Abstract
Background
Huntington’s disease (HD) is an autosominal dominant neurodegenerative condition that leads to progressive loss of motor and cognitive functions. Early symptoms in HD include subtle executive dysfunction related to white and grey matter loss in cortico-striatal-thalamic loops. There is no cure for HD and hence a significant need for early intervention with the potential to delay the clinical onset of the disease.
Objective
The objective of the present pilot study was to devise a novel behavioural intervention involving drumming and rhythm exercises that targets early dysexecutive problems, such as difficulties in sequence and reversal learning, response speed, timing, and dual tasking.
Method
One preclinical person and nine people with early to advanced stages of HD were recruited of whom five completed the two months intervention. The effects of rhythm exercise on executive function, basal ganglia volume, and white matter microstructure in the anterior corpus callosum, the anterior thalamic radiation, and the cortico-spinal tract were assessed post- relative to pre-training.
Results
After two months training, improvements in executive function and changes in white matter microstructure, notably in the genu of the corpus callosum that connects prefrontal cortices of both hemispheres, were observed. No changes in basal ganglia volume were present.
Conclusion
This pilot study provides novel preliminary evidence that carefully targeted behavioural stimulation in HD can result in cognitive enhancement and improvements in callosal white matter microstructure.
Keywords: Huntington’s disease, training, rhythm exercise, brain plasticity, executive function, white matter microstructure, diffusion magnetic resonance imaging, corpus callosum, anterior thalamic radiation, corticospinal tract
Introduction
Huntington’s disease (HD) is a neurodegenerative disease that leads to a progressive decline in motor abilities, mood, and cognition. HD is caused by an autosomal dominant mutation of the Huntingtin gene with expanded trinucleotide CAG repeats on chromosome 4. By convention, the clinical onset of HD is formally recognised by the development of movement symptoms. However, disease related structural changes in the brain (1-6) as well as in cognition (7) are usually observed many years prior to the onset of movement symptoms.
HD causes atrophy in the basal ganglia, which receive input from cortical areas and project back via the thalamus to the cortex (8, 9). Information transfer within these cortico-striatalthalamic loops as well as with other regions of the brain is supported by white matter connections (10). Presymptomatic and early stage HD have been associated with not only striatal atrophy (1, 3) but also with white matter microstructural alterations in major fibre bundles such as the corpus callosum, the internal capsule, that maintains striatal-thalamiccortical projections, and the cortico-spinal tract, the main motor output projection (4, 5, 11, 12). Early cognitive problems, due to disruptions of frontal-striatal networks, are often manifest as executive dysfunctions and include impairments in motor planning, response speed, sequence learning, inhibition, and multi-tasking, for instance, walking and talking (13).
There is no disease modifying treatment for HD, but accumulating evidence indicates that environmental and behavioural intervention may have the potential to slow disease progression. Van Dellen et al. (14-17) were the first to report, in a transgenic HD mouse model, a delay of the onset of motor symptoms after exposure to environmental stimulation relative to standard housing. Furthermore, environmental stimulation and training have been shown to improve the functional efficacy of striatal graft transplants in mice and enhance postsurgical prognosis (18).
Behavioural interventions for individuals with HD have focused primarily on improving movement and balance symptoms with physiotherapy and exercise (19, 20). Ideally, however, an intervention may aim to delay or even prevent clinical onset and disease progression, and hence should start as early as possible in the course of the disease. The well-characterised profile of HD provides an excellent opportunity to study the efficacy of interventions that aim to delay disease progression. Very little is known about potential effects of behavioural training on early executive symptoms or about the underlying mechanisms of brain plasticity in grey and white matter in HD patients. Thus, the aim of the present pilot study was to devise a novel behavioural intervention involving drumming and rhythm exercises that targets dysexecutive problems associated with disruptions of cortico-striatal loops, such as difficulties in sequence and reversal learning, response speed, timing, and dual tasking (13, 21-23).
To assess the feasibility of the training for individuals at different stages of the disease, one presymptomatic individual and nine individuals with early to advanced stages of HD were recruited (see Table 1). Out of these ten people, five individuals completed the two months intervention. Potential training effects on cognition and brain structure were studied using a longitudinal design. More specifically, basal ganglia volume measures were extracted from the caudate, putamen, and pallidum on both hemispheres. Diffusion tensor imaging (DTI) based white matter microstructural indices of fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity (24, 25) were derived from pathways known to be affected early in the disease, i.e. the anterior portions of the corpus callosum and the anterior limb of the internal capsule including the anterior thalamic radiation as well as the motor corticospinal tract. Based on accumulating evidence of training specific cognitive improvements and plasticity in grey and white matter in the healthy and diseased brain (26-28) it was hypothesized that drumming training would lead to micro- and macrostructural alterations in the basal ganglia and the selected white matter pathways as well as to improvements in executive functioning.
Table 1.
Summary of patients’ demographic and clinical background information. Age is displayed in years. CAG repeat is given for allele 1 and 2. FAS = Functional Assessment Score out of 25 maximum (the higher the score the better performance), MOCA = Montreal Cognitive Assessment out of 30 (the higher the score the better the performance), TMS = Total Motor Score out of 124 (the higher the score the more severe the presentation).
| Age | Sex | CAG | FAS | TMS | MOCA | Intervention | |
|---|---|---|---|---|---|---|---|
| P01 | 42 | female | 18/45 | 21 | 41 | 24 | completed |
| P02 | 46 | female | 20/40 | 24 | 9 | 23 | completed |
| P03 | 24 | male | 21/43 | 25 | 2 | 29 | withdrawn |
| P04 | 38 | female | n/a | n/a | n/a | 22 | withdrawn |
| P05 | 60 | male | 18/43 | 17 | 48 | 25 | completed |
| P06 | 45 | female | n/a | 16 | 46 | 27 | completed |
| P07 | 55 | male | 18/43 | 13 | 82 | 19 | Too difficult |
| P08 | 49 | male | 18/42 | 23 | 7 | n/a | withdrawn |
| P09 | 64 | male | 17/41 | 23 | 19 | 21 | completed |
| P10 | 44 | female | 19/47 | 1 | 72 | 21 | Too difficult |
Methods
Participants
N = 9 right-handed individuals with HD and one right-handed presymptomatic individual were recruited from local National Health Service (NHS) HD clinics in Cardiff and Bristol. The study was approved by the local NHS Research Ethics Committee. In accordance with the Declaration of Helsinki (29) informed written consent was obtained from all participants.
