Abstract
Aims
Data regarding the effects of regular alcohol consumption on cardiac anatomy and function are scarce. Therefore, we sought to determine the relationship between regular alcohol intake and cardiac structure and function as evaluated with cardiac magnetic resonance (CMR) imaging.
Methods and results
Participants of the UK Biobank with full manual contouring of CMR data were enrolled in our analysis. Data regarding regular alcohol consumption were obtained from questionnaires filled in by the study participants. Exclusion criteria were poor image quality, missing or incongruent data regarding alcohol drinking habits, prior drinking, presence of heart failure or angina, prior myocardial infarction or stroke. 4,335 participants (61.5±7.5 years, 47.6% male) were analyzed. We used multivariate linear regression models adjusted for age, ethnicity, body mass index, smoking, hypertension, diabetes mellitus, physical activity, cholesterol level and Townsend deprivation index to examine the relationship between regular alcohol intake and cardiac structure and function. In men, alcohol intake was independently associated with marginally increased left ventricular end-diastolic volume (ß=0.14; [95%CI=0.05-0.24]; p=0.004), left ventricular stroke volume (ß=0.08; [95%CI=0.03-0.14]; p=0.005), and right ventricular stroke volume (ß=0.08; [95%CI=0.02-0.13]; p=0.006). In women, alcohol consumption was associated with increased LA volume (ß=0.14; [95%CI=0.04-0.23]; p=0.006).
Conclusion
In a sample of participants of the UK Biobank study, alcohol consumption is independently associated with a marginal increase in left and right ventricular volumes in men, but not in women, while alcohol intake showed an association with increased LA volume in women. Our results suggest that there is only minimal relationship between regular alcohol consumption and cardiac morphology and function in an asymptomatic middle-aged population.
Keywords: alcohol consumption, cardiac morphology and structure, magnetic resonance imaging, UK Biobank
Introduction
Alcoholic cardiomyopathy is a well-known phenomenon in heavy drinkers, however controversial results have been reported regarding potential effects of lower levels of alcohol intake on cardiovascular diseases.(1–4) Moreover, sex-specific differences on the effects of regular alcohol intake remains relatively unclear. Previous publications have described a protective role of light-to-moderate alcohol intake when compared with abstainers or heavy drinkers, whereas other population-based studies revealed the opposite, with neutral or worse cardiovascular outcomes.(5–8) However, the outcome variables of previous investigations were based on self-reported history of physician-diagnosed heart failure, or hospital discharge diagnoses based on symptoms, radiographic or physical examinations.(5, 7, 9, 10) Furthermore, in studies utilizing imaging, the cardiac function and structure was evaluated with echocardiography.(6, 11, 12) Cardiac magnetic resonance (CMR) is considered as reference standard for the assessment of cardiac function and structure, since it provides more accurate and reproducible measurements as compared to echocardiography, especially for right ventricular (RV) assessment and in patients with suboptimal image quality.(13, 14) However, no studies have evaluated the relationship between regular alcohol consumption and cardiac anatomy and function as depicted by CMR in a large population-based study.
Therefore, we sought to assess the association between regular alcohol consumption and cardiac chamber sizes and mechanical function derived from CMR imaging in the UK Biobank Population Study.(15, 16)
Methods
Study sample
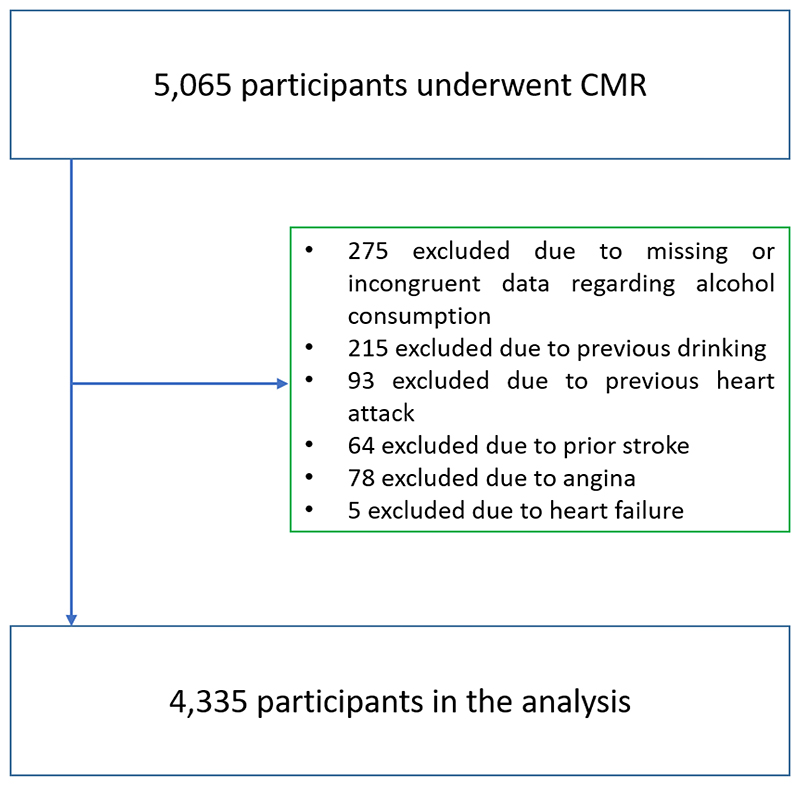
The UK Biobank is a prospective cohort study which collected questionnaire data, physical measurements and biological samples from half a million 40-69 year-old individuals in the United Kingdom.(15) In total, 100,000 participants are being recalled to undergo comprehensive imaging of the brain, heart, whole body, carotid artery, bone and joints. Imaging of the heart is performed by CMR.(17) CMR examinations of 5,065 consecutive participants have already been manually contoured and analyzed. These patients formed the recruitment cohort for our analysis. Exclusion criteria of the current study were poor image quality, refusal to report alcohol drinking habits, missing or incongruent data regarding alcohol consumption (e.g.: as patients indicating current drinking but stated zero alcohol consumption or patients indicating ‘never drinker’ status but with more than zero unit of alcohol per week), former drinking (defined as any prior regular alcohol intake), heart failure, angina, prior myocardial infarction or stroke.
