Abstract
Malnutrition has historically been researched and addressed within two distinct silos, focusing either on undernutrition, food insecurity, and micronutrient deficiencies, or on overweight, obesity, and dietary excess. However, through rapid global nutrition transition, an increasing proportion of individuals are exposed to different forms of malnutrition during the life course and have the double burden of malnutrition (DBM) directly. Long-lasting effects of malnutrition in early life can be attributed to interconnected biological pathways, involving imbalance of the gut microbiome, inflammation, metabolic dysregulation, and impaired insulin signalling. Lifecourse exposure to early undernutrition followed by later overweight increases the risk of non-communicable disease, by imposing a high metabolic load on a depleted capacity for homoeostasis, and in women increases the risk of childbirth complications. These life-course trajectories are shaped both by societal driving factors—ie, rapidly changing diets, norms of eating, and physical activity patterns—and by broader ecological factors such as pathogen burden and extrinsic mortality risk. Mitigation of the DBM will require major societal shifts regarding nutrition and public health, to implement comprehensive change that is sustained over decades, and scaled up into the entire global food system.
Introduction
Undernutrition and overweight have historically been considered separate challenges affecting distinct populations, and with contrasting risk factors. Undernutrition was linked with poverty, food insecurity, and infection, whereas obesity was linked with affluence, dietary richness, and sedentary behaviour. Increasingly, the two forms of malnutrition co-occur within communities, families, and even individuals, such as those who are both stunted and overweight.1 The current manifestation of this global double burden of malnutrition (DBM) is summarised in the first paper of this Series.2 Obesogenic environments are expanding while the causes of undernutrition persist,2 and an increasing proportion of individuals who are overweight were undernourished earlier in life.3 To understand the implications of the DBM for health at the individual level, the explanatory framework must shift from descriptive epidemiology to biology.
In their Lancet Commission on the global syndemic of obesity, undernutrition, and climate change, Swinburn and colleagues4 reconceptualised the two extremes of malnutrition within a single ecological framework, relating them to common drivers that also underlie climate breakdown. Here, we develop this overarching perspective, focusing on the biological interconnections between undernutrition and overweight. First, we describe the cause of malnutrition across life courses and generations. Both undernutrition and overweight can propagate long-term effects, especially if they develop early in life, and each might increase risk of the other occurring. Moreover, increasing numbers of people are being exposed to both forms of malnutrition at different points in the life course, due to the rapid nature of global nutrition transition. Second, we show that individuals who experience the DBM through their life course have increased risk of diverse forms of ill health. Third, we examine why the DBM is affecting more people worldwide, and highlight populations with high susceptibility. We provide an evolutionary perspective that can help to understand these biological interactions and their health consequences in different settings. Our framework might help to identify effective strategies for double-duty actions that address both forms of malnutrition, as discussed in subsequent papers in this Series.5,6
Life-course manifestation of malnutrition
Malnutrition is a complex phenotype that manifests across the life course in different ways (appendix pp 2−5), yet its categorisation remains unsophisticated. Regarding undernutrition, simple anthropometry is used to categorise low birthweight, stunting (low height for age) or wasting (low weight for age) during infancy or childhood, and short stature or low body-mass index (BMI) in adulthood. Assessed thus, undernutrition is most prevalent among younger age groups. Undernutrition can also be assessed in terms of depleted stores or circulating concentrations of nutrients, reflecting dietary inadequacy. Micronutrient deficiencies remain prevalent in adults and are of particular concern among women of reproductive age.7
Excess weight can likewise emerge in late fetal life (macrosomia), but usually develops from early childhood through cumulative exposure to obesogenic factors acting on both individuals and societies.4 Many studies link elevated adiposity, especially abdominal fat, with ill health. Despite being weakly associated with adiposity, the simple anthropometric BMI provides a useful metabolic risk marker for populations.8 The main limitation of BMI is its inconsistent association with non-communicable disease risk across populations.9 As with undernutrition, indices of nutritional excess extend beyond the body to traits such as diet composition and physical inactivity, both of which can perturb metabolism.
