Abstract
Short-term outcomes in kidney transplantation are marred by progressive transplant failure and mortality secondary to immunosuppression toxicity. Immune modulation with autologous polyclonal regulatory T cell (Treg) therapy may facilitate immunosuppression reduction promoting better long-term clinical outcomes. In a Phase I clinical trial, 12 kidney transplant recipients received 1–10 × 106 Treg per kg at Day +5 posttransplantation in lieu of induction immunosuppression (Treg Therapy cohort). Nineteen patients received standard immunosuppression (Reference cohort). Primary outcomes were rejection-free and patient survival. Patient and transplant survival was 100%; acute rejection-free survival was 100% in the Treg Therapy versus 78.9% in the reference cohort at 48 months posttransplant. Treg therapy revealed no excess safety concerns. Four patients in the Treg Therapy cohort had mycophenolate mofetil withdrawn successfully and remain on tacrolimus monotherapy. Treg infusion resulted in a long-lasting dose-dependent increase in peripheral blood Tregs together with an increase in marginal zone B cell numbers. We identified a pretransplantation immune phenotype suggesting a high risk of unsuccessful ex-vivo Treg expansion. Autologous Treg therapy is feasible, safe, and is potentially associated with a lower rejection rate than standard immunosuppression. Treg therapy may provide an exciting opportunity to minimize immunosuppression therapy and improve long-term outcomes.
Keywords: clinical research/practice, clinical trial, immune regulation, immunosuppression/immune modulation, immunosuppressive regimens – minimization/withdrawal, kidney transplantation/nephrology, kidney transplantation: living donor, monitoring: immune, translational research/science
1. Introduction
There is increasing interest in the use of cell-based therapies as a means of modulating the immune response to organ and tissue transplants to potentially allow modification of standard immunosuppression protocols.1–4 Recipients with a functioning kidney transplant require life-long immunosuppressive therapy to prevent immunological rejection and graft loss. While initial clinical outcomes are excellent, long-term patient and transplant survival rates are compromised by the cumulative toxicity of immunosuppression resulting in increased serious infection, cardiovascular events and malignancy.5,6 New approaches are required to explore ways of minimizing the burden of immunosuppression and reducing the associated significant long-term side effects with the potential to improve kidney transplantation outcomes.7,8
Regulatory T cells (Tregs) are naturally occuring immune modulatory cells that are critical to immune homeostasis and have the capacity to reduce the inflammatory response to alloantigens.9 Animal models have demonstrated the ability of infused Tregs to prevent the development of acute rejection of skin grafts and endothelial vascular rejection of human arterial transplants.10,11 Mounting evidence indicates that Tregs act within tissues to directly control immune responses at the site of potential injury.12
Recent developments have allowed the ex vivo expansion of autologous Tregs making clinical trials of Treg therapy in solid organ transplantation feasible.13 Pilot studies of polyclonal autologous Treg therapy in kidney and liver transplantation have demonstrated good short-term safety data with no significant serious adverse reactions to Treg infusions, no increased toxicity and no early increased risk of infection or neoplasia.2–4,14
We report the next step in the evaluation of autologous Treg therapy as a safe potential therapeutic adjunct in clinical kidney transplantation to allow immunosuppression minimization. This clinical trial was part of the ONE Study Consortium which aimed to test the feasibility and safety of several different immune modulating types of cell therapy using a single standardized immunosuppression protocol and comparator Reference cohort.14 In this study we compare the long-term clinical outcomes and associated immune monitoring of 12 living donor kidney transplant recipients who received ex vivo-expanded autologous Treg therapy with a reference cohort receiving standard immunosuppression.
