Abstract
Canonical CRISPR-Cas systems provide adaptive immunity against mobile genetic elements1. However, type I-F, I-B and V-K systems have been adopted by Tn7-like transposons to direct RNA-guided transposon insertion2–7. Type V-K CRISPR-associated transposons rely on the pseudonuclease Cas12k, the transposase TnsB, the AAA+ ATPase TnsC, and the zinc-finger protein TniQ7, but the molecular mechanism of RNA-directed DNA transposition has remained elusive. Here, we report cryoelectron microscopic structures of a Cas12k-guide RNA-target DNA complex and a DNA-bound, polymeric TnsC filament. The Cas12k complex structure reveals an intricate guide RNA architecture and critical interactions mediating RNA-guided target DNA recognition. TnsC helical filament assembly is ATP-dependent and accompanied by structural remodeling of the bound DNA duplex. In vivo transposition assays corroborate key features of the structures, and biochemical experiments show that TniQ restricts TnsC polymerization, while TnsB interacts directly with TnsC filaments to trigger their disassembly upon ATP hydrolysis. Together, these results suggest that RNA-directed target selection by Cas12k primes TnsC polymerization and DNA remodeling, generating a recruitment platform for TnsB to catalyze site-specific transposon insertion. Insights from this work will inform development of CRISPR-associated transposons as programmable site-specific gene insertion tools.
In prokaryotes CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated) adaptive immune systems target mobile genetic elements, including transposons, for nucleolytic degradation using Cas proteins and CRISPR RNA (crRNA) guides1,8. However, several nuclease-deficient type I-F, I-B, and V-K systems have been co-opted by Tn7-like transposons to direct transposon DNA insertion into specific target sites2–7. In these systems, DNA targeting involves transposon-encoded Cas effectors, either a Cas3-less multisubunit complex (termed Cascade) in type I systems3 or a single catalytically inactive Cas12k protein in type V-K systems7 and results in transposon insertion at a fixed distance from the target site specified by the crRNA. Cas12k-mediated DNA targeting requires a crRNA guide and a trans-activating CRISPR RNA (tracrRNA), and is coupled to the concerted activities of three transposon proteins - the transposase TnsB, the AAA+ ATPase TnsC, and the zinc-finger protein TniQ (Fig. 1a) - to direct transposon insertion downstream of the target site. In the prototypical Escherichia coli Tn7 transposon9, the DDE-type TnsB transposase catalyses the 5'-DNA strand breakage and joining reactions required for transposon DNA excision from a donor locus and integration into target DNA10,11, while TnsC helps recruit TnsB to the target site and stimulates its activity12–14. The Tn7 core transposition machinery relies on DNA-binding cofactors TnsD and TnsE to direct transposition to specific DNA sequences15 and structures16,17, respectively. In CRISPR-associated transposons, TnsD and TnsE are replaced by the crRNA-guided effectors and the putative DNA binding protein TniQ. DNA targeting by type I-F3 CRISPR-transposon systems has been elucidated through structural studies of Cascade-TniQ complexes18–21. However, the mechanistic basis of RNA-guided DNA targeting in type V-K systems remains unknown, hindering the development of crRNA-directed transposition for programmable, homology-independent DNA insertion. Here, to understand target DNA recognition and transpososome assembly, we carried out structural studies of the Scytonema hofmanni type V-K CRISPR-associated transposon (ShCAST) system7, determining cryo-electron microscopy (cryo-EM) structures of a ternary Cas12k-guide RNA-target DNA complex and DNA-bound TnsC.
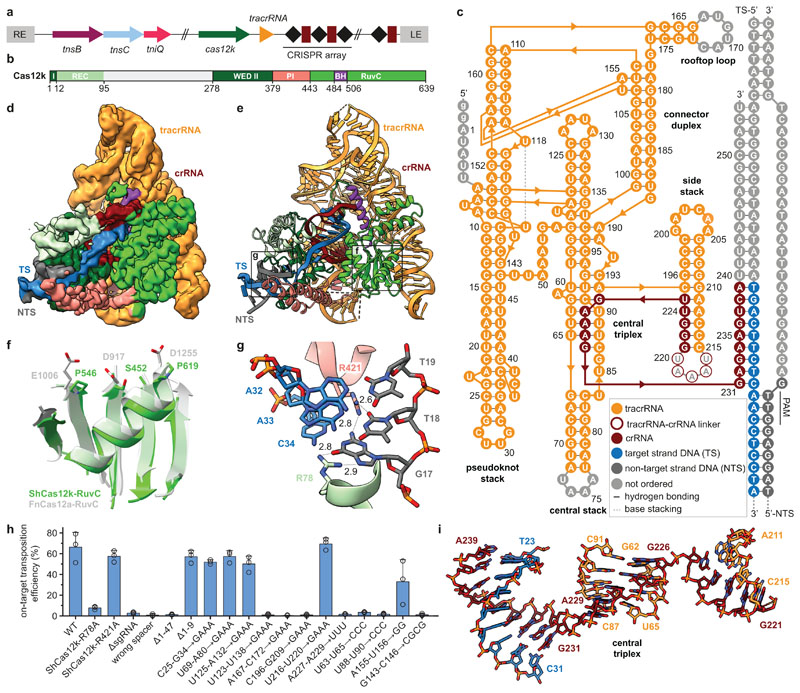
Fig. 1. Cryo-EM structure of the ShCas12k-sgRNA-target DNA complex.
a, Schematic of the type V-K CRISPR-associated transposon system from Scytonema hofmanni. LE, RE - left and right transposon ends. b, Domain organization of ShCas12k. REC, recognition lobe. WED, wedge domain. PI, PAM interacting domain. BH, bridging helix. c, Schematic of the sgRNA structure. d-e, Electron density map (d) and structural model (e) of the ShCas12k-sgRNA-target DNA complex. TS, target DNA strand; NTS, non-target DNA strand. f, Detail of the inactivated ShCas12k RuvC domain overlaid with the FnCas12a RuvC domain (PDB: 6i1k)30. g, Detailed view of PAM recognition. Hydrogen bonds are depicted with dotted lines. Distances are indicated in Å. h, Site-specific transposition activity in E. coli of ShCAST systems containing mutations in PAM interacting residues, as determined by droplet digital PCR (ddPCR) analysis. Data are presented as mean ± s.d. (n=3 biologically independent replicates).i, Details of the sgRNA central triplex.
Target DNA recognition by Cas12k
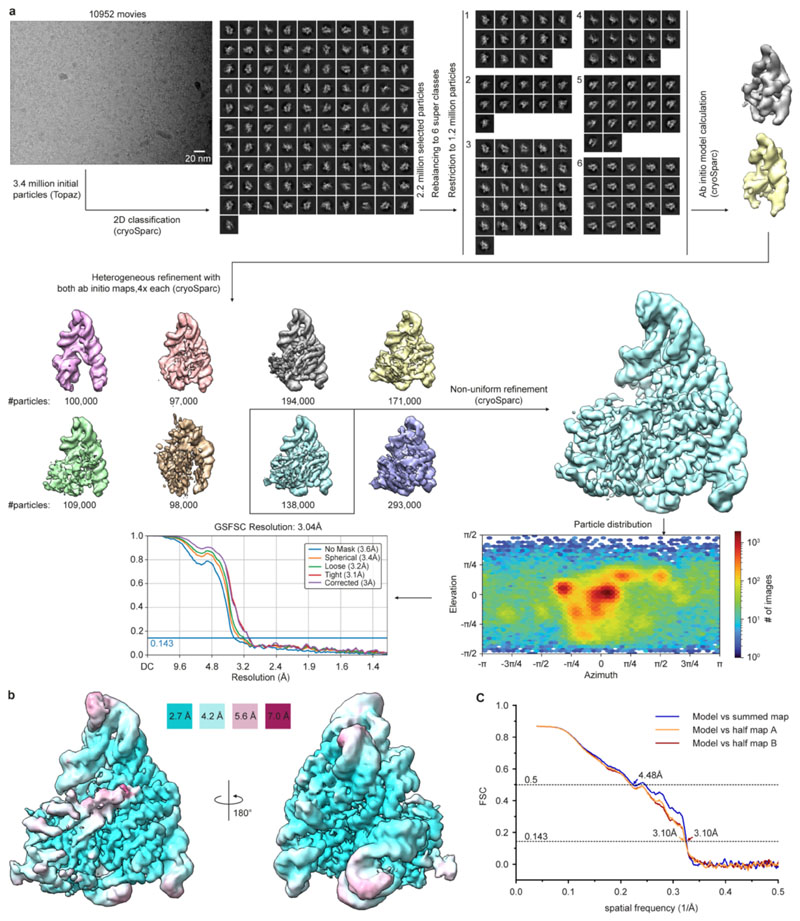
To understand how Cas12k recognizes its DNA target, we analyzed a ternary complex comprising ShCas12k, 254-nucleotide single-guide RNA (sgRNA, a crRNA-tracrRNA fusion containing a 24-nucleotide guide segment) and 50-base pair (bp) target double-stranded (ds)DNA using single-particle cryo-EM, obtaining a reconstruction at an overall resolution of 3.0 Å (Fig. 1b-e, Extended Data Fig. 1,2, Extended Data Table 1). Three-dimensional classification and refinement revealed that the ShCas12k complexes exhibit considerable conformational heterogeneity (Extended Data Fig. 2). While the majority of sgRNA nucleotides could be modeled, only a part of the R-loop structure formed by the sgRNA and target dsDNA could be resolved, corresponding to a 9-bp duplex of the target (TS) and non-target DNA strands (NTS) containing the protospacer adjacent motif (PAM), and a downstream 9-bp heteroduplex formed by the sgRNA and PAM-proximal region of the TS DNA (Fig. 1c).
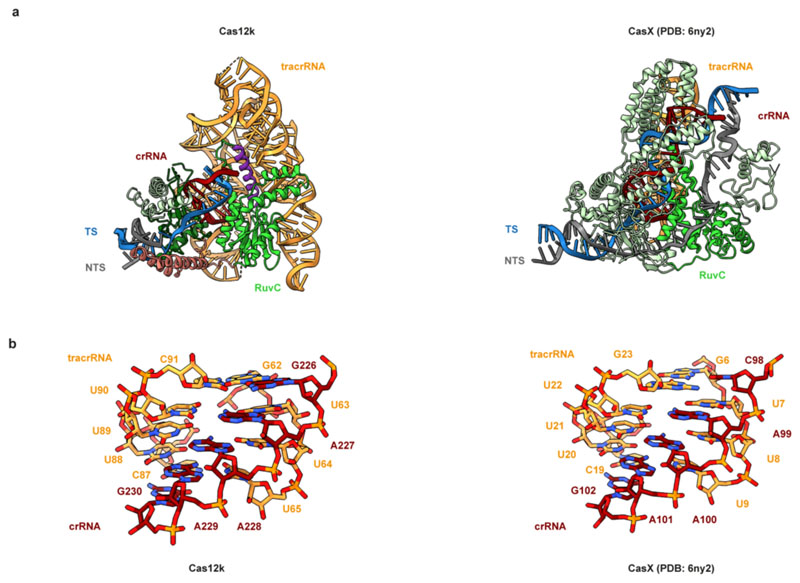
The molecular architecture of Cas12k comprises WED, REC1, REC2, PI (PAM interacting) and RuvC domains, similar to other Cas12-family effectors (Fig. 1b,e). The RuvC domain adopts a canonical fold and superimposes closely with the corresponding domains of other Cas12-family proteins (Fig. 1f). However, the Cas12k RuvC active site lacks the canonical Asp-Glu-Asp motif involved in Mg2+ binding, instead featuring Ser452ShCas12k, Pro546ShCas12k and Pro619ShCas12k (Fig. 1f), confirming that Cas12k is a pseudonuclease incapable of cleaving target DNA. The sgRNA-TS DNA heteroduplex is nested in a cleft formed by the REC1 and RuvC domains and terminates after 9 bp as it contacts the Cas12k bridge helix (Extended Data Fig. 3a, b), suggesting that full sgRNA-TS DNA hybridization requires additional conformational rearrangements in Cas12k.
crRNA-guided DNA recognition in the ShCAST system depends on a 5’-GTN-3’ PAM upstream of the target site7. In the ternary complex structure, the PAM-containing DNA duplex is contacted by the WED, REC1, and PI domains. The G-C base pair at the -3 PAM position is recognized via a bidentate hydrogen bond between the side chain of Arg78ShCas12k and the major groove edge of the guanine base (Fig. 1g). Arg421ShCas12k contributes to PAM recognition via hydrogen bonds to the minor groove edges of the guanine and thymine bases at the -3 and -2 PAM positions, respectively (Fig. 1g). To quantify the effect of Cas12k mutations on transposition efficiency in E. coli, we utilized a droplet digital PCR (ddPCR) assay. The R78A mutation nearly abolished transposition in vivo (Fig.1h), while the R421A mutation did not affect transposition frequency, indicating that Arg78ShCas12k is the major structural determinant of PAM recognition in ShCAST.
