Summary
Background
Use of cell-based medicinal products (CBMPs) represents a state-of-the-art approach for reducing general immunosuppression in organ transplantation. We tested multiple regulatory CBMPs in kidney transplant trials to establish the safety of regulatory CBMPs when combined with reduced immunosuppressive treatment.
Methods
The ONE Study consisted of seven investigator-led, single-arm trials done internationally at eight hospitals in France, Germany, Italy, the UK, and the USA (60 week follow-up). Included patients were living-donor kidney transplant recipients aged 18 years and older. The reference group trial (RGT) was a standard-of-care group given basiliximab, tapered steroids, mycophenolate mofetil, and tacrolimus. Six non-randomised phase 1/2A cell therapy group (CTG) trials were pooled and analysed, in which patients received one of six CBMPs containing regulatory T cells, dendritic cells, or macrophages; patient selection and immunosuppression mirrored the RGT, except basiliximab induction was substituted with CBMPs and mycophenolate mofetil tapering was allowed. None of the trials were randomised and none of the individuals involved were masked. The primary endpoint was biopsyconfirmed acute rejection (BCAR) within 60 weeks after transplantation; adverse event coding was centralised. The RTG and CTG trials are registered with ClinicalTrials.gov, NCT01656135, NCT02252055, NCT02085629, NCT02244801, NCT02371434, NCT02129881, and NCT02091232.
Findings
The seven trials took place between Dec 11, 2012, and Nov 14, 2018. Of 782 patients assessed for eligibility, 130 (17%) patients were enrolled and 104 were treated and included in the analysis. The 66 patients who were treated in the RGT were 73% male and had a median age of 47 years. The 38 patients who were treated across six CTG trials were 71% male and had a median age of 45 years. Standard-of-care immunosuppression in the recipients in the RGT resulted in a 12% BCAR rate (expected range 3·2-18·0). The overall BCAR rate for the six parallel CTG trials was 16%. 15 (40%) patients given CBMPs were successfully weaned from mycophenolate mofetil and maintained on tacrolimus monotherapy. Combined adverse event data and BCAR episodes from all six CTG trials revealed no safety concerns when compared with the RGT. Fewer episodes of infections were registered in CTG trials versus the RGT.
Interpretation
Regulatory cell therapy is achievable and safe in living-donor kidney transplant recipients, and is associated with fewer infectious complications, but similar rejection rates in the first year. Therefore, immune cell therapy is a potentially useful therapeutic approach in recipients of kidney transplant to minimise the burden of general immunosuppression.
Funding
The 7th EU Framework Programme.
Introduction
Combinations of general immunosuppressive drugs have enabled the widespread application of life-saving organ transplantation; however, transplant survival is shortened by chronic rejection and immunosuppression sideeffects, and has plateaued over the past decade.1 Organ rejection can mean that secondary transplantations are needed when there is already an inadequate number of organs available for first-time transplantation, while the morbidity and economic costs associated with life-long general immunosuppression accrue. To address this problem, the organ transplantation community urgently needs new strategies to decrease our dependency on immunosuppressive drugs to prevent allograft rejection.2 Indeed, international networks have been established with this explicit purpose, notably including a series of EU-funded programmes and, in North America, the Immune Tolerance Network. Research from these expert networks, and from numerous research laboratories worldwide, consistently call for novel therapies that will reduce our reliance on full immunosuppression to prevent organ rejection. At least two general strategies have been considered, including a deletional approach based on establishment of donor bone-marrow chimerism to reduce donor-reactive immune cells, and an immune regulation-based approach that takes advantage of regulatory cells or pathways that control immunity and restrain immune responses to autologous antigens.3 Although protocols to create chimerism in recipients of organ transplants have been trialled for more than a decade, finding conditioning regimens with acceptable toxicity and avoiding the problem of graft-versus-host disease has been a persistent obstacle. Regarding the second strategy of building immune regulation, a therapeutic means to augment these cellular networks has only recently come of age for clinical testing.3
Regulatory cell therapy has emerged as one attractive therapeutic approach to establish immune regulation aimed at protecting organ allografts.4–6 The overall principle of this approach is to expand specific regulatory immune cell populations ex vivo in the form of cell-based medicinal products (CBMPs), which can then be infused into transplant recipients. Toward this aim, an EU-funded consortium called The ONE Study was initiated to develop a range of CBMPs and to test the cell products in early-phase clinical trials. The six CBMPs developed and tested in six parallel cell therapy group (CTG) trials in The ONE Study included two polyclonal T regulatory (pTreg-1 and pTreg-2), two donor-antigen reactive Treg (darTreg-CSB and darTreg-sBC), one tolerogenic dendritic cell (autologous tolerogenic dendritic cell [ATDC]), and one regulatory macrophage (Mreg) cell product. Central to the concept of the study was that all CBMPs be tested with the equivalent patient population of recipients of living-donor kidney transplants, who received identical background immunosuppressive treatment, placing testing of the six CBMPs on a directly comparable basis. Also fundamental to this study was that a larger reference group trial (RGT) be done with an equivalent patient population using standard-of-care immunosuppression. Although the RGT is not strictly a true control group because of inclusion of basiliximab in place of cell therapy, it serves two purposes. First, since we have applied our CBMPs under similar, but reduced, immunosuppression, the RGT provides a recognised standard-of-care benchmark to assess whether currently expected outcomes are generally attainable with regulatory cell therapy and less immunosuppression. Second, with a standard-of-care RGT, performance of centralised immune monitoring allows for reliable detection of potential immunological changes caused by cell therapy. Here, we present the novel study design, clinical data, safety results, and immune monitoring data for The ONE Study RGT and combined CTG trials, which is intended as a foundation for further regulatory cell therapy trials in organ transplantation.
We aimed to explore the safety and immunological effects of regulatory cell-based therapy as an adjunct immunosuppressive treatment in recipients of a livingdonor kidney transplant through a series of clinical trials sharing the same general design.
Methods
Study design and participants
We did seven single-arm trials and a harmonised analysis to compare the CTG trials with standard-of-care treatment. The first trial was a single-arm multicentre RGT done at all clinical sites that were also planning to do an individual cell therapy trial. The RGT formed the basis for the other six individual trials testing CBMPs (the CTG trials). Enrolment for the RGT was completed before any of the CTG trials commenced. The RGT was initiated while regulatory approvals for the CTG trials and cell manufacturing procedures were being obtained.
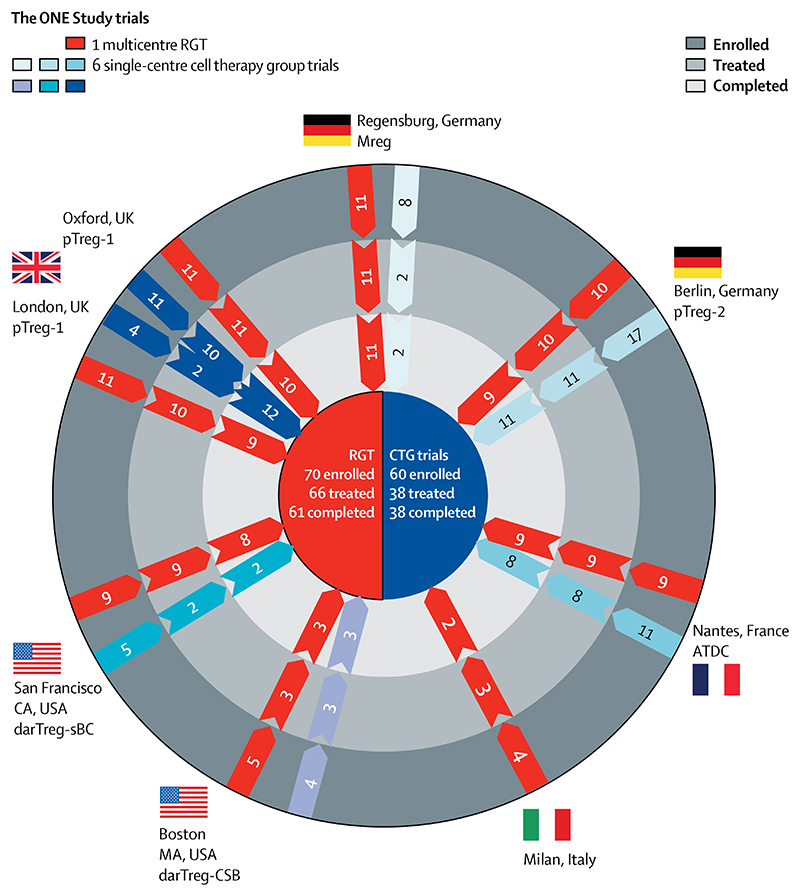
The multicentre RGT was done at eight international hospitals, including the University Hospital Regensburg (Regensburg, Germany), Charité (Berlin, Germany), Centre Hospitalier Universitaire Nantes (Nantes, France), Ospedale San Raffaele (Milan, Italy), Oxford University Hospitals NHS Foundation Trust (Oxford, UK), Guy’s Hospital (London, UK), Massachusetts General Hospital (Boston, MA, USA) and University of California, San Francisco Medical Center (San Francisco, CA, USA; figure 1). After completing enrolment for the RGT, seven centres did a separate CTG trial with one of the six regulatory cell products: pTreg-1 (Oxford and London), pTreg-2 (Berlin), darTreg-CSB (Boston, MA), darTreg-sBC (San Francisco, CA), ATDCs (Nantes), or Mreg (Regensburg). Unlike the five centres that recruited patients into their respective single-centre CTG trials, the Oxford and London sites joined forces to recruit patients into one CTG trial (pTreg-1). Notably, the Milan site participated only in the RGT, because their cell product was not approved for clinical trial testing during The ONE Study. None of the trials were randomised and none of the individuals involved in the study were masked.