2. Methods
The study was designed as a prospective cohort study performed in two centers in the United Kingdom to explore the feasibility, safety and potential efficacy of Treg therapy in living donor kidney transplant recipients (Figure 1). Treg-treated patients were compared with a standard-of-care reference cohort. The objective was to determine whether administration of autologous ex vivo-expanded polyclonal Tregs to recipients of living-donor kidney transplants is safe and able to regulate the immunological response of the recipient allowing avoidance of induction therapy and potential minimization of maintenance immunosuppression. Primary endpoints were the incidence of biopsy-confirmed acute rejection (Banff criteria) and transplant survival within 60 weeks of transplantation.14 The impact of Treg therapy on the host immune cell composition was assessed by whole blood flow cytometric analysis and Quantibrite analysis of HLA-DR expression.15
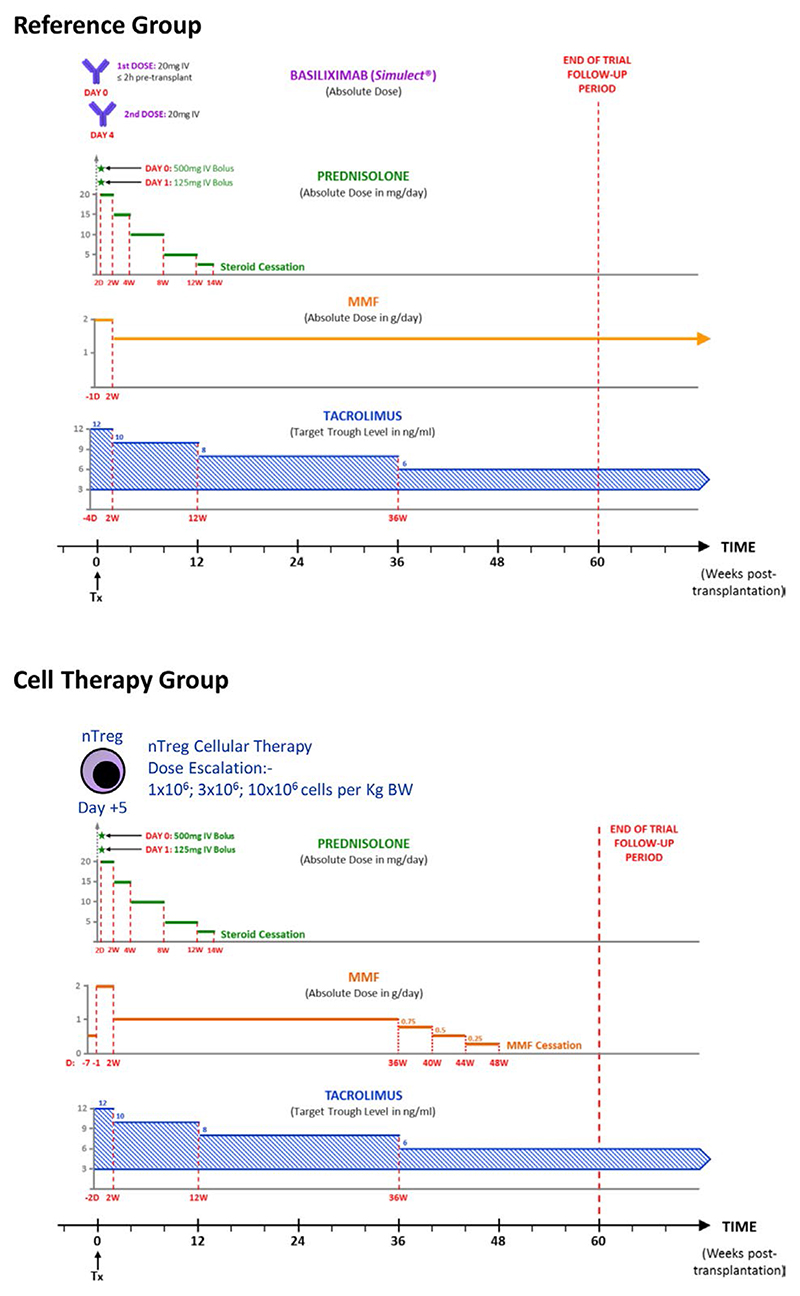
Figure 1.
ONE Study immunosuppression protocol for Reference and Treg Therapy kidney transplant recipients. MMF, mycophenolate mofetil; nTreg, naturally occurring Treg; Tx, transplantation; BW, body weight [Color figure can be viewed at wileyonlinelibrary.com]
2.1. Patients
A prospective cohort of renal transplant recipients were recruited and received a standard immunosuppression protocol similar to the Symphony Trial (Reference cohort, Figure 1A, EuDra CT No 2011-004301-24).16 This Reference cohort generated baseline clinical and immunological outcome data to act as comparator to the Treg Therapy cohort. A second cohort of patients was recruited who underwent transplantation with Treg therapy in lieu of induction immunosuppression and subsequent reduction of maintenance immunosuppression (Treg Therapy cohort). Individual patient follow-up in both cohorts was scheduled for 60 weeks posttransplantation, as almost all treatment failures defined by the primary endpoint would be expected to occur within this period of time. Additional follow-up for patient and graft outcomes was continued until 48 months posttransplantation in both cohorts. Living donor kidney transplant recipients and donors were recruited according to the same inclusion and exclusion criteria in both the Reference and Treg Therapy cohorts (Table 1). Each enrolled patient had 11 trial study visits scheduled relative to the day of transplantation (Day 0). Patient demographics are shown in Table 2. In the cell therapy cohort 50% of transplanted kidneys were from live related living donors and 50% from live unrelated donors; all were first kidney transplant recipients and two recipients had minor sensitization with a PRA of 2 and 4% the remainder had no HLA antibodies at transplantation. Twenty-five percent of patients were CMV negative recipients receiving a CMV positive transplant. No recipients developed CMV infection posttransplantation over the 48 months follow-up period.
