Abstract
Oenocytes have intrigued insect physiologists since the nineteenth century. Many years of careful but mostly descriptive research on these cells high-lights their diverse sizes, numbers, and anatomical distributions across In-secta. Contemporary molecular genetic studies in Drosophila melanogaster and Tribolium castaneum support the hypothesis that oenocytes are of ectodermal origin. They also suggest that, in both short and long germ-band species, oenocytes are induced from a Spalt major/Engrailed ectodermal zone by MAPK signaling. Recent glimpses into some of the physiological functions of oenocytes indicate that they involve fatty acid and hydrocarbon metabolism. Genetic studies in D. melanogaster have shown that larval oenocytes synthesize very-long-chain fatty acids required for tracheal water-proofing and that adult oenocytes produce cuticular hydrocarbons required for desiccation resistance and pheromonal communication. Exciting areas of future research include the evolution of oenocytes and their cross talk with other tissues involved in lipid metabolism such as the fat body.
Keywords: oenocytes, fat body, lipid metabolism, very-long-chain fatty acids, pheromones, cuticular hydrocarbons
Introduction
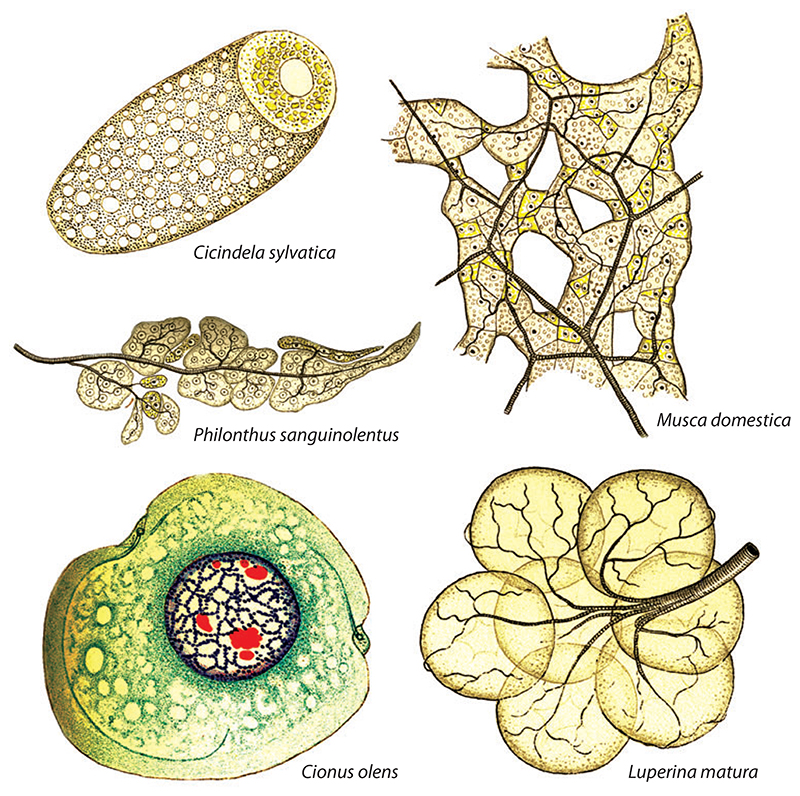
Oenocytes are secretory cells that are found in most, if not all, pterygote insects. These mysterious cells were documented as early as 1856 and have been termed unicellular glands, intermingled cells (eingesprengte Zellen), and respiration cells (Respirationzellen) (32, 42, 69). The currently accepted term, oenocytes (originally oenocythen), was coined in 1886 to highlight the wine yellow (weingelb) color of these cells in Chironomus midges (132). Even though oenocytes are yellow in some insect species, in others they can be red, green, or almost colorless (49) (Figure 1). Numerous analyses at both the light and electron microscope levels indicate that the morphology of oenocytes is characteristic of a specialized secretory cell (64, 76, 103, 106, 133, 135).
Figure 1.
The diversity of oenocytes across Insecta. Hollande’s beautiful drawings show an oenocyte associated with a larger fat body cell from a wood tiger beetle imago (Cicindela sylvatica) multiple oenocytes intermingled with fat body cells and trachea in the house fly imago (Musca domestica) and in a species of rove beetle (Philonthus sanguinolentus), a single larval oenocyte showing penetration by a tracheole in a weevil (Cionus olens), and a larval oenocyte cluster with associated tracheal network from a moth caterpillar (Luperina matura). Adapted from Reference 49.
Oenocyte.
an insect cell type of ectodermal developmental origin that utilizes hemolymph lipophorin; functions include the synthesis of VLCFAs and/or hydrocarbons
The size, number, and anatomical locations of oenocytes all vary widely between insect species (reviewed in 41, 80, 118). Although a systematic phylogenetic analysis of oenocyte features in arthropods is currently lacking, partial coverage indicates that there are dramatic differences across Insecta. Oenocytes have also been reported in arachnids and crustaceans (110, 122), but it is not yet known whether these are homologous to insect oenocytes and so are not discussed further. Although oenocytes in many insect species tend to be large polyploid cells organized into discrete clusters restricted to the abdominal segments, there are many exceptions. For example, oenocytes are present in the abdomen and thorax of some parasitic wasps (Torymus nigricornis) and aquatic Hemiptera (95, 128). The location of oenocytes within a segment also varies considerably between species. In some species they remain contiguous with the epidermis but project into the body cavity, in Blatta cockroaches they are sandwiched between the epidermis and the basement membrane, in tachinid and Drosophila fly larvae they lie beneath the epidermis in conspicuous cell clusters, whereas in Chrysomela beetles they are scattered throughout the fat body (10, 89, 129, 134). Oenocytes also come in a wide range of different sizes. Larval oenocytes tend to be very large cells with diameters typically 60–100 μm, but they can reach an impressive 150 μm in Cynipidae gall wasps (111). Adult oenocytes tend to be smaller but more numerous than their larval namesakes; for example, they are ∼15 μm in diameter in Culex mosquitoes (50). In those species that have discrete segmental clusters of larval oenocytes, there are also striking differences in the number of cells per cluster. Those of D. melanogaster contain an average of 6 cells, those of the parasitic wasp Phaenoserphus viator contain more than 20 cells, whereas those of the leopard moth, Zeuzera pyrina, can contain up to 50 cells per cluster (10, 30, 40).
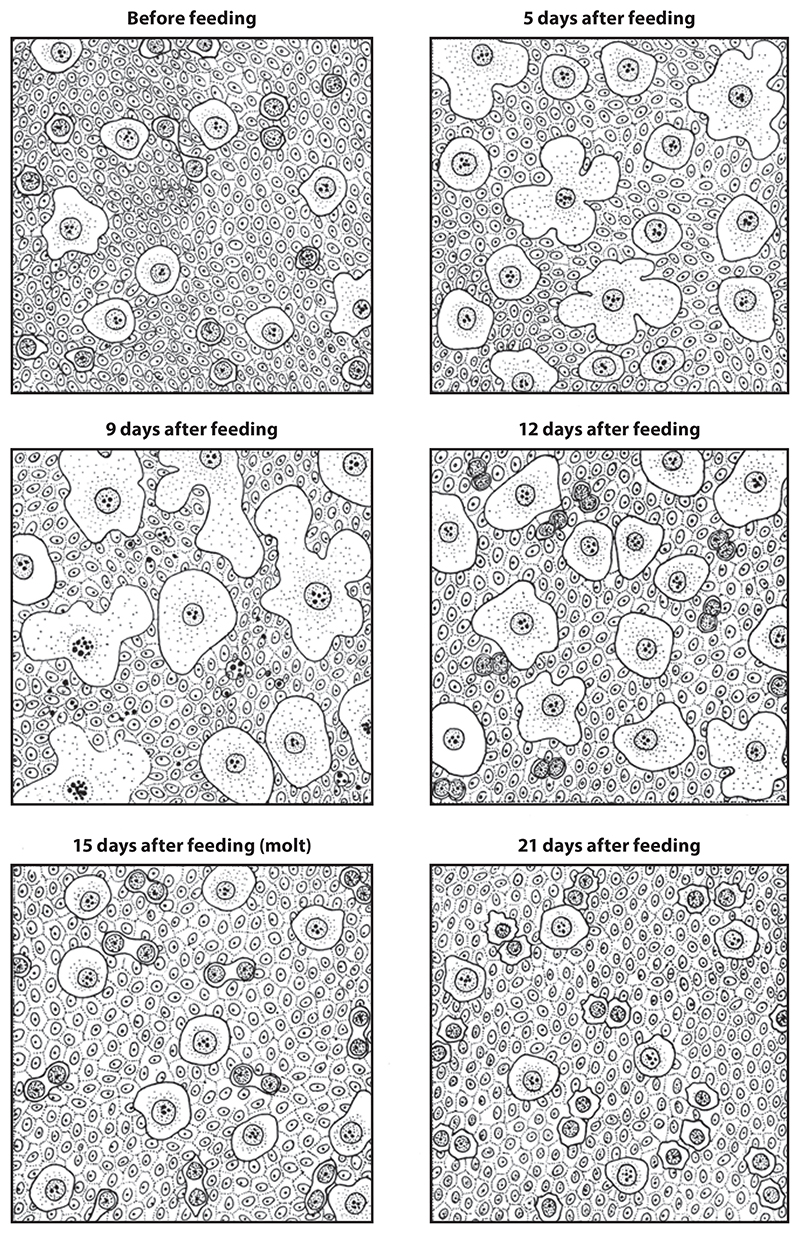
In addition to evolutionary variation, the size, number, and distribution of oenocytes can also change dramatically within a given species during development. For example, a new generation of oenocytes may develop at each molt, as described by Wigglesworth in the heteropteran Rhodnius prolixus (133) (Figure 2). Differences also exist as a function of polyphenism such that, in larvae of Apis mellifera, the average oenocyte cell diameter reaches 80 μm in the worker, 100 μm in the drone, and 110 μm in the queen (115). Importantly, in dipterans such as D. melanogaster, there are two separate larval and adult (imaginal) generations of oenocytes, each of which is distinct with respect to cell size and number (10, 62, 66, 126) (Figure 3) and, as discussed below, perhaps also in terms of functions. For both oenocyte generations, cell size increases after formation, but this is much more dramatic during the larval growth phase than during adult aging (10, 56). Genetic studies in D. melanogaster demonstrate that the progenitors of larval and adult oenocytes are different (31, 70) and that larval oenocytes fragment and disappear long before adult eclosion, presumably via apoptosis (44). The separation between larval and adult oenocyte progenitors is less clear in other holometabolous insects, with early descriptions in Formica rufa, Lasius flavus, and Galerucella luteola suggesting that larval oenocytes are the progenitors of adult oenocytes in some hymenopterans and coleopterans (58, 91, 97).
