Abstract
Objective
Targeted electrical energy applied to wounds has been shown to improve wound-healing rates. However, the mechanisms are poorly understood. The aim of this study was to identify genes that are responsive to electrical stimulation (ES) in healthy subjects with undamaged skin.
Methods
To achieve this objective, study authors used a small, non-invasive ES medical device to deliver a continuous, specific, set sequence of electrical energy impulses over a 48-hour period to the skin of healthy volunteers and compared resultant gene expression by microarray analysis.
Main Results
Application of this specific ES resulted in differential expression of 105 genes, the majority of which were downregulated. Post microarray analyses revealed there was commonality with a small number of genes that have previously been shown to be upregulated in skin wounds including venous leg ulcers.
Conclusions
The specific sequence of ES applied continuously for 48 hours to the skin of healthy patients has the effect of modifying expression in a number of identified genes. The identification of the differential expression in this subset of genes in healthy subjects provides new potential lines of scientific inquiry for identifying similar responses in subjects with slow or poorly healing wounds.
Keywords: epidermis, electrical stimulation, gene expression, healthy skin, microarray, wound healing
Introduction
The use of externally applied electrical energy to promote the healing of complex wounds is a concept introduced more than 40 years ago.1 There are various types of electrical stimulation (ES) devices. Despite variation in application mode, dose, and duration of therapy, the majority of trials show significant improvement in wound healing or wound area reduction with ES therapy compared with control treatment or standard care.2,3 The use of continuous direct current4–7 of 200 to 800 μA and pulsed current8,9 improved healing of chronic wounds in a number of studies. However, the mechanisms of ES-mediated wound healing in vivo are poorly understood; a number of different explanations have been provided for the clinical outcomes reported.
Clinical Problem Addressed
Identifying how ES can regulate or modify gene expression in skin is critical in improving the understanding of why ES treatment improves healing rates in chronic wounds in some individuals and not in others. Further, identifying ES-responsive genes might provide new potential lines of scientific inquiry for promoting similar, beneficial gene responses in subjects with slow or poorly healing wounds. It also might be possible to measure expression in responders and non-responders alike to predict treatment efficacy in different patient groups and to select ES treatment parameters that yield the best wound healing outcomes.
Materials and Methods
A recent systematic review concluded that the ideal ES device needs to be noninvasive, portable, cost-effective, and provide minimal interference with patients’ daily life.3 In addition, a device that delivers a program of fixed parameters and duration is important in order to standardize findings and to make comparisons and draw conclusions from studies or clinical applications that use it.
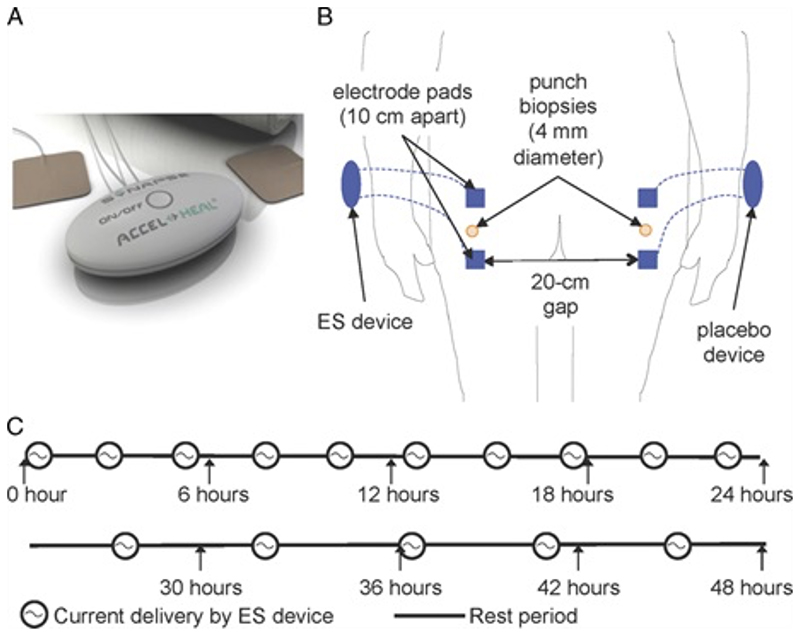
The device selected for this study is a class IIA medical device (under medical directive 93/42/EEC, which has full Medical Devices Directorate approval under ISO 13485:2003). The device delivers a proprietary frequency of pulsed electrical energy at the microcurrent level directly to the skin via 2 off-the-shelf treatment electrode pads placed directly onto intact skin for a duration of 48 hours (Figure 1).