The concept of malnutrition should also incorporate the gut microbiome, representing millions of genes from microorganisms. The microbiome generates a collective metabolic activity that affects and responds to the human host. Diverse forms of malnutrition are associated with dysbiosis, propagating adverse metabolic consequences (appendix pp 6−9), although findings for obesity are heterogeneous. The microbiome shows resilience within individuals, with implications for health maintenance and disease risk,10 but can also respond to interventions (appendix pp 6−9).11
Malnutrition harms health throughout life, but its early emergence has particularly harmful consequences. Development is characterised by a succession of sensitive periods or so-called critical windows, when phenotype is particularly responsive to nutritional influences. Physiological mechanisms characterising these periods include the differential growth of organs and tissues, establishment of hormonal set points and epigenetic variability, telomere attrition, and microbiome maturation (panel 1). Crucially, these mechanisms respond to both inadequate and excessive levels of nutritional supply in early life, meaning that they contribute to intergenerational effects in both contexts. Such physiological sensitivity explains why early nutrition and growth have major implications for immediate survival, long-term health, and human capital.21,22
Many critical windows close early during development, reducing the sensitivity of specific traits to environmental influences. For example, some epigenetic effects are restricted to the periconceptional period,23 others to early infancy.18 Likewise, from late infancy linear growth becomes less sensitive to nutritional intake,24 hence the environmental contribution to short adult stature is primarily attributable to early stunting. However, other traits subsequently become plastic, and adolescence represents a key period of sensitivity to nutritional factors, especially relating to reproductive biology.
At the individual level, the DBM can thus be assessed through diverse somatic, dietary, and behavioural traits, as well as the microbiome, all of which might be targeted by appropriate interventions. Through the mechanisms of plasticity we have highlighted, different forms of malnutrition interact through the life course and across generations.
Intergenerational emergence of the DBM
Although malnutrition manifests within the life course, the causes of this condition span generations. For example, early sensitive periods fall within pregnancy and lactation, making maternal phenotype the key nutritional factor shaping early development.19,23,25 Through rapid nutrition transition, increasing numbers of people in low-income and middle-income countries (LMICs) are exposed to both nutritional deficiencies and fuel excess at different ages, a scenario termed double teratogenesis.26
Many LMIC populations have experienced chronic undernutrition, characterised by intergenerational cycles of disadvantage. Maternal undernutrition compromises fetal growth and increases the risk of childhood under-weight, stunting, and micronutrient deficiency (figure 1; appendix pp 13−18). Stunting is a cumulative process, often apparent by birth but worsening until around the age of 2 years when growth becomes canalised.27 Faltering of linear growth during infancy is exacerbated by episodes of wasting,28 which helps explain why stunting is associated with an elevated risk of mortality. Such intergenerational cycles have proven difficult to disrupt through interventions: maternal supplementation from mid-pregnancy to term with both macronutrients and micronutrients has modest effects on birthweight, but does not benefit growth in the longer term.29–31
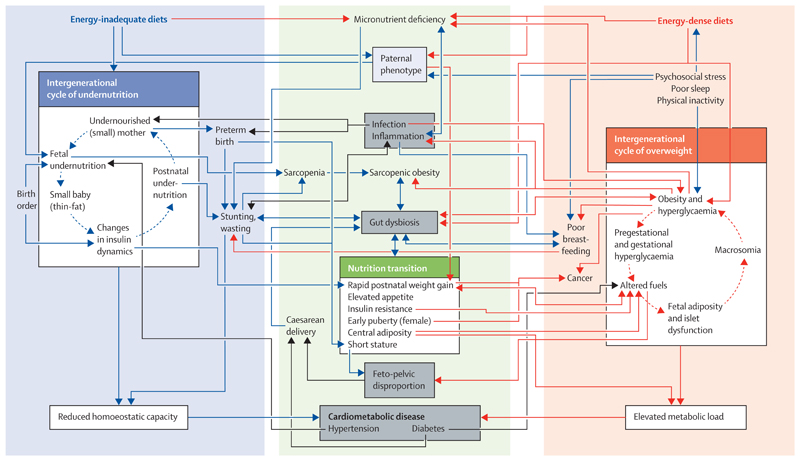
Figure 1. Complex interconnections between intergenerational cycles of undernutrition and nutritional excess and the effect of nutrition transition.
The intergenerational cycle of undernutrition (blue) associated with energy-inadequate diets and micronutrient deficiencies constrains growth and reduces the metabolic capacity for homoeostasis. The intergenerational cycle of overnutrition (red) associated with energy-dense diets is characterised by excess metabolic fuel and elevated adiposity, each of which challenges homoeostasis. Both cycles of malnutrition contribute to a wide range of adverse health outcomes (grey boxes), and specific diseases also increase the risk of malnutrition (black arrows). Through nutrition transition, individuals shift between these cycles within the life course, both increasing the risk and exacerbating the magnitude of health consequences. This framework helps identify how nutrition transition generates biological connections between many different forms of ill health (eg, low birthweight, stunting, central obesity, diabetes, and caesarean delivery).