Table 1. Inclusion and exclusion criteria.
|
Inclusion criteria
|
|
Exclusion criteria
|
Table 2. Demographics of study participants.
| Reference | Cell therapy |
|
|---|---|---|
| Recipient age | ||
| Mean | 48 | 47 |
| Median (range) | 46 (31–67) | 44 (28–69) |
| Sex (n) | ||
| Male (%) | 14 (74) | 10 (83) |
| Female (%) | 5 (26) | 2 (17) |
| Ethnicity (n) | ||
| White (%) | 15 (79) | 10 (83) |
| Asian (%) | 4 (21) | 1 (8) |
| Black (%) | 0 | 1 (8) |
| Primary renal disease (n) | ||
| APKD (%) | 5 (26) | 3 (25) |
| Glomerulonephritis (%) | 7 (37) | 4 (33) |
| Urological (%) | 3 (16) | 1 (8) |
| Other (%) | 4 (21) | 4 (33) |
| RRT (n) | ||
| HD (%) | 5 (26) | 3 (25) |
| PD (%) | 5 (26) | 0 |
| Pre-emptive (%) | 9 (47) | 9 (75) |
| Donor age | ||
| Mean | 56 | 45 |
| Median (range) | 59 (32–68) | 45 (28–71) |
| Donor relationship (n) | ||
| Living related (%) | 15 (79) | 6 (50) |
| Living unrelated (%) | 4 (21) | 6 (50) |
| Mismatches (n) | ||
| 1–2 (%) | 9 (47) | 5 (42) |
| 3–4 (%) | 8 (42) | 6 (50) |
| 5–6 (%) | 2 (11) | 1 (8) |
| PRA baseline (n) | ||
| 0 (%) | 16 (84) | 10 (83) |
| <10 (%) | 2 (11) | 2 (16) |
| 10–30 (%) | 1 (5) | 0 (0) |
| CMV status (n) | ||
| Donor – recipient – (%) | 2 (11) | 3 (25) |
| Donor – recipient + (%) | 4 (21) | 5 (42) |
| Donor + recipient + (%) | 10 (53) | 1 (8) |
| Donor + recipient – (%) | 3 (16) | 3 (25) |
Abbreviations: APKD, adult polycystic kidney disease; CMV, cytomegalovirus; HD, hemodialysis; PD, peritoneal dialysis; PRA, panel reactive antibody; RRT, renal replacement therapy.
2.2. Manufacture of Treg
Three hundred and eighty milliliter of whole blood was obtained from each potential living donor kidney transplant recipient in the Treg Therapy cohort and transported to a central GMP manufacturing unit in London. Polyclonal Tregs were extracted and expanded in cell culture using a previously validated protocol and the final cell product was cryopreserved in vapor phase liquid nitrogen.13 Release criteria included purity, sterility, and suppressive function.
Treg therapy was administered on day 5 posttransplant by central venous infusion. The trial adopted a traditional 3 + 3 dose-escalation design with three patients at each dose receiving 1 × 106, 3 × 106, 6 × 106, or 10 × 106 cells per kg body weight. A minimum period of 2 weeks was observed before escalation to the next higher dose to ensure there were no adverse reactions or toxicity resulting from infusion. The Treg product was thawed at the bedside and delivered by syringe into 150–200 ml 4.5% sterile isotonic human serum albumin contained in a glass bottle. The final dispersed cell product was then infused intravenously at a rate of 2 ml/min. On the day of Treg infusion, patients also commenced treatment for 48 h or until discharge with unfractionated heparin (5000 units) given subcutaneously twice daily to reduce the risk of pulmonary embolus. In addition, a prophylactic dose of paracetamol 1 g orally and an anti-histamine was given to mitigate against potential allergic febrile reactions. Monitoring including ECG, pulse rate, respiratory rate, blood pressure, oxygen saturations, and body temperature were recorded at 15 min intervals during the infusion and for 12 h following completion of the infusion.
2.3. Immunosuppression
Immunosuppression in the Reference cohort followed a standard protocol in the United Kingdom comprising induction with basiliximab and triple therapy with tacrolimus, mycophenolate mofetil, and a reducing dose of prednisolone (Figure 1A).16 Basiliximab was not included in the Treg Therapy cohort because of the potential for inhibition of infused Tregs.17 Patients in this cohort received no induction immunosuppression but otherwise the same initial triple therapy immunosuppression regimen was followed (Figure 1B). The investigators had the option to taper MMF to cessation over a 12-week period in the Treg Therapy cohort if the protocol biopsy revealed no evidence of rejection or inflammation (Figure 1B). All clinical trial data including any adverse events up until the first 60 weeks posttransplant were recorded in a bespoke electronic clinical trial database (Koehler eClinical).
