Abstract
RNA-protein interactions play a pivotal role in cell homeostasis and disease, but current approaches to study them require a considerable amount of starting material, favor the recovery of only a subset of RNA species or are complex and time-consuming. We recently developed orthogonal organic phase separation (OOPS): a quick, efficient and reproducible method to purify cross-linked RNA-protein adducts in an unbiased way. OOPS avoids molecular tagging or the capture of polyadenylated RNA. Instead, it is based on sampling the interface of a standard TRIzol extraction to enrich RNA-binding proteins (RBPs) and their cognate bound RNA. OOPS specificity is achieved by digesting the enriched interfaces with RNases or proteases to release the RBPs or protein-bound RNA, respectively. Here we present a step-by-step protocol to purify protein-RNA adducts, free protein and free RNA from the same sample. We further describe how OOPS can be applied in human cell lines, Arabidopsis thaliana, Schizosaccharomyces pombe and Escherichia coli and how it can be used to study RBP dynamics.
Introduction
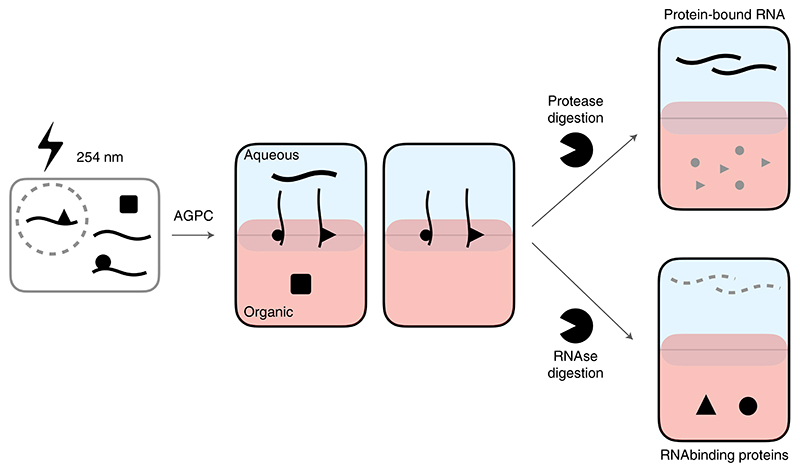
RNA-protein interactions regulate all steps of RNA life, from transcription to maturation, transport, localization and degradation1. Dynamic interactions between RNA and RBPs form functional ribo-nucleo-protein (RNP) entities that then carry out a broad spectrum of cellular functions. Characterizing the intricate crosstalk between RNAs and RBPs is thus of paramount importance to understand the RNA-mediated regulation of cellular physiology. Indeed, dysregulation of RNA-protein interactions has been implicated in a wide range of diseases, including spinal muscular atrophy2, amyotrophic lateral sclerosis3 and a variety of cancers4, representing promising targets for nucleic-acid-based therapies. Unfortunately, the absence of methods to characterize such interactions from an unbiased system-wide perspective has limited our knowledge of their implications in cell homeostasis. We recently developed the OOPS method to address this shortfall in ability to fully analyze the RNA-binding proteome in an unbiased manner5. OOPS combines UV cross-linking to stabilize RNA−protein interactions, with acidic guanidine phenol chloroform (AGPC, commercially known as TRIzol) extractions to enrich RNA-protein adducts. Under normal AGPC extraction conditions, non-cross-linked RNA partitions to the upper aqueous phase, whereas non-cross-linked proteins partition to the lower organic phase. The cross-linked RNA-protein adducts share the physicochemical properties of both RNA and protein and are thus retained at the interface and can be further enriched by repeated AGPC extractions. Enriched protein-bound RNAs (PBRs) or RBPs are retrieved by enzymatic digestion of the counterpart of the adduct (Fig. 1).
Fig. 1. Schematic representation of OOPS workflow.
Cells are UV cross-linked (254 nm) and RNA, proteins and RNA-protein adducts are separated according to their physicochemical properties. RNA-binding proteins or protein-bound RNA can be retrieved digesting the counterpart of the adduct. RNA is represented as strings, proteins as solid forms.
OOPS is directly applicable to any biological system that can be subjected to UV cross-linking, independently of the polyadenylation state of their RNAs, and is therefore suitable to study noncoding RNA-protein interactions and the RBPomes of prokaryotic organisms. Moreover, owing to its low input requirement (see ‘Starting cell culture material’ in ‘Reagent setup’ section) and reliability, OOPS can be coupled with different quantitative proteomic techniques to characterize RBP variations under dynamic conditions.
Development of the method
The development of OOPS is detailed in Queiroz et al.5. To characterize any potential bias of OOPS when recovering RBPs, we sequenced the RNA that partitioned to the interface upon UV cross-linking and AGPC extraction and observed that the full long (>200 nucleotides) transcriptome is protein bound, suggesting no bias toward any particular RNA species. From the protein perspective, the specificity of the method was tested by quantitative proteomics using stable isotope labeling by amino acids in cell culture (SILAC). We observed that UV cross-linking increases protein abundance at the interface and that repeating the AGPC extractions on the interface further enriches UV cross-linked proteins compared to free protein content of the first organic phase. To specifically retrieve the proteins that are in the interface because of their interaction with RNA, we treated the interfaces with RNases and recovered proteins released to the organic phase of a subsequent phase separation. Notably, the presence of over 95% of the proteins in the interface is RNase sensitive, strongly suggesting a highly specific enrichment in the RBPome.
We applied OOPS to obtain the RBPome of embryonic (HEK293), tumor-derived (U2OS) and non-tumor-derived (MCF10A) human cell lines, as well as bacteria (E. coli), retrieving one of the first RBPomes of prokaryotic organisms and proving the broad utility of OOPS. We proved the applicability of OOPS on dynamic frameworks by recovering both the total proteome and the RBPome (from the same sample) under microtubule-depolymerization cell-arrest conditions. Moreover, by comparing changes in the total proteome and RBPome, we were able to distinguish changes in RNA-binding activity from changes in RNA binding that could be explained simply by changes in RBP abundance.
Applications of the OOPS method
OOPS recovers free (non-cross-linked) RNA and proteins, as well as UV cross-linked RNA–protein adducts from the same sample. The most obvious applications of this technique are for the characterization of these interactions from a system-wide perspective by RNA sequencing and mass spectrometry (MS)-based proteomics. Nevertheless, cross-linked protein−RNA adducts might also be used as starting material for more specific studies. In combination with partial protein digestion and silica-based enrichment of peptide–RNA adducts, the cross-linked peptide can be inferred to determine the RNA-binding domain or region5. Moreover, interface fractions can be used in combination with other techniques (e.g. CLIP-based technology) where use of a pure sample of cross-linked protein−RNA simplifies the protocol6,7. Furthermore, because OOPS recovers all RNA that is cross-linked to proteins, it can be coupled with oligo(dT)-based mRNA-binding protein recovery methods, referred to as RNA interactome capture (RIC)8,9, to distinguish mRNA-binding proteins from non-coding RNA-binding proteins.
Comparison with other methods
The high-throughput identification of RBPs has been largely determined by RIC-based methods. RIC has proven valuable for cataloguing RBPs in different eukaryotic systems10, but it is explicitly biased toward the recovery of poly-adenylated RNA-binding proteins, precluding the comprehensive cataloguing of RBPs or applications where poly-adenylation is scarce (e.g. studies of bacterial RBPs or long non-coding RNA-binding proteins). Alternative methods to extract RBPs using UV crosslinking and phase separation have been recently published—protein-cross-linked RNA extraction (XRNAX)6 and Phenol Toluol extraction (PTex)7—although there are some important differences between all three methods (reviewed in Smith et al.11). OOPS requires the least starting material (for human cells: OOPS ~3 × 106, PTex ~5 × 106 and XRNAX ~8 × 107); however, it is not possible to determine the relative strengths and weaknesses of each method because a direct comparison has yet to be performed.