Intergenerational effects are equally relevant to mothers with obesity or perturbed metabolism. Maternal obesity is associated with elevated fetal adiposity, especially when compounded by gestational diabetes (figure 1; appendix pp 13−18). More generally, a high amount of nutrition in early life (greater gestational weight gain, higher birthweight, faster postnatal weight gain) is associated with greater risk of obesity, abdominal adiposity, and insulin resistance in adulthood.
However, changes in food systems are breaking down the separation between these intergenerational cycles of nutritional deficiency and excess; we summarise evidence for interactions between these cycles (figure 1 and appendix pp 13−18). We highlight numerous ways in which exposure to undernutrition alters the consequences of subsequent exposure to obesity; for example rapid gain in BMI following early undernutrition might predispose an individual to central adiposity and non-communicable diseases. We show that the reverse pathway is also relevant; for example, the offspring of mothers who are obese might have poor growth and development in early life,32 although heterogeneity within each of these examples is substantial.
Whether early undernutrition predisposes to later adiposity depends on postnatal patterns of growth and nutrition, including complementary feeding patterns. Growth faltering in early pregnancy might induce a catch up in fat mass for the fetus before birth,33 and individuals who were small when born often undergo accelerated weight gain in infancy or childhood.34 This catch up might induce elevated adiposity, and in high-income countries rapid infant weight gain is associated with later obesity.35 However, studies in LMICs typically associate faster infant weight gain with greater adult height and lean mass,36 and in these settings, rapid weight gain seems to promote adiposity after about age 2 years,22 although the pattern of association might change in concert with nutrition transition.
Associations between stunting and later body composition are complex. In the short term, compensatory weight gain immediately following undernutrition might prioritise accretion of fat over lean body mass, through mechanisms of energy sparing.37 In some studies in South America, early stunting was found to predict excess abdominal adiposity, mediated by changes in fuel metabolism (panel 2).41 However, stunting was not associated with impaired fat oxidation in young children aged 2−6 years from Cameroon,42 and in Peru, the height of children aged 3·0−8·5 years was positively associated with adiposity at low altitude, but inversely associated at high altitude.43 In malnourished young children aged 6−23 months from Burkino Faso, 93·5% of weight gained during a food supplementation programme consisted of lean tissue.44 These findings indicate complex developmental links between growth patterns and adiposity, whereby growth might either be accelerated across all traits, or characterised by trade-offs between traits. Regardless of whether early stunting elevates abdominal adiposity, a consistent finding is that early undernutrition permanently reduces lean mass and its functional correlates such as grip strength.45–47
Although maternal obesity is associated with higher birthweight, it is also associated with micronutrient deficiencies that might impair offspring development, and maternal hypertension is associated with increased risk of offspring having a low birthweight, a scenario exacerbated by antihypertensive pharmaceutical agents.48 Obesity is generally associated with worse micronutrient status,49 mediated by chronic inflammation and nutrientpoor diets, and maternal obesity might also contribute to dysbiosis in the offspring.50
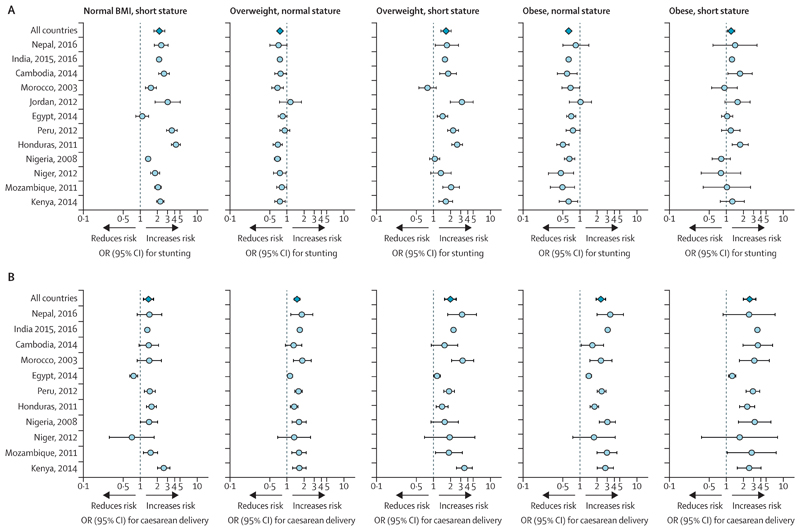
Using Demographic and Health Survey data from 12 LMICs we analysed how markers of maternal malnutrition (short stature, overweight, and obesity) interact in association with the risk of stunting in the offspring. Although short maternal stature (defined as having a height in the bottom quartile of the population sample) increases the risk of stunting, maternal overweight or obesity typically reduces this risk relative to normal BMI, providing the mother does not have a short stature. However, this protective effect disappears if the mother is both overweight and short (figure 2A). The consequences of maternal obesity for the next generation therefore depend on the mother’s own developmental experience. In a Swedish study, the intergenerational transmission of obesity was three times greater among mothers who were obese and born small for gestational age than in mothers of normal birthweight.51
Figure 2. Risk of stunting and caesarean delivery according to maternal phenotype in selected LMIC populations relative to mothers of normal height and normal BMI.