Figure 1. Trial profile.
RGT=reference group trial. CTG=cell therapy group. pTreg=polyclonal T regulatory cells. ATDCs=autologous tolerogenic dendritic cells. darTreg=donor-antigen reactive Treg. Mreg=regulatory macrophages.
Recipients of living-donor kidney transplants were selected for inclusion into all seven trials. Living donors were chosen for these trials to allow for maximal planning logistics regarding obtaining informed consent, having a medically stable recipient population, coordinating regulatory cell manufacturing from donor or recipient cells (in the CTG trials), and obtaining pretransplant immune monitoring samples. The core inclusion and exclusion criteria that were common to all trials for both the donors and recipients are listed in the appendix (p 1). The main exclusion criteria were patients transplanted previously, recipients with panel-reactive antibody more than 40%, and HLA identical donorrecipient mismatches (0-0-0 mismatches); all patients were aged 18 years or older. Ethical approval was given for all trials by the local ethics committee or institutional review board, and written informed consent was obtained from all enrolled trial participants.
Procedures
In the course of The ONE Study project, six regulatory cell products were approved for manufacture and therapeutic testing in the CTG trials by the national competent authority in each participating country. Two of the six cell products consisted of pTreg cells approved in the UK (pTreg-1)7 and Berlin (pTreg-2).8 The third and fourth cell products consisted of Treg, but were generated in the presence of donor antigen during manufacturing; one product was exposed under conditions of costimulatory blockade in the presence of donor peripheral blood mononuclear cells in Boston (MA, USA)9 referred to as costimulatory blockade darTreg-CSB, and the other product was developed in San Francisco (CA, USA), where Tregs sorted from peripheral blood mononuclear cells were stimulated with donor B cells that had been activated with K562 cells expressing human CD40L (referred to as donor alloantigen-reactive darTreg-sBC).10 The fifth and sixth cell products were derived from peripheral blood monocytes, in which monocytes were stimulated in Nantes, France with granulocyte macrophage colonystimulating factor to produce ATDCs,11 or in Regensburg with macrophage colony-stimulating factor and IFNγ to produce regulatory macrophages (Mreg-UKR).12 All six regulatory cell products were derived from recipient leucocytes (blood or leucopheresates), with the exception that Mreg-UKR were donor-derived. An overview of the overall characteristics of the CBMPs, including a reference to cell production methods is provided in the appendix (p 2).
The ONE Study group of clinicians developed the RGT immunosuppression design based on their own local standard-of-care protocols, which included some features of the ELITE-Symphony study,13 for the selected non-high risk kidney transplant patient population. The study protocol (NCT01656135) consisted of: basiliximab administration 2 h or less before transplant surgery and on day 4 after surgery (20 mg intravenously); prednisolone starting on day 0 (day of kidney transplant) and gradually tapered away by week 15; mycophenolate mofetil at 2 g per day from day −1 to day +14 and 1·5 g per day thereafter; and tacrolimus starting on day −4 at 3–12 ng/mL and gradually reduced over 9 months to 3–6 ng/mL. A diagram showing the exact dosing scheme is shown in the appendix (p 9). Patient follow-up was continued for 60 weeks. The target recruitment number for the RGT was 60 patients.
The clinical protocol for the six CTG trials closely followed that of the RGT (appendix p 9). All cell products were delivered once intravenously between day −7 and day +10 relative to the day of kidney transplant; within this timeframe, monocyte-derived cell products were administered before kidney transplant and T-cell derived products were given after kidney transplant. The exact cell numbers infused will be provided in the individual CTG trial descriptions to be reported elsewhere, but ranged from 0·5 to 10 × 106 cells per kg bodyweight for all cell products except darTreg-CSB, in which a range between 2×103 to 2×106 cells per kg bodyweight was targeted. Pharmacological immunosuppression and dosing were the same as with the RGT, except that basiliximab induction therapy was omitted, and at 9 months post-kidney transplant an option was included to completely taper away mycophenolate mofetil by 1 year post-kidney transplant; with mycophenolate mofetil cessation, tacrolimus was continued as a monotherapy. Tapering of mycophenolate mofetil was not allowed if a biopsy at 9 months post-transplant showed signs of subclinical rejection or there was evidence of declining renal function. Patient follow-up continued for approximately 60 weeks, after which time immunosuppressive treatment was decided by the local transplant physician. The number of patients given cell therapy did not exceed 12 in any individual CTG trial.
We used a mixed model of locally and centrally performed assays to compare pre-transplant and posttransplant immune status of patients in the RGT and CTG trials.14 The following analyses were done as detailed in the appendix (p 13): immune cell composition by whole blood flow cytometry, Treg-specific demethylated region demethylation and gene expression (appendix p 13), and anti-donor as well as anti-cytomegalovirus IFNγ EliSpot. To reveal differences in peripheral blood immune cell composition between patients with endstage renal disease (RGT and CTG before transplantation) and healthy individuals, we did comparative analyses with age-matched and sex-matched healthy controls from our published cohort dataset.15
Outcomes
Biopsy-confirmed acute rejection (BCAR) was the primary endpoint. Histopathological grading of kidney transplant biopsies was done by a central pathologist (ISDR, Oxford University) for all trials within The ONE Study, with the standard assessment done according to the Banff criteria.16 Notably, a case of borderline histological change in a for-cause biopsy with clinical evidence of acute rejection was considered as BCAR. However, histological changes consistent with acute rejection that were not accompanied by clinical evidence of rejection were not recorded as BCAR, but were logged as a secondary endpoint. Estimated glomerular filtration rate (eGFR using the Modification of Diet in Renal Disease method) was recorded as a secondary endpoint.
Statistical analysis
For the RGT, we estimated a BCAR rate of approximately 10% after 60 weeks under standard immunosuppressive therapy in our study population. With this assumption, a two-sided 95% CI for a single proportion of 0·106 predicts a rejection rate ranging from 3·2-18·0% with a sample size of 66 patients; a BCAR rate falling outside this interval would suggest that the rejection rate is atypical.
Clinical data from all trials were entered into a web-based data capture platform consisting of electronic case report forms custom-made for The ONE Study (Koehler eClinical, Freiburg, Germany). A core set of clinical data were collected from all trials to ensure that these parameters could be directly compared. Selected data items for evaluation of the study endpoints were verified for accuracy against source documents during on-site monitoring visits. Additionally, data were reviewed, queried, and cleaned remotely by a central team of data managers using both automatic and manual data validation checks. All adverse events and serious adverse events were coded centrally using version 20.1 of the Medical Dictionary for Regulatory Activities and quality-controlled to ensure consistency of coding across all trials and study sites. To compare safety events reported from cohorts of different sizes, serious adverse event and adverse event frequencies were normalised using a cohort-specific patient study-years denominator. Patient study-years are the cumulative amount of time spent by trial participants in study follow-up and were calculated and applied for the RGT and CTGs separately. A safety advisory board received serious adverse event reports for all CTG trials as they occurred and reviewed all safety data twice per year. To be sure of open communication within the trial series, safety alerts or conclusions from the safety advisory board were shared with all centres doing CTG trials.