Experimental design
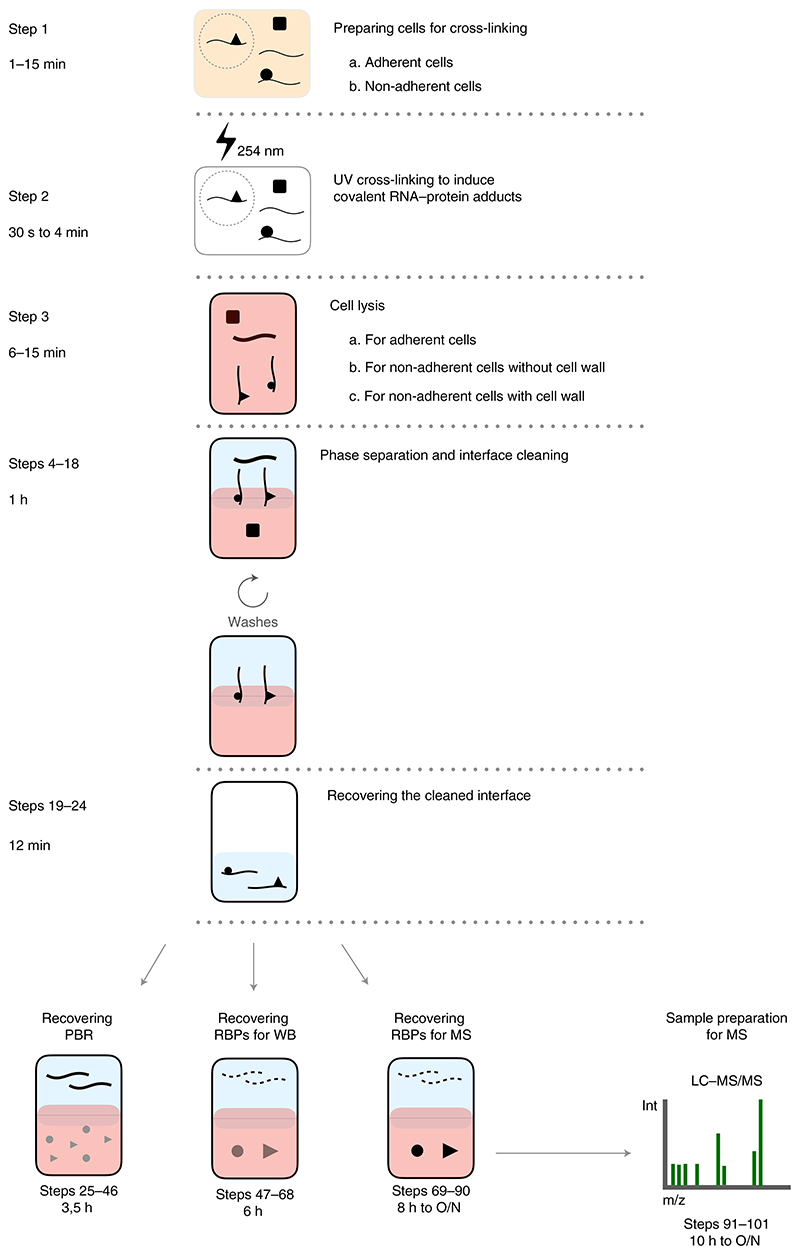
OOPS was developed for two major purposes: to comprehensively catalogue RBPs in any organism and to study RBPome dynamics. It has been applied to eukaryotic and prokaryotic cells, in adherent and non-adherent cell cultures, and to cells with and without a cell wall5. A typical OOPS experiment involves UV irradiation to form protein–RNA adducts, cell lysis, repeated rounds of phase separation to enrich the adducts and, finally, recovery of the protein and/or RNA. All the modifications required to implement the protocol in these different biological systems, as well as the key OOPS steps, are detailed in the step-by-step protocol (Fig. 2).
Fig. 2. Graphical index of OOPS workflow.
The steps of the protocol and the timing to complete them (right) are represented in combination with a graphical description of the RNA and protein behavior along OOPS (center), together with a brief description of the steps and sub-steps of the protocol (right). The time required to complete each step depends on the type of cell (Steps 1−3) and the choice of short or overnight digestions (Steps 69−90 and 91−101).
Starting sample
In general, OOPS is easier to perform when the interface contains more material. The interface content depends on the quantity of starting material, RNA-protein cross-linking efficiency and extent of cell lysis, which can be optimized during the protocol implementation if required. An excessive volume interface, however, will be difficult to disaggregate, clean and digest.
When implementing OOPS in a new species or tissue, optimization of UV dosage and starting material is recommended (see Supplementary Information ‘UV cross-linking in non-human models’). Furthermore, in organisms with a cell wall, cell lysis in an RNA- and protein-compatible fashion will require optimization (see Supplementary Information ‘Extra controls for organisms with a cell wall’).
Controls
OOPS modularity allows the incorporation of different controls to enhance the reliability of the identified RBPs or the changes in their RNA-binding behaviour. When cataloguing RBPs in new biological models, SILAC controls are preferred. Comparing the abundance of OOPS-enriched proteins from SILAC-labeled UV cross-linked cells and non-cross-linked cells, mixed at the cell lysate stage, constitutes the classical approach for cataloguing RBPs (Fig. 3, top). This control facilitates the assessment of to what extent the protein extraction using OOPS is UV dependent. OOPS recovers a consistent quantity of RNA between biological replicates5. Thus, an alternative control of RNA degradation can be performed using SILAC by mixing independent OOPS interfaces after RNase +/− treatment (Fig. 3, bottom). This control facilitates the assessment of to what extent the protein presence in the final cleaned interface is dependent on RNA. For biological systems where incorporation of stable isotope-coded amino acids is not a suitable option, a single final OOPS interface can be split into RNAse +/− samples and analyzed by label-free quantitation (LFQ) to provide quantitative evidence of the RNA-binding state of the OOPS-enriched proteins, albeit less precisely than SILAC. Similarly, separate non-cross-link and RNase negative-control samples can be analyzed by western blotting.
Fig. 3. Representation of different SILAC controls compatible with OOPS workflow.
SILAC controls for UV cross-linking and/or RNase can be added at their respective stages in the protocol. Red dotted arrows represent the steps in which samples can be combined (Step 3 for non-cross-link controls and Step 75 for RNase-negative controls). For the non-cross-link control, cells are mixed at the cell lysis stage (top). For the RNase-negative control, cleaned interfaces are mixed before the final phase separation (bottom).
RNA binding protein dynamics
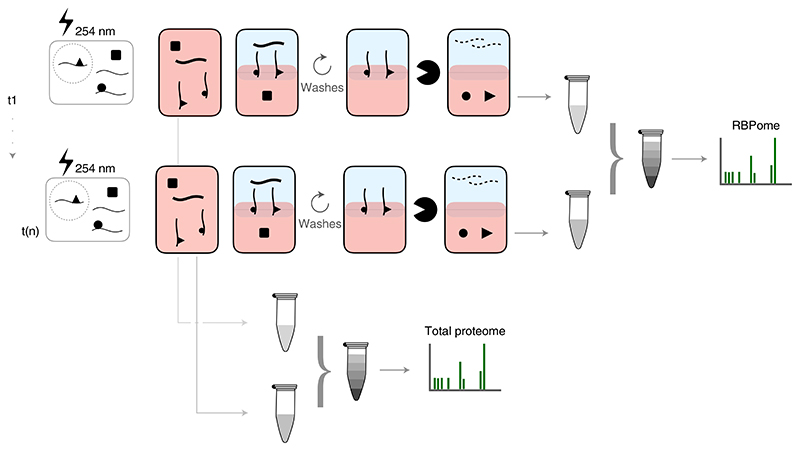
OOPS can be easily adapted to interrogate RBP dynamics by quantitative comparisons of RBPs in different conditions. For example, tandem mass tag (TMT)-based analyses were previously employed to characterize RBP dynamics under microtubule-depolymerization cell-arrest conditions5. TMT allows high multiplexing capability, reducing the mass spectrometric analysis time and the number of missing values in comparison to independent liquid chromatography with tandem mass spectrometry (LC-MS/MS) runs for each sample. RBPome dynamic studies were carried out by simultaneous quantification of total protein and RNA-bound protein abundances to specifically identify RBPs with altered RNA binding, as opposed to simply identifying RBPs with differential abundance (Fig. 4). Obtaining both total proteome and RBPome from the same sample is advisable to reduce confounding factors and batch effects. This experimental framework may be implemented to study RBPome dynamics in other experimental conditions. Alternatively, if up to three conditions are analyzed, SILAC quantification of the total and RNA-bound protein in different conditions is an easy-to-implement alternative to TMT quantification.
Fig. 4. Schematic of TMT experimental design.
Different treatments (t) can be combined in the same TMT experiment. Total proteomes are obtained from the total cell lysates, TMT labeled and combined into a single sample. Different RBPomes are obtained independently, labeled and combined into a second sample.
Technical considerations for MS
OOPS is a highly efficient RBP enrichment technique that yields no detectable signal in negative controls (non-UV cross-linked or RNase negative) for hundreds of proteins. When using SILAC to quantify the relative abundance of RBPs in UV cross-linked and non-cross-linked controls, many peptides are observed only in the cross-linked sample. We therefore suggest that MS settings are adjusted so that the detection of SILAC pairs trigger MS2 fragmentation to avoid missing these peptides, which are very highly enriched in cross-linked samples.
Commercially available TMT kits are designed to label a greater quantity of peptides than typically obtained by RBP enrichment. In our hands, splitting a 0.80-mg TMT tag vial into two aliquots to label the RBPs and the total proteome extracts (~10−25 μg and 15−40 μg, respectively) provides satisfactory results. When dealing with low reaction volumes for TMT labeling, it is important to maintain the correct concentrations of TMT tags and peptides12. It is also worth mentioning that TMT quantification can lead to underestimation of fold changes, which is alleviated by quantification using an Orbitrap mass spectrometer compatible with SPS-MS3 13 and conservative exclusion of peptide spectra matches with evidence of co-isolation.
Level of expertise needed to implement the protocol
Any laboratory that uses phenol/chloroform or AGPC methods to obtain DNA, RNA or protein will find OOPS straightforward to implement. Indeed, OOPS will not represent a challenge to any scientist with basic molecular biology laboratory skills.
Prior knowledge of MS is not required for the OOPS method itself. However, when preparing RBP samples for MS, prior consultation with a researcher experienced with sample preparation for MS is strongly recommended, as keratin, polymer or detergent contamination can have severe detrimental effects on the quality of data obtained.
For researchers not proficient with high-throughput data analysis, RStudio (RStudio Team (2015)) (http://www.rstudio.com/) provides an accessible environment for R-based analyses.
Limitations
All methods that retrieve RNA−protein interactions in denaturing conditions require prior stabilization of these interactions. UV-C cross-linking is currently the ‘gold standard’ approach to covalently stabilize RNA–protein interactions at ‘zero’ distance14. Nevertheless, it has been reported that, at dosages above 1 J/cm2, UV-C can induce single-strand DNA-protein15 and protein-protein16 adducts; however, these adducts should not be retained in the OOPS interfaces because both halves of the adduct preferentially separate to the organic phase. We recommend evaluating the optimal UV required by the chosen biological system (see Step 2). Because OOPS requires RNA-protein interaction stabilization, it is only applicable to biological systems that can be subjected to UV cross-linking.