(A) Associations between stunting and maternal nutritional phenotype in 12 populations. (B) Associations between caesarean section and maternal nutritional phenotype in 11 populations. Based on Demographic and Health Survey data. All models adjust for wealth, parity, and offspring sex. Full details are given in the appendix (p 21). BMI=body-mass index. LMIC=low-income and middle-income countries. OR=odds ratio.
Although evidence remains scarce, paternal metabolic phenotype can also affect offspring development. For example, paternal smoking and dietary intake during adolescence have been associated with offspring BMI,52 mediated by imprinting of the sperm.53 Paternal genes might be especially relevant in early life because they contribute to placental function. Bariatric surgery in men has been associated with remodelling of sperm DNA methylation, in particular of genes associated with appetite control.53 However, beyond father−child correlations in height and BMI,54 the understanding of paternal biological contributions to the DBM in LMICs is minimal.
The life-course and intergenerational physiological pathways that we have summarised underlie associations of the DBM with several forms of ill health, as we discuss next.
The DBM and the risk of non-communicable disease
Associations of adult obesity and unhealthy lifestyle with non-communicable diseases are well recognised,55 but the evidence that exposure to undernutrition in early life exacerbates these relationships is compelling. To elucidate this interconnection, we present a capacityload conceptual model.56,57
Initially, associations of the risk of non-communicable disease with birthweight were attributed to long-term consequences of fetal undernutrition. The so-called thrifty phenotype hypothesis proposed that inadequate fetal nutrition reduced growth of some organs (eg, pancreas, liver, kidney) to protect the brain. Later, such individuals would be more likely to develop ill health as a result of obesity and energy-dense diets, elevating the risk of non-communicable diseases.38 However, birthweight is inversely associated with the risk of non-communicable disease across most of its range,58,59 although infants who are macrosomic have an increased risk of non-communicable disease.60 This variability refutes the notion that fetal undernutrition is the primary developmental mechanism of non-communicable diseases. The capacity-load model addresses continuous associations of both developmental and adult traits with the risk of noncommunicable disease, and can be applied to diverse traits through the life course and to various noncommunicable disease outcomes (appendix pp 22−26). Metabolic capacity refers to traits that are strongly dependent on growth and metabolic exposures during early life, and that have life-long implications for the capacity for homoeostasis.56 Relevant traits include pancreatic β-cell mass and function, nephron number, organ and tissue mass, airway and blood vessel diameter, and cardiac structure. All these traits scale with the magnitude of growth during the period of hyperplasic growth. Environmentally induced epigenetic variability and microbiome development can be considered within the same conceptual framework,61,62 although the extent to which early variability in these traits persists long term remains uncertain.63 Size at birth and early postnatal growth patterns act as useful, though imperfect, composite markers of metabolic capacity.
Metabolic load refers to traits that challenge homoeostasis, including excess adiposity, physical inactivity, lipogenic diet, smoking, infection, and psychosocial stress.56,57 These traits broadly show dose−response associations with the risk of non-communicable disease, and are all associated with increased oxidative damage. Metabolic load can increase early in life in association with catch-up growth, which elevates not only adiposity but also molecular markers of non-communicable disease risk (epigenetic effects, telomere attrition).64 Macrosomic infants already have elevated adiposity (high metabolic load), and potentially also low metabolic capacity, by birth.
