Summary
Epithelial stem cells accumulate mutations throughout life. Some of these mutants increase competitive fitness and may form clones that colonize the stem cell niche and persist to acquire further genome alterations. After a transient expansion, mutant stem cells must revert to homeostatic behavior so normal tissue architecture is maintained. Some positively selected mutants may promote cancer development while others inhibit carcinogenesis. Factors that shape the mutational landscape include wild type and mutant stem cell dynamics, competition for the niche, and environmental exposures. Understanding these processes may give new insight into the basis of cancer risk and opportunities for cancer prevention.
Introduction
Aging is accompanied by mutation (1). From the first division after conception, somatic cells acquire mutations (2–4). This progressive increase in the number of mutations in human stem cells is unavoidable, but on its own cannot explain the diverse mutational landscapes that develop across normal tissues. Differences in the structure of the stem cell niche, wild type and mutant stem cell dynamics, and environmental exposures all play a part in determining the prevalence of particular mutant genes in normal tissues. Understanding these processes sheds light on the biology of stem cells, the impact of environmental exposures and the nature of the precancer state. In turn, these may guide interventions to reduce cancer risk, as it is from this mutated landscape that the founder clones of cancers emerge over years or decades (5,6).
Progress in transgenic mouse and primary cell culture research has given insights into the behavior of wild type and mutant stem cells that inform the interpretation of mutational data (7,8). Here we will first briefly consider the approaches used to detect mutations and then consider insights into epithelial stem cells and mutational selection that apply across tissues. Epithelia which have been studied in depth will then be reviewed, and finally we draw together common principles in this nascent field and consider the complex relationship between normal tissue mutations and carcinogenesis.
The challenge of finding normal epithelial mutants
Cancers are clonal with subclones within them, so that as long as a sample is of sufficient purity, sequencing at normal depth will detect the founder clone and large subclones (9). An equivalent mass of normal epithelia is highly polyclonal, containing many different mutant clones (Figure 1A). For a given gene, almost all reads will be wild type, so that most mutants are likely to be below the lower limit of reliable detection of standard sequencing and hence be missed. Several strategies have been developed to get around this challenge.
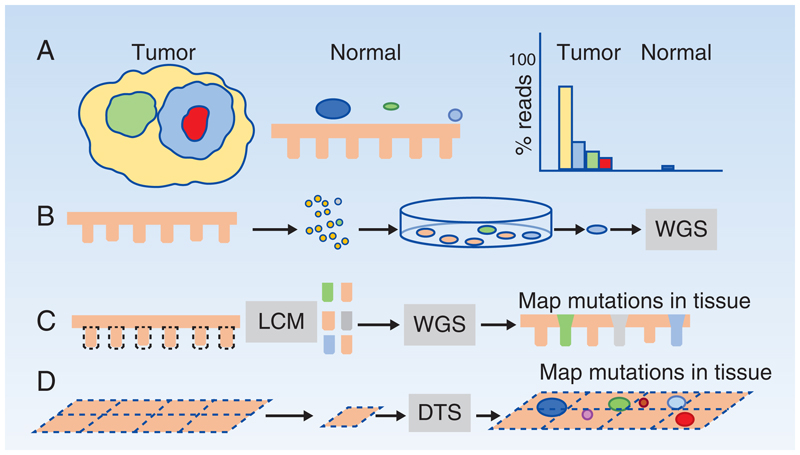
Figure 1. Detecting somatic mutants in normal epithelia.
A: Clonality and somatic mutant detection in tumors and normal epithelia. Tumors are clonal yellow) with sub-clonal mutations (green, blue and red). Normal epithelia harbor scattered somatic mutant clones (colored circles) in a wild type background). When sequenced at standard depth, the founder clone and common subclones in a tumor are readily identified, whereas only the largest mutant clones in normal tissue exceed the lower limit of detection.
B-D: Methods to detect somatic mutant clones in normal tissues
B: A single cell suspension is generated from normal epithelium, cultured at clonal density and individual colonies whole genome sequenced (WGS).
C: Laser capture microdissection (LCM) is used to isolate areas of tissue such as individual stem cell niches like the colonic crypt. Special protocols are used to perform WGS, whole exome or targeted sequencing and mutations can then be located within the tissue section from which they came.
D: Larger areas of epithelia can be dissected into a gridded array of typically 2mm2 samples, deep targeted sequencing (DTS) performed, and a statistical approach used to call rare somatic mutants in each sample, which can then be mapped (colored circles) within the sample grid.
A simple approach is to generate single cell derived clonal cultures from the tissue of interest (1,10,11) (Figure 1B). This allows amplification of single genomes in live cells to generate enough DNA for reliable whole genome sequencing (WGS). The disadvantages are a loss of spatial information as the tissue is disaggregated when cultured, the potential for biased sampling as some wild type or mutant cells may not grow in culture, the introduction of new mutations while cells are being cultured, and the substantial labor involved.
An alternative strategy is to use laser capture microdissection (LCM) to remove a microscopic piece of tissue and perform either exome or whole genome sequencing (12). Laser capture of a histologically defined stem cell niche such as a colonic crypt is quite likely to yield an oligo clonal or clonal population (13) (Figure 1C). Crucially, LCM retains spatial information, a major advantage allowing mutant clones to be mapped across an epithelium by taking multiple samples from serial sections. Optimization of multiple steps in small sample sequencing protocols has enabled WGS of as few as 100 cells with minimal sequencing errors (12). LCM overcomes the potential selective bias of the cell culture approach, has an acceptable technical failure rate, and has become the most widely used approach in the field. It has yielded a rich harvest of information on single nucleotide variants, structural alterations, burden of synonymous mutations and mutational signatures in mutant clones in normal tissues. However, LCM remains labor intensive and the area of tissue and number of individual donors that can be analyzed in such studies is small, limiting the statistical robustness of studies.
More recent methods for the analysis of small samples are based on detecting somatic mutations in single DNA molecules, using an extremely accurate protocol called duplex sequencing (14). The latest iteration of this approach achieved an error rate of less than five errors per billion bases and has been used to map the mutational burden and signatures across multiple human tissues (15). The technique randomly samples about a third of the genome, so cannot give information about specific mutant genes, but is scalable to allow large numbers of individuals to be sampled in epidemiological studies to assess links between cancer risk and mutational burden/signatures for example. A key insight from duplex sequencing is that there is little relationship between the rate of cell division in a tissue and somatic mutation burden, for example post-mitotic neurons accumulate somatic mutations at a similar rate to proliferating tissues (15)
To map mutations in epithelia without a clearly defined niche, an alternative method relies on deep targeted sequencing (DTS) (16–18) (Figure 1D). Sheets of epithelia, such as skin or esophagus, can be detached from the underlying stroma and dissected into a grid of samples (1-2mm2 each in the case of the skin or esophagus)(19,20). These are then sequenced at several hundredfold coverage. To identify rare variant alleles, a ‘reference library’ of very high depth (over 10,000-fold) sequence data of normal DNA is used to measure the technical sequencing error rate at each base in each gene. This is then used to estimate the probability that the variant observed in the experimental sample is a true mutation (20). By collecting samples in a grid, clones spanning adjacent samples can be merged and low spatial resolution maps of mutant clones generated (19,20). The advantage of DTS is that large areas can be rapidly surveyed at low cost, and large numbers of mutant clones collected, orders of magnitude more than with the LCM approach. However, in most cases the combinations of mutants within a clone are not revealed, copy number information is limited, and estimates of synonymous mutation burden and signature may be biased in targeted data.
Finally, mutational information may be extracted from RNA sequencing data. An analysis of the Gtex study, which took a single sample from 30 tissues in hundreds of donors revealed somatic mutants in multiple epithelia, with skin, lung and esophagus the most highly mutated and colon, stomach and bladder the least (21). Mutational signatures and copy number alterations were detected and agreed with DNA sequencing results where these were available. However, the samples contain a complex mixture of cell types and varying proportions of wild type and mutant cells and the expression level of mutant genes may vary across lineages, so the sensitivity of mutant detection and ability to reliably estimate proportions of mutant cells in tissues is unclear. Nevertheless, this study confirms that somatic mutations are widespread across all tissues and suggests their abundance may vary substantially between different epithelia.
In summary, there is no single solution to mapping mutant clones in epithelia. The structure of the tissue and knowledge about the stem cell niche are invaluable in guiding which approach should be selected. WGS of clones is clearly the gold standard for many analyses, but is limited to small areas of tissue in small numbers of donors. DTS mapping has revealed much about the nature of competitive selection of mutants at a scale unmatched by any other method. Duplex sequencing has great promise for studying small biopsies in large numbers of individuals. In exploring a previously unstudied tissue, a hybrid approach seems wise, using DTS to assess the prevalence and size of mutant clones, guiding more costly but informative WGS sampling (22).
Normal stem cell dynamics and somatic mutations
We now turn to key principles for interpreting the effects of somatic mutations on normal epithelia. These are stem cell dynamics and how mutations that alter cell behavior may be identified.
A critical feature of a normal tissue is cellular homeostasis, so that the number of cells in the tissue as a whole and in the proliferating compartment is stable over time. Even in highly mutated normal epithelia, the rate of cell loss by processes such as shedding or apoptosis must match cell production. Furthermore, within the stem cell niche, on average, each division must produce one stem cell and one cell that will leave the niche to differentiate, so that the number of stem cells remains constant. In transgenic mice, a specific reporter mutation can be induced in scattered single cells to allow clones to be tracked. When combined with statistical modeling or more recently live imaging this has shown how epithelial stem cells achieve this homeostatic balance (23–27).
There are two distinct cellular mechanisms to achieve homeostasis used by stem cell populations in epithelia. The first, exemplified by the intestinal crypt, is likely to operate in most epithelia organized into spatially defined proliferative compartments. Stem cells divide to generate two stem cell daughters, but division is limited by the finite space within the niche. Stem cells can only divide when a neighboring stem cell leaves the niche as it differentiates (23,26,28,29). In other epithelia where the stem cells lie within an uninterrupted cell layer, the probability of generating stem cell and differentiating daughter cells is balanced so that across the stem cell population the average division results in 50% stem cells and 50% differentiating cells, ensuring homeostasis (24,27,28,30–32). Both of these mechanisms result in neutral drift in lineage tracing experiments (23,25,28).
