Abstract
Purpose
There is altered breastmilk composition among mothers with gestational diabetes and conflicting evidence on whether breastfeeding is beneficial or detrimental to their offspring’s cardiometabolic health. We aimed to investigate associations between breastfeeding and offspring’s cardiometabolic health across the range of gestational glycemia.
Methods
We included 827 naturally-conceived, term singletons from a prospective mother-child cohort. We measured gestational (26-28weeks) fasting plasma glucose (FPG) and 2-hour plasma glucose (2hrPG) after an oral glucose tolerance test as continuous variables. Participants were classified into 2 breastfeeding categories (high/intermediate vs. low) according to their breastfeeding duration and exclusivity. Main outcome measures included magnetic resonance imaging (MRI)-measured abdominal fat, intramyocellular lipids (IMCL), and liver fat, quantitative magnetic resonance (QMR)-measured body fat mass, blood pressure, blood lipids, and insulin resistance at 6 years old (all continuous variables). We evaluated if gestational glycemia (FPG & 2hrPG) modified the association of breastfeeding with offspring outcomes after adjusting for confounders using a multiple linear regression model that included a ‘gestational glycemia x breastfeeding’ interaction term.
Results
With increasing gestational FPG, high/intermediate (vs. low) breastfeeding was associated with lower levels of IMCL (p-interaction=0.047), liver fat (p-interaction=0.033), and triglycerides (p-interaction=0.007), after adjusting for confounders. Specifically, at 2 standard deviations above the mean gestational FPG level, high/intermediate (vs. low) breastfeeding was linked to lower adjusted mean IMCL (0.39% of water signal [0.29,0.50] vs. 0.54% of water signal [0.46,0.62]), liver fat (0.39% by weight [0.20,0.58] vs. 0.72% by weight [0.59,0.85]), and triglycerides (0.62mmol/L [0.51,0.72] vs. 0.86mmol/L [0.75,0.97]). 2hrPG did not significantly modify the association between breastfeeding and childhood cardiometabolic risk.
Conclusion
Our findings suggest breastfeeding may confer protection against adverse fat partitioning and higher triglyceride concentration among children exposed to increased glycemia in utero.
Keywords: Breastfeeding, Gestational diabetes, Glycemia, Cardiometabolic risk, Fat partitioning, Adiposity
Introduction
The World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of life. Breastfeeding has been shown to protect against infections and improve child cognition. Breastfeeding also appears to protect against offspring obesity and cardiometabolic risk later in life [1, 2]. However, the findings on whether breastfeeding by mothers with gestational diabetes also protects their offspring against childhood obesity and adverse cardiometabolic risk are equivocal [3–8]. Impaired glucose regulation in mothers with gestational diabetes typically does not resolve immediately after delivery and could last weeks or months postpartum [9–12]. The concentrations of glucose and insulin found in breastmilk are influenced by maternal plasma glucose and insulin levels [13–15]. Studies have reported that mothers with diabetes or gestational diabetes had increased glucose [15] and insulin [16, 17] levels in breastmilk. Breastmilk glucose had been positively associated with offspring weight, fat mass, and lean mass, while higher insulin concentration in the milk had been linked to lower weight and lean mass [18]. Other than increased glucose and insulin, reduced breastmilk concentrations of hormones such as adiponectin [16] and nesfatin-1 [19] had been reported among mothers with gestational diabetes, which might increase appetite and promote weight gain [16, 20]. Alterations in macronutrient content, fatty acid composition, and other bioactive components of breastmilk from mothers with diabetes have also been reported [21, 22] and it is unclear how such complex changes in breastmilk composition programs the offspring’s cardiometabolic health later in life. Plagemann, et al. found that increased volume of breastmilk consumed from mothers with either type 1 diabetes or gestational diabetes was linked to risk of overweight at 2 years old [3], while other studies have shown a positive impact of breastfeeding on obesity among children of women with gestational diabetes [4–6, 23–25]. This might be due to the positive effect of breastfeeding on postpartum glucose control of women with gestational diabetes [26], which might in turn improve breastmilk composition. Given these mixed findings, there is an urgent need to provide clarity on whether longer breastfeeding is protective or detrimental to cardiometabolic health of offspring born to mothers with increased gestational glycemia.
Most of the works on breastfeeding among women with gestational diabetes had focused on the improvement in maternal metabolic health [26, 27]. There is a paucity of data on the potential impacts on childhood body composition and metabolic health. To the best of our knowledge, no previous studies have separately investigated associations between breastfeeding and offspring cardiometabolic health across the range of gestational fasting plasma glucose (FPG) and 2-hour plasma glucose (2hrPG) levels after an oral glucose tolerance test, which might be important due to different pathophysiologies behind elevated FPG compared to elevated 2hrPG [28]. Furthermore, it is important to investigate FPG and 2hrPG on a continuum because there is increasing evidence that maternal glycemia across the entire continuum is significantly associated with offspring adiposity, even if diagnostic criteria for gestational diabetes were not met [29–31]. Providing appropriate infant-feeding guidance to mothers for optimizing offspring cardiometabolic health is pertinent due to the rapid rise in the prevalence of increased gestational glycemia worldwide, especially in Asia [32]. Using a prospective, deeply phenotyped Asian mother-offspring cohort, we sought to elucidate whether gestational FPG and 2hrPG modify the associations between breastfeeding duration and cardiometabolic risk markers in prepubertal children (body fat partitioning, general adiposity, blood pressure and metabolic markers).
Materials and methods
Study population
This sub-study included 827 mother-offspring dyads from the prospective cohort study, Growing Up in Singapore Towards healthy Outcomes (GUSTO). From June 2009 to October 2010, pregnant women in their first trimester from KK Women’s and Children’s Hospital (KKH) and National University Hospital (NUH) who were Singapore citizens/permanent residents aged at least 18 years, of homogenous parental ethnic background, planned to deliver in KKH or NUH and reside in Singapore for the next 5 years, and willing to donate birth tissues at delivery, were recruited. Women receiving chemotherapy, on psychotropic drugs, or having type 1 diabetes, were excluded. For this sub-study, we also excluded women who conceived through in vitro fertilization, delivered preterm infants, delivered twins, used donor breastmilk, received insulin treatment for gestational diabetes, or were lacking data on gestational glycemia or breastfeeding (Supplementary figure 1). Written informed consent and approval from the National Healthcare Group Domain Specific Review Board and SingHealth Centralized Institutional Review Board were obtained.
Exposure
Breastfeeding practices and duration were captured using interviewer-administered questionnaires administered from postnatal visit week 3, every quarter from month 3 to month 18 (with monthly breastfeeding practices within the 3-month interval recorded at each postnatal visit), and yearly from year 2 up to year 4. During each interview, the mothers were asked if they were exclusively breastfeeding (only breastmilk is given but the baby may also receive oral rehydration solution, medicines, and vitamin or mineral drops), predominantly breastfeeding (breastmilk is given as the predominant source of nourishment but water, water-based drinks, oral rehydration solution, medicines, and vitamin or mineral drops may be given), partially breastfeeding (sometimes breastmilk is given while other times formula milk is given), or not breastfeeding. We classified participants into 3 categories according to their breastfeeding duration and exclusivity – “high breastfeeding” (exclusively/predominantly breastfed till 4 months and at least partially breastfed till 6 months), “low breastfeeding” (exclusively formula-fed before 3 months), “intermediate breastfeeding” (breastfed beyond 3 months but did not meet the criteria for high breastfeeding) [33]. Due to limited sample size in the “high breastfeeding” group, we grouped participants from the “high” and “intermediate” categories together, forming the “high/intermediate breastfeeding” group (infants received breastmilk for at least 3 months) and “low breastfeeding” group (infants were exclusively formula-fed before age 3 months). As a sensitivity analysis, we also explored grouping participants into 3 categories based on breastfeeding intensity in the first 3 months after birth: Exclusive/predominant breastfeeding vs. mixed feeding (a combination of breastfeeding and formula feeding) vs. exclusive formula feeding.