A statistical analysis plan defined the conventions and analyses, and emphasised the exploratory nature of the study; accordingly the proposed statistical examination of clinical data was descriptive. The reported comparative analyses of changes in immune cell composition and functionality between patients in the RGT and CTG were done as post-hoc analyses.
For clinical data, results for baseline characteristics, safety, and transplant function or rejection endpoints were summarised descriptively. No formal testing was done. In addition to crude rejection rates, time to first BCAR was analysed using Kaplan-Meier methods. The primary BCAR endpoint is reported descriptively for the intention-to-treat population (66 for RGT, 38 for CTG); the time-to-event Kaplan-Meier BCAR analysis is presented for both the intention-to-treat (66 for RGT, 38 for CTG) and per-protocol (47 for RGT, 32 for CTG) populations. All other variables (donor-specific antibody [DSA], eGFR, tacrolimus levels) are summarised for the number of patients who were tested at the relevant study timepoints. Incidence of adverse events normalised per 100 patient study-years was calculated and based on the intention-to-treat population.
Differences in immune monitoring results between patients in the RGT before transplantation and healthy controls were analysed applying Kruskal Wallis tests followed by Dunn-Bonferroni tests. Changes between pre-transplant and post-transplant timepoints of the same patient were analysed applying Wilcoxon matched-pairs signed rank test. To reveal differences in immune cell composition or Treg-specific demethylated region changes after transplantation between patients in the RGT and CTG trials, we used a Kruskal Wallis and a post-hoc Dunn’s multiple comparison test. p values less than 0·05 were considered significant. The RTG and CTG trials are registered with ClinicalTrials.gov, NCT01656135, NCT02252055, NCT02085629, NCT02244801, NCT02371434, NCT02129881, and NCT02091232.
Role of the funding source
The funders of the study had no role in data collection, data analysis, data interpretation, or writing of the report. EKG, as The ONE Study Consortium FP7 project coordinator, had full access to all the data in the study; BS and BJ had full access to all the data in the study. As a group, members of this FP7 consortium discussed the publication plans, and therefore were involved in the decision to submit the manuscript; EKG and BS had final responsibility for the decision to submit for publication.
Results
Of 782 patients assessed for eligibility, 130 (17%) patients were enrolled and 104 were treated and included in the analysis. Recruitment to the RGT began on Dec 11, 2012, with the last patient’s last visit on Dec 29, 2015. Figure 1 shows that 70 patients were enrolled in the RGT, with 66 (94%) receiving a kidney transplant. Of the four prekidney transplant withdrawals, two patients had their transplant postponed, one patient needed treatment for DSA that did not allow further inclusion into the study protocol, and one patient withdrew consent. 61 patients in the RGT completed the study: of the five who were noncompleters, one patient withdrew consent (at 8 days), one patient was lost to follow-up (at 33 weeks), one patient had a major vascular complication and graft loss (at 8 days), one patient received anti-thymocyte globulins instead of basiliximab induction therapy (discovered on day 11), and one patient violated the eligibility criteria (noted at 24 weeks). None of these five patients registered a primary endpoint. In the RGT, median follow-up time was 60·1 weeks (IQR 1·3). Figure 1 summarises patient recruitment into the six individual CTG trials (non-red arrow bars), in which 60 patients were recruited into the various trials, with the first patient’s first visit done on May 13, 2014, and the last patient’s last visit done on Nov 14, 2018. Of the 60 enrolled patients, 38 received a kidney transplant and the designated cell therapy. All of these patients completed the 60-week follow-up planned in the study. The causes for withdrawal of 22 patients were: cell manufacturing failures (n=14), early development of acute rejection before the planned cell infusion (n=5), discovery of ineligibility criteria after enrolment (n=2), or requirement for a second abdominal surgery shortly after kidney transplant (n=1). Cell manufacturing failures were because of not meeting release criteria (n=9), cancellation (n=2), microbiology testing positive (n=2), and leucapheresis side-effects (n=1). No trial was stopped due to lack of manufacturing feasibility. In the CTG, median follow-up time was 60·0 weeks (IQR 0·6). A summary of the recipient and donor demographic data for the RGT and CTG trials is provided in the appendix (pp 3–4). Data for recipient and donor age, sex, ethnicity, renal replacement therapy, relationship of donor and recipient, and underlying diagnosis show that the RGT and combined CTG trials were well balanced when compared with each other. Median age of recipients in the RGT was 47 years, compared with 45 years in the CTG trials; median donor age was 53 years in the RGT, versus 51 years in the CTG trials. Notably, 73% of recipients in the RGT were male, with a similar overrepresentation of male recipients (71%) in the CTG trials. Because sex-related effects are known in transplantation, the greater number of male recipients should be taken into consideration when interpreting the results.
A set of per-protocol criteria were defined based mostly on overall adherence to the planned immunosuppression regime in both the RGT and CTG trials (appendix p 5). In the RGT, 47 (71%) of 66 kidney transplant patients received treatment that closely followed the clinical protocol, whereas 32 (84%) of the 38 patients in the CTG trials were treated with close adherence to the protocol. Reasons for non-adherence varied widely among the trials, but were mostly related to adjustments or switching of immunosuppression that the treating physician deemed necessary. Furthermore, ONE Study physicians doing the CTG trials tapered immunosuppression to tacrolimus monotherapy (optional) in 17 (45%) of 38 patients. The immunosuppression was successfully tapered in all but two cases, in which triple therapy was later reinstated due to a BCAR and detection of recurrent IgA nephropathy.
BCAR rate in the RGT was 12% (eight of 66), which is within the expected range of 3·2–18·0%. BCAR occurred in six (16%) of 38 of the patients receiving cell therapy within the combined CTG trials, which was within the expected range calculated for the RGT. The Kaplan-Meier curves in figure 2A highlight the early incidence of BCARs in all trials. The severity of the first BCAR by Banff scoring was distributed similarly between the RGT and the group of CTG trials (appendix p 6); one patient in the RGT had a second BCAR episode, but other BCARs in all trials were single episodes and were successfully treated. Only one of eight first BCAR episodes in the RGT occurred more than 2 weeks after kidney transplant; similarly, four of six episodes of BCAR in the CTG trials occurred within 3 weeks after kidney transplant. Specific BCAR data from individual sites will be published separately for each CTG trial. We also did a Kaplan-Meier analysis for the per-protocol patients in the RGT and CTG trials (figure 2B), which shows that the rate and timing of the BCAR episodes were essentially the same.
Figure 2. Primary endpoint (BCAR) data.
(A) Kaplan-Meier estimates of the cumulative BCAR-free survival probability in the RGT (n=66) and CTG (n=38) intention-to-treat analysis sets (88% vs 84% at 60 weeks). (B) Kaplan-Meier estimates of the cumulative BCAR-free survival probability in the RGT (n=47) and CTG (n=32) per-protocol analysis sets (83% vs 81% at 60 weeks). Censored patients are indicated with ticks. RGT=reference group trial. CTG=cell therapy group. BCAR=biopsy-confirmed acute rejection.