Extensive guide RNA scaffold supports Cas12k function
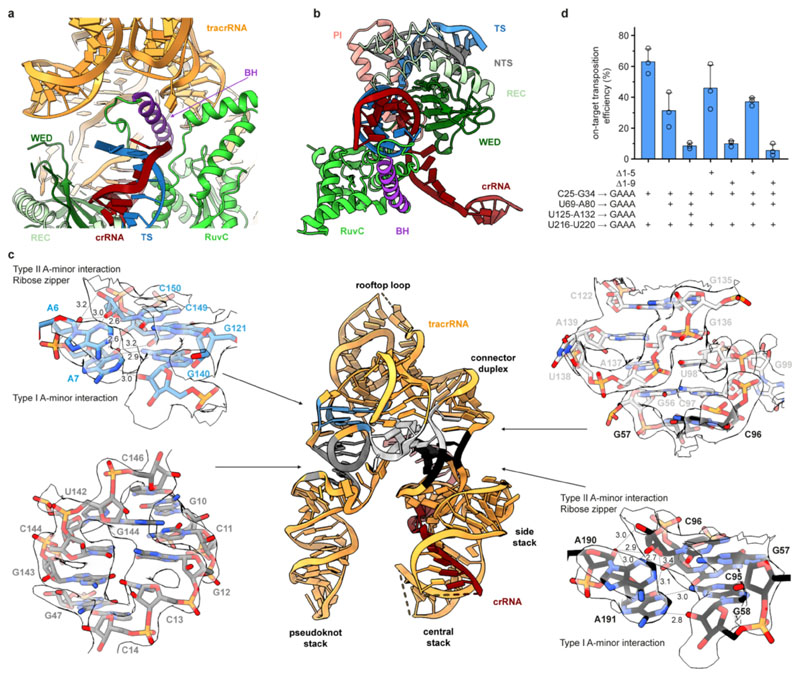
The ShCas12k sgRNA has an intricate pseudoknot topology comprising two coaxial duplex stacks that clamp the Cas12k RuvC domain and a smaller side stack containing the crRNA-tracrRNA repeat/anti-repeat duplex (Fig. 1c, Extended Data Figure 3c). The central element of the sgRNA is a triplex region (nucleotides G226-A229) derived from the crRNA repeat sequence, which contacts the WED domain and connects the repeat/anti-repeat duplex of the sgRNA with the sgRNA-TS DNA duplex (Fig. 1i). The central triplex is conserved in the Cas12e (CasX) sgRNA22, which is otherwise structurally divergent from the Cas12k sgRNA scaffold (Extended Data Fig. 4). The sgRNA also includes: a triplex region involving the 5’-terminal segment of the tracrRNA (nucleotides A6-A9) forming A-minor interactions with the pseudoknot duplex stack; a pseudoknot duplex between nucleotides G10-C13 and G143-C146; a “rooftop” stem-loop and a “connector” duplex that bridge the pseudoknot and central stacks; and a triplex junction featuring ribose-zipper interactions between sgRNA nucleotides C96-C97 and A190-A191 (Fig. 1c, Extended Data Fig. 3c). The tracrRNA provides a scaffold that extensively interacts with the Cas12k WED and RuvC domains to ensure their correct orientation and presents the guide segment of the crRNA for base-pairing interactions with the target DNA.
We tested the effects of structure-based sgRNA mutations on transposition efficiency in vivo using ddPCR. Mutations of the central triplex (nucleotides U88-U90), rooftop loop (A167-C172), pseudoknot duplex (G143-C146), and truncation of the sgRNA 5’-terminal region (nucleotides 1-47) all resulted in substantial reduction or complete loss of transposition (Fig. 1h, Extended Data Fig. 3d). In contrast, substitution of the peripheral stem-loops of the tracrRNA scaffold (nucleotides C25-G34, U69-A80, U125-A132, U216-U220) with GAAA tetraloops did not perturb transposition efficiency (Fig. 1h). These results confirm the role of key sgRNA structural elements in the transposition activity of ShCAST and suggest ways to minimize the sgRNA for genome engineering applications.
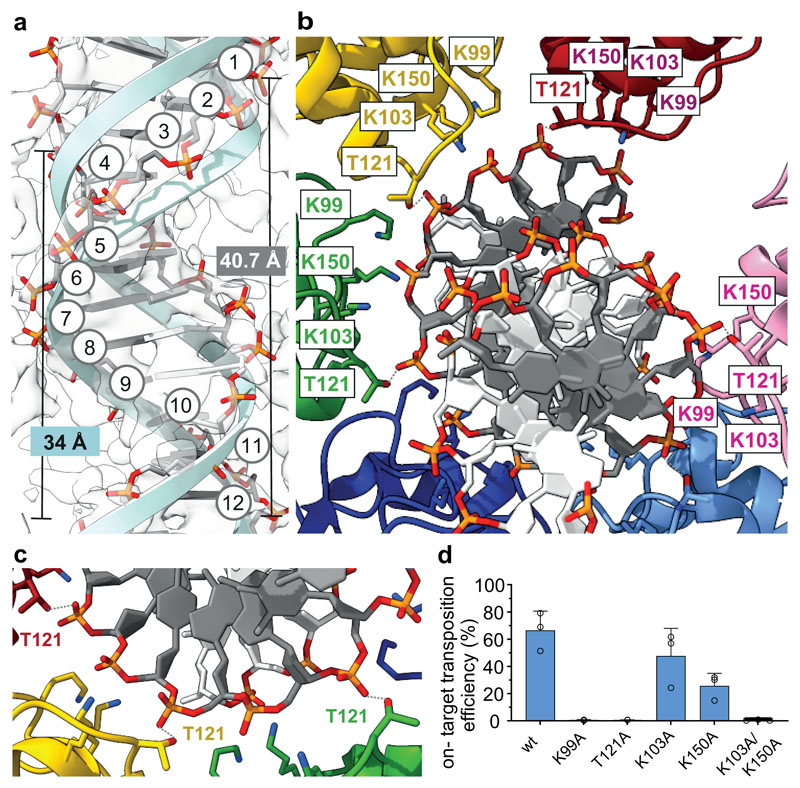
Fig. 3. DNA binding and remodeling by the ShTnsC filament.
a, Overview of the bound DNA duplex (backbone in stick representation; tracking strand, dark grey; opposite strand, white) and comparison with an ideal B form dsDNA (light blue cartoon backbone). Backbone phosphates of the tracking strand are numbered. b-c, Close-up views of key DNA-interacting residues. ShTnsC protomers are shown in distinct colors. Hydrogen bonds are indicated with grey dashed lines. d, Site-specific transposition activity in E. coli of ShCAST systems containing mutations in the DNA binding interface of ShTnsC, as determined by droplet digital PCR (ddPCR) analysis. Data are presented as mean ± s.d. (n=3 biologically independent replicates).
TnsC forms helical filaments
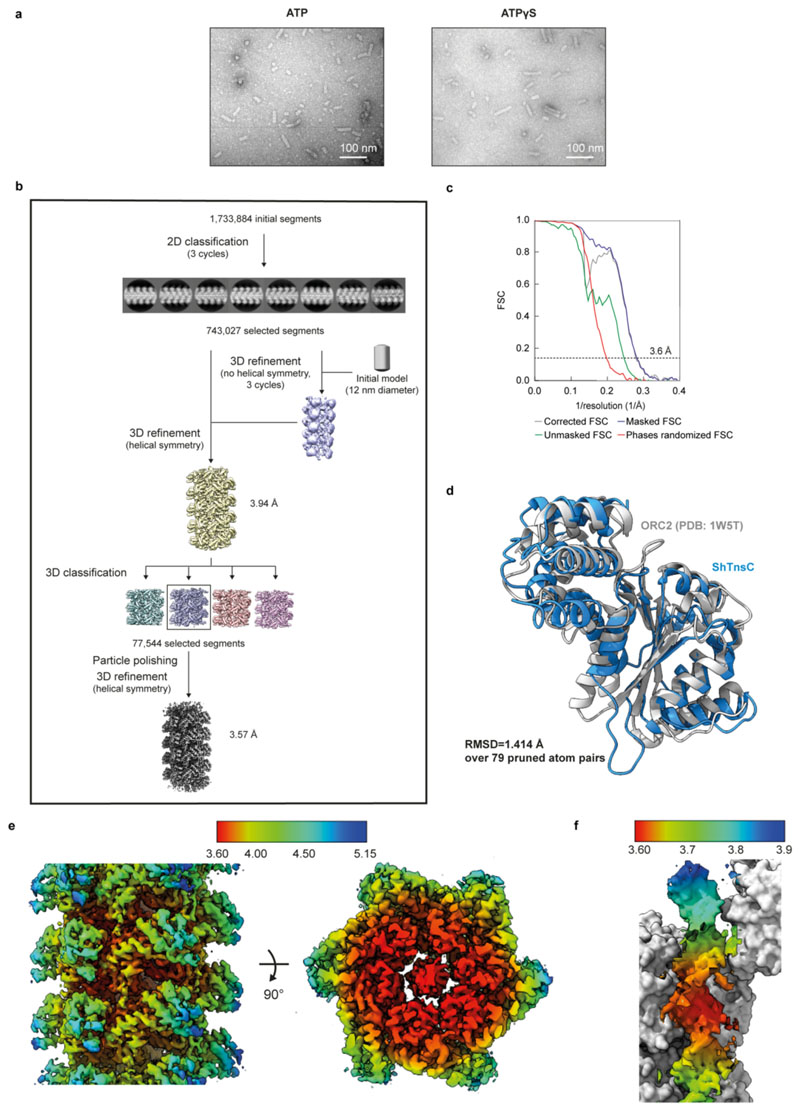
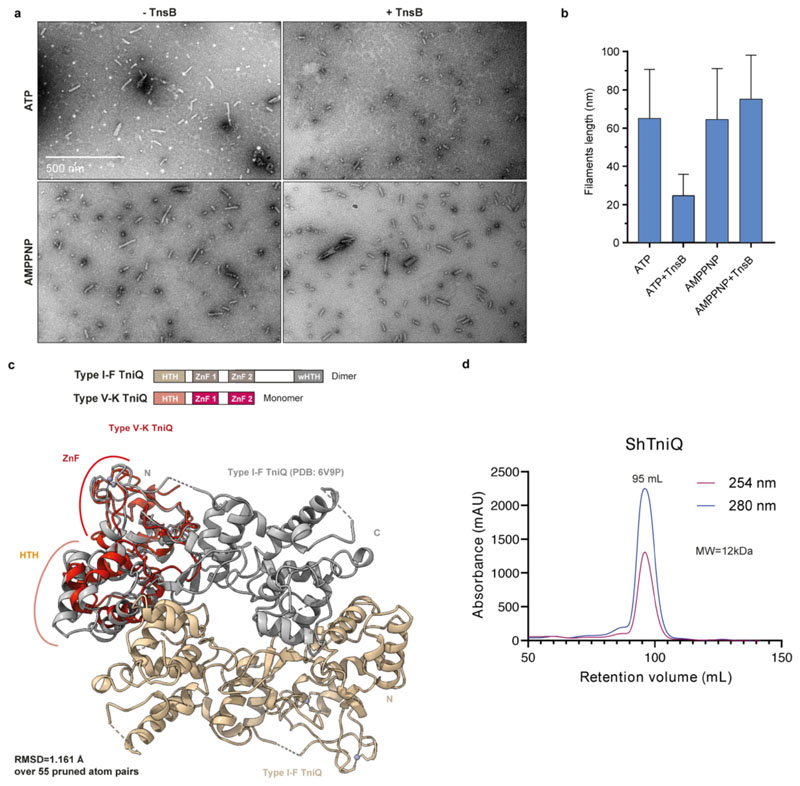
The TnsC component of type V CRISPR-associated transposons is a putative AAA+ ATPase (Fig. 2a) postulated to link Cas12-mediated target site recognition to transpososome recruitment7,23. Transposon-associated AAA+ ATPases such as MuB and IstB are known to oligomerize in the presence of ATP24,25. To determine whether TnsC behaves similarly, we used negative-stain EM to directly observe oligomerization of purified ShTnsC. Upon incubation with a linear dsDNA in the presence of ATP (Fig. 2b) or the non-hydrolyzable analog ATPγS (Extended Data Fig. 5a), ShTnsC formed polymeric filaments with an average length of 70 nm. Filament formation did not occur in the absence of dsDNA or ATP. Thus, TnsC polymerization is a DNA-dependent process that requires ATP binding but not hydrolysis, similar to the multimerization of other transposon-associated AAA+ ATPases24,25.
