Abstract
Faithful assembly of newly-synthesized proteins into functional oligomers is crucial for cell activity. Here, we asked whether direct interactions of two nascent proteins, emerging from nearby ribosomes (co-co assembly), is a general mechanism for oligomer formation. We used a proteome-wide screen to detect nascent chain-connected ribosome pairs and identified hundreds of homomer subunits that co-co assemble in human cells. Interactions were mediated by five major domain classes, among which N-terminal coiled coils were the most frequent. We were able to reconstitute co-co assembly of nuclear lamin in E. coli, demonstrating that dimer formation was independent of dedicated assembly machineries. Co-co assembly may thus constitute an efficient way to limit protein aggregation risks posed by diffusion-driven assembly routes and ensure isoform-specific homomer formation.
Sophisticated mechanisms have evolved to ensure efficient and accurate protein complex biogenesis, including the fine-tuning of subunit expression to match complex stoichiometries (1), the employment of general or dedicated chaperones to guide oligomerization (2–4), the co-localization of subunit synthesis (5–7), and the timely oligomerization by coupling translation and subunit interactions (co-translational assembly) (3, 8, 9). Selective Ribosome Profiling (SeRP) has provided mechanistic details of co-translational assembly for Vibrio harveyi luciferase expressed in E. coli (3) and several heteromeric complexes in yeast (8). In all cases studied, a freely diffusing, presumably folded protein engages its nascent partner subunit (co-post assembly).
Here, we tested whether co-translational assembly of protein complexes may also occur via association of two nascent subunits concurrently translated by two ribosomes (co-co assembly). A priori, co-co assembly may involve nascent chains synthesized on two different mRNAs (in trans) or, for homo-oligomer assembly, on the same mRNA (in cis). Importantly, cis-assembly does not require that distinct mRNA molecules co-localize in the cytosol and enables transcript-specific homomeric complex generation, avoiding undesired interactions between closely related proteins or wildtype and mutant alleles (10). Although co-co assembly has already been proposed for individual protein complexes in different organisms (10–14), direct experimental evidence that two ribosome-nascent chain complexes interact is still missing, and we lack any information on the prevalence, molecular mechanisms and relevance of this proposed assembly process. Here, we developed an unbiased, proteome-wide screen based on ribosome profiling (15), termed Disome Selective Profiling (DiSP), to reveal the co-co assembly proteome in human cells.
DiSP reveals widespread disome formation mediated by nascent chain interactions
To identify co-co assembling complexes across the proteome, we reasoned that ribosome pairs (disomes) connected by their exposed nascent chains will remain connected even upon mRNA digestion. Thus, it should be possible to detect co-co assembly candidates by RNase treatment of cell lysates, followed by separation of monosomes and disomes in sucrose gradients and deep-sequencing of 30 nt ribosomal footprints from both fractions (DiSP, Fig. 1A and S1A). The disome fraction will also contain RNase-resistant disomes that form upon collision of ribosomes translating the same mRNA; however, these disomes will protect double length (60 nt) mRNA fragments (16) and are not analyzed by DiSP. Translating ribosomes engaged in co-co assembly will shift from the monosome to the disome fraction upon nascent chain dimerization, which could be detected by analyzing the relative footprint density of both samples (separately or as enrichment of disome over monosome) along a gene's coding sequence (Fig. 1A). In contrast to SeRP, which has been used to explore co-post assembly of selected protein complexes (3, 8), DiSP can provide proteome-wide interaction profiles of all translating ribosomes.
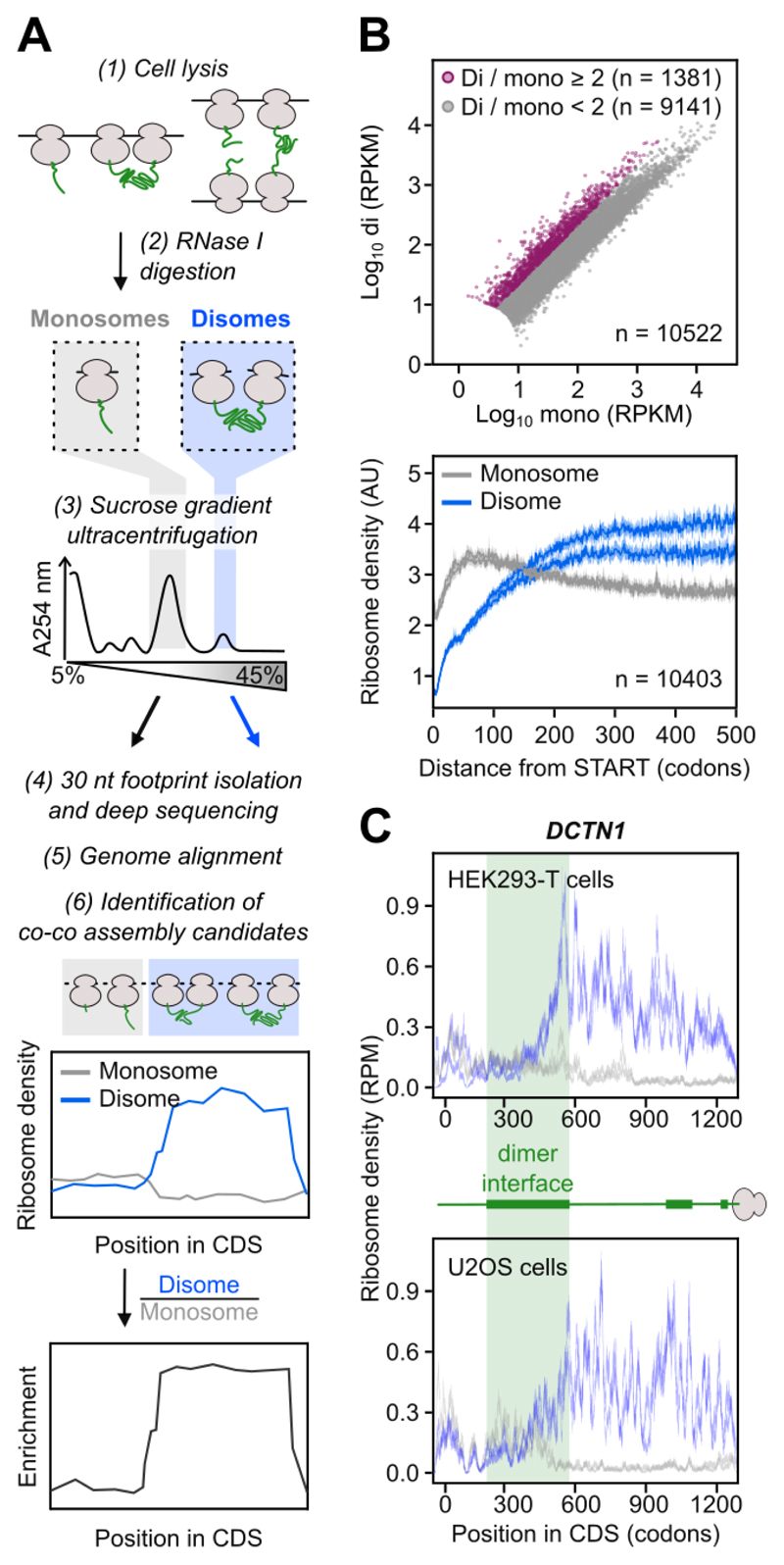
Fig. 1. Disome Selective Profiling (DiSP) reveals widespread disome formation.