Individuals were eligible to participate if their CAG expansion of the HD gene was confirmed by genetic testing and/or a clinical diagnosis of HD had been confirmed by neurological examination and family history. Participants also had to be capable of giving informed consent and had to be on a stable medication regime for at least four weeks prior to taking part in the study. At the time of the study participants were taking the following medication: P01: Olanzepine 5mg, Citalopram 20 mg, Pregablin 50 mg, Depakote 250mg, Piraton; P04: Amitriptyline 75mg, Omeprazole 10mg, Prochlorperazine 10mg; P05: Olanzepine 12mg; P06: Natrilix 1.5mg, Rosuvasatin 10mg, Atenolol 25mg, Carioplan 5mg, Quetiapine 700mg, Metformin 500mg, Indomethocin 25mg, Pendopril 4mg; P07: Olanzapine 20mg, Propanolol 30mg, Cetrizine, Fortisips, Temazepam 10mg; P08: Paracetamol; P09: Citalopram 40mg; P10: Trazodone 300mg, Abilify 5mg, TEmazepam 10mg, Pregabalin 150mg. P02 and P03 were on no medication.
Individuals were excluded from the study if they had a history of other neurological conditions, head injury, stroke or cerebral haemorrhage, and of MRI contra-indications, such as pacemakers, metal clips, stents, claustrophobia, and significant chorea that would have prevented them from lying still in the MRI scanner. Table 1 summarises participants’ demographic information as well as their Unified Huntington’s Disease Rating Scale (UHDRS) Total Motor Score (TMS) and Functional Assessment Score (FAS) [24] and their performance on the Montreal Cognitive Assessment (MoCA) (30). Participants varied in disease stages from preclinical level (P03) to advanced stages (P07 and P10). While ten participants underwent baseline scanning and cognitive assessment and started the drumming training, only five completed the intervention successfully.
Rhythm exercise and Drumming Training
In collaboration with a professional musician (JC), a bespoke training program comprising twenty-two 15 min training sessions of rhythm exercises was recorded. Participants were provided with these sessions on CDs, a pair of Bongo drums, and a training diary. They were asked to exercise for 15 min per day, 5 times per week, for 2 months at home and to record the date and time of each exercise in their diary. Each training session introduced a drumming pattern based on one of the following rhythms: Brazilian samba, Spanish rumba, West-African kuku and Cuban son. After a brief warm up, trainees were encouraged to drum along with the instructor, initially with each hand separately and then with both hands alternating, starting with the dominant hand first and then reversing the order of the hands. The first exercises were based on very simple, slow, and regular patterns but the level of complexity and speed increased over the training sessions. Importantly, each individual progressed through the training adaptively at their own pace (31) i.e., as long as they exercised for the specified time they could repeat each session as often as they felt necessary to master it. To maintain engagement and motivation, the training incorporated pieces of music based on rhythms participants had learned and could drum along to. The researcher (CMB) supervised the first training sessions and then remained in regular telephone contact (at least once a week) with each participant throughout the intervention. Whenever possible, carers and/or spouses were involved in the study to support the training.
Cognitive assessment
Different aspects of executive functions including multi-tasking, attention switching and inhibition, and processing speed were assessed with standard neuropsychological assessment tools before and after the training. To account for practice effects, parallel test versions were used at the two time points. Parallel versions were counterbalanced across patients and time of assessment at baseline.
(1) The ability to multi-task was assessed with a standard dual task requiring the simultaneous crossing out of boxes and repeating digit sequences at an individual’s level of verbal short term memory span (32); (2) The ability to switch attention was assessed with a version of the trails test (VT) which requires the verbal generation of alternating letter and digit sequences (32). Switching costs were assessed relative to generating letter or digit sequences only; (3) The ability to suppress distracting and response incongruent information was tested with the Stroop task requiring the reading of colour words as baseline and naming their incongruent ink colour as interference condition (33, 34). (4) The Digit Symbol Substitution Test (DSST) from the Wechsler Adult Intelligence Scale was employed as a measure of working memory and processing speed (35). Verbal and category fluency from the Delis and Kaplan executive function battery were assessed using the letter cues “F”, “A”, “S” and “M”, “C”, “R” as well as the categories of “animals” and “boys’ names” and “supermarket items” and “girls’ names” respectively (36). The performance scores in these tasks were based on the total number of correctly generated responses and the number of errors. In addition, cognitive processing speed was estimated by the response times in the baseline conditions of the Stroop, the verbal trails test, and the DSST.
MRI data acquisition
MRI data were acquired on a 3 Tesla General Electric HDx MRI system (GE Medical Systems, Milwaukee) using an eight channel receive-only head RF coil at the Cardiff University Brain Research Imaging Centre (CUBRIC). High-resolution T1 weighted anatomical images (FSPGR) (256 × 256 matrix, TR = 7.8 ms, TE = 2.9 ms, flip angle = 20, 172 slices, 1mm slice thickness, FOV = 23c, 7 min acquisition time) were acquired as well as cardiac-gated high angular resolution diffusion MRI images that employed an optimised 60 direction gradient vector scheme [b-value 1200s/mm2, 60 slices (2.4mm), FoV 24 cm, matrix 96×96, TE 87ms, 30 min acquisition time].