Given the conservation of stem cell behavior across evolution, it is to be expected that human epithelial stem cells will also fall into one or other of these two classes of homeostatic regulation (28,33). An implication of these dynamics is that single nucleotide variants that do not alter cell behavior may be lost by neutral drift before the second allele is disrupted. A further constraint on mutant clones in tissues organized into clonal units, such as the colon, is that once the niche is fully occupied its further expansion is restricted unless it can transgress the normal boundaries of the compartment (23,29). In squamous epithelia on the other hand, the niche is a two-dimensional sheet and there are no limits to clonal expansion within the plane of proliferating cells (24,31). As well as setting a potential upper bound on clone size, the niche also defines the dynamics of competition between mutants and mutant selection as discussed below.
A key challenge, that parallels the analysis of mutations in cancer genomes, is to discriminate ‘driver’ mutations that alter cell behavior from neutral ‘passenger’ mutants. A simple method to resolve the two that is widely used in somatic mutation studies is to compute the ratio of protein altering (dN) to silent (dS) mutations in the coding region of a gene. This approach avoids pitfalls such as the variation in mutation rates across the genome and variations in sequencing coverage and has recently been enhanced by using the mutational spectrum to estimate the expected frequency for every possible nucleotide substitution in the gene (17,34). The dN:dS ratio under the assumption of neutrality is one, whereas positively selected mutant genes in epithelia may have dN:dS ratios of up to 50 or more. Negative selection is much harder to detect, requiring a much larger sample size, but has been observed in highly competitive tissues such as the skin (20).
It should be noted that dN:dS ratios have some limitations. For example, a mutant gene such as PIK3CA with both inactivating nonsense and missense mutations and gain of function ‘hot spot’ mutations can have a net dN:dS ratio close to 1 (20). Some synonymous mutations may have functional consequences and so cannot be assumed to be neutral (35). Simple comparisons of dN:dS ratios across tissues or studies cannot be relied upon as indicators of the strength of selection of a mutant gene in different contexts. Furthermore, sufficient numbers of mutations are required so this approach is not suitable for small studies or sparsely mutated tissues. Nevertheless, the approach is a simple and robust method to identify mutant genes likely to have function impact on cell dynamics.
As we will discuss below, there are several examples of positive selection of mutant genes in humans that have been studied in mouse models, which show the mutant gene confers a proliferative advantage on stem cells. In each case, the competitive advantage of mutant stem cells is transient, as following colonization of a niche, such as a colonic crypt, or a region of squamous epithelium, the mutant cells revert back from expansion towards homeostasis. This behavioral reversion is critical for the maintenance of normal histology and function of aging tissues but its molecular basis is poorly understood.
In epithelia, a mutant cell immediately comes into direct contact with wild type neighbors and must compete with them for space in the tissue. Wild type cells are able to sense cells with a markedly abnormal phenotype, such as those overexpressing high levels of oncogenic mutants from strong synthetic promoters, and extrude them from the epithelium (36–38). However, the phenotype of the same mutants expressed from their own promoter is subtler allowing the mutant cell to persist (39,40). Positively selected mutants confer a proliferative advantage on the mutant cell itself, but some ‘super competitors’ also act on adjacent wild type cells, inducing their differentiation. For example, in mice Apc null stem cells in the intestinal crypt secrete the WNT inhibitor NOTUM which drives adjacent wild type stem cells to differentiate and leave the crypt, creating space for mutant clone expansion (41,42). This increases the chances that the Apc mutant cells will completely occupy the crypt and go on to found an adenoma. Interestingly the efficiency of crypt takeover by Apc null cells is reduced by a calorie restricted diet which increases the number of wild type cells and level of competition in the niche (43). A further example is of Notch1 null cells in the mouse esophagus, which induce the differentiation and upwards migration of adjacent wild type cells in the proliferative layer, apparently by activation of Notch signalling in the wild type cell (44–46).
We now explore these themes in individual epithelia firstly considering those organised into discrete clonal units where mutants compete within a niche, such as the colon, and then tissues where there is no defined niche, squamous epithelia and the bladder.
Colon
The colonic epithelium is a classic example of a tissue organized into spatially discrete proliferative units, defined by the colonic crypt and an area of adjacent epithelium that it supports, each maintained by a separate population of stem cells (47,48). Wild type stem cells compete neutrally for space in the niche, with stem cell division being linked to the exit of a cell as it begins the process of differentiation (Figure 2A). Lineage tracing in mice shows crypts becoming monoclonal through neutral competition, with the colonizing clone being no fitter than the stem cells it displaces (49) (Figure 2B).
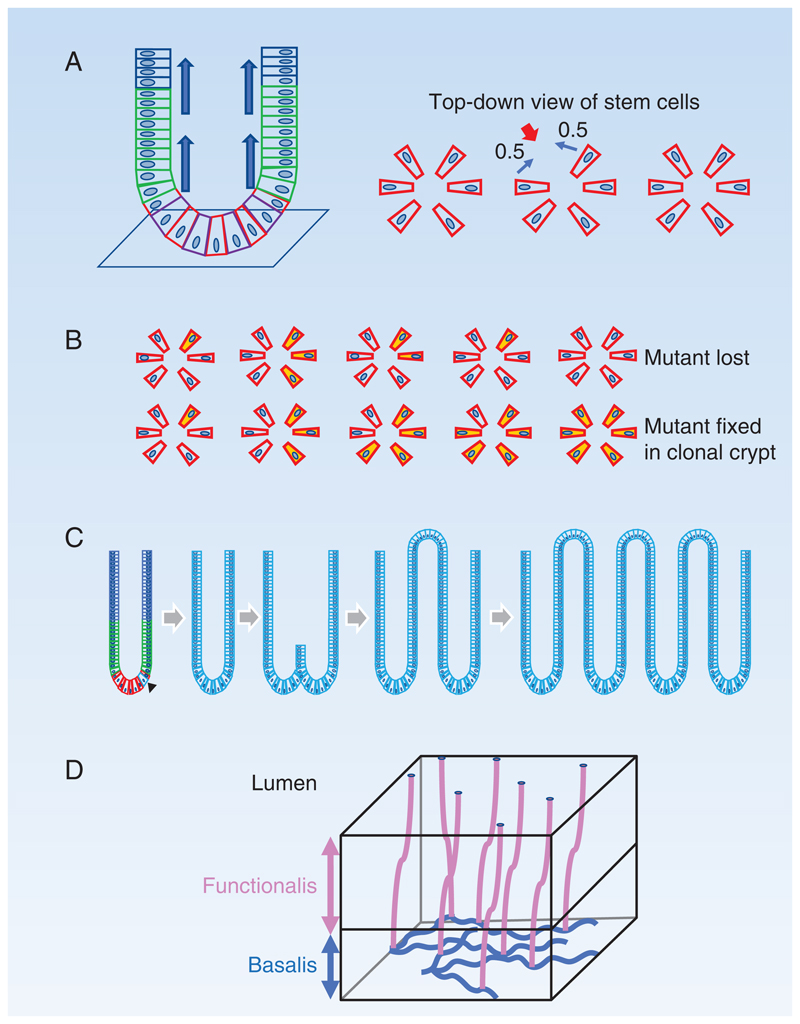
Figure 2. Colon and endometrium.
A: Colonic crypt: Stem cells (red) reside in a niche at the base of the crypt. Differentiation of stem cells generates short lived proliferating cells (green) which migrate up into the crypt and differentiate into post-mitotic differentiated cells (blue). Right hand panels show top-down views of stem cells in the crypt base. When a stem cell differentiates and exits the niche (red arrow), it is replaced by division of one of its neighboring stem cells with equal probability (0.5).
B: Possible outcomes of a neutral mutation in a crypt stem cell: A new mutation that does not alter stem cell fate (yellow) may generate a clone that is outcompeted by wild type cells and lost or replaces other stem cells so the mutant becomes fixed in the crypt as no non-mutant cells remain. Positively selected mutations confer a greater than 50% chance of replacing a wild type neighbor and a high likelihood of taking over a crypt.
C: Crypt Fission: Crypt fission occurs rarely in wild type crypts, but the rate is greatly accelerated by activating KRAS and nonsense STAG2 mutations, which spread to colonize the crypt and then induce the crypt to split, creating two independent crypts. The process may repeat, allowing a mutation to spread widely across the epithelium.
D: Structure of human endometrial epithelium: The basalis layer (blue) contains a branching network of interconnected glands through which clonal mutations may spread. The overlying functionalis layer (pink) contains glands connected with the basalis layer and undergoes cyclical growth, apoptosis, and shedding between menarche and menopause.
More recently spontaneous mutations have been used as lineage markers to infer the dynamics of wild type stem cells in normal human colonic crypts. Somatic mutations in the enzyme O-acetyl transferase (OAT) can be detected by histochemical staining allowing the visualization of clones within colonic crypts (50). A mixed population of clonal, partially colonized and non-mutated crypts is seen, which when analyzed leads to estimates of 7 stem cells per crypt that divide every 9 months on average, with a median time for a crypt to drift to monoclonality of 6 years, other studies reaching similar conclusions (13,50,51). The rate of stem cell turnover is far slower than in the mouse colon, though other aspects of stem cell competition appear to be conserved (39,49,50). Human colonic epithelium is thus an array of small spatially separated stem cell pools undergoing slow turnover and a process of neutral drift.
The insight that many colonic crypts in aging humans will carry clonal mutations motivated the large-scale sequencing of normal human colonic crypts by LCM (13). Over 500 clonal crypts from middle aged or older donors were subject to WGS. Signature analysis indicated the bulk of mutations were due to cell intrinsic processes (signatures SBS1 and 5) or oxidative DNA damage (SBS18) which were found in all samples (13,52). In addition, some mutational signatures were restricted to either individuals or crypts. For example, a patient who had received chemotherapy had a unique signature reflecting this and two crypts had the signature of the APOBEC cytidine deaminases. The WGS analysis was augmented by targeted sequencing for known colon cancer drivers. Mutant AXIN2 and STAG2 were identified as under positive selection and gain of function ‘hot spot’ mutations detected in ERBB2, ERBB3, PIK3CA and FBXW7. Overall, these mutant genes are found in about 1% of crypts of a typical 50 year old (13). About 50% of crypts harbor mutations likely to have an impact on protein function in genes of the Cancer Gene Census, but the absence of selection argues these do not substantially alter the behavior of colon stem cells (53).
