Abstract
Purpose
Hearing and visual impairment have been associated with psychosis. Mechanisms behind this are poorly understood. We tested whether i) self-reported hearing and visual impairments are associated with psychotic symptoms in the 2014 UK Adult Psychiatric Morbidity Survey; ii) the odds of having psychotic symptoms vary with self-perceived degree of impairments; and iii) reduced social functioning partially explains these associations.
Methods
We analysed cross-sectional data using logistic regression. Hearing and visual impairment were the exposures, and screening positive on the Psychosis Screening Questionnaire was the outcome. We used structural equation modelling to assess mediation by social functioning, measured by the Social Functioning Questionnaire.
Results
Psychotic symptoms were strongly associated with visual impairment (Adjusted Odds Ratio (AOR) 1.81, 95% Confidence Intervals (CI) 1.33 to 2.44), especially moderate visual impairment (AOR 2.75, 95% CI 1.78 to 4.24, p < .001). Psychotic symptoms were associated with a severe degree of hearing impairment (AOR 4.94, 95% CI 1.66 to 14.67, p=.004), and weakly associated with hearing impairment overall (AOR 1.50, 95% CI 1.10 to 2.04, p=.010). Social functioning accounted for approximately 50% of associations with both types of sensory impairment, but the confidence intervals around these estimates were broad.
Conclusions
Our findings suggest an association between psychosis and visual impairment, with the strongest evidence for moderate visual impairment; the findings also support a linear relationship between psychosis and degree of hearing impairment. Social functioning may mediate these relationships and be a potential target for intervention, alongside sensory correction. These should be investigated longitudinally.
Keywords: Visual impairment, Hearing impairment, Social functioning, Psychotic symptoms
1. Introduction
In the UK, one in eight people have hearing impairment (Action on Hearing Loss, Updated 2015). Incidence and prevalence of hearing impairment increase with age, with age-related cochlea damage the single greatest cause (Action on Hearing Loss, Updated 2015; Fortnum et al., 2001). Hearing impairment, defined as an inability to hear sounds below 40 dB, can affect social participation (Action on Hearing Loss, Updated 2015). There are nearly 2 million people living with sight loss in the UK; around 360,000 are registered blind or partially-sighted (NHS, Reviewed 2018). The World Health Organisation (WHO) defines visual impairment as a visual acuity below 0.3 or a visual field <30° (World Health Organisation, 2007).
Psychotic symptoms may constitute a disorder such as schizophrenia, or be below the threshold at which illness is diagnosed. A 2016 systematic review found that people with hearing impairment were at greater risk of psychotic symptoms than those without (Linszen et al., 2016). One study found an age-specific association between hearing impairment and psychosis due to disruption during a critical developmental phase in adolescence (van der Werf et al., 2011). More severe and chronic hearing impairment might carry the greatest risk of psychosis (Eastwood et al., 1985; van der Werf et al., 2007). The degree to which self-perceived impairment is correctable might also alter its impact on psychosis (van der Werf et al., 2007). There are case reports of new onset auditory hallucinations when hearing declines (Bernardini et al., 2017); in some cases improving or resolving with hearing aids (Coebergh et al., 2015; Sommer et al., 2014). Several studies found no association between hearing impairment and psychotic symptoms, but had small samples (Eastwood et al., 1985; Livingston et al., 2001). The few studies reporting reduced odds of psychosis in hearing impaired people included very small numbers of participants with psychotic disorders (Brodaty et al., 1999; Mason et al., 2008; Moore, 1981).
A 2013 WHO survey of over 20,000 people showed that visual problems were associated with psychotic symptoms (Moreno et al., 2013). Both near and distance visual impairment are implicated (Viertiö et al., 2007). In one study, one-eighth of middle-aged, severely mentally ill patients had distance visual impairment (Zheng et al., 2015). This was towards the higher end of the estimated range for the local population. There is a known association between visual processing difficulties and both the negative symptoms of psychotic illnesses, and schizotypal features (Kéri et al., 2005; Türközer et al., 2018).
Several hypotheses might explain the apparent association between sensory impairment and psychosis. Sensory impairment could be a biomarker of psychotic illness. Oxidative stress, NMDA receptor damage and deafferentation can all underlie both sensory deficits and schizophrenia (Geiser et al., 2017). Some studies suggest an association between schizophrenia and reduced retinal fibre layer thickness (Adams and Nasrallah, 2017; Pan et al., 2018), or visual processing abnormalities (Császár et al., 2018; Silverstein and Rosen, 2015). Visual problems can result from antipsychotic medication (Yue Chen et al., 2003).
Alternatively sensory impairment might cause psychosis. Sensory deprivation can induce psychotic symptoms in healthy individuals (Mason and Brady, 2009) (Bernardini et al., 2017). Early evidence implicates visual difficulties in the aetiology of psychotic illnesses. A 2018 longitudinal study of over 1,000,000 Swedish military conscripts showed that men with severe visual impairment had an increased subsequent risk of psychotic disorder (Hayes et al., 2018). The hazard ratios were highest in those whose vision could not be corrected to normal, suggesting a possible cortical origin to the deficit.
An absence of known cases of schizophrenia in congenitally, cortically blind individuals has led to a proposed ‘Protection against Schizophrenia’ (PaSZ) model (Landgraf and Osterheider, 2013; Silverstein and Rosen, 2015). This suggests that incorrect interpretation of visual information caused by partial visual impairment increases the risk of schizophrenia; in contrast, perfect and absent vision protect against schizophrenia (Landgraf and Osterheider, 2013). This model is supported by evidence from one (Hayes et al., 2018), but not a second observational study (Caspi et al., 2009). By contrast there are many reported cases of psychosis in congenital profound hearing impairment, so the theory that severe sensory impairment may be protective is not supported for hearing impairment (Atkinson et al., 2007).
Another possible explanation for an association between sensory impairments and psychosis is that sensory impairment reduces opportunities for social participation, thereby increasing isolation and loneliness, causing stress that increases the risk of psychosis (Linszen et al., 2016). Sensory impairments can contribute to loneliness, depression and reduced social participation in older adults (Contrera et al., 2017; Han et al., 2018; Jayakody et al., 2018; McManus et al., 2018; Stam et al., 2016). Younger adults may experience discrimination and reduced educational, social and occupational opportunities (Du Feu and Fergusson, 2003). These experiences can be captured by measuring social functioning.
