Chronic hepatitis C virus infection (HCV) affects approximately 71 million individuals worldwide [1], being a major etiological factor for the development of liver cirrhosis and hepatocellular carcinoma (HCC). Acute HCV infection often progresses to chronicity and is characterized by a non-resolving liver inflammation leading to a broad range of alterations in the tissue microenvironment. About ninety percent of HCC cases arise in the context of chronic liver inflammation, highlighting the central role of this persistent immune response in disease pathogenesis [2]. Despite efficient antiviral therapy by direct acting antivirals (DAA), the risk of HCC development cannot be fully eliminated in patients with advanced liver disease [3]. In this regard, accumulating evidence suggests a potentially persisting proto-oncogenic environment created by virus-induced changes in the cell signaling [4–7]. Therefore, even in the DAA era, the understanding of virus-host interactions during chronic HCV-associated inflammation is key to identify and treat patients at high risk to develop HCC.
In this context, a recent article in Journal of Hepatology by Johannes G. Bode’s laboratory at the Heinrich-Heine University in Germany provides a novel mechanism by which HCV infection contributes to this pathologic inflammatory response [8]. Aiming to identify chemokines regulated by HCV, the authors performed a functional screen using an HCV subgenomic replicon system and identified an HCV-induced upregulation of C-X-C motif chemokine receptor 2 (CXCR2) ligands (CXCLs) 1, 2, 3 and 8. Consistently, similar results were obtained upon HCV infection using the cell culture-derived strain JC1. Having previously shown that HCV infection enhances epidermal growth factor (EGF) signaling, the authors next explored the possible involvement of this pathway on CXCR2 ligand expression. EGFR perturbation studies combining RNAi knockdown of EGF and the use of MAPK inhibitors, confirmed an HCV-induced upregulation of CXCL8 via EGFR and the MAP kinase kinase MEK1. Additionally, knockdown of the p65 subunit of the NF-κB complex was sufficient to abrogate basal and EGF-induced CXCL8 expression in replicon-expressing cells, while in HCV-infected cells this mainly affected basal CXCL8 levels. This suggests that the observed enhancement of chemokine expression during HCV infection not only depends on the EGFR pathway but also on the activation of additional transcription factors such as NF-κB. The in vivo relevance of the data is emphasized by an association of HCV viral load with CXCL8 serum levels in chronically infected patients. Similarly, serum levels of EGF and CXCL8 tend to positively correlate although this did not reach statistical significance in their study cohort.
In a previous study, the authors demonstrated that HCV enhances EGFR signaling via NS3/4A-mediated proteolytic cleavage of T-cell protein tyrosine phosphatase (TC-PTP), one of the major negative regulators of EGFR tyrosine-kinase activity [9]. Indeed, here they demonstrate that NS3/4A expression alone enhances EGF-inducible CXCL8 expression, an effect that can be mimicked by knocking down TC-PTP. As the major role of chemokines is the recruitment of immune cells to the site of inflammation, the authors next evaluated if in the context of HCV replication EGF-induced release of chemokines influences leukocyte migration. Remarkably, the authors demonstrate that media from EGF-treated cell lines expressing the HCV subgenomic replicon enhances the migration of neutrophils, an effect that was not observed with EGF-conditioned media alone. This suggests that HCV infection modulates chemoattraction of immune cells to the liver via EGF-regulated chemokine secretion.
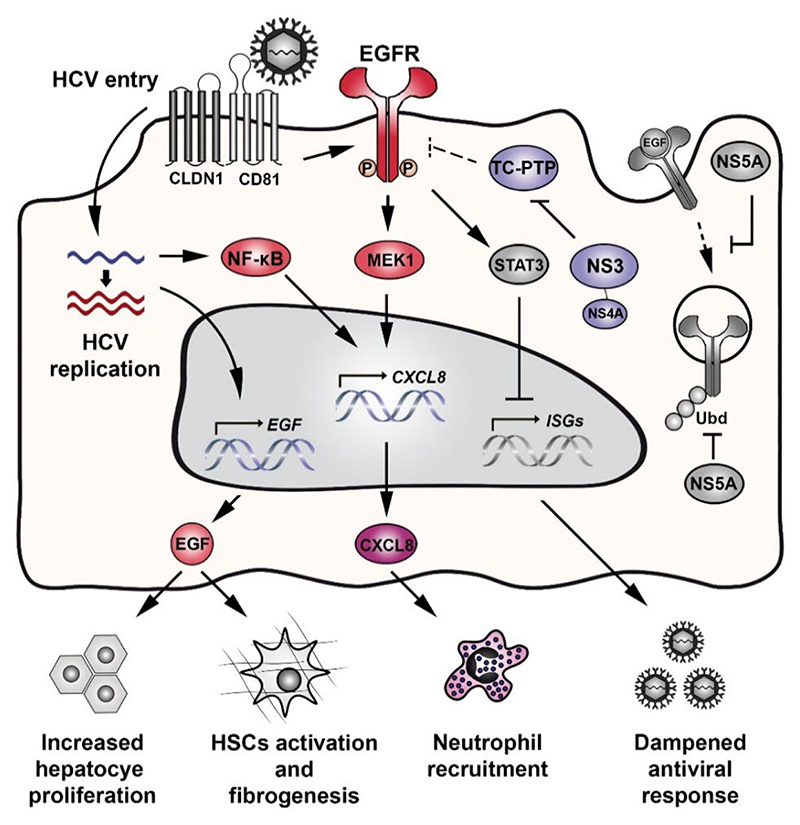