How do mutants alter stem cell dynamics? STAG2 lies on the X chromosome, so a protein truncating mutant will lead to loss of protein expression (50). This allows the visualization of mutant cells in crypts, revealing a 10-fold higher ratio of monoclonal to partially colonized crypts than is seen with neutral mutations. A STAG2 mutant stem cell has a decisive competitive advantage over wild type cells, as when a differentiated cell leaves the crypt, it is much more likely to be replaced by a STAG2 mutant than a wild type cell, the former having a 99% probability of taking over its crypt (50).
Copy number changes and/or structural variants were much more common than positively selected mutations in normal colon, being detected in about a fifth of evaluable crypts (13). The commonest events were large deletions and tandem duplications, whole chromosome copy number increases were seen more rarely. The events observed were not recurrent, are not known to be linked to cancer and were confined to single crypts. The level of copy number alterations in normal epithelium is far lower than in cancer (13).
Almost all clonal genomic events are confined within a single crypt. However, rarely crypts may split into two, a process termed crypt fission, first demonstrated in the normal human colon by visualizing clones carrying somatic mitochondrial mutations (54). Mouse models have argued that oncogenic mutants such as Kras may spread by accelerating the rate of crypt fission, allowing them to break out of the imprisonment of a single crypt (Figure 2C) (55). In humans the fission rate of wild type crypts is low, estimated at 0.7%/year. STAG2 mutants increase this rate three-fold and for mutant KRAS the rate of fission may be up to 10-fold higher (50). The effect is to create areas of multiple clonal crypts within the normal epithelium. As well as crypt fission, recent mouse studies hint that oncogenic Kras mutants secrete short range signals that may have detrimental effects on wild type stem cells in adjacent crypts in the small intestine in mice, though it remains to be seen if such mechanisms operate to promote clone spread in humans (56).
Whilst our focus is on normal epithelium, it is worth noting that recent studies have shown the dramatic impact of non-malignant disease, specifically inflammatory bowel disease (IBD), on the selection and dynamics of mutant clones in the colon. These disorders are characterized by episodes of inflammation, ulceration and healing, lasting over decades. An LCM based study found that IBD increased mutational burden, substantially elevated the proportion of crypts with copy number alterations and generated multiple clones extended over millimeters, presumably due to crypt fission (57). Genes under positive selection in IBD epithelium included 2 known cancer drivers ARID1A and FBXW7, but also PIGR and ZC3H12A genes implicated in inflammation specifically selected in IBD epithelium. These findings were extended by studies using both single and bulk crypt sequencing and clonal organoid sequencing, confirming the selection of multiple mutant genes in the IL17 pathway that drives ulcerative colitis (58,59). Functional studies confirm that the selected mutants protect against IL17 driven apoptosis (58). In an elegant study of patients with Crohn’s disease, LCM of crypts was performed on biopsies of normal tissue over 8 years prior to the development of a tumor requiring resection (60). These revealed the dramatic expansion of TP53 mutant clones from the ascending to descending colon. Interestingly, in mouse models, while Apc and Kras mutant cells out compete wild type cells to take over crypts Trp53 mutant cells compete neutrally in normal epithelium, only gaining a competitive advantage when the intestine is inflamed (39). Thus, strong selective pressures can lead to the selection of specific somatic mutants unrelated to cancer in inflamed bowel.
Normal colon thus tolerates both copy number altered and mutant crypts, and particular oncogenic mutants have the potential to expand across large areas of epithelium by driving crypt fission, in response to selection driven by environmental cues such as inflammation. Common cancer driver mutants such as APC and KRAS are rarely found in normal epithelium, whereas some mutant genes that are more common in normal epithelium such as ERBB2 are comparatively infrequent in colonic cancer. The ability of a mutant to alter stem cell dynamics to increase the likelihood of crypt colonization is thus separable from oncogenicity.
Stomach and small intestine
Less information is available on somatic mutations in the stomach and small intestine and rectum, but a recent study examined multiple organs by LCM and sequencing in five human donors giving a preliminary view of the mutational landscape of the stomach and small intestine in which stem cells are also arranged into glands (stomach) or crypts (intestine) (22). The mutational burden at both sites is similar to the colon, despite the extreme rarity of cancer in the small intestine. Signatures are also similar, although the signature of the carcinogen aristolochic acid (AA) was identified in the stomach (22).
Endometrium
A second tissue that contains histologically restricted clonal units but differs dramatically from the colon is the endometrium, which consists of an epithelial sheet punctuated by glands. Between menarche and the menopause, the human endometrium undergoes cyclical apoptosis, shedding, regeneration and remodeling of its functionalis layer (61–63) (Figure 2D). The regeneration of the epithelium is thought to depend on stem cells that persist in the highly branched endometrial glands that lie in the basalis layer (64). LCM and WGS of human basalis glands shows that over 90% are clonal (65). As many clonal gland sections do not carry mutants under positive selection, this may reflect a drift to monoclonality due to neutral competition, as seen in the colonic crypt. The mutation burden was proportional to age, in keeping with the predominant mutational signatures being the clock-like SBS1, SBS5 and SBS40, which is similar to SBS5, and SBS18 reflecting oxidative damage (65). Twelve mutant genes were under positive selection in normal endometrial glands. These included the growth factor receptors ERBB2 and ERBB3, signal transduction components KRAS, PIK3CA, PIK3R1, ARHGAP35 and PPP2R1A, steroid hormone response genes ZFHX3 and FOXA2 and also FBXW7, CHD4 and SPOP. 60 percent of glands had one or more selected mutant gene (65). Consistent results have been obtained in other studies using LCM of epithelium and glands with a targeted sequencing approach (66–68). Somatic copy-number changes and structural variants were rarer and found in about a sixth of normal endometrial glands, almost all of which had only a single alteration (65). Some clones appeared to extend across multiple glands, consistent with the interconnected branching structure of the glands (64,65,68). The mutants that are commonly selected in normal epithelium differ from those frequently mutated in cancer. Endometrial cancer driver genes are rarely mutated in glands, and are not under selection in the endometrium. Only 2% of glands harbored cancer drivers (heterozygous TP53 and ARID1A mutants) (65). As with the colon, the normal tissue does not provide conditions that favor strong selection of oncogenic mutant clones.
It is interesting to speculate if the same mutants would be selected in the same tissue even if it grows in a different body site. Endometriosis, which occurs in about 10% of women, is a condition in which endometrial like epithelium grows outside of the uterus. LCM followed by targeted sequencing has revealed recurrent PIK3CA, PIK3R1 KRAS, FBXW7 and PPP2R1A mutant clones in endometriosis lesions consistent with convergent selection of mutants in the same environment albeit at a different location (66,69).
Epidermis
The outermost layer of the skin, the epidermis, consists of a sheet of keratinocytes, interspersed with hair follicles and sweat ducts (33). The keratinocytes are organized into layers. Proliferating cells are confined to the deepest, basal layer. Dividing cells generate daughters that either go on to divide themselves or differentiate, exiting the basal layer and migrating through the overlying layers until they reach the surface of the skin from which they are shed (Figure 3A). Shedding and proliferation continue throughout life. One consequence of the structure of the epidermis is that there is no barrier to limit the lateral spread of clones, which can extend over a centimeter in diameter (17). Transgenic mouse research and live imaging of primary human cell cultures indicate that in normal epidermis proliferating cells are a single population which has a simple pattern of behavior (27,30,45). The average cell division generates dividing and differentiating daughter cells with equal probability (Figure 3A), achieving cellular homeostasis across the population of proliferating cells (Figure 3A). A consequence of these normal cell dynamics is that while most cells that acquire a neutral mutation generate short lived clones that are lost by differentiation within a few rounds of cell division, by chance, a minority of clones will expand and persist longer term in the tissue (Figure 3B).
Figure 3. Epidermis.
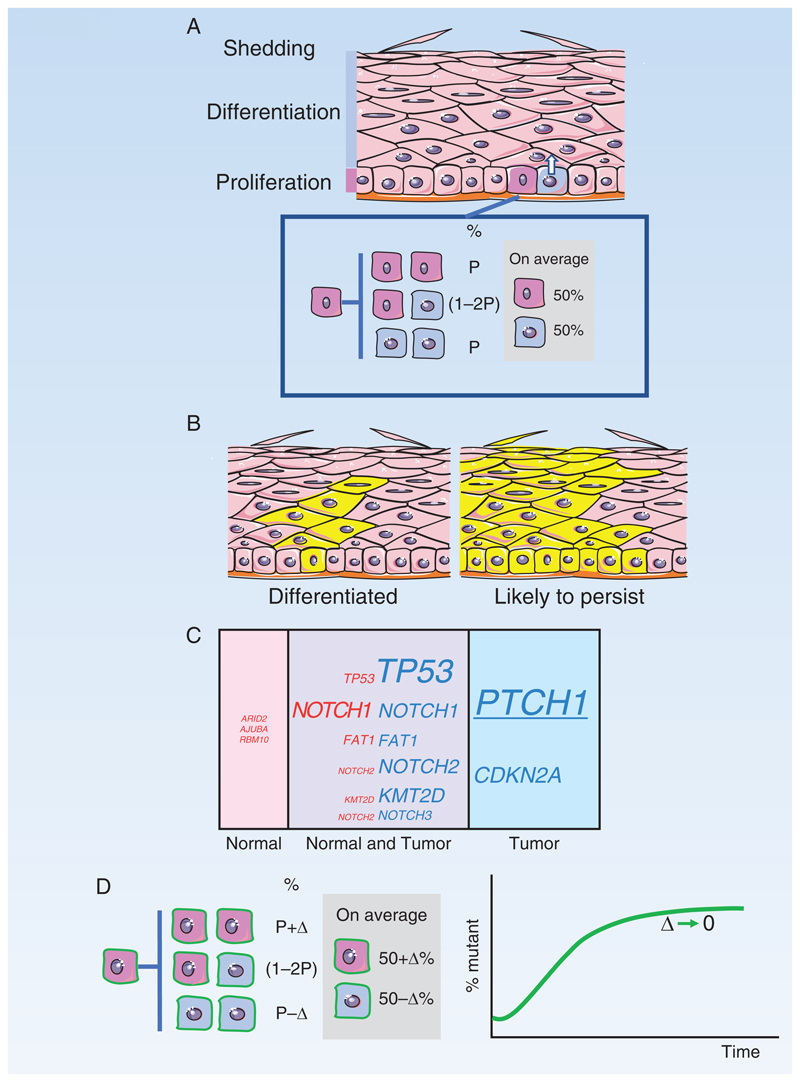
A: Structure of the epidermis. Proliferating cells are confined to the basal cell layer. On commitment to differentiation, cells exit the basal layer and migrate to the surface. Division of a basal stem cell (dark pink) is coupled to the exit of an adjacent cell (blue) from the basal layer. Inset: Stem cell division results in two stem cells (pink), two differentiating cells (blue), or one cell of each type. The likelihoods of each division outcome (P) are balanced so that an average division generates 50% stem cells and 50% differentiating cells.
