Abstract
Synchronous and anti-synchronous activity between neural elements at rest reflects the physiological processes underlying complex cognitive ability. Regional and pairwise-connectivity investigations suggest perturbations in these activity patterns may relate to widespread cognitive impairments seen in bipolar disorder (BD). Here we take a network-based perspective to more meaningfully capture interactions among distributed brain regions compared to focal measurements and examine network-cognition relationships across a range of commonly affected cognitive domains in BD in relation to healthy controls.
Resting-state networks were constructed as matrices of correlation coefficients between regionally averaged resting-state time series from 86 cortical/subcortical brain regions (FreeSurferv5.3.0). Cognitive performance measured using Weschler Adult Intelligence Scale, Cambridge Automated Neuropsychological Test Battery (CANTAB) and Reading the Mind in the Eyes tests was examined in relation to whole-brain connectivity measures and patterns of connectivity using a permutation-based statistical approach.
Faster response times in controls (n=49) related to synchronous activity between frontal, parietal, cingulate, temporal and occipital regions while similar response times in BD (n=35) related to anti-synchronous activity between regions of this subnetwork. Across all subjects, anti-synchronous activity between frontal, parietal, temporal, occipital, cingulate, insula and amygdala regions related to improved memory performance. No resting-state subnetworks related to intelligence, executive function, short-term memory or social cognition performance in the overall sample or in a manner that would explain deficits in these facets in BD.
Our results demonstrate alterations in the intrinsic connectivity patterns underlying response timing in BD that are not specific to performance or errors on the same tasks. Across all individuals, no strong effects of resting-state global topology on cognition are found, while distinct functional networks supporting episodic and spatial memory highlight intrinsic inhibitory influences present in the resting-state that facilitate memory processing.
Manuscript keywords: cognition, resting-state, bipolar disorder, network analysis, graph theory
Acronyms
- AFNI
Analysis of Functional NeuroImages
- BD
Bipolar disorder
- BOLD
Blood-oxygen level dependent
- CANTAB
Cambridge Automated Neuropsychological Test Battery
- DMS
Delayed Match to Sample
- TE
Echo Time
- EPI
Echo-planar imaging
- FDR
False-discovery rate
- FWER
Family-wise error rate
- pFWE
Family-wise error rate corrected p-values
- FSL
FMRIB Software Library
- FLIRT
FMRIB's Linear Image Registration Tool
- HRB
Health Research Board
- HC
Healthy controls
- ICA
Independent component analysis
- IQ
Intelligence
- IED
Intra/Extra Dimensional Shift
- KMO
Kaiser-Meyer-Oklin
- MRI
Magnetic Resonance Imaging
- MANCOVA
Multivariate analysis of covariance
- NBS
Network-based statistic
- PAL
Paired-Associates Learning
- PCA
Principal component analysis
- TR
Repetition Time
- SRM
Spatial Recognition Memory
- SD
Standard deviation
- SCID
Structured Clinical Interview for DSM
- DSM
The Diagnostic and Statistical Manual for Mental Disorders
- HAM-D
The Hamilton Rating Scale for Depression
- YMRS
The Young Mania Rating Scale
- WAIS-III
Weschler Adult Intelligence Scale
Introduction
Bipolar disorder (BD) is a chronic mood disorder associated with widespread impairments in cognitive function, including general intelligence, executive function, memory, social cognition and response timing (Bora, Bartholomeusz, & Pantelis, 2016; Bourne et al., 2013; Brotman, Rooney, Skup, Pine, & Leibenluft, 2009; Mann-Wrobel, Carreno, & Dickinson, 2011). Cognitive impairments contribute to poorer functional and quality of life outcomes (Andreou & Bozikas, 2013; Baune & Malhi, 2015), persist during mood symptom remission (Mann-Wrobel et al., 2011; Volkert et al., 2016), and are not solely accounted for by medication use (Vrabie et al., 2015) and thus represent an important yet poorly understood target for future therapies.
Brain imaging studies to date have placed focus on regional activation and pairwise-connectivity-based understandings of these impairments. However, how cognitive processing emerges from a whole brain of interacting elements may be more meaningfully probed using a network-theory framework, which enables examination of multivariate patterns of functional connectivity across the brain. The application of this framework to intrinsic resting-state connectivity, which reflects the most frequent coupling patterns between regions (Cole, Bassett, Power, Braver, & Petersen, 2014), can provide a context-independent marker of cognitive impairment that potentially represents core functional circuit dysfunction in BD.
Consistent findings from localized approaches, which involve the specification of regions of interest, find functional dysconnectivity between and within regions of the prefrontal cortex and limbic system in BD (Chase & Phillips, 2016; Syan et al., 2018). Parallel to these seed-based analyses, findings from whole-brain network approaches suggest altered functional integration and segregation globally (Dvorak et al., 2019; Spielberg et al., 2016; Y. Wang et al., 2017), and locally among frontal cortex, limbic and default-mode areas (Doucet, Bassett, Yao, Glahn, & Frangou, 2017; Dvorak et al., 2019; He et al., 2016; Roberts et al., 2017; Spielberg et al., 2016; Y. Wang et al., 2017; Ying Wang et al., 2017, 2016; Zhao et al., 2017).
There is corroborative evidence of underlying anatomical dysconnectivity both in terms of whole-brain integration and segregation (L Nabulsi et al., 2019; O’Donoghue et al., 2017; Ying Wang et al., 2019) and connectivity between prefrontal, basal ganglia and limbic regions (L Nabulsi et al., 2019; O’Donoghue, Holleran, Cannon, & McDonald, 2016; Perry, Roberts, Mitchell, & Breakspear, 2018) suggesting a structural foundation for altered functional connectivity in the illness. Recent work suggests impairments in intelligence (IQ), executive function and processing speed associate with global anatomical network integration, segregation and interhemispheric connectivity in BD (Ajilore et al., 2015; McPhilemy et al., 2019). However, an investigation of intrinsic functional network-level interactions relating to cognitive deficits in BD has yet to provide further context to understand their neural basis.
In BD, activation and seed-based resting-state approaches have investigated executive function (Alonso-Lana et al., 2016; Diler et al., 2013; Favre et al., 2013; Nguyen et al., 2017; Pompei et al., 2011), episodic memory (Oertel-Knöchel et al., 2015) and social cognition (Grant, Hassel, Bobyn, Hall, & MacQueen, 2018; Kim et al., 2009; Malhi et al., 2008); implicating specific patterns of functional dysconnection within and between fronto-parietal, striatal, cingulate and temporal areas. These approaches have been useful in understanding the neural underpinnings of these cognitive impairments in isolated brain regions but are insensitive to complex functional interactions between neural elements across the brain and have often been restricted in using few cognitive performance measures. Here, we aim to determine resting-state network-level connectivity associated with cognition across a broad range of domains in healthy control and BD samples, to determine shared or differential relationships indicative of a breakdown in the resting-state network architecture supporting cognitive functioning in BD.