OOPS can be used to obtain the RNA-bound proteome and/or protein-bound transcriptome by enzymatic hydrolysis of the cognate macromolecule. Nevertheless, the bound transcriptome and proteome retain signatures of their counterparts, which affects the downstream analysis. For example, after protease digestion, one or more amino acids might still be cross-linked to the protein-bound transcriptome. Remaining amino acids interfere with many reverse transcriptases17, affecting cDNA synthesis and ultimately RNA quantification. This is of particular consideration if full-length cDNA is required. Reverse transcription with fragmented RNA is therefore recommended. Nevertheless, a loss of coverage at the cross-linked sites will still be detectable, affecting the transcript quantitation. This limitation can also provide useful information if the researcher wants to infer the sites of cross-linking from the loss of read coverage5. From the RBPome perspective, the cross-linked proteins migrate differently in size-based electrophoretic analysis unless the RNA is fully digested. Moreover, the most commonly used peptide spectra matching algorithms cannot handle the plethora of amino acids and nucleotides cross-linking moieties that a cross-linked peptide might possess. As a result, the crosslinked peptide is not directly detectable. Again, this limitation can also be considered as an advantage because it allows the use of bespoke open search engines to directly identify the cross-linked peptide and, thus, the site of cross-linking on the protein6,18.
Finally, we observed that phase separation methods enrich a subset of glycoproteins at the interface, independently of their RNA-binding status5. These glycoproteins can be identified by use of RNase and/or cross-link negative-control samples, but the exclusion of glycoproteins during the data analysis is recommended.
Materials
Biological materials
Cell line used to generate data shown in ‘Anticipated results’:
U-2 OS (ATCC cat. no. HTB-96, RRID:CVCL_0042) Additional cell lines and species that have been successfully used with OOPS:
MCF10A (ATCC cat. no. CRL-1573, RRID:CVCL_0045)
HEK293 RRID (ATCC cat. no. CRL-10317, RRID:CVCL_0598)
Schizosaccharomyces pombe (provided by J. Mata, University of Cambridge)
Arabidopsis cell suspension culture line (cv. L. erecta; provided by H. T. Parson, University of Cambridge)
DH5α Escherichia coli (New England BioLabs, cat. no. C2987I)
Reagents
Reagents for OOPS
TRIzol (Thermo Fisher Scientific, cat. no. 15596018) ! CAUTION Toxic if swallowed, in contact with skin or inhaled; causes severe skin burns and eye damage; may cause respiratory irritation; suspected of causing genetic defects; may cause damage to organs through prolonged or repeated exposure; harmful to aquatic life with long-lasting effects. Use it under the fume extraction hood. Wear appropriate gloves and safety glasses.
Chloroform (Fisher, cat. no. 11408123) ! CAUTION Harmful if swallowed; toxic if inhaled; causes skin irritation; causes serious eye irritation; may cause drowsiness or dizziness; suspected of causing cancer; suspected of damaging the unborn child; causes damage to organs through prolonged or repeated exposure; causus cardiac and respiratory depression; overexposure may cause decreased heart rate, decreased blood pressure, heart block and cardiac failure. Use it under the fume extraction hood. Wear appropriate gloves and safety glasses.
2-Propanol (Sigma-Aldrich, cat. no. I9516−500ML) ! CAUTION Highly flammable liquid and vapor; causes serious eye irritation; may cause drowsiness or dizziness. Wear appropriate gloves and safety glasses. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources.
UltraPure phenol:chloroform:isoamyl alcohol (25:24:1 (vol/vol); Thermo Fisher Scientific, cat. no. 15593031) ! CAUTION Toxic if swallowed, in contact with skin or inhaled; causes severe skin burns and eye damage; suspected of causing genetic defects; suspected of causing cancer; suspected of damaging fertility or the unborn child; causes damage to organs through prolonged or repeated exposure; toxic to aquatic life with long-lasting effects. Use it under the fume extraction hood. Wear appropriate gloves and safety glasses.
Ethanol (Honeywell, cat. no. 24194−2.5L) ! CAUTION Highly flammable liquid and vapor; causes serious eye irritation. Wear appropriate gloves and safety glasses. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources.
Methanol (Fisher, cat. no. 10675112) ! CAUTION Highly flammable liquid and vapor; toxic if swallowed; toxic when in contact with skin; toxic if inhaled; causes damage to organs. Wear appropriate gloves and safety glasses. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources.
Dulbecco’s phosphate-buffered saline (PBS; Sigma-Aldrich, cat. no. D8537−500ML)
Proteinase K (Thermo Fisher Scientific, cat. no. EO0491) ! CAUTION May cause allergy or asthma symptoms or breathing difficulties if inhaled. Wear appropriate gloves and safety glasses.
UltraPure DEPC-treated water (Thermo Fisher Scientific, cat. no. 750024)
Sodium acetate (3 M, pH 5.5; Thermo Fisher Scientific, cat. no. AM9740)
EDTA (Merck Millipore, cat. no. 324506−100ML) ! CAUTION Causes serious eye irritation. Wear appropriate gloves and safety glasses.
RNase A/T1 mix (Thermo Fisher Scientific, cat. no. EN0551)
MgCl2 (1 M; Thermo Fisher Scientific, cat. no. AM9530G)
Tris (1 M, pH 8.0; Thermo Fisher Scientific, cat. no. AM9855G)
SDS 10% (wt/vol) solution (Thermo Fisher Scientific, cat. no. AM9822) ! CAUTION Causes skin irritation; causes serious eye irritation. Wear appropriate gloves and safety glasses.
Triethylammonium bicarbonate (TEAB) buffer (1 M, pH 8.5; Sigma-Aldrich, cat. no. T708)
Qubit RNA BR (Broad-Range) Assay Kit (Thermo Fisher Scientific, cat. no. Q10210)
Agilent RNA 6000 Nano Kit (Agilent, cat. no. 5067−1511)
Glass beads, acid-washed (Sigma-Aldrich, cat. no. G8772−100G)
Reagents for MS
DL-dithiothreitol (DTT) (Sigma-Aldrich, cat. no. 43815) ! CAUTION Harmful if swallowed. Harmful to aquatic life with long-lasting effects. If swallowed, call a poison center or doctor if feeling unwell. Rinse mouth.
Formic acid (Fluka, cat. no. 94318) ! CAUTION Causes severe skin burns and eye damage. Toxic if inhaled. Corrosive to the respiratory tract. Wear protective gloves, protective clothing, eye protection and face protection. If inhaled, remove the affected user to fresh air and keep at rest in a position comfortable for breathing. If in eyes, rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do. Continue rinsing.
High-purity water obtained from a Milli-Q purification system (Millipore)
Iodoacetamide (Sigma-Aldrich, cat. no. I6125) ! CAUTION May cause an allergic skin reaction. May cause allergy or asthma symptoms or breathing difficulties if inhaled. Avoid breathing dust. Wear protective gloves.
Trypsin (Promega, cat. no. V5111)
Acetonitrile (ACN), LC-MS grade (Thermo Fisher Scientific, cat. no. 51101) ! CAUTION Highly flammable liquid and vapor; toxic if swallowed; toxic when in contact with skin/eye; toxic if inhaled; may cause serious eye damage and eye irritation. Wear appropriate gloves and safety glasses. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. Use only in a well-ventilated area.
Trifluoroacetic acid (Thermo Fisher Scientific, cat. no. 28904) ! CAUTION Causes severe skin burns and eye damage. Harmful if inhaled. Harmful to aquatic life with long-lasting effects. The solutions must be prepared in a fume hood. Wear protective gloves, protective clothing, eye protection and face protection. Avoid release to the environment by following institutional guidelines for safe disposal.
Pierce peptide de-salting spin columns (Thermo Fisher Scientific, cat. no. 89851)
Pierce High pH Reversed-Phase Peptide Fractionation Kit (Thermo Fisher Scientific, cat. no. 84868)
Qubit Protein Assay Kit (Thermo Fisher Scientific, cat. no. Q33212)
TMT10plex Isobaric Label Reagent Set, 1 ×0.8MG, 10-RXN SET (Thermo Fisher Scientific, cat. no. 90110)
SILAC Protein Quantitation Kit (trypsin), DMEM (Thermo Fisher Scientific, cat. no. A33972)
Equipment
Fume extraction hood
Cross-linker with shortwave 254-nm UV lamps (UVP, CL-1000 ultraviolet crosslinker)
Benchtop centrifuge (Eppendorf refrigerated benchtop centrifuge, 5415R)
Thermoshaker (Thermal Shake lite, VWR, cat. no. 460−0249)
NanoDrop (Thermo Fisher Scientific, NanoDrop 2000)
Qubit (Thermo Fisher, Qubit 2.0)
Speedvac vacuum centrifuge with cold trap (Labconco, Refrigerated CentriVap, Concentrator)
Bioruptor Plus sonicator device (Diagenode, cat. no. B01020001)
Bioanalyzer (Agilent, 2100 Bioanalyzer system)
RNase-free 1.5-ml microfuge tubes (Ambion, cat. no. AM12400)
1.7-ml protein low-binding microfuge tubes (Sorenson Bioscience, cat. no. 39640T)
Scraper (Cellstar, cat. no. 541070)
Cell culture coated dishes, 90 mm (Falcon, cat. no. 353003)
MP FastPrep-24 5 G tissue homogenizer (MP Biomedicals, cat. no. SKU 116005500)
IKA Matrix Orbital Delta F2.0 (IKA, cat. no. 0030000753)
MS equipment
Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific)
Software and data analysis tools
Proteome Discoverer (version 2.3 or higher; Thermo Fisher Scientific, www.thermofisher.com)
R (version 3.6 or higher) (https://www.r-project.org/)
Rstudio (https://rstudio.com/)
R packages MSnbase (https://bioconductor.org/packages//MSnbase)
LIMMA (https://bioconductor.org/packages/release/bioc/html/limma.html)
Reagent setup
The starting cell culture material
The starting cell culture material for OOPS should be optimized for every cell line and organism. When working with human adherent cell lines, 3 × 106 cells should be sufficient for SILAC and LFQ cataloguing of RBPs and 8 × 106 cells for TMT characterization of RBP RNA-binding dynamics. If cells have a lower volume or RNA or protein content than a typical human adherent cell line (such as HEK293 or U2OS cells), additional material will be required. Culture cells in standard conditions. An average of 45 ml at 0.4 OD (600 nm) for yeast (~6 × 108 cells) and 20 ml of 0.4 OD (600 nm) bacterial culture (~ 6 × 109 cells) are recommended. Cells need to be plated on dishes (cat. no. 353004) instead of flasks to allow a direct exposure to UV irradiation. RNA−protein cross-linking and cellular lysis are the most limiting steps when optimizing the RBP recovery (refer to Table 1 (Troubleshooting) and Supplementary Information).