MRI data processing
Extraction of subcortical basal ganglia volume from T1 weighted anatomical images Subcortical volumes in mm3 of the left and right caudate, putamen, and pallidum were automatically extracted from the T1 weighted images with the FMRIB Software Library (FSL) FIRST registration and segmentation tool (37) (www.fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST). Each individuals’ T1 weighted image was registered to the Montreal Neurological Institute (MNI) standard space using affine registration and voxels outside subcortical regions were excluded using an MNI subcortical mask. FIRST then applied a Bayesian model of shape recognition for the segmentation of subcortical structures. This model was trained on manually segmented images from the Center for Morphometric Analysis, Boston, and utilized a priori information about shape and volume intensities to fit deformable meshes of subcortical structures to the T1 weighted images. Each boundary voxel was subsequently classified as belonging to the segmentation or not and the final segmentation was corrected for any voxels that was categorized as not belonging to the segmented structure. All segmented images were uploaded on the original T1 weighted images and were visually inspected for correct registration for all participants. Volume measures of the corrected basal ganglia segmentations were extracted using the FSL statistics tool.
Diffusion MRI and tractography and extraction of DTI indices of white matter microstructure
Images were corrected for motion and distortion with re-orientation of gradient directions (38). The tensor was estimated in each voxel and damped Richardson-Lucy spherical deconvolution was used to extract voxelwise peaks in the fibre orientational density function (fODF) (39). Data were corrected for cerebrospinal fluid based partial volume artefacts with the Free Water Elimination method (40, 41). Whole brain tractography was performed by seeding in all voxels and following the fODF peaks using ExploreDTI (42). Putative 3D pathways belonging to segment 1 (the genu of the corpus callosum that maintains prefrontal connections) and segment 2 (maintaining connections between premotor and supplementary motor areas of both hemispheres) of the corpus callosum (43), the anterior limb of the internal capsule and the cortico-spinal tract (44, 45) were selected from the whole brain tracking results using ‘waypoint’ regions of interest and reproducible landmarking techniques (see Figure 1). Tract-specific DTI measures of median fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) were subsequently generated.
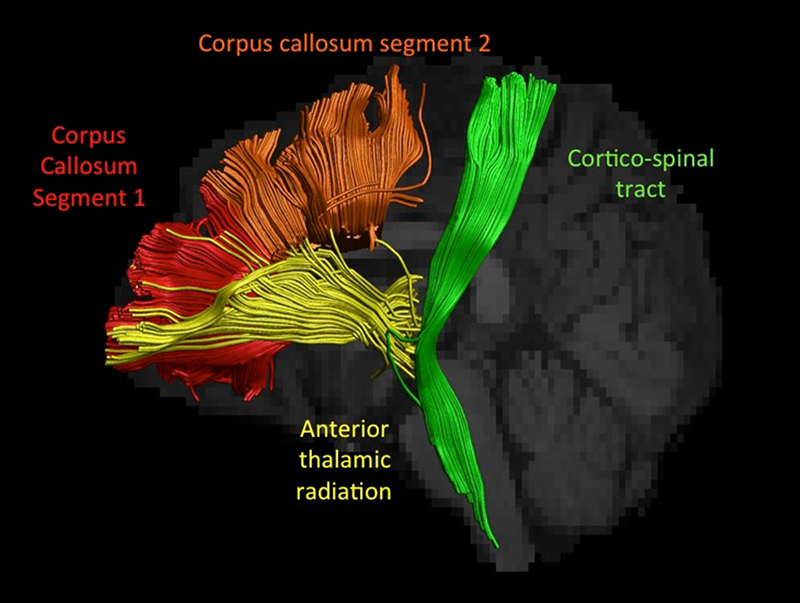
Figure 1.
displays a sagittal view of the reconstructed white matter pathways for one patient co-registered with that patient’s T1 weighted image. The cortico-spinal tract is displayed in green, fibres passing through the anterior limb of the internal capsule including the anterior thalamic radiation in yellow, segment 1 of the corpus callosum in red and segment 2 of the corpus callosum in orange.
Statistical analyses and results
Feasibility of the training
Five out of the ten participants withdrew from the study for the following reasons: The two most severely impaired individuals (P07 and P10) found the training too demanding. P08 lived on his own without carer’s support and was excluded because of non-compliance with the training schedule, P04 withdrew due to apathy and depression, and the research and clinical team lost contact with the presymptomatic person P03 during the course of the study. The remaining five participants (P01, P02, P05, P06 and P09) were compliant with the exercise schedule, as evidenced by their entries in the diaries provided. All individuals with HD who completed the training were at early to moderate disease stages and three of them had support from their spouses. All five individuals reported that they had enjoyed the training and four wished to continue with the drumming exercises after the end of the study.
Relationship between white matter microstructure, grey matter macrostructure, and executive function at baseline
All statistical analyses were carried out in SPSS Version 20 (46). To reduce the number of multiple comparisons, first principal component analysis (PCA) based on all cognitive function scores at baseline was conducted. A PCA procedure was used with orthogonal rotation (varimax) of the factor matrix, ensuring that the extracted components were independent from one another. The factor loadings obtained (see Table 2) reflect the strength of each variable in defining the factor with negative loadings indicating that a variable related inversely to the other components. The higher the factor loading of a variable (positive or negative), the more variance is explained by that variable on the factor. Per convention variables were included as first principal components when their loading exceeded a value of 0.5. Individual regression scores from the PCA were used as best estimates of individual cognitive performance (see Table 2) in further analysis.
Table 2.
