Abstract
Endometrial cancer incidence is rising, with 435,000 global cases in 2019. An effective, low cost primary prevention strategy is required to reduce disease burden. Obesity, insulin resistance and inflammation contribute to endometrial carcinogenesis and physical activitytargets these pathways.This study sought to quantify the amount of physical activity required to impact upon endometrial cancer risk.
Physical activity data from 222,031 female participants with an intact uterus in the UK Biobank studywere analysed using a multi-variable Cox proportional hazards model. A systematic review of the literature was performed, searching CENTRAL, Embase and MEDLINE databases up to 19/04/2021. Studies including participants withand without endometrial cancerinvestigating the effect of physical activity measured in MET-h/week on disease risk were included. Two reviewers independently selected studies, extracted data and evaluated the risk of bias.
Within the UK Biobank, each 1 MET-h/week increase in total physical activity was associated with a 0.2% (95%CI 0.1-0.4%, p=0.020) reduction in endometrial cancer risk, equating to a 10.4% reduction if performing 50 MET-h/week or 7 hours of jogging/week. Eleven cohort and 12 case-control studieswere identified in the systematic review, including821,599 participants. One study reported a non-significant effect of 1 MET-h/week increases in physical activity on endometrial cancer risk (OR 1.00, 95%CI 0.99-1.00). Eight studies found significant reductions in disease risk of 15-53%, but only in the mostphysically active individuals.
Physical activity reduces endometrial cancer risk, but the effect size appears small. Regular vigorous activity should be encouraged to maximise the health benefit observed.
Keywords: endometrial cancer, risk, prevention, physical activity, quantify
Introduction
Endometrial cancer accounts for3% of all new cancer cases diagnosed in the UK and 3.5% of all new cancer cases in the USA (1,2). A woman’s lifetime risk of endometrial cancer is currently estimated to be 3.1% (3), although for some women their individual risk is substantially greater than this. Risk factors include increasing age, obesity, insulin resistance and lifetime oestrogen exposure (4). As a consequence of the increasing prevalence of these risk factors within the population, endometrial cancer case numbers are rising, with a doubling in diagnoses in the UK over the last 30 years and a 0.5% increase in age-adjusted rates year-on-year in the USA (1,2). The increase in disease incidence is not purely limited to high socio-demographic index (SDI) nations, however, with rising case numbers observed in nearly all global regions(5). Whilst early presentation with postmenopausal bleeding means that the majority of cases are diagnosed at an early stage and are potentially curable with surgery, endometrial cancer deaths are also rising and are projected to become the 6th most common cause of death in women in the UK by 2035 (6). Treatment for endometrial cancer is also not without its risks, particularly in an increasingly elderly and obese population with multiple co-morbid conditions, and in younger women where surgery will result in loss of fertility.
Strategies aimed at reducing the risk of endometrial cancer are, therefore, urgently required not only to negate the physical and psychological impact of an endometrial cancer diagnosis and its treatment, but also to reduce the costs of this disease to national health services. For primary disease prevention to be effective at a population level, it should not only be effective, but also inexpensive, accessible and associated with minimal side effects.Physical activity, by reducing adiposity, improving insulin sensitivity, decreasing serum oestradiol levels and modulating the immune response, could fulfil these criteria (7–9).Epidemiological evidence suggests that active individuals could have a 16-25% lower risk of developing endometrial cancer compared with more sedentary women (10–13). Previously conducted meta-analyseshave, however, frequently only compared the most with the least physically active, with limited attempts to establish a dose-response relationship. Indeed, any such relationship may even be lost once adiposityis adjusted for (12). The close association between endometrial cancer and obesity may provide some explanation for the significant heterogeneity in effect size observed between studies, which could be compounded by the simultaneous analysis of cohort and case-control studies, with the latter at risk of recall bias. As a result, umbrella reviews of the literature have concluded, on the basis of their stringent methodological criteria, that there is only ‘probable’ evidence of an association between physical activity and endometrial cancer prevention (14,15).Before physical activity can be incorporated into any future endometrial cancer prevention strategies, the optimal duration and intensity of exercise for cancer risk reduction needs to be determined.(12), in their meta-analysis in 2020, concluded that there was likely to be a linear relationship between increasing physical activity and a reduction in endometrial cancer risk, but were unable to comment on whether moderate and vigorous activity was more beneficial due to a small sample size. The authors based their analysis on data from only eight prospective cohort studies that had contributed to the National Cancer Institute Cohort Consortium, all of which were conductedin the USA, Europe and Australia. By considering only leisure time activity, they also failed to consider other domains of physical activity that can contribute significantly to an individual’s total daily physical activity levels, including transportation and occupational activity. Subsequent data from the UK Biobank, a large prospective cohort study of over half a million UK adults, was also suggestive of a linear inverse relationship between total physical activity levels and endometrial cancer risk (16). The authors here, however, failed to include almost half of the potential incident endometrial cancer cases within the dataset in their analysis by excluding individuals with a history of any malignancy and those with incomplete physical activity data. They alsodid not consider a number of potential confounding variables including waist circumference and diabetes status and did not adequately control for women who had undergone a hysterectomy and were, therefore, no longer at risk of endometrial cancer.
This study, therefore, aims to quantify the amount of total physical activity needed to significantly impact upon endometrial cancer risk using data from the UK Biobank study,adjusted for all potential confounding risk factors in the at-risk population. In addition, it seeks to compare these results with previously published data identified through a systematic review of the literature. As a secondary objective, it aims to identify the types and domains of activity associated with the greatest reduction in endometrial cancer risk and the age at which such activity is most beneficial.
Materials and Methods
UK Biobank study
The UK Biobank is a major national and international health resource, created to improve the prevention, diagnosis and treatment of serious and life-threatening illnesses, including cancer(17). The female cohort consists of 273,384 individuals aged between 39 and 71 years, after exclusion of withdrawals from the study. Health, demographic and anthropometric data were collected using standardised questions posed through computer terminals and by trained nurses and were supplemented with the donation of biological samples, including blood, saliva and urine. Cancer diagnoses were ascertained through linkage to national cancer registries in England, Scotland and Wales. Deaths were ascertained through linkage to death registries. Complete follow-up was available through to 31st March 2016 for England and Wales and 31st October 2015 for Scotland. Full details can be found at https://www.ukbiobank.ac.uk.
The study was approved by the North West Multi-Centre Research Ethics Committee (16/NW/0274), Patient Information Advisory Group (England and Wales) and the Community Health Index Advisory Group (Scotland). All participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
All cancers were recorded within the UK Biobank using either the International Classification of Diseases 9 or 10 or self-reported data. Identification of endometrial cancer cases was performed using all three of these sources. Within the database, each participant had 9 follow-up time point records for ICD10, 11 follow-up time point records for ICD9 and 9 follow-up points for self-reported cancer status. Cases were characterised as incident or prevalent using the age when they attended the UK Biobank centre and the age at diagnosis of endometrial cancer. Cases were regarded as incident if the age of cancer diagnosis was greater than the age at which they first attended the centreand prevalent if the reverse were true. If there was a discrepancy between the self-reported age of cancer diagnosis and that recorded by the cancer registry, the age documented by the cancer registry was used. Only incident endometrial cancer cases occurring at least two years after recruitment into the UK Biobank study were considered for this analysis, to minimize the risk of reverse causality. Female participants were defined as controls if they had no record of endometrial cancer and had not previously undergone a hysterectomy. Data were censored at date of endometrial cancer diagnosis, hysterectomy, death or last data collection.
Individuals self-reported their physical activity levels by answering adapted questions from the validated short International Physical Activity Questionnaire (IPAQ), which covers the frequency and duration of walking, moderate and vigorous activity. Responses were considered to be greater than zero if activity was performed for at least 10 minutes and limited to 180 mins per day, as it was deemed unlikely that individuals would be undertaking physical activity for longer than this in any one 24 hour period. Time spent undertaking activities of differing intensity were weighted by the energy expended for each of these categories using the IPAQ data processing rules and expressed in MET-hours per week (MET-h/week) (18). A MET-h is a ratio of an individual’s working metabolic rate compared to a standard resting rate of 1 kcal/kg/hr (defined as quiet sitting for 1 hour). The Compendium of Physical activities provides a list of specific physical activity types and their MET values (19). Walking was considered to have a MET value of 3.3, moderate activity a MET value of 4.0 and vigorous activity a MET value of 8.0. The effect of each category of variable intensity physical activity on endometrial cancer risk was considered as a continuous variable expressed in MET-h/week. Total physical activity represented the summation of each individual category of physical activity intensity and was also considered as a continuous variable.
Systematic review
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (20).
Data sources and searches
Acomprehensive literature search was conducted using CENTRAL, Ovid Embase, and Ovid MEDLINE databases. The databases were searched from date of inception to 19th April 2021. Search terms were “endometrial cancer” and physical activity” with associated Medical Subject Headings (MeSH). The full search strategy for each database can be found in Supplementary methods 1.In addition, grey literature including conference proceedings, internal gynaecological oncology journals, clinical trial databases and reference lists of included studies were hand searched for eligible publications.