This study was covered by the ethical approval for UK Biobank studies from the National Health Service (NHS) National Research Ethics Service on 17th June 2011 (Ref 11/NW/0382) and extended 10th May 2016 (Ref 16/NW/0274).
Measurement of baseline covariates, potential cofounders and alcohol consumption
Standardized and validated questionnaires included the assessment of smoking, current medications, presence of any disease diagnosed by physician. Data regarding alcohol consumption were obtained from the questionnaire filled in by the study participants. Participants reported alcohol drinker status as ’never’, ’previous’, or ’current’ drinkers. Current drinkers were questioned regarding the amount of consumed alcoholic beverages per week. In calculating the average alcohol intake per week, we used standard units of alcohol. It was assumed that a standard glass (175 ml) of red or white wine is equal to 2.1 units, a pint of beer or cider is equal to 2 units, a single small shot of spirits or any other type of alcohol is equal to 1 unit, based on NHS guide.(18) One standard unit of alcohol contains 8 grams of pure alcohol.(18) Detailed questions of the UK Biobank questionnaires can be found in UK Biobank Data Showcase (https://www.ukbiobank.ac.uk/data-showcase/).
CMR protocol and image analysis
The UK Biobank CMR protocol has been described in detail previously.(14, 19) Briefly, all examinations were performed on a 1.5 Tesla scanner (MAGNETOM Aera, Syngo Platform VD13A, Siemens Healthcare, Erlangen, Germany). For cardiac function, long-axis cines and a complete short-axis stack of balanced steady-state free precession (bSSFP) cines were acquired covering the left and right ventricle.
Analysis of the left ventricle (LV), RV, left atrium (LA), and right atrium (RA) for all CMR examinations were performed manually by eight observers under the supervision of three principal investigators across two core laboratories in London and Oxford according to pre-approved standard operating procedures using dedicated post-processing software (cvi42, Version 5.1.1, Circle Cardiovascular Imaging Inc., Calgary, Canada). LV papillary muscles were included in the LV end-diastolic volume (LVEDV) and end-systolic volume (LVESV). For the measurement of RV parameters, manual tracing of the end-diastolic and end-systolic endocardial borders were carried out in the short-axis view. Thin-walled structures with no trabeculation were excluded and volumes below the pulmonary valves were included as part of the RV. LA and RA end-diastolic volumes (EDV) and end-systolic volumes (ESV), stroke volumes (SV) and ejection fractions (EF) were calculated based on the manually traced endocardial atrial contours in 4-chamber view. Detailed descriptions of analysis methodology, including exemplar contours and intra- and inter-observer variability, have been previously described.(14)
Data analysis and statistics
Summary statistics for independent variables were calculated as means and standard deviation (SD) for continuous variables. Categorical variables were expressed as frequencies and percentages.
To assess the relationship between the amount of alcohol intake and LV, RV, LA and RA anatomy and function in men and women, the various CMR parameters were analyzed using unadjusted and multivariate linear regression analysis. Adjustment was made for age, ethnicity, body mass index, smoking, hypertension, diabetes mellitus, physical activity, cholesterol level, ethnicity and Townsend deprivation index. Definitions for these covariates were previously described.(20, 21)
Missing data were imputed by multiple imputation by chained equations approach to create 50 complete datasets. We use predictive mean matching for continuous variables, logistic regression for binary variables, and polytomous regression for categorical variables. All variables were included in the imputation models.
In order to assess the association between regular alcohol consumption and CMR parameters in the healthy population, we ran a sub-analysis of participants without any condition known to affect cardiac chamber size and function, such as any kind of cardiovascular, respiratory, haematological, renal, rheumatological disease. Moreover, we also excluded those with non-Caucasian ethnicity, hypertension, hyperlipidaemia, diabetes mellitus, malignancy, dyspnoea, those with BMI over 30 kg/m2 and recent or former smokers, as previously described.(14)
In order to correct for multiple testing Galwey method was applied to adjust the p-value. A p-value below 0.007 was considered statistically significant. Statistical analysis was performed using R (version 3.6.1) Statistical Software.(22)
Results
CMR examinations of 5,065 consecutive participants have been manually contoured and analyzed. Figure 1 shows the flowchart with exclusion criteria and the final study sample. After exclusion, 4,335 individuals were included in our study. At the time of the CMR examinations, their mean age was 61.5±7.5 years and 47.6% of the participants were male. Estimated average alcohol intake was 12.8±13.1 standard units per week. Table 1 illustrates the characteristics of the study population.
Figure 1. Flowchart with exclusion criteria and definition of the study population.
CMR=Cardiac magnetic resonance.
The relationship between cardiac structure and function and alcohol consumption
Average CMR values of the left ventricular parameters and results of the linear regression analysis are shown in Table 2. In the unadjusted model, each additional alcohol unit intake per week significantly increased LVEDV by 0.18 ml (95%CI=0.08-0.27 ml; p<0.001), left ventricular stroke volume (LVSV) by 0.10 ml (95%CI=0.04-0.15 ml; p<0.001) and LV mass by 0.13 g (95%CI=0.07-0.19 g; p<0.001) in men, but not in women. After adjustment for age, ethnicity, body mass index, smoking, hypertension, diabetes mellitus, physical activity, Townsend deprivation index and cholesterol level, only the association between alcohol consumption and LVEDV (ß=0.14; [95%CI=0.05-0.24]; p=0.004) and LVSV (ß=0.08; [95%CI=0.03-0.14]; p=0.005) remained significant among male participants.