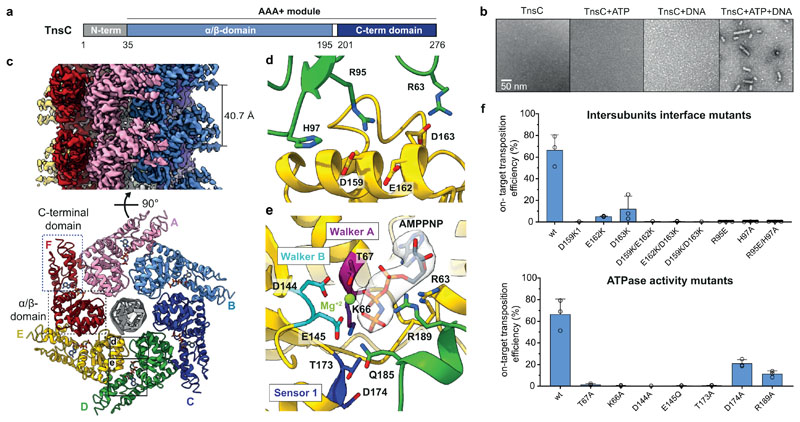
Fig. 2. Cryo-EM structure of the ShTnsC-AMPPNP-dsDNA filaments.
a, Domain organization of ShTnsC. b, Representative negative stain electron micrographs of ShTnsC incu the presence or absence of dsDNA and/or ATP. Magnification, 68,000 x. Experiment was repeated two times independently with similar results. c, Cryo-EM density map and structural model of the dsDNA- and AMPPMP-bound ShTnsC filament at 3.6 Å overall resolution (side and top views). Protein subunits are uniquely colored. dsDNA is shown in grey. d–e, Detailed views of the inter-protomer interface and of the ATPase catalytic site. Density corresponding to AMPPNP is shown (contour level of 4.0 σ). f, Site-specific transposition activity in E. coli of ShCAST systems containing mutations at the inter-protomer interface and in the catalytic pocket of ShTnsC, as determined by droplet digital PCR (ddPCR) analysis. Data are presented as mean ± s.d. (n=3 biologically independent replicates).
We used cryo-EM to determine the structure of a DNA-bound ShTnsC filament in the presence of the non-hydrolyzable analog AMPPNP and Mg2+ at an overall resolution of 3.6 Å (Fig. 2 c-e, Extended Data Fig. 5b-f, Extended Data Table 1). The structure reveals that TnsC forms a righthanded helix with a pitch of 40.7 Å and rise of 6.8 Å, accommodating six ShTnsC copies per turn (Fig. 2c). The ShTnsC protomers contain a canonical bipartite AAA+ ATPase module, composed of an α/β domain comprising a five-stranded beta sheet flanked by alpha helices on both sides, and an α-helical C-terminal domain (Fig. 2c, Extended Data Fig. 5d). The ATPase active site is located in a cleft between the α/β and C-terminal domains and contains the canonical AAA+ structural motifs involved in ATP binding and hydrolysis, including Walker A and Walker B (Fig. 2e). The active site is completed by the insertion of the arginine finger Arg189ShTnsC and Gln185ShTnsC from the adjacent ShTnsC protomer, thereby coupling ATP binding to ShTnsC polymerization. Helical assembly of ShTnsC protomers is further enabled by additional salt-bridge interactions involving Arg63ShTnsC, Arg95ShTnsC and His97ShTnsC from one protomer and Asp159ShTnsC, Asp163ShTnsC and Glu162ShTnsC from another (Fig. 2d).
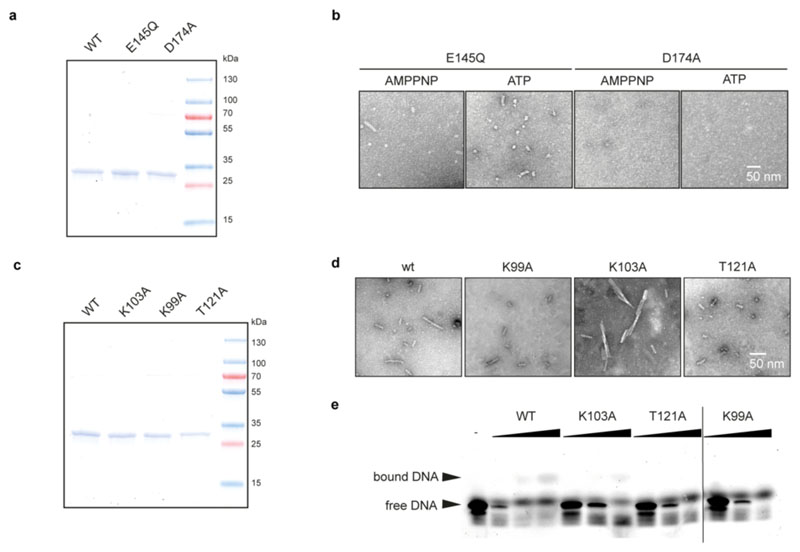
To validate our structural findings, we analyzed the polymerization behavior of ShTnsC mutant proteins by negative-stain EM (Extended Data Fig. 6a,b). The E145Q Walker B mutant protein, predicted to be deficient in ATP hydrolysis but not ATP binding, supported filament formation in the presence of ATP or AMPPNP. In contrast, ShTnsC containing the D174A mutation in the Sensor 1 motif was incapable of filament formation, confirming that ATP binding, but not hydrolysis, is required for ShTnsC assembly. Mutations of key residues in the ATP binding pocket or at the inter-protomer interface resulted in a substantial reduction or complete loss of transposition in vivo (Fig. 2f). Thus, both TnsC oligomerization and ATP hydrolysis by TnsC are critical for the activity of ShCAST.
TnsC filaments assemble on remodeled DNA
The ShTnsC filament encloses the bound DNA duplex, which is structurally distorted to match the filament helical symmetry. The duplex has 12 bp per helical turn, a pitch of 40.7 Å, a rise of 3.4 Å per bp along the helical axis, and a narrower diameter (17.6 Å) compared to a canonical B-form DNA (20 Å) (Fig. 3a). The right-handed ShTnsC filament makes continuous interactions with the deoxyribosephosphate backbone of only one DNA strand (the tracking strand), with each protomer straddling the duplex minor groove and contacting two consecutive backbone phosphate groups on the tracking strand via a salt bridge with Lys103ShTnsC and a hydrogen bond with Thr121ShTnsC (Fig. 3b, c). The other DNA strand is not contacted directly by ShTnsC, however, its backbone phosphate groups are adjacent to Lys99ShTnsC and Lys150ShTnsC (Fig. 3b). T121A and K99A ShTnsC mutant proteins generated substantially shorter filaments in vitro, while filament formation was not perturbed by the K103A mutation (Extended Data Fig. 6c-d). Corroborating these results, the DNA binding activities of T121A, and K99A ShTnsC mutants were substantially reduced (Extended Data Fig. 6e). ShTnsC mutations resulting in defective filament formation also abolished or significantly reduced transposition in vivo (Fig. 3d), validating the functional importance of DNA-dependent TnsC polymerization and TnsC-induced DNA remodeling for the transposition activity of type V CRISPR-associated transposons.
TnsB triggers filament disassembly
In Tn7 and Mu transposons, the transposases TnsB and MuA interact with and stimulate the ATPase activity of their cognate AAA+ ATPases 26–28. To test whether this also occurs in ShCAST, we performed pull-down experiments using affinity-tagged ShTnsB. In the presence of DNA, ShTnsB efficiently coprecipitated ShTnsC when ATP or AMPPNP was also present, but not in the presence of ADP (Fig. 4a). A truncated ShTnsB protein comprising the catalytic and C-terminal domains was sufficient to mediate the interaction; however, a TnsB construct lacking the C-terminal domain (residues Leu494-Phe584ShTnsB) was unable to co-precipitate ShTnsC. Thus, ShTnsB interacts via its C-terminal region with ShTnsC filaments but not with monomeric ShTnsC. Negative-stain EM revealed that ATP-bound ShTnsC filaments disassembled in the presence of ShTnsB (Fig. 4b, Extended Data Fig. 7a,b), while AMPPNP-bound filaments remained intact, indicating that ShTnsB-driven disassembly was dependent on ATP hydrolysis. These results suggest that ShTnsB interacts with polymerized ShTnsC and stimulates its ATPase activity, triggering filament disassembly.
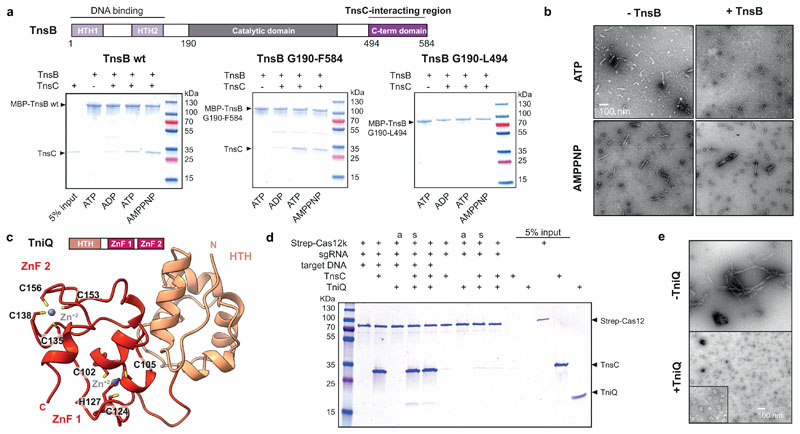
Fig. 4. Functional interactions and roles of ShCAST components.
a, Domain organization of ShTnsB (top panel). HTH, helix turn helix motif. Co-precipitation of ShTnsC by ShTnsB constructs fused to maltose binding protein (MBP) in the presence of different nucleotides (bottom panel). b, Representative negative stain EM micrographs of ShTnsC-dsDNA complexes in the presence of ShTnsB and different nucleotides. Magnification, 98,000 x. c, Top: Domain architecture of ShTniQ. ZnF, zinc-finger motif. Bottom: Crystal structure of ShTniQ. Side chains of zinc-coordinating residues are shown as sticks. d, Co-precipitation of ShTnsC and ShTniQ by immobilized StrepII-fused Cas12k-sgRNA-target DNA complex. a, TnsC and TniQ components were added together; s, sequential addition. e, Representative negative stain EM micrographs of ShTnsC in the presence of dsDNA, ATP and ShTniQ. Magnification of 68,000 x for main micrographs, 120,000 x for insert. Experiments in a, b, d and e were repeated twice independently with similar results. For gel source data, see Supplementary Figure 1.
