Abstract
Synthetic materials are an everyday component of modern healthcare yet often fail routinely as a consequence of medical device-centred infections. The incidence rate for catheter-associated urinary tract infections is between 3 and 7% for each day of use, which means that infection is inevitable when resident for sufficient time. The O’Neill Review on antimicrobial resistance estimates that, left unchecked, 10 million people will die annually from drug-resistant infections by 2050. Development of biomaterials resistant to bacterial colonisation can play an important role in reducing device-associated infections. However, rational design of new biomaterials is hindered by the lack of quantitative structure-activity relationships (QSAR). Here we report the development of a predictive QSAR for bacterial biofilm formation on a range of polymers, using calculated molecular descriptors of monomer units to discover and exemplify novel, biofilm-resistant (meth)acrylate-based polymers. These predictions are validated successfully by the synthesis of new monomers which we polymerize to create coatings found to be resistant to biofilm formation by six different bacterial pathogens: Pseudomonas aeruginosa, Proteus mirabilis, Enterococcus faecalis, Klebsiella pneumoniae, Escherichia coli and Staphylococcus aureus.
Keywords: low-fouling, polymer microarray, QSAR, transesterification, biofilms
Graphic abstract. A quantitative structure activity relationship is extrapolated to develop a biomaterial capable of reducing biofilm formation outperforming previously identified hit biomaterials.
A high efficacy for reducing biofilm formation of six clinically relevant bacterial species to catheter associated urinary tract infections is observed with an average 55-fold reduction compared to clinically used silicone catheters.
The concept of the post-antibiotic era is becoming a reality, with patients presenting with infections from bacterial pathogens that resist multiple last line antibiotics while at the same time, very few novel antimicrobial drugs are coming onto the market.[1–5] As multi-antibiotic resistance becomes widespread,[6] the prevention of bacterial infections has become an urgent healthcare priority in order to reduce morbidity and mortality.[7–9] Medical device-related infections are a consequence of planktonic bacteria attaching to the surface of implanted devices and developing into surface-associated ‘slime’ layers called biofilms, which have been reported to be 1000 times more resistant to antibiotic and host immune system clearance.[10–13]
High throughput materials discovery screens have been utilised to discover polymers that reduce bacterial surface colonization in order to circumvent our poor understanding of material surface-bacteria interactions that leaves us ill-equipped to design new materials from first principles.[14] Novel coatings were identified by screening a commercially available (meth)acrylate monomer library for polymers that successfully reduced biofilm formation by P. aeruginosa, S. aureus and E. coli in laboratory cultures in vitro and in vivo in a foreign body mouse infection model.[15,16] Such screening experiments facilitate the rapid assessment of readily available monomers to identify hit materials for a particular application, such as reducing bacterial fouling of surfaces in industry and healthcare, the expansion of pluripotent stem cells, increasing the maturation of cardiomyocyte derived from stem cells, and providing bio-instructive implant materials.[16–19]
To maximize the productivity of materials discovery screening campaigns, the monomer building blocks should ideally be cheap, easy to process and readily accessible, i.e. not requiring time consuming bespoke synthesis. Acrylate/acrylamide monomers that are commercially available with wide chemical diversity (hundreds of different compounds) are suitable for this as they are readily printable and amenable to in situ polymerization.[20,21] To synthesize variants of these materials in-house, we have used a single step, transesterification synthesis (see scheme Figure S1a) that utilises commercially available alcohols (thousands are readily available) to create bespoke monomer building blocks that significantly expand the chemical space relative to the commercial (meth)acrylates used to date. Typical polymer microarray approaches use unbiased screening of as wide a range of materials or chemical space as possible to maximize the chances of identifying hit materials that surpass the performance of existing material solutions. To date, this process has been very successful in identifying a class of monomers that surpass conventional silicone catheters for preventing catheter-associated urinary tract infections, resulting in the granting of a CE mark for a urinary catheter device.[16,19,22] To guide synthesis beyond the commercially available compounds computational modelling has been used to generate structure-function relationships that can predict the biological performance of virtual materials. [23–25] In the case of bacterial biofilm formation a simple parameter combining the partition coefficient (logP) and the number of rotatable bonds for hydrocarbon acrylate pendant groups pointed towards a route to a more targeted approach for materials discovery, although until now this has not been experimentally validated (Figure 1a). [26] Here, we validate a modification of that QSAR by extrapolating from the correlation identified between the bacterial biofilm formation and a monomer molecular descriptor parameter, alpha, to predict novel biofilm resistant monomers which were not included in the initial library.