Table 1. Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 3A(iv) and 3B(viii) | Aggregates in the TRIzol homogenate for cells without a cell wall | Insufficient homogenization or TRIzol saturation | Vortex the sample for longer. If aggregates are still present, pass the sample through a 23−27-G syringe needle to further homogenize the sample. If aggregates are still present, freeze the sample at −80 °C, thaw and vortex again. If the previous steps do not disperse the aggregates, it is likely that the TRIzol is saturated. Scale down the number of cells. If aggregates are unavoidable, spin down the sample at <5,000g to pellet the aggregates and transfer the supernatant to a new tube |
| Cells containing a high nuclear:cytosolic ratio (e.g., lymphocytes) can be difficult to resuspend in TRIzol once pelleted. Resuspend the cross-linked cell pellet in 50−100 μl of 1% (wt/vol) SDS RNase-free water before adding 1 ml of TRIzol. | |||
| 3C(xi) | Aggregates in TRIzol lysis step for cells with a cell wall | Inefficient cell lysis or incomplete removal of cellular debris | Inefficient cellular disruption might lead to remnants of the cell wall, which will populate the interphase during phase separation. If the cellular lysis has already been optimized, increase the speed at which you pellet the cellular debris. This spin takes place after cell lysis and resuspension in TRIzol and should not surpass 12,000g |
| 46 | Nucleic acids in the interface of a non-cross-linked sample (amount of RNA >5% of the RNA obtained in the interface of a cross-linked sample) | Inefficient lysis (mainly for organisms with a cell wall) or insufficient interface washing | If the cell lysis has already been optimized, increase the number of phase separation steps, paying special attention in the interface homogenization. If nucleic acids are still present, scale down the starting material |
| Low cross-linking efficacy | UV at 254 nm has low penetrance. Any shadowing of lower layer of cells by upper layers can affect the cross-linking efficacy | Ensure cells are as close as possible to a monolayer. Diminish the volume of liquid covering the cells. If possible, cross-link your cells when dry | |
| Media absorbance of UV light at 254 nm | Any pigments within colored cell growth media might absorb UV light at 245 nm. Check if the media is lowering the UV cross-link efficiency (see Supplementary Information) | ||
| RNA degradation | RNAse contamination | Use RNase-free reagents in all steps. The presence of RNases in the cell culture media, proteinase K buffer or RNase-free water is the most probable source of RNA degradation. Be sure that the cell culture media is fully removed before cross-linking. Pre-incubate proteinase K buffer with the proteinase K for 15 min at 50 °C before adding the mixture to the precipitated interfaces to inactivate all potential RNases in the buffers. Periodically check for RNase contamination in RNase-free water and working stock by incubating newly extracted RNA with the buffers for 15 min at 37 °C and evaluating the RNA integrity by Bioanalyzer or 1% (wt/vol) agarose gel electrophoresis or use a new stock. Avoid sonication and sample boiling if RNA integrity is required | |
| 68 and 90 | Proteins in the non-cross-linked control interface | Some RBPs (mainly ribosomal proteins) can be found in the interface in the absence of cross-linking. The presence of a high amount of proteins in the non-cross-linked interface could reflect problems in the cell lysis, too much starting material or inefficient interface washes | The interface has to be completely homogenized when doing the repeated chloroform/TRIzol phase separations. An inefficient interface homogenization impedes the release of the interface contaminants from the interface in the washing steps. Check the cellular lysis (steps Supplementary Information ‘Extra controls for organisms with a cell wall’). If the cell lysis and interface homogenization are not likely to be the source of the problem, scale down the starting material |
| Proteins in the RNase-negative control | Insufficient interface cleaning or RNA degradation | Avoid the sonication and interface boiling steps. Both of them degrade the RNA. Add RNase inhibitors to the control buffers. Avoid incubations over 8 h. Check for RNase contaminations | |
| 97 | Less than 10 μg of sample is recovered | Insufficient digestion of the RNA | Use a new batch of RNase |
| Low RBP yield in the chosen biological system | Start a new experiment and use 0.1 μg of modified trypsin per sample to avoid saturation of the MS with trypsin signal Scale-up the starting material |
TRIzol
TRIzol is commercially available. However, if desired, the equivalent solution can be prepared as described by Chomczynski & Sacchi19.
Proteinase K buffer
10 mM Tris-HCl, pH 8, 10 mM EDTA. 300 μl is required per reaction. The buffer can be stored at room temperature (RT; 20–25 °C) for 1 month.
Ethanol
Ethanol (70% (vol/vol)) diluted in RNase-free water. The solution can be stored at RT for 1 d.
WB buffer (5×)
0.5 M Tris-HCl, 0.5 M DTT, 346 mM, 10% (wt/vol) SDS, 7.5 mM Bromophenol blue, 5.4 M Glycerol. The buffer can be stored at 4 °C for 1 month.
TEAB buffer
Dilute the stock solution of 1 M TEAB buffer, pH 8.5, in milli-Q water to a final concentration of 100 mM.
Protein reduction reagent
DTT stock solution. Prepare DTT stock solution by dissolving DTT in 100 mM TEAB, pH 8.5, to a final concentration of 300 mM ▲ CRITICAL DTT is susceptible to oxidation and should be freshly prepared.
Protein alkylation reagent
Iodoacetamide (IAA) stock solution. Prepare IAA stock solution by dissolving IAA in 100 mM TEAB, pH 8.5, to a final concentration of 640 mM ▲ CRITICAL IAA is sensitive to light and should be freshly prepared and kept in the dark. To avoid lysine and histidine alkylation, maintain pH above 7.520.
Trypsin solution
Resuspend 20 μg of trypsin in 200 μl of 100 mM TEAB, pH 8.5. This buffer is best prepared on the day of use. For longer storage conditions of trypsin, see the manufacturer’s recommendations.
C18 conditioning/washing solution (0.1% (vol/vol) trifluoroacetic acid in water)
Conditioning/washing solution is 0.1% (vol/vol) trifluoroacetic acid (TFA) in HPLC grade water. Freshly prepare on the day of use and keep at RT.
C18 elution solution (50% (vol/vol) ACN)
50% (vol/vol) ACN in HPLC grade water. Freshly prepare on the day of use and keep at RT. The elution solution is prepared without acid to avoid confounding protein estimation with Qubit.
0.1% (vol/vol) formic acid
Dilute the stock solution of formic acid in milli-Q water to a final concentration of 0.1% (vol/vol). The solution can be kept at RT for a week.
Equipment setup
▲ CRITICAL Unless stated otherwise, we recommend performing all centrifugation steps at 4 °C. Pre-cooling the centrifuge is recommended before the first centrifugation.
Mass spectrometer setup
The parameters we recommend for the Orbitrap Fusion Lumos Tribrid MS for TMT-labeled samples using SPS-MS3 acquisition8 and for SILAC or LFQ samples using CHarge Ordered Parallel Ion aNalysis (CHOPIN)11 acquisitions are listed in Supplementary Tables 1 and 2, respectively.
Procedure
Preparing cells for cross-linking ● Timing 1 min for adherent cells and 15 min for non-adherent cells
-
1 Any component of the cell culture media able to absorb light at 254 nm needs to be removed to maximize RNA–protein cross-linking (see Supplementary Information: How to check if the media absorbs light at 254 nm). Moreover, many types of cell culture media are supplemented with cell extracts or contain dying cells that release RNases to the media. Once cross-linked, cells become permeable, and RNases from the media can degrade the RNA. The removal of RNase-containing media before cross-linking is therefore required to maintain RNA integrity. If the cells to be cross-linked are adherent, refer to option A. For non-adherent cells, the contaminants of the media need to be removed, and the exposure of the cells to the UV light needs to be enhanced, to maximize the cross-link. For non-adherent cells, follow option B.
▲ CRITICAL STEP Phenol red containing cell culturing media and Luria–Bertani broth absorb UV at 254 nm, hindering the RNA–protein cross-link. See Supplementary Information.
▲ CRITICAL STEP Cell culture media supplemented with fetal bovine serum or equivalent contains RNases.-
(A)Adherent cells
-
(i)Remove the media by aspiration.
-
(ii)Wash the cells with equivalent volume of PBS.
-
(iii)Remove and discard the PBS.
-
(iv)Repeat the washing Step 1A(ii and iii) for a total of two PBS washes.
-
(v)Remove completely the PBS (use a pipette if necessary) and proceed immediately to cross-link the cells (Step 2).
-
(i)
-
(B)Non-adherent cells▲ CRITICAL Different cells and organisms require different pelleting speeds and cross-linking dosage. Use the same speed used to pellet cells as when culturing the cells (e.g. ~300 g for human cell lines or 6,000g for E. coli).
-
(i)If the media used to grow the cells does not contain any component that absorbs UV light at 254 nm or RNases, proceed directly to cross-link the cell. Otherwise:
-
(ii)Pellet down the cells for 5 min.