TniQ restricts TnsC polymerization
ShTniQ is required for the transposition activity of ShCAST in vivo7. In type I-F CRISPR-transposon systems, a TniQ dimer interacts directly with the crRNA-guided Cascade 18–21, but whether TniQ plays an analogous role in type V systems is unknown. To investigate TniQ function in type V systems, we determined the crystal structure of ShTniQ at a resolution of 1.3 Å (Fig. 4c, Extended Data Table 2). The structure reveals a conserved domain architecture comprising an N-terminal helix-turn-helix (HTH) motif followed by two zinc-finger domains (Fig. 4c), previously observed in the structure of TniQ from the Vibrio cholerae type I-F CRISPR-associated transposon18–21 (Extended Data Fig. 7c). However, unlike the dimeric V. cholerae TniQ, ShTniQ lacks the winged HTH domain involved in dimerization, consistent with the observation that ShTniQ is monomeric in solution (Extended Data Fig. 7d).
We next tested the interactions between ShTniQ, ShCas12k and ShTnsC using pull-down assays. We assembled StrepII-tagged ShCas12k with a sgRNA and a target DNA and immobilized the resulting complex on Strep-tactin beads. TnsC was efficiently co-precipitated by the ShCas12k-sgRNA-target DNA complex in the presence of ATP, but did not bind in the absence of target DNA (Fig. 4d), indicating that the interaction is a result of ATP-dependent ShTnsC oligomerization on the target DNA. The interaction of ShTnsC with Cas12k was neither enhanced nor inhibited by the addition of ShTniQ (Fig. 4d), implying that TniQ is not required for Cas12k-dependent recruitment of TnsC to target DNA. Furthermore, ShTniQ was only co-precipitated by ShCas12k in the presence of DNA-bound TnsC (Fig. 4d). Together, these results suggest a direct interaction between ShTniQ and ShTnsC and imply that unlike in type I CRISPR-transposon systems, the interaction of TniQ with the CRISPR effector in type V-K systems is indirect. To probe the effect of TniQ on TnsC oligomerization, we examined ShTnsC filament formation in the presence of ShTniQ. Incubation of ShTniQ and ShTnsC (Fig. 4e) in the precence of ATP and dsDNA yielded substantially shorter ShTnsC filaments or ring-shaped assemblies, indicating that TniQ inhibits TnsC polymerization.
Mechanism of Cas12k-mediated transposition
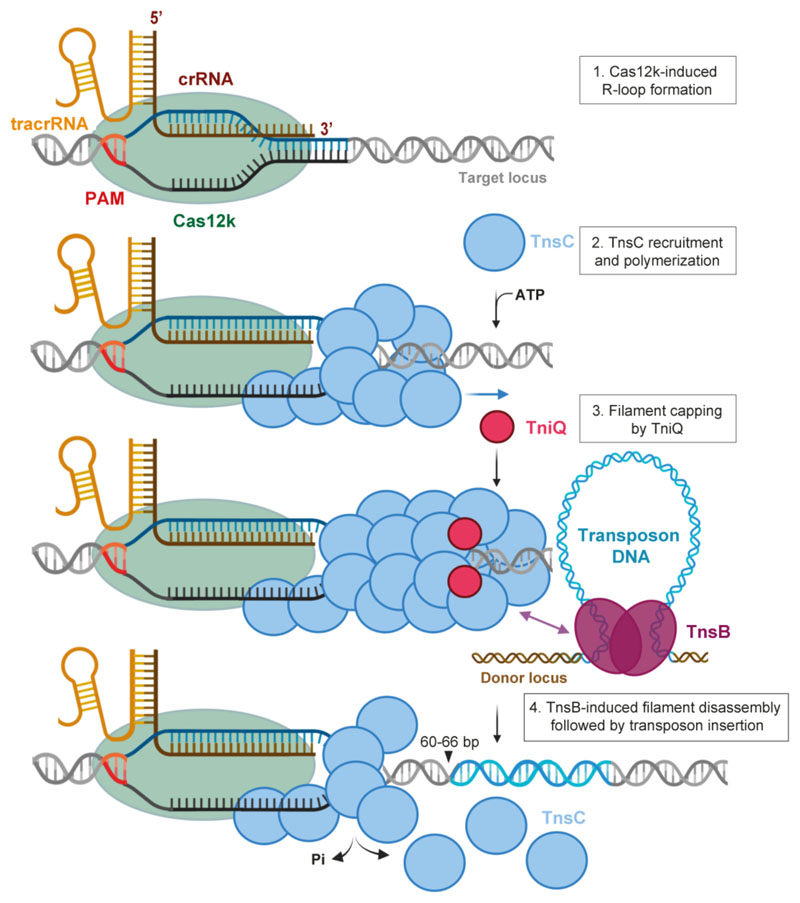
Our structural and functional analysis of the DNA-bound Cas12k effector complex reveals an extensive tracrRNA scaffold that appears to compensate for the compact size of Cas12k, reminiscent of other small type V CRISPR-associated effectors22. The Cas12k complex forms an incomplete R-loop structure, consistent with the reported minimal requirements for guide RNA-target complementarity in ShCAST7. This draws parallels with the Cascade-TniQ complex from I-F CRISPR-transposon systems18 and suggests that recruitment of downstream transposon factors, including TnsC, might allosterically regulate Cas12k to promote R-loop completion, serving as an activation checkpoint. The structure of TnsC filaments shows that ATP-dependent TnsC polymerization occurs on remodeled, underwound DNA, suggesting that DNA unwinding and R-loop formation by Cas12k may help nucleate TnsC filament assembly, although R-loop formation alone is not sufficient for ShCAST activity7. Based on our findings, we propose a mechanistic model of Cas12k-dependent DNA transposition in type V-K CRISPR-transposon systems (Extended Data Fig. 8), in which target site recognition by Cas12k initiates local TnsC polymerization to generate an interaction platform for the transposase TnsB. TniQ inhibits TnsC polymerization, consistent with a recent study showing that TniQ caps one end of the TnsC filament29. In contrast to a previously proposed model29, our interaction data, together with the observation that TniQ is not essential for the transposition activity of ShCAST in vitro7, suggest that TniQ might restrict TnsC polymerization to the vicinity of the target site by capping the Cas12k-distal end of the filament. Upon recruitment, TnsB stimulates the ATPase activity of TnsC to trigger filament disassembly, which exposes the target site and enables transposon insertion at a fixed distance from Cas12k, as suggested by the structure of DNA-bound ShTnsC in the post-hydrolysis state29. Altogether, this work provides mechanistic insights into type V CRISPR-associated transposons and provides a structural framework for their development as an RNA-guided insertion technology for genome engineering.
Methods
Plasmid DNA constructs
The DNA sequences of Scytonema hofmanni Cas12k (WP_029636312.1), TnsC (WP_029636336.1), TniQ (WP_029636334.1) and TnsB (WP_084763316.1) proteins were codon optimized for heterologous expression in Escherichia coli (E. coli) and synthesized by GeneArt (Thermo Fisher Scientific). The ShCas12k gene was inserted into the 1B (Addgene 29653) and 1R (Addgene 29664) plasmids using ligation-independent cloning (LIC), resulting in constructs carrying an N-terminal hexahistidine tag followed by a tobacco etch virus (TEV) protease cleavage site and a N-terminal hexahistidine-StrepII tag followed by a TEV cleavage site, respectively. The ShTnsB gene and truncated derivatives were inserted by LIC into 1B (Addgene 29653) and 1C (Addgene 29659) plasmids, generating constructs carrying an N-terminal hexahistidine tag and a hexahistidine-maltose binding protein (MBP) tag followed by a TEV cleavage site. The ShTnsC gene was inserted into the 1S (Addgene 29659) plasmid to produce a construct carrying a N-terminal hexahistidine and hexahistidine-SUMO tag followed by a TEV cleavage site and the ShTniQ was cloned into a 1C vector. Point mutations were introduced by Gibson assembly using gBlock gene fragments synthetized by IDT or annealed oligonucleotides provided by Sigma as inserts. The pDonor (Addgene 127921), pHelper (Addgene 127924), and pTarget (Addgene 127926) plasmids7 used in droplet digital PCR experiments were sourced from Addgene. The PSP1-targeting spacer was cloned into pHelper by Gibson assembly, yielding pHelper-PSP1. Mutations in the sgRNA or in the sequence of the ShCas12k and ShTnsC genes were introduced into the pHelper plasmid by Gibson assembly. Plasmids were cloned and propagated in Mach I cells (Thermo Fisher Scientific) with the exception of pHelper, which was grown in One Shot PIR1 cells (Thermo Fisher Scientific). Plasmids were purified using the GeneJET plasmid miniprep kit (Thermo Fisher Scientific) and verified by Sanger sequencing. All sequence of primers, gBlocks and oligonucleotides used for cloning are provided in Supplementary Table 1.
Protein expression and purification
For expression of ShCas12k constructs, hexahistidine-Strep II-tagged and hexahistidine-tagged ShCas12k proteins were expressed in E. coli BL21 Star (DE3) cells. Cell cultures were grown at 37 °C shaking at 100 rpm until reaching an OD600 of 0.6 and protein expression was induced with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and continued for 16 h at 18 °C. Harvested cells were resuspended in 20 mM Tris-HCl pH 8.0, 500 mM NaCl, 5 mM Imidazole, 1 μg/mL Pepstatin, 200 μg/mL AEBSF and lysed in a Maximator Cell homogenizer at 2,000 bar and 4 °C. The lysate was cleared by centrifugation at 40,000 x g for 30 min at 4 °C and applied to two 5 mL Ni-NTA cartridges connected in tandem. The column was washed with 50 mL of 20 mM Tris-HCl pH 8.0, 500 mM NaCl, 5 mM Imidazole before elution with 25 mL of 20 mM Tris-HCl pH 8.0, 500 mM NaCl, 250 mM Imidazole. Elution fractions were pooled and dialyzed overnight against 20 mM HEPES-KOH pH 7.5, 250 mM KCl, 1 mM EDTA, 1 mM DTT in the presence of tobacco etch virus (TEV) protease. Dialyzed proteins were loaded onto a 5 mL HiTrap Heparin HP column (GE Healthcare) and eluted with a linear gradient of 20 mM HEPES-KOH pH 7.5, 1 M KCl. Elution fractions were pooled, concentrated using 30,000 molecular weight cutoff centrifugal filters (Merck Millipore) and further purified by size exclusion chromatography using a Superdex 200 (16/600) column (GE Healthcare) in 20 mM HEPES-KOH pH 7.5, 250 mM KCl, 1 mM DTT yielding pure, monodisperse proteins. Purified proteins were concentrated to 10-15 mg mL−1, flash frozen in liquid nitrogen and stored at -80 °C until further usage.
Expression of wild-type ShTnsC, ShTnsC mutants, ShTnsB, ShTnsB G190-F584, ShTnsB G190-L494 and ShTniQ was performed in E. coli BL21 Rosetta2 (DE3) cells. Cells were grown in LB medium until reaching an OD600 of 0.6 and expression was induced by addition of 0.4 mM IPTG. Proteins were expressed at 18 °C for 16 h. For ShTnsC, the cells were harvested and resuspended in lysis buffer containing 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 5% glycerol and 10 mM imidazole supplemented with EDTA-free protease inhibitor (Roche). The cell suspension was lysed by ultrasonication and the lysate was cleared by centrifugation at 40,000 x g for 40 min. Cleared lysate was applied to a 5 mL Ni-NTA cartridge (Qiagen). The column was washed in two steps with lysis buffer supplemented with 25 mM and 100 mM imidazole, and bound proteins were eluted with 25 mL of same buffer supplemented with 500 mM imidazole pH 7.5. Eluted proteins were dialysed overnight against 20 mM Tris-HCl pH 7.5, 250 mM NaCl, 5% glycerol, 1 mM DTT in the presence of TEV protease. The proteins was further purified using a 5 mL HiTrap HP Heparin column (GE Healthcare) and eluted with a buffer containing 20 mM Tris-HCl pH 7.5, 700 mM NaCl, 5% glycerol and 1 mM DTT. The eluted fraction was concentrated and further purified by size exclusion chromatography using an S200 increase (10/300 GL) column (GE Healthcare) in 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 1 mM DTT, yielding pure, monodisperse proteins. Purified ShTnsC was concentrated to 1-2 mg mL−1 using 30,000 kDa molecular weight cut-off centrifugal filters (Merck Millipore) and flash-frozen in liquid nitrogen. All mutant ShTnsC proteins were purified using the same protocol as for the wild-type protein.