Figure 1.
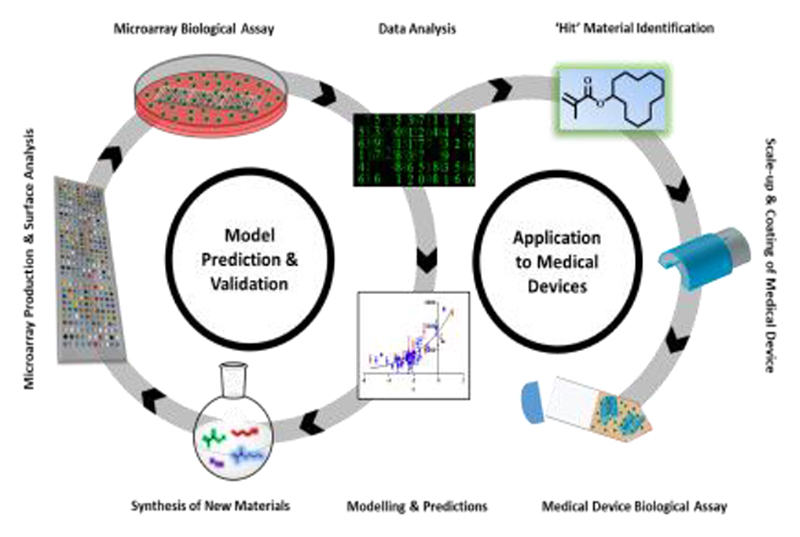
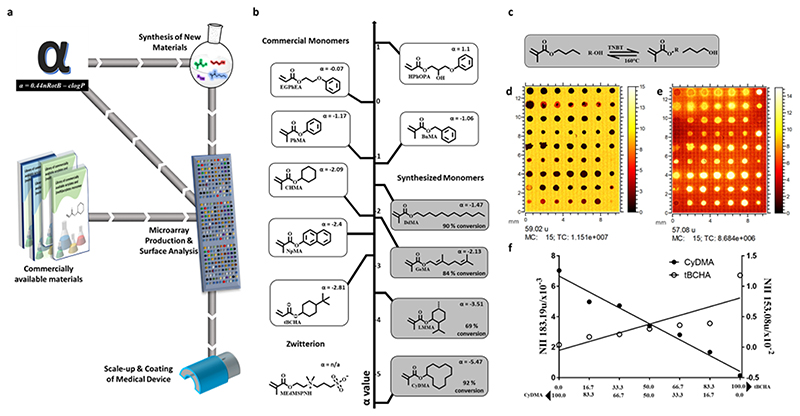
(a) Process for developing and validating a predictive model at the microscale level including testing on a medical device. Hundreds of commercially available materials are assessed for their ability to reduce bacterial biofilm formation via high throughput screening. Processed data is used to identify a ‘hit’ material and scaled-up to coat medical devices for confirmatory in vitro studies. Analysed data together with material properties are used to generate models that predict new untested materials which are synthesized and reincorporated into the materials library for further screening. This repeated cycle refines the theoretical model and makes it a more powerful predictor of ‘hit’ performance. (b) Materials library made up of 12 monomers positioned along the alpha value vertical axis. (c) Reaction scheme for the transesterification synthesis reaction at 160 °C with titanium butoxide (TNBT) catalyst. (d and e) ToF SIMS ion images from one replicate area of the microarray corresponding to (d) C2H3O2- (m/z 59.02) and (e) C4H9+ (m/z 57.07) both displayed from 0-15 normalized counts. (f) Graphs of normalized ion intensity (NII) for representative SIMS ions for copolymers of tBCHA with CyDMA, The content (%) of each monomer is indicated below graph. The representative ion for tBCHA is C10H17O- indicative of tBCHA (R2 = 0.68) and C12H23O- indicative of CyDMA (R2 = 0.97).
