Abstract
Rationale
Fluid resuscitation is widely considered a life-saving intervention in septic shock; however, recent evidence has brought both its safety and efficacy in sepsis into question.
Objectives
In this study, we sought to compare fluid resuscitation with vasopressors with the use of vasopressors alone in a hyperdynamic model of ovine endotoxemia.
Methods
Endotoxemic shock was induced in 16 sheep, after which they received fluid resuscitation with 40 ml/kg of 0.9% saline or commenced hemodynamic support with protocolized noradrenaline and vasopressin. Microdialysis catheters were inserted into the arterial circulation, heart, brain, kidney, and liver to monitor local metabolism. Blood samples were recovered to measure serum inflammatory cytokines, creatinine, troponin, atrial natriuretic peptide, brain natriuretic peptide, and hyaluronan. All animals were monitored and supported for 12 hours after fluid resuscitation.
Measurements and Main Results
After resuscitation, animals that received fluid resuscitation required significantly more noradrenaline to maintain the same mean arterial pressure in the subsequent 12 hours (68.9 mg vs. 39.6 mg; P = 0.04). Serum cytokines were similar between groups. Atrial natriuretic peptide increased significantly after fluid resuscitation compared with that observed in animals managed without fluid resuscitation (335 ng/ml [256−382] vs. 233 ng/ml [144−292]; P = 0.04). Cross-sectional time-series analysis showed that the rate of increase of the glycocalyx glycosaminoglycan hyaluronan was greater in the fluid-resuscitated group over the course of the study (P = 0.02).
Conclusions
Fluid resuscitation resulted in a paradoxical increase in vasopressor requirement. Additionally, it did not result in improvements in any of the measured microcirculatory- or organ-specific markers measured. The increase in vasopressor requirement may have been due to endothelial/glycocalyx damage secondary to atrial natriuretic peptide−mediated glycocalyx shedding.
Keywords: sepsis, septic shock, fluid resuscitation, endotoxemia
Conservative estimates place the annual incidence of sepsis at 19 million cases, with global estimates of costs upward of 16 billion dollars (USD) (1, 2). Mortality remains high, with estimates of greater than 20% in the developed world (3).
Fluid resuscitation is widely regarded as an essential life-saving intervention in sepsis, and early fluid resuscitation is recommended in all iterations of the international Surviving Sepsis Campaign guidelines (4, 5).
The onset of sepsis is believed to result in pathological tissue hypoperfusion, which if untreated leads to progressive organ dysfunction (6). Fluid resuscitation is used to increase cardiac output to reverse pathological hypoperfusion and thus prevent organ injury (4, 7). Although this approach has been widely adopted, emerging evidence is calling into question both the safety and efficacy of fluid resuscitation (8–10). Importantly, before 2011, no randomized control trials (RCTs) had directly examined the impact of fluid resuscitation on mortality in sepsis.
The FEAST (Fluid Expansion as Supportive Therapy) trial was published in 2011 and remains the only RCT of fluid resuscitation in sepsis (11). Children with severe sepsis were randomized to receive fluid resuscitation with either 0.9% saline or 4% albumin, or supportive care. The trial demonstrated harm from fluid resuscitation, with both resuscitation arms having a 45% relative increase in mortality (11). Similarly, Andrews and colleagues conducted an RCT of early protocolized resuscitation with fluids and vasopressors in Zambian patients presenting with sepsis, and found increased mortality in the resuscitated patients, suggesting that there may be harm associated with the traditional resuscitation paradigm (8). The results of these trials are in direct contrast to more than 50 years of medical theory and call into question the safety of this common intervention, and suggest that it may in fact be harmful.
In a secondary analysis of the FEAST trial, Maitland and colleagues demonstrated that the excess mortality in the fluid resuscitation arms was due to delayed cardiovascular collapse, a potentially novel mechanism of harm (12). Consequently, we aimed to examine the effectiveness of fluid resuscitation for the treatment of septic shock in an animal model of endotoxemia. We hypothesized that fluid resuscitation would not result in substantial improvements in endotoxemic shock and would lead to an increased need for cardiovascular support after fluid resuscitation. The primary outcome of the study was vasopressor requirements in the post-fluid-resuscitation period. Additionally, because fluid resuscitation is used to maintain tissue perfusion, we used tissue microdialysis of the vital organs to assess for pathological tissue hypoperfusion. Serum proinflammatory cytokines and biochemistry were measured throughout the study to assess for a putative mechanistic connection between fluid resuscitation and the inflammatory state and the development of organ failure. Given the observation of delayed cardiovascular collapse in the FEAST study, plasma atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), troponin, and glycocalyx products were measured to assess for potential cardiac and endothelial damage.
Limited hemodynamic and microdialysis data from the non−fluid-resuscitated (NR) control group have been published previously (13).
Methods
This study was approved by the Animal Research and Ethics Committee of the Queensland University of Technology and the University of Queensland (approval No. 1,400,000,032), and adhered to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th Edition (14).
Animal Preparation
Sixteen Merino ewes (43.5 ± 6.2 kg) were fasted overnight for the study. Procedures involving animal care, data capture, and anesthetic and surgical techniques, including placement of microdialysis catheters and microdialysis, were done in accordance with previously described methods (13). At the commencement of the study, all animals were anesthetized with midazolam (0.5 mg/kg; Pfizer), buprenorphine (300 μg; Reckitt Benckiser Healthcare), and alfaxalone (3 mg/kg; Jurox). Anesthesia was maintained with an infusion of alfaxalone (6 mg/kg/h; Jurox), midazolam (0.25 mg/kg/h; Pfizer), fentanyl (15 μg/kg/h; Hameln Pharmaceuticals), and ketamine (10 mg/kg/h; Troy Laboratories). All anesthetic and analgesic medications were titrated to maintain adequate surgical anesthesia.
A central venous catheter (Arrow International), facial artery arterial catheter (Arterial Leadercath, Vygon), and pulmonary artery catheter (Swan-Ganz CCOmbo, Edwards Lifesciences) were inserted to facilitate drug administration and cardiovascular monitoring.
Microdialysis catheters (CMA 63 and 70 MD probes) were surgically inserted into the femoral artery, brain, heart, liver, and left kidney.
Throughout the study, all animals received protocolized ventilation to maintain an SaO2 of >94% and an end-tidal CO2 of 35−45 mm Hg.