Effect modifier
At the 26-28wk pregnancy visit, all mothers, not just those with suspected gestational diabetes, were invited to undergo a 75g 2-hour oral glucose tolerance test [7]. FPG and 2hrPG were measured by colorimetry [Advia 2400 Chemistry system (Siemens Medical Solutions Diagnostics, Deerfield, IL, USA) and Beckman LX20 Pro analyser (Beckman Coulter, USA)]. Mothers diagnosed with gestational diabetes (n=131) according to the 1999 WHO criteria (FPG ≥7.0mmol/L or 2hrPG ≥7.8mmol/L) were placed under either a diet treatment (88.6%), insulin treatment (6.4%), or no treatment (5%). The minority of participants placed under insulin treatment were excluded from the study due to potential significant biological effects of insulin treatment which might confound the findings. Gestational FPG and 2hrPG were each independently assessed as a measure of gestational glycemia and used as a continuous variable.
Outcomes: Cardiometabolic risk markers in 6-year-old offspring
Due to differential rates of consent for different cardiometabolic measures to be taken, outcome measures were available in different subsets of the 827 included children. All measured outcome variables were taken as continuous variables. Standing height (SECA 213 stadiometer) and weight (SECA 803 Weighing Scale) were measured using standardized protocols [7], and were used to calculate BMI. Sex- and age-standardized z-scores of BMI (z-BMI) was calculated using the WHO child growth standards [34]. To measure overall adiposity, Quantitative Magnetic Resonance (QMR) (EchoMRI-Adolescent Humans Body Composition Analyzer, EchoMRI Corporation, Singapore) with a low magnetic field (0.007 Tesla) was performed [35]. We calculated body fat % using the following formula: [total body fat (kg) / total body weight (kg)]*100%.
To assess body fat partitioning, abdominal subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) were obtained by abdominal MRI using the Siemens Skyra 3T MR scanner performed using a water-suppressed HASTE sequence (repetition time: 1000 ms, echo time: 95 ms) [36]. SAT and VAT compartments were identified using a fully automated segmentation algorithm [37] and volumes of each fat compartment were calculated by multiplying the image resolution by the sum of the voxels (a three-dimensional pixel). Intramyocellular lipids (IMCL) in the soleus muscle was assessed by proton magnetic resonance spectroscopy (1H-MRS) performed by the point-resolved spectroscopy (PRESS) sequence (with TR=2000ms, TE =33ms and Nacq=24) following T1-weighted axial localization. LC-Model [38] was used to quantify the water and lipid spectra, with T2 correction of peaks using T2 values reported in literature [39] and normalization of intramyocellular lipid (IMCL) peak area to the water peak from a water-unsuppressed scan. Liver fat was assessed by 1H-MRS using a PRESS sequence with respiratory gating (TR/TR=30/2000ms, Signal Averages=4), to account for respiratory motion. Area of the water resonance (4.7 ppm) and lipid resonances (0.5-3 ppm) in the liver spectrum were quantified using LC-Model [38] with T2 correction of the water and lipid peaks [40] to estimate liver fat percentage by weight using validated methods reported in literature [41, 42]. A correction factor of 0.914 was used to account for the fact that lipid peaks between 0.5-3ppm represent 91.4% of the total lipid peak area [43].
Peripheral systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in duplicate from the right upper arm (Dinamap CARESCAPE V100, GE Healthcare, Milwaukee, WI) according to standardized protocols [44]. Venous plasma glucose was measured after an overnight fast using the hexokinase enzymatic method (Abbott Architect c8000 analyzer at KKH and Beckman AU5800 analyzer at NUH). Concentrations of fasting insulin were measured using a sandwich immunoassay (Beckman DXL800 analyzer, Beckman Coulter), triglycerides and total cholesterol using the colorimetric method (Beckman AU5800 analyzer, Beckman Coulter), and HDL using the enzymatic method (Beckman AU5800 analyzer, Beckman Coulter). LDL level was calculated as: Total cholesterol (mmol/L) – HDL (mmol/L) – triglycerides (mmol/L) / 2.2 [45]. We also calculated homeostasis model assessment of insulin resistance (HOMA-IR) using the following formula: [fasting insulin (mU/L) * fasting glucose (mmol/L)] / 22.5 [46].
Covariates
Maternal age, highest educational attainment, ethnicity, and self-reported pre-pregnancy weight were collected at recruitment through interviewer-administered questionnaires. Gestational age (in weeks) was calculated based on first trimester ultrasound scans conducted by trained ultrasonographers.
At the 26-28 week antenatal study visit, maternal height was measured using a calibrated stadiometer (SECA213 Stadiometer, SECA Corp, Hamburg, Germany). Interviewer-administered tobacco exposure questionnaires and objectively measured plasma cotinine by liquid chromatography-tandem mass spectrometry as previously described [47] were used to classify mothers into three tobacco exposure groups: No exposure (no self-reported tobacco exposure and no detectable cotinine), exposure (self-reported tobacco exposure but no detectable cotinine), and exposure with detectable cotinine (self-reported tobacco exposure and detectable cotinine).
Infant birthweight, sex, and parity were obtained from medical records. Cohort-specific birthweight percentiles, adjusted for sex and gestational age, were derived [48]. We classified an infant as small-for-gestational-age (SGA) if the infant had a birthweight less than the 10th centile for gestational age.
Statistical analysis
All analyses were performed using the Stata16.0 software (StataCorp LP, TX) with statistical significance set at p < 0.05. We evaluated the association between breastfeeding (dichotomously categorized as high/intermediate vs. low) and each of the child cardiometabolic risk markers (z-BMI, body fat %, SAT, VAT, IMCL, liver fat, SBP, DBP, triglycerides, HDL, LDL, HOMA-IR) measured as continuous variables across the range of gestational FPG using multiple linear regression models with an interaction term (gestational FPG x breastfeeding). All models were adjusted for the following confounders: maternal age, education, ethnicity, pre-pregnancy BMI, tobacco exposure, child birth size, and child sex. To investigate the influence of breastfeeding intensity, the regression analysis described above was repeated using breastfeeding intensity in the first 3 months after birth (3 category variable: exclusive/predominant breastfeeding vs. mixed feeding vs. exclusive formula feeding) in place of the original breastfeeding classification (high/intermediate vs. low).
To visualize these regression models, we used the margins command to estimate the adjusted means (adjusted for confounders) of each cardiometabolic outcome for the high/intermediate and low breastfeeding groups, over the entire range of FPG measured in this study population. We plotted these using the marginsplot command. For outcomes where we found a statistically significant interaction between FPG and breastfeeding, we also plotted the adjusted mean and 95% confidence interval of the outcomes stratified by breastfeeding categories at the following discrete FPG levels: mean, mean+SD and mean+2SD. We repeated the analyses using 2hrPG in place of FPG.