Figure 2.
Oenocytes during the molting cycle in Rhodnius prolixus. Wigglesworth’s drawings show that, prior to feeding, oenocytes are present as large single cells combined with smaller doublets of cells interconnected by a cytoplasmic strand. Feeding stimulates oenocyte growth, but average cell size has reduced somewhat by the time of molting at 15 days. After 21 days, oenocyte size does not change markedly until another meal initiates a new molting cycle. Adapted from Reference 133.
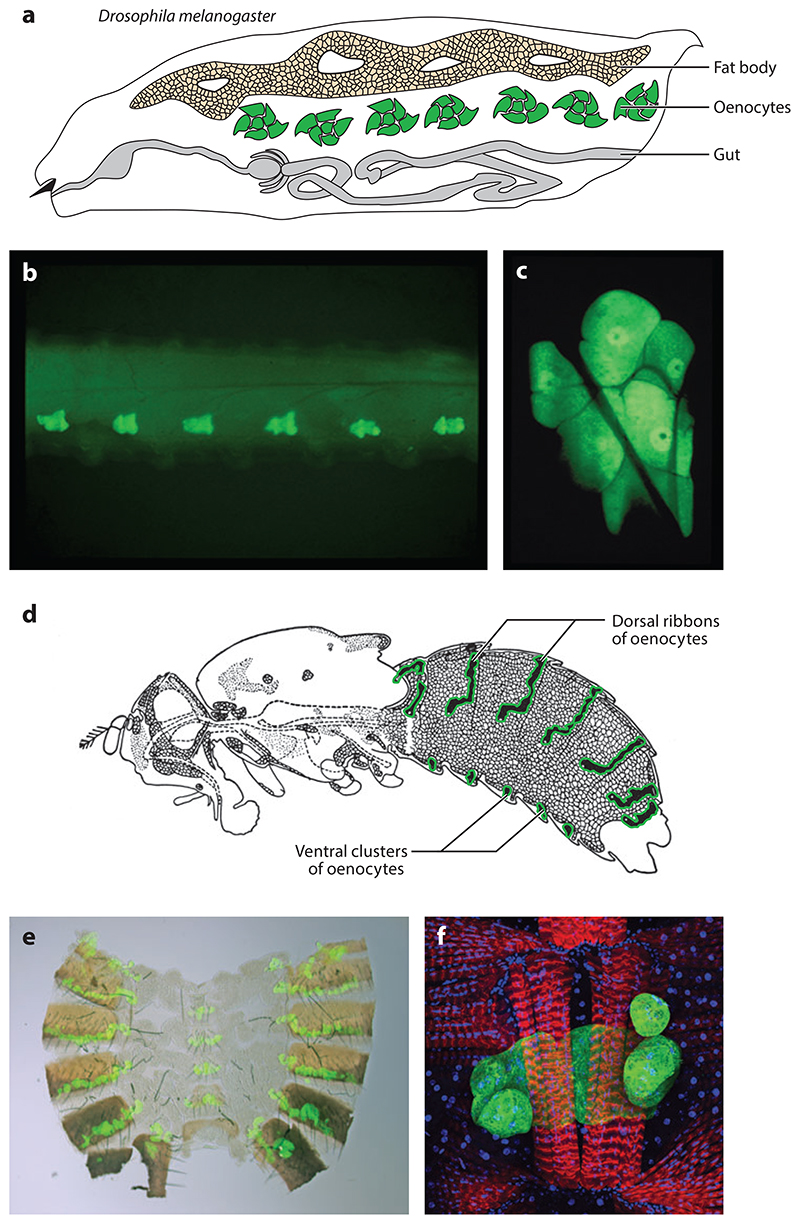
Figure 3. Larval and adult oenocytes in Drosophila melanogaster.
(a) Drawing of a larva showing oenocytes, the fat body, and the gut. (b) Lateral view of a larva showing oenocyte clusters (green) in A1-A6 (A7 is not shown). (c) Single confocal section of one larval oenocyte cluster of six cells. The lateral chordotonal organ and tracheoles are visible as shadows crossing the surface of the oenocytes. (d) Drawing of an adult fly showing the abdominal dorsal ribbons and ventral clusters of oenocytes (green). (e) A filleted adult abdomen showing the segmental dorsal stripes and ventral clusters of oenocytes (green).(f) Confocal image of a ventral adult oenocyte cluster (green) associated with muscles (red) with nuclei also shown (blue). Adapted with permission from Reference 44, and adapted from 84.
Fat body.
the major lipid storage organ of insects, regulating the balance between lipogenesis and lipolysis in a dietary nutrient-dependent manner
Holometabolous insects.
members of the Endopterygota superorder that undergo complete metamorphosis, developing via four stages: embryo, larva, pupa, and adult (imago)
Oenocyte Formation By Induction From The Ectoderm
Hints as to the developmental origin of oenocytes were provided by observations that they develop in close proximity to known ectodermal derivatives such as spiracles (43, 65, 111, 129). However, a molecular genetic mechanism for their specification from the ectoderm is currently available only in Drosophila. For the oenocytes of the imago, it was originally suggested that they share a common developmental precursor with the epidermis and fat body (62). Subsequent cell lineage analyses showed that adult oenocytes and abdominal epidermis arise from a common pool of histoblasts but that the adult fat body has a separate mesodermal origin (70, 71). Surprisingly for such an intensively studied model organism, the underlying mechanism by which adult Drosophila oenocytes are specified from histoblasts is still unclear.
COP.
chordotonal organ precursor
EGFR.
epidermal growth factor receptor
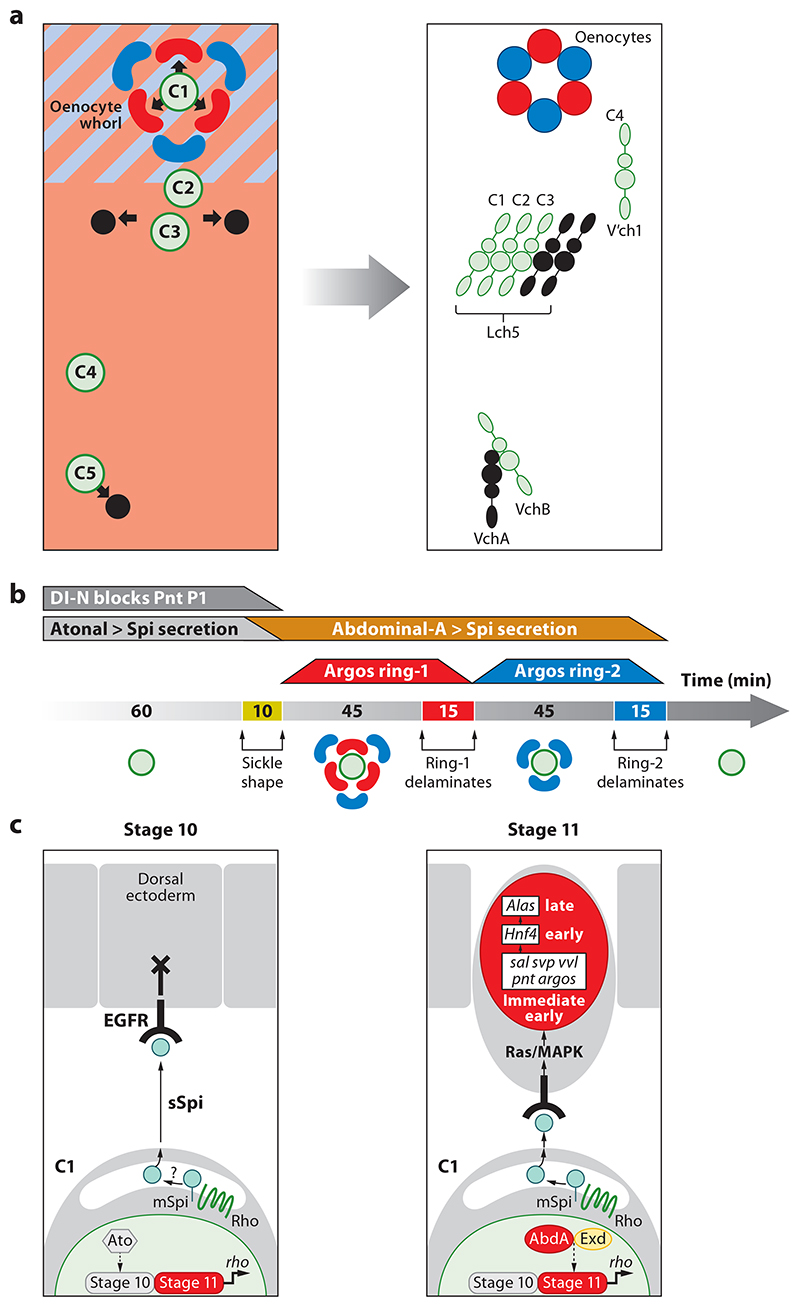
In contrast to the adult oenocytes of Drosophila, much more is known about the formation of their larval counterparts. The observation that larval oenocytes, as well as neural precursors, are overproduced in Notch mutant embryos suggested that they may originate from the ectoderm (47). Subsequently, a molecular mechanism for oenocyte induction from the ectoderm during the extended germ-band stages of embryogenesis was identified (reviewed in 41). Central to this process is Spitz secretion by one of the five primary chordotonal organ precursors (COPs) that locally activates the epidermal growth factor receptor (EGFR) in the overlying dorsal ectoderm, thus inducing ∼6 oenocyte precursors (31, 113). Three or four out of the five primary COPs are thought to secrete Spitz. However, only the most dorsal COP (C1) induces oenocytes, whereas the other two or three (C3, C5, and possibly C2) induce the alternative fate of secondary COPs (31, 86, 141). These findings, together with chordotonal organ lineage studies (12, 13), provide evidence for an embryonic fate map for the complete set of all 14 oenocytes and chordotonal organs per abdominal hemisegment (Figure 4a).
Figure 4. Oenocyte induction in Drosophila melanogaster.