Figure 1. Electrical Stimulation (ES) Application to Skin of Healthy Participants.
(A) The ES device. (B) Two ES devices were applied to the skin overlying the buttocks (1 on each side). The electrode pads of each device were 10 cm apart and there was a 20 cm gap between the electrode pads of the different devices. (C) Program specification of the ES device.
Study Design and Participants
The study was an investigator-initiated, double-blinded, randomized, placebo-controlled trial undertaken at Bispebjerg Hospital in Copenhagen, Denmark. The study was reported to the Danish register (Datatilsynet), and was performed in accordance with Danish law (Lov om behandling af personoplysninger). This included an approval from the Regional Ethical Committee for the Hospital Region of Greater Copenhagen (Region H, H-3-2012-158).
Only healthy, nonsmoking male Caucasians, 20 to 30 years old, with a BMI of between 19 and 25 and no history of cancer, diabetes, or vascular disease, who undertook similar levels of moderate physical activity per week were recruited.
Two ES devices identical in appearance (1 active and 1 placebo), attached to pairs of electrodes identical in appearance, were placed on intact healthy skin on the buttocks of 12 healthy male subjects. The electrode pads of each device were placed 10 cm apart and there was a 20 cm gap between the electrode pads of the different devices (Fig. S1B) Only a third independent person had knowledge of which device was active, making the study was double-blinded. After 48 hours, full-thickness 4-mm skin biopsies were excised from a region midway between the 2 electrodes.
Comparative Microarray
Subjects’ RNA was isolated from skin biopsies and cells using Invitrogen TRIzol reagent (ThermoFisher Scientific, Carlsbad, California) following manufacturer protocols.10 The RNA concentrations and integrity were measured using Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California). The RNA was amplified and hybridized to Human Genome U133 2.0 GeneChip