Based on previously reported associations in controls, we hypothesised that executive function, episodic memory and IQ would associate positively with global efficiency and negatively with characteristic path length (Baum et al., 2017; Sheffield et al., 2015; van Den Heuvel, Stam, Kahn, & Hulshoff Pol, 2009) and that IQ would associate with specific patterns of functional connectivity between fronto-parietal and default-mode regions (Hearne, Mattingley, & Cocchi, 2016). Given the established deficit in these cognitive domains and alterations in both global efficiency, characteristic path length and subnetwork connectivity in BD, we hypothesised differential brain-cognition relationships would exist in BD that may explain these cognitive deficits present in the disorder (Malhi et al., 2008; McPhilemy et al., 2019; Nguyen et al., 2017). In contrast, we explored relationships between short-term memory, spatial memory, social cognition and the above cognitive domains with other commonly used measures of global integration and segregation and explored the variance in connectivity patterns underlying executive function, memory, social cognition and a general factor of response time, all commonly impaired in BD.
This study represents an important extension of our previous work that examined neuroanatomical network features supporting cognition in this BD cohort (McPhilemy et al., 2019) and may provide a resting-state marker of functional connectivity useful in understanding cognitive deficits and brain function in BD.
Materials and Methods
Participants
Individuals with a diagnosis of BD and psychiatrically healthy individuals between 18 and 65 years of age were recruited through mental health services of the Western region of Ireland. The Diagnostic and Statistical Manual (DSM-V-TR) criteria for BD were confirmed by a psychiatrist using the Structured Clinical Interview for DSM-V (SCID) (American Psychiatric Association, 2013). Healthy volunteers had no personal history of psychiatric illness confirmed using the SCID, non-patient edition and no first-degree family history. Exclusion criteria included neurological disorders, learning disability, comorbid substance or alcohol abuse, history of head injury resulting in a loss of consciousness for over 5 minutes, or any illnesses potentially affecting cognitive function. The Hamilton Rating Scale for Depression (HAM-D-21) (Hamilton, 1959) and Young Mania Rating Scale (YMRS) (Young, Biggs, Ziegler, & Meyer, 1978) assessed mood symptoms on the day of scanning and cognitive testing; euthymia was defined as scores of <8 and <7 respectively. All participants provided fully informed written consent. Ethical approval was obtained from The Clinical Research Ethics Committees of Galway University Hospital and St James’s Hospital Dublin.
Cognitive Testing
Selected subtests of the Wechsler Adult Intelligence Scale (WAIS-III) (vocabulary, similarities, block design and matrix reasoning) were combined to obtain full-scale IQ (Wechsler, 1997). The Cambridge Neuropsychological Test Automated Battery (CANTAB) was used to measure executive function (Intra/Extra Dimensional shift, IED), episodic memory (Paired-Associates Learning, PAL), short-term memory (Delayed Match to Sample, DMS) and spatial recognition memory (Spatial Recognition Memory, SRM) (Cognition, 2018). The ‘Reading the Mind in the Eyes’ assessed social cognition (Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001). Principal component analysis (PCA) was used to identify variability factors from four available CANTAB response time measures; motor screening task (MOT) mean latency, DMS mean correct latency, IED total latency and SRM mean correct latency. Bartlett’s test of sphericity was significant (Approx. chi square=79.87, p=3.81 x 10-15) and the Kaiser–Meyer–Oklin (KMO) value of 0.76 exceeded the defined 0.6, suggesting a underlying latent structure in the response time data (Kaiser, 1974). One component exceeding an eigenvalue of 1 and explaining 58.34% of the total variance in response time was used to generate a factor score for subsequent analysis (Supplementary Figure 1). Comparison of cognitive performance between diagnostic groups used multivariate analysis of covariance (MANCOVA) or Mann-Whitney U (SPSS v23).
Imaging Acquisition and Processing
Resting-state functional images were acquired with a single-shot echo-planar imaging (EPI) sequence (repetition time 2 seconds; echo time 28 ms; flip angle 90 degrees; field of view 240x240x133 mm; a 3 mm2 in-plane resolution; 38 ascending slices covering the whole brain; 3.2 mm slice thickness with a 0.3 mm gap) using a 3T Philips Achieva scanner (Philips, The Netherlands) at the Centre for Advanced Medical Imaging, St. James’s Hospital, Dublin, prior to the acquisition of any task-based functional images in the same scanning session. A total of 180 volumes were acquired along with 4 dummy scans to establish a steady state longitudinal magnetization. Individuals were instructed to lie still with eyes open focusing on a red cross on the MRI screen for the 6-minute scan duration. To register resting-state scans, T1-weighted images were acquired using a 3D magnetization-prepared rapid gradient echo sequence (TR/TE 8.5/3.9 ms; flip angle 8°; TI 804.3 ms; 1 mm3 isotropic voxel size). Pre-processing of resting-state functional images was performed to obtain functional correlation estimates with minimal sensitivity to motion, modelled on recommendations from Jo et al., (2013) including: 1) despiking (3dDespike, AFNI v18.1.09 http://afni.nimh.nih.gov/afni); 2) motion correction registering all resting-state volumes to the first (3dvolreg, AFNI); 3) linear registration to T1-weighted images (FLIRT, FSL v5.0.4 https://fsl.fmrib.ox.ac.uk/fsl/fslwiki); 4) nuisance regression, bandpass filtering (0.01 – 0.1 Hz) and motion scrubbing in a single regression model (3dTproject, AFNI) and 5) slice-timing correction (3dTshift, AFNI, Jo et al., 2013). ANATICOR regressors included 6 motion parameters and tissue-based averages of white matter and lateral ventricle signals. Motion-corrupted volumes with >0.5 mm framewise displacement (Euclidean norm) were removed and individuals with >30 volumes corrupted were excluded (n=3 BD), ensuring all subjects had the equivalent of at least 5 minutes of resting-state data. Supplementary Figure 2 presents an examination of mean framewise displacement pre-censoring and the number of censored volumes between groups. Quality assessment involved careful visual inspection of resting-state-to-T1 registration and resulted in the removal of 1 further case (n=1 BD).
Functional Network Reconstruction
Eighty-six regions (34 cortical, 8 subcortical per hemisphere and bilateral cerebellar hemispheres) were defined in a subject-specific manner (FreeSurfer v5.3.0, http://surfer.nmr.mgh.harvard.edu/) and underwent manual correction where necessary (Fischl et al., 2002). Regionally averaged blood-oxygen-level dependent (BOLD) signal time series were used to define functional connectivity between each region-pair using Pearson’s and partial correlation with in-house scripts (MATLAB r2017b), resulting in 86x86 resting-state functional connectivity matrices for each individual. Quality assessment involved careful visual inspection for widespread and inflated positive correlation values.