(a) Fate map for oenocytes and chordotonal organs. Panels represent a single abdominal hemisegment at (left) early (stage 11) and (right) late (stage 16) embryogenesis. At stage 11, a whorl of approximately six sickle-shaped oenocyte precursors (red and blue) is induced by the most dorsal primary COP, C1 (green) from dorsoposterior ectoderm expressing Spalt major (light blue) and Engrailed (pink). More ventrally positioned primary COPs lying outside the Spalt domain (C3, C5, and possibly C2) induce secondary COPs (black) giving rise to chordotonal organs. By stage 16, all oenocytes and chordotonal organs have delaminated beneath the ectoderm and differentiated within their characteristic clusters. Adapted with permission from References 14, 15, and 41. (b) Embryonic timeline (minutes) showing two three-by-three pulses of oenocyte delamination during stage 11. Above the line, trapezoids indicate the duration of Atonal-dependent Spi secretion from C1 (gray), inhibition by Notch (dark gray), and AbdA-dependent secretion from C1 (orange) as well as the strong EGFR response in oenocyte precursors marked by Argos expression in ring-1 (red) and ring-2 (blue). (c) Model for oenocyte specification by AbdA. A primary COP (lower cell) signaling to the dorsal ectoderm via rbo-dependent Spi secretion is depicted. During stage 10, the EGFR response is blocked by Notch signaling and probably other factors. During stage 11, AbdA and Exd maintain rbo transcription, thus converting membrane-bound (mSpi) to secreted Spitz (sSpi), activating the EGFR and thus triggering immediate-early, early, and late gene expression in differentiating oenocytes. Abbreviations: AbdA, Abdominal-A; COP, chordotonal organ precursor; EGFR, epidermal growth factor receptor.
Patterning of the embryonic ectoderm prior to induction accounts for why Spitz induces oenocytes at dorsal locations but secondary COPs more ventrally. In each abdominal segment, a dorsoposterior zone of competence for oenocyte induction is defined by the intersection of dorsal Spalt and posterior Engrailed expression (14, 15, 31, 113). Two key experimental findings here are that, in spalt major (salm) mutants, C1 induces secondary COPs rather than oenocytes (31, 113) and that excessive Spitz secretion can convert most or all cells of the Salm/Engrailed zone from an epidermal fate into supernumerary oenocytes (15). During normal embryogenesis, oenocyte precursors are first distinguishable as a characteristic whorl of approximately six sickle-shaped cells overlying C1. The whorl expresses Salm more strongly than the surrounding dorsal ectoderm and consists of two concentric rings of approximately three cells (31, 113). The inner ring, closest to the C1 Spitz source, most strongly expresses activated mitogen-activated protein kinase (MAPK) and high-threshold EGFR targets such as seven-up and argos (15). Once it has delaminated, the oenocyte precursors of the outer ring are brought closer to C1, upregulate high-threshold EGFR targets, and then also delaminate. This three-by-three mechanism of oenocyte delamination requires EGFR negative feedback, mediated by secreted Argos, and usually occurs over only two pulses (Figure 4b). However, artificially prolonging Spitz secretion can sustain the pulsatile mechanism for up to eight cycles of delamination (15)
AbdA.
Abdominal-A
HNF4.
hepatocyte nuclear factor 4
Lipophorin.
extracellular lipoprotein transport particles present in the insect hemolymph, containing the shuttle protein apo-Lipophorin complexed with diglycerides and other lipids
The Hox/homeotic gene abdominal-A (abd-A) is required for the induction of Drosophila larval oenocytes (14, 41). The restricted anteroposterior pattern of AbdA expression in the ectoderm of A1-A7 thus accounts for why larval oenocytes are formed only in abdominal segments. AbdA acts in the C1 precursor to maintain Spitz activity until the developmental stage at which a Notch-dependent block to EGFR signaling is lifted from the dorsal ectoderm (14) (Figure 4b,c). Spitz activity is initiated in C1, under the control of the proneural factor Atonal, in both thoracic and abdominal segments, but this is not maintained sufficiently long enough to induce productive EGFR signaling unless AbdA is also expressed (14, 45, 138). Hox mutant rescue assays argue that the only principal target for the AbdA transcription factor during oenocyte induction is the rhomboid gene (14), encoding a protease that is rate limiting for processing Spitz into an active secreted form (72, 124). AbdA binds directly to a C1-specific cis-regulatory module of rhomboid as part of a transcriptional activation complex with the cofactors Homothorax, Extradenticle, and Pax2 (74, 75, 138).
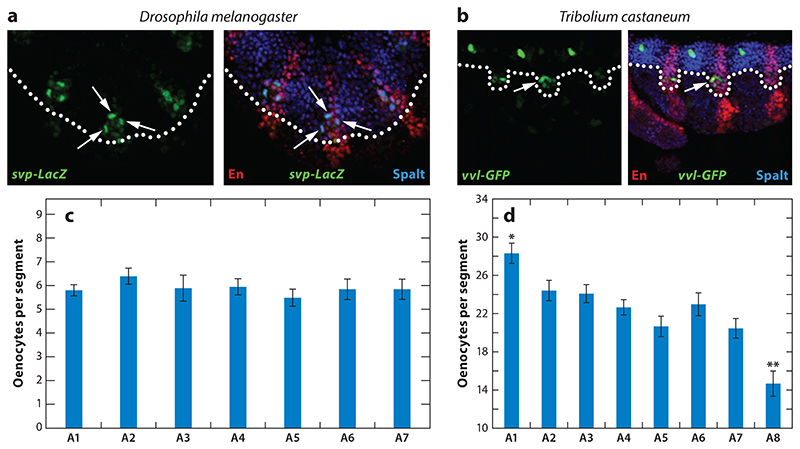
Outside Diptera, at least some key elements of the Drosophila oenocyte induction program are conserved, as illustrated by a recent oenocyte study in the red flour beetle, Tribolium castaneum (16). T. castaneum differs from D. melanogaster in that it develops via the short not the long germ-band mode, growing abdominal segments sequentially rather than by partitioning a nongrowing blastoderm (112). Despite this difference, the segments expressing abdominal Hox proteins also bear oenocytes in T. castaneum, although there are eight rather than seven of these. Moreover, Tribolium oenocyte precursors also develop from dorsoposterior embryonic ectoderm, at the intersection of the ventral edge of the dorsal Spalt domain with the posterior segmental stripe of Engrailed (Figure 5a,b). Although the mechanism of oenocyte formation in T. castaneum has yet to be dissected by genetic analysis, there are hints that it may involve receptor tyrosine kinase-mediated induction similar to that in D. melanogaster. Thus, early oenocyte precursors express activated MAPK in both species, and in D. melanogaster, it is known to be triggered by EGFR (15, 16, 31). Dipteran-coleopteran parallels also extend to the maturation of oenocytes from their precursors, a process that occurs without any cell division and involves the expression of ventral veins lacking and hepatocyte nuclear factor 4 (hnf4) in both orders (16, 41, 44). One clear species difference worthy of further study is oenocyte number. Oenocyte clusters contain an average of 22 cells in T. castaneum (16), compared with only ∼6 in D. melanogaster (15, 31). Perhaps owing to the sequential nature of abdominal segment formation in T. castaneum, there is also a marked anterior-to-posterior decrease in the number of oenocytes per cluster (16) (Figure 5c,d). It will be interesting to explore whether the species-to-species and segment-to-segment variations in cell number are accounted for by different numbers of cycles of oenocyte induction/delamination.
Figure 5. Oenocytes derive from Spalt/Engrailed-expressing ectoderm.
In both (a) Drosophila melanogaster and (b) Tribolium castaneum embryos, oenocyte precursors (labeled with svp-lacZ or vvl-GFP) (arrows) arise within ectoderm expressing both Engrailed (En) (marks posterior compartment) and Spalt (marks dorsal territory above dotted line). (c) The average number of cells per oenocyte cluster in Drosophila embryos is ∼6 in A1-A7. (d) This number is dramatically increased in Tribolium embryos, with a transient graded distribution from ∼26 cells in A1 to ∼14 cells in A8. Asterisks indicate significant (p < 0.05) differences in the number of oenocytes in A1 (*) and A8 (**) compared to all other segments. Adapted with permission from Reference 16.
Regulation and Functions of Oenocytes
Since the discovery of oenocytes more than 150 years ago (32), their functions have remained mysterious. Systemic roles are suggested by the extensive infoldings of the oenocyte plasma membrane, known as the plasma reticular system, which present a large surface area to the hemolymph (52, 77, 82). Elegant but largely descriptive early studies implicated them in a bewildering variety of processes, including larval growth and nutrition (1, 120), the histolysis of larval tissues (1), oxygen uptake/respiration (69, 129), the elimination of toxic waste products (4, 5, 65, 89, 111, 116, 128), the regulation of hemolymph composition (53, 120), cuticle synthesis (133, 135), and the production of ecdysteroids (29, 76, 106, 108). Several of these long-standing proposed roles for oenocytes have yet to be thoroughly tested at the functional level. However, the more recent molecular and genetic evidence reviewed below now provides strong support for a subset of the classic hypotheses as well as for some new ones. A common denominator linking at least some of the apparently diverse bona fide functions of oenocytes is lipid metabolism. Indeed, oenocytes were once referred to as wax-producing cells (cérodécytes), because histological stains and organic extractions suggested that they contain particles of waxes or other lipids (49). Oenocytes also contain abundant smooth endoplasmic reticulum suggestive of a role in the synthesis, processing, and/or secretion of lipids (52, 77). Consistent with this, oenocytes express an extensive battery of lipid-synthesizing and -catabolizing enzymes and other proteins including lipophorin receptors, acetyl-CoA carboxylase (Acc), fatty acid synthase (Fas), fatty acid desaturases, fatty acid elongases, cytochrome P450 monooxygenases, NADPH cytochrome P450 reductase, fatty acid β-oxidation enzymes, and activated sterol-response element binding protein (24, 44, 68, 78, 81, 90, 98). They also strongly express two key conserved transcriptional regulators of lipid metabolism, HNF4 and the chicken ovalbumin upstream promoter transcription factor ortholog Seven Up (Svp) (44, 87).