According to this conceptual model, the risk of non-communicable disease decreases in association with metabolic capacity and increases in association with metabolic load. Substantial evidence supports the model,58,59 but the majority is from studies in high-income countries. Evidence from Asian and sub-Saharan African populations is summarised in the appendix (p 27−31), focusing on anthropometric markers of low capacity and elevated load.
One caveat is that the specific role of linear growth in this model varies by outcome. For cardiovascular disease, diabetes, and hypertension, linear growth promotes metabolic capacity, indicated by elevated risk of these non-communicable diseases among individuals with poor early growth or short adult stature (appendix p 32). This association is probably explained by height being a good proxy for organ growth and development through the life course. However, for many forms of cancer, linear growth might better be considered a marker of metabolic load than of metabolic capacity, because faster growth and taller height are associated with elevated cancer risk (appendix pp 33−34).65,66 These associations indicate that efforts to reduce low birthweight and stunting in LMICs might increase future cancer incidences.
This conceptual model helps explain why the DBM is strongly associated with the risk of non-communicable disease. Low birthweight, childhood stunting, and wasting all deplete components of metabolic capacity, whereas overweight and unhealthy environmental exposures exacerbate metabolic load. Importantly, the extent to which early undernutrition leads to overt non-communicable diseases depends strongly on subsequent nutritional status. For example, survivors of severe malnutrition during early life in Malawi had long-term deficits in height, lean mass, and grip strength; however, the risk of non-communicable disease was negligibly affected, most probably because these children remained relatively thin and had low metabolic load.47 The combination of poor early growth and subsequent elevated BMI associated with nutrition transition appears to be the important factor contributing to the risk of adults developing noncommunicable diseases.67
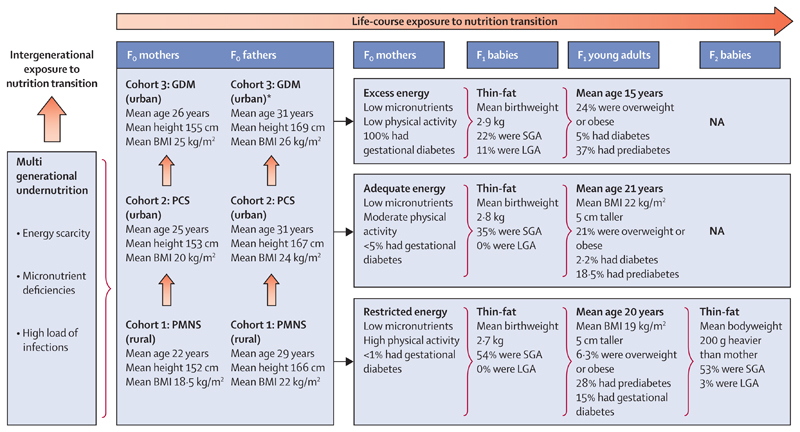
figure 3 illustrates how nutrition transition is driving the epidemic of non-communicable disease in Pune, India, combining data from three cohorts at different stages of economic development.68–73 Following multigenerational exposure to energy scarcity and micronutrient deficiencies, the rural cohort (recruited 1993−96) has shown a secular increase in adult size compared with parents, but 190 (28·7%) of 663 young adults have developed prediabetes, and 15% of 100 young mothers have gestational diabetes. These trends are more extreme in the matched urban cohort of 357 offspring (recruited 1987−89), with 75 (21·0%) developing overweight or obese, 66 (18·5%) developing prediabetes, and 8 (2·2%) developing diabetes by the age of 21 years. Finally among an urban cohort born to mothers with diabetes in pregnancy (recruited 1990−2010), 48 (24%) of 200 children were overweight or obese, 30 (37%) of 81 tested had prediabetes, and 4 (5%) had diabetes at mean age of 15 years.
Figure 3. Rapid transition and evolution of the double burden of malnutrition in Pune, India, over the past 40 years, based on data from rural and urban cohorts.
