Abstract
Replacement of regular salt with potassium-enriched substitutes reduces blood pressure in controlled situations, mainly among people with hypertension. We report on a population-wide implementation of this strategy in a stepped wedge cluster randomized trial (NCT01960972). The regular salt of enrolled households was retrieved and replaced, free of charge, with a combination of 75% NaCl and 25% of KCl. A total of 2376 participants were enrolled in six villages in Tumbes, Peru. The fully adjusted intention-to-treat analysis showed an average reduction of 1.29 mm Hg (95% confidence interval [95% CI] -2.17; - 0.41) in systolic and of 0.76 mm Hg (95% CI -1.39; -0.13) in diastolic blood pressure. Among participants without hypertension at baseline, in the time and cluster adjusted model, the use of the salt substitute was associated with a 51% (95% CI 29% - 66%) reduced risk of developing hypertension compared with the control group. In 24-hour urine samples, there was no evidence of differences in sodium levels (mean difference -0.01; 95% CI -0.25; 0.23), but potassium levels were higher at the end of the study compared to those at baseline (mean difference -0.63; 95% CI -0.78; -0.47). Our results support a case for implementing a pragmatic population-wide salt substitute strategy in reducing blood pressure and hypertension incidence.
Reducing salt intake has been identified as one of the most cost-effective measures to improve health outcomes.1–3 Different studies have reported the benefit of salt reduction interventions in decreasing blood pressure and cardiovascular events.4–6 Results from meta-analysis show that modest reductions in salt intake are followed by a decrease in blood pressure levels among both hypertensive and normotensive subjects.7 Nevertheless, the evidence of the effectiveness of population-level behaviour change interventions on reducing salt intake is inconsistent, suggesting that education and awareness-raising interventions alone are not sufficient for reducing population salt intake.8
Salt substitutes, i.e., salt enriched with potassium or other similar components such as magnesium or aluminum, have been reported to be effective for reducing both systolic (SBP) and diastolic (DBP) blood pressure.9–11 Under controlled conditions, salt substitution strategies can reduce SBP up to 5 mm Hg and DBP up to 1.5 mm Hg, and this effect was larger among individuals with hypertension than normotensive subjects.12 There is limited evidence however studying the population-level effect of these salt substitution interventions. A cluster-randomized trial conducted in China, evaluating the effect of a community-based sodium reduction programme using a salt substitute on salt consumption and blood pressure, found reductions in urinary sodium excretion but not in blood pressure.13
Currently, an increasing number of countries have adopted national salt reduction strategies.14 Salt substitution initiatives could aid such strategies in settings where added salt during cooking is the main source of salt intake, particularly in low- and middle-income countries where hypertension rates are increasing at a fast rate.15 The aim of this study was to assess the efficacy of a pragmatic intervention using a salt substitution strategy to reduce blood pressure, as well as its impact on the incidence of hypertension, at the population level using a stepped wedge cluster trial in Peru.
Results
Population characteristics
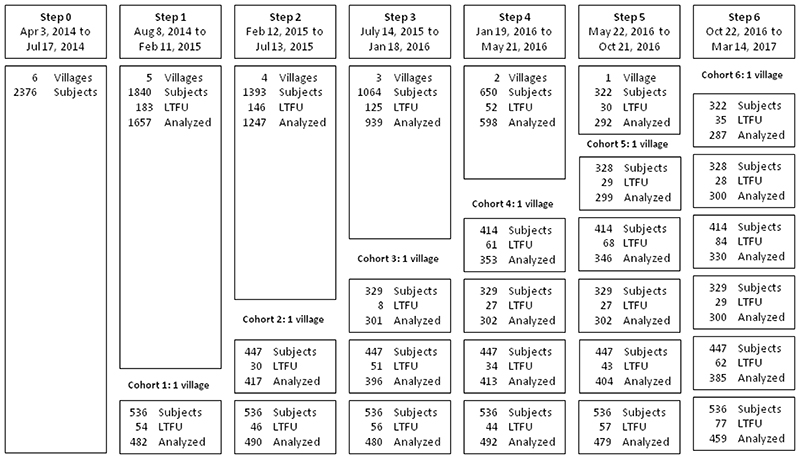
Figure 1 shows the detail of participants’ enrollment, including dates, number of subjects assessed, those lost to follow-up and analyzed for each step of the trial. A total of 2376 (91.2%) out of 2605 eligible subjects in the six villages were enrolled in the study from Apr 3 to Jul 17, 2014; 49.6% females, mean age 43.3 ± 17.2 years.
Figure 1. Flowchart diagram of participants in the stepped wedge trial.
LTFU = Lost to follow-up. Randomization of villages occurred after baseline assessment.
Of note, only 18.9% of the individuals had 12 or more years of education, 68.1% were on the overweight or obesity range with a BMI ≥25 kg/m2, and 18.3% had a diagnosis of hypertension. Table 1 shows the characteristics of the study population at baseline and a comparison between the control and intervention periods. There were differences between villages in the distribution of age, education, wealth index, BMI, SBP, DBP, and hypertension (Supplementary Table 1).