The principal component loadings of the three principal component analyses. The first column summarises the principal components based on the baseline cognitive data. Please note that P07 could not perform the Stroop interference condition and the DSST and P10 could not perform DSST, box crossings, and VT switching. These patients were hence excluded from the principal component analysis (n = 8 patients). The second and third columns summarise the principal component loadings for the baseline and outcome cognitive data (n = 5 patients who completed the intervention) and the change in cognition measured as differences between post- and pretraining performance (n = 5). High loadings > 0.5 are highlighted in bold.
| Cognitive scores | Principal component loadings based on | ||
|---|---|---|---|
| Baseline performance (n = 8) | Baseline and outcome performance (n = 5) | Change in outcome versus baseline performance (n = 5) | |
| Single task digit sequences attempted | 0.853 | 0.387 | -0.836 |
| Single task correct digit sequences | 0.783 | 0.429 | -0.680 |
| Dual task digit sequences attempted | 0.831 | 0.659 | 0.691 |
| Dual task correct digit sequences | 0.712 | 0.540 | 0.945 |
| Single task boxes correct | 0.739 | 0.863 | 0.921 |
| Dual task boxes correct | 0.811 | 0.802 | 0.932 |
| Proportional digit score | 0.150 | - | - |
| Proportional box score | 0.474 | - | - |
| Stroop baseline response time | -0.897 | -0.636 | 0.497 |
| Stroop baseline score | 0.444 | - | - |
| Stroop interference score | 0.803 | 0.836 | 0.874 |
| DSST | 0.940 | 0.823 | 0.790 |
| VT baseline response time | -0.714 | -0.810 | -0.764 |
| VT baseline errors | -0.151 | - | - |
| VT switching response time | -0.906 | -0.821 | -0.833 |
| VT switching response time cost | -0.806 | -0.469 | -0.408 |
| VT switching errors | -0.168 | - | - |
| Verbal Fluency total correct | 0.796 | 0.829 | 0.843 |
| Verbal Fluency errors | -0.131 | - | - |
| Category Fluency total correct | 0.893 | 0.910 | 0.747 |
| Category Fluency errors | 0.437 | - | - |
In addition, DTI indices for two tracts were collapsed across hemipsheres, since there were no differences between the median DTI indices extracted from the left and the right anterior thalamic radiation [FA: t(9) = 0.64, p = 0.54; MD: t(9) = 1.31, p = 0.22; AD: t(9) = 0.85, p = 0.42; RD: t(9) = 1.68, p=0.13] and the left and right cortico-spinal tract [FA: t(9) = 0.28, p = 0.79; MD: t(9) = 0.74, p = 0.48; AD: t(9) = 0.51, p = 0.62; RD: t(9) = 0.55, p=0.60]. Similarly, there were no differences between average volume indices between left and right caudate [t(9) = 1.23, p=0.25] and left and right pallidum [t(9) = 1.38, p=0.20] and these measures were also collapsed across hemispheres. Average volume in the left putamen was significantly larger than in the right putamen [t(9) = 2.64, p = 0.03] and were therefore not combined.
Hierarchical linear regression analysis was used to investigate the baseline relationships between brain structure and cognition i.e. between participants’ white matter microstructural indices in the four pathways (collapsed anterior thalamic radiation, collapsed cortico-spinal tract and segments 1 and 2 of the corpus callosum), volume measures in the four basal ganglia structures (collapsed caudate, collapsed pallidum, left and right putamen) and their cognitive performance in the executive function tasks. The individual PCA regression scores of cognitive performance were used as the dependent variable and all twenty macro- and microstructural indices (FA, MD, AD, RD for four tracts each and volume measures of four basal ganglia structures) were entered in a stepwise fashion into the regression model. This analysis demonstrated that individual variation at baseline in volume of the collapsed pallidum accounted for 62% of the variance in the cognitive principal component indices [adjusted R2 = 0.62; SE = 0.66; F(1,7) = 9.81 p = 0.02).
Pre- versus post-training changes in cognition
First PCA based on the cognitive data from both pre- and post-training assessments for the five individuals with HD who completed the intervention was conducted (see Table 2 for PC loadings and Table 3 for mean cognitive performance scores the PCs were derived from). This analysis was based on those cognitive scores that loaded highest i.e. > 0.5 in the baseline PCA (see Table 2). A paired t-test between each individual’s regression scores from the first PCA based on pre- and posttraining sessions demonstrated a significant improvement in cognition, t(4) = 3.30, p ≤ .03 (see Figure 2a).
Table 3.
summarises the mean and standard deviation of the baseline and outcome performance in the cognitive tasks that were included in the principal component analysis for the five patients who completed the training. Response speed is measures in seconds.
| Baseline (n = 5) | Outcome (n = 5) | |
|---|---|---|
| Single task digit sequences attempted | 13.6 (0.5) | 18.8 (5.9) |
| Single task correct digit sequences | 10.2 (1.5) | 17.2 (5.8) |
| Dual task digit sequences attempted | 13.2 (0.8) | 17.2 (1.3) |
| Dual task correct digit sequences | 9.4 (3.1) | 14.0 (3.7) |
| Single task boxes correct | 97.6 (53.1) | 107.0 (35.8) |
| Dual task boxes correct | 87.2 (52.1) | 95.6 (52.8) |
| Stroop baseline response time | 111.0 (20.1) | 93.8 (24.1) |
| Stroop baseline score | 98.2 (15.4) | 112.8 (4.3) |
| Stroop interference score | 66.8 (31.8) | 77.4 (28.1) |
| DSST | 42.4 (15.4) | 43.6 (13.6) |
| VT baseline response time | 39.0 (18.9) | 31.4 (9.4) |
| VT switching response time | 121.2 (32.6) | 106.0 (14.8) |
| VT switching response time cost | 82.2 (21.9) | 74.6 (16.9) |
| Verbal Fluency total correct | 28.0 (10.4) | 39.8 (8.8) |
| Category Fluency total correct | 30.4 (11.3) | 39.2 (15.4) |
Figure 2.
