Abstract
Remote detection and monitoring of the vegetation responses to stress became relevant for sustainable agriculture. Ongoing developments in optical remote sensing technologies have provided tools to increase our understanding of stress-related physiological processes. Therefore, this study aimed to provide an overview of the main spectral technologies and retrieval approaches for detecting crop stress in agriculture. Firstly, we present integrated views on: i) biotic and abiotic stress factors, the phases of stress, and respective plant responses, and ii) the affected traits, appropriate spectral domains and corresponding methods for measuring traits remotely. Secondly, representative results of a systematic literature analysis are highlighted, identifying the current status and possible future trends in stress detection and monitoring. Distinct plant responses occurring under shortterm, medium-term or severe chronic stress exposure can be captured with remote sensing due to specific light interaction processes, such as absorption and scattering manifested in the reflected radiance, i.e. visible (VIS), near infrared (NIR), shortwave infrared, and emitted radiance, i.e. solar-induced fluorescence and thermal infrared (TIR). From the analysis of 96 research papers, the following trends can be observed: increasing usage of satellite and unmanned aerial vehicle data in parallel with a shift in methods from simpler parametric approaches towards more advanced physically-based and hybrid models. Most study designs were largely driven by sensor availability and practical economic reasons, leading to the common usage of VIS-NIR-TIR sensor combinations. The majority of reviewed studies compared stress proxies calculated from single-source sensor domains rather than using data in a synergistic way. We identified new ways forward as guidance for improved synergistic usage of spectral domains for stress detection: (1) combined acquisition of data from multiple sensors for analysing multiple stress responses simultaneously (holistic view); (2) simultaneous retrieval of plant traits combining multi-domain radiative transfer models and machine learning methods; (3) assimilation of estimated plant traits from distinct spectral domains into integrated crop growth models. As a future outlook, we recommend combining multiple remote sensing data streams into crop model assimilation schemes to build up Digital Twins of agroecosystems, which may provide the most efficient way to detect the diversity of environmental and biotic stresses and thus enable respective management decisions.
Keywords: Precision agriculture multi-modal solar-induced fluorescence satellite hyperspectral multispectral biotic and abiotic stress
1. Introduction
One of the most challenging concerns of today is finding answers to the question “How to feed the world?”, as formulated in the 2nd Sustainable Development Goal (SDG), FAO (2021). The task is huge as the world population is expected to increase to 9.8 billion by 2050 (UN, 2017). Thus, global food production needs to be expanded by 70% in order to feed the future population, maintain nutritional security, account for changing diets and the increasing demand for biological products from the construction and energy sectors (Galieni et al., 2021; Mueller et al., 2012; Tester and Langridge, 2010). In this context, the environmental impact of agriculture must be minimized to protect water, climate, soil, and biodiversity resources (Gomiero et al., 2011) while reducing production risks for farmers. Climate change is adding pressure, as the frequency of extreme weather events as well as major shifts in precipitation and temperature patterns are expected to increase worldwide (Cogato et al., 2019; Gomiero et al., 2011). As a consequence, abiotic (e.g., heat and drought) and/or biotic (e.g., diseases and pests) stresses and their combinations (Govender et al., 2009), will also become more frequent and lead, without swift and effective management responses, to decreases in crop productivity (Atzberger, 2013). Thus, early detection of crop stress prior to irreversible damage is essential to be able to respond with suitable agrotechnical solutions and thus minimize yield loss.
For the detection and quantification of biotic and abiotic stresses in agricultural crops, destructive and non-destructive methods can be used. Some quantitative methods provide sensitive analyses of molecules in biological systems, also known as high-throughput-omic techniques (Fiehn, 2001; Llanes et al., 2018). However, these methods are not only destructive but also time-consuming and costly, limiting their use in continuous monitoring and scalable research (Galieni et al., 2021). As for non-destructive quantitative methods, remote sensing (RS) technologies have been established, such as imaging spectroscopy, fluorescence spectroscopy, thermal and microwave remote sensing, which all provide insights into the effects of stress in plants. In contrast to omic techniques, RS can be applied at larger spatial scales, with high revisit frequency, hence enabling a cost-effective detection of crop stress status and spatiotemporal dynamics across cultivated landscapes. Furthermore, RS is suitable for global coverage and can thus potentially contribute to enhanced food security in developing countries (e.g., Rembold et al., 2000).
Driven by new platforms and sensors with enhanced spatial, temporal and spectral capacities, RS studies focusing on agricultural applications grew exponentially in the last decades (Weiss et al., 2020). Together with improvements in computing power and machine learning (ML), unprecedented possibilities are offered for precision agriculture and other agricultural applications, going along with major improvements in crop stress detection. The most common crop stress type analysed by means of RS techniques is drought, i.e., water deficit stress Damm et al., 2022; Gago et al., 2015; Gerhards et al., 2016; Govender et al., 2009; Ihuoma and Madramootoo, 2017; Parkash and Singh, 2020; Virnodkar et al., 2020). Also, other stress factors have been studied with RS, such as insects (Herrmann et al., 2017) and pathogens (Gold et al., 2020; Herrmann et al., 2018; Mahlein, 2016; Sishodia et al., 2020) nutrient deficiency (Baret et al., 2007; Herrmann et al., 2010; Mahajan et al., 2021) or soil contamination (Gholizadeh and Kopacková, 2019).
Optical remote sensing covers the wavelengths from the visible to the shortwave infrared (VSWIR, 400–2500 nm) and the thermal infrared (TIR, 8–14 μm), collecting radiation reflected and emitted from the observed surfaces. When it comes to the detection of plant responses to diverse stresses, visible (VIS, 400–700 nm), near-infrared (NIR, 700–1300 nm) and shortwave infrared (SWIR, 1300–2500 nm) reflectance, but also TIR and solar-induced fluorescence (SIF, often at 687 nm and 760 nm, or over the full emission wavelength between 650 and 800 nm) have been the most exploited passive sensing signals (Gerhards et al., 2019). In these spectral domains, information on plant morphology and structure can also be derived by means of active sensing devices, such as LiDAR (light detection and ranging; Madec et al., 2017), and through stereophotogrammetry using multi-spectral imagery (St-Onge et al., 2008). Note that this review explicitly excludes microwave technologies. Passive microwave remote sensing operates at spatial scales (typically at 0.25°) being too coarse for studies at the farm or field scale. Active microwave remote sensing (RADAR) has been shown useful in obtaining crop type information (Gella et al., 2021) and surface soil moisture, with potentially high relevance for assessing crop water availability. However, we restrict this review to crop traits for stress detection and exclude soil attributes.
Beyond usage of single RS domains, observations in multiple spectral domains are strongly suggested, potentially providing a deeper understanding of the complex interactions of stressors and affected crop traits (Damm et al., 2018; Jiao et al., 2021). Optical multi-sensor synergies for stress-related research in agriculture have been identified for precision agriculture (Chaerle et al., 2006; Gerhards et al., 2019; Maes and Steppe, 2019), but also for plant breeding projects (Herrmann et al., 2020; Pineda et al., 2020; Singh et al., 2016; Yang et al., 2017a). The synergistic use of data from various electromagnetic domains goes back to the launch of the Thematic Mapper (TM) onboard Landsat-4 in 1982. However, the rather coarse spectral and spatial resolutions of the sensor and its successors prevented a deeper look into plant conditions at subfield scales. By the end of the 1990s, plant scientists promoted nearrange sensing for single leaves and plants using thermography, VIS and NIR, as well as fluorescence imaging, to improve understanding of plant physiological states and related stresses (Chaerle and Van Der Straeten, 2000; Lichtenthaler et al., 1998), but challenges in precise image alignment and data calibration prevented synergistic usage of such multi-domain data. First synergistic use of multi-domain acquisitions has been realized using piloted aircraft (Gerhards et al., 2018; Mohammed et al., 2019; Panigada et al., 2014) while miniaturization in sensor design has led to the development of drone systems allowing for multi-sensor integration at the field level (Aasen et al., 2018). For the first time, the FLuorescence EXplorer-Sentinel 3 (FLEX-S3) tandem mission will provide concurrent observations of VIS, NIR, SWIR and TIR along with SIF at satellite level. (e.g., Drusch et al., 2017).
Recent studies propose efficient early stress detection using multi-scale UAVs and satellite observations. Within such frameworks, the advantages of both platforms can be explored, such as the availability of higher temporal and spatial resolution data (Alvarez-Vanhard et al., 2021; Sagan et al., 2019). However, the integration of multi-scale approaches goes beyond the scope of this review, which is explicitly constrained to synergistic exploitation of multiple spectral domains for stress detection in agriculture.
While the developments in sensor and airborne platform technologies allow for coincident recordings from multiple (spectral) domains (e. g., Timmermans et al., 2015), studies exploring these data by applying integrative methods to assess plant stress are still rare. Before a multi-domain retrieval approach can be defined, all acquired remotely sensed signals must be translated into meaningful values (proxies) related to stress (i.e. affected plant traits). In this respect, a multitude of modeling and retrieval approaches have been investigated (Verrelst et al., 2015, 2019a). The majority of proposed methods relied on parametric regressions, i.e. use of spectral bands, vegetation indices (VIs) or spectral ratios and their relationships with functional traits linked to plant stress (e.g. Gerhards et al., 2016; Govender et al., 2009; Herrmann et al., 2020). To better understand cause–effect relationships, and to simultaneously use the full set of spectral variables, physically-based methods opened attractive pathways. For instance, a promising approach is provided by the soil-canopy observation, photochemistry and energy fluxes (SCOPE) model (Van der Tol et al., 2009; Yang et al., 2021a) and its vertically heterogeneous versions mSCOPE (Yang et al., 2017b) and senSCOPE (Pacheco-Labrador et al., 2021), which combines radiative transfer approaches with photosynthesis and energy balance modeling. In contrast to physically-based approaches, other studies investigated methods of reduced complexity but increased efficiency via implementation of data-driven approaches, in particular ML regression algorithms (Gewali et al., 2019; Singh et al., 2016; Virnodkar et al., 2020). To provide a scientific foundation to the pros and cons of different approaches, Sishodia et al. (2020) recently reviewed stress-related applications of RS in precision agriculture, including water stress and disease detection. The authors stated that advanced approaches using process-based physical models should be pursued to complement ML regression algorithms, as for instance demonstrated by Reichstein et al. (2019) in the context of surface energy balance (SEB) modeling. A pioneering example is provided by Zarco-Tejada et al. (2018) who used multi-sensor acquisitions (airborne hyperspectral and thermal) to analyse trees undergoing early stress caused by infection with the vector-transmitted bacterial plant pathogen Xylella Fastidiosa. The authors estimated fluorescence efficiency by exploring a threedimensional radiative transfer model (RTM) in a multi-step inversion scheme. Also, thermal stress indicators were derived, and canopy structural and leaf biochemical traits were estimated from RTM inversion. In addition, narrow-band spectral indices known to be sensitive to certain functional plant traits (i.e., chlorophylls, carotenes and xanthophylls) were calculated. All traits and indicators were processed within a multivariate analysis based on ML algorithms for the classification of the (pre-visual) disease incidence and severity at the landscape scale. Another example of early stress detection by multiple spectral domains is provided by Hernández-Clemente et al. (2019). The authors reviewed the capability of remotely sensed physiological indicators, such as canopy temperature, chlorophyll fluorescence, photosynthesis and pigments to monitor the early responses of plants to a variety of stressors. They pointed towards the main challenges for the RS community in detecting stress, being: (1) the availability of high spatial, spectral and temporal resolution data, (2) the validation of diverse retrieval methods, (3) upscaling of physiological traits from leaf to canopy-level, and (4) capturing the temporal dynamics and interaction of traits as stress proxies.
Altogether, despite these advances, critical knowledge and methodological gaps for crop stress detection remain. The main gap is that currently no concept exists that uses multiple spectral domains in an integrated way for a complete view of crop conditions to differentiate between crop stress types and to understand the degree of stress severity. This shortcoming can be addressed by leveraging the full potential of spectral information provided by multi-domain RS data sources in a synergistic way. Therefore, we aim to elaborate and propose a new methodological concept for the assessment of multiple plant stresses in agriculture through optical RS synergies. To achieve this, we review, synthesize and integrate current knowledge across spectral domains, remote sensing platforms and retrieval methods for the identification of crop stress.
The paper is organized as follows: in Chapter 2, we present a general introduction on plant stress, physiological strain as well as adaptations to stress at different temporal stages. This is followed by RS of stress using distinct proxies and sensor systems in Chapter 3. Chapter 4 concentrates on the systematic literature review about multi-domain and multi-sensor studies, and Chapter 5 elaborates the proposed multisensor synergy concept.
2. Stress definitions and physiology
2.1. Stress definitions
Plants are sessile organisms exposed to biotic and abiotic pressures, i. e. the physical, chemical and biological environment characterizing their habitat (Jones and Jones, 1989). Abiotic stress includes, among others, radiation, salinity, flooding (waterlogging), water stress, temperature extremes, nutrient shortage and heavy metals (Gull et al., 2019; Larcher, 2003). Second, plants are influenced by their biotic surroundings, referring to other co-occurring organisms. Specifically, biotic stress involves inter- and intra-specific competitions as well as diseases caused by fungal and bacterial pathogens (Lichtenthaler, 1996), weeds, insect pests and/or damage to plants by nematodes, protists, viruses, and vi-roids (Madani et al., 2019).
Both the biotic and abiotic interactions can impact the survival or success of plant species. Thus, understanding the impacts of different stress factors on plants have been the focus of plant physiologists (Larcher, 2003), particularly important in crop sciences (Blum, 2016). In general terms, plant stress can be defined according to Lichtenthaler (1996) as: ‘any unfavourable condition or substance that affects or blocks a plant’s metabolism, growth or development’.
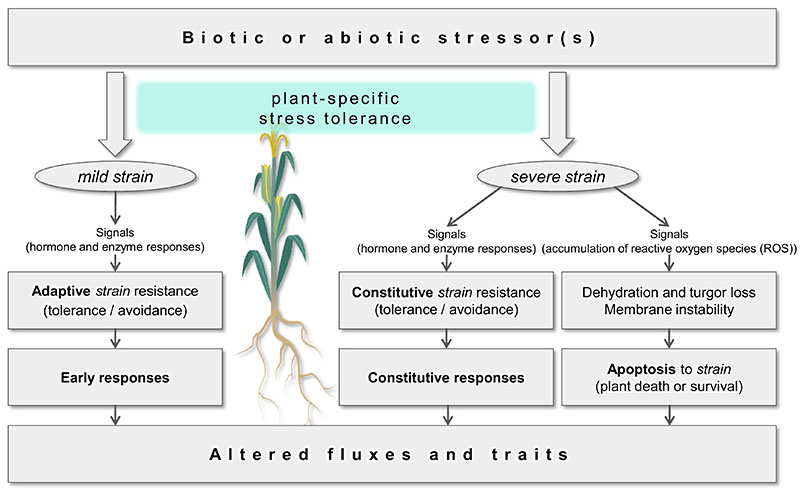
A simplified description of the effect of various stress factors on a plant is outlined in Fig. 1 (Blum, 2016; Kranner et al., 2010). Accordingly, plants respond to a changing environment by a coordinated shortterm acclimation. The response is initiated by biochemical triggers like phytohormones or enzymes and leads to a damage and repair cycle, activating the plant metabolism usually with small or non-visible phenotypic adaptation (reversible and adaptive strain tolerance). With increased severity and duration of the stress exposure, plants change their phenotypic expressions through long-term adaptations. This affects, for instance, leaf size and thickness, stomatal density, or function of chloroplasts depending on high or low light conditions. Depending on the stress factor, the adaptation may take days or weeks, while persistent and severe (long-term) stress may arise in the final stage and even lead to apoptosis (Lichtenthaler, 1996). The ability of plants to vary their phenotypic expression in response to environmental conditions is also described as “phenotypic plasticity” (Sultan, 2000), which can result in altered phenotypic traits and fluxes.
Fig. 1.
Scheme of plant stress, strain, and signaling, leading to plant responses and resistance. Adapted from (Blum, 2016).
It has to be noted that the impact of any stress factor usually results in a complex interplay between the plant’s genes and its environment often leading to multiple strains, not explicitly shown in Fig. 1. For example, high light stress affects fluorescence parameters, impacts photosynthesis, increases non-photochemical quenching (NPQ), and enhances leaf temperature leading to increased transpiration and changes in leaf angle. Likewise, it must be remarked that the effect of a single and/or multiple stress factors, as well as the combination of biotic and/or abiotic stresses, can often lead to very similar physiological responses in the plants (Blum, 2016; Kranner et al., 2010), see Fig. 1.
It is also important to consider the timing of stress impacts. For example, short-term environmental changes may be reflected in changing flux rates of photosynthesis, respiration, and transpiration (Damm et al., 2018). Stress occurring over long-term periods during different development stages may affect growth variously. For example, drought stress in wheat may affect leaf expansion in the initial phase, the number of tillers in the tillering phase, plant height in the stem elongation phase, and grain development during the flowering stage, with the period between stem elongation and flowering being the most sensitive to drought (Sarto et al., 2017).
2.2. Stages of plant stress responses
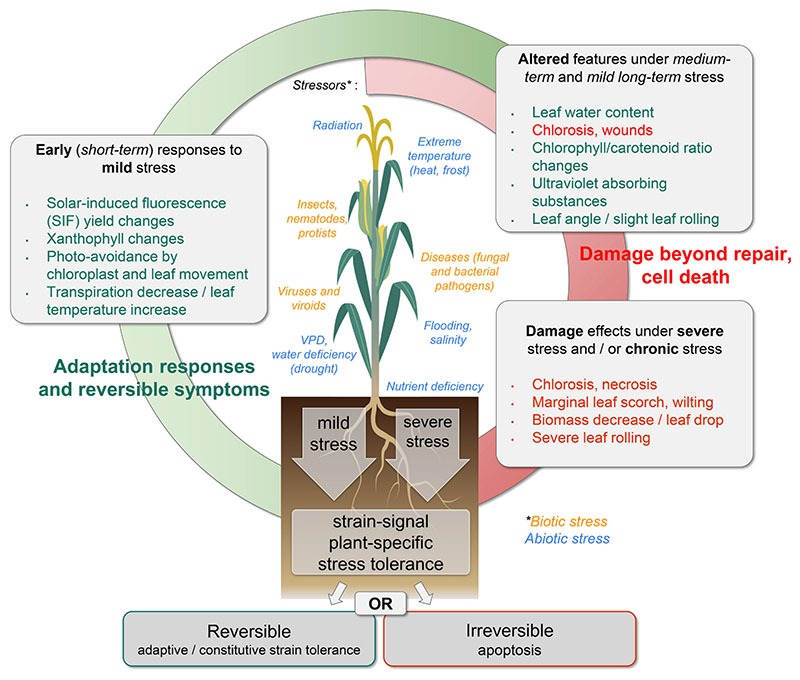
Reactions to biotic and abiotic stresses usually are continuous but often also non-linear processes (Kranner et al., 2010). In general, three phases of the plant stress response may be identified with respect to the severity and duration of one or multiple stressors. We exemplary demonstrate the phases (or stages) of drought stress and subsequent plant response in Fig. 2. very mild drought stress usually has little impact on the plant, i.e., initial stomatal closure may occur, but the photosynthetic capacity remains high and no phenotypic changes are observed. Under increasing stress levels, however, stomatal closure reduces the internal CO2 availability, which often triggers the production of reactive oxygen species (ROS) and lowers the photosynthetic rates. Moreover, leaf temperature increases and changes in leaf angle or leaf rolling are observed. As a result, plant growth may be inhibited. A stronger decrease in water availability will result in a reduced stomatal conductance affecting the leaf turgor (Abdullah et al., 2019). Over-production and accumulation of ROS and membrane instability will lead to cellular damage beyond repair and thus to necrotic spots. Under severe drought stress, growth usually stops, and continuous damage may eventually lead to apoptosis (see Fig. 1, Fig. 2).
Fig. 2.
Biotic and abiotic stress factors and the plants’ responses to stress as a function of dose and exposure time: early (mild), medium-term or mild long-term, and severe/chronic exposure.
These stress phases may vary for diverse or combined stressors. This is particularly important for agriculture, where often even mild stress may reduce crop growth, and finally the yield, particularly when it occurs at crucial growth stages (Fahad et al., 2017). Consequently, it is essential to detect and quantify crop stress at the earliest possible stage.