Table 2. Association between LV CMR parameters and alcohol consumption.
| Average value | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| Mean±SD | β (95% CI) | p | β (95% CI) | p | |
| LVEDV (ml) | |||||
| Total participants | 144.3±34.3 | 0.55 (0.48-0.63) | <0.001 | 0.13 (0.06-0.19) | <0.001 |
| Men | 164.2±33.4 | 0.18 (0.08-0.27) | <0.001 | 0.14 (0.05-0.24) | 0.004 |
| Women | 126.2±23.2 | 0.10 (0.00-0.21) | 0.046 | 0.09 (-0.01-0.20) | 0.065 |
| LVESV (ml) | |||||
| Total participants | 59.2±20.1 | 0.27 (0.22-0.31) | <0.001 | 0.05 (0.01-0.09) | 0.020 |
| Men | 69.2±21.1 | 0.08 (0.02-0.14) | 0.008 | 0.06 (0.00-0.12) | 0.053 |
| Women | 50.0±13.9 | 0.03 (-0.03-0.09) | 0.328 | 0.02 (-0.04-0.08) | 0.483 |
| LVSV (ml) | |||||
| Total participants | 85.1±19.5 | 0.29 (0.24-0.33) | <0.001 | 0.07 (0.03-0.12) | <0.001 |
| Men | 94.9±19.4 | 0.10 (0.04-0.15) | <0.001 | 0.08 (0.03-0.14) | 0.005 |
| Women | 76.2±14.5 | 0.07 (0.01-0.14) | 0.027 | 0.07 (0.01-0.14) | 0.027 |
| LVEF (%) | |||||
| Total participants | 59.4±6.4 | -0.03 (-0.04 to - 0.01) | <0.001 | 0.00 (-0.02-0.02) | 0.946 |
| Men | 58.1±6.5 | -0.01 (-0.02-0.01) | 0.528 | 0.00 (-0.02-0.02) | 0.810 |
| Women | 60.6±6.0 | 0.01 (-0.02-0.03) | 0.556 | 0.01 (-0.02-0.04) | 0.405 |
| LV mass (g) | |||||
| Total participants | 89.6±24.7 | 0.45 (0.40-0.51) | <0.001 | 0.07 (0.03-0.11) | 0.002 |
| Men | 106.7±21.9 | 0.13 (0.07-0.19) | <0.001 | 0.05 (-0.01-0.11) | 0.076 |
| Women | 74.0±14.9 | 0.05 (-0.02-0.12) | 0.136 | 0.06 (0.00-0.12) | 0.062 |
Adjustment was made for age, ethnicity, body mass index, smoking, hypertension, diabetes mellitus, physical activity, Townsend deprivation index and cholesterol level.
CI=Confidence interval; LVEDV=Left ventricular end-diastolic volume; LVEF=Left ventricular ejection fraction; LVESV=Left ventricular end-systolic volume; LVSV=Left ventricular stroke volume.
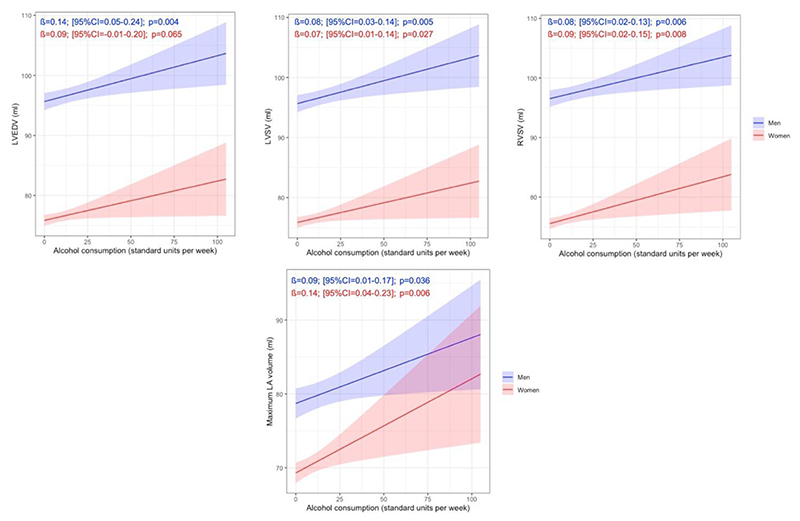
Uni- and multivariable models describing the relationship between alcohol consumption and RV parameters are presented in Table 3. In men, significantly higher RVEDV (ß=0.15; [95%CI=0.05-0.24]; p=0.004) and right ventricular stroke volume (RVSV) (ß=0.09; [95%CI=0.04-0.15]; p<0.001) were measured in association with increasing alcohol consumption in the univariate model. After adjustment, alcohol intake was independently associated with higher RVSV in men (ß=0.08; [95%CI=0.02-0.13]; p=0.006). In women, only LA volume was associated with regular alcohol consumption in the multivariate model (ß=0.14; [95%CI=0.04-0.23]; p=0.006). Atrial parameters can be seen in Table 4. All other associations were not significant neither in men nor women. The sex-specific multivariate linear associations between regular alcohol intake and LVEDV, LVSV, RVSV and maximal LA volume are presented in Figure 2.
Figure 2. Association between regular alcohol consumption and cardiac parameters by sex.