In this paper we explore the relationship between sensory impairment and psychosis. This is, to our knowledge, the first study to test whether reduced social functioning is a mechanism linking sensory impairment to psychosis; and to assess whether psychotic symptoms, rather than diagnoses, are associated with visual impairment in a dose-dependent manner in a nationally representative sample. We tested hypotheses that:
People with hearing or visual impairment have greater odds of reporting psychotic symptoms than people without, and this varies according to the type (hearing or vision) and degree of sensory impairment.
The association between sensory impairment and psychotic symptoms is partially accounted for by reduced social functioning.
2. Methods
2.1. Sample
The UK Adult Psychiatric Morbidity Survey (APMS) is a cross-sectional household survey, conducted every 7 years since 1993 (McManus et al., 2016). The 2014 APMS sample contains 7546 members of the English population aged over 16 (McManus et al., 2016). It is a stratified, multi-stage probability sample. Participants were selected randomly from households, which had been randomly selected from postal areas stratified according to socioeconomic variables. The response rate was 57%. Data are weighted to account for non-response and selection probability. Questions on sensory impairment were included for the first time in the 2014 survey. Permission to use the data for this study was granted by NHS Digital.
2.2. Outcome measures
The Psychosis Screening Questionnaire (PSQ) is a tool to detect presence of psychotic symptoms (Bebbington and Nayani, 1995). It has high sensitivity and specificity for identifying psychosis in general practice (Bebbington and Nayani, 1995). It originally included 6 question clusters about 6 symptoms: hypomania, thought interference, persecution, perceptual abnormalities, ‘strange experiences’ and hallucinosis; 20 questions in total. In APMS the hypomania cluster was excluded, leaving 5 clusters and 17 questions in total. Endorsing one or more clusters is considered indicative of clinically significant psychotic symptoms; this requires a specific answer to more than one question (Johns et al., 2004). We used this standard interpretation of the result.
2.3. Exposure variables
Participants were asked whether they had difficulty hearing or wore a hearing aid as one question with a yes/no response. We analysed this as a binary exposure variable, commensurate with previous studies examining presence of sensory impairment (Linszen et al., 2016; Moreno et al., 2013). Participants who responded yes were asked: (With your hearing aid) how would you describe your difficulty hearing? - No difficulty, mild difficulty, moderate difficulty, severe difficulty, or cannot do. We analysed severity of hearing impairment as a second exposure variable. We combined the severe difficulty and cannot do categories due to low numbers and to increase statistical power and precision of estimates.
People were asked how much difficulty they had: reading a newspaper (near vision impairment), and seeing a face across the room (distance vision impairment), even with visual aids. They gave their answers as a degree of difficulty using Likert-scale answers (No difficulty, mild difficulty, moderate difficulty, severe difficulty, or cannot do). We combined near and distance types of visual impairment. For each participant, the more severe degree of visual impairment in either category was used. We combined the severe and cannot do categories. We analysed presence of any degree of visual impairment as a binary exposure variable. We also analysed degree of visual impairment as a second exposure.
2.4. Potential mediator
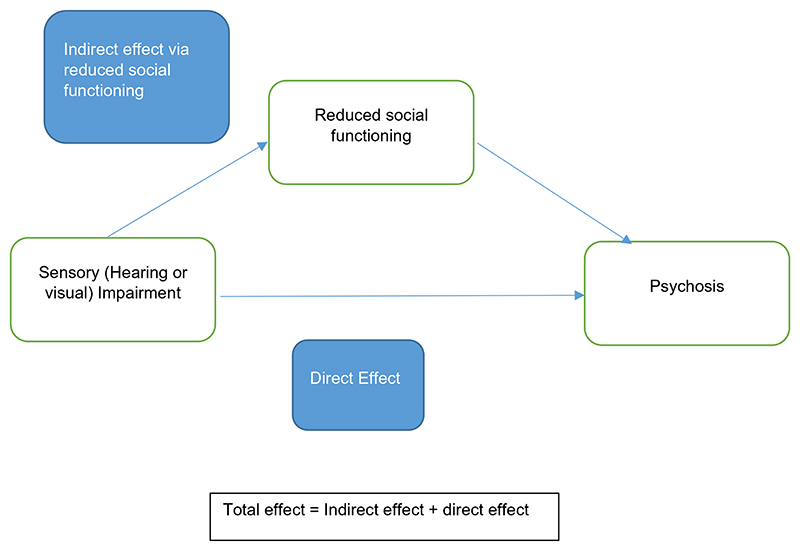
Reduced social functioning was considered as a potential mediator of the relationship between sensory impairment and psychotic symptoms (Fig. 1). The Social Functioning Questionnaire (SFQ) is a validated eight-item scale measuring perceived social functioning with a score range from 0 to 24 (Tyrer et al., 2005). Lower scores represent better functioning. Feelings of loneliness, stress, relationships, and enjoyment of leisure time are among the items covered. We used total SFQ score as a continuous measure.
Fig. 1. Mediation model. Total effect = Indirect effect + direct effect.
2.5. Confounders
We included age (in 10 year brackets), gender, ethnicity, and housing tenure and highest educational qualification (as markers of socioeconomic status) as potential confounding variables in the models testing associations between sensory impairment and psychosis. Ethnicity was coded as either White British, White Other; Black/African/Caribbean/Black British; Asian/Asian British; or mixed/multiple ethnic groups/other ethnic groups. Highest educational qualification was coded as either Degree, Teaching/Higher National Diploma/Nursing, A Level, GCSE or equivalent, Foreign/other, or No Qualifications. Tenure was coded as Own Outright, Buying it with the help of a mortgage or loan, Pay part rent and part mortgage (shared ownership), Rent it, Live here rent free, or squatting. We also included Alcohol Use Disorders Identification Test (AUDIT) score (Saunders et al., 1993). These variables were included as potential confounders if they were differentially distributed among those exposed and unexposed and those with and without the outcome, or if they were previously recognised to be associated with the exposure and outcome and not on the causal pathway.
