Abstract
Introduction
Coverage of health interventions usually shows social gradients with higher levels among wealthy than among poor individuals. Owing to the upsurge of vaccine hesitancy in high-income countries, the authors hypothesized that the social gradient may also be changing over time in the low- and middle-income countries and set out to test this hypothesis.
Methods
In January 2020, surveys conducted from 2010 to 2018 in 86 low- and middle-income countries were analyzed to assess full immunization coverage in children aged 12–23 months. The authors calculated full immunization coverage point estimates and 95% CIs for each country and wealth quintile. To explore wealth-related inequalities, the authors estimated the slope index of inequality and calculated the Pearson correlation coefficient between these values and per capita gross domestic product. Time trends were analyzed in 10 countries with recent evidence of hesitancy.
Results
Pro-poor patterns were defined as significant slope index of inequality values with higher coverage among poor children, and pro-rich patterns were defined as the reverse pattern. A total of 11 countries showed pro-poor patterns in the most recent survey, accounting for 20% of upper middle– and 7% of low-income countries. The correlation between the slope index of inequality and log per capita gross domestic product was −0.38 (p<0.001). Among the 10 countries with recent evidence of hesitancy, 5 showed full immunization coverage declines over time in the wealthiest quintiles, and 4 switched from pro-rich to pro-poor patterns throughout the years.
Conclusions
Lower full immunization coverage was found among the wealthy than among the poor in 10 countries, especially in the upper middle—income group, consistent with the emergence of vaccine hesitancy.
Introduction
In 2019, the WHO declared reluctance or refusal to vaccinate, known as vaccine hesitancy, as 1 of the 10 threats to global health.1 According to the Strategic Advisory Group of Experts working group on vaccine hesitancy, Vaccine hesitancy refers to delay in acceptance or refusal of vaccination despite availability of vaccination services. Vaccine hesitancy is complex and context specific, varying across time, place and vaccines. It is influenced by factors such as complacency, convenience and confidence.2
Although not a recent phenomenon, vaccine hesitancy is garnering attention notably because it is spreading rapidly through social media.3
Great progress has been achieved in vaccinating children against target diseases. Currently, 85% of children in the world have received the 8 doses of recommended vaccines by WHO: 1 dose each of bacille Calmette–Guérin (BCG) and measles-containing vaccines (MCVs) and 3 doses each of diphtheria–pertussis–tetanus (DPT) and polio vaccines.4,5 Nevertheless, the coverage of these recommended vaccines has remained stable since 2010 and in many countries at levels that are below the goal of 90% established by WHO.5
Suboptimal vaccination coverage may be due to poorly structured health systems that do not achieve efficient distribution and administration of vaccines. This may affect children from poor families more than it affects the wealthy ones, explaining the prevailing inequality pattern of higher vaccination coverage among children from wealthy families—referred to as pro-rich patterns.6,7
However, in the face of increasing loss of public confidence in vaccination plus the rising attention given to anti-vaccination movements, a second reason for suboptimal coverage has emerged: parents intentionally refuse to vaccinate their children, even where vaccines are available and affordable.1,8–10 There is growing evidence that vaccine-hesitant groups may be geographically clustered or characterized by social conditions such as high educational level and socioeconomic position.2,11,12
Vaccine hesitancy is a well-known phenomenon in high-income countries. Thus, the authors hypothesize that it might also be detected in low- and middle-income countries (LMICs), especially in the latter. This study analyzes whether the classic pro-rich pattern of vaccination coverage changed over time in LMICs. This study has 2 main objectives: (1) to analyze the current status of inequalities by wealth on immunization coverage and (2) to verify the trends in the immunization coverage in both the poorest and wealthiest groups.
Methods
Study Sample
The authors analyzed nationally representative data sets from the Demographic and Health Surveys (DHS) and the Multiple Indicator Cluster Surveys (MICS). Both DHS and MICS are cross-sectional surveys that use standardized and comparable questionnaires.13 The institutions that administered the surveys at the national level were responsible for ethical clearance.
This analysis used data from the most recent survey carried out between 2010 and 2018 in LMICs with available data on BCG, DPT, polio, and MCV vaccinations and on wealth index, for all countries with such surveys.
Measures
The main outcome was full immunization coverage (FIC), defined as the percentage of children aged 12–23 months (or 15–26 or 18–29 months depending on the vaccination calendar of the country) who received 1 dose each of BCG and MCV and 3 doses each of vaccines against DPT and polio, excluding the polio dose given at birth. This information was collected from vaccination cards or the mother’s report if a card was not available. Children without information from either a card or maternal report were excluded from the analyses.
To explore inequalities, socioeconomic position was assessed through the household wealth index, which is calculated by the DHS and MICS teams and included in the survey data sets. The index is based on principal component analysis from variables describing ownership of household assets such as TVs and refrigerators; building characteristics such as materials used for the walls, floors, and roofs; water supply and sanitary facility; and variables related to economic status.14,15 A total of 2 separate analyses were conducted for urban and rural households to account for differences in assets and their importance, and they were later combined into a single score.16 This score was then divided into 5 groups of equal population size (quintiles). The first quintile included the households with the poorest 20% of the population, and the fifth quintile included the wealthiest 20% of the sample.