Experimental Protocol
After the completion of surgical instrumentation, a 60-minute interval was allowed before commencement of the experimental protocol for physiological stabilization of the animal.
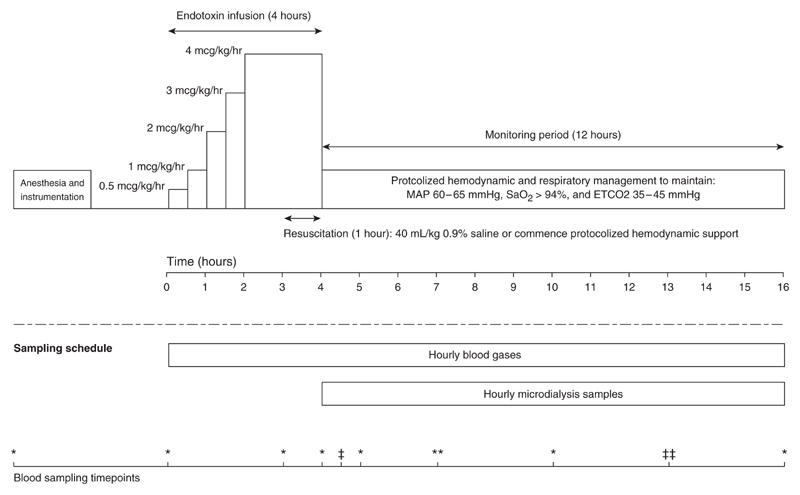
To avoid profound pulmonary hypertension, LPS infusion (Escherichia coli serotype O55:B5) was commenced at 0.5 μg/kg/h and escalated to 4 μg/kg/h over 4 hours (Figure 1). Resuscitation occurred in the last hour of the endotoxin infusion. All animals had a mean arterial pressure (MAP) below 60 mm Hg before resuscitation. Animals received either fluid resuscitation with 40 ml/kg of normal saline (FR; n = 8) given over 1 hour or commenced vasopressor support (NR; n = 8). After fluid resuscitation, noradrenaline 60 μg/ml in 5% dextrose (Hospira) was commenced to maintain an MAP of 60−65 mm Hg. In both groups, the starting noradrenaline rate was 5 μg/min and it was titrated every 5 minutes to maintain the MAP in the target range. If the noradrenaline dose reached a predetermined 20 μg/min, vasopressin (PPC) was commenced at 0.8 U/h and increased to a maximum of 1.6 U/h if hypotension persisted. Controlled administration of noradrenaline and vasopressin was achieved using a Gemini infusion pump (Alaris Medical Systems) and the administration protocol was the same for both groups. Animals were monitored for 12 hours after the end of the endotoxin infusion and received a total of 13 hours of hemodynamic support (Figure 1).
Figure 1.
Schematic representation of the experimental protocol. Anesthesia and surgical instrumentation were followed by a 1-hour stabilization period during which no interventions were performed. Endotoxemic shock was induced with a 4-hour escalating dose of endotoxin infusion. Resuscitation occurred in the last hour of the endotoxin infusion, with animals either receiving a 40 ml/kg bolus of 0.9% saline or commencing protocolized hemodynamic support. After resuscitation, all animals were monitored for a further 12 hours, during which time both groups received protocolized hemodynamic and respiratory support. Blood gases were taken hourly during the 16 hours of the experiment and analyzed immediately on an ABL800 Flex (Radiometer). During the monitoring period, hourly microdialysis samples were recovered and analyzed for lactate and pyruvate on an ISCUS clinical microdialysis analyzer (Hammarby Fabriksvag). The bottom scale indicates the blood-sampling time points throughout the experiment. *Time points at which blood was taken for serum cytokines (IL-1 β, Il-6, IL-8, IL-10, and tumor necrosis factor α [TNF-α]), troponin, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), hyaluronan, and creatinine. *Time points at which blood was taken to measure hyaluronan only. **Time points at which blood was taken to measure serum cytokines and hyaluronan. **Time points at which blood was taken to measure serum cytokines. Cytokine measurements were performed using an in-house ELISA for IL-1β, IL-6, IL-8, IL-10, and TNF-α. The methodology of the cytokine analysis has been described previously (13). Cardiac troponin I was measured using the Unicel DxI AccuTnI+3 immunoassay (Beckman Coulter). Hyaluronan was measured using a hyaluronan Quantikine ELISA Kit (R&D Systems). Serum ANP and BNP were measured using a custom ovine radioimmunoassay (Endolab). Creatinine was measured using a COBAS Integra 400 blood chemistry analyzer (Roche Diagnostics). ETCO2 = end-tidal CO2; MAP = mean arterial pressure.
Statistical Analysis
Data are presented as mean (SD) for normally distributed variables and median (interquartile range) for non-normally distributed variables. Only the MAP, serum lactate, base excess, and serum hyaluronan were normally distributed, and values at each time point were compared with a two-sample t test. All other variables were not normally distributed, and values at individual time points were compared using the Wilcoxon rank-sum test. P < 0.05 was considered statistically significant.
Repeated-measures regression analysis was performed for hyaluronan, noradrenaline, and vasopressin infusion rate over time to test for differences between groups. Fixed-effect modeling was used throughout. Confirmation of the validity of this methodology was assessed using the Hausman test. The independent variables used were resuscitation group (FR or NR) and time in hours, and all models were tested for significant interactions between the independent predictors. Model diagnostics included testing the error terms (residuals) for normality using the Shapiro-Wilk test, and homoscedasticity using the Breusch-Pagan test. Outliers and high leverage points were identified using leverage to squared residual plots, and influential points were identified using Cook’s distance method.
Statistical analyses were performed using the STATA statistical software package (version 13; StataCorp). One animal in the FR group was killed 90 minutes before the end of the experiment because it was deemed unsupportable by the investigators due to metabolic acidosis and refractory hypotension (pH < 6.9 and MAP < 60 mm Hg despite 240 μg/min of noradrenaline).