Alpha comprises the log of the calculated partition coefficient (clogP) and the number of rotatable bonds (nRotB), alpha = 0.44nRotB – clogP for each monomer previously developed from a polymer microarray library.[26] Using this as a starting point, a polymer of the novel monomer cyclododecyl methacrylate (CyDMA) was predicted to offer improved prevention of biofilm formation by virtue of its low (-5.47) alpha value. Here we confirm this prediction both using polymer microarray screening experiments and subsequent multi-gram synthesis of both monomer and polymer. The alpha parameter was also shown to be valid for a range of other bespoke materials synthesised with values outside of the previously accessed range using commercially available monomers (of -2.81 to 1.10). Upon scale-up, pCyDMA produced an average 55-fold reduction in individual surface coverage for all six bacterial pathogens compared with silicone catheters, and 14-fold compared to silver hydrogel coated catheters. pCyDMA coatings on average showed a 4-fold reduction in biofilm formation for the six pathogens compared to a previously identified hit polymer coating (poly(ethylene glycol dicyclopentyl ether acrylate-co-diethyleneglycol methacrylate p(EGDPEA-co-DEGMA)).
The molecular structures of several monomers with low alpha parameter values were identified for synthesis, shown in Figure 1b, to expand the chemical space in order to identify improved materials. This also tested the validity of correlation between bacterial biofilm formation and the alpha parameter beyond the initial library limited to commercially available compounds. An alpha value of -5.47 was calculated for CyDMA and -3.51 for 5-methyl-2-(1-methylethyl) cyclohexyl methacrylate (LMMA), which is lower than all materials previously tested (alpha = -3 to 1).[26]
Four monomers were successfully synthesized using the one-step transesterification route (Figure S1a), (CyDMA, trans-3, 7-dimethyl-2, 6-octadienyl methacrylate (GeMA), LMMA, Dodecyl methacrylate (DdMA)) outlined in Figure 1b, with conversions of 92, 82, 69 and 92% respectively (see Figure S1-5 for NMR spectroscopic studies). In addition to the four synthesized monomers, eight commercially available monomers that had been previously tested were also included for comparison. A polymer microarray was printed using 11monomers with an alpha value range of -5.47 to 1.1 mixed pairwise with tert-butyl cyclohexyl acrylate (tBCHA) to produce unique polymer spots printed in triplicate on a single slide after photo polymerisation with an alpha value range of -5.47 to 1.1 (Figure 1b). Time-of-flight secondary ion mass spectrometry (ToF-SIMS) was employed to monitor spot printing fidelity and provide surface analysis to detect any surface segregation due to de-mixing of monomers (Figure 1d-f).[27] Unique ions were identified for most monomers and used to monitor the relationship between monomer feed ratio and the surface composition of the polymer product. The ion intensities indicate linear trends for the SIMS ion intensity versus composition when mixing the two monomers, cyclododecyl methacrylate (CyDMA) and tert-butyl cyclohexyl acrylate (tBCHA) (Figure 1f) with correlation coefficients of R2 = 0.97 and R2 = 0.68 respectively. In both cases the pure monomers exhibit slightly higher intensity than predicted by a straight-line fit to all the data, suggesting possible matrix effect non-linearity. All the copolymer series were found to approximate to the bulk ratios apart from 6-octadienyl methacrylate (GeMA) and ethylene glycol phenyl ether acrylate (EGPhEA) where surface segregation when mixed with tert-butyl cyclohexyl acrylate was inferred (Figure S8). This is likely due to differences in the miscibility of the monomers causing phase separation before polymerisation is complete -copolymers which exhibited this were not studied further herein. [28]
To determine whether unreacted reagents (monomers and their associated alcohols) could inhibit bacterial growth and hence reduced bacterial attachment and biofilm formation, P. aeruginosa was cultured over 24 h in RPMI media dosed with monomers and the associated alcohols at concentrations of 0.01 to 0.1% (v/v) (Figure S9 and S10). Further bacterial growth studies were conducted on the catalysts and the scaled-up monomers for all six pathogens (Figure S11 and S12). No bacteriostatic or bactericidal effects were observed, with the exception of 5-methyl-2-(1-methylethyl) cyclohexyl methacrylate (LMMA) which caused a 50% reduction in growth compared with the untreated control. Therefore, materials containing LMMA were omitted from the subsequent polymer microarray analysis. It is also possible that esterase enzymes produced by bacteria could cleave and release pendant alcohol groups that contribute to the lack of biofilm formation on resistant materials. To investigate whether this was a factor, degradation of benzyl methacrylate polymer sample was quantified after treatment with fast-acting porcine liver esterase (PLE) over 2 h; no degradation was observed for the polymers, in contrast to the monomers which were readily cleaved (Figure S13-16). It is likely that the increased steric hindrance and/or the electronic stability of the ester moiety in poly(acrylates) prevented cleavage of the polymer side chains. These results suggest that the biofilm inhibition observed on all polymers (excepting LMMA) was not influenced by soluble compounds released from the materials, but instead by the response of the bacterial to the polymer surface.