A) Experimental procedure of DiSP: cell lysates are RNase-treated (1, 2), monosomes and disomes are separated by sucrose gradient ultracentrifugation (3) and ribosome footprints with a length of about 30 nucleotides are extracted, converted into a DNA library and sequenced (4). Co-co assembly candidates are identified by a shift of the footprint density from monosome to disome fraction, or by a disome over monosome enrichment profile (5, 6).
B) Comparison of disome (di) and monosome (mono) footprint density (RPKM = Reads Per Kilobase per Million mapped reads) of all detected genes in HEK293-T cells (top, one replicate shown). Average footprint density along the coding sequence of all detected genes (metagene) aligned to translation start (bottom, n = 2).
C) Monosome (grey) and disome (blue) footprint density along the coding sequence (CDS) of DCTN1 (RPM = Reads Per Million). Cartoon shows exposed nascent chain segments during translation, green bars indicate dimerization interfaces. DiSP data of HEK293-T (n = 2) and U2OS cells (n = 2) are compared.
We initially performed DiSP of HEK293-T cells. To identify co-co assembly candidates, we first compared gene-specific footprint densities in the disome and monosome fractions, revealing over 1300 genes with a disome over monosome enrichment higher than two (Fig. 1B, top). A metagene profile of the averaged monosome and disome density along all coding sequences showed that early during translation, when nascent chains are short, ribosomes mostly migrated as monosomes, followed by a steady disome enrichment that leveled out at about 200 codons (Fig. 1B, bottom). The monosome to disome shift of translating ribosomes occurred only on a subset of genes, supporting the assumption that it depended on interaction properties of nascent chains (Fig. 1B, top and S1B). One example among the two-fold disome enriched genes is DCTN1, encoding p150glued, a subunit of the dynactin motor complex. Ribosomes translating DCTN1 convert from monosomes to disomes near codon 430, when about 400 amino acids of nascent p150glued are exposed on the ribosomal surface. This N-terminal segment includes major parts of the coiled coil dimerization domain, suggesting the disome shift was caused by co-translational homodimerization (Fig. 1C, top). Repeating DiSP in U2OS cells, we found a large overlap of disome enriched genes and robustly correlated enrichment profiles (Fig. 1C and S1B, C), demonstrating that disome formation is a general feature of a specific subset of nascent proteins across different cell types.
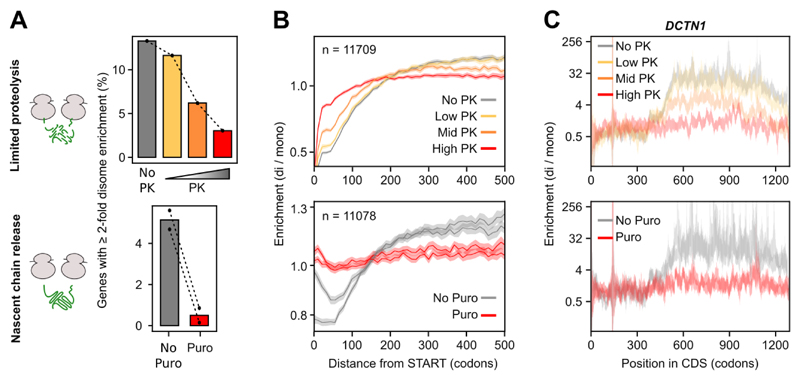
To challenge our model that disome formation is mediated by nascent proteins, we explored whether disome shifts were sensitive to release or degradation of nascent chains. Treating lysates with Puromycin (Puro) or increasing concentrations of Proteinase K (PK) efficiently suppressed the shift of footprints from monosome to disome. This was apparent from a general reduction of the disome enrichment (Fig. 2A) and a flattening of enrichment profiles at both, the metagene level (Fig. 2B) and for individual genes (Fig. 2C and S1D-G). Thus, the stability of DiSP-detected disomes critically depends on the integrity of nascent chains, in agreement with the model of co-co assembly.
Fig. 2. Disome formation is nascent chain dependent.
A) DiSP was performed on lysates treated with increasing Proteinase K (PK, n = 1) concentrations or with Puromycin (Puro, n = 2) to degrade or release nascent chains. Both treatments resulted in a large depletion of genes with ≥ 2-fold higher footprint density in the disome compared to the monosome fraction.
B) Metagene enrichment profiles (disome / monosome) aligned to translation start of all detected genes in PK (top) and Puro (bottom) DiSP experiments.
C) Enrichment profiles (disome / monosome) of DCTN1 of untreated DiSP samples and samples treated with increasing concentrations of Proteinase K (PK, top) or with Puromycin (Puro, bottom).
