (alpha-synuclein, caspase-1), differ from those related to cognitive progression and clinical heterogeneity (endotoxin, TREM2, TLR4, classical monocytes, HLA-DR), with mechanistic and therapeutic implications. Alpha-synuclein and caspase-1 are associated, suggesting inflammasome involvement common to all PD, while bacterial translocation associated changes may contribute towards progression to Parkinson’s dementia. Additionally, HLA-DR-associated variations in antigen presentation/clearance may modulate existing clinical disease.
Keywords: Parkinson’s disease, heterogeneity, innate immune system, monocyte, alpha-synuclein, endotoxin, caspase-1
1. Introduction
Parkinson’s disease (PD) is clinically and pathologically heterogeneous (Rajput et al., 2009)(Kehagia et al., 2010)(Greenland et al., 2019). Clinical and genetic factors at diagnosis are known to predict the rate of progression to dementia, which affects around 50% of patients by 10 years (Williams-Gray et al., 2013). However, the biological drivers of heterogeneity in progression rates are not fully understood, and are likely to be complex, arising from interactions between genetic and environmental risk factors acting at multiple levels.
Mounting evidence from several fields has implicated involvement of the immune system in PD, however the precise components and mechanisms involved and their relationships with clinical heterogeneity and progression to dementia remain poorly understood. Genetic studies have indicated associations between immune related gene variants and PD risk (e.g. Human Leukocyte Antigen-DR (HLA-DR), Triggering Receptor Expressed on Myeloid cells-2 (TREM2), Toll-like receptor 4 (TLR4) (Nalls et a l., 2011)(Hamza et al., 2010)(Rayaprolu et al., 2013)(Zhao et al., 2015)), while established PD related genes such as Leucine Rich Repeat Kinase 2 (LRRK2) have demonstrated involvement in the innate immune system (H. Lee et al., 2017) and immune-mediated conditions such as inflammatory bowel disease (Dzamko, 2017). Epidemiological studies have suggested a decreased risk of PD with anti-inflammatory and immunosuppressant drug use (X. Gao et al., 2011)(Ju et al., 2019)(Racette et al., 2018) and increased risk with immune-related conditions such as autoimmune diseases (Chang et al., 2018) and infections (Pakpoor et al., 2017)(Vlajinac et al., 2013). Animal models have also suggested involvement of the immune system in driving PD pathology, with systemic lipopolysaccharide (LPS) administration (H.-M. Gao et al., 2011) worsening alpha-synuclein pathology. Mice lacking mature T and B lymphocytes (Brochard et al., 2009) have decreased cell loss in a toxin-based model of PD and Central Nervous System (CNS) infiltration of C-C motif chemokine Receptor2+ (CCR2+) monocytes (Harms et al., 2017) has also been shown to influence disease pathology and expression.
In the human PD brain, microglial activation has been demonstrated, both at post mortem (McGeer and McGeer, 2008) and in-vivo using Positron Emission Tomography (PET) neuroimaging (Gerhard et al., 2006). Several studies have also identified peripheral immune changes in PD patients, including increases in serum and secreted cytokines (e.g. Tumour Necrosis Factor (TNF)-α, Interleukin (IL)-1β, IL-2 and IL-10) (Williams-Gray et al., 2016)(Qin et al., 2016)(Sulzer et al., 2017), and associations between a more ‘pro-inflammatory’ cytokine profile at diagnosis and faster motor progression and impaired cognition over 3 years follow-up (Williams-Gray et al., 2016).
Many immune components are likely to play a role, and studies have demonstrated changes in adaptive immune factors such as variations in T lymphocyte subtypes and function (Baba et al., 2005)(Cen et al., 2017)(Sulzer et al., 2017)(Williams-Gray et al., 2018), decreased B lymphocytes (Stevens et al., 2012) and alterations in serum antibody levels (Scott et al., 2018) in PD. However, many of the key genetic links (HLA-DR, TREM2, TLR4) and imaging evidence have implicated specific involvement of the ‘innate’ immune system. In addition to central microglial activation, innate immune abnormalities have been seen in the cerebrospinal fluid (Schröder et al., 2018) and the periphery, with changes in monocyte subtype and marker expression (Grozdanov et al., 2014)(Funk et al., 2013). Studies have specifically found increased numbers of classical monocytes, increased monocyte CCR2, TLR2 and TLR4 expression (Funk et al., 2013)(Drouin-Ouellet et al., 2015), enhanced phagocytosis (Grozdanov et al., 2014)(Gardai et al., 2013)(Wijeyekoon et al., 2018) and reduced viability in culture (Nissen et al., 2019).
There is also increasing evidence that alpha-synuclein, the key pathological protein in PD, can be influenced by, and exert influence on, innate immune pathways and related microbial factors. Microbial involvement has been shown to influence disease pathology (Sampson et al., 2016) and alpha-synuclein itself may be produced, altered or trafficked in response to microbial/immune related challenges including bacterial endotoxin (Forsyth et al., 2011)(Stolzenberg et al., 2017)(Wang et al., 2016). Caspase-1, a key component of the innate immune inflammasome pathway (which is activated by a range of damage and pathogen associated molecular patterns (DAMPs/PAMPs)), can cleave alpha-synuclein and make it more aggregable, while Lewy bodies have been found to stain for caspase-1 together with alpha-synuclein (Wang et al., 2016). Forms of alpha-synuclein are also capable of activating the TLR and inflammasome pathways, leading to further cytokine production and inflammation (Codolo et al., 2013)(Gustot et al., 2015)(Kim et al., 2013)(White et al., 2018)(Grozdanov et al., 2019).
Although these lines of evidence implicate the innate immune system in PD, there has been limited characterisation of systemic innate immune and associated factors and their relevance to clinical heterogeneity and disease progression rate, in clinical PD cases. Consequently, we sought to pursue such an investigation, by characterising relevant peripheral innate immune components in the blood of a cohort of early-moderate stage PD cases stratified around risk for early dementia and paired matched controls without neurological disease. We also investigated blood samples for the presence of factors implicated in driving the innate immune changes in PD, including alpha-synuclein and bacterial endotoxin. We used a data driven approach to explore the relationships between these factors and innate immune changes and assessed links with disease status and dementia risk.
2. Materials and Methods
2.1. Participants
Ethical approval was obtained from the Cambridgeshire Research Ethics Committee and written consent was obtained from participants in compliance with the Declaration of Helsinki. Parkinson’s cases were recruited from the PD Research Clinic at the John van Geest Centre for Brain Repair, University of Cambridge.
Inclusion criteria comprised satisfying UK Brain Bank Criteria for Parkinson’s disease, age 55-80 and Hoehn and Yahr (HY) stage ≥2.
In order to classify patients a priori according to their risk of progression to dementia, the study utilised factors previously identified from the CamPaIGN longitudinal cohort study (semantic fluency score <20, impaired pentagon copying and H1/H1 MAPT haplotype)(Williams-Gray et al., 2009)(Williams-Gray et al., 2013). Each factor contributes significantly to increased dementia risk at 10 years, with a hazard ratio of 3.05 for semantic fluency <20, 2.55 for impaired pentagon copying and 3.08 for Microtubule Associated Protein Tau (MAPT) H1/H1(Williams-Gray et al., 2013).
The ‘Higher Dementia Risk’ (HR) group had at least one of these factors at diagnosis, while the ‘Lower Dementia Risk’ (LR) group had none.
Age, gender and MAPT-genotype matched controls, with no history of neurological disease, self-reported memory problems or depression were recruited via the Cambridge Bioresource(http://www.cambridgebioresource.org.uk).
Exclusion criteria for all participants consisted of the presence of-: another neurodegenerative, chronic inflammatory or autoimmune disorder, current clinically significant infection, surgery within last month, vaccinations in the last 3 weeks, use of steroids (within last 3 months), aspirin >75mg or ibuprofen/nonsteroidal anti-inflammatory drugs (within 2 weeks) or long-term immunosuppressant drugs (within 1 year).
2.2. Clinical data acquisition and sample collection
Participants attended for three visits at monthly intervals. Data gathered included demographic data, medical and drug history and comorbidity status (Cumulative Illness Rating Scale (CIRS)(Parmelee et al., 1995)). Verbal screening for inter-current infections was performed to avoid sampling during periods of illness. Clinical assessments included the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), HY scale, Addenbrooke’s Cognitive Examination-Revised (ACE-R), semantic fluency (animals in 90s) and pentagon copying.
Venous blood (up to 50ml) was sampled at each visit and used for serum extraction and/or Peripheral Blood Mononuclear Cell (PBMC) isolation and subsequent ex-vivo immunocytochemistry/flow cytometry or CD14+ cell separation. All samples were collected between 9.00 and 11.00am, with no imposed medication changes. PD/control samples paired by age, gender and genotype were processed in parallel on the same day.
2.3. Sample Processing
2.3.1. PBMC isolation, Immunocytochemistry and Flow Cytometry
PBMCs were extracted using the standard Ficoll gradient method and subjected to immunocytochemistry and flow cytometry as previously described (Appendix A) (Wijeyekoon et al., 2018), to measure key monocyte cell surface markers.
The antibody panel used for flow cytometry consisted of CD14 – APC-H7, CD16 – PerCP-Cy5.5, HLA-DR – BV605, TREM2 –APC, TLR2 – PE, TLR4 – BV421 (Table A.1). Monocytes were gated and analysed as described in the literature (Ziegler-Heitbrock and Hofer, 2013)(Fig. 1A) and as detailed in Appendix A.
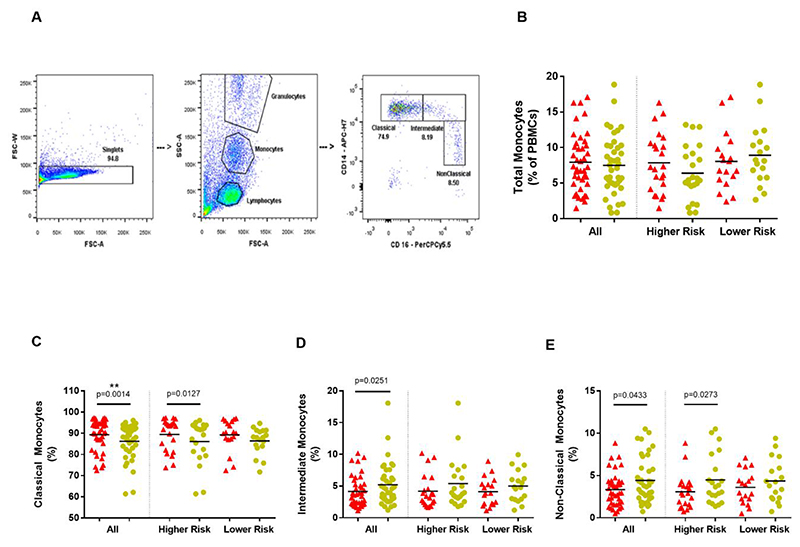
Figure 1. Monocyte subtypes.
(A) - Flow cytometry gating strategy for monocytes and monocyte subtypes Singlets are identified by plotting Forward Scatter-Area (FSC-A) versus Forward Scatter-Width (FSC-W) and excluding cells with multiples of a single width size. Monocyte are broadly distinguished using tight gates based on FSC-A (size) and Side Scatter-Area (SSC-A) (granularity/internal complexity). Monocyte subtypes are distinguished based on CD14 and CD16 expression –Classical (CD14 high, CD16 negative); Intermediate (CD14 high, CD16 positive); Non-Classical (CD14 low, CD16 high).