Results
Hemodynamic Variables
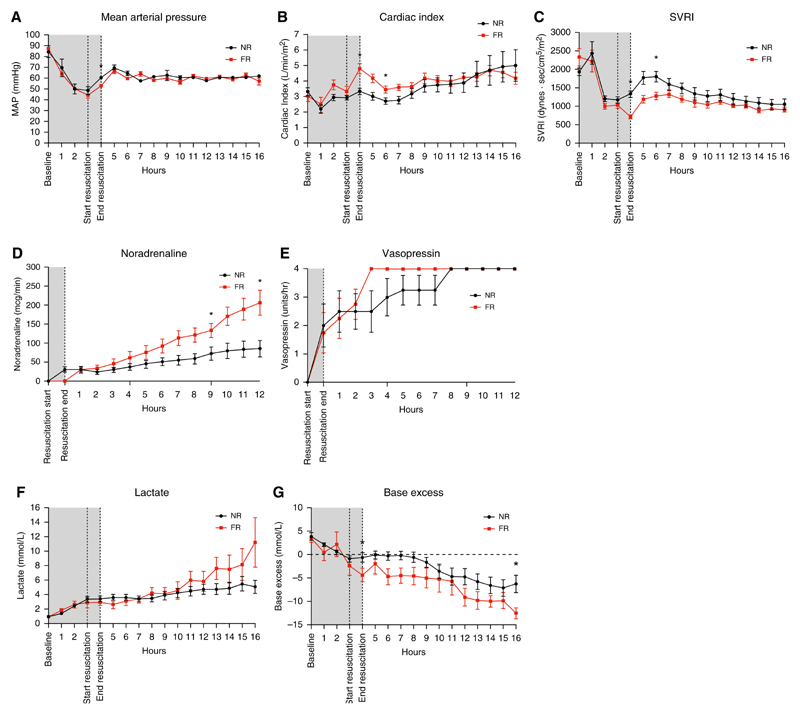
At baseline, hemodynamic variables were similar between the two groups. Endotoxemia produced a similar reduction in blood pressure in both groups, with a mean MAP of 44−49 mm Hg before resuscitation (Table 1 and Figure 2A). Endotoxemia produced a temporary decrease in the cardiac index 1 hour after infusion that recovered to normal or supranormal levels before resuscitation (Table 1, Figure 2B). Fluid resuscitation produced a significant increase in the cardiac index, increasing from a median of 3.42 (interquartile range, 2.52−3.72) to 4.94 (3.91−5.62) (P = 0.005). The systemic vascular resistance index (SVRI) was similar between groups at baseline and before resuscitation (Figure 2C). Fluid resuscitation resulted in a significant decrease in SVRI from 1,110 (950−1230) to 714 (630−807) (P = 0.02) associated with a relatively large increase in cardiac output and a concomitant modest increase in blood pressure.
Table 1. Systemic Hemodynamic Variables at Baseline and throughout the Experimental Period in Fluid-resuscitated and Non−Fluid-resuscitated Animals.
| Mean Arterial Pressure (mm Hg) (Mean ± SD) | Cardiac Index (L/min/m2) (Median and IQR) | Systemic Vascular Resistance Index (dyn · s/cm5) (Median and IQR) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Point | FR Group (n = 8) | NR Group (n = 8) | P Value | FR Group (n = 8) | NR Group (n = 8) | P Value | FR Group (n = 8) | NR Group (n = 8) | P Value |
| TO, baseline, start endotoxin infusion | 87.1 + 8.4 | 84.5 + 15.3 | 0.68 | 2.78 (2.61−3.18) | 3.23 (2.78−3.74) | 0.29 | 2,270 (1,918−2,815) | 2,000 (1,757−2,111) | 0.21 |
| T3, start resuscitation | 44.6 ± 9.4 | 48.5 ± 9.7 | 0.43 | 3.42 (2.52−3.72) | 2.91 (2.70−3.04) | 0.41 | 1,110 (950−1,230) | 1,155 (1,029−1,340) | 0.53 |
| T4, end resuscitation/endotoxin | 52.8 + 4.4 | 60.4 + 2.3 | <0.001* | 4.94 (3.91−5.62) | 3.08 (2.94−3.70) | 0.006* | 714 (630−807) | 1,434 (1,146−1,492) | <0.001* |
| T6, 2 h after resuscitation | — | — | — | 3.52 (3.08−3.96) | 2.53 (2.27−3.28) | 0.046* | 1,236 (1,043−1,379) | 1,858 (1,425−2,176) | 0.02* |
| T8, 4 h after resuscitation | 60.1 + 2.6 | 57.8 + 4.2 | 0.20 | 3.62 (2.92−4.28) | 2.98 (2.59−3.66) | 0.40 | 1,212 (982−1,372) | 1,518 (1,248−1,722) | 0.09 |
| T12, 8 h after resuscitation | — | — | — | 4.17 (3.62−4.98) | 3.10 (2.76−4.71) | 0.21 | 1,006 (871−1,149) | 1,412 (806−1,513) | 0.21 |
| T16, 12 h after resuscitation | 56.9 ± 3.4 | 61.9 ± 2.4 | 0.15 | 4.02 (3.14−5.26) | 3.85 (3.47−5.53) | 0.99 | 910 (814−1,036) | 1,147 (827−1,269) | 0.25 |
Definition of abbreviations: FR = fluid-resuscitated; IQR = Interquartile range; NR = non−fluid-resuscitated.
Statistically significant difference, P value < 0.05.
Figure 2. Hemodynamic, vasopressor, and acid/base variables during the experiment in both fluid-resuscitated (FR) and non−fluid-resuscitated (NR) animals.
(A) Mean arterial pressure during endotoxemia, resuscitation, and the postresuscitation monitoring period. Gray shading corresponds to the time period of endotoxin infusion. Results are expressed as mean ± SD. (B and C) Cardiac index and systemic vascular resistance during the experiment. Results are expressed as median values with interquartile range (IQR). (D) Noradrenaline infusion rate during the experiment, median, and IQR. Linear regression analysis demonstrated the rate of increase to be significantly greater in the FR group. (E) Vasopressin infusion rates during the experiment (median and IQR). There was no significant difference in vasopressin infusion rates. All animals required the maximal dose of vasopressin by the end of the study. (F and G) Lactate and base excess over the experiment; data are represented as mean ± SD. *Time points with a statistically significant difference (P < 0.05). MAP = mean arterial pressure; SVRI = systemic vascular resistance index.