Table 1. Description of the study population at baseline by control and intervention periods.
| Variables | Baseline N=2376 | Control (person-years) | Intervention (person-years) |
|---|---|---|---|
| Sex | |||
| Female | 1197 (50.4%) | 1335.2 | 1768.4 |
| Male | 1179 (49.6%) | 1212.0 | 1836.9 |
| Age | |||
| Mean (SD) | 43.3 (17.2) | ||
| 18-29 years | 633 (26.6%) | 595.6 | 703.0 |
| 30-44 years | 780 (32.8%) | 880.2 | 1226.9 |
| 45-64 years | 656 (27.6%) | 715.3 | 1129.2 |
| ≥65 years | 307 (12.9%) | 356.1 | 546.3 |
| Wealth Index | |||
| Bottom | 689 (29.6%) | 629.4 | 1137.8 |
| Middle | 785 (33.7%) | 866.5 | 1180.6 |
| Top | 855 (36.7%) | 1001.1 | 1232.5 |
| Education | |||
| <7 years | 836 (35.2%) | 909.0 | 1281.0 |
| 7-11 years | 1090 (45.9%) | 1185.3 | 1636.6 |
| ≥12 years | 450 (18.9%) | 452.9 | 687.6 |
| Study Site (village) | |||
| A | 536 (22.6%) | 1.7 | 1366.1 |
| B | 447 (18.8%) | 286.9 | 883.1 |
| C | 329 (13.9%) | 329.0 | 518.3 |
| D | 414 (17.4%) | 542.1 | 460.2 |
| E | 328 (13.8%) | 637.0 | 256.3 |
| F | 322 (13.6%) | 750.6 | 121.3 |
| BMI | |||
| Mean (SD) | 27.2 (4.6) | ||
| Normal Weight | 758 (32.7%) | 762.3 | 1160.1 |
| Overweight | 985 (42.5%) | 1093.0 | 1492.2 |
| Obese | 573 (24.7%) | 629.1 | 887.0 |
| Blood Pressure | |||
| SBP [mean (SD)] | 113.1 (17.0) | ||
| DBP [mean (SD)] | 72 (10.1) | ||
| Hypertension | |||
| No | 1914 (81.7%) | 2038.0 | 2925.6 |
| Yes | 428 (18.3%) | 476.1 | 646.2 |
Effect of the salt substitute on blood pressure levels
In the intent-to-treat analysis, adjusting only for clustering and time effects, there was an average reduction of 1.23 mm Hg (95% CI 0.38; 2.07; p = 0.004) in SBP and of 0.72 mm Hg (95% CI 0.10; 1.34; p = 0.022) in DBP among the participants who received the salt substitute compared with controls. These results remained consistent after further adjustment for sex, age, years of education, wealth index, and BMI measured at baseline (Table 2).
Table 2. Overall effect of the intervention on blood pressure levels.
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
|---|---|---|---|---|
| Main analysis | ||||
| Systolic BP | -1.23 (-2.07; -0.38) | 0.004 | -1.29 (-2.17; -0.41) | 0.004 |
| Diastolic BP | -0.72 (-1.34; -0.10) | 0.022 | -0.76 (-1.39; -0.13) | 0.017 |
Linear mixed effects regression model were used for analyses (n = 2376 biologically independent individuals and 16632 samples in total).
Adjusted for time and clustering, as per study design.
Adjusted by time and clustering, but also by age, sex, education, wealth index, and body mass index.
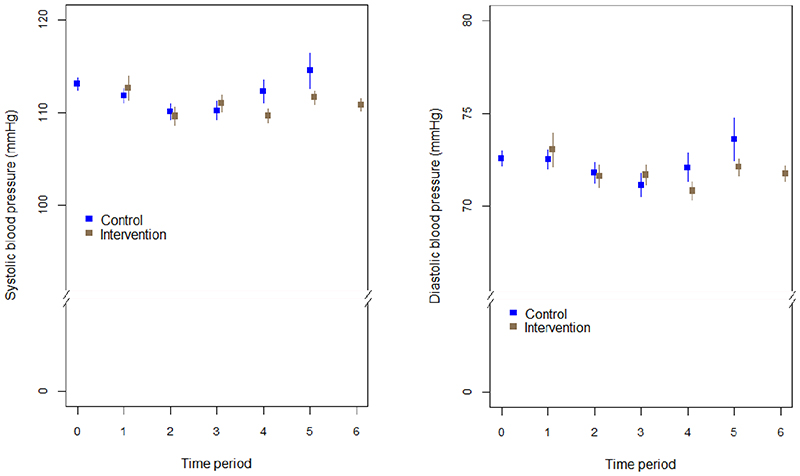
Variations in SBP and DBP mean levels over the intervention and control periods are shown in Figure 2. There was no evidence that the effect of the intervention was modified over time (p = 0.14 for SBP, and p = 0.46 for DBP). Mean levels of SBP and DBP by village and intervention period are available in the supplemental files (Extended Data Fig 1 and Extended Data Fig 2, respectively).
Figure 2. Trends in mean SBP (A) and DBP (B) and their respective 95% confidence intervals by intervention and control group.
Time periods are 5-month analysis periods occurring before the initiation of the intervention in each wave (n = 2072).
Results from exploratory analyses, shown in Table 3, showed no evidence of an interaction effect by sub-groups. When the analysis was stratified by hypertension status at baseline, an average reduction in SBP of 1.92 mm Hg (95% CI 0.54; 3.29) and 1.18 mm Hg (95% CI 0.08; 2.29) in DBP among individuals with hypertension at baseline was observed. Among individuals without hypertension, corresponding average reductions in SBP and DBP were 1.15 (95% CI 0.34; 1.96) and 0.63 mm Hg (95% CI -0.01; 1.28), respectively. In addition, participants did not report medication changes during the duration of the study (10.5% at baseline vs. 10.1% at the end of the study (p = 0.73). In terms of age sub-groups, the effect on blood pressure among those aged ≥60 years was a reduction of 2.17 mm Hg (95% CI 0.68; 3.67) in SBP and of 1.18 mm Hg (95% CI 0.22; 2.14) in DBP (Table 3). As a sensitivity analysis, we evaluated for a possible interaction of duration of exposure and intervention effect, and we did not find evidence of delayed effects (Supplementary Table 2).