Only responses affecting the biophysical and functional properties and thus, traits of the plants may be detected by RS. Stress factors such as water scarcity, water logging, salinity, heat and excessive light often induce changes in the rates of photosynthesis, respiration, transpiration and stomatal conductance in the early stress stage. This is usually followed by changes in amounts and ratios of the photosynthetic pigments (chlorophylls and carotenoids), and the concentration of different metabolites at later stages (Lichtenthaler, 1996). While the remote detection of altered phenotypic traits, such as pigments and water content, is prevalent when monitoring medium-term, mild long-term and severe or chronic stress, altered photosynthesis and transpiration fluxes and their possible feedback mechanisms already show subtle responses under the first occurring stress (e.g., Gerhards et al., 2016; Jonard et al., 2020). In fact, daily occurring cycles of stress are common for crops as diurnal cycles in radiation and temperature show strong variation, often reaching excessive energy inputs at solar noon. In general, electromagnetic energy absorbed by plants is used to fuel photosynthesis, but under excessive irradiation the photosynthetic apparatus needs to immediately adapt energy pathways to avoid photo-damage. Increasing stress that inhibits the photosynthetic light reactions induces an increasing dissipation of energy by NPQ and reduces the fluorescence yield. The latter is detectable over vast vegetated areas as solar-induced fluorescence (SIF), a subtle energy flux emitted by vegetation that may be used to detect the onset and progress of stress (Ač et al., 2015; Demmig-Adams et al., 2020; Gerhards et al., 2019). Dynamic adjustments under early stress are also observed for transpiration, an energy-demanding process that decreases leaf surface temperature. Therefore, sensing the canopy surface temperature became a useful tool for assessing early changes in plant-water and plant-health status (Idso et al., 1981; Jackson et al., 1988; Maes and Steppe, 2012).
2.3. Common symptoms of crop stress
In the following, we describe the causalities and illustrate several examples of stress occurrence in crops (Fig. 3).
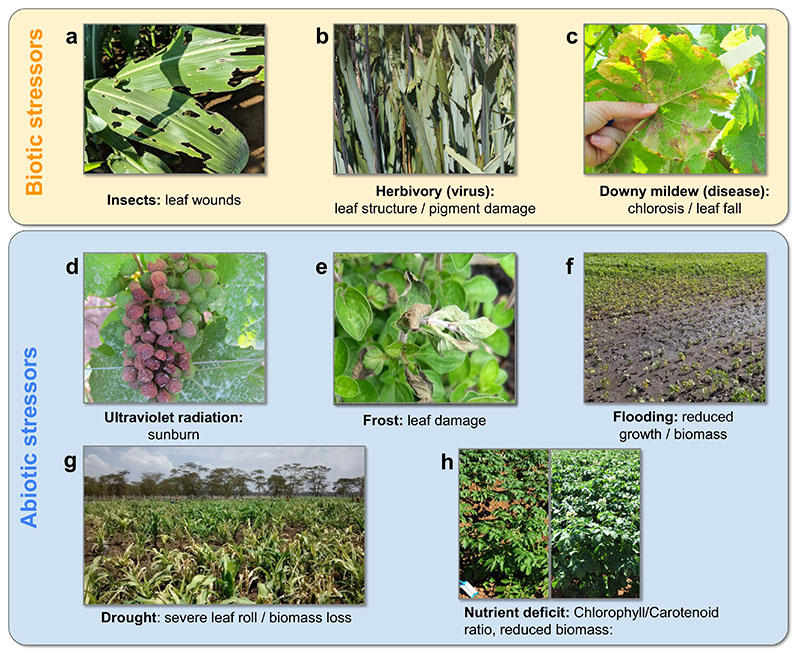
Fig. 3.
Examples of distinct biotic (upper box) and abiotic (lower box) stressors (bold) and corresponding affected traits/symptoms on crops (mainly). Photographs were provided by the authors.
Biotic stress responses result from complex interactions between plants and other biological organisms and can be very diverse. Often plant responses are induced directly by the biotic agents. For instance, in the (common) case of herbivory (see Fig. 3(a)), the immediate effect is loss of water content due to wounding and loss of functional leaf area combined with the necrosis of the wounded leaf parts (Meza-Canales et al., 2017). In this way, biotic pathogens often affect a plant locally and then induce a systemic plant response. This may include the activation of signal pathways to reduce stomatal conductance (Aldea et al., 2005) or increased production of volatile organic compounds (VOCs) as defence mechanisms (Holopainen and Gershenzon, 2010; Kessler, 2001).
Viruses can also lead to biotic stress as shown in Fig. 3(b) for an example of Maize Lethal Necrosis (MLN). MLN causes mostly pigment damage but also some structural wilting in advanced stages due to necrosis and viral replication related to phosphorous deficiencies.
A broad diversity in fungal pathogens exists. Some pathogens, such as yellow rust, can stimulate photosynthesis during early infection stages as the fungus benefits from the increase in sugar production (Chang et al., 2013). However, at a later stress stage it may cause decreases in photosynthetic capacity, leading to visible foliar lesions (Vergara-Diaz et al., 2015). The diversity of fungal pathogens may cause plant diseases, such as anthracnose, leaf spot, rust, or wilt (for an overview, see Jain et al. (2019)). Many of these diseases are manifested as changes in optical reflectance properties of the canopy, which may be specific to the pathogen. Such changes in optical properties can either be detected as radiometric changes or can be identified at leaf level through computer vision approaches for classification of plant diseases (Singh et al., 2020). Similar symptoms can be caused by microbes, as shown in the example of downy mildew in Fig. 3(c).
Radiation stress or excessive exposure to ultraviolet (UV) sunlight also disturbs plant growth when the absorbed excess energy cannot dissipate. It results in an overproduction of ROS that may be detoxified or eventually disturb the overall cellular homeostasis of the plant (Singh et al., 2020). In Fig. 3(d), the effects of radiation stress are shown for an exemplary vineyard.
As illustrated in Fig. 3(e), frost or cold stress is another serious threat to crops (Yadav, 2010). Moderate cold stress reduces plant growth and development, but severe cold stress may lead to stronger symptoms. When cold stress coincides with high light intensities, it leads to yellowing of leaves (i.e., chlorosis), reduced leaf expansion and wilting, and finally necrosis, i.e. apoptosis.
Waterlogging is among the major abiotic stressors for crops. Fig. 3(f) shows a previously flooded field. Waterlogging induces a number of alterations in soil properties, such as soil pH, redox potential and oxygen level. Thus, plants growing on waterlogged soil face a stressful environment in terms of hypoxia (deficiency of O2) or anoxia (absence of O2), which may substantially reduce plant growth, development and survival (Tewari and Mishra, 2018). One very frequent source of abiotic stress is drought, as demonstrated in Fig. 3(g) for a very severe drought stress event.
Plants require a number of essential nutrients for growth, which are supplied either from soil minerals and soil organic matter, or as organic and inorganic fertilizers as part of agricultural management. The deficiency of one or multiple nutrients may lead to manifestations, as demonstrated in Fig. 3(h).
These few selected examples illustrate that: (i) diverse stressors may lead to the same symptoms, and (ii) the same stressor may cause distinct symptoms under varying environmental conditions. This also depends on the scale of observation and the actual development stage of the crops. To better relate stressors and the corresponding symptoms, a deeper understanding about species-specific plant physiological responses is required. Ultimately, all abiotic and biotic stress factors have in common that they pose a large risk for the agricultural sector: major yield losses and thus decrease in agricultural productivity.
3. Remote sensing of stress: Time domains and methodologies
From a sensing point of view and in accordance with the dose and duration of stress exposure, we group the detection of plant stress responses into three main categories. Table 1 lists the stress phases (short-term, medium-term and longer-term) along with the (i) physiological responses of the plants, (ii) the primarily affected or adapted traits or stress proxies, and (iii) typical or optimal sensing domains. In accordance with that and to associate the stress proxies to these three temporal phases of stress, we grouped the affected traits into fluxes, biochemical and structural, as delineated in Fig. 4. In addition, the Figure illustrates the interplay of “optimal” spectral domains to estimate these stress proxies for an example of decreasing crop water availability. The resulting health status and potential yield losses are delineated below as a chronological function of stress duration and severity. Before a multi-sensor concept can be defined, the potential of each of these spectral domains has to be understood. Hence, in the following subsections, we will discuss the capabilities of various RS technologies to track plant stress responses as a function of dose and exposure times, providing an overview of commonly used methodologies.
Table 1.
Short-medium- and long-term effects of plant stress, with physiological responses, primarily affected traits (examples) that act as stress proxies and optimal spectral domains to detect the traits. Exemplary references exploring the different stress proxies with defined sensing domains are also given. Spectral responses to stress exposure are further related to plant genotypes, not being considered here (cf., Galieni et al. (2021) for an overview of such responses).
| Stress phase | Physiological response | Primarily affected traits | Optimal spectral domains | References |
|---|---|---|---|---|
| Early responses (minutes to hours) | Downregulation of photosynthetic electron transport, | SIFyield | Normalized fluorescence, VIS/NIR | Magney et al. (2019) |
| ⋮ | Activation of NPQ | Xanthophylls | 500–600 nm (quick) | Acebron et al. (2021) Van Wittenberghe et al. (2021) |
| ⋮ | (and chlorophyll) | 500–750 nm (slow) | Van Wittenberghe et al. (2019) | |
| ⋮ | Photo-avoidance through | Absorption by | VIS | Kasahara et al. (2002), |
| ⋮ | chloroplast movement | chloroplast pigments | Brugnoli and Bjorkman (1992) | |
| ⋮ | Reduced stomatal conductance, transpiration, stomatal closures | Leaf/canopy temperature | TIR | Gerhards et al. (2016) |
| Medium-term responses: | Leaf turgor loss | Leaf water content | NIR/SWIR | Seelig et al. (2008) |
| (hours to days) | Changes in pigment contents | Leaf chlorophyll and | VIS/NIR | Baret et al. (2007) |
| ⋮ | (chlorosis) | carotenoid contents | Gitelson (2020) | |
| Longer-term responses | Leaf movement | Leaf angle, fraction of | VIS/NIR/SWIR | Spišić et al. (2022) |
| (days to weeks): | and rolling | photosynthetically active radiation | ⋮ | Baret et al. (2018) |
| ⋮ | Reduced leaf expansions | Leaf area index, biomass | ⋮ | Berni et al. (2009b) |
| ⋮ | ⋮ | ⋮ | ⋮ | Hazaymeh and Hassan (2017) |
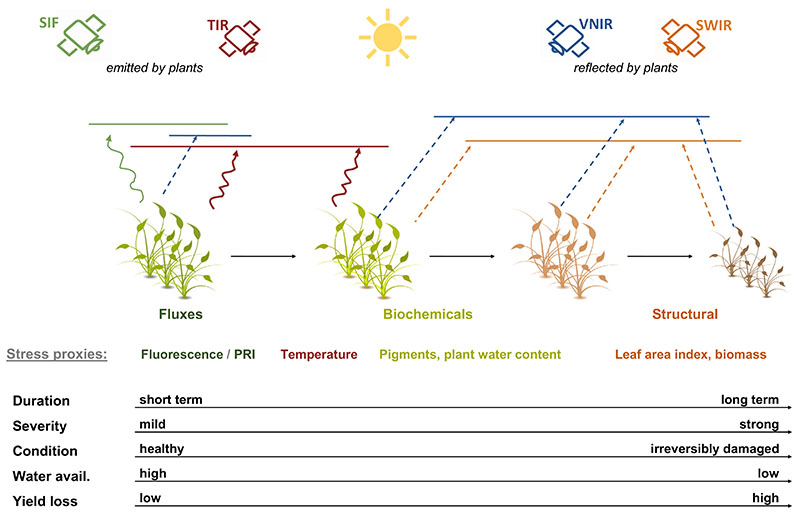
Fig. 4.
Integrated concept of plant responses to (drought) stress with optimal sensing domains (i.e. solar-induced fluorescence (SIF), thermal infrared (TIR) visible to near infrared (VNIR) and shortwave infrared (SWIR)) for estimating trait groups (i.e., fluxes, biochemicals and structural) and exemplary stress proxies (PRI: photochemical reflectance index). Changing plant health conditions and yield loss probability are delineated as a chronological function of stress duration and severity through decreasing water availability.
3.1. Remote sensing methods for early and mild stress detection
3.1.1. Solar-induced chlorophyll fluorescence
During the diurnal course of the day, vegetation is perceiving a large gradient of incoming solar energy, fluctuating between low light conditions up to excessive amounts of incident radiation. The energy in the photosynthetically active radiation (PAR, 380–710 nm) region triggering the photosynthetic light reactions is, therefore, easily reaching harmful doses of energy requiring dissipation. SIF corresponds to the energy directly emitted from the photosynthetic machinery when the photosynthetic pigments get excited by the incoming radiance. The harvested light triggering this SIF emission depends on the amount of photosynthetic pigments and their composition within the photosystem II antenna complexes. Passive fluorescence techniques quantify the absolute values of SIF emission. Hereby, retrieval methods disentangle surface reflectance from the fluorescence signal using data from very high spectral resolution spectroradiometers. By means of these techniques, fluorescence emission can be retrieved as scalar values in the oxygen absorption regions or within the Fraunhofer lines, where the reflected radiance is strongly retained due to absorption of the incoming sunlight (Meroni et al., 2009). By exploiting distinct absorption features in the red and NIR spectral window, it is possible to reconstruct the entire spectrum of chlorophyll fluorescence. There are two peaks around 685 nm and 740 nm, which are mechanistically related to the photosynthetic electron transport within the two photosystems of plant’s photosynthetic apparatus (Mohammed et al., 2019). Recent approaches model the entire fluorescence spectrum (Cogliati et al., 2019), which will open the path towards a more complete signal analysis.
The intensity of the SIF signal is in its first order related to the total PAR, which is absorbed by the canopy. Secondly, structural canopy properties determine the escape probability of the emitted SIF signal (Guanter et al., 2014). Recent studies developed methods to correct the SIF emission for scattering (Yang and van der Tol, 2018; Zeng et al., 2019) and re-absorption effects (Liu et al., 2020) by determining the escape probability and thus made it possible to down-scale SIF from the canopy to the leaf level. To further disentangle illumination related variations from the underlying physiological information carried by the down-scaled SIF signal, the calculation of an (apparent) yield can be employed by further normalizing for the amount of chlorophyll (Goulas et al., 2017) or radiation absorbed by chlorophyll (Yang et al., 2020a). This step allows the comparison between species, independent of their pigment pool sizes and facilitates the quantitative detection of variations in plant physiology (Migliavacca et al., 2017; Van der Tol et al., 2016).
Mechanistically, SIFyield, i.e. the SIF emission normalized by the absorbed energy triggering the emission, is closely related to the efficiency of the photosynthetic electron transport. Thus it can be used as an early stress indicator when photosynthetic electron transport is affected by the specific stress reaction. However, as a matter of fact, SIFyield is not linearly related to photosynthetic electron transport, as the dynamic nature of NPQ mechanisms renders these relationships non-linear. Plants have evolved a variety of protection mechanisms, which can be actively regulated to optimize light harvesting especially in stressful times to avoid over-energization of the photosynthetic apparatus. These NPQ mechanisms act as energy quenchers and, as a consequence, may also affect the intensity of SIF (Bilger and Bjorkman, 1990). Thus, for stress detection, SIFyield needs to be further constrained by an independent measure of NPQ. Some NPQ processes are linked to a reversible conversion of the xanthophyll pigment pool (Jahns and Holzwarth, 2012), which may result in consequential optical reflectance change in the 500–600 nm range (Acebron et al., 2021; Gamon and Surfus, 1999; Van Wittenberghe et al., 2021), or even further changes in the VIS–NIR range (Van Wittenberghe et al., 2019). To circumvent the above described complexities, the Photochemical Reflectance Index (PRI) has been frequently used to detect these instantaneous optical changes and to exploit the modification in the light use efficiency behaviour under excessive radiation (Garbulsky et al., 2011). However, the PRI is also known to be affected by both facultative and constitutive pigment effects (Gamon and Berry, 2012; Moncholi-Estornell et al., 2022). These multiple underlying pigments effects illustrate the complexity of the pigment dynamics affecting the PAR on both short and longer time scales.
3.1.2. Thermal infrared sensing: Temperature
There has been a long interest in using canopy temperature as a remote indicator of plant water stress (Jones and Vaughan, 2010). Although RS-based surface energy balance (SEB) approaches allow an estimation of the actual evapotranspiration (indirectly as the residual term of the SEB equation, using thermal data to calculate heat flux), they require vast meteorological input data and parameterisation of conductance (Tolomio and Casa, 2020). Other than SEB models, the direct use of thermal data in water stress indices has been promoted. TIR radiance measurements obtained over vegetation depend mainly on the temperatures of the (sunlit or shaded) leaves and the soil, the leaf and soil spectral emissivities, and eventual contributions from the surroundings and the atmosphere (Gerhards et al., 2019; Norman and Becker, 1995). Temperatures and emissivities of vegetated surfaces can be retrieved from a variety of algorithms that use - depending on the number of spectral bands - different approaches to correct for atmospheric effects and to separate temperature and emissivities from each other (Li et al., 2013).
At a given ambient air temperature, canopy temperature (Tc) is inversely related to the rate of water loss from the canopy. The transpiration itself is closely linked to stomatal conductance (Jones and Vaughan, 2010). To take into account the effects of weather conditions on Tc, the crop water stress index (CWSI) has been developed as a simplified means to quantify water stress. Besides the effects of air temperature (Ta) the index also considers atmospheric relative humidity, expressed as vapour pressure deficit (VPD). CWSI is based on a simple scaling operation, where Tc is normalized by the Ts of a fully transpiring crop (Tsmax) and the Ts of a crop that is not transpiring at all (Tsmin). Different approaches to calculate CWSI were developed (see Maes and Steppe, 2012 for a comprehensive review): The empirical CWSI (CWSIe) establishes a relationship between Tc–Ta and VPD from which Tsmax is derived (Idso et al., 1981). Hence, it requires only three input measurements (Tc, Ta, and VPD) for its application. However, the lower limit is sensitive to changes in radiation and wind speed (Gonzalez-Dugo et al., 2014) and therefore cannot be transferred across growing seasons. The theoretical CWSI (CWSIt) calculates the upper and lower limits (Tsmax and Tsmin) from equations based on a combination of the Penman-Monteith and the energy balance equations. The CWSI can be used in day-to-day water stress monitoring, but requires measurement of additional atmospheric variables (i.e., net radiation, wind speed, canopy resistance) and the challenging estimation of aerodynamic resistance (rA) (Jackson et al., 1981, 1988). To overcome this limitation, the use of an empirical upper limit together with the theoretical lower limit has been suggested for practical purposes (Agam et al., 2013; Rud et al., 2014). This so-called hybrid CWSh (Ekinzog et al., 2022) eliminates the need for estimating rA and achieved comparable accuracies as CWSIt and CWSIe. The development and early application of CWSI was done on point based Tc measurements, but has been adapted and applied to high resolution thermal imagery for assessing the spatial variability of crop water status using the direct or image based CWSI (Agam et al., 2013). Hereby, the fully and non-transpiring Ts along with Tc are measured directly using wet and dry artificial reference surfaces placed within the image (Gerhards et al., 2018). CWSI showed to exhibit good relationships with in situ soil based measures such as soil water content (DeJonge et al., 2015; Taghvaeian et al., 2014) and plant based measures, such as leaf water potential and stomatal conductance (Berni et al., 2009a; Bian et al., 2019; Han et al., 2018). The index proved to be robust in many arid and semi-arid regions around the globe and for various agricultural crops (Cohen et al., 2017; DeJonge et al., 2015), and more recently also in humid regions (Ekinzog et al., 2022; Hoffmann et al., 2016).
Moreover, CWSI requires a fully closed canopy and this limitation has been tried to overcome using the water deficit index (WDI, Moran et al., 1994), relating Tc–Ta with the fractional cover or a vegetation index (VI). The empirical version of WDI is derived from a Ts – VI scatterplot, without requiring any ground-based weather measurement and is thus widely applied in thermal RS (Maes and Steppe, 2012).
3.1.3. Thermal infrared sensing: Emissivity
Analysing the thermal infrared spectral region, relatively little attention has been paid so far to the spectral emissivity of plants (leaf and canopy), which responds to environmental stresses. This could be explained with the following reasons (Ribeiro da Luz and Crowley, 2007): (i) hyperspectral TIR instruments were lacking until a few years ago, (ii) gap in knowledge about the origin of complex spectral emissivity variations, (iii) low signal-to-noise-ratio (SNR) together with low spectral and spatial resolution of the available sensors prevented to detect these subtle changes in spectral emissivity, (iv) lack of advanced pre-processing algorithms (i.e., atmospheric correction and Temperature-Emissivity-Separation). However, Salisbury (1986) and the ground-breaking studies by Ribeiro da Luz and Crowley (2010), Ribeiro da Luz and Crowley (2007) and Neinavaz et al. (2016) showed that differences in the spectral emissivity among plant species are related to differences in structural and biochemical leaf surface and canopy properties. These findings, in combination with recent advances in sensor technologies, e.g., spatially enhanced broadband array spectrograph system (SEBASS, Vaughan et al., 2003), Telops Hyper-Cam LW (Schlerf et al., 2012), or hyperspectral thermal emission spectrometer (HyTES, Meerdink et al., 2019), opened the field to study the application of spectral emissivity for plant stress detection. So far, only two studies pursued the question if spectral emissivity can be used for the detection of plant responses to environmental stresses. Based on laboratory measurements, Buitrago et al. (2016) were able to use spectral emissivity for the detection of cold and water stress on European beech (Fagus syl-vatica) and rhododendron (Rhododendron cf. catawbiense) leaves. Gerhards et al. (2016) successfully showed in a greenhouse experiment that the spectral emissivity of potato plants (Solanum tuberosum L. Cilena) is equally sensitive to water stress as compared to temperature based indices (e.g., CWSI). However, further research is needed to better understand the linkages between stress, leaf traits and spectral emissivity features. Overall, using emissivity as an indicator of thermal stress is challenging as it requires TIR measurements at high spectral resolution, correction of atmospheric effects and the separation of temperature and emissivity (Gerhards et al., 2019).