Acc.
acetyl-CoA carboxylase
Metabolic Regulation of a Fat Body–Oenocyte Axis
The insect fat body carries out many of the nutrient-sensing, fat and glycogen storage, and lipid metabolism functions related to those of the mammalian adipose tissue and liver (reviewed in 2, 17, 26, 61, 123). There is also growing evidence that the fat body is metabolically linked to larval oenocytes in a dietary nutrient-dependent manner. Early observations that insect oenocytes are nutrient responsive came from Apis mellifera and the lepidopterans Aglais io and Aglais urticae, in which lipid-rich “grains” within oenocytes increase in abundance during starvation (49). These grains may correspond to lipid droplets, the major intracellular site for the storage and lipolysis of neutral lipids such as triglycerides and cholesteryl esters (reviewed in 11, 67). Indeed, using the neutral lipid stain Oil Red O, researchers later demonstrated that the larval oenocytes of D. melanogaster are highly unusual larval cells since they specifically accumulate lipid droplets as a normal response to fasting, unlike most other tissues, which tend to lose them (44) (Figure 6a,b). This readout of oenocyte steatosis has also been used to reveal abnormal lipid metabolism in fed Drosophila larvae that are mutant for the energy sensor AMP-activated protein kinase, the glucose sensor Bride of Sevenless, the sugar/fat responsive cytokine Unpaired 2, or HNF4 (which regulates fatty acid mobilization and β-oxidation) (55, 63, 83, 87, 99). Although not well studied, the adult namesakes of larval oenocytes are also likely to be responsive to dietary nutrients. For example, in A. mellifera, amino acid supplementation can increase the expression of Insulin-like peptide 1, one of two insulin-like peptides expressed in adult oenocytes (85).
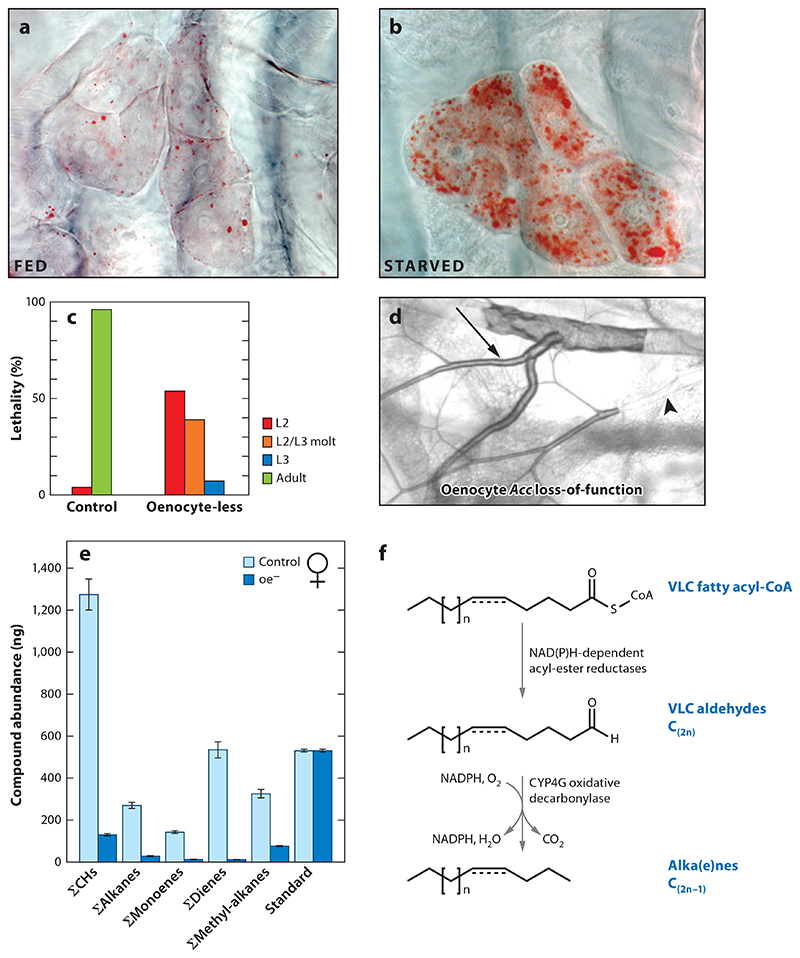
Figure 6. Oenocytes regulate the tracheal system and cuticle in Drosophila melanogaster.
Compared with (a) fed controls, (b) oenocytes of early L3 larvae starved for 14 h show strong accumulation of neutral lipids in droplets detected with Oil Red O staining (red). Adapted with permission from Reference 44. (c) Oenocyte-less larvae show polyphasic lethality during the second (L2) and third (L3) instars, compared with control larvae. Adapted with permission from Reference 44. (d) Larval tracheal branches from an oenocyte-specific acetyl-CoA carboxylase (Acc) loss-of-function mutant at the L3 stage. Arrow and arrowhead point to air- and liquid-filled regions, respectively. Adapted with permission from Reference 90. (e) Quantitation of adult female cuticular hydrocarbon classes in control versus oenocyte-less D. melanogaster. Adapted with permission from Reference 7. (f) Biosynthetic pathway for cuticular hydrocarbons in adult oenocytes. Cyp4g family enzymes from Drosophila (Cyp4g1) and Musca domestica (Cyp4g2) convert very-long-chain (VLC) aldehydes to alkanes and alkenes via oxidative decarbonylation. Adapted with permission from Reference 98.
An important issue in insect integrative physiology is the extent to which different fat-handling cell types regulate each other. One poorly understood aspect of this is whether oenocytes play a significant role in regulating lipid metabolism in fat body cells (also called trophocytes). Cell ablations demonstrate clearly that larval oenocytes are required for maximal mobilization of fat body triglycerides during fasting (44). This effect, however, may be very indirect, mediated by tracheal flooding, which in turn leads to larval hypoxia and thus reduced fatty acid β-oxidation (90). Nevertheless, Drosophila larvae lacking the activity of the oenocyte cytochrome P450, Cyp4g1, do not display tracheal flooding, yet they do show an elevated whole-animal oleic acid/stearic acid ratio (C18:1/C18:0) that is specific to the global triglyceride rather than to the phospholipid pool, thus likely reflecting altered fat body lipid metabolism (44). In addition, marked changes in total triglyceride content are also seen in adult Drosophila flies following oenocyte-specific knockdown of glycerol kinase, a fatty acid elongase, or several other genes with predicted roles in lipid metabolism (96).
Lipid droplet.
intracellular organelle composed of a core of neutral lipids surrounded by a phospholipid monolayer associated with numerous proteins that regulate lipolysis
Lipolysis.
the hydrolysis of triglycerides and related lipids catalyzed by Brummer and other lipases
Triglycerides.
neutral storage lipids formed from three fatty acids esterified to a glycerol backbone
Steatosis.
physiological or pathological overload of neutral lipids within a cell in the form of lipid droplets
There is clearer evidence for the opposite direction of interorgan communication: from fat body to oenocytes. The normal response of larval oenocytes to starvation is strikingly reminiscent of physiological steatosis in mammals, a hallmark of fasting hepatocytes (37, 44). Moreover, the mechanism of lipid accumulation in mammalian hepatocytes during fasting has provided clues for understanding the regulation of oenocyte steatosis. In mammals, the activation of multiple lipases in adipocytes releases fatty acids into the circulation during fasting (reviewed in 21). These are then captured by hepatocytes and metabolized via complex processes such as mitochondrial β-oxidation to provide fuel for other tissues (48). Similarly, during nutrient deprivation in Drosophila larvae, there is a reciprocal relationship between triglyceride loss in the fat body and lipid droplet accumulation in oenocytes (44). Moreover, attenuating fat body lipolysis using tissue-specific manipulations of perilipin-related lipid droplet proteins (Plin1/Lsd1 knockdown or Plin2/Lsd2 overexpression) reduces lipid droplet accumulation in oenocytes during larval fasting (6, 23, 44). Conversely, the mobilization of lipids from the fat body, via tissue-specific overexpression of the adipose triglyceride lipase-related enzyme Brummer, is sufficient to induce oenocyte steatosis in fed larvae (23, 44). Experiments inactivating the amino acid transporter Slimfast or its downstream TOR signaling pathway in the fat body, also under fed conditions, support the idea that the sensing of decreased amino acid levels by fat body cells is sufficient to induce oenocyte steatosis (25, 44).
Lipids are likely to be transported from the fat body to the larval oenocytes by the hemolymph, largely in the form of diacylglycerol-rich lipoprotein particles containing the apolipoprotein B-like molecule apolipophorin (Lpp) (reviewed in 18, 125). Recent work on larval lipophorin transport indicates that the midgut is coupled with the fat body in a bidirectional manner, contributing to the loading of sterols and diacylglycerol onto fat body-synthesized Lpp (88). This suggests that the midgut may also act as an intermediate between the fat body and larval oenocytes. Nevertheless, during nutrient restriction, midgut lipid droplets are exhausted rapidly (44), suggesting that their contribution to oenocyte steatosis in this context is minor. Consistent with the idea that oenocytes can take up lipids from hemolymph Lipophorin, clonal knockdown experiments show that Lipophorin receptor 2 (Lpr2) is required in an oenocyte-autonomous manner for starvation-induced steatosis (90). Clonal analysis also indicates that the enzyme generating the fatty acid precursor malonyl CoA, Acc, is required in oenocytes to suppress inappropriate Lpr2-dependent accumulation of oenocyte lipid droplets in fed larvae (90). Hence, steady-state oenocyte triglyceride levels are not only increased by hemolymph lipophorin uptake, but also reduced by cell-autonomous Acc activity. The mechanism of this Acc-dependent regulation is not yet clear, but a related role for mammalian Fas has been observed in the liver (20).
Lpp.
apolipophorins
Lpr2.
Lipophorin receptor 2
20E.
20-hydroxyecdysone
In summary, the fat body regulates lipid metabolism in larval oenocytes in a nutrient-dependent manner. Some aspects of this regulation are also broadly similar to those of the mammalian adipose-liver axis. In contrast to their hepatocyte-like regulation and gene expression, most of the known physiological functions of larval and adult oenocytes are suggestive of an insect-specific, rather than a conserved hepatocyte-like, role.
Lipid Metabolism Links the Functions of Larval and Adult Oenocytes
Studies of many different insects have demonstrated that oenocyte morphology varies during the molting cycle (19, 27, 76, 104, 106, 119, 133, 139). Moreover, such cyclical oenocyte changes are blocked in a nonmolting strain of the silkworm, Bombyx mori (140). Maximal oenocyte size, smooth endoplasmic reticulum, and lipid content tend to be reached just prior to the time of cuticle deposition (76, 102, 103, 133). This cyclical correlation has prompted many investigations into oenocyte roles in molting: either in the production of the molting hormone, 20-hydroxyecdysone (20E), or in the downstream response to it during cuticle synthesis. The prothoracic gland plays a central endocrine role, triggering each molt by synthesizing bursts of the steroid lipid ecdysone (reviewed in 101). Prothoracic gland cells synthesize ecdysone from its precursor cholesterol, or related sterols, via a pathway involving cytochrome P450 encoding genes of the Halloween family (reviewed in 39). In addition, larval oenocytes in Musca domestica abdominal explants incorporate labeled cholesterol from the culture medium (121). Although larval oenocytes can metabolize cholesterol, it is unlikely that they play a major prothoracic gland-like role in ecdysone synthesis in most insects. Instead, oenocytes may act to metabolize ecdysone downstream of its synthesis in the prothoracic gland (76). Direct support for this comes from Tenebrio molitor organ cocultures, in which oenocytes can convert prothoracic gland-derived ecdysone into the biologically active molting hormone 20E (108, 109). Studies in D. melanogaster and Manduca sexta now clearly indicate that many different peripheral tissues express the P450 20-monooxygenase, Shade, which converts ecdysone into 20E (92, 100). Nevertheless, it remains to be determined whether larval oenocytes also express Shade and/or make a significant in vivo contribution to the overall circulating levels of 20E.