-
(iii)Remove the media and re-solubilize the pellet in 10 ml of PBS by gentle pipetting.
-
(iv)Repeat Step 1A(ii and iii) for a total of two washes.
-
(v)Resuspend the cells in 10 ml of RNase-free media that does not absorb UV light at 254 nm (e.g., PBS).
-
(vi)Distribute the cells on a new cell culture dish, minimizing the cell overlap (90-mm dishes are recommended for 107 cells), and proceed immediately to cross-link the cells (Step 2).
-
(i)
-
(A)
UV cross-linking to induce covalent RNA−protein adducts ● Timing 30 s−4 min
-
2
Place the dish of cells (without the lid) into the UV cross-linker and irradiate the cells at the appropriate dosage to cross-link at least 50% of the RNA. 150−400 mJ/cm2 represents a normal dosage for many human cells. Once finished, proceed immediately with the lysis step (Step 3).
▲ CRITICAL STEP UV cross-linking is dependent on exposure to UV light. Maximize the UV exposure by avoiding any 254-nm UV light absorbance from the media and allowing a proper exposure of all cells in culture to the UV light. The UV dose used can have a significant effect on the number of proteins cross-linked18. The UV dose can be optimized by analyzing the proportion of RNA that is retained at the interface upon different UV exposure times, in relation to the RNA that remains free on the first aqueous phase (see ‘Anticipated results’ section). Avoid over cross-linking the samples once the amount of PBR is saturated to minimize non-physiological protein–RNA cross-linking18. For human adherent cell lines, ~75% of RNA should be present in the interface between 150 and 400 mJ/cm2 (see ‘Anticipated results’ section). Different cells, organisms and tissues require different UV dosages to efficiently generate RNA−protein adducts (see Supplementary Information: UV cross-linking in non-human models).
Cell lysis and preparing cells for phase separation ● Timing 6−15 min
-
3
Cell lysis is one of the most variable steps depending on the chosen biological system. To ensure a proper release of all the cellular content, follow the appropriate subsection. For lysing adherent cells without a cell wall, follow option A; for lysing non-adherent cells without a cell wall, follow option B; for lysing non-adherent cells with a cell wall, follow option C.
! CAUTION All steps involving TRIzol and/or chloroform must be performed in a fume hood.-
(A)Cell lysis of adherent cells without a cell wall (e.g. mammalian cell lines):
-
(i)Add 1 ml of TRIzol to the cross-linked dry 90-mm dish. If working with a larger area, scale up accordingly.
-
(ii)Scrape the dish content using a cell scraper and transfer the cross-linked cells to a new 1.5-ml RNase-free tube.
-
(iii)Completely homogenize the sample by pipetting it five times and vortexing for 15 s at maximum speed.▲ CRITICAL STEP If performing a SILAC cross-link versus non-cross-link controlled experiment, scrape each in 0.5 ml of TRIzol and mix.
-
(iv)Incubate for 5 min at RT to dissociate non-cross-linked RNA−protein interactions. 10% of the sample can be removed and precipitated (Step 59) to analyze the total proteome. Alternatively, the free proteome (precipitated from the first organic phase, Step 9) can be used for the same purpose.▲ CRITICAL STEP Sample should appear homogeneous.◼ PAUSE POINT Samples can be stored at −80 °C for 1 year.? TROUBLESHOOTING
-
(i)
-
(B)Cell lysis of non-adherent cells without a cell wall:
-
(i)Use a cell scraper to detach the cross-linked cells (cells attach to the dish after crosslinking).
-
(ii)Transfer the cells to a new tube.
-
(iii)Spin down the cross-linked cells for 5 min at 300g.▲ CRITICAL STEP The cell pelleting speed will depend on the organism. Use the same speed used for culturing cells.
-
(iv)Remove and discard the supernatant, leaving a maximum of 100 μl.
-
(v)Homogenize the cells in the remaining volume by vortexing for 15 s.
-
(vi)Add 1 ml of TRIzol to the tube.
-
(vii)Completely homogenize the sample by pipetting it five times and vortexing for 15 s at maximum speed.▲ CRITICAL STEP If performing a SILAC cross-link versus non-cross-link controlled experiment, mix them in the same 1 ml of TRIzol.
-
(viii)Incubate for 5 min at RT to dissociate non-cross-linked RNA−protein interactions. 10% of the sample can be removed and precipitated (Step 59) to analyze the total proteome. Alternatively, the free proteome (precipitated from the first organic phase, Step 9) can be used for the same purpose.▲ CRITICAL STEP Sample should appear homogeneous.◼ PAUSE POINT Samples can be stored at −80 °C for 1 year.? TROUBLESHOOTING
-
(i)
-
(C)Non-adherent cells with a cell wall (e.g. E. coli or S. pombe):▲ CRITICAL Efficient cellular lysis, essential for the isolation of RBPs, might be hindered by the presence of a cell wall. RNA extraction can be optimized by analyzing the proportion of RNA that is retained at the interface between non-cross-linked and cross-linked samples (see ‘Anticipated results’ section and Supplementary Information: Extra controls for organisms with a cell wall).
-
(i)Collect the suspension of cross-linked cells to a new 1.5-ml tube.
-
(ii)Spin down the cross-linked cells for 5 min at the appropriate speed for the organism.▲ CRITICAL STEP The cell pelleting speed will depend on the organism. Use the same speed used for culturing cells.
-
(iii)Remove and discard the supernatant as much as possible without disrupting pellet.
-
(iv)Add 50 μl of TRIzol to the pellet.
-
(v)Add glass beads to the 0.5-ml mark.
-
(vi)Lyse cells in tissue homogenizer for 1 min at 6 m/s. Immediately place on ice.▲ CRITICAL STEP Tissue disruptor settings might require optimization depending on the organisms of study (see Supplementary Information: Extra controls for organisms with a cell wall).
-
(vii)Add 1 ml of TRIzol to the tube.
-
(viii)Shake on table-top shaker for 5 min at 1,000 r.p.m.
-
(ix)Transfer 1 ml of supernatant to a new tube.
-
(x)Spin down for 5 min at the same speed used to pellet the cells in Step 3C(ii).
-
(xi)Transfer supernatant to a new tube. If the volume is less than 1 ml, complete to this value with TRIzol. 10% of the sample can be removed and precipitated (Steps 59−62) to analyze the total proteome. Alternatively, the free proteome (precipitated from the first organic phase, Step 9) can be used for the same purpose.▲ CRITICAL STEP Sample should appear homogeneous.◼ PAUSE POINT Samples can be stored at −80 °C for 1 year.? TROUBLESHOOTING
-
(i)
-
(A)
Phase separation and interface cleaning ● Timing 1h
! CAUTION All steps involving TRIzol and/or chloroform must be performed in a fume hood.
-
4
Add 1:5 (vol/vol, chloroform:TRIzol) of chloroform to the TRIzol homogenate.
-
5
Vortex the sample for 15 s at maximum speed.
▲ CRITICAL STEP The sample should appear homogeneously light-pink after vortexing. If a transparent portion remains present at the bottom of the tube, repeat the vortexing step.
-
6
Centrifuge for 15 min at 12,000g at 4 °C.
-
7
Transfer the upper (transparent) aqueous phase to a new tube. Avoid the interface. If part of the interface is taken, return it slowly to the tube. This transferred aqueous phase contains the non-cross-linked free RNA. The sample can be stored on ice for a short period of time (up to 2 h) or at −80 °C for 1 year. To recover the free RNA, follow Steps 33−45 (without adding the sodium acetate (NaAc)).
-
8
Pass through the interface with the tip of the pipette to the bottom of the tube. See Supplementary Video 1.
-
9
Transfer the lower (pink) organic phase to a new tube. Avoid taking the interface. This phase contains the non-cross-linked free protein. The sample can be stored on ice for a short period of time (up to 2 h) or at −20 °C for 1 month. To precipitate the free protein, follow Steps 59−62.
▲ CRITICAL STEP TRIzol-to-sample ratio should be at least 10:1 (vol/vol). Leave a maximum of 100 μl of final volume in the tube after removing the upper and lower phases. If a higher volume remains in the tube, remove more aqueous and/or organic phases.
-
10
Add 1 ml of TRIzol to the interface.
-
11
Vortex the sample for 15 s at maximum speed.
▲ CRITICAL STEP The interface should be completely homogenized. If traces of the interface are still visible, repeat the vortexing step or pipette the sample until the sample is completely homogeneous.
-
12
Add 1:5 (vol/vol, chloroform:TRIzol) of chloroform to the TRIzol homogenate.
-
13
Vortex the sample for 15 s at maximum speed.
▲ CRITICAL STEP The sample should look homogeneously light-pink after vortexing. If a transparent portion remains present at the bottom of the tube, repeat the vortexing step.
-
14
Centrifuge for 15 min at 12,000 g at 4 °C.
-
15
Remove and discard the upper aqueous phase.
-
16
Pass through the interface with the tip of the pipette to the bottom of the tube.
-
17
Remove and discard the lower organic phase.
▲ CRITICAL STEP A maximum of 100 μl of final volume should remain in the tube after removing the upper and lower phases. If a higher volume remains in the tube, remove more aqueous and/or organic phases.
-
18
Repeat Steps 10−17 for a third-round phase separation.
! CAUTION Phase separation rounds required to clean the interface could need optimization if more starting material is used.
Recovering the cleaned interface ● Timing 12 min
! CAUTION The interface can be precipitated immediately or further cleaned. Fast precipitation of the interface (described in Steps 19−24 below) is the most consistent method and is recommended when comparing between samples. Nevertheless, precipitated interfaces contain traces of phenol that can inhibit enzymatic activity downstream. If the interface will be used as a starting material for further applications, or if highly phenol-sensitive enzymes need to be used, we recommend further steps are taken to clean the interface (see How to solubilize the interface in Supplementary Information). Unfortunately, these steps might introduce more variability between samples due to the further handling steps.