3. Results
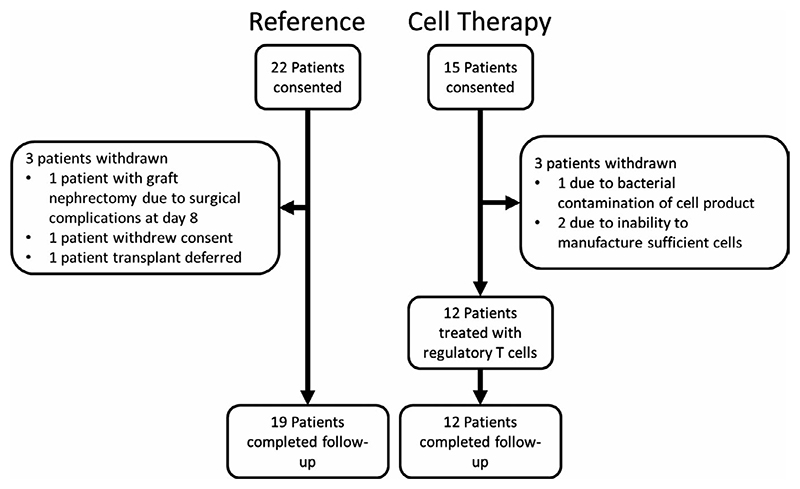
Recruitment for the Reference cohort was undertaken between 2011 and 2013 and the Treg Therapy cohort between 2014 and 2016. Twenty-two patients were recruited into the Reference cohort. One patient was withdrawn at day 8 posttransplant due to renal vein thrombosis and early graft lost secondary to surgical technical issues. One further patient did not progress to transplantation and a third patient withdrew consent (Figure 2). Nineteen patients underwent transplantation, completed follow-up and were included for analysis. Fifteen patients were recruited to the Treg Therapy cohort. Three patients had to be withdrawn due to failure to sufficiently expand the Treg cell numbers required (n = 2) and bacterial contamination during manufacture (n = 1). In a further participant, T cell expansion in culture was rapid and cells died prematurely due to lack of nutritional support. This participant agreed to delay transplantation and provided a second unit of blood for cell isolation which was successfully expanded with a reduction in the stimulation phases. Twelve patients were treated with Tregs, all completed follow-up and were included for analysis.
Figure 2. Trial cohorts.
3.1. Clinical outcomes
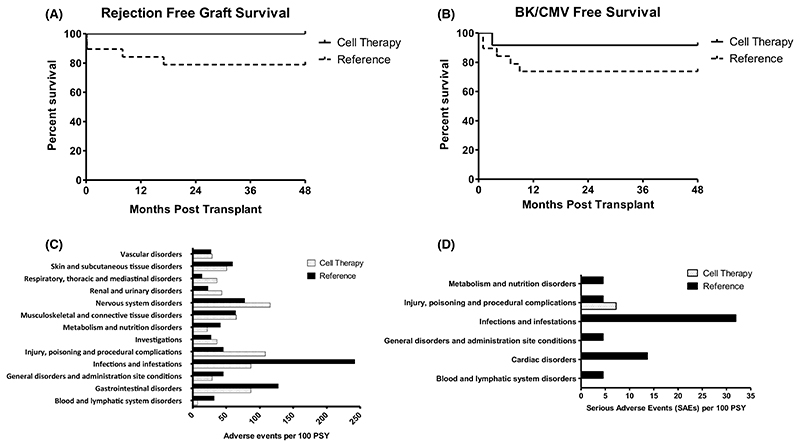
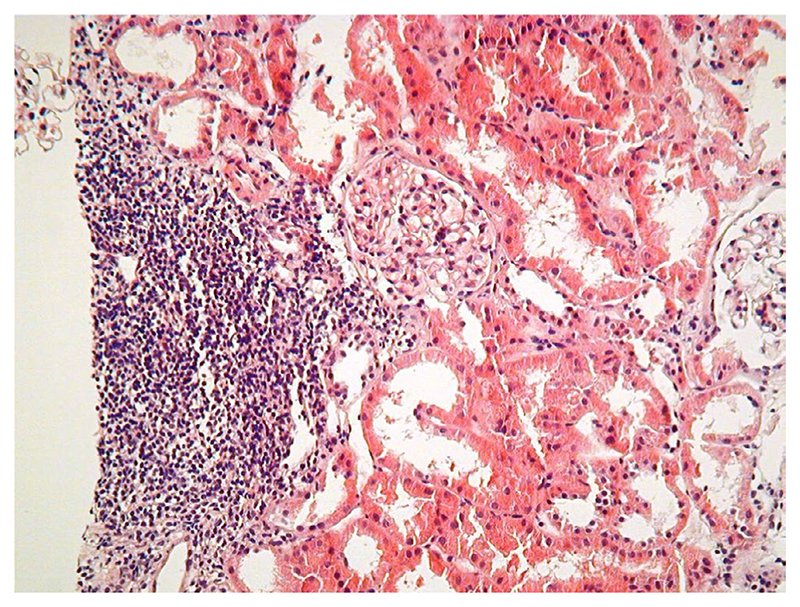
Transplant recipients were well matched in both Reference and Treg Therapy cohorts for age, ethnicity, cause of kidney failure and degree of HLA match (Table 2), but there were more male and fewer dialysis-dependent individuals in the Treg Therapy cohort. There were no hemodynamic or inflammatory reactions to the infusion of the Treg product and overall fewer adverse events and serious adverse events in the Treg Therapy cohort compared to the Reference cohort over the initial 60-week follow-up period (Figure 3C,D). In both the Reference and Treg Therapy cohorts there was 100% transplant survival at 48 months. Median (IQR) serum creatinine levels of 1.5 (1.2-1.9) vs. 1.4 (1.2-1.7) mg/dl, CKD-EPI eGFR of 53 (41.5-65.5) vs. 54.5 (48.5-72.3) and tacrolimus levels of 5.4 (5.3-6.2) vs. 5.8 (5.0-7.4) ng/ml were similar in reference and Treg Therapy cohorts respectively at 48 months posttransplant. There was a trend approaching significance suggesting a lower rate of acute rejection in Treg-treated patients compared with the Reference cohort (0% vs. 21.1%), despite there being a lower immunosuppression load and no induction therapy (Figure 3A). In protocol biopsies histological findings of focal inflammatory infiltrates in the setting of stable transplant function were detected in the first five recipients that were not typical of acute allograft rejection (Figure 4). The presence of these infiltrates led to caution in tapering of immunosuppression in the initial five patients who remained on dual immunosuppressive therapy. Despite these changes transplant function remained very stable until 60 weeks transplant in the first five patients. Immunohistochemistry revealed that 5%–10% of cells within the observed infiltrates were FOXP3+. We subsequently proceeded to minimization of immunosuppression to tacrolimus monotherapy at 8 months posttransplantation in four recipients despite similar biopsy appearances with 100% success and no adverse outcomes. Immunosuppression was not tapered in two patients who refused a protocol biopsy at 8 months posttransplantation due to the stability of their transplant function and a further patient in whom the biopsy sample was insufficient. Donor-specific antibody screening remained negative in 90% of recipients receiving cell therapy at 60 weeks posttransplant (10% not tested). A lower incidence of composite opportunistic infection with polyoma virus or cytomegalovirus in individuals who received Treg cell therapy was observed (Figure 3B). One patient developed polyoma virus nephropathy confirmed on a for cause transplant biopsy at 4 months posttransplant. There was a good response to reduction of immunosuppression resulting in resolution of polyoma virus-related inflammation on the 8 months protocol biopsy and stable transplant function at 48 months posttransplant.
Figure 3. Clinical outcomes.
(A) Rejection-free graft survival at 4 years posttransplant. (B) CMV- and BK-free survival over 4 years posttransplant. BK and CMV infection defined by blood PCR titers meeting criteria for immunosuppression adjustment or evidence of tissue invasive disease. (C) Adverse events per 100 patient study years over first 60 weeks posttransplant (censored for events occurring at >20 per 100 PSY). (D) Serious adverse events per 100 patient study years over first 60 weeks posttransplant. CMV, cytomegalovirus; BKV, BK virus
Figure 4.
Representative H&E section from a renal transplant biopsy in cell therapy-treated patients at 8 months posttransplant. Focal infiltrates as demonstrated, that were not typical of rejection, were seen in the first five patients treated with regulatory T cells with otherwise stable graft function [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Immunological outcomes
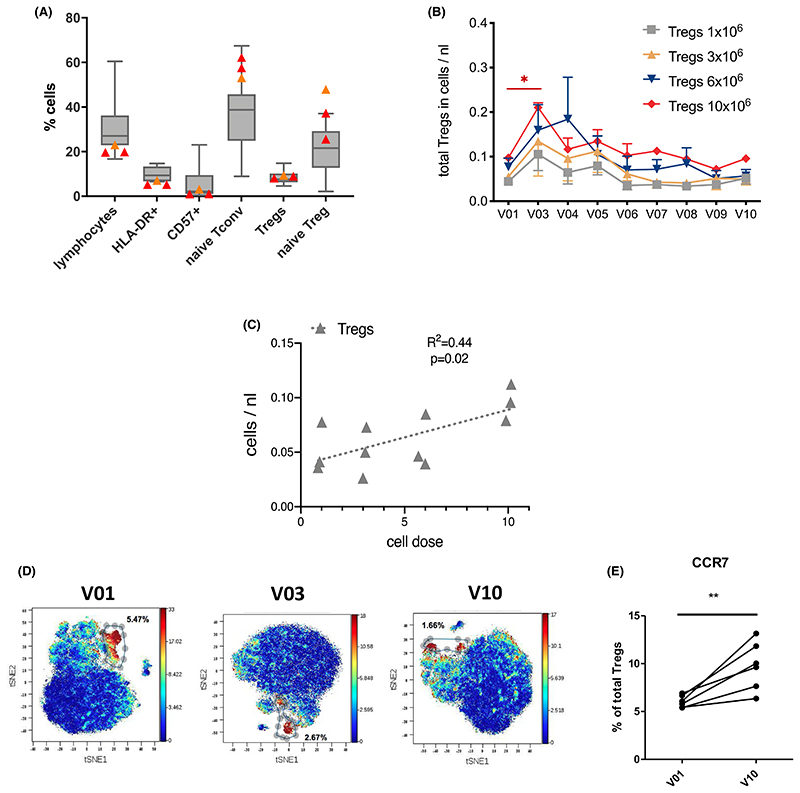
In the patients for whom Treg expansion failed or did not reach the required numbers for therapy, there was a suggestion of a reduced frequency of lymphocytes and a higher proportion of naïve Tregs compared to patients in whom expansion was successful (Figure 5A).