VLCFA.
very-long-chain fatty acid
Hydrocarbons.
lipids composed only of hydrogen and carbon atoms deposited on the epicuticle and functioning as pheromones and barriers to transpiration
A function for larval oenocytes in molting has been directly demonstrated in D. melanogaster by cell ablation using a GAL4/UAS-reaper system (44). Ablating oenocytes in the late embryo produces polyphasic lethality during subsequent larval stages (Figure 6c). Many oenocyte-less larvae show defective and duplicated cuticular structures such as mouth hooks and trachea and fail to complete the second-to-third instar molt (44). However, exogenous ecdysone or 20E does not rescue the molt, suggesting that a lack of molting hormone is not the sole underlying reason for this phenotype (44). Oenocyte-less larvae also fail to grow during the second larval instar, which correlates with the onset of food avoidance, a behavioral phenotype resembling that seen in hypoxic larvae (44, 137). This intriguing similarity was accounted for by a recent study showing that developing oenocyte-less larvae become anoxic as a result of compromised tracheal air-filling (90) (Figure 6d). The air-filling phenotype was thought not to depend on abnormal molting but to result from liquid ingress into the tracheal system via defective spiracular waterproofing (90). The same study used cell-type-specific gene deletions and knockdowns to demonstrate that spiracular water tightness is dependent on the oenocyte activities of enzymes in the very-long-chain fatty acid (VLCFA) synthetic pathway: Acc, Fas, and a fatty acid elongase. Spiracles are thought to obtain waterproofing lipids via long ducts from specialized spiracular gland cells (reviewed in 54, 105), but in oenocyte-ablated or Acc loss-of-function larvae, these ducts no longer fill with Oil Red O-stained material (90). Accordingly, the authors concluded that larval oenocytes synthesize a VLCFA-dependent remote signal that controls lipid transfer within the spiracles and thus waterproofs the respiratory system.
Numerous descriptive and functional studies together provide evidence that oenocytes also play a more general role in the synthesis of lipid components of the epicuticle. Studies in the cockroach Leucophaea maderae and in Rhodnius prolixus showed that both the size of oenocytes and features of their secretory morphology, including the smooth endoplasmic reticulum, vary with the molting cycle (102, 133). These classic papers also showed that oenocytes are contacted by epidermal processes and that, in D. melanogaster, cytoplasmic strands from the oenocytes project into the epidermis (10, 102, 133). These cell-to-cell contacts may also facilitate lipid or lipoprotein transfer from the oenocytes to the epidermis and, hence, to the cuticle prior to molting (136, 139). Lipids that coat the epicuticle of both larval and adult insects are a complex cocktail of hydrocarbons, sterols, fatty acids, fatty alcohols, triglycerides, wax esters, and other species (reviewed in 46). Cuticular hydrocarbons are species- and sex-specific mixes of straight-chain alkanes, methyl-branched alkanes, alkenes, and their derivatives that function as a barrier to water transpiration and in pheromonal communication (reviewed in 9, 35, 38, 51).
A role for oenocytes as a major site of synthesis for hydrocarbons and other cuticular lipids is supported by experiments using radioactive acetate, glucose, and propionate tracers in diverse species including A. mellifera, Blattella germanica, D. melanogaster, Locusta migratoria, Periplaneta americana, Schistocerca gregaria, and T. molitor (28, 33, 59, 60, 93, 94, 107). Many of these studies also suggest that oenocytes transport hydrocarbons to the epidermis in the form of hemolymph lipophorin particles, rather than via direct cell-to-cell contacts (28, 33, 59, 60, 93). Genetic evidence that adult oenocytes are required for the in vivo synthesis of cuticular hydrocarbons comes from D. melanogaster. Male and female flies in which the adult but not larval oenocytes have been ablated using the GAL4/UAS system show dramatic reductions in most of the 20 or so cuticular hydrocarbon species analyzed (7) (Figure 6e). Insect hydrocarbons are synthesized from fatty acid precursors that are then mono- and di-unsaturated by acyl-CoA desaturases, chain lengthened by fatty acid elongases to give VLCFAs, and then subjected to oxidative decarbonylation to generate hydrocarbons (reviewed in 8, 130). Recently, it was shown that the aldehyde oxidative decarbonylation step is catalyzed by the insect-specific cytochrome P450 enzyme Cyp4g1 and its redox partner Cpr, both of which are strongly expressed in oenocytes (44, 78, 98) (Figure 6f). Null mutants for Cyp4g1 are lethal at late pupal or adult eclosion stages (44). High mortality at adult emergence is also seen with oenocyte-specific knockdown of Cyp4g1 or Cpr, with surviving adult flies showing reduced levels of most cuticular hydrocarbons and a concomitant accumulation of esters and fatty acids, not normally found on the epicuticle (98). Importantly, oenocyte-specific knockdown of Cyp4g1 significantly reduces desiccation resistance in both sexes and, in females, alters pheromone-driven courtship elicited from control males (98). Not all aspects of the Cyp4g1 courtship phenotype are the same as in oenocyte-less female flies. For example, mating latency time increases in the former but decreases in the latter (7, 98). Nevertheless, cell ablations and Cyp4g1/Cpr knockdowns together provide strong evidence that adult oenocytes are critical for the synthesis of cuticular hydrocarbons with barrier and pheromonal roles.
In the absence of hydrocarbon pheromones, oenocyte-less male or female flies hyperstimulate other males to court, suggesting that this is the default behavior (7). Moreover, oenocyte-less D. melanogaster females mate with wild-type Drosophila simulans males, indicating that adult oenocytes are required for the normal pheromone-dependent inhibition to interspecies courtship and copulation (7). In D. melanogaster, the predominant hydrocarbon sex pheromones are alkenes. Males express high levels of the female receptivity monoalkene (Z)-7-tricosene, whereas females produce the male aphrodisiac dienes (7Z,11Z)-heptacosadiene and (7Z,11Z)-nonacosadiene (reviewed in 35, 130). Oenocytes play a central role in pheromone precursor desaturation by expressing two enzymes, Desat1 and DesatF/Fad2. Desat1 catalyzes the first fatty acid desaturation event, converting palmitate/stearate into palmitoleate/oleate. Thus, its activity in adult oenocytes is required to synthesize all alkene hydrocarbons (79, 131). DesatF promotes the second desaturation event, and in D. melanogaster, it is expressed only in female adult oenocytes where it is essential for producing dienes (22, 131). In an elegant demonstration of the sex specificity of oenocytes in pheromone synthesis, these cells were selectively feminized in males using transformer expression, a manipulation that triggered 7,11-diene synthesis and elicited homosexual courtship from other males (36, 114). The reciprocal manipulation, masculinizing the oenocytes of females by knocking down transformer, did not prevent copulation but induced inappropriate aggression from males (34). This response is not triggered in oenocyte-less females, arguing that, unlike courtship, normal male-to-male aggression requires male oenocyte-derived pheromones rather than just the absence of female pheromones (7, 34). Perfuming oenocyte-less males with the male-enriched cuticular hydrocarbon (Z)-7-tricosene (7-T) was sufficient to restore normal levels of aggression and to suppress homosexual courtship (127). Thus, a specific oenocyte-derived male hydrocarbon regulates male-male aggression and courtship suppression. Interestingly, pheromone synthesis in oenocytes has been subject to rapid evolution in Insecta. Across the genus Drosophila, genetic variation at the desatF locus can account for losses, gains, and swaps in the sexually dimorphic expression of diene hydrocarbon pheromones in oenocytes (73, 117). In some other species, hydrocarbon precursors are chemically modified to form more complex pheromones. For example, the female gypsy moth, Lymantria dispar, produces an epoxide called disparlure (2-methyl-7R, 8S-epoxy-octadecane), which attracts males from long distances. Deuterium tracer experiments and gas chromatography-mass spectrometry strongly suggest that oenocytes synthesize the alkene precursor (2-methyl-Z7-octadecene), which is then transported via the hemolymph to a specialized pheromone gland for conversion into the active epoxide disparlure (57).
Future Directions
It has taken more than 150 years of insect research to obtain the first glimpses of the development and functions of oenocytes. On both fronts, many fascinating, yet tractable, research areas remain virtually unexplored. From an evo-devo viewpoint, oenocytes provide a rich source of variation across Insecta at the level of a single and easily identifiable cell type. They are therefore well suited for addressing questions related to how cell specification, cell morphology, cell number, and cell functions are modified during arthropod evolution. From a functional perspective, it is interesting that the synthesis of VLCFAs and their derivatives provides a common theme that unites the tracheal waterproofing and cuticular hydrocarbon roles for Drosophila larval and adult oenocytes, respectively. Larval oenocytes also play a distinct role in the Drosophila embryo, secreting Semaphorin 2a, which signals through Plexin receptors to inhibit inappropriate axonal extensions of lateral chordotonal and other sensory organs (3). However, the mechanism accounting for the observed requirement of larval oenocytes in molting remains obscure. Moreover, it is highly likely that additional physiological functions for oenocytes remain to be uncovered. In this regard, it will be interesting to see whether the role of cytochrome P450 reductase in sensitivity to the insecticide permethrin in Anopheles gambiae (78) maps to adult oenocytes rather than to another highly expressing tissue. Finally, an important area of future research is the characterization of the metabolic cross talk between oenocytes and other tissues such as the fat body, midgut, and epidermis.
Summary Points.
Oenocytes are a cell type of ectodermal origin that display marked evolutionary variations in size, number, and anatomical location across Insecta.
Oenocytes are specialized for the synthesis and metabolism of lipids, including VLCFAs and hydrocarbons.
In D. melanogaster, a holometabolous insect, larval and adult generations of oenocytes are morphologically distinct ectodermal derivatives with separate developmental origins.