The human pathogenic bacteria P. aeruginosa, Pr. mirabilis and S. aureus were chosen for initial biofilm formation experiments with polymer microarrays, shown in Figure 2, as they include both gram-positive (S. aureus) and gram-negative (P. aeruginosa, Pr. mirabilis) bacterial species, and are frequently associated with healthcare-associated infections including catheter-associated urinary tract infections (CAUTI).[29]
Figure 2.
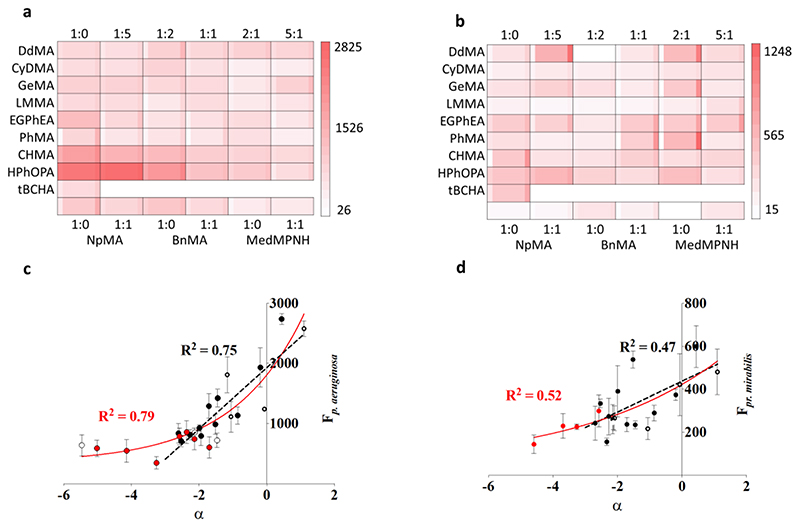
(a) Results from polymer microarray biological assay with mCherry tagged P. aeruginosa. Monomer identity is organised into rows and mixing ratio columns with tert-Butyl cyclohexyl acrylate (tBCHA). The center square is the fluorescence value for attachment of P. aeruginosa (red indicating high bacterial adhesion and white low bacterial adhesion), whilst the narrow columns to the left or right indicate ± 1 sd unit respectively (N = 3). (b) Results from the same experiment as (a) but with dsRed tagged Pr. mirabilis (n = 3). (c) Fluorescence intensity measured from P. aeruginosa attachment to the polymer microarray. The unfilled/solid black symbols represent materials used to build the alpha model (homopolymers/copolymers respectively), the dashed black line is the linear relationship from -3 to 1.1, R2 = 0.75. The red unfilled/solid symbols are materials used to extend alpha range (homopolymers/copolymers respectively) and red solid line is exponential relationship with values from -5.47 to 1.1, R2 = 0.79, error bars shown are ± 1 sd (n = 3) (d) Fluorescence from Pr. mirabilis attachment to the polymer library using the same display conventions as for (c).