To summarize wealth-related inequalities, the slope index of inequality (SII) was calculated with logistic regression, using information from the whole distribution of coverage by wealth. The index represents the absolute difference in coverage between the fitted values at the extremes of the wealth distribution.17 It is expressed on a scale from −100 to +100 percentage points; negative values represent a pro-poor inequality pattern, that is, higher coverage among the poorest, and positive values indicate pro-rich patterns. Zero values represent the absence of inequality.
In addition, the authors gathered information on per capita gross domestic product (GDP) (purchasing power parity, constant 2011 international dollars) and income level from the World Bank Open Data repository.18 This study considered the income level in 2014, the median year of surveys in the analysis.
Among the countries included in the cross-sectional analyses, the 10 countries with significant pro-poor inequality patterns (i.e., negative SII values in the most recent survey since 2010) were selected, which also had ≥1 other survey carried out between 2000 and 2009 to assess time trends.
Statistical Analysis
Analyses were conducted in January 2020 using Stata, version 16, and R, version 3.6.1. The databases were harmonized by the International Center for Equity in Health, and results were checked for consistency against the published national survey reports.19 The analyses accounted for the multistage survey design, including sampling weights.
In accordance with the World Bank national income level classifications, countries were separated into 3 groups: low, lower middle, and upper middle income. Point estimates and 95% CIs of FIC were calculated for each country and wealth quintile. Pearson correlation coefficients between SII and per log capita GDP were calculated using countries as the units. In addition, the median coverage and IQRs for each of the 4 vaccines were calculated for each country’s income group level.
For the time trend analyses, FIC was estimated by quintile on the basis of all the surveys available from each country. Using this information, the authors estimated the SII for each survey and performed variance-weighted least squares regression to estimate annual changes in FIC by quintile. Pro-rich coverage patterns were identified when the SII was positive and its 95% CI did not include the value of 0. Conversely, pro-poor patterns were characterized by a negative SII, also with a CI that did not include 0. Graphical presentation of the time trends allowed the comparison of inequality patterns in the earliest and most recent surveys in the 10 countries.
Results
The authors analyzed data for 199,702 children from 86 countries, encompassing 62.8% of all LMICs. These countries account for 82.4% of the world’s low-income countries (28 of 34 countries), 70.2% of all lower middle–income countries (33 of 47 countries), and 44.6% of all upper middle–income countries (25 of 56 countries). Other LMICs could not be included because DHS or MICS surveys with information on wealth were not available.
The median values of FIC at the national level were 61.8% (IQR=44.0–76.4), 70.0% (IQR=62.3–;80.8), and 70.8% (IQR=62.0–85.0) in low-, lower middle−, and upper middle–income countries, respectively. The median coverage levels with vaccines that are part of the FIC definition, stratified by country income level, are presented in Appendix Table 1 (available online).