(B) - Total monocytes (as a percentage of PBMCs) in all patients and controls; overall and within dementia risk groups. (Parkinson’s disease=red; Controls=yellow).
(C),(D),(E)-Monocyte Subtypes. (C) Classical, (D) Intermediate and (E) Non-Classical monocytes (as percentage of total monocytes) in Parkinson’s disease patients and controls; overall and within dementia risk groups. (Parkinson’s disease=red; Controls=yellow). **significance withstood Bonferroni correction for multiple testing within the relevant category.
2.3.2. Serum sample processing and assays
Blood samples for serum collection were left to clot for 15 minutes and centrifuged at 2000rpm for 15 minutes at room temperature. The separated serum was stored in 200-400 μl aliquots, frozen at -80°C and thawed before use. The following assays were performed (see Appendix A for further details).
Mesoscale Discovery (MSD) platform electrochemiluminescence assays
Samples from each of the 3 visits were processed in duplicate according to the manufacturer’s instructions for the MSD V-Plex 10-spot Pro-inflammatory panel 1 assay (Interferon(IFN)-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13 and TNFα; 1:2 dilution; 50ul per well); MSD V-Plex Human C-Reactive Protein (CRP) assay (1:1000 dilution; 25ul per well); MSD Human Alpha-Synuclein Assay (1:10 dilution; 25ul per well).
Readings were obtained using the MSD SECTOR Imager. Data was exported and analysed using the MSD Discovery Workbench software.
Additional assays -: Caspase-1, soluble CD14, soluble TREM2 and bacterial endotoxin
Additional markers were measured from remaining serum aliquots and included-: caspase-1 (Wang et al., 2016)(Codolo et al., 2013); bacterial endotoxin (a primary ligand for TLR4) and soluble CD14, as markers of microbial translocation (Morris et al., 2015)(Kelesidis et al., 2012); and soluble TREM2, as an indicator of TREM2 shedding (Feuerbach et al., 2017).
Samples were analysed in duplicate using ELISA assays for caspase-1(R and D Systems) (2:3 dilution), soluble TREM2(Cloud-Clone Corp.)(1:2 dilution), and soluble CD14(R and D systems) (1:800 dilution), following the manufacturer’s instructions (Appendix A).
Serum endotoxin was measured using the Pierce Limulus Amoebocyte Lysate (LAL) Chromogenic Endotoxin Quantitation Kit (Thermo Scientific) (40 samples) and the LAL Chromogenic Endpoint Assay (Hycult Biotech) (36 samples), due to supplier shortages of the former during the course of the study. Samples from PD and control pairs were analysed using the same plate and kit. The results from both batches covered similar ranges (Thermo Scientific kit – 0.46 – 6.32 EU/ml; Hycult Biotech kit – 0.46 – 5.00 EU/ml). All samples were assayed in duplicate according to the manufacturer’s instructions, with 1:50 dilution.
2.3.3. Monocyte separation
Monocytes were separated using MACS® CD14 magnetic beads (Miltenyi Biotec) as per the manufacturer’s instructions and as previously detailed (Appendix A)(Wijeyekoon et al., 2018).
2.3.4. Fluorescent alpha-synuclein endocytosis assays
Monocyte uptake of recombinant human alpha-synuclein (1-140) HiLyte™ Fluor 488 (Anaspec) was assessed in standard medium (clear Roswell Park Memorial Institute (RPMI) culture medium and 10% Foetal Calf Serum (FCS)), and in autologous serum (Appendix A). Titration and time course experiments were performed prior to study commencement, to optimise concentrations and end time points. Final assays were run using 10,000ng/ml of alpha-synuclein for an incubation period of 90 minutes. Alpha-synuclein uptake was assessed and quantified using flow cytometry. Representative post-uptake monocyte samples were used for imaging using fluorescence microscopy.
2.3.5. Monocyte alpha-synuclein and caspase-1 secretion assays
Separated monocytes were cultured in RPMI and 10% FCS under standard conditions at a concentration of 1x106 cells per ml per well, with and without LPS (1ng/ml). Paired PD and control cultures, with and without LPS, were performed in parallel. The supernatant was collected at 24h, aliquoted and stored at -80°C.
Supernatants were analysed for alpha-synuclein and caspase-1 according to the manufacturer’s instructions as detailed above and in Appendix A. Supernatants were diluted 1:10 for alpha-synuclein and 2:3 for caspase-1 in the appropriate buffers and were assayed in duplicate.
2.3.6. Monocyte Lysates
Monocytes were lysed in homogenisation solution (Appendix A), and their total protein concentration was measured using a Bicinchoninic acid (BCA) assay (Appendix A). Alpha-synuclein and caspase-1 levels were measured in monocyte lysates using the assays and dilutions described above. Western blots for alpha-synuclein and caspase-1 were performed on the monocyte lysates, with β-actin as loading control (Appendix A).
2.4. Statistical analysis
Data was analysed using IBM SPSS version 25 or GraphPad Prism 7. Experimental outliers >3 standard deviations (SD) above or below the mean were excluded and normality was assessed using the Shapiro-Wilk test prior to analysis. Due to the paired experimental methodology, group comparisons were performed for PD cases overall and within each a priori determined risk group versus paired controls using paired two-tailed t-tests (parametric) or Wilcoxon matched-pairs tests (non-parametric) as appropriate. Bonferroni correction for multiple testing was used across each assay category (e.g. monocyte subtypes, monocyte surface expression markers, all serum assays). All monocyte markers and serum markers with a PD-Control uncorrected significant difference of p<0.05 were included in a Principal Components Analysis (PCA).
Relationships between monocyte/serum markers and clinical variables (MDS-UPDRS motor, ACE-R and semantic fluency scores) were assessed using bivariate correlation analysis. Markers reaching significance (p<0.05) were included in multiple regression analyses for each clinical variable with appropriate confounders. Correlations between PCA component scores and clinical variables were compared using similar methods.
2.5. Plasma markers and disease progression
Stored baseline plasma samples were available for another cohort of 93 patients (Incidence of Cognitive Impairment in Cohorts with Longitudinal Evaluation in Parkinson’s Disease (ICICLE-PD) Cambridge cohort) who were newly diagnosed with PD and recruited between 2009 and 2011 from community/outpatient clinics in Cambridge, United Kingdom. Details of recruitment, assessment and follow up of the patients have been previously published (Yarnall et al., 2014). Subjects were assessed clinically as previously described (Williams-Gray et al., 2016), at baseline, 18 and 36 months.
Venous blood samples (EDTA tubes) were obtained at baseline entry to the study and plasma extracted by centrifugation (2000rpm for 15 minutes), with storage at -80°C. On use, plasma was thawed and processed for alpha-synuclein and caspase-1(1:50 dilution)(Appendix A).
Relationships between baseline plasma markers and longitudinal clinical progression were analysed using simple bivariate correlation analyses. Significant correlations were further examined using multiple regression analyses with relevant confounders.
3. Results
3.1. Participants
41 Parkinson’s disease cases and 41 paired matched controls were recruited (23 pairs HR group and 18 pairs LR group) (Table 1).
Table 1. Demographic details of participant groups.
(Mean ± Standard Deviation). *p<0.05. MDS-UPDRS – Movement Disorder Society Unified Parkinson’s Disease Rating Scale; ACE-R – Addenbrooke’s Cognitive Examination- Revised; CIRS – Cumulative Illness Rating Scale.
| Variable | Higher Risk (HR) Group | Lower Risk (LR) Group | HR vs LR Parkinson’s disease P | ||||
|---|---|---|---|---|---|---|---|
| Parkinson’s disease | Paired Controls | p | Parkinson’s disease | Paired Controls | p | ||
| Number (n) | 23 | 23 | 18 | 18 | |||
| Age (years) | 70.13± 5.96 | 69.43± 5.40 | 0.680 | 66.33 ± 6.36 | 66.39± 5.71 | 0.978 | 0.056 |
| Gender (% male) | 73.9 | 73.9 | 0.631 | 61.1 | 61.1 | 0.633 | 0.295 |
| Disease Duration (years) | 4.26 ± 1.09 | 4.26 ± 1.23 | 0.994 | ||||
| MDS-UPDRS motor score | 38.35± 12.71 | 31.00 ± 10.78 | 0.062 | ||||
| Equivalent Levodopa dose | 493.34 ± 269.78 | 717.03 ± 279.27 | 0.013* | ||||
| ACE-R score | 89.96± 9.94 | 96.88±2.31 | 0.008* | ||||
| CIRS Total Score | 4.30 ± 2.05 | 5.39 ± 2.99 | 0.177 | ||||
3.2. Monocyte markers
There were no significant differences in total monocytes (as a percentage of PBMCs) between PD and paired controls, overall (t(40)=0.5446, p=0.5891) nor in both risk groups (Fig.1B). PD cases had a statistically significant (withstanding Bonferroni correction for multiple testing) higher proportion of classical monocytes (W=-445, p=0.0014), with trends (uncorrected significance) towards correspondingly lower intermediate (t(37)=2.334, p=0.0251) and non-classical (W=278, p=0.0433) monocytes, compared to paired controls (Fig.1C-E). Risk group analysis revealed a trend towards higher classical monocyte percentages in the HR PD group (W= -141, p=0.0127) compared to paired controls, but not the LR group (Fig. 1C).
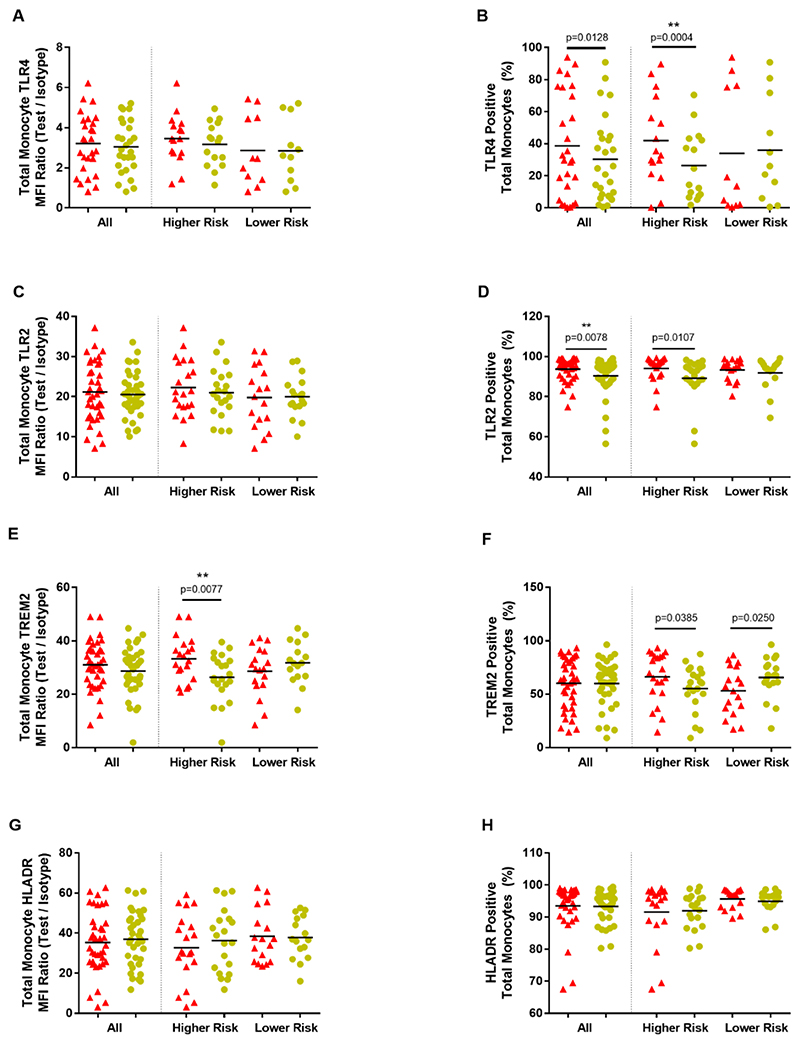
TLR4+ monocytes constituted a higher percentage in PD compared to controls (W= -204, p=0.0128), with statistical significance in the HR PD group (W= -124, p=0.0004). The percentage of TLR2+ monocytes was also significantly higher in all PD cases (W= -347, p=0.0078)compared to controls and within the HR PD group (W= -134, p=0.0107) (Fig.2A-D).