The lines and shaded areas represent the marginal means and 95% confidence intervals of the cardiac parameters. Marginal means were estimated from the linear regression models adjusted for age, ethnicity, body mass index, smoking, hypertension, diabetes mellitus, physical activity, Townsend deprivation index and cholesterol level using the first imputed dataset.
LA=Left atrium; LVEDV=Left ventricular end-diastolic volume; LVSV=Left ventricular stroke volume; RVSV=Right ventricular stroke volume.
Sensitivity analysis
In order to assess the association between regular alcohol consumption and CMR parameters in patients without any known medical condition that might affect cardiac structure and function, we divided the study population into ‘healthy’ and ‘non-healthy’ categories based on the presence of any kind of medical condition that might affect cardiac anatomy and function. Demographic data are shown in Supplementary Table 1. After adjustment for age, ethnicity, cholesterol level, BMI, seven-day average acceleration and Townsend deprivation index, alcohol intake was independently associated with higher LVEDV (ß=0.11; [95%CI=0.04-0.19]; p=0.002), LVSV (ß=0.06; [95%CI=0.02-0.11]; p=0.004), RVSV (ß=0.07; [95%CI=0.02-.011]; p=0.002) and LA volume (ß=0.10; [95%CI=0.03-0.16]; p=0.004) only in the ‘non-healthy’ participants. Results of the uni- and multivariate models can be seen in Supplementary Table 2.
Discussion
This is the first study to systematically assess the sex-specific relationship between alcohol consumption and cardiac structure and function as evaluated by CMR. Our results suggest that increasing amount of regularly consumed alcohol is independently associated with marginally elevated measures of LVEDV, LVSV, RVSV in men and higher LA volume in women after adjustment for the main cardiovascular risk factors as assessed in a general population with no history of cardiovascular disease.
Heavy alcohol consumption is a known and important risk factor for cardiac chamber enlargement and increased LV mass.(1, 23) Approximately one third of the patients diagnosed with dilated cardiomyopathy report excessive alcohol consumption.(24, 25) Moreover, a close relationship between alcohol consumption and hypertension has been established, which may also lead to changes in cardiac structure and function.(26) However, when examining the sex-based differences, this association between any level of alcohol intake and hypertension is only present in men, but not in women.(27)
Previously published studies show controversial results regarding the effect of alcohol consumption on systolic and diastolic LV function.(28–34) Some authors have found preserved, while others report impaired LV filling and systolic function. (28–34) These conflicting results may be due to the different methods used to assess cardiac function. Since CMR is the most accurate non-invasive imaging test to assess cardiac structure and function, our study permits more detailed and robust analysis of the cardiac effects of alcohol consumption.(35) Nonetheless, alcohol-related cardiac effects are likely to differ between men and women. Some publications support that women have a higher risk of alcohol-related cardiomyopathy and CV mortality for the same amount of alcohol.(36, 37)
A recent echocardiography study demonstrated that alcohol intake is an independent predictor of larger LV diastolic and systolic diameters and larger left atrial diameter in both sexes and alcohol consumption was associated with greater LV mass in men and lower LVEF in women.(6) In line with these findings, we measured significantly higher LVEDV, LVSV, LV mass and LA volume in the overall population after adjustment for the main cardiovascular risk factors. However, we did not find any association between LVESV, LVEF and amount of alcohol intake. When assessing the sex-related differences, alcohol intake was associated with higher LVEDV and LVSV in men, and with LA enlargement in women. However, all effect sizes were marginal. A previous age- and gender-matched CMR study of 165 participants, who underwent ventricular T1 mapping has shown no significant difference in left end right ventricular dimensions, LVEF or LVM between light-to-moderate drinkers and non-drinking controls. However, moderate alcohol intake was associated with reduction in native T1 times suggestive of a reduction in diffuse ventricular fibrosis.(38)
There are limited data available regarding the effect of alcohol on RV. In animal models moderate-to-high doses of alcohol (from 1 g/kg to 1.5 g/kg) were associated with RV dysfunction in a dose-dependent way.(39) Conversely, Cameli et al. reported that low doses of alcohol may lead to RV end-diastolic dilatation and reduced function measured by echocardiography in healthy young individuals.(40) Consistent with these findings, we measured significantly higher RVEDV and RVSV in the overall population. The association between modest alcohol exposure and atrial structure remains largely undetermined. Echocardiographic studies provide evidence that alcohol may lead to left atrial enlargement,(6, 41) but little is known regarding the effects of alcohol on the RA. In line with the literature, we observed increased LA volume in the overall population and in women, but not in men.
The sex-specific differences in cardiac structure cannot be explained by BMI, smoking, hypertension, diabetes mellitus, cholesterol level, physical activity or ethnicity. However, weekly alcohol intake was significantly higher in men than in women. Contrary to previous studies where women seemed to be more sensitive, in our study alcohol intake was independently associated with increased ventricular end-diastolic and stroke volumes only in men, but not in women. However, LA enlargement was specific only for women. These differences between the two sexes might be explained by the fact that there is a difference in alcohol absorption and metabolism between the two sexes.(42) Moreover, since alcohol plays a role in the metabolism of the sex hormones, these sex-specific differences might be explained by the higher circulating levels of androgens.(43)
The association between alcohol consumption and cardiac structure and function on an epidemiological scale highlights the importance of public health level preventive actions and education. On an individual level, the timely diagnosis of alcohol related changes in cardiac structure and function may facilitate improved preventative actions and contribute to better care of our patients. The sensitivity analysis has shown a significant association between alcohol consumption and cardiac structure and function, while in healthy subjects we could not demonstrate any association between alcohol intake and cardiac parameters.