For ShTnsB, cells were harvested and resuspended in lysis buffer containing 20 mM Tris-HCl pH 8.0, 500 mM NaCl, 5% glycerol and 5 mM Imidazole supplemented with EDTA-free protease inhibitor (Roche) and lysed by ultrasonication. Lysate was clarified by centrifugation at 40,000 x g for 40 min. The lysate was applied to a 5 mL Ni-NTA cartridge (Qiagen) and the column was washed with lysis buffer supplemented with 25 mM imidazole. The protein was eluted with 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 5% glycerol and 200 mM Imidazole. Eluted proteins were dialysed overnight against 20 mM Tris-HCl pH 8.0, 200 mM NaCl, 1 mM DTT in the presence of TEV protease and subsequently purified using a 5 mL HiTrap HP Heparin column (GE Healthcare), eluting with a buffer containing 20 mM Tris-HCl pH 8.0, 400 mM NaCl, and 1 mM DTT. The eluted fraction was concentrated and further purified by size exclusion chromatography using a Superdex (16/600) column (GE Healthcare) in 20 mM Tris-HCl pH 8.0, 400 mM NaCl, and 1 mM DTT. Purified ShTnsB was concentrated to 7 mg mL−1, and flash-frozen in liquid nitrogen. For the ShTnsB G190-F584 and ShTnsB G190-L494 constructs, cells were harvested and resuspended in 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 5% glycerol and 5 mM imidazole supplemented with EDTA-free protease inhibitor (Roche) and lysed by ultrasonication. Cleared lysate was applied to a 5 mL Ni-NTA cartridge (Qiagen) and the column was washed in two steps with lysis buffer supplemented with 25 and 50 mM imidazole. The proteins were eluted with 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 5% glycerol and 125 mM imidazole and dialysed overnight against 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 5% glycerol and 4 mM beta-mercaptoethanol in the presence of TEV. Dialysed proteins were passed through a 5 mL Ni-NTA cartridge and washed with buffer containing 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 5% glycerol and 25 mM imidazole, and further purified by size exclusion chromatography using an Superdex (16/600) column (GE Healthcare) in 20 mM Tris-HCl pH 7.5, 250 mM NaCl, 1 mM DTT. Purified ShTnsB proteins were concentrated to 13–24 mg mL−1 and flash-frozen in liquid nitrogen. For the purification of the MBP-tagged constructs used in pull-downs experiments (MBP-ShTnsB, MBP-ShTnsB G190-F584 and MBP-ShTnsB G190-L494), eluted fraction were set aside after the first step of Ni affinity chromatography and directly loaded on a Superdex 200 Increase (10/300 GL) column (GE Healthcare) and eluted in 20 mM Tris-HCl pH 7.5, 250 mM NaCl and 1 mM DTT. Appropriate fractions were concentrated to 3-0.7 mg mL−1 using 30,000 kDa molecular weight cut-off centrifugal filters (Merck Millipore) and flash-frozen in liquid nitrogen.
For ShTniQ, cells were harvested and resuspended in 20 mM Tris-HCl pH 7.8, 500 mM NaCl, 5% glycerol and 5 mM imidazole supplemented with EDTA-free protease inhibitor (Roche) and lysed by ultrasonication. The cleared lysate was applied to a 5 mL Ni-NTA cartridge (Qiagen) and the column was washed in two steps with lysis buffer supplemented with 25 and 50 mM imidazole. The protein was eluted with lysis buffer supplemented with 300 mM imidazole. Eluted protein was dialysed overnight against 20 mM Tris-HCl pH 7.8, 500 mM NaCl, 1 mM DTT in the presence of TEV protease. Dialysed protein was passed through a 5 mL MBPTrap column (GE Healthcare). The flow-through fraction was concentrated and further purified by size exclusion chromatography using a Superdex 200 (16/600) column (GE Healthcare) in 20 mM Tris-HCl pH 7.8, 250 mM NaCl, 1 mM DTT. Size exclusion chromatography allowed to estimate the oligomeric state of the protein (monomer), as shown in Extended Data Fig. 7d. Purified ShTniQ was concentrated to 10 mg mL−1 using 10,000 kDa molecular weight cut-off centrifugal filters (Merck Millipore) and flash-frozen in liquid nitrogen.
sgRNA preparation
DNA sequence encoding the T7 RNA polymerase promoter upstream of the ShCas12k-sgRNA was sourced as a gBlock (IDT), cloned into a pUC19 plasmid using restriction digest with BamHI and EcoRI, and confirmed by Sanger sequencing. The sequence encoding the T7 RNA polymerase promoter and sgRNA was amplified by PCR and purified by ethanol precipitation for use as template for in vitro transcription with T7 RNA polymerase as described previously31. The transcribed RNA was gel purified, precipitated with 70 % (v/v) ethanol, dried and dissolved in nuclease-free water.
TnsC filament formation
For the reconstitution of ShTnsC filaments, wild-type ShTnsC protein was diluted to a final concentration of 0.5 μM in a buffer containing 20 mM HEPES-KOH pH 7.5, 200 mM KCl, 10 mM MgCl2, 1 mM DTT and mixed at a ratio of 1:5 with a double-stranded 92-bp duplex oligo (Supplementary Table 1) in a 24 μl reaction volume. Protein and DNA were incubated for 10 min at 25 °C. Upon addition of nucleotide (ATP, ATPγS or AMPPNP) at a final concentration of 1 mM, reactions were further incubated for 20 min at 37 °C. To analyse filament assembly in the presence of TnsB, TnsC filaments were formed using the same procedure described above, followed by addition of TnsB at a final concentration of 10 μM (1:2 ratio TnsC:TnsB) in a 60 μl final reaction volume. To probe filament formation in the presence of ShTniQ, ShTnsC was diluted to a final concentration of 3 μM in a buffer containing 20 mM HEPES-KOH pH 7.5, 200 mM KCl, 10 mM MgCl2, 1 mM DTT and 1 mM ATP and mixed with a double-stranded 69-bp duplex oligo (Supplementary Table 1) at a ratio of 1:25 (DNA:TnsC) in a 20 μl reaction volume. ShTniQ was added to the reactions at a final concentration of 6 μM and incubated for 1 hour at 37 °C. Prepared samples were then analysed by negative stain electron microscopy.
Negative stain electron microscopy
Samples (4 μL) were applied to glow-discharged continuous carbon film supported copper grids (CF300-CU-50 grids, Electron Microscopy Sciences). After 1 min incubation, the grids were washed once with 4 μl of 2 % (w/v) uranyl formate and stained in a 4 μL drop of 2 % uranyl formate during a total of 1 min prior blotting. Negatively-stained specimens were imaged using a FEI Tecnai G2 Spirit transmission electron microscope operated at an acceleration voltage of 120 kV. Data were acquired at a nominal magnification of 68,000, 98,000, 120,000 or 180,000 x, as indicated in figure legends. The length of the filaments was measured manually with the Gatan Digital micrograph software.
Transposition assay and droplet digital PCR analysis
Transposition assay were conducted as in6, with minor modifications. All transposition experiments were performed in One Shot PIR1 E. coli cells (Thermo Fisher Scientific). The strain was first cotransformed with 20 ng each of pDonor and pTarget, and transformants were isolated by selective plating on double antibiotic LB-agar plates. Liquid cultures were then inoculated from single colonies, and the resulting strains were made chemically competent using standard methods, aliquoted and snap frozen. pHelper plasmids (20 ng) were then introduced in a new transformation reaction by heat shock, and after recovering cells in fresh LB medium at 37 °C for 1 h, cells were plated on triple antibiotic LB-agar plates containing 100 μg mL−1 carbenicillin, 50 μg mL−1 kanamycin, and 33 μg mL−1 chloramphenicol. After overnight growth at 37 °C for 16 h, colonies were harvested from the plates, resuspended in 15 μL Lysis buffer (TE with 0.1 % Triton X 100) and heated for 5 min at 95 °C. 60 μL of water were added to the samples before centrifugation for 10 min at 16,000 x g. The supernatant was then transferred and the nucleic acid concentration adjusted to 0.3 ng μL−1. 0.75 ng of template DNA were used for subsequent investigation by droplet digital PCR (ddPCR). A mixture of five primers (900 nM final concentration each), two probes (250 nM final each) (Supplementary Table 1) and template DNA were combined with ddPCR Supermix for Probes (No dUTP) (BioRad) in a final 20 μL reaction volume. Droplets were generated with 70 μL of Droplet Generation Oil for Probes (BioRad) using the QX200 Droplet Generator (BioRad). 40 μL of final sample were transferred to 96 well plates for amplification by PCR (1 cycle, 95 °C, 10 min; 40 cycles, 94 °C, 30 s, 58 °C, 1 min; 1 cycle, 98 °C, 10 min; 4 °C hold). Samples were read with a QX200 Droplet Reader, and data analysed using the QuantaSoft to determine the concentration of inserts and template in each reaction (Abs counting mode). On-target transposition efficiencies were calculated as inserts/(inserts+targets). All ddPCR measurements presented in the text and figures were determined from three independent biological replicates and measured in technical duplicates.
Pull-down experiments
For pull-down experiments using ShCas12k as bait, sgRNA was first mixed with hexahistidine-StrepII-tagged ShCas12k (Strep-Cas12k) in assembly buffer (20 mM HEPES-KOH pH 7.5, 250 mM KCl, 10 mM MgCl2, 1 mM DTT), and incubated 20 min at 37 °C to allow complex formation. A dsDNA target (Supplementary Table 1) was then added to the reaction in a Strep-Cas12k:sgRNA:dsDNA molar ratio of 1:1.2:2 and incubated for 20 min at 37 °C. The final 20 μL reaction contained 2.5 μg (at a concentration of 1.6 μM) Strep-Cas12k, 1.9 μM sgRNA, and 3.2 μM DNA in assembly buffer. Samples were mixed with 12.5 μL Strep-Tactin beads (iba) equilibrated in pull-down wash buffer 1 (20 mM HEPES-KOH pH 7.5, 250 mM KCl, 10 mM MgCl2, 1 mM DTT, 0.05 % Tween20) and incubated 20 min at 4 °C on a rotating wheel. The beads were washed three times with pull-down wash buffer 1 to remove excess nucleic acids. The beads were resuspended in 250 μL of pull-down wash buffer 1 and ShTniQ and/or ShTnsC was added in 10-fold molar excess together with AMPPNP (1 mM final concentration). Samples were incubated 20 min at 37 °C, then washed twice with pull-down wash buffer 2 (20 mM HEPES-KOH pH 7.5, 250 mM KCl, 10 mM MgCl2, 1 mM DTT, 0.05 % Tween20, 1 mM AMPPNP) and eluted with pull-down elution buffer (20 mM HEPES-KOH pH 7.5, 250 mM KCl, 10 mM MgCl2, 1 mM DTT, 1 mM AMPPNP, 5 mM desthiobiotin). Eluted samples were analyzed by SDS-PAGE using Any kDa gradient polyacrylamide gels (Bio-Rad) and stained with Coomassie Brillian Blue.