Figure 2. Monocyte surface markers.
Total monocyte marker expression in Parkinson’s disease cases versus paired controls; overall and within risk groups. Graphs showing total monocyte MFI (Median Fluorescence Intensity) ratios (Test/Isotype) ((A), (C), (E), (G)) and percentage monocytes positive ((B), (D), (F), (H)). (Parkinson’s disease=red; Controls=yellow). **significance withstood Bonferroni correction for multiple testing within the relevant category.
Monocyte TREM2 and HLA-DR measures did not differ between PD cases and controls. However, HR group PD cases had significantly higher monocyte TREM2 expression (t(19)=2.977, p=0.0077) compared to paired controls and a trend towards a higher percentage of TREM2+ monocytes (t(20)=2.216, p=0.0385), while LR PD cases demonstrated a trend towards a lower percentage of TREM2+ monocytes (t(17)=2.457, p=0.0250) (Fig. 2E-H).
Additional analyses performed on the Classical monocyte sub-population indicated similar patterns of changes to Total monocytes in all measured markers, but with decreased significance overall, possibly due to smaller cell numbers within the subpopulation (Appendix A, Figure A.5).
3.3. Monocyte markers and clinical variables
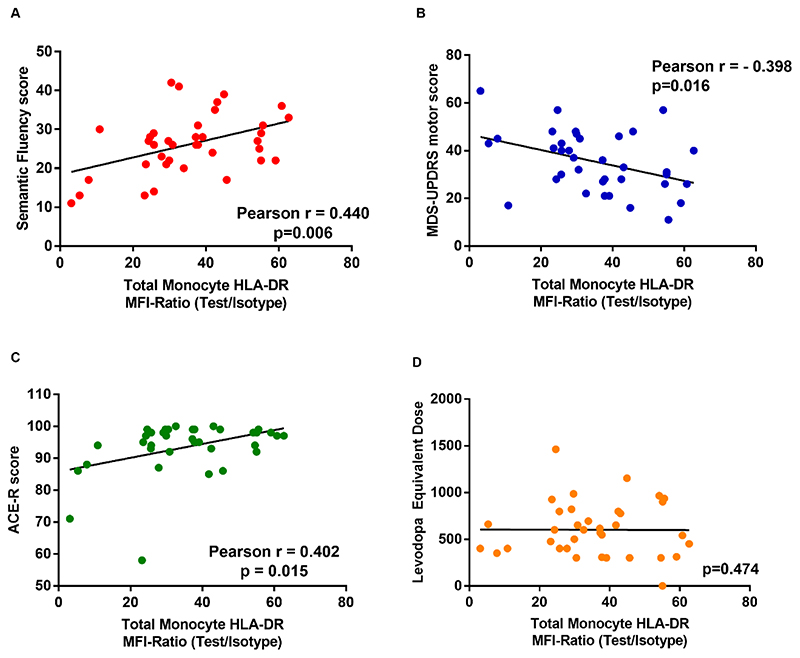
Monocyte HLA-DR surface expression levels correlated with better cognitive function (higher ACE-R (r=0.402, p=0.015) and semantic fluency (r=0.440, p=0.006)) and motor function (lower UPDRS motor score (r=-0.398, p=0.016)). There were no correlations with levodopa equivalent dose, suggesting the associations were unlikely to be medication driven (Fig.3). There were no significant correlations between clinical scores and other monocyte marker measures (data not shown).
Figure 3. Monocyte HLA-DR expression and clinical data.
Relationships between Total Monocyte HLA-DR expression (Test/Isotype MFI Ratio) and clinical data - (A) Semantic Fluency. (B) MDS-UPDRS III motor score. (C) ACE-R score. (D) Absence of correlation with Levodopa equivalent dose.
Multivariate regression analysis with the UPDRS motor score, semantic fluency or ACE-R scores as the dependent variables, and age, disease duration, levodopa equivalent dose and CIRS comorbidity score as potential confounders, confirmed the relationships with total monocyte HLA-DR surface expression level (Table A.2).
3.4. Serum markers
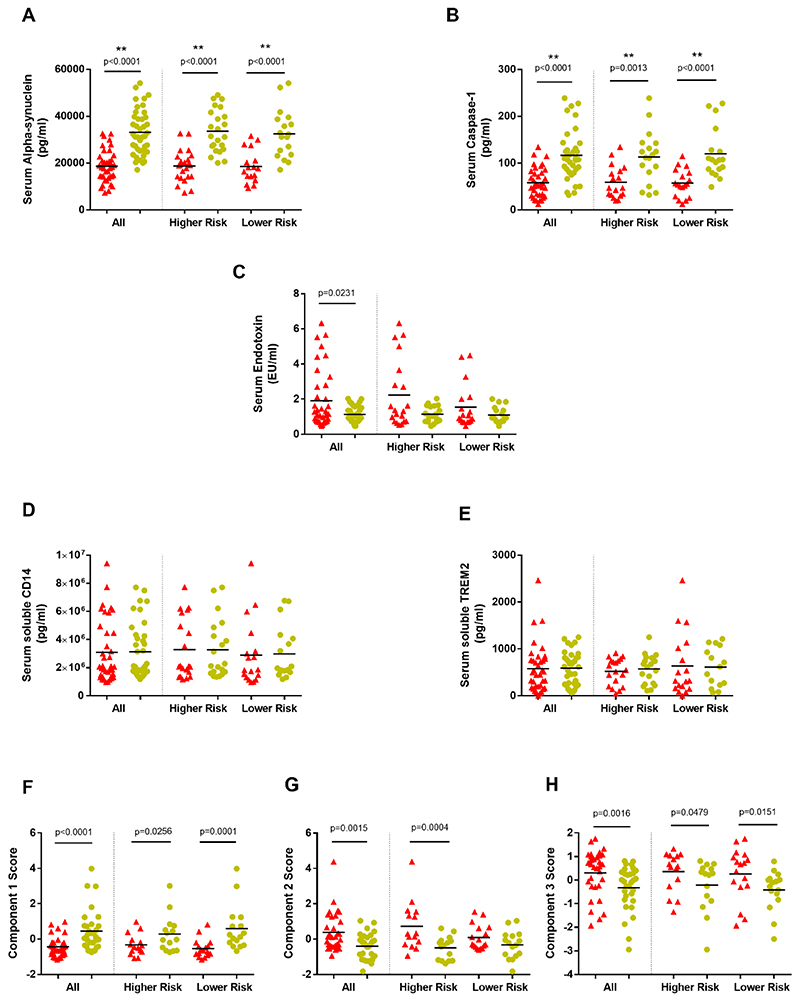
Serum alpha-synuclein concentration was significantly lower in PD cases compared to controls, regardless of risk group (Overall W=810, p<0.0001; HR W=276, p<0.0001; LR W=147, p<0.0001)(Fig. 4A).
Figure 4. Serum markers and Principal Components Analysis.
(A)-(E) - Concentrations of serum (A) alpha-synuclein, (B) caspase-1, (C) endotoxin, (D) soluble CD14 and (E) soluble TREM2 in Parkinson’s disease patients and controls and within Parkinson’s dementia risk groups. (Patients=red; Controls=yellow). **significance withstood Bonferroni correction for multiple testing within the relevant category.
(F)-(H) - Summary of Principal Components Analysis (PCA) component score comparisons between all Parkinson’s patients and paired controls and within risk groups. (Patients=red; Controls=yellow).
Component 1 - (+)serum alpha-synuclein, (+)caspase-1 and (-)TLR2+ monocytes; Component 2 - (+)serum endotoxin, (+)monocyte TREM2 and (-)serum alpha-synuclein; Component 3 - (+)classical monocyte percentage and (-)serum alpha-synuclein
Serum caspase-1 was also significantly lower in PD compared to controls, regardless of risk group (Overall W=596, p<0.0001; HR W=139, p=0.0013; LR W=161, p<0.0001) (Fig. 4B).
Serum endotoxin was higher in PD compared to controls (W=-287, p=0.0231), with a non-significant trend in the HR group (W=-79, p=0.0898) (Fig. 4C).
Serum IL-2 and IFN-γ displayed trends towards decrease in the HR-PD group, but there were no statistically significant case-control differences in the serum concentrations of measured cytokines, CRP, soluble CD14 or soluble TREM2 in patients compared to controls (Tables A.3 and A.4; Fig. 4D,E).
For both monocyte markers and serum variables, gender-stratified paired comparisons indicated overall similar trends in all markers within both genders, though significance was greater in the male group, which may relate to the larger sample size (>68% male)(data not shown).
3.5. Serum markers and clinical variables
MDS-UPDRS motor scores correlated negatively with serum alpha-synuclein (r=-0.407; p=0.010) (Fig.A.8, but this did not remain significant in a multiple regression analysis with age, disease duration, Levodopa equivalent dose and CIRS total score as potential confounders (Table A.5).
3.6. Principal Component Analysis (PCA)
In order to explore relationships between key markers, a PCA was performed, using all participant data on all monocyte markers, and serum markers with uncorrected significant results on overall PD-Control paired analysis (Appendix A).
A three-component solution cumulatively explained 67.64% of the total variance. The principal component loadings of the rotated solution are shown in Table 2. Component 1 (32.48%) was mainly driven by (+)serum alpha-synuclein, (+)caspase-1 and (-)TLR2+ monocytes.
Table 2. Principal component loadings for the rotated solution of the PCA.
Coefficients <0.3 were suppressed and are shown in brackets.
| Variable | Component 1 | Component 2 | Component 3 | Communalities |
|---|---|---|---|---|
| Total Monocyte TLR2+ percentage | -0.880 | (-0.093) | (-0.042) | 0.785 |
| Serum Caspase-1 | 0.801 | (-0.275) | (-0.093) | 0.726 |
| Serum Endotoxin | (-0.120) | 0.759 | (0.056) | 0.594 |
| Total Monocyte TREM2 MFI ratio | (0.058) | 0.673 | (-0.244) | 0.515 |
| Serum Alpha-synuclein | 0.401 | -0.536 | -0.330 | 0.558 |
| Classical Monocyte percentage | (-0.012) | (-0.072) | 0.936 | 0.881 |
Component 2 (19.48%) was mainly driven by (+)serum endotoxin, (+)monocyte TREM2 and less by (-)serum alpha-synuclein. Component 3 (15.67%) was mainly driven by (+)classical monocyte percentage and (-)serum alpha-synuclein.