A set of tests was done at study end (60 weeks) to further assess outcomes in the trials, including DSA detection, eGFR, and tacrolimus blood concentrations. At study end, DSA testing revealed that seven (14%) of 51 of the RGT recipients who were tested had a DSA, with five (15%) of 33 of those tested showing DSA in the combined CTG trials. Of the patients in the CTG trials tapered to monotherapy, two (13%) of the 15 tested had a new DSA. Regarding kidney function (appendix p 10), eGFR measurements in the RGT and CTG trials showed a similar increase over the study period (20% in the RGT and 21% in the CTG) when comparing median eGFR at 60 weeks post-kidney transplant to median eGFR at 1 week post-kidney transplant. As a reflection of immunosuppressive load at study end, tacrolimus trough concentrations were similar in the RGT and combined CTG trials, at a mean of 6·1 ng/mL (SD 2·1; n=44 tested) in the RGT and a mean of 6·6 ng/mL (1·6; n=32 tested) in the combined CTG trials. Furthermore, immunosuppressive burden with tacrolimus (trough concentration) and mycophenolate mofetil (dose) was similar or even tended to be lower throughout the study period in the patients in the CTG trials versus the RGT (appendix p 11). Together, these data should be considered with the understanding that 15 (40%) of 38 patients in the CTG trials were on tacrolimus monotherapy at study end, whereas 60 (98%) of 61 patients in the RGT continued on at least dual immunosuppression.
The normalised incidence in the RGT (n=66) for treatment-emergent serious adverse events was 91·2 per 100 patient study-years and for adverse events was 1614·6 per 100 patient study-years. The normalised incidence for the CTG trials (n=38) for serious adverse events was 70·7 per 100 patient study-years and for adverse events was 1452·0 per 100 patient study-years. These results indicated no increase in adverse events with cell therapy (appendix p 7). In the CTG trials, there was special attention given to identifying serious adverse events and adverse events related to cell therapy infusion. Overall, there were 12 adverse events reported with a possible relationship to the cell infusion, only one of which was a serious adverse event (increased creatinine; appendix p 8). All potentially related adverse events only occurred once, so no specific pattern was exposed in the 38 patients given CBMPs. No deaths were reported in any of the trials.
A descriptive analysis of normalised data comparing Medical Dictionary for Regulatory Activities-coded serious adverse events in the RGT versus the combined data from the CTG trials revealed that most serious medical problems were similar in frequency (figure 3A). However, there was one substantial difference that emerged, which is worth considering in detail. The incidence of serious adverse events in the RGT related to infections and infestations was nearly six-times higher than the combined CTG trials. After examining all infection-related adverse events recorded in the trials, this pattern of decreased infections in the CTG trials was consistently observed across each of the CTG trials (figure 3B) and was evident during the entire post-kidney transplant observation period (figure 3C). Also, we found that the main difference was a reduced number of viral infections in the CTG trials (figure 3D). Notably, there was also an appreciable difference in the number of infections recorded without specifying the pathogen, but numbers of bacterial and fungal infections were essentially the same. Breaking the data down even further regarding adverse events, the main decreases in viral infections in the CTG trials were for cytomegalovirus, herpes (including herpes simplex, herpes-zoster, oral herpes, nasal herpes, and varicellazoster), and polyoma virus (figure 3E). The decreased rate of viral infection in the CTG was not due to more preventive measures, since 43 (65%) of 66 patients in the RGT and 20 (53%) of 38 patients in the CTG trials received antiviral prophylaxis in the first 3 months after kidney transplant. Also, notably, the percentage of cytomegalovirus-positive to cytomegalovirus-negative donor to recipient transplants was 18% (12 of 66) in the RGT and 21% (eight of 38) in the CTG trials. Therefore, patients in the CTG trials in general developed fewer viral infections than patients in the RGT.
Figure 3. Normalised safety data.
(A) Incidence of treatment-emergent serious adverse events by MedDRA primary system organ class. (B) Incidence of treatment-emergent infections (all adverse events) by study site. (C) Incidence proportion of treatment-emergent infections (all adverse events) over time. (D) Incidence of treatment-emergent infections (all adverse events) by MedDRA high-level group term. (E) Incidence of treatment-emergent viral infections (all adverse events) by MedDRA high-level term. All adverse events coded using MedDRA version 20.1. Treatment-emergent events are events with onset date equal to or after first dose of any study drug. All events coded to the MedDRA preferred term transplant rejection were excluded as rejection was measured as the primary efficacy endpoint. RGT=reference group trial. CTG=cell therapy group. MedDRA=Medical Dictionary for Regulatory Activities.
Identical standardised immune monitoring testing of peripheral blood cells was done in all patients in the seven trials. In general, principal component analyses showed that before kidney transplant, patients in the RGT had major alterations in absolute and relative blood immune cell population composition compared with age-matched and sex-matched healthy controls (figure 4A). Populations contributing most to those alterations were granulocytes, CD16+ myeloid dendritic cells, and CD14highCD16+ intermediate monocytes, which were increased in RGT patient samples, but also plasmacytoid dendritic cells, marginal zone-like B cells, and CD8+CD28+ T cells, which were higher in samples of healthy controls (figure 4B). Postkidney transplant longitudinal analysis revealed only moderate normalisation of CD16-expressing monocytes and no normalisation of marginal zone-like B cells (figure 4C). Furthermore, although composition of conventional CD4+ T-cell subsets remained normal and similar to healthy controls, CD8+ T-cell subset composition showed major alterations over the post-kidney transplant course. Although naive T cells increased early after transplantation, we observed a skewing towards terminal differentiation of CD8+ T cells in the long-term, starting at 24 weeks post-kidney transplant (figure 4C).
Figure 4. Leucocyte subset alterations in patients with end-stage renal disease and time-dependent changes after kidney transplantation.
(A) PCA revealing the differences in leucocyte subsets between whole blood samples from end-stage renal disease (n=70) and healthy controls (n=98). (B) Box-and-whiskers plots of absolute numbers from leucocyte subpopulations with highest influence at the PCA shown in A. (C) Time-dependent changes from visit 1 before transplantation (V01) to visit 10 at 60 weeks post-transplant (V10) of monocyte, B cell, CD4+, and CD8+ T cell subset composition (stacked bars of mean proportions) in whole blood samples of patients in the RGT (n=59). Statistical analysis by Kruskal-Wallis-Test. PCA=principal component analysis. PC=principal component. mDCs=myeloid dendritic cells.
Examining immunophenotyping results from the RGT and combined CTG trials, we did not observe significant differences in numbers or proportions of CD4+CD25highCD127low Tregs between the groups at 60 weeks post-kidney transplant (figure 5A). A significant reduction in Treg-specific demethylated region demethylation occurred in patients in the RGT, but not in patients in the CTG trials. Furthermore, only patients in the RGT showed a significant increase in CD8+ TEMRA cells and CD8+CD57+ chronically activated T cells (figure 5B), whereas in samples from patients in the CTG trials, we observed more CD8+CD28+ T cells. Both patient groups showed a reduction of donorspecific IFNγ producing memory T cells after kidney transplant (appendix p 12). However, patients in the RGT, in contrast to patients in the CTG trials, showed higher anti-cytomegalovirus T-cell responses (appendix p 12), which correlated with absolute CD8+ TEMRA numbers (appendix p 12). This increase is well known in patients with a kidney transplant and is probably related to inflammation triggered reactivation of cytomegalovirus, which we also only observed in patients in the RGT but not the CTG trials (figure 3E). Although both patient groups had more plasmacytoid dendritic cells 60 weeks post-kidney transplant, we only observed a normalisation of marginal zone-like B-cell numbers and a significant reduction of CD14highCD16+ monocytes in patients in the CTG trials (figure 5C). In addition, patients in the CTG trials showed increased mRNA expression of genes described to be high in immunosuppression-free operationally tolerant patients with a kidney transplant (eg, Ms4A1) and co-inhibitory molecules (CD200), but reduced expression of rejection-associated genes (HMMR, appendix p 12). Together, these data suggest that our CTG trial patients show a healthy control-like restoration of immune cell composition.
Figure 5. Differences in post-transplant changes between patients in the RGT and CTG trials.
(A) Differences in post-transplant changes in regulatory T cells. Box and whisker plots of absolute numbers and proportions of CD4+CD25highCD127low Tregs as well as the percentage of CD4+ T cells with demethylated Treg-specific demethylated region in whole blood samples collected pre-transplant (V01) and at the end of the observation period (60 weeks post-transplant, V10) from patients in the RGT (n=59) and CTG trials (n=38). (B) Differences in post-transplant changes in CD8+ T cell subpopulations. Box and whisker plots of absolute numbers of CD8+CD28+, CD8+CD45RA+CCR7-TEMRA and CD8’CD57’ chronically activated cells in whole blood samples collected pre-transplant (V01) and at the end of the observation period (60 weeks post-transplant, V10) from patients in the RGT (n=59) and CTG trials (n=38). (C) Differences in post-transplant changes in marginal zone-like B cells and dendritic cell subpopulations. Box and whisker plots of absolute numbers and proportions of marginal zone-like B cells, CD16+ myeloid dendritic cells, and plasmacytoid dendritic cells in whole blood samples collected pre-transplant (V01) and at the end of the observation period (60 weeks post-transplant, V10) from patients in the RGT (n=59) and CTG trials (n=38). Statistical analysis by Wilcoxon matched-pairs signed rank and Dunn’s multiple comparison test. RGT=reference group trial. CTG=cell therapy group. *p<0·05. †p<0·01. ‡p<0·001. §p<0·0001.