2.6. Statistical analysis
We undertook complete case analyses in Stata version 15 (StataCorp, 2017). The original APMS survey weighting was preserved in our study using the Stata svy command. Where missing data meant that a stratum contained only one primary sampling unit, the observations within that unit were moved to an adjacent stratum to allow the variation within each strata to be calculated (McDowell, n.d.). This was essential to preserve the weighting without losing data. We report weighted percentages, but unweighted absolute numbers. We explored differences between those with and without missing data.
We used logistic regression to assess whether people with hearing or visual impairment (as binary exposures) had increased odds of screening positive on the PSQ relative to those without. The exposure variable was either presence of hearing impairment or presence of visual impairment. The outcome was whether the participant screened positive on the PSQ.
We also carried out logistic regressions with degree of hearing or visual impairment as separate exposures and screening positive on the PSQ as the outcome. Univariable models were run initially, followed by multivariable models including relevant confounding variables.
Finally, we built structural equation models using the Stata gsem command, appropriate for binary outcomes, to test to what extent reduced social functioning might account for associations between sensory impairment and screening positive on the PSQ. An estimated percentage of mediation was obtained by dividing the indirect effect of the exposure on the outcome, via the mediator, by the total effect of the exposure on the outcome (including any direct effect). We used additive log coefficients in these calculations, but present their exponentials in the results section. This mediation analysis was exploratory, due to the limitations of cross-sectional data.
2.7. Sensitivity analyses
We did not adjust for estimated verbal IQ in our main analyses, because this would restrict the analytic sample to native English speakers (McManus et al., 2018). It could also produce misleading results, as reduced IQ can be intrinsic to psychosis (Kahn and Keefe, 2013). We ran separate analyses adjusting for estimated verbal IQ however.
We did not adjust for antipsychotic medication use in primary analyses since it is a result of the outcome, but we performed a separate sensitivity analysis to assess the impact of this. Current use of antipsychotic medication was decided by showing participants a list of such medications and asking if they were taking any.
We performed a sensitivity analysis in which participants who reported screening positive on the PSQ purely due to the presence of auditory or visual hallucinations were removed from the analytic sample, to exclude hallucinations resulting directly from sensory deprivation as the sole driver of any association. We used logistic regression to test for an unadjusted association between reporting visual or auditory hallucinations (outcome) and visual and hearing impairment (exposures).
We tested separately for an interaction between age (in 10 year brackets) and sensory deprivation in the association with PSQ result in case older and younger people are affected differently by sensory impairment.
Finally, we performed a sensitivity analysis in which reporting having received a diagnosis of psychosis or schizophrenia was the outcome.
3. Results
3.1. Sample demographics
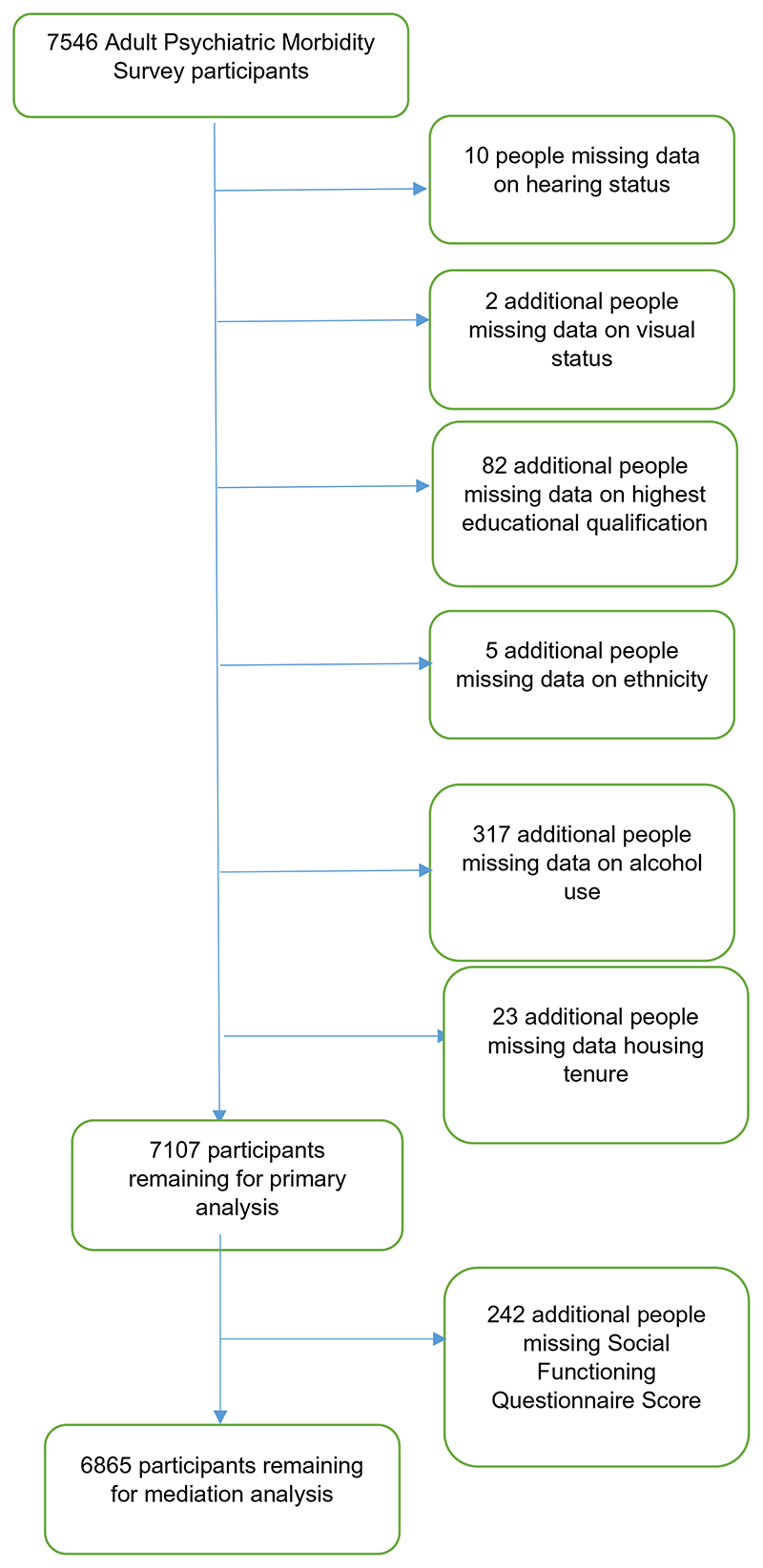
Of 7546 people who participated in the AMPS, 7107 had provided all relevant data and constituted the analytic sample. Table 1 shows the demographics of this sample according to sensory impairment. 435 (6.4%) of people included screened positive on the PSQ. 1207 (14.3%) reported hearing impairment, 934 (11.9%) reported visual impairment, and 241 (2.6%) reported both.