Figure 5.
(A) Pre-expansion phenotype predicts feasibility of successful Treg expansion. (B–E) Treg detection post-infusion. (A) Patients for whom Treg expansion failed (red triangles) or was difficult (yellow triangle) showed reduced frequencies of lymphocytes, HLA-DR+ or CD57+ activated CD4+ T cells as well as higher proportions of naïve conventional and regulatory T cells prior to transplantation as compared to patients with successful expansion (n = 11). (B) Dose-dependent increase in absolute numbers of CD4+CD25highCD127low regulatory T cells upon Treg infusion 2 weeks after transplantation determined by whole blood flow cytometry in samples collected from patients receiving either 1 × 106, 3 × 106, 6 × 106, or 10 × 106 Tregs/kg (n = 3 per dose) prior to transplantation (V01), at 2 (V03), 4 (V04), 8 (V05), 12 (V06), 24 (V07), 36 (V08), 48 (V09), and 60 (V10) weeks posttransplant. Statistical analysis by mixed-effects analysis and Tukey's multiple comparison test.*p < .05. (C) Trend toward dose-dependent increase in absolute numbers of CD4+CD25highCD127low regulatory T cells at the end of the observation period (60 weeks posttransplant). Statistical analysis by linear regression. (D) Cytometry time of flight (CyTOF) example tSNE plots from one patient pretransplantation (V01), and at 2 (V03) and 60 (V10) weeks posttransplantation, highlighting distinct Treg clusters. (E) CCR7 expression on peripheral Tregs pretransplant (V01) and at 60 weeks posttransplant (V10) in 6 Treg-treated patients by CyTOF. Paired t test, **p < .01 [Color figure can be viewed at wileyonlinelibrary.com]
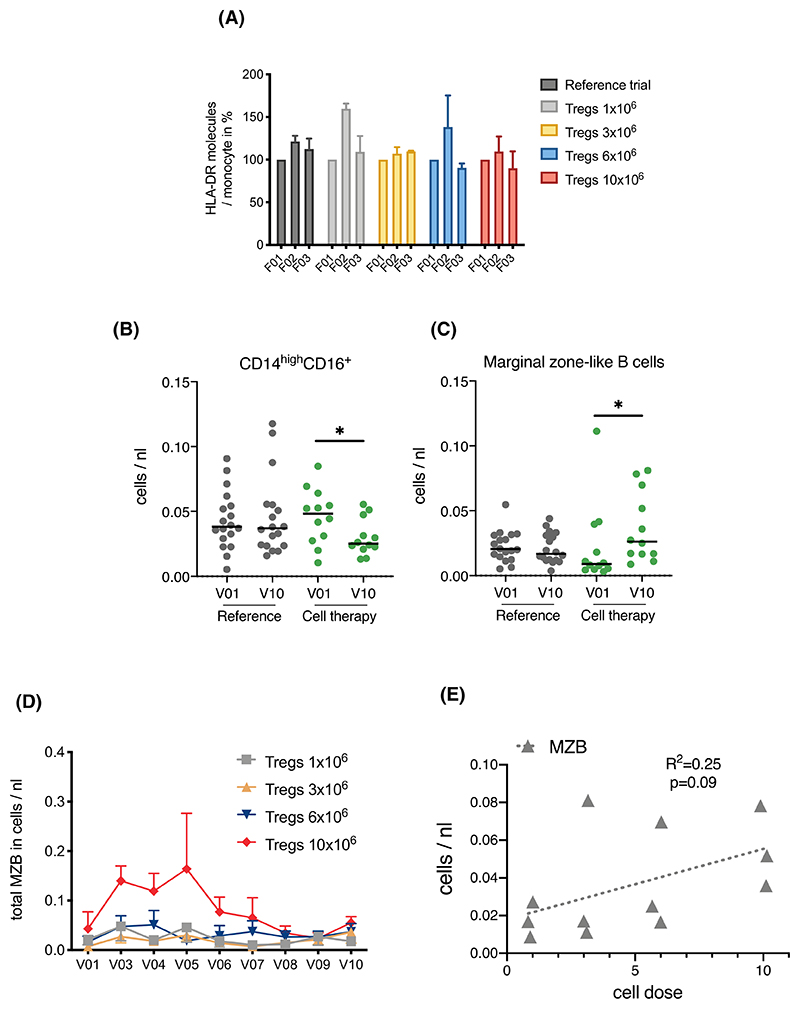
Early expression of HLA-DR on monocytes was assessed as a measure of immune activation in response to Treg infusion. Reassuringly, infusion at all doses did not result in any significant increase in HLA-DR expression up to 7 days post-infusion (Figure 6A), while overall CD14highCD16+ pro-inflammatory monocyte numbers were reduced over time in Treg-treated patients (Figure 6B). Importantly, infusion of Tregs resulted in a dose-dependent increase in absolute numbers of peripheral blood CD4+CD25highCD127low cells 2 weeks after transplantation, suggesting survival of the infused cell product (Figure 5B). A trend for a dose-dependent increase in peripheral Treg numbers was maintained up to the end of the observation period (60 weeks, Figure 5C). Distinct Treg clustering could also be observed by mass cytometry (Figure 5D), which also revealed an increase in Treg CCR7 expression (Figure 5E), a known marker of Treg function.18,8 Interestingly, marginal zone B (MZB) cells, which have been shown to have a regulatory role through the production of IL-10,19,9 were increased in patients receiving Treg therapy (Figure 6B). This increase was dose dependent and maintained until the end of the observation period (Figure 6D,E).