The highest FIC was found in Turkmenistan in 2015 (94.3%, 95% CI=92.2, 95.9), and the lowest was found in South Sudan in 2010 (7.3%, 95% CI=5.8, 9.2). Regarding absolute inequality, Nigeria (2016) had the most marked pro-rich inequality pattern with SII=56.4 percentage points (95% CI=51.5, 61.4), whereas Tunisia (2018) had the most marked pro-poor pattern with SII= −41.2 percentage points (95% CI= −51.4, −28.3). Full results for FIC and SII by country can be found in Appendix Table 2 (available online).
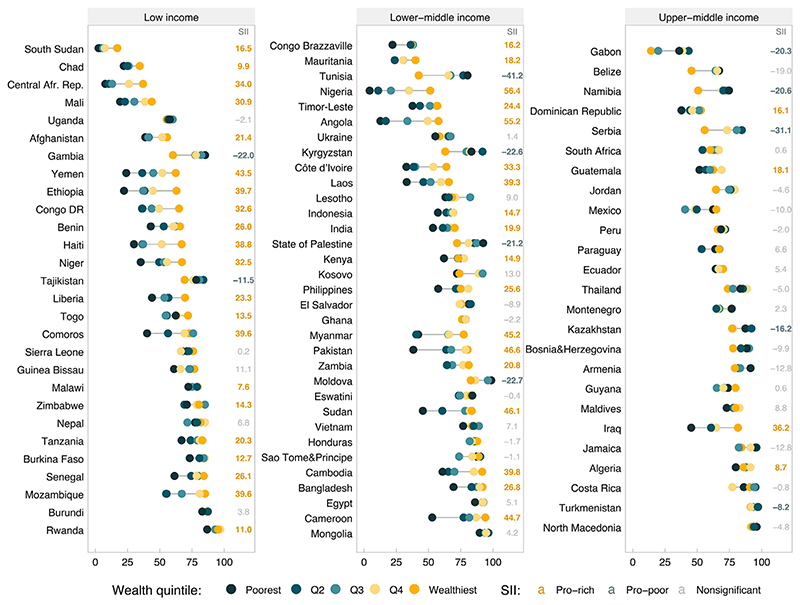
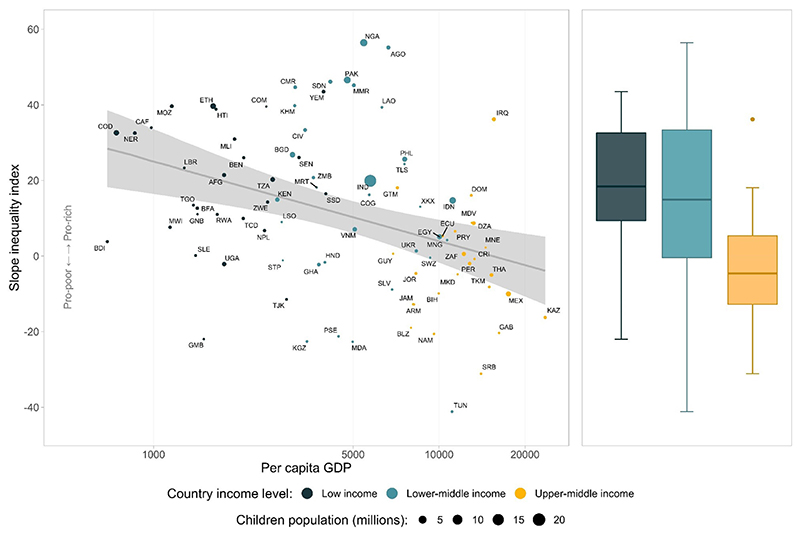
Figure 1 shows FIC by wealth quintiles in all the surveys, stratified by country income level. The percentages of countries with significant pro-poor patterns were 7.1% in low-income countries, 12.1% in lower middle –income countries, and 20.0% in upper middle–income countries. A moderate negative correlation of −0.38 (p<0.001) was observed between SII and log per capita GDP (Figure 2), which was strongly driven by the extremes of GDP. This finding is consistent with a lower median SII in upper middle–income countries when compared with those of other groups (Figure 2). Despite similar median values, lower middle–income countries showed more variability than low-income countries (Figure 2). Full results for FIC and SII can be found in Appendix Table 2 (available online).
Figure 1. FIC stratified by wealth quintiles and corresponding SII for 86 countries according to income groups.
Note: Pro-rich values of SII (yellow font) indicate higher coverage among the wealthy, whereas pro-poor values (blue font) indicate higher coverage among the poor. Statistical significance was ascertained with 95% CIs.
Afr., African; DR, Democratic Republic; FIC, full immunization coverage; Q, quintile; Rep., Republic; SII, slope index of inequality.
Figure 2. Scatter diagram of the SII for FIC and per capita GDP in 86 countries and box plot by country income groups.
Note: Logistic regression line and its 95% CI interval are in shaded gray.
FIC, full immunization coverage; GDP, gross domestic product; SII, slope index of inequality; AFG, Afghanistan; AGO, Angola; ARM, Armenia; BDI, Burundi; BEN, Benin; BFA, Burkina Faso; BGD, Bangladesh; BIH, Bosnia and Herzegovina; BLZ, Belize; CAF, Central African Republic; CIV, Cote d’Ivoire; CMR, Cameroon; COD, Democratic Republic of the Congo; COG, Congo Brazzaville; COM, Comoros; CRI, Costa Rica; DOM, Dominican Republic; DZA, Algeria; ECU, Ecuador; EGY, Egypt; ETH, Ethiopia; GAB, Gabon; GHA, Ghana; GMB, Gambia; GNB, Guinea Bissau; GTM, Guatemala; IRQ, Iraq; GUY, Guyana; HND, Honduras; HTI, Haiti; IDN, Indonesia; IND, India; JAM, Jamaica; JOR, Jordan; KAZ, Kazakhstan; KGZ, Kyrgyzstan; KHM, Cambodia; KEN, Kenya; LAO, Laos; LBR, Liberia; LSO, Lesotho; MDA, Moldova; MDV, Maldives; MEX, Mexico; MKD, North Macedonia; MLI, Mali; MMR, Myanmar; MNE, Montenegro; MNG, Mongolia; MOZ, Mozambique; MRT, Mauritania; MWI, Malawi; NAM, Namibia; NER, Niger; NGA, Nigeria; NPL, Nepal; PAK, Pakistan; PER, Peru; PHL, Philippines; PRY, Paraguay; PSE, State of Palestine; RWA, Rwanda; SDN, Sudan; SEN, Senegal; SLE, Sierra Leone; SLV, El Salvador; SRB, Serbia; SSD, South Sudan; STP, Sao Tome and Principe; SWZ, Eswatini; TCD, Chad; TGO, Togo; THA, Thailand; TJK, Tajikistan; TKM, Turkmenistan; TLS, Timor Leste; TUN, Tunisia; TZA, Tanzania; UGA, Uganda; UKR, Ukraine; VNM, Vietnam; XKX, Kosovo; YEM, Yemen; ZAF, South Africa; ZMB, Zambia; ZWE, Zimbabwe.