Study selection
Studies investigating the effect of physical activity on endometrial cancer risk as either a primary or secondary outcome were eligible for inclusion. Whilst all domains of physical activity were considered, including recreational and occupational activity, sufficient information must have been collected during the study about the type and duration of physical activity performed to allow MET-h/week to be calculated. No limits were placed on the age or BMI of study participants. All study designs were included in order to ensure a comprehensive analysis of the literature. Studies were required to include a reference population who did not develop endometrial cancer for comparison. Searches were restricted to English language publications.
Data extraction
Titles and abstracts were collated into Microsoft Excel 2016. Duplicate publications were removed using Endnote 20. All titles and abstracts were screened independently by two reviewers (OA and JP). Conflicts were resolved by agreement with a third reviewer (SK). Those studies identified as meeting the inclusion criteria underwent full text review and data extraction by two independent reviewers (OA and JP).
Baseline data extracted included study design, selection criteria, number of participants and endometrial cancer cases, setting, follow-up, demographic data, domain of exercise studied and risk estimates, such as odds ratios and hazards ratios with corresponding 95%CIs and the adjustment variables in multivariable analyses. Study authors were contacted for additional information where this was not provided in the original publication.
A risk of bias (RoB) assessment was undertaken independently by two reviewers (OA and JP), based on the ROBINS-1 tool (21)(Supplementary methods 1), with discrepancies resolved through discussion with a third reviewer (SK). The RoB assessment included the risk of confounding, selection, information, deviation from intended intervention, missing data, detection and reporting bias.
Statistical analysis
Continuous and categorical variables in the UK Biobank dataset were compared using a Mann-Whitney U and Chi-square test, respectively. Multiple imputationwas performed to deal with missing data in the UK Biobank dataset, which was assessed to be missing at random. The proportion of missing data for each variable is reported in table 1. A Cox proportional hazardsmodel was used to determine the association between total physical activity and endometrial cancer risk, using time from baseline assessment as the underlying time variable.Hazard ratios and corresponding 95% confidence intervals were calculated after checking the proportional hazards assumption graphically with log-log plots and by examiningSchoenfoeld’s residuals. A multivariable model was generated, adjusting for potential confounders of endometrial cancer risk including age (logarithmic), body mass index (BMI), waist circumference, age at menarche (squared), age at last birth (squared), age at menopause (<55 years or ≥55 years), HRT use (current, never/prior), oral contraceptive pill use (never/use for <5 years, use for ≥5 years), tamoxifen use (current, never/prior), type 2 diabetes mellitus (yes, no) and smoking (never, current/prior).Data on family history of endometrial cancer was, unfortunately, not collected from the UK Biobank cohort.A family history of bowel cancer in at least one first degree relative has been shown to be associated with a statistically significant increase in the risk of endometrial cancer because of shared genetic (Lynch syndrome) and lifestyle factors (22). A family history of bowel cancer (none, one or more first degree relatives diagnosed) was, therefore, also included in the multivariable model. The impact of transformation of predictor variables and restricted cubic splines on model fit was assessed using themvrs program in Stata. Sensitivity analyses were undertaken to examine the effect of excluding self-reported endometrial cancer cases and of imputing missing data.
Table 1.
Demographic and baseline characteristic data for endometrial cancer cases and controls in the UK Biobank cohort, including proportion of missing data. Results given as median (IQR) or n (%).
| Characteristic | Endometrial cancer cases (n=902) | Controls (n=221,129) |
|---|---|---|
| Age at recruitment, years | 61 (55-65) | 56 (49-62) |
| Duration of follow-up, years | 4.1 (2.6-5.7) | 7.2 (6.4-7.7) |
| BMI, kg/m2 | ||
| <25.0 | 204 (22.6) | 92048 (41.6) |
| 25.0-29.9 | 300 (33.3) | 79448 (35.9) |
| 30.0-34.9 | 190 (21.1) | 32373 (14.6) |
| 35.0-39.9 | 106 (11.8) | 11092 (5.0) |
| ≥40.0 | 94 (10.4) | 4983 (2.3) |
| Missing | 8 (0.9) | 1185 (0.5) |
| Ethnicity | ||
| White | 848 (94.0) | 207755 (94.0) |
| Black or Black British | 8 (0.9) | 3651 (1.7) |
| Mixed | 4 (0.4) | 1591 (0.7) |
| Indian | 14 (1.6) | 2514 (1.1) |
| Pakistani | 2 (0.2) | 634 (0.3) |
| Bangladeshi | 0 (0.0) | 62 (0.03) |
| Chinese | 4 (0.4) | 879 (0.4) |
| Other Asian | 3 (0.3) | 724 (0.3) |
| Other ethnic background | 14 (1.6) | 2192 (1.0) |
| Missing | 5 (0.6) | 1127 (0.5) |
| Family history of colorectal cancer | ||
| Yes | 117 (13.0) | 23037 (10.4) |
| No | 785 (87.0) | 198092 (89.6) |
| Smoking | ||
| Never | 586 (65.0) | 132288 (59.8) |
| Current/previous | 310 (34.4) | 87599 (39.6) |
| Missing | 6 (0.7) | 1242 (0.6) |
| Waist circumference, cm | 90 (81-101) | 82 (75-91) |
| Missing | 4 (0.4) | 904 (0.4) |
| Age at menarche, years | ||
| <12 | 233 (25.8) | 40685 (18.4) |
| ≥12 | 605 (67.1) | 160601 (72.6) |
| Missing | 64 (7.1) | 19843 (9.0) |
| Age at menopause, years | ||
| Pre-menopausal at study entry | 146 (16.2) | 72740 (32.9) |
| <55 | 568 (63.0) | 126502 (57.2) |
| ≥55 | 188 (20.8) | 21887 (9.9) |
| Age at last birth, years | 27 (18-31) | 29 (23-33) |
| Missing | 6 (0.7) | 1345 (0.6) |
| HRT use | ||
| Never/prior use | 790 (87.6) | 198695 (89.9) |
| Current use | 62 (6.9) | 12303 (5.6) |
| Missing | 50 (5.5) | 10131 (4.6) |
| Oral contraceptive pill use | ||
| <5 years or never use | 465 (51.6) | 79849 (36.1) |
| ≥5 years | 354 (39.3) | 121244 (54.8) |
| Missing | 83 (9.2) | 20036 (9.1) |
| Tamoxifen use | ||
| Current | 15 (1.7) | 1320 (0.6) |
| Never/prior use | 887 (98.3) | 219809 (99.4) |
| Type 2 diabetes | ||
| Yes | 73 (8.1) | 5587 (2.5) |
| No | 825 (91.5) | 214517 (97.0) |
| Missing | 4 (0.4) | 1025 (0.5) |
| Walking MET-h/week | 8.3 (4.4-23.1) | 11.6 (5.5-23.1) |
| Missing | 211 (23.4) | 48782 (22.1) |
| Moderate activity MET-h/week | 7.0 (1.3-18.7) | 8.0 (2.0-20.0) |
| Missing | 211 (23.4) | 48782 (22.1) |
| Vigorous MET-h/week | 2.0 (0.0-10.7) | 2.7 (0.0-12.0) |
| Missing | 211 (23.4) | 48782 (22.1) |
All analyses were performed using STATA version 14(23). A p value <0.05 was considered statistically significant.
Public and patient involvement
The research question was developed in collaboration with clinicians, patients and the general public as part of a James Lind Alliance Priority Setting Partnership, in which the development of a personalized risk score to reflect an individual’s risk of endometrial cancer and the identification of prevention strategies were identified as the most important unanswered research question (24).
Results
UK Biobank
In total, 902 cases and 221,129 controls were eligible for analysis. No incident ICD-9 coded endometrial cancer cases were identified. Eight cases were based on a self-reported diagnosis of endometrial cancer which could not be verified in the linked cancer registry data. In total, 5817 and 479 women in the control group died and/or underwent a hysterectomy during follow-up, respectively.The baseline characteristics of cases and controls within the UK Biobank are described in table 1. As anticipated, cases were older at the time of recruitment into the study, had a higher BMI, were more likely to have type 2 diabetes and to have longer periods of endogenousoestrogen exposure (p<0.0001). Women who did not develop endometrial cancer during follow-up were significantly more physically active at study recruitment than those subsequently diagnosed with the disease (median MET-h/week 28.7 (IQR 13.3-55.8) vs. 23.4 (10.6-49.8), p<0.0001). A linear dose-response relationship between increasing physical activity levels and endometrial cancer risk was observed.A 1 MET-h/week increase in physical activity was associated with a 0.4% (95%CI 0.2-0.6, p<0.0005) reduction in endometrial cancer risk, after adjusting for age alone. In a multivariable analysis taking baseline BMI into account, the effect size was reduced, with each 1 MET-h/week increase in total physical activity associated with a 0.2% (95%CI 0.01-0.4, p=0.020) reduction in endometrial cancer risk. This effect equated to a 10.4% (95%CI 1.7-18.3, p=0.020) decrease in endometrial cancer risk for each additional 50 MET-h/week of physical activity or 7 hours of jogging (assuming jogging is equivalent to 7.0 MET-h)(19). Walking and moderate intensity physical activity were associated with statistically significant decreases in endometrial cancer risk, with each 1 MET-h/week increase associated with a decrease in endometrial cancer risk of 0.5% (95%CI 0.1-0.9%, p=0.027) and0.4% (95%CI 0.1-0.8, p=0.042), respectively. Increasing levels of vigorous activity were associated with a smaller, non-significant, reduction in endometrial cancer risk (HR 0.998, 95%CI 0.994-1.003, p=0.427).