arrays (Affymetrix, Santa Clara, California). Microarray data are available in the ArrayExpress database under accession number E-MTAB-3935. Technical quality control and outlier analysis was performed with dChip (V2005)11 using the default settings. Background correction, quantile normalization, and gene expression analysis were performed using RMA in Bioconductor (Buffalo, New York).12
To assess whether ES altered gene expression in the skin, an analysis of variance linear model was employed to evaluate where the variation within the data set originated (Partek Genomics Solution version 6.5; Partek Inc, St Charles, Missouri). Differential expression analysis was performed for subjects who were exposed to ES using a paired model in Limma (Bioconductor) using the functions lmFit and eBayes.13 A gene list of differentially expressed genes was formed from probe sets that had a greater than 1.5-fold change (P < .05).
Data Analysis
Gene ontology analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID Bioinformatics Resources, version 6.8) as well as Ingenuity Pathway Analysis (Qiagen, Hilden, Germany) showed that there is an enrichment of receptors for advanced glycation end products (RAGE)-binding proteins: S100A7, S100A8, and S100A9. A comparison was performed with wound-regulated genes in a microarray data set generated by Cooper et al14 under the ArrayExpress accession number E-MTAB-3836(14) from genes differentially regulated in venous leg ulcer wound edges.15
Results
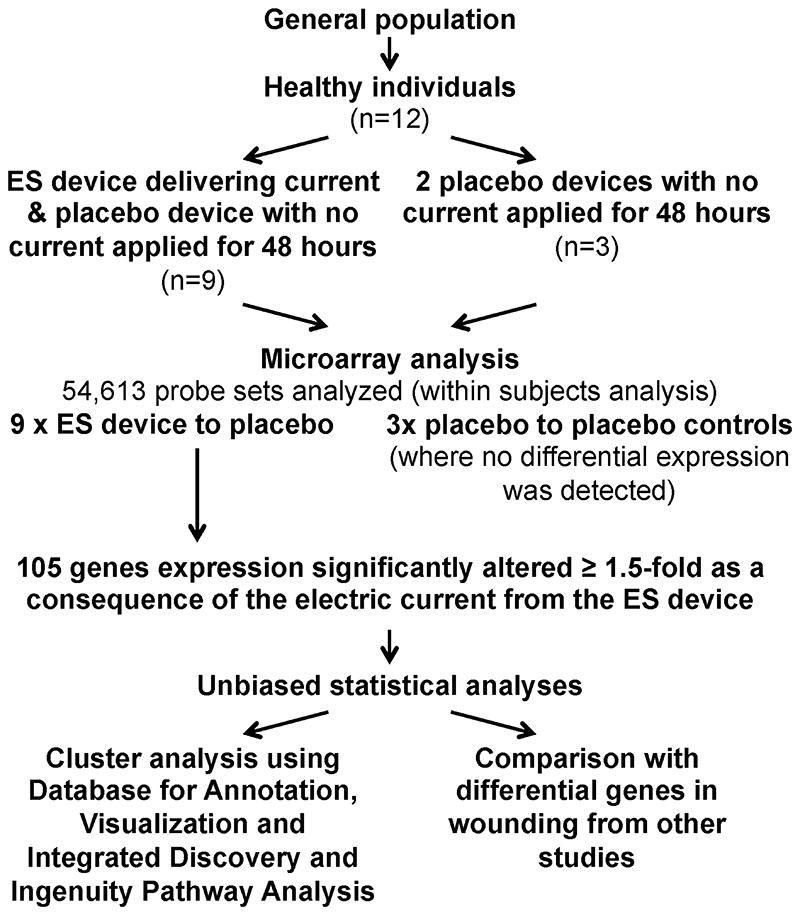
The study included 12 participants, of whom 9 received 1 activated and 1 placebo ES device and 3 of whom received only placebo devices (Figure 2). The activated device delivered a series of preset programs of varying amplitude (40-500 μA), frequency (10-900 Hz), and polarity (alternating every 0.1 seconds). The electrical pulses were delivered in repeated 240-minute treatment sessions with a 2-hour resting period between each in the first 24 hours and a 4-hour resting period between each in the second 24 hours.