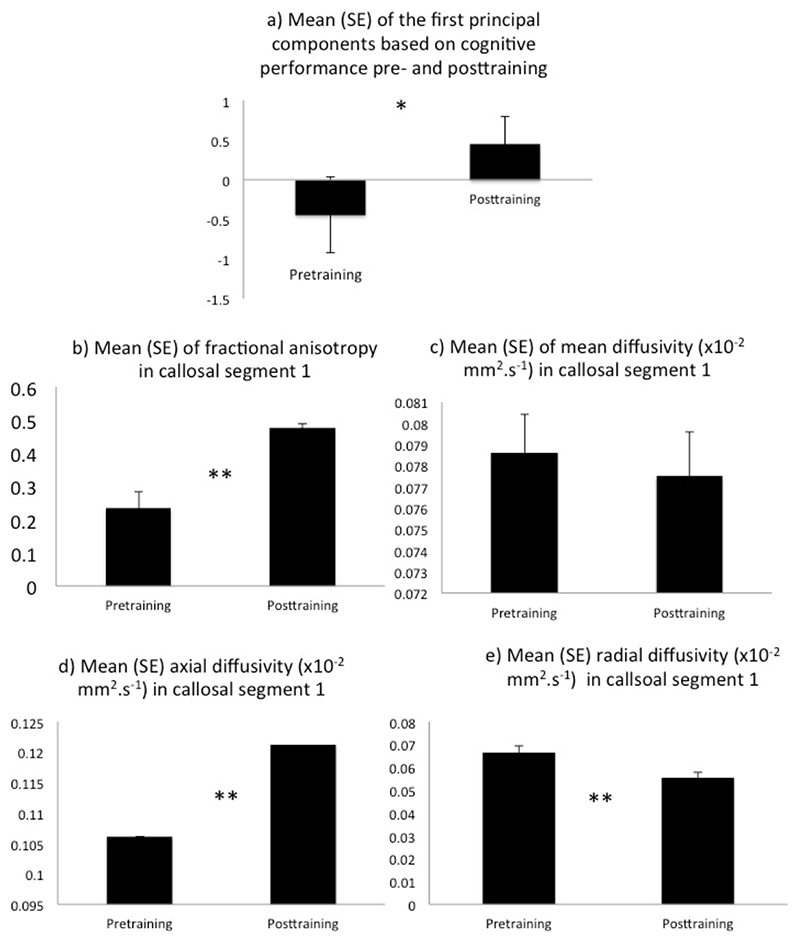
displays the pre- versus posttraining differences between (a) the average regression scores of the first principal components based on the cognitive performance as well as between (b) average fractional anisotropy, (c) mean diffusivity, (d) axial diffusivity, and (e) radial diffusivity in segment 1 of the corpus callosum. * p ≤ 0.05, ** p ≤ 0.01.
Pre-versus post-training changes in subcortical volume of the basal ganglia
To investigate any potential structural changes in the basal ganglia, paired t-tests were conducted between the mean volume in mm3 of the caudate, putamen and pallidum on both hemispheres before and after the training. There were no volumetric changes in the left caudate [t(4) = 1.2, p = 0.29], right caudate [t(4) = 0.89, p = 0.42], left putamen [t(4) = 0.42, p = 0.69], right putamen [t(4) = 0.85, p = 0.44], left pallidum [t(4) = 0.75, p = 0.50] or right pallidum [t(4) = 1.1, p = 0.35].
Pre- versus post-training changes in white matter microstructure
To investigate any potential changes in white matter microstructure, paired t-tests were carried out between the median DTI indices at baseline versus outcome across the four tracts. Multiple comparisons for each of the microstructural indices (FA, AD, RD, MD) across the four pathways were controlled for type I error with the Bonferonni correction, necessitating a significance level of p ≤ 0.0125 to comply with a family-wise significance level of 5%.
Significant microstructural changes post- relative to pretraining were observed in segment 1 of the corpus callosum, with increases in FA [t(4) = 5.2, p = 0.006] (Figure 2b) and AD [t(4) = 5.1, p = 0.007] (Figure 2d) and a decrease in radial diffusivity [t(4) = -4.6, p = 0.01] (Figure 2e) but no change in mean diffusivity [t(4) = 1.63, p = 0.16] (Figure 2c). Trends were observed for increases in FA [t(4) = 3.3, p = 0.03] and AD [t(4) = 2.9, p = 0.04] and a decrease of RD [t(4) = -4.2, p = 0.014] in the cortico-spinal tract, increases of FA [t(4) = 3.7, p = 0.02] and AD [t(4) = 4.04, p = 0.016] in the anterior thalamic radiation and an increase in AD in segment 2 of the corpus callosum [t(4) = 3.03, p = 0.04]. No other comparisons were significant.