Sensitivity analysis showed no effect of excluding self-reported endometrial cancer cases (HR 0.998, 95%CI 0.996-0.999, p=0.023). Whilst the effect size was unchanged when only un-imputed data was considered, the result was no longer statistically significant (HR 0.998, 95%CI 0.996-1.001, p=0.161).
Systematic review
Study selection and characteristics
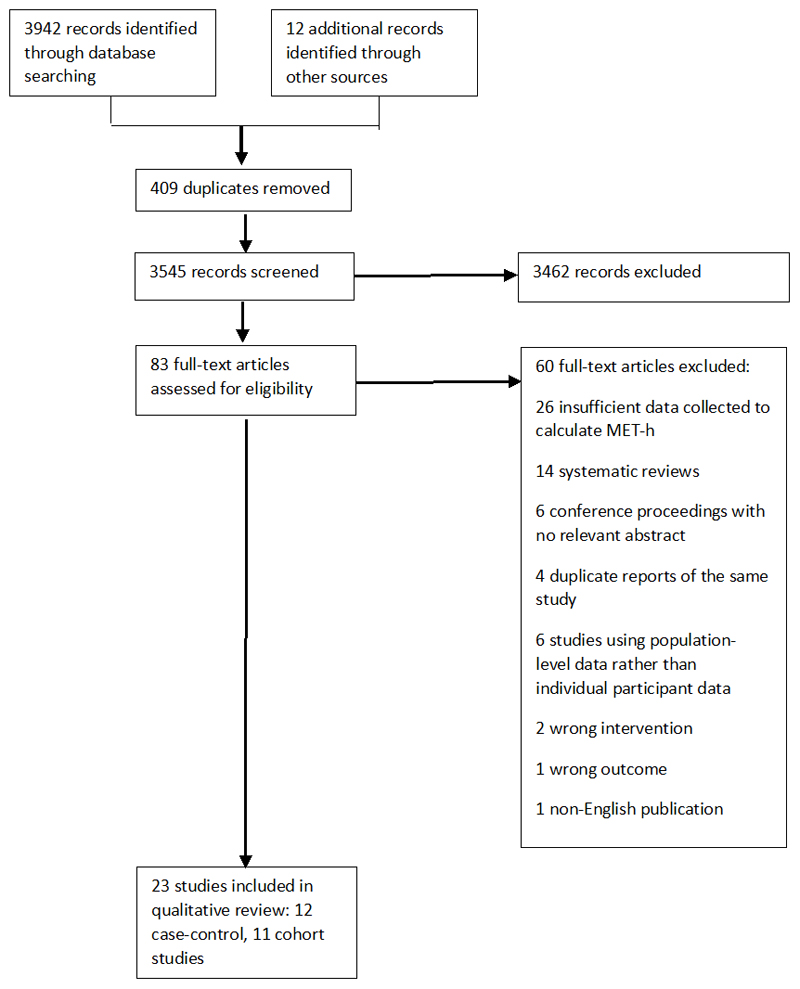
Database and hand searching initially identified 3954 articles, of which3871 were excluded due to duplicate publications (n=409) or irrelevance (n=3462). Of the 83 full text articles reviewed, 23 met the eligibility criteria for this review and were included, including 12 case-control studies and 11 cohort studies (figure 1)(25–47). A detailed summary of the characteristics of the included studies is shown in table 2.
Figure 1.
Flow diagram of study selection. Of the 3954 records identified through a systematic search of the literature, 83 full text articles were assessed for eligibility, of which 23 were included in the qualitative analysis.
Table 2. Characteristics of included studies.
| Study | Study period | Study design | Study population | Number of participants | Age of participants (years) | Physical activity questionnaire | Method of administering questionnaire | Follow-up duration |
|---|---|---|---|---|---|---|---|---|
| Arem et al. 2011 | 2004-2008 | Case-control study | Residents of Connecticut, USA | 1329 (667 EC cases) | 35-79 | Kriska’s modifiable Activity Questionnaire (MAQ)-reliable and validated | In-person interview | n/a |
| Barberio et al. 2018 | 2000-2016 | Cohort study | Residents of Alberta, Canada | 15368 women (90 EC cases) | 35-69 | Past Year Total Physical Activity Questionnaire (PYTPAQ)-reliable and validated | Self-administered | Mean 6.6 years cases, 11.1 years controls |
| Colbert et al. 2003 | 1987-1998 | Cohort study | Participants in Breast Cancer Detection Demonstration Project (BCDDP), USA | 23374 (253 EC cases) | Unknown, mean age 60-62 years | Non-validated questionnaire, reliability not checked | Self-administered | Average 8.2 years |
| Conroy et al. 2009 | 1992-2007 | Cohort study | Health professionals enrolled into Women’s Health Study, USA | 19917 (264 EC cases) | 45+ | Non-validated questionnaire, reliability not checked | Self-administered | Mean 8.8 years |
| Dieli-Conwright et al. 2013 | 1995-2007 | Cohort study | Public school teachers/administrators enrolled into California Teachers Study, USA | 93888 (976 EC cases) | 20+ | Non-validated questionnaire, reliability confirmed | Self-administered | Median 12.1 years |
| Du et al. 2014 | 1986-2008 | Cohort study | Registered nurses enrolled in Nurses’ Health Study, USA | 71570 (777 EC cases) | 30-55 | Non-validated questionnaire, reliability confirmed | Self-administered | 1,235,880 person-years |
| Friberg et al. 2006 | 1997-2005 | Cohort study | Residents of Uppsala and Västmanland counties, Sweden | 33723 (199 EC cases) | 50-83 | Non- validated questionnaire, reliability confirmed | Self-administered | Mean 7.25 years |
| Friedenreich et al. 2007 | 1992-2004 | Cohort study | General adult population of 10 European countries enrolled into EPIC study | 253023 (689 EC cases) | 35-70 | Modified version of Baecke questionnaire, reliability confirmed, not validated | Self-administered | Mean 6.6 years |
| Friedenreich et al. 2010 | 2002-2006 | Case-control study | Residents of Alberta, Canada | 1574 (542 EC cases) | 30-79 | Non-validated questionnaire, reliability confirmed | In-person interview | n/a |
| Gierach et al. 2009 | 1995-2003 | Cohort study | Members of the American Association of Retired Persons who participated in NIH-AARP Diet and Health Study, USA | 109621 (1052 EC cases) | 50-71 | Non-validated questionnaire, reliability not checked | Self-administered | Mean 3.8 years cases, 7.0 years for controls |
| Goodman et al. 1997 | 1985-1993 | Case-control study | Residents of Oahu, Hawaii of Japanese, Caucasian, Native Hawaian, Filipino and Chinese ethnicity | 843 (332 EC cases) | 18-84 | Non-validated questionnaire, reliability not checked | In-person interview | n/a |
| John et al. 2010 | 1996-1999 | Case-control study | Non-hispanic white, Hispanic and African American residents of San Francisco bay area, USA | 970 (500 EC cases) | 35-79 | Non-validated questionnaire, reliability not checked | In-person interview | n/a |
| Kwon et al. 2012 | 2009 | Case-control study | Participants in Behavioral Risk Factor Surveillance System (BFRSS), USA | 144012 (1080 EC cases) | 40-79 | Non-validated questionnaire, reliability not checked | Computer-assisted telephone interviews | n/a |
| Littman et al. 2001 | 1985-1991 | Case-control study | Residents of King, Pierce and Snohomish counties, Washington, USA | 1933 (822 EC cases) | 45-74 | Modified version of Minnesota Leisure Time Physical Activity questionnaire, validated | In-person interviews | n/a |
| Matthews et al. 2005 | 1997-2001 | Case-control study | Residents of Shanghai, China | 1678 (832 EC cases) | 30-69 | Non-validated questionnaire, reliability confirmed | In-person interview | n/a |
| Modesitt et al. 2012 | 2007-2009 | Case-control study | Overweight and obese postmenopausal women due for hysterectomy for EC or benign indications, USA | 38 (22 EC cases) | Mean age 58.3 | Modified version of Aerobics Center Longitudinal Study Physical Activity Questionnaire, reliability not checked and validity in determining MET-h/week unknown | Self-administered | n/a |
| Olson et al. 1997 | 1986-1991 | Case-control study | Residents of New York state, USA | 863 (232 EC cases) | 40-85 | Non-validated questionnaire, reliability not checked | In-person interview | n/a |
| Patel et al. 2008 | 1992-2003 | Cohort study | Participants in Cancer Prevention II study Nutrition Cohort, USA | 42672 (466 EC cases) | 50-74 | Non-validated questionnaire, reliability not checked | Self-administered | Not reported |
| Plagens-Rotman et al. 2020 | 2011-2013 | Case-control study | Patients at the Gynaecological and Obstetric Hospital of the Medical University of Poznan, Poland | 751 (68 EC cases) | 21-84 | Non-validated questionnaire, reliability not checked | Self-administered | n/a |
| Robsahm et al. 2010 | 1953-2007 | Cohort study | Norwegian world class athletes | 1424 (3 EC cases) | 18+ | Non-validated questionnaire, reliability not checked | Self-administered | Not reported |
| Salazar-Martinez et al. 2000 | 1995-1997 | Case-control study | Patients attending Castelazo Ayala Hospital, Mexico | 753 (85 EC cases) | <40->71 | Non-validatedquestionnaire, reliability not checked | Self-administered | n/a |
| Schouten et al. 2004 | 1986-1995 | Cohort study | Participants in The Netherlands Cohort Study on Diet and Cancer, Netherlands | 1739 (226 EC cases) | 55-69 | Non-validated questionnaire, reliability not checked | Self-administered | Average 9.3 years |
| Shu et al. 1993 | 1988-1990 | Case-control study | Residents of the Shanghai metropolitan area, China | 536 (268 EC cases) | 18-74 | Non-validated questionnaire, reliability not checked | In-person interview | n/a |