Average age in index pregnancy, height, and BMI of mothers and fathers are shown for three cohorts set up in Pune, India in the 1980−90s.68–73 Rural mothers (Fo generation, cohort 1: PMNS, recruited 1993−96) show the legacy of multigenerational undernutrition (stunted and underweight, low energy intake and excess physical activity from subsistence farming, multiple micronutrient deficiencies, and low prevalence of gestational diabetes). Parents in the contemporary urban cohort (cohort 2: PCS, recruited 1987−89) were somewhat taller and had a higher BMI. Parents in the diabetic pregnancy cohort (cohort 3: GDM, recruited 1990−2010) were the tallest and heaviest, almost half being overweight or obese (BMI>25 kg/m2), reflecting the effects of socioeconomic transition. F1 babies born to cohort 1 mothers had low average weight and a characteristic thin-fat composition (ie, low lean mass but high fat mass compared with European babies). F1 babies in cohort 2 had somewhat higher birthweight but 35% were still SGA by INTERGROWTH criteria.74 Babies born to mothers in cohort 3 were the heaviest (11% LGA), but 22% were still SGA. F1 young adults in cohort 1 were taller and heavier than their parents, although still thin (low BMI) but adipose (high body fat). F2 babies were 200 g heavier at birth than their mothers’ birthweight, highlighting an intergenerational effect of the DBM. In cohort 2, F1 children were similarly taller and heavier than their parents, with 75 (21%) of 357 being overweight or obese. In cohort 3, these intergenerational effects were more marked, with 48 (24%) of 200 F1 children being overweight or obese. BMI=body-mass index. DBM=double burden of malnutrition. GDM=gestational diabetes. LGA=large for gestational age. NA=not available. PCS=Pune Children’s Study. PMNS=Pune Maternal Nutrition Study. SGA=small for gestational age. *Husbands of women with gestational diabetes.
The increased toxicity of obesity among individuals who were initially undernourished has been shown in diverse populations (appendix pp 27−31).58,75 Studies in Brazil have revealed some of the physiological mechanisms through which childhood stunting might predispose that individual to central fat deposition and the risk of non-communicable disease (panel 2). Notably, however, a combination of better diet quality and prevention of infections appears to be able to reverse these effects.39
Likewise, nutritional supplementation in early life might potentially promote metabolic capacity and thus reduce the risk of non-communicable disease. Supplementation during pregnancy reduces the risk of low birthweight29,76 but propagates few beneficial effects into childhood,31,77 whereas a community food supplementation programme provided to both pregnant women and their offspring in early childhood improved childhood growth and was associated with greater height and lean mass but not adiposity in early adulthood.78 However, the longer-term consequences of this intervention for the risk of non-communicable disease were mixed,79 possibly because the intervention spanned several different developmental stages and might have affected both metabolic capacity and load.
The optimal timing in early life for interventions to prevent the DBM therefore needs further research. We suggest that alongside the preconception period and pregnancy,23 early infancy might be another crucial window of opportunity.5 For example, the period of exclusive breastfeeding is simultaneously a developmental period when many mechanisms of plasticity respond to nutritional influences (appendix pp 35−36), an important period for the development of metabolic capacity, and a period when metabolic load can be suppressed.57 However, the success of breastfeeding is itself threatened by maternal malnutrition (panel 3), indicating that interventions targeting mothers who are breastfeeding might simultaneously improve maternal health while also mitigating the DBM in the next generation.
Although malnutrition damages health in all populations, the manifestation and physiological consequences of the DBM vary. First, the prevalence and consequences of malnutrition often differ between the sexes. Low adult BMI and childhood stunting tend to be slightly more common in male individuals than female individuals in LMICs,86,87 whereas adult women show higher prevalence than men for obesity and anaemia.88,89 Moreover, the lifecourse development of the risk of non-communicable disease also differs by sex.90
Second, ethnicity contributes to variability in the health consequences of the DBM. Human morphology and physiology vary with geography, in ways that also change through the life course.91 For example, south Asian populations have a high prevalence of low birthweight and stunting and relatively short adult stature, all indicative of reduced metabolic capacity, but also a high fat-to-lean ratio and abdominal adiposity, indicative of elevated metabolic load for a given BMI value.92,93 These traits are strongly implicated in the elevated susceptibility of non-communicable disease in south Asian populations, although whether these traits arise from genetic factors or mechanisms of intergenerational plasticity is unclear. However, these traits are also overlaid by economic and cultural factors, including diet preferences, migration patterns, and social inequality.
Malnutrition and inflammation
The full consequences of the DBM for ill health relate not only to physical phenotype (growth and nutritional status), but also to the local ecological setting. The lifecourse manifestation of malnutrition varies markedly across ecological settings. In high-income countries, the obesity epidemic developed in the context of low burdens of communicable disease and childhood undernutrition. In LMICs, however, both extremes of malnutrition coexist with persistent burdens of infections. Both undernutrition and overweight are associated with inflammation,94,95 effectively generating a triple challenge to metabolic health with major implications for the risk of non-communicable disease.