Relationship between changes in cognition and white matter microstructure
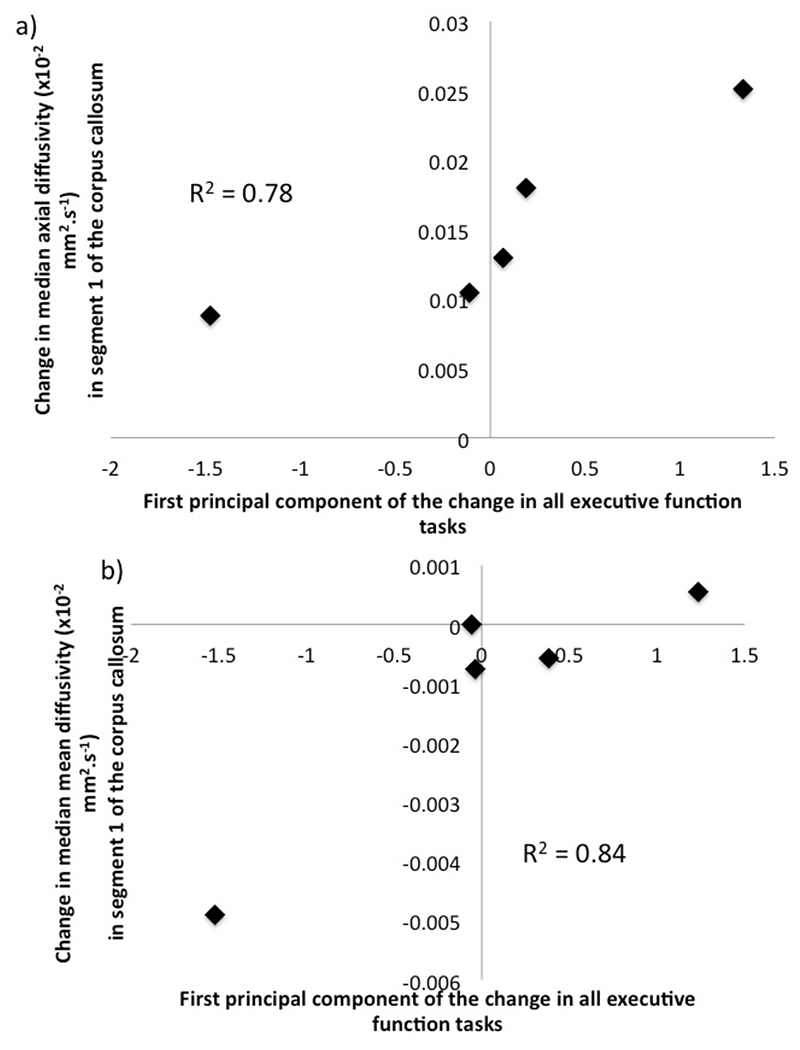
To explore whether the changes in executive function were related to alterations in the microstructural indices, the difference between pre- and posttraining in each executive function score and between the microstructural indices (FA, MD, AD, RD) in each tract were calculated. First PCA of the cognitive difference scores was conducted (see Table 2) and Pearson correlation coefficients were calculated between the individual PCA regression scores and the change in median FA, MD, AD, and RD for each tract. There were positive relationships between change in cognition and change in MD (r = 0.92, p = 0.02) (Figure 3a) and change in AD (r = 0.89, p = 0.05) (Figure 3b) in segment 1 of the corpus callosum (Table 4). Trends for positive correlations between cognitive change and change in MD and AD respectively were also observed for segment 2 of the corpus callosum as well as with RD in both callosal segments. No correlations were present between changes in cognition and changes in FA for any tracts nor were any correlations present for the anterior thalamic radiation and the cortico-spinal tract (see Table 4).
Figure 3.
displays the positive correlations between the principal components of the change in cognition and the change in (a) mean diffusivity and (b) axial diffusivity in segment 1 of the corpus callosum.
Table 4.
displays the Pearson product moment correlations between the individual regression coefficients of the first PCA on change in cognition and white matter microstructural indices of fractional anisotropy, mean diffusivity, axial diffusivity and radial diffusivity in segment 1 and 2 of the corpus callosum, the anterior thalamic radiation and the cortico-spinal tract collapsed across hemispheres. Significant correlations are highlighted in bold. PCA = Principal Component Analysis, * p ≤ 0.05, ** p ≤ 0.01.
| Regression coefficients of first PCA of change in executive functions (n = 5) | ||
|---|---|---|
| Change in Fractional Anisotropy | ||
| Segment 1 of corpus callosum | -0.01 | |
| Segment 2 of corpus callosum | -0.01 | |
| Anterior thalamic radiation | 0.23 | |
| Cortico-spinal tract | -0.25 | |
| Change in Mean Diffusivity | ||
| Segment 1 of corpus callosum | 0.92* | |
| Segment 2 of corpus callosum | 0.59 | |
| Anterior thalamic radiation | -0.04 | |
| Cortico-spinal tract | -0.12 | |
| Change in Axial Diffusivity | ||
| Segment 1 of corpus callosum | 0.89* | |
| Segment 2 of corpus callosum | 0.38 | |
| Anterior thalamic radiation | -0.10 | |
| Cortico-spinal tract | -0.39 | |
| Change in Radial Diffusivity | ||
| Segment 1 of corpus callosum | 0.57 | |
| Segment 2 of corpus callosum | 0.31 | |
| Anterior thalamic radiation | 0.16 | |
| Cortico-spinal tract | 0.06 | |
Discussion
The present pilot study investigated i) the feasibility and ii) the effects of a novel rhythm exercise intervention on cognition and brain micro- and macrostructure. Potential plastic changes in the brain were investigated for volume measures of the basal ganglia as well as for white matter microstructural indices of the corpus callosum, the cortico-spinal tract and the anterior thalamic radiation, all known to be affected early in the disease.
The main results of the study were improved performance in executive function and alterations in white matter microstructure after two months of drumming training. There was also some evidence for relationships between executive function improvement and microstructural changes in the genu of the corpus callosum, which connects the prefrontal cortices of both hemispheres.
More specifically, participants exhibited increases in fractional anisotropy and axial diffusivity, and decreases in radial diffusivity in segment 1 of the corpus callosum after two months of drumming. These results add to accumulating evidence of training and experience induced white matter microstructural alterations in the healthy and diseased brain (47, 48) ((49). For instance, piano lessons over a period of 15 months were shown to lead to macro- and microstructural changes in the motor cortex and the corpus callosum that were related to improvements in performance in a motor sequence task in children (50). White matter pathways such as the corpus callosum may be of particular importance because they allow the temporal co-ordination of sensory-motor processes between distant brain structures including the basal ganglia and cortical regions.