The strengths of our study are firstly, the large cohort of asymptomatic population with no prevalent cardiovascular disease with prospectively collected data, physical measurements and biological samples; secondly, the use of CMR to assess cardiac structure and function. Our study’s limitations are important to be acknowledged. First, the alcohol intake was assessed by questionnaires, therefore recall bias may lead to inaccuracy. Inaccurate information on alcohol consumption is a common problem in such studies, because many alcohol-drinkers tend to underestimate their weekly alcohol consumption. Furthermore, current alcohol consumption habits might not reflect accurately the total lifetime alcohol consumption, especially in older people. To mitigate these effects, UK Biobank used validated and standardized questionnaires to collect information on alcohol consumption. Second, our investigation has a cross-sectional study design, therefore we cannot infer causality. However, the observed dose-response relationship between the amount of alcohol consumption and ventricular function indicate unfavorable cardiac effects on an epidemiological level despite the observed small effect sizes. Third, the clinical outcome data to support the hypothesis that alcohol consumption leads to increased risk for cardiovascular events is still pending due to the design of the study. However, the main goal of our study was to decipher the relationship between regular alcohol intake and cardiac function and morphology as assessed by the clinical gold standard CMR imaging.
In this large population imaging study we found a significant association between alcohol consumption and left and right ventricular and left atrial enlargement. In men, alcohol intake was independently associated with higher LVEDV, LVSV and RVSV, while in women, alcohol consumption was related to LA enlargement in the multivariate model. Moreover, we did not find any association between regular alcohol consumption and cardiac structure and function in healthy participants. Even though these associations between regular alcohol intake and cardiac structure were relatively weak, our results highlight the importance of public health measures, education and prevention.
Supplementary Material
Table 1. Demographic data.
| Total participants | Men | Women | p | |
|---|---|---|---|---|
| n=4335 | n=2065 | n=2270 | … | |
| Weekly consumed standard units of alcohol | 12.8±13.1 | 16.7±15.3 | 9.3±9.4 | <0.001 |
| Age (years) | 61.5±7.5 | 62.2±7.6 | 60.9±7.5 | <0.001 |
| Ethnic minority | 2.9% | 3.4% | 2.4% | 0.046 |
| Hypertension | 26.0% | 31.4% | 21.1% | <0.001 |
| Diabetes mellitus | 4.1% | 5.3% | 3.1% | <0.001 |
| Cholesterol | 5.8±1.1 | 5.6±1.1 | 5.9±1.0 | <0.001 |
| Smoker (current, past) | 38.8% | 42.1% | 35.8% | <0.001 |
| Body mass index (kg/m2) | 26.6±4.2 | 27.1±3.8 | 26.1±4.5 | <0.001 |
| Seven-day average acceleration, milli-gravity | 3.2±0.5 | 3.2±0.5 | 3.2±0.5 | 0.290 |
| Townsend deprivation index | -2.0±2.7 | -2.0±2.7 | -2.1±2.6 | 0.288 |
Continuous variables are reported as mean ± standard deviation, whereas categorical variables as frequencies and percentages.
Table 3. Association between RV CMR parameters and alcohol consumption.
| β (95% CI) | p | β (95% CI) | p | ||
|---|---|---|---|---|---|
| RVEDV (ml) | |||||
| Total participants | 153.0±37.5 | 0.61 (0.53-0.69) | <0.001 | 0.10 (0.03-0.17) | 0.004 |
| Men | 177.1±34.3 | 0.15 (0.05-0.24) | 0.004 | 0.12 (0.02-0.22) | 0.014 |
| Women | 131.2±24.8 | 0.08 (-0.04-0.19) | 0.184 | 0.08 (-0.02-0.19) | 0.131 |
| RVESV (ml) | |||||
| Total participants | 67.7±22.5 | 0.31 (0.26-0.36) | <0.001 | 0.03 (-0.02-0.07) | 0.205 |
| Men | 81.3±21.6 | 0.05 (-0.01-0.11) | 0.101 | 0.05 (-0.02-0.11) | 0.151 |
| Women | 75.9±14.8 | -0.01 (-0.07-0.06) | 0.856 | 0.00 (-0.07-0.06) | 0.897 |
| RVSV (ml) | |||||
| Total participants | 85.4±19.5 | 0.30 (0.25-0.34) | <0.001 | 0.07 (0.03-0.11) | <0.001 |
| Men | 95.8±19.0 | 0.09 (0.04-0.15) | <0.001 | 0.08 (0.02-0.13) | 0.006 |
| Women | 75.9±14.5 | 0.08 (0.02-0.15) | 0.014 | 0.09 (0.02-0.15) | 0.008 |
| RVEF (%) | |||||
| Total participants | 56.4±6.5 | -0.03 (-0.04 to - 0.01) | <0.001 | 0.01 (0.00-0.03) | 0.104 |
| Men | 54.4±6.3 | 0.01 (-0.01-0.03) | 0.306 | 0.01 (-0.01-0.03) | 0.437 |
| Women | 58.2±6.1 | 0.02 (0.00-0.05) | 0.075 | 0.03 (0.00-0.05) | 0.067 |
Adjustment was made for age, ethnicity, body mass index, smoking, hypertension, diabetes mellitus, physical activity, Townsend deprivation index and cholesterol level.
CI=Confidence interval; RVEDV=Right ventricular end-diastolic volume; RVEF=Right ventricular ejection fraction; RVESV=Right ventricular end-systolic volume; RVSV=Right ventricular stroke volume.