Discussion
The ONE Study consortium has taken the unique approach of side-by-side trialling of different T cell, dendritic cell, and macrophage regulatory cell products in recipients of kidney transplants of low to medium risk for early graft loss. In this coordinated group of six international early phase clinical trials (the CTG trials), we showed that CBMP application in this patient population is feasible for multiple regulatory cell types, and their categorical application near the time of kidney transplant revealed no apparent safety concerns. Furthermore, 15 (40%) of the 38 patients given CBMPs were successfully weaned to tacrolimus monotherapy during the 60-week observation period. The reference trial (the RGT) by the same clinical sites collecting matching clinical information and immune monitoring data provided a standard-of-care benchmark to confidently assess essential safety and immunological parameters, and also to evaluate whether reduction of immunosuppression through CBMP application could have potential benefits to patients. Remarkably, the rate of infections was considerably lower in patients given regulatory cell products than in standard-of-care treatment, particularly for viral infections. Furthermore, centralised immune monitoring of peripheral blood leucocyte populations suggested a return of CBMP-treated (CTG), but not conventionally treated (RGT) recipients towards a state of immune homoeostasis. Therefore, our results have established a fundamental basis for further testing of regulatory CBMP therapy in organ transplantation, and provide initial evidence that reducing general immuno-suppressive burden through cell therapy could potentially decrease serious side-effects in recipients of kidney transplants.
This initial report focuses only on the CTG trials as a combined group, and not on results from the individual CTG trials. While each of the six individual CTG trials followed the same clinical treatment protocol regarding background immunosuppression, thus allowing for a comprehensive analysis of the CTG trials as a whole group, there are important details from each of those trials that deserve in-depth reporting and explanations in additional follow-up publications. Forthcoming details from the individual cases will provide insight into feasibility, safety aspects, and effects of each specific cell therapy product. These results will permit examination of issues such as cell production methods, CBMP characterisation, cell dosing, infusion scheduling, clinical outcomes, immunological features from kidney transplant biopsy specimens, and a comprehensive set of central immune monitoring results. Nonetheless, the current analysis of results from the combined CTG trials provides a uniquely broad evaluation of the safety and outlook perspective for cell therapy in organ transplantation, and shows that cell therapy was feasible in terms of logistics and cell manufacturing in the majority (38 [73%] of 52) of patients ready to receive the therapy.
One of the main motivations for seeking new therapies in organ transplantation is to reduce the need for general immunosuppressive drugs, which have substantial toxicities and incrementally expose recipients to dangers inherent from a suppressed immune system, most commonly infections. A set of guidelines and comprehensive review by Fishman17 highlights the extent of the infection problem, and its direct relationship with immunosuppressive load. Results from the CTG trials indicate that lowering immunosuppression does appear to decrease the risk for viral infections. This finding was also supported by the immune monitoring results, as only patients in the RGT showed a tendency towards increased proportions of cytomegalovirus-specific memory T cells correlating with signs of chronic CD8+ T-cell activation at the end of the observation period, as previously described.18–20 What remains unknown is whether decreased infections were simply due to less immunosuppression in the CTG trials, or were related in some way to the cell therapy action itself; neither possibility can be ruled out yet. It should be noted that immunosuppressive burden was lower in the early stages after kidney transplant (no basiliximab induction), and in some patients, 9 months after kidney transplant (mycophenolate mofetil tapering), but that the infection rates were consistently lower across the spectrum of CTG trials during the entire observation period (figure 3C). Although reduction of mycophenolate mofetil treatment is within the prophylactic guidelines for patients at risk for developing viral infection,17 the gap in reported infections did not show evidence of widening between the RGT and CTG trials after 9 months, leaving this issue unresolved. Nonetheless, our data encourage prospective randomised clinical trials to confirm an infectious disease benefit from regulatory cell therapy protocols.
Our immune monitoring results showed that patients with end-stage renal failure had major alterations in their peripheral immune cell composition compared with age-matched and sex-matched healthy controls, most likely reflecting their increased inflammatory state.21–23 Standard immunosuppressive therapy in patients in the RGT did not reverse these alterations, but rather led to further immune cell imbalance as evidenced by a significant reduction in markers for stable Tregs.24 Importantly, regulatory cell therapy mitigated this Treg reduction and was associated with a healthy control-like restoration of immune cell composition. In particular, marginal zone-like B-cell numbers, also discussed to have anti-inflammatory or regulatory function,25,26 were increased in patients in the CTG trials at the end of the observation period. Thus, although both patients in the RGT and CTG trials had a reduction in donor-specific IFNγ-producing memory T cells, only the patients given cell therapy tended to have a re-establishment of immune cell homoeostasis, which is a major goal in organ transplantation. Importantly, these immune-related differences were independent of potential confounding factors such as donor relationships. Whether this effect is related to cell therapy itself, or is due to reduced immunosuppressive load in the CTG trials, will need to be investigated further in future trials.
To date, few reports have been published on the use of regulatory cell therapy in human organ transplantation, some of which were pilot trials done previously by The ONE Study investigators. Hutchinson and colleagues have tested different preparations of regulatory macrophages in recipients of kidney transplants,27–29 which provided essential lessons for designing the CTG trials. Additionally, polyclonal Tregs have been administered by the UCSF group to three recipients of kidney transplant with biopsy-proven subclinical inflammation 6 months after transplantation, showing that cell therapy is feasible in this circumstance.30 Late administration of expanded polyclonal Tregs has also been reported by the Northwestern group in nine lymphodepleted recipients of kidney transplants.31 In liver transplantation, Todo and colleagues have infused costimulatory blockade conditioned lymphocytes similar to those used by the Massachusetts General Hospital group in The ONE Study, and were able to achieve complete immunosuppression withdrawal in seven of the ten liver transplant recipients who were splenectomised and conditioned with cyclophosphamide.32 Unfortunately, these pilot studies are highly variable in design, and did not incorporate a parallel trial with a similar group of patients not receiving cells to better appraise whether cell therapy is safe or shows indications of discernable effects. Importantly, The ONE Study trials were developed with the fundamental viewpoint that a reference trial, and also comparison to healthy control data, is absolutely necessary to make practical conclusions about regulatory cell therapy testing. Therefore, to advance the cell therapy field in organ transplantation, we aimed to evaluate cell therapy against a recognised standard-of-care (RGT) treatment by infusing different CBMPs near the time of kidney transplant as a replacement for conventional induction treatment (omitting basiliximab induction). Into this design we incorporated an option to wean mycophenolate mofetil starting at 9 months to further offer potential benefit to patients by reducing general immunosuppression, and to stress-test this cell therapy protocol under rigorous clinical monitoring. With this overall study strategy, and by doing the RGT as a multicentre study together with the CTG trials as parallel individual trials at the same sites, we uniquely delivered meaningful and reliable information about regulatory cell therapy to the organ transplantation community. Based on The ONE Study, the UK group has already initiated a randomised trial called the TWO Study with their polyclonal Treg cell product (ISRCTN11038572), and other ONE Study partners (Massachusetts General Hospital: NCT03577431 and UCSF Medical Center: NCT02188719) are doing trials in transplant recipients with cell products used in The ONE Study. Opening the way to these and other more advanced clinical trials was the unifying philosophy of The ONE Study.
Supplementary Material
Research in context.