For pull-downs using ShTnsB as bait, MBP-ShTnsB and the truncated derivatives ShTnsB G190-F584 and ShTnsB G190-L494 (62.5 μmol) were immobilized on 25 μl amylose agarose beads (New England Biolabs) in pull-down buffer (20 mM HEPES-KOH pH 7.5, 200 mM KCl, 10 mM MgCl2, 1 mM DTT) by incubation for 30 min at 4 °C. The beads were resuspended and washed three times with 200 μL of pull-down buffer. In parallel, to allow filament formation, TnsC was first diluted to a final concentration of 8 μM in pull-down buffer and mixed at a molar ratio of 5:1 (TnsC:DNA) with a double-stranded 92-bp DNA duplex (Supplementary Table 1) in a 24 μL reaction volume and incubated for 10 min at 25 °C. Upon addition of nucleotide (ADP, ATP, or AMPPNP) at a final concentration of 1 mM, the 25 μL reactions were further incubated for 20 min at 37 °C. The 25 μL mixture containing ShTnsC was then added to the amylose beads containing TnsB as bait, followed by incubation for 15 min at 37 °C. Beads were then washed three times with 200 μL of pull-down buffer. Proteins were eluted from the beads by adding SDS loading buffer, and analyzed on 4–15% gradient polyacrylamide gel (Bio-Rad).
DNA binding assays
Increasing concentrations of wild-type or mutant ShTnsC (0 nM, 1.3 μM, 2.5 μM, 8 μM) were incubated with a fluorescently labeled double-stranded 27 bp duplex oligonucletide (20 nM final concentration) (Supplementary Table 1) for 30 min at 37 °C in a buffer containing 20 mM HEPES-KOH pH 7.5, 200 mM KCl, 10 mM MgCl2, 1 mM DTT and 1 mM AMPPNP, in a final volume of 20 μL. Reactions were resolved on a 4–20% native polyacrylamide gel (Bio-Rad) and imaged using a Typhoon scanner (GE Helthcare).
Crystallization and structure determination of TniQ
Crystals of ShTniQ were grown using the hanging drop vapor diffusion method. ShTniQ at a concentration of 6.3 mg mL−1 was mixed in a 1:1 ratio (1 μL + 1 μL) with reservoir solution containing 100 mM HEPES-KOH pH 7.5, 17–25 % PEG6000 and 250 mM NaCl. Crystals were cryoprotected with reservoir solution supplemented with 25 % (v/v) ethylene glycol and flash-cooled in liquid nitrogen. Data were collected at a temperature of 100 K at the beamline PXIII of the Swiss Light Source (Paul Scherrer Institute, Villigen, Switzerland) using the DA+ data collection software and processed with XDS32. The crystals belonged to space group P212121 and contained four copies of ShTniQ per asymmetric unit. Experimental phases were obtained by the single-wavelength anomalous diffraction (SAD) method from data measured at a wavelength of 1.0000 Å, utilizing the anomalous signal of Zn+2 scatters present in the protein. Initial phases were calculated using Phenix33 (Autosol34) and density modification of experimental phases in AutoSHARP35 resulted in an interpretable map which was used for initial model building using ARP/wARP36. The atomic model was completed by iterative building in Coot37 and refined with Phenix.refine38 (Supplementary Table 2).
ShCas12k-sgRNA-DNA ternary complex sample preparation and cryo-EM data collection
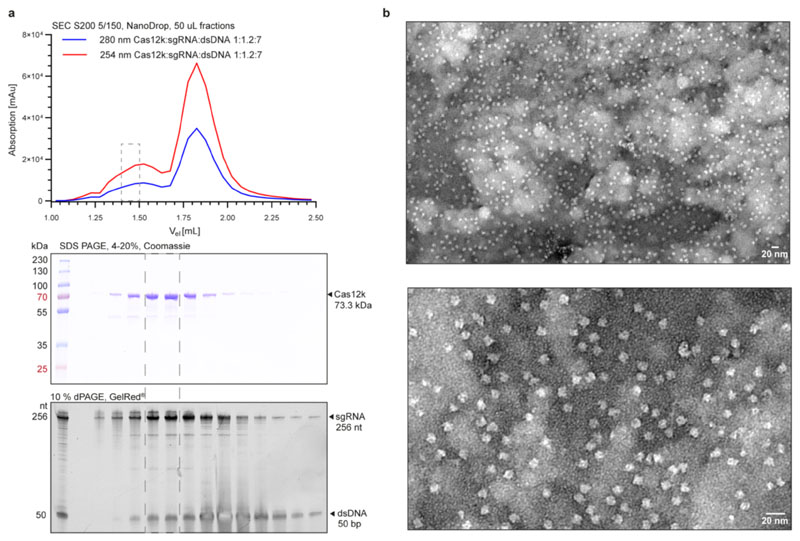
The target strand and non-target strand DNAs were sourced as synthetic, HPLC-purified oligonucleotides (IDT) and annealed in a 1:1 molar ratio. sgRNA was pre-incubated with ShCas12k for 20 min at 37 °C in assembly buffer (20 mM HEPES-KOH pH 7.5, 50 mM KCl, 10 MgCl2, 1 mM DTT). dsDNA was added and the sample was further incubated for 20 min at 37 °C. The final sample contained 47.8 μM ShCas12k, 57.4 μM sgRNA and 334.6 μM dsDNA (1:1.2:7 molar ratio) in 20 mM HEPES-KOH pH 7.5, 50 mM KCl, 10 mM MgCl2, 1 mM DTT. The sample was purified by size exclusion chromatography using a Superdex 200 (5/150) column. Eluted fractions were analysed by SDS-PAGE on an Any kDa polyacrylamide gel (Bio-Rad, stained with Coomassie) and by denaturing PAGE on a 10 % polyacrylamide- 7 M urea gel upon proteinase K digestion for 15 min at 37 °C. 2.5 μL of selected elution fractions were applied to a 300-mesh holey carbon grid (Au R1.2/1.3, Quantifoil Micro Tools), blotted 2 s at 75 % humidity, 4 °C, plunge frozen in liquid ethane (using a Vitrobot Mark IV plunger, FEI) and stored in liquid nitrogen. Cryo-EM data collection was performed on a FEI Titan Krios microscope operated at 300 kV equipped with a Gatan K3 direct electron detector using counting mode (EMBL Heidelberg, Germany). A total of 10,952 micrographs were recorded at a calibrated magnification of 130,000 x with a pixel size of 0.64 Å. Each movie comprises 40 subframes with a total dose of 51.81 e− Å−2. Data acquisition was performed automatically using SerialEM39 with four shots per hole at −0.9 μm to −1.9 μm defocus (0.1 μm steps).
Image processing and model building of the Cas12k-sgRNA-DNA complex
ShCas12k-sgRNA-dsDNA data was processed using cryoSPARC40. 10,952 movies were imported and motion-corrected (patch motion correction (multi)). Upon patch CTF estimation (multi), exposures were selected by estimated resolution (better than 3.5 Å) and defocus (<2 μm), yielding 10,866 movies. Particles were picked on denoised micrographs using Topaz41 with the pretrained model ResNet16 (64 units). Particle picks were inspected and selected particles (3.3 million) extracted in a box size of 300 x 300 pixel (fourier cropped to 100 x 100 pixel) and subjected to 2D classification. Particle-containing classes were selected (101 classes, 2.2 million particles) and rebalanced to 6 super-classes each containing a maximum of 200,000 particles, yielding a final set of 1.2 million particles. The particle stack was used to calculate ab initio models. Two ab initio models matching the appearance of 2D classes were selected as volumes for heterogeneous refinement with the particle stack, resulting in reconstructions of eight 3D classes. Classes were inspected visually, respective particles re-extracted in unbinned form and subjected to non-uniform refinement using the respective size-corrected volume from the heterogeneous refinement as input. The local resolution was calculated based on the resulting map using the local resolution functionality in cryoSPARC and plotted on the map using UCSF Chimera42. The structure model for the ShCas12k-sgRNA-target DNA complex was built in Coot37. The sgRNA and target DNA were built de novo exclusively. The model was refined in Coot using restraints for the nucleic acids calculated with the LibG43 script (base pair, stacking plane and sugar pucker restraints) in ccp4 and finally refined using Phenix33,38. Real space refinement was performed with the global minimization and atomic displacement parameter (ADP) refinement options selected. Secondary structure restraints, side chain rotamer restraints, and Ramachandran restraints were used. Key refinement statistics are listed in Extended Data Table 1. The final atomic model includes Cas12k residues 1-43, 56-70, 76-94, and 278-636, sgRNA nucleotides 5-72, 77-164, 174-215, and 221-239, 17 nucleotides of TS DNA and 10 nucleotides of NTS DNA. The quality of the atomic model, including basic protein and DNA geometry, Ramachandran plots, clash analysis and model cross-validation, was assessed with MolProbity44,45 and the validation tools in Phenix. Structural superposition was performed in Coot using the secondary structure matching46 (SSM) function. Figure preparation for maps and models and calculation of map contour levels was performed using UCSF ChimeraX47.
ShTnsC-dsDNA complex cryo-EM sample preparation and data collection
Purified ShTnsC was diluted to a final concentration of 6.4 μM in a buffer containing 20 mM HEPES-KOH pH 7.5, 200 mM KCl, 10 mM MgCl2, 1 mM DTT and mixed with a 92 nt dsDNA oligonucleotide containing two mismatched regions (Supplementary Table 1) at a ratio of 5:1 (TnsC:DNA) in a 21.4 μL reaction volume and incubated for 10 min at 25 °C. Upon addition of AMPPNP at a final concentration of 1 mM, the sample was further incubated for 20 min at 37 °C. Sample vitrification was performed with a Leica EM GP2 plunge freezer at 10 °C and at 70 % humidity. 3.5 μL sample were applied onto a freshly glow-discharged 200-mesh copper 2 nm C R1.2/1.3 grids (Quantifoil Micro Tools). The grids were blotted for 1 s and plunge-vitrified in liquid ethane. Cryo-EM data collection was performed on a FEI Titan Krios G3i microscope (University of Zurich, Switzerland) operated at 300 kV equipped with a Gatan K3 direct electron detector in super-resolution counting mode. A total of 5,055 micrographs were recorded at a calibrated magnification of 130,000 × resulting in super-resolution pixel size of 0.325 Å. Each movie comprised 36 subframes with a total dose of 66.39 e− Å−2. Data acquisition was performed with EPU Automated Data Acquisition Software for Single Particle Analysis (ThermoFisher) with three shots per hole at −1.0 μm to −2.4 μm defocus (0.2 μm steps).
Image processing, model building and validation of the ShTnsC-dsDNA complex
All movies were motion-corrected and dose-weighted with MotionCor248. Aligned, non-dose-weighted micrographs were then used to estimate the contrast transfer function (CTF) with Gctf49. All subsequent image processing steps were performed using helical reconstruction in RELION50,51. Approximately 1,100 segments were manually picked in RELION. One round of reference-free 2D classification was performed to produce templates for reference-dependent auto-picking. A limited resolution E-step (low-pass filter) of 10 Å was applied to prevent overfitting. Using the resulting 2D classes as templates, overlapping helical filament segments were automatically picked with an inter-box distance of 10 Å. A total of 1,733,884 segments were extracted with a box size of 352 pixels from the full dataset and subjected to three cycles of reference-free 2D classification to remove low-quality filaments, yielding 743,027 particles. Three iterative cycles of 3D auto-refinement in RELION using a cylinder with a 12 nm diameter as the initial model were conducted to produce initial reconstructions. A spherical mask with a diameter equal to 90 % of the box size was applied. Selected 2D classes were then used with the best ab initio model to perform 3D auto-refinement with a local search of helical symmetry (twist 58°–62° and rise 6–8 Å). The helical parameters were estimated based on a pitch of 41 Å calculated from the power spectra and assuming a number of 6 units/turns. The central Z-height parameter in RELION was set to 30 %. The output volume (3.94 Å resolution) was used as the reference model for 3D classification. The particles were divided into four classes in 3D classification. A 3D class with the most clearly discernible secondary structure features (59.44° twist and 6.65 Å rise and 77,544 particles) was selected and 3D auto-refined. A single round of Bayesian polishing, further helical refinement and post-processing with a soft mask applied around the filament produced a volume with final reported resolution of 3.57 Å, final twist 59.72° and rise 6.78 Å. The 3D reconstruction was sharpened with a B-factor of −79.50 Å−2. The overall map resolution reported in Extended Data Table 1 was derived from Fourier shell correlation (FSC) calculations between reconstructions from two independently refined half-maps, and reported resolutions are based on the gold-standard criterion. Local resolution was estimated with RELION. For model building, a TnsC homology model based on the crystal structure of the DNA replication initiator factor ORC2 (PDB:1W5T)52 was generated using the Phyre2 server53 and the two main domains (α/β domain and C-terminal domain) were manually docked separately as rigid bodies in the cryo-EM density map with UCSF Chimera42, followed by real space fitting with the Fit in Map function. The atomic model of a ShTnsC protomer was manually rebuilt in Coot37. The coordinated AMPPNP molecules and magnesium ions were manually docked in Coot. The DNA duplex was manually built in Coot and then subjected to refinement in Coot using base pair, stacking plane and sugar pucker restraints generated in LibG43 in ccp4. The sequence of the dsDNA was randomly selected and is purely representative as any DNA sequence information was lost during helical symmetry averaging. The adjacent subunits in the filament were then generated by applying the helical symmetry from RELION to the rebuilt atomic model. Real-space refinement of the resulting model was performed in Phenix33,38 with the global minimization and atomic displacement parameter (ADP) refinement options selected. The following restraints were used in real-space refinement: secondary structure restraints, non-crystallographic symmetry (NCS) restraints between the protein subunits, side chain rotamer restraints, and Ramachandran restraints. Key refinement statistics are listed in Extended Data Table 1. The final atomic model includes 7 copies of ShTnsC (one helical filament turn plus an additional TnsC copy), each bound to a single Mg2+ ion and AMPPNP, and a 22-bp DNA duplex. The quality of the atomic model, including basic protein and DNA geometry, Ramachandran plots, and clash analysis, was assessed and validated with MolProbity44,45 and validation tools as implemented in Phenix. Structural superposition was performed in Coot using the secondary structure matching46 (SSM) function. Figure preparation for maps and models and calculation of map contour levels was performed using UCSF ChimeraX47.