Paired comparisons of PCA components between PD and controls and within the risk groups (Fig.(4F),(G),(H)), indicated that Component 1 was significantly lower in all PD versus controls (W=397, p<0.0001) and in both HR (W=78, p=0.0256) and LR (W=118, p=0.0001) groups, while Component 2 was significantly higher in all PD versus controls (W=-299, p=0.0015) and within the HR group (W=-112, p=0.0004). Component 3 was also significantly higher in all PD versus controls (W=-297, p=0.0016), with elevation within the LR (W=-84, p=0.0151) and the HR (W=-70, p=0.0479) groups. Bivariate correlation analyses did not show any associations between PCA components and measured clinical variables.
Key relationships identified through PCA were further explored, including the relationship between endotoxin and TREM2 (component 2) and between alpha-synuclein and caspase-1 (component 1).
3.7. Endotoxin and TREM2
Serum endotoxin did not directly correlate with monocyte surface TREM2. However, it demonstrated a significant, but weak positive correlation with soluble TREM2 (r=0.1613, p=0.0005), which remained significant on multivariate linear regression analysis, with monocyte TREM2 and age as potential confounders (Fig.A.9; Table A.6).
3.8. Alpha-synuclein and Caspase-1
In addition to clustering within the PCA (component 1), serum alpha-synuclein and caspase-1 were significantly positively correlated with each other (r =0.382, p=0.001)(Fig. 5I). In order to further investigate potential mechanistic factors relating to the serum changes seen in these proteins (e.g. differences in cellular uptake and release), additional functional assays and analyses were performed using ex-vivo monocytes from subsets of participants from the cohort.
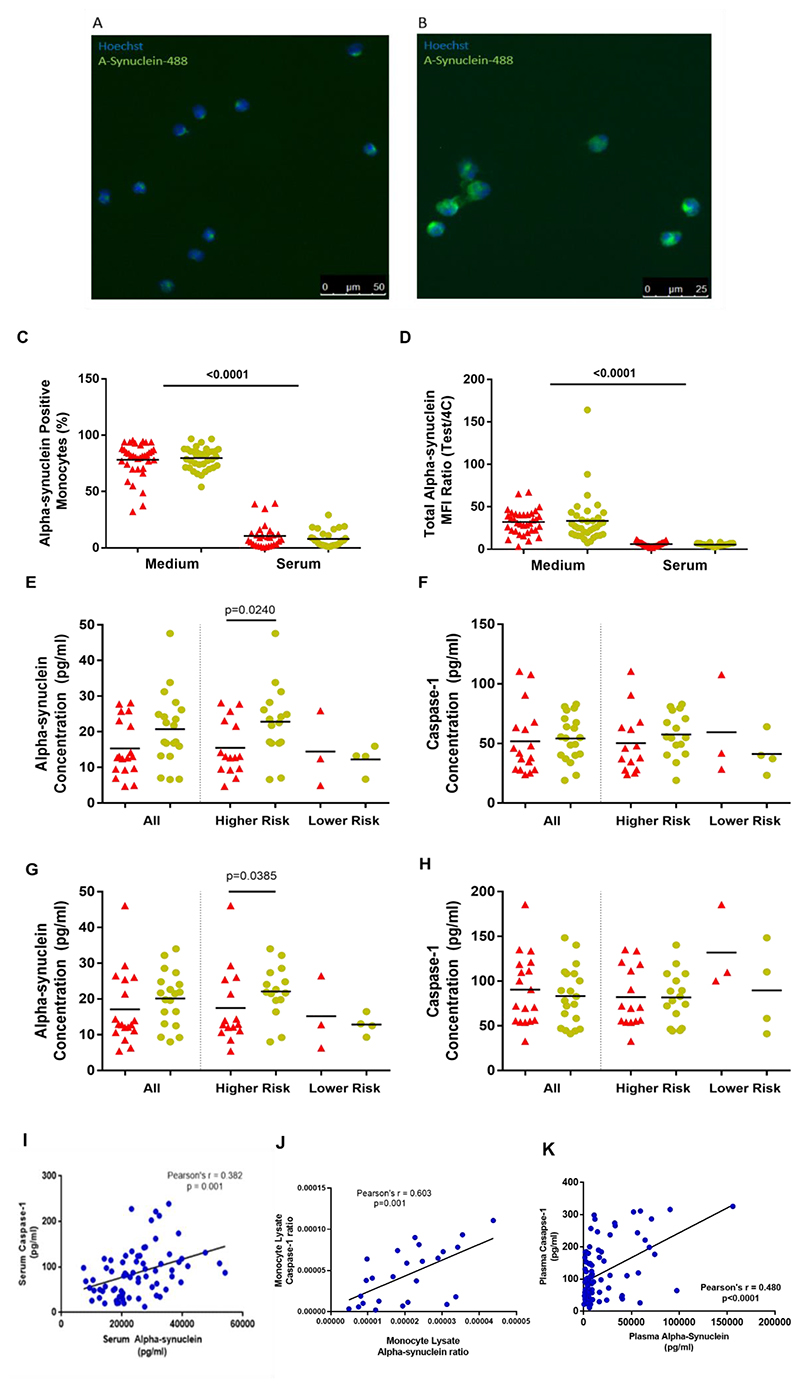
Figure 5. Alpha-synuclein and Caspase-1.
(A), (B) - Fluorescence microscope images of monocytes which have taken up fluorescent alpha-synuclein-HiLyte™ Fluor 488 at 90minutes. Hoechst staining identifies cell nuclei. (a) 20X; (b) 40X.
(C), (D) - Monocyte fluorescent alpha-synuclein uptake in standard medium and in autologous serum – (C) percentage alpha-synuclein positive monocytes; (D) total monocyte MFI ratio. (Medium- Patients=35, Controls=35; Serum- Patients=27, Controls=28) (Patients=red; Controls=yellow).
(E),(F),(G),(H) - Alpha-synuclein and caspase-1 in monocyte 24 hour culture supernatants without (E)(F) and with (G)(H) LPS, in all participants and within risk groups. (Patients = red, Controls= yellow).
(All participants-Patients=18, Controls=20; Higher Risk Patients=15, Controls=16; Lower Risk Patients=3, Controls=4) (Patients=red; Controls=yellow).
(I), (J) Graphs showing significant relationship between alpha-synuclein and caspase-1 in serum (I) and monocyte lysates (J).
(K) ICICLE-Cambridge cohort - Relationship between alpha-synuclein and caspase-1 in plasma samples collected at study baseline.
3.8.1. Monocyte fluorescent alpha-synuclein uptake
Monocyte uptake of fluorescent monomeric alpha-synuclein was assessed using standard medium, as well as an autologous serum environment (Fig. 5A-D). Autologous serum was used to better represent the in-vivo intravascular environment (Wijeyekoon et al., 2018), while the standard medium was used to study the intrinsic uptake ability of the monocytes independent of serum factors.
There were no significant differences in fluorescent alpha-synuclein uptake in standard medium between patients and controls (% positive monocytes t(35)=0.7691, p=0.4470)(Fig. 5C,D). In autologous serum, uptake was decreased across all groups, compared to standard medium (t(50)=46.45, p<0.0001), but no significant case-control differences were seen (t(27)=1.685, p=0.1034).
3.8.2. Monocyte alpha-synuclein and caspase-1 secretion
Monocyte secretion of alpha-synuclein and caspase-1 were not significantly different between all PD and controls (Fig. 5E-H). However alpha-synuclein secretion was decreased in HR cases compared to paired controls, with (t(12)=2.324, p=0.0385) and without LPS stimulation (t(13)=2.555, p=0.0240)(Fig. 5E,G). The number of LR group pairs were insufficient for analysis.
Additionally, LPS led to increased secretion of caspase-1 overall, with no relative difference in PD versus controls (Figure 5F,H). However, alpha-synuclein secretion did not increase with LPS stimulation (Figure 5E,G).
3.8.3. Monocyte lysate alpha-synuclein and caspase-1
Western blot analysis indicated the presence of higher molecular weight alpha-synuclein and of caspase-1 in monocyte lysates in both PD and controls (Fig.A.10A,B). Measurement of total alpha-synuclein and caspase-1 levels in monocyte lysates (relative to total protein) using MSD and ELISA assays, found no significant case-control differences (Fig.A.10C,D)).
However, similar to the serum findings, monocyte lysate alpha-synuclein and caspase-1 were also positively correlated with each other (r=0.603, p=0.001)(Fig. 5J).
3.9. Alpha-synuclein and caspase-1 in a separate, larger incident PD cohort
Alpha-synuclein and caspase-1 were measured in baseline plasma samples from the ICICLEC-ambridge cohort (n=93; mean(standard deviation)-:age=66.73(6.92);disease duration =0.81(0.57); 60.2% male).
The two proteins again demonstrated a significant positive correlation with each other (Pearson’s r=0.480, p<0.001;Fig. 5K), replicating findings in the primary study cohort. There were no significant correlations between the baseline levels of either marker and baseline or longitudinal cognitive/motor measures over 36 months (data not shown).
4. Discussion
This study evaluated key peripheral innate immune markers linked to PD, in a cohort of dementia risk stratified patients and carefully matched controls. The findings confirmed that there are significant peripheral innate immune changes in PD, with increased classical monocytes and TLR positive monocytes, and further demonstrated that these changes are most marked in those individuals at increased early dementia risk. Monocyte TREM2 expression was also elevated in the higher dementia risk group, while higher monocyte HLA-DR expression correlated with better existing motor and cognitive performance.
The study has also uniquely shown that innate immune changes in PD are accompanied by elevated serum levels of bacterial endotoxin, suggesting that this may be playing a critical role in driving the innate immune response and associated pathology. In addition, serum alpha-synuclein was significantly decreased in PD, irrespective of dementia risk group and had a close relationship with serum caspase-1-a key component of the inflammasome pathway.
PCA confirmed the clustering of markers with similar patient-control group variations. In particular, component 1 was mainly driven by alpha-synuclein and caspase-1 plus monocyte TLR2 and demonstrated significant PD-control differences overall and in both risk groups. Component 2 was driven by serum endotoxin, monocyte TREM2 and serum alpha-synuclein, with significant overall PD-control differences, predominant within the HR group. Component 3 was driven by the classical monocyte percentage and serum alpha-synuclein, with significant PD-control differences also overall and within both groups.
4.1. Monocyte changes in PD
The increase in classical monocytes (with corresponding decrease in non-classical monocytes), and monocyte TLR2+ and TLR4+ monocytes observed in this PD cohort confirms previous findings (Grozdanov et al., 2014)(Drouin-Ouellet et al., 2015). However, this study further demonstrates that these changes are mainly driven by those at increased risk of dementia.
Classical monocytes (up to 85% of total monocytes) are mainly involved in phagocytosis, antigen presentation to adaptive immune cells and cytokine secretion in response to TLR stimulation (Wong et al., 2012)(Mukherjee et al., 2015), although exact subtype functions may vary under different clinical conditions (J. Lee et al., 2017)(Boyette et al., 2017). Intermediate and non-classical monocytes may also be more senescent cells, that increase with age (Ziegler-Heitbrock and Hofer, 2013) and derive from further differentiation of classical monocytes in the bone marrow (Ong et al., 2018)(Patel et al., 2017). At a systemic level, LPS stimulation leads to depletion of monocyte numbers and on recovery, classical monocytes appear to rise first (Tak et al., 2017). Thus, the increased classical monocytes seen in PD compared to controls, most prominent in the HR group, may reflect chronic exposure to the increased endotoxin, in these patients. The data is also consistent with our previous finding of reduced senescence markers in T lymphocytes in PD cases in this cohort (Williams-Gray et al., 2018).