Table 3. Effect of the salt substitute on blood pressure according hypertension status at baseline and age groups (sub-group analysis).
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
|---|---|---|---|---|
| Hypertension*** | ||||
| Among individuals without hypertension | ||||
| Systolic BP | -1.13 (-1.93; -0.33) | 0.006 | -1.15 (-1.96; -0.34) | 0.005 |
| Diastolic BP | -0.62 (-1.23; 0.00) | 0.051 | -0.63 (-1.28; 0.01) | 0.053 |
| Among individuals with hypertension | ||||
| Systolic BP | -1.74 (-3.04; -0.44) | 0.009 | -1.92 (-3.29; -0.54) | 0.006 |
| Diastolic BP | -1.25 (-2.24; -0.27) | 0.013 | -1.18 (-2.29; -0.08) | 0.036 |
| Age**** | ||||
| Among individuals <40 years | ||||
| Systolic BP | -0.91 (-1.51; -0.31) | 0.003 | -0.94 (-1.54; -0.34) | 0.002 |
| Diastolic BP | -0.25 (-0.79; 0.30) | 0.38 | -0.27 (-0.80; 0.27) | 0.33 |
| Among individuals 40-59 years | ||||
| Systolic BP | -1.20 (-2.02; -0.38) | 0.004 | -1.17 (-1.98; -0.35) | 0.005 |
| Diastolic BP | -1.04 (-1.70; -0.39) | 0.002 | -1.01 (-1.67; -0.36) | 0.002 |
| Among individuals ≥60 years | ||||
| Systolic BP | -1.95 (-3.44; -0.45) | 0.01 | -2.17 (-3.67; -0.68) | 0.004 |
| Diastolic BP | -1.13 (-2.09; -0.18) | 0.02 | -1.18 (-2.14; -0.22) | 0.02 |
Linear mixed effects regression model were used for analyses (n = 2376 biologically independent individuals and 16632 samples in total).
Adjusted for time and clustering, as per study design.
Adjusted by time and clustering, but also by age, sex, education, wealth index, and body mass index. Age was excluded as confounder when analyses were stratified by age.
p-values for the interaction of hypertension status and intervention were 0.858 and 0.951 for the systolic and diastolic BP models.
p-values for the interaction of age groups and intervention were 0.211 and 0.279; and 0.379 and 0.015 for the systolic and diastolic BP models.
Effect of the salt substitute intervention on the incidence of hypertension
After excluding patients with hypertension at baseline (n = 428), the data for 1891 of the 1914 subjects were available for analysis with 4673.4 person-years of follow-up. The overall incidence of hypertension was estimated as 5.1 per 100 person-years (95% CI 4.5; 5.8). In the time and cluster adjusted model, the participants in the intervention period were 51% less likely (HR = 0.49, 95% CI 0.34; 0.71, p < 0.001) to develop hypertension compared to the control period (Extended Data Fig 3 and Supplementary Table 3). The Schoenfeld residual test for non-proportional hazards was not significant (p = 0.40). The observed effect of the intervention remained consistent after further adjustment for sex, age, education, wealth index, and BMI (HR = 0.45; 95% CI 0.31; 0.66, p < 0.001).
Effect of the salt substitute intervention on urinary sodium and potassium
The levels of sodium in 24-hour urine samples (Supplementary Table 4) at the end of the study and baseline were 3.95 g (SD ±1.83) and 3.94 g (SD ±1.86), respectively, mean difference -0.01 (95% CI -0.25; 0.23). These results were similar across all the study villages. In contrast, the levels of potassium were higher at the end of the study (2.60 g, SD ±1.20) than at baseline (1.97 g; SD ±1.20), mean difference -0.63 (95% CI -0.78; -0.47).
Harms
No severe adverse effects were reported during the duration of the study.
Discussion
Evidence has been inconclusive regarding the efficacy of population-wide salt reduction interventions on blood pressure. In this study, we report the findings of a pragmatic strategy to reduce blood pressure and hypertension incidence at the population level. Overall, there was a decrease in SBP and DBP following the intervention, with a larger effect observed among those individuals with hypertension at baseline. In addition to the effect on blood pressure levels, the risk of incidence of hypertension was halved among those who received the intervention. These findings are supported by the analysis of urine samples, at baseline and follow-up, which showed an increase in mean potassium levels but no changes in sodium levels, indicating that the salt substitute intervention was accepted and adopted. We did not find evidence of a delayed effect suggesting that the effect is sustainable, independent of the duration of the intervention given the nature of the stepped wedge design.
The absolute reductions in blood pressure may appear modest, yet they carry major implications. The Prospective Studies Collaboration conducted a meta-analysis of 61 observational studies of blood pressure and vascular disease in adults and found that for each 2 mm Hg decrease in systolic blood pressure, stroke mortality and cardiovascular mortality decreased by 10% and 7%, respectively, an effect that was observed in reductions of systolic blood pressure levels up to 115 mm Hg.16 This indicates that small reductions in blood pressure at the population level could result in large public health gains, in line with the approaches to shift the entire distribution of a given risk factor.17 The main challenge until now, however, has been how best to introduce and achieve these changes under real life conditions. Our study demonstrates that such benefits can be introduced on a population-wide level.