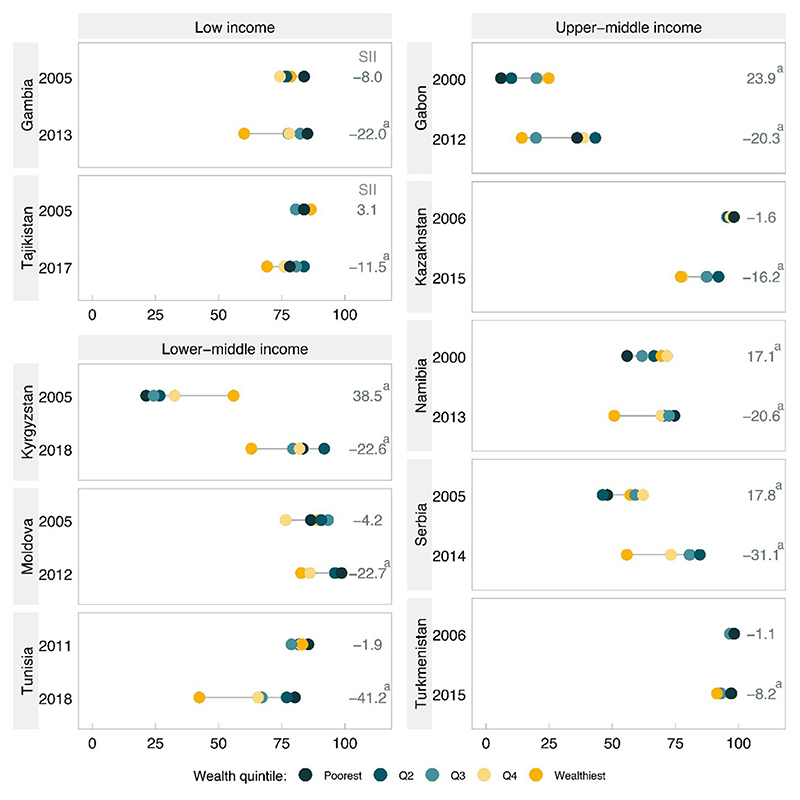
A total of 10 countries were eligible for trend analyses, including 2 low-, 3 lower middle−, and 5 upper middle–income countries, because they presented significant pro-poor patterns in the most recent survey (Figure 3 and Table 1). Of the 10 countries, none had significant pro-poor patterns in the earlier survey and 6 did not present a significant pattern. The remaining 4 countries switched from a pro-rich to a pro-poor pattern over time: Kyrgyzstan, Gabon, Serbia, and Namibia. The changes in pattern were due to a combination of reduced coverage among the rich with increased coverage among the poor (Table 1): in Namibia, there was a combination of a significant increase in coverage among the poor and a significant decline among the rich, but the other 3 countries showed significant increases among the poor combined with stagnation among the rich.
Figure 3. FIC stratified by wealth Qs for 10 countries with pro-poor inequality patterns in the most recent survey, according to income group.
aSignificant SII value according to its 95% CI.
FIC, full immunization coverage; Q, quintile; SII, slope index of inequality.
Table 1. Annual Change in FIC From 2000 in Countries With Pro-Poor Coverage Patterns.
| Poorest quintile | Wealthiest quintile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Surveys, n | Initial year | Final year | FIC in the initial year, % | FIC in the final year, % | Annual change, pp (95% Cl) | FIC in the initial year, % | FIC in the initial year, % | Annual change, pp (95% Cl) | |
| Low income | ||||||||||
| Gambia | 3 | 2005 | 2013 | 83.9 | 85.2 | 0.3 (-0.4,1.0) | 78.5 | 60.1 | 1.1 (-0.2, 2.3) | |
| Tajikistan | 3 | 2005 | 2017 | 83.9 | 78.2 | -0.4 (-1.3, 0.4) | 86.5 | 69.2 | -1.2 (-1.9,-0.5) | |
| Lower middle income | ||||||||||
| Kyrgyzstan | 4 | 2005 | 2018 | 21.3 | 83.3 | 4.9 (4.0, 5.8) | 55.9 | 63.0 | 1.1 (0.0, 2.3) | |
| Moldova | 2 | 2005 | 2012 | 86.5 | 98.7 | 1.7 (0.3, 3.2) | 89.8 | 82.7 | -1.0 (-2.4, 0.4) | |
| Tunisia | 2 | 2011 | 2018 | 85.6 | 80.2 | -0.8 (-2.2, 0.6) | 83.2 | 42.4 | 5.8 (8.0, -3.7) | |
| Upper middle income | ||||||||||
| Gabon | 2 | 2000 | 2012 | 5.9 | 36.1 | 2.5 (1.9, 3.1) | 24.8 | 14.2 | -0.9 (-2.0, 0.2) | |
| Kazakhstan | 3 | 2006 | 2015 | 98.1 | 87.4 | -1.3 (-1.9, -0.8) | 96.8 | 77.1 | -2.3 (-3.1, -01.5) | |
| Namibia | 3 | 2000 | 2013 | 55.9 | 74.6 | 1.6 (0.7, 2.6) | 69.4 | 50.7 | -1.5 (-2.4, -0.5) | |
| Serbia | 2 | 2005 | 2014 | 48.0 | 80.7 | 3.6 (1.7, 5.6) | 57.1 | 55.7 | -0.2 (-2.0,1.7) | |
| Turkmenistan | 2 | 2006 | 2015 | 98.3 | 97.2 | -0.1 (-0.6, 0.4) | 97.4 | 91.5 | -0.7 (-1.3, -0.1) | |
Note: Boldface indicates statistical significance. A total of 10 countries with pro-poor coverage patterns in the most recent survey.