Results
Cohort description
Of 1000 participants who fulfilled the inclusion criteria for this study, we had 827 mother-offspring dyads with both gestational glycemia and breastfeeding data available (Supplementary figure 1). Among the participants, gestational FPG levels ranged from 2.9 mmol/L to 7.9 mmol/L while 2hrPG levels ranged from 2.9 mmol/L to 12.1 mmol/L. Included participants were classified into the “low breastfeeding” (n = 409) and “high/intermediate breastfeeding” (n = 418) groups. Table 1 & Supplementary Table 1 show the maternal/offspring characteristics and offspring cardiometabolic assessments at age 6y, respectively, stratified by the breastfeeding levels. Mothers in the high/intermediate breastfeeding group were more likely to be older, of higher education attainment, of Chinese ethnicity, have no tobacco exposure, have lower pre-pregnancy BMI, and less likely to have small-for-gestational-age offspring compared to mothers in the low breastfeeding group.
Table 1. Demographic and clinical characteristics of participants.
Abbreviations: SD = standard deviation; ppBMI = pre-pregnancy body mass index; FPG = fasting plasma glucose; 2hrPG = 2-hour plasma glucose after a 75g oral glucose tolerance test; GDM = gestational diabetes mellitus; SGA = small-for-gestational-age
| All (n = 827) | Low breastfeeding (n = 409) | High/intermediate breastfeeding (n = 418) | |||||
|---|---|---|---|---|---|---|---|
| n | % / mean ± SD | n | % / mean ± SD | n | % / mean ± SD | p | |
| Maternal characteristics | |||||||
| Maternal age (yr) | 827 | 31.1 ± 5.1 | 409 | 30.1 ± 5.4 | 418 | 32.0 ± 4.7 | <0.001 |
| Maternal education | <0.001 | ||||||
| University | 288 | 35.2% | 60 | 14.8% | 228 | 55.2% | |
| Post-secondary | 280 | 34.2% | 166 | 41.0% | 114 | 27.6% | |
| Secondary or lower | 250 | 30.6% | 179 | 44.2% | 71 | 17.2% | |
| Ethnicity | <0.001 | ||||||
| Chinese | 467 | 56.5% | 200 | 48.9% | 267 | 63.9% | |
| Malay | 212 | 25.6% | 144 | 35.2% | 68 | 16.3% | |
| Indian | 148 | 17.9% | 65 | 15.9% | 83 | 19.9% | |
| Parity | 0.839 | ||||||
| Primiparous | 357 | 43.2% | 178 | 43.5% | 179 | 42.8% | |
| Multiparous | 470 | 56.8% | 231 | 56.5% | 239 | 57.2% | |
| Tobacco exposure | <0.001 | ||||||
| No exposure | 427 | 54.2% | 142 | 37.2% | 285 | 70.2% | |
| Exposure | 223 | 28.3% | 139 | 36.4% | 84 | 20.7% | |
| Exposure with detectable cotinine | 138 | 17.5% | 101 | 26.4% | 37 | 9.1% | |
| Maternal height (cm) | 815 | 158.2 ± 5.6 | 402 | 157.9 ± 5.7 | 413 | 158.4 ± 5.6 | 0.163 |
| ppBMI (kg/m2) | 763 | 22.7 ± 4.4 | 377 | 23.2 ± 5.0 | 386 | 22.1 ± 3.6 | <0.001 |
| FPG (mmol/L) | 827 | 4.33 ± 0.43 | 409 | 4.35 ± 0.46 | 418 | 4.31 ± 0.40 | 0.188 |
| 2hrPG (mmol/L) | 827 | 6.41 ± 1.38 | 409 | 6.24 ± 1.38 | 418 | 6.57 ± 1.35 | <0.001 |
| GDM status | 0.196 | ||||||
| No GDM | 696 | 84.2% | 351 | 85.8% | 345 | 82.5% | |
| GDM | 131 | 15.8% | 58 | 14.2% | 73 | 17.5% | |
| Child characteristics | |||||||
| Sex | 0.801 | ||||||
| Female | 402 | 48.6% | 197 | 48.2% | 205 | 49.0% | |
| Male | 425 | 51.4% | 212 | 51.8% | 213 | 51.0% | |
| Gestational age (wk) | 827 | 39.1 ± 1.0 | 409 | 39.0 ± 1.0 | 418 | 39.1 ± 1.0 | 0.084 |
| Birthweight (kg) | 827 | 3.14 ± 0.40 | 409 | 3.12 ± 0.40 | 418 | 3.17 ± 0.39 | 0.042 |
| Birth size | 0.046 | ||||||
| Not SGA | 736 | 89.0% | 355 | 86.8% | 381 | 91.1% | |
| SGA | 91 | 11.0% | 54 | 13.2% | 37 | 8.9% | |
Effect modification by FPG
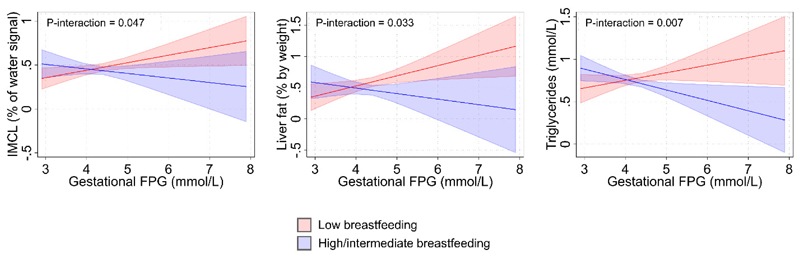
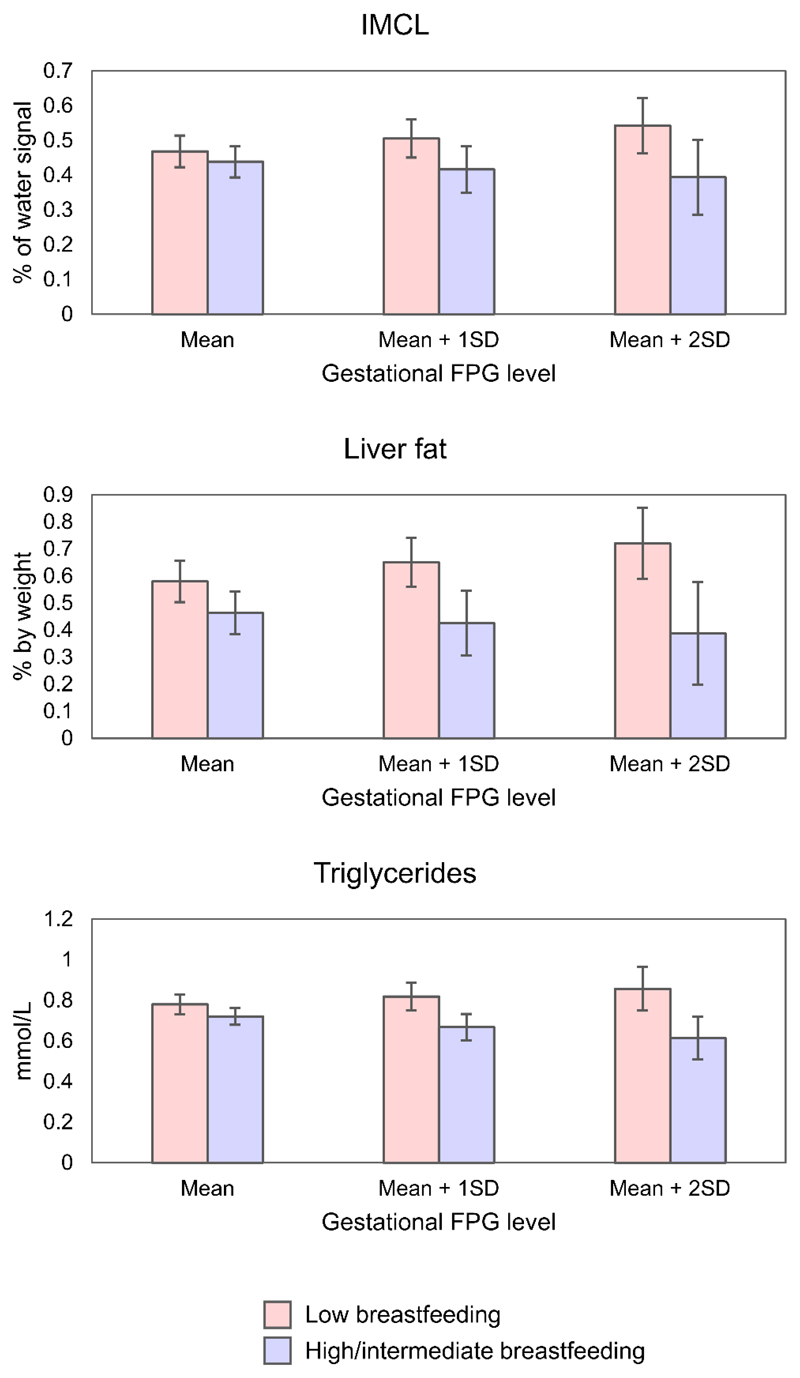