Table 1. Sociodemographics of analytic sample.
| Characteristic N = 7107 | No hearing impairment n (weighted %) | Hearing impairment n (weighted %) | No visual impairment n (weighted %) | Visual impairment n (weighted %) |
|---|---|---|---|---|
| Total (and % of total sample) | 5900 (85.7%) | 1207 (14.3%) | 6173 (88.1%) | 934 (11.9%) |
| Number scoring Positive on Psychosis Screening Questionnaire (PSQ) | 368 (6.4%) | 67 (6.3%) | 348 (5.9%) | 87 (9.6%) |
| Male | 2280 (47.8%) | 594 (55.2%) | 2509 (49.1%) | 365 (47.4%) |
| Aged over 65 | 1330 (15.9%) | 713(50.2%) | 1662 (19.3%) | 378 (32.0%) |
| Ethnicity | ||||
| White British | 4928 (79.2%) | 1112 (89.6%) | 5216 (80.2%) | 824 (84.1%) |
| Any other ethnic background | 972 (20.8%) | 95 (10.4%) | 957 (19.8%) | 110 (15.8%) |
| Marital status | ||||
| Married or cohabiting | 3303 (61.7%) | 645 (65.1%) | 3521 (63.0%) | 427 (56.4%) |
| Single | 1355 (26.3%) | 138 (12.2%) | 1314 (24.7%) | 179(21.3%) |
| Divorced or separated | 761 (7.6%) | 162 (9.0%) | 765 (7.4%) | 158 (10.8%) |
| Widowed | 481 (4.4%) | 262 (13.8%) | 573 (5.0%) | 170 (11.6%) |
| Highest educational qualification | ||||
| Degree | 1533 (26.7%) | 210 (19.5%) | 1576 (26.6%) | 167 (19.1%) |
| Teaching/higher national diploma/nursing | 485 (7.8%) | 95 (7.9%) | 509 (7.9%) | 71 (7.3%) |
| A levels | 1000 (19.4%) | 153 (14.5%) | 1041 (19.4%) | 112 (14.0%) |
| GCSE or equivalent | 1457 (25.8%) | 227 (20.2%) | 1466 (24.9%) | 218 (25.8%) |
| Foreign/other | 180 (2.6%) | 71 (5.6%) | 211 (3.0%) | 40 (3.5%) |
| No qualifications | 1245 (17.6%) | 451 (32.5%) | 1370 (18.3%) | 326 (30.3%) |
| Tenure | ||||
| Own outright | 1908 (27.8%) | 651 (49.8%) | 2201 (30.7%) | 358 (32.8%) |
| Buying with help of mortgage or loan/shared ownership scheme | 1925 (35.2%) | 207 (22.3%) | 1922 (34.3%) | 210 (26.6%) |
| Renting | 1897 (32.9%) | 318 (25.3%) | 1876 (31.2%) | 339 (36.2%) |
| Living rent-free (including squatting) | 170 (4.1%) | 31 (2.7%) | 174(3.8%) | 27 (4.4%) |
| Currently taking antipsychotic medications | 66 (1.0%) | 18 (1.3%) | 58 (0.8%) | 26 (2.3%) |
| Alcohol Use Disorders (AUDIT) scorea | 4.2 (4.6) | 3.7 (4.2) | 4.1 (4.4) | 4.0 (5.3) |
| Verbal Intelligence Quotient (IQ)a | 103.4(15.3) | 102.9 (16.2) | 103.7 (15.1) | 100.5 (17.1) |
| Social Functioning Questionnaire (SFQ) scorea | 4.2 (3.5) | 3.9 (3.4) | 4.0 (3.4) | 5.1 (4.1) |
| Reported having ever been diagnosed with psychosis or schizophrenia | 31 (<0.1%) | 6 (<0.1%) | 29 (<0.1%) | 8 (<0.1%) |
This is the analytic sample for the primary analyses.
n = Number in group (unweighted) weighted % = percentage of group weighted.
For these variables, the mean (standard deviation) is shown.
People with sensory impairment were more likely to be aged over 65 and widowed, and less likely to have a degree.
45 (<0.1%) of people in the sample reported ever having been diagnosed with schizophrenia or psychosis. 53.4% of this group screened positive on the PSQ, compared to 6.2% who reported no diagnosis of psychosis.
3.2. Missing data
Participants with missing data were less likely to be aged over 65 (37% vs 20.8%). They were less likely to be Asian or Asian British (3.8% vs 7.1%), and more likely to be from a mixed or ‘other’ ethnic group (4.8% vs 2.5%). They were more likely to have no qualifications (34.1% vs 19.7%), and to own their home outright (41.6% vs 31%). Similar proportions of people with and without missing data scored positive on the PSQ (5.5% with vs 6.4% without). People with missing data were more likely to report hearing impairment (23.1% vs 14.3%) and visual impairment (18.7% vs 11.9%).
For mediation analysis, a further 242 people were excluded because they had not completed the SFQ (Fig. 2).
Fig. 2. Missing data.
3.3. Visual impairment and psychosis
We found evidence of an association between visual impairment and a positive PSQresult, even after controlling for potential confounders (Adjusted Odds Ratio (AOR) 1.81, 95% confidence intervals (CI) 1.33–2.44, p <.001). (Table 2). Moderate visual impairment was associated with screening positive on the PSQ, including following adjustment (AOR 2.75, 95% CI 1.78 to 4.24, p <.001). There was weak evidence for an association between a positive PSW result and mild (AOR 1.49, 95% CI 0.99 to 2.24, p=.054), but not severe visual impairment (AOR 1.43, 95% CI 0.60 to 3.42, p=.423) (Table 3). The mediation analysis attributed 50% (95% CI 18% to 81%, p=.002) of the association between visual impairment and psychotic experiences to reduced social functioning (Table 4).