The TLR2+ monocyte percentage varied inversely to alpha-synuclein and caspase-1 in PCA component 1. TLR2 is an innate immune receptor to DAMPs/PAMPs(e.g. bacterial-derived lipids, amyloids) and endogenous ligands including forms of alpha-synuclein (Bryant et al., 2015) (Kim et al., 2013)(Kim et al., 2016a)(Codolo et al., 2013), and can also facilitate activation of the inflammasome pathway (Rapsinski et al., 2015) and caspase-1.
TLR4 has a major role in the transduction of the cellular response to bacterial endotoxin and its stimulation leads to activation of Nuclear factor-kappa B (NF-κB) and transcription of cytokine precursors (e.g.IL-6, IL-1β) and inflammasome components (Lu et al., 2008), potentially leading to a relative ‘priming’ effect on inflammasome activation in patients.
Thus, the TLR2 and TLR4 monocyte changes seen may reflect increased potential to respond to DAMPS/PAMPS including alpha-synuclein, with subsequent promotion of caspase-1 activation, intracellular accumulation/aggregation and inflammation, mainly in the HR patients.
Significant elevation of monocyte TREM2 was also observed in the HR patient group. TREM2 is involved in bacterial clearance during sepsis (Chen et al., 2013) and may have a role in regulating TLR pathway signalling (Hamerman et al., 2006)(Kober and Brett, 2017). Increased monocyte TREM2 expression is seen in Alzheimer’s disease (Hu et al., 2014) and TREM2 can act as a microglial receptor for amyloid-beta, with involvement in phagocytosis and clearance of Alzheimer’s pathology (Zhao et al., 2018)(Lee et al., 2018). Interestingly, this may suggest that TREM2 may be raised in response to chronic TLR stimulation and indicates possible immunological similarities between high dementia risk PD and Alzheimer’s disease.
Monocyte HLA-DR expression did not differ between PD cases and controls, but did significantly correlate with clinical variables in disease (lower levels associated with worse cognitive/motor function). Low monocyte HLA-DR is a key feature of monocyte tolerance, typically known to occur subsequent to stimuli such as LPS/endotoxin or sepsis, and is associated with decreased antigen presentation, cytokine production and changes in phagocytosis, and mediated by epigenetic and metabolic factors (Pfortmueller et al., 2017)(Saeed et al., 2014)(Cheng et al., 2014). These immune disruptions associated with low HLA-DR may contribute to impaired clearance of pathological proteins, neuronal dysfunction and subsequent cognitive and motor impairment. In contrast, PD animal models involving acute toxin/protein injections/pathology suggest deleterious effects of increased HLA-DR (Williams et al., 2018)(Harms et al., 2013). This may indicate that in the acute situation seen in animal models, the detrimental effects of HLADR (e.g.pro-inflammatory cytokine release) outweigh the beneficial effects of increased antigen presentation/clearance, whereas in the chronic clinical situation, the beneficial effects of increased HLA-DR mediated pathology clearance outweigh the detrimental effects. However, more detailed prospective studies will be required to further investigate these effects and hypotheses.
The PD-control marker changes seen in total monocytes may mainly reflect changes seen in the pre-dominant classical monocyte subtype. However, analysis of the more limited data from Intermediate and Non-Classical monocytes also indicated broadly similar trends in these subtypes overall (Appendix A). Further detailed studies specifically investigating monocyte subtype and related innate immune cell (e.g. dendritic cell) changes in PD will be important in future work.
4.2. Serum Endotoxin
To our knowledge, this is the first study to directly demonstrate elevated serum endotoxin in PD, particularly in patients with increased risk for early dementia. Endotoxin is a principal component of the outer membrane of gram-negative bacteria and its major source in serum is translocation of gram-negative bacterial components across gastrointestinal/other mucosal membranes (Bischoff et al., 2014) (Kelesidis et al., 2012)(Alexopoulou et al., 2017) and thus the findings indicate greater bacterial translocation in PD, particularly in the HR group.
Accordingly, previous studies have demonstrated greater gastrointestinal permeability in PD patients, with lower levels of LPS binding protein (LBP) (indicating less LPS neutralisation) and increased gut staining for Escherichia Coli bacteria (Forsyth et al., 2011)(Perez-Pardo et al., 2019). One smaller study (Hasegawa et al., 2015) found no difference in serum endotoxin, but lower LBP (Pal et al., 2015)(Vreugdenhil et al., 2003) in PD patients, suggesting an LPS-LBP imbalance with increased LPS activity. Intestinal microbiome differences are also seen in PD (Scheperjans et al., 2015) and may modulate disease pathology and manifestation (Sampson et al., 2016)(Dodiya et al., 2018).
Low level chronic elevation of serum endotoxin in PD may be an important mediator of innate immune changes. At a cellular level, endotoxin/LPS acts via TLR4, activating NF-κB and transcription of pro-inflammatory cytokines and proteins including pro-IL-1β and NLRP3 (Lu et al., 2008). Intracellular LPS can also activate the non-canonical NLRP3 inflammasome pathway via caspase-11 (caspase-4 or -5 in humans), leading to caspase-1 activation (Man and Kanneganti, 2015)(Stowe et al., 2015). Thus, endotoxin would stimulate both TLR and inflammasome pathways, potentially resulting in cumulative pathogenic effects.
Endotoxin also has direct effects on alpha-synuclein, stimulating increased production in macrophages (Tanji et al., 2002) and specific fibril formation (Kim et al., 2016b)(Bhattacharyya et al., 2019). Furthermore, in-vivo studies have demonstrated synergistic deleterious effects of LPS and alpha-synuclein on PD related pathology and neuronal survival (H.-M. Gao et al., 2011)(Zhang et al., 2018). Amyloid beta and tau pathology, which are additionally related to cognitive impairment, have also been linked to endotoxin-associated inflammation (Asti and Gioglio, 2014)(Kitazawa et al., 2005), with LPS stimulation leading to increased tau production (Bhaskar et al., 2010)(Gardner et al., 2016).
Peripheral endotoxin may also lead to neuroinflammation in the brain, as measured using PET imaging of microglial activation in human subjects following peripheral intravenous injection of low dose LPS (Sandiego et al., 2015). It is unclear whether the microglial activation is a direct consequence of endotoxin in CNS tissues, or of secondary immune cell/protein CNS entry. However, post-mortem studies have found LPS/microbial proteins in relation to amyloid-beta plaques in Alzheimer’s disease brains, indicating the presence of endotoxin itself within the brain (Zhan et al., 2016).
As discussed above, variable extent and duration of serum endotoxin exposure (Morris et al., 2015) may also subsequently influence disease pathology through monocyte tolerance and effects on HLA-DR/protein clearance functions (Mukherjee et al., 2015)(Kobayashi et al., 2016)(de Lima et al., 2014), in addition to driving an innate immune response and promoting aggregation, particularly in the HR group.
PCA Component 2 indicated a possible relationship between endotoxin and monocyte TREM2. As discussed above, TREM2 is involved in bacterial clearance (Chen et al., 2013) and may be raised in response to elevated endotoxin and TLR signalling in PD (Hamerman et al., 2006)(Kober and Brett, 2017). Opposite to this elevation seen in HR PD, monocyte TREM2 is decreased in LR PD relative to controls, consistent with the lack of rise in endotoxin/TLR in LR PD. Furthermore, serum endotoxin was positively correlated with sTREM2, which would be consistent with the sTREM2 rise seen with infection related immune activation (Gisslén et al., 2019).
4.3. Alpha-synuclein and Caspase-1
Serum alpha-synuclein and caspase-1 (major factors in PCA component 1), were significantly lower in PD compared to controls regardless of risk group. The study has also discovered a positive relationship between these proteins in serum, plasma and monocyte lysates. This would be consistent with co-accumulation/aggregation and/or parallel changes in production and breakdown.
Alpha-synuclein is physiologically produced, released and taken up by many cells, including peripheral blood cells (Shin et al., 2000)(Barbour et al., 2008)(Tyson et al., 2016) and decreased levels are seen in PD blood (Gupta et al., 2015)(Li et al., 2007)(Ishii et al., 2015) and cerebrospinal fluid (CSF) (Eusebi et al., 2017). This may suggest alpha-synuclein is being sequestered out of bio-fluids by aggregation and/or intracellular accumulation. Higher patient plasma alpha-synuclein found in some studies (Lin et al., 2017)(Duran et al., 2010), may relate to red cell/platelet leakage during centrifugation (Shi et al., 2010).
The decreased monocyte alpha-synuclein release seen in the HR patient group may be an additional contributor to lower serum alpha-synuclein in this group and could relate to exocytosis dysfunction in PD (Lautenschlager et al., 2017)(Logan et al., 2017). Further, increased aggregated alpha-synuclein species found in PD (Parnetti et al., 2019) may also cause confounding issues with detection by the immunoassays used.
Caspase-1, a key component of the inflammasome pathway (Strowig et al., 2012), is produced by many cell types, including monocytes (Shamaa et al., 2015). Activation of inflammasome complexes (e.g. Nucleotide-binding domain, leucine rich repeat containing receptor family pyrin domain containing-3(NLRP3)) (Strowig et al., 2012)(Man and Kanneganti, 2015), triggered by a variety of stimuli related to homeostatic disruption (e.g. microbial/viral RNA/DNA components, ATP, uric acid) (Man and Kanneganti, 2015), leads to activation of caspase-1, which subsequently cleaves other proteins (e.g. pro-IL-1β, pro-IL-18), leading to inflammation.
Activated caspase-1 can also cleave alpha-synuclein, making it more aggregable (Wang et al., 2016) and our observation of decreased serum caspase-1 and its correlation with alpha-synuclein may suggest they are both being sequestered out of serum, possibly into cells or aggregates, as a common process in PD. Studies, including our own work (White et al., 2018), have also shown that alpha-synuclein can activate inflammasome pathways causing inflammatory cytokine production (Codolo et al., 2013)(Zhou et al., 2016). Hence both alpha-synuclein and caspase-1 may interact in a bidirectional loop, which contributes to both increased inflammation and intracellular alpha-synuclein aggregation in PD.
In contrast to the current study, Zhou et al. found increased caspase-1 in a smaller cohort of twelve patients compared to controls (Zhou et al., 2016). However, differences in patient/control demographics and longer delays prior to higher speed centrifugation (increasing leakage risk of intracellular caspase-1) in that study, may have contributed to the differences.
The alpha-synuclein and caspase-1 related monocyte assays may additionally suggest functional impairment of monocytes in PD. Previous studies have found TLR4-mediated stimulation of microglial phagocytosis and alpha-synuclein uptake (Fellner et al., 2013)(Venezia et al., 2017) and increased caspase-1 release on endotoxin stimulation of PBMCs (White et al., 2018). However, PD monocytes in this study demonstrated no increased alpha-synuclein uptake or LPS-induced caspase-1 secretion, contrary to what might be expected given their higher TLR4+ percentage, compared to controls.
4.4. Relevance of peripheral immune changes to PD pathology
Peripheral changes in alpha-synuclein and innate immune and microbial related molecules may provide relevant insights into PD pathogenesis and heterogeneity. In particular, multiple routes of communication between the CNS and periphery, (including lymphatic routes (Louveau et al., 2015), blood/brain barrier, choroid plexus, CSF, meninges and the peripheral/autonomic nervous system (Su and Federoff, 2014)), indicate that peripheral changes could be influenced by and/or influence CNS pathology. Importantly, peripheral LPS injection in humans leads to rapid central microglial activation on PET imaging and highlights the strength of influence of peripheral immune factors on the CNS (Sandiego et al., 2015).