3.2. Quantification of mild long-term and severe stress using optical reflective approaches
Mild long-term, chronic or severe stress induces visible symptoms on crop leaves and canopies. Those symptoms are also captured by remote sensing data in the reflective optical domain, including the VIS, NIR and SWIR. The first remote sensing studies for linking remote sensing in the optical domain to stressed vegetation canopies started in the early 1970’s (see Fig.1 in Houborg et al., 2015) with the milestone publication by Knipling (1970). This study related leaf and canopy spectral signatures to effects of physiological stress. With this, Knipling (1970) identified the two main groups of traits, which are affected first by medium-term and mild long-term stress - the biochemical compounds - and second, those affected by chronic and severe stress, i.e. the structural variables. Here we refer to Fig. 2 (the two blocks on the right), and Table 1 (second and third lines). The use of these traits as stress proxies has since been confirmed by numerous studies. According to Baret et al. (2007), for instance, retrieval of leaf chlorophyll content (Cab) and leaf area index (LAI) can serve to diagnose plant (nitrogen) stress: whereas Cab decreases due to limited synthesis or destruction of chloroplasts, losses in leaf area (i.e., LAI) indicates a decrease of leaf production going along with an increase in senescence. The study of Carter and Knapp (2001), linking a variety of stressors to physiological responses, observed that consistent stress induces changes in leaf reflectance within the visible range due to changing Cab. In the study of Linke et al. (2008), relative leaf water content decreased during water stress periods going along with changes in spectral leaf reflectance. It was also found that recovery from stress was not reflected by leaf reflectance as expected: still some differences remained between the formerly stressed plant and the control plant, suggesting that secondary effects followed the stress, such as changes in cell structure and biochemistry. This points towards the need to retrieve multiple plant traits instead of a single one to accurately diagnose medium or longer lasting stress events in crops. To derive traits from optical reflective measurements, it is necessary to convert the measured signals into semantic values. Four broad methodological categories have been identified recently, and were summarized in the review studies of Verrelst et al. (2015, 2019a) (for general vegetation properties retrievals) and specifically for nitrogen in Berger et al. (2020):
parametric regressions, referring to the use of VIs;
nonparametric regressions, which include chemometric methods and ML regression algorithms,
mechanistic or physically-based, using radiative transfer modeling (RTM), and
hybrid approaches, combining ML regression algorithms with RTM.
The first studies evaluating the use of optical reflective RS for nitrogen stress quantification were mainly based on empirical relationships employing spectral indices sensitive to chlorophyll content (Penuelas et al., 1994). Later, the study of Lebourgeois et al. (2012) used different VIs to investigate crop nitrogen status in cases of combined nitrogen and water stresses. Further, hyperspectral data opened the opportunity for the development of narrowband VIs (Broge and Leblanc, 2001), which were used for estimation of plant water status and stress (Gerhards et al., 2019; Zhang and Zhou, 2019).
According to Singh et al. (2016), ML regression algorithms emerged as one of the most promising tools for the retrieval of vegetation traits in the context of stress. ML allows analysing huge data sets to detect patterns by simultaneously looking at multiple factors instead of analysing each predictor and/or feature (trait) individually.
As an example of RTM exploitation, the study by Richter et al. (2008) used a look-up table (LUT) inversion of the PROSAIL model to estimate LAI, Cab and a soil brightness factor for the diagnosis of drought risk zones within wheat fields. Hereby, crop growth variability was caused by the sandy soil type, leading to limited water availability for the crops, which was reflected in lower LAI and Cab values compared to other soil types.
Merging physically-based and data-driven methods to hybrid approaches seems ideal for functional traits retrieval and thus stress identification due to the complementary nature: RTMs provide physical constraints and domain knowledge to fast and efficient ML algorithms (Verrelst et al., 2015, 2021). The efficiency of these hybrid methods can be even enhanced by exploring active learning for optimisation of ML training samples (Berger et al., 2021). These methods can be powerful for the detection of stress levels, for instance, by investigating the ratio of Cab and leaf carotenoid contents (Hendry and Price, 1993; Sonobe et al., 2020).
Recently, Lassalle (2021) reviewed the advances in optical reflective hyperspectral remote sensing of plant stress over the last five decades. The author pointed out that for most stressors, high to very-high spatial resolution is preferable. Optimally, the VNIR spectral region should be exploited using VIs and ML methods. In some cases, the SWIR region improved the performance of the methods in the analysed studies. Nevertheless, we believe that exploiting multiple domains provided by diverse sensors (SIF, TIR, VIS-SWIR) may be an advantage over using only hyperspectral optical reflectance. Such a “multi-view” is required to obtain more complete insights about stressors, crop physiological responses and affected traits. We therefore examined the existing literature to identify the current state of the art with respect to multi-domain and multi-sensor approaches.
4. Review of multi-domain and multi-sensor studies
4.1. Identification of relevant literature
The main purpose of the systematic review was to identify all published studies detecting agricultural stress by exploring synergies of different optical domains. To collect all relevant studies, we followed the approach by Cronin et al. (2008). The literature analysis included four main steps:
identification of relevant literature by means of well-defined keywords,
screening of the overall suitability of the selected records,
evaluation of the eligibility and inclusion, i.e. fully scanning the records, and
extraction of meta-information.
In the identification step (1.), the keywords “remote sensing & (fusion OR synergy OR multi OR multi-sensor) & stress & (agriculture OR crops)” were searched for in ISI Web of Science and similar search engines within the period from 1994 to 2021. Finally, a large pool of records was collected. The identification step followed a first rough checking to remove duplicates. This resulted in approximately 300 papers eligible for further investigation. In the screening step (2.), at first, non-peer reviewed records being considered irrelevant (i.e., conference proceedings or reports), were removed. Reading of titles and abstracts led further to the exclusion of all records that either focused on other than agricultural applications, employed active RS data or were review studies. The screening step resulted in a total of 133 studies. In the eligibility part (3.), these 133 studies underwent intense screening, i.e. full reading, required to extract the criteria listed below. Hereby, records that mentioned stress but focused on yield or soil properties, or used only one sensor (or domain) for stress evaluation were excluded. From the remaining 96 records, detailed information was extracted, including the following variables:
Meta-information: authors, title, journal, year;
Site: location, studied vegetation (crop, orchard);
Stress type (biotic, abiotic) and specification;
Analysed traits (individual and according to the three trait groups, see chapter 2);
Time scale of stress period (short-, medium- and long-term);
Observational levels (leaf-level, top-of-canopy or top-of-atmosphere);
Observational scales (micro/plant, local, regional, global);
Platforms (handheld, gantry, tower, unmanned aerial vehicle (UAV), airborne, satellite);
Spectral domains (VIS, SIF, NIR, SWIR, TIR);
Algorithms (classification, regression, anomaly detection);
Methodologies (parametric, nonparametric, RTMs, hybrid, SEBM).
Note that with the performed literature search we do not claim completeness. There is a high certainty of additional potentially relevant records, which may be missed due to deviating keywords. However, due to the high number of co-workers and repetitive literature searches, we believe to have covered the essential studies exploring multiple spectral sensing domains to assess stress in agricultural research.
4.2. Factor analysis
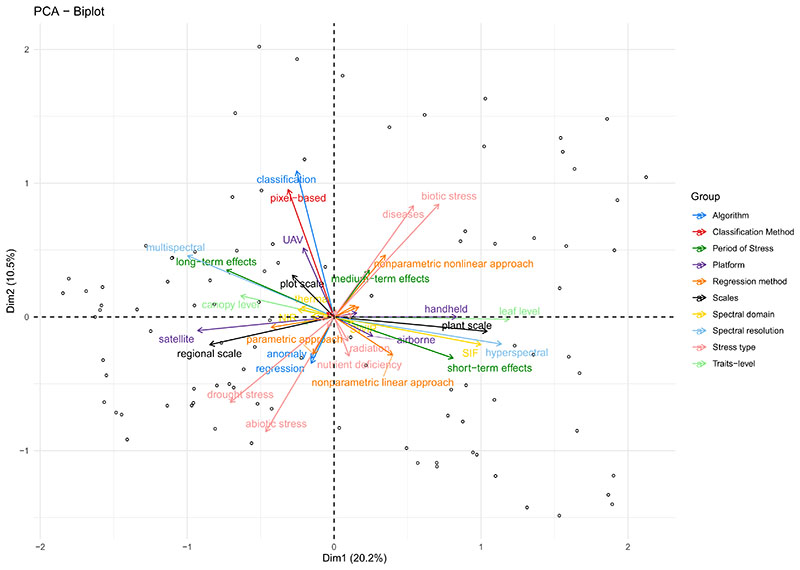
In a first step, we analysed the contribution of the different variables via principal component analysis (PCA), see Fig. 5. A PCA reveals the strongest patterns between all variables and thus provides an attractive method to explore general trends within the database. In the first dimension (x-axis), the largest discrepancies can be found between platforms (satellite vs handheld), spectral resolutions (multispectral vs hyperspectral), as well as the duration of the stress periods (long-term vs short-term stress). These patterns can be interpreted with the investigation of either one or the other aspect, but rarely both together. The second dimension (y-axis) indicates, among others, the exclusive use of one stress type (biotic or abiotic). In addition, parametric and nonparametric nonlinear approaches point towards opposite directions.
Fig. 5.
Principal component analysis of literature variables (first two dimensions). Positively correlated variables point to the same direction and negatively correlated variables to the opposite. The groups refer to the variable categories searched for in the reviewed studies (algorithm, classification method, period of stress, platform, regression method, scales, spectral domain, spectral resolution, stress type, traits level).
On the other hand, variables pointing in the same direction may indicate a joint usage, for instance, the estimation of biotic stresses (e.g., diseases) by means of nonparametric nonlinear approaches (i.e., machine learning), or the estimation of abiotic (i.e., drought stress) by parametric methods (e.g., vegetation indices). Since this PCA analysis can only give an approximate idea of the complex interplay of all these variables, the following sub-chapters describe and illustrate the findings for the individual criteria.
4.3. Platforms and sensors
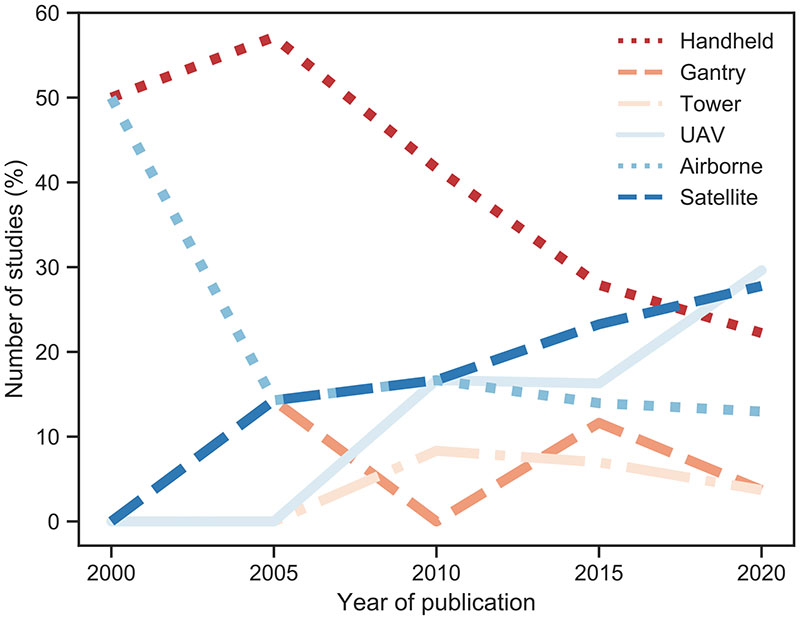
Aggregation of the publications over 5-year periods reveals clear trends in platform usage over time (Fig. 6). While only handheld and airborne platforms prevailed during the period 2000–2005, their usage continuously decreased from 2005 onwards. At the same time, researchers made increasing use of sensors onboard UAVs (e.g., Di Gennaro et al., 2017; Joalland et al., 2018) and satellites (e.g., Anderson et al., 2018; Bayat et al., 2018). With respect to UAVs, this can be largely explained by the miniaturization of sensor technologies for (hyper-spectral) VNIR, SWIR and TIR, and recently also for SIF cameras on drones (Gonzalez Toro and Tsourdos, 2021). Regarding satellites, multispectral satellite images, such as Sentinel-2 A/B (from June 2015) and Landsat (from October 2008), are easily accessible nowadays. The analysis further revealed that the majority of UAV spectral domain combinations were composed of VIS-NIR-TIR sensors. As SIF measurements are not yet available from satellite sensors in spatial resolutions required for precision agriculture applications, studies using SIF data mainly explored airborne data sets.
Fig. 6.
Change in platform usage for the detection of stress in agriculture over 5-year periods. Note that the years refer to a 5-years period, e.g. 2005 stands for 2003–2007.
4.4. Algorithms and methodologies
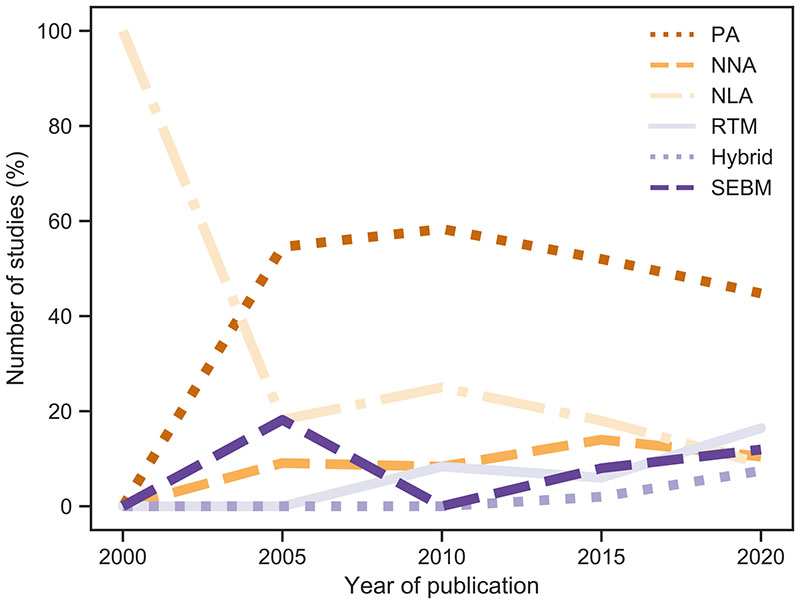
As discussed in section 3, the detection of stress conditions in crops often relies on the estimation of traits linked to the crop status. In the investigated studies, most focused on quantifying traits using regression or RTM methods, whereas the usage of classification or anomaly detection was less pronounced. Among the papers reviewed in this study, about 72% were based on parametric approaches (e.g., Gerhards et al., 2016; Guan et al., 2017; Panigada et al., 2014), 22% on nonparametric linear approaches (e.g., Ainsworth et al., 2014; Sobejano-Paz et al., 2020; Thomas et al., 2017), about 17% on nonparametric nonlinear approaches (e.g., Camino et al., 2021; Gao et al., 2009; Zarco-Tejada et al., 2018), nearly 15% on surface energy balance models (e.g., Bayat et al., 2018; Bhattarai et al., 2019; Zhuang et al., 2020), 16% used radiative transfer models (e.g., Camino et al., 2018; Celesti et al., 2018) and only a few on hybrid approaches (e.g., De Grave et al., 2020; Delalieux et al., 2014). Note that several studies employed multiple methods. Fig. 7 indicates the use of different methodologies in studies published from 1999 to 2021. Though a clear trend was missing, we noticed that on a percentual basis, the use of parametric and nonparametric linear approaches has been slightly decreasing, while SEBMs, RTMs and hybrid approaches gained popularity. With respect to machine learning regression algorithms, mainly conventional shallow learning methods were employed, such as support vector machine (regression), random forest or neural network approaches. Although it may be promising for stress detection, the exploration of deep learning algorithms is still missing. RTMs and hybrid approaches have been receiving attention in recent years due to improved performance in computational speed, flexibility and generic applicability (Verrelst et al., 2019a). Also, they have been demonstrated to be attractive methods as regards to mapping large areas, especially in operational and global contexts (Verrelst et al., 2015). Accurate and fast mapping of crop traits over large cultivated areas by means of these hybrid methods reveals potential for the quantification and monitoring of stress-related proxies.
Fig. 7.
Change of retrieval methods usage over 5-year periods to infer vegetation traits as stress proxies. PA: parametric regressions (e.g., vegetation indices), NNA: nonparametric nonlinear approaches (e.g., machine learning), NLA: nonparametric linear approaches (e.g., principal component regression), RTM: radiative transfer models (inversion), Hybrid: hybrid methods (i.e., combination of RTM and NNA methods), SEBM: surface energy balance models. Note that the years refer to a 5-years period, e.g. 2005 stands for 2003–2007.
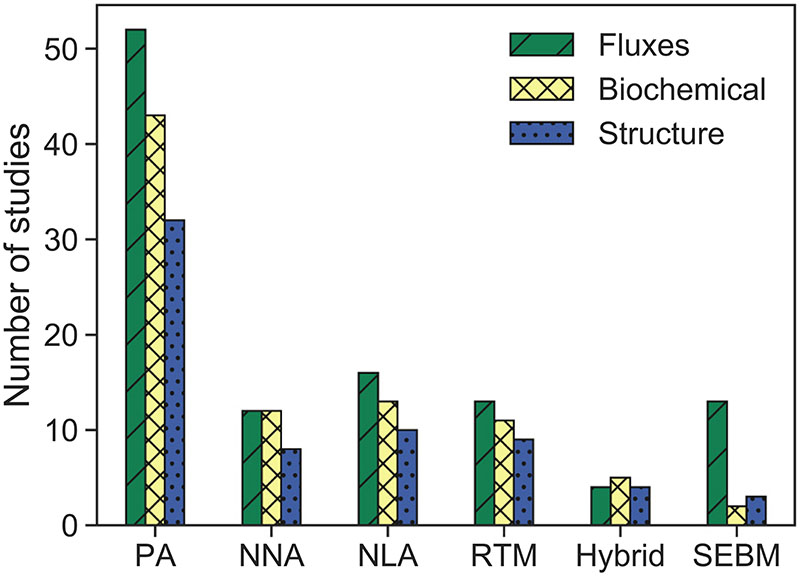
Regarding the retrieved traits, Fig. 8 shows that the highest percentage of the studies focused on the retrieval of traits related to fluxes (mostly ET), followed by those related to biochemical traits and finally structural traits, independently of the method used. The only exception are hybrid approaches, which were mainly applied to retrieve biochemical traits, rather than those related to fluxes.
Fig. 8.
Applied retrieval methods as function of distinct trait groups. PA: parametric regressions (e.g., vegetation indices), NNA: nonparametric nonlinear approaches (e.g., machine learning), NLA: nonparametric linear approaches (e.g., principal component regression), RTM: radiative transfer models (inversion), Hybrid: hybrid methods (i.e., combination of RTM and NNA methods), SEBM: surface energy balance models.
4.5. Observational levels and scales
Regarding observational levels and spatial scales considered in the selected studies, mostly the top-of-canopy (TOC) level was addressed with 72%, followed by the leaf level with 45% or both. Only six studies addressed (exclusively) the top-of-atmosphere (TOA) level (Bhuiyan et al., 2017; Knipper et al., 2019; Mladenova et al., 2017; Navarro et al., 2016). The majority of the TOA studies investigated regional scale stress levels. In fact, most of the papers considered the field/plot scale (67%), but multiple studies addressed the micro/plant scale (i.e., 31%) with little overlap between these categories. As illustrated by regional-scale TOA studies, there was a strong relationship between the spatial scales and observational levels. For example, all but one out of the plant scale studies focused on leaf level traits, with the exception of Kim and Glenn (2017), where the authors proposed a new multi-modal stress detection system for plants by integrating off-the shelf sensors in a custom-made platform and by developing software for processing data flows.