Measures of global functional network connectivity
As hypothesised, executive function, IQ and episodic memory were investigated in relation to global efficiency and characteristic path length. Exploratory investigations included relationships between these cognitive measures and positive/negative strength, global clustering, betweenness centrality and assortativity and between short-term and spatial memory and social cognition and all global network measures (Brain Connectivity Toolbox v2017-15-01, http://www.brain-connectivity-toolbox.net). We chose these additional global connectivity measures due to their common use in BD resting-state network literature. Global measures excepting negative strength were calculated from resting-state networks thresholded to retain positive connection weights (r>0) and improve interpretation of path-based measures such as global efficiency. Relationships between measures of global functional network connectivity and cognitive performance and interactions effects between global connectivity and diagnosis on cognitive performance were examined using a general linear model covarying for age, gender and diagnosis (SPSS v23). Uncorrected p-values are presented for this analysis unless otherwise stated.
Subnetwork patterns of resting-state connectivity
Resting-state connectivity patterns associated with IQ, executive function, episodic, short-term and spatial memory, social cognition and the PCA-derived response time factor were investigated using a permutation-based method that controls for the family-wise error rate (FWER) at the network-level, covarying for age, gender and diagnosis (network-based statistic, NBS v1.2) (Zalesky, Fornito, & Bullmore, 2010). Unthresholded resting-state matrices retaining both positive and negative weights were used in this analysis. Interactions between cognitive performance and diagnosis were modelled to test for resting-state connectivity patterns associated with diagnosis-based variation in cognitive performance. A T-statistic representing the main effect of cognitive performance or interaction between cognitive performance and diagnosis for each connection was calculated and thresholded using T-statistic values of 1.5, 2, 2.5 and 3. FWER-corrected p-values (pFWE) were assigned to resulting subnetworks using a null distribution of maximum component size obtained via 5000 permutations.
Results
Participants
Analysis included 35 BD outpatient participants (28 BD type-I and 7 BD type-II) and 49 psychiatrically healthy individuals (HC) balanced for age, gender and education (Table 1). Of the 35 BD participants, 24 met criteria for euthymia; 11 met criteria for mild-moderate depression (31%; HAM-D mean=15.64, SD=4.80, range=11-26) and 2 met criteria for hypomania (YMRS score=10 for both individuals). Mood scores did not differ between the day of scanning and cognitive testing (HAM-D; T=-0.12, p=0.91, YMRS; T=-0.77. p=0.45). At cognitive testing, 20 BD participants were treated with mood stabilizers (11 lithium); 20 antipsychotic medications (19 atypical antipsychotics); 11 antidepressant medications; 1 benzodiazepine; 6 other psychotropic medications and 3 antiepileptic mood-stabilizing medication and 4 were medication free (Supplementary Table 2). Over half of individuals with BD (n = 23) were taking a combination of at least two of the above classes of drugs. The time between scanning and cognitive testing was similar between diagnostic groups (HC mean ± SD=5.73 ± 5.24 months, range=0-20 months; BD mean ± SD=6.83 ± 5.57 months, range=0-16 months; T=-0.92, p=0.36).
Table 1. Demographic and Clinical Characteristics.
| Control group | Bipolar group | Statistical comparison | ||
|---|---|---|---|---|
| n = 49 | n = 35 | Test stat (t, X2) | p | |
| Age, Mean (SD) | 41 (14) | 44 (13) | -0.8 | 0.43 |
| Gender, Male/Female, n | 20/29 | 16/19 | 0.2 | 0.66 |
| Level of Education, n1 | 9.57 | 0.09 | ||
| Junior high school | 1 | 1 | ||
| Some high school | 1 | 2 | ||
| High school graduate | 3 | 5 | ||
| Some college or technical school, at least one year | 10 | 9 | ||
| College graduate | 16 | 15 | ||
| Graduate training | 18 | 3 | ||
| Hamilton Depression Rating Scale, mean score (SD), range | 1.08 (1.72), 0-7 | 6.86 (6.90), 0-26 | -4.85 | 2.3×10–5* |
| Young Mania Rating Scale, mean score (SD), range | 0.76 (1.48), 0-6 | 1.94 (2.72), 0-10 | -2.35 | 0.02* |
SD, standard deviation. Mood scores provided are from the day of scanning.
Significant difference at p<0.05
Cognitive differences between diagnostic groups
BD participants exhibited poorer neurocognitive performance than controls in executive function (U=619.00, p=0.03), episodic memory (F=4.79, p=0.03), short-term memory (F=6.15, p=0.02) and social cognition (F=5.91, p=0.02). No difference was demonstrated between groups for IQ (F=3.14, p=0.08), PCA-derived response time (F=1.01, p=0.32) or spatial memory accuracy (F=2.08, p=0.15) or response time (F=2.36, p=0.13; Supplementary Table 1).
Global resting-state connectivity
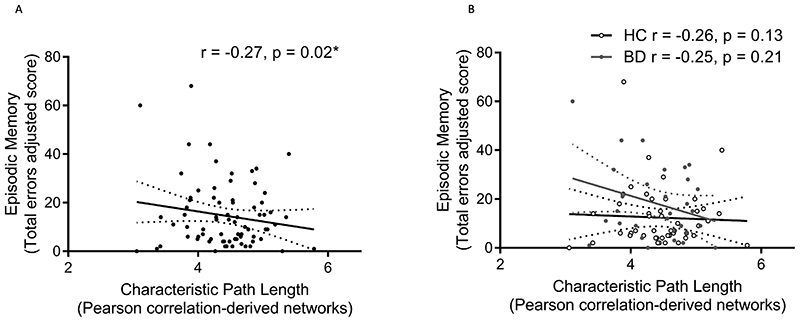
We found an inverse relationship between episodic memory errors and characteristic path length but not global efficiency that did not survive FDR-correction and no relationships between IQ or executive function and these global network measures [Figure 1](Table 2). No interaction effects between diagnosis and global network measures on episodic memory, IQ or executive function were detected (Table 3). Exploratory analysis found direct relationships between executive functioning errors and positive (F=6.32, p=0.01) and negative (F=5.81, p=0.02) strength; direct relationships between episodic memory score and positive (F=5.96, p=0.02) and negative (F=8.26, p=0.01) strength; direct and inverse relationships between episodic memory total errors and positive strength (F= 4.48, p=0.04) and assortativity (F=4.31, p=0.04) respectively; and a direct relationship between spatial memory accuracy and betweenness centrality (F=3.89, p=0.05), all of which did not survive FDR-correction. Group x global measure interactions were found for episodic memory errors and assortativity (F=4.08, p=0.05, HC r=-0.06, BD r=0.24) and episodic memory score and betweenness centrality (F=11.12, p=0.001; HC r=0.22, BD r=-0.51), with the latter surviving FDR-correction.
Figure 1.
Relationship between episodic memory performance (total errors) and characteristic path length computed from Pearson-correlation-derived resting-state networks (A) across all individuals and (B) separated by diagnostic group.