Hemodynamic Support
Vasopressor use differed significantly between the groups. Noradrenaline requirements increased for both groups throughout the monitoring period, with a statistically significant difference between groups at both 8 and 12 hours of the monitoring period (Figure 2D). The median noradrenaline rate 8 hours after resuscitation was 140 μg/min (96.5−172.5) in the FR group and 62 μg/min (31.0−110.0) in the NR group (P = 0.03). At the end of the monitoring period, the difference between groups had increased and was 240.0 μg/min (104.0, 270.0) in the FR group compared with 73.0 μg/min (33.0, 137.5) in the NR group (P = 0.02). Repeated-measures regression analysis demonstrated that the rate of increase was significantly greater for the FR group than for the NR group (β of 18.74 μg/min/h [P = 0.001] vs. 6.51 μg/min/h [P = 0.001], with the group time interaction term P < 0.001) (Figure 2D). With the exception of the noradrenaline dose, no interactions were noted, and the regression error terms were normally distributed and homoscedastic. All animals required a full dose of vasopressin (0.03 U/min), and there was no significant difference in vasopressin use between the groups (Figure 2E).
Biochemistry
Serum lactate increased in both groups throughout the study. Although no statistically significant difference was observed at any point (Figure 2F), the mean lactate did diverge over the course of the monitoring period, with a final mean lactate of 11.22 (±7.65) in the FR arm and 5.07 (±2.02) in the NR resuscitation group (P = 0.09). Serum base excess decreased in both groups throughout the study (Figure 2G) and was greater in the FR group than in the NR group (P = 0.048). This effect persisted throughout the study, with a serum base excess of −12.52 (±2.82) in the FR group and −6.26 (±4.94) at the end of the monitoring period (P = 0.02).
Microdialysis
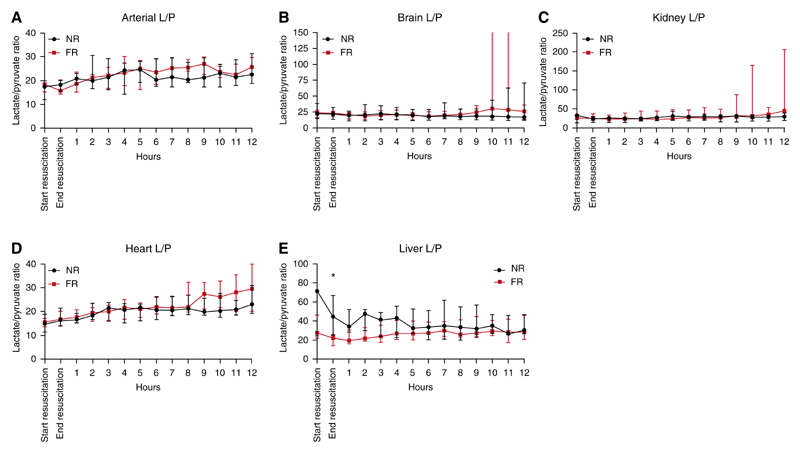
The lactate/pyruvate (L/P) ratios of both resuscitation strategies were similar across all organs (Figure 3). For the arterial, brain, heart, and kidney microdialysis samples, there were no statistically significant differences between the groups at any time point. The liver microdialysis samples did demonstrate a statistically significant difference after resuscitation; however, the groups had large differences in baseline L/P ratios before resuscitation (Figure 3E). None of the other time points in the liver microdialysis demonstrated significant differences.
Figure 3. Individual organ lactate/pyruvate (L/P) ratios for the period after resuscitation in fluid-resuscitated (FR) and non−fluid-resuscitated (NR) animals.
(A) Arterial L/P ratios during the postresuscitation period. Values were similar between the groups throughout. (B) Brain L/P ratios during the postresuscitation period. The variance seen in interquartile range (IQR) after hour 10 was due to a rapid increase in the L/P ratio in a single animal in the final 3 hours of the experiment to >3,400. (C) Kidney L/P ratio during the same period. There was no significant difference between the groups at any time point. (D) Heart L/P ratio during the same period. There was no significant difference between the groups at any time point. (E) Liver L/P ratios during the period after resuscitation. Values started at a higher baseline for the NR group than for the FR group. After resuscitation, values were significantly greater for the NR animals than for the FR animals. All data are represented as median values with IQR. *P < 0.05.
Inflammatory Cytokines
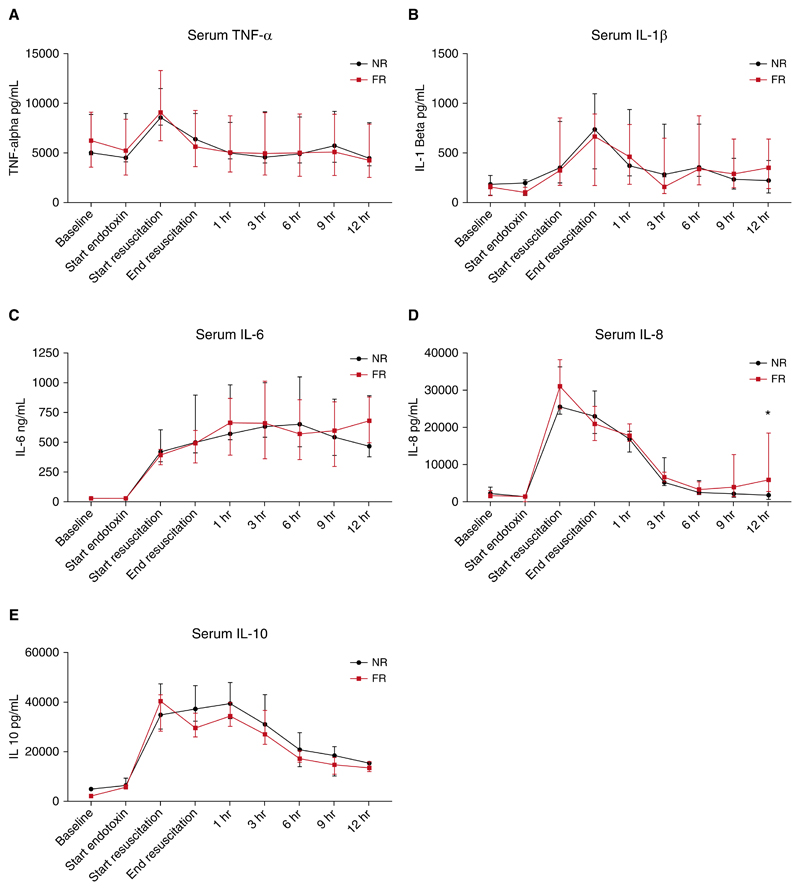
Endotoxemia produced large increases in all of the measured serum cytokines (Figure 4). Tumor necrosis factor α (TNF-α), IL-1β, IL-8, and IL-10 all exhibited a similar pattern of response, with levels peaking at or near the end of the endotoxin infusion before decreasing to near baseline values. IL-6 increased during endotoxemia and remained near maximal values throughout the monitoring period. The pattern of cytokine response between groups was remarkably similar, with no significant differences at any of the time points for TNF-α, IL-1β, IL-6, and IL-10. IL-8 values were similar between groups throughout the experiment; however, the final values at the end of the monitoring period were significantly different (1,779 pg/ml [759−5,051] in the NR group compared with 5,903 pg/ml [3,519−14,591] in the FR group; P = 0.04) (Figure 4).
