Abstract
Álvarez-Buylla and colleagues provide an alternative interpretation of some of the data included in our manuscript and question whether well-validated markers of adult hippocampal neurogenesis (AHN) are related to this phenomenon in our study. In Terreros-Roncal et al., reconstruction of the main stages of human AHN revealed its enhanced vulnerability to neurodegeneration. Here we clarify ambiguities raised by these authors.
Álvarez-Buylla and colleagues asserted undetectable neurogenesis in the adult human dentate gyrus (DG) as they did not visualize well-validated AHN markers (such as Nestin or Doublecortin (DCX)) in this structure (1). The authors now acknowledge the presence of cells positive for these and other markers (2) while disbelieving that they are related to AHN in our study (3). No published data support their suggestion that the human DCX+ dentate granule cells (DGCs) described in numerous studies, including ours, (3-5) have a developmental origin. Conversely, the adult-born nature of DCX+ cells is consistent with BrdU and C14 birthdating approaches (6, 7), which support the continuous addition of new neurons to the human DG. Our study (3) reveals the presence of DCX+ immature DGCs at distinct differentiation stages in this structure. DCX+ cells co-labeled with Calretinin, Neuronal nuclei, or Calbindin (which identify sequential stages of AHN) show morphologies and positioning (Figs. 1F – J and S2H (3)) matching those observed in rodents. Contrary to Álvarez-Buylla’s view that DCX+ cells in (3) are large, the size of these cells varies during their maturation (Figs. 1J and S2H (3)) but remains significantly smaller than that of mature DGCs (Fig. 2m (5) and Fig. 6K (4)). Álvarez-Buylla et al. allege that DCX+ DGCs in (3) are exclusively located in the granule cell layer (GCL), despite our quantitative data revealing the abundant presence of these cells in the subgranular zone (SGZ). In fact, DCX+ cells are distributed in a maturation gradient between the GCL and SGZ, the most immature (those that co-express Calretinin or Polysialylated-neural cell adhesion molecule (PSA-NCAM)) being located in the SGZ (Figs. 1G and S2B (3)). These data support the notion that human DCX+ DGCs undergo a dynamic maturation process characteristic of AHN in numerous mammalian species.
Fig. 1.
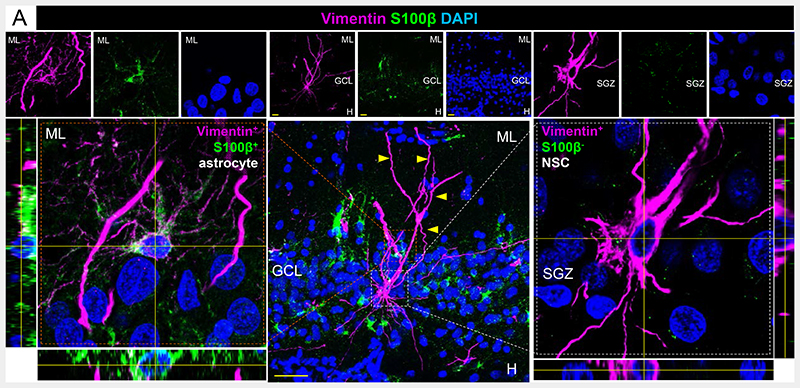
Vimentin and S100β staining in the human DG. ML: Molecular layer. GCL: Granule cell layer. SGZ: Subgranular zone. Yellow scale bar: 10 μm.
Álvarez-Buylla et al. postulate that the putative presence of DCX+ cells in non-neurogenic regions challenge the authenticity of human AHN. However, a recent study (8) revealed that the DCX signal observed in the macaque cortex is artefactual, thereby calling for caution when interpreting DCX staining in non-neurogenic regions of the primate brain. Conversely, control experiments showed that DCX protein is present in neurogenic niches of macaques (8) and humans (see the use of monoclonal antibodies and pre-adsorption with blocking peptides in Extended Data Figure 4 of (5)). Their suggestion that DCX is re-expressed by mature DGCs goes against experimental evidence (9). The expression of the DGC marker Prox1 by ~90% DCX+ cells (Fig. 1E (3)) and absence of DCX protein in glia, vasculature, and interneurons (Fig. S5 (3)) shown in (3) also contest Álvarez-Buylla’s view that DCX may be detected in non-DGCs.