Evidence before this study
New therapies that limit exposure to general immunosuppression in recipients of kidney transplants are needed to advance the field. With this aim, we united a group of European and American investigators to test the hypothesis that immunoregulation induced through cellular immunotherapy is safe and could benefit recipients of kidney transplants. The types of cell therapies tested in six parallel trials included different T regulatory (Treg) and monocyte-derived (dendritic cell, Mreg) cell products; each trial was done with only one cell product, but in the same living-donor patient population using identical baseline immunosuppression and with the option to minimise immunosuppression to tacrolimus monotherapy. For comparison, a multicentre reference trial was also done in the same patient population by all centres involved, using standard immunosuppression without minimisation. To establish the current evidence base in PubMed, we referred to a recent publication summarising Treg and monocyte-derived cell therapy kidney transplant trials. We also searched Clinicaltrials.gov by combining the terms “Treg”, “dendritic cell”, or “Mreg” with “kidney transplantation”. These sources revealed five clinical trials, with one being of an unknown status, three currently recruiting, and one completed. None of these trials are being run comparatively, nor do they include a multicentre or other comparator group to assess outcomes, changes in immune parameters, or adverse event differences. Therefore, no immune cell therapy trial to date has, to our knowledge, been able to comparatively evaluate outcomes, safety and benefits, and immunological effects of multiple regulatory immune cell therapies in kidney transplantation.
Added value of this study
We showed that immune regulatory cell therapies as a whole are safe and, importantly, we provided the first data that recipients of kidney transplants receiving immune cell therapy have fewer episodes of common viral infections, which often cause clinically significant comorbidities in organ transplantation. We also provide the first evidence that nearly all of the patients on cell therapy, in whom minimisation of immunosuppression was attempted, could be successfully weaned within the first year post-transplantation to monotherapy. Furthermore, immune monitoring data from the cell therapy trials, in comparison to patients on standard immunosuppression, show no loss of Treg-specific demethylated region demethylation as an indicator of stable Tregs, no increase of CD8+ terminally differentiated effector memory cells, and a healthy control-like restoration of immune cell composition (eg, marginal zone-like B cells), providing the first evidence that cell therapy has positive systemic immunological effects. Our study not only provides guidance for clinical trials introducing different immune cell therapies in organ transplantation, it is also relevant for similar immune cell-based therapy trials outside the field of transplantation, including those involving autoimmune diseases.
Implications of all the available evidence
Our study suggests that immune cell therapy is a potentially useful therapeutic approach in recipients of living-donor kidney transplants to minimise the burden of general immunosuppression. Furthermore, our results provide evidence that cell therapy can lead to a restoration of the immune system towards more normal, non-inflammatory levels, thereby decreasing adverse side-effects of conventional immunosuppressive drugs, such as reactivation of harmful persistent infections. Therefore, immune cell therapy in transplantation warrants further study in larger clinical trials.
Acknowledgments
This work was also supported by funds from IHU-CESTI (Investissement d’Avenir ANR-10-IBHU-005, Région Pays de la Loire and Nantes Métropole), the Labex IGO project (ANR-11-LABX-0016-01), and a donation from John Lang and Nancy Merrell. We also thank the Cell and Gene Therapy Manufacturing facility from Centre Hospitalier Universitaire de Nantes for the ATDC production. In addition, we thank apceth Biopharma (Munich, Germany) for Mreg production in the study. We thank the nurses, physicians, and patients who contributed to the study. The research leading to these results has received funding from the EU Seventh Framework Programme (FP7) under grant agreement number 260687.
Footnotes
Contributors
BS, EKG, PNH, PR, AM, JAH, DSG, CAB, JFM, TB, RC, WP, NA, HJS, PJF, RH, MB, BJ, JBvdN, MPH-F, UK, SJK, JG, PJM, LB, LAT, RIL, AB, JAB, GL, KJW, MCC, ASe, BB, GB, S-MK, and H-DV contributed to the study design. PNH, PR, JAH, DSG, CAB, JFM, TB, RC, WP, NA, HJS, PJF, RH, JBvdN, ASe, BB, GB, S-MK, NMO, and RÖ managed patient care. PR, AM, JAH, MB, AB, JAB, GL, KJW, MCC, QT, CS, ECG, LC-R, KC, ME, SK, and AS were involved in cell production. BS, EKG, PNH, PR, AM, JAH, MB, BJ, JBvdN, MPH-F, AB, MCC, H-DV, QT, CS, ECG, LC-R, KC, WJB, JLH, IM, FI, ISDR, MS, RJ, CB, ND, MK, and TM did biomarker development and data collection. BS, EKG, AM, JAH, BJ, MPH-F, AB, S-MK, QT, CS, WJB, JLH, IM, FI, ISDR, MS, RJ, CM, and SS did data analysis. BJ, CM, SS, and KJ were study statisticians. BS, EKG, PNH, PR, UK, SJK, JG, PJM, LB, LAT, RIL, AB, JAB, GL, KJW, MCC, ASc, BB, GB, S-MK, H-DV, ASe, ISDR, MS, RJ, CM, SS, and KJ interpreted data. EKG, BJ, and BS wrote the manuscript, which was reviewed by JAH, SS, and KJ, and all other authors. EKG was The ONE Study EU FP7 project coordinator.
Declaration of interests
BS, PR, AM, JAH, DSG, QT, ECG, MB, WJB, ISDR, MS, RJ, JFM, CB, BJ, LC-R, RC, IM, NMO, MPH-F, CM, SK, LAT, JAB, RJL, HJS, MCC, SS, S-MK, BB, GB, H-DV, GL, KJW, and EKG report grants from the EU (FP7 ONE Study) during the conduct of the study. PR and H-DV report grants from the BMBF, outside the submitted work. JAH reports other support from Trizell, personal fees from Finvector Oy during the conduct of the study. DSG reports non-financial support and other from Sandoz, non-financial support and other from Chiesi, non-financial support and other from Astellas, outside the submitted work. QT has a patent US14/382,537 issued and she is a co-founder of Sonoma. MB is one inventor of a patent for in-vitro generation and expansion of CD4+CD25+ T regulatory cells by rapamycin (# WO 2006/090291A2). The patent was licensed for non-exclusive usage to Miltenyi Biotech to develop a commercial kit for the ex-vivo expansion of Treg cells with rapamycin. ND reports other from Beckman Coulter Life Sciences, during the conduct of the study; other from Beckman Coulter Life Sciences, outside the submitted work. MK reports other from Beckman Coulter Life Sciences, during the conduct of the study; other from Beckman Coulter Life Sciences, outside the submitted work. MPH-F reports other from UCB Pharma, outside the submitted work. LAT reports personal fees from Third Rock Ventures, personal fees from Rheos Medicine, outside the submitted work; LAT is employed by Rubius Therapeutics. JAB has a patent US 7722862 B2 issued, a patent US 20080131445 A1, 9,012,1 issued, and a patent US 20150110761 A1 issued and is a founder and current CEO of Sonoma Biotherapeutics, which works on Tregs as therapeutics. HJS reports grants and personal fees from Novartis Pharma, grants and personal fees from Chiesi, outside the submitted work. TM reports other from Beckman Coulter Life Sciences, during the conduct of the study and outside the submitted work. RH reports personal fees and non-financial support from Chiesi, outside the submitted work. EKG reports grant support from Trizell and speaking fees from Novartis Pharma and Chiesi, outside the submitted work. All other authors declare no competing interests.
For the Immune Tolerance Network see https://www.immunetolerance.org/
For the common controlled access principles see https://www.ukri.org/funding/information-for-award-holders/data-policy/
Contributor Information
Birgit Sawitzki, Institute of Medical Immunology, Charité, Universitätsmedizin Berlin, Berlin, Germany.
Paul N Harden, Oxford Transplantation Centre, Oxford University Hospitals NHS Foundation Trust, University of Oxford, Oxford, UK.
Prof Petra Reinke, BeCAT, BCRT, and Department of Nephrology & Intensive Care, Charité Universitätsmedizin Berlin, and Berlin Institute of Health, Berlin, Germany.
Aurélie Moreau, Centre de Recherche en Transplantation et Immunologie, Nantes Université, Inserm, Nantes, France; Institute of Transplantation Urology Nephrology, Nantes, France.
James A Hutchinson, Department of Surgery, University of Regensburg, University Hospital Regensburg, Regensburg, Germany.
David S Game, Guy's & St Thomas' NHS Foundation Trust, Guy's Hospital, London, UK.