Figure 2. Flow Chart of Study to Identify Genes in Skin that are Regulated By Electrical Stimulation.
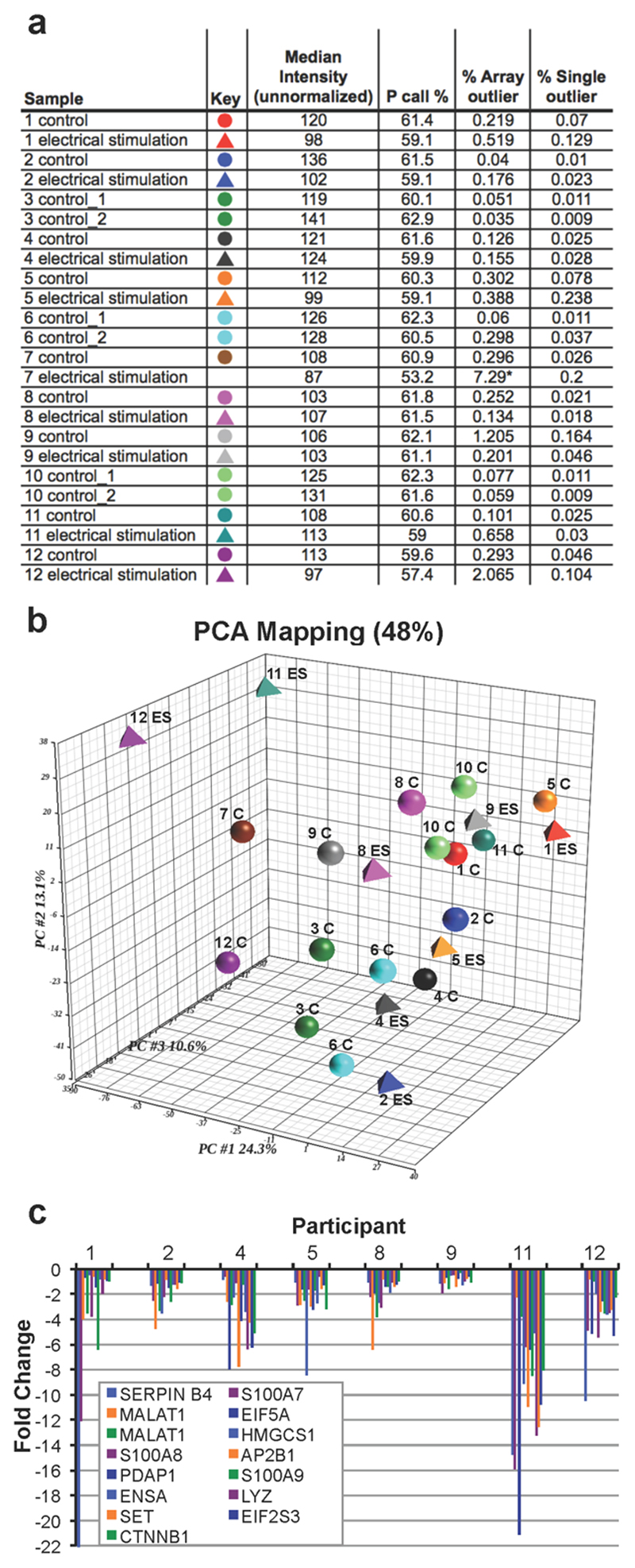
Unbiased statistical analyses showed that 1 of the 24 RNA samples was an outlier and was excluded from subsequent analyses (Figure 4A). Both principal component analysis and linear model evaluation suggested that the largest source of variation in global gene expression changes was from differences among individuals (Figure 4B). No significant differential expression was detected between biopsies from the placebo group in any of the 3 control participants. A within-subject analysis of ES-treated versus placebo-treated skin produced a significant variation score of 1.47, as illustrated by the 15 most differentially regulated (Figure 3). As a result, alterations in levels of gene expression could be attributed to the 48-hour ES treatment.
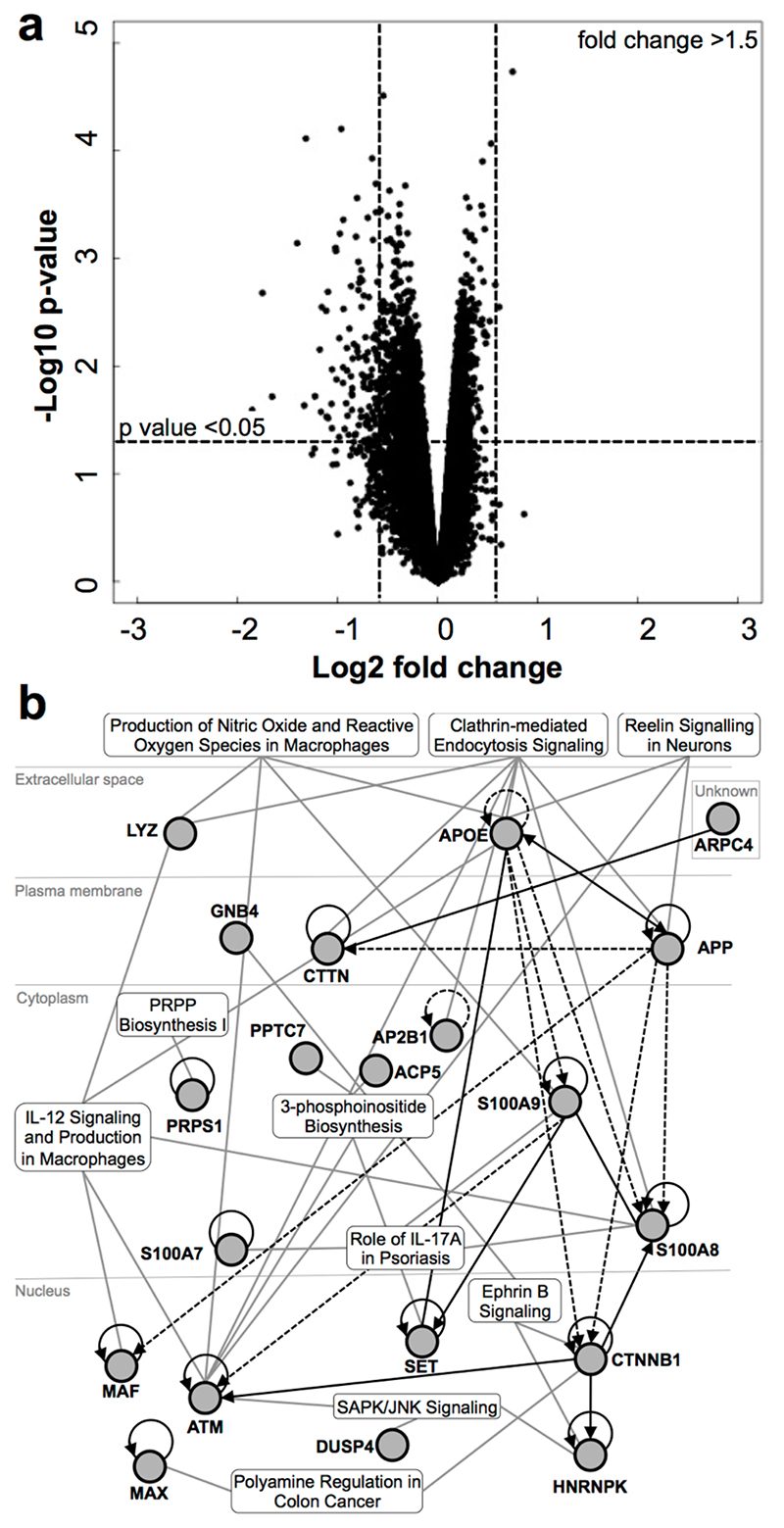
Figure 4. Electrical Stimulation Treatment to Healthy Skin Leads to Downregulation of Gene Expression.
(A) Volcano box plot to illustrate the expression changes of all genes. The 105 genes whose expression was significantly altered more than 1.5-fold (P < .05) are shown in the top left and top right boxes. (B) Top 10 canonical pathways identified in ES-regulated genes in skin by Ingenuity Pathway Analysis.
Figure 3. Variation Among Individuals.
(A) dChip analysis of samples obtained from the 12 participants of the study. Subject 8’s ES treatment was an array outlier, and was subsequently excluded from further analyses. (B) Principal component analysis was used to provide a statistical summary of the array samples, and the first 3 principal components are shown. C = control; MC = microcurrent. (C) The top 15 differentially regulated genes (as ranked by fold change) of the 8 participants who were treated with a placebo and the ES device. Note that SERPINB4 expression in subject 1 is not included in the graph but it showed a fold change of over -90 with treatment.