Associations between the 2 breastfeeding groups and child cardiometabolic risk markers across the range of gestational FPG can be visualized in Figure 1 and Supplementary Figure 2. There was a significant negative interaction term between gestational FPG and breastfeeding for IMCL (p-interaction=0.047), liver fat (p-interaction=0.033), and triglycerides (p-interaction=0.007) (Table 2). This can be visualized in Figure 1 with a significantly more negative gradient in the graph for the high/intermediate breastfeeding group compared to the reference low breastfeeding group, which shows lowered cardiometabolic risk markers in the high/intermediate (vs. low) breastfeeding group when there is increased gestational glycemia. With increasing gestational FPG, high/intermediate (vs. low) breastfeeding was linked to decreasing levels of IMCL (-0.14% of water signal per mmol/L increase in FPG [-0.27, 0.00]), liver fat (-0.25% by weight per mmol/L increase in FPG [-0.48, -0.02]), and triglycerides (-0.21mmol/L per mmol/L increase in FPG [-0.37, -0.06]). Specifically, at an elevated gestational FPG level 2 standard deviations above the mean (FPG mean + 2SD = 5.19mmol/L), high/intermediate (vs. low) breastfeeding was linked to lower adjusted mean IMCL (0.39% of water signal [0.29,0.50] vs. 0.54% of water signal [0.46,0.62]), liver fat (0.39% by weight [0.20,0.58] vs. 0.72% by weight [0.59,0.85]), and triglycerides (0.62mmol/L [0.51,0.72] vs. 0.86mmol/L [0.75,0.97]) (Figure 2). We detected no significant interactions between gestational FPG and breastfeeding for all other outcomes.
Fig. 1.
Adjusted mean cardiometabolic risk markers at 6 years old for the 2 breastfeeding groups, over the range of gestational fasting plasma glucose measured. Models were adjusted for maternal age, maternal education, ethnicity, tobacco exposure, pre-pregnancy BMI, child birth size, and child sex. 95% confidence interval bands were shown. Only models with statistically significant interaction terms are shown. Abbreviations: FPG = fasting plasma glucose; z-BMI = body mass index z-score; SDS = standard deviation score; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; IMCL = intramyocellular lipid; SBP = systolic blood pressure; DBP = diastolic blood pressure; HDL = high-density lipoproteins; LDL = low-density lipoproteins; HOMA-IR = homeostasis model assessment of insulin resistance
Table 2.
Association between gestational fasting plasma glucose and breastfeeding with cardiometabolic risk markers at age 6 years, with low breastfeeding as the reference group. Models were adjusted for maternal age, maternal education, ethnicity, tobacco exposure, pre-pregnancy BMI, child birth size, and child sex. Abbreviations: FPG = fasting plasma glucose; z-BMI = body mass index z-score; SDS = standard deviation score; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; IMCL = intramyocellular lipid; SBP = systolic blood pressure; DBP = diastolic blood pressure; HDL = high-density lipoproteins; LDL = low-density lipoproteins; HOMA-IR = homeostasis model assessment of insulin resistance
| N | Main effect: FPG b (95% CI) | Main effect: Breastfeeding b (95% CI) | Interaction: FPG x Breastfeeding b (95% CI) | p-interaction | |
|---|---|---|---|---|---|
| Adiposity | |||||
| z-BMI (SDS) | 573 | 0.32 (0.02, 0.62) | 1.94 (-0.08, 3.96) | -0.43 (-0.89, 0.04) | 0.072 |
| Body fat % | 258 | 2.57 (0.58, 4.55) | 11.16 (-2.58, 24.90) | -2.49 (-5.63, 0.65) | 0.119 |
| SAT (cc) | 281 | 11.59 (-126.47, 149.65) | -221.64 (-1,114.14, 670.86) | 50.02 (-155.49, 255.53) | 0.632 |
| VAT (cc) | 284 | 6.54 (-17.02, 30.11) | -14.00 (-167.62, 139.62) | 2.14 (-33.20, 37.48) | 0.905 |
| IMCL (% of water signal) | 267 | 0.09 (0.01, 0.16) | 0.56 (-0.03, 1.15) | -0.14 (-0.27, 0.00) | 0.047 |
| Liver fat (% by weight) | 271 | 0.16 (0.03, 0.30) | 0.97 (-0.03, 1.98) | -0.25 (-0.48, -0.02) | 0.033 |
| Cardiovascular Markers | |||||
| SBP (mmHg) | 477 | 0.56 (-1.65, 2.77) | 1.09 (-14.18, 16.36) | -0.17 (-3.69, 3.35) | 0.926 |
| DBP (mmHg) | 477 | 0.09 (-1.45, 1.63) | 5.59 (-5.05, 16.23) | -1.33 (-3.78, 1.12) | 0.288 |
| Metabolic Markers | |||||
| Triglycerides (mmol/L) | 267 | 0.09 (-0.02, 0.20) | 0.86 (0.20, 1.52) | -0.21 (-0.37, -0.06) | 0.007 |
| HDL (mmol/L) | 267 | 0.03 (-0.08, 0.15) | 0.17 (-0.50, 0.83) | -0.04 (-0.19, 0.11) | 0.606 |
| LDL (mmol/L) | 267 | -0.19 (-0.47, 0.10) | -0.31 (-1.94, 1.33) | 0.07 (-0.31, 0.44) | 0.731 |
| HOMA-IR (units) | 266 | 0.12 (-0.10, 0.35) | 0.69 (-0.62, 1.99) | -0.13 (-0.43, 0.17) | 0.398 |
Fig. 2.