The findings of Christina Groepper and co-workers are not just relevant for our understanding of HCV-EGFR interaction but most importantly provide insight into the pathologic consequences of derailed EGF signaling for liver inflammation and HCC development (Fig. 1). EGFR is a host factor for HCV by facilitating the assembly of the host entry complex, viral glycoprotein-dependent membrane fusion and cell-to-cell transmission of the virus [7]. HCV requires EGFR signaling to maintain its life cycle but also induces these signals itself during binding to the receptor complex [6, 10]. Moreover, during HCV infection the non-structural protein NS5A prolongs EGFR signaling by perturbing its internalization and subsequent degradation [11, 12]. This leads to a persistent EGFR activation during chronic HCV infection that potentially contributes to an impaired antiviral response by modulating interferon alpha signaling via STAT3 [13].
Figure 1. Refined model of HCV-EGFR modulation and its impact on liver disease development.
HCV binding to the HCV entry receptor complex (i.e. CD81, CLDN1) at the cell surface induces EGFR phosphorylation and downstream signaling. EGFR activity is prolonged by the NS5A-mediated perturbation of EGFR internalization and degradation. As a consequence, prolonged EGFR activity is associated with an increased hepatocyte proliferation, HSCs activation, fibrogenesis and a dampened antiviral response via modulation of STAT3. Groepper et al., (colored pathway) demonstrated that HCV replication enhances the expression of CXCR2 ligands (e.g. CXCL8) by intermediary of an EGF-dependent mechanism and activation of the NF-κB signaling pathway. This is further favored via the proteolytic cleavage of TC-PTP by NS3/4A, resulting in increased EGFR activation. Upon EGF stimulation, the production of CXCL8 during HCV replication promotes the recruitment of neutrophils.
Their finding that HCV replication promotes EGF expression is highly relevant in the study of HCV-induced chronic liver disease, as the EGF pathway is a key driver associated with progression towards cirrhosis [14] and HCC development [15]. Equally interesting is the observation that HCV-induced EGF expression is a regulator of CXCR2 ligands. For example, HCV infection has been previously described to promote CXCL8 expression, which inhibits interferon antiviral activity and facilitates viral infection [16]. Hepatic CXCL8 is detected at low maintenance levels during acute HCV infection, although marked increases in serum and hepatic levels have been observed in HCV-infected patients with progressive inflammation and cirrhosis [17]. Indeed, CXCL8, which is associated with poor outcome in HCC patients, has been suggested as HCC biomarker [18]. Here, Groepper and co-workers validated a mechanistic concept between EGFR signaling and CXCL8 during HCV infection, that has been previously proposed for hepatomas [19]. Moreover, they provide a previously undescribed mechanism linking EGFR signaling to chemoattraction of immune cells. In macrophages EGFR knockout attenuates HCC development in mice [20]. EGF-mediated recruitment of neutrophils during HCV infection is potentially relevant for liver pathobiology, since it has detrimental effects on the host by contributing to the necro-inflammatory process [21].
Although further studies in larger patient cohorts are needed to consolidate the model proposed by Groepper and co-workers, the impact of their findings for liver disease and its association to EGF signaling is evident [22]. In future studies, it would be very interesting and potentially relevant to follow up HCV-induced EGF expression pattern in liver tissue and blood samples before and after sustained viral response and to compare them to liver fibrosis scores. Furthermore, does HCV genotype influences EGF and chemokine expression profiles since genotype 3 is associated with more severe liver disease manifestations? Taken together, this paper represents a further corroboration for the clinical potential of HCC chemo-preventive strategies based on regulators of signal transduction. Indeed, EGFR which is phosphorylated in hepatic stellate cells (HSCs) has been successfully targeted by the clinical EGFR inhibitor erlotinib in animal models, demonstrating proof of concept that EGF-based therapies attenuates chemically induced liver fibrosis and HCC nodules [14]. Therefore, EGFR or MAPK modulators could be part of a personalized immuno-therapeutic strategy modulating chemokine profiles and inflammatory responses associated with liver disease progression.