A high confidence list of co-co assembly candidates enriched for homomers
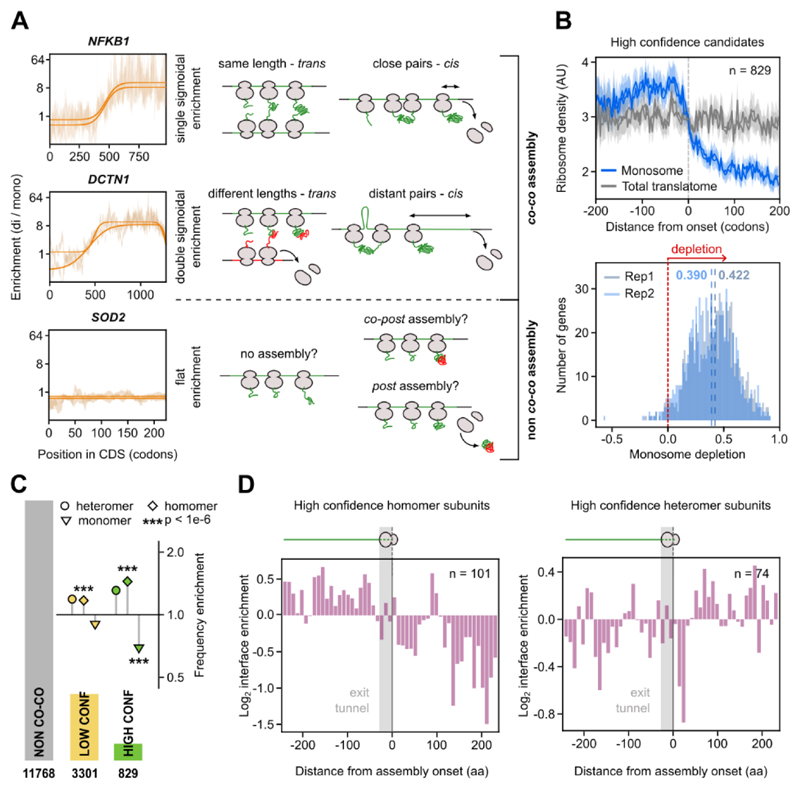
We developed an unbiased bioinformatics selection regime to classify proteins based on their proficiency to co-co assemble. Accordingly, a protein qualified as high confidence candidate if all of the following criteria were fulfilled: (i) The gene's enrichment profile had a sigmoidal shape, indicating that with progressing translation, ribosomes shifted from the monosome to the disome fraction. If one of the interacting ribosomes terminates earlier, the other ribosome in the pair will shift back to the monosome fraction before it reaches the end of the coding sequence, resulting in a double sigmoidal shift (Fig. 3A). (ii) The enrichment profile becomes less sigmoidal upon treatment of the lysate with Puromycin and (iii) similarly with PK. (iv) The mature protein localizes to either the cytoplasm or the nucleus. We decided to categorize translocated protein candidates as low confidence because we cannot formally exclude the possibility that these ribosomes interact with membrane components of the translocation machinery and therefore migrate in the disome fraction. In addition, our validation experiments focused on cytosolic and nuclear candidates (Fig. 5 and S4) and poor structural annotation of membrane proteins complicates the downstream bioinformatics analysis. Out of a total of 15898 detected genes, 829 fulfilled all criteria and were classified as high confidence co-co assembly candidates (Table S1). A large number of genes (3301) fulfilled the important criterion (i) but not all of criteria (ii) to (iv) and were therefore categorized as low confidence candidates (Table S1). The low confidence list included 1404 proteins that are translocated across or inserted into organelle membranes, mainly the Endoplasmic Reticulum (ER), of which 443 fulfilled all other criteria. The latter fraction reflects the general frequency of ER-translocated proteins in the human proteome, indicating that co-co assembly may be an equally important mechanism to assemble cytosolic/nuclear and ER complexes, in agreement with previous experimental indications (17–19). The disome shift of ribosomes synthesizing membrane proteins frequently occurs after exposure of the first transmembrane domain (TMD) (Fig. S2A), which may suggest that co-co assembly involves interactions of two TMDs in the ER membrane.
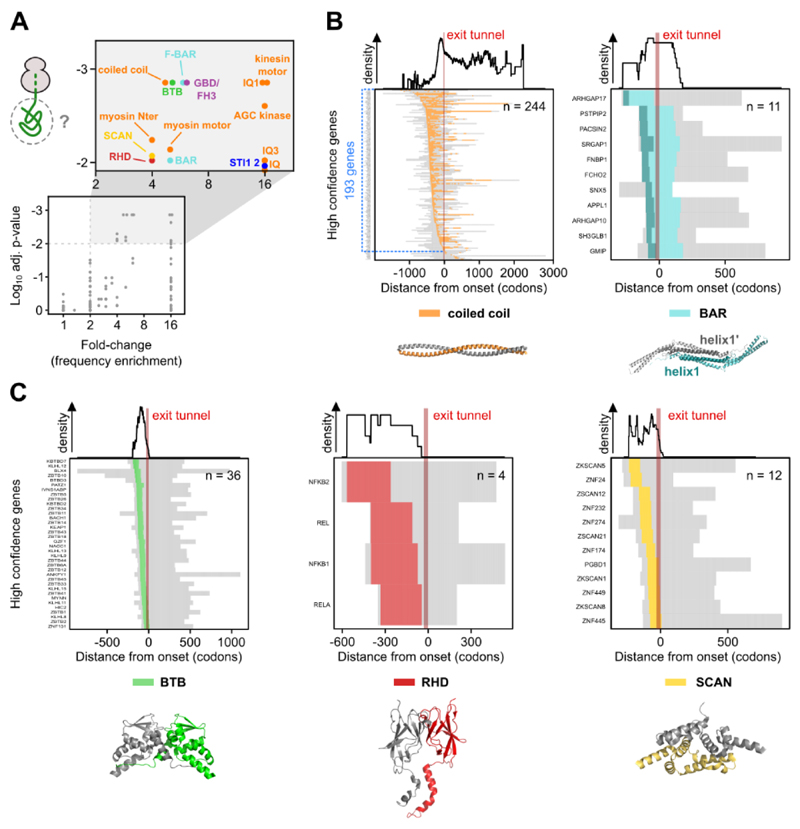
Fig. 3. High confidence co-co assembly proteins are enriched in homo-oligomers.
A) Examples of gene-specific disome over monosome enrichment profiles (DiSP data, in the background, n = 2) and the corresponding fitting (solid lines) for each of the three possible shapes of DiSP enrichments. The single sigmoid agrees with nascent chain-connected ribosomes that terminate translation simultaneously, either by co-co assembly in trans if the mRNA segments translated by both ribosomes after co-co assembly have similar lengths, or in cis, with ribosomes that closely follow each other on the same mRNA (top). The double sigmoid agrees with co-co assembly involving two ribosomes that do not terminate at the same time; this may occur in trans if the mRNA segments translated by both ribosomes after co-co assembly have different lengths, or in cis, if the leading ribosome is distant from the trailing one (middle). Flat enrichment profiles indicate that nascent proteins do not co-co assemble.
B) Metagene profiles of all high confidence candidates aligned to assembly onset (top). Footprint density in the monosome fraction and the total translatome are shown (n = 2). Gene-specific quantification of the efficiency of co-co assembly, calculated as the relative depletion of footprint density in monosome compared to total translatome after assembly onset (bottom). The median monosome depletion for each replicate is indicated by blue dashed lines.
C) Frequency enrichment of annotated subunits of protein complexes in high and low confidence lists compared to the whole proteome (absolute and relative numbers are provided in Table S2) (33). The number of genes included in each assembly class is indicated in the bar plot. The p-values were calculated using an enrichment test adjusted for expression bias (33, 34).
D) Distribution of residues forming the inter-subunit interface of protein complexes determined from available crystal structures. The position of interface residues on the proteins' primary sequence is aligned to assembly onset of high confidence homomers (left) or heteromers (right).