Adjusted mean cardiometabolic risk markers at 6 years old for the 2 breastfeeding groups, at 3 different levels of gestational fasting plasma glucose (mean, mean + 1SD, mean + 2SD), with 95% confidence intervals shown. Models were adjusted for maternal age, maternal education, ethnicity, tobacco exposure, pre-pregnancy BMI, child birth size, and child sex. Only models with significant interaction terms were shown. Abbreviations: FPG = fasting plasma glucose; SD = standard deviation; IMCL = intramyocellular lipid
In sensitivity analysis using breastfeeding intensity categories in place of the original breastfeeding classification, findings on increased breastfeeding and lowered cardiometabolic risk markers were consistent. There were significant negative interaction terms between gestational FPG and breastfeeding intensity (mixed feeding vs. exclusive formula feeding) on several cardiometabolic risk markers: z-BMI (p-interaction<0.001), IMCL (p-interaction=0.038), and liver fat (p-interaction=0.018) (Supplementary Table 2). There was also a significant negative interaction term between gestational FPG and breastfeeding intensity (exclusive/predominant breastfeeding vs. exclusive formula feeding) for z-BMI (p-interaction=0.011). These mean that with increasing gestational FPG, mixed feeding (vs. exclusive formula feeding) was linked to decreasing levels of z-BMI (-1.48SDS per mmol/L increase in FPG [-2.16, -0.79]), IMCL (-0.17% of water signal per mmol/L increase in FPG [-0.33, -0.01]), and liver fat (-0.33% by weight per mmol/L increase in FPG [-0.60, -0.06]), while exclusive/predominant breastfeeding (vs. exclusive formula feeding) was linked to decreasing levels of z-BMI (-1.29SDS per mmol/L increase in FPG [-2.28, -0.30]).
Effect modification by 2hrPG
Associations between breastfeeding and childhood cardiometabolic risk markers were not altered over the range of gestational 2hrPG, with no statistically significant interaction terms reported (Table 3). In sensitivity analysis using breastfeeding intensity categories in place of the original breastfeeding classification, findings were generally consistent, with no significant interaction terms except for a significant negative interaction term between gestational 2hrPG and breastfeeding intensity (mixed feeding vs. exclusive formula feeding) on z-BMI (p-interaction=0.013). This means that with increasing gestational 2hrPG, mixed feeding (vs. exclusive formula feeding) was linked to decreasing levels of z-BMI (-0.47SDS per mmol/L increase in 2hrPG [-0.84, -0.10]).
Table 3.
Association between gestational 2-hour glucose and breastfeeding with cardiometabolic risk markers at age 6 years, with low breastfeeding as the reference group. Models were adjusted for maternal age, maternal education, ethnicity, tobacco exposure, pre-pregnancy BMI, child birth size, and child sex. Abbreviations: 2hrPG = 2-hour plasma glucose after a 75g oral glucose tolerance test; z-BMI = body mass index z-score; SDS = standard deviation score; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; IMCL = intramyocellular lipid; SBP = systolic blood pressure; DBP = diastolic blood pressure; HDL = high-density lipoproteins; LDL = low-density lipoproteins; HOMA-IR = homeostasis model assessment of insulin resistance
| N | Main effect: 2hrPG b (95% CI) | Main effect: Breastfeeding b (95% CI) | Interaction: 2hrPG x Breastfeeding b (95% CI) | p-interaction | |
|---|---|---|---|---|---|
| Adiposity | |||||
| z-BMI (SDS) | 573 | -0.01 (-0.12, 0.09) | 0.13 (-0.83, 1.09) | -0.01 (-0.15, 0.14) | 0.937 |
| Body fat % | 258 | 0.24 (-0.49, 0.97) | -0.75 (-7.69, 6.19) | 0.14 (-0.93, 1.22) | 0.796 |
| SAT (cc) | 281 | 7.95 (-36.15, 52.05) | -26.65 (-424.36, 371.06) | 2.79 (-58.17, 63.75) | 0.928 |
| VAT (cc) | 284 | 2.88 (-4.55, 10.30) | -18.00 (-86.08, 50.08) | 1.95 (-8.46, 12.36) | 0.713 |
| IMCL (% of water signal) | 267 | 0.01 (-0.02, 0.04) | 0.08 (-0.22, 0.37) | -0.02 (-0.06, 0.03) | 0.457 |
| Liver fat (% by weight) | 271 | 0.02 (-0.04, 0.07) | -0.04 (-0.54, 0.45) | -0.01 (-0.09, 0.06) | 0.749 |
| Cardiovascular Markers | |||||
| SBP (mmHg) | 477 | -0.64 (-1.42, 0.14) | -5.12 (-12.01, 1.78) | 0.86 (-0.19, 1.90) | 0.110 |
| DBP (mmHg) | 477 | -0.19 (-0.73, 0.36) | 0.51 (-4.30, 5.33) | -0.10 (-0.83, 0.64) | 0.799 |
| Metabolic Markers | |||||
| Triglycerides (mmol/L) | 267 | 0.01 (-0.02, 0.04) | -0.02 (-0.32, 0.27) | -0.01 (-0.05, 0.04) | 0.824 |
| HDL (mmol/L) | 267 | 0.01 (-0.02, 0.05) | 0.14 (-0.15, 0.43) | -0.02 (-0.07, 0.02) | 0.301 |
| LDL (mmol/L) | 267 | -0.04 (-0.12, 0.05) | -0.25 (-0.96, 0.47) | 0.04 (-0.07, 0.14) | 0.510 |
| HOMA-IR (units) | 266 | 0.03 (-0.03, 0.10) | 0.55 (-0.01, 1.11) | -0.07 (-0.15, 0.02) | 0.128 |
Discussion
We found breastfeeding to be associated with improvements in several cardiometabolic biomarkers (decreased IMCL, liver fat, and triglycerides) in children at the age of 6 years whose mothers had elevated levels of gestational FPG. These cardiometabolic biomarkers have been linked to cardiometabolic risk in adults as well as children [49, 50]. These changes occurred without significant alterations in BMI, which is regarded as a practical, albeit crude, proxy for adiposity and cardiometabolic risk. Despite emerging evidence linking increased gestational glycemia with altered breastmilk composition [16, 20–22], we did not find breastfeeding to be significantly associated with any adverse cardiometabolic risk biomarkers among children born to mothers with elevated gestational glycemia (FPG or 2hrPG) across the numerous cardiometabolic biomarkers investigated and sensitivity analyses performed in this study; on the contrary, breastfeeding is associated with a protective effect. Hence, our results support the current breastfeeding recommendations to encourage breastfeeding, even among mothers with increased gestational glycemia.