-
19
Add 9:1 (vol/vol, methanol:sample) of methanol to the interfaces (e.g. for a 100-μl sample, add 900 μl of methanol).
-
20
Invert the tubes three times.
-
21
Vortex the tubes for 15 s at maximum speed.
-
22
Pellet the interface by centrifugation for 10 min at 14,000g at RT (or 4°C).
-
23
Completely remove the supernatant and discard it.
-
24
(Optional) Add 1 ml of 100% methanol, repeat the 10-min centrifugation at 14,000g and discard the supernatant to further clean the interface.
! CAUTION Visually inspect the sample to assess whether it is contaminated with organic phase traces (e.g. pink in color). Samples contaminated with phenol will not be efficiently cleaned with C18 de-salting and might jeopardize LC-MS/MS.
◼ PAUSE POINT Precipitated interfaces can be stored at −80 °C for 1 year.
Recovering protein-bound RNA ● Timing 3.5 h
▲ CRITICAL Follow this part of the protocol only if you want to recover the protein-bound RNA. Protein-bound RNA is used to estimate the cross-linking efficacy.
-
25
Prepare a mix of proteinase K solution by adding 15−30 μl of proteinase K to 300 μl of proteinase K buffer (per sample).
-
26
Incubate the enzyme and buffer mix in the thermoshaker for 15 min at 50 °C at 400 r.p.m.
▲ CRITICAL STEP Incubation of the enzyme and buffer mix removes any RNase contamination of the buffers. This is an important step considering the temperature and length of the incubation.
-
27
Add 300 μl of proteinase K and buffer mix to the precipitated interface from Step 24.
▲ CRITICAL STEP The interface is really sticky at this point. Do not try to solubilize it with the pipette. Proteinase K digests the proteins present in the interface without the need to solubilize it beforehand.
-
28
Incubate in the thermoshaker for 2 h at 400 r.p.m. at 50 °C.
-
29
Add 300 μl of phenol:chloroform:isoamyl alcohol (vol/vol, 25:24:1).
! CAUTION Any step including phenol:chloroform:isoamyl alcohol needs to be performed in a fume hood.
-
30
Vortex for 15 s at maximum speed.
-
31
Centrifuge for 15 min at 12,000g at 4 °C.
-
32
Transfer the upper aqueous phase to a new tube.
▲ CRITICAL STEP Avoid the interface.
-
33
Add 50 μl of NaAc and 600 μl of 100% isopropanol.
-
34
Vortex for 15 s at maximum speed.
-
35
(Optional) Incubate for 10 min in ice. Ice incubation helps nucleic acid precipitation.
-
36
Precipitate the protein-bound RNA by centrifugation at maximum speed for 10 min at 4 °C.
-
37
Remove and discard all the supernatant by pipette.
! CAUTION The pellet might be translucent at this step. Do not disturb it.
-
38
Add 900 μl of 100% ethanol without disturbing the pellet.
-
39
Centrifuge for 5 min at maximum speed at 4 °C.
-
40
Remove and discard all the supernatant by pipette.
-
41
Add 900 μl of 70% (vol/vol) ethanol.
-
42
Centrifuge for 5 min at maximum speed at 4 °C.
-
43
Remove and discard all the supernatant by pipette.
-
44
Air dry for 5 min at RT.
-
45
Resuspend the pellet in the appropriate volume of RNase-free water (e.g. 100 μl of water for a starting material of 8 × 106 human cells).
◼ PAUSE POINT Protein-bound RNA can be stored at −80 °C for 1 year.
-
46
RNA integrity can be determined with a bioanalyzer (following manufacturer instructions) or in 1% agarose gel (wt/vol). RNA can be stored at −80 °C for up to 1 year
? TROUBLESHOOTING
Recovering RNA-binding proteins for western blotting ● Timing 6 h
▲ CRITICAL Protein analysis by electrophoresis-based methods requires integrity of the proteins. This part of the protocol has been optimized to maintain protein integrity at the expense of a potential reduced protein recovery. Follow this part of the protocol only for analysis by western blotting.
-
47
Add 100 μl of 100 mM TEAB and 1% (wt/vol) SDS to the pellets (Step 24).
! CAUTION Pellets are difficult to resuspend. Pay careful attention to any observed portion of pellet stuck on the walls of the tube. If present, pipette the buffer onto the tube wall until all portions of the stuck pellet are in contact with the buffer. Complete solubilization by pipetting is not required.
-
48
Sonicate in cold water for 15 min using the high setting and 30-s sonication/rest cycles. This will start to break the nucleic acid chains present in the interface.
▲ CRITICAL STEP Sonication degrades RNA. Avoid this step for the RNase-negative control.
-
49
Incubate samples at 95 °C for 5 min. 95-°C incubation helps degrade the RNA of the sample.
▲ CRITICAL STEP Incubation at 95 °C degrades RNA. Avoid this step for the RNase-negative control because the incubation might degrade RNA and increase the detection of RBPs in the negative control.
-
50
Cool samples on ice for 2 min.
-
51
Resuspend the pellets by pipetting. If part of the pellet is still visible, incubate again at 95 °C for 30 s−2 min and cool samples on ice for 2 min.
▲ CRITICAL STEP Incubation at 95 °C degrades RNA. Avoid this step for the RNase-negative control.
-
52
Add 2 μl of RNase A/T1 mix and incubate at 37 °C for 4 h. If an RNase-negative control is performed, do not add RNases to the sample.
-
53
Add 1 ml of TRIzol to the sample.
-
54
Homogenize by vortexing for 15 s at maximum speed.
◼ PAUSE POINT Samples can be stored at −20 °C for 1 month.
-
55
Add 200 μl of chloroform.
-
56
Homogenize by vortexing for 15 s at maximum speed.
-
57
Centrifuge at 12,000g for 15 min at 4 °C.
-
58
Remove and discard the upper aqueous phase and interface.
▲ CRITICAL STEP The interface contains undigested nucleic acids and other interface contaminants. Remove it completely. Alternatively, transfer the lower organic phase, without taking the interface, to a new tube. If the second option is preferred, leave 50−80 μl of organic phase with the interface in the tube to ensure that no interface is transferred to a new tube. Whichever option is chosen, be consistent when comparing between experimental conditions and/or replicates.
-
59Precipitate the organic phase. We have previously used methanol to precipitate proteins5. Ethanol might be preferred owing to lower toxicity. To precipitate the organic phase using methanol, follow option A; to precipitate the organic phase using ethanol, follow option B.
-
(A)Organic phase precipitation using methanol.
-
(i)Transfer 150 μl of the organic phase to a new tube.
-
(ii)Add 1,350 μl of 100% methanol.
-
(iii)Mix the sample by vortexing for 15 s at maximum speed.
-
(iv)Centrifuge at maximum speed for 10 min at 4°C.
-
(v)Remove and discard the supernatant. If visible, the pellet should be small.
-
(vi)Repeat Step 59A(i-v) until all the collected organic phase is precipitated. Use the same tube from Step 59A(i) for all precipitations, transferring 150 μl of the organic phase each time per round of centrifugation.
-
(i)
-
(B)Interface precipitation using ethanol.
-
(i)Transfer 225 μl of the organic phase to a new tube.
-
(ii)Add 900 μl of 100% ethanol.
-
(iii)Mix the sample by vortexing for 15 s at maximum speed.
-
(iv)Centrifuge at maximum speed for 10 min at 4 °C.
-
(v)Remove and discard the supernatant. The pellet should be small if visible.
-
(vi)Repeat Step 59B(i-v) until all the collected organic phase is precipitated. Use the same tube from Step 59B(i) for all precipitations, transferring 225 μl of the organic phase each time per round of centrifugation.
-
(i)
-
60Wash the precipitated sample by adding 1 ml of 80% ethanol. Do not vortex.
-
61Centrifuge at maximum speed for 10 min at 4 °C.
-
62Remove and discard the supernatant and leave tubes to air dry for 2 min.! CAUTION It is common at this stage to not be able to see the pellet.
-
63Add 80−100 μl of 100 mM TEAB and 1% (wt/vol) SDS to the tubes.
-
64Pipette up and down vigorously, making sure to direct the buffer at the walls of the tube.
-
65(Optional) Take 5−10-μl aliquots to measure protein concentration.
-
66Add WB buffer (5×) to a final concentration of 1× and DTT to a final concentration of 50 mM to each sample.
-
67Incubate samples at 75 °C for 10 min (or at 95 °C for 3 min).
-
68Cool samples for 5 min on ice.◼ PAUSE POINT Samples can be stored at -20°C for up to 1 year.? TROUBLESHOOTING
-
(A)
Recovering RNA-binding proteins for LC−MS/MS ● Timing 8−22 h
▲ CRITICAL Protein analysis by MS does not require high levels of protein integrity. This part of the protocol has been optimized to maximize protein recovery at the expense of potential partial protein degradation. Follow this part of the protocol only if the RBP will be analyzed by MS-based techniques.
-
69
Add 100 μl of 100 mM TEAB and 1% (wt/vol) SDS to the pellets from Step 24.
-
70
(Optional) Sonicate in ice water for 15 min using the high setting and 30-s sonication/rest cycles. Sonication starts breaking the nucleic acid chains present in the interface.
▲ CRITICAL STEP Sonication degrades the RNA. Avoid this step for the RNase-negative control. RNA degradation will increase detection of RBPs in the negative control.
-
71
Incubate at 95 °C for 20 min. 1 mM MgCl2 (final concentration) can be added to enhance the RNA hydrolysis, minimally affecting the RNase activity.