Larval oenocytes are induced from the embryonic ectoderm in both short (T. castaneum) and long (D. melanogaster) germ-band species. In Drosophila, the induction mechanism requires EGFR signaling in dorsoposterior ectoderm expressing Spalt major and Engrailed.
Adult Drosophila oenocytes likely derive from pupal histoblasts, but the molecular mechanism underlying their formation is unknown.
The fat body is an important nutrient-dependent regulator of lipid metabolism in larval oenocytes. Lipophorin is transported from the fat body to the larval oenocytes in the hemolymph. Lipid droplets accumulate in larval oenocytes during fasting and in other contexts when fat body lipolysis is increased.
Larval oenocytes are essential for molting and waterproofing the tracheal system. The molting role is poorly understood, but the tracheal waterproofing function requires VLCFA biosynthesis in larval oenocytes to maintain the water tightness of spiracles.
Adult oenocytes synthesize sex- and species-specific mixes of cuticular hydrocarbons from VLCFAs via a pathway requiring a cytochrome P450 aldehyde oxidative decarbonylase. Cuticular hydrocarbons are essential for adult desiccation resistance and pheromonal communication.
Acknowledgments
We apologize to those whose work was not directly cited here because of space constraints. We thank the British Library and University College London for supplying historical articles and NIMR Photographics for assistance with figures. We also acknowledge Panayotis Pachnis for kindly providing Figure 3e,f and Iris Salecker for translation of papers from German. A.P.G., E.C., and R.M. were supported by the Medical Research Council (U117584237) and E.C. by an EMBO Long-Term Fellowship.
Footnotes
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Anglas J. Observations sur les métamorphoses internes de la guêpe et de l’abeille. Bull Sci Fr Belg. 1900;34:363–473. [Google Scholar]
- 2.Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–25. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates KE, Whitington PM. Semaphorin 2a secreted by oenocytes signals through plexin B and plexin A to guide sensory axons in the Drosophila embryo. Dev Biol. 2007;302:522–35. doi: 10.1016/j.ydbio.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Berlese A. Osservazioni su Fenomeni che Avvengono Durante la Ninfosi degli Insetti Metabolici, Part 1. Florence: Ferdinando Mariani; 1899. [Google Scholar]
- 5.Berlese A. Osservazioni su Fenomeni che Avvengono Durante la Ninfosi degli Insetti Metabolici, Part 2. Florence: Ferdinando Mariani; 1902. [Google Scholar]
- 6.Bi J, Xiang Y, Chen H, Liu Z, Gronke S, et al. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. J Cell Sci. 2012;125:3568–77. doi: 10.1242/jcs.101329. [DOI] [PubMed] [Google Scholar]
- 7.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–91. doi: 10.1038/nature08495. [ 7. Demonstrates that adult oenocytes are required to synthesize cuticular hydrocarbons such as 7,11-HD in D. melanogaster and to define a reproductive barrier between this and other Drosophila species. ] [DOI] [PubMed] [Google Scholar]
- 8.Blomquist GJ. Biosynthesis of cuticular hydrocarbons. 2010:35–52. See Ref. 9. [Google Scholar]
- 9.Blomquist GJ, Bagneres A-G, editors. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge, UK: Cambridge Univ. Press; 2010. [Google Scholar]
- 10.Bodenstein D. In: Biology of Drosophila. Demerec M, editor. New York: Wiley; 1950. The postembryonic development of Drosophila; pp. 275–367. [Google Scholar]
- 11.Brasaemle DL, Wolins NE. Packaging of fat: an evolving model of lipid droplet assembly and expansion. J Biol Chem. 2012;287:2273–79. doi: 10.1074/jbc.R111.309088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewster R, Bodmer R. Origin and specification of type II sensory neurons in Drosophila. Development. 1995;121:2923–36. doi: 10.1242/dev.121.9.2923. [DOI] [PubMed] [Google Scholar]
- 13.Brewster R, Bodmer R. Cell lineage analysis of the Drosophila peripheral nervous system. Dev Genet. 1996;18:50–63. doi: 10.1002/(SICI)1520-6408(1996)18:1<50::AID-DVG6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Brodu V, Elstob PR, Gould AP. abdominal A specifies one cell type in Drosophila by regulating one principal target gene. Development. 2002;129:2957–63. doi: 10.1242/dev.129.12.2957. [DOI] [PubMed] [Google Scholar]
- 15.Brodu V, Elstob PR, Gould AP. EGF receptor signaling regulates pulses of cell delamination from the Drosophila ectoderm. Dev Cell. 2004;7:885–95. doi: 10.1016/j.devcel.2004.10.016. [ 15. Shows Drosophila oenocytes delaminate from ectoderm in bursts of three cells; delamination begins when Notch inhibition is lifted and requires activation of high-threshold EGFR targets. ] [DOI] [PubMed] [Google Scholar]
- 16.Burns KA, Gutzwiller LM, Tomoyasu Y, Gebelein B. Oenocyte development in the red flour beetle Tribolium castaneum. Dev Genes Evol. 2012;222:77–88. doi: 10.1007/s00427-012-0390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterworth FM, Bodenstein D, King RC. Adipose tissue of Drosophila melanogaster I. An experimental study of larval fat body. J Exp Zool. 1965;158:141–53. doi: 10.1002/jez.1401580203. [DOI] [PubMed] [Google Scholar]
- 18.Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu Rev Nutr. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- 19.Cassier P, Fain-Maurel MA. Caractéres infrastructurelles et cytochimiques des oenocytes de Locusta migratoria migratorioides en rapport avec les mues et les cycles ovariens. Arch Anat Microsc. 1972;61:357–80. [Google Scholar]
- 20.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, et al. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–22. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Chaves VE, Frasson D, Kawashita NH. Several agents and pathways regulate lipolysis in adipocytes. Biochimie. 2011;93:1631–40. doi: 10.1016/j.biochi.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Chertemps T, Duportets L, Labeur C, Ueyama M, Wicker-Thomas C. A female-specific desaturase gene responsible for diene hydrocarbon biosynthesis and courtship behaviour in Drosophila melanogaster. Insect Mol Biol. 2006;15:465–73. doi: 10.1111/j.1365-2583.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 23.Chien CH, Chen WW, Wu JT, Chang TC. Investigation of lipid homeostasis in living Drosophila by coherent anti-Stokes Raman scattering microscopy. J Biomed Opt. 2012;17:126001. doi: 10.1117/1.JBO.17.12.126001. [DOI] [PubMed] [Google Scholar]
- 24.Chung H, Sztal T, Pasricha S, Sridhar M, Batterham P, Daborn PJ. Characterization of Drosophila melanogaster cytochrome P450 genes. Proc Natl Acad Sci USA. 2009;106:5731–36. doi: 10.1073/pnas.0812141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–49. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 26.Dean RL, Locke M, Collins JV. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut GA, Gilbert LI, editors. New York: Pergamon; 1985. Structure of the fat body; pp. 155–210. [Google Scholar]
- 27.Delachambre J. Remarques sur l’histophysiologie des oenocytes épidermiques de la nymphe de Tenebrio molitor. C R Acad Sci. 1966;263:764–67. [Google Scholar]
- 28.Diehl PA. Synthesis and release of hydrocarbons by the oenocytes of the desert locust, Schistocerca gregaria. J Insect Physiol. 1975;21:1237–46. [Google Scholar]
- 29.Dorn A, Romer F. Structure and function of prothoracic glands and oenocytes in embryos and last larval instars of Oncopeltus fasciatus Dallas (Insecta, Heteroptera) Cell Tissue Res. 1976;171:331–50. doi: 10.1007/BF00224658. [DOI] [PubMed] [Google Scholar]
- 30.Eastham L. The post-embryonic development of Phaenoserphus viator Hal. (Proctotrypoidea), a parasite of the larva of Pterostichus niger (Carabidae), with notes on the anatomy of the larva. Parasitology. 1929;21:1–21. [Google Scholar]
- 31.Elstob PR, Brodu V, Gould AP. spalt-dependent switching between two cell fates that are induced by the Drosophila EGF receptor. Development. 2001;128:723–32. doi: 10.1242/dev.128.5.723. [ 31. Identifies the genetic program of larval oenocyte induction from the Spalt major expressing-dorsal embryonic ectoderm. This process involves Spitz/EGFR signaling. ] [DOI] [PubMed] [Google Scholar]
- 32.Fabre JH. Etude sur l’instinct les métamorphoses des Sphégiens. Ann Sci Nat Zool. 1856;6:137–183. [Google Scholar]
- 33.Fan Y, Zurek L, Dykstra MJ, Schal C. Hydrocarbon synthesis by enzymatically dissociated oenocytes of the abdominal integument of the German cockroach, Blattella germanica. Naturwissenschaften. 2003;90:121–26. doi: 10.1007/s00114-003-0402-y. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K, et al. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferveur JF. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–95. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 36.Ferveur JF, Savarit F, O’Kane CJ, Sureau G, Greenspan RJ, Jallon JM. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997;276:1555–58. doi: 10.1126/science.276.5318.1555. [ 36. Provides evidence that adult oenocytes synthesize hydrocarbons that function as sex-specific pheromones. ] [DOI] [PubMed] [Google Scholar]
- 37.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57. doi: 10.1016/s1388-1981(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs AG, Rajpurohit S. Cuticular lipids and water balance. 2010:100–20. See Ref. 9. [Google Scholar]
- 39.Gilbert LI. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol Cell Endocrinol. 2004;215:1–10. doi: 10.1016/j.mce.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Glaser R. A contribution to our knowledge of the function of the oenocytes of insects. Biol Bull. 1912;23(3):213–24. [Google Scholar]