Our findings contradict studies which suggested that breastmilk from mothers with pre-existing diabetes or gestational diabetes might be detrimental to the offspring’s obesity and cardiometabolic risk. Among 112 offspring of mothers with either type 1 diabetes (n=83) or gestational diabetes (n=29), increased volume of diabetic breastmilk (vs. nondiabetic banked donor breastmilk) consumed in the first 7 days after birth was associated with increased risk of overweight at 2 years old [3], Differences in our findings might be due to differences in the metabolic status of mothers, since our cohort does not include mothers with type 1 diabetes, as well as differences in the racial/ethnic groups between the two study populations. Additionally, there were differences in exposures investigated, where we compared breastfeeding to formula feeding rather than nondiabetic banked donor breastmilk which is less accessible, and we studied breastfeeding duration and intensity over a longer period of time (≥3 months). Our findings also contradict earlier reports which suggested that breastmilk from mothers with pre-existing diabetes or gestational diabetes might have an altered composition and be detrimental to the offspring’s obesity and cardiometabolic risk. There could be several explanations behind this. Firstly, although a few studies have found that breastmilk from mothers with pre-existing diabetes or gestational diabetes had increased glucose [15] and insulin levels [16, 17], this association might be dependent on postpartum glycemic control and other factors. For instance, van Beusekom and colleagues (1993) reported no associations between well-controlled maternal diabetes and glucose levels in breastmilk [51]. Secondly, the association between glucose levels in breastmilk and infant adiposity or later obesity risk is unclear. Although a study had reported increased infant adiposity [18] as a result of consuming breastmilk with increased glucose levels, another study reported no associations [52]. In fact, increased glucose or insulin in the colostrum or breastmilk might protect the offspring of mothers with gestational diabetes from postpartum hypoglycemia which they are vulnerable to [53]. Thirdly, although breastmilk from mothers with gestational diabetes had decreased adiponectin which might increase appetite and had been associated with increased infant growth in the first three months [16], other studies suggest that there might be a reversal in associations beyond early infancy. Specifically, studies have associated decreased adiponectin with decreased skinfold thickness, decreased risk of overweight, and slower growth up to 2 years of age [54–56]. These studies suggest that decreased adiponectin in breastmilk from mothers with gestational diabetes might eventually be linked to reduced obesity risk later in life, which offspring of mothers with gestational diabetes are vulnerable to. Taken together with our findings, breastmilk from mothers with pre-existing diabetes or gestational diabetes seems to be “personalized” to the offspring’s nutritional needs by protecting offspring of mothers with diabetes or gestational diabetes against early postpartum hypoglycemia and increased obesity risk which they are particularly vulnerable to. More studies need to be done in this nascent field to understand the complex alterations in breastmilk composition among mothers with different metabolic status and their role in programming offspring’s cardiometabolic health.
Similar to two other Asian cohort studies which reported no association between breastfeeding and childhood BMI among offspring born to mothers with gestational diabetes [7, 8], we did not find high/intermediate breastfeeding to be linked to significantly altered z-BMI among offspring of mothers with elevated gestational glycemia. However, there might be protective effects for cardiometabolic risk markers despite no significant associations with BMI, which we have shown in the current study.
Our findings were consistent with studies which also found that longer breastfeeding duration or exclusivity among mothers with gestational diabetes was associated with decreased offspring metabolic risk, though ours is the first to report decreased IMCL and liver fat and to report findings across the continuum of gestational FPG and 2hrPG separately. In a retrospective study involving 60 overweight or obese Hispanic children of mothers with self-reported gestational diabetes, infants breastfed for at least 1 month had decreased risk of being diagnosed with prediabetes or metabolic syndrome from 8-13 years old [57]. Another retrospective study with 89 children exposed to diabetes in utero reported that longer duration of breastfeeding (≥6 breastmilk months) was associated with lower VAT and SAT at 6-13 years, which suggests decreased metabolic risk [58]. Similarly, in 564 offspring (84 exposed to gestational diabetes) from the Exploring Perinatal Outcomes among Children study, longer duration of breastfeeding (≥6 breastmilk months) attenuated the association between gestational diabetes and increased VAT and SAT [59]. Several studies have also linked breastfeeding to reduced risk of overweight/obesity in offspring born to mothers with gestational diabetes [5, 6, 23–25]. For instance, Vandyousefi et al. have shown a negative association between breastfeeding and childhood obesity (2-5y) in more than 800 children exposed to gestational diabetes in utero [5].
Interestingly, longer duration of breastfeeding was associated with improvements in several cardiometabolic risk markers at elevated gestational FPG, but not 2hrPG. We propose 2 potential explanations for the lack of significant associations with 2hrPG. First, in our cohort, most mothers who were diagnosed with gestational diabetes (based on the WHO 1999 criteria, which was in use at the time of study recruitment) had elevated gestational 2hrPG (≥7.8mmol/L), rather than elevated gestational FPG (≥7mmol/L). These women were placed on a diet treatment, which could have attenuated the associations between 2hrPG and offspring cardiometabolic outcomes. The second potential explanation is that protective effects of breastfeeding compared to formula feeding for offspring cardiometabolic health might be more easily detected in the “metabolically unhealthier” group, which is the group prone to greater metabolic risk. We found that the benefit of longer duration of breastfeeding was not as obvious at lower levels of FPG and only became more obvious with increasing gestational FPG, which represents poorer maternal metabolic health. Similarly, larger effect sizes for gestational FPG compared to gestational 2hrPG suggest that in our cohort, the former might be more indicative of maternal metabolic health and transmission of metabolic risk to the offspring. These are supported by previous studies which have also found larger effect sizes for gestational FPG compared to gestational 2hrPG on neonatal overall adiposity, neonatal abdominal adiposity, and abdominal adiposity at age 4.5 years [29, 60]. Furthermore, Schaefer-Graf et al. found that being in the 4th quartile (vs. 1st quartile) of fasting glucose level during pregnancy was the best predictor of postpartum diabetes mellitus (21-fold increased odds ratio), followed by the severity of glucose intolerance (3- to 4-fold increased odds ratio) [61].
Our study has several strengths and limitations. To the best of our knowledge, the current study is the first to investigate the effects of breastfeeding on offspring cardiometabolic profile over the range of FPG and 2hrPG values in an Asian cohort. First, we assessed objectively measured cardiometabolic markers in childhood, many of which could indicate an adverse cardiometabolic phenotype, undetected by conventional measures such as weight and BMI. Assessing ectopic fat deposition to the intra-abdominal tissue, liver, and muscles enables detection of early “adipose tissue overflow” which is especially pertinent in South Asians who are at higher cardiometabolic risk [62]. Second, we examined the associations over a continuous range of gestational glycemia, rather than using a discrete cut-off, as the effects of gestational glycemia on child outcomes exist on a continuum [31, 60, 63]. We also investigated gestational FPG and 2hrPG as separate markers of gestational glycemia which may be pathophysiologically distinct [28]. Third, the prospective design of our cohort and frequent administration of breastfeeding questionnaires reduced recall bias. Fourth, we collected an extensive range of sociodemographic and clinical confounders to reduce confounding biases. However, our study is limited by the lack of information on the postpartum glucose control of mothers, breastmilk composition, or volume of breastmilk consumed, which might be potential mediators and shed light on the potential mechanisms at play. We are also limited by the lack of sample size to classify participants into more specific categories of breastfeeding duration and intensity. Gestational glycemia might not be sufficiently characterized by measurement only at one timepoint (26-28 weeks of gestation) and future studies measuring gestational glycemia at multiple timepoints might be useful. Missing exposure and outcome data due to differential rates of consent and loss to follow up could lead to potential selection bias and relatively small sample sizes for many of the outcomes might cause us to be underpowered to detect significant interactions. Due to the observational study design, it is difficult to infer causality and to exclude the possibility of residual confounding from unmeasured confounders such as environmental conditions and food insecurity. Findings in our multi-ethnic Asian cohort might not be generalizable to other populations and other racial/ethnic groups.
In conclusion, greater breastfeeding exposure was linked to reduced childhood cardiometabolic risk (reduced ectopic fat accumulation and lower triglycerides) in children who were exposed to increased levels of gestational FPG. This suggests that breastfeeding might have some metabolically protective effects in this vulnerable group of children who are at increased cardiometabolic risk. Our findings provide an impetus for more intensive investigations on breastfeeding as a potential modifiable factor to reduce cardiometabolic risk among offspring of mothers with increased gestational glycemia, and to reduce the intergenerational transmission of cardiometabolic risk.