Figure 4. Plasma cytokine levels for both fluid-resuscitated (FR) and non−fluid-resuscitated (NR) animals throughout the experiment.
(A) Serum tumor necrosis factor-α (TNF-α) increased during endotoxemia, reaching peak values in both groups at the beginning of resuscitation before returning to near baseline levels. (B) Serum IL-1β increased during endotoxemia in both groups, reaching maximal values at the end of resuscitation. (C) Endotoxemia produced large increases in IL-6 in both groups that were sustained throughout the study. (D) Serum IL-8 reached maximal values in both groups at the beginning of resuscitation. IL-8 decreased in both groups after resuscitation; however, there was a late increase in IL-8 in the FR animals, and the final IL-8 values were statistically significantly different between the groups (*P = 0.04). (E) Endotoxin produced a large increase in IL-10 in both groups that slowly decreased throughout the monitoring period. Results are expressed as median values and interquartile range.
Cardiac, Endothelial, and Renal Biomarkers
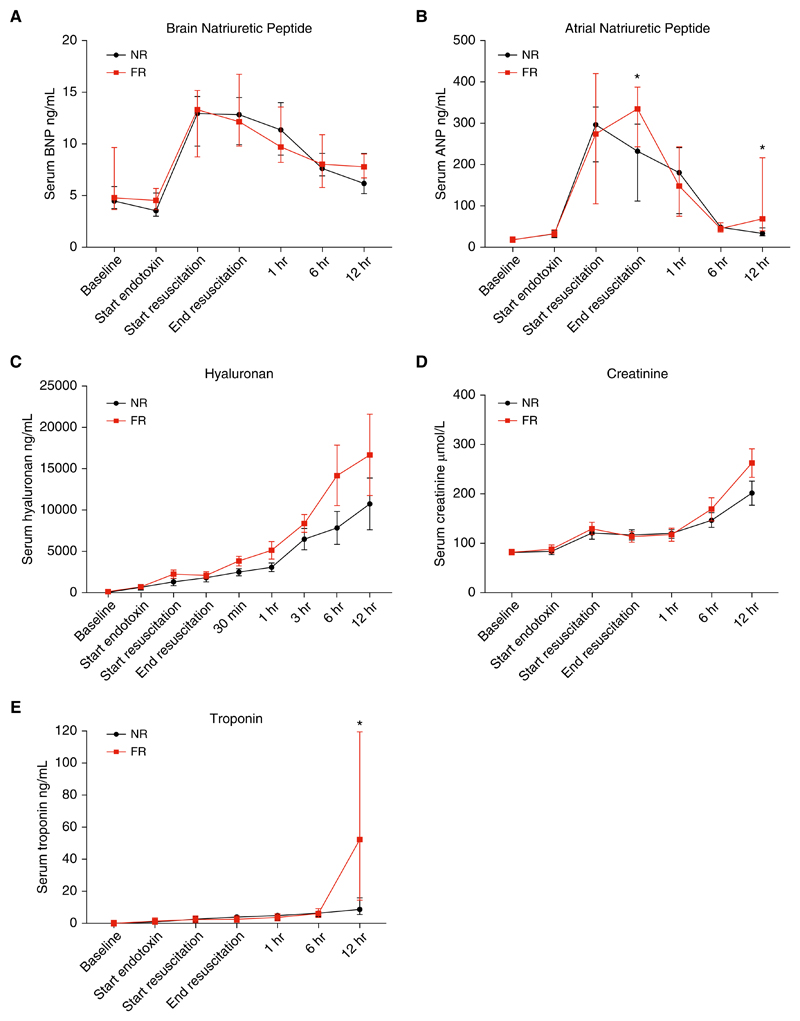
BNP increased during endotoxemia in both groups and reached maximal values at 3 hours. There were no significant differences in BNP values between the groups at any time point (Figure 5A). Endotoxemia resulted in a large increase in serum ANP in both groups (Figure 5B). In the NR group, ANP peaked at a median of 297 ng/ml (227−331) 3 hours into the endotoxin infusion and then declined throughout the rest of the experimental period. In the FR group, ANP reached a similar value after 3 hours (274 ng/ml [142−418]); however, it increased further after fluid resuscitation to 335 ng/ml (256−382). There was a significant difference in the median ANP between the two groups after resuscitation (335 ng/ml [256−382] in the FR group vs. 233 ng/ml [144−292] in the NR group; P = 0.02). There was a large difference in high-sensitivity serum troponin at the end of the study, with median values of 8.60 ng/ml (5.85−15.50) in the NR group compared with 52.5 ng/ml (18−104) in the FR group (P = 0.03) (Figure 5E).
Figure 5. Cardiovascular and renal biomarkers during the experiment in both fluid-resuscitated (FR) and non−fluid-resuscitated (NR) animals.
(A) Endotoxemia produced a large increase in plasma brain natriuretic peptide (BNP) in both groups that then declined throughout the observation period in both groups (median and interquartile range [IQR]). (B) Serum atrial natriuretic peptide (ANP) increased in response to endotoxemia in both groups. Plasma ANP peaked at the initiation of resuscitation in the NR group. In the FR group, plasma ANP rose with endotoxemia and increased further after resuscitation. There was a statistically significant difference in plasma ANP between the groups after resuscitation (*P < 0.05) (median and IQR). (C) Hyaluronan increased in both groups during the study (mean ± SD). (D) Serum creatinine was not significantly different at any time point and increased in both groups throughout the study (median and IQR). (E) Serum troponin values were similar between the groups until the end of the experiment, when there was a large increase in the FR animals (median and IQR) (*P = 0.03).