To accommodate the large individual variation, a paired test was used, resulting in 105 annotated genes with a significant differential regulation (at least 1.5-fold [P < .05]) in ES-treated skin compared with placebo treatment. A volcano plot of the microarray data shows that the majority of these differentially regulated genes were reduced in expression, with the exception of 2 genes (Figure 4).
Study authors subjected this list of ES-regulated genes to unbiased functional annotation analyses using DAVID and Ingenuity Pathway Analysis. Functional gene ontology analysis showed that there was an overrepresentation of cellular compartment genes, including focal adhesion (ANXA6, PDIA3, TPM4, LPP), protein-DNA complex (CTNNB1, HNRNPK, JUP), and endoplasmic reticulum-Golgi intermediate compartment membrane (TMED9, RAB2A, ERGIC1), and in genes encoding ribonucleoprotein (TEP1, HNRNPH1, MRPS16, MRPL30), gene activators (SUPT4H1, SMARCA2, MAX, RORC, EBF1), and RAGE-binding proteins (S100A7, S100A8, S100A9). Pathway analysis showed that these ES-regulated genes were implicated in canonical pathways, including regulation of macrophage production of IL-12, nitric oxide and reactive oxygen species, and IL-17A in psoriasis (Figure 4).
Next, study authors compared the list of ES-regulated genes to 2 publically available array datasets that identified genes regulated by wounding. First, the new dataset was compared with over 2600 probe sets differentially regulated 24 hours after wounding in vivo identified in a mouse skin injury model.14 In common were 25 genes that were upregulated in wounding but downregulated with ES; 1 gene (RAD23B) was similarly downregulated in both microarray studies (Table). Next, the new dataset was compared with the top 50 most upregulated and top 50 most downregulated genes identified in the wound edge of venous leg ulcers.15 All 3 S100 genes and SERPINB4 were upregulated in the venous leg ulcer wound edge but were downregulated in ES-treated skin (Table).