B: Typical outcomes of a neutral mutation in a stem cell (yellow). Most clones are lost by differentiation and shedding, after all basal layer cells differentiate (left hand panel). By chance, a few clones will become large and are likely to persist (right hand panel).
C: Mutant genes in normal epidermis and keratinocyte tumors
Percentage by area occupied by cells carrying mutant genes in epidermis of normal typical sun-exposed skin (pink) compared with percentage of tumors carrying mutants (blue). Mutations that appear in both normal skin and tumor are also indicated (purple). Font size indicates percentage. PTCH1, underlined, is only commonly selected in basal cell carcinoma.
D: Dynamics of Trp53 mutant clones in mouse epidermis
Induction of a missense dominant negative Trp53 mutation in single cells in mouse epidermis tilts the normal balance of stem cell fate towards proliferation, with the probability of divisions resulting in two stem cell daughters increasing by Δ above the likelihood of divisions producing two differentiating daughters. This increases the odds of mutant clones persisting rather than being lost by differentiation and produces an exponential increase in the proportion of Trp53 mutant cells in the epidermis. However, once areas of the epidermis have been colonized, the mutant cells within them revert towards balanced cell production (green curve, Δ decreases), so the tissue remains histologically normal and functional.
Early studies on somatic mutation in the epidermis used immunostaining for TP53 to detect clusters of cells in which the protein was stabilized by mutation, which could then be sequenced. TP53 mutant clones were identified in sun exposed normal skin (70). More recently DTS has been used to map mutations in sheets of normal skin in multiple body sites, uncovering a high density of mutant clones particularly in sites regularly or intermittently exposed to sunlight (16–18,20). The majority of these mutations are C to T (mutational signature SBS7) and CC to TT substitutions consistent with UV induced mutation, the remaining mutations are attributable to the clock like SBS5 (20). The density of mutant clones varies widely between individuals at the same body site and across the body.
Positively selected genes in the epidermis include NOTCH1, NOTCH2, NOTCH3, FAT1, TP53, TP63, ARID1A, AJUBA, KMT2D, RB1 and RBM10 (20) (Figure 3C). In addition, canonical ‘hotspot’ activating mutations were found in the receptor tyrosine kinases EGFR, ERBB2, ERBB3 and FGFR3 and the downstream signaling components KRAS, HRAS, AKT1, and PIK3CA, along with the transcription factor NFE2L2 which regulates the oxidative stress response. The skin is also the first tissue in which evidence of negative selection, of missense CUL3 and DICER1 mutations and nonsense PIK3CA mutations, has emerged, which may reflect the fierce clonal selection in the skin (20).
How do mutant genes drive clonal expansion? In the case of Trp53 insight comes from a mouse model in which the equivalent of the commonest missense mutant in human skin (TP53R248W) can be induced in single cells and the resultant clones tracked by virtue of expressing a fluorescent reporter. Proliferating mutant cells produce slightly more dividing than differentiating daughters in each cell division on average (40) (Figure 3D). This gives mutant cells a proliferative advantage over wild type cells, and an increased chance persisting and spreading through the epidermis compared with a neutral mutant. The mutant clones expand and colonize large areas of epidermis, which soon appear thickened and express stress markers. However, as months pass, the behavior of the mutant cells changes, reverting back towards balanced cell production and mutant and wild type epidermis become histologically indistinguishable (40) (Figure 3D). A similar mechanism of a transient competitive advantage followed by reversion to homeostatic behavior in driver mutant clones in human epidermis would help to explain how the epidermis can carry a high burden of positively selected mutants within normal epithelium.
Surveying skin across the body reveals intriguing differences between sites. The UV mutational signature is subtly different in facial skin (SBS7d), which is exposed to UV light on a daily basis, from other locations, reflecting distinct DNA damage and/or repair processes (20,52,71). Selection of mutant genes also varies. Mutant TP53 is preferentially selected and mutant FAT1 is less competitive in facial skin compared with other locations. In contrast, in the lower leg, mutant NOTCH1 and NOTCH2 are more strongly selected than other mutant genes (20). These differences in selection may result from the frequency and intensity of UV light exposure as UV light may alter the behavior of existing mutant clones. For example, in mice, repeated exposure to sub-sunburn doses of UV light dramatically expands Trp53 mutant clones compared with unirradiated skin (40,72,73). As a result, the bulk of the Trp53 mutant population in sun exposed skin is generated by UV light induced growth of pre-existing clones rather than de novo mutations (72). The impact of UV light on other mutant genes remains to be studied, but even if Trp53 was the only gene affected, the landscape may be changed as other clones are displaced by mutant Trp53 clonal expansion.
By old age, whole genome sequencing of micro-biopsies reveals normal epidermis to be a dense patchwork of mutant clones, carrying up to 30-40,000 mutations per genome (20). Single clones may have several driver mutations, with one or more having loss of heterozygosity (LOH). The most frequent gene undergoing LOH is NOTCH1, followed by PTCH1, the driver of basal cell carcinoma, which lies close to NOTCH1. FAT1 and TP53 LOH are also frequent.
Collectively, mutant NOTCH1 and FAT1 clones each occupy about a third of aged sun exposed epidermis, about twice the area colonized by TP53 and NOTCH2 mutants (20). It is striking that the proportion of keratinocyte cancers carrying NOTCH1 and FAT1 mutants is similar to that in normal skin, whereas these tumors are substantially enriched in mutant TP53 and NOTCH2 compared with normal tissue. This suggests that while mutant TP53 and NOTCH2 promote cancer development, mutant NOTCH1 and FAT1 may make little contribution to transformation (20).
In summary, normal epidermis tolerates a remarkably high burden of clones carrying multiple mutations under strong positive selection. UV light both generates the bulk of mutations and shapes the mutational landscape, particularly by expanding the TP53 mutant population.
Esophagus
Like the epidermis, the squamous esophagus consists of layers of keratinocytes, but differs in several respects. The lower cell layers contain dividing cells, cells exit the cell cycle and migrate towards the surface, but retain their nuclei until they are shed (74,75). Continual cell turnover is required to maintain tissue integrity, the majority of cell divisions occurring in the 2-3 layers immediately above the basal cell layer (76). The esophagus has rare glands, which appear to be almost entirely quiescent (77). As in the epidermis, there are no barriers to restrict the lateral expansion of clones within the proliferative compartment, which can expand to millimeter scale (19). In terms of stem cell dynamics, mouse studies argue the proliferating cells are a single population with similar properties to those in the epidermis, so that cells with a neutral mutation will follow neutral drift (24,78). In human esophagus, evidence for stem cell behavior is indirect, inferred from proliferation marker expression and cell culture, but all proliferating cells seem to have similar potential to generate cultures and reconstitute esophageal epithelium in xenograft studies, consistent what would be expected from mouse studies (74,75).
It might be expected that a lower proportion of esophageal epithelium would be mutated compared with epidermis, given the lifelong exposure of the latter to mutagenic UV light. However, this is not the case (10,19,21,22). Human esophagus progressively acquires mutations with age, the predominant mutational signatures being the clock-like SBS1 and 5, with the addition of the alcohol mutational signature SBS16 in some individuals. Mutant genes under positive selection include NOTCH1, NOTCH2, NOTCH3, TP53, FAT1, ARID1A, KMT2D, CUL3, AJUBA, PIK3CA, ARID2, TP63, NFE2L2, CCND1, and PPM1D (10,19) (Figure 4A). WGS shows copy neutral LOH of NOTCH1 is frequent but other genome alterations are rare. By old age, esophageal epithelium is one of the most mutated tissues in the body, with mutant clones occupying the majority of the epithelium.
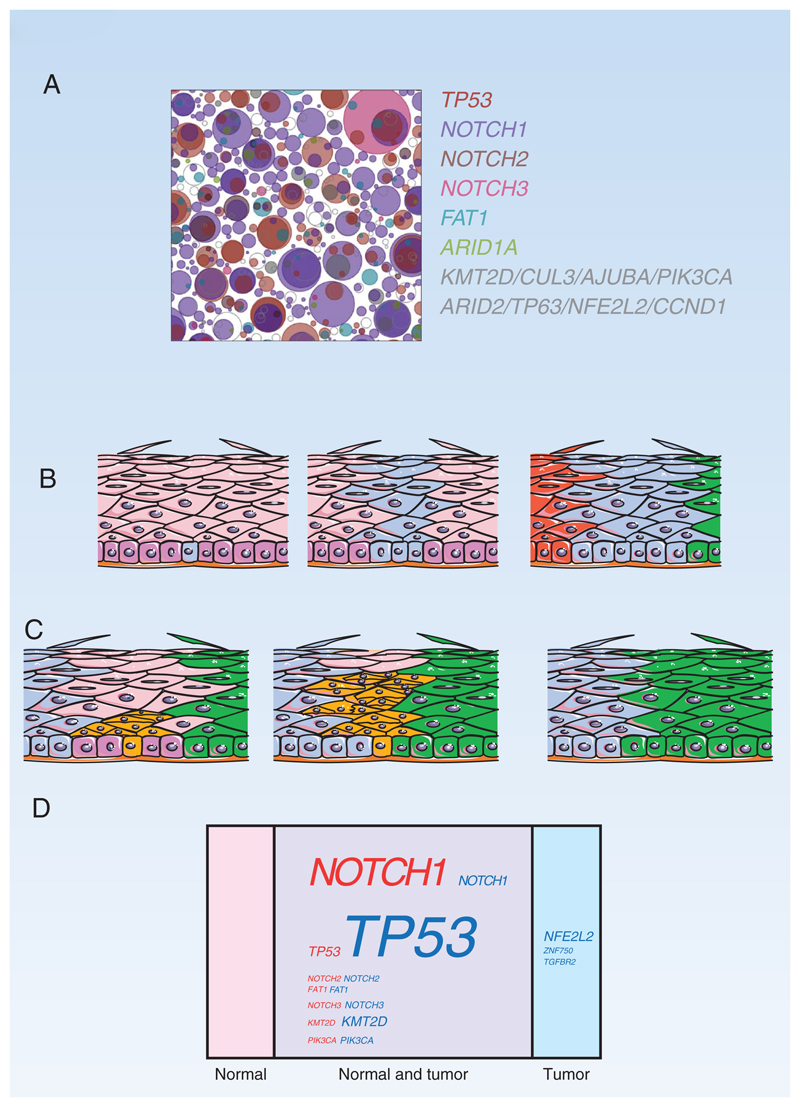
Figure 4.