Nestin+ cells are present in the adult human DG (10). In (3), we observed a population of Nestin+ cells that express a panel of radial glia-like cell markers (such as SRY-Box Transcription Factor 2 (Sox2) and Vimentin) while lacking S100 calcium-binding protein-B (S100β) expression. Although these criteria are widely used to phenotypically identify neural stem cells (NSCs) by immunohistochemistry (11), Álvarez-Buylla suggests that the Nestin+ S100β- cells identified in (3) are astrocytes. The morphology of Nestin+ S100β- NSCs differs from that of Nestin+ S100β+ astrocytes (Fig. 1 and Fig. S3 (3)). Moreover, it is consistent with that of hippocampal NSCs in aged rodents (12). Nestin+ S100β- cells show distally branched (Fig. S3E (3)) long apical processes that transverse the GCL (Fig. S3A, C, D, F (3)), and ~97% of their somas located in the SGZ (Fig. S3G (3))—features also evident with Vimentin staining (Fig. 1). Furthermore, the number of Nestin+ cells does not exhibit variations correlative to those of S100β+ astrocytes either in control or diseased individuals (Fig. S16E (3)). These observations refute Álvarez-Buylla and colleagues’ interpretation that the Nestin+ S100β- NSCs identified in (3) are astrocytes.
Álvarez-Buylla et al. challenge the nature of the proliferative cells observed in (3), suggesting that ~10000 phospho-histone 3+ mitotic cells per mm3 do not constitute an actual proliferative niche. These authors overlooked the fact that, in (3), we also used ELAV-Like Proteins HuC/HuD (which are transiently expressed by intermediate progenitors and proliferative neuroblasts immediately after cell division (13)) to phenotypically characterize human DG proliferative cells. About 90% of these cells expressed DCX and ~85% were located in the SGZ (Fig. S4 (3)). These data contest their suggestion that HuC/HuD labels mature neurons and support the notion that most proliferative cells in the human DG correspond to transit-amplifying progenitors and neuroblasts located in the SGZ. With respect to Calretinin+ cells, only those double-labeled with DCX were studied in (3) and (5), thereby excluding putative Calretinin+ interneurons.
Several interpretations by Alvarez-Buylla et al. with respect to neurodegenerative diseases are inaccurate. Regarding frontotemporal dementia (FTD), they mention two studies that found DGC loss in patients with FTD-Tau, which is characterized by DG nuclear inclusions and atrophy. However, these features are far from constant in other FTD variants (14). Given the absence of patients with FTD-Tau in (3), this comment is irrelevant to our study. These authors mention a study suggesting absence of major DG alterations in patients with Dementia with Lewy bodies. However, our study included patients not only with that condition but also with Parkinson’s disease. These α-synucleinopathies have different clinical, neuropathological, and molecular features, thereby triggering distinct hippocampal signatures (3). Álvarez-Buylla et al., suggest that the fluctuations in the number of RGL and proliferative cells observed in neurodegenerative diseases point to these cells not being related to AHN. The altered neuronal differentiation and exacerbated apoptosis (Fig. S8 (3)) likely accounts for unchanged numbers of mature neurons even in the presence of increased proliferative cells and/or NSCs in diseased individuals. Moreover, independent regulation of individual AHN stages has been extensively demonstrated (15).
We appreciate the interest that our new data has raised with our colleagues and are confident that our study will contribute to a greater understanding of how AHN persists throughout human life.
Funding
-
-
European Research Council (ERC) (ERC-CoG-2020-101001916 (MLLM)
-
-
Spain’s Ministry of Economy and Competitiveness (PID2020-113007RB-I00, SAF-2017-82185-R and RYC-2015-171899 (MLLM)
-
-
The Alzheimer’s Association (2015-NIRG-340709, AARG-17-528125, and AARG-17-528125-RAPID) (MLLM)
-
-
The Association for Frontotemporal Degeneration (2016 Basic Science Pilot Grant Award (MLLM))
-
-
Center for Networked Biomedical Research on Neurodegenerative Diseases (CIBERNED, Spain) (MLLM).