Table 4. Association between atrial CMR parameters and alcohol consumption.
| β (95% CI) | p | β (95% CI) | p | ||
|---|---|---|---|---|---|
| LA maximal volume (ml) | |||||
| Total participants | 74.2±24.8 | 0.19 (0.13-0.25) | <0.001 | 0.10 (0.04-0.16) | <0.001 |
| Men | 78.8±27.1 | 0.09 (0.01-0.17) | 0.022 | 0.09 (0.01-0.17) | 0.036 |
| Women | 70.1±21.7 | 0.13 (0.03-0.22) | 0.009 | 0.14 (0.04-0.23) | 0.006 |
| LA EF (%) | |||||
| Total participants | 60.1±9.4 | -0.03 (-0.06 to - 0.01) | 0.003 | -0.01 (-0.03-0.01) | 0.014 |
| Men | 58.8±9.8 | -0.01 (-0.03-0.02) | 0.699 | 0.00 (-0.03-0.03) | 0.026 |
| Women | 61.3±8.9 | -0.01 (-0.05-0.03) | 0.483 | -0.02 (-0.05-0.02) | 0.024 |
| RA maximal volume (ml) | |||||
| Total participants | 79.6±26.5 | 0.30 (0.24-0.36) | <0.001 | 0.06 (0.00-0.11) | 0.068 |
| Men | 91.6±28.5 | 0.06 (-0.02-0.15) | 0.127 | 0.07 (-0.01-0.16) | 0.088 |
| Women | 68.7±18.6 | 0.03 (-0.05-0.12) | 0.448 | 0.03 (-0.06-0.12) | 0.488 |
| RA EF (%) | |||||
| Total participants | 42.4±10.6 | -0.08 (-0.11 to - 0.06) | <0.001 | -0.02 (-0.05-0.01) | 0.120 |
| Men | 39.4±10.8 | -0.03 (-0.06-0.00) | 0.067 | -0.02 (-0.06-0.01) | 0.180 |
| Women | 45.1±9.7 | -0.01 (-0.05-0.03) | 0.638 | -0.01 (-0.05-0.03) | 0.657 |
Adjustment was made for age, ethnicity, body mass index, smoking, hypertension, diabetes mellitus, physical activity, Townsend deprivation index and cholesterol level.
CI=Confidence interval; EF=Ejection fraction; LA=Left atrium; RA=Right atrium; SV=Stroke volume.
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application 2964. We thank all UK Biobank participants and staff.
Funding
SEP, SN and SKP acknowledge the British Heart Foundation for funding the manual analysis to create a cardiovascular magnetic resonance imaging reference standard for the UK Biobank imaging resource in 5000 CMR scans (www.bhf.org.uk; PG/14/89/31194). JS, MK and PMH were supported by Project no. NVKP_16-1-2016-0017 (‘National Heart Program’) provided from the National Research, Development and Innovation Fund of Hungary, financed under the NVKP_16 funding scheme. The research was financed by the Higher Education Institutional Excellence Programme of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapeutic Development thematic programme of the Semmelweis University. SEP acts as a paid consultant to and acknowledges receipt of research support to unrelated work and owns stock from Circle Cardiovascular Imaging Inc., Calgary, Canada and Servier. NA is supported by a Wellcome Trust Research Training Fellowship (wellcome.ac.uk; 203553/Z/Z). KF is supported by the Medical College of Saint Bartholomew’s Hospital Trust, an independent registered charity that promotes and advances medical and dental education and research at Barts and The London School of Medicine and Dentistry. AL and SEP acknowledge support from the National Institute for Health Research (NIHR Biomedical Research Centre at Barts and from the “SmartHeart” EPSRC programme grant (www.nihr.ac.uk; EP/P001009/1). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 825903. SN and SKP are supported by the Oxford NIHR Biomedical Research Centre and the Oxford British Heart Foundation Centre of Research Excellence. This project was enabled through access to the MRC eMedLab Medical Bioinformatics infrastructure, supported by the Medical Research Council (www.mrc.ac.uk; MR/L016311/1). JK was funded by the Fulbright Visiting Researcher Grant (E0583118) and the Rosztoczy Foundation throughout the time of the study.
Footnotes
Conflicts of interest
Dr. Petersen provides consultancy to Circle Cardiovascular Imaging Inc, Calgary, Canada. The other authors report no conflicts.