Table 2. Odds ratios with 95% confidence intervals for screening positive on the Psychosis Screening Questionnaire (PSQ) according to presence of hearing and visual impairment.
| Type of sensory impairment n = 7107 | Unadjusted Odds Ratio relative to no difficulty (95% CI) | p-Value | Adjusteda Odds Ratio relative to no difficulty (95% CI) | p-Value |
|---|---|---|---|---|
| Hearing impairment | 0.98 (0.74 to 1.31) | 0.911 | 1.50 (1.10 to 2.04) | 0.010 |
| Visual impairment | 1.68 (1.26 to 2.24) | <0.001 | 1.81 (1.33 to 2.44) | <0.001 |
n = total number of participants in analysis.
Adjusted for age (in 10 year brackets), gender, ethnicity, highest educational qualification, tenure and Alcohol Use Disorders Identification Test (AUDIT) score.
Table 3. Odds of screening positive on Psychosis Screening Questionnaire (PSQ) by degree of sensory impairment.
| Degree of sensory impairment | Unadjusted Odds Ratio relative to no difficulty (95% CI) | p-Value | Adjusteda Odds Ratio relative to no difficulty (95% CI) | p-Value |
|---|---|---|---|---|
| Hearing impairment | ||||
| No difficulty (reference category) N = 6116 | 1 | 1 | ||
| Mild difficulty n = 630 | 1.10 (0.75 to 1.60) | 0.630 | 1.59 (1.07 to 2.35) | 0.022 |
| Moderate difficulty N = 310 | 0.94 (0.58 to 1.53) | 0.800 | 1.56 (0.92 to 2.64) | 0.097 |
| Severe difficulty or cannot do N = 51 | 2.36 (0.87 to 6.39) | 0.091 | 4.94 (1.66 to 14.67) | 0.004 |
| Visual impairment | ||||
| No difficulty (reference category) N = 6173 | 1 | 1 | ||
| Mild difficulty N = 519 | 1.45 (0.97 to 2.16) | 0.070 | 1.49 (0.99 to 2.24) | 0.054 |
| Moderate difficulty N = 274 | 2.45 (1.62 to 3.71) | <0.001 | 2.75 (1.78 to 4.24) | <0.001 |
| Severe difficulty or cannot do N = 141 | 1.20 (0.56 to 2.59) | 0.637 | 1.43 (0.60 to 3.42) | 0.423 |
n = total number of participants in analysis.
Adjusted for age, gender, ethnicity, employment type, highest educational qualification, housing tenure and Alcohol Use Disorders Identification Test (AUDIT) score.
Table 4.
Mediation analysis: assessment of mediation of association of visual impairment with odds of screening positive on Psychosis Screening Questionnaire (PSQ) as binary outcome by Social Functioning Questionnaire (SFQ) score.
| Analytic sample n = 6865 | Unadjusted Odds Ratio in visual impairment (95% confidence interval) | p-Value | Adjusteda Odds Ratio in visual impairment (95% confidence interval) | p-Value |
|---|---|---|---|---|
| Total effect of visual impairment on odds of positive PSQscreen | 1.59 (1.14 to 2.22) | 0.006 | 1.78 (1.28 to 2.48) | 0.001 |
| Indirect effect via SFQ score | 1.31 (1.21 to 1.42) | <0.001 | 1.33 (1.24 to 1.43) | <0.001 |
| Direct effect of visual impairment on odds of positive PSQ screen | 1.22 (0.86 to 1.72) | 0.261 | 1.34 (0.95 to 1.89) | 0.095 |
| Analytic sample | Unadjusted Odds Ratio in hearing | p-Value | Adjusteda Odds Ratio in hearing | p-Value |
| n = 6865 | impairment | impairment | ||
| (95% confidence interval) | (95% confidence interval) | |||
| Total effect ofhearing impairment on odds of positive PSQ | 0.99 (0.72 to 1.35) | 0.937 | 1.45 (1.05 to 2.00) | 0.024 |
| screen | ||||
| Indirect effect via SFQ score | 0.98 (0.92 to 1.04) | 0.524 | 1.17 (1.10 to 1.24) | <0.001 |
| Direct effect of hearing impairment on odds of positive PSQ | 1.01 (0.74 to 1.37) | 0.963 | 1.24 (0.90 to 1.70) | 0.186 |
| screen | ||||
n = Number in analysis.
Total effect = Combined estimated direct effect of sensory impairment on odds of screening positive on PSQ + indirect effect mediated by SFQ score.
Indirect effect = Estimated effect of sensory impairment on odds of screening positive on PSQ via effect on SFQ score.
Direct effect = Estimated direct effect of sensory impairment on odds of screening positive on PSQ (not via SFQscore).
Estimated degree of mediation was obtained by dividing the indirect effect by the total effect.
Direct effect of sensory impairment on Psychosis Screening Questionnaire (PSQ) result adjusted for age, gender, ethnicity, highest educational qualification, housing tenure, and Alcohol Use Disorders Identification Test (AUDIT) score. Effect of sensory impairment on SFQ result adjusted for age, gender, ethnicity, highest educational qualification, housing tenure, and Alcohol Use Disorders Identification Test (AUDIT) score. Effect of SFQscore on PSQresult adjusted for age, gender, ethnicity, housing tenure and Alcohol Use Disorders Identification Test (AUDIT) score.
3.4. Hearing impairment and psychosis
There was weak evidence for an association between any hearing impairment and screening positive on the PSQ following adjustment (AOR 1.50, 95% C11.10 to 2.04, p=.010) (Table 2). Severe hearing impairment was associated with screening positive on the PSQ, although the confidence intervals were wide (AOR 4.94, 95% C11.66 to 14.67, p=.004). We found weak evidence that mild hearing impairment was associated with a positive PSQ result (AOR 1.59, 95% C11.07 to 2.35, p=.022), but there was insufficient statistical evidence for an association with moderate hearing impairment (AOR 1.56, 95% CI 0.92 to 2.64, p=.097). Odds of psychosis were highest in severe hearing impairment (Table 3).
Mediation analysis suggested that reduced social functioning accounted for 42% of the association between any hearing impairment and PSQ result, although the 95% confidence intervals were wide (95% CI 5% to 79%, p =.026). (Table 4).