The glycocalyx glycosaminoglycan (GAG) hyaluronan increased in both groups throughout the study (Figure 5C). Maximal mean values of 10,734 (± 8,940) in the NR group and 16,681 (±13,903) in the FR group (P = 0.33) were reached at the end of the experiment. The values were not significantly different between the groups at any of the measured time points (Figure 5C). Univariate time-series cross-sectional analysis demonstrated that the rate of increase was significantly greater in the FR group than in the NR group (P = 0.02). Similarly, multivariate regression analysis confirmed that FR was a predictor of an increased rate of increase of hyaluronan.
Serum creatinine increased throughout the study in both groups, reaching maximal median values of 263 μmol/L (191−335) in the FR group and 208 μmol/L (148−240) in the NR group (P = 0.12) at the end of the study (Figure 5D).
Discussion
Fluid resuscitation is promoted as a critical life-saving intervention in sepsis and has been strongly recommended as the first-line resuscitative therapy in all iterations of the international Surviving Sepsis Campaign guidelines (4). It is believed that adequate fluid resuscitation in the early phase of sepsis improves shock and results in reduced vasopressor requirements later in a patient’s illness (7). The key finding of this study was that fluid resuscitation given for endotoxemic shock produced short-lived hemodynamic improvements that were then followed by significantly increased vasopressor requirements in the subsequent 12 hours. These results challenge the current paradigm of resuscitation and warrant a detailed discussion.
First, our study has a number of important differences in comparison with previous animal models that demonstrated a benefit of fluid resuscitation in sepsis and endotoxemia. Our model was developed to produce hyperdynamic/distributive shock with a progressive decline in SVRI while maintaining near-normal cardiac output at the initiation of resuscitation. These are the changes most often encountered in both human septic shock and endotoxemia (15–18). Previous studies that supported fluid resuscitation in animals used hypodynamic models of illness, with rapid and severe reductions in cardiac output being the dominant cause of hypotension (19–22). In a porcine model of endotoxemic shock, Oi and colleagues demonstrated improved mortality using fluid resuscitation with hypertonic saline-dextran (22). However, after the administration of endotoxin, untreated animals developed a profound and persistent low-cardiac-output state, with a mortality of 77% at 5 hours (the end of the experiment). Similarly, Bressack and colleagues were able to show improved outcomes with fluid resuscitation in a porcine model of septic shock; however, animals in that study also developed severe cardiogenic shock (19). As hyperdynamic shock appears to be the most commonly seen presentation in human sepsis (16–18), the rapid development of a life-threatening low-cardiac-output state in these models makes it difficult to extrapolate their findings to the clinical arena. The incremental increase in the rate of endotoxin infusion used in our study minimized the cardiac dysfunction that has hampered other models, and allowed our model to generate a hyperdynamic response similar to that seen in clinical sepsis. Therefore, we believe that this model better reflects the actual effect of fluid resuscitation in the clinical setting.
In addition, previous studies of fluid resuscitation did not use an alternative hemodynamic support strategy and compared fluid resuscitation with untreated shock (19–22). The relevance of such models is a concern because clinically, fluid resuscitation is one element of a larger resuscitation strategy, and short-term studies of it in isolation do not reflect how it is used clinically and may miss important interactions with other therapies. The advantage of this study is that it compares fluid resuscitation as part of a resuscitation strategy that is similar to current guideline recommendations with a viable alternative. This model better addresses the question of the likely impact of fluid resuscitation on current patients treated with septic shock, and will inform clinical studies of alternative strategies to manage septic shock.
As expected, the FR animals had a large increase in cardiac output immediately after the fluid bolus was administered. Although the MAP improved over the hour of resuscitation, the magnitude of the increase was modest compared with the increase in cardiac output. This was due to a concurrent decrease in the SVRI of the resuscitated animals. Such fluid resuscitation−induced vasodilation has been observed clinically in a number of studies. Monge García and colleagues described the immediate effects of fluid bolus resuscitation on arterial load in 81 patients with sepsis (23). They found that 67% of the patients had an increase in cardiac output with fluid resuscitation; however, this only resulted in an increase in MAP in 44% of the patients. Overall fluid resuscitation resulted in a decrease in SVRI that was most marked among patients who showed an increase in cardiac output. In a similar observational study in 51 patients with sepsis, Pierrakos and colleagues found that in patients with a significant increase in cardiac output, fluid resuscitation resulted in a decrease in SVRI (24). The underlying mechanism of this vasodilation is unclear, but it is believed to involve a combination of baroreceptor-mediated reduction in sympathetic tone and flow-mediated vascular relaxation secondary to endothelial nitric oxide release (23).
The novel finding of this study is the divergent trends in vasopressor requirement after resuscitation. Interestingly, this was not apparent in the immediate postresuscitation period, and noradrenaline requirements were similar between the groups for the first 3−4 hours. After 3−4 hours, the noradrenaline requirement diverged, plateauing in the NR animals and increasing rapidly in the FR animals. This was reflected in a statistically significant difference between the groups in the repeated-measures linear regression analysis, and differences between the groups at both 9 and 12 hours after resuscitation. This result has some parallels to Maitland and colleagues’s exploratory analysis of the increased mortality seen in the landmark FEAST trial (12). In a post hoc analysis of the excess mortality, the authors noted that the largest contributor to the excess mortality seen with fluid resuscitation was delayed cardiovascular collapse occurring 2−11 hours after resuscitation. This mode of death alone accounted for more than half of the additional deaths seen in the FR groups (12). The results of our study support the hypothesis that fluid resuscitation produces delayed vascular injury that manifests as vascular hyporeactivity hours after the initial bolus is given.
One might expect the above differences seen in the progression of shock to be attributable to alterations in inflammatory signaling and increased systemic inflammation due to fluid resuscitation. However, the measured serum cytokines do not support this hypothesis. Although endotoxemia produced large increases in all of the measured cytokines, fluid resuscitation had little additional effect, and the two groups had very similar cytokine profiles over the entire course of the study.