Extended Data
Extended Data Fig. 1. ShCas12k-sgRNA-target DNA complex assembly.
a, Top: Size exclusion chromatography analysis of the ShCas12k-sgRNA-target DNA complex. Middle: SDS-PAGE analysis of fractions from a. Proteins were visualised by Coomassie blue staining. Bottom: denaturing PAGE analysis of fractions from a. Nucleic acids were stained with a fluorescent dye (Gel Red). b, Representative negative stain EM micrographs of the ShCas12k-sgRNA-dsDNA complex at 68,000x magnification (top) and 180,000x magnification (bottom). Experiment was repeated three times independently with similar results. For gel source data, see Supplementary Figure 1.
Extended Data Fig. 2. ShCas12k cryo-EM image processing workflow and model validation.
a, Cryo-EM image processing workflow for the ShCas12k-sgRNA-target DNA complex. Fourier Shell Correlations (FSC) of ShCas12k reconstruction from two independently refined half-maps. The gold-standard cutoff (FSC = 0.143) is marked with a blue line. b, Final electron density map colored according to the local resolution. c, Validation of Cas12k-sgRNA-target DNA structure model.
Extended Data Fig. 3. Structural comparison between Cas12k and CasX.
a, Structural model of Cas12k-sgRNA-target DNA (left) and CasX-sgRNA-target DNA (right, PDB: 6ny222) complexes. The structures were superimposed using secondary structure matching46 of the RuvC domains and are shown in identical orientations. b, Zoom-in views of the tracrRNA-crRNA triplexes.
Extended Data Fig. 4. Structural features and functional analysis of ShCAST sgRNA.
a, Close-up view of the RNA:DNA duplex in proximity of the Bridge Helix (BH). b, Close-up view of the sgRNA-TS DNA heteroduplex in the ShCas12k-sgRNA-target DNA complex. c, Structural model of the ShCas12k sgRNA with detailed views of the 5’ terminal segment (top left, 6.6 σ contour level) and the triplex junction (bottom right, 9.0 σ contour level) of the tracrRNA forming ribose-zipper and A-minor interactions, the pseudoknot duplex (bottom left, 9.0 σ contour level) and the central stack junction (top right, 7.6 σ contour level). d, Droplet digital PCR (ddPCR)-based analysis of the transposition activity of structure-based sgRNA scaffold mutants in the ShCAST system. Data are presented as mean ± s.d. (n=3 biologically independent replicates).
Extended Data Fig. 5. ShTnsC filament formation and cryo-EM image processing of ShTnsC•AMPPNP•dsDNA helical filaments.
a, Representative negative-stain EM micrographs of ShTnsC in the presence of a 92 bp dsDNA and ATP or ATPγS. Scale bars, 100 nm. Magnification, 120,000x. Experiment was repeated twice independently with similar results. b, Cryo-EM data processing workflow for the ShTnsC-dsDNA complex. Selected 2D class averages used in image reconstruction and intermediate and final reconstructions are shown. c, Fourier Shell Correlations (FSC) of ShTnsC filament reconstruction from two independently refined half-maps. The gold-standard cutoff (FSC = 0.143) is marked with dashed lines. The resolution value of the FSC corrected curve at this level is indicated. d, Structural alignment of the cryo-EM structure of ShTnsC (blue) and the crystal structure of the Aeropyrum pernix ORC2 protein (grey; PDB ID: 1W5T52) used as initial homology model for model building (single protomers). The root mean square deviation (RMSD) between the structures is 1.41 Å over 79 pruned atom pairs, as calculated in Chimera. e, Local resolution estimation (Å) for the cryo-EM volumes of the ShTnsC•AMPPNP•dsDNA helical filaments. f, Local resolution estimation (Å) of the bound DNA duplex.
Extended Data Fig. 6. Biochemical analysis of ShTnsC mutants.
a, SDS-PAGE analysis of purified wild-type and ATPase activity mutant ShTnsC proteins. b, Representative negative-stain EM micrographs of ShTnsC ATPase activity mutants incubated in the presence of dsDNA and ATP or AMPPNP. Magnification, 98,000x. Experiment was repeated twice independently with similar results. c, SDS-PAGE analysis of purified wild-type and DNA binding mutant ShTnsC proteins. d, Representative negative stain EM micrographs of ShTnsC in the presence of AMPPNP and dsDNA and with mutations in the DNA binding interface. Magnification, 98,000x. Experiment was repeated twice independently with similar results. e, Electromobility shift assay using fluorophore-labeled 27-bp dsDNA in the presence of AMPPNP and either wild-type or DNA binding mutant TnsC proteins. -, no protein control. Experiment was repeated twice independently with similar results. For gel source data, see Supplementary Figure 1.
Extended Data Fig. 7. Role of ShTnsB in ShTnsC filament disassembly and crystal structure of ShTniQ.
a, Representative negative stain EM micrographs of dsDNA-bound ShTnsC filaments in the presence of ShTnsB and AMPPNP or ATP. Magnification, 98,000x. b, Quantification of filament length in the indicated samples. Data represents the average length ± s.d. of 50 randomly selected filaments in each sample. Experiment in a and quantification in b were repeated twice independently with similar results. c, Top panel: Domain architecture of TniQ proteins. HTH, helix turn helix motif. wHTH, winged HTH. ZnF, zinc-finger motif. Bottom panel: Structural alignment of the crystal structures of type V ShTniQ (red) and type I-F Vibrio cholerae TniQ (grey and beige protomers; PDB ID: 6V9P19) (bottom panel). The root mean square deviation (RMSD) between the structures is 1.16 Å over 55 pruned atom pairs, as calculated in Chimera. d, Size exclusion chromatography analysis of ShTniQ. The retention volume corresponds to that of a protein of 12 kDa, as calculated based on molecular weight standards. The theoretical molecular weight of ShTniQ is 19 kDa.
Extended Data Fig. 8. Mechanistic model of Cas12k-directed transposition.
Schematic diagram depicting transposition of type V-K CRISPR-associated transposons. Cas12k in association with a crRNA-tracrRNA dual guide RNA recognizes target DNA sequences, forming a partial R-loop structure. Full R-loop is formed upon recruitment of TnsC by interactions with DNA-bound Cas12k, which nucleates ATP-dependent formation of a helical filament around structurally remodeled DNA. Filament growth is restricted by TniQ capping the Cas12k-distal end of the TnsC filament. The TnsC filament serves as a recruitment platform for TnsB, which interacts directly with TnsC and stimulates its ATPase activity. This leads to filament disassembly, making DNA downstream of the PAM accessible for transposon insertion. In the post-hydrolysis state, TnsC forms a single-turn helical hexamer around target DNA, possibly acting as a molecular ruler to define the spacing between the Cas12k binding and transposon insertion sites (60-66 nt downstream of the PAM).
Extended Data Table 1. Cryo-EM data collection, refinement and validation statistics for the ShCas12k-sgRNA-target DNA complex structure and the ShTnsC-AMPPNP-dsDNA structure.
| ShCas12k-sgRNA-dsDNA (EMDB-13486) (PDB 7PLA) |
ShTnsC-AMPPNP-dsDNA (EMDB-13489) (PDB 7PLH) |
|
|---|---|---|
| Data collection and processing | ||
| Magnification | 130,000 | 130,000 |
| Voltage (kV) | 300 | 300 |
| Electron exposure (e–/Å2) | 51.82 | 66.39 |
| Defocus range (μm) | -0.9 to -1.9 (0.1 steps) | -1.0 to -2.4 (0.2 steps) |
| Pixel size (Å) | 0.64 | 0.325 |
| Symmetry imposed | C1 | C1 |
| Initial particle images (no.) | 10,952 | 5,055 |
| Final particle images (no.) | 10,866 | 4,988 |
| Map resolution (Å) | 3.04 | 3.58 |
| FSC threshold | 0.143 | 0.143 |
| Map resolution range (Å) | 2.7-7.0 | 3.58-5.05 |
| Refinement | ||
| Initial model used (PDB code) | n. a. | 1W5T |
| Model resolution (Å) | 4.5 | 3.9 |
| FSC threshold | 0.5 | 0.5 |
| Map sharpening B factor (Å2) | -113.9 | -79.50 |
| Model composition | ||
| Non-hydrogen atoms | 8701 | 15542 |
| Protein residues | 437 | 1820 |
| Nucleotide residues | 244 | 44 |
| Ligands | 0 | MG :7 AMPPNP :7 |
| B factors (Å2) min/max/mean | ||
| Protein | 50.00/288.87/177.33 | 17.40/95.74/39.30 |
| Nucleotide | 20.00/30.00/29.96 | 20.00/20.00/20.00 |
| Ligand | 39.53/40.64/40.61 | |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.008 | 0.010 |
| Bond angles (°) | 1.152 | 1.659 |
| Validation | ||
| MolProbity score | 1.71 | 2.36 |
| Clashscore | 9.53 | 13.30 |
| Poor rotamers (%) | 0.26 | 8.98 |
| Ramachandran plot | ||
| Favored (%) | 96.74 | 98.06 |
| Allowed (%) | 3.26 | 1.94 |
| Disallowed (%) | 0.00 | 0.00 |
| Ramachandran Z score | ||
| whole | -0.03 | -2.29 |
| helix | 1.11 | -0.98 |
| sheet | -1.19 | -1.95 |
| loop | -1.32 | -2.19 |
Extended Data Table 2. X-ray crystallographic data collection and refinement statistics for ShTniQ.
| ShTniQ (PDB 7OXD) |
|
|---|---|
| Data collection | |
| Space group | P 21 21 21 |
| Cell dimensions | |
| a, b, c (Å) | 41.636 49.875 86.8 |
| α, β, γ (°) | 90 90 90 |
| Resolution (Å) | 43.40 - 1.3 (1.346 - 1.3) |
| R merge | 0.03864 (0.9239) |
| I / σI | 23.26 (1.84) |
| Completeness (%) | 99.75 (99.72) |
| Redundancy | 6.7 (6.3) |
| Refinement | |
| Resolution (Å) | 43.24 - 1.3 |
| No. reflections | 45126 (4426) |
| Rwork / Rfree | 0.1881 (0.2866)/ 0.1952 (0.2872) |
| No. atoms | 1470 |
| Protein | 1232 |
| Ligand/ion | 2 |
| Water | 236 |
| B-factors | |
| Protein | 18.97 |
| Ligand/ion | 17.19 |
| Water | 30.86 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.005 |
| Bond angles (°) | 0.92 |
Supplementary Material
Acknowledgements
We are grateful to Marta Sawicka and Simona Sorrentino (University of Zurich Center for Microscopy and Image Analysis) for assistance with cryo-EM sample preparation and data collection. We acknowledge the Cryo-Electron Microscopy Service Platform at EMBL Heidelberg for instrument access and are grateful to Felix Weis for assistance with data collection. We are grateful to Rodolfo Ciuffa for advice on helical reconstruction, Martin Pacesa for help with cryo-EM data processing and Franziska Boneberg for technical assistance. We thank Beat Blattmann at the Protein Crystallization Center (University of Zurich) for assistance with crystallization screening; Meitian Wang, Vincent Olieric, and Takashi Tomizaki at the Swiss Light Source (Paul Scherrer Institute, Villigen, Switzerland) for assistance with X-ray diffraction measurements. We thank Susanne Kreutzer and the ETH Genome Engineering and Measurement Lab for assistance with ddPCR assays. We are grateful to Guy Riddhough (Life Science Editors) for assistance with manuscript editing. This work was supported by Swiss National Science Foundation Project Grant 31003A_182567 and European Research Council (ERC) Consolidator Grant no. ERC-CoG-820152. I.Q. was supported by FEBS and EMBO (ALTF 296-2020) long-term postdoctoral fellowships. M.J. is an International Research Scholar of the Howard Hughes Medical Institute, and Vallee Scholar of the Bert L & N Kuggie Vallee Foundation.