Table. Genes Common With Those Differentially Regulated in Skin 24 hours Postwounding and in Venous Leg Ulcer Wound Edge.
| Gene Symbol | Therapy Versus Placebo (Fold Change) | Acute Wound Versus Control (Fold Change) | Ulcer Wound Edge Versus Control (Fold Change)15 |
|---|---|---|---|
| SERPINB4 | -3.62 | - | 94.96 |
| S100A7 | -3.37 | - | 105.80 |
| EIF5A | -2.52 | 1.62 | - |
| S100A8 | -2.26 | 90.42 | 95.11 |
| A02B1 | -2.24 | 1.47 | - |
| PDAP1 | -2.17 | 1.52 | - |
| S100A9 | -2.16 | 97.95 | 119.00 |
| LYZ | -2.14 | 1.24 | - |
| SET | -2.09 | 1.48 | - |
| VCAN | -1.98 | 8.59 | - |
| CPR124 | -1.93 | 2.72 | - |
| CD47 | -1.92 | 1.60 | - |
| RAD23B | -1.92 | -1.26 | - |
| SPP1 | -1.90 | 7.52 | - |
| CALD1 | -1.88 | 1.52 | - |
| ARPC4 | -1.87 | 1.77 | - |
| LCN2 | -1.81 | 44.12 | - |
| PDIA3 | -1.67 | 1.56 | - |
| ERGIC1 | -1.67 | 1.70 | - |
| RNF125 | -1.66 | 1.27 | - |
| KRT17 | -1.64 | 2.72 | - |
| KRT6B | -1.63 | 146.32 | 37.66 |
| APP | -1.62 | - | -9.17 |
| TPM4 | -1.55 | 1.7 | - |
| RBM3 | -1.53 | 1.53 | - |
| SOX7 | -1.53 | 1.23 | - |
| GNB4 | -1.52 | 1.51 | - |
| AQP3 | -1.52 | 2.80 | - |
| GNG12 | -1.50 | 1.36 | - |
Discussion
The primary finding of this study is that ES delivered with a preset, fixed program and duration to healthy human skin reduces the expression of a specific set of genes that are upregulated in skin inflammation. This study used healthy volunteers instead of patients with chronic wounds primarily because of the risk that biopsy could have adverse effects on wound healing. It is unclear from the results of this study, but possible to hypothesize, that the same ES device applied in the same manner would have the same effects on healthy wound edge skin adjacent to a chronic wound. However, many patients with chronic and nonhealing wounds commonly present with comorbidities so this hypothesis would have to be fully tested in a variety of patients and the results compared with this study to draw a safe conclusion.
The 48 hour duration of treatment was chosen to investigate any immediate differential gene expression because it constitutes 1 application of the device’s full delivery cycle. Because the device was applied over the buttocks, study authors suggest that 48 hours was an acceptable length of time for participants to withhold from showering, bathing, and activities that could cause excessive sweating.
Analysis of the sources of variation reinforced the fact that there is extensive variation among participants, but did indicate that ES significantly affected the expression of specific genes. While some variables could be controlled (eg, age, smoking status), other variations in the participants’ lifestyle (eg, activity and diet) or underlying medical conditions were unknown. However, the participants were all healthy young individuals without any signs of abnormal conditions or diseases. The participant profiles of the most regulated genes varied among participants, suggesting that some of the participants could be viewed as either responders or nonresponders to the ES treatment.
Functional annotation analysis of the 105 ES-regulated genes showed that there is an enrichment of RAGE-binding proteins: S100A7, S100A8, and S100A9. Study authors could compare these findings with only 2 other publically available array datasets produced from wound-related studies14,15; however, the comparisons confirmed that S100 genes are upregulated in both acute and chronic wounds. These proteins are expressed at low levels in keratinocytes in healthy skin but are upregulated upon wounding. The expression of S100 genes is crucial for reepithelialization of the wound,16 recruitment of inflammatory cells,17,18 regeneration of the hair follicle,19 and regulating keratinocyte growth and differentiation.20 Controversially, S100 proteins are also readily detected in wound exudates21,22 and upregulated in chronic wounds15,23 and psoriasis,24,25 which suggests that their expression is a biomarker for nonhealing wounds. Overexpression of S100 genes in HaCat keratinocytes impaired collective migration in the cell sheet immediately behind the migrating front in an in vitro scratch wound assay, and staining of β-catenin showed cells adjacent to gaps in the cell sheet had no positive staining in the periphery but accumulated cell junction proteins in the cytosol when S100 genes were over expressed (data not shown).