Recently, the role of the endothelial glycocalyx in the pathophysiology of sepsis has received increased attention. The glycocalyx, a thin gel structure lining the vascular endothelium, is believed to play a critical role in maintaining plasma oncotic pressure, preventing leukocyte adhesion, inhibiting coagulation activation, and preserving vascular responsiveness (25, 26). It has been demonstrated to be shed in both sepsis and endotoxemia, and circulating levels of glycocalyx fragments have been associated with mortality (27, 28). ANP has been shown to be a potent trigger for glycocalyx shedding in experimental models of exogenous ANP administration (29, 30). Similarly, transgenic mice with knockout inactivation of the endothelial guanylyl cyclase-A ANP receptor demonstrated decreased vascular permeability compared with controls and did not have the same immediate increase in hematocrit from vascular leak in response to exogenous ANP (31). Furthermore, hypervolemia has been shown to be a potent trigger for glycocalyx shedding, potentially mediated by ANP release (32). In our model, endotoxemia was associated with a large increase in ANP (more than 14-fold in both groups). Fluid resuscitation resulted in a further rise in ANP, whereas levels in the NR group had already begun to decline. This additional increase in ANP may be pathological and cause increased glycocalyx shedding, which may exacerbate vascular dysfunction. This notion is supported by the increased rate of increase of the glycosaminoglycan hyaluronan in the FR animals.
One of the main aims of fluid resuscitation in sepsis is to reverse pathological tissue hypoperfusion and thus prevent the onset of tissue ischemia and organ dysfunction (6, 7). We aimed to use tissue microdialysis to detect potentially favorable effects of fluid resuscitation on the microcirculation of various vital organs. The L/P ratio is a sensitive marker of the redox state of a tissue, with high levels being associated with hypoperfusion and impaired oxidative metabolism (33). One would expect that if fluid resuscitation had reversed or improved pathological hypoperfusion, this would be reflected in a comparatively lower L/P ratio in the respective organ. This was not demonstrated in the arterial circulation, heart, brain, or kidney, and remarkably similar L/P ratios were observed throughout the experimental period. The liver results are notable for the large difference in baseline values before resuscitation, with the hepatic L/P ratio being nearly threefold higher for the NR group than for the FR group. There was a significant difference in the hepatic L/P ratios after resuscitation; however, the significance of this must be questioned given the large differences in baseline values. The authors are not able to explain the discordant baseline values, given the identical treatment of the experimental subjects before resuscitation. It is notable, however, that the hepatic L/P ratios in the NR group decreased throughout the observation period and were approximately the same as those observed in the FR group by the end of the study.
Similarly, if fluid resuscitation improved perfusion and prevented organ injury, one might expect to see a difference in serum biomarkers (creatinine and troponin) over time between the groups. In this study, there was no difference in serum creatinine between the groups throughout the experiment, with both groups developing evidence of acute kidney injury at a similar rate. Of note, although serum troponin was similar between the groups for the majority of the study, a dramatic late rise was seen in the FR group. This finding is of interest because increases in serum troponin have been observed in patients with sepsis and no obstructive coronary disease, and are associated with increased mortality (34). The mechanism underlying this phenomenon is unclear, but a number of potential contributing mechanisms have been proposed, including mitochondrial oxidative stress, microvascular thrombi, and increased sarcolemma membrane permeability (34, 35). Catecholamines have been shown to increase myocardial oxidative stress in experimental models (36), and in a murine model of sepsis, Haileselassie and colleagues demonstrated that increased oxidative stress was a central factor in the development of septic-induced myocardial dysfunction (37). The observed increase in troponin is a concerning find because it suggests that the increased vasopressor requirement is not a benign epiphenomenon and may produce secondary cardiac injury.
Arterial lactate has been shown to be associated with increased mortality in sepsis (38). Furthermore, a serial decrease in arterial lactate during treatment is associated with improved outcomes (39). In this study, fluid resuscitation did not result in significantly lower serum lactate in the immediate postresuscitation period or at any time point. Contrary to what would be expected, there was a trend toward higher arterial lactate in the FR group as the monitoring period progressed, with a final median lactate of 9.1 mmol/L in the FR group and 4.9 mmol/L in the NR group. Similarly, metabolic acidosis was worse in the FR sheep throughout the postresuscitation period. Neither of these results supports the conclusion that fluid resuscitation had favorable effects on the progression of metabolic derangements associated with endotoxemia.
It is important to recognize the limitations of this study. The observed association between increased ANP and hyaluronan and an increased need for vasopressors is interesting given the current understanding of the function of the glycocalyx in vascular function. However, in this study, we were not able to quantify the degree to which the observed differences in both ANP and hyaluronan accounted for the differences in vasopressor requirements. Future studies exploring this phenomenon using either ANP inhibition or exogenous ANP are required to establish the degree to which changes in glycocalyx function are responsible for changes in vascular reactivity. Additionally, because this trial investigated the harms seen in the FEAST study, the same fluid bolus dose (40 ml/kg) was used. This is more than is recommended by the Surviving Sepsis Campaign guidelines (4), and the same effects may not be seen with different volumes or rates of administration.
Conclusions
In conclusion, we found that fluid resuscitation had a detrimental effect on endotoxemic shock, with an increased vasopressor requirement in the 12 hours after resuscitation. This did not appear to be attributable to increased inflammatory signaling, as the inflammatory-cytokine results suggested a noninflammatory mechanism for the observed difference. Fluid resuscitation was associated with an increase in plasma ANP and the glycocalyx glycosaminoglycan hyaluronan, suggesting that it resulted in iatrogenic damage to the glycocalyx. This may have been a causative factor in the need for higher doses of vasopressor after resuscitation. Additionally, we were not able to demonstrate any beneficial effects of fluid resuscitation with respect to individual organ metabolism or any of the measured markers of injury or function.
At A Glance Commentary.
Scientific Knowledge on the Subject
Fluid resuscitation is a common therapy used for the treatment of septic shock.
What This Study Adds to the Field
This study tests the effectiveness of the therapy in an animal model of sepsis and finds that it is actually harmful.
Acknowledgment
The authors thank the Australian Red Cross Blood Service for its support in conducting the study. J.F.F. received a fellowship from the Queensland Health Office of Health and Medical Research.
Footnotes
Supported by grants from the Australian National Health and Medical Research Council, the Emergency Medicine Foundation, and The Prince Charles Hospital Foundation.
Author disclosures are available with the text of this article at www.atsjournals.org.