▲ CRITICAL STEP 95 °C degrades the RNA. Avoid this step for the RNase-negative control. Do not add MgCl2 to the RNase-negative controls.
-
72
Cool samples on ice for 2 min.
-
73
Add 1 μl of RNase A/T1 mix and incubate at 37 °C for 2−4 h. Do not add RNase to RNase-negative control samples.
-
74
Add 1 μl of RNase A/T1 mix and incubate at 37 °C for 8−16 h. If the long incubation needs to be avoided (e.g., when handling parallel RNase-negative controls), digest for 4 h with 2 μl of RNase A/T1 mix.
-
75
Add 1 ml of TRIzol to the sample.
▲ CRITICAL STEP If performing a SILAC RNAse +/− experiment, mix samples in the same 1 ml of TRIzol.
-
76
Homogenize by vortexing for 15 s at maximum speed.
◼ PAUSE POINT Samples can now be stored at −80 °C for 1 year.
-
77
Add 200 μl of chloroform.
-
78
Homogenize by vortexing for 15 s at maximum speed.
-
79
Centrifuge at 12,000g for 15 min at 4 °C.
-
80
Remove and discard the upper aqueous phase and the interface.
▲ CRITICAL STEP The interface contains undigested nucleic acids and other interface contaminants. Remove it completely. Alternatively, leave it in the tube, pass the pipette tip through it and transfer the lower organic phase, without taking the interface, to a new protein low-binding tube. If the second option is preferred, take 450 μl of organic phase from the bottom of the tube, leaving 50−80 μl of organic phase with the interface to ensure that no interface is transferred to the new protein low-binding tube.
-
81
Transfer 150 μl of the organic phase to a new protein low-binding tube.
-
82
Add 1,350 μl of 100% methanol.
-
83
Mix the sample by vortexing for 15 s at maximum speed.
-
84
Centrifuge at maximum speed for 10 min at 4 °C.
-
85
Remove the supernatant. The pellet should be small if visible.
-
86
Repeat Steps 81−85 until all the collected organic phase is precipitated. Use the same tube from Step 81 for all precipitations, transferring 150 μl of the organic phase each time per round of centrifugation.
-
87
(Optional) The organic phase can be precipitated after Step 80 using ethanol precipitation, as explained in Step 59B(i−iv).
-
88
(Optional) Wash the precipitated sample by adding 1 ml of methanol. Do not vortex.
-
89
Centrifuge at maximum speed at 4 °C for 10 min.
-
90
Remove the supernatant and leave tubes to air dry for 2 min. Proceed with sample preparation for MS (Step 90) or store samples at −20 °C.
! CAUTION It is common at this stage to not be able to see the pellet.
◼ PAUSE POINT Samples can be stored at −20 °C for 1 month.
? TROUBLESHOOTING
Sample preparation for MS ● Timing 10-20 h
-
91
Add 50−100 μl of 100 mM TEAB to the sample from Step 90.
-
92
Add DTT from the 300 mM stock solution to obtain a final concentration of 10 mM, vortex vigorously and incubate at 56 °C for 45 min.
-
93
Bring solution to RT and add IAA to a 20 mM final concentration, vortex vigorously and incubate at RT in the dark for at least 45 min.
-
94
Add 1 μg of trypsin to each sample and incubate for 8−16 h at 37 °C.
▲ CRITICAL STEP 1 μg of trypsin is sufficient for up to 50 μg of protein.
▲ CRITICAL STEP When performing quantitative proteomics, ensure that the same amount of enzyme is used in all samples to avoid biasing the quantification. The proportion of enzyme suggested here constitutes up to 5% (wt/wt) of the final sample.
-
95
Quench the reaction by acidification with TFA to a final concentration of 0.5% (vol/vol).
-
96
De-salt the peptides with C18 spin columns according to the manufacturer’s instructions, eluting to a new protein low-binding tube using 50% (vol/vol) ACN.
▲ CRITICAL STEP The 50% ACN elution solution should not contain 0.1% TFA to avoid the acid interfering with Qubit quantitation (or any other peptide quantitation method) when using an aliquot straight from the C18 elution.
-
97
Take 10 μl of the eluted peptides to perform peptide quantity estimation using Qubit protein kit or another peptide quantitation method following the manufacturer’s instructions.
▲ CRITICAL STEP Qubit protein estimation is designed for quantification of proteins rather than peptides. The method is simple and quick and performs well in samples comprised of de-salted peptides in the C18 elution solution with 50−70% ACN without acid and when 10 μl of sample is used per measurement.
▲ CRITICAL STEP When relative quantitation is intended, the peptide quantification should be estimated in parallel for all samples to avoid batch effects.
▲ CRITICAL STEP The enzyme:substrate ratio suggested here constitutes an excess of enzyme compared to the manufacturer’s instructions. This is intended to avoid the need for further cleanup steps to ensure complete removal of phenol, which can affect the enzyme activity.
? TROUBLESHOOTING
-
98
Aliquot the volume required for TMT-labeling (5−50 μg) or LC–MS/MS analysis (up to 2 μg per run) to a new protein low-binding tube.
▲ CRITICAL STEP Ensure that equal quantities of peptides are obtained across all conditions and replicates to avoid bias.
-
99
Lyophilize the de-salted peptides by vacuum centrifugation without heating for 30 min–2 h.
◼ PAUSE POINT Samples can be stored at −20 °C for 1 month.
-
100
(Optional) Proceed with TMT labeling (following the manufacturer’s instructions) followed by pre-fractionation with Pierce High pH Reversed-Phase Peptide Fractionation Kit following the manufacturer’s protocols.
-
101
Resuspend the samples in 0.1% formic acid (vol/vol) and submit to LC-MS/MS.
Troubleshooting
Troubleshooting advice can be found in Table 1.
Timing
Step 1, preparing cells for cross-linking: 1 min for adherent cells and 15 min for non-adherent cells
Step 2, UV cross-linking to induce covalent RNA−protein adducts: 30 s−4 min depending on the length of the UV dosage
Step 3, cell lysis: 6 min for adherent and non-adherent cells without a cell wall and 15 min for non-adherent cells with a cell wall
Steps 4−18, phase separation and interface cleaning: 1 h
Steps 19−24, recovering the cleaned interface: 12 min
Steps 25−46, recovering the protein-bound RNA: 3.5 h
Steps 47−68, recovering RNA-binding proteins for western blotting: 6 h
Steps 69−90, recovering RNA-binding proteins for LC-MS/MS: 8−22 h if the enzymatic treatment is done overnight
Steps 91−101, sample preparation for MS: 10−20 h if the enzymatic treatment is done overnight
A detailed timing visualization is shown (Fig. 2).
Anticipated results
This protocol can be used to recover protein-cross-linked RNA (Step 46) and non-cross-linked RNA (Step 7), as well as RNA-cross-linked protein (Step 90) and non-cross-linked proteins (Step 9), all from the same sample.
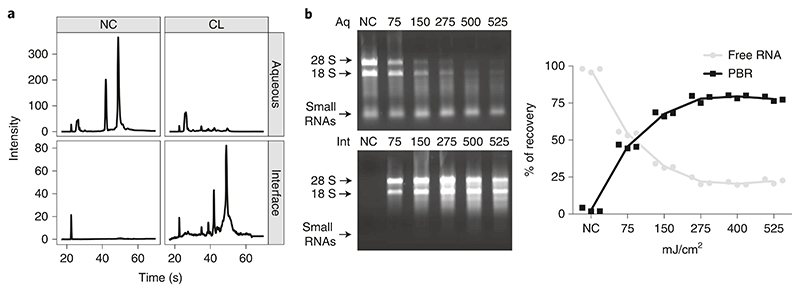
After proteinase K treatment, the RNA profile on an agarose gel or Bioanalyzer for the cross-linked and non-cross-linked RNA should look similar (Fig. 5a). Nevertheless, the area of the ribosomal RNA peaks might be broader because of the remaining amino acids attached to the RNA. Minor peaks might appear below the 18S when cross-linking. This does not affect transcriptomic results. The amount of RNA present in the interface of a non-cross-linked sample should not represent more than 2−5% of the RNA in the interface of a cross-linked sample (Fig. 5b). If more than 5% is detected, refer to ‘Troubleshooting’ (Table 1).
Fig. 5. OOPS RNA recovery.
a, Representative Bioanalyzer RNA profile in the aqueous phase and interfaces of non-cross-link (NC) and cross-link (CL) samples (adapted from ref. 5), after proteinase-K digestion. b, Left: representative image of a 1% agarose (wt/vol) gel showing the main ribosomal RNA bands; UV dosage in mJ/cm2 is indicated per column (top: first aqueous phase (Aq; free RNA); bottom: interfaces (Int; protein-bound RNA)). Right: relative RNA cross-link proportion at different UV dosage measured using Nanodrop (n = 3), adapted from ref. 5.
One of the most limiting steps to obtain RNP complexes is the optimization of the cross-linking efficacy. Cross-linking efficacy can be inferred by the proportion of RNA that is contained in the interface upon different length of exposure to UV.