- 41.Gould AP, Elstob PR, Brodu V. Insect oenocytes: a model system for studying cell-fate specification by Hox genes. J Anat. 2001;199:25–33. doi: 10.1046/j.1469-7580.2001.19910025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graber V. Ueber den propulsatorischen Apparat der Insecten. Archiv Mikrosk Anat. 1873;9:129–96. [Google Scholar]
- 43.Graber V. Zur Embriologie der Insecten. Zool Anz. 1891;14:286–291. [Google Scholar]
- 44.Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–80. doi: 10.1038/nature05382. [ 44. Shows that larval oenocytes express numerous lipid metabolic genes including Cyp4g1, accumulate lipid droplets upon fasting, are regulated by the fat body, and are required for molting. ] [DOI] [PubMed] [Google Scholar]
- 45.Gutzwiller LM, Witt LM, Gresser AL, Burns KA, Cook TA, Gebelein B. Proneural and abdominal Hox inputs synergize to promote sensory organ formation in the Drosophila abdomen. Dev Biol. 2010;348:231–43. doi: 10.1016/j.ydbio.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadley NF. Cuticular lipids of terrestrial plants and arthropods: a comparison of their structure, composition, and waterproofing function. Biol Rev Camb Philos Soc. 1981;56:23–27. [Google Scholar]
- 47.Hartenstein AY, Rugendorff A, Tepass U, Hartenstein V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development. 1992;116:1203–20. doi: 10.1242/dev.116.4.1203. [DOI] [PubMed] [Google Scholar]
- 48.Hodson L, Frayn KN. Hepatic fatty acid partitioning. Curr Opin Lipidol. 2011;22:216–24. doi: 10.1097/MOL.0b013e3283462e16. [DOI] [PubMed] [Google Scholar]
- 49.Hollande AC. Les cérodécytes ou “oenocytes” des insectes considérés au point de vue biochimique. Arch Anat Microsc. 1914;16:1–66. [Google Scholar]
- 50.Hosselet C. Cytology of insects. C R Acad Sci. 1925;180:399–401. [Google Scholar]
- 51.Howard RW, Blomquist GJ. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50:371–93. doi: 10.1146/annurev.ento.50.071803.130359. [DOI] [PubMed] [Google Scholar]
- 52.Jackson A, Locke M. The formation of plasma membrane reticular Systems in the oenocytes of an insect. Tissue Cell. 1989;21:463–73. doi: 10.1016/0040-8166(89)90059-1. [DOI] [PubMed] [Google Scholar]
- 53.Janet C. Sur I’Ontogenese de I’Insecte. Limoges, France: Ducourtieux et Gout; 1909. [Google Scholar]
- 54.Jarial MS, Engstrom L. Fine structure of the spiracular glands in larval Drosophila melanogaster (Meig.) (Diptera: Drosophilidae) Int J Insect Morphol Embryol. 1995;24:1–12. [Google Scholar]
- 55.Johnson EC, Kazgan N, Bretz CA, Forsberg LJ, Hector CE, et al. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PLoS One. 2010;5:312799. doi: 10.1371/journal.pone.0012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson MB, Butterworth FM. Maturation and aging of adult fat body and oenocytes in Drosophila as revealed by light microscopic morphometry. J Morphol. 1985;184:51–59. doi: 10.1002/jmor.1051840106. [DOI] [PubMed] [Google Scholar]
- 57.Jurenka RA, Subchev M, Abad JL, Choi MY, Fabrias G. Sex pheromone biosynthetic pathway for disparlure in the gypsy moth, Lymantria dispar. Proc Natl Acad Sci USA. 2003;100:809–14. doi: 10.1073/pnas.0236060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karawaiew W. Die nachembryonale Entwicklung von Lasius flavus. Z Wiss Zool. 1898;16:385–478. [Google Scholar]
- 59.Katase H, Chino H. Transport of hydrocarbons by the lipophorin of insect hemolymph. Biochim Biophys Acta. 1982;710:341–48. [Google Scholar]
- 60.Katase H, Chino H. Transport of hydrocarbons by haemolymph lipophorin in Locusta migratoria. Insect Biochem. 1984;14:1–6. [Google Scholar]
- 61.Keeley LL. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut GA, Gilbert LI, editors. New York: Pergamon; 1985. Physiology and biochemistry of the fat body; pp. 211–48. [Google Scholar]
- 62.Koch J. Die Oenocyten von Drosophila melanogaster. Rev Suisse Zool. 1945;52:415–20. [Google Scholar]
- 63.Kohyama-Koganeya A, Kim YJ, Miura M, Hirabayashi Y. A Drosophila orphan G protein–coupled receptor BOSS functions as a glucose-responding receptor: loss of boss causes abnormal energy metabolism. Proc Natl Acad Sci USA. 2008;105:15328–33. doi: 10.1073/pnas.0807833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koller G. Die innere Sekretion bei Wirbellosen Tieren. Biol Rev. 1929;4:269–306. [Google Scholar]
- 65.Koschevnikov G. Uber den Fettkörper und die Œnocyten der honigbiene (Apis mellifica) Zool Anz. 1900;23:337–53. [Google Scholar]
- 66.Krupp JJ, Levine JD. Dissection of oenocytes from adult Drosophila melanogaster. J Vis Exp. 2010;41:22–42. doi: 10.3791/2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuhnlein RP. The contribution of the Drosophila model to lipid droplet research. Prog Lipid Res. 2011;50:348–56. doi: 10.1016/j.plipres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Kunte AS, Matthews KA, Rawson RB. Fatty acid auxotrophy in Drosophila larvae lacking SREBP. Cell Metab. 2006;3:439–48. doi: 10.1016/j.cmet.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 69.Landois L. Über die Function des Fettkörper. Z Wiss Zool. 1865;15:371–72. [Google Scholar]
- 70.Lawrence PA, Johnston P. Cell lineage of the Drosophila abdomen: the epidermis, oenocytes and ventral muscles. J Embryol Exp Morphol. 1982;72:197–208. [PubMed] [Google Scholar]
- 71.Lawrence PA, Johnston P. Observations on cell lineage of internal organs of Drosophila. J Embryol Exp Morphol. 1986;91:251–66. [ 71. Demonstrates, using Drosophila genetic mosaics, that adult oenocytes share a lineage with adult epidermis rather than with fat body. ] [PubMed] [Google Scholar]
- 72.Lee JR, Urban S, Garvey CF, Freeman M. Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell. 2001;107:161–71. doi: 10.1016/s0092-8674(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 73.Legendre A, Miao XX, Da Lage JL, Wicker-Thomas C. Evolution of a desaturase involved in female pheromonal cuticular hydrocarbon biosynthesis and courtship behavior in Drosophila. Insect Biochem Mol Biol. 2008;38:244–55. doi: 10.1016/j.ibmb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Li-Kroeger D, Cook TA, Gebelein B. Integration of an abdominal Hox complex with Pax2 yields cell-specific EGF secretion from Drosophila sensory precursor cells. Development. 2012;139:1611–19. doi: 10.1242/dev.077842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li-Kroeger D, Witt LM, Grimes HL, Cook TA, Gebelein B. Hox and senseless antagonism functions as a molecular switch to regulate EGF secretion in the Drosophila PNS. Dev Cell. 2008;15:298–308. doi: 10.1016/j.devcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Locke M. The ultrastructure of the oenocytes in the molt/intermolt cycle of an insect. Tissue Cell. 1969;1:103–54. doi: 10.1016/s0040-8166(69)80009-1. [DOI] [PubMed] [Google Scholar]
- 77.Locke M, Huie P. A function for plasma membrane reticular Systems. Tissue Cell. 1983;15:885–902. doi: 10.1016/0040-8166(83)90056-3. [DOI] [PubMed] [Google Scholar]
- 78.Lycett GJ, McLaughlin LA, Ranson H, Hemingway J, Kafatos FC, et al. Anopheles gambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol Biol. 2006;15:321–27. doi: 10.1111/j.1365-2583.2006.00647.x. [DOI] [PubMed] [Google Scholar]
- 79.Marcillac F, Bousquet F, Alabouvette J, Savarit F, Ferveur JF. A mutation with major effects on Drosophila melanogaster sex pheromones. Genetics. 2005;171:1617–28. doi: 10.1534/genetics.104.033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martins GF, Ramalho-Ortigao JM. Oenocytes in insects. Invertebr Surviv J. 2012;9:139–52. [Google Scholar]
- 81.Martins GF, Ramalho-Ortigao JM, Lobo NF, Severson DW, McDowell MA, Pimenta PF. Insights into the transcriptome of oenocytes from Aedes aegypti pupae. Mem Inst Oswaldo Cruz. 2011;106:308–15. doi: 10.1590/s0074-02762011000300009. [DOI] [PubMed] [Google Scholar]
- 82.Martins GF, Serrao JE, Ramalho-Ortigao JM, Pimenta PF. Histochemical and ultrastructural studies of the mosquito Aedes aegypti fat body: effects of aging and diet type. Microsc Res Tech. 2011;74:1032–39. doi: 10.1002/jemt.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maynard JC, Pham T, Zheng T, Jockheck-Clark A, Rankin HB, et al. Gp93,the Drosophila GRP94 ortholog, is required for gut epithelial homeostasis and nutrient assimilation-coupled growth control. Dev Biol. 2010;339:295–306. doi: 10.1016/j.ydbio.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller A. In: Biology of Drosophila. Demerec M, editor. New York: Wiley; 1950. The internal anatomy and histology of the imago of Drosophila melanogaster; pp. 420–534. [Google Scholar]
- 85.Nilsen KA, Ihle KE, Frederick K, Fondrk MK, Smedal B, et al. Insulin-like peptide genes in honey bee fat body respond differently to manipulation of social behavioral physiology. J Exp Biol. 2011;214:1488–97. doi: 10.1242/jeb.050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okabe M, Okano H. Two-step induction of chordotonal organ precursors in Drosophila embryogenesis. Development. 1997;124:1045–53. doi: 10.1242/dev.124.5.1045. [DOI] [PubMed] [Google Scholar]
- 87.Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–39. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, et al. Lipoproteins in Drosophila melanogaster: assembly, function, and influence on tissue lipid composition. PLoS Genet. 2012;8:e1002828. doi: 10.1371/journal.pgen.1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pantel J. Le Trixion halidayanum Rend. Essai monographique sur les caractéres exterieurs, la biologie et l’anatomie d’une larve parasite des tachinaires. Cellule. 1893;15:5–290. [Google Scholar]
- 90.Parvy JP, Napal L, Rubin T, Poidevin M, Perrin L, et al. Drosophila melanogaster acetyl-CoA-carboxylase sustains a fatty acid-dependent remote signal to waterproof the respiratory system. PLoS Genet. 2012;8:e1002925. doi: 10.1371/journal.pgen.1002925. [ 90. Identifies a role for larval oenocytes in preventing tracheal flooding; uses genetic manipulations to show that this function requires the VLCFA synthetic pathway in oenocytes. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pérez C. Contribution a l’étude des métamorphoses. Bull Sci Fr Belg. 1902;37:195. [Google Scholar]