Figure 6. Effect of Treg therapy on immune cell composition.
(A) Treg infusion of either 1 × 106, 3 × 106, 6 × 106, and 10 × 106 cells/kg. (n = 3 patients/dose) did not lead to a significant increase in HLA-DR expression on CD14+ monocytes 1 day (F02) or 7 days later (F03). (B,C) Differences in posttransplant changes in CD14highCD16+ monocytes and marginal zone-like B cells. Scatter plots of absolute numbers in whole blood samples collected pretransplant (V01) and at the end of the observation period (60 weeks posttransplant, V10) from reference (n = 19) and cell therapy trial patients (n = 12). Statistical analysis by Wilcoxon matched-pairs signed rank and Dunn's multiple comparison test. *p < .05. (D) Trend toward dose-dependent increase in absolute numbers of marginal zone-like B cells upon Treg infusion 2 weeks after transplantation determined by whole blood flow cytometry in samples collected from patients receiving either 1 × 106, 3 × 106, 6 × 106, or 10 × 106 cells/kg. (n = 3 patients per dose) prior to transplantation (V01), at 2 (V03), 4 (V04), 8 (V05), 12 (V06), 24 (V07), 36 (V08), 48 (V09), and 60 (V10) weeks posttransplant. (E) Trend toward dose-dependent increase in absolute numbers of marginal zone-like B cells at the end of the observation period (60 weeks posttransplant, V10). Statistical analysis by linear regression [Color figure can be viewed at wileyonlinelibrary.com]
4. Discussion
This study confirms the feasibility of expansion and cryopreservation of autologous polyclonal Treg isolated from peripheral blood for therapeutic administration in kidney transplant recipients. We identified a useful lymphocyte phenotype which could identify patients pretransplantation at high risk of unsuccessful ex vivo Treg expansion. The cell product was successfully transported to two clinical sites using a dry cold storage shipper making a future multicenter trial of efficacy feasible. Infusion of the cell product was uncomplicated with no adverse reactions making outpatient day case infusion of Treg cell products a practical possibility in the future. Similar immediate safety observations were observed in a pilot Phase I study (TRACT Trial) involving a dose escalation of ex vivo-expanded polyclonal Tregs (0.5, 1, and 5 × 109 cells per recipient) administered 2 months posttransplantation in nine living donor kidney transplant recipients.2 In that study, cell infusion was associated with a 9–20-fold increase in circulating Treg cell concentration. Autologous Tregs have also been shown to be successfully expanded posttransplantation, allowing treatment of three kidney transplant recipients who had sub-clinical inflammation on a 6-month protocol surveillance biopsy.4 Recently we reported nine liver transplant recipients who received autologous polyclonal Tregs (three recipients received 1 × 106/kg Tregs 4 months posttransplant; six recipients received 4.5 × 106/kg Tregs 333-505 days posttransplant).3 No adverse events were observed in the cohort infused with 1 × 106/kg Tregs, only one of the six patients infused with 4.5 × 106/ kg Tregs developed a transient fever, neutropenia, lymphopenia, and raised cytokines without hemodynamic instability which resolved completely by day 7. In the study reported here, we have the additional benefit of a reference cohort of living donor kidney recipients as a comparator which has demonstrated comparable clinical outcomes of safety and efficacy of Treg therapy in the absence of induction immunosuppression with 48 months of follow-up. The stability of transplant function in the Treg Therapy cohort permitted minimization of immunosuppression in all patients in whom it was attempted. Immune monitoring revealed evidence for Treg survival together with changes associated with immune regulation and a significant reduction of inflammatory cell populations. The combination of observed feasibility, safety, and ability to minimize immunosuppression with Treg therapy is very promising and requires further exploration in a randomized controlled trial.