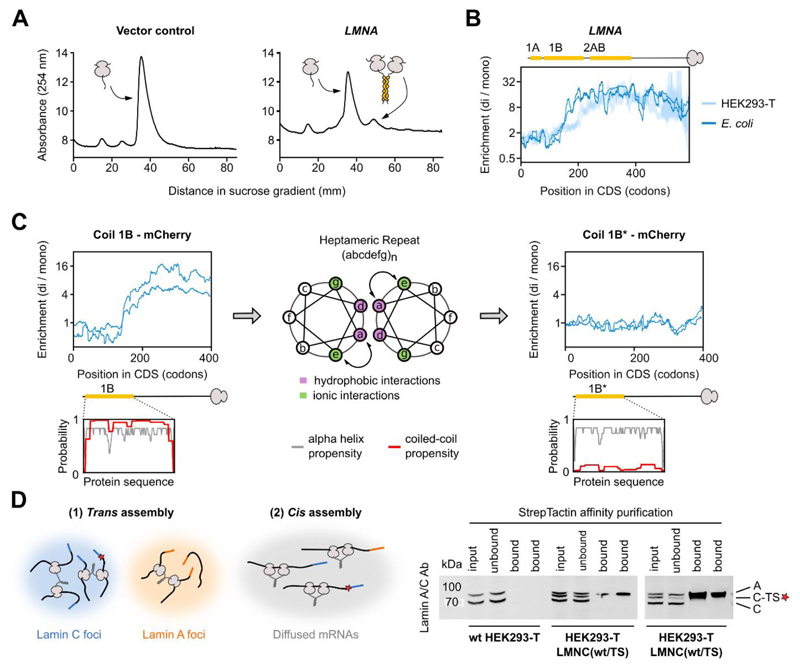
Fig. 5. Co-co assembly does not rely on eukaryote-specific factors and facilitates native biogenesis of lamin C homodimers.
A) Sucrose gradient sedimentation analysis of E. coli ribosomes from cells transformed with a control plasmid (left) or a plasmid enclosing human LMNA encoding lamin C (right), lacking the unstructured N-terminal head domain (33).
B) Disome over monosome enrichment profile of plasmid-encoded LMNA expressed in E. coli (dark blue, n = 2), and endogenously expressed LMNA in HEK293-T cells (light blue, n = 2). The ribosome-exposed coiled coil interfaces are indicated by yellow bars.
C) Disome over monosome enrichment profiles of LMNA encoding lamin coil 1B (left) or the a↔e swapped version of 1B (1B*, right) fused N-terminally to mCherry and expressed in E. coli (n = 2). The ribosome-exposed coiled coil interfaces are indicated by yellow bars. Helical wheel projection shows residue arrangements (a-g) of the heptad repeat (middle). Coiled coil (red) and alpha-helical (grey) probability predictions are shown for both wild type and mutant 1B (insets).
D) Hypothetical models of co-co assembly supporting isoform-specific homodimerization (left). A red star represents the TwinStrep tag (TS). Affinity purification of tagged lamin C (C-TS) from wild type or heterozygous LMNC(wt/TS) HEK293-T cells (bottom, technical replicates shown). Bands are labeled: A (lamin A), C (lamin C), C-TS (lamin C – TwinStrep).
Our next aim was to quantitatively assess what fraction of each high confidence candidate assembles co-translationally (from hereon named “efficiency” of co-co assembly). The efficiency was estimated by determining the reduction of footprints in the monosome fraction after initiation of co-co assembly compared to the total translatome (including all translating ribosomes, determined by classical ribosome profiling (15, 20)). Metagene analyses of footprint densities of all high-confidence genes aligned to the onset of assembly revealed a reduction of footprints in the monosome fraction from a DiSP experiment but not in the total translatome (Fig. 3B, top). This confirmed that the monosome depletion was caused by a shift of ribosomes to the disome fraction. The median monosome footprint reduction after the detected co-co assembly onset of high confidence genes was around 40%, and for some genes even exceeded 90%, indicating that in many cases the majority of nascent chains assembled co-translationally (Fig. 3B, bottom). Although to a smaller extent, monosome depletion was also observed for many low confidence candidates, suggesting that this list includes additional proteins that employ co-co assembly as a main route for complex formation (Fig. S2B, C). Importantly, the calculated depletion value most likely under-estimates the in vivo co-co assembly efficiency due to (i) the inevitable slight cross-contamination between the monosome and disome fractions and (ii) the possibility of a partial loss of disomes during sucrose gradient centrifugation that are connected by comparably weak nascent chain interactions. Supporting this notion, the three proteins featuring the highest efficiency (≥ 90% depletion, namely TPR, EEA1 and CLIP1) contained extremely long coiled coil homodimerization domains (between 1000 and 1500 amino acids, compared to a median coiled coil length of 66 amino acids in the cellular proteome) suggesting high stability.
We went on to analyze the features of proteins included in the high and low confidence lists. Consistently, annotated monomeric proteins were depleted in both lists of co-co assembly candidates, most strongly among the high confidence proteins (Fig. 3C, Table S2). Both classes showed a significant enrichment of homomers while heteromers were not significantly enriched. Furthermore, we often found only one subunit of a heterodimer in our candidate list, suggesting that this subunit rather formed a homo-oligomer or co-co assembled with a so far unknown partner subunit.
We used available crystal structures of protein complexes to determine the position of residues involved in subunit interaction at the onset of the disome shift. This analysis showed that the onset of assembly often coincided with the emergence of nascent chain segments that form the interfaces for the homo-oligomers (Fig. 3D, left). This correlation was not detected for heteromeric high confidence candidates (Fig. 3D, right). While these findings do not exclude the possibility that individual heteromers co-co assemble, as previously reported (13, 14, 19), they rather suggest that co-co assembly is predominantly employed for the formation of homomeric protein complexes.
Co-co assembly is driven by exposure of conserved N-terminal homodimerization domains
Most detected co-co assembly interactions were established at early translation stages (Fig. S3A). Consistently, homodimerization interfaces are enriched in the N-terminal halves of high confidence candidates (Fig. S3B, left). This is different in the human proteome, where homodimerization interfaces are more often located in the C-terminal half of the protein, as previously reported (21) (Fig. S3B, right).
We next aimed to identify protein motifs or folds that mediate co-co assembly, by studying the enrichment of exposed domains at the onset of assembly. This analysis identified seven domain clusters mediating co-co assembly (color-coded in Fig. 4A), of which five are established homodimerization units.
Fig. 4. Co-co assembly is coordinated with exposure of five major dimerization domain classes.
A) Analysis of protein domains on nascent chain segments exposed at assembly onset. The frequency of each domain in the high confidence class is compared to their general frequency in the proteome (33). We used a Monte-Carlo simulation of the null hypothesis to calculate the p-value (33) and the Benjamini-Yekutieli procedure to correct for multiple testing. The adjusted p-value is plotted against the respective fold-change (frequency enrichment). Domains passing a significance (p-adj. ≥ 0.01) and fold-change (≥ 2) threshold are shown in the magnified rectangle and further analyzed.
B) Heatmaps of partially exposed domains: coiled coil (left) and BAR (right). In the heatmaps, nascent chain segments left from the ribosome exit tunnel (approximated to 30 codons, shown by a red bar) are exposed when assembly starts. The subset of genes exposing a coiled coil segment on the nascent chain at the onset of assembly is highlighted in blue (n = 193). Residues forming helix1 of BAR domains are colored dark green in the heatmap and in the exemplary structure. Corresponding domain density profiles shown on top. Representative structures are PDB: 1D7M, 3Q0K.