References
- 1.Regan TJ. Alcohol and the cardiovascular system. JAMA. 1990 Jul 18;264(3):377–81. Epub 1990/07/18. [PubMed] [Google Scholar]
- 2.Emrich IE, Bohm M, Mahfoud F. The 2018 ESC/ESH Guidelines for the management of arterial hypertension: A German point of view. Eur Heart J. 2019 Jun 14;40(23):1830–1. doi: 10.1093/eurheartj/ehz381. Epub 2019/06/15. [DOI] [PubMed] [Google Scholar]
- 3.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016 Aug 1;37(29):2315–81. doi: 10.1093/eurheartj/ehw106. Epub 2016/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016 Jul 14;37(27):2129–200. doi: 10.1093/eurheartj/ehw128. Epub 2016/05/22. [DOI] [PubMed] [Google Scholar]
- 5.Beulens JW, Algra A, Soedamah-Muthu SS, Visseren FL, Grobbee DE, van der Graaf Y, et al. Alcohol consumption and risk of recurrent cardiovascular events and mortality in patients with clinically manifest vascular disease and diabetes mellitus: the Second Manifestations of ARTerial (SMART) disease study. Atherosclerosis. 2010 Sep;212(1):281–6. doi: 10.1016/j.atherosclerosis.2010.04.034. Epub 2010/06/12. [DOI] [PubMed] [Google Scholar]
- 6.Goncalves A, Jhund PS, Claggett B, Shah AM, Konety S, Butler K, et al. Relationship Between Alcohol Consumption and Cardiac Structure and Function in the Elderly The Atherosclerosis Risk in Communities Study. Circ-Cardiovasc Imag. 2015 Jun;8(6) doi: 10.1161/CIRCIMAGING.114.002846. English, PubMed PMID: WOS:000369714500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh CR, Larson MG, Evans JC, Djousse L, Ellison RC, Vasan RS, et al. Alcohol consumption and risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2002 Feb 5;136(3):181–91. doi: 10.7326/0003-4819-136-3-200202050-00005. Epub 2002/02/06. [DOI] [PubMed] [Google Scholar]
- 8.Hart CL, Smith GD, Hole DJ, Hawthorne VM. Alcohol consumption and mortality from all causes, coronary heart disease, and stroke: results from a prospective cohort study of scottish men with 21 years of follow up. BMJ. 1999;318(7200):1725–9. doi: 10.1136/bmj.318.7200.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramson JL, Williams SA, Krumholz HM, Vaccarino V. Moderate alcohol consumption and risk of heart failure among older persons. JAMA. 2001 Apr 18;285(15):1971–7. doi: 10.1001/jama.285.15.1971. Epub 2001/04/20. [DOI] [PubMed] [Google Scholar]
- 10.Klatsky AL, Chartier D, Udaltsova N, Gronningen S, Brar S, Friedman GD, et al. Alcohol drinking and risk of hospitalization for heart failure with and without associated coronary artery disease. Am J Cardiol. 2005 Aug 1;96(3):346–51. doi: 10.1016/j.amjcard.2005.03.073. Epub 2005/08/02. [DOI] [PubMed] [Google Scholar]
- 11.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980 Nov;62(5):1054–61. doi: 10.1161/01.cir.62.5.1054. Epub 1980/11/01. [DOI] [PubMed] [Google Scholar]
- 12.Kupari M, Koskinen P, Suokas A. Left ventricular size, mass and function in relation to the duration and quantity of heavy drinking in alcoholics. Am J Cardiol. 1991 Feb 1;67(4):274–9. doi: 10.1016/0002-9149(91)90559-4. Epub 1991/02/01. [DOI] [PubMed] [Google Scholar]
- 13.Gardner BI, Bingham SE, Allen MR, Blatter DD, Anderson JL. Cardiac magnetic resonance versus transthoracic echocardiography for the assessment of cardiac volumes and regional function after myocardial infarction: an intrasubject comparison using simultaneous intrasubject recordings. Cardiovasc Ultrasound. 2009 Aug 18;7:38. doi: 10.1186/1476-7120-7-38. Epub 2009/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JM, et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson. 2017 Feb 3;19(1):18. doi: 10.1186/s12968-017-0327-9. Epub 2017/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015 Mar;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. Epub 2015/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Littlejohns TJ, Sudlow C, Allen NE, Collins R. UK Biobank: opportunities for cardiovascular research. Eur Heart J. 2019 Apr 7;40(14):1158–66. doi: 10.1093/eurheartj/ehx254. Epub 2017/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen SE, Matthews PM, Bamberg F, Bluemke DA, Francis JM, Friedrich MG, et al. Imaging in population science: cardiovascular magnetic resonance in 100,000 participants of UK Biobank - rationale, challenges and approaches. J Cardiovasc Magn Reson. 2013 May 28;15:46. doi: 10.1186/1532-429X-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NHS. Alcohol units. 2018. 27 May 2020, date last accessed, Available from: https://www.nhs.uk/live-well/alcohol-support/calculating-alcohol-units/#table.
- 19.Petersen SE, Matthews PM, Francis JM, Robson MD, Zemrak F, Boubertakh R, et al. UK Biobank’s cardiovascular magnetic resonance protocol. J Cardiovasc Magn Reson. 2016 Feb 1;18:8. doi: 10.1186/s12968-016-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen SE, Sanghvi MM, Aung N, Cooper JA, Paiva JM, Zemrak F, et al. The impact of cardiovascular risk factors on cardiac structure and function: Insights from the UK Biobank imaging enhancement study. PLoS One. 2017;12(10):e0185114. doi: 10.1371/journal.pone.0185114. Epub 2017/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty A, Jackson D, Hammerla N, Plotz T, Olivier P, Granat MH, et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS One. 2017;12(2):e0169649. doi: 10.1371/journal.pone.0169649. Epub 2017/02/02, study. PO has previously been a director of Axivity Ltd. NH has previously consulted for Axivity Ltd. The partners of DJ and PO own shares in Axivity. This does not alter our adherence to PLOS ONE policies on sharing data and materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team RC. R: A language and environment for statistical computing. 2016. 27 May 2020, date last accessed, Available from: https://www.r-project.org/