3.5. Sensitivity analyses
Further adjustment for estimated verbal IQ weakened the evidence of association between mild visual impairment and PSQ (OR 1.39, 95% CI 0.90 to 2.15, p = .137). It did not markedly affect the evidence of association with moderate visual impairment (OR 2.69, 95% CI 1.74 to 4.15, p <.001). It weakened the evidence of an association between PSQ and mild hearing impairment (OR 1.28, 95% CI 0.85 to 1.93, p = .235), and hearing impairment overall (OR 1.36, 95% CI 1.00 to 1.87, p = .053). It strengthened the evidence of association between moderate hearing impairment and PSQ(OR 1.71, 95% CI 1.02 to 2.88, p = .043). Of note, there were no participants with an intellectual disability (estimated verbal IQ lower than 70) in the sample.
Adjusting for whether participants reported currently using antipsychotic medications did not markedly alter the adjusted results in which vision was the exposure variable.
Excluding participants who screened positive on the PSQ solely due to reporting hallucinations did not significantly affect the associations with overall visual impairment. It did however slightly weaken the association of severe hearing impairment with screening positive on the PSQ (AOR 4.01, 95% CI 1.23 to 13.06, p = .021), and the association with hearing impairment overall (AOR 1.47, 95% CI 1.06 to 2.05, p = .022). It significantly weakened the association between mild visual impairment and screening positive on the PSQ (AOR 1.38, 95% CI 0.90 to 2.11, p = .145). Reporting hallucinations was associated with visual impairment (OR 1.63, 95% CI 1.18 to 2.26, p=.003), but not with hearing impairment (OR 1.18, 95% CI 0.85 to 1.65, p = .314).
There was no evidence of association between either hearing or visual impairment and reporting a diagnosis of schizophrenia or psychosis.
There was also no evidence of an interaction between age and sensory impairment in influencing PSQ result.
4. Discussion
4.1. Main findings
We found that visual impairment was associated with screening positive on the PSQ, and moderate visual impairment was more strongly associated than mild or severe impairment. This was not driven purely by hallucinosis. Half of the association between visual impairment and psychotic symptoms might be accounted for by reduced social functioning. Hearing impairment overall was weakly associated with screening positive on the PSQ, but a more severe degree of hearing impairment was strongly associated.
4.2. Comparison with existing literature
Our finding that hearing impairment is associated with psychosis is consistent with previous literature (Linszen et al., 2016). Our finding that severe hearing impairment is the most strongly associated is consistent with a straightforward, linear relationship between these variables.
The finding that visual impairment overall is associated with psychosis accords with results from one large previous longitudinal study (Hayes et al., 2018), but is at odds with a study of over 600,000 Israeli military conscripts. The latter found refractive errors to be associated with a reduced risk of later schizophrenia (Caspi et al., 2009). This may be because the prevalence of refractive error in that sample was lower than would usually be expected (Hayes et al., 2018). This was also a young sample, who may have been affected differently to the older population with sensory impairment in the APMS sample.
The finding that moderate visual impairment is the most strongly associated is at odds with the concept of a dose-response relationship, and requires consideration. If visual impairment is a biomarker of psychosis, then perhaps the pathological process involved does not cause eyesight to deteriorate to a severe level. Another explanation is that visual impairment caused by reduced ability to self-care in psychosis falls in the moderate category. It should also be noted that we used corrected sensory impairments as the exposure. Therefore visual processing difficulties, which might be part of the developmental process of psychosis and cannot be corrected by aids, could account for the association.
Alternatively, our finding is consistent with the ‘Protection against Schizophrenia’ model whereby reduced or absent vision are protective against schizophrenia. Lastly, participants with severe visual impairment reported better median social functioning than those with moderate impairment; so mediation by social functioning remains plausible.
4.3. Strengths and limitations
Strengths of this study include the large, nationally representative sample and adjustment for a broad range of potential confounders. The inclusion of people who were not in contact with clinical services widens the remit of the investigation. Use of a screening tool for psychosis meant that we examined a broad population. Our study included females, unlike the two studies of military conscripts (Caspi et al., 2009; Hayes et al., 2018).
There are also limitations. Our analyses were cross-sectional, so we cannot determine temporal associations between the outcomes, exposures and mediator. We cannot therefore draw conclusions as to whether sensory impairments are a cause of psychosis, a biomarker, or a consequence of people with psychosis having poorer general health. The associations may be bidirectional. Reduced social functioning could result from psychosis or sensory impairment, and visual perceptual impairments may be part of the developmental process leading to psychosis. Our model is exploratory and only one of several possible models to link these variables.
Additionally, sensory impairment was self-reported rather than objectively assessed. People with psychotic symptoms in particular may be less able to assess their own sensory capabilities accurately, for example if they perceive their vision to be poor due to processing difficulties, or if they have less social contact and therefore do not realise they are less able to hear speech. The same may apply to social functioning.
We do not know how many participants had congenital versus acquired sensory impairment, and these have been shown to be differently associated with psychosis (Atkinson et al., 2007). Neither did we know how many people in our study were British Sign Language (BSL) users. Using sign language early in life facilitates development of language as well as psychosocial and emotional development in deaf children, which might protect against mental health problems (Austen and Crocker, 2004).
Being a household survey, the APMS excluded people who were hospitalised due to severe psychotic illness. Selection bias might therefore have led to an underestimation of associations between psychosis and sensory impairment. Nevertheless it has been estimated that people living in communal establishments make up fewer than 2% of the overall UK population, so this effect should have been minimal (McManus et al., 2016). People with significant sensory impairment or psychosis might also have been less likely to participate in the survey. However, the number of people unable to participate is likely to have been small. An unavoidable limitation of our study is the relatively smaller number of participants with severe sensory impairments.
Although we have made efforts to include all relevant confounders, residual confounding is possible. For example, cognitive impairment was not measured in participants aged under 60 and therefore could not be included (Livingston et al., 2017).
Lastly, the confidence intervals around the estimates for the percentage of the association between hearing and visual impairments and screening positive on the PSQ that was mediated by social functioning were very wide. The same is true of the association between severe hearing impairment and PSQ result; in this case due to small numbers. Ideally these findings would be replicated in larger longitudinal samples.