Alpha-synuclein pathology is present in the gut and periphery as well as the brain in PD (Forsyth et al., 2011) and some of the peripheral immune changes observed may be directly reflective of such pathology. Also, peripheral factors such as the microbiome (e.g. gut) may have parallel peripheral immune and CNS effects (Marizzoni et al., 2017), including in PD (Scheperjans et al., 2015)(Unger et al., 2016).
In addition to potential direct contributions towards the pathology, peripheral monocytes constitute an easily repeatedly accessible source of cells from a living patient and may provide insights into generic cellular processes disrupted in PD. While monocytes are considered to contribute towards choroid plexus macrophages (Kierdorf et al., 2019), genetic variations in central microglia function have been paralleled in related peripheral monocytes (Bradshaw et al., 2013), indicating that monocytes could act as peripheral proxies or models for particular aspects of study of microglia and CNS innate immune cells in PD.
4.5. Limitations
Our sample size was limited by the feasibility of collecting patient-control samples paired by age, gender and MAPT haplotype for parallel processing of samples. Although this was advantageous in terms of ensuring that each patient-control pair experienced minimal variations due to methodological issues, it limited power which may explain our inability to detect previously observed differences in inflammatory cytokines(Williams-Gray et al., 2016)(Qin et al., 2016). In addition, despite practical corrections for multiple comparison testing, we cannot exclude the possibility of Type I errors.
Dopamine may influence immune cell properties (Papa et al., 2017) and Levodopa/dopaminergic medications could therefore cause potential confounding effects. However, none of the measured markers had any relationship with the Levodopa equivalent dose.
4.6. Conclusions
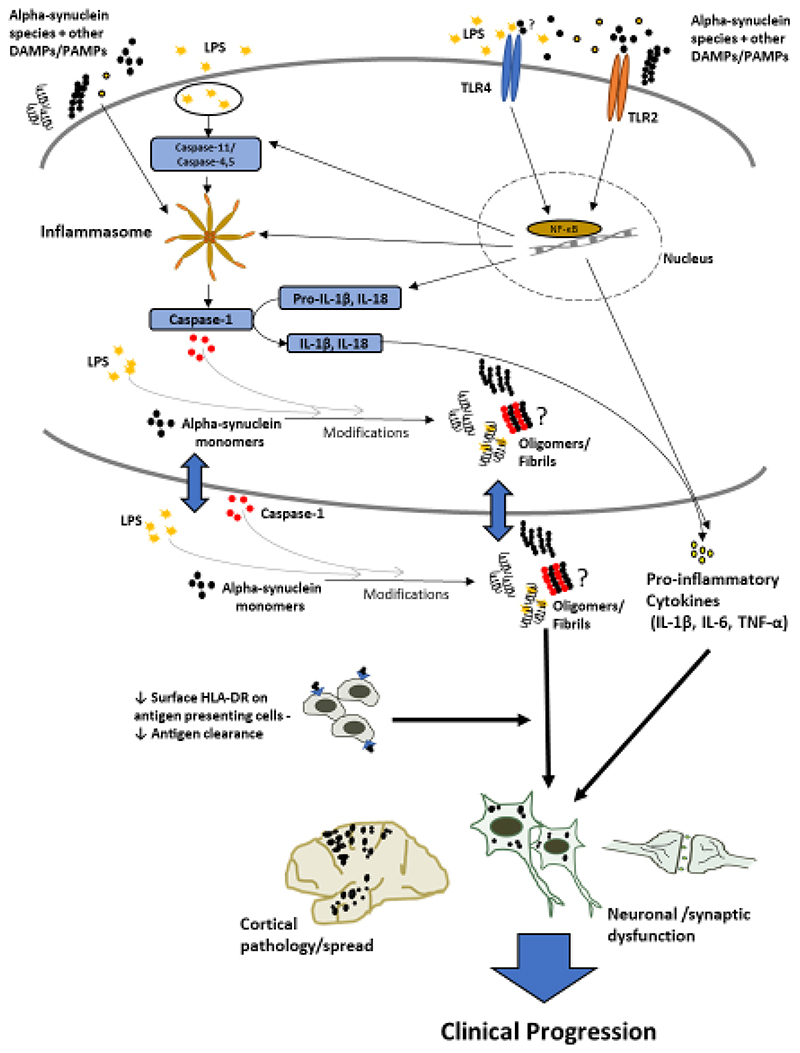
The observed changes in monocyte and serum markers indicate significant innate immune involvement in early-moderate PD, with differential and more marked effects in those with greater clinical impairment and at increased risk for early cognitive decline, suggesting that certain immune changes may be relevant to faster disease progression. Relevant mechanistic pathways and hypothesized interactions are summarised in Fig.6.
Figure 6. Summary.
Schematic diagram of key innate immune related markers, pathological pathways and hypothesised interactions relevant to clinical progression in Parkinson’s disease, based on insights from this study and previous literature. The cellular section represents any cell in which the relevant markers/processes are present. The outcomes of these processes, together with decreased clearance and circulatory spread, could ultimately contribute towards increased neuronal/synaptic dysfunction, cortical pathology, and consequent cognitive/clinical progression.
LPS – Lipopolysaccharide (Endotoxin); DAMPs/PAMPs – Damage/Pathogen Associated Molecular Patterns; NF-κB – Nuclear Factor Kappa B; TREM2- Triggering Receptor Expressed on Myeloid cells-2; HLA-DR- Human Leukocyte Antigen-DR subtype; TLR-Toll Like Receptor; IL- Interleukin; TNF – Tumour Necrosis Factor.
Higher endotoxin in those patients with higher dementia risk suggests that greater microbial translocation may be an important factor in driving pathology, whilst the association between low monocyte HLA-DR and poor clinical status suggests that impaired antigen presentation may also contribute to increased pathology and neurodegeneration.
Alpha-synuclein and caspase-1 appear to be closely associated and may have reciprocal effects which contribute to inflammasome activation and intracellular alpha-synuclein aggregation in all PD.
The findings need to be replicated and the extent to which the clinical differences are simply correlated with, or are a consequence of, immune changes will require further prospective longitudinal studies. Measurement of serum LBP and other microbial translocation markers (e.g. bactericidal-permeability increasing protein (BPI) (Alexopoulou et al., 2017)), epigenetic markers associated with monocyte endotoxin tolerance and other inflammasome pathway components (e.g. NLRP3 and Apoptosis-associated Speck-like protein containing a C-terminal caspase-recruitment domain (ASC) specks (Gordon et al., 2018)(Franklin et al., 2018)) would provide further insights into involvement of these pathways in PD.
A clearer mechanistic understanding of how innate immune pathways contribute to disease progression and dementia may open up new therapeutic avenues and enable targeted trials of specific immune/microbial related therapies (e.g. caspase-1 /inflammasome inhibitors (Bassil et al., 2016)(Flores et al., 2018)(Coll et al., 2015), TLR antagonists (Lucas and Maes, 2013)(Peri and Piazza, 2012)(Kouli et al., 2019), and gut bacterial translocation reduction therapies (Fukui, 2017)(Gangarapu et al., 2015)) in particular subgroups. Improving constipation/gastrointestinal health, and prevention/early treatment of infections may also be of relevance, while specific markers may have additional value as biomarkers to monitor target engagement of immune-directed therapies. Further detailed investigation of the components and relationships highlighted in this study will be essential for progression towards such clinical and therapeutic applications.
Supplementary Material
Highlights.
-
*
Disease relevant innate immune monocyte markers are altered in Parkinson’s disease
-
*
The monocyte marker changes are accompanied by elevated serum bacterial endotoxin
-
*
These findings are most pronounced in Parkinson’s cases at higher dementia risk
-
*
Alpha-synuclein and caspase-1 are correlated and are lower in Parkinson’s serum
Acknowledgements
We gratefully acknowledge the participation of all our patient and control volunteers and NIHR Cambridge BioResource volunteers and thank the NIHR Cambridge BioResource centre and staff for their contribution. We thank the National Institute for Health Research and NHS Blood and Transplant. We also acknowledge the support of the Cambridge NIHR BRC Cell Phenotyping Hub and the Core Biochemical Assay Laboratory at Cambridge University Hospitals.
Funding
Funding for this work was provided by Addenbrooke’s Charitable Trust (PF15/CWG), the Rosetrees Trust (M369-F1) and the NIHR Cambridge Biomedical Research Centre (146281). RSW was supported by a Fellowship from Addenbrooke’s Charitable Trust (RG77199, PF19/CWG). DKV was supported by a Junior Research Fellowship from Homerton College, Cambridge. KMS was supported by a Fellowship from the Wellcome Trust. WLK is supported by the MRC/UKRI fellowship (MR/S005528/1). DPB is supported by a Wellcome Clinical Research Career Development Fellowship. JJ is supported by the Wellcome Trust (RG79413). RAB is an NIHR Senior Investigator (NF-SI-0616-10011) and is supported by the Wellcome Trust-MRC Cambridge Stem Cell Institute. CHWG is supported by a RCUK/UKRI Research Innovation Fellowship awarded by the Medical Research Council (MR/R007446/1) and by the Cambridge Centre for Parkinson-Plus.
Abbreviations
- ACE-R
Addenbrooke’s Cognitive Examination – Revised
- CIRS
Cumulative Illness Rating Scale
- CNS
Central Nervous System
- CSF
Cerebrospinal fluid
- HR
Higher Dementia Risk
- HY
Hoehn and Yahr
- LR
Lower Dementia Risk
- MDS-UPDRS
Movement Disorder Society – Unified Parkinson’s Disease Rating Scale.
- PBMC
Peripheral blood mononuclear cells
- PCA
Principal Components Analysis
- PD
Parkinson’s Disease
Footnotes
Competing Interests
The authors report no competing interests.
Data availability
Data related to the findings of this study will be available from the corresponding author, upon reasonable request.