A linear correlation (R2 = 0.77) was observed between the bacterial load measured as fluorescence intensity of P. aeruginosa versus alpha between -3 and 1.1 (Figure 2c).[26] However, when materials in the extended range of alpha of -5.47 to 1.1 were considered, a poorer linear correlation (R2 = 0.61) was observed. As alpha decreased, bacterial fluorescence approached a lower limit under these microarray assay conditions. An exponential fit better described the relationship between alpha and P. aeruginosa attachment for the full polymer library dataset (R2 = 0.79) (Figure 2c). While weaker correlation was observed for Pr.mirabilis (R2 = 0.52) (Figure 2d), these correlations suggest that both species respond to the physicochemical properties described by the alpha value (hydrophilicity and molecular rigidity). Attachment of S. aureus, a non-motile gram-positive bacterial species, showed no correlation with alpha suggesting that other factors are dominant (Figure S17). As the lowest alpha parameter materials were statistically similar for both bacterial species, the CyDMA homopolymer was taken forward for further investigation as a coating on silicone catheter segments in order to carry out initial comparison with the biofouling performance of existing devices.
Polymerisation of CyDMA was undertaken using thermal polymerisation to produce a polymer solution with which to dip-coat silicone catheter segments. For comparison using the same scale-up test, a high performing acrylate copolymer material previously reported in the literature to prevent bacterial biofilm formation, poly(ethylene glycol dicyclopentyl ether acrylate-co-diethyleneglycol methacrylate p(EGDPEA-co-DEGMA), was similarly polymerised and coated onto silicone catheter segments.[16] An example of a high alpha value polymer, neopentyl glycol propoxylate diacrylate (NGPDA) was polymerised using the same route and coated onto catheter sections: NGPDA has an alpha value of 2.96 compared to CyDMA (α = -5.47). Uncoated silicone catheter and silver hydrogel coated catheters were commercially sourced and used as comparators that are applied in clinical practice. To mimic environmental conditions associated with CAUTI, bacteria were cultured in artificial urine (AU) with the 5 different surfaces to quantify both single and mixed species bacterial biofilm formation with 6 clinically relevant bacterial species: P. aeruginosa, Pr. mirabilis, E. faecalis, K. pneumoniae, E. coli and S. aureus (Figure 3).
Figure 3.
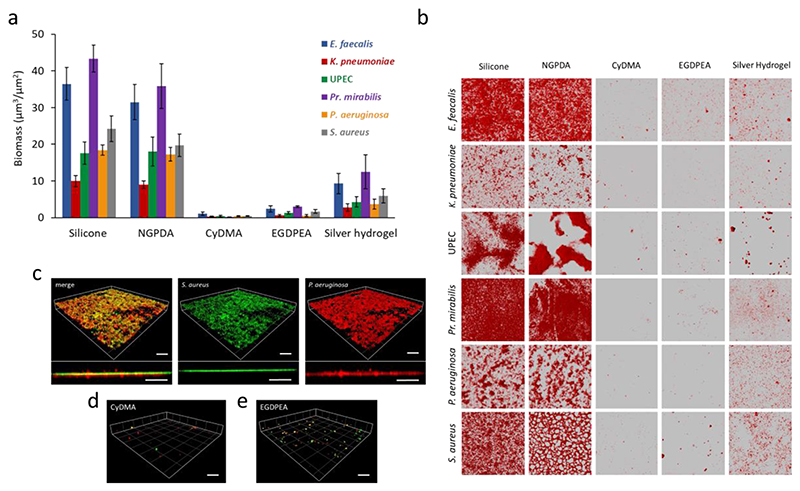
(a) Surface coverage by single species (E. faecalis, K. pneumoniae, E. coli, Pr. mirabilis, P. aeruginosa and S. aureus) biofilms quantified after 72 h incubation on silicone, silver hydrogel, pNGPDA, pCyDMA and p(EGDPEA-co-DEGMA) (labelled EGDPEA) coated silicone catheter segments in AU. Error bars equal ± 1 sd unit, n = 3. (b) The corresponding confocal microscopy images for Syto64 stained E. faecalis, K. pneumoniae, E. coli, Pr. mirabilis, P. aeruginosa and S. aureus growing on each polymer surface. Each image is 160 x 160 μm. (c) 3D representation and transverse view of a dual-species biofilm formed on glass: GFP-tagged S. aureus SH1000 (green) and mCherry labelled P. aeruginosa (red) in a 10:1 ratio. (d)-(e) Three dimensional representation and transverse view showing the lack of mature biofilm on pCyDMA and pEGDPEA. Scale bars represent 50μm.