It is thought that a prolonged inflammatory phase and the increased expression of proteolytic enzymes prevent wounds from progressing into the proliferative phase, and are major factors that contribute to nonhealing wounds.26 Pathway analysis indicated that the ES application dampened the release of proinflammatory cytokine interleukin-12, nitric oxide, and reactive oxygen species by macrophages responsible for the breakdown of connective tissues.27 Reduction of S100A7, S100A8, and S100A8 and activation of macrophages by ES could potentially improve wound healing by dampening these pathways in chronic wounds.
In summary, study authors hypothesize that ES-mediated healing of chronic wounds could be achieved via downregulation of certain gene pathways that are known to compromise wound healing, enabling an improved rate of repair.
Limitations
This study was performed on a small number of participants, limiting the generalizability of results. Further, it cannot be inferred from these results that the same ES device applied in the same manner to the periwound skin of a poorly healing wound would have the same effect as on the skin of healthy volunteers. Findings may be different in different areas of the body where skin thickness varies.
There are no known or anticipated adverse reactions from this electroceutical application. A single type of ES device was used for this investigation, delivering a fixed pulse sequence and duration of ES. Therefore, it is impossible to know whether the same changes in gene expression reported here would be seen if a different set of ES parameters were used.
Conclusions
This study is a first step toward a mechanistic understanding of the potential benefits of ES for improving skin healing in pathologic conditions. The identification of these changes in gene expression, albeit in healthy skin, provide potential novel therapeutic targets for individuals who present with delayed skin healing. Identifying higher levels of these proteins may help to identify the patients in this group who are predisposed to poor healing. However, because a single type of ES device was used for this investigation, further investigation is required to see if the same changes in gene expression result from a different set of ES parameters.
Acknowledgements
The authors would like to thank the University of Manchester Bioimaging Facility and Genomics Technologies Facility, especially Michal Smiga. This work was funded by Synapse Electroceutical Ltd. Synapse Electroceutical Ltd had no part in study design, data collection, or writing of this paper. The authors have disclosed no other financial relationships related to this article. Submitted May 4, 2017; accepted in revised form October 16, 2017.
Contributor Information
Chloe Lallyett, Bolton St Catherine’s Academy, Bolton, United Kingdom.
Ching-Yan Chloé Yeung, Institute of Sports Medicine Copenhagen, Denmark.
Rie Harboe Nielson, Department of Rheumatology, Aarhus University Hospital, Denmark.
Leo A. H. Zeef, University of Manchester, United Kingdom.
David Chapman-Jones, Institute of Healthcare Policy and Practice, University of the West of Scotland, United Kingdom.
Michael Kjaer, University of Copenhagen, Denmark.
Karl E. Kadler, University of Manchester, United Kingdom.