Salt substitutes have been previously tested, mostly in China and mainly on patients with established hypertension,12 and show reductions in blood pressure with a larger effect observed among individuals with hypertension. Similar results have been obtained using home blood pressure measurements.18 Our study further expands the current literature using a pragmatic population-wide intervention that included a heterogeneous sample, i.e., delivered to the general population irrespective of hypertension status (i.e. aware or not aware of diagnosis), and perhaps due to this, the effect was modest (i.e. differential effect of salt substitute in subjects with hypertension diagnosis compared to those with recent diagnosis and those without the diagnosis). These features account for the potential scalability of our results to large populations and their influence on public health policies. Our study introduced a salt substitute containing NaCl (75%) and KCl (25%);19 however, previous reports have also included other minerals (e.g., MgSO2).12, 18 Potassium has also been shown to have benefits on blood pressure, irrespective of the sodium lowering,20–22 especially among individuals with hypertension with high consumption of sodium.23 Hence, the potassium contained in the salt substitute might contribute to explaining the benefits observed in our study. This hypothesis is further supported by the higher levels of potassium excretion, but not sodium, in the urine samples at the end of the study. A higher intake of potassium could be achieved through a combined strategy of a salt substitute intervention together with health education programs that focus on promoting the consumption of fresh vegetables and fruits to increase the potassium intake.
Our results point also to a lower incidence of hypertension in the participants receiving the intervention, a key clinical and public health finding. Whether this is a short-term effect, (i.e., the intervention did not prevent hypertension onset but delayed it) remains to be further studied. As the endocrine system in charge of salt regulation, the renin-angiotensin-aldosterone system, continues to receive larger amounts of sodium or lower amounts of potassium, it is likely that blood pressure will start to increase until it reaches hypertensive thresholds.24 Longer follow-ups, with and without intervention, are required to assess whether the endocrine system develops salt resistance. However, our findings show no evidence of an interaction effect between time and intervention.
From a pragmatic implementation angle, we provide evidence for the ability to introduce a salt substitute to the entire participating communities following a social marketing campaign designed to improve its acceptance. With evidence that antihypertensive medication is often unavailable or unaffordable in many low- and middle-income settings,25 the implementation of similar primary prevention strategy could reduce the burden associated with hypertension and its cardiovascular complications. The cost of the salt substitute should be also considered. During our study, before the intervention the cost of 1 kg of the salt substitute to the general public was 35 PEN (~$10 USD), and through the project we were able to get a price reduction to 14 PEN (~4 USD), further indicating opportunities for scaling-up implementation efforts.
Current hypertension guidelines advocate for non-pharmacological treatment, even in low-risk stage-1 hypertensive patients.26 Our results provide evidence of a pragmatic approach that reduces blood pressure and, secondarily, also halves the incidence of hypertension. These guidelines have also lowered the threshold for hypertension, meaning that more people will receive this diagnosis and will need to incorporate essential hypertension management strategies, making it difficult for health systems to provide pharmacological treatment and counselling to new patients. Moreover, given the alarming rates of non-adherence to medication of hypertension globally, non-pharmacological measures at the population level to improve blood pressure control are urgently needed. This population-wide intervention has the potential to contribute to reduce overall blood pressure levels without additional congestion of the healthcare system, potentially saving health-care costs.27
In Peru, and in many other resource-constrained settings, there are different venues through which a salt substitute can be introduced to replace the current salt including, for example, community kitchens for people of low socioeconomic status,28 or different national program for providing nutritious breakfasts for children attending public schools in rural areas, or elderly people. Therefore, the logistics underlying this process are already established and could be adapted to provide patients with hypertension and their families with a salt substitute. Similar scenarios may be present in other countries, signalling a window of opportunity to introduce a seemingly effective tool to reduce blood pressure. Other countries, including high-income countries, could also accommodate a similar salt substitution approach using existing channels and various venues, including school feeding programs.
This is an intervention study that provides a high level of evidence. The randomized allocation of the intervention removes several biases that exist in non-randomized studies, even after adjusting for potential confounders. The stepped-wedge design guaranteed a pragmatic scheme where randomization of the intervention was protected, allowing a large population to be reached. In some villages a reduction in blood pressure was observed before the intervention and potential explanations for this could be a community-like white coat effect, which in the case of rural or semi-urban areas where access to healthcare is limited, can also be present. Another explanation could be regression to the mean. In both circumstances, the repeated measurements would be the best way to overcome such potential weaknesses. The repeated follow-up visits within short and equally spaced periods, a characteristic of the stepped-wedge design, augmented the statistical power and afforded additional strength to address regression to the mean. Additionally, uptake of the intervention was objectively assessed with urine samples that demonstrated more potassium excretion at the end of the intervention, suggesting that the intervention was indeed well received and the salt substitute was used. Consistently, the intervention included a social marketing campaign to guarantee adoption of the new salt. From a public health perspective, the delivery of the intervention at the population level following a pragmatic methodology could inform prevention guidelines and policies to control the rising burden of increased blood pressure worldwide. Nevertheless, limitations of the study must also be acknowledged. First, the absence of a dietary assessment of other sources of sodium and potassium could have an impact on our results; however, this factor should be negligible because of the population-wide approach used. We provided whole villages with the salt substitute, also targeting families who prepare and sell food as street vendors. Therefore, it seems unlikely that other sources of sodium could have contaminated the intervention. Similarly, it is also unlikely that other sources of potassium confounded the intervention. To prevent any potential harm and thus protect the safety of the study population, we did not include people with kidney disease or those receiving digoxin (used as a proxy of cardiovascular disease). Although this exclusion warrants close follow-up of these patients, including regular check-ups with their physicians or tailored diets, it does not affect the implementation of wider population-wide benefits aimed to lower blood pressure. Finally, despite the inclusion of urine data from individuals with a complete 24-hour urine sample, the creatinine levels were in the normal range and their variation was within the range of dispersion (SD) of measurements.