However, when interpreting changes in DTI indices it is important to bear in mind that they provide sensitive but not specific markers of white matter microstructural properties (51). Increased fractional anisotropy may reflect training related changes in the coherence of tracts within a fiber bundle potentially due to numerous mechanisms including increased myelination, increased axonal density, or selective pruning of inactive axons and hence reduced fibre complexity. Similarly, reductions in radial diffusivity are not specific but may arise from mechanisms such as enhanced myelination, strengthening of the axonal membrane, or increases in axonal density due to glia cell swelling (48).
We observed that changes in mean and axial diffusivity in the genu of the corpus callosum appeared to be related to functional improvements in cognition. Although correlational analyses based on small sample sizes have to be interpreted with caution, such relationships seemed to be more prevalent for the anterior portions of the corpus callosum than the anterior thalamic radiation or the cortico-spinal tract (Table 4). The anterior portions of the corpus callosum connect prefrontal (segment 1) as well as frontal premotor and supplementary motor areas (segment 2) of both hemispheres. Given the well established role of the prefrontal cortex in executive functioning (52-54), one may speculate that training induced improvements in callosal white matter may have increased information transfer between the frontal cortices, which may in turn have resulted in benefits for executive functioning.
The findings from the baseline data of the present study also informed about the relationship between cognitive performance and brain micro- and macrostructure in Huntington’s disease. Individual differences in the volume of the globus pallidum accounted for 62% of the variation in the PCA regression scores derived from participants’ performance in the executive tasks. This result is consistent with the notion of the basal ganglia being not only important for motor but also for cognitive functions. A recent study, for instance, reported that the extent of atrophy in the globus pallidum was related to response inhibition deficits in Huntington’s disease (55).
In contrast to the changes observed in white matter microstructure, no alterations in volumetric measures of the basal ganglia were present. There are several possible interpretations of this finding. The first concerns the small sample size and hence power of the study, which may have prevented the detection of any potential plastic effects in basal ganglia volume. It may also be the case that basal ganglia plasticity is hampered in HD due to atrophy and that other learning mechanisms such as the above discussed alterations in callosal white matter may compensate for this loss. Future studies including larger sample sizes and adequate control conditions are required to disentangle these different hypotheses. The inclusion of a healthy matched control group, for instance, will aid in addressing questions of compensatory mechanisms of brain plasticity in HD. It would also allow the quantification of any potential practice effects arising from repeated testing of cognition. The present study employed parallel versions of all cognitive tasks to minimize practice effects, however, without a control group practice effects cannot be completely ruled out.
Drumming is a demanding task, even for people without neurogenerative disease, and we observed a very large drop out rate suggesting that this kind of training may only be suitable for patients at early disease stages who have support and encouragement from their carer and/or family. Despite this caveat, the present results, whilst based on a small number of patients and hence preliminary in nature, are the first to demonstrate: 1) cognitive improvement; 2) alterations in white matter microstructure; and 3) some evidence for relationships between the two after eight weeks of rhythm exercise intervention in HD.
In conclusion, the results of this pilot study suggest that behavioural stimulation, when specifically targeted at early executive problems caused by cortico-striatal dysfunction in HD, may not only improve performance in animal models, but may have a very positive outcome in patients with early Huntington’s disease.
Acknowledgements
This study was funded by a seedcorn grant from the Neuroscience and Mental Health Research Institute, Cardiff University, awarded to Claudia Metzler-Baddeley.
Footnotes
Conflict of interest
The authors have no conflict of interest to report.
References
- 1.Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004 Jul;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 2.Aylward EH, Codori AM, Rosenblatt A, Sherr M, Brandt J, Stine OC, et al. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington’s disease. Mov Disord. 2000 May;15(3):552–60. doi: 10.1002/1531-8257(200005)15:3<552::AID-MDS1020>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Aylward EH, Liu D, Nopoulos PC, Ross CA, Pierson RK, Mills JA, et al. Striatal volume contributes to the prediction of onset of Huntington disease in incident cases. Biol Psychiatry. 2012 May;71(9):822–8. doi: 10.1016/j.biopsych.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, et al. Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical “disconnection”. Neuroimage. 2010 Feb;49(4):2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006 Sep;21(9):1317–25. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 6.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005 Sep;65(5):745–7. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 7.Robins Wahlin TB, Lundin A, Dear K. Early cognitive deficits in Swedish gene carriers of Huntington’s disease. Neuropsychology. 2007 Jan;21(1):31–44. doi: 10.1037/0894-4105.21.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Afifi AK. Basal ganglia: functional anatomy and physiology. Part 2. J Child Neurol. 1994 Oct;9(4):352–61. doi: 10.1177/088307389400900403. [DOI] [PubMed] [Google Scholar]
- 9.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994 Sep;265(5180):1826–31. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 10.Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008 Jul;28(28):7143–52. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips O, Squitieri F, Sanchez-Castaneda C, Elifani F, Griguoli A, Maglione V, et al. The Corticospinal Tract in Huntington’s Disease. Cereb Cortex. 2014 Apr; doi: 10.1093/cercor/bhu065. [DOI] [PubMed] [Google Scholar]