4.6. Spectral domains
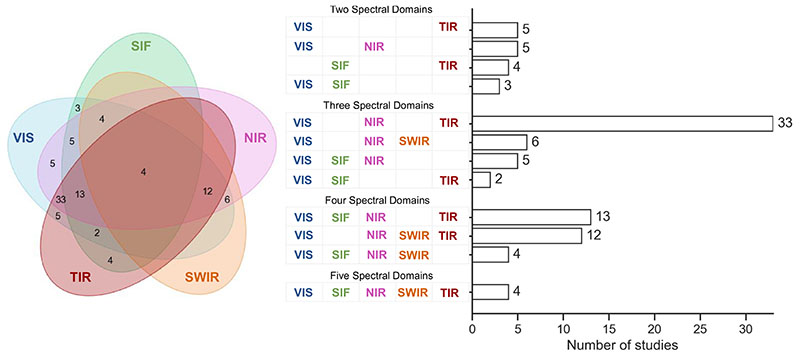
Overall, the number of publications between 1999 and 2021 increased approximately by factor of four. With respect to the explored wavelengths, this increase was reflected by all domains, though with higher absolute numbers of NIR, VIS and TIR studies compared to SWIR and SIF. The utilization and synergistic use of different spectral domains within the selected studies is demonstrated by a Venn diagram (Fig. 9). Here, we observe that the combination of VIS-NIR-TIR domains represents the largest category (N = 33). Regarding the two-domain combinations, the VIS-NIR and VIS-TIR were the most often used (N = 5). In addition, the VIS domain was employed by the majority of studies. As two spectral regions in the optical reflective domain are insufficient to detect short-term stress, the combination with TIR sensors proved to be useful. For instance, the study by Baluja et al. (2012) examined crop water status using multispectral VIS and NIR sensors along with thermal imagery. The authors found that with the latter technology it was possible to detect short-term responses. However, normalized difference vegetation index (NDVI) and two other VIs calculated from the VIS-NIR data were rather reflecting the results of cumulative water deficits on the observed crop, hence its response to chronic stress exposure.
Fig. 9.
Venn diagram visualizing the different combinations of used spectral domains (VIS, SIF, NIR, SWIR, and TIR) in the reviewed studies (left). Number of studies using different sensor combinations (two to five) with respect to the different spectral domains (right).
In total, only four studies concurrently exploited all five main domains (i.e., VIS, SIF, NIR, SWIR and TIR) (Camino et al., 2021; Gerhards et al., 2018; Mahlein et al., 2019; Zarco-Tejada et al., 2018). This gap could be explained, among others, by the still limited (for SWIR) and missing (for SIF) availability of high spatial resolution satellites, and rather high prizes for sensors, such as UAV-suitable cameras. In fact, for the detection of very early stresses, the optimal spectral domains could mainly be provided by a combination of SIF and TIR, which however, was realized by only a few studies (N = 4) (Chaerle et al., 2004, 2009; Ni et al., 2015; Pérez-Bueno et al., 2015). All in all, these findings suggest that many study designs were mainly driven by sensor availability and practical economical reasons, respectively, leading to a common usage of VIS-NIR sensors. This was confirmed by a study analysing costs of multi-sensor systems (Appeltans et al., 2020), calculating, for instance, total costs of 200.000 euros for a combination of a hyperspectral, thermal and fluorescence sensor.
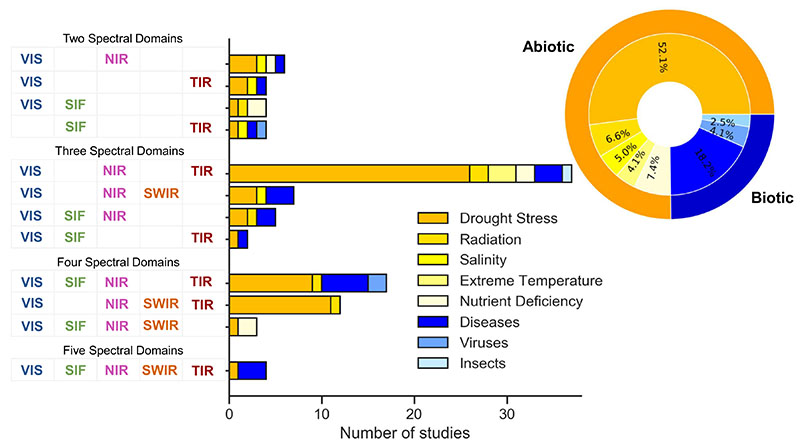
4.7. Stress types and plant responses
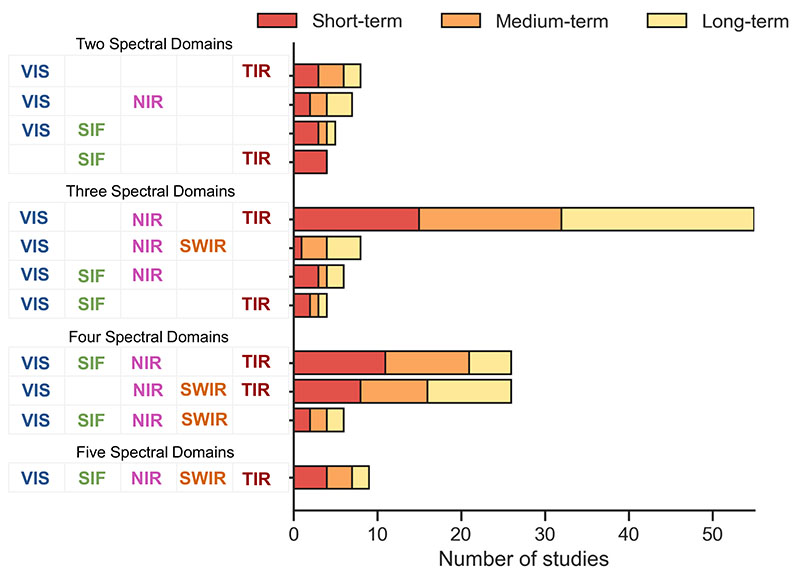
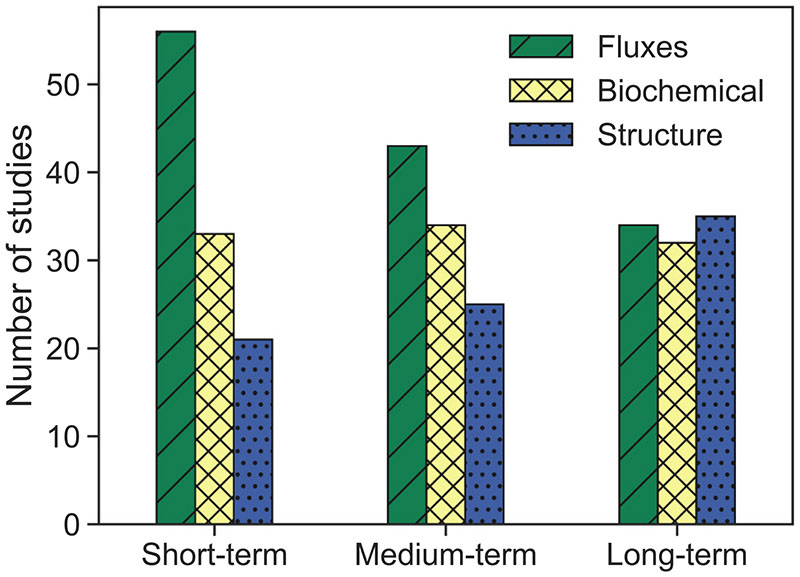
Fig. 10 clearly demonstrates that the majority of the selected studies investigated abiotic stress, in particular drought stress. On a short-term scale, drought stress influences transpiration through stomatal closure (see chapter 2.2). Consequently, the analysis of TIR data (i.e., temperature) may provide the most useful information. However, TIR sensors struggle with lower spatial and spectral resolutions leading to weaker SNR in comparison to sensors measuring optical reflective signals (VSWIR). This means if the detectors of both measurement types are of comparable size, less energy is available in the TIR than for VSWIR, which limits the applicability of TIR in precision farming contexts (Gerhards et al., 2019). Moreover, the TIR signal is highly influenced by environmental factors, such as wind turbulence and background cover (e.g., weed or soil type) (Döpper et al., 2020). Thus, a combination of TIR data with the VNIR/SWIR spectral domains is most common (see Fig. 10). Within this combination, the majority of studies investigated long-term stress, as such data enable studying different traits with the evolution from short-term fluxes to longer-term biochemical and structural changes. Fig. 11 demonstrates the behaviour of the three trait groups as a function of stress duration or severity. As expected, variations of fluxes have been related most often to short-term stress, whereas the structural traits are mainly used as proxies for longer term or chronic stress. Interestingly, there is a balance of all three trait groups for the long-term stress category (see Fig. 12). It may be explained by the fact that the same traits are observed over longer study periods, instead of using only the most responsive trait at a certain stress stage.
Fig. 10. Usage of diverse spectral sensor combinations with respect to targeting biotic and abiotic stresses.
Fig. 11. Usage of diverse spectral sensor combinations with respect to targeting short-, medium-, and long-term stresses.
Fig. 12.
Detection of different trait groups acting as proxies for crop stress in the reviewed studies, aggregated to time scales (short-, medium and long-term stress). The traits refer to fluxes-related, such as transpiration or SIF yield, biochemicals, i.e. leaf water or pigment contents, and structure-related traits, e. g. leaf inclination, LAI or biomass.
In particular, the combination of VIS/NIR with TIR sensors can increase the accuracy of the long-term stress assessment since the same traits are observed based on distinct principles (e.g., Chaerle and VanDer Straeten, 2000; Gerhards et al., 2016). As stated above, this specific wavelength domain combination is also driven by the availability and comparatively low costs of the sensors. In contrast to TIR data, being applied to any time scale, SIF data was almost exclusively employed to analyse short-term (flux-related) responses (Campbell et al., 2007; Ni et al., 2015; Pérez-Bueno et al., 2015; Zarco-Tejada et al., 2018). Similarly to the analysis of TIR data, the SIF signal might be weak and interpretability can be increased by combining the data with measurements from other spectral domains (e.g., Alonso et al., 2017; Celesti et al., 2018; Chaerle et al., 2009). Overall, we found that studies including SIF data were rather underrepresented.
A smaller number of studies focused on biotic stress (see Fig. 10), mainly disease detection (e.g., Bendel et al., 2020; Mahlein et al., 2019; Savian et al., 2020). Here we observed a large variety in the combination of spectral domains. However, it must be remarked that the number of analysed traits in those studies was also large, including structural, biophysical and biochemical traits.
In conclusion, we observed that the majority of reviewed studies was rather restricted to a comparison of the outcomes by diverse sensors and spectral domains as opposed to a synergistic usage of the multi-domain data. Only a few studies were working on synergistic integration of RS data from various spectral domains (e.g., Zarco-Tejada et al., 2018) targeting stress detection.
4.8. Disentangling abiotic and biotic stress sources
Since plant pathogens pose an enormous threat to global food security causing yield losses exceeding 30% in selected regions or even up to 100% on a local scale (Savary et al., 2019; Zarco-Tejada et al., 2021), we address this specific topic here. The differentiation of biotic and abiotic stressors is a challenging task, as symptoms triggered by pathogens may be confounded with abiotic-induced responses. Also, the distinction between multiple biotic-induced stressors remains to be investigated, in order to provide quantitative tools for pre-symptomatic disease detection allowing respective measures and adaptation of management practices. So far, only a few studies focused on disentangling infection sources with similar symptoms (e.g., Fallon et al., 2020; Gold et al., 2020; Moshou et al., 2014; Poblete et al., 2021). Gold et al. (2020), for instance, sampled hyperspectral measurements of hemibiotrophic and necrotrophic pathogen-affected potato crops, estimating multiple traits over the optical reflective domains, which were synergistically explored using chemometric models. This approach allowed to provide a pre- and post-symptomatic differentiation of the two foliar diseases. In Poblete et al. (2021), olive trees affected by two pathogenes were investigated, restricting water and nutrient flow through the xylem, which leads to symptoms similar to those caused by drought stress. The authors applied three-stage machine learning algorithms to airborne hyperspectral and thermal imagery to discriminate the two diseases successfully. Zarco-Tejada et al. (2021) explored thermal and hyperspectral reflectance data revealing the existence of divergent pathogen- and host-specific spectral pathways through uncoupling of specific biotic and abiotic spectral effects. All in all, these studies concluded that a multi-sensor approach is absolutely necessary for in-depth stress analysis, and presented in a pioneering way how synergy of different domains and sensors (mainly with hyperspectral resolutions in the VIS/NIR/SWIR domain) can be used to predict and distinguish biotic and abiotic stressors. However, it needs to be mentioned that differentiation is still vague in many cases and advanced methods such as machine learning should be explored (Behmann et al., 2015; Neupane and Baysal-Gurel, 2021). The review by Zhang et al. (2019) about monitoring plant diseases and pests using RS also concluded that the detection of abiotic stress is still challenging and under-exploited through the simultaneous appearance of multiple stressors. Hence, we need to advance near real-time detection at larger spatial scales, especially for pests, pathogens and diseases, causing significant global production losses (Savary et al., 2019). This is even more evident, as our literature analysis revealed that the majority of available studies in this domain rather concentrated on single crops and pathogens at local and experimental levels.
5. New concepts of sensor synergies
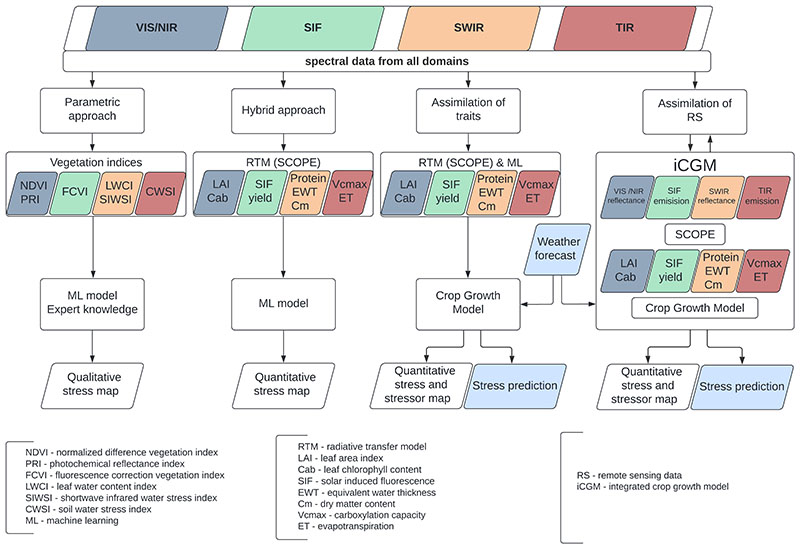
Combining distinct spectral domains acquired from one or multiple sensors leads to more data for an improved (post-)processing of signals or quantities to derive plant physiological, biochemical and structural information. In addition, it offers advanced possibilities to observe a complete range of plant responses (i.e., traits), which can be directly related to stress. This review has clearly indicated a possibly strong - and currently largely underexploited - potential of using spectral information from multiple sensors or domains in a synergistic way for crop stress detection, monitoring and management. Based on these insights, we propose four ways forward to increasing complexity by employing multi-domains data, as delineated in Fig. 13, fostering:
Combined acquisition and analysis of spectral data from multiple sensors representing multiple modalities and using parametric regressions (see Section 5.1);
Simultaneous retrieval of plant traits using multi-domain RTMs and ML methods (see Section 5.2);
Assimilation of estimated plant traits based on data from different spectral domains into mechanistic models (see Section 5.3);
Conceptual framework: Development of fully integrated mechanistic and radiative transfer models directly simulating the full variety of spectral measurements that can thereafter be assimilated (see Section 5.4).
Fig. 13.
Four concepts of sensor synergies for stress detection with increasing complexity. The colors blue, green, orange and red refer to the spectral domains VIS/ NIR, SIF, SWIR, and TIR, respectively, and with respect to the derived indices or traits. The indices and traits in the small coloured boxes are some selected representative examples. The box on the right shows the concept of our proposed conceptual framework, the iCGM - integrated crop growth model. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5.1. Combined acquisition and analysis of multi-sensor data
In the short run - and directly feasible with existing methodologies and sensors - we believe that stress detection towards operational monitoring should employ at least one sensor and one parametric regression or classification method per trait group (i.e. functional, biochemical, structural), typically representing plant adaptation responses at varying time scales (see Fig.13, left). The synergistic database can then be used to train machine learning regression algorithms for generating crop stress prediction models. This procedure may allow: i) to better understand the sensitivity of various spectral indices to stress occurrence, which will ultimately enable ii) to distinguish between diverse stress severity (e.g. mild to severe water stress). For instance, Zarco-Tejada et al. (2021) obtained several spectral traits from multisensor data and used the derived spectral fingerprints for distinguishing different diseases. Another study by Damm et al. (2022) investigated temporal sensitivity of multi-sensor derived vegetation information for evolving water stress. Since such kinds of studies are still scarce, we advocate more research aiming to obtain a holistic view of plants under biotic and abiotic stress through synergistic usage of spectral information from multiple sensors or domains. However, the differentiation between distinct types of stresses (e.g. water-deficit or nutrient stress) will remain a challenge with purely RS-based approaches because distinct stressors often lead to similar stress reactions (Poblete et al., 2021; Zhang et al., 2019).
5.2. Simultaneous retrieval of plant traits employing multi-sensor data, multi-domain RTMs and hybrid retrieval methods
As a second strategy, we encourage exploiting multi-domain radiative transfer models for simultaneous usage of VNIR/SWIR hyper-spectral, TIR and SIF observations (Yang et al., 2020b). One advantage of using a unified radiative transfer scheme for these spectral domains is that the retrieval can be better constrained. For example, several studies retrieved crop traits and stress responses by combining the VNIR/SWIR and SIF signals with the model SCOPE (Celesti et al., 2018; Van der Tol et al., 2016; Yang et al., 2019) and FluorFlight (Hernández-Clemente et al., 2017). The analogy of radiative transfer among these spectral domains and between reflected and emitted radiation enabled the decomposition of SIF patterns into structure related effects (such as leaf inclinations) and physiological stress responses. Some of the difficulties of using TIR data can also be overcome with multi-domain radiative transfer modeling. For example, Duffour et al. (2015) used vegetation traits that can be retrieved from VNIR/SWIR data in SCOPE to simulate radiative transfer in the TIR domain as well as the directionality of the TIR signal. The improved understanding of the influence of the geometry dependent fraction of sunlit and shaded leaves and soil supports the interpretation of TIR for stress detection.
The inclusion of photosynthesis and non-radiative heat exchange (evaporative and convective cooling) in a RTM opens further possibilities for multi-domain retrieval. Stomatal and photochemical responses, for example, can simultaneously respond to variations in incident solar radiation, both affecting the TIR and SIF emissions and radiation absorption in the VIS. With such integrated approach, the joint retrieval from the VNIR and TIR domains (Bayat et al., 2018) or from the VNIR and SIF (Pacheco-Labrador et al., 2019b) enables the estimation of photochemical parameters and stomatal aperture. Suarez et al. (2021) retrieved photosynthetic parameters from SCOPE, but using hyper-spectral data of the VNIR region including subtle responses in the 530–570 nm range caused by xanthophyll pigment changes associated with non-photochemical quenching in response to excess light as modelled by Vilfan et al. (2018).
These studies demonstrate the benefit of multi-domain and multi-process models, but analysis of such datasets can be complex and computationally intensive. For larger datasets, the capabilities of these models can be preserved without additional computational burden by using machine learning or deep learning approaches: the retrieval of proxies could be accomplished by means of hybrid approaches which combine machine learning with RTMs (Verrelst et al., 2019a), as delineated in Fig. 13 (second left).
5.3. Assimilation of remotely derived crop traits into crop growth models
For efficient stress detection and prognosis, the assimilation of spatially derived trait maps from RS observations into crop growth models (CGM) (e.g., van Diepen et al., 1989; Hank et al., 2015; Jones et al., 2003) would be beneficial. These mechanistic models also account for weather and soil variables or agricultural management practices, which could help to identify the cause of the stress (Baret et al., 2007).
The continuous description of crop growth and development using mechanistic models offers means to summarize current knowledge about the main processes in the soil-plant-atmosphere continuum, which also include stress related processes and phenomena (Weiss et al., 2020). Therefore, we propose to explore this concept (see also Fig.13, second right), which can serve for the development of a digital twin (DT) of the farming system (Verdouw et al., 2021). Such DTs are digital equivalents of real-life objects, virtually mirroring the behaviour and states of physical systems over their whole lifetime (Grieves and Vickers, 2016). In relation to precision agriculture applications, current state-of-the-art CGMs include advanced crop growth models (Peng et al., 2018), crop-specific parameterizations (Boas et al., 2021; Sulis et al., 2015), and crop management practices like irrigation and nitrogen fertilization (Lombardozzi et al., 2020). The synergistic use of CGMs and RS is already being explored for building operational monitoring and prediction platforms capable of delivering useful information for agricultural applications and decisions (e.g., McNally et al., 2017; Peters-Lidard et al., 2021). These platforms are mainly based on the assimilation of water cycle measurements (e.g., soil moisture) and remotely sensed vegetation variables (e.g., LAI), obtaining clear improvements in the estimation of vegetation biomass, evapotranspiration, root zone soil moisture, and carbon fluxes. More complex and realistic representation of physical processes recently implemented in CGMs and the exploitation of the different optical domains in RS open the opportunity to predict better a wider range of crop processes, most notably to capture the onset of crop water and heat stress, reducing also the uncertainty in crop model variable estimation. The assimilation of multiple data streams into CGMs and thus the establishment of DTs, represents also a viable approach for getting an improved understanding about physical mechanisms and feedback controlling the development, duration, and impacts of stress on plant growth and yield. However, significant challenges have to be addressed in order to fully exploit this potential. These challenges include consistency checks between diverse RS observations and between the retrieval algorithms and the crop model parameteri-zations, as well as the robust quantification of the uncertainties along traceable processing chains.