On silicone catheter segments a mean bacterial biomass (averaging all 6 strains) of 25 ± 2.9 μm3/μm2 was observed compared with the reduced load of 6.4 ± 2.2 μm3/μm2 on a commercial silver hydrogel coated catheter (Figure 3a). In contrast, the pCyDMA coated catheter segment successfully prevented biofilm formation by all six individual bacterial species with a mean biomass of only 0.5 ± 0.2 μm3/μm2 as shown in Figure 3a-b. The p(EGDPEA-co-DEGMA) [16] coating also performed well, with a mean biomass of 1.6 ± 0.4 μm3/μm2, although this was surpassed in performance by the pCyDMA discovered in this QSAR process. The high alpha value polymer, pNGPDA, performance was consistent withthe microarray relationships identified, with an average biomass of 21.9 ± 3.4 μm3/μm2, showing an approximate 44-fold greater biomass compared with pCyDMA.
Most CAUTI infections are polymicrobial.[30] To test the biofilm resistance of a dual species biofilm, we used GFP-labelled S. aureus SH1000 (green) and mCherry labelled P. aeruginosa (red) inoculated onto the catheter segments in a 10:1 ratio. Figure 3c-e shows that for silicone, pCyDMA and p(EGDPEA-co-DEGMA) after 72h incubation, the dual species biofilm completely covered the silicone surface, whereas very little biofilm coverage was observed on p(EGDPEA-co-DEGMA) and was almost completely absent on pCyDMA and the quantification of this mixed biofilm can be found in Figure S18.
In summary, we have used a QSAR model (alpha) to successfully predict bacterial biofilm formation on a new polymer (pCyDMA) which we synthesised along with others using a polymer microarray. Coating of catheter segments with pCyDMA reduced biofilm formation by 6 commonly associated urinary tract pathogens by 32 to 300 fold (average 55 fold) compared with an uncoated silicone catheter and 9 – 85 fold (average 14 fold) compared with a commercial silver hydrogel coated catheter. This polymer coating outperformed our previously identified best polymer (pEGDPEA) determined by experimental screening using a high throughput discovery approach with an average 4-fold improvement. Furthermore, pCyDMA was also shown to prevent the formation of dual-species biofilm in artificial urine, exemplifying the uses of the material in a more realistic medical device associated infection scenario. This illustrates how validated QSAR models with simple physical molecular descriptions can be used to predict novel materials that improve on previously established materials that have great potential to reduce medical device associated infections.
Experimental Section
Materials
All materials were used as supplied without further purification unless otherwise stated in the preparations detailed below
Transesterification Experiments
In a typical reaction butyl methacrylate (Sigma-Aldrich) (14 g, 98.5 mmol) was introduced into a 50 ml vessel along with the required quantity of individual target alcohols [cyclododecanol (VWR) (13 g, 70.5 mmol), trans-3,7-dimethyl-2,6-octadien-1-ol (10.8 g, 70.3 mmol) 5-methyl-2-(1-methylethyl) cyclohexanol (11.2 g, 71.6 mmol) or 1-dodecanol (sigma-aldrich) (13.1 g, 70.3 mmol)] to form a 7:5 molar ratio. Then titanium butoxide (Sigma-Aldrich) catalyst at a concentration of 1% by molar ratio (relative to butyl methacrylate) and 1000 ppm of 4-methoxyphenol (Sigma-Aldrich) inhibitor were added. The reaction was heated to 160 °C and stirred for 45 min at which point a nitrogen gas sparge was introduced to increase the rate of removal of butanol by-product. Butyl methacrylate has been chosen as the methacrylate precursor for these reactions to allow these elevated temperatures to drive the reaction kinetics and equilibrium toward full completion in a short timescale. The reaction was sampled every 15 min and these samples were quenched in a freezer prior to NMR analysis.