A total of 821,599participants were included in this review, including 10,445endometrial cancer cases, with the age of participants ranging from 18 to >84 years. The included studies were conductedin a wide range of geographical locations, including USA, Canada, Mexico, UK, Norway, Sweden, Poland,The Netherlands, Europeand China. Cohort studies frequently recruited women from the general population, with the exception of those studies focusing on health professionals (28,30) and teachers (29). (44)conducted a study ofworld-class professional Norwegian athletes and compared their incidence of cancer with that of the general population. Data were collected by self-administered questionnaire in 14 studies and by interview in the remaining nine.None of the studies measured physical activity objectively, instead relying on patient recall.The studies considered a range of domains of physical activity including recreational, occupational, household activity and physical activity for transportation, either singularly or in combination as ‘total physical activity’. Whilst the majority of studies asked participants to report the number of hours of each activity performed in a specific time period, (27) and (31) also collected data on sleep duration to allow the calculation of total MET-h of activity in a 24 hour period. As a result, the median and total reported MET-h/week varied dramatically between studies, with the most active individuals in the study by (31)undertaking more than 46 MET-h/day. Reported physical activity levelswere also influenced by whether studies considered all intensities of physical activity undertaken or solely reported on moderate and vigorous activity (25,29,34,37,40). Endometrial cancer risk estimates were based on long-term physical activity levels in seven studies(25,29,30,33,36,39,41), with the others considering short term snap shots of activity only. With the exception of the study by (43), all studies provided adjusted estimates of endometrial cancer risk. The majority of studies adjusted for many of the most important endometrial cancer risk factors, including age, BMI, parity, age at menarche and menopause, oral contraceptive pill and hormone replacement therapy use and family history of endometrial and/or colorectal cancer. Fourteen studies also reported estimates of endometrial cancer risk without BMI adjustment (25–28,30–35,38,42,44,47)
Primary outcome
A summary of the results of the 23 included studies is provided in table 3. Only one study published data on the effect of 1 MET-h/week on endometrial cancer risk, finding a non-significant odds ratio of 1.00 (95%CI 0.99-1.00) (33). The authors of the remaining 22 studies were contacted for this information but either did not reply (n=10) or were unfortunately unable to access the original study data for re-analysis (n=12). For this reason, alongside the significant variability in study design, a meta-analysis could not be performed.
Table 3. Summary of results of included studies.
| Study | Domains/types of physical activity studied | Comparison | Age-adjusted relative effect ratio (95%CI) | Age-adjusted p-trend | Adjusted variables | Adjusted relative effect ratio (95%CI) | Multi-variate adjusted p-trend |
|---|---|---|---|---|---|---|---|
| Barberioet al. 2018 | Employment, transportation-related, household and recreational activities of all intensities | ≤113.7 vs. ≥201.2 MET-h/week | HR=0.54 (0.30-0.98) | <0.01 | Age, ethnicity, marriage status, highest level of education, area of residence, smoking, alcohol use, mean energy intake, self-reported BMI, cardiovascular and respiratory history, family history, menopausal status. Exclusion of cancers within 2 years of baseline assessment | HR=0.71 (0.36-1.40) | 0.13 |
| Colbert et al. 2003 | All types and intensities of physical activity including sleep. Entire 24 hour period considered. | Low (Q1) vs. high Median MET-h/week 8.0 vs. 56.0 | RR=0.8 (0.5-1.1) | 0.24 | Age, parity, education | RR=0.8 (0.5-1.1) | 0.24 |
| Conroy et al. 2009 | Walking, jogging, running, bicycling, use of stationary machines, aerobic exercise, aerobic dance, use of exercise machines, tennis/squash or racquetball, lap swimming, yoga/stretching/toning | Total energy expenditure ≥20.4 vs. <2.7 MET-h/week | RR=1.42 (1.01-1.98) | 0.02 | Age, BMI, smoking status, alcohol use, saturated fat intake, fibre intake, fruit/vegetable intake, parity, use and type of hormone therapy, menopausal status | RR=1.15 (0.79-1.67) | 0.39 |
| Dieli-Conwrightet al. 2013 | Longterm moderate and strenuous recreational activity | ≤0.5 vs. ≥5.5 hours/week/year | not reported | not reported | Age, race, BMI | RR=0.85 (0.68-1.06) | 0.03 |
| Du et al. 2014 | Cumulative average walking, jogging, running, bicycling, lap swimming, tennis, calisthenics/aerobics/aerobic dance/rowing machine, squash/racquetball | <3 vs. ≥27 MET-h/week | RR=0.81 (0.62-1.05) | 0.17 | Age at menarche, OCP use, parity and age at first birth, age at last birth, menopausal status, age at menopause, HT use, HT type, BMI at age 18, pack-years of smoking, family history of endometrial cancer, family history of colorectal cancer, alcohol intake, caffeine intake, recent BMI | RR=1.10 (0.84-1.45) | 0.32 |
| Friberget al. 2006 | Occupational, household, leisure time activity, walking/biking, watching TV/sitting, sleep. Entire 24 hour period considered. | <38.9 vs. ≥45.9 MET-h/day | RR=0.74 (0.50-1.09) | 0.14 | Age, parity, diabetes, total fruit and vegetable intake, education, BMI | RR=0.79 (0.53-1.17) | 0.27 |
| Friedenreichet al. 2007 | Occupational, recreational, household activity | Age, centre, BMI, age at menarche, menopausal status, age at menopause, number of full-term pregnancies, age at birth of last child, ever use of OCP, ever use of HT, education, smoking status, hypertension, diabetes, fruit and vegetable intake, fibre intake, carbohydrate intake, energy intake | |||||
| Recreational <12.01 vs. ≥41.26 MET-h/week | Recreational HR=0.92 (0.74-1.15) | 0.38 | Recreational HR=0.94 (0.75-1.18) | 0.47 | |||
| Gierach et al. 2009 | Vigorous activity at work or home in past 12 months | Never/rarely vs. 5+ times/week | RR=0.60 (0.49-0.73) | <0.0001 | Age, race, smoking status, parity, OCP use, age at menopause, HT use, BMI | RR=0.77 (0.63-0.95) | 0.02 |
| Patel et al. 2008 | Recreational, non-recreational activity (baseline) | Recreational <7 vs. ≥31.5 MET-h/week Non-recreational <5.0 vs. ≥18.5 MET-h/week | Age, age at menarche, age at menopause, duration OCP use, parity, smoking, total caloric intake, diabetes, postmenopausal HT use, BMI | ||||
| Non-recreational RR=0.78 (0.60-1.01) | ns | Non-recreational RR=0.83 (0.64-1.07) | 0.13 | ||||
| Robsahm et al. 2010 | Physical activity of any intensity | Athletes that developed EC vs. general Norwegian population | SIR=0.79 (0.16-2.30) | n/a | Age | SIR=0.79 (0.16-2.30) | n/a |
| Schouten et al. 2004 | Transportation to work, household, recreational activity | <30 min/day vs. ≥90 min/day | not reported | not reported | Age, BMI, age at menarche, OCP use, age at menopause, parity, smoking status | RR=0.54 (0.34-0.85) | 0.002 |
| Aremet al. 2011 | Moderate and vigorous sport/recreational activity (2-5 years before interview) | 0 vs. 7.5 MET-h/week | OR=0.46 (0.36-0.60) | <0.001 | Age, BMI, race, number of live births, menopausal status, OCP use, hypertension, smoking status | OR=0.66 (0.50-0.87) | 0.002 |