A: Top down view of mutant clones in 1cm2 of normal esophagus from a 75-year old non-smoker mapped by DTS. Clones containing mutant genes under positive selection are represented by colored circles. From Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science (New York, NY) 2018;362(6417):911-7 doi 10.1126/science.aau3879. Reprinted with permission from AAAS
B: Clonal competition in the mouse esophagus. A stem cell carrying a positively selected mutant (cyan) grows into a mutant clone due to a proliferative advantage conferred by the mutation. The clone expands laterally until it encounters neighboring clones of similar fitness (red and green), at which point the mutant cells revert to neutral competition and balanced production of stem and differentiated daughters (79).
C: Elimination of intra-epithelial tumor by expanding clones. In a mouse model, highly competitive mutant clones (green and blue) in normal wild type epithelium (pink) have been shown to remove microscopic tumors (orange) from the esophagus as they expand by displacing them from the proliferating cell layer (magenta) (80).
D: Mutant genes in normal esophagus and squamous cell carcinoma
Percentage by area occupied by cells carrying mutant genes in the middle third of the esophagus (pink) compared with percentage of esophageal squamous cell carcinoma cells carrying mutations (blue). Mutations that appear in both normal esophagus and tumor cells are also indicated (purple). Font size indicates percentage (19,81).
Mouse models argue the mechanism of clonal selection in the esophagus is competition for space within the proliferative compartment (79). Lineage tracing in mutagen treated mouse esophagus with a patchwork of mutations very similar to that in humans indicates mutant clones expand, displacing wild type and less fit mutants until they encounter mutants of similar fitness (Figure 4B). At this point, clones revert towards homeostatic behavior and compete neutrally, explaining both selection and the ability of the tissue to retain normal structure and cell dynamics with such a high burden of mutant cells (79). The intense competition for space in highly mutated epithelia such as the esophagus also poses a challenge for early tumors, as highly competitive clones within the normal epithelium may remove microscopic lesions before they can progress further (Figure 4C) (80).
In terms of the area of the esophagus colonized, NOTCH1 mutants are predominant, and in combination with LOH this means that by middle age the majority of the esophagus has lost both alleles of NOTCH1 (20,44). In mouse models, transgenic inhibition of Notch signaling confers a strong competitive advantage on clones in normal esophagus and Notch1 is haploinsufficient, so mutation of a single allele confers a competitive advantage, increased by loss of the second allele (44,45). However, despite being very competitive in normal esophagus, NOTCH1 mutants are poorly oncogenic, being found in under 10% of cancers (81) (Figure 4D). Indeed, the depletion of mutants in cancer compared to normal tissue argues that NOTCH1 loss may protect against transformation. In contrast to NOTCH1, TP53 mutation with LOH is found in almost all squamous carcinomas of the esophagus. In normal tissue, heterozygous mutant clones are found in 10% of the tissue by middle age, rising up to 30% for those in their 70s (10,19) (Figure 4D). This suggests that TP53 mutants confer a competitive advantage on the cells that carry them, but unlike NOTCH1, TP53 mutant clones with LOH are very rare in normal epithelium (19,82).
Less is known about how environmental factors shape the normal esophageal landscape. High alcohol intake is a risk factor for squamous esophageal cancer and is associated with an increased mutational burden with an alcohol mutational signature and a higher density of clones carrying TP53 and NOTCH1 mutations (10). Almost all squamous esophageal cancers carry TP53 mutations, and in drinkers they are likely to be caused by alcohol generating a TP53 mutant clone in normal epithelium, as evidenced by the alcohol mutational signature (83). In mice, it has been shown that exposure to low dose ionizing radiation, 50mGy, equivalent to 3-4 CT scans, is able to promote the expansion of pre-existing Trp53 mutant clones by a DNA damage independent mechanism (84). The exposure results in redox stress, causing wild type cells to differentiate. Mutant cells express high levels of antioxidant genes and are protected, and so are able to expand into the space vacated by wild type cells (84). This is an example of an environmental exposure that leaves no mutational signature but might potentially increase cancer risk by expanding the population of oncogenic mutants in a normal tissue. Such factors might explain the absence of different mutational signatures in esophageal squamous cancers from high and low incidence parts of the world (83).
Urothelium
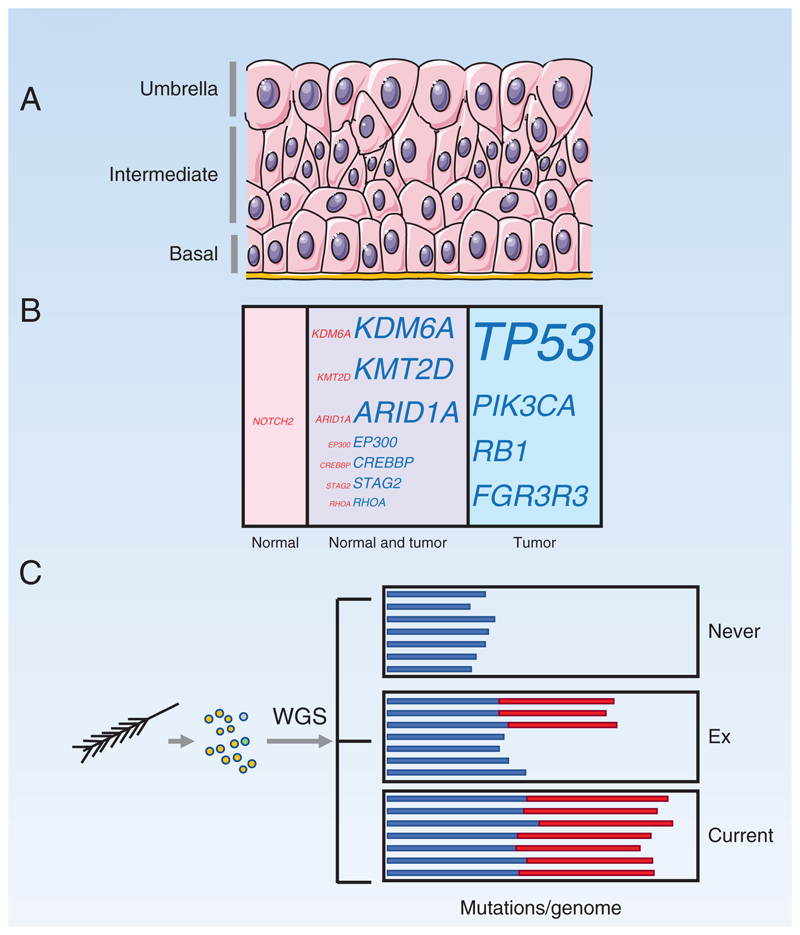
The urothelium that lines the bladder and ureters is a continuous multilayered sheet of cells which normally have a very low rate of cell division with less than one in a thousand cells expressing proliferation markers (85,86) (Figure 5A). However, in response to injury basal and possibly the overlying intermediate layer cells rapidly proliferate to reconstitute the epithelial surface (87–89). Such proliferative potential may be exploited by mutations and as with stratified epithelia, there are no barriers to restrict the expansion of mutant clones, so although most mutant clones are submillimeter in scale they can extend beyond a centimeter (90–92).
Figure 5. Bladder and Bronchus.
A: Urothelium. Proliferation is very rare. The basal layer is thought to contain stem cells, but following injury, cells in the intermediate layer may be recruited into cycle. The upper layer of ‘umbrella’ cells is binucleate and post mitotic.
B: Mutant genes in normal urothelium and bladder cancer. Proportion by area occupied by cells carrying mutant genes in normal urothelium (pink) compared with percentage of bladder cancer cells carrying mutations (blue). Mutations that appear in both normal urothelium and tumor cells are also indicated (purple). Font size indicates percentage.
C: Analysis of mutant cells in bronchial epithelium. Single cells from a small area were sampled via a brush biopsy, cultured, and WGS of single colonies performed. Bar chart depicts mutational burden, with colors showing age-correlated mutational signatures (blue) and tobacco-linked signatures (red), in subjects who have never smoked, ex-smokers, and current smokers. The data implies that the epithelium in ex-smokers becomes colonized by lightly mutated cells protected from the effects of smoke exposure.
Two recent studies have used LCM to examine mutations in urothelium. The first used a combination of targeted sequencing and whole exome sequencing, subsequently performing standard depth whole genome sequencing on samples found to be clonal in normal bladder urothelium from transplant donors and cancer patients from the UK (91). The mutation burden rose with age and was similar to that in other tissues. Mutational signature analysis was complicated by marked variation between donors, so de novo signature discovery was performed. This revealed the presence of APOBEC cytidine deaminase mutagenesis, which is rare in other normal tissues (91). The most frequently positively selected mutant genes were the epigenetic regulators KMT2D, KDM6A and ARID1A, while common bladder cancer drivers were rarely mutated (Figure 5B) (91,93). Copy number alterations were absent from the majority of clones, the most common changes being gains of whole chromosomes or chromosome arms.
A second study studied both ureters and bladders from a Chinese population, sampling larger areas of urothelium and performing whole exome sequencing on histologically normal epithelium distant from urothelial cancers (92). The larger area of urothelium sampled and relatively shallow 140-fold coverage meant smaller mutant clones were not detected. De novo mutational signature analysis revealed an ‘aging’ SBS1 and SBS5 like signature, an APOBEC cytidine deaminase signature resembling SBS2 and SBS 13 and most surprisingly and aristolochic acid (AA), SBS22 like signature, which was present in 60% of female and 25% of male samples. AA is a powerful mutagen present in traditional herbal medicines that is associated with an increased risk of urological cancers (94,95). The mutational burden of normal epithelium was low in patients without the AA signature, but significantly higher in those with AA exposure, dramatically so in some individuals. Copy number alterations were rare. Mutant clone sizes were larger in the AA exposed subjects. Positively selected mutant genes in normal urothelium were again KMT2D and KDM6A but also TP53. AA emerges as not just a mutagen but an agent able to alter mutant clonal dynamics, with the largest clone found in AA exposed tissue extending over several square centimeters (92).