4.4. Implications
Our results suggest that visual impairment, particularly moderate impairment, is associated with psychotic symptoms. These results are more consistent the ‘Protection against Schizophrenia’ (PaSZ) model, whereby moderate visual impairment might carry the highest risk of psychosis, than with a linear dose response relationship (Landgraf and Osterheider, 2013). This remains inconclusive however as we do not report data on congenitally blind individuals. More longitudinal research is needed to establish whether correcting refractory errors and increasing uptake of hearing aids, and otherwise reducing the incidence of sensory impairment are credible interventions for reducing the impact of psychosis. Regardless, these associations highlight the importance of assisting people with psychosis to attend opticians' and audiology appointments and address conditions that may cause sensory impairment.
Reduced social functioning might be one mechanism underlying a relationship between sensory impairments and psychosis: this finding requires to be tested in longitudinal studies. Social isolation and loneliness affect many people with mental illness, and may be preventable or amenable to modification through increasing social connection and support.
Acknowledgements
Supported by the NIHR University College London Hospitals Biomedical Research Centre.
Permission to use data from the 2014 Adult Psychiatric Morbidity Survey was granted by NHS Digital.
Funding
NIHR University College London Hospitals Biomedical Research Centre. No formal funding was obtained for this paper.
Footnotes
Contributors
N Shoham, G Lewis, J Hayes and C Cooper conceptualised and designed the study. N Shoham and G Lewis carried out the statistical analysis. N Shoham and R Kiani were responsible for drafting the manuscript. All authors critically revised and contributed to the manuscript and have accepted the final version.
Declaration of competing interest
There are no conflicts of interest to declare.
References
- Action on Hearing Loss Updated 2015. Hearing Matters.
- Adams SA, Nasrallah HA. Multiple retinal anomalies in schizophrenia. Schizophr Res. 2017;195:3–12. doi: 10.1016/j.schres.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Atkinson JR, Gleeson K, Cromwell J, O’Rourke S. Exploring the perceptual characteristics of voice-hallucinations in deaf people. Cognitive neuropsychiatry. 2007;12(4):339–361. doi: 10.1080/13546800701238229. [DOI] [PubMed] [Google Scholar]
- Austen S, Crocker SR. Deafness in Mind: Working Psychologically With Deaf People Across the Lifespan. Wiley; 2004. [Google Scholar]
- Bebbington P, Nayani T. The psychosis screening questionnaire. Int J Methods Psychiatr Res. 1995;5(1):11–19. [Google Scholar]
- Bernardini F, Attademo L, Blackmon K, Devinsky O. Musical hallucinations: a brief review of functional neuroimaging findings. CNS spectrums. 2017;22(5):397–403. doi: 10.1017/S1092852916000870. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Sachdev P, Rose N, Rylands K, Prenter L. Schizophrenia with onset after age 50 years: I: phenomenology and risk factors. Br J Psychiatry. 1999;175(5):410–415. doi: 10.1192/bjp.175.5.410. [DOI] [PubMed] [Google Scholar]
- Caspi A, Vishne T, Reichenberg A, Weiser M, Dishon A, Lubin G, Shmushkevitz M, Mandel Y, Noy S, Davidson M. Refractive errors and schizophrenia. Schizophr Res. 2009;107(2-3):238–241. doi: 10.1016/j.schres.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Nakayama K, Matthysse S, Holzman PS. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. American Journal of Psychiatry. 2003;160(10):1795–1801. doi: 10.1176/appi.ajp.160.10.1795. Published Online:1 Oct 2003. [DOI] [PubMed] [Google Scholar]
- Coebergh JA, Lauw R, Bots R, Sommer I, Blom J. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi: 10.3389/fpsyg.2015.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrera KJ, Betz J, Deal J, Choi JS, Ayonayon HN, Harris T, Helzner E, Martin KR, Mehta K, Pratt S. Association of hearing impairment and anxiety in older adults. Journal of Aging and Health. 2017;29(1):172–184. doi: 10.1177/0898264316634571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Császár N, Kapócs G, Bókkon I. A possible key role of vision in the development of schizophrenia. Rev Neurosci. 2018;30:359–379. doi: 10.1515/revneuro-2018-0022. [DOI] [PubMed] [Google Scholar]
- Du Feu M, Fergusson K. Sensory impairment and mental health. Adv Psychiatr Treat. 2003;9(2):95–103. [Google Scholar]
- Eastwood M, Corbin S, Reed M, Nobbs H, Kedward H. Acquired hearing loss and psychiatric illness: an estimate of prevalence and co-morbidity in a geriatric setting. Br J Psychiatry. 1985;147(5):552–556. doi: 10.1192/bjp.147.5.552. [DOI] [PubMed] [Google Scholar]
- Fortnum HM, Davis A, Summerfield AQ, Marshall DH, Davis AC, Bamford JM, Yoshinaga-Itano C, Hind S. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment studyCommentary: universal new-born hearing screening: implications for coordinating and developing services for deaf and hearing impaired children. Bmj. 2001;323(7312):536. doi: 10.1136/bmj.323.7312.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser E, Retsa C, Knebel J-F, Ferrari C, Jenni R, Fournier M, Alameda L, Baumann PS, Clarke S, Conus P. The coupling of low-level auditory dysfunction and oxidative stress in psychosis patients. Schizophr Res. 2017;190:52–59. doi: 10.1016/j.schres.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Han J, Lee H, Jung J, Park E-C. Effects of self-reported hearing or vision impairment on depressive symptoms: a population-based longitudinal study. Epidemiology and Psychiatric Sciences. 2018:1–13. doi: 10.1017/S2045796018000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JF, Picot S, Osborn DP, Lewis G, Dalman C, Lundin A. Visual acuity in late adolescence and future psychosis risk in a cohort of 1 million men. Schizophr Bull. 2018;45:571–578. doi: 10.1093/schbul/sby084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakody DM, Almeida OP, Speelman CP, Bennett RJ, Moyle TC, Yiannos JM, Friedland PL. Association between speech and high-frequency hearing loss and depression, anxiety and stress in older adults. Maturitas. 2018;110:86–91. doi: 10.1016/j.maturitas.