| Friedenreichet al. 2010 | Occupational, recreational, household activity (lifetime) | Per MET-h/week/year | OR=1.00 (0.99-1.00) | ns | Age | OR=1.00 (0.99-1.00) | ns |
| Goodman et al. 1997 | Recreational and non-recreational activity | BMI | |||||
| Non-recreational 0 vs. 20089 lifetime hours since age 15 | Non-recreational OR=0.8 (not given) | 0.27 | Non-recreational OR=0.7 (not given) | 0.08 | |||
| John et al. 2010 | Lifetime transportation to work, occupational, household, recreational activity of any intensity | <43.2 vs. ≥91.9 MET-h/week | not reported | not reported | Age, ethnicity, education, family history of endometrial cancer, age at menarche, parity, duration OCP use, duration HT use, menopausal status, BMI, height | OR=0.61 (0.43-0.87) | 0.01 |
| Kwon et al. 2012 | Non-occupational moderate and vigorous activity | Non cancer vs. endometrial cancer Means of weekly moderate intensity physical activity equivalents (MIE, minutes per week) |
not reported | not reported | Age, ethnicity, education, annual household income | Age 40-64 91 (90-93) vs. 80 (66-97) Age 65-79 61 (58-63) vs. 59 (48-74) | ns |
| Littman et al. 2001 | Non-occupational activity, including transportation to work, of all intensities | Low vs. high (no exercise vs. Q5) | OR=0.65 (0.46-0.92) | 0.23 | Age, county, unopposed oestrogen use and duration, income, BMI | OR=0.78 (0.55-1.11) | 0.38 |
| Matthews et al. 2005 | Total adult occupational, walking/cycling for transportation, household activity | <3.64 vs. >8.81 MET-h/day | not reported | not reported | Age, age at menarche, menopausal status and age, parity, OCP use, current smoking, ever drinking, family history of cancer, education, height, BMI | OR=0.71 (0.52-0.96) | <0.01 |
| Modesittet al. 2012 | Sleep, watching TV, moderate and vigorous physical activity | Activity data not reported | |||||
| Olson et al. 1997 | Walking, occupational, vigorous recreational activity (10 years ago) | Vigorous activity None vs. ≥100 hours/year | not reported | not reported | Age, education, BMI, diabetes, smoking, parity, age at menarche, menopausal status, use of unopposed oestrogen | OR=0.72 (0.43-1.19) | 0.10 |
| Plagens-Rotmanet al. 2020 | Work-related physical activity | <10 hours vs. >30 hours per week | not reported | not reported | None | OR=1.65 (0.72-3.79) | ns |
| Salazar-Martinez et al. 2000 | Not stated | ≤29 vs. ≥38 MET-h/week | not reported | not reported | Age, anovulatory index, smoking, menopausal status, hypertension, diabetes, BMI | OR=0.47 (0.26-0.86) | 0.01 |
| Shu et al. 1993 | Occupational, recreational activity of all intensities | Occupational Inactive (Q1) vs. Active (Q4) | Occupational RR=0.8 (0.5-1.4)) | ns | Age, parity, BMI, caloric intake | Occupational RR=0.9 (0.6-1.6) | ns |
| Non-occupational age ≥60 Inactive (Q1) vs. Active (Q4) | Non-occupational RR=0.6 (0.3-1.4) | ns | Non-occupational RR=0.5 (0.2-1.3) | ns |
OCP Oral Contraceptive Pill HT Hormone Therapy HR Hazard Ratio CI Confidence Interval RR Risk ratio SIR Standardised Incidence Ratio OR Odds Ratio ns not significant
Of the 11 cohort studies appraised, five found a statistically significant reduction in endometrial cancer risk with increasing physical activity, withrisk estimates ranging from 0.54-0.85(26,28,29,34,46). These risk estimates were based on performing 10.5 hours of total physical activity or five hours of moderate and vigorous activity each week compared with individuals performing less than 30 minutes of total physical activity each day or no moderate or vigorous activity.Whilst the risk estimates were reducedin each of these studies following BMI adjustment, the range remained the same, althoughonly three studies demonstrated statistically significant effects of physical activity on endometrial cancer risk in multivariable analyses (25, 30, 42).Five of the 12 case-control studies also found statistically significant reductions in endometrial cancer risk with increasing physical activity levels in both age-adjusted and multivariable analyses(25,35,36,39,45). BMI-unadjusted odds ratios ranged from 0.46-0.65, when comparing those in the most active with those in the least active groups, with slightly higher odds ratios observed following BMI adjustment (0.47-0.71). The greatest reduction in endometrial cancer risk was seen in the study by (45), where ≥38 MET-h/week or 5 hours of vigorous physical activity each week was associated with a 53% (95%CI 14-74%) reduction in endometrial cancer risk compared with women undertaking ≤29 MET-h/week or 3.5 hours of vigorous activity each week, after adjusting for age, anovulatory index, smoking, menopausal status, hypertension, diabetes and BMI.Theremaining studies found no statistically significant effect of increasing physical activity levels on endometrial cancer risk, with the exception of the study by (40), which failed to report the effect of physical activity on endometrial cancer risk, despite collecting the relevant data.
Secondary outcome
No studies reported the effect of different types of physical activity on the risk of endometrial cancer in continuous MET-h/week. Eighteen of the 23 included studies undertook at least one analysis to determine whether the domain or intensity of physical activity performed impacted upon endometrial cancer risk (25,27–39,41,42,46,47). Four of the 10 studies that assessed vigorous or moderate and vigorous activity together found a statistically significant reduction in endometrial cancer risk for the most active compared with the least active group (25,29,34,36). Endometrial cancer risk reductions ranged from 23-36% in those undertaking between three and eight hours of vigorous activity per week compared with women who never or rarely undertook physical activity of this intensity. Two studies noted a decrease in endometrial cancer risk in association with increasing levels of light physical activity only, with up to a 40% reduction in endometrial cancer risk for women undertaking more than 3.7 hours of walking each week compared with those performing no physical activity(33,38).
Two studies found a statistically significant reduction in endometrial cancer risk in women undertaking regular recreational activity, with risk reductions of 36-46% for women performing at least 90 minutes of recreational activity each day or 16.9 MET-h/week compared with those performing minimal recreational activity (33,46). (39)found a statistically significant decrease in endometrial cancer risk in women walking daily for transportation for more than one hour (OR 0.64, 95%CI 0.47-0.87, p-trend<0.01) and in those performing more than three hours of household chores each day (OR 0.62, 95%CI 0.46-0.85, p-trend<0.01). No other statistically significant relationships between domain or intensity of physical activity and endometrial cancer riskwere noted in the remaining studies.
Eleven studies investigated the effect of either long term physical activity or physical activity levels at different points in a woman’s lifetime on endometrial cancer risk (27,29,30,33–36,39,41,42,47). The findings were inconsistent, with three studies finding increased benefit with sustained high physical activity levels (12,36,42) and the others noting no demonstrable difference (27,29,30,33–35,41,47).