Bronchial Epithelium
Finally, we consider bronchial epithelium, which contains basal cells and multiple specialized cell types. Mitochondrial mutant clones demonstrate the potential for extensive lateral expansion involving all cell types extending at least 1mm in diameter and are argued to exhibit features of neutral competition, although this is controversial (82,96). The mutational landscape has been studied by WGS of clonal cultures of cells from brush biopsies of never, current, and former smokers (Figure 5C) (11). The efficiency of culture generation was 15-40%, but the extent of selection during the establishment of cultures is not known. All subjects accumulated mutations with age at a low rate, circa 20 mutations/year, but this is dwarfed by the effect of smoking, with over 5000 mutations/cell in current smokers and half this in former smokers, where about half the cells had a mutational burden close to that of non-smokers (11).
Mutational signatures included the age-correlated SBS1 and SBS5, particularly dominant in non-smokers, and tobacco-linked signatures SBS4 and SBS16 in current and ex-smokers. Of particular interest, the cells with normal mutation burden in ex-smokers had little SBS4 (11).
Mutant genes under positive selection were TP53 and NOTCH1, both present in over 30% of colonies, and more rarely PTEN, ARID1A and ARID2, which are selected in carcinoma of the lung, and also FAT1 and CHEK2 (11,34,97). As the brush biopsy samples cells in a small area, some mutations were shared between colonies from a given donor, and 75% shared the same TP53 mutation. The proportion of colonies carrying a selected mutant was under 10% in never smokers but ranged from 25 to over 50% in current smokers, a few of which carried 2 or 3 selected genes (11).
These findings demonstrate the huge impact of smoking on the mutational landscape of bronchial epithelium, which is to be expected in light of the link of smoking in lung cancer risk (98). Less expected however, and something that was detected by the single cell genome analysis that is a feature of this study, is the emergence of a population of cells in ex-smokers that carry few tobacco-induced mutations (11). These cells seem to outcompete their heavily mutated neighbors in the absence of the selective pressure exerted by smoke exposure and may contribute to the decrease in cancer risk that follows the cessation of smoking, though their nature and the mechanism by which they evade mutation remains to be determined (11,98).
Discussion
All cells age and mutate but the mutations that found clones which expand to outcompete their neighbors, persist, and colonize the stem cell niche vary across epithelia. For example, mutant NOTCH1 and TP53 are strongly selected and colonize a large proportion of skin, esophagus, and lung but are comparatively rare in other epithelia (10,11,19,20). Both these genes are keratinocyte stem cell regulators, promoting differentiation, and mutant clones gain a proliferative advantage through a bias in cell fate from differentiation to proliferation and spread widely (40,45,46,79). It is tempting to hypothesize that many normal tissue mutants are similarly key parts of the regulatory networks that control stem cell dynamics in the tissues in which they are selected.
Epithelial resilience, continuing to function normally while carrying clones with multiple driver mutants, is widespread but is currently unexplained. Candidate mechanisms include the highly conserved ability of epithelial cells to sense local density, which in squamous epithelia can trigger cell differentiation and exit from the proliferative compartment, and is observed coincident with a return towards normal cell behavior in mouse models (40,45,99). Epithelial cells balance division and cell extrusion via mechanosensitive ion channels such as Piezo1, alterations in the dynamics of MAPK signalling, epithelial calcium waves and cell-cell junctions all of which may play a role in density-dependent regulation of mutant cells (100–104). The reversion from clonal expansion towards homeostasis occurs for every mutant clone in our normal tissues, bar the one that escapes to cause cancer, so this is a critical area for future research.
Mutational signatures are compelling evidence of environmental exposures that may alter normal tissue landscapes such as AA and tobacco exposure, and can identify cell populations not exposed to mutagenesis (11,92). However, as shown with UV light, a mutagen can have a major impact by driving clonal expansion independent of its effect on generating mutations (40,72). Other factors, such as low dose radiation may cause clonal expansion and leave no mutational signature (84). Only 3 of 20 known or suspected human chemical carcinogens were found to be mutagenic when administered to mice (105). Thus, the main effects of environmental factors on cancer risk may be by reshaping the mutational landscape of normal tissues rather than by mutagenesis. A combination of human and model system studies will be needed to resolve the mechanisms of action of potential carcinogens on aging mutated tissues.
Little is known about how germline variation impacts normal tissue landscapes. A recent study of normal intestinal crypts from patients with germline POLE/POLD1 mutations that cause cancer predisposition found an increased mutational burden but no evidence of other genome changes or abnormal tissue function (106). An outstanding task is to extend normal tissue studies into diverse populations that vary in their genetics, environmental exposures, and risk of cancer.
It is noteworthy how many mutant genes that are selected in cancer are not enriched in normal tissue and vice versa, indicative of the different processes of competition that operate in the spatial zero-sum game of normal tissue compared with an expanding tumor (107,108). It seems clear that in the future cancer driver genes should not be defined in terms of their frequency in cancer alone, but rather their relative frequency in tumors versus normal tissues (20). A mutant gene with the same prevalence in normal tissue as a tumor may have no role in carcinogenesis, while a mutant depleted in cancer compared with a normal tissue may inhibit transformation (19,20). Other key differences between normal tissue clones and cancer are a vast increase in mutational burden in most cancers, additional mutational signatures indicating mutagenic processes not present in normal tissue, and a great increase in copy number alterations.
There seems no simple way to predict cancer risk from the normal tissue landscape. Some very low-risk tissues, such as the small intestine seem to have a similar mutational burden to the comparatively high-risk colon (13,22). A very high prevalence of mutant clones such as in the esophagus need not translate into a high cancer risk as the most prevalent mutant, NOTCH1, may even be anti-oncogenic (19,80). By old age, epithelia harbour billions of cells carrying mutations associated with cancer, and yet in most cases no cancers form within a given tissue (53). Learning to decipher the metrics that predict cancer risk within the normal landscape of each tissue is a key challenge for the future.
Finally, can the somatic mutational landscape be manipulated to reduce cancer risk? Data from mouse models suggests this may be feasible. Treatment of mice with the WNT activator Lithium Chloride reduced the competitive advantage of Apc null cells in the intestine and hence the number of adenomas they generate (42). Manipulating redox stress can deplete the population of Trp53 mutant cells in the mouse esophageal epithelium and treatment with the anti-diabetes drug Metformin reduces the fitness of Pik3ca mutant cells in the same tissue (84,109). The challenges of designing long-term studies to test such interventions in humans are considerable but rapid progress in this field gives hope that both candidate agents and means to validate them may soon be developed.
Significance.
Recent advances in sequencing have found somatic mutations in all epithelial tissues studied to date. Here we review how the mutational landscape of normal epithelia is shaped by clonal competition within the stem cell niche combined with environmental exposures. Some of the selected mutant genes are oncogenic while others may be inhibitory of transformation. Discoveries in this area leave many open questions, such as the definition of cancer driver genes, the mechanisms by which tissues constrain a high proportion of oncogenic mutant cells, and whether clonal fitness can be modulated to decrease cancer risk.
Acknowledgements
We thank Peter Campbell, Mike Stratton and Inigo Martincorena for insightful discussions. This work was supported by a grant from the Wellcome Trust to the Wellcome Sanger Institute (296194) and a Cancer Research UK Programme Grant to P.H.J. (C609/A27326).
Footnotes
Conflict of interest Disclosure
The authors declare no competing interests.