5.4. Proposed conceptional framework: Integration of crop growth and radiative transfer models
Future stress research should focus on the integration of suitable multi-domain radiative transfer models (e.g. SCOPE) with dynamic crop growth models to build up DTs of agroecosystems. Such integrated models (iCGMs) directly simulate remotely observed signals based on the status of the underlying CGM at any given point in time (see Fig.13, right). This means the iCGM simulates the spectral signatures of the canopy (400–2500 nm), the SIF emission as well as the thermal properties, along with physiological processes (Delécolle et al., 1992; Moulin et al., 1998).
The integration of CGMs and RTMs has several compelling advantages. On the one hand, the CGM can benefit from all available satellite data while the assimilation scheme can weigh the value of observations by using information about their uncertainties. However, combining data with various spatial and temporal resolution needs to be considered carefully in the conceptual design (Huang et al., 2019). Traits that appear both as a status variable in the CGM and as an input of the RTM have to permit the RTM to simulate spectral properties across all domains. For the number of traits, being sensitive in the different spectral domains, assimilation strategies need to be developed taking into account the different availability (timing) of sensor data. The assimilation needs to allow for a model update at different times, where only a part of the variables are assimilated, with model-intern constraints and links to further variables. On the other hand, the RTM could benefit from the background information of the CGM. Satellite derived time series of vegetation traits can be noisy due to atmospheric, orbital and geometric effects. The use of retrievals in close proximity in time as background information has shown to be effective in making the satellite data smoother (Mousivand et al., 2015; Yang et al., 2021b).
CGMs provide continuous series of temporal growth dynamics which can be used to detect outliers and to improve the construction of smooth time-series from heterogeneous satellite data. In this respect, the use of a CGM assimilating these data is a step up. Furthermore, by inserting a morphological sub-model, one could simulate the physiological appearance of plants, which lead - using a suitable sensor model - to temporal evolution of point clouds observable through photogrammetry or Lidar (Liu et al., 2019). Expanding existing RTMs from the optical spectral domain to the microwave range would similarly lead to the simulation of temporal sequences of (active and passive) microwave signatures (e.g. using a RADAR backscatter model). The combined set of spectral properties would be simulated simultaneously for each time step of the iCGM, which ideally should be hourly. For executing iCGMs over large areas in high temporal and spatial resolutions, this physically based modeling could be combined with machine learning to estimate traits or stress proxies within a hybrid manner. The complex models can also be partly replaced through surrogate models or emulators speeding up the forward simulations (Verrelst et al., 2019b). As CGMs are driven by weather variables, it is also easy to conceive the integration of weather forecasts and/or weather scenarios in such iCGM (see Section 5.3). This dynamic nature would meet the requirements of a DT of agroecosystems, which besides the representation of current behaviour, also predicts their future behaviour (Verdouw et al., 2021). Therefore, we consider the iCGM as the most promising approach and this section is meant as a way to stimulate further discussion about DTs within the community. Once developed, such iCGMs facilitate tackling various research gaps needed to define future sensitive Earth observational approaches and stimulate the development of applications in the realm of precision agriculture.
In the near future, we will have a multitude of spectral data available to feed the proposed iCGM. Besides the recently available and upcoming spaceborne imagine spectrometer data, such as the Environmental Mapping and Analysis Program (EnMAP) (Guanter et al., 2015), PRe-cursore IperSpettrale della Missione Applicativa (PRISMA) (Cogliati et al., 2021; Loizzo et al., 2019), Copernicus Hyperspectral Imaging Mission for the Environment (Rast et al., 2021) or the ECOsystem Spaceborne Thermal Radiometer Experiment on Space Station (ECO-STRESS) data (Fisher et al., 2020), UAVs became a crucial platform with respect to sensor synergies. Several light multi- and hyperspectral VIS, NIR and SWIR, thermal and SIF sensors are available nowadays (overviews provided by Aasen et al. (2018); Awais et al. (2022); Herrmann and Berger (2021); Messina and Modica (2020); Neupane and Baysal-Gurel (2021); Yang et al. (2017a)), which can be explored for model development or validation of physiological traits related to crop stress. Besides spectral synergies, the exploitation of multi-scale multi-sensor data allows to fill temporal gaps and improve spatial resolutions. Hence, such upcoming multi-scale approaches are foreseen to play a more dominant role in future studies (Alvarez-Vanhard et al., 2021; Inoue et al., 2018; Machwitz et al., 2021; Sagan et al., 2019).
Some applications and use cases of our proposed conceptional framework are laid out below.
Sensitivity assessment of state-of-the-art approaches
Remote sensing measurements and retrieved crop information can be subject to insensitivity or uncertainties caused by various sources, e.g., instrumental effects and calibration issues (Pacheco-Labrador et al., 2019a), spatial-spectral-temporal sampling design (Aasen et al., 2019), retrieval assumptions (Cendrero-Mateo et al., 2019), cf. Buman et al. (2022) for an overview of related uncertainties on SIF. Such effects can result in a mismatch between expected and observed crop behaviour (e.g. spatio-temporal dynamics of crop state variables). The proposed conceptual framework (section 5.4) can be applied to facilitate sensitivity analysis aiming to assess potential crop responses and their spatio-temporal dynamics. Resulting insights allow us to identify sensitive wavelength regions, sampling schemes, and sensor modalities for crop stress symptoms. In this way, artificially modulated dynamics inherent to state-of-the art observational approaches, e.g., trends caused by sensor degradation, anomalies, and covariance between traits due to imperfect retrievals, can be unraveled.
Benchmarking and uncertainty assessment for mission development
Experimental or operational satellite missions require dedicated tools to define scientific needs and requirements (Malenovský et al., 2012). The conceptual framework holds the capacity to support the evaluation of such requirements. One example is to parameterize the framework with densely sampled multi-sensor data to assess subtle stress responses of crops, such as photosynthesis, gas exchange or pigment degradation (Jin et al., 2018). In an ablation study, data scarcity can be simulated to quantify uncertainties related to e.g. reduced spectral, temporal and spatial resolutions. Resulting insights allow assessing critical sampling dimensions for various stress symptoms and to make recommendations towards new experimental or operational crop stress missions or to design dedicated in situ or close range approaches.
Stress detection via anomalies
The framework allows to calculate potential crop states and functioning (e.g. growth rates, gas exchange) as induced by environmental drivers (Huang et al., 2019). The comparison of actual estimates of crop state and functioning against its simulated potential offers a pathway to identify anomalous crop conditions (e.g. caused by crop stress).
Forecasting of crop stress and tools
The framework allows to learn - through simulation - how ecosystems behave under current and future environmental conditions, also known as scenario analysis (Machwitz et al., 2018). The coupling also enables forecasting of crop stress responses when upcoming growth conditions can be reasonably well simulated. The ultimate goal of developing advanced crop monitoring and forecasting tools is to provide reliable and usable information to public and private stakeholders in the agricultural sector. This goal can be achieved by targeting distinct spatial scales (i.e., from field to the regional scale) and temporal horizons (from short-range to seasonal) that match with farmers’ activities and planning. In this context, the seamless integration of CGM + RTM data assimilation cycles into operational weather forecast chains that cover the full temporal spectrum of farmers’ decisions appears the most straightforward approach in order to accomplish this objective. The additional use of meteorological information relevant during the distinct crop development stages (e.g., accumulated heat time, probability of heat/cold stress) also has the advantage to improve the interpretation of environmental stresses for a given crop development stage (McNally et al., 2017; Peters-Lidard et al., 2021). The wealth of numerical information extracted from such monitoring and forecasting tools needs to be translated into crop-specific stress indicators to create an interface between scientific advancements and the decision-making process. Finally, the probabilistic character of the meteorological forecasts and the uncertainties in RS observations and numerical models formulations must be accounted for.
6. Conclusions
Timely and efficient detection of crop stress remains a challenge but can be approached by exploring multiple RS data sources. In addition, the portfolio of available tools and retrieval methods for the identification of specific stresses has been increasing in the last decade. In this review, we focused on the use of optical sensor synergies for improving spectral sampling to better characterize and interpret crop stresses in support of sustainable agriculture. We identified three different phases (stages) of stress depending on duration and severity: (1) early and short-term, (2) medium-term and mild (3) chronic and severe. The three phases can best be detected by optical RS domains through specifically affected traits, such as fluxes at early stages sensed by SIF and TIR, and biochemicals and structural properties at later stress stages, measured with reflective optical systems in the VIS, NIR and SWIR wavelength ranges. Our review revealed that the combination of VIS, NIR and TIR domains was employed by the majority of studies addressing crop stress.
However, this choice was mainly driven by economical and practical reasons, i.e. the availability of sensors. Furthermore, in most publications, the exploration of diverse spectral domains was rather based on a comparison of respective methods than on synergistic and integrated methodological concepts. An efficient crop stress detection framework has to be versatile, easy to operate, and inexpensive so that it can be easily integrated into precision agriculture and breeding systems. To achieve this, we pursue four ways forward of increasing complexity. These possible concepts involve: (i) Combined analysis of data from multiple sensors using representative parametric regressions and ML approaches, leading to qualitative stress maps. (ii) Simultaneous retrieval of plant traits using multi-domain RTMs and ML methods, providing quantitative stress maps. (iii) Assimilation of estimated plant traits based on data from different spectral domains into CGMs, and integrated dynamic CGMs (iv), which both lead to quantitative stress and stressor maps also enabling stress prediction. In this way, the digital twinning of agroecosystems provides a new perspective for real-life, highly accurate and holistic views of stress and stressors, allowing to act on time and thus contributing to a sustainable agricultural production needed to feed the future world population. Hence, we suggest that the community progresses towards such a DT design for stress effects, causes and prediction in a joint initiative. Such a synergistic approach has the potential to extract essential information about the plant stress status and may reveal the causes of biophysical, physiological and photochemical changes over cultivated areas in space and time.
Acknowledgments
The research was mainly supported by the Action CA17134 SENSECO (Optical synergies for spatiotemporal sensing of scalable ecophysiological traits) funded by COST (European Cooperation in Science and Technology, http://www.cost.eu/ (accessed on 16/03/2022)). The publication is also the result of the project implementation: “Scientific support of climate change adaptation in agriculture and mitigation of soil degradation” (ITMS2014+313011W580) supported by the Integrated Infrastructure Operational Programme funded by the ERDF. We also thank the two reviewers for their fundamental suggestions.
Funding
This research was funded by the EnMAP scientific preparation program under the DLR Space Administration with resources from the German Federal Ministry of Economic Affairs and Energy, grant number 50EE1923 (K. Berger). This research was also funded by the European Research Council (ERC) under the ERC-2017-STG SENTIFLEX project (grant agreement 755617) (K. Berger) and Ramón y Cajal Contract (Spanish Ministry of Science, Innovation and Universities) (J. Verrelst). S.C. Kefauver is supported by the Ramon y Cajal RYC-2019-027818-I research fellowship from the Ministerio de Ciencia e InnovaciÃ3n, Spain. S. Van Wittenberghe is supported by the European Research Council (ERC) under the ERC-2021-STG PHOTOFLUX project (grant agreement 101041768). M. Celesti was supported by a Living Planet Fellowship (ESA/Contract No. 4000125442/18/I-NS) of the European Space Agency. V. Sobejano Paz PhD thesis was supported by the AgWIT JPI project ERA-NET Co-fund Water Works 2015 Call and a SDC grant (Sino Danish Council).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Credit author statement
Conceptualisation: KB, MM, MK, SK, JV, UR, AD, MSc; Investigation: KB, MM, MK, ETG, MR, MF, BS, AA, GT, IP, MG, ET, OR, LF, GS, MSc; Methodology: KB; Visualisation: KB, MM, SVW, VSP, MG, GK, MF, EP, ET, TH, MSc; Software: KB, MC, EP; Writing - original draft: KB, MM, MK, SVW, UR, IH, CA, MG, AH, GK, CvT, MR, MF, BS, GT, SB, AD, MSu, ET, OR, MSc; Writing - review and editing: KB, MM, SK, MC, JV, IH, CA, SF, RP, CvT, MR, HA, AD, MLB, RD, TH, MSc; Project administration: KB, MM, MSc.
References
- Aasen H, Honkavaara E, Lucieer A, Zarco-Tejada PJ. Quantitative remote sensing at ultra-high resolution with UAV spectroscopy: a review of sensor technology, measurement procedures, and data correction workflows. Remote Sens. 2018;10:1091. doi: 10.3390/rs10071091. [DOI] [Google Scholar]
- Aasen H, Van Wittenberghe S, Sabater Medina N, Damm A, Goulas Y, Wieneke S, Hueni A, Malenovský Z, Alonso L, Pacheco-Labrador J, Cendrero-Mateo MP, et al. Sun- induced chlorophyll fluorescence II: review of passive measurement setups, protocols, and their application at the leaf to canopy level. Remote Sens. 2019;11:927. doi: 10.3390/rs11080927. [DOI] [Google Scholar]
- Abdullah H, Skidmore AK, Darvishzadeh R, Heurich M. Timing of red-edge and shortwave infrared reflectance critical for early stress detection induced by bark beetle (Ips typographus, L.) attack. Int J Appl Earth Obs Geoinf. 2019;82:101900. doi: 10.1016/j.jag.2019.101900. [DOI] [Google Scholar]
- Ač A, Malenovský Z, Olejníčková J, Gallé A, Rascher U, Mohammed G. Meta-analysis assessing potential of steady-state chlorophyll fluorescence for remote sensing detection of plant water, temperature and nitrogen stress. Remote Sens Environ. 2015;168:420–436. doi: 10.1016/j.rse.2015.07.022. [DOI] [Google Scholar]
- Acebron K, Matsubara S, Jedmowski C, Emin D, Muller O, Rascher U. Diurnal dynamics of nonphotochemical quenching in Arabidopsis npq mutants assessed by solar-induced fluorescence and reflectance measurements in the field. New Phytol. 2021;229:2104–2119. doi: 10.1111/nph.16984. [DOI] [PubMed] [Google Scholar]
- Agam N, Cohen Y, Alchanatis V, Ben-Gal A. How sensitive is the CWSI to changes in solar radiation? Int J Remote Sens. 2013;34:6109–6120. doi: 10.1080/01431161.2013.793873. [DOI] [Google Scholar]
- Ainsworth EA, Serbin SP, Skoneczka JA, Townsend PA. Using leaf optical properties to detect ozone effects on foliar biochemistry. Photosynth Res. 2014;119:65–76. doi: 10.1007/s11120-013-9837-y. [DOI] [PubMed] [Google Scholar]
- Aldea M, Hamilton JG, Resti JP, Zangerl AR, Berenbaum MR, Elucia EHD. Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant Cell Environ. 2005;28:402–411. doi: 10.1111/j.1365-3040.2005.01279.x. [DOI] [Google Scholar]
- Alonso L, Van Wittenberghe S, Amorós-López J, Vila-Francés J, Gómez-Chova L, Moreno J. Diurnal cycle relationships between passive fluorescence, pri and npq of vegetation in a controlled stress experiment. Remote Sens. 2017;9 doi: 10.3390/rs9080770. URL: https://www.mdpi.com/2072-4292/9/8/770. [DOI] [Google Scholar]
- Alvarez-Vanhard E, Corpetti T, Houet T. UAV & satellite synergies for optical remote sensing applications: a literature review. Sci Remote Sens. 2021;3:100019. doi: 10.1016/j.srs.2021.100019. [DOI] [Google Scholar]
- Anderson M, Gao F, Knipper K, Hain C, Dulaney W, Baldocchi D, Eichelmann E, Hemes K, Yang Y, Medellin-Azuara J, Kustas W. Field-scale assessment of land and water use change over the California delta using remote sensing. Remote Sens. 2018;10:889. doi: 10.3390/rs10060889. [DOI] [Google Scholar]
- Appeltans S, Guerrero A, Nawar S, Pieters J, Mouazen AM. Practical recommendations for hyperspectral and thermal proximal disease sensing in potato and leek fields. Remote Sens. 2020;12:1939. doi: 10.3390/rs12121939. [DOI] [Google Scholar]
- Atzberger C. Advances in remote sensing of agriculture: context description, existing operational monitoring systems and major information needs. Remote Sens. 2013;5:949–981. doi: 10.3390/rs5020949. [DOI] [Google Scholar]
- Awais M, Li W, Cheema MJM, Zaman QU, Shaheen A, Aslam B, Zhu W, Ajmal M, Faheem M, Hussain S, Nadeem AA, et al. UAV- based remote sensing in plant stress imagine using high-resolution thermal sensor for digital agriculture practices: a meta-review. Int J Environ Sci Technol. 2022:1–18. doi: 10.1007/s13762-021-03801-5. [DOI] [Google Scholar]
- Baluja J, Diago MP, Balda P, Zorer R, Meggio F, Morales F, Tardaguila J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV) Irrig Sci. 2012;30:511–522. doi: 10.1007/s00271-012-0382-9. [DOI] [Google Scholar]
- Baret F, Houles V, Guérif M. Quantification of plant stress using remote sensing observations and crop models: the case of nitrogen management. J Exp Bot. 2007;58:869–880. doi: 10.1093/jxb/erl231. [DOI] [PubMed] [Google Scholar]
- Baret F, Madec S, Irfan K, Lopez J, Comar A, Hemmerlé M, Dutartre D, Praud S, Tixier MH. Leaf-rolling in maize crops: from leaf scoring to canopy-level measurements for phenotyping. J Exp Bot. 2018;69:2705–2716. doi: 10.1093/jxb/ery071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat B, Van der Tol C, Verhoef W. Integrating satellite optical and thermal infrared observations for improving daily ecosystem functioning estimations during a drought episode. Remote Sens Environ. 2018;209:375–394. doi: 10.1016/j.rse.2018.02.027. [DOI] [Google Scholar]