Poor nutrition in early life (fetal growth faltering, postnatal stunting, suboptimal breastfeeding) has been associated with elevated markers of inflammation in childhood and young adulthood, and obesity is also a chronic inflammatory condition.94,96–98 Although research from LMICs is scarce, studies from high-income countries indicate that the inflammatory load of obesity might be exacerbated by undernutrition in early life.99,100
An unfavourable gut microbiome might contribute to these associations (appendix pp 6−9). Microbiota immaturity, increased enteropathogen burden, and gut barrier dysfunction are inter-related factors associated with inflammation in early life.101 The microbiota of Indian children with stunting was depleted in probiotic species and enriched in inflammogenic taxa, relative to controls.102 In adulthood, dysbiosis contributes to associations of obesity with insulin resistance and systemic inflammation.103,104 Among adults who are obese with similar BMI, those with greater dysbiosis have a higher risk of non-communicable disease,105 and dysbiosis also contributes to the inflammatory process associated with sarcopenia.106 Thus, in LMICs the nutrition transition could exacerbate the inflammatory burden of malnutrition.
Manipulation of the microbiome—eg, by providing probiotics or faecal transplantation—might beneficially modify markers for the risk of non-communicable disease (appendix pp 6−9), but further research is needed to understand how this treatment could achieve long-lasting effects mitigating both forms of malnutrition.
The DBM and childbirth complications
Although much emphasis has been placed on the implications of the DBM for the risk of non-communicable disease, both short stature and overweight are also independent risk factors for obstructed labour, related to maternal mortality.107 The DBM might therefore affect health outcomes of mothers and offspring related to childbirth. For women who are short, the primary underlying mechanism is likely to be reduced pelvic dimensions, whereas for women who are overweight a key mechanism is perturbed fuel metabolism, increasing birthweight.107 Globally, many mothers who were stunted in early life become overweight before reproducing, and women who are overweight are also more likely to develop gestational diabetes if previously stunted.107 The combined effect is predicted to increase the risk of obstructed labour.
We analysed Demographic and Health Surveys from 11 LMICs. Both maternal stunting and overweight each increase risk of caesarean delivery (a correlate of obstructed labour); however, the risk tends to be further amplified among women who are stunted and overweight or obese (figure 2B). Although increases in the number of caesareans have been linked with indices of wealth, the financial incentives of health-care providers, and defensive medicine to minimise litigation, 108,109 the emerging DBM might be an additional factor.3
Given the profound health consequences of the DBM in individuals, we need to understand what is driving the global epidemic. We show in the next section that biological susceptibility interacts with societal factors and provide an evolutionary perspective to help understand how we might combat the DBM most effectively.
Societal driving factors
The biological interlinkages between undernutrition and overweight that we have described can only be fully understood in the context of broader societal drivers, which mediate differential exposure to the causes of malnutrition. The global DBM is closely associated with rapid economic development and increased income per capita,2 but also incorporates a constellation of societal trends acting across culture, behaviour, and technology.4 Many of the individuals most exposed to these trends are not the wealthiest and are the least empowered to resist adverse societal and corporate influences.5,57 The metabolic consequences of unhealthy diets that are both energy dense and micronutrient poor110 are exacerbated by increases in sedentary behaviour,111 and exposure to psychosocial stress has been associated with unhealthy food choices and eating patterns, and with perturbed appetite, weight gain, and central adiposity.112,113
Many factors reduce individual agency. For example, at a societal level, gender inequality exacerbates female exposure to the DBM.114,115 From a political perspective, governments struggle to restrain commercial activities in the interests of population health, but might also contribute to the DBM through the promotion of international trade and national economic development, and the opening of domestic markets to multinational corporations. Policy makers are increasingly exploring strategies to reduce malnutrition that place less emphasis on economic growth, by addressing issues such as food sovereignty, gender equality, education, and healthy food systems.114,116–118 Nevertheless, the emerging DBM is a stark indication of how a large proportion of the global population, especially in LMICs, is poorly protected from multiple factors driving malnutrition in all its forms.
Groups at high risk
Although the DBM affects many countries,2,4 various groups are at high risk of both undernutrition and overweight and merit attention. This susceptibility relates to diverse factors spanning both biology and environmental stressors.