References
- 1.Blokzijl F, de Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538(7624):260–4. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju YS, Martincorena I, Gerstung M, Petljak M, Alexandrov LB, Rahbari R, et al. Somatic mutations reveal asymmetric cellular dynamics in the early human embryo. Nature. 2017;543(7647):714–8. doi: 10.1038/nature21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S, Mali NM, Kim R, Choi JW, Lee J, Lim J, et al. Clonal dynamics in early human embryogenesis inferred from somatic mutation. Nature. 2021;597(7876):393–7. doi: 10.1038/s41586-021-03786-8. [DOI] [PubMed] [Google Scholar]
- 4.Coorens THH, Moore L, Robinson PS, Sanghvi R, Christopher J, Hewinson J, et al. Extensive phylogenies of human development inferred from somatic mutations. Nature. 2021;597(7876):387–92. doi: 10.1038/s41586-021-03790-y. [DOI] [PubMed] [Google Scholar]
- 5.Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, et al. The evolutionary history of 2,658 cancers. Nature. 2020;578(7793):122–8. doi: 10.1038/s41586-019-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dujon AM, Aktipis A, Alix-Panabières C, Amend SR, Boddy AM, Brown JS, et al. Identifying key questions in the ecology and evolution of cancer. Evol Appl. 2021;14(4):877–92. doi: 10.1111/eva.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Post Y, Clevers H. Defining Adult Stem Cell Function at Its Simplest: The Ability to Replace Lost Cells through Mitosis. Cell stem cell. 2019;25(2):174–83. doi: 10.1016/j.stem.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nature Reviews Molecular Cell Biology. 2020;21(10):571–84. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dentro SC, Leshchiner I, Haase K, Tarabichi M, Wintersinger J, Deshwar AG, et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell. 2021;184(8):2239–54.:e39. doi: 10.1016/j.cell.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama A, Kakiuchi N, Yoshizato T, Nannya Y, Suzuki H, Takeuchi Y, et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565(7739):312–7. doi: 10.1038/s41586-018-0811-x. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida K, Gowers KHC, Lee-Six H, Chandrasekharan DP, Coorens T, Maughan EF, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578(7794):266–72. doi: 10.1038/s41586-020-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis P, Moore L, Sanders MA, Butler TM, Brunner SF, Lee-Six H, et al. Reliable detection of somatic mutations in solid tissues by laser-capture microdissection and low-input DNA sequencing. Nat Protoc. 2021;16(2):841–71. doi: 10.1038/s41596-020-00437-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574(7779):532–7. doi: 10.1038/s41586-019-1672-7. [DOI] [PubMed] [Google Scholar]
- 14.Hoang ML, Kinde I, Tomasetti C, McMahon KW, Rosenquist TA, Grollman AP, et al. Genome-wide quantification of rare somatic mutations in normal human tissues using massively parallel sequencing. Proc Natl Acad Sci U S A. 2016;113(35):9846–51. doi: 10.1073/pnas.1607794113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abascal F, Harvey LMR, Mitchell E, Lawson ARJ, Lensing SV, Ellis P, et al. Somatic mutation landscapes at single-molecule resolution. Nature. 2021;593(7859):405–10. doi: 10.1038/s41586-021-03477-4. [DOI] [PubMed] [Google Scholar]
- 16.Lynch MD, Lynch CNS, Craythorne E, Liakath-Ali K, Mallipeddi R, Barker JN, et al. Spatial constraints govern competition of mutant clones in human epidermis. Nat Commun. 2017;8(1):1119. doi: 10.1038/s41467-017-00993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science (New York, NY) 2015;348(6237):880–6. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei L, Christensen SR, Fitzgerald ME, Graham J, Hutson ND, Zhang C, et al. Ultradeep sequencing differentiates patterns of skin clonal mutations associated with sun-exposure status and skin cancer burden. Science Advances. 2021;7(1):eabd7703. doi: 10.1126/sciadv.abd7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science (New York, NY) 2018;362(6417):911–7. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler JC, King C, Bryant C, Hall M, Sood R, Ong SH, et al. Selection of oncogenic mutant clones in normal human skin varies with body site. Cancer Discovery. 2021;11(2):340–361. doi: 10.1158/2159-8290.CD-20-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yizhak K, Aguet F, Kim J, Hess JM, Kubler K, Grimsby J, et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science (New York, NY) 2019;364(6444) doi: 10.1126/science.aaw0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Di L, Li J, Fan W, Liu Y, Guo W, et al. A body map of somatic mutagenesis in morphologically normal human tissues. Nature. 2021;597(7876):398–403. doi: 10.1038/s41586-021-03836-1. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a neutral drift. Science (New York, NY) 2010;330:822–5. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 24.Doupe DP, Alcolea MP, Roshan A, Zhang G, Klein AM, Simons BD, et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science (New York, NY) 2012;337(6098):1091–3. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–9. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 26.Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507(7492):362–5. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rompolas P, Mesa KR, Kawaguchi K, Park S, Gonzalez D, Brown S, et al. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science (New York, NY) 2016;352(6292):1471–4. doi: 10.1126/science.aaf7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138(15):3103–11. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- 29.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–44. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Roshan A, Murai K, Fowler J, Simons BD, Nikolaidou-Neokosmidou V, Jones PH. Human keratinocytes have two interconvertible modes of proliferation. Nature cell biology. 2016;18(2):145–56. doi: 10.1038/ncb3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doupe DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell. 2010;18(2):317–23.:S1534-5807(10)00015-8. doi: 10.1016/j.devcel.2009.12.016. [pii] [DOI] [PubMed] [Google Scholar]
- 32.Jones KB, Furukawa S, Marangoni P, Ma H, Pinkard H, D'Urso R, et al. Quantitative Clonal Analysis and Single-Cell Transcriptomics Reveal Division Kinetics, Hierarchy, and Fate of Oral Epithelial Progenitor Cells. Cell stem cell. 2019;24(1):183–92.:e8. doi: 10.1016/j.stem.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wabik A, Jones PH. Switching roles: the functional plasticity of adult tissue stem cells. Embo j. 2015;34(9):1164–79. doi: 10.15252/embj.201490386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martincorena I, Raine KM, Gerstung M, Dawson KJ, Haase K, Van Loo P, et al. Universal Patterns of Selection in Cancer and Somatic Tissues. Cell. 2017;171(5):1029–41.:e21. doi: 10.1016/j.cell.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma Y, Miladi M, Dukare S, Boulay K, Caudron-Herger M, Groß M, et al. A pan-cancer analysis of synonymous mutations. Nat Commun. 2019;10(1):2569. doi: 10.1038/s41467-019-10489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kon S, Ishibashi K, Katoh H, Kitamoto S, Shirai T, Tanaka S, et al. Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nature cell biology. 2017;19(5):530–41. doi: 10.1038/ncb3509. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki A, Nagatake T, Egami R, Gu G, Takigawa I, Ikeda W, et al. Obesity Suppresses Cell-Competition-Mediated Apical Elimination of RasV12-Transformed Cells from Epithelial Tissues. Cell reports. 2018;23(4):974–82. doi: 10.1016/j.celrep.2018.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe H, Ishibashi K, Mano H, Kitamoto S, Sato N, Hoshiba K, et al. Mutant p53-Expressing Cells Undergo Necroptosis via Cell Competition with the Neighboring Normal Epithelial Cells. Cell reports. 2018;23(13):3721–9. doi: 10.1016/j.celrep.2018.05.081. [DOI] [PubMed] [Google Scholar]
- 39.Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science (New York, NY) 2013;342(6161):995–8. doi: 10.1126/science.1243148. [pii] 342/6161/995. [DOI] [PubMed] [Google Scholar]
- 40.Murai K, Skrupskelyte G, Piedrafita G, Hall M, Kostiou V, Ong SH, et al. Epidermal Tissue Adapts to Restrain Progenitors Carrying Clonal p53 Mutations. Cell stem cell. 2018;23(5):687–99.:e8. doi: 10.1016/j.stem.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flanagan DJ, Pentinmikko N, Luopajärvi K, Willis NJ, Gilroy K, Raven AP, et al. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature. 2021;594(7863):430–5. doi: 10.1038/s41586-021-03525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Neerven SM, de Groot NE, Nijman LE, Scicluna BP, van Driel MS, Lecca MC, et al. Apc-mutant cells act as supercompetitors in intestinal tumour initiation. Nature. 2021;594(7863):436–41. doi: 10.1038/s41586-021-03558-4. [DOI] [PubMed] [Google Scholar]
- 43.Bruens L, Ellenbroek SIJ, Suijkerbuijk SJE, Azkanaz M, Hale AJ, Toonen P, et al. Calorie Restriction Increases the Number of Competing Stem Cells and Decreases Mutation Retention in the Intestine. Cell reports. 2020;32(3):107937. doi: 10.1016/j.celrep.2020.107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abby E, Dentro SC, Hall MWJ, Fowler JC, Ong SH, Sood R, et al. <em>Notch1</em> mutation drives clonal expansion in normal esophageal epithelium but impairs tumor growth. bioRxiv. 2021:2021.06.18.448956. doi: 10.1101/2021.06.18.448956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alcolea MP, Greulich P, Wabik A, Frede J, Simons BD, Jones PH. Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nature cell biology. 2014;16(6):615–22. doi: 10.1038/ncb2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcolea MP, Jones PH. Cell competition: Winning out by losing notch. Cell Cycle. 2015;14(1):9–17. doi: 10.4161/15384101.2014.988027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 48.Sugimoto S, Ohta Y, Fujii M, Matano M, Shimokawa M, Nanki K, et al. Reconstruction of the Human Colon Epithelium In Vivo. Cell stem cell. 2018;22(2):171–6.:e5. doi: 10.1016/j.stem.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, et al. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell stem cell. 2013;13(5):626–33. doi: 10.1016/j.stem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson AM, Olpe C, Hoyle A, Thorsen AS, Rus T, Colombe M, et al. Fixation and Spread of Somatic Mutations in Adult Human Colonic Epithelium. Cell stem cell. 2018;22(6):909–18.:e8. doi: 10.1016/j.stem.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stamp C, Zupanic A, Sachdeva A, Stoll EA, Shanley DP, Mathers JC, et al. Predominant Asymmetrical Stem Cell Fate Outcome Limits the Rate of Niche Succession in Human Colonic Crypts. EBioMedicine. 2018;31:166–73. doi: 10.1016/j.ebiom.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578(7793):94–101. doi: 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans EJ, Jr, DeGregori J. Cells with Cancer-associated Mutations Overtake Our Tissues as We Age. Aging Cancer. 2021;2(3):82–97. doi: 10.1002/aac2.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greaves LC, Preston SL, Tadrous PJ, Taylor RW, Barron MJ, Oukrif D, et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:714–9. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 2014;15(1):62–9. doi: 10.1002/embr.201337799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yum MK, Han S, Fink J, Wu SS, Dabrowska C, Trendafilova T, et al. Tracing oncogene-driven remodelling of the intestinal stem cell niche. Nature. 2021;594(7863):442–7. doi: 10.1038/s41586-021-03605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olafsson S, McIntyre RE, Coorens T, Butler T, Jung H, Robinson PS, et al. Somatic Evolution in Non-neoplastic IBD-Affected Colon. Cell. 2020;182(3):672–84.:e11. doi: 10.1016/j.cell.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nanki K, Fujii M, Shimokawa M, Matano M, Nishikori S, Date S, et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature. 2020;577(7789):254–9. doi: 10.1038/s41586-019-1844-5. [DOI] [PubMed] [Google Scholar]