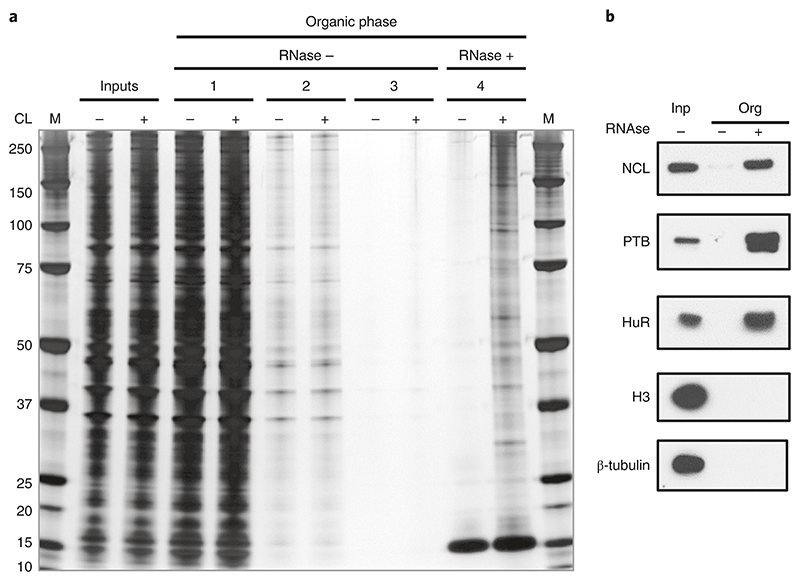
With 75% of the RNA cross-linked, more than 10 μg of RBPs are usually obtained from 1 × 107 human adherent cells, whereas a non-cross-linked control should retrieve minimal (if detectable) protein content (Fig. 6). As a guide, ~1,500 RNA-interacting proteins (excluding glycoproteins) are detected per OOPS experiment with human cell lines, starting from more than 10 μg of RBPs.
Fig. 6. Silver staining illustrating different stages of the OOPS protocol.
a, The OOPS protocol was performed on non-cross-linked (CL-) and cross-linked (CL+) U2OS cells, and protein samples were obtained from the following stages: cell lysates before separation (inputs), organic phases after each of the first three rounds of TRIzol separation (organic phases 1, 2 and 3) and the organic phases after RNase and final TRIzol separation (organic phase 4). Samples were run, along with a protein ladder (M), on a 4−12% Bis-Tris gel, which was then silver stained. b, Representative western blots for positive RBP controls (NCL; Nucleolin, 100KDa, PTB; 60KDa and HuR; 36KDa) and negative-control proteins (H3; Histone-H3; 18KDa and β-tubulin; 55 KDa). Anti-nucleolin (1:1,000, Clone 4E2, GeneTex, GeneTex, cat. no. GTX13541, RRID:AB_372550); anti-PTB (1:1,000, RRID:AB_2827814); #GTX13541); anti-HuR (1:1,000, Santa Cruz Biotechnology, cat. no. sc-5261, RRID:AB_627770); anti-histone H3 (1:1,000, Bethyl, cat. no. A300−823A, RRID:AB_2118462); and anti-β-tubulin (1:1,000, Cell Signaling Technology, cat. no. 2146, RRID: AB_2210545). Adapted from ref. 5.
When performing LC-MS/MS analysis using SILAC, we recommend not demanding the detection of SILAC pairs as a prerequisite for MS2 fragmentation. Following this advice, three populations of proteins are expected: proteins with no enrichment over the RNase- or crosslink-negative control (contaminants); proteins with enrichment over the negative control (RBPs); and proteins with no detectable signal in the negative control (highly enriched RBPs). OOPS is a highly efficient RBP enrichment technique that frequently yields no detectable signal in negative-control samples. If this is consistent between replicates, these proteins can be considered as an RBP. Figure 7a shows the expected SILAC ratios for crosslink +/− and RNase +/− experiments.
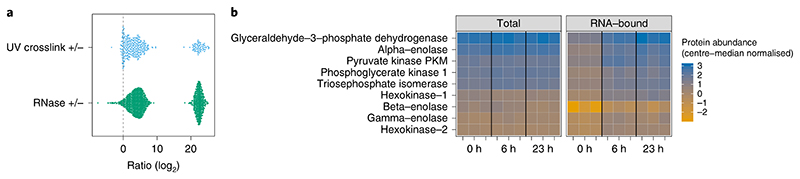
Fig. 7. OOPS MS results.
a, Expected results for SILAC cross-link (CL) +/− and RNase +/− experiments using U2OS cells. Proteins with no detectable signal in the negative-control sample are given a pseudo-value equivalent to the average intensity in the CL or RNase samples, respectively. These pseudo-values represent proteins highly enriched in the CL or RNase conditions. log ratios close to zero represent proteins without clear enrichment, which might be considered to be non-RNA-binding. Adapted from ref. 5, n = 4. b, Visualization of parallel total and RNA-bound protein abundances in a nocodazole arrest (0 h) and release (6 h and 23 h) experiment. Protein abundances are center-median normalized within each sample and are thus relative to the total protein abundance in a given sample and only comparable within total and RNA-bound quantifications, not between them. Shown here are the members of the glycolysis pathway. A clear increase in RNA-bound protein abundance between arrest and release time points is observed for many pathway members without any appreciable change in total protein abundance. n = 3 for each time point. Data presented are taken from ref. 5 and made available alongside plotting code in an R markdown notebook (see Supplementary Data Set 1).
When quantifying RBPome dynamics across conditions using parallel quantification of total protein abundance and RNA-bound protein abundance with OOPS, one can apply a linear model framework to identify proteins with a significant change in RNA binding, while taking into account any concurrent change in protein abundance (see Supplementary Information and Supplementary Data Set 1). For most biological stimuli, the proteins of interest will be those with little or no change in total protein abundance and a reproducible change in RNA-bound abundance. Figure 7b shows the total and RNA-bound abundances of glycolysis pathway proteins in a previously published data set5.
Supplementary Material
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Acknowledgements
E.V., T.S., R.Q., R.F.H., M.P., M.R., V.D., M.M. and M.E. are supported by the Medical Research Council, grant/award no. 5TR00, and by the Wellcome Trust, grant/award nos. 110170/Z/15/Z and 110071/Z/15/Z.
Footnotes
Author contributions
All author contributions are based on CRediT standards. E.V.: conceptualization, methodology, writing—original draft, visualization, project administration and writing—review and editing. T.S.: conceptualization, methodology, writing—original draft, visualization, data curation, formal analysis and writing—review and editing. R.M.L.Q.: conceptualization, methodology, writing—original draft and writing—review and editing. M.M.: conceptualization, methodology, investigation, visualization, writing—original draft and writing— review and editing. M.P.: conceptualization, methodology, investigation, visualization and writing—original draft. M.E.: writing—original draft. V.D.: writing—original draft. R.F.H.: writing—original draft. M.R.: writing—original draft. A.E.W.: writing—original draft, project administration and funding acquisition. K.S.L.: conceptualization, writing—original draft, project administration and funding acquisition.
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data availability
All data presented herein are taken from ref. 5. The specific data sets presented in Fig. 7 are additionally made available alongside the plotting code in Supplementary Data Set 1.
References
- 1.Harvey RF, et al. Trans-acting translational regulatory RNA binding proteins. Wiley Interdiscip Rev RNA. 2018;9:e1465. doi: 10.1002/wrna.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossoll W, et al. Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: a role for Smn in RNA processing in motor axons? Hum Mol Genet. 2002;11:93–105. doi: 10.1093/hmg/11.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Hanson KA, Kim SH, Tibbetts RS. RNA-binding proteins in neurodegenerative disease: TDP-43 and beyond. WIREs RNA. 2012;3:265–285. doi: 10.1002/wrna.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira B, Billaud M, Almeida R. RNA-binding proteins in cancer: old players and new actors. Trends Cancer. 2017;3:506–528. doi: 10.1016/j.trecan.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Queiroz RML, et al. Comprehensive identification of RNA–protein interactions in any organism using orthogonal organic phase separation (OOPS) Nat Biotechnol. 2019;37:169–178. doi: 10.1038/s41587-018-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trendel J, et al. The human RNA-binding proteome and its dynamics during translational arrest. Cell. 2019;176:391–403. doi: 10.1016/j.cell.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Urdaneta EC, et al. Purification of cross-linked RNA–protein complexes by phenol-toluol extraction. Nat Commun. 2019;10:990. doi: 10.1038/s41467-019-08942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castello A, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Baltz AG, et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018;19:327–341. doi: 10.1038/nrm.2017.130. [DOI] [PubMed] [Google Scholar]
- 11.Smith T, et al. Organic phase separation opens up new opportunities to interrogate the RNA-binding proteome. Curr Opin Chem Biol. 2020;54:70–75. doi: 10.1016/j.cbpa.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Zecha J, et al. TMT labeling for the masses: a robust and cost-efficient, in-solution labeling approach. Mol Cell Proteomics. 2019;18:1468–1478. doi: 10.1074/mcp.TIR119.001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAlister GC, et al. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem. 2014;86:7150–7158. doi: 10.1021/ac502040v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashev IG, Dimitrov SI, Angelov D. Crosslinking proteins to nucleic acids by ultraviolet laser irradiation. Trends Biochem Sci. 1991;16:323–326. doi: 10.1016/0968-0004(91)90133-g. [DOI] [PubMed] [Google Scholar]
- 15.Steube A, Schenk T, Tretyakov A, Saluz HP. High-intensity UV laser ChIP-seq for the study of protein–DNA interactions in living cells. Nat Commun. 2017;8:1303. doi: 10.1038/s41467-017-01251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leo G, et al. Ultraviolet laser-induced cross-linking in peptides: UV laser-induced cross-linking in peptides. Rapid Commun Mass Spectrom. 2013;27:1660–1668. doi: 10.1002/rcm.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.König J, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol. 2010;17:909–915. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shchepachev V, et al. Defining the RNA interactome by total RNA-associated protein purification. Mol Syst Biol. 2019;15:e8689. doi: 10.15252/msb.20188689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 20.Boja ES, Fales HM. Overalkylation of a protein digest with iodoacetamide. Anal Chem. 2001;73:3576–3582. doi: 10.1021/ac0103423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented herein are taken from ref. 5. The specific data sets presented in Fig. 7 are additionally made available alongside the plotting code in Supplementary Data Set 1.