Table 2. Main effect of resting-state global efficiency on cognitive performance.
| Task | Outcome measure | Pearson correlation-derived networks | Partial correlation-derived networks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Global efficiency | Characteristic Path Length | Global efficiency | Characteristic Path Length | ||||||
| F | p | F | p | F | p | F | p | ||
| Full-scale IQ | IQ score | 0.03 | 0.86 | 0.02 | 0.89 | 0.01 | 0.94 | 4 x 10-5 | 0.99 |
| Intra/Extra Dimensional Shift | Total errors adjusted | 0.20 | 0.66 | 0.75 | 0.39 | 3.24 | 0.08 | 3.33 | 0.07 |
| Paired Associates Learning | First trial memory score | 0.56 | 0.46 | 0.68 | 0.41 | 1.93 | 0.17 | 2.24 | 0.14 |
| Total errors adjusted | 1.67 | 0.20 | 5.41 | 0.02* | 0.18 | 0.68 | 0.24 | 0.63 | |
Significant difference at p<0.05
Table 3. Interaction effects between diagnosis and global functional connectivity measures on cognitive performance.
| Task | Outcome measure | Pearson correlation-derived networks | Partial correlation-derived networks | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Global efficiency | Characteristic Path Length | Global efficiency | Characteristic Path Length | ||||||
| F | p | F | p | F | p | F | p | ||
| Full-scale IQ | IQ score | 0.07 | 0.79 | 0.33 | 0.57 | 0.63 | 0.43 | 0.80 | 0.37 |
| Intra/Extra Dimensional Shift | Total errors adjusted | 0.20 | 0.66 | 1.83 | 0.18 | 2.25 | 0.14 | 0.30 | 0.59 |
| Paired Associates Learning | First trial memory score | 0.56 | 0.46 | 0.08 | 0.78 | 0.09 | 0.76 | 0.36 | 0.55 |
| Total errors adjusted | 1.67 | 1.42 | 0.24 | 0.10 | 0.75 | 1.12 | 0.30 | 0.79 | |
Resting-state Subnetwork Connectivity
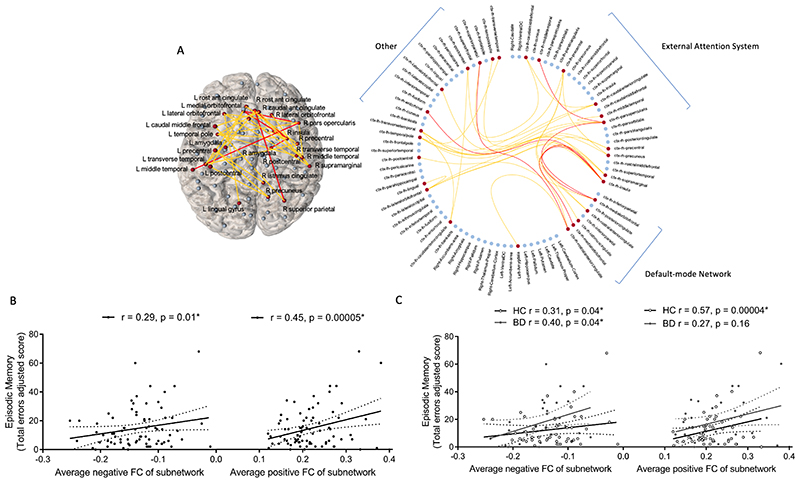
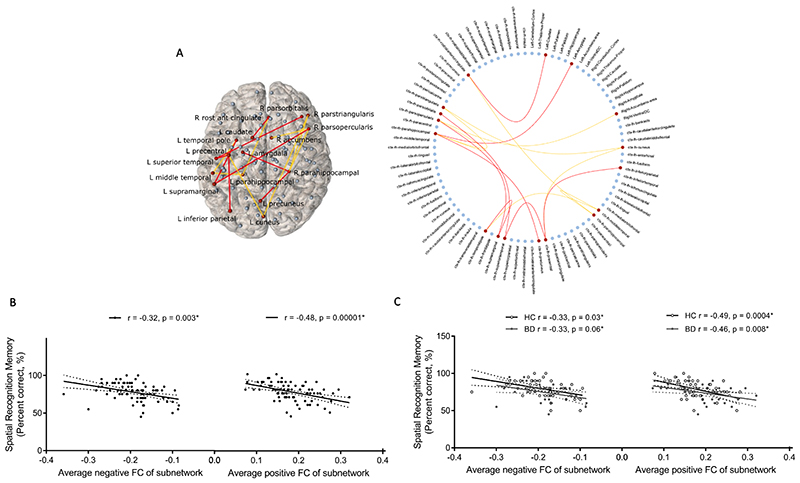
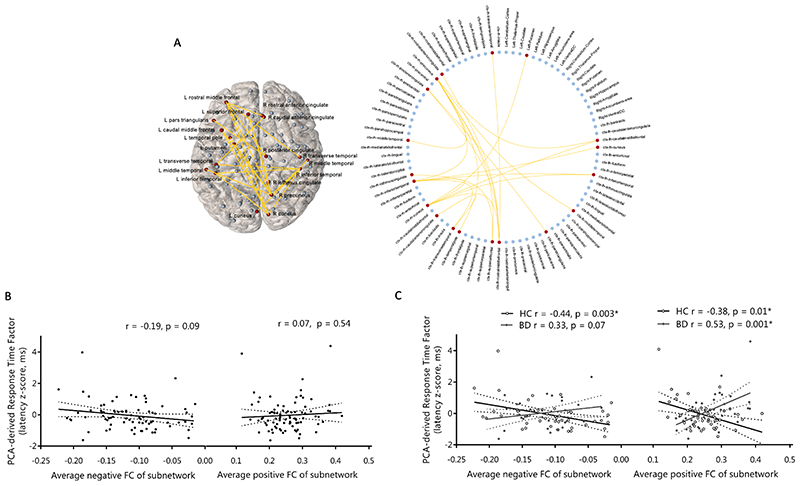
Hypoconnectivity (decreased positive/increased negative connectivity) between distinct frontal, parietal, temporal, occipital, cingulate, insula and amygdala regions related to lower episodic memory errors (T=3, pFWE=0.02; Figure 2) and higher spatial recognition memory accuracy (T=2.5, pFWE=0.02; Figure 3) in the overall sample and similarly in BD compared to controls. We found no resting-state subnetworks related to IQ, executive function, short-term memory or social cognition in the overall sample or in a manner that would explain deficits in these facets in BD. Resting-state connectivity between frontal, parietal, cingulate, temporal and occipital regions had a significantly different relationship with the PCA-derived response time factor in BD compared to controls (T=3, pFWE=0.03; Figure 4); greater connectivity (increased positive/decreased negative) related to faster response times in the control group and an inverse relationship was detected in BD.
Figure 2.