-
-
Institutional grants from the Fundación Ramón Areces and Banco de Santander to CBMSO are also acknowledged.
-
-
The salary of JTR was supported by a Doctoral fellowship from the Universidad Autónoma de Madrid (FPI-UAM 2017 program).
-
-
The salary of EPMJ was supported by a 2018 Neuroscience Doctoral fellowship from the Fundación Tatiana Pérez de Guzmán.
-
-
The salary of MFG was supported by a Formación de Personal Investigador (FPI) contract, associated with the SAF-2017-82185-R grant (MLLM), supported by the Spain’s Ministry for Economy and Competitiveness (PRE2018-085233).
-
-
The salary of CBRM is supported by “Subvenciones para la promoción de empleo joven e Implantación de la garantía juvenil en I+D+i 2018” (PEJ2018-001725-A) awarded by the Spain’s Ministry of Economy and Competitiveness and the AARG-17-528125-RAPID grant awarded by the Alzheimer’s Association to MLLM.
-
-
The salary of BMV was supported by a Consejo Nacional de Ciencia y Tecnología (CONACYT) of Mexican Government (CVU Number 385084) and a Secretaría de Educación, Ciencia Tecnología e Innovación (SECTEI) de la Ciudad de México (CDMX) postdoctoral fellowships.
References
- 1.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. published online Epub Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Cebrian-Silla A, Sorrells SF, Nascimento MA, Paredes MF, Farcía-Verdugo JM, Yang Z, Huang EJ. Impact of neurodegenerative diseases on human adulthippocampal neurogenesis. Science. 2022 doi: 10.1126/science.abn8861. Comment on XX, XX published online EpubXX (XXXX) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terreros-Roncal J, Moreno-Jimenez EP, Flor-Garcia M, Rodriguez-Moreno CB, Trinchero MF, Cafini F, et al. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science. 2021;374:1106–1113. doi: 10.1126/science.abl5163. published online Epub Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flor-Garcia M, Terreros-Roncal J, Moreno-Jimenez EP, Avila J, Rabano A, Llorens-Martin M. Unraveling human adult hippocampal neurogenesis. Nat Protoc. 2020;15:668–693. doi: 10.1038/s41596-019-0267-y. published online Epub Feb. [DOI] [PubMed] [Google Scholar]
- 5.Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. published online Epub Apr. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. published online Epub Nov. [DOI] [PubMed] [Google Scholar]
- 7.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. published online Epub Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu RX, Ma J, Wang B, Tian T, Guo N, Liu SJ. No DCX-positive neurogenesis in the cerebral cortex of the adult primate. Neural regeneration research. 2020;15:1290–1299. doi: 10.4103/1673-5374.272610. published online Epub Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. The Journal of comparative neurology. 2003;467:1–10. doi: 10.1002/cne.10874. published online Epub Dec 1. [DOI] [PubMed] [Google Scholar]
- 10.Cipriani S, Ferrer I, Aronica E, Kovacs GG, Verney C, Nardelli J, et al. Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults. Cerebral cortex. 2018;28:2458–2478. doi: 10.1093/cercor/bhy096. published online Epub Jul 1. [DOI] [PubMed] [Google Scholar]
- 11.Encinas JM, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods in cell biology. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Suarez S, Valero J, Muro-Garcia T, Encinas JM. Phenotypical and functional heterogeneity of neural stem cells in the aged hippocampus. Aging cell. 2019;18:e12958. doi: 10.1111/acel.12958. published online Epub Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marichal N, Garcia G, Radmilovich M, Trujillo-Cenoz O, Russo RE. Enigmatic central canal contacting cells: immature neurons in “standby mode”? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10010–10024. doi: 10.1523/JNEUROSCI.6183-08.2009. published online Epub Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bocchetta M, Iglesias JE, Scelsi MA, Cash DM, Cardoso MJ, Modat M, et al. Hippocampal Subfield Volumetry: Differential Pattern of Atrophy in Different Forms of Genetic Frontotemporal Dementia. J Alzheimers Dis. 2018;64:497–504. doi: 10.3233/JAD-180195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC neuroscience. 2006;7:77. doi: 10.1186/1471-2202-7-77. published online Epub Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]