FIC, full immunization coverage; pp, percentage point.
Among the 6 countries where the pattern was not inverted, 4 showed significant reductions in coverage among the rich: in Kazakhstan, this was accompanied by a significant decline among the poor, but in Tajikistan, Tunisia, and Turkmenistan, the decline was restricted to the rich. Altogether, 7 of the 10 countries presented either a switch in the pattern from pro-rich to pro-poor or faster decline among the rich than was observed among the poor (Table 2).
Table 2. Annual Change in SII From 2000 in Countries With Pro-Poor Coverage Patterns.
| Country | Surveys, n | Initial year | Final year | SII in the initial year | SII in the final year | Annual change, pp (95% CI) | Inequality pattern |
|---|---|---|---|---|---|---|---|
| Low income | |||||||
| Gambia | 3 | 2005 | 2013 | −8.0 | −22.0 | 0.1 (−1.5,1.8) | Pro-poor pattern increased over time |
| Tajikistan | 3 | 2005 | 2017 | 3.1 | −11.5 | −1.2 (−2.4, 0.0) | Pattern changed from pro-rich to pro-poor |
| Lower middle income | |||||||
| Kyrgyzstan | 4 | 2005 | 2018 | 38.5 | −22.6 | −4.1 (−5.8, −2.3) | Pattern changed from pro-rich to pro-poor |
| Moldova | 2 | 2005 | 2012 | −4.2 | −22.7 | −2.6 (−5.2, −0.1) | Pro-poor pattern increased over time |
| Tunisia | 2 | 2011 | 2018 | −1.9 | −41.2 | −5.6 (−8.5, −2.7) | Pro-poor pattern increased over time |
| Upper middle income | |||||||
| Gabon | 2 | 2000 | 2012 | 23.9 | −20.3 | −3.7 (−5.1, −2.3) | Pattern changed from pro-rich to pro-poor |
| Kazakhstan | 3 | 2006 | 2015 | −1.6 | −16.2 | −1.5 (−2.5, −0.4) | Pro-poor pattern increased over time |
| Namibia | 3 | 2000 | 2013 | 17.1 | −20.6 | −3.2 (−4.7, −1.8) | Pattern changed from pro-rich to pro-poor |
| Serbia | 2 | 2005 | 2014 | 17.8 | −31.1 | −5.4 (−8.2, −2.7) | Pattern changed from pro-rich to pro-poor |
| Turkmenistan | 2 | 2006 | 2015 | −1.1 | −8.2 | −0.8 (−1.7, 0.1) | Pro-poor pattern increased over time |
Note: Boldface indicates statistical significance. A total of 10 countries with pro-poor coverage patterns in the most recent survey.
pp, percentage points; SII, slope index of inequality.
Discussion
The Sustainable Development Goals stand to reduce inequalities (Goal 10) and ensure universal health coverage by providing access to safe vaccines for all (Goal 3).20 These analyses show a wide range of FIC levels among LMICs such that some countries have achieved the WHO targets for immunization coverage, whereas others still have a long way to go. In particular, the authors observed that the proportion of LMICs exhibiting the classic pro-rich inequality pattern for FIC declined as national income increased and conversely that pro-poor patterns were more common in the upper middle–income group. Further evidence was provided by the finding that most of the 10 countries where propoor patterns were observed in the most recent survey did not present such patterns in the past.
Tudor–Hart’s inverse care law21 states that the availability of good medical care tends to vary inversely with the need for it in the population served. In terms of vaccine coverage, this is reflected by the presence of higher coverage among children from rich than among those from poor families in LMICs, which has been documented repeatedly for most countries.22,23 The current finding that this classic pattern of inequality is becoming less common, especially among upper middle–income countries, suggests the emergence of vaccine hesitancy.7
There are several studies indicating that vaccine hesitancy is a major problem in high-income countries, where lower rates of vaccine uptake have been found among the wealthiest children.24 This pattern has been identified in several countries including Canada, England, France, Italy, and the U.S.12,25–28 In the English study, Middleton and Baker28 suggested that vaccine hesitancy may be partly explained by the inverse equity hypothesis–a corollary to the inverse care law–which states that health interventions and behaviors tend to reach the more privileged groups of the society first before trickling down to the rest of the population.29,30 They suggested that the hypothesis also applies to unhealthy behaviors–such as refusal to vaccinate–which are wrongly perceived to be appropriate. If this is true, then the emergence of hesitancy among the better-offs may be expected to become more widespread over time.
Although the phenomenon of vaccine hesitancy has been recognized as a global issue, evidence from LMICs is still incipient.1,31 In Brazil, an upper middle–income country recognized for having reached high coverage and low levels of inequality in most of its states, a recent study showed that the pattern of inequality switched from pro-rich to pro-poor in the city of Pelotas, in the Southern region.32 In addition, in 2011, an ethnographic study described hesitant behavior regarding child immunization among highly educated and affluent Brazilian parents.33 A study of 21 national surveys carried out from 2000 to 2013 had identified declines in DPT coverage over time in 4 countries (Benin, Mozambique, Peru, and Vietnam), but these analyses were not disaggregated by family wealth.34 The present trend analysis revealed that pro-poor pattern became more accentuated in 5 countries and recent changes from pro-rich to pro-poor patterns in FIC in another 5, thus indicating that this is a contemporary phenomenon in these countries.