A resting-state functional subnetwork (Pearson-correlation-derived networks) was correlated with episodic memory total errors over all subjects covarying for age, gender and diagnosis (T = 3, p = 0.02), while no subnetwork differently related to this measure between diagnostic groups: (A) Visualisation of significant subnetwork in anatomical space and a circular representation including all network nodes. Positive functional connections are coloured yellow, negative functional connections are coloured red and brain regions in the significant subnetwork are coloured red. In order to compare to previous literature brain regions are ordered according to networks defined using independent component analysis as per Fornito et al. (2012). (B) Relationship between average positive and average negative functional connectivity of this subnetwork and episodic memory total errors in the overall sample and (C) separated by diagnostic group, with partial correlations including age and gender as covariates. HC, healthy control; BD, bipolar disorder.
Figure 3.
A resting-state functional subnetwork (Partial-correlation-derived networks) correlated with spatial recognition memory accuracy over all subjects covarying for age, gender and diagnosis (T = 2.5, p = 0.02), while no subnetwork differently related to this measure between diagnostic groups: (A) Visualisation of significant subnetwork in anatomical space and a circular representation including all network nodes. Positive functional connections are coloured yellow, negative functional connections are coloured red and brain regions in the significant subnetwork are coloured red. (B) Relationship between average negative and average positive functional connectivity of this subnetwork and spatial memory accuracy in the overall sample and (C) separated by diagnostic group, with partial correlations including age and gender as covariates. HC, healthy control; BD, bipolar disorder.
Figure 4. Resting-state functional network (Pearson-correlation-derived networks) significantly different in its relationship with the PCA-response time factor in BD versus controls.
(A) Visualisation of significant subnetwork in anatomical space and a circular representation including all network nodes. Positive functional connections are coloured yellow and brain regions in the significant subnetwork are coloured red. (B) Relationship between average negative and average positive functional connectivity of this subnetwork and the PCA-derived response time factor in the overall sample and (C) separated by diagnostic group, with partial correlations including age and gender as covariates. HC, healthy control; BD, bipolar disorder.
Discussion
We present for the first time evidence of alterations in the resting-state patterns underlying response timing in BD, which may prove important for understanding cognitive impairment generally in the disorder. Across all subjects, episodic memory related to global resting-state network integration and episodic and spatial memory to anti-synchronous activity within distinct resting-state subnetworks; no relationships globally or in terms of subnetwork patterns explained variance in intelligence, executive function or social cognition. Despite the presence of expected cognitive deficits in BD in executive function, episodic and short-term memory and social cognition, we find no evidence implicating altered intrinsic functional connectivity patterns at the global or subnetwork-level in these deficits.
Response timing
We find alterations in the resting-state connectivity patterns underlying response time variation in BD. Generally inconsistent with previous work (Emre Bora, Vahip, & Akdeniz, 2006; Brotman et al., 2009; Gallagher et al., 2015; Teixeira et al., 2013) reaction times were not significantly increased in the present BD cohort compared to controls, suggesting reaction time deficits in BD may be sensitive to the attentional demands of the task (Townsend et al., 2012). Our findings corroborate previous task-based imaging of response time variation in controls that show activation in prefrontal, cingulate and posterior parietal cortices, and suggest attentional mechanisms in the underlying neurobiology of these deficits in BD (Bellgrove, Hester, & Garavan, 2004; Johnson et al., 2015; Simmonds et al., 2007; Yarkoni, Barch, Gray, Conturo, & Braver, 2009). Synchronous activity between these regions appears to support efficient response timing in healthy individuals, however in BD it is antisynchronous activity that confers similar levels of functioning. Lower synchronous and greater antisynchronous activity between regions of this system has been identified in BD previously (Chase & Phillips, 2016) and in the present cohort (Nabulsi et al., 2020). Taken together this suggests compensatory support due to a framework of functionally dysconnected regions underlying response timing in BD.
Intelligence and Executive function
Neither global efficiency nor distinct functional subnetworks related to intelligence or executive function generally or explained deficits in these facets BD. We are consistent with a recent large-scale analysis reporting that resting-state efficiency does not explain variance in intelligence (Kruschwitz, Waller, Daedelow, Walter, & Veer, 2018), and find that, while IQ and executive function impairments in BD may relate to altered integration and segregation within anatomical networks (McPhilemy et al., 2019) this does not extend to implicate resting-state connectivity. This may be expected given that the brain’s intrinsic functional connections generally overlap with but are not identical to anatomical connections (Honey et al., 2009; Stam et al., 2015) and are not equally suited to the application of path-based measures when compared to anatomical networks (Honey et al., 2009; Petersen & Sporns, 2015; Stam et al., 2015). We were unable to detect expected network-level relationships with intelligence involving fronto-parietal and default-mode regions, as recently reported in 317 healthy individuals using the same statistical approach (Hearne et al., 2016); or with executive function in overlapping neural systems detected using a priori and independent component analysis (ICA) approaches (Reineberg, Gustavson, Benca, Banich, & Friedman, 2018; Vaidya & Gordon, 2013). Given brain-wide correction for multiple comparisons in network-approaches, larger samples may be required to detect network-level relationships and subtle alterations in BD for these complex and integrative facets. Furthermore, a recent preliminary investigation of executive function deficits indicates underlying functional connectivity differences may not be detected with aggregate connectivity patterns but rather connectivity dynamics (Nguyen et al., 2017).
Memory
We find distinct resting-state subnetworks supporting episodic and spatial memory across all subjects and none that explain cognitive deficits extant in BD. Longer average path length within resting-state networks related to less episodic memory errors; we note that the effect size of this relationship is moderate not surviving FDR-correction, however potentially reflective of the specific inhibitory and not necessarily most topologically efficient pathways facilitating better memory performance. These findings corroborate a common inhibitory influence between external attention and default-mode systems supporting memory (Kelly, Uddin, Biswal, Castellanos, & Milham, 2008) although is not exclusive to these systems at the network-level and may not generalise to other actively engaging tasks. Specific to spatial memory performance, direct antisynchronous co-activation patterns between the right prefrontal, left parietal and select limbic regions detected here reflect the separation of information transfer between distinct anatomical subnetworks that have been shown to relate to faster and slower response times in this spatial memory task previously in an overlapping cohort (see Supplementary Figure 3 for visual comparison of these anatomical and functional networks) (McPhilemy et al., 2019). It is important to note that the precise nature of antisynchronous activity within functional networks remains unclear. While the present results may be indicative of inhibitory or competitive functional interactions between these brain areas (Fox et al. 2005) they may also represent the different spatiotemporal structures being produced on the underlying anatomical network rather than direct antagonistic relationships (Deco et al. 2011).