Acknowledgments
This work was supported by the ONE Study which was funded by the 7th EU Framework Programme (award 260687). We thank the ONE Study consortium partners for their support, including the international clinical teams and Beckman Coulter Diagnostics for support of the immune monitoring and Koehler eClinical for eCRF support. A special thanks to Ben James of the central clinical trials team in Regensburg, Germany. We would like to thank the GMP team at Guys Hospital, London composed of Andrew Hope, Christopher Fisher, Henrieta Fraser, Sarah Thirkell, Katie Lowe, and Gilliam Lewis for all their work in generating all the cell products used in this clinical trial. In addition for statistical advice from Saskia Eddy and Dr Sama Ayis at the School of Population Health and Environmental Sciences, Kings College, London. We are grateful to David Ahern of NDORMS in Oxford for the CyTOF analysis. This research was partially funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The Welcome Trust as a senior fellowship grant funded the immune monitoring and analysis component of the trial.
Funding information
7th EU Framework Programme, Grant/ Award Number: 260687; National Institute for Health Research (NIHR); Guy's and St Thomas’ NHS Foundation Trust; King's College London; NIHR Clinical Research Facility; The Welcome Trust
Abbreviations
- APKD
adult polycystic kidney disease
- CMV
cytomegalovirus
- ECG
electrocardiogram
- GMP
good manufacturing practice
- HD
hemodialysis
- HLA
human leucocyte antigen
- IL
interleukin
- IQR
interquartile range
- MMF
mycophenolate Mofetil
- MZB
marginal zone B cells
- PD
peritoneal dialysis
- PRA
panel reactive antibody
- RRT
renal replacement therapy
- Treg
regulatory T cell
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Professor Giovanna Lombardi is a founder of Quell Therapeutics Ltd. Professor Robert Lechler is a non-executive director of Quell Therapeutics Ltd. The other authors have no conflict of interest to disclose.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, (PNH), upon reasonable request.
References
- 1.Safinia N, Grageda N, Scotta C, et al. Cell therapy in organ transplantation: our experience on the clinical translation of regulatory T cells. Front Immunol. 2018;9:354. doi: 10.3389/fimmu.2018.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew JM, Voss JH, LeFever A, et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep. 2018;8(1):7428. doi: 10.1038/s41598-018-25574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Fueyo A, Whitehouse G, Grageda N, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20(4):1125–1136. doi: 10.1111/ajt.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandran S, Tang Q, Sarwal M, et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant. 2017;17(11):2945–2954. doi: 10.1111/ajt.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao NN, Coates PT. Cardiovascular disease after kidney transplant. Semin Nephrol. 2018;38(3):291–297. doi: 10.1016/j.semnephrol.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Au E, Wong G, Chapman JR. Cancer in kidney transplant recipients. Nat Rev Nephrol. 2018;14(8):508–520. doi: 10.1038/s41581-018-0022-6. [DOI] [PubMed] [Google Scholar]
- 7.Sayegh MH, Remuzzi G. Clinical update: immunosuppression minimisation. Lancet. 2007;369(9574):1676–1678. doi: 10.1016/S0140-6736(07)60762-4. [DOI] [PubMed] [Google Scholar]
- 8.Karpe KM, Talaulikar GS, Walters GD. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst Rev. 2017;7:CD006750. doi: 10.1002/14651858.CD006750.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50(2):302–316. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, Wood K. Ex vivo-ex-panded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90(12):1321–1327. doi: 10.1097/TP.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadig SN, Wieckiewicz J, Wu DC, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16(7):809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miragaia RJ, Gomes T, Chomka A, et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity. 2019;50(2):493–504.:e497. doi: 10.1016/j.immuni.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser H, Safinia N, Grageda N, et al. A rapamycin-based GMP-compatible process for the isolation and expansion of regulatory T cells for clinical trials. Mol Ther Methods Clin Dev. 2018;8:198–209. doi: 10.1016/j.omtm.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawitzki B, Harden P, Reinke P, et al. The ONE study: evaluation of regulatory cell therapy in kidney transplantation using a harmonized trial design. Lancet. 2020;395(10237):1627–1639. doi: 10.1016/S0140-6736(20)30167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streitz M, Miloud T, Kapinsky M, et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplant Res. 2013;2(1):17. doi: 10.1186/2047-1440-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekberg H, Bernasconi C, Tedesco-Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9(8):1876–1885. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouvy AP, Klepper M, Kho MM, et al. The impact of induction therapy on the homeostasis and function of regulatory T cells in kidney transplant patients. Nephrol Dial Transplant. 2014;29(8):1587–1597. doi: 10.1093/ndt/gfu079. [DOI] [PubMed] [Google Scholar]
- 18.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204(4):735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wortel CM, Heidt S. Regulatory B cells: phenotype, function and role in transplantation. Transpl Immunol. 2017;41:1–9. doi: 10.1016/j.trim.2017.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, (PNH), upon reasonable request.