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Johns LC, Cannon M, Singleton N, Murray RM, Farrell M, Brugha T, Bebbington P, Jenkins R, Meltzer H. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry. 2004;185(4):298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Keefe RS. E. Schizophrenia Is a Cognitive Illness: Time for a Change in Focus. JAMA Psychiatry. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. Published October 2013. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kiss I, Kelemen O, Benedek G, Janka Z. Anomalous visual experiences, negative symptoms, perceptual organization and the magnocellular pathway in schizophrenia: a shared construct? Psychological Medicine. 2005 June 29;35(10):1445–1455. doi: 10.1017/S0033291705005398. 2005 Published online by Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- Landgraf S, Osterheider M. “To see or not to see: that is the question.” the “Protection-Against-Schizophrenia” (PaSZ) model: evidence from congenital blindness and visuo-cognitive aberrations. Front Psychol. 2013;4:352. doi: 10.3389/fpsyg.2013.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linszen MM, Brouwer RM, Heringa SM, Sommer IE. Increased risk of psychosis in patients with hearing impairment: review and meta-analyses. Neurosci Biobehav Rev. 2016;62:1–20. doi: 10.1016/j.neubiorev.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Livingston G, Kitchen G, Manela M, Katona C, Copeland J. Persecutory symptoms and perceptual disturbance in a community sample of older people: the Islington study. International Journal of Geriatric Psychiatry. 2001;16(5):462–468. doi: 10.1002/gps.362. [DOI] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Mason OJ, Brady F. The psychotomimetic effects of short-term sensory deprivation. J Nerv Ment Dis. 2009;197(10):783–785. doi: 10.1097/NMD.0b013e3181b9760b. [DOI] [PubMed] [Google Scholar]
- Mason P, Rimmer M, Richman A, Garg G, Johnson J, Mottram PG. Middle-ear disease and schizophrenia: case-control study. BrJ Psychiatry. 2008;193(3):192–196. doi: 10.1192/bjp.bp.108.052795. [DOI] [PubMed] [Google Scholar]
- McDowell A. What do I do when one of the survey estimators returns an error message, “stratum with only one PSU detected”? Stata Resources and Support. n.d. [Google Scholar]
- McManus S, Bebbington P, Jenkins R, Brugha T. Mental Health and Wellbeing in England: Adult Psychiatric Morbidity Survey 2014: A Survey Carried Out for NHS Digital by NatCen Social Research and the Department of Health Sciences, University of Leicester. NHS Digital; 2016. [Google Scholar]
- McManus S, Ali A, Bebbington P, Brugha T, Cooper C, Rai D, Saunders C, Strydom A, Hassiotis A. Inequalities in health and service use among people with borderline intellectual impairment. NatCen; London: 2018. p. 38. [Google Scholar]
- Moore N. Is paranoid illness associated with sensory defects in the elderly? J Psychosom Res. 1981;25(2):69–74. doi: 10.1016/0022-3999(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Moreno C, Nuevo R, Chatterji S, Verdes E, Arango C, Ayuso-Mateos JL. Psychotic symptoms are associated with physical health problems independently of a mental disorder diagnosis: results from the WHO World Health Survey. World Psychiatry. 2013;12(3):251–257. doi: 10.1002/wps.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS. Reviewed Blindness and Vision Loss. 2018.
- Pan J, Zhou Y, Xiang Y, Yu J. Retinal nerve fiber layer thickness changes in schizophrenia: a meta-analysis of case-control studies. Psychiatry Res. 2018;270:786–791. doi: 10.1016/j.psychres.2018.10.075. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophrenia Research: Cognition. 2015;2(2):46–55. doi: 10.1016/j.scog.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Roze C, Linszen M, Somers M, van Zanten G. Hearing loss; the neglected risk factor for psychosis. Schizophr Res. 2014;158(1):266–267. doi: 10.1016/j.schres.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Stam M, Smit JH, Twisk JW, Lemke U, Smits C, Festen JM, Kramer SE. Change in psychosocial health status over 5 years in relation to adults’ hearing ability in noise. Ear Hear. 2016;37(6):680–689. doi: 10.1097/AUD.0000000000000332. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; College Station, TX: 2017. [Google Scholar]
- Türközer HB, Hasoğlu T, Chen Y, Norris LA, Brown M, Delaney-Busch N, Kale EH, Pamir Z, Boyaci H, Kuperberg G, Lewandowski KE, et al. Dost: Integrated assessment of visual perception abnormalities in psychotic disorders and relationship with clinical characteristics. Psychological Medicine. 2018 September 04;49(10):1740–1748. doi: 10.1017/S0033291718002477. Published online by Cambridge University Press: 2018. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Nur U, Crawford M, Karlsen S, MacLean C, Rao B, Johnson T. The social functioning questionnaire: a rapid and robust measure of perceived functioning. Int J Soc Psychiatry. 2005;51(3):265–275. doi: 10.1177/0020764005057391. [DOI] [PubMed] [Google Scholar]
- Viertiö S, Laitinen A, Perälä J, Saarni SI, Koskinen S, Lönnqvist J, Suvisaari J. Visual impairment in persons with psychotic disorder. Soc Psychiatry Psychiatr Epidemiol. 2007;42(11):902. doi: 10.1007/s00127-007-0252-6. [DOI] [PubMed] [Google Scholar]
- van der Werf M, van Boxtel M, Verhey F, Jolles J, Thewissen V, van Os J. Mild hearing impairment and psychotic experiences in a normal aging population. Schizophr Res. 2007;94(1-3):180–186. doi: 10.1016/j.schres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- van der Werf M, Thewissen V, Dominguez M, Lieb R, Wittchen H, van Os J. Adolescent development of psychosis as an outcome of hearing impairment: a 10-year longitudinal study. Psychol Med. 2011;41(3):477–485. doi: 10.1017/S0033291710000978. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. Global Initiative for the Elimination of Avoidable Blindness: Action Plan 2006-2011. 2007 [Google Scholar]
- Zheng W, Tang L-R, Correll CU, Ungvari GS, Chiu HF, Xiang Y-Q, Xiang Y-T. Frequency and correlates of distant visual impairment in patients with schizophrenia, bipolar disorder, and major depressive disorder. East Asian Arch Psychiatr. 2015;25(3):115. [PubMed] [Google Scholar]