Social cognition
Despite an expected deficit in social cognition in BD, we find no resting-state network-level connectivity patterns explaining this, building on our previous investigation, which identified no anatomical network-level basis (McPhilemy et al., 2019). Studies focusing on functional activation during social cognition tasks in BD implicate altered frontal, temporal, parietal and insular cortex activation (Grant et al., 2018; Kim et al., 2009; Malhi et al., 2008; Willert et al., 2015) and lower seed-based functional connectivity between medial prefrontal and temporal cortices (Willert et al., 2015). It is therefore possible that co-activation patterns important for social cognition deficits are specific to task conditions and will be detected on examination of dynamic network configurations.
Methodological strengths, limitations & future directions
In this study we implement head motion correction and removal of white matter signal, cerebrospinal signal and high motion time-points from resting-state data, designed to mitigate physiological noise and head motion which can spuriously contribute to variance in functional connectivity measures and thus sensitivity to detect relationships with cognitive ability (Satterthwaite et al., 2019). To guard against the problem of multiple comparisons, we addressed primary a priori hypotheses with additional exploratory analyses and used PCA dimensionality-reduction to analyse a parsimonious factor capturing most of the predictive information from the response time data. An important caveat to the present work is the influence that medication can have on both cognitive performance and fMRI measures in BD (Dandash et al., 2018; Gitlin, 2016; Hafeman, Chang, Garrett, Sanders, & Phillips, 2012; Torrent et al., 2011), although the advantage of this is that any findings presented are generalizable to a natural population of individuals with bipolar disorder that are normally taking combinations of different medications (Dandash et al., 2018; Gitlin, 2016; Hafeman, Chang, Garrett, Sanders, & Phillips, 2012; Torrent et al., 2011). Importantly, our approach considers a single static network structure represented as the average resting-state connectivity over the course of scanning. We are therefore unable to assess how dynamic changes during the resting-state or task-specific network changes (Cole et al., 2014) relate to cognition and underlie cognitive deficits in BD. Functional network dynamics have been shown to represent an important driver of cognitive performance (D S Bassett, Wymbs, Rombach, Porter, & Mucha, 2013; Danielle S. Bassett et al., 2011; Cohen, 2018) and there is preliminary evidence to suggest a reduced ability to reconfigure functional network architecture relates to processing speed and executive functioning deficits in BD (Nguyen et al., 2017). This is an exciting avenue for future neuroimaging investigations of cognitive impairments in BD.
Conclusions
Our findings suggest commonly reported alterations in intrinsic connectivity patterns in BD relate to a specific breakdown in the support of response timing in contrast to performance or errors on the same executive function and memory tasks or intelligence or social cognition performance. Independent of BD, we identify distinct inhibitory resting-state patterns underlying memory performance, demonstrating the promise of network-approaches in characterizing complex cognitive processing in the brain.
Supplementary Material
Acknowledgements
We gratefully acknowledge the participants, the support of the Welcome-Trust HRB Clinical Research Facility, the Centre for Advanced Medical Imaging at St. James Hospital Dublin and funding support from the Irish Research Council Government of Ireland Postgraduate Scholarship. This research was funded by the Health Research Board (HRA-POR-324) awarded to Dara M. Cannon, PhD.
Footnotes
Authors’ Contributions Statement
DMC designed, obtained funding for and supervised data collection, analysis and interpretation; BH and CMcD contributed to recruitment and intellectual content; LN and GMP recruited and collected data, processed the fMRI data, performed MRI data quality checks and developed code to extract network measures; JRW and KM trained GMP on the image analysis approach; LK contributed matlab based quality control and reconstruction scripts; FM and GMP conducted statistical analyses; GMP conducted all analyses and wrote the manuscript; all authors reviewed the findings and their interpretation.
Author Disclosure Statement
No competing financial interests exist.
Contributor Information
Leila Nabulsi, Email: l.nabulsi1@nuigalway.ie.
Liam Kilmartin, Email: liam.kilmartin@nuigalway.ie.
Joseph R. Whittaker, Email: WhittakerJ3@cardiff.ac.uk.
Fiona M. Martyn, Email: f.martyn6@nuigalway.ie.
Brian Hallahan, Email: brian.hallahan@nuigalway.ie.
Colm McDonald, Email: colm.mcdonald@nuigalway.ie.
Kevin Murphy, Email: murphyk2@cardiff.ac.uk.
Dara M. Cannon, Email: dara.cannon@nuigalway.ie.
References
- Ajilore O, Vizueta N, Walshaw P, Zhan L, Leow A, Altshuler LL. Connectome signatures of neurocognitive abnormalities in euthymic bipolar I disorder. Journal of Psychiatric Research. 2015;68:37–44. doi: 10.1016/j.jpsychires.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Lana S, Goikolea JM, Bonnin CM, Sarró S, Segura B, Amann BL, Monté GC, Moro N, Fernandez-Corcuera P, Maristany T, Salvador R, et al. Structural and functional brain correlates of cognitive impairment in euthymic patients with bipolar disorder. PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0158867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th. New York American Psychiatric Press Inc; 2013. [Google Scholar]
- Andreou C, Bozikas VP. The predictive significance of neurocognitive factors for functional outcome in bipolar disorder. Current Opinion in Psychiatry. 2013;26(1):54–59. doi: 10.1097/YCO.0b013e32835a2acf. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Rombach MP, Porter MA, Mucha PJ. Task-Based Core-Periphery Organization of Human Brain Dynamics. PLoS Comput Biol. 2013;9(9):1003171. doi: 10.1371/journal.pcbi.1003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett Danielle S, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(18):7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, Kahn AE, Vandekar SN, Rupert PE, Quarmley M, Cook PA, et al. Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Current Biology. 2017;27(11):1561–1572.:e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Malhi GS. A review on the impact of cognitive dysfunction on social, occupational, and general functional outcomes in bipolar disorder. Bipolar Disorders. 2015;17(S2):41–55. doi: 10.1111/bdi.12341. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia. 2004;42(14):1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bora E, Bartholomeusz C, Pantelis C. Meta-analysis of Theory of Mind (ToM) impairment in bipolar disorder. Psychological Medicine. 2016;46(02):253–264. doi: 10.1017/S0033291715001993. [DOI] [PubMed] [Google Scholar]
- Bora Emre, Vahip S, Akdeniz F. Sustained attention deficits in manic and euthymic patients with bipolar disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(6):1097–1102. doi: 10.1016/j.pnpbp.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Bourne C, Aydemir O, Balanzá-Martínez V, Bora E, Brissos S, Cavanagh JTO, Clark L, Cubukcuoglu Z, Dias VV, Dittmann S, Ferrier IN, et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: An individual patient data meta-analysis. Acta Psychiatrica Scandinavica. 2013;128(3):149–162. doi: 10.1111/acps.12133. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rooney MH, Skup M, Pine DS, Leibenluft E. Increased Intrasubject Variability in Response Time in Youths With Bipolar Disorder and At-Risk Family Members. J Am Acad Child Adolesc Psychiatry. 2009;48(6):628–635. doi: 10.1097/CHI.0b013e3181a27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Phillips ML. Elucidating Neural Network Functional Connectivity Abnormalities in Bipolar Disorder: Toward a Harmonized Methodological Approach. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1(3):288–298. doi: 10.1016/j.bpsc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognition C. CANTAB® [Cognitive assessment software] 2018. All rights reserved. http://www.cantab.com.