C) Heatmaps of completely exposed domains: BTB (left), RHD (middle) and SCAN (right). Corresponding domain density profiles shown on top. Representative structures are PDB: 1BUO, 1K3Z, 3LHR.
Among our high confidence candidates, coiled coils were the most prevalent annotated domain class that is exposed on the ribosome surface at assembly onset (193 of 829 proteins according to UniprotKB, Fig. 4B, left). Furthermore, the DeepCoil prediction tool (22) identified coiled coil segments on the exposed nascent chains in 408 genes (Fig. S3C), suggesting that up to 50% of high confidence candidates employ this fold for co-co assembly. In many cases, the coiled coil is only partially exposed at assembly onset (Fig. 4B, left). The number of exposed residues involved in coiled coil formation varied (median of 111 residues in the high confidence class, Fig. S3D), which may suggest that different lengths of the coiled coil are needed to form a stable dimer.
We found seven additional domains that are generally positioned N-terminally to coiled coil domains in myosins, kinesins and AGC-kinases (orange in Fig. 4A), and were therefore exposed at the onset of co-co assembly. However, disome enrichment generally required the partial or complete exposure of the coiled coil segment, suggesting that these domains do not contribute to oligomerization.
A second domain class that was often only partially exposed at the onset of assembly are BAR domains (named after Bin, Amphiphysin and Rvs, Fig. 4B, right); conserved dimerization domains found in many proteins mediating membrane curvature. They consist of three (classical BAR) to five (F-BAR) bent antiparallel alpha-helices. According to our dataset, co-co assembly generally required the exposure of the most N-terminal alpha-helix (helix1, Fig. 4B, right), that interacts with its partner helix1’ in an antiparallel fashion.
All other enriched domain classes were globular and fully exposed at assembly onset, implying that their co-translational folding was required for assembly, including BTB (Broad-Complex, Tramtrack and Bric a brac), RHD (Rel Homology Domain) and SCAN (SRE-ZBP, CTfin51, AW-1 and Number 18 cDNA) domains (Fig. 4C). BTBs are highly conserved globular dimerization domains located at the N-termini of many transcription factors, ion channels and E3 ligase subunits, and found in 36 of our high confidence candidates (Fig. 4C, left). The less abundant RHDs are found at the N-terminus of proteins involved in nuclear factor kappa-B (NF-kB) complex formation and create the interface of homo- and heteromeric interactions. According to our DiSP, all NF-kB homologs co-co assemble, confirming earlier indications that proteins encoded by NFKB1 may co-translationally assemble in cis and that early assembly is required for native biogenesis of the p50 transcription factor (12, 23) (Fig. 4C, middle, Fig. S1B, right). This very likely also holds true for the RELB encoded homolog, however, because RELB is low expressed in HEK293-T cells, we cannot make a definite statement.
The high confidence list also included 12 transcription factors that employ SCAN domains for co-co assembly (Fig. 4C, right). SCAN domains are leucine-rich, N-terminal motifs composed of five packed alpha-helices that mediate homo- and hetero-oligomerization of a large family of C2H2 zinc finger proteins by intercalating helix 2 of one monomer between helices 3 and 5 of the opposing monomer.
Comparing the co-co assembly efficiency of these five major dimerization domains, we found that coiled coils conferred the highest, yet very variable stability to the nascent chain interactions, followed by BTB, BAR, RHD and SCAN domains (Fig. S3E).
Finally, our dataset included two less characterized domains that were significantly enriched (Fig. 4A). The first were STI1 repeats of ubiquilin proteins. This domain mediates homo- and hetero-dimerization of ubiquilin 1 and 2 (24), which both were high confidence candidates that fully exposed the second STI1 repeat (STI1 2) at the assembly onset (Fig. S3F).
The second are GBD/FH3; conserved N-terminal regulatory elements in Diaphanous-related formins, a protein class involved in nucleation and remodeling of the actin cytoskeleton. The FH3 domain has been implicated in dimerization of the mouse homologue of human DIAPH1 (25). We found six human formins among our high confidence proteins and in all cases the FH3 domain was exposed at assembly onset, suggesting that formins may co-translationally assemble via the FH3 domain (Fig. S3G).
Co-co assembly is independent of eukaryotic assembly factors
We next examined whether ribosome exposure of co-co assembly-competent nascent chains suffices for disome formation, and if it could occur outside the eukaryotic folding environment. To investigate this question, we performed DiSP of E. coli synthesizing human lamin C (LMNA), one of the mammalian intermediate filaments that were all high confidence candidates of our DiSP screen. Lamins form homodimers in the cytosol and assemble into higher-order polymers in the nucleus. Dimerization involves the N-terminal rod domain, a long discontinuous coiled coil that includes three segments named coil 1A, 1B, and 2AB. LMNA overexpression generated a disome peak in the RNase-digested lysate (Fig. 5A). DiSP revealed that these disomes were enriched with ribosomes translating LMNA (Fig. 5B), indicating nascent lamin C can co-translationally dimerize in bacteria. The minimal length of nascent lamin C mediating the disome shift in E. coli was close to that of the endogenously expressed lamin C in mammalian cells (Fig. 5B). Likewise, overexpression of DCTN1 generated a disome peak that was enriched with ribosomes exposing the coiled coil of p150glued, and the assembly onset was similar to human cells (Fig. S4A). This indicates that co-co assembly of coiled coils is independent of eukaryote-specific assembly factors or mRNA subcellular localization.
To test our hypothesis that the formation of a coiled coil between two nascent chains is minimally required and sufficient to induce disome shifts in bacteria, we used coil 1B of lamin C as a paradigm. First, we employed an established in vivo dimerization assay based on a lambda repressor fusion system (26) to show that the isolated 1B efficiently dimerized in E. coli (Fig. S4B). Second, we performed DiSP to verify that nascent 1B, N-terminally fused to mCherry, efficiently mediated co-co assembly (Fig. 5C, left). Third, we perturbed the periodicity of nonpolar and charged amino acids required for coiled coil formation of 1B by swapping the position 'a' and 'e' of the coiled coil heptameric repeats (1B*; Fig. 5C, middle). These swaps do not change the overall amino acid composition, nor the hydrophobicity, or the predicted propensity to form alpha helices, but eliminated the proficiency of 1B to form a coiled coil (Fig. 5C, insets). In contrast to 1B, the mutated 1B* did not confer co-translational disome formation in E. coli (Fig. 5C, right), further supporting that DiSP detects productive, in vivo interactions between nascent chains that drive protein oligomer formation.