- 23.Mathews EC, Jr, Gardin JM, Henry WL, Del Negro AA, Fletcher RD, Snow JA, et al. Echocardiographic abnormalities in chronic alcoholics with and without overt congestive heart failure. Am J Cardiol. 1981 Mar;47(3):570–8. doi: 10.1016/0002-9149(81)90540-3. Epub 1981/03/01. [DOI] [PubMed] [Google Scholar]
- 24.Fauchier L, Babuty D, Poret P, Casset-Senon D, Autret ML, Cosnay P, et al. Comparison of long-term outcome of alcoholic and idiopathic dilated cardiomyopathy. Eur Heart J. 2000 Feb;21(4):306–14. doi: 10.1053/euhj.1999.1761. [DOI] [PubMed] [Google Scholar]
- 25.Gavazzi A, De Maria R, Parolini M, Porcu M. Alcohol abuse and dilated cardiomyopathy in men. Am J Cardiol. 2000 May 1;85(9):1114–8. doi: 10.1016/s0002-9149(00)00706-2. Epub 2000/04/27. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertension. 2001 May;37(5):1242–50. doi: 10.1161/01.hyp.37.5.1242. Epub 2001/05/23. [DOI] [PubMed] [Google Scholar]
- 27.Roerecke M, Tobe SW, Kaczorowski J, Bacon SL, Vafaei A, Hasan OSM, et al. Sex-Specific Associations Between Alcohol Consumption and Incidence of Hypertension: A Systematic Review and Meta-Analysis of Cohort Studies. J Am Heart Assoc. 2018 Jun 27;7(13) doi: 10.1161/JAHA.117.008202. Epub 2018/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerqueira MD, Harp GD, Ritchie JL, Stratton JR, Walker RD. Rarity of preclinical alcoholic cardiomyopathy in chronic alcoholics less than 40 years of age. Am J Cardiol. 1991 Jan 15;67(2):183–7. doi: 10.1016/0002-9149(91)90442-n. Epub 1991/01/15. [DOI] [PubMed] [Google Scholar]
- 29.Dancy M, Bland JM, Leech G, Gaitonde MK, Maxwell JD. Preclinical left ventricular abnormalities in alcoholics are independent of nutritional status, cirrhosis, and cigarette smoking. Lancet. 1985 May 18;1(8438):1122–5. doi: 10.1016/s0140-6736(85)92431-6. Epub 1985/05/18. [DOI] [PubMed] [Google Scholar]
- 30.Friedman HS, Vasavada BC, Malec AM, Hassan KK, Shah A, Siddiqui S. Cardiac function in alcohol-associated systemic hypertension. Am J Cardiol. 1986 Feb 1;57(4):227–31. doi: 10.1016/0002-9149(86)90896-9. Epub 1986/02/01. [DOI] [PubMed] [Google Scholar]
- 31.Kino M, Imamitchi H, Morigutchi M, Kawamura K, Takatsu T. Cardiovascular status in asymptomatic alcoholics, with reference to the level of ethanol consumption. Br Heart J. 1981 Nov;46(5):545–51. doi: 10.1136/hrt.46.5.545. Epub 1981/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spodick DH, Pigott VM, Chirife R. Preclinical cardiac malfunction in chronic alcoholism. Comparison with matched normal controls and with alcoholic cardiomyopathy. N Engl J Med. 1972 Oct 5;287(14):677–80. doi: 10.1056/NEJM197210052871401. Epub 1972/10/05. [DOI] [PubMed] [Google Scholar]
- 33.Urbano-Marquez A, Estruch R, Navarro-Lopez F, Grau JM, Mont L, Rubin E. The effects of alcoholism on skeletal and cardiac muscle. N Engl J Med. 1989 Feb 16;320(7):409–15. doi: 10.1056/NEJM198902163200701. Epub 1989/02/16. [DOI] [PubMed] [Google Scholar]
- 34.Silberbauer K, Juhasz M, Ohrenberger G, Hess C. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in young alcoholics. Cardiology. 1988;75(6):431–9. doi: 10.1159/000174413. Epub 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 35.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015 Apr 18;17:29. doi: 10.1186/s12968-015-0111-7. Epub 2015/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Sola J, Estruch R, Nicolas JM, Pare JC, Sacanella E, Antunez E, et al. Comparison of alcoholic cardiomyopathy in women versus men. Am J Cardiol. 1997 Aug 15;80(4):481–5. doi: 10.1016/s0002-9149(97)00399-8. Epub 1997/08/15. [DOI] [PubMed] [Google Scholar]
- 37.Urbano-Marquez A, Estruch R, Fernandez-Sola J, Nicolas JM, Pare JC, Rubin E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA. 1995 Jul 12;274(2):149–54. doi: 10.1001/jama.1995.03530020067034. Epub 1995/07/12. [DOI] [PubMed] [Google Scholar]
- 38.Voskoboinik A, Costello BT, La Gerche A, Prabhu S, Wong G, Flannery MD, et al. Relation of Alcohol Consumption to Left Ventricular Fibrosis Using Cardiac Magnetic Resonance Imaging. Am J Cardiol. 2019 Feb;123(3):460–5. doi: 10.1016/j.amjcard.2018.10.026. eng, Epub 2018/11/06. [DOI] [PubMed] [Google Scholar]
- 39.Kettunen R, Timisjarvi J, Saukko P. The acute dose-related effects of ethanol on right ventricular function in anesthetized dogs. Alcohol. 1992 Mar-Apr;9(2):149–53. doi: 10.1016/0741-8329(92)90026-7. Epub 1992/03/01. [DOI] [PubMed] [Google Scholar]
- 40.Cameli M, Ballo P, Garzia A, Lisi M, Bocelli A, Mondillo S. Acute effects of low doses of ethanol on left and right ventricular function in young healthy subjects. Alcohol Clin Exp Res. 2011 Oct;35(10):1860–5. doi: 10.1111/j.1530-0277.2011.01530.x. Epub 2011/07/19. [DOI] [PubMed] [Google Scholar]
- 41.Singh KJ, C B, Na B, Regan M, Schiller NB, Whooley MA. Alcohol consumption and 5- year change in left atrial volume among patients with coronary heart disease: results from the Heart and Soul study. J Card Fail. 2013;19:183–9. doi: 10.1016/j.cardfail.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990 Jan 11;322(2):95–9. doi: 10.1056/NEJM199001113220205. Epub 1990/01/11. [DOI] [PubMed] [Google Scholar]
- 43.Gordon GG, Altman K, Southren AL, Rubin E, Lieber CS. Effect of alcohol (ethanol) administration on sex-hormone metabolism in normal men. N Engl J Med. 1976 Oct 7;295(15):793–7. doi: 10.1056/NEJM197610072951501. Epub 1976/10/07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.