- Cohen JR. The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. NeuroImage. 2018;180:515–525. doi: 10.1016/j.neuroimage.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O, Yücel M, Daglas R, Pantelis C, McGorry P, Berk M, Fornito A. Differential effect of quetiapine and lithium on functional connectivity of the striatum in first episode mania. Translational Psychiatry. 2018;8(1) doi: 10.1038/s41398-018-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diler RS, Segreti AM, Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Phillips ML, Pan L. Neural Correlates of Treatment in Adolescents with Bipolar Depression During Response Inhibition. Journal of Child and Adolescent Psychopharmacology. 2013;23(3):214–221. doi: 10.1089/cap.2012.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S. The role of intrinsic brain functional connectivity in vulnerability and resilience to bipolar disorder. American Journal of Psychiatry. 2017;174(12):1214–1222. doi: 10.1176/appi.ajp.2017.17010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J, Hilke M, Trettin M, Wenzler S, Hagen M, Ghirmai N, Müller M, Kraft D, Reif A, Oertel V. Aberrant brain network topology in fronto-limbic circuitry differentiates euthymic bipolar disorder from recurrent major depressive disorder. Brain and Behavior. 2019:e01257. doi: 10.1002/brb3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre P, Baciu M, Pichat C, De Pourtalès M-A, Fredembach B, Garçon S, Bougerol T, Polosan M. Modulation of fronto-limbic activity by the psychoeducation in euthymic bipolar patients. A functional MRI study. Psychiatry Research: Neuroimaging. 2013;214(3):285–295. doi: 10.1016/j.pscychresns.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Nilsson J, Finkelmeyer A, Goshawk M, Macritchie KA, Lloyd AJ, Thompson JM, Porter RJ, Young AH, Ferrier IN, McAllister-Williams RH, et al. Neurocognitive intra-individual variability in mood disorders: effects on attentional response time distributions. Psychological Medicine. 2015;45(14):2985–2997. doi: 10.1017/S0033291715000926. [DOI] [PubMed] [Google Scholar]
- Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. International Journal of Bipolar Disorders. 2016;4:27. doi: 10.1186/s40345-016-0068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K, Hassel S, Bobyn JA, Hall GBC, MacQueen GM. A novel task for examining the neural basis of Theory of Mind deficits in bipolar disorder. Psychiatry Research: Neuroimaging. 2018;282:143–150. doi: 10.1016/j.pscychresns.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: An updated review. Bipolar Disorders. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. The British Journal of Medical Psychology. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- He H, Yu Q, Du Y, Vergara V, Victor TA, Drevets WC, Savitz JB, Jiang T, Sui J, Calhoun VD. Resting-state functional network connectivity in prefrontal regions differs between unmedicated patients with bipolar and major depressive disorders. Journal of Affective Disorders. 2016;190:483–493. doi: 10.1016/j.jad.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne LJ, Mattingley JB, Cocchi L. Functional brain networks related to individual differences in human intelligence at rest. Scientific Reports. 2016;6 doi: 10.1038/srep32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Honey CJ, Sporns O, Sporns O, Cammoun L, Cammoun L, Gigandet X, Gigandet X, Thiran JP, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. Journal of Applied Mathematics. 2013;2013 doi: 10.1155/2013/935154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BP, Pinar A, Fornito A, Nandam LS, Hester R, Bellgrove MA. Left anterior cingulate activity predicts intra-individual reaction time variability in healthy adults. Neuropsychologia. 2015 doi: 10.1016/j.neuropsychologia.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. An index of factorial simplicity. Psychometrika. 1974;39 [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim E, Jung YC, Ku J, Kim JJ, Lee H, Kim SY, Kim SI, Cho HS. Reduced activation in the mirror neuron system during a virtual social cognition task in euthymic bipolar disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(8):1409–1416. doi: 10.1016/j.pnpbp.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Kruschwitz JD, Waller L, Daedelow LS, Walter H, Veer IM. General, crystallized and fluid intelligence are not associated with functional global network efficiency: A replication study with the human connectome project 1200 data set. NeuroImage. 2018;171:323–331. doi: 10.1016/j.neuroimage.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Das P, Moss K, Berk M, Coulston CM. A functional MRI study of Theory of Mind in euthymic bipolar disorder patients. Bipolar Disorders. 2008;10(8):943–956. doi: 10.1111/j.1399-5618.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: An update and investigation of moderator variables. Bipolar Disorders. 2011;13(4):334–342. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- McPhilemy G, Nabulsi L, Kilmartin L, O’Donoghue S, Tronchin G, Costello L, Najt P, Ambati S, Neilsen G, Creighton S, Byrne F, et al. Neuroanatomical network features associated with cognitive dysfunction in bipolar disorder; Proceedings of the 74th Annual Meeting of the Society for Biological Psychiatry; Chicago, United States of America: 2019. [Google Scholar]
- Nabulsi L, McPhilemy G, Kilmartin L, O’Hora D, O’Donoghue S, Forcellini G, Najt P, Ambati S, Costello L, Byrne F, McLoughlin J, et al. Bipolar Disorder and Gender are Associated with Fronto-limbic and Basal Ganglia Dysconnectivity: A Study of Topological Variance Using Network Analysis. Brain Connectivity. 2019 doi: 10.1089/brain.2019.0667. [DOI] [PubMed] [Google Scholar]
- Nabulsi Leila, McPhilemy G, Kilmartin L, Whittaker JR, Martyn FM, Hallahan B, McDonald C, Murphy K, Cannon DM. Frontolimbic, Frontoparietal, and Default Mode Involvement in Functional Dysconnectivity in Psychotic Bipolar Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2020;5(2):140–151. doi: 10.1016/j.bpsc.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Kovacevic S, Dev SI, Lu K, Liu TT, Eyler LT. Dynamic functional connectivity in bipolar disorder is associated with executive function and processing speed: A preliminary study. Neuropsychology. 2017;31(1):73–83. doi: 10.1037/neu0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue S, Holleran L, Cannon DM, McDonald C. Anatomical Dysconnectivity in Bipolar Disorder Compared with Schizophrenia: A selective review of structural network analyses using diffusion MRI. Journal of Affective Disorders. 2016;209:217–228. doi: 10.1016/j.jad.2016.11.015. [DOI] [PubMed] [Google Scholar]
- O’Donoghue S, Kilmartin L, O’Hora D, Emsell L, Langan C, McInerney S, Forde NJ, Leemans A, Jeurissen B, Barker GJ, McCarthy P, et al. Anatomical integration and rich-club connectivity in euthymic bipolar disorder. Psychological Medicine. 2017:1–15. doi: 10.1017/S0033291717000058. [DOI] [PubMed] [Google Scholar]