In high-income countries such as Canada, Australia, and the UK, for example, First Nation, Indigenous, and ethnic minority populations, respectively, typically show higher levels of low birthweight and childhood undernutrition than do the general population, but also increased risk of obesity and non-communicable diseases in later life (appendix pp 39−42). Similarly, African Americans show persisting deficits in birthweight relative to people of European ancestry, additionally, Hispanic and African Americans have greater prevalence of adult obesity than Americans of European descent.119 Similar patterns increasingly apply to minority groups within LMICs, such as tribal populations in India.120
To track such population-specific susceptibility, ethnic differences in physique and metabolism should be addressed. In the UK, for example, adjusting for ethnic differences in the relationship between BMI and adiposity reveals that obesity is most prevalent and increasing fastest in children of south Asian ethnicity compared with other ethnic groups.121 Moreover, body fat is more toxic in children of south Asian descent than in those of European descent, since it has a stronger association with insulin resistance.122 These patterns help explain why some ethnic groups show high susceptibility to noncommunicable diseases in early adulthood, even at relatively low BMI thresholds.
As noted in the first paper of this Series,2 within LMICs, rural to urban migration exposes increasing numbers of people to drastic changes in diet, physical activity, and living conditions. Rural populations have a high prevalence of childhood stunting,123 whereas migration to cities is typically associated with rapid increases in BMI and abdominal fat. This adiposity elevates the risk of non-communicable disease in comparison with the rural population, but typically to lower levels than in established urban populations.124 These health consequences might increase through lengthier urban residence and at older ages; however, research on malnutrition in older people (aged >65 years) in LMICs remains very scarce.125,126
Adolescents are another particularly important group because of the imminence of reproduction. Surveys indicate high prevalences of underweight, overweight, and anaemia in adolescents aged 10−19 years, although they vary by country.127,128Adolescents are also among the first groups to adopt new diets and lifestyles, in part through their tendency to migrate in search of new economic opportunities. The combination of overweight and anaemia in adolescent women is difficult to address, because obesity-mediated inflammation might impede iron absorption and reduce the efficacy of supplementation programmes.127 Finally, infants are susceptible to complementary foods that are fattening and also deficient in micronutrients.129
An evolutionary perspective
The profound health risks associated with life-course exposure to the DBM might seem puzzling. First, why does nutrition transition not resolve the effects of multigenerational undernutrition? Why do children with stunting often remain short in adulthood and become overweight, rather than growing tall and remaining lean? Second, why is the combination of early stunting and later overweight so detrimental to health? An evolutionary perspective might help explain why different forms of malnutrition interact and shape the risk of noncommunicable disease. Evolutionary life-history theory assumes that every organism allocates energy between four competing functions: maintenance, growth, reproduction, and defence, resulting in trade-offs between these functions.130 The optimal allocation strategy for maximising reproductive fitness is expected to vary in association with developmental trajectory and ecological conditions.131
A key factor influencing these allocation decisions is extrinsic mortality risk. In high-risk environments, selection favours discounting the future, diverting energy away from maintenance and growth towards defence (short-term survival) and reproduction. This insight helps understand the combination of high fertility and lower birthweights in chronically undernourished populations with high infectious burdens: fitness is maximised by producing more offspring, but investing less in each. Suboptimal fetal nutrition not only constrains the development of metabolic capacity, but also is associated with long-term central adiposity and inflammation, promoting immune function through the life course.57,98 Economic development increases dietary energy availability and alters life history strategy; however, the nature of this change depends on both extrinsic mortality risk and diet composition. In high-pathogen and food-insecure environments, if energy supply increases during childhood it is too late to allocate this energy to maintenance because the physiological critical window has already closed. Instead, the surplus is primarily diverted to survival (pro-inflammatory state, energy stores) and reproduction (gaining weight during adolescence). This strategy helps to explain why individuals who were initially undernourished do not entirely resolve their growth deficit, and are prone to central adiposity and elevated inflammation.99,100,132 Reduced fertility among women who are obese133 suggests that these trade-offs evolved in ancestral environments characterised by energy scarcity, and in contemporary settings they might be exacerbated through exposure to processed foods high in energy but low in protein and micronutrients. Inflammation disrupts many components of homoeostasis relevant to the risk of non-communicable disease, such as appetite, sleep, insulin metabolism, arterial health, and oxidative balance.134 The result