References
- Alexopoulou A, Agiasotelli D, Vasilieva LE, Dourakis SP. Bacterial translocation markers in liver cirrhosis. Ann Gastroenterol. 2017;30:486–497. doi: 10.20524/aog.2017.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asti A, Gioglio L. Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation? J Alzheimers Dis. 2014;39:169–79. doi: 10.3233/JAD-131394. [DOI] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Park Relat Disord. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ. Red Blood Cells Are the Major Source of Alpha-Synuclein in Blood. Neurodegener Dis. 2008;5:55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- Bassil F, Fernagut P-O, Bezard E, Pruvost A, Leste-Lasserre T, Hoang QQ, Ringe D, Petsko GA, Meissner WG. Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy. Proc Natl Acad Sci. 2016;113:9593–9598. doi: 10.1073/pnas.1609291113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. Regulation of Tau Pathology by the Microglial Fractalkine Receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya D, Mohite GM, Krishnamoorthy J, Gayen N, Mehra S, Navalkar A, Kotler SA, Ratha BN, Ghosh A, Kumar R, Garai K, et al. Lipopolysaccharide from Gut Microbiota Modulates a-Synuclein Aggregation and Alters Its Biological Function. ACS Chem Neurosci. 2019:acschemneuro.8b00733. doi: 10.1021/acschemneuro.8b00733. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke J-D, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12:e0176460. doi: 10.1371/journal.pone.0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, The Alzheimer Disease Neuroimaging, I et al. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16:848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CE, Gay NJ, Heymans S, Sacre S, Schaefer L, Midwood KS. Advances in Toll-like receptor biology: Modes of activation by diverse stimuli. Crit Rev Biochem Mol Biol. 2015;50:359–379. doi: 10.3109/10409238.2015.1033511. [DOI] [PubMed] [Google Scholar]
- Cen L, Yang C, Huang S, Zhou M, Tang X, Li K, Guo W, Wu Z, Mo M, Xiao Y, Chen X, et al. Peripheral Lymphocyte Subsets as a Marker of Parkinson’s Disease in a Chinese Population. Neurosci Bull. 2017;33:493–500. doi: 10.1007/s12264-017-0163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-C, Lin T-M, Chang Y-S, Chen W-S, Sheu J-J, Chen Y-H, Chen J-H. Autoimmune rheumatic diseases and the risk of Parkinson disease: a nationwide population-based cohort study in Taiwan. Ann Med. 2018;50:83–90. doi: 10.1080/07853890.2017.1412088. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang K, Jin Y, Zhu T, Cheng B, Shu Q, Fang X. Triggering Receptor Expressed on Myeloid Cells-2 Protects against Polymicrobial Sepsis by Enhancing Bacterial Clearance. Am J Respir Crit Care Med. 2013;188:201–212. doi: 10.1164/rccm.201211-1967OC. [DOI] [PubMed] [Google Scholar]
- Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, et al. mTOR- and HIF-1 -mediated aerobic glycolysis as metabolic basis for trained immunity. Science (80-) 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M. Triggering of Inflammasome by Aggregated α–Synuclein, an Inflammatory Response in Synucleinopathies. PLoS One. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima TM, Sampaio SC, Petroni R, Brigatte P, Velasco IT, Soriano FG. Phagocytic activity of LPS tolerant macrophages. Mol Immunol. 2014;60:8–13. doi: 10.1016/j.molimm.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Dodiya HB, Forsyth CB, Voigt RM, Engen PA, Patel J, Shaikh M, Green SJ, Naqib A, Roy A, Kordower JH, Pahan K, et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol Dis. 2018 doi: 10.1016/j.nbd.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, St-Amour I, Saint-Pierre M, Lamontagne-Proulx J, Kriz J, Barker RA, Cicchetti F. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran R, Barrero FJ, Morales B, Luna JD, Ramirez M, Vives F. Plasma a-synuclein in patients with Parkinson’s disease with and without treatment. Mov Disord. 2010;25:489–493. doi: 10.1002/mds.22928. [DOI] [PubMed] [Google Scholar]
- Dzamko NL. LRRK2 and the Immune System. Advances in Neurobiology. 2017:123–143. doi: 10.1007/978-3-319-49969-7_7. [DOI] [PubMed] [Google Scholar]
- Eusebi P, Giannandrea D, Biscetti L, Abraha I, Chiasserini D, Orso M, Calabresi P, Parnetti L. Diagnostic utility of cerebrospinal fluid a-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord. 2017;32:1389–1400. doi: 10.1002/mds.27110. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for a-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–60. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerbach D, Schindler P, Barske C, Joller S, Beng-Louka E, Worringer KA, Kommineni S, Kaykas A, Ho DJ, Ye C, Welzenbach K, et al. ADAM17 is the main sheddase for the generation of human triggering receptor expressed in myeloid cells (hTREM2) ectodomain and cleaves TREM2 after Histidine 157. Neurosci Lett. 2017;660:109–114. doi: 10.1016/j.neulet.2017.09.034. [DOI] [PubMed] [Google Scholar]
- Flores J, Noël A, Foveau B, Lynham J, Lecrux C, LeBlanc AC. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat Commun. 2018;9:3916. doi: 10.1038/s41467-018-06449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BS, Latz E, Schmidt FI. The intra- and extracellular functions of ASC specks. Immunol Rev. 2018;281:74–87. doi: 10.1111/imr.12611. [DOI] [PubMed] [Google Scholar]
- Fukui H. Gut Microbiome-based Therapeutics in Liver Cirrhosis: Basic Consideration for the Next Step. J Clin Transl Hepatol. 2017;5:249–260. doi: 10.14218/JCTH.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk N, Wieghofer P, Grimm S, Schaefer R, Bühring H-J, Gasser T, Biskup S. Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov Disord. 2013;28:392–5. doi: 10.1002/mds.25300. [DOI] [PubMed] [Google Scholar]
- Gangarapu V, Ince AT, Baysal B, Kayar Y, Klg U, Gök Ö, Uysal Ö, Şenturk H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840–845. doi: 10.1097/MEG.0000000000000348. [DOI] [PubMed] [Google Scholar]
- Gao H-M, Zhang F, Zhou H, Kam W, Wilson B, Hong J-S. Neuroinflammation and a-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119:807–14. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76:863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, Mao W, Schüle B, Babcock M, Schoebel S, Lorenzana C, Alexander J, Kim S, Glick H, Hilton K, Fitzgerald JK, et al. Elevated Alpha-Synuclein Impairs Innate Immune Cell Function and Provides a Potential Peripheral Biomarker for Parkinson’s Disease. PLoS One. 2013;8:e71634. doi: 10.1371/journal.pone.0071634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LE, White JD, Eimerbrink MJ, Boehm GW, Chumley MJ. Imatinib methanesulfonate reduces hyperphosphorylation of tau following repeated peripheral exposure to lipopolysaccharide. Neuroscience. 2016;331:72–77. doi: 10.1016/j.neuroscience.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gisslén M, Heslegrave A, Veleva E, Yilmaz A, Andersson L-M, Hagberg L, Spudich S, Fuchs D, Price RW, Zetterberg H. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol - Neuroimmunol Neuroinflammation. 2019;6:e512. doi: 10.1212/NXI.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O’Neill LA, Kanthasamy AG, et al. Inflammasome inhibition prevents a-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018;10:eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland JC, Williams-Gray CH, Barker RA. The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur J Neurosci. 2019;49:328–338. doi: 10.1111/ejn.14094. [DOI] [PubMed] [Google Scholar]
- Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, Ludolph AC, et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014;128:651–63. doi: 10.1007/s00401-014-1345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov V, Bousset L, Hoffmeister M, Bliederhaeuser C, Meier C, Madiona K, Pieri L, Kiechle M, McLean PJ, Kassubek J, Behrends C, et al. Increased Immune Activation by Pathologic a-Synuclein in Parkinson’s Disease. Ann Neurol. 2019:ana.25557. doi: 10.1002/ana.25557. [DOI] [PubMed] [Google Scholar]
- Gupta V, Garg RK, Khattri S. Serological Analysis of Alpha-synuclein and NF- κB in Parkinson’s Disease Patients. J Clin DIAGNOSTIC Res. 2015;9:BC01-4. doi: 10.7860/JCDR/2015/12545.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert J-M, Raussens V. Amyloid fibrils are the molecular trigger of inflammation in Parkinson’s disease. Biochem J. 2015;471:323–333. doi: 10.1042/BJ20150617. [DOI] [PubMed] [Google Scholar]
- Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–5. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33:9592–600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A, West AB. α-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol Commun. 2017;5:85. doi: 10.1186/s40478-017-0494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K, et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS One. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Tan M-S, Yu J-T, Sun L, Tan L, Wang Y-L, Jiang T, Tan L. Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. J Alzheimers Dis. 2014;38:497–501. doi: 10.3233/JAD-130854. [DOI] [PubMed] [Google Scholar]
- Ishii R, Tokuda T, Tatebe H, Ohmichi T, Kasai T, Nakagawa M, Mizuno T, El-Agnaf OMA. Decrease in Plasma Levels of a-Synuclein Is Evident in Patients with Parkinson’s Disease after Elimination of Heterophilic Antibody Interference. PLoS One. 2015;10:e0123162. doi: 10.1371/journal.pone.0123162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju U-H, Liu F-C, Lin C-S, Huang W-Y, Lin T-Y, Shen C-H, Chou Y-C, Lin C-L, Lin K-T, Kao C-H, Chen C-H, et al. Risk of Parkinson disease in Sjogren syndrome administered ineffective immunosuppressant therapies. Medicine (Baltimore) 2019;98:e14984. doi: 10.1097/MD.0000000000014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia Aa, Barker Ra, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of Microbial Translocation and Macrophage Activation: Association With Progression of Subclinical Atherosclerosis in HIV-1 Infection. J Infect Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Masuda T, Jordão MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20:547–562. doi: 10.1038/s41583-019-0201-x. [DOI] [PubMed] [Google Scholar]
- Kim C, Ho D-H, Suk J-E, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee H-J, Lee S-J. Neuron-released oligomeric a-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Lee H-J, Masliah E, Lee S-J. Non-cell-autonomous Neurotoxicity of a-synuclein Through Microglial Toll-like Receptor 2. Exp Neurobiol. 2016a;25:113. doi: 10.5607/en.2016.25.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Lv G, Lee JS, Jung BC, Masuda-Suzukake M, Hong C-S, Valera E, Lee H-J, Paik SR, Hasegawa M, Masliah E, et al. Exposure to bacterial endotoxin generates a distinct strain of a-synuclein fibril. Sci Rep. 2016b;6:30891. doi: 10.1038/srep30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-Induced Inflammation Exacerbates Tau Pathology by a Cyclin-Dependent Kinase 5-Mediated Pathway in a Transgenic Model of Alzheimer’s Disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Inagawa H, Kohchi C, Okazaki K, Zhang R, Soma G-I. Effect of Lipopolysaccharide Derived from Pantoea agglomerans on the Phagocytic Activity of Amyloid β by Primary Murine Microglial Cells. Anticancer Res. 2016;36:3693–8. [PubMed] [Google Scholar]
- Kober DL, Brett TJ. TREM2-Ligand Interactions in Health and Disease. J Mol Biol. 2017;429:1607–1629. doi: 10.1016/j.jmb.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouli A, Horne CB, Williams-Gray CH. Toll-like receptors and their therapeutic potential in Parkinson’s disease and a-synucleinopathies. Brain Behav Immun. 2019 doi: 10.1016/j.bbi.2019.06.042. [DOI] [PubMed] [Google Scholar]