- 59.Kakiuchi N, Yoshida K, Uchino M, Kihara T, Akaki K, Inoue Y, et al. Frequent mutations that converge on the NFKBIZ pathway in ulcerative colitis. Nature. 2020;577(7789):260–5. doi: 10.1038/s41586-019-1856-1. [DOI] [PubMed] [Google Scholar]
- 60.Galandiuk S, Rodriguez–Justo M, Jeffery R, Nicholson AM, Cheng Y, Oukrif D, et al. Field Cancerization in the Intestinal Epithelium of Patients With Crohn's Ileocolitis. Gastroenterology. 2012;142(4):855–64.:e8. doi: 10.1053/j.gastro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaitu'u-Lino TJ, Ye L, Gargett CE. Reepithelialization of the uterine surface arises from endometrial glands: evidence from a functional mouse model of breakdown and repair. Endocrinology. 2010;151(7):3386–95. doi: 10.1210/en.2009-1334. [DOI] [PubMed] [Google Scholar]
- 62.Salamonsen LA, Hutchison JC, Gargett CE. Cyclical endometrial repair and regeneration. Development. 2021;148(17) doi: 10.1242/dev.199577. [DOI] [PubMed] [Google Scholar]
- 63.Jin S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc Natl Acad Sci U S A. 2019;116(14):6848–57. doi: 10.1073/pnas.1814597116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tempest N, Jansen M, Baker AM, Hill CJ, Hale M, Magee D, et al. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J Pathol. 2020;251(4):440–51. doi: 10.1002/path.5478. [DOI] [PubMed] [Google Scholar]
- 65.Moore L, Leongamornlert D, Coorens THH, Sanders MA, Ellis P, Dentro SC, et al. The mutational landscape of normal human endometrial epithelium. Nature. 2020;580(7805):640–6. doi: 10.1038/s41586-020-2214-z. [DOI] [PubMed] [Google Scholar]
- 66.Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell reports. 2018;24(7):1777–89. doi: 10.1016/j.celrep.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 67.Lac V, Nazeran TM, Tessier-Cloutier B, Aguirre-Hernandez R, Albert A, Lum A, et al. Oncogenic mutations in histologically normal endometrium: the new normal? J Pathol. 2019;249(2):173–81. doi: 10.1002/path.5314. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi M, Nakaoka H, Suda K, Yoshihara K, Ishiguro T, Yachida N, et al. Spatiotemporal dynamics of clonal selection and diversification in normal endometrial epithelium. Nat Commun. 2022;13(1):943. doi: 10.1038/s41467-022-28568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noë M, Horlings HM, et al. Cancer-Associated Mutations in Endometriosis without Cancer. New England Journal of Medicine. 2017;376(19):1835–48. doi: 10.1056/NEJMoa1614814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jonason AS, Kunala S, Price GJ, Restifo RJ, Spinelli HM, Persing JA, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci U S A. 1996;93(24):14025–9. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volkova NV, Meier B, González-Huici V, Bertolini S, Gonzalez S, Vöhringer H, et al. Mutational signatures are jointly shaped by DNA damage and repair. Nat Commun. 2020;11(1):2169. doi: 10.1038/s41467-020-15912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein AM, Brash DE, Jones PH, Simons BD. Stochastic fate of p53-mutant epidermal progenitor cells is tilted toward proliferation by UV B during preneoplasia. Proc Natl Acad Sci U S A. 2010;107(1):270–5.:0909738107. doi: 10.1073/pnas.0909738107. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang W, Remenyik E, Zelterman D, Brash DE, Wikonkal NM. Escaping the stem cell compartment: sustained UVB exposure allows p53-mutant keratinocytes to colonize adjacent epidermal proliferating units without incurring additional mutations. Proc Natl Acad Sci U S A. 2001;98(24):13948–53. doi: 10.1073/pnas.241353198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barbera M, di Pietro M, Walker E, Brierley C, MacRae S, Simons BD, et al. The human squamous oesophagus has widespread capacity for clonal expansion from cells at diverse stages of differentiation. Gut. 2015;64(1):11–9. doi: 10.1136/gutjnl-2013-306171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan Q, Nicholson AM, Barr H, Harrison LA, Wilson GD, Burkert J, et al. Identification of lineage-uncommitted, long-lived, label-retaining cells in healthy human esophagus and stomach, and in metaplastic esophagus. Gastroenterology. 2013;144(4):761–70.:S0016-5085(12)01848-3. doi: 10.1053/j.gastro.2012.12.022. [pii] [DOI] [PubMed] [Google Scholar]
- 76.Madissoon E, Wilbrey-Clark A, Miragaia RJ, Saeb-Parsy K, Mahbubani KT, Georgakopoulos N, et al. scRNA-seq assessment of the human lung, spleen, and esophagus tissue stability after cold preservation. Genome Biology. 2019;21(1):1. doi: 10.1186/s13059-019-1906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowicki-Osuch K, Zhuang L, Jammula S, Bleaney CW, Mahbubani KT, Devonshire G, et al. Molecular phenotyping reveals the identity of Barrett’s esophagus and its malignant transition. Science (New York, NY) 2021;373(6556):760–7. doi: 10.1126/science.abd1449. [DOI] [PubMed] [Google Scholar]
- 78.Piedrafita G, Kostiou V, Wabik A, Colom B, Fernandez-Antoran D, Herms A, et al. A single-progenitor model as the unifying paradigm of epidermal and esophageal epithelial maintenance in mice. Nat Commun. 2020;11(1):1429. doi: 10.1038/s41467-020-15258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colom B, Alcolea MP, Piedrafita G, Hall MW, Wabik A, Dentro S, et al. Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium. Nature Genetics. 2020;52:604–14. doi: 10.1038/s41588-020-0624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colom B, Herms A, Hall MWJ, Dentro SC, King C, Sood RK, et al. Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature. 2021;598(7881):510–4. doi: 10.1038/s41586-021-03965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Consortium T. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169–75. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hall MWJ, Jones PH, Hall BA. Relating evolutionary selection and mutant clonal dynamics in normal epithelia. Journal of the Royal Society Interface. 2019;16(156):20190230. doi: 10.1101/480756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moody S, Senkin S, Islam SMA, Wang J, Nasrollahzadeh D, Cortez Cardoso Penha R, et al. Mutational signatures in esophageal squamous cell carcinoma from eight countries with varying incidence. Nat Genet. 2021;53(11):1553–63. doi: 10.1038/s41588-021-00928-6. [DOI] [PubMed] [Google Scholar]
- 84.Fernandez-Antoran D, Piedrafita G, Murai K, Ong SH, Herms A, Frezza C, et al. Outcompeting p53-Mutant Cells in the Normal Esophagus by Redox Manipulation. Cell stem cell. 2019;25(3):329–41. doi: 10.1016/j.stem.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dalghi MG, Montalbetti N, Carattino MD, Apodaca G. The Urothelium: Life in a Liquid Environment. Physiological Reviews. 2020;100(4):1621–705. doi: 10.1152/physrev.00041.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Limas C, Bigler A, Bair R, Bernhart P, Reddy P. Proliferative activity of urothelial neoplasms: comparison of BrdU incorporation, Ki67 expression, and nucleolar organiser regions. Journal of Clinical Pathology. 1993;46(2):159–65. doi: 10.1136/jcp.46.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell. 2013;26(5):469–82. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A. KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun. 2016;7:11914. doi: 10.1038/ncomms11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–4. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaisa NT, Graham TA, McDonald SA, Cañadillas-Lopez S, Poulsom R, Heidenreich A, et al. The human urothelium consists of multiple clonal units, each maintained by a stem cell. J Pathol. 2011;225(2):163–71. doi: 10.1002/path.2945. [DOI] [PubMed] [Google Scholar]
- 91.Lawson ARJ, Abascal F, Coorens THH, Hooks Y, O'Neill L, Latimer C, et al. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science (New York, NY) 2020;370(6512):75–82. doi: 10.1126/science.aba8347. [DOI] [PubMed] [Google Scholar]
- 92.Li R, Du Y, Chen Z, Xu D, Lin T, Jin S, et al. Macroscopic somatic clonal expansion in morphologically normal human urothelium. Science (New York, NY) 2020;370(6512):82–9. doi: 10.1126/science.aba7300. [DOI] [PubMed] [Google Scholar]
- 93.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171(3):540–56.:e25. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ng AWT, Poon SL, Huang MN, Lim JQ, Boot A, Yu W, et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Sci Transl Med. 2017;9(412) doi: 10.1126/scitranslmed.aan6446. [DOI] [PubMed] [Google Scholar]
- 95.Poon SL, Pang ST, McPherson JR, Yu W, Huang KK, Guan P, et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Sci Transl Med. 2013;5(197):197ra01. doi: 10.1126/scitranslmed.3006086. [DOI] [PubMed] [Google Scholar]
- 96.Teixeira VH, Nadarajan P, Graham TA, Pipinikas CP, Brown JM, Falzon M, et al. Stochastic homeostasis in human airway epithelium is achieved by neutral competition of basal cell progenitors. Elife. 2013;2:e00966. doi: 10.7554/eLife.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48(6):607–16. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine. 2013;368(4):341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 99.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien C-B, Morcos PA, et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484(7395):546–9. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543(7643):118–21. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holt JR, Zeng W-Z, Evans EL, Woo S-H, Ma S, Abuwarda H, et al. Spatiotemporal dynamics of PIEZO1 localization controls keratinocyte migration during wound healing. Elife. 2021;10:e65415. doi: 10.7554/eLife.65415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas M, Ladoux B, Toyama Y. Desmosomal Junctions Govern Tissue Integrity and Actomyosin Contractility in Apoptotic Cell Extrusion. Current Biology. 2020;30(4):682–90.:e5. doi: 10.1016/j.cub.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Takeuchi Y, Narumi R, Akiyama R, Vitiello E, Shirai T, Tanimura N, et al. Calcium Wave Promotes Cell Extrusion. Current Biology. 2020;30(4):670–81.:e6. doi: 10.1016/j.cub.2019.11.089. [DOI] [PubMed] [Google Scholar]
- 104.Aikin TJ, Peterson AF, Pokrass MJ, Clark HR, Regot S. MAPK activity dynamics regulate non-cell autonomous effects of oncogene expression. Elife. 2020;9:e60541. doi: 10.7554/eLife.60541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riva L, Pandiri AR, Li YR, Droop A, Hewinson J, Quail MA, et al. The mutational signature profile of known and suspected human carcinogens in mice. Nat Genet. 2020;52(11):1189–97. doi: 10.1038/s41588-020-0692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robinson PS, Coorens THH, Palles C, Mitchell E, Abascal F, Olafsson S, et al. Increased somatic mutation burdens in normal human cells due to defective DNA polymerases. Nat Genet. 2021;53(10):1434–42. doi: 10.1038/s41588-021-00930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nam AS, Chaligne R, Landau DA. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nature Reviews Genetics. 2021;22(1):3–18. doi: 10.1038/s41576-020-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zahir N, Sun R, Gallahan D, Gatenby RA, Curtis C. Characterizing the ecological and evolutionary dynamics of cancer. Nature Genetics. 2020;52(8):759–67. doi: 10.1038/s41588-020-0668-4. [DOI] [PubMed] [Google Scholar]
- 109.Herms A, Colom B, Piedrafita G, Murai K, Ong SH, Fernandez-Antoran D, et al. Levelling out differences in aerobic glycolysis neutralizes the competitive advantage of oncogenic <em>PIK3CA</em> mutant progenitors in the esophagus. bioRxiv. 2021:2021.05.28.446104. doi: 10.1101/2021.05.28.446104. [DOI] [Google Scholar]