Bacterial strains and growth conditions
Pr. mirabilis strain Hauser 1885, P. aeruginosa strain PAO1 (Washington sub-line, Nottingham collection), Staphylococcus aureus SH1000, Enterococcus faecalis NCTC12697, Klebsiella pneumoniae NCIMP10104 and uropathogenic Escherichia coli (UPEC) were routinely grown at 37°C in lysogeny broth (LB) with shaking at 200 rpm or on LB agar (2% w/v). Where required, plasmids for constitutively expressing fluorescent proteins GFP (pBK-miniTn7-egfp) and mCherry (pMMR) were introduced into the relevant host strain by conjugation or electroporation.
Supplementary Material
Acknowledgements
A.A.D. and O.S. contributed equally to this work. M.R.A., D.J.I., and P.W. are the joint senior authors. The authors would like to kindly acknowledge Emma Daffern and Samara Dawson for their assistance in the production of polymer microarrays. This work was supported by the Engineering and Physical Sciences Research Council [grant numbers EP/N006615/1] and the Wellcome Trust [grant numbers 103882 and 103884]. The University of Nottingham is kindly acknowledged for funding Dr Hook’s Nottingham Research Fellowship. All relevant data are available from the University of Nottingham’s Research Data Management Repository.
Contributor Information
Dr Adam A. Dundas, Advanced Medical and Healthcare Technologies School of Pharmacy University of Nottingham Nottingham NG7 2RD, UK.
Olutoba Sanni, Advanced Medical and Healthcare Technologies School of Pharmacy University of Nottingham Nottingham NG7 2RD, UK; Department of Chemical and Environmental Engineering Faculty of Engineering University of Nottingham Nottingham NG7 2RD, UK.
Dr Jean-Frédéric Dubern, Centre of Biomolecular Sciences School of Life Sciences University of Nottingham Nottingham NG7 2RD, UK.
Dr Georgios Dimitrakis, Department of Chemical and Environmental Engineering Faculty of Engineering University of Nottingham Nottingham NG7 2RD, UK.
Dr Andrew L. Hook, Advanced Medical and Healthcare Technologies School of Pharmacy University of Nottingham Nottingham NG7 2RD, UK.
Derek J. Irvine, Department of Chemical and Environmental Engineering Faculty of Engineering University of Nottingham Nottingham NG7 2RD, UK.
Paul Williams Alexander, Centre of Biomolecular Sciences School of Life Sciences University of Nottingham Nottingham NG7 2RD, UK.
Morgan R. Alexander, Advanced Medical and Healthcare Technologies School of Pharmacy University of Nottingham Nottingham NG7 2RD, UK.
References
- [1].Tyers M, Wright GD. Nat Rev Microbiol. 2019;17:141. doi: 10.1038/s41579-018-0141-x. [DOI] [PubMed] [Google Scholar]
- [2].Lee JYH, Monk IR, Gonçalves da Silva A, Seemann T, Chua KYL, Kearns A, Hill R, Woodford N, Bartels MD, Strommenger B, Laurent F, et al. Nat Microbiol. 2018;3:1175. doi: 10.1038/s41564-018-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Renwick MJ, Brogan DM, Mossialos E. J Antibiot (Tokyo) 2016;69:73. doi: 10.1038/ja.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alanis AJ. Arch Med Res. 2005;36:697. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [5].Schneider EK, Reyes-Ortega F, Velkov T, Li J. Essays Biochem. 2017;61:115. doi: 10.1042/EBC20160058. [DOI] [PubMed] [Google Scholar]
- [6].Torumkuney D, Mayanskiy N, Edelstein M, Sidorenko S, Kozhevin R, Morrissey I. J Antimicrob Chemother. 2018;73:14. doi: 10.1093/jac/dky065. [DOI] [PubMed] [Google Scholar]
- [7].Percival SL, Suleman L, Vuotto C, Donelli G. J Med Microbiol. 2015;64:323. doi: 10.1099/jmm.0.000032. [DOI] [PubMed] [Google Scholar]