Author Contributions: Study design, conduct of experiments, and manuscript generation: L.B. and N.G.O. Study design and conduct of experiments: S.D.D. and K.R.D. Conduct of experiments and sample analysis: M.R.P., A.-C.B., and L.S.H. Conduct of experiments: S.P. and M.H.F. Conduct of experiments and data analysis: L.P.P. Study design, data analysis, and manuscript editing: F.V.H. Study design, statistical analysis, and manuscript editing: C.M.A. Study design, data analysis, and manuscript editing: L.C., K.S., and K.M. Study design, sample analysis, and manuscript editing: J.-P.T. Project oversight, study design, data analysis, and manuscript editing: J.F.F.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Hodgin KE, Moss M. The epidemiology of sepsis. CurrPharm Des. 2008;14:1833–1839. doi: 10.2174/138161208784980590. [DOI] [PubMed] [Google Scholar]
- 3.Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care. 2004;8:222–226. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 6.Rivers EP, Jaehne AK, Eichhorn-Wharry L, Brown S, Amponsah D. Fluid therapy in septic shock. Curr Opin Crit Care. 2010;16:297–308. doi: 10.1097/MCC.0b013e32833be8b3. [DOI] [PubMed] [Google Scholar]
- 7.Rivers EP, Coba V, Visbal A, Whitmill M, Amponsah D. Management of sepsis: early resuscitation. Clin Chest Med. 2008;29:689–704. doi: 10.1016/j.ccm.2008.06.005. ix-x. [DOI] [PubMed] [Google Scholar]
- 8.Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMAs. 2017;318:1233–1240. doi: 10.1001/jama.2017.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 10.Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock the effect of increasing fluid balance on mortality. J Intensive Care Med. 2014;29:213–217. doi: 10.1177/0885066613478899. [DOI] [PubMed] [Google Scholar]
- 11.Maitland K, Kiguli S, Opoka RO, Engoru C, OlupoOlupot-t P, Akech SO, et al. FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 12.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, et al. FEAST trial group. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne L, Obonyo NG, Diab S, Dunster K, Passmore M, Boon AC, et al. An ovine model of hyperdynamic endotoxemia and vital organ metabolism. Shock. 2018;49:99–107. doi: 10.1097/SHK.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Health and Medical Research Council. Australian code of practice for the care and use of animals for scientific purposes. 2004. Available from: https://www.nhmrc.gov.au/guidelines-publications/ea16.
- 15.Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, et al. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 16.Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med. 1987;15:923–929. doi: 10.1097/00003246-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Winslow EJ, Loeb HS, Rahimtoola SH, Kamath S, Gunnar RM. Hemodynamic studies and results of therapy in 50 patients with bacteremic shock. Am J Med. 1973;54:421–432. doi: 10.1016/0002-9343(73)90038-7. [DOI] [PubMed] [Google Scholar]
- 18.Villazon SA, Sierra UA, Lopez SF, Rolando MA. Hemodynamic patterns in shock and critically ill patients. Crit Care Med. 1975;3:215–221. doi: 10.1097/00003246-197511000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Bressack MA, Morton NS, Hortop J. Group B streptococcal sepsis in the piglet: effects of fluid therapy on venous return, organ edema, and organ blood flow. Circ Res. 1987;61:659–669. doi: 10.1161/01.res.61.5.659. [DOI] [PubMed] [Google Scholar]
- 20.Ottosson J, Dawidson I, Brandberg A, Idvall J, Sandor Z. Cardiac output and organ blood flow in experimental septic shock: effect of treatment with antibiotics, corticosteroids, and fluid infusion. Circ Shock. 1991;35:14–24. [PubMed] [Google Scholar]
- 21.Ottosson J, Persson T, Dawidson I. Oxygen consumption and central hemodynamics in septic shock treated with antibiotics, fluid infusions, and corticosteroids. Crit Care Med. 1989;17:772–779. doi: 10.1097/00003246-198908000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Oi Y, Aneman A, Svensson M, Ewert S, Dahlqvist M, Haljamäe H. Hypertonic saline-dextran improves intestinal perfusion and survival in porcine endotoxin shock. Crit Care Med. 2000;28:2843–2850. doi: 10.1097/00003246-200008000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Monge García MI, Guijo Gonzalez P, Gracia Romero M, Gil Cano A, Oscier C, Rhodes A, et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 2015;41:1247–1255. doi: 10.1007/s00134-015-3898-7. [DOI] [PubMed] [Google Scholar]
- 24.Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38:422–428. doi: 10.1007/s00134-011-2457-0. [DOI] [PubMed] [Google Scholar]
- 25.Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 26.Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69:777–784. doi: 10.1111/anae.12661. [DOI] [PubMed] [Google Scholar]
- 27.Marechal X, Favory R, Joulin O, Montaigne D, Hassoun S, Decoster B, et al. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock. 2008;29:572–576. doi: 10.1097/SHK.0b013e318157e926. [DOI] [PubMed] [Google Scholar]
- 28.Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30:623–627. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 29.Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1993–H1999. doi: 10.1152/ajpheart.00218.2005. [DOI] [PubMed] [Google Scholar]
- 30.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108:347. doi: 10.1007/s00395-013-0347-z. [DOI] [PubMed] [Google Scholar]
- 31.Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, et al. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18:538. doi: 10.1186/s13054-014-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellander B-M, Cantais E, Enblad P, Hutchinson P, Nordstrom CH, Robertson C, et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166–2169. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- 34.Bessiere F, Khenifer S, Dubourg J, Durieu I, Lega J-C. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med. 2013;39:1181–1189. doi: 10.1007/s00134-013-2902-3. [DOI] [PubMed] [Google Scholar]
- 35.Maeder M, Fehr T, Rickli H, Ammann P. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129:1349–1366. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 36.Neri M, Cerretani D, Fiaschi AI, Laghi PF, Lazzerini PE, Maffione AB, et al. Correlation between cardiac oxidative stress and myocardial pathology due to acute and chronic norepinephrine administration in rats. J Cell Mol Med. 2007;11:156–170. doi: 10.1111/j.1582-4934.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haileselassie B, Su E, Pozios I, Niño DF, Liu H, Lu DY, et al. Myocardial oxidative stress correlates with left ventricular dysfunction on strain echocardiography in a rodent model of sepsis. Intensive Care Med Exp. 2017;5:21. doi: 10.1186/s40635-017-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakker J, Coffernils M, Leon M, Gris P, Vincent J-L. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest. 1991;99:956–962. doi: 10.1378/chest.99.4.956. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]