Acknowledgments
TFB acknowledges support by the European Union Horizon 2020 research and innovation program (ERC AdG HEPCIR – No. 667273; H2020 HEP-CAR – No. 667273; ERC POC PRELICAN – No. 755460), the NCI of the National Institutes of Health (1R21CA209940-01A1), the French National Research Agency (LABEX ANR-10-LABX-0028 HEPSYS) and the Office of the Assistant Secretary of Defense for Health Affairs (No. W81XWH-16-1-0363). JL and AARS acknowledge the French Agence Nationale de Recherche sur le Sida et les Hépatites Virales (ANRS) (ECTZ4236; ECTZ4446). All authors thank Atish Mukherji (Inserm U1110, Strasbourg, France) for critical reading of the manuscript.
Footnotes
Conflict of interest:
The authors declared that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- [1].Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- [2].Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- [3].Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719–726. doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- [4].Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61:S79–90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Van Renne N, Roca Suarez AA, Duong FH, Gondeau C, Calabrese D, Fontaine N, et al. miR-135a-5p-mediated downregulation of protein tyrosine phosphatase receptor delta is a candidate driver of HCV-associated hepatocarcinogenesis. Gut. 2017 doi: 10.1136/gutjnl-2016-312270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mailly L, Xiao F, Lupberger J, Wilson GK, Aubert P, Duong FHT, et al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33:549–554. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Groepper C, Rufinatscha K, Schröder N, Stindt S, Ehlting C, Albrecht U, et al. HCV modifies EGF signaling and upregulates production of CXCR2 ligands: role in inflammation and antiviral immune response. Journal of Hepatology. 2018;68 doi: 10.1016/j.jhep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- [9].Brenndorfer ED, Karthe J, Frelin L, Cebula P, Erhardt A, Schulte am Esch J, et al. Nonstructural 3/4A protease of hepatitis C virus activates epithelial growth factor-induced signal transduction by cleavage of the T-cell protein tyrosine phosphatase. Hepatology. 2009;49:1810–1820. doi: 10.1002/hep.22857. [DOI] [PubMed] [Google Scholar]
- [10].Diao J, Pantua H, Ngu H, Komuves L, Diehl L, Schaefer G, et al. Hepatitis C virus induces epidermal growth factor receptor activation via CD81 binding for viral internalization and entry. J Virol. 2012;86:10935–10949. doi: 10.1128/JVI.00750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Plissonnier ML, Lahlali T, Michelet M, Lebosse F, Cottarel J, Beer M, et al. Epidermal Growth Factor Receptor-Dependent Mutual Amplification between Netrin-1 and the Hepatitis C Virus. PLoS Biol. 2016;14:e1002421. doi: 10.1371/journal.pbio.1002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mankouri J, Griffin S, Harris M. The hepatitis C virus non-structural protein NS5A alters the trafficking profile of the epidermal growth factor receptor. Traffic. 2008;9:1497–1509. doi: 10.1111/j.1600-0854.2008.00779.x. [DOI] [PubMed] [Google Scholar]
- [13].Lupberger J, Duong FH, Fofana I, Zona L, Xiao F, Thumann C, et al. Epidermal growth factor receptor signaling impairs the antiviral activity of interferon-alpha. Hepatology. 2013;58:1225–1235. doi: 10.1002/hep.26404. [DOI] [PubMed] [Google Scholar]
- [14].Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Polyak SJ, Khabar KS, Paschal DM, Ezelle HJ, Duverlie G, Barber GN, et al. Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J Virol. 2001;75:6095–6106. doi: 10.1128/JVI.75.13.6095-6106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Neuman MG, Benhamou JP, Marcellin P, Valla D, Malkiewicz IM, Katz GG, et al. Cytokine--chemokine and apoptotic signatures in patients with hepatitis C. Transl Res. 2007;149:126–136. doi: 10.1016/j.trsl.2006.11.002. [DOI] [PubMed] [Google Scholar]
- [18].Ren Y, Poon RT, Tsui HT, Chen WH, Li Z, Lau C, et al. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res. 2003;9:5996–6001. [PubMed] [Google Scholar]
- [19].Huang P, Xu X, Wang L, Zhu B, Wang X, Xia J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J Cell Mol Med. 2014;18:218–230. doi: 10.1111/jcmm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, et al. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;16:972–977. doi: 10.1038/ncb3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wisniewska-Ligier M, Wozniakowska-Gesicka T, Glowacka E, Lewkowicz P, Banasik M, Tchorzewski H. Involvement of innate immunity in the pathogenesis of chronic hepatitis C in children. Scand J Immunol. 2006;64:425–432. doi: 10.1111/j.1365-3083.2006.01800.x. [DOI] [PubMed] [Google Scholar]
- [22].Nakagawa S, Wei L, Song WM, Higashi T, Ghoshal S, Kim RS, et al. Molecular Liver Cancer Prevention in Cirrhosis by Organ Transcriptome Analysis and Lysophosphatidic Acid Pathway Inhibition. Cancer Cell. 2016;30:879–890. doi: 10.1016/j.ccell.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]