- Behmann J, Mahlein AK, Rumpf T, Römer C, Plümer L. A review of advanced machine learning methods for the detection of biotic stress in precision crop protection. Precis Agric. 2015;16:239–260. doi: 10.1007/s11119-014-9372-7. [DOI] [Google Scholar]
- Bendel N, Kicherer A, Backhaus A, Klück HC, Seiffert U, Fischer M, Voegele RT, Töpfer R. Evaluating the suitability of hyper- and multispectral imaging to detect foliar symptoms of the grapevine trunk disease Esca in vineyards. Plant Methods. 2020;16:142. doi: 10.1186/s13007-020-00685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, Verrelst J, Féret JB, Wang Z, Wocher M, Strathmann M, Danner M, Mauser W, Hank T. Crop nitrogen monitoring: recent progress and principal developments in the context of imaging spectroscopy missions. Remote Sens Environ. 2020;242:8. doi: 10.1016/j.rse.2020.111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, Rivera Caicedo JP, Martino L, Wocher M, Hank T, Verrelst J. A survey of active learning for quantifying vegetation traits from terrestrial earth observation data. Remote Sens. 2021;13:287. doi: 10.3390/rs13020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni J, Zarco-Tejada P, Sepulcre-Cantó G, Fereres E, Villalobos F. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sens Environ. 2009a;113:2380–2388. doi: 10.1016/j.rse.2009.06.018. URL: https://linkinghub.elsevier.com/retrieve/pii/S0034425709002090. [DOI] [Google Scholar]
- Berni JAJ, Zarco-Tejada PJ, Suarez L, Fereres E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans Geosci Remote Sens. 2009b;47:722–738. doi: 10.1109/TGRS.2008.2010457. [DOI] [Google Scholar]
- Bhattarai N, Mallick K, Stuart J, Vishwakarma BD, Niraula R, Sen S, Jain M. An automated multi-model evapotranspiration mapping framework using remotely sensed and reanalysis data. Remote Sens Environ. 2019;229:69–92. doi: 10.1016/j.rse.2019.04.026. [DOI] [Google Scholar]
- Bhuiyan C, Saha AK, Bandyopadhyay N, Kogan FN. Advances in remote sensing and GIS-based drought monitoring analyzing the impact of thermal stress on vegetation health and agricultural drought - a case study from Gujarat, India. GI- Sci Remote Sens. 2017;54:678–699. doi: 10.1080/15481603.2017.1309737. [DOI] [Google Scholar]
- Bian J, Zhang Z, Chen J, Chen H, Cui C, Li X, Chen S, Fu Q. Simplified evaluation of cotton water stress using high resolution unmanned aerial vehicle thermal imagery. Remote Sens. 2019;11 doi: 10.3390/rs11030267. [DOI] [Google Scholar]
- Bilger W, Bjorkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves ofHedera canariensis. Photosynth Res. 1990;25:173–185. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- Blum A. Stress, strain, signaling, and adaptation –not just a matter of definition. J Exp Bot. 2016;67:562–565. doi: 10.1093/jxb/erv497. [DOI] [PubMed] [Google Scholar]
- Boas T, Bogena H, Grünwald T, Heinesch B, Ryu D, Schmidt M, Vereecken H, Western A, Hendricks Franssen HJ. Improving the representation of cropland sites in the community land model (clm) version 5.0. Geosci Model Dev. 2021;14:573–601. doi: 10.5194/gmd-14-573-2021. URL: https://gmd.copernicus.org/articles/14/573/2021/ [DOI] [Google Scholar]
- Broge NH, Leblanc E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens Environ. 2001;76:156–172. doi: 10.1016/S0034-4257(00)00197-8. [DOI] [Google Scholar]
- Brugnoli E, Bjorkman O. Chloroplast movements in leaves: influence on chlorophyll fluorescence and measurements of light-induced absorbance changes related to pH and zeaxanthin formation. Photosynth Res. 1992;32:23–35. doi: 10.1007/BF00028795. [DOI] [PubMed] [Google Scholar]
- Buitrago MF, Groen TA, Hecker CA, Skidmore AK. Changes in thermal infrared spectra of plants caused by temperature and water stress. ISPRS J Photogramm Remote Sens. 2016;111:22–31. doi: 10.1016/j.isprsjprs.2015.11.003. [DOI] [Google Scholar]
- Buman B, Hueni A, Colombo R, Cogliati S, Celesti M, Julitta T, Burkart A, Siegmann B, Rascher U, Drusch M, Damm A. Towards consistent assessments of in situ radiometric measurements for the validation of fluorescence satellite missions. Remote Sens Environ. 2022;274:112984. doi: 10.1016/j.rse.2022.112984. [DOI] [Google Scholar]
- Camino C, Zarco-Tejada PJ, Gonzalez-Dugo V. Effects of heterogeneity within tree crowns on airborne-quantified sif and the cwsi as indicators of water stress in the context of precision agriculture. Remote Sens. 2018;10 doi: 10.3390/rs10040604. URL: https://www.mdpi.eom/2072-4292/10/4/604. [DOI] [Google Scholar]
- Camino C, Calderón R, Parnell S, Dierkes H, Chemin Y, Román-Écija M, Montes-Borrego M, Landa BB, Navas-Cortes JA, Zarco-Tejada PJ, Beck PSA. Detection of Xylella fastidiosa in almond orchards by synergic use of an epidemic spread model and remotely sensed plant traits. Remote Sens Environ. 2021;260:112420. doi: 10.1016/j.rse.2021.112420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PKE, Middleton EM, McMurtrey JE, Corp LA, Chap-pelle EW. Assessment of vegetation stress using reflectance or fluorescence measurements. J Environ Qual. 2007;36:832–845. doi: 10.2134/jeq2005.0396. [DOI] [PubMed] [Google Scholar]
- Carter GA, Knapp AK. Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am J Bot. 2001;88:arXiv:11302854. [PubMed] [Google Scholar]
- Celesti M, Van der Tol C, Cogliati S, Panigada C, Yang P, Pinto F, Rascher U, Miglietta F, Colombo R, Rossini M. Exploring the physiological information of Sun-induced chlorophyll fluorescence through radiative transfer model inversion. Remote Sens Environ. 2018;215:97–108. doi: 10.1016/j.rse.2018.05.013. [DOI] [Google Scholar]
- Cendrero-Mateo MP, Wieneke S, Damm A, Alonso L, Pinto F, Moreno J, Guanter L, Celesti M, Rossini M, Sabater N, Cogliati S, et al. Sun-induced chlorophyll fluorescence III: benchmarking retrieval methods and sensor characteristics for proximal sensing. Remote Sens. 2019;11:962. doi: 10.3390/rs11080962. [DOI] [Google Scholar]
- Chaerle L, Van Der Straeten D. Imaging techniques and the early detection of plant stress. Trends Plant Sci. 2000;5:495–501. doi: 10.1016/S1360-1385(00)01781-7. [DOI] [PubMed] [Google Scholar]
- Chaerle L, Hagenbeek D, De Bruyne E, Valcke R, Van Der Straeten D. Thermal and chlorophyll-fluorescence imaging distinguish plant- pathogen interactions at an early stage. Plant Cell Physiol. 2004;45:887–896.:arXiv:15295072. doi: 10.1093/pcp/pch097. [DOI] [PubMed] [Google Scholar]
- Chaerle L, Leinonen I, Jones HG, Van Der Straeten D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J Exp Bot. 2006;58:773–784. doi: 10.1093/jxb/erl257. [DOI] [PubMed] [Google Scholar]
- Chaerle L, Lenk S, Leinonen I, Jones HG, Straeten DVD, Buschmann C. Multi-sensor plant imaging: towards the development of a stress-catalogue. Biotechnol J. 2009;4:1152–1167. doi: 10.1002/biot.200800242. [DOI] [PubMed] [Google Scholar]
- Chang Q, Liu J, Wang Q, Han L, Liu J, Li M, Huang L, Yang J, Kang Z. The effect of Puccinia striiformis f. sp. tritici on the levels of water-soluble carbohydrates and the photosynthetic rate in wheat leaves. Physiol Mol Plant Pathol. 2013;84:131–137. doi: 10.1016/j.pmpp.2013.09.001. [DOI] [Google Scholar]
- Cogato A, Meggio F, De Antoni Migliorati M, Marinello F. Extreme weather events in agriculture: a systematic review. Sustainability. 2019;11:2547. doi: 10.3390/su11092547. [DOI] [Google Scholar]
- Cogliati S, Celesti M, Miglietta F, Genesio L, Julitta T, Schuettemeyer D, Drusch M, Rascher U, Jurado P, Colombo R. A spectral fitting algorithm to retrieve the fluorescence spectrum from canopy radiance. Remote Sens. 2019;11:1840. doi: 10.3390/rs11161840. [DOI] [Google Scholar]
- Cogliati S, Sarti F, Chiarantini L, Cosi M, Lorusso R, Lopinto E, Miglietta F, Genesio L, Guanter L, Damm A, Pérez-López S, et al. The PRISMA imaging spectroscopy mission: overview and first performance analysis. Remote Sens Environ. 2021;262:112499. doi: 10.1016/j.rse.2021.112499. [DOI] [Google Scholar]
- Cohen Y, Alchanatis V, Saranga Y, Rosenberg O, Sela E, Bosak A. Mapping water status based on aerial thermal imagery: comparison of methodologies for upscaling from a single leaf to commercial fields. Precis Agric. 2017;18:801–822. doi: 10.1007/s11119-016-9484-3. [DOI] [Google Scholar]
- Cronin P, Ryan F, Coughlan M. Undertaking a literature review: a step-by-step approach. Br J Nurs. 2008;23:17, 38–43.:arXiv:18399395. doi: 10.12968/bjon.2008.17.1.28059. [DOI] [PubMed] [Google Scholar]
- Damm A, Paul-Limoges E, Haghighi E, Simmer C, Morsdorf F, Schneider F, Van der Tol C, Migliavacca M, Rascher U. Remote sensing of plant-water relations: An overview and future perspectives. J Plant Physiol. 2018;227:3–19. doi: 10.1016/j.jplph.2018.04.012. URL: https://www.sciencedirect.com/science/article/pii/S0176161718301172 from aqua-porin to ecosystem: Plants in the water cycle. [DOI] [PubMed] [Google Scholar]
- Damm A, Cogliati S, Colombo R, Fritsche L, Genangeli A, Genesio L, Hanus J, Peressotti A, Rademske P, Rascher U, Schuettemeyer D, et al. Response times of remote sensing measured sun-induced chlorophyll fluorescence, surface temperature and vegetation indices to evolving soil water limitation in a crop canopy. Remote Sens Environ. 2022;273:112957. doi: 10.1016/j.rse.2022.112957. [DOI] [Google Scholar]
- De Grave C, Verrelst J, Morcillo-Pallarés P, Pipia L, Rivera-Caicedo J, Amin E, Belda S, Moreno J. Quantifying vegetation biophysical variables from the sentinel-3/flex tandem mission: Evaluation of the synergy of olci and floris data sources. Remote Sens Environ. 2020;251:112101. doi: 10.1016/j.rse.2020.112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJonge KC, Taghvaeian S, Trout TJ, Comas LH. Comparison of canopy temperature-based water stress indices for maize. Agric Water Manag. 2015;156:51–62. doi: 10.1016/j.agwat.2015.03.023. URL: https://linkinghub.elsevier.com/retrieve/pii/S0378377415001067. [DOI] [Google Scholar]
- Delalieux S, Zarco-Tejada PJ, Tits L, Jimenez Bello MA, Intrigliolo DS, Somers B. Unmixing-based fusion of hyperspatial and hyperspectral airborne imagery for early detection of vegetation stress. IEEE J Select Top Appl Earth Observ Remote Sens. 2014;7:25712582. doi: 10.1109/JSTARS.2014.2330352. [DOI] [Google Scholar]
- Delécolle R, Maas SJ, Guérif M, Baret F. Remote sensing and crop production models: present trends. ISPRS J Photogramm Remote Sens. 1992;47:145–161. doi: 10.1016/0924-2716(92)90030-D. [DOI] [Google Scholar]
- Demmig-Adams B, Stewart J, López-Pozo M, Polutchko S, Adams W. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules. 2020;25:5825. doi: 10.3390/molecules25245825. URL: https://www.mdpi.com/1420-3049/25/24/5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gennaro SF, Matese A, Gioli B, Toscano P, Zaldei A, Palliotti A, Genesio L. Multisensor approach to assess vineyard thermal dynamics combining high-resolution unmanned aerial vehicle (UAV) remote sensing and wireless sensor network (WSN) proximal sensing. Sci Hortic. 2017;221:83–87. doi: 10.1016/j.scienta.2017.04.024. [DOI] [Google Scholar]
- Döpper V, Gränzig T, Kleinschmit B, Förster M. Challenges in UAS-based TIR imagery processing: image alignment and uncertainty quantification. Remote Sens. 2020;12:1552. doi: 10.3390/rs12101552. [DOI] [Google Scholar]
- Drusch M, Moreno J, Del Bello U, Franco R, Goulas Y, Huth A, Kraft S, Middleton E, Miglietta F, Mohammed G, Nedbal L, et al. The fluorescence explorer mission concept- esa’s earth explorer 8. IEEE Trans Geosci Remote Sens. 2017;55:1273–1284. doi: 10.1109/TGRS.2016.2621820. [DOI] [Google Scholar]
- Duffour C, Olioso A, Demarty J, Van der Tol C, Lagouarde JP. An evaluation of scope: a tool to simulate the directional anisotropy of satellite-measured surface temperatures. Remote Sens Environ. 2015;158:362–375. doi: 10.1016/j.rse.2014.10.019. [DOI] [Google Scholar]
- Ekinzog K, Schlerf M, Kraft M, Werner FAR, Rock G, Mallick K. Revisiting crop water stress index based on potato field experiments in Northern Germany. Agric Water Manag. 2022;269:107664. doi: 10.1016/j.agwat.2022.107664. [DOI] [Google Scholar]
- Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, et al. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon B, Yang A, Lapadat C, Armour I, Juzwik J, Montgomery RA, Cavender-Bares J. Spectral differentiation of oak wilt from foliar fungal disease and drought is correlated with physiological changes. Tree Physiol. 2020;40:377–390. doi: 10.1093/treephys/tpaa005. [DOI] [PubMed] [Google Scholar]
- FAO. Sustainable Food And Home Food And Agriculture - PDF Free Download. 2021. [accessed 25 Feb 2021]. Online. URL: https://zbook.org/sustainable-food-and-home-food-and-agriculture_MjUzODMw.html.
- Fiehn O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp Funct Genom. 2001;2:155. doi: 10.1002/cfg.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JB, Lee B, Purdy AJ, Halverson GH, Dohlen MB, Cawse-Nicholson K, Wang A, Anderson RG, Aragon B, Arain MA, Baldocchi DD, et al. ECOSTRESS: NASA’s next generation mission to measure evapotranspiration from the international space station. Water Resour Res. 2020;56 doi: 10.1029/2019WR026058e2019WR026058. [DOI] [Google Scholar]
- Gago J, Douthe C, Coopman R, Gallego P, Ribas-Carbo M, Flexas J, Escalona J, Medrano H. Uavs challenge to assess water stress for sustainable agriculture. Agric Water Manag. 2015;153:9–19. doi: 10.1016/j.agwat.2015.01.020. URL: https://www.sciencedirect.com/science/article/pii/S0378377415000293. [DOI] [Google Scholar]
- Galieni A, D’Ascenzo N, Stagnari F, Pagnani G, Xie Q, Pisante M. Past and future of plant stress detection: an overview from remote sensing to positron emission tomography. Front Plant Sci. 2021;11 doi: 10.3389/fpls.2020.609155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamon J, Berry J. Facultative and constitutive pigment effects on the photochemical reflectance index (pri) in sun and shade conifer needles. Israel J Plant Sci. 2012;60:85–95. doi: 10.1560/IJPS.60.1-2.85. [DOI] [Google Scholar]
- Gamon J, Surfus J. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999;143:105–117. doi: 10.1046/j.1469-8137.1999.00424.x. [DOI] [Google Scholar]
- Gao BC, Montes MJ, Davis CO, Goetz AF. Atmospheric correction algorithms for hyperspectral remote sensing data of land and ocean. Remote Sens Environ. 2009;113:S17–S24. doi: 10.1016/j.rse.2007.12.015. [DOI] [Google Scholar]
- Garbulsky MF, Peñuelas J, Gamon J, Inoue Y, Filella I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: a review and metaanalysis. Remote Sens Environ. 2011;115:281–297. doi: 10.1016/j.rse.2010.08.023. [DOI] [Google Scholar]
- Gella GW, Bijker W, Belgiu M. Mapping crop types in complex farming areas using SAR imagery with dynamic time warping. ISPRS J Photogramm Remote Sens. 2021;175:171–183. doi: 10.1016/j.isprsjprs.2021.03.004. [DOI] [Google Scholar]
- Gerhards M, Rock G, Schlerf M, Udelhoven T. Water stress detection in potato plants using leaf temperature, emissivity, and reflectance. Int J Appl Earth Obs Geoinf. 2016;53:27–39. doi: 10.1016/j.jag.2016.08.004. [DOI] [Google Scholar]
- Gerhards M, Schlerf M, Rascher U, Udelhoven T, Juszczak R, Alberti G, Miglietta F, Inoue Y. Analysis of airborne optical and thermal imagery for detection of water stress symptoms. Remote Sens. 2018;10:1139. doi: 10.3390/rs10071139. [DOI] [Google Scholar]
- Gerhards M, Schlerf M, Mallick K, Udelhoven T. Challenges and future perspectives of multi—/hyperspectral thermal infrared remote sensing for crop water-stress detection: a review. Remote Sens. 2019;11 doi: 10.3390/rs11101240. URL: https://www.mdpi.com/2072-4292/11/10/1240. [DOI] [Google Scholar]
- Gewali UB, Monteiro ST, Saber E. Machine learning based hyperspectral image analysis: A survey. 2019:arXiv:1802.08701 [Google Scholar]
- Gholizadeh A, Kopackovaé V. Detecting vegetation stress as a soil contamination proxy: a review of optical proximal and remote sensing techniques. Int J Environ Sci Technol. 2019;16:2511–2524. doi: 10.1007/s13762-019-02310-w. [DOI] [Google Scholar]
- Gitelson A. Towards a generic approach to remote non-invasive estimation of foliar carotenoid-to-chlorophyll ratio. J Plant Physiol. 2020;252:153227. doi: 10.1016/j.jplph.2020.153227. arXiv:32683162. [DOI] [PubMed] [Google Scholar]
- Gold KM, Townsend PA, Chlus A, Herrmann I, Couture JJ, Larson ER, Gevens AJ. Hyperspectral measurements enable pre-symptomatic detection and differentiation of contrasting physiological effects of late blight and early blight in potato. Remote Sens. 2020;12:286. doi: 10.3390/rs12020286. [DOI] [Google Scholar]
- Gomiero T, Pimentel D, Paoletti MG. Environmental impact of different agricultural management practices: conventional vs. organic agriculture Crit Rev Plant Sci. 2011;30:95–124. doi: 10.1080/07352689.2011.554355. [DOI] [Google Scholar]
- Gonzalez Toro F, Tsourdos A. [accessed 9. Oct. 2021];UAV Sensors for Environmental Monitoring - CORE. 2021 Online. URL: https://core.ac.uk/display/286407874. [Google Scholar]
- Gonzalez-Dugo V, Zarco-Tejada PJ, Fereres E. Applicability and limitations of using the crop water stress index as an indicator of water deficits in citrus orchards. Agric For Meteorol. 2014:198–199. doi: 10.1016/j.agrformet.2014.08.003. 94-104. [DOI] [Google Scholar]
- Goulas Y, Fournier A, Daumard F, Champagne S, Ounis A, Marloie O, Moya I. Gross primary production of a wheat canopy relates stronger to far red than to red solar-induced chlorophyll fluorescence. Remote Sens. 2017;9:97. doi: 10.3390/rs9010097. [DOI] [Google Scholar]
- Govender M, Govender PJ, Weiersbye IM, Witkowski E, Ahmed F. Review of commonly used remote sensing and ground-based technologies to measure plant water stress. 2009;1:35. doi: 10.4314/wsa.v35i5.49201. [DOI] [Google Scholar]
- Grieves M, Vickers J. Transdisciplinary Perspectives on Complex Systems: New Findings and Approaches. Springer; Cham, Switzerland: 2016. Digital twin: mitigating unpredictable, undesirable emergent behavior in complex systems; pp. 85–113. [DOI] [Google Scholar]
- Guan K, Wu J, Kimball JS, Anderson MC, Frolking S, Li B, Hain CR, Lobell DB. The shared and unique values of optical, fluorescence, thermal and microwave satellite data for estimating large-scale crop yields. Remote Sens Environ. 2017;199:333–349. doi: 10.1016/j.rse.2017.06.043. URL: https://www.sciencedirect.com/science/article/pii/S0034425717303024. [DOI] [Google Scholar]
- Guanter L, Zhang Y, Jung M, Joiner J, Voigt M, Berry JA, Frankenberg C, Huete AR, Zarco-Tejada P, Lee JE, Moran MS, et al. Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc Natl Acad Sci. 2014;111:E1327–E1333. doi: 10.1073/pnas.1320008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guanter L, Kaufmann H, Segl K, Foerster S, Rogass C, Chabrillat S, Kuester T, Hollstein A, Rossner G, Chlebek C, Straif C, et al. The EnMAP spaceborne imaging spectroscopy mission for Earth observation. Remote Sens. 2015;7:8830. doi: 10.3390/rs70708830. [DOI] [Google Scholar]
- Gull A, Lone A, Wani N. Biotic and Abiotic Stresses in Plants. 2019 doi: 10.5772/intechopen.85832. [DOI] [Google Scholar]