Our results provide evidence that a population-based intervention to replace regular salt with a low-sodium potassium-enriched salt reduces SBP and DBP, particularly in people with hypertension. In addition, the intervention halved the incidence of hypertension. This pragmatic intervention could be adapted and scaled-up to counter the high burden of elevated blood pressure observed worldwide.
Methods
The study protocol and methods have been described previously,29 and a summary is provided below. The CONSORT statement for randomized cluster trials30 and recent literature on reporting results of stepped wedge cluster trials 31, 32 were utilized.
Study design
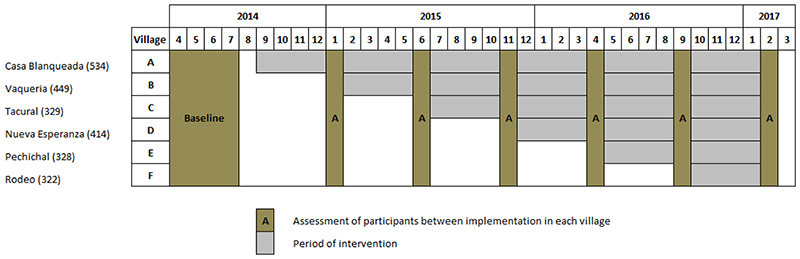
A stepped-wedge cluster randomized controlled trial was conducted, in which the six participating villages (clusters) crossed over from the control to the intervention phase during the study.33 The order of switchover for each cluster was determined by randomization, and all villages received the salt substitute by the end of the study. The structure of the stepped wedge is provided in Figure 3, where the intervention periods (village implementation phases, grey colour) lasted 4 months, and blood pressure measurements were made every 5 months after the baseline period. The study was undertaken between April 2014 (start of baseline assessment) and March 2017 (last measurement and assessment).
Figure 3. Structure and time framework of the stepped wedge cluster randomized trial.
* Assessment of participants included a short questionnaire and weight and blood pressure measurements.
Study setting
Tumbes, a coastal region in northern Peru, bordering Ecuador, was the setting selected for this study as hypertension prevalence and incidence rates are above the national average.34,35 According to official estimates of the Tumbes population,36 in 2017 there were 243,362 inhabitants with a life expectancy of 75 years, 20% of the population did not have any health insurance, and 12% were below the poverty line. The semiurban area of the region, with approximately 100 villages of varying size and with approximately 80,000 inhabitants, was the area chosen for the study. Mid-sized villages with 350 to 700 individuals (~130 to 250 households), were initially selected for the study. Of the 20 villages available with these characteristics, six were randomly selected. Enough distance between them was also guaranteed (i.e. a median of 14 Km [IQR: 7.1 – 17.1] between them) to avoid contamination by verifying villages selection in maps.
Participants and recruitment
Potentially eligible subjects were identified from the most updated census in the area (2010, updated in 2014). All males and females aged 18 years and over from the six selected villages, who were capable of understanding procedures, capable of providing informed consent and full-time residents in the area were eligible. Individuals with a self-reported history of chronic kidney disease and heart disease who were undergoing treatment with digoxin were excluded from the study.
Participant recruitment, as well as the initial assessment, was performed during the first four months of the study (April to July 2014). Individuals were contacted through home visits aiming to enrol all members of the household members of the villages who met the selection criteria.
Randomization and blinding
The selected villages were randomly assigned to one of the six sequences (one village = one cluster) for time cross-over from control to intervention. For this, a computer-generated list of random numbers was used and information was kept in a password-protected computer. The order of villages to be implemented was revealed one by one as required according to the nature of the study. Due to the pragmatic nature of the intervention, the participants were not blinded; however, the primary study outcome was objectively measured using standardized techniques. A team of fieldworkers, differing from those involved with the implementation of the intervention, was responsible for periodic assessments of participants using automated devices to reduce observer bias.
Intervention
Applying social marketing strategies,37 a campaign was developed targeting women responsible of food preparation at home. The purpose of the marketing campaign was to introduce the salt substitute as a new product in the intervention villages and enhance its acceptance. Thus, the common salt (sodium chloride, NaCl) of enrolled households was retrieved and replaced, free of charge, with a salt substitute using a combination of 75% NaCl and 25% of potassium chloride (KCl) based on previous research.19 Iodine, in addition to fluorine, was also part to the salt substitute as part of Peruvian regulations.38 As the usual cost of a bag of 1 kg of common salt in the region was between 0.15-0.17 USD (about 0.50 PEN), we provided the salt substitute free of charge to the participants in their respective homes (the amounts of salt were estimated based on self-reported monthly household consumption).
The time to provide a salt replacement was planned to occur over a period of five months in each village, however, there was a delay in salt substitute delivery of, on average, 15 days. The intervention considered the salt delivery to families, as well as to owners of small shops, bakeries and community kitchens,28 and food vendors including street vendors and restaurants. This approach was used to guarantee the full replacement of salt in the entire village. Additional salt substitute packs were also made freely available during the study period in case any household required additional salt.