Co-co assembly in cis may ensure isoform-specific coiled coil formation
Lamin A and C are isoforms encoded by the same gene but translated on two alternatively spliced transcripts. Although they share the same N-terminal rod dimerization domain, lamin A and C exclusively form homodimers in vivo (27). How this isoform-specificity is achieved in the cellular environment is not known. Co-co assembly may provide a simple answer to this conundrum: isoform-specific assembly may be achieved by co-co assembly in trans on co-localized mRNAs of the same kind (which might segregate in the cytosol due to their unique 3'UTRs), or - even simpler - in cis, facilitated by interaction of nascent proteins synthesized by neighboring ribosomes organized in a polysome (Fig. 5D, left).
To discriminate between these possibilities, we generated a heterozygous HEK293-T cell line, in which one LMNA allele encodes a C-terminally TwinStrep-tagged lamin C. We performed a series of affinity purification experiments which revealed that tagged lamin C never co-purified the untagged counterpart, even though both proteins derive from identically spliced mRNAs with identical UTRs (Fig. 5D, right). This result supports the model that co-co assembly in cis facilitates isoform-specific lamin dimerization in human cells.
Discussion
We provide a comprehensive analysis of co-translational protein complex assembly mediated by two nascent subunits. The ribosome profiling-based approach developed here (DiSP) allowed us to identify hundreds of high confidence and thousands of low confidence candidates in human cells, revealing co-co assembly as a major route to complex formation.
We decided to include all translocated proteins into the low confidence list. Many of them are membrane proteins that are often partially or fully resistant to PK but sensitive to Puromycin, in particular small proteins (up to 35 kDa) with multiple annotated TMDs. PK resistance may be conferred by ribosome docking to the translocon that limits the access of PK to the nascent protein. We speculate that docking of ribosomes that closely follow each other in a polysome may spatially organize translocons in the membrane and facilitate homomer assembly.
Our data show that predominantly homodimers co-co assemble. We did not find clear evidence that heteromers co-co assemble in trans, because our high confidence list in most cases only contained one of the subunits of an annotated heteromer. The absence of a known partner subunit may be caused by the less complete structural characterization of heteromeric complexes.
We also did not find clear evidence that the recently described assembly of TAF6-TAF9 nuclear complex includes nascent chain interactions (14). Both subunits are included in the low confidence list, but the length of the disome shift and the enrichment efficiency is very different between the two proteins, which does not agree with a model of co-co assembly in trans.
Co-co assembly of homomers in cis may be facilitated by a generally high ribosome occupancy to ensure close proximity of the interacting nascent chains. In addition, both heteromer assembly (in trans) and homomer assembly (in cis or in trans) may benefit from the slowdown of ribosomes at the onset of assembly, to allow the trailing ribosome translating the same mRNA to catch up or to provide an extended timeframe to establish the interaction with another nascent chain translated on a distinct mRNA (13).
We discovered two different types of nascent chain dimerization. First, a zipper-like formation of coiled coils and BAR domains. In these cases, the interaction strength may gradually increase as both nascent chains grow, until enough residues involved in dimerization are ribosome-exposed to drive the co-co assembly of stable dimers.
The second type of nascent chain dimerization may require the prior folding of a fully emerged, globular interaction domain, i.e. BTB, RHD and SCAN domains, a feature already reported for co-post assembly (3, 8).
Homodimerization contact regions are evolutionarily selected to be enriched in C-terminal halves of proteins, supposedly to ensure that folding occurs undisturbed by the vicinity of another identical, incompletely folded subunit (28). Our analysis supports this C-terminal enrichment for the majority of the human proteome, except for the proteins enclosed in our high confidence list. For the latter proteins, the selective pressure to assemble early apparently overrules the penalty that is inferred by enhanced folding problems of yet to be synthesized C-terminal domains. We speculate that productive folding of the native dimer, beyond co-co assembly, is likely supported by extensive, finely tuned intervention of molecular chaperones.
There are multiple reasons that may create selective pressure against diffusion-driven assembly and favoring co-co assembly. (i) Co-co assembly may increase the efficiency and rate of complex formation. This advantage is most evident for the cis assembly mode where dimerizing nascent chains are already adjacent within polysomes. (ii) Synthesis-coupled assembly may suppress unproductive interactions and facilitate native folding, by limiting the exposure of aggregation-prone dimerization interfaces to the crowded cellular environment. (iii) Cis assembly creates mRNA-specific homomers. Coiled coils and BTB domains are recurrent dimerization modules in the human proteome, bearing high potential for promiscuous, potentially deleterious heteromeric interactions (29, 30). Such interactions would be efficiently prevented by in cis assembly, including the mix of splicing-derived isoforms that share identical dimerization domains, as in the case of human lamin A and C (27, 29). Misassembled subunits that failed to co-co assemble may be recognized by a recently described pathway, that specifically detects and eliminates complexes of aberrant composition (DQC - Dimerization Quality Control (31)). Interestingly, DQC has been reported as a surveillance mechanism for BTB complexes, but a similar molecular machinery may exist that monitors the composition of other complexes, including coiled coils. Here, our proteome-wide study reveals that co-translational interactions between nascent subunits are a general and efficient strategy to guide the isoform-specific formation of protein complexes.
Materials and Methods
Detailed materials and methods can be found in the supplementary materials.
Human osteosarcoma U2OS (ATCC Cat# HTB-96), human embryonal kidney HEK293-T (DSMZ Cat# ACC 635) and E. coli Rosetta cells (Novagene) were employed for DiSP experiments.
All ribosome profiling libraries were prepared as described (20) and sequenced on a NextSeq550 (Illumina) according to the manufacturer's protocol, except for libraries of U2OS samples, which were prepared as described (8) and sequenced on a HiSeq 2000 (Illumina).
DiSP with PK treatment included incubation of the cell lysates for 30 minutes at 4°C with following PK to total protein amounts: (i) Low PK = 1:20000; (ii) Mid PK = 1:6000; (iii) High PK = 1:2000; (iv) Very High PK = 1:200.
DiSP with Puromycin omitted cycloheximide from all buffers; cell lysates were incubated for 25 minutes with 2 mM Puromycin and crosslinked with 0.5% formaldehyde.
Supplementary Material
One Sentence Summary.
Co-translational homomer assembly occurs by N-terminal dimerization of two nascent proteins and supports isoform-specificity.
Acknowledgments
We thank all members of B.B.'s laboratory, for discussions and advice; David Coombs for help with optimization of ribosome separation on sucrose gradients; Ulrike Friedrich for help on establishing pipelines for processing ribosome profiling data; Simon Anders for valuable advice concerning development of DiSP data analysis tools; the ZMBH Flow Cytometry & FACS Core Facility, the DKFZ Sequencing Core Facility and the DKFZ Vector and Clone Repository for support of experimental work. M.B., K.F. and J.S. are members of the Heidelberg Biosciences International Graduate School (HBIGS).