References
- 1.Assimacopoulos D. Wound healing promotion by the use of negative electric current. Am Surg. 1968;34(6):423–31. [PubMed] [Google Scholar]
- 2.Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle. 2013;4 doi: 10.3402/dfa.v4i0.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashrafi M, Alonso-Rasgado T, Baguneid M, Bayat A. The efficacy of electrical stimulation in lower extremity cutaneous wound healing: a systematic review. Exp Dermatol. 2017;26(2):171–8. doi: 10.1111/exd.13179. [DOI] [PubMed] [Google Scholar]
- 4.Carley PJ, Wainapel SF. Electrotherapy for acceleration of wound healing: low intensity direct current. Arch Phys Med Rehabil. 1985;66(7):443–6. [PubMed] [Google Scholar]
- 5.Gault WR, Gatens PF., Jr Use of low intensity direct current in management of ischemic skin ulcers. Phys Ther. 1976;56(3):265–9. doi: 10.1093/ptj/56.3.265. [DOI] [PubMed] [Google Scholar]
- 6.Wolcott LE, Wheeler PC, Hardwicke HM, Rowley BA. Accelerated healing of skin ulcer by electrotherapy: preliminary clinical results. South Med J. 1969;62(7):795–801. doi: 10.1097/00007611-196907000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Wood JM, Evans PE, 3rd, Schallreuter KU, et al. A multicenter study on the use of pulsed low-intensity direct current for healing chronic stage II and stage III decubitus ulcers. Arch Dermatol. 1993;129(8):999–1009. [PubMed] [Google Scholar]
- 8.Junger M, Zuder D, Steins A, Hahn M, Klyscz T. Treatment of venous ulcers with low frequency pulsed current (Dermapulse): effects on cutaneous microcirculation. Hautarzt. 1997;48(12):897–903. doi: 10.1007/s001050050682. [DOI] [PubMed] [Google Scholar]
- 9.Young S, Hampton S, Tadej M. Study to evaluate the effect of low-intensity pulsed electrical currents on levels of oedema in chronic non-healing wounds. J Wound Care. 2011;20(8):368. doi: 10.12968/jowc.2011.20.8.368. 70-3. [DOI] [PubMed] [Google Scholar]
- 10.Yeung CY, Zeef LA, Lallyett C, Lu Y, Canty-Laird EG, Kadler KE. Chick tendon fibroblast transcriptome and shape depend on whether the cell has made its own collagen matrix. Sci Rep. 2015;5:13555. doi: 10.1038/srep13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98(1):31–6. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 14.Cooper NH, Balachandra JP, Hardman MJ. Global gene expression analysis in PKCα-/- mouse skin reveals structural changes in the dermis and defective wound granulation tissue. J Invest Dermatol. 2015;135(12):3173–82. doi: 10.1038/jid.2015.338. [DOI] [PubMed] [Google Scholar]
- 15.Stojadinovic O, Pastar I, Vukelic S, et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med. 2008;12(6B):2675–90. doi: 10.1111/j.1582-4934.2008.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voss A, Gescher K, Hensel A, Nacken W, Zanker KS, Kerkhoff C. Double-stranded RNA induces S100 gene expression by a cycloheximide-sensitive factor. FEBS Lett. 2012;586(2):196–203. doi: 10.1016/j.febslet.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274(46):32672–9. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 18.Jinquan T, Vorum H, Larsen CG, et al. Psoriasin: a novel chemotactic protein. J Invest Dermatol. 1996;107(1):5–10. doi: 10.1111/1523-1747.ep12294284. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Kizawa K. Expression of calcium-binding S100 proteins A4 and A6 in regions of the epithelial sac associated with the onset of hair follicle regeneration. J Invest Dermatol. 2001;116(6):956–63. doi: 10.1046/j.0022-202x.2001.01369.x. [DOI] [PubMed] [Google Scholar]
- 20.Voss A, Bode G, Sopalla C, et al. Expression of S100A8/A9 in HaCaT keratinocytes alters the rate of cell proliferation and differentiation. FEBS Lett. 2011;585(2):440–6. doi: 10.1016/j.febslet.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Lee KC, Eckert RL. S100A7 (Psoriasin)--mechanism of antibacterial action in wounds. J Invest Dermatol. 2007;127(4):945–57. doi: 10.1038/sj.jid.5700663. [DOI] [PubMed] [Google Scholar]
- 22.Edsberg LE, Wyffels JT, Brogan MS, Fries KM. Analysis of the proteomic profile of chronic pressure ulcers. Wound Repair Regen. 2012;20(3):378–401. doi: 10.1111/j.1524-475X.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 23.Dressel S, Harder J, Cordes J, et al. Differential expression of antimicrobial peptides in margins of chronic wounds. Exp Dermatol. 2010;19(7):628–32. doi: 10.1111/j.1600-0625.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 24.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. 2004;123(1):23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- 25.Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem. 2003;51(5):675–85. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen. 2009;17(6):832–9. doi: 10.1111/j.1524-475X.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 27.Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the culprits: macrophages-versatile regulators of wound healing. Adv Wound Care. 2013;2(7):357–68. doi: 10.1089/wound.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]