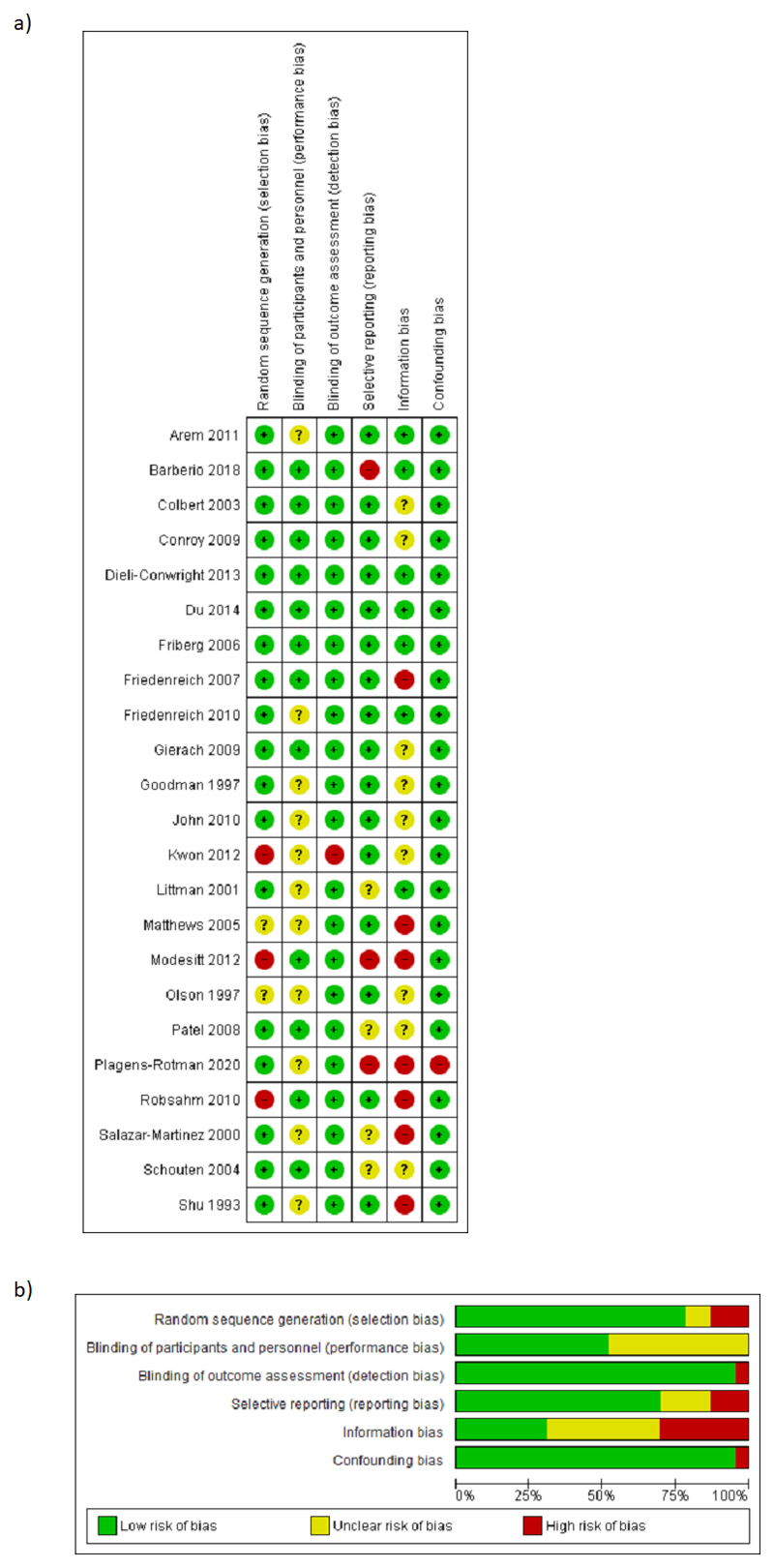
Risk of bias in included studies
The risk of bias summary and assessment for each individual study is shown in figure 2. Overall, the 11 cohort studies were considered at moderate risk of bias whilst the 12 case-control studies were considered at high risk of bias, predominately due to the risk of recall bias in a number of studies.
Figure 2.
Risk of bias summary a) per individual study b) per domain. a) Cohort studies were considered to be at moderate risk of bias overall whilst case-control studies were generally considered at high risk of bias due to the potential for recall bias. b) At least one study was considered to be at high risk of bias in all of the domains considered, with the exception of performance bias.
Of the included studies, 18were considered to be at low risk of selection bias as participants were selected from the general population and physical activity levels were assessed after participant recruitment(25–36,38,42,43,45–47). (44)assessed cancer risk in Norwegian world-class athletes, thereby placing this study at high risk of selection bias. (40)restricted their inclusion criteria to obese women and compared their physical activity levels with women undergoing a hysterectomy for benign indications and whose activity levels may have been affected by their underlying pathology. The study by (12) was deemed to be at unclear risk of selection bias as there was a 12% lower response rate from controls than cases, which may have impacted upon the results observed. A further study was also assessed to be at unclear risk of selection bias due to the identification of controls from lists of driving license holders, who may have been less physically active than women without a driving license and who relied on walking or bicycling for transportation (41).
Eleven studies were deemed at unclear risk of performance bias as they did not report whether study personnel were blinded to endometrial cancer diagnosis at the time of participant interview (25,33,35–39,41,43,45,47). This was because of the potential risk of recall bias and influence in interviews of lifestyle factors on cancer diagnosis.
Only one study was considered at high risk of detection bias as endometrial cancer diagnoses were based on patient report only (43). All other studies were considered at low risk due to endometrial cancer case ascertainment through cancer registries and histological confirmation.
None of the included studies published their protocols prospectively. Seven studies were considered to be at unclear or high risk of reporting bias as they failed to report all data collected (26,38,40,42,43,45,46).
Threestudies were considered at low risk of information bias as data were collectedusing questionnaires whose reliability had been checked through comparison with other well described questionnaires or within the same individuals over time and had been validated against objective measurements of physical activity using accerolometers(25,26,38). Four further studies were also considered at low risk of information bias as the questionnaires used had been shown to be reliable although their validity had not been assessed (29–31,33). This decision was taken based on the relatively modest correlation between all subjective assessments of physical activity tested and accelerometer-measured activity levels.Nine studies were deemed to be at unclear risk of information bias as the questionnaires used did not appear to have been assessed for reliability and had not been validated (26–31,34–37,40–42,46). Whilst there is a risk of recall error in case-control studies, which, by their nature, require participants to retrospectively recall information whilst being aware of their outcome status, such studies were not considered at increased risk of information bias if a broad range of data on potential endometrial cancer risk factors were collected at the time of interview and/or the study authors utilised techniques to minimize the possibility of systemic over or under-reporting of physical activity levels.Seven studies in total were assessed to be at high risk of information bias (32,39,40,43–45,47). Three studies used non-validated questionnaires whose reliability had not been assessed and did not attempt to mitigate the risk of biased recall of physical activity levels (43,45,47). The study by (40) utilized a questionnaire designed to quantify fitness levels and which has not been assessed for use in determining physical activity levels. The questionnaire used by (32)had previously been shown to satisfactorily rank participants in terms of their activity levels, but information about duration and frequency of some activity types were lacking with a risk of measurement error in other domains. (39)reported occupational activity based on job title only and (44)used next-of-kin in 416 instances to quantify physical activity levels as the subjects themselves were deceased.
All but one study wasconsidered at low risk of confounding bias as they had adjusted for at least one variable in their analysis (43).
Discussion
In this study we assessed the impact of physical activity levels on endometrial cancer risk in a primary analysis of participants of the UK Biobank and through a systematic review of the literature.Data from the UK Biobank revealed a statistically significant reduction in endometrial cancer risk with each 1 MET-h/week increase in total physical activity. Only one previously published study, by(33), was identified through the systematic review to have reported on the impact of a 1 MET-h/week increase in physical activity on endometrial cancer risk, finding no significant effect of increasing lifetime physical activity. Tenof the cohort and case-control studies reviewed found a statistically significant reduction in endometrial cancer risk but only in the group of most active individuals, who wereundertaking regular vigorous physical activity for at least five hours each week, equating to approximately 40 MET-hours/week. Whilst only eight of these studies retained statistically significant results following BMI adjustment, suggesting some obscuring of the true impact of physical activity on endometrial cancer prevention, the overall range of effect size remained unchanged. Vigorous and sustained physical activity over an individual’s lifetime may be associated with a greater reduction in endometrial risk, although study findings were inconsistent. These results suggest that large amounts of physical activitymay be neededfor an individual’s endometrial cancer risk to be reducedsignificantly. Currently, there is limited evidence on which to base firm recommendations about the type and amount of physical activity associated with the greatest reduction in endometrial cancer risk. More robust studies aimed at quantifying the impact of physical activity in MET-h/week on endometrial cancer risk are required to standardise findings and allow for inter-study comparisons.
The World Health Organisation (WHO) and US Physical Activity Guidelines Advisory committee recently concluded that there was moderate to high-certainty evidence that high physical activity levels were associated with a reduction in endometrial cancer risk, based on their appraisal of a number of systematic reviews and meta-analyses that have been conductedon the topic to date (48,49). The largest of these meta-analyses found that compared with individuals who undertook ‘low’ levels of physical activity, those that participated in ‘high’ levels had a 20% lower risk of endometrial cancer (RR 0.80, 95% CI 0.75 – 0.85)(10). This meta-analysis was conducted, however, by pooling the results of 33 studies that had variably defined ‘high’ and ‘low’ physical activity levels and incorporated different types and intensities of physical activity. There also appeared to be a disparity between the level of cancer risk reduction observed between cohort and case-control studies, with only a 16% reduction found in the more methodologically robust cohort studies.Neither the WHO nor the US Physical Activity Guidelines Advisory committee were able to comment on the nature of any dose-response relationship between physical activity and endometrial cancer risk or advise on the optimal type and intensity of activity to be undertaken, which this study aimed to address.(50) had previously suggested that a 3 MET-h/week increase in leisure time activity could be associated with a non-significant 2% (95%CI 0-5%) reduction in endometrial cancer risk based on their review of three cohort and three case-control studies, which are also included in this review (25,28,32,33,39,42). The authors found moderate heterogeneity between studies, a likely reflection of differences in study design and approach to calculation of physical activity levels, and had usedmodelled risk estimates based on limited reported data, with the inherent inaccuracies associated with this statistical approach (51). (12)alsoattempted to address the question of the dose-response relationship between increasing physical activity levels and endometrial cancer risk and found, as in this study, that the association was approximately linear. Unlike in this study, however, they noted, that the relationship was no longer statistically significant after BMI was taken into account (HR 1.02, 95%CI 0.91-1.14). This may reflect the fact that total physical activity levels were considered in the present study compared with only leisure-time activity in the earlier meta-analysis.