Outcomes and data collection
The primary outcomes were SBP and DBP, assessed as continuous variables (in mm Hg) evaluated in the period between the end of each wedge and start of the next one. Blood pressure assessments were performed with the participants seated, after a 5-minute resting period, using an automated device (OMRON HEM-780, Illinois, US) that had been previously validated in adult populations.39 Three different measurements, at least one minute apart, were carried out, and the average of the second and third measurements was used for the analyses.
The secondary outcomes included progression toward hypertension (incidence) and, in a random sub-sample of participants, changes in levels of sodium and potassium excretion in 24-hour urine. Hypertension at baseline was defined as SBP ≥140 mm Hg, DBP ≥90 mm Hg, a self-reported physician diagnosis or current treatment for hypertension.40 During follow-up, hypertension was defined based in two ways: considering only the study measurements (average of the second and third measurements, with the participant in seated position, after resting five minutes, and at least one minute apart between measures), taking advantage of their repeated assessment conducted every five months, as well as using the same definition as in the baseline.
After providing consent, each participant was given a unique code. At baseline, detailed information regarding socio-demographics (e.g., age, sex, education, wealth index), lifestyle behaviours (smoking, alcohol consumption, and physical activity), self-reported personal medical history and medication (hypertension and type 2 diabetes mellitus), anthropometric measurements (height, weight, and blood pressure), and health-care utilization and expenditures was collected using paper-based formats. Follow-up assessments were conducted in all participants and included some lifestyle behaviours (smoking and alcohol consumption), anthropometric measurements (weight and blood pressure), and health-care utilization and expenditures.
Urine samples were retrieved in a random sub-sample of 600 participants after baseline and in another randomly selected sub-sample of 600 participants at the end of the study. Only one participant per household was included in the urine assessments. Urine samples were collected over a 24-hour period, and all samples were assessed in a central laboratory facility. These samples were used to extract information about levels of creatinine, sodium and potassium. Sodium and potassium were assessed using the ion-selective electrode method, whereas creatinine was assessed with the compensated kinetic Jaffe method.
Statistical methods
All statistical procedures were conducted using Stata for Windows v15.0 (StataCorp, College Station, TX, US) and R statistical software,41 and a per protocol intent-to-treat analysis was performed. A pre-specified linear mixed effects regression analysis was performed to model SBP and DBP using an identity link, an unstructured working correlation, including covariates for intervention status and time period which was considered as a factor, and random effects for village, family, and repeated observations of the same individual over time,42,43 and robust variances were computed. Thus, the following model was used:
where Yijkl is the SBP (or DBP) measured for individual i, in family j, at cluster k, in time l; μ is the mean outcome in the control group at baseline; ai is a random intercept of individual i; γj is a random intercept for family j; φk is a random intercept for cluster k; is the effect of time l, Xil is an indicator of the treatment mode in village k at time l; and θ is the overall effect of the intervention.
We also evaluated a priori, as a sensitivity analysis, whether there was evidence of a delayed effect, i.e., an interaction between duration of exposure and intervention,42 and estimated the effect of the intervention on SBP and DBP controlling by a priori defined possible confounders: age, sex, education, wealth index, and body mass index (BMI) at baseline. Furthermore, we conducted exploratory sub-group analyses by hypertension status and age group defined at baseline.
For incidence calculations, Cox proportional hazard modelling on a calendar time axis to account for time trends with random effects that follow gamma distribution for village-level (shared frailty), was considered to compare the instantaneous risk of hypertension for both the intervention and controls.44 The Schoenfeld residuals were used to test for the non-proportional hazard without considering the frailty term.45 Time and cluster adjusted Cox models were constructed for the primary analysis, and fully adjusted models were generated to account for confounding variables such as age, sex, education, wealth index, and BMI at baseline. Calculations (i.e. Hazard ratios) were estimated taking into account the clustering of villages; and in addition, a time-varying binary covariate tracking intervention status was fit using definitions of times-at-risk in each period described above.
Finally, changes in the 24-hour urine concentrations of sodium and potassium were also evaluated (at the end of the study and after baseline). For the analysis, we included only individuals with a complete 24-hour urine sample, defined as a) at least 500 ml, and b) creatinine <4 mmol per day in women or <6 mmol per day in men.46,47 Comparisons were conducted using the t test for independent samples.
Ethics
This project was registered in clinicaltrials.gov (Identifier: NCT01960972). The protocol and informed consent forms used in this project were reviewed and approved by the institutional review boards of the Universidad Peruana Cayetano Heredia, Lima, Peru, and Johns Hopkins University, Baltimore, MD, USA. Given that the intervention was implemented at the village level but the outcome was measured at the individual level, we involved all the members of the recruited families in the study. For this, we initially engaged with authorities and leaders from the villages, and an initial presentation and explanation of the study at the village level was conducted before starting the research activities. Then, family members aged 18 years and over were contacted for individual informed consent. Since hypertension is not common among children, we did not include children and adolescents, i.e. any family member <18 years old, in the study. Participants with a history of terminal or severe chronic kidney disease (any form of dialysis) or those taking digoxin or potassium-sparing diuretics (for heart disease) with their families were excluded from this study.
Supplementary Material
Acknowledgements
This study was supported by the National Heart, Lung, and Blood Institute (Project 1 U01 HL114180-01), United States, under The Global Alliance for Chronic Diseases (GACD) hypertension programme. A.B.-O. was supported by a Wellcome Trust Research Training Fellowship in Public Health and Tropical Medicine (Grant number: 103994/Z/14/Z). V.G.S.y.R. was funded by the Dirección de Gestión de la Investigación at the PUCP (Grant number: DGI-2017-496).