- 12.Phillips O, Sanchez-Castaneda C, Elifani F, Maglione V, Di Pardo A, Caltagirone C, et al. Tractography of the corpus callosum in Huntington’s disease. PLoS One. 2013;8(9):e73280. doi: 10.1371/journal.pone.0073280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group P-HIotHS. Harrington DL, Smith MM, Zhang Y, Carlozzi NE, Paulsen JS. Cognitive domains that predict time to diagnosis in prodromal Huntington disease. J Neurol Neurosurg Psychiatry. 2012 Jun;83(6):612–9. doi: 10.1136/jnnp-2011-301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass M, van Dellen A, Blakemore C, Hannan AJ, Faull RL. Delayed onset of Huntington’s disease in mice in an enriched environment correlates with delayed loss of cannabinoid CB1 receptors. Neuroscience. 2004;123(1):207–12. doi: 10.1016/s0306-4522(03)00595-5. [DOI] [PubMed] [Google Scholar]
- 15.van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington’s in mice. Nature. 2000 Apr;404(6779):721–2. doi: 10.1038/35008142. [DOI] [PubMed] [Google Scholar]
- 16.Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004 Mar;24(9):2270–6. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregatedensity in a mouse model of Huntington’s disease. BMC Neurosci. 2008;9:34. doi: 10.1186/1471-2202-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunnett SB. Neural tissue transplantation, repair, and rehabilitation. Handb Clin Neurol. 2013;110:43–59. doi: 10.1016/B978-0-444-52901-5.00004-6. [DOI] [PubMed] [Google Scholar]
- 19.Busse ME, Khalil H, Quinn L, Rosser AE. Physical therapy intervention for people with Huntington disease. Phys Ther. 2008 Jul;88(7):820–31. doi: 10.2522/ptj.20070346. [DOI] [PubMed] [Google Scholar]
- 20.Khalil H, Quinn L, van Deursen R, Dawes H, Playle R, Rosser A, et al. What effect does a structured home-based exercise programme have on people with Huntington’s disease? A randomized, controlled pilot study. Clin Rehabil. 2013 Jul;27(7):646–58. doi: 10.1177/0269215512473762. [DOI] [PubMed] [Google Scholar]
- 21.Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Wang C, Stout JC, et al. “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci. 2010;22(2):196–207. doi: 10.1176/appi.neuropsych.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin HH. The sensorimotor striatum is necessary for serial order learning. J Neurosci. 2010 Nov;30(44):14719–23. doi: 10.1523/JNEUROSCI.3989-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holl AK, Wilkinson L, Tabrizi SJ, Painold A, Jahanshahi M. Selective executive dysfunction but intact risky decision-making in early Huntington’s disease. Mov Disord. 2013 Jul;28(8):1104–9. doi: 10.1002/mds.25388. [DOI] [PubMed] [Google Scholar]
- 24.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996 Jun;111(3):209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 25.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996 Dec;201(3):637–48. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi H, Taki Y, Kawashima R. Effects of working memory training on cognitive functions and neural systems. Rev Neurosci. 2010;21(6):427–49. doi: 10.1515/revneuro.2010.21.6.427. [DOI] [PubMed] [Google Scholar]
- 27.Holman C, de Villers-Sidani E. Indestructible plastic: the neuroscience of the new aging brain. Front Hum Neurosci. 2014;8:219. doi: 10.3389/fnhum.2014.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bürki CN, Ludwig C, Chicherio C, de Ribaupierre A. Individual differences in cognitive plasticity: an investigation of training curves in younger and older adults. Psychol Res. 2014 Mar; doi: 10.1007/s00426-014-0559-3. [DOI] [PubMed] [Google Scholar]
- 29.WMA. Declaration of Helsinki - Ethical principles for medical research involving human Subjects. 2008. http://www.wma.net . [PubMed]
- 30.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. eng. [DOI] [PubMed] [Google Scholar]
- 31.Metzler-Baddeley C, Baddeley R. Does Adaptive Training Work? Applied Cognitive Psychology. 2009;23(2):254–66. [Google Scholar]
- 32.Baddeley AD. Exploring the Central Executive. Quarterly Journal of Experimental Psychology. 1996;49A(1):5–28. [Google Scholar]
- 33.Stroop J. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935:643–62. [Google Scholar]
- 34.Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop Neuropsychological Screening Test. Psychological Assessment Ressources; Odessa, FL: 1989. [Google Scholar]
- 35.Wechsler D. Wechsler Adult Intelligence Scale-3rd UK Edition (WAIS-III UK) Psychological Corporation and Pearson Assessment; Oxford, UK: 1999. [Google Scholar]
- 36.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) Pearson Assessment; Oxford, UK: 2001. [Google Scholar]
- 37.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011 Jun;56(3):907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009 Jun;61(6):1336–49. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- 39.Dell’acqua F, Scifo P, Rizzo G, Catani M, Simmons A, Scotti G, et al. A modified damped Richardson-Lucy algorithm to reduce isotropic background effects in spherical deconvolution. Neuroimage. 2010 Jan;49(2):1446–58. doi: 10.1016/j.neuroimage.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 40.Metzler-Baddeley C, O’Sullivan MJ, Bells S, Pasternak O, Jones DK. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage. 2012 Jan 16;59(2):1394–403. doi: 10.1016/j.neuroimage.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 41.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009 Sep;62(3):717–30. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- 42.Leemans A, Jeurissen B, Sijbers J, DK J. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data; 17th Annual Meeting of Intl Soc Mag Reson Med; Hawaii, USA. 2009; 3537 [Google Scholar]
- 43.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006 Sep;32(3):989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 44.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007 Jul;36(3):630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004 Jan;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 46.IBM. SPSS Statistics, Version 200. IBM Corp; Armonk, NY: 2011. [Google Scholar]
- 47.Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008 Sep;192(1):137–42. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012 Apr;15(4):528–36. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herholz SC, Zatorre RJ. Musical training as a framework for brain plasticity: behavior, function, and structure. Neuron. 2012 Nov;76(3):486–502. doi: 10.1016/j.neuron.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J Neurosci. 2013 Jan;33(3):1282–90. doi: 10.1523/JNEUROSCI.3578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007 Jul;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society London B. 1982:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 53.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–34. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 54.Norman DA, Shallice T. Attention to action. Springer; 1986. [Google Scholar]
- 55.Rao JA, Harrington DL, Durgerian S, Reece C, Mourany L, Koenig K, et al. Disruption of response inhibition circuits in prodromal Huntington disease. Cortex. 2014 Jun;58C:72–85. doi: 10.1016/j.cortex.2014.04.018. ENG. [DOI] [PMC free article] [PubMed] [Google Scholar]