The current study, as well as providing an up to date review of the literature on the effect of physical activity on endometrial cancer risk, also presents novel data from the UK Biobank, a large biomedical resource containing detailed demographic and anthropometric information on over 250,000 women with linkage to the national cancer registry. Multiple imputation was utilised to deal with the modest amount of missing physical activity data and sensitivity analyses performed to determine the impact of this on the results observed. Appropriate statistical analysis techniques, including cubic spline analysis, were also employed to investigate the dose-response relationship between physical activity and endometrial cancer risk. Whilst thephysical activity levels studied here were self-reported, they were determined using a widely utilised questionnaire, validated against objectively measured physical activity levels as determined by an accelerometer (52,53). Although the correlation between self-reported and accelerometer measured physical activity levels is relatively modest with all surveys in current use, self-reported data do allow the accurate ranking of individuals within a population and hence the determination of a dose-response relationship, which was the focus of this study. Thus whilst the use of self-reported physical activity levels may have underestimated effects on disease risk, these data were used in preference to those based on accelerator-measured physical activity as accelerometers were worn by only 80,000 participants in the UK Biobank study and had shorter follow-up duration. The UK Biobank, like many cohort studies, has been shown to include a preferentially healthy population, of lower BMI and with fewer co-morbidities than the general population (54). The disease-exposure relationships observed in this analysis are in keeping with those previously published in the literature and along with the heterogeneity in physical activity levels observed within the cohort means that the findings reported here are generalizable to other populations.
The systematic review was conducted in accordance with the gold-standard methodology proposed by The Cochrane Collaboration and included a wide and systematic search of the literature to identify eligible studies, including hand searching of the grey literature and no limitations on publication or study type, with the exception of language. The risk of bias in the review process was minimised by having two authors independently screening titles, abstracts and full texts, with consensus reached in the presence of a third assessor in the case of disagreements. In addition, two authors worked independently to extract the data and to assess the risk of bias of included studies.
Alimitation of this study is the fact that it was not possible to complete a dose-response assessment of the effect of physical activity on endometrial cancer risk across more of the studies identified in the systematic review. Despite making contact, many study authors were unable to accessthe primary data due to the length of time since completion of their studies. The significant differences between studies in the methodology employed to calculate MET-h/week of physical activity, including the types of physical activity assessed and whether this incorporated all activity within a 24-hour period including sleep, would anyway have meant that any meta-analysis would have had to combine smaller numbers of similarly conducted studies only. It was also not possible to assess the most beneficial type or intensity of physical activity or the age at which it has maximal impact on endometrial cancer risk for the same reasons. Norandomised controlled trials have been performed to investigate the effect of physical activity on endometrial cancer risk and are unlikely to ever be undertaken given that in excess of 35,000 high-risk women would potentially need to be recruited and followed up for 5 years for any benefit to be observed according to calculations performed using similar data for breast cancer prevention(55). Any conclusions about the effect of physical activity on endometrial cancer riskwill, therefore, continue to be basedon observational data only. Whilst this review included 23 studies and over 10,000 endometrial cancer cases, there were concerns about the moderate to high risk of bias in included studies and inconsistency in reported results, which reduces the level of certainty around the evidence.
This study, like previous meta-analyses, has considered the impact of physical activity on endometrial cancer risk in isolation, without taking into account its important preventive effect on weight gain and the development of obesity and the potential value it may add to a dietary intervention as part of a weight loss strategy. This has not been possible as the majority of included primary studies have collected data on physical activity and BMI at study entry only, which has usually been in mid-life, and have not documented long term changes in physical activity and BMI levels. When this has been explored within the NIH-AARP Diet and Health study using mediation analysis, it appears that the majority (56-63%) of the benefit from increased physical activity in preventing endometrial cancer is mediated through a reduction inthe risk of obesity (56). Encouragingly, the authors of that study were also able to demonstrate that previously inactive individuals gained a substantial benefit from increasing their physical activity levels in mid-life, suggesting that it is never too late to take up physical activity. This study did, however, rely on retrospectively recalled physical activity and BMI data and included only 1468 endometrial cancer cases, resulting in wide confidence intervals.
Any future studies of physical activity and endometrial cancer risk need to take the effect of physical activity on BMI into account and should, ideally,be prospectively conducted using standardised methodology to allow for pooling of results and a meaningful meta-analysis. Theyshould incorporate objective measurement of physical activity levels using accelerometers to reduce the risk of information bias. Categorisation of data should be avoided, wherever possible, to maximize information gathering. Studies should focus on the effects of different types of physical activity on endometrial cancer risk and the age at which such activity has maximal beneficial effect. Until more evidence is available, women of all ages should continue to be encouraged to undertake 150 minutes of moderate to vigorous physical activity each week for its broader health benefits in line with World Health Organisation recommendations(49).
Conclusions
There is a paucity of high quality evidence to determine the dose-response relationship between physical activity and endometrial cancer risk. The available data indicate a weak inverse linear relationship, withfrequent prolonged periods of physical activity associated with greatest endometrial cancer riskreduction. Regular vigorous physical activity is encouraged to maximise the health benefit observed, in line with WHO recommendations.
Supplementary Material
Prevention relevance statement.
Effective, low cost primary prevention strategies are urgently needed to tackle the rapid global increase in endometrial cancer. We sought to quantify the effect of physical activity on endometrial cancer risk, noting a linear inverse relationship influenced by BMI. The most beneficial type and amount of activity remains unclear.
Acknowledgements
We would like to express our appreciation to UK Biobank participants and staff for providing the research community with this valuable source of data. This work used data from the application number 5791.
Financial support
S.J. Kitson is a NIHR Academic Clinical Lecturer and the recipient of a Wellbeing of Women Postdoctoral Research Fellowship (PRF101). E.J. Crosbie is supported by a National Institute for Health Research (NIHR) Advanced Fellowship (NIHR300650) and the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007). A. Lophatananon and K.R. Muirare supported by the European Union's funded Project iHELP under grant agreement 101017441.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Data availability
The data analysed in this study were obtained from the UK Biobank under application number 5791. The dataset is available to researchers through an open application at https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access.
References
- 1.Cancer Research UK. Uterine Cancer Statistics. 2022. Jan 13th, [Accessed 2022 13th January]. < https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer#heading-Zero>.
- 2.SEER. Cancer Stat Facts: Uterine Cancer. National Cancer Institute; Bethesda, MD: 2021. Jan 13th, [Accessed 2022 13th January.]. https://seer.cancer.gov/statfacts/html/corp.html . [Google Scholar]
- 3.Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–28. doi: 10.1016/S0140-6736(22)00323-3.. [DOI] [PubMed] [Google Scholar]
- 4.Kitson SJ, Evans DG, Crosbie EJ. Identifying High-Risk Women for Endometrial Cancer Prevention Strategies: Proposal of an Endometrial Cancer Risk Prediction Model. Cancer Prev Res (Phila) 2017;10(1):1–13. doi: 10.1158/1940-6207.Capr-16-0224. [DOI] [PubMed] [Google Scholar]
- 5.Gu B, Shang X, Yan M, Li X, Wang W, Wang Q, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels 1990-2019. Gynecol Oncol. 2021;161(2):573–80. doi: 10.1016/j.ygyno.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147–55. doi: 10.1038/bjc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 8.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3119–30. doi: 10.1158/1055-9965.EPI-10-0832. [DOI] [PubMed] [Google Scholar]
- 9.Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biologic mechanisms. Mol Oncol. 2021;15(3):790–800. doi: 10.1002/1878-0261.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30(5):397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 11.Voskuil DW, Monninkhof EM, Elias SG, Vlems FA, van Leeuwen FE. Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiol Biomarkers Prev. 2007;16(4):639–48. doi: 10.1158/1055-9965.Epi-06-0742. [DOI] [PubMed] [Google Scholar]
- 12.Matthews CE, Moore SC, Arem H, Cook MB, Trabert B, Hakansson N, et al. Amount and Intensity of Leisure-Time Physical Activity and Lower Cancer Risk. J Clin Oncol. 2020;38(7):686–97. doi: 10.1200/JCO.19.02407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med Sci Sports Exerc. 2019;51(6):1252–61. doi: 10.1249/MSS.0000000000001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezende LFM, Sa TH, Markozannes G, Rey-Lopez JP, Lee IM, Tsilidis KK, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52(13):826–33. doi: 10.1136/bjsports-2017-098391. [DOI] [PubMed] [Google Scholar]
- 15.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer. 2019;145(7):1719–30. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 16.Murray JM, Coleman HG, Hunter RF. Physical activity and cancer risk: Findings from the UK Biobank, a large prospective cohort study. Cancer Epidemiol. 2020;68:101780. doi: 10.1016/j.canep.2020.101780. [DOI] [PubMed] [Google Scholar]
- 17.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) – Short and Long Forms. [Accessed 2022 13th January]. 2005 13th January. < https://biobank.ndph.ox.ac.uk/ukb/ukb/docs/ipaq_analysis.pdf>.