Funding
M.B. and K.F. were supported by a HBIGS PhD fellowship. M.B. was additionally supported by a Boehringer Ingelheim Fonds (BIF) PhD fellowship. F.W. received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement No 745798. This work was supported by the Helmholtz-Gemeinschaft (DKFZ NCT3.0 Integrative Project in Cancer Research (DysregPT_Bukau 1030000008 G783)), the Deutsche Forschungsgemeinschaft (SFB 1036), the European Research Council (ERC Advanced grant (743118)) and the Klaus Tschira Foundation. Work in the Tans laboratory was supported by the Netherlands Organization for Scientific Research (NWO).
Footnotes
Authors contributions:
Conceptualization: M.B., K.F., F.W., J.S., S.T., B.B., and G.K.
Methodology: M.B., K.F., J.A., B.B. and G.K.
Investigation: M.B., K.F.
Software: I.K., F.T., M.B. and K.F.
Formal Analysis, Data curation and Visualization: M.B., K.F., I.K., F.T., B.B. and G.K.
Writing – Original Draft: M.B., K.F., I.K. and G.K.
Writing – Review & Editing: all authors
Supervision: S.T., B.B. and G.K.
Competing interests: All authors declare no competing interests.
Data and materials availability
All sequencing data reported in this study are available at GEO under accession number GSE151959. Explicit Julia code is available as supplementary material; explicit R code will be made available upon request. Data analysis of ribosome profiling datasets were performed with RiboSeqTools (available at https://github.com/ilia-kats/RiboSeqTools and (32)).
References and Notes
- 1.Li G-W, Burkhardt D, Gross C, Weissman JS. Quantifying Absolute Protein Synthesis Rates Reveals Principles Underlying Allocation of Cellular Resources. Cell. 2014;157:624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian G, Lewis SA, Feierbach B, Stearns T, Rommelaere H, Ampe C, Cowan NJ. Tubulin Subunits Exist in an Activated Conformational State Generated and Maintained by Protein Cofactors. J Cell Biol. 1997;138:821–832. doi: 10.1083/jcb.138.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shieh YW, Minguez P, Bork P, Auburger JJ, Guilbride DL, Kramer G, Bukau B. Operon structure and cotranslational subunit association direct protein assembly in bacteria | Science. Science. 2015;350:678–680. doi: 10.1126/science.aac8171. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau A, Bertolotti A. Regulation of proteasome assembly and activity in health and disease. Nat Rev Mol Cell Biol. 2018;19:697–712. doi: 10.1038/s41580-018-0040-z. [DOI] [PubMed] [Google Scholar]
- 5.Mingle LA. Localization of all seven messenger RNAs for the actin-polymerization nucleator Arp2/3 complex in the protrusions of fibroblasts. J Cell Sci. 2005;118:2425–2433. doi: 10.1242/jcs.02371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzinga M, Bates C, Lui J, Forte G, Morales-Polanco F, Linney E, Knotkova B, Wilson B, Solari CA, Berchowitz LE, Portela P, et al. Translation factor mRNA granules direct protein synthetic capacity to regions of polarized growth. J Cell Biol. 2019;218:1564–1581. doi: 10.1083/jcb.201704019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampoelz B, Schwarz A, Ronchi P, Bragulat-Teixidor H, Tischer C, Gaspar I, Ephrussi A, Schwab Y, Beck M. Nuclear Pores Assemble from Nucleoporin Condensates During Oogenesis. Cell. 2019;179:671–686.:e17. doi: 10.1016/j.cell.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiber A, Doring K, Friedrich U, Klann K, Merker D, Zedan M, Tippmann F, Kramer G, Bukau B. Cotranslational assembly of protein complexes in eukaryotes revealed by ribosome profiling. Nature. 2018;561:268–272. doi: 10.1038/s41586-018-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer G, Shiber A, Bukau B. Mechanisms of Cotranslational Maturation of Newly Synthesized Proteins. Annu Rev Biochem. 2019;88:337–364. doi: 10.1146/annurev-biochem-013118-111717. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls CD, McLure KG, Shields MA, Lee PWK. Biogenesis of p53 Involves Cotranslational Dimerization of Monomers and Posttranslational Dimerization of Dimers: IMPLICATIONS ON THE DOMINANT NEGATIVE EFFECT. J Biol Chem. 2002;277:12937–12945. doi: 10.1074/jbc.M108815200. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore R, Coffey MC, Leone G, McLure K, Lee PW. Co-translational trimerization of the reovirus cell attachment protein. EMBO J. 1996;15:2651–2658. [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L, DeMartino GN, Greene WC. Cotranslational dimerization of the Rel homology domain of NF-κB1 generates p50–p105 heterodimers and is required for effective p50 production. EMBO J. 2000;19:4712–4722. doi: 10.1093/emboj/19.17.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panasenko OO, Somasekharan SP, Villanyi Z, Zagatti M, Bezrukov F, Rashpa R, Cornut J, Iqbal J, Longis M, Carl SH, Peña C, et al. Co-translational assembly of proteasome subunits in NOT1-containing assemblysomes. Nat Struct Mol Biol. 2019;26:110–120. doi: 10.1038/s41594-018-0179-5. [DOI] [PubMed] [Google Scholar]
- 14.Kamenova I, Mukherjee P, Conic S, Mueller F, El-Saafin F, Bardot P, Garnier J-M, Dembele D, Capponi S, Timmers HTM, Vincent SD, et al. Co-translational assembly of mammalian nuclear multisubunit complexes. Nat Commun. 2019;10:1–15. doi: 10.1038/s41467-019-09749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science (80-) 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han P, Shichino Y, Schneider-Poetsch T, Mito M, Hashimoto S, Udagawa T, Kohno K, Yoshida M, Mishima Y, Inada T, Iwasaki S. Genome-wide Survey of Ribosome Collision. Cell Rep. 2020;31:107610. doi: 10.1016/j.celrep.2020.107610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redick SD, Schwarzbauer JE. Rapid intracellular assembly of tenascin hexabrachions suggests a novel cotranslational process. J Cell Sci. 1995;108:1761–1769. doi: 10.1242/jcs.108.4.1761. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Robinson JM, Edwards D, Deutsch C. T1–T1 Interactions Occur in ER Membranes while Nascent Kv Peptides Are Still Attached to Ribosomes †. Biochemistry. 2001;40:10934–10946. doi: 10.1021/bi010763e. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Jones DK, de Lange WJ, Robertson GA. Cotranslational association of mRNA encoding subunits of heteromeric ion channels. Proc Natl Acad Sci. 2016;113:4859–4864. doi: 10.1073/pnas.1521577113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MGlincy NJ, Ingolia NT. Transcriptome-wide measurement of translation by ribosome profiling. Methods. 2017;126:112–129. doi: 10.1016/j.ymeth.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natan E, Endoh T, Haim-Vilmovsky L, Flock T, Chalancon G, Hopper JTS, Kintses B, Horvath P, Daruka L, Fekete G, Pál C, et al. Cotranslational protein assembly imposes evolutionary constraints on homomeric proteins. Nat Struct Mol Biol. 2018;25:279–288. doi: 10.1038/s41594-018-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwiczak J, Winski A, Szczepaniak K, Alva V, Dunin-Horkawicz S. DeepCoil-a fast and accurate prediction of coiled-coil domains in protein sequences. Bioinformatics. 2019;35:2790–2795. doi: 10.1093/bioinformatics/bty1062. [DOI] [PubMed] [Google Scholar]