- [8].Nicolle LE. Antimicrob Resist Infect Control. 2014;3:1. doi: 10.1186/2047-2994-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].O ’Neill J. Comm by UK Gov. 2016 [Google Scholar]
- [10].Costerton JW, Stewart PS, Greenberg EP. Science. 1999;284:1318. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- [11].Bayston R, Fisher LE, Weber K. Biomaterials. 2009;30:3167. doi: 10.1016/j.biomaterials.2009.02.028. [DOI] [PubMed] [Google Scholar]
- [12].Donlan RM. Emerg Infect Dis. 2001;7:277. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hall CW, Mah T. FEMS Microbiol Rev. 2017;41:276. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- [14].Kohn J, Road T. Nat Mater. 2004;3:745. doi: 10.1038/nmat1249. [DOI] [PubMed] [Google Scholar]
- [15].Hook AL, Chang C-Y, Yang J, Luckett J, Cockayne A, Atkinson S, Mei Y, Bayston R, Irvine DJ, Langer R, Anderson DG, et al. Nat Biotechnol. 2012;30:868. doi: 10.1038/nbt.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hook AL, Chang C-Y, Yang J, Atkinson S, Langer R, Anderson DG, Davies MC, Williams P, Alexander MR. Adv Mater. 2013;25:2542. doi: 10.1002/adma.201204936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Patel AK, Tibbitt MW, Celiz AD, Davies MC, Langer R, Denning C, Alexander MR, Anderson DG. Curr Opin Solid State Mater Sci. 2016;20:202. [Google Scholar]
- [18].Tourniaire G, Collins J, Campbell S, Mizomoto H, Ogawa S, Thaburet J-F, Bradley M. Chem Commun. 2006;20:2118. doi: 10.1039/b602009g. [DOI] [PubMed] [Google Scholar]
- [19].Celiz AD, Smith JGW, Patel AK, Hook AL, Rajamohan D, George VT, Flatt L, Patel MJ, Epa VC, Singh T, Langer R, et al. Adv Mater. 2015;27:4006. doi: 10.1002/adma.201501351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Celiz AD, Smith JGW, Patel AK, Langer R, Anderson DG, Barrett DA, Young LE, Davies MC, Denning C, Alexander MR. Biomater Sci. 2014;2:1604. doi: 10.1039/c4bm00054d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Anderson DG, Levenberg S, Langer R. Nat Biotechnol. 2004;22:863. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- [22].Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, Cho S-W, Mitalipova M, Pyzocha N, Rojas F, Van Vliet KJ, et al. Nat Mater. 2010;9:768. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Epa VC, Hook AL, Chang C, Yang J, Langer R, Anderson DG, Williams P, Davies MC, Alexander MR, Winkler DA. Adv Funct Mater. 2014;24:2085 [Google Scholar]
- [24].Vasilevich AS, Carlier A, De Boer J, Singh S. Trends Biotechnol. 2017;35:743. doi: 10.1016/j.tibtech.2017.05.007. [DOI] [PubMed] [Google Scholar]
- [25].Lin S, Ryu S, Tokareva O, Gronau G, Jacobsen MM, Huang W, Rizzo DJ, Li D, Staii C, Pugno NM, Wong JY, et al. Nat Commun. 2015;6:6892. doi: 10.1038/ncomms7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sanni O, Chang CY, Anderson DG, Langer R, Davies MC, Williams PM, Williams P, Alexander MR, Hook AL. Adv Healthc Mater. 2015;4:695. doi: 10.1002/adhm.201400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hook AL, Scurr DJ, Burley JC, Langer R, Anderson DG, Davies MC, Alexander MR. J Mater Chem B. 2013;1:1035. doi: 10.1039/c2tb00379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hook AL, Yang J, Chen X, Roberts CJ, Mei Y, Anderson DG, Langer R, Alexander MR, Davies MC. Soft Matter. 2011;7:7194. doi: 10.1039/C1SM06063E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Donlan RM, Costerton JW. ClinMicrobiol Rev. 2002;15:167. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Römling U, Balsalobre C. J Intern Med. 2012;272:541. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.