- Han M, Zhang H, DeJonge KC, Comas LH, Gleason S. Comparison of three crop water stress index models with sap flow measurements in maize. Agric Water Manag. 2018;203:366–375. doi: 10.1016/j.agwat.2018.02.030. URL: https://linkinghub.elsevier.com/retrieve/pii/S0378377418301252. [DOI] [Google Scholar]
- Hank TB, Bach H, Mauser W. Using a remote sensing-supported hydroagroecological model for field-scale simulation of heterogeneous crop growth and yield: application for wheat in Central Europe. Remote Sens. 2015;7:3934–3965. doi: 10.3390/rs70403934. [DOI] [Google Scholar]
- Hazaymeh K, Hassan QK. A remote sensing-based agricultural drought indicator and its implementation over a semi-arid region, Jordan. J Arid Land. 2017;9:319–330. doi: 10.1007/s40333-017-0014-6. [DOI] [Google Scholar]
- Hendry G, Price A. Stress indicators chlorophylls and carotenoids, methods incomparative plant ecology. 1993 [Google Scholar]
- Hernaández-Clemente R, North PR, Hornero A, Zarco-Tejada PJ. Assessing the effects of forest health on sun-induced chlorophyll fluorescence using the fluorflight 3-d radiative transfer model to account for forest structure. Remote Sens Environ. 2017;193:165–179. doi: 10.1016/j.rse.2017.02.012. [DOI] [Google Scholar]
- Hernaández-Clemente R, Hornero A, Mottus M, Penuelas J, Hernández V, Jiménez JC, Suárez L, Alonso L, Zarco-Tejada PJ. Early diagnosis of vegetation health from high-resolution hyperspectral and thermal imagery: lessons learned from empirical relationships and radiative transfer modelling. Curr Forestry Rep. 2019;5:169–183. doi: 10.1007/s40725-019-00096-1. [DOI] [Google Scholar]
- Herrmann I, Berger K. Remote and proximal assessment of plant traits. Remote Sens. 2021;13:1893. doi: 10.3390/rs13101893. [DOI] [Google Scholar]
- Herrmann I, Karnieli A, Bonfil DJ, Cohen Y, Alchanatis V. SWIR-based spectral indices for assessing nitrogen content in potato fields. Int J Remote Sens. 2010;31:5127–5143. doi: 10.1080/01431160903283892. [DOI] [Google Scholar]
- Herrmann I, Berenstein M, Paz-Kagan T, Sade A, Karnieli A. Spectral assessment of two-spotted spider mite damage levels in the leaves of greenhouse-grown pepper and bean. Biosyst Eng. 2017;157:72–85. doi: 10.1016/j.biosystemseng.2017.02.008. [DOI] [Google Scholar]
- Herrmann I, Vosberg SK, Ravindran P, Singh A, Chang HX, Chil-vers MI, Conley SP, Townsend PA. Leaf and canopy level detection of fusarium virguliforme (sudden death syndrome) in soybean. Remote Sens. 2018;10:426. doi: 10.3390/rs10030426. [DOI] [Google Scholar]
- Herrmann I, Bdolach E, Montekyo Y, Rachmilevitch S, Townsend PA, Karnieli A. Assessment of maize yield and phenology by drone-mounted superspectral camera. Precis Agric. 2020;21:51–76. doi: 10.1007/s11119-019-09659-5. [DOI] [Google Scholar]
- Hoffmann H, Jensen R, Thomsen A, Nieto H, Rasmussen J, Friborg T. Crop water stress maps for an entire growing season from visible and thermal UAV imagery. Biogeosciences. 2016;13:6545–6563. doi: 10.5194/bg-13-6545-2016. URL: https://bg.copernicus.org/articles/13/6545/2016/ [DOI] [Google Scholar]
- Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant vocs. Trends Plant Sci. 2010;15:176–184. doi: 10.1016/j.tplants.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Houborg R, Fisher J, Skidmore A. Advances in remote sensing of vegetation function and traits. Int J Appl Earth Obs Geoinf. 2015;43:1–6. doi: 10.1016/j.jag.2015.06.001. [DOI] [Google Scholar]
- Huang J, Gómez-Dans JL, Huang H, Ma H, Wu Q, Lewis PE, Liang S, Chen Z, Xue JH, Wu Y, Zhao F, et al. Assimilation of remote sensing into crop growth models: current status and perspectives. Agric For Meteorol. 2019:276–277.:107609. doi: 10.1016/j.agrformet.2019.06.008. [DOI] [Google Scholar]
- Idso SB, Jackson RD, Pinter PJ, Reginato RJ, Hatfield JL. Normalizing the stress-degree-day parameter for environmental variability. Agric Meteorol. 1981;24:45–55. doi: 10.1016/0002-1571(81)90032-7. [DOI] [Google Scholar]
- Ihuoma SO, Madramootoo CA. Recent advances in crop water stress detection. Comput Electron Agric. 2017;141:267–275. doi: 10.1016/j.compag.2017.07.026. [DOI] [Google Scholar]
- Inoue Y, Darvishzadeh R, Skidmore A. Biophysical and Biochemical Characterization and Plant Species Studies. CRC Press; Boca Raton, FL, USA: 2018. Hyperspectral assessment of ecophysiological functioning for diagnostics of crops and vegetation; pp. 25–71. [DOI] [Google Scholar]
- Jackson RD, Idso SB, Reginato RJ, Pinter PJ. Canopy temperature as a crop water stress indicator. Water Resour Res. 1981;17:1133–1138. doi: 10.1029/wr017i004p01133. [DOI] [Google Scholar]
- Jackson RD, Kustas WP, Choudhury BJ. A reexamination of the crop water stress index. Irrig Sci. 1988;9:309–317. doi: 10.1007/BF00296705. [DOI] [Google Scholar]
- Jahns P, Holzwarth AR. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta (BBA) - Bioenergetics. 2012;1817:182–193. doi: 10.1016/j.bbabio.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Jain A, Sarsaiya S, Wu Q, Lu Y, Shi J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered. 2019;10:409–424. doi: 10.1080/21655979.2019.1649520. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jiao W, Wang L, McCabe MF. Multi-sensor remote sensing for drought characterization: current status, opportunities and a roadmap for the future. Remote Sens Environ. 2021;256:112313. doi: 10.1016/j.rse.2021.112313. [DOI] [Google Scholar]
- Jin X, Kumar L, Li Z, Feng H, Xu X, Yang G, Wang J. A review of data assimilation of remote sensing and crop models. Eur J Agron. 2018;92:141–152. doi: 10.1016/j.eja.2017.11.002. [DOI] [Google Scholar]
- Joalland S, Screpanti C, Varella HV, Reuther M, Schwind M, Lang C, Walter A, Liebisch F. Aerial and ground based sensing of tolerance to beet cyst nematode in sugar beet. Remote Sens. 2018;10:787. doi: 10.3390/rs10050787. [DOI] [Google Scholar]
- Jonard F, De Cannière S, Brüggemann N, Gentine P, Gianotti DJS, Lobet G, Miralles DG, Montzka C, Pagán BR, Rascher U, Vereecken H. Value of sun-induced chlorophyll fluorescence for quantifying hydrological states and fluxes: current status and challenges. Agric For Meteorol. 2020;291:108088. doi: 10.1016/j.agrformet.2020.108088. [DOI] [Google Scholar]
- Jones HG, Jones MB. Plants under stress. Introduction: some terminology and common mechanisms ciety for Experime. 1989 URL: https://agris.fao.org/agris-search/search.do?recordID=US201302682914. [Google Scholar]
- Jones H, Vaughan R. Remote Sensing of Vegetation: Principles, Techniques, and Applications. OUP Oxford; 2010. URL: https://books.google.it/books?id=sTmcAQAAQBAJ. [Google Scholar]
- Jones JW, Hoogenboom G, Porter CH, Boote KJ, Batchelor WD, Hunt LA, Wilkens PW, Singh U, Gijsman AJ, Ritchie JT. The DSSAT cropping system model. Eur J Agron. 2003;18:235–265. doi: 10.1016/S1161-0301(02)00107-7. [DOI] [Google Scholar]
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420:829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- Kessler A. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Kim JY, Glenn DM. Multi-modal sensor system for plant water stress assessment. Comput Electron Agric. 2017;141:27–34. doi: 10.1016/j.compag.2017.07.009. [DOI] [Google Scholar]
- Knipling EB. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens Environ. 1970;1:155–159. doi: 10.1016/S0034-4257(70)80021-9. [DOI] [Google Scholar]
- Knipper KR, Kustas WP, Anderson MC, Alsina MM, Hain CR, Alfieri JG, Prueger JH, Gao F, McKee LG, Sanchez LA. Using high-spatiotemporal thermal satellite ET retrievals for operational water use and stress monitoring in a California vineyard. Remote Sens. 2019;11 doi: 10.3390/rs11182124. [DOI] [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010;188:655–673. doi: 10.1111/j.1469-8137.2010.03461.x. URL: [DOI] [PubMed] [Google Scholar]
- Larcher W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups. Springer-Verlag; Berlin Heidelberg: 2003. URL: https://www.springer.com/gp/book/9783540435167. [DOI] [Google Scholar]
- Lassalle G. Monitoring natural and anthropogenic plant stressors by hyperspectral remote sensing: recommendations and guidelines based on a metareview. Sci Total Environ. 2021;788:147758. doi: 10.1016/j.scitotenv.2021.147758. [DOI] [PubMed] [Google Scholar]
- Lebourgeois V, Bégué A, Labbé S, Houlès M, Martín JF. A light-weight multi-spectral aerial imaging system for nitrogen crop monitoring. Precis Agric. 2012;13:525–541. doi: 10.1007/s11119-012-9262-9. [DOI] [Google Scholar]
- Li ZL, Tang BH, Wu H, Ren H, Yan G, Wan Z, Trigo IF, Sobrino JA. Satellite-derived land surface temperature: Current status and perspectives. Remote Sens Environ. 2013;131:14–37. doi: 10.1016/j.rse.2012.12.008. [DOI] [Google Scholar]
- Lichtenthaler HK. Vegetation stress: an introduction to the stress concept in plants. J Plant Physiol. 1996;148:4–14. doi: 10.1016/S0176-1617(96)80287-2. [DOI] [Google Scholar]
- Lichtenthaler HK, Wenzel O, Buschmann C, Gitelson A. Plant stress by reflectance and fluorescence. Ann N Y Acad Sci. 1998;851:271–285. doi: 10.1111/j.1749-6632.1998.tb09002.x. URL: [DOI] [Google Scholar]
- Linke R, Richter K, Haumann J, Schneider W, Weihs P. Occurrence of repeated drought events: can repetitive stress situations and recovery from drought be traced with leaf reflectance? Period Biol. 2008;110:219–229. URL: https://hrcak.srce.hr/32569. [Google Scholar]
- Liu J, Wang T, Skidmore AK, Jones S, Heurich M, Beudert B, Premier J. Comparison of terrestrial lidar and digital hemispherical photography for estimating leaf angle distribution in european broadleaf beech forests. ISPRS J Photogramm Remote Sens. 2019;158:76–89. doi: 10.3390/rs13163325. [DOI] [Google Scholar]
- Liu X, Liu L, Hu J, Guo J, Du S. Improving the potential of red sif for estimating gpp by downscaling from the canopy level to the photosystem level. Agric For Meteorol. 2020;281:107846. doi: 10.1016/j.agrformet.2019.107846. URL: https://www.sciencedirect.com/science/article/pii/S0168192319304629. [DOI] [Google Scholar]
- Llanes A, Andrade A, Alemano S, Luna V. Plant Metabolites and Regulation Under Environmental Stress. Academic Press; Cambridge, MA, USA: 2018. Metabolomic approach to understand plant adaptations to water and salt stress; pp. 133–144. [DOI] [Google Scholar]
- Loizzo R, Daraio M, Guarini R, Longo F, Lorusso R, Dini L, Lopinto E. Prisma mission status and perspective; IGARSS 2019–2019 IEEE International Geoscience and Remote Sensing Symposium; 2019. pp. 4503–4506. [DOI] [Google Scholar]
- Lombardozzi DL, Lu Y, Lawrence PJ, Lawrence DM, Swenson S, Oleson KW, Wieder WR, Ainsworth EA. Simulating agriculture in the community landmodel version 5. J Geophys Res Biogeosci. 2020;125:e2019JG005529 [Google Scholar]
- Machwitz M, Haß E, Junk J, Udelhoven T, Schlerf M. CropGIS – A web application for the spatial and temporal visualization of past, present and future crop biomass development. Comput Electron Agric. 2018;161 doi: 10.1016/j.compag.2018.04.026. [DOI] [Google Scholar]
- Machwitz M, Pieruschka R, Berger K, Schlerf M, Aasen H, Fahrner S, Jiménez-Berni J, Baret F, Rascher U. Bridging the gap between remote sensing and plant phenotyping—challenges and opportunities for the next generation of sustainable agriculture. Front Plant Sci. 2021;0 doi: 10.3389/fpls.2021.749374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani B, Mirshekari A, Imahori Y. In: Postharvest Physiology and Biochemistry of Fruits and Vegetables. Yahia EM, editor. Woodhead Publishing; 2019. Chapter 19 - Physiological responses to stress; pp. 405–423. URL: https://www.sciencedirect.com/science/article/pii/B9780128132784000208. [DOI] [Google Scholar]
- Madec S, Baret F, de Solan B, Thomas S, Dutartre D, Jezequel S, Hemmerleé M, Colombeau G, Comar A. High-throughput phenotyping of plant height: comparing unmanned aerial vehicles and ground LiDAR estimates. Front Plant Sci. 2017;8 doi: 10.3389/fpls.2017.02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes WH, Steppe K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: a review. J Exp Bot. 2012;63:4671–4712. doi: 10.1093/jxb/ers165. [DOI] [PubMed] [Google Scholar]
- Maes W, Steppe K. Perspectives for remote sensing with unmanned aerial vehicles in precision agriculture. Trends Plant Sci. 2019;24:152–164. doi: 10.1016/j.tplants.2018.11.007. URL: https://biblio.ugent.be/publication/8642827. [DOI] [PubMed] [Google Scholar]
- Magney TS, Frankenberg C, Köhler P, North G, Davis TS, Dold C, Dutta D, Fisher JB, Grossmann K, Harrington A, Hatfield J, et al. Disentangling changes in the spectral shape of chlorophyll fluorescence: implications for remote sensing of photosynthesis. J Geophys Res Biogeosci. 2019;124:1491–1507. doi: 10.1029/2019JG005029. [DOI] [Google Scholar]
- Mahajan GR, Das B, Murgaokar D, Herrmann I, Berger K, Sahoo RN, Patel K, Desai A, Morajkar S, Kulkarni RM. Monitoring the foliar nutrients status of mango using spectroscopy-based spectral indices and PLSR-combined machine learning models. Remote Sens. 2021;13:641. doi: 10.3390/rs13040641. [DOI] [Google Scholar]
- Mahlein AK. Plant Disease Detection by Imaging Sensors – Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2016 doi: 10.1094/PDIS-03-15-0340-FE. [DOI] [PubMed] [Google Scholar]
- Mahlein AK, Alisaac E, Al Masri A, Behmann J, Dehne HW, Oerke EC. Comparison and combination of thermal, fluorescence, and hyperspectral imaging for monitoring fusarium head blight of wheat on Spikelet scale. Sensors. 2019;19:2281. doi: 10.3390/s19102281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenovský Z, Rott H, Cihlar J, Schaepman M, Garcia J, C.A-Santos G, Fernandes R, Berger M. Sentinels for science: potential of Sentinel-1, — 2,and — 3 missions for scientific observations of ocean, cryosphere, and land. RemoteSens Environ. 2012;120:91–101. [Google Scholar]
- McNally A, Arsenault K, Kumar S, Shukla S, Peterson P, Wang S, Funk C, Peters-Lidard CD, Verdin JP. A land data assimilation system for sub-Saharan Africa food and water security applications. Scientific Data. 2017;4 doi: 10.1038/sdata.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerdink S, Roberts D, Hulley G, Gader P, Pisek J, Adamson K, King J, Hook SJ. Plant species’spectral emissivity and temperature using the hyperspectral thermal emission spectrometer (HyTES) sensor. Remote Sens Environ. 2019;224:421–435. doi: 10.1016/j.rse.2019.02.009. [DOI] [Google Scholar]
- Meroni M, Rossini M, Guanter L, Alonso L, Rascher U, Colombo R, Moreno J. Remote sensing of solar-induced chlorophyll fluorescence: review of methods and applications. Remote Sens Environ. 2009;113:2037–2051. doi: 10.1016/j.rse.2009.05.003. URL: http://linkinghub.elsevier.com/retrieve/pii/S003442570900162X http://www.sciencedirect.com/science/article/pii/S003442570900162X . [DOI] [Google Scholar]
- Messina G, Modica G. Applications of UAV thermal imagery in precision agriculture: state of the art and future research outlook. Remote Sens. 2020;12:1491. doi: 10.3390/rs12091491. [DOI] [Google Scholar]
- Meza-Canales ID, Meldau S, Zavala JA, Baldwin IT. Herbivore perception decreases photosynthetic carbon assimilation and reduces stomatal conductance by engaging 12-oxo-phytodienoic acid, mitogen- activated protein kinase 4 and cytokinin perception. Plant Cell Environ. 2017;40:1039–1056. doi: 10.1111/pce.12874. [DOI] [PubMed] [Google Scholar]
- Migliavacca M, Perez-Priego O, Rossini M, El-Madany TS, Moreno G, Van der Tol C, Rascher U, Berninger A, Bessenbacher V, Burkart A, Carrara A, et al. Plant functional traits and canopy structure control the relationship between photosynthetic co2 uptake and far-red sun-induced fluorescence in a mediterranean grassland under different nutrient availability. New Phytol. 2017;214:1078–1091. doi: 10.1111/nph.14437. URL: [DOI] [PubMed] [Google Scholar]
- Mladenova IE, Bolten JD, Crow WT, Anderson MC, Hain CR, Johnson DM, Mueller R. Intercomparison of soil moisture, evaporative stress, and vegetation indices for estimating corn and soybean yields over the US. IEEE J Select Top Appl Earth Observ Remote Sens. 2017;10:1328–1343. doi: 10.1109/JSTARS.2016.2639338. [DOI] [Google Scholar]
- Mohammed GH, Colombo R, Middleton EM, Rascher U, Van der Tol C, Nedbal L, Goulas Y, Péerez-Priego O, Damm A, Meroni M, Joiner J, et al. Remote sensing of solar-induced chlorophyll fluorescence (sif) in vegetation: 50 years of progress. Remote Sens Environ. 2019;231 doi: 10.1016/j.rse.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncholi-Estornell A, Van Wittenberghe S, Cendrero-Mateo MP, Alonso L, Malenovský Z, Moreno J. Impact of structural, photochemical and instrumental effects on leaf and canopy reflectance variability in the 500–600 nm range. Remote Sens. 2022;14:56–70. doi: 10.3390/rs14010056. [DOI] [Google Scholar]
- Moran MS, Clarke TR, Inoue Y, Vidal A. Estimating crop water deficit using the relation between surface-air temperature and spectral vegetation index. Remote Sens Environ. 1994;49:246–263. doi: 10.1016/0034-4257(94)90020-5. [DOI] [Google Scholar]
- Moshou D, Pantazi XE, Kateris D, Gravalos I. Water stress detection based on optical multisensor fusion with a least squares support vector machine classifier. Biosyst Eng. 2014;117(15–22) doi: 10.1016/j.biosystemseng.2013.07.008. 4th International Workshop on Computer Image Analysis in Agriculture, held at CIGR- AgEng, Valencia, SPAIN, JUL 08–12, 2012. [DOI] [Google Scholar]
- Moulin S, Bondeau A, Delecolle R. Combining agricultural crop models and satellite observations: From field to regional scales. Int J Remote Sens. 1998;19:1021–1036. doi: 10.1080/014311698215586. [DOI] [Google Scholar]
- Mousivand A, Menenti M, Gorte B, Verhoef W. Multi-temporal, multi-sensorretrieval of terrestrial vegetation properties from spectral- directional radiometricdata. Remote Sens Environ. 2015;158:311–330. [Google Scholar]
- Mueller ND, Gerber JS, Johnston M, Ray DK, Ramankutty N, Foley JA. Closing yield gaps through nutrient and water management. Nature. 2012;490:254–257. doi: 10.1038/nature11420. [DOI] [PubMed] [Google Scholar]
- Navarro A, Rolim J, Miguel I, Catalao J, Silva J, Painho M, Vekerdy Z. Crop monitoring based on spot-5 take-5 and sentinel-1a data for the estimation ofcrop water requirements. Remote Sens. 2016;8:525. [Google Scholar]
- Neinavaz E, Darvishzadeh R, Skidmore AK, Groen TA. Measuring the response of canopy emissivity spectra to leaf area index variation using thermal hyperspectral data. Int J Appl Earth Obs Geoinf. 2016;53:40–47. doi: 10.1016/j.jag.2016.08.002. [DOI] [Google Scholar]
- Neupane K, Baysal-Gurel F. Automatic identification and monitoring of plant diseases using unmanned aerial vehicles: a review. Remote Sens. 2021;13:3841. doi: 10.3390/rs13193841. [DOI] [Google Scholar]
- Ni Z, Liu Z, Huo H, Li ZL, Nerry F, Wang Q, Li X. Early water stress detection using leaf-level measurements of chlorophyll fluorescence and temperature data. Remote Sens. 2015;7:3232–3249. doi: 10.3390/rs70303232. URL: https://www.mdpi.com/2072-4292/7/3/3232. [DOI] [Google Scholar]
- Norman JM, Becker F. Terminology in thermal infrared remote sensing of natural surfaces. Agric. For. Meteorol. 1995;77:153–166. doi: 10.1016/0168-1923(95)02259-Z. [DOI] [Google Scholar]