Supplementary Material
Acknowledgements
We thank all GUSTO participants as well as the GUSTO study group, which includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Chung Ning Law, Evelyn Xiu Ling Loo, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira., Jonathan Y. Bernard, Joshua J. Gooley, Kenneth Kwek, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Mark Hanson, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael Meaney, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, See Ling Loy, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, and Yin Bun Cheung.
Funding
This work was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore [NMRC/TCR/004-NUS/2008, NMRC/TCR/012-NUHS/2014]. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group (17/63/154) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus+ Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP and ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174).
Declarations
Conflicts of interest: YSC, KMG, and SYC are part of an academic consortium that has received research funding from companies selling nutritional products. KMG and SYC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. All other authors have nothing to disclose.
Ethics approval
Written informed consent and approval from the National Healthcare Group Domain Specific Review Board and SingHealth Centralized Institutional Review Board were obtained (Ethics approval number: NHG DSRB Ref: 2009/00021). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Freely-given, informed consent to participate in the study was obtained from participants (or their parent or legal guardian in the case of children under 16)
Consent for publication
Not applicable
Availability of data and material
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Code availability
Not applicable.
References
- 1.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:30–37. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 2.Ma J, Qiao Y, Zhao P, et al. Breastfeeding and childhood obesity: A 12-country study. Matern Child Nutr. 2020;16:e12984. doi: 10.1111/mcn.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plagemann A, Harder T, Franke K, Kohlhoff R. Long-Term Impact of Neonatal Breast-Feeding on Body Weight and Glucose Tolerance in Children of Diabetic Mothers. Diabetes Care. 2002;25:16–22. doi: 10.2337/diacare.25.1.16. [DOI] [PubMed] [Google Scholar]
- 4.Dugas C, Perron J, Kearney M, et al. Postnatal Prevention of Childhood Obesity in Offspring Prenatally Exposed to Gestational Diabetes mellitus: Where Are We Now? Obes Facts. 2017;10:396–406. doi: 10.1159/000477407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandyousefi S, Davis JN, Gunderson EP. Association of infant diet with subsequent obesity at 2-5 years among children exposed to gestational diabetes: the SWIFT study. Diabetologia. 2021;64:1121–1132. doi: 10.1007/s00125-020-05379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shearrer GE, Whaley SE, Miller SJ, et al. Association of gestational diabetes and breastfeeding on obesity prevalence in predominately Hispanic low-income youth. Pediatr Obes. 2015;10:165–171. doi: 10.1111/ijpo.247. [DOI] [PubMed] [Google Scholar]
- 7.Aris IM, Soh SE, Tint MT, et al. Associations of infant milk feed type on early postnatal growth of offspring exposed and unexposed to gestational diabetes in utero. Eur J Nutr. 2017;56:55–64. doi: 10.1007/s00394-015-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui LL, Li AM, Nelson EaS, et al. In utero exposure to gestational diabetes and adiposity: does breastfeeding make a difference? Int J Obes (Lond) 2018;42:1317–1325. doi: 10.1038/s41366-018-0077-2. [DOI] [PubMed] [Google Scholar]
- 9.Saucedo R, Zarate A, Basurto L, et al. Relationship Between Circulating Adipokines and Insulin Resistance During Pregnancy and Postpartum in Women with Gestational Diabetes. Archives of Medical Research. 2011;42:318–323. doi: 10.1016/j.arcmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Zheng W, Huang W, et al. Risk factors for abnormal postpartum glucose outcome in women with gestational diabetes mellitus diagnosed by modified The International Association of the Diabetes and Pregnancy Study Groups criteria. Journal of Obstetrics and Gynaecology Research. 2019;45:1545–1552. doi: 10.1111/jog.14009. [DOI] [PubMed] [Google Scholar]
- 11.Rehder PM, Borovac-Pinheiro A, de Araujo ROMB, et al. Gestational Diabetes Mellitus and Obesity are Related to Persistent Hyperglycemia in the Postpartum Period. Rev Bras Ginecol Obstet. 2021;43:107–112. doi: 10.1055/s-0040-1721356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClean S, Farrar D, Kelly CA, et al. The importance of postpartum glucose tolerance testing after pregnancies complicated by gestational diabetes. Diabetic Medicine. 2010;27:650–654. doi: 10.1111/j.1464-5491.2010.03001.x. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic-Peterson L, Fuhrmann K, Hedden K, et al. Maternal milk and plasma glucose and insulin levels: studies in normal and diabetic subjects. Journal of the American College of Nutrition. 1989;8:125–131. doi: 10.1080/07315724.1989.10720287. [DOI] [PubMed] [Google Scholar]
- 14.Whitmore TJ, Trengove NJ, Graham DF, Hartmann PE. Analysis of insulin in human breast milk in mothers with type 1 and type 2 diabetes mellitus. Int J Endocrinol. 2012;2012:296368. doi: 10.1155/2012/296368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butte NF, Garza C, Burr R, et al. Milk composition of insulin-dependent diabetic women. J Pediatr Gastroenterol Nutr1. 1987;6:936–941. doi: 10.1097/00005176-198711000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Rong SS, Sun X, et al. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: a longitudinal study. Br J Nutr. 2018;120:1380–1387. doi: 10.1017/S0007114518002933. [DOI] [PubMed] [Google Scholar]
- 17.Ley SH, Hanley AJ, Sermer M, et al. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. The American Journal of Clinical Nutrition. 2012;95:867–874. doi: 10.3945/ajcn.111.028431. [DOI] [PubMed] [Google Scholar]
- 18.Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatric Obesity. 2012;7:304–312. doi: 10.1111/j.2047-6310.2012.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31:2236–2240. doi: 10.1016/j.peptides.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Badillo-Suárez PA, Rodríguez-Cruz M, Nieves-Morales X. Impact of Metabolic Hormones Secreted in Human Breast Milk on Nutritional Programming in Childhood Obesity. J Mammary Gland Biol Neoplasia. 2017;22:171–191. doi: 10.1007/s10911-017-9382-y. [DOI] [PubMed] [Google Scholar]
- 21.Peila C, Gazzolo D, Bertino E, et al. Influence of Diabetes during Pregnancy on Human Milk Composition. Nutrients. 2020;12 doi: 10.3390/nu12010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen L, Wu Y, Yang Y, et al. Gestational Diabetes Mellitus Changes the Metabolomes of Human Colostrum, Transition Milk and Mature Milk. Med Sci Monit. 2019;25:6128–6152. doi: 10.12659/MSM.915827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaefer-Graf UM, Hartmann R, Pawliczak J, et al. Association of Breast-feeding and Early Childhood Overweight in Children From Mothers With Gestational Diabetes Mellitus. Diabetes Care. 2006;29:1105–1107. doi: 10.2337/dc05-2413. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, et al. Breast-Feeding and Risk for Childhood Obesity: Does maternal diabetes or obesity status matter? Diabetes Care. 2006;29:2231–2237. doi: 10.2337/dc06-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandyousefi S, Whaley SE, Widen EM, et al. Association of breastfeeding and early exposure to sugar-sweetened beverages with obesity prevalence in offspring born to mothers with and without gestational diabetes mellitus. Pediatric Obesity. 2019;14:e12569. doi: 10.1111/ijpo.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and Postpartum Glucose Regulation Among Women With Prior Gestational Diabetes: A Systematic Review. J Hum Lact. 2020;36:723–738. doi: 10.1177/0890334420950259. [DOI] [PubMed] [Google Scholar]