Footnotes
Author contributions
I.Q., M.S. and M.J. conceived the study and designed experiments. I.Q. determined the structure of ShTnsC. M.S. conducted cryo-EM analysis of Cas12k. M.S. and I.Q. carried out biochemical and ddPCR functional experiments and negative-stain EM analysis. S.O. expressed and purified ShTnsC mutant proteins. C.C. assisted with sample preparation for biochemical and ddPCR assays. I.Q., M.S. and M.J. analyzed the data and wrote the manuscript.
Competing interest
The authors declare no competing interests.
Additional information
Correspondence and requests for materials should be addressed to M.J.
Data availability
Maps and atomic coordinates of the reported cryo-EM structures have been deposited in the Electron Microscopy Data Bank under accession codes EMD-13486 (ShCas12k-sgRNA-DNA complex) and EMD-13489 (ShTns-DNA filament), and the Protein Data Bank with accession codes 7PLA (ShCas12k-sgRNA-DNA complex) and 7PLH (ShTns-DNA filament). Structure factors and atomic coordinates of the reported X-ray crystallographic structure of ShTniQ has been deposited in the Protein Data Bank with accession code 7OXD.
References
- 1.Sorek R, Lawrence CM, Wiedenheft B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu Rev Biochem. 2013;82:237–266. doi: 10.1146/annurev-biochem-072911-172315. [DOI] [PubMed] [Google Scholar]
- 2.Faure G, et al. CRISPR-Cas in mobile genetic elements: counter-defence and beyond. Nat Rev Microbiol. 2019;17:513–525. doi: 10.1038/s41579-019-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klompe SE, Vo PLH, Halpin-Healy TS, Sternberg SH. Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature. 2019;571:219–225. doi: 10.1038/s41586-019-1323-z. [DOI] [PubMed] [Google Scholar]
- 4.Petassi MT, Hsieh SC, Peters JE. Guide RNA Categorization Enables Target Site Choice in Tn7-CRISPR-Cas Transposons. Cell. 2020;183:1757–1771.:e1718. doi: 10.1016/j.cell.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters JE, Makarova KS, Shmakov S, Koonin EV. Recruitment of CRISPR-Cas systems by Tn7-like transposons. Proc Natl Acad Sci U S A. 2017;114:E7358–E7366. doi: 10.1073/pnas.1709035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito M, et al. Dual modes of CRISPR-associated transposon homing. Cell. 2021;184:2441–2453.:e2418. doi: 10.1016/j.cell.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strecker J, et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science. 2019;365:48–53. doi: 10.1126/science.aax9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koonin EV, Makarova KS, Wolf YI. Evolutionary Genomics of Defense Systems in Archaea and Bacteria. Annu Rev Microbiol. 2017;71:233–261. doi: 10.1146/annurev-micro-090816-093830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters JE, Craig NL. Tn7: smarter than we thought. Nat Rev Mol Cell Biol. 2001;2:806–814. doi: 10.1038/35099006. [DOI] [PubMed] [Google Scholar]
- 10.May EW, Craig NL. Switching from cut-and-paste to replicative Tn7 transposition. Science. 1996;272:401–404. doi: 10.1126/science.272.5260.401. [DOI] [PubMed] [Google Scholar]
- 11.Sarnovsky RJ, May EW, Craig NL. The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products. EMBO J. 1996;15:6348–6361. [PMC free article] [PubMed] [Google Scholar]
- 12.Choi KY, Spencer JM, Craig NL. The Tn7 transposition regulator TnsC interacts with the transposase subunit TnsB and target selector TnsD. Proc Natl Acad Sci U S A. 2014;111:E2858–2865. doi: 10.1073/pnas.1409869111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronning DR, et al. The carboxy-terminal portion of TnsC activates the Tn7 transposase through a specific interaction with TnsA. EMBO J. 2004;23:2972–2981. doi: 10.1038/sj.emboj.7600311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stellwagen AE, Craig NL. Gain-of-function mutations in TnsC, an ATP-dependent transposition protein that activates the bacterial transposon Tn7. Genetics. 1997;145:573–585. doi: 10.1093/genetics/145.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra R, McKenzie GJ, Yi L, Lee CA, Craig NL. Characterization of the TnsD-attTn7 complex that promotes site-specific insertion of Tn7. Mob DNA. 2010;1:18. doi: 10.1186/1759-8753-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parks AR, et al. Transposition into replicating DNA occurs through interaction with the processivity factor. Cell. 2009;138:685–695. doi: 10.1016/j.cell.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolkow CA, DeBoy RT, Craig NL. Conjugating plasmids are preferred targets for Tn7. Genes Dev. 1996;10:2145–2157. doi: 10.1101/gad.10.17.2145. [DOI] [PubMed] [Google Scholar]
- 18.Halpin-Healy TS, Klompe SE, Sternberg SH, Fernandez IS. Structural basis of DNA targeting by a transposon-encoded CRISPR-Cas system. Nature. 2020;577:271–274. doi: 10.1038/s41586-019-1849-0. [DOI] [PubMed] [Google Scholar]
- 19.Jia N, Xie W, de la Cruz MJ, Eng ET, Patel DJ. Structure-function insights into the initial step of DNA integration by a CRISPR-Cas-Transposon complex. Cell Res. 2020;30:182–184. doi: 10.1038/s41422-019-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Zhang H, Xiao RJ, Chang LF. Cryo-EM structure of a type I-F CRISPR RNA guided surveillance complex bound to transposition protein TniQ. Cell Res. 2020;30:179–181. doi: 10.1038/s41422-019-0268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang BB, Xu WH, Yang H. Structural basis of a Tn7-like transposase recruitment and DNA loading to CRISPR-Cas surveillance complex. Cell Res. 2020;30:185–187. doi: 10.1038/s41422-020-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JJ, et al. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature. 2019;566:218–223. doi: 10.1038/s41586-019-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li ZC, NL, Peters JE. In: Bacterial integrative mobile genetic elements. Roberts AP, Mullany P, editors. Landes Bioscience; Austin, Texas: 2013. pp. 1–32. [Google Scholar]
- 24.Arias-Palomo E, Berger JM. An Atypical AAA+ ATPase Assembly Controls Efficient Transposition through DNA Remodeling and Transposase Recruitment. Cell. 2015;162:860–871. doi: 10.1016/j.cell.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno N, et al. MuB is an AAA+ ATPase that forms helical filaments to control target selection for DNA transposition. Proc Natl Acad Sci U S A. 2013;110:E2441–2450. doi: 10.1073/pnas.1309499110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stellwagen AE, Craig NL. Avoiding self: two Tn7-encoded proteins mediate target immunity in Tn7 transposition. EMBO J. 1997;16:6823–6834. doi: 10.1093/emboj/16.22.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene EC, Mizuuchi K. Target immunity during Mu DNA transposition. Transpososome assembly and DNA looping enhance MuA-mediated disassembly of the MuB target complex. Mol Cell. 2002;10:1367–1378. doi: 10.1016/s1097-2765(02)00733-5. [DOI] [PubMed] [Google Scholar]
- 28.Greene EC, Mizuuchi K. Dynamics of a protein polymer: the assembly and disassembly pathways of the MuB transposition target complex. EMBO J. 2002;21:1477–1486. doi: 10.1093/emboj/21.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JU, et al. Structural basis for target site selection in RNA-guided DNA transposition systems. Science. 2021;373:768–774. doi: 10.1126/science.abi8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swarts DC, Jinek M. Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a. Mol Cell. 2019;73:589–600.:e584. doi: 10.1016/j.molcel.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders C, Niewoehner O, Jinek M. In Vitro Reconstitution and Crystallization of Cas9 Endonuclease Bound to a Guide RNA and a DNA Target. Methods Enzymol. 2015;558:515–537. doi: 10.1016/bs.mie.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liebschner D, et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol. 2019;75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terwilliger TC, et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr D Biol Crystallogr. 2009;65:582–601. doi: 10.1107/S0907444909012098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vonrhein C, Blanc E, Roversi P, Bricogne G. Automated structure solution with autoSHARP. Methods Mol Biol. 2007;364:215–230. doi: 10.1385/1-59745-266-1:215. [DOI] [PubMed] [Google Scholar]
- 36.Langer G, Cohen SX, Lamzin VS, Perrakis A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc. 2008;3:1171–1179. doi: 10.1038/nprot.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afonine PV, et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D Struct Biol. 2018;74:531–544. doi: 10.1107/S2059798318006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de la Cruz MJ, Martynowycz MW, Hattne J, Gonen T. MicroED data collection with SerialEM. Ultramicroscopy. 2019;201:77–80. doi: 10.1016/j.ultramic.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 41.Bepler T, et al. Positive-unlabeled convolutional neural networks for particle picking in cryoelectron micrographs. Res Comput Mol Biol. 2018;10812:245–247. [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 43.Brown A, et al. Tools for macromolecular model building and refinement into electron cryomicroscopy reconstructions. Acta Crystallogr D Biol Crystallogr. 2015;71:136–153. doi: 10.1107/S1399004714021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prisant MG, Williams CJ, Chen VB, Richardson JS, Richardson DC. New tools in MolProbity validation: CaBLAM for CryoEM backbone, UnDowser to rethink “waters,” and NGL Viewer to recapture online 3D graphics. Protein Sci. 2020;29:315–329. doi: 10.1002/pro.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 47.Pettersen EF, et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang K. Gctf: Real-time CTF determination and correction. J Struct Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He S, Scheres SHW. Helical reconstruction in RELION. J Struct Biol. 2017;198:163–176. doi: 10.1016/j.jsb.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheres SH. A Bayesian view on cryo-EM structure determination. J Mol Biol. 2012;415:406–418. doi: 10.1016/j.jmb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singleton MR, et al. Conformational changes induced by nucleotide binding in Cdc6/ORC from Aeropyrum pernix. J Mol Biol. 2004;343:547–557. doi: 10.1016/j.jmb.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 53.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Maps and atomic coordinates of the reported cryo-EM structures have been deposited in the Electron Microscopy Data Bank under accession codes EMD-13486 (ShCas12k-sgRNA-DNA complex) and EMD-13489 (ShTns-DNA filament), and the Protein Data Bank with accession codes 7PLA (ShCas12k-sgRNA-DNA complex) and 7PLH (ShTns-DNA filament). Structure factors and atomic coordinates of the reported X-ray crystallographic structure of ShTniQ has been deposited in the Protein Data Bank with accession code 7OXD.