Qizhi Tang, Division of Transplantation, Department of Surgery, University of California, San Francisco, San Francisco, CA, USA.
Prof Eva C Guinan, Department of Radiation Oncology, Dana-Farber Cancer Institute and Harvard Medical School, Boston MA, USA.
Manuela Battaglia, Diabetes Research Institute, Istituto di Ricovero e Cura a Carattere Scientifico, San Raffaele Scientific Institute, Milan, Italy.
William J Burlingham, Division of Transplantation, Department of Surgery, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, USA.
Prof Ian S D Roberts, Department of Cellular Pathology, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
Mathias Streitz, Institute of Medical Immunology, Charité, Universitätsmedizin Berlin, Berlin, Germany; BIH Center for Regenerative Therapies, Charité and Berlin Institute of Health, Charité, Universitätsmedizin Berlin, Berlin, Germany.
Prof Régis Josien, Centre de Recherche en Transplantation et Immunologie, Nantes Université, Inserm, Nantes, France; Institute of Transplantation Urology Nephrology, Nantes, France.
Carsten A Böger, Department of Nephrology, University of Regensburg, University Hospital Regensburg, Regensburg, Germany.
Cristiano Scottà, MRC Centre for Transplantation, Peter Gorer Department of Immunobiology, School of Immunology & Microbial Sciences, King's College London, London, UK.
Prof James F Markmann, Center for Transplantation Sciences, Mass General Hospital, Boston, MA, USA.
Joanna L Hester, Transplantation Research and Immunology Group, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK.
Karsten Juerchott, BIH Center for Regenerative Therapies, Charité and Berlin Institute of Health, Charité, Universitätsmedizin Berlin, Berlin, Germany.
Cecile Braudeau, Centre de Recherche en Transplantation et Immunologie, Nantes Université, Inserm, Nantes, France; Institute of Transplantation Urology Nephrology, Nantes, France; Laboratoire d'Immunologie, Cimna, Centre Hospitalier Universitaire, Nantes, France.
Ben James, Department of Surgery, University of Regensburg, University Hospital Regensburg, Regensburg, Germany; Division of Personalized Tumor Therapy, Fraunhofer Institute for Experimental Medicine and Toxicology, Regensburg, Germany.
Laura Contreras-Ruiz, Department of Experimental Medicine, DFCI, Boston, MA, USA.
Jeroen B van der Net, Oxford Transplantation Centre, Oxford University Hospitals NHS Foundation Trust, University of Oxford, Oxford, UK.
Tobias Bergler, Department of Nephrology, University of Regensburg, University Hospital Regensburg, Regensburg, Germany.
Rossana Caldara, Transplant Medicine, Istituto di Ricovero e Cura a Carattere Scientifico, San Raffaele Scientific Institute, Milan, Italy.
William Petchey, Oxford Transplantation Centre, Oxford University Hospitals NHS Foundation Trust, University of Oxford, Oxford, UK.
Matthias Edinger, Department of Internal Medicine III, University of Regensburg, University Hospital Regensburg, Regensburg, Germany; Regensburg Center for Interventional Immunology, University of Regensburg, Regensburg, Germany.
Nathalie Dupas, Beckman Coulter Life Sciences, Immunotech, Marseille, France.
Michael Kapinsky, Beckman Coulter, Krefeld, Germany.
Ingrid Mutzbauer, Department of Surgery, University of Regensburg, University Hospital Regensburg, Regensburg, Germany; Division of Personalized Tumor Therapy, Fraunhofer Institute for Experimental Medicine and Toxicology, Regensburg, Germany.
Natalie M Otto, BeCAT, BCRT, and Department of Nephrology & Intensive Care, Charité Universitätsmedizin Berlin, and Berlin Institute of Health, Berlin, Germany.
Robert Öllinger, Department of Surgery, Charité Campus Mitte, Campus Virchow Klinikum, Charité Universitätsmedizin, Berlin, Germany.
Maria P Hernandez-Fuentes, MRC Centre for Transplantation, Peter Gorer Department of Immunobiology, School of Immunology & Microbial Sciences, King's College London, London, UK.
Fadi Issa, Transplantation Research and Immunology Group, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK.
Norbert Ahrens, Institute for Clinical Chemistry and Laboratory Medicine, Transfusion Medicine, University of Regensburg, University Hospital Regensburg, Regensburg, Germany.
Christoph Meyenberg, KOEHLER eClinical, Freiburg, Germany.
Sandra Karitzky, Miltenyi Biotec, Bergisch Gladbach, Germany.
Prof Ulrich Kunzendorf, Clinic for Nephrology and Hypertension, Christian Albrechts University, University Clinic Schleswig-Holstein, Kiel, Germany.
Prof Stuart J Knechtle, Department of Surgery, Duke Transplant Center, Duke University Medical Center, Durham, NC, USA.
Prof Josep Grinyó, Kidney Transplant Unit, Nephrology Department, Bellvitge University Hospital, IDIBELL, Barcelona University, Barcelona, Spain.
Prof Peter J Morris, Centre for Evidence in Transplantation, Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK; Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK.
Prof Leslie Brent, St Mary's Hospital Transplant Unit, Paddington, London, UK.
Andrew Bushell, Transplantation Research and Immunology Group, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK.
Prof Laurence A Turka, Center for Transplantation Sciences, Mass General Hospital, Boston, MA, USA.
Jeffrey A Bluestone, UCSF Diabetes Center, University of California, San Francisco, San Francisco, CA, USA.
Prof Robert I Lechler, MRC Centre for Transplantation, Peter Gorer Department of Immunobiology, School of Immunology & Microbial Sciences, King's College London, London, UK.
Prof Hans J Schlitt, Department of Surgery, University of Regensburg, University Hospital Regensburg, Regensburg, Germany.
Prof Maria C Cuturi, Centre de Recherche en Transplantation et Immunologie, Nantes Université, Inserm, Nantes, France; Institute of Transplantation Urology Nephrology, Nantes, France.
Stephan Schlickeiser, Institute of Medical Immunology, Charité, Universitätsmedizin Berlin, Berlin, Germany; BIH Center for Regenerative Therapies, Charité and Berlin Institute of Health, Charité, Universitätsmedizin Berlin, Berlin, Germany.
Prof Peter J Friend, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK.
Tewfik Miloud, Beckman Coulter Life Sciences, Immunotech, Marseille, France.
Prof Alexander Scheffold, Institute for Immunology, Christian Albrechts University, University Clinic Schleswig-Holstein, Kiel, Germany.
Antonio Secchi, Vita-Salute San Raffaele University Milan, Istituto di Ricovero e Cura a Carattere Scientifico, San Raffaele Scientific Institute, Milan, Italy.
Kerry Crisalli, Center for Transplantation Sciences, Mass General Hospital, Boston, MA, USA.
Sang-Mo Kang, Division of Transplantation, Department of Surgery, University of California, San Francisco, San Francisco, CA, USA.
Rachel Hilton, Guy's & St Thomas' NHS Foundation Trust, Guy's Hospital, London, UK.
Prof Bernhard Banas, Department of Nephrology, University of Regensburg, University Hospital Regensburg, Regensburg, Germany.
Prof Gilles Blancho, Centre de Recherche en Transplantation et Immunologie, Nantes Université, Inserm, Nantes, France; Institute of Transplantation Urology Nephrology, Nantes, France.
Prof Hans-Dieter Volk, Institute of Medical Immunology, Charité, Universitätsmedizin Berlin, Berlin, Germany; BIH Center for Regenerative Therapies, Charité and Berlin Institute of Health, Charité, Universitätsmedizin Berlin, Berlin, Germany.
Prof Giovanna Lombardi, MRC Centre for Transplantation, Peter Gorer Department of Immunobiology, School of Immunology & Microbial Sciences, King's College London, London, UK.
Prof Kathryn J Wood, Transplantation Research and Immunology Group, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK.
Prof Edward K Geissler, Department of Surgery, University of Regensburg, University Hospital Regensburg, Regensburg, Germany; Division of Personalized Tumor Therapy, Fraunhofer Institute for Experimental Medicine and Toxicology, Regensburg, Germany; Regensburg Center for Interventional Immunology, University of Regensburg, Regensburg, Germany.