Footnotes
Contributions
A.B.-O. and J.J.M. drafted the first version of the manuscript with inputs from R.M.C.-L. A.B.-O., R.H.G., K.A.S., and J.J.M. conceived and designed the overall study. V.G.S.y.R. and A.B.-O. developed the statistical analysis plan and conducted the statistical analysis. V.P.-L. led the social marketing campaign. M.K.C. designed the strategy for the cost-effectiveness analysis. F.D.-C. and M.A.P. conducted qualitative work during the intervention as part of a process evaluation. All of the authors contributed to the revising of the manuscript for important content and gave their final approval of the version submitted for publication.
Competing Interests
None declared.
Data Availability Statement
Anonymized clinical and anthropometric data are available upon request, subject to an internal review by J.J.M., R.H.G. and A.B.-O. to ensure that the participants’ anonymity and confidentiality are protected, completion of a data sharing agreement, and in accordance with the Universidad Peruana Cayetano Heredia and Johns Hopkins University’s institutional review boards and institutional guidelines. Material requests, i.e. marketing campaign information, or economics data requests will be considered based on a proposal review, completion of a material transfer agreement and/or a data use agreement. Please submit requests for participant-related clinical and other data to A.B.-O. (Antonio.Bernabe@upch.pe) copying J.J.M. (Jaime.Miranda@upch.pe).
References
- 1.Wang G, Bowman BA. Recent economic evaluations of interventions to prevent cardiovascular disease by reducing sodium intake. Curr Atheroscler Rep. 2013;15(9):349. doi: 10.1007/s11883-013-0349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Salt reduction: Fact sheet. http://www.who.int/mediacentre/factsheets/fs393/en/
- 3.Kontis V, Cobb LK, Mathers CD, Frieden TR, Ezzati M, Danaei G. Three Public Health Interventions Could Save 94 Million Lives in 25 Years Global Impact Assessment Analysis. Circulation. 2019 doi: 10.1161/CIRCULATIONAHA.118.038160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melander O, von Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, Hulthen UL. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N-terminal atrial natriuretic peptide in plasma. J Hypertens. 2007;25(3):619–27. doi: 10.1097/HJH.0b013e328013cd50. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Lauer JA, Hutubessy RC, Niessen L, Tomijima N, Rodgers A, Lawes CM, Evans DB. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet. 2003;361(9359):717–25. doi: 10.1016/S0140-6736(03)12655-4. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 7.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 8.Trieu K, McMahon E, Santos JA, Bauman A, Jolly KA, Bolam B, Webster J. Review of behaviour change interventions to reduce population salt intake. Int J Behav Nutr Phys Act. 2017;14(1):17. doi: 10.1186/s12966-017-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salt substitution: a low-cost strategy for blood pressure control among rural Chinese. A randomized, controlled trial. J Hypertens. 2007;25(10):2011–8. doi: 10.1097/HJH.0b013e3282b9714b. [DOI] [PubMed] [Google Scholar]
- 10.Geleijnse JM, Witteman JC, Bak AA, den Breeijen JH, Grobbee DE. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. Bmj. 1994;309(6952):436–40. doi: 10.1136/bmj.309.6952.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou B, Wang HL, Wang WL, Wu XM, Fu LY, Shi JP. Long-term effects of salt substitution on blood pressure in a rural north Chinese population. J Hum Hypertens. 2013;27(7):427–33. doi: 10.1038/jhh.2012.63. [DOI] [PubMed] [Google Scholar]
- 12.Peng YG, Li W, Wen XX, Li Y, Hu JH, Zhao LC. Effects of salt substitutes on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100(6):1448–54. doi: 10.3945/ajcn.114.089235. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Yan LL, Niu W, Yao C, Feng X, Zhang J, Shi J, Zhang Y, Zhang R, Hao Z, Chu H, et al. The Effects of a Community-Based Sodium Reduction Program in Rural China - A Cluster-Randomized Trial. PLoS One. 2016;11(12):e0166620. doi: 10.1371/journal.pone.0166620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trieu K, Neal B, Hawkes C, Dunford E, Campbell N, Rodriguez-Fernandez R, Legetic B, McLaren L, Barberio A, Webster J. Salt Reduction Initiatives around the World - A Systematic Review of Progress towards the Global Target. PLoS One. 2015;10(7):e0130247. doi: 10.1371/journal.pone.0130247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet (London, England) 2017;389(10064):37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 17.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14(1):32–8. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Zhao L, Thompson B, Zhang Y, Wu Y. Effects of salt substitute on home blood pressure differs according to age and degree of blood pressure in hypertensive patients and their families. Clin Exp Hypertens. 2018:1–9. doi: 10.1080/10641963.2018.1425415. [DOI] [PubMed] [Google Scholar]
- 19.Saavedra-Garcia L, Bernabe-Ortiz A, Gilman RH, Diez-Canseco F, Cardenas MK, Sacksteder KA, Miranda JJ. Applying the Triangle Taste Test to Assess Differences between Low Sodium Salts and Common Salt: Evidence from Peru. PLoS One. 2015;10(7):e0134700. doi: 10.1371/journal.pone.0134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2015;33(8):1509–20. doi: 10.1097/HJH.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 21.Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, Lear S, Ah STL, Wei L, Diaz R, Avezum A, et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018;392(10146):496–506. doi: 10.1016/S0140-6736(18)31376-X. [DOI] [PubMed] [Google Scholar]