- Oertel-Knöchel V, Reinke B, Matura S, Prvulovic D, Linden DEJ, van de Ven V. Functional connectivity pattern during rest within the episodic memory network in association with episodic memory performance in bipolar disorder. Psychiatry Research - Neuroimaging. 2015;231(2):141–150. doi: 10.1016/j.pscychresns.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Perry A, Roberts G, Mitchell PB, Breakspear M. Connectomics of bipolar disorder: a critical review, and evidence for dynamic instabilities within interoceptive networks. Molecular Psychiatry. 2018;1 doi: 10.1038/s41380-018-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Sporns O. Brain Networks and Cognitive Architectures. Neuron. 2015;88(1):207–219. doi: 10.1016/j.neuron.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei F, Jogia J, Tatarelli R, Girardi P, Rubia K, Kumari V, Frangou S. Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. NeuroImage. 2011;56(3):1677–1684. doi: 10.1016/j.neuroimage.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Reineberg AE, Gustavson DE, Benca C, Banich MT, Friedman NP. The relationship between resting state network connectivity and individual differences in executive functions. Frontiers in Psychology. 2018;9:1–14. doi: 10.3389/fpsyg.2018.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G, Lord A, Frankland A, Wright A, Lau P, Levy F, Lenroot RK, Mitchell PB, Breakspear M. Functional Dysconnection of the Inferior Frontal Gyrus in Young People With Bipolar Disorder or at Genetic High Risk. Biological Psychiatry. 2017;81(8):718–727. doi: 10.1016/j.biopsych.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Ciric R, Roalf DR, Davatzikos C, Bassett DS, Wolf DH. Motion artifact in studies of functional connectivity: Characteristics and mitigation strategies. Human Brain Mapping. 2019;40(7):2033–2051. doi: 10.1002/hbm.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW, Daniel Ragland J, Silverstein SM, Godwin D, Barch DM. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Beall EB, Hulvershorn LA, Altinay M, Karne H, Anand A. Resting State Brain Network Disturbances Related to Hypomania and Depression in Medication-Free Bipolar Disorder. Neuropsychopharmacology. 2016;41:3016–3024. doi: 10.1038/npp.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Hillebrand A, Tewarie P, Van Dellen E, Meier J, van Straaten ECW, Gong G, Van Mieghem P. The relation between structural and functional connectivity patterns in complex brain networks. International Journal of Psychophysiology. 2015;103:149–160. doi: 10.1016/j.ijpsycho.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Syan SK, Smith M, Frey BN, Remtulla R, Kapczinski F, Hall GBC, Minuzzi L. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: A systematic review. Journal of Psychiatry and Neuroscience. 2018;43(5):298–316. doi: 10.1503/jpn.170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira S, Machado S, Paes F, Velasques B, Silva J, Sanfim A, Minc D, Anghinah R, Menegaldo L, Salama M, Cagy M, et al. Time Perception Distortion in Neuropsychiatric and Neurological Disorders. CNS & Neurological Disorders - Drug Targets. 2013;12(5):567–582. doi: 10.2174/18715273113129990080. [DOI] [PubMed] [Google Scholar]
- Torrent C, Martinez-Arán A, Daban C, Amann B, Balanzá-Martínez V, Del Mar Bonnín C, Cruz N, Franco C, Tabarés-Seisdedos R, Vieta E. Effects of atypical antipsychotics on neurocognition in euthymic bipolar patients. Comprehensive Psychiatry. 2011;52(6):613–622. doi: 10.1016/j.comppsych.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Townsend JD, Bookheimer SY, Foland-Ross LC, Moody TD, Eisenberger NI, Fischer JS, Cohen MS, Sugar CA, Altshuler LL. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task HHS Public Access. Bipolar Disord. 2012;14(4):442–450. doi: 10.1111/j.1399-5618.2012.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Gordon EM. Phenotypic Variability in Resting-State Functional Connectivity: Current Status. Brain Connectivity. 2013;3(2):99–120. doi: 10.1089/brain.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. Journal of Neuroscience. 2009;29(23):7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert J, Schiele MA, Kazmaier J, Glaser F, Zierhut KC, Kopf J, Kittel-Schneider S, Reif A. Cognitive deficits in bipolar disorder: from acute episode to remission. European Archives of Psychiatry and Clinical Neuroscience. 2016;266(3) doi: 10.1007/s00406-015-0657-2. [DOI] [PubMed] [Google Scholar]
- Vrabie M, Marinescu V, Talaşman A, Tăutu O, Drima E, Micluţia I. Cognitive impairment in manic bipolar patients: important, understated, significant aspects. Annals of General Psychiatry. 2015;14:41. doi: 10.1186/s12991-015-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang J, Jia Y, Zhong S, Zhong M, Sun Y, Niu M, Zhao L, Zhao L, Pan J, Huang L, et al. Topologically convergent and divergent functional connectivity patterns in unmedicated unipolar depression and bipolar disorder. Translational Psychiatry. 2017;7(7) doi: 10.1038/tp.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Ying Deng F, Jia Y, Wang J, Zhong S, Huang H, Chen L, Chen G, Hu H, Huang L, Huang R. Disrupted rich club organization and structural brain connectome in unmedicated bipolar disorder. Psychological Medicine. 2019;49(3):510–518. doi: 10.1017/S0033291718001150. [DOI] [PubMed] [Google Scholar]
- Wang Ying, Wang J, Jia Y, Zhong S, Niu M, Sun Y, Qi Z, Zhao L, Huang L, Huang R. Shared and Specific Intrinsic Functional Connectivity Patterns in Unmedicated Bipolar Disorder and Major Depressive Disorder. Scientific Reports. 2017;7(1):3570. doi: 10.1038/s41598-017-03777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Ying, Zhong S, Jia Y, Sun Y, Wang B, Liu T, Pan J, Huang L. Disrupted Resting-State Functional Connectivity in Nonmedicated Bipolar Disorder. Radiology. 2016;280(2):529–536. doi: 10.1148/radiol.2016151641. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: administration and scoring manual: Wechsler Adult Intelligence Scale. Psychological Corporation; 1997. [Google Scholar]
- Willert A, Mohnke S, Erk S, Schnell K, Romanczuk-Seiferth N, Quinlivan E, Schreiter S, Spengler S, Herold D, Wackerhagen C, Romund L, et al. Alterations in neural Theory of Mind processing in euthymic patients with bipolar disorder and unaffected relatives. Bipolar Disorders. 2015;17(8):880–891. doi: 10.1111/bdi.12352. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PLoS ONE. 2009;4(1) doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133(11):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. NeuroImage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang Y, Jia Y, Zhong S, Sun Y, Qi Z, Zhang Z, Huang L. Altered interhemispheric functional connectivity in remitted bipolar disorder: A Resting State fMRI Study. Scientific Reports. 2017;7(1):4698. doi: 10.1038/s41598-017-04937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.