- 92.Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci USA. 2003;100:13773–78. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pho DB, Pennanec’h M, Jallon JM. Purification of adult Drosophila melanogaster lipophorin and its role in hydrocarbon transport. Arch Insect Biochem Physiol. 1996;31:289–303. doi: 10.1002/(SICI)1520-6327(1996)31:3<289::AID-ARCH4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 94.Piek T. Synthesis of wax in the honey bee Apis mellifera. J Insect Physiol. 1964;10:563–72. [Google Scholar]
- 95.Poisson R. Contribution a l’étude des hémiptères aquatiques. Bull Biol Fr Belg. 1924;58:49–306. [Google Scholar]
- 96.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–60. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 97.Poyarkoff E. Recherches histologiques sur la métamorphose d’un coléoptère (la galeruque de l’orme) Arch Anat Microsc. 1910;12:333–474. [Google Scholar]
- 98.Qiu Y, Tittiger C, Wicker-Thomas C, Le Goff G, Young S, et al. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc Natl Acad Sci USA. 2012;109:14858–63. doi: 10.1073/pnas.1208650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajan A, Perrimon N. Drosophila cytokine Unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–37. doi: 10.1016/j.cell.2012.08.019. [ 99. Demonstrates that Musca Cyp4g2 and Drosophila Cyp4g1 can catalyze the oxidative decarbonylation of very-long-chain aldehydes to produce hydrocarbon alkanes/alkenes. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. Developmental expression of Manduca shade, the P450 mediating the final step in molting hormone synthesis. Mol Cell Endocrinol. 2006;247:166–74. doi: 10.1016/j.mce.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 101.Riddiford LM. In: The Development of Drosophila melanogaster. Bate M, Martinez Arias A, editors. New York: Cold Spring Harbor Lab. Press; 1993. Hormones and Drosophila development; pp. 899–939. [Google Scholar]
- 102.Rinterknecht E. Cuticulogenesis correlated with ultrastructural changes in oenocytes and epidermal cells in the late cockroach embryo. Tissue Cell. 1985;17:723–43. doi: 10.1016/0040-8166(85)90007-2. [DOI] [PubMed] [Google Scholar]
- 103.Rinterknecht E, Matz G. Oenocyte differentiation correlated with the formation of ectodermal coating in the embryo of a cockroach. Tissue Cell. 1983;15:375–90. doi: 10.1016/0040-8166(83)90070-8. [DOI] [PubMed] [Google Scholar]
- 104.Rinterknecht E, Perolini M, Porter A, Joly P. Sur les variations ultrasturcturelles des oenocytes au cours du cycle et aprés ablation des glandes prothoraciques chez Locusta migratoria. C R Acad Sci. 1973;276:2827–30. [Google Scholar]
- 105.Rizki MTM. The cytophysiology of the spiracular glands of Drosophila melanogaster. J Morphol. 1956;98:497–511. [Google Scholar]
- 106.Romer F. Ultrastructural changes of the oenocytes of Gryllus bimaculatus DEG (Saltatoria, Insecta) during the moulting cycle. Cell Tissue Res. 1974;151:27–46. doi: 10.1007/BF00222032. [DOI] [PubMed] [Google Scholar]
- 107.Romer F. Histochemical and biochemical investigations concerning the function of larval oenocytes of Tenebrio molitor L. (Coleoptera, Insecta) Histochemistry. 1980;69:69–84. doi: 10.1007/BF00508368. [DOI] [PubMed] [Google Scholar]
- 108.Romer F, Bressel HU. Secretion and metabolism of ecdysteroids by oenocyte-fat body complexes (OEFC) in adult males of Gryllus bimaculatus DEG (Insecta) Z Naturforsch. 1994;49:871–80. [Google Scholar]
- 109.Romer F, Emmerich H, Nowock J. Biosynthesis of ecdysones in isolated prothoracic glands and oenocytes of Tenebrio molitor in vitro. J Insect Physiol. 1974;20:1975–87. doi: 10.1016/0022-1910(74)90105-x. [DOI] [PubMed] [Google Scholar]
- 110.Romer F, Gnatzy W. Arachnid oenocytes: ecdysone synthesis in the legs of harvestmen (Opilion-idae) Cell Tissue Res. 1981;216:449–53. doi: 10.1007/BF00233631. [DOI] [PubMed] [Google Scholar]
- 111.Rössig H. Von welchen Organen der Gallwespenlarven geht der Reiz zur Bildung der Pflanzengalle aus? Untersuchung der Drüsenorgane der Gallwespenlarven, zugleich ein Beitrag zur postembryonalen Entwicklung derselben. Zool Jahrb. 1904;20:19–90. [Google Scholar]
- 112.Roth S, Hartenstein V. Development of Tribolium castaneum. Dev Genes Evol. 2008;218:115–18. doi: 10.1007/s00427-008-0215-2. [DOI] [PubMed] [Google Scholar]
- 113.Rusten TE, Cantera R, Urban J, Technau G, Kafatos FC, Barrio R. Spalt modifies EGFR-mediated induction of chordotonal precursors in the embryonic PNS of Drosophila promoting the development of oenocytes. Development. 2001;128:711–22. doi: 10.1242/dev.128.5.711. [DOI] [PubMed] [Google Scholar]
- 114.Savarit F, Ferveur JF. Genetic study of the production of sexually dimorphic cuticular hydrocarbons in relation with the sex-determination gene transformer in Drosophila melanogaster. Genet Res. 2002;79:23–40. doi: 10.1017/s0016672301005481. [DOI] [PubMed] [Google Scholar]
- 115.Schnelle H. Über dem feineren Bau des Fettkörpers der Honigbiene. Zool Anz. 1923;57:172–79. [Google Scholar]
- 116.Sémichon L. Recherches morphologiques sur quelques melliféres solitaires. Bull Sci Fr Belg. 1906;15:281–439. [Google Scholar]
- 117.Shirangi TR, Dufour HD, Williams TM, Carroll SB. Rapid evolution of sexpheromone-producing enzyme expression in Drosophila. PLoS Biol. 2009;7:e1000168. doi: 10.1371/journal.pbio.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Snodgrass RE. Principles of Insect Morphology. New York/London: McGraw-Hill; 1935. [Google Scholar]
- 119.Soranzo L. Development and ultrastructure of oenocytes of the cattle grub: Hypoderma sp. (Diptera, Oestridae) Ann Sci Nat Zool Biol Anim. 1980;2:35–50. [Google Scholar]
- 120.Stendell W. Beiträge zur Kenntnis der Oenocyten von Ephestia kuehniella Zeller. Z Wiss Zool. 1912;102:137–68. [Google Scholar]
- 121.Studinger G, Willig A. Biosynthesis of α and β ecdysone in isolated abdomens of larvae of Musca domestica. J Insect Physiol. 1975;21:1793–98. doi: 10.1016/0022-1910(75)90243-7. [DOI] [PubMed] [Google Scholar]
- 122.Symonova R, Smrz J. First record of hemocytes and oenocytes in freshwater ostracodes. J Crustacean Biol. 2009;29:18–25. [Google Scholar]
- 123.Tennessen JM, Thummel CS. Coordinating growth and maturation: insights from Drosophila. Curr Biol. 2011;21:R750–57. doi: 10.1016/j.cub.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsruya R, Schlesinger A, Reich A, Gabay L, Sapir A, Shilo BZ. Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev. 2002;16:222–34. doi: 10.1101/gad.214202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van der Horst DJ, Roosendaal SD, Rodenburg KW. Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol Cell Biochem. 2009;326:105–19. doi: 10.1007/s11010-008-0011-3. [DOI] [PubMed] [Google Scholar]
- 126.Verson E. Beitrag zur Oenocytenlitteratur. Zool Anz. 1900;23:657–61. [Google Scholar]
- 127.Wang L, Han X, Mehren J, Hiroi M, Billeter JC, et al. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–62. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weissenberg R. Über Œnocyten von Torymus nigricornis Boh. mit besonderer Berücksichtigung der Metamorphose. Zool Jahrb Anat. 1907;23:231–68. [Google Scholar]
- 129.Wheeler W. Concerning the “blood-tissue” of the Insecta. Psyche J Entomol. 1892;6:216–58. [Google Scholar]
- 130.Wicker-Thomas C, Chertemps T. Molecular biology and genetics of hydrocarbon production. 2010:53–74. See Ref. 9. [Google Scholar]
- 131.Wicker-Thomas C, Guenachi I, Keita YF. Contribution of oenocytes and pheromones to courtship behaviour in Drosophila. BMC Biochem. 2009;10:21. doi: 10.1186/1471-2091-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wielowiejski H. Uber das Blutgewebe der Insekten. Z Wiss Zool. 1886;43:512–36. [Google Scholar]
- 133.Wigglesworth VB. The physiology of the cuticle and of ecdysis in Rhodnius prolixus (Triatomidae, Hemiptera); with special reference to the function of the oenocytes and of the dermal glands. QJ Microsc Sci. 1933;76:269–318. [ 133. Describes the changing structure of the oenocytes and their relationship to the cuticle during molting in R. proxilus. ] [Google Scholar]
- 134.Wigglesworth VB. The Principles of Insect Physiology. London: Methuen; 1950. [Google Scholar]
- 135.Wigglesworth VB. Structural lipids in the insect cuticle and the function of the oenocytes. Tissue Cell. 1970;2:155–79. doi: 10.1016/s0040-8166(70)80013-1. [DOI] [PubMed] [Google Scholar]
- 136.Wigglesworth VB. The source of lipids and polyphenols for the insect cuticle: the role of fat body, oenocytes and oenocytoids. Tissue Cell. 1988;20:919–32. doi: 10.1016/0040-8166(88)90033-x. [DOI] [PubMed] [Google Scholar]
- 137.Wingrove JA, O’Farrell PH. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell. 1999;98:105–14. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Witt LM, Gutzwiller LM, Gresser AL, Li-Kroeger D, Cook TA, Gebelein B. Atonal, Senseless, and Abdominal-A regulate rhomboid enhancer activity in abdominal sensory organ precursors. Dev Biol. 2010;344:1060–70. doi: 10.1016/j.ydbio.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wolfe L. The deposition of the third instar larval cuticle of Calliphora erythrocephala. Q J Microsc Sci. 1954;95:49–66. [Google Scholar]
- 140.Yokoyama T. Histological observations on a non-moulting strain of silkworm. Proc R Entomol Soc Lond A. 1936;11:35–44. [Google Scholar]
- 141.zur Lage P, Jan YN, Jarman AP. Requirement for EGF receptor signalling in neural recruitment during formation of Drosophila chordotonal sense organ clusters. Curr Biol. 1997;7:166–75. doi: 10.1016/s0960-9822(97)70087-3. [DOI] [PubMed] [Google Scholar]