Data sharing
We will follow the common controlled access principles outlined by the Medical Research Council Clinical Trials Unit. According to those principles, we will acknowledge that data with long-term value be preserved, and usable for future research. We do, however, want to ensure that there are legal, ethical, and commercial constraints maintained on the release of research data according to the following code. Research teams are entitled to receive appropriate recognition for their efforts in collecting and analysing data and should be given at least a limited period of sole access to use and publish the data, before key trial data are open for use by other researchers. If such requests are made to access the data, resources need to be available to process the request and prepare the data in a timely manner, if possible. Because of these demands, there must be an important scientific objective behind each request. Especially in the case of our international project, The ONE Study, any request must comply with regulations set by the competent authorities in the relevant countries that govern data security policies.
References
- 1.Wekerle T, Segev D, Lechler R, Oberbauer R. Strategies for long-term preservation of kidney graft function. Lancet. 2017;389:2152–62. doi: 10.1016/S0140-6736(17)31283-7. [DOI] [PubMed] [Google Scholar]
- 2.Bamoulid J, Staeck O, Halleck F, et al. The need for minimization strategies: current problems of immunosuppression. Transpl Int. 2015;28:891–900. doi: 10.1111/tri.12553. [DOI] [PubMed] [Google Scholar]
- 3.Rickert CG, Markmann JF. Current state of organ transplant tolerance. Curr Opin Organ Transplant. 2019;24:441–50. doi: 10.1097/MOT.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 4.Safinia N, Grageda N, Scottà C, et al. Cell therapy in organ transplantation: our experience on the clinical translation of regulatory T cells. Front Immunol. 2018;9:354. doi: 10.3389/fimmu.2018.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marín E, Cuturi MC, Moreau A. Tolerogenic dendritic cells in solid organ transplantation: where do we stand? Front Immunol. 2018;9:274. doi: 10.3389/fimmu.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson JA, Geissler EK. Now or never? The case for cell-based immunosuppression in kidney transplantation. Kidney Int. 2015;87:1116–24. doi: 10.1038/ki.2015.50. [DOI] [PubMed] [Google Scholar]
- 7.Fraser H, Safinia N, Grageda N, et al. A rapamycin-based gmp-compatible process for the isolation and expansion of regulatory T cells for clinical trials. Mol Ther Methods Clin Dev. 2018;8:198–209. doi: 10.1016/j.omtm.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landwehr-Kenzel S, Zobel A, Hoffmann H, et al. Ex vivo expanded natural regulatory T cells from patients with end-stage renal disease or kidney transplantation are useful for autologous cell therapy. Kidney Int. 2018;93:1452–64. doi: 10.1016/j.kint.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Guinan EC, Cole GA, Wylie WH, et al. Ex vivo costimulatory blockade to generate regulatory t cells from patients awaiting kidney transplantation. Am J Transplant. 2016;16:2187–95. doi: 10.1111/ajt.13725. [DOI] [PubMed] [Google Scholar]
- 10.Putnam AL, Safinia N, Medvec A, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant. 2013;13:3010–20. doi: 10.1111/ajt.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin E, Bouchet-Delbos L, Renoult O, et al. Human tolerogenic dendritic cells regulate immune responses through lactate synthesis. Cell Metab. 2019;30:1075–90.:e8. doi: 10.1016/j.cmet.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson JA, Ahrens N, Geissler EK. MITAP-compliant characterization of human regulatory macrophages. Transpl Int. 2017;30:765–75. doi: 10.1111/tri.12988. [DOI] [PubMed] [Google Scholar]
- 13.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–75. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 14.Streitz M, Miloud T, Kapinsky M, et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplant Res. 2013;2:17. doi: 10.1186/2047-1440-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kverneland AH, Streitz M, Geissler E, et al. Age and gender leucocytes variances and references values generated using the standardized ONE-Study protocol. Cytometry A. 2016;89:543–64. doi: 10.1002/cyto.a.22855. [DOI] [PubMed] [Google Scholar]
- 16.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856–79. doi: 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- 18.Meijers RW, Litjens NH, de Wit EA, et al. Cytomegalovirus contributes partly to uraemia-associated premature immunological ageing of the T cell compartment. Clin Exp Immunol. 2013;174:424–32. doi: 10.1111/cei.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijers RW, Litjens NH, Hesselink DA, Langerak AW, Baan CC, Betjes MG. Primary Cytomegalovirus Infection Significantly Impacts Circulating T Cells in Kidney Transplant Recipients. Am J Transplant. 2015;15:3143–56. doi: 10.1111/ajt.13396. [DOI] [PubMed] [Google Scholar]
- 20.Makwana N, Foley B, Fernandez S, et al. CMV drives the expansion of highly functional memory T cells expressing NK-cell receptors in renal transplant recipients. Eur J Immunol. 2017;47:1324–34. doi: 10.1002/eji.201747018. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich C, Heine GH, Gerhart MK, Köhler H, Girndt M. Proinflammatory CD14+CD16+ monocytes are associated with subclinical atherosclerosis in renal transplant patients. Am J Transplant. 2008;8:103–10. doi: 10.1111/j.1600-6143.2007.02035.x. [DOI] [PubMed] [Google Scholar]
- 22.Vereyken EJ, Kraaij MD, Baan CC, et al. A shift towards pro-inflammatory CD16+ monocyte subsets with preserved cytokine production potential after kidney transplantation. PLoS One. 2013;8:e70152. doi: 10.1371/journal.pone.0070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Bosch TPP, Hilbrands LB, Kraaijeveld R, et al. Pretransplant numbers of CD16+ monocytes as a novel biomarker to predict acute rejection after kidney transplantation: a pilot study. Am J Transplant. 2017;17:2659–67. doi: 10.1111/ajt.14280. [DOI] [PubMed] [Google Scholar]
- 24.Braza F, Dugast E, Panov I, et al. Central role of CD45RA-Foxp3hi memory regulatory T cells in clinical kidney transplantation tolerance. J Am Soc Nephrol. 2015;26:1795–805. doi: 10.1681/ASN.2014050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray M, Gray D. Regulatory B cells mediate tolerance to apoptotic self in health: implications for disease. Int Immunol. 2015;27:505–11. doi: 10.1093/intimm/dxv045. [DOI] [PubMed] [Google Scholar]
- 26.Appelgren D, Eriksson P, Ernerudh J, Segelmark M. Marginal-zone B-cells are main producers of IgM in humans, and are reduced in patients with autoimmune vasculitis. Front Immunol. 2018;9:2242. doi: 10.3389/fimmu.2018.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchinson JA, Brem-Exner BG, Riquelme P, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl Int. 2008;21:742–54. doi: 10.1111/j.1432-2277.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- 28.Hutchinson JA, Riquelme P, Brem-Exner BG, et al. Transplant acceptance-inducing cells as an immune-conditioning therapy in renal transplantation. Transpl Int. 2008;21:728–41. doi: 10.1111/j.1432-2277.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 29.Riquelme P, Haarer J, Kammler A, et al. TIGIT+ iTregs elicited by human regulatory macrophages control T cell immunity. Nat Comms. 2018;9:2858. doi: 10.1038/s41467-018-05167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandran S, Tang Q, Sarwal M, et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant. 2017;17:2945–54. doi: 10.1111/ajt.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathew JM, H-Voss J, LeFever A, et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep. 2018;8:7428. doi: 10.1038/s41598-018-25574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todo S, Yamashita K, Goto R, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–43. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We will follow the common controlled access principles outlined by the Medical Research Council Clinical Trials Unit. According to those principles, we will acknowledge that data with long-term value be preserved, and usable for future research. We do, however, want to ensure that there are legal, ethical, and commercial constraints maintained on the release of research data according to the following code. Research teams are entitled to receive appropriate recognition for their efforts in collecting and analysing data and should be given at least a limited period of sole access to use and publish the data, before key trial data are open for use by other researchers. If such requests are made to access the data, resources need to be available to process the request and prepare the data in a timely manner, if possible. Because of these demands, there must be an important scientific objective behind each request. Especially in the case of our international project, The ONE Study, any request must comply with regulations set by the competent authorities in the relevant countries that govern data security policies.