- Pacheco-Labrador J, Hueni A, Mihai L, Sakowska K, Julitta T, Kuusk J, Sporea D, Alonso L, Burkart A, Cendrero-Mateo MP, Aasen H, et al. Sun-Induced chlorophyll fluorescence I: instrumental considerations for proximal spectro- radiometers. Remote Sens. 2019a;11:960. doi: 10.3390/rs11080960. [DOI] [Google Scholar]
- Pacheco-Labrador J, Perez-Priego O, El-Madany TS, Julitta T, Rossini M, Guan J, Moreno G, Carvalhais N, Martín MP, Gonzalez-Cascon R, Kolle O, et al. Multiple-constraint inversion of SCOPE. Evaluating the potential of GPP and SIF for the retrieval of plant functional traits. Remote Sens Environ. 2019b;234:111362. doi: 10.1016/j.rse.2019.111362. URL: https://linkinghub.elsevier.com/retrieve/pii/S0034425719303815. [DOI] [Google Scholar]
- Pacheco-Labrador J, El-Madany TS, Van der Tol C, Martin MP, Gonzalez-Cascon R, Perez-Priego O, Guan J, Moreno G, Carrara A, Reichstein M, Migliavacca M. senSCOPE: Modeling mixed canopies combining green and brown senesced leaves. Evaluation in a Mediterranean Grassland Remote Sens Environ. 2021;257:112352. doi: 10.1016/j.rse.2021.112352. [DOI] [Google Scholar]
- Panigada C, Rossini M, Meroni M, Cilia C, Busetto L, Amaducci S, Boschetti M, Cogliati S, Picchi V, Pinto F, Marchesi A, et al. Fluorescence, PRI and canopy temperature for water stress detection in cereal crops. Int J Appl Earth Obs Geoinf. 2014;30:167–178. doi: 10.1016/j.jag.2014.02.002. [DOI] [Google Scholar]
- Parkash V, Singh S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability. 2020;12 doi: 10.3390/su12103945. URL: https://www.mdpi.com/2071-1050/12/10/3945. [DOI] [Google Scholar]
- Peng B, Guan K, Chen M, Lawrence DM, Pokhrel Y, Suyker A, Arkebauer T, Lu Y. Improving maize growth processes in the community land model: Implementation and evaluation. Agric For Meteorol. 2018;250-251:64–89. doi: 10.1016/j.agrformet.2017.11.012. URL: https://www.sciencedirect.com/science/article/pii/S0168192317303854. [DOI] [Google Scholar]
- Penuelas J, Gamon JA, Fredeen AL, Merino J, Field CB. Reflectanceindices associated with physiological changes in nitrogen- and water-limitedsunflower leaves. Remote Sens Environ. 1994;48:135–146. [Google Scholar]
- Pérez-Bueno ML, Pineda M, Díaz-Casado E, Baron M. Spatial and temporal dynamics of primary and secondary metabolism in phaseolus vulgaris challenged by pseudomonas syringae. Physiol Plant. 2015;153:161–174. doi: 10.1111/ppl.12237. [DOI] [PubMed] [Google Scholar]
- Peters-Lidard CD, Mocko DM, Su L, Lettenmaier DP, Gentine P, Barlage M. Advances in land surface models and indicators for drought monitoring and prediction. Bull Am Meteorol Soc. 2021;102:E1099–E1122. doi: 10.1175/BAMS-D-20-0087.1. URL: https://journals.ametsoc.org/view/journals/bams/102/5/BAMS-D-20-0087.1.xml. [DOI] [Google Scholar]
- Pineda M, Barón M, Pérez-Bueno ML. Thermal imaging for plant stress detection and phenotyping. Remote Sens. 2020;13:68. doi: 10.3390/rs13010068. [DOI] [Google Scholar]
- Poblete T, Navas-Cortes JA, Camino C, Calderon R, Hornero A, Gonzalez-Dugo V, Landa BB, Zarco-Tejada PJ. Discriminating Xylella fastidiosa from verticillium dahliae infections in olive trees using thermal- and hyperspectral-based plant traits. ISPRS J Photogramm Remote Sens. 2021;179:133–144. doi: 10.1016/j.isprsjprs.2021.07.014. [DOI] [Google Scholar]
- Rast M, Nieke J, Adams J, Isola C, Gascon F. Copernicus Hyperspectral Imaging Mission for the Environment (Chime); 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS; 2021. pp. 108–111. [DOI] [Google Scholar]
- Reichstein M, Camps-Valls G, Stevens B, Jung M, Denzler J, Carvalhais N, Prabhat Deep learning and process understanding for data-driven earthsystem science. Nature. 2019;566:195–204. doi: 10.1038/s41586-019-0912-1. [DOI] [PubMed] [Google Scholar]
- Rembold F, Carnicelli S, Nori M, Ferrari GA. Use of aerial photographs, Landsat TM imagery and multidisciplinary field survey for land-cover change analysis in the lakes region (Ethiopia) Int J Appl Earth Obs Geoinf. 2000;2:181–189. doi: 10.1016/S0303-2434(00)85012-6. [DOI] [Google Scholar]
- Ribeiro da Luz B, Crowley JK. Spectral reflectance and emissivity features of broad leaf plants: Prospects for remote sensing in the thermal infrared (8.0–14.0 μm) Remote Sens Environ. 2007;109:393–405. doi: 10.1016/j.rse.2007.01.008. [DOI] [Google Scholar]
- Ribeiro da Luz B, Crowley JK. Identification of plant species by using high spatial and spectral resolution thermal infrared (8.0–13.5μm) imagery. Remote Sens Environ. 2010;114:404–413. doi: 10.1016/j.rse.2009.09.019. [DOI] [Google Scholar]
- Richter K, Rischbeck P, Eitzinger J, Schneider W, Suppan F, Weihs P. Plant growth monitoring and potential drought risk assessment by means of Earth observation data. Int J Remote Sens. 2008;29:4943–4960. doi: 10.1080/01431160802036268. [DOI] [Google Scholar]
- Rud R, Cohen Y, Alchanatis V, Levi A, Brikman R, Shenderey C, Heuer B, Markovitch T, Dar Z, Rosen C, Mulla D, et al. Crop water stress index derived from multi-year ground and aerial thermal images as an indicator of potato water status. Precis Agric. 2014;15:273–289. doi: 10.1007/s11119-014-9351-z. [DOI] [Google Scholar]
- Sagan V, Maimaitijiang M, Sidike P, Maimaitiyiming M, Erkbol H, Hartling S, Peterson KT, Peterson J, Burken J, Fritschi F. UAV/SATELLITE Multiscale DATA Fusion for Crop Monitoring and Early Stress Detection. International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences XLII-2-W13. 2019:715–722. doi: 10.5194/isprs-archives-XLII-2-W13-715-2019. [DOI] [Google Scholar]
- Salisbury JW. Preliminary measurements of leaf spectral reflectance in the 8–14 μmregion. Int J Remote Sens. 1986;7:1879–1886. doi: 10.1080/01431168608948981. [DOI] [Google Scholar]
- Sarto MVM, Sarto JRW, Rampim L, Rosset JS, Bassegio D, Costa PFd, Inagaki AM. Wheat phenology and yield under drought: a review. Aust J Crop Sci. 2017;11:941–946. URL: https://www.cabdirect.org/cabdirect/abstract/20183392190. [Google Scholar]
- Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- Savian F, Martini M, Ermacora P, Paulus S, Mahlein AK. Prediction of the kiwifruit decline syndrome in diseased orchards by remote sensing. Remote Sens. 2020;12 doi: 10.3390/rs12142194. URL: https://www.mdpi.com/2072-4292/12/14/2194. [DOI] [Google Scholar]
- Schlerf M, Rock G, Lagueux P, Ronellenfitsch F, Gerhards M, Hoffmann L, Udelhoven T. A hyperspectral thermal infrared imaging instrument for natural resources applications. Remote Sens. 2012;4:3995–4009. doi: 10.3390/rs4123995. [DOI] [Google Scholar]
- Seelig HD, Hoehn A, Stodieck LS, Klaus DM, Adams WW, Emery WJ. The assessment of leaf water content using leaf reflectance ratios in the visible, near-, and short-wave-infrared. Int J Remote Sens. 2008;29:3701–3713. doi: 10.1080/01431160701772500. [DOI] [Google Scholar]
- Singh A, Ganapathysubramanian B, Singh AK, Sarkar S. Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci. 2016;21:110–124. doi: 10.1016/j.tplants.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Singh S, Fatima A, Tiwari S, Prasad SM. Plant Life Under Changing Environment. Academic Press; Cambridge, MA, USA: 2020. Plant responses to radiation stress and its adaptive mechanisms; pp. 105–122. [DOI] [Google Scholar]
- Sishodia RP, Ray RL, Singh SK. Applications of remote sensing in precision agriculture: A review. Remote Sens. 2020;12 doi: 10.3390/rs12193136. URL: https://www.mdpi.com/2072-4292/12/19/3136. [DOI] [Google Scholar]
- Sobejano-Paz V, Mikkelsen TN, Baum A, Mo X, Liu S, Köppl CJ, Johnson MS, Gulyas L, García M. Hyperspectral and thermal sensing of stomatal conductance, transpiration, and photosynthesis for soybean and maize under drought. Remote Sens. 2020;12:3182. doi: 10.3390/rs12193182. [DOI] [Google Scholar]
- Sonobe R, Yamashita H, Mihara H, Morita A, Ikka T. Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens. 2020;12:3265. doi: 10.3390/rs12193265. [DOI] [Google Scholar]
- Spišić J, Šimić D, Balen J, Jambrović A, Galić V. Machine learning in the analysis of multispectral reads in maize canopies responding to increased temperatures and water deficit. Remote Sens. 2022;14:2596. doi: 10.3390/rs14112596. [DOI] [Google Scholar]
- St-Onge B, Vega C, Fournier RA, Hu Y. Mapping canopy height using a combination of digital stereo-photogrammetry and lidar. Int J Remote Sens. 2008;29:3343–3364. doi: 10.1080/01431160701469040. [DOI] [Google Scholar]
- Suarez L, González-Dugo V, Camino C, Hornero A, Zarco-Tejada PJ. Physical model inversion of the green spectral region to track assimilation rate inalmond trees with an airborne nano-hyperspectral imager. Remote Sens Environ. 2021;252:112147 [Google Scholar]
- Sulis M, Langensiepen M, Shrestha P, Schickling A, Simmer C, Kollet SJ. Evaluating the influence of plant-specific physiological parameterizations on the partitioning of land surface energy fluxes. J Hydrometeorol. 2015;16:517–533. doi: 10.1175/JHM-D-14-0153.1. URL: https://journals.ametsoc.org/view/journals/hydr/16/2/jhm-d-14-0153_1.xml. [DOI] [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000;5:537–542. doi: 10.1016/S1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Taghvaeian S, Comas L, DeJonge KC, Trout TJ. Conventional and simplified canopy temperature indices predict water stress in sunflower. Agric Water Manag. 2014;144:69–80. doi: 10.1016/j.agwat.2014.06.003. URL: https://linkinghub.elsevier.com/retrieve/pii/S0378377414001796. [DOI] [Google Scholar]
- Tester M, Langridge P. Breeding technologies to increase crop production in achanging world. Science. 2010;327:818–822. doi: 10.1126/science.1183700. [DOI] [PubMed] [Google Scholar]
- Tewari S, Mishra A. Plant Metabolites and Regulation Under Environmental Stress. Academic Press; Cambridge, MA, USA: 2018. Flooding stress in plants and approaches to overcome; pp. 355–366. [DOI] [Google Scholar]
- Thomas S, Wahabzada M, Kuska MT, Rascher U, Mahlein AK. Observation of plant-pathogen interaction by simultaneous hyperspectral imaging reflection and transmission measurements. Funct Plant Biol. 2017;44:23–34. doi: 10.1071/FP16127. [DOI] [PubMed] [Google Scholar]
- Timmermans WJ, Van der Tol C, Timmermans J, Ucer M, Chen X, Alonso L, Moreno J, Carrara A, Lopez R, de la Cruz Tercero F, Corcoles HL, et al. An overview of the regional experiments for landatmosphere exchanges 2012 (REFLEX 2012) campaign. Acta Geophys. 2015;63:1465–1484. doi: 10.2478/s11600-014-0254-1. [DOI] [Google Scholar]
- Tolomio M, Casa R. Dynamic crop models and remote sensing irrigation decision support systems: a review of water stress concepts for improved estimation of water requirements. Remote Sens. 2020;12:3945. doi: 10.3390/rs12233945. [DOI] [Google Scholar]
- UN. World Population Prospects: The 2017 Revision | Multimedia Library - United Nations Department of Economic and Social Affairs. 2017. [accessed 13. Jun. 2022]. Online. URL: https://www.un.org/development/desa/publications/world-population-prospects-the-2017-revision.html.
- Van der Tol C, Verhoef W, Timmermans J, Verhoef A, Su Z. An integrated model of soil-canopy spectral radiances, photosynthesis, fluorescence, temperature and energy balance. Bio geo sciences. 2009;6:31093129. doi: 10.5194/bg-6-3109-2009. [DOI] [Google Scholar]
- Van der Tol C, Rossini M, Cogliati S, Verhoef W, Colombo R, Rascher U, Mohammed G. A model and measurement comparison of diurnal cycles of sun-induced chlorophyll fluorescence of crops. Remote Sens Environ. 2016;186:663–677. doi: 10.1016/j.rse.2016.09.021. URL: https://www.sciencedirect.com/science/article/pii/S0034425716303649. [DOI] [Google Scholar]
- van Diepen CA, Wolf J, van Keulen H, Rappoldt C. WOFOST: a simulation model of crop production. Soil Use Manag. 1989;5:16–24. doi: 10.1111/j.1475-2743.1989.tb00755.x. [DOI] [Google Scholar]
- Van Wittenberghe S, Alonso L, Malenovsky Z, Moreno J. In vivo photo protection mechanisms observed from leaf spectral absorbance changesshowing vis-nir slow-induced conformational pigment bed changes. Photosynth Res. 2019;142:283–305. doi: 10.1007/s11120-019-00664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wittenberghe S, Laparra V, Garcia-Plazaola J, Fernandez-Marin B, Moreno J. Combined dynamics of the 500–600 nm leaf absorption and chlorophyllfluorescence changes in vivo: evidence for the multifunctional energy quenching roleof xanthophylls. Biochim Biophys Acta Bioenerg. 2021;1862:148351. doi: 10.1016/j.bbabio.2020.148351. [DOI] [PubMed] [Google Scholar]
- Vaughan RG, Calvin WM, Taranik JV. SEBASS hyperspectral thermal infrared data: surface emissivity measurement and mineral mapping. Remote Sens Environ. 2003;85:48–63. doi: 10.1016/S0034-4257(02)00186-4. [DOI] [Google Scholar]
- Verdouw C, Tekinerdogan B, Beulens A, Wolfert S. Digital twins in smart farming. Agric Syst. 2021;189:103046. doi: 10.1016/j.agsy.2020.103046. [DOI] [Google Scholar]
- Vergara-Diaz O, Kefauver SC, Elazab A, Nieto-Taladriz MT, Araus JL. Grain yield losses in yellow-rusted durum wheat estimated using digital and conventional parameters under field conditions. Crop J. 2015;3:200–210. doi: 10.1016/j.cj.2015.03.003. URL: https://www.sciencedirect.com/science/article/pii/S2214514115000355. special Issue: Breeding to Optimize Agriculture in a Changing World. [DOI] [Google Scholar]
- Verrelst J, Camps Valls G, Munoz-Marí J, Rivera J, Veroustraete F, Clevers J, Moreno J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties - a review. ISPRS J Photogramm Remote Sens. 2015;108:273–290. [Google Scholar]
- Verrelst J, Malenovský Z, Van der Tol C, Camps-Valls G, Gastellu-Etchegorry JP, Lewis P, North P, Moreno J. Quantifying vegetation biophysical variables from imaging spectroscopy data: a review on retrieval methods. Surv Geophys. 2019a;40:589–629. doi: 10.1007/s10712-018-9478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrelst J, Vicent J, Rivera-Caicedo JP, Lumbierres M, Morcillo-Pallarés P, Moreno J. Global sensitivity analysis of leaf-canopy-atmosphere RTMs:implications for biophysical variables retrieval from top-of-atmosphere radiancedata. Remote Sens. 2019b;11 doi: 10.3390/rs11161923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrelst J, Rivera-Caicedo JP, Reyes-Muñoz P, Morata M, Amin E, Tagliabue G, Panigada C, Hank T, Berger K. Mapping landscape canopy nitrogen content from space using PRISMA data. ISPRS J Photogramm Remote Sens. 2021;178:382–395. doi: 10.1016/j.isprsjprs.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilfan N, Van der Tol C, Yang P, Wyber R, Malenovský Z, Robinson SA, Verhoef W. Extending fluspect to simulate xanthophyll driven leaf reflectance dynamics. Remote Sens Environ. 2018;211:345–356. [Google Scholar]
- Virnodkar SS, Pachghare VK, Patil VC, Jha SK. Remote sensing andmachine learning for crop water stress determination in various crops: a criticalreview. Precis Agric. 2020;21:1121–1155. [Google Scholar]
- Weiss M, Jacob F, Duveiller G. Remote sensing for agricultural applications: ameta-review. Remote Sens Environ. 2020;236:111402 [Google Scholar]
- Yadav SK. Cold stress tolerance mechanisms in plants. A review Agron Sustain Dev. 2010;30:515–527. doi: 10.1051/agro/2009050. [DOI] [Google Scholar]
- Yang P, van der Tol C. Linking canopy scattering of far-red sun- induced chlorophyll fluorescence with reflectance. Remote Sens Environ. 2018;209:456–467. doi: 10.1016/j.rse.2018.02.029. [DOI] [Google Scholar]
- Yang G, Liu J, Zhao C, Li Z, Huang Y, Yu H, Xu B, Yang X, Zhu D, Zhang X, Zhang R, et al. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: current status and perspectives. Front Plant Sci. 2017a;8:1111. doi: 10.3389/fpls.2017.01111. arXiv:28713402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Verhoef W, van der Tol C. The mSCOPE model: a simple adaptation to the SCOPE model to describe reflectance, fluorescence and photosynthesis of vertically heterogeneous canopies. Remote Sens Environ. 2017b;201:1–11. doi: 10.1016/j.rse.2017.08.029. URL: https://www.sciencedirect.com/science/article/pii/S0034425717303954 https://linkinghub.elsevier.com/retrieve/pii/S0034425717303954. [DOI] [Google Scholar]
- Yang P, van der Tol C, Verhoef W, Damm A, Schickling A, Kraska T, Muller O, Rascher U. Using reflectance to explain vegetation biochemical and structural effects on sun-induced chlorophyll fluorescence. Remote Sens Environ. 2019;231:110996 [Google Scholar]
- Yang P, van der Tol C, Campbell PK, Middleton EM. Fluorescence Correction Vegetation Index (FCVI): a physically based reflectance index to separate physiological and non-physiological information in far- red sun-induced chlorophyll fluorescence. Remote Sens Environ. 2020a;240:111676. doi: 10.1016/j.rse.2020.111676. URL: https://linkinghub.elsevier.com/retrieve/pii/S0034425720300456. [DOI] [Google Scholar]
- Yang P, Verhoef W, Van der Tol C. Unified four-stream radiative transfertheory in the optical-thermal domain with consideration of fluorescence for multilayer vegetation canopies. Remote Sens. 2020b;12:3914 [Google Scholar]
- Yang P, Prikaziuk E, Verhoef W, van der Tol C. SCOPE 2.0: a model to simulate vegetated land surface fluxes and satellite signals. Geosci Model Dev. 2021a;14:4697–4712. doi: 10.5194/gmd-14-4697-2021. URL: https://gmd.copernicus.org/articles/14/4697/2021/ [DOI] [Google Scholar]
- Yang P, Verhoef W, Prikaziuk E, van der Tol C. Improved retrieval of land surface biophysical variables from time series of Sentinel-3 OLCI TOA spectral observations by considering the temporal autocorrelation of surface and atmospheric properties. Remote Sens Environ. 2021b;256:112328. doi: 10.1016/j.rse.2021.112328. [DOI] [Google Scholar]
- Zarco-Tejada PJ, Camino C, Beck PSA, Calderon R, Hornero A, Hernandez-Clemente R, Kattenborn T, Montes-Borrego M, Susca L, Morelli M, Gonzalez-Dugo V, et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant trait alterations. Nat Plants. 2018;4:432–439. doi: 10.1038/s41477-018-0189-7. [DOI] [PubMed] [Google Scholar]
- Zarco-Tejada PJ, Poblete T, Camino C, Gonzalez-Dugo V, Calderon R, Hornero A, Hernandez-Clemente R, Román-Écija M, Velasco-Amo MP, Landa BB, Beck PSA, et al. Divergent abiotic spectral pathways unravel pathogen stress signals across species. Nat Commun. 2021;12:1–11. doi: 10.1038/s41467-021-26335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Badgley G, Dechant B, Ryu Y, Chen M, Berry J. A practical approach for estimating the escape ratio of near-infrared solar- induced chlorophyll fluorescence. Remote Sens Environ. 2019;232:111209. doi: 10.1016/j.rse.2019.05.028. URL: https://www.sciencedirect.com/science/article/pii/S0034425719302226. [DOI] [Google Scholar]
- Zhang F, Zhou G. Estimation of vegetation water content using hyperspectral vegetation indices: a comparison of crop water indicators in response to water stress treatments for summer maize. BMC Ecol. 2019;19:1–12. doi: 10.1186/s12898-019-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Huang Y, Pu R, MGonzalez-oreno P, Yuan L, Wu K, Huang W. Monitoring plant diseases and pests through remote sensing technology: a review. Comput Electron Agric. 2019;165:104943. doi: 10.1016/j.compag.2019.104943. [DOI] [Google Scholar]
- Zhuang Q, Wang H, Xu Y. Comparison of remote sensing based multi-source et models over cropland in a semi-humid region of china. Atmosphere. 2020;11 doi: 10.3390/atmos11040325. URL: https://www.mdpi.eom/2073-4433/11/4/325. [DOI] [Google Scholar]