In a context where several countries still have not achieved the WHO immunization target, the emergence of vaccine-hesitant groups makes the problem more complicated to tackle. Interventions such as health system strengthening and conditional cash transfers are effective in reaching vulnerable children and raising immunization coverage.7,35 However, reaching vaccine-hesitant parents is not a matter of geographic or financial accessibility. A total of 3 major sets of determinants have been proposed to explain hesitancy: confidence (trust in the vaccines themselves as well as in health systems and health policies), complacency (how important families consider vaccination in their context), and convenience (including the availability and affordability of vaccines, quality of vaccination services, and related aspects).2 A systematic review conducted in 2014 showed that factors related to vaccine hesitancy are present in several LMICs, especially the belief that vaccines may be harmful to children.31
Several elements may have contributed to the emergence of vaccine hesitancy, including a loss of confidence in vaccine safety. Social media may play an important role in this sense, acting as a source of general information about vaccines, including adverse reactions, for the public.36,37 Bearing this in mind, the provided information may sometimes be alarmist and inaccurate.38
Another factor is how important the population perceives that vaccines are to children’s health. The eradication, or at least control of, vaccine-preventable diseases that have killed millions of children in the past, such as smallpox, measles, and polio, led to a feeling that vaccines are no longer needed. People with access to better health services, which themselves lead to better health outcomes, and people from the European region are those that least agree that vaccines are important for children.10,39
The Strategic Advisory Group of Experts working group on vaccine hesitancy suggests 3 main strategies to address vaccine hesitancy: understand its root causes, engage civil society and other partners to support immunization, and develop and promote tools to address vaccine hesitancy.40
It is very important to account for particularities in each country when evaluating and addressing vaccine hesitancy. Although the authors are pointing out the well-off as a vaccine-hesitant cluster, there may be others. In Sudan, for example, hesitant groups appear to be clustered by religion and ethnicity; in India, they are clustered by low educational status.41,42
The strengths of the study include that it was based on nationally representative surveys with large sample sizes and high comparability with each other. The study used a complex measure of inequality (SII) to provide a more accurate inequality estimation because it considers the whole population instead of a simple measure such as the difference between the wealthiest and the poorest quintiles.17 In addition, the extended period covered by the surveys provides a unique opportunity for examining time trends and the state of children’s immunization in recent years.
It is fundamental to raise and maintain immunization coverage at high levels to ensure community immunity that safeguards all children, even those who cannot be vaccinated owing to medical exemptions, and to avert outbreaks of vaccine-preventable diseases around the globe. Every vaccine demands a minimum level of coverage to secure community immunity; for example, for polio vaccine, the level varies from 80% to 86%, and for measles vaccine, it varies from 93% to 95%.43
Limitations
These analyses have limitations. It is assumed that vaccine hesitancy is present when coverage among children from wealthier families is lower than that among those from poorer families, without measuring hesitancy directly. Nevertheless, a cohort study carried out in the U.S. showed that a questionnaire designed to identify vaccine-hesitant parents predicted lower rates of up-to-date vaccination status among their children.44
Because the DHS and MICS questionnaires do not inquire specifically about vaccine hesitancy, the authors believe that it is reasonable to presume that when the well-offs are not vaccinating their children, it is not due to a lack of access to vaccination services but rather a concern about vaccines. Considering that vaccine-hesitant parents may arrange for their children to receive the basic vaccines yet remain concerned about them, in the worst-case scenario, these analyses only captured those vaccine-hesitant parents who refused vaccination, which would underestimate the problem.45
In addition, when the child’s vaccination card was not available, information on immunization was based on the mother’s recall, a potential source of recall bias. Nonetheless, this is how the WHO recommends calculating the FIC indicator. Moreover, a given country’s complex immunization schedule may increase the risk of recall bias.46
Conclusions
This study found that the classic pro-rich inequality pattern, in which the wealthy present better coverage than the poor, appears to be changing, especially in upper middle—income countries. The authors propose that this shift may be due to vaccine hesitancy among the wealthy families.
Immunization is a major advance in public health, and special attention is required to prevent vaccine hesitancy from affecting coverage in LMICs, with continued efforts to secure access to vaccination.
Supplementary Material
Acknowledgments
The authors acknowledge and thank the Global Institute for Vaccine Equity at the University of Michigan School of Public Health for financial support of this supplement. The findings and conclusions of articles in this supplement are those of the authors and do not necessarily represent the official position of the University of Michigan.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
This paper was made possible with funds from the Wellcome Trust (Grant Number: 101815/Z/13/Z), Bill & Melinda Gates Foundation (Grant Number: OPP1199234), and Associação Brasileira de saúde Coletiva and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance Code 001).
All authors participated in the preparation of the manuscript and approved its final version for submission. BDOCP and TMS conducted the analyses under the supervision of FCW and CGV. BDOCP and CGV wrote the manuscript. FCW and AJDB supervised the interpretation of the findings as well as the writing of the paper. All authors contributed to the interpretation of the analyses.
No financial disclosures were reported by the authors of this paper.