- Lautenschlager J, Kaminski CF, Kaminski Schierle GS. α-Synuclein – Regulator of Exocytosis, Endocytosis, or Both? Trends Cell Biol. 2017;27:468–479. doi: 10.1016/j.tcb.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Lee CYD, Daggett A, Gu X, Jiang L-L, Langfelder P, Li X, Wang N, Zhao Y, Park CS, Cooper Y, Ferando I, et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron. 2018;97:1032–1048.:e5. doi: 10.1016/j.neuron.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, James WS, Cowley SA. LRRK2 in peripheral and central nervous system innate immunity: its link to Parkinson’s disease. Biochem Soc Trans. 2017;45:131–139. doi: 10.1042/BST20160262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Tam H, Adler L, Ilstad-Minnihan A, Macaubas C, Mellins ED. The MHC class II antigen presentation pathway in human monocytes differs by subset and is regulated by cytokines. PLoS One. 2017;12:e0183594. doi: 10.1371/journal.pone.0183594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q-X, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, Culvenor JG, Horne MK. Plasma a-synuclein is decreased in subjects with Parkinson’s disease. Exp Neurol. 2007;204:583–588. doi: 10.1016/j.expneurol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Lin C-H, Yang S-Y, Horng H-E, Yang C-C, Chieh J-J, Chen H-H, Liu B-H, Chiu M-J. Plasma α-synuclein predicts cognitive decline in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88:818–824. doi: 10.1136/jnnp-2016-314857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan T, Bendor J, Toupin C, Thorn K, Edwards RH. a-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci. 2017;20:681–689. doi: 10.1038/nn.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lucas K, Maes M. Role of the Toll Like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway. Mol Neurobiol. 2013;48:190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Kanneganti T-D. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marizzoni M, Provasi S, Cattaneo A, Frisoni GB. Microbiota and neurodegenerative diseases. Curr Opin Neurol. 2017;30:630–638. doi: 10.1097/WCO.0000000000000496. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008 doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- Morris MC, Gilliam EA, Li L. Innate Immune Programing by Endotoxin and Its Pathological Consequences. Front Immunol. 2015;5:680. doi: 10.3389/fimmu.2014.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin U-M, Saad M, Simón-Sanchez J, Schulte C, Lesage S, Sveinbjörnsdóttir S, Stefánsson K, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SK, Shrivastava K, Schulte C, Otzen DE, Goldeck D, Berg D, Møller HJ, Maetzler W, Romero-Ramos M. Alterations in Blood Monocyte Functions in Parkinson’s Disease. Mov Disord. 2019:mds.27815. doi: 10.1002/mds.27815. [DOI] [PubMed] [Google Scholar]
- Ong S-M, Hadadi E, Dang T-M, Yeap W-H, Tan CT-Y, Ng T-P, Larbi A, Wong S-C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9:266. doi: 10.1038/s41419-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakpoor J, Noyce A, Goldacre R, Selkihova M, Mullin S, Schrag A, Lees A, Goldacre M. Viral hepatitis and Parkinson disease. Neurology. 2017;88:1630–1633. doi: 10.1212/WNL.0000000000003848. [DOI] [PubMed] [Google Scholar]
- Pal GD, Shaikh M, Forsyth CB, Ouyang B, Keshavarzian A, Shannon KM. Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Front Neurosci. 2015;9:306. doi: 10.3389/fnins.2015.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa I, Saliba D, Ponzoni M, Bustamante S, Canete PF, Gonzalez-Figueroa P, McNamara HA, Valvo S, Grimbaldeston M, Sweet RA, Vohra H, et al. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature. 2017;547:318–323. doi: 10.1038/nature23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, Calabresi P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019;18:573–586. doi: 10.1016/S1474-4422(19)30024-9. [DOI] [PubMed] [Google Scholar]
- Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017;214:1913–1923. doi: 10.1084/jem.20170355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, Voigt RM, Naqib A, Green SJ, Kordower JH, Shannon KM, et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut. 2019;68:829–843. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- Peri F, Piazza M. Therapeutic targeting of innate immunity with Toll-like receptor 4 (TLR4) antagonists. Biotechnol Adv. 2012;30:251–260. doi: 10.1016/j.biotechadv.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Pfortmueller CA, Meisel C, Fux M, Schefold JC. Assessment of immune organ dysfunction in critical illness: utility of innate immune response markers. Intensive Care Med Exp. 2017;5:49. doi: 10.1186/s40635-017-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X-Y, Zhang S-P, Cao C, Loh YP, Cheng Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease. JAMA Neurol. 2016;73:1316. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- Racette BA, Gross A, Vouri SM, Camacho-Soto A, Willis AW, Searles Nielsen S. Immunosuppressants and risk of Parkinson disease. Ann Clin Transl Neurol. 2018;5:870–875. doi: 10.1002/acn3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A. Course in parkinson disease subtypes: A 39-year clinicopathologic study. Neurology. 2009;73:206–212. doi: 10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]
- Rapsinski GJ, Wynosky-Dolfi MA, Oppong GO, Tursi SA, Wilson RP, Brodsky IE, Tükel Ç. Toll-Like Receptor 2 and NLRP3 Cooperate To Recognize a Functional Bacterial Amyloid, Curli. Infect Immun. 2015;83:693–701. doi: 10.1128/IAI.02370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu S, Mullen B, Baker M, Lynch T, Finger E, Seeley WW, Hatanpaa KJ, Lomen-Hoerth C, Kertesz A, Bigio EH, Lippa C, et al. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener. 2013;8:19. doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, Cheng S-C, Ratter J, Berentsen K, van der Ent MA, Sharifi N, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science (80-) 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480.:e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, Matuskey D, Lee J-Y, O’Connor KC, Huang Y, Carson RE, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci. 2015;112:12468–12473. doi: 10.1073/pnas.1511003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- Schröder JB, Pawlowski M, Meyer zu Horste G, Gross CC, Wiendl H, Meuth SG, Ruck T, Warnecke T. Immune Cell Activation in the Cerebrospinal Fluid of Patients With Parkinson’s Disease. Front Neurol. 2018;9:1081. doi: 10.3389/fneur.2018.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Kouli A, Yeoh SL, Clatworthy MR, Williams-Gray CH. A Systematic Review and Meta-Analysis of Alpha Synuclein Auto-Antibodies in Parkinson’s Disease. Front Neurol. 2018;9:815. doi: 10.3389/fneur.2018.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamaa OR, Mitra S, Gavrilin MA, Wewers MD. Monocyte Caspase-1 Is Released in a Stable, Active High Molecular Weight Complex Distinct from the Unstable Cell Lysate-Activated Caspase-1. PLoS One. 2015;10:e0142203. doi: 10.1371/journal.pone.0142203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Zabetian CP, Hancock AM, Ginghina C, Hong Z, Yearout D, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, et al. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosci Lett. 2010;480:78–82. doi: 10.1016/j.neulet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EC, Cho SE, Lee DK, Hur MW, Paik SR, Park JH, Kim J. Expression patterns of alpha-synuclein in human hematopoietic cells and in Drosophila at different developmental stages. Mol Cells. 2000;10:65–70. doi: 10.1007/s10059-000-0065-x. [DOI] [PubMed] [Google Scholar]
- Stevens CH, Rowe D, Morel-Kopp M-C, Orr C, Russell T, Ranola M, Ward C, Halliday GM. Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol. 2012;252:95–99. doi: 10.1016/j.jneuroim.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Stolzenberg E, Berry D, Yang D, Lee EY, Kroemer A, Kaufman S, Wong GCLL, Oppenheim JJ, Sen S, Fishbein T, Bax A, et al. A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity. J Innate Immun. 2017;9:456–463. doi: 10.1159/000477990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe I, Lee B, Kayagaki N. Caspase-11: arming the guards against bacterial infection. Immunol Rev. 2015;265:75–84. doi: 10.1111/imr.12292. [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Su X, Federoff HJ. Immune Responses in Parkinson’s Disease: Interplay between Central and Peripheral Immune Systems. Biomed Res Int. 2014;2014:1–9. doi: 10.1155/2014/275178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, et al. T cells from patients with Parkinson’s disease recognize a-synuclein peptides. Nature. 2017;546:656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak T, van Groenendael R, Pickkers P, Koenderman L. Monocyte Subsets Are Differentially Lost from the Circulation during Acute Inflammation Induced by Human Experimental Endotoxemia. J Innate Immun. 2017;9:464–474. doi: 10.1159/000475665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Mori F, Imaizumi T, Yoshida H, Matsumiya T, Tamo W, Yoshimoto M, Odagiri H, Sasaki M, Takahashi H, Satoh K, et al. Upregulation of alpha-synuclein by lipopolysaccharide and interleukin-1 in human macrophages. Pathol Int. 2002;52:572–7. doi: 10.1046/j.1440-1827.2002.01385.x. [DOI] [PubMed] [Google Scholar]
- Tyson T, Steiner JA, Brundin P. Sorting out release, uptake and processing of alpha-synuclein during prion-like spread of pathology. J Neurochem. 2016;139:275–289. doi: 10.1111/jnc.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann K-U, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer K-H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK, Stefanova N. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal a-synucleinopathy. Mol Neurodegener. 2017;12:52. doi: 10.1186/s13024-017-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajinac H, Dzoljic E, Maksimovic J, Marinkovic J, Sipetic S, Kostic V. Infections as a risk factor for Parkinson’s disease: a case–control study. Int J Neurosci. 2013;123:329–332. doi: 10.3109/00207454.2012.760560. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil ACE, Rousseau CH, Hartung T, Greve JWM, van ‘t Veer C, Buurman WA. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol. 2003;170:1399–405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- Wang W, Nguyen LTT, Burlak C, Chegini F, Guo F, Chataway T, Ju S, Fisher OS, Miller DW, Datta D, Wu F, et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein a-synuclein. Proc Natl Acad Sci. 2016;113:9587–9592. doi: 10.1073/pnas.1610099113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Wijeyekoon RS, Scott KM, Gunawardana NP, Hayat S, Solim IH, McMahon HT, Barker RA, Williams-Gray CH. The Peripheral Inflammatory Response to Alpha-Synuclein and Endotoxin in Parkinson’s Disease. Front Neurol. 2018;9:946. doi: 10.3389/fneur.2018.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeyekoon RS, Kronenberg-Versteeg D, Scott KM, Hayat S, Jones JL, Clatworthy MR, Floto RA, Barker RA, Williams-Gray CH. Monocyte Function in Parkinson’s Disease and the Impact of Autologous Serum on Phagocytosis. Front Neurol. 2018;9:870. doi: 10.3389/fneur.2018.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84:1258–64. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Wijeyekoon R, Yarnall AJ, Lawson RA, Breen DP, Evans JR, Cummins GA, Duncan GW, Khoo TK, Burn DJ, Barker RA, et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD) Mov Disord. 2016;31:995–1003. doi: 10.1002/mds.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Wijeyekoon RS, Scott KM, Hayat S, Barker RA, Jones JL. Abnormalities of age-related T cell senescence in Parkinson’s disease. J Neuroinflammation. 2018;15:166. doi: 10.1186/s12974-018-1206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GP, Schonhoff AM, Jurkuvenaite A, Thome AD, Standaert DG, Harms AS. Targeting of the class II transactivator attenuates inflammation and neurodegeneration in an alpha-synuclein model of Parkinson’s disease. J Neuroinflammation. 2018;15:244. doi: 10.1186/s12974-018-1286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Yeap WH, Tai JJY, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ, Nombela C, Winder-Rhodes S, Evans JR, Rowe JB, Mollenhauer B, et al. Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD Study. Neurology. 2014;82:308–316. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Stamova B, Jin L-W, DeCarli C, Phinney B, Sharp FR. Gramnegative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87:2324–2332. doi: 10.1212/WNL.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gao J-H, Yan Z-F, Huang X-Y, Guo P, Sun L, Liu Z, Hu Y, Zuo L-J, Yu S-Y, Cao C-J, et al. Minimally Toxic Dose of Lipopolysaccharide and a-Synuclein Oligomer Elicit Synergistic Dopaminergic Neurodegeneration: Role and Mechanism of Microglial NOX2 Activation. Mol Neurobiol. 2018;55:619–632. doi: 10.1007/s12035-016-0308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Han X, Xue L, Zhu K, Liu H, Xie A. Association of TLR4 gene polymorphisms with sporadic Parkinson’s disease in a Han Chinese population. Neurol Sci. 2015;36:1659–1665. doi: 10.1007/s10072-015-2227-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wu X, Li X, Jiang L-L, Gui X, Liu Y, Sun Y, Zhu B, Pina-Crespo JC, Zhang M, Zhang N, et al. TREM2 Is a Receptor for P-Amyloid that Mediates Microglial Function. Neuron. 2018;97:1023–1031.:e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lu M, Du R-H, Qiao C, Jiang C-Y, Zhang K-Z, Ding J-H, Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Hofer TPJ. Toward a refined definition of monocyte subsets. Front Immunol. 2013 doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data related to the findings of this study will be available from the corresponding author, upon reasonable request.