- 23.Lin L, DeMartino GN, Greene WC. Cotranslational biogenesis of NF-κB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- 24.Ford DL, Monteiro MJ. Dimerization of ubiquilin is dependent upon the central region of the protein: evidence that the monomer, but not the dimer, is involved in binding presenilins. Biochem J. 2006;399:397–404. doi: 10.1042/BJ20060441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435:513–518. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- 26.Hu JC, O'Shea EK, Kim PS, Sauer RT, O’Shea EK, Kim PS, Sauer RT. Sequence requirements for coiled-coils: analysis with lambda repressor-GCN4 leucine zipper fusions. Science. 1990;250:1400–1404. doi: 10.1126/science.2147779. [DOI] [PubMed] [Google Scholar]
- 27.Kolb T, Maass K, Hergt M, Aebi U, Herrmann H. Lamin A and lamin C form homodimers and coexist in higher complex forms both in the nucleoplasmic fraction and in the lamina of cultured human cells. Nucleus. 2011;2:425–433. doi: 10.4161/nucl.2.5.17765. [DOI] [PubMed] [Google Scholar]
- 28.Natan E, Endoh T, Haim-Vilmovsky L, Flock T, Chalancon G, Hopper JTS, Kintses B, Horvath P, Daruka L, Fekete G, Pál C, et al. Teichmann, Cotranslational protein assembly imposes evolutionary constraints on homomeric proteins. Nat Struct Mol Biol. 2018;25:279–288. doi: 10.1038/s41594-018-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye Q, Worman HJ. Protein-protein interactions between human nuclear lamins expressed in yeast. Exp Cell Res. 1995;219:292–298. doi: 10.1006/excr.1995.1230. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber G, Keating AE. Protein binding specificity versus promiscuity. Curr Opin Struct Biol. 2011;21:50–61. doi: 10.1016/j.sbi.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mena EL, Kjolby RAS, Saxton RA, Werner A, Lew BG, Boyle JM, Harland R, Rape M. Dimerization quality control ensures neuronal development and survival. Science (80-) 2018;362:eaap8236. doi: 10.1126/science.aap8236. [DOI] [PubMed] [Google Scholar]
- 32.ilia-kats ilia-kats/RiboSeqTools: v0.1. 2020 doi: 10.5281/ZENODO.4016066. [DOI] [Google Scholar]
- 33.Materials and methods are available as supplementary materials at the Science website.
- 34.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeliseev A, Zoubak L, Gawrisch K. Use of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expr Purif. 2007;53:153–163. doi: 10.1016/j.pep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paix A, Folkmann A, Goldman DH, Kulaga H, Grzelak MJ, Rasoloson D, Paidemarry S, Green R, Reed RR, Seydoux G. Precision genome editing using synthesis-dependent repair of Cas9-induced DNA breaks. Proc Natl Acad Sci. 2017;114:E10745–E10754. doi: 10.1073/pnas.1711979114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shivashankar GV. Nuclear Mechanics and Genome Regulation. Vol. 98. Academic Press; 2010. pp. 111–112. [DOI] [PubMed] [Google Scholar]
- 38.Blobel G, Sabatini D. Dissociation of Mammalian Polyribosomes into Subunits by Puromycin. Proc Natl Acad Sci U S A. 1971;68:390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galmozzi CV, Merker D, Friedrich UA, Döring K, Kramer G. Selective ribosome profiling to study interactions of translating ribosomes in yeast. Nat Protoc. 2019;14:2279–2317. doi: 10.1038/s41596-019-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Döring K, Ahmed N, Riemer T, Suresh HG, Vainshtein Y, Habich M, Riemer J, Mayer MP, O’Brien EP, Kramer G, Bukau B. Profiling Ssb-Nascent Chain Interactions Reveals Principles of Hsp70-Assisted Folding. Cell. 2017;170:298–311.:e20. doi: 10.1016/j.cell.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 42.Becker AH, Oh E, Weissman JS, Kramer G, Bukau B. Selective ribosome profiling as a tool for studying the interaction of chaperones and targeting factors with nascent polypeptide chains and ribosomes. Nat Protoc. 2013;8:2212–2239. doi: 10.1038/nprot.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaefer J, Jovanovic G, Kotta-Loizou I, Buck M. Single-step method for β-galactosidase assays in Escherichia coli using a 96-well microplate reader. Anal Biochem. 2016;503:56–57. doi: 10.1016/j.ab.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiedler U, Weiss V. A common switch in activation of the response regulators NtrC and PhoB: phosphorylation induces dimerization of the receiver modules. EMBO J. 1995;14:3696–3705. doi: 10.1002/j.1460-2075.1995.tb00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bezanson J, Edelman A, Karpinski S, Shah VB. Julia: A Fresh Approach to Numerical Computing. SIAM Rev. 2017;59:65–98. [Google Scholar]
- 46.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agresti A, Coull BA. Approximate Is Better than “Exact” for Interval Estimation of Binomial Proportions. Am Stat. 1998;52:126. [Google Scholar]
- 49.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 50.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM, Bäckström A, et al. A subcellular map of the human proteome. Science (80-) 2017;356:eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 51.UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sprenger J, Fink JL, Karunaratne S, Hanson K, Hamilton NA, Teasdale RD. LOCATE: a mammalian protein subcellular localization database. Nucleic Acids Res. 2008;36:D230–D233. doi: 10.1093/nar/gkm950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chou K-C, Wu Z-C, Xiao X. iLoc-Euk: A Multi-Label Classifier for Predicting the Subcellular Localization of Singleplex and Multiplex Eukaryotic Proteins. PLoS One. 2011;6:e18258. doi: 10.1371/journal.pone.0018258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giurgiu M, Reinhard J, Brauner B, Dunger-Kaltenbach I, Fobo G, Frishman G, Montrone C, Ruepp A. CORUM: the comprehensive resource of mammalian protein complexes-2019. Nucleic Acids Res. 2019;47:D559–D563. doi: 10.1093/nar/gky973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data reported in this study are available at GEO under accession number GSE151959. Explicit Julia code is available as supplementary material; explicit R code will be made available upon request. Data analysis of ribosome profiling datasets were performed with RiboSeqTools (available at https://github.com/ilia-kats/RiboSeqTools and (32)).