References
- 1.WHO. Ten threats to global health in 2019. Geneva, Switzerland: WHO; [Accessed January 4, 2020]. https://www.who.int/vietnam/news/feature-stories/detail/ten-threats-to-global-health-in-2019 Published March 21, 2019. [Google Scholar]
- 2.MacDonald NE. SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Burki T. Vaccine misinformation and social media. Lancet. 2019;1(6):E258–E259. doi: 10.1016/S2589-7500(19)30136-0. [DOI] [Google Scholar]
- 4.WHO. Immunization coverage. [Accessed February 3, 2020]. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage Updated July 15, 2020.
- 5.The expanded programme on immunization. WHO; [Accessed February 5, 2020]. https://www.who.int/immunization/programmes_systems/supply_chain/benefits_of_immunization/en/ Updated December 1, 2013. [Google Scholar]
- 6.Bryce J, Terreri N, Victora CG, et al. Countdown to 2015: tracking intervention coverage for child survival. Lancet. 2006;368(9541):1067–1076. doi: 10.1016/S0140-6736(06)69339-2. [DOI] [PubMed] [Google Scholar]
- 7.Gwatkin DR, Bhuiya A, Victora CG. Making health systems more equitable. Lancet. 2004;364(9441):1273–1280. doi: 10.1016/S0140-6736(04)17145-6. [DOI] [PubMed] [Google Scholar]
- 8.Measles: epidemiological alerts and updates. Pan American Health Organization, WHO; [Accessed February 9, 2020]. https://www.paho.org/hq/index.php?option=-com&_topics&view=rdmore&cid=2204&item=measles&type=alerts&I-temid=40899&lang=en Updated February 28, 2020. [Google Scholar]
- 9.Davis MM, Shah SK. Outbreaks of vaccine-preventable diseases: responding to system failure with national vaccination requirements. JAMA. 2019;322(1):33–34. doi: 10.1001/jama.2019.8251. [DOI] [PubMed] [Google Scholar]
- 10.Larson HJ, de Figueiredo A, Xiahong Z, et al. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi: 10.1016/j.ebiom.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360(19):1981–1988. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 12.Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315(11):1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancioglu A, Arnold F. Measuring coverage in MNCH: tracking progress in health for women and children using DHS and MICS household surveys. PLoS Med. 2013;10(5):e1001391. doi: 10.1371/journal.pmed.1001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 15.Rutstein SO, Johnson K. DHS comparative reports 6: the DHS wealth index. Calverton, MD: ORC Macro; [Accessed January 10, 2020]. https://dhsprogram.com/pubs/pdf/cr6/cr6.pdf Published August 2004. [Google Scholar]
- 16.Rutstein SO. DHS woking papers. The DHS wealth index: approaches for rural and urban areas. Calverton, Maryland: Macro International Inc; [Accessed January 10, 2020]. https://dhsprogram.com/pubs/pdf/WP60/WP60.pdf Published October 2008. [Google Scholar]
- 17.Barros AJ, Victora CG. Measuring coverage in MNCH: determining and interpreting inequalities in coverage of maternal, newborn, and child health interventions. PLoS Med. 2013;10(5):e1001390. doi: 10.1371/journal.pmed.1001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health nutrition and population statistics: population estimates and projections. The World Bank; [Accessed January 10, 2020]. https://databank.worldbank.org/reports.aspx?source=health-nutrition-and-population-statistics:-population-estimates-and-projections Updated September 19, 2019. [Google Scholar]
- 19.International Center for Equity in Health. [Accessed March 4, 2020]. http://www.equidade.org .
- 20.The 17 goals. United Nations, Department of Economic and Social Affairs, Sustainable Development Goals; [Accessed February 29, 2020]. https://sdgs.un.org/goals Updated April 20, 2018. [Google Scholar]
- 21.Hart JT. The inverse care law. Lancet. 1971;1(7696):405–412. doi: 10.1016/s0140-6736(71)92410-x. [DOI] [PubMed] [Google Scholar]
- 22.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66(7):e14. doi: 10.1136/jech.2010.124651. [DOI] [PubMed] [Google Scholar]
- 23.WHO. State of inequality: childhood immunization. WHO; Geneva, Switzerland: [Accessed August 27, 2020]. https://www.who.int/gho/health_equity/report_2016_immunization/en/ Published 2016. [Google Scholar]
- 24.Bocquier A, Ward J, Raude J, Peretti-Watel P, Verger P. Socioeconomic differences in childhood vaccination in developed countries: a systematic review of quantitative studies. Expert Rev Vaccines. 2017;161(11):1107–1118. doi: 10.1080/14760584.2017.1381020. [DOI] [PubMed] [Google Scholar]