- 27.Shub A, Miranda M, Georgiou HM, et al. The effect of breastfeeding on postpartum glucose tolerance and lipid profiles in women with gestational diabetes mellitus. Int Breastfeed J. 2019;14:46. doi: 10.1186/s13006-019-0238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer C, Pimenta W, Woerle HJ, et al. Different Mechanisms for Impaired Fasting Glucose and Impaired Postprandial Glucose Tolerance in Humans. Diabetes Care. 2006;29:1909–1914. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- 29.Tint M-T, Sadananthan SA, Soh S-E, et al. Maternal glycemia during pregnancy and offspring abdominal adiposity measured by MRI in the neonatal period and preschool years: The Growing Up in Singapore Towards healthy Outcomes (GUSTO) prospective mother-offspring birth cohort study. Am J Clin Nutr. 2020 doi: 10.1093/ajcn/nqaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan WL, Lin J, Kramer MS, et al. Maternal glycaemia during pregnancy and child carotid intima media thickness, pulse wave velocity and augmentation index. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown FM, Isganaitis E, James-Todd T. Much to HAPO FUS About: Increasing Maternal Glycemia in Pregnancy Is Associated With Worsening Childhood Glucose Metabolism. Diabetes Care. 2019;42:393–395. doi: 10.2337/dci18-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy and Childbirth. 2018;18:494. doi: 10.1186/s12884-018-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai S, Pang WW, Low YL, et al. Infant feeding effects on early neurocognitive development in Asian children. Am J Clin Nutr. 2015;101:326–336. doi: 10.3945/ajcn.114.095414. [DOI] [PubMed] [Google Scholar]
- 34.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height for age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age, methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 35.Chen L-W, Tint M-T, Fortier MV, et al. Body composition measurement in young children using quantitative magnetic resonance: a comparison with air displacement plethysmography. Pediatr Obes. 2018;13:365–373. doi: 10.1111/ijpo.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semelka RC, Kelekis NL, Thomasson D, et al. HASTE MR imaging: description of technique and preliminary results in the abdomen. J Magn Reson Imaging. 1996;6:698–699. doi: 10.1002/jmri.1880060420. [DOI] [PubMed] [Google Scholar]
- 37.Sadananthan SA, Prakash B, Leow MK-S, et al. Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. Journal of Magnetic Resonance Imaging. 2015;41:924–934. doi: 10.1002/jmri.24655. [DOI] [PubMed] [Google Scholar]
- 38.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 39.Kautzky-Willer A, Krssak M, Winzer C, et al. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes. 2003;52:244–251. doi: 10.2337/diabetes.52.2.244. [DOI] [PubMed] [Google Scholar]
- 40.Chabanova E, Bille DS, Thisted E, et al. MR spectroscopy of liver in overweight children and adolescents: investigation of 1H T2 relaxation times at 3T. Eur J Radiol. 2012;81:811–814. doi: 10.1016/j.ejrad.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 42.Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011;24:784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aris IM, Bernard JY, Chen L-W, et al. Postnatal height and adiposity gain, childhood blood pressure and prehypertension risk in an Asian birth cohort. International Journal of Obesity. 2017;41:1011–1017. doi: 10.1038/ijo.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 46.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 47.Ng S, Aris IM, Tint MT, et al. High Maternal Circulating Cotinine During Pregnancy is Associated With Persistently Shorter Stature From Birth to Five Years in an Asian Cohort. Nicotine Tob Res. 2019;21:1103–1112. doi: 10.1093/ntr/nty148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikolajczyk RT, Zhang J, Betran AP, et al. A global reference for fetal-weight and birthweight percentiles. The Lancet. 2011;377:1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang HH, Lee DK, Liu M, et al. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr Gastroenterol Hepatol Nutr. 2020;23:189–230. doi: 10.5223/pghn.2020.23.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larson-Meyer DE, Newcomer BR, Ravussin E, et al. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia. 2011;54:869–875. doi: 10.1007/s00125-010-2022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Beusekom CM, Zeegers TA, Martini IA, et al. Milk of patients with tightly controlled insulin-dependent diabetes mellitus has normal macronutrient and fatty acid composition. Am J Clin Nutr. 1993;57:938–943. doi: 10.1093/ajcn/57.6.938. [DOI] [PubMed] [Google Scholar]
- 52.Fields DA, George B, Williams M, et al. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes. 2017;12(Suppl 1):78–85. doi: 10.1111/ijpo.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalsgaard BT, Rodrigo-Domingo M, Kronborg H, Haslund H. Breastfeeding and skin-to-skin contact as non-pharmacological prevention of neonatal hypoglycemia in infants born to women with gestational diabetes; a Danish quasi-experimental study. Sexual & Reproductive Healthcare. 2019;19:1–8. doi: 10.1016/j.srhc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Brunner S, Schmid D, Zang K, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. 2015;10:67–73. doi: 10.1111/j.2047-6310.2014.222.x. [DOI] [PubMed] [Google Scholar]
- 55.Weyermann M, Brenner H, Rothenbacher D. Adipokines in Human Milk and Risk of Overweight in Early Childhood: A Prospective Cohort Study. Epidemiology. 2007;18:722–729. doi: 10.1097/ede.0b013e3181567ed4. [DOI] [PubMed] [Google Scholar]
- 56.Woo JG, Guerrero ML, Guo F, et al. Human milk adiponectin affects infant weight trajectory during the second year of life. J Pediatr Gastroenterol Nutr. 2012;54:532–539. doi: 10.1097/MPG.0b013e31823fde04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandyousefi S, Goran MI, Gunderson EP, et al. Association of breastfeeding and gestational diabetes mellitus with the prevalence of prediabetes and the metabolic syndrome in offspring of Hispanic mothers. Pediatr Obes. 2019;14:e12515. doi: 10.1111/ijpo.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crume TL, Ogden L, Maligie M, et al. Long-Term Impact of Neonatal Breastfeeding on Childhood Adiposity and Fat Distribution Among Children Exposed to Diabetes In Utero. Diabetes Care. 2011;34:641–645. doi: 10.2337/dc10-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sauder KA, Bekelman TA, Harrall KK, et al. Gestational diabetes exposure and adiposity outcomes in childhood and adolescence: An analysis of effect modification by breastfeeding, diet quality, and physical activity in the EPOCH study. Pediatric Obesity. 2019;14:e12562. doi: 10.1111/ijpo.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aris IM, Soh SE, Tint MT, et al. Effect of Maternal Glycemia on Neonatal Adiposity in a Multiethnic Asian Birth Cohort. J Clin Endocrinol Metab. 2014;99:240–247. doi: 10.1210/jc.2013-2738. [DOI] [PubMed] [Google Scholar]
- 61.Schaefer-Graf UM, Buchanan TA, Xiang AH, et al. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. American Journal of Obstetrics and Gynecology. 2002;186:751–756. doi: 10.1067/mob.2002.121895. [DOI] [PubMed] [Google Scholar]
- 62.Sniderman AD, Bhopal R, Prabhakaran D, et al. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–225. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- 63.Aris IM, Soh SE, Tint MT, et al. Associations of gestational glycemia and prepregnancy adiposity with offspring growth and adiposity in an Asian population. Am J Clin Nutr. 2015;102:1104–1112. doi: 10.3945/ajcn.115.117614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Not applicable.