- 22.Poorolajal J, Zeraati F, Soltanian AR, Sheikh V, Hooshmand E, Maleki A. Oral potassium supplementation for management of essential hypertension: A meta-analysis of randomized controlled trials. PLoS One. 2017;12(4):e0174967. doi: 10.1371/journal.pone.0174967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filippini T, Violi F, D’Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int J Cardiol. 2017;230:127–135. doi: 10.1016/j.ijcard.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 24.Perez V, Chang ET. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv Nutr. 2014;5(6):712–41. doi: 10.3945/an.114.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attaei MW, Khatib R, McKee M, Lear S, Dagenais G, Igumbor EU, AlHabib KF, Kaur M, Kruger L, Teo K, Lanas F, et al. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet Public Health. 2017;2(9):e411–e419. doi: 10.1016/S2468-2667(17)30141-X. [DOI] [PubMed] [Google Scholar]
- 26.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):2199–2269. [Google Scholar]
- 27.Aminde LN, Takah NF, Zapata-Diomedi B, Veerman JL. Primary and secondary prevention interventions for cardiovascular disease in low-income and middle-income countries: a systematic review of economic evaluations. Cost Eff Resour Alloc. 2018;16:22. doi: 10.1186/s12962-018-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garret JL. Comedores Populares: Lessons for Urban programming from Peruvian Community Kitchens. CARE-USA; Atlanta, Georgia, US: 2001. [Google Scholar]
- 29.Bernabe-Ortiz A, Diez-Canseco F, Gilman RH, Cardenas MK, Sacksteder KA, Miranda JJ. Launching a salt substitute to reduce blood pressure at the population level: a cluster randomized stepped wedge trial in Peru. Trials. 2014;15:93. doi: 10.1186/1745-6215-15-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. Bmj. 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 31.Davey C, Hargreaves J, Thompson JA, Copas AJ, Beard E, Lewis JJ, Fielding KL. Analysis and reporting of stepped wedge randomised controlled trials: synthesis and critical appraisal of published studies, 2010 to 2014. Trials. 2015;16:358. doi: 10.1186/s13063-015-0838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. Bmj. 2015;350:h391. doi: 10.1136/bmj.h391. [DOI] [PubMed] [Google Scholar]
- 33.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernabe-Ortiz A, Carrillo-Larco RM, Gilman RH, Checkley W, Smeeth L, Miranda JJ. Contribution of modifiable risk factors for hypertension and type-2 diabetes in Peruvian resource-limited settings. J Epidemiol Community Health. 2016;70(1):49–55. doi: 10.1136/jech-2015-205988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernabe-Ortiz A, Carrillo-Larco RM, Gilman RH, Checkley W, Smeeth L, Miranda JJ. Impact of urbanisation and altitude on the incidence of, and risk factors for, hypertension. Heart. 2017;103(11):827–833. doi: 10.1136/heartjnl-2016-310347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Instituto Nacional de Estadistica e Informatica. Censos Nacionales 2017: XI de Población y VIde Vivienda. http://www.inei.gob.pe/estadisticas/censos/
- 37.French J, Blair-Stevens C, Merritt R, McVey D. Social Marketing and Public Health: Theory and Practice. Oxford University Press; 2010. [Google Scholar]
- 38.Pretell EA, Higa AM. Peru celebrates 25 years of sustained elimination of IDD. IDD; Lima, Peru: 2009. [Google Scholar]
- 39.Coleman A, Steel S, Freeman P, de Greeff A, Shennan A. Validation of the Omron M7 (HEM-780-E) oscillometric blood pressure monitoring device according to the British Hypertension Society protocol. Blood Press Monit. 2008;13(1):49–54. doi: 10.1097/MBP.0b013e3282cb57b6. [DOI] [PubMed] [Google Scholar]
- 40.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 41.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. URL https://www.r-project.org. [Google Scholar]
- 42.Hughes JP, Granston TS, Heagerty PJ. Current issues in the design and analysis of stepped wedge trials. Contemp Clin Trials. 2015;45(Pt A):55–60. doi: 10.1016/j.cct.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott JM, deCamp A, Juraska M, Fay MP, Gilbert PB. Finite-sample corrected generalized estimating equation of population average treatment effects in stepped wedge cluster randomized trials. Stat Methods Med Res. 2017;26(2):583–597. doi: 10.1177/0962280214552092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durovni B, Saraceni V, Moulton LH, Pacheco AG, Cavalcante SC, King BS, Cohn S, Efron A, Chaisson RE, Golub JE. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13(10):852–8. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41. [Google Scholar]
- 46.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovsky J, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. Jama. 2011;305(17):1777–85. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 47.Swanepoel B, Schutte AE, Cockeran M, Steyn K, Wentzel-Viljoen E. Sodium and potassium intake in South Africa: an evaluation of 24-hour urine collections in a white, black, and Indian population. J Am Soc Hypertens. 2016;10(11):829–837. doi: 10.1016/j.jash.2016.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized clinical and anthropometric data are available upon request, subject to an internal review by J.J.M., R.H.G. and A.B.-O. to ensure that the participants’ anonymity and confidentiality are protected, completion of a data sharing agreement, and in accordance with the Universidad Peruana Cayetano Heredia and Johns Hopkins University’s institutional review boards and institutional guidelines. Material requests, i.e. marketing campaign information, or economics data requests will be considered based on a proposal review, completion of a material transfer agreement and/or a data use agreement. Please submit requests for participant-related clinical and other data to A.B.-O. (Antonio.Bernabe@upch.pe) copying J.J.M. (Jaime.Miranda@upch.pe).