- 25.Carpiano RM, Polonijo AN, Gilbert N, Cantin L, Dubé E. Socioeconomic status differences in parental immunization attitudes and child immunization in Canada: findings from the 2013 Childhood National Immunization Coverage Survey (CNICS) Prev Med. 2019;123:278–287. doi: 10.1016/j.ypmed.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Gualano MR, Bert F, Voglino G, et al. Attitudes towards compulsory vaccination in Italy: results from the NAVIDAD multicentre study. Vaccine. 2018;36(23):3368–3374. doi: 10.1016/j.vaccine.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Bocquier A, Fressard L, Cortaredona S, et al. Social differentiation of vaccine hesitancy among French parents and the mediating role of trust and commitment to health: a nationwide cross-sectional study. Vaccine. 2018;36(50):7666–7673. doi: 10.1016/j.vaccine.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 28.Middleton E, Baker D. Comparison of social distribution of immunisation with measles, mumps, and rubella vaccine, England, 1991 — 2001. BMJ. 2003;326(7394):854. doi: 10.1136/bmj.326.7394.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victora CG, Vaughan JP, Barros FC, Silva AC, Tomasi E. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet. 2000;356(9235):1093–1098. doi: 10.1016/S0140-6736(00)02741-0. [DOI] [PubMed] [Google Scholar]
- 30.Victora CG, Joseph G, Silva ICM, et al. The inverse equity hypothesis: analyses of institutional deliveries in 286 national surveys. Am J Public Health. 2018;108(4):464–471. doi: 10.2105/AJPH.2017.304277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobos Muñoz D, Monzón Llamas L, Bosch-Capblanch X. Exposing concerns about vaccination in low-and middle-income countries: a systematic review. Int J Public Health. 2015;60(7):767–780. doi: 10.1007/s00038-015-0715-6. [DOI] [PubMed] [Google Scholar]
- 32.Silveira MF, Buffarini R, Bertoldi AD, et al. The emergence of vaccine hesitancy among upper-class Brazilians: results from four birth cohorts, 1982-2015. Vaccine. 2020;38(3):482–488. doi: 10.1016/j.vaccine.2019.10.070. [DOI] [PubMed] [Google Scholar]
- 33.Couto MT, Barbieri CLA. Care and (non)-vaccination in the context of high-income and well-schooled families in São Paulo in the state of São Paulo, Brazil. Cien Saude Colet. 2015;20(1):105–114. doi: 10.1590/1413-81232014201.21952013. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinpoor AR, Bergen N, Schlotheuber A, et al. State of inequality in diphtheria—tetanus—pertussis immunisation coverage in low-income and middle-income countries: a multicountry study of household health surveys. Lancet Glob Health. 2016;4(9):e617–e626. doi: 10.1016/S2214-109X(16)30141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinman AR, McKinlay MA. Immunization equity. Vaccine. 2015;33(suppl 4):D72–D77. doi: 10.1016/j.vaccine.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 36.Gellin BG, Maibach EW, Marcuse EK. Do parents understand immunizations? A national telephone survey. Pediatrics. 2000;106(5):1097–1102. doi: 10.1542/peds.106.5.1097. [DOI] [PubMed] [Google Scholar]
- 37.Pareek M, Pattison HM. The two-dose measles, mumps, and rubella (MMR) immunisation schedule: factors affecting maternal intention to vaccinate. Br J Gen Pract. 2000;50(461):969–971. [PMC free article] [PubMed] [Google Scholar]
- 38.Bradshaw AS, Treise D, Shelton SS, et al. Propagandizing anti-vaccination: analysis of Vaccines Revealed documentary series. Vaccine. 2020;38(8):2058–2069. doi: 10.1016/j.vaccine.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Vanderslott S, Dadonaite B, Roser M. Vaccination. Our World in Data; [Accessed Feb 16, 2020]. https://ourworldindata.org/vaccination Updated December 2019. [Google Scholar]
- 40.Eskola J, Duclos P, Schuster M, MacDonald NE. Sage Working Group on Vaccine Hesitancy. How to deal with vaccine hesitancy? Vaccine. 2015;33(34):4215–4217. doi: 10.1016/j.vaccine.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 41.Sabahelzain MM, Moukhyer M, Dubé E, Hardan A, van den Borne B, Bosma H. Towards a further understanding of measles vaccine hesitancy in Khartoum state, Sudan: a qualitative study. PLoS One. 2019;14(6):e0213882. doi: 10.1371/journal.pone.0213882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasgupta P, Bhattacherjee S, Mukherjee A, Dasgupta S. Vaccine hesitancy for childhood vaccinations in slum areas of Siliguri, India. Indian J Public Health. 2018;62(4):253–258. doi: 10.4103/ijph.IJPH_397_17. [DOI] [PubMed] [Google Scholar]
- 43.Kwong A, Ambizas EM. Measles and the MMR vaccine. [Accessed March 20 2020];U S Pharm. 2019 44(7):8–13. https://www.uspharmacist.com/article/measles-and-the-mmr-vaccine . [Google Scholar]
- 44.Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 2013;167(11):1065–1071. doi: 10.1001/jamapediatrics.2013.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards KM, Hackell JM. the Committee on Infectious Diseases, the Committee on Practice and Ambulatory Medicine. Countering vaccine hesitancy. Pediatrics. 2016;138(3):e20162146. doi: 10.1542/peds.2016-2146. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Harmonizing vaccination coverage measures in household surveys: a primer. Geneva, Switzerland: WHO Expanded Programme on Immunization in the Department of Immunization, Vaccines and Biologics; [Accessed August 27, 2020]. https://www.who.int/immunization/monitoring_surveillance/Surveys_White_Paper_immunization_2019.pdf?ua=1 Published May 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.