- 19.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, et al. The Compendium of Physical Activities Tracking Guide. Healthy Lifestyles Research Center, College of Nursing & Health Innovation, Arizona State University; [Accessed 2022 13th January]. 2022 13th January. < https://sites.google.com/site/compendiumofphysicalactivities/>. [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Hernán MA, McAleenan A, Reeves BC, JPT H. Cochrane Handbook for Systematic Reviews of Interventions. 6.2 ed. 2021. Chapter 25: Assessing risk of bias in a non-randomized study. [Google Scholar]
- 22.Lucenteforte E, Talamini R, Montella M, Dal Maso L, Pelucchi C, Franceschi S, et al. Family history of cancer and the risk of endometrial cancer. Eur J Cancer Prev. 2009;18(2):95–9. doi: 10.1097/CEJ.0b013e328305a0c9. [DOI] [PubMed] [Google Scholar]
- 23.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 24.Wan YL, Beverley-Stevenson R, Carlisle D, Clarke S, Edmondson RJ, Glover S, et al. Working together to shape the endometrial cancer research agenda: The top ten unanswered research questions. Gynecol Oncol. 2016;143(2):287–93. doi: 10.1016/j.ygyno.2016.08.333. [DOI] [PubMed] [Google Scholar]
- 25.Arem H, Irwin ML, Zhou Y, Lu L, Risch H, Yu H. Physical activity and endometrial cancer in a population-based case-control study. Cancer Causes Control. 2011;22(2):219–26. doi: 10.1007/s10552-010-9689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barberio AM, Friedenreich CM, Lynch BM, Campbell KL, Arora P, Brenner DR. Physical Activity and Cancer Incidence in Alberta's Tomorrow Project: Results from a Prospective Cohort of 26,538 Participants. Cancer Epidemiol Biomarkers Prev. 2018;27(8):945–54. doi: 10.1158/1055-9965.EPI-17-1124. [DOI] [PubMed] [Google Scholar]
- 27.Colbert LH, Lacey JV, Jr, Schairer C, Albert P, Schatzkin A, Albanes D. Physical activity and risk of endometrial cancer in a prospective cohort study (United States) Cancer Causes Control. 2003;14(6):559–67. doi: 10.1023/a:1024866827775. [DOI] [PubMed] [Google Scholar]
- 28.Conroy MB, Sattelmair JR, Cook NR, Manson JE, Buring JE, Lee IM. Physical activity, adiposity, and risk of endometrial cancer. Cancer Causes Control. 2009;20(7):1107–15. doi: 10.1007/s10552-009-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieli-Conwright CM, Ma H, Lacey JV, Jr, Henderson KD, Neuhausen S, Horn-Ross PL, et al. Long-term and baseline recreational physical activity and risk of endometrial cancer: the California Teachers Study. Br J Cancer. 2013;109(3):761–8. doi: 10.1038/bjc.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du M, Kraft P, Eliassen AH, Giovannucci E, Hankinson SE, De Vivo I. Physical activity and risk of endometrial adenocarcinoma in the Nurses' Health Study. Int J Cancer. 2014;134(11):2707–16. doi: 10.1002/ijc.28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friberg E, Mantzoros CS, Wolk A. Physical activity and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2136–40. doi: 10.1158/1055-9965.EPI-06-0465. [DOI] [PubMed] [Google Scholar]
- 32.Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, et al. Physical activity and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Int J Cancer. 2007;121(2):347–55. doi: 10.1002/ijc.22676. [DOI] [PubMed] [Google Scholar]
- 33.Friedenreich CM, Cook LS, Magliocco AM, Duggan MA, Courneya KS. Case-control study of lifetime total physical activity and endometrial cancer risk. Cancer Causes Control. 2010;21(7):1105–16. doi: 10.1007/s10552-010-9538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gierach GL, Chang SC, Brinton LA, Lacey JV, Hollenbeck AR, Jr, Schatzkin A, et al. Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2009;124(9):2139–47. doi: 10.1002/ijc.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman MT, Hankin JH, Wilkens LR, Lyu LC, McDuffie K, Liu LQ, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997;57(22):5077–85. [PubMed] [Google Scholar]
- 36.John EM, Koo J, Horn-Ross PL. Lifetime physical activity and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1276–83. doi: 10.1158/1055-9965.Epi-09-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon S, Hou N, Wang M. Comparison of physical activity levels between cancer survivors and non-cancer participants in the 2009 BRFSS. J Cancer Surviv. 2012;6(1):54–62. doi: 10.1007/s11764-011-0204-8. [DOI] [PubMed] [Google Scholar]
- 38.Littman AJ, Voigt LF, Beresford SA, Weiss NS. Recreational physical activity and endometrial cancer risk. Am J Epidemiol. 2001;154(10):924–33. doi: 10.1093/aje/154.10.924. [DOI] [PubMed] [Google Scholar]
- 39.Matthews CE, Xu WH, Zheng W, Gao YT, Ruan ZX, Cheng JR, et al. Physical activity and risk of endometrial cancer: a report from the Shanghai endometrial cancer study. Cancer Epidemiol Biomarkers Prev. 2005;14(4):779–85. doi: 10.1158/1055-9965.EPI-04-0665. [DOI] [PubMed] [Google Scholar]
- 40.Modesitt SC, Geffel DL, Via J, W AL. Morbidly obese women with and without endometrial cancer: are there differences in measured physical fitness, body composition, or hormones? Gynecol Oncol. 2012;124(3):431–6. doi: 10.1016/j.ygyno.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Olson SH, Vena JE, Dorn JP, Marshall JR, Zielezny M, Laughlin R, et al. Exercise, occupational activity, and risk of endometrial cancer. Ann Epidemiol. 1997;7(1):46–53. doi: 10.1016/s1047-2797(96)00071-3. [DOI] [PubMed] [Google Scholar]
- 42.Patel AV, Feigelson HS, Talbot JT, McCullough ML, Rodriguez C, Patel RC, et al. The role of body weight in the relationship between physical activity and endometrial cancer: results from a large cohort of US women. Int J Cancer. 2008;123(8):1877–82. doi: 10.1002/ijc.23716. [DOI] [PubMed] [Google Scholar]
- 43.Plagens-Rotman K, Piskorz-Szymendera M, PIĘTA B. Lifestyle with particular emphasis on physical activity and genital carcinomas in women. Eur J Gynaecol Oncol. 2020;41(2):233–9. 10.31083. [Google Scholar]
- 44.Robsahm TE, Hestvik UE, Veierod MB, Fagerlie A, Nystad W, Engebretsen L, et al. Cancer risk in Norwegian world class athletes. Cancer Causes Control. 2010;21(10):1711–9. doi: 10.1007/s10552-010-9600-z. [DOI] [PubMed] [Google Scholar]
- 45.Salazar-Martinez E, Lazcano-Ponce EC, Lira-Lira GG, Escudero-De los Rios P, Salmeron-Castro J, Larrea F, et al. Case-control study of diabetes, obesity, physical activity and risk of endometrial cancer among Mexican women. Cancer Causes Control. 2000;11(8):707–11. doi: 10.1023/a:1008913619107. [DOI] [PubMed] [Google Scholar]
- 46.Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst. 2004;96(21):1635–8. doi: 10.1093/jnci/djh291. [DOI] [PubMed] [Google Scholar]
- 47.Shu XO, Hatch MC, Zheng W, Gao YT, Brinton LA. Physical activity and risk of endometrial cancer. Epidemiology. 1993;4(4):342–9. doi: 10.1097/00001648-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 48.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S.: Department of Health and Human Services; 2018. pp. F4–18. [Google Scholar]
- 49.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keum N, Ju W, Lee DH, Ding EL, Hsieh CC, Goodman JE, et al. Leisure-time physical activity and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2014;135(3):682–94. doi: 10.1002/ijc.28687. [DOI] [PubMed] [Google Scholar]
- 51.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 52.Guo W, Key TJ, Reeves GK. Accelerometer compared with questionnaire measures of physical activity in relation to body size and composition: a large cross-sectional analysis of UK Biobank. BMJ Open. 2019;9(1):e024206. doi: 10.1136/bmjopen-2018-024206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 54.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186(9):1026–34. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballard-Barbash R, Hunsberger S, Alciati MH, Blair SN, Goodwin PJ, McTiernan A, et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101(9):630–43. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saint-Maurice PF, Sampson JN, Michels KA, Moore SC, Loftfield E, McClain K, et al. Physical Activity From Adolescence Through Midlife and Associations With Body Mass Index and Endometrial Cancer Risk. JNCI Cancer Spectr. 2021;5(4) doi: 10.1093/jncics/pkab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analysed in this study were obtained from the UK Biobank under application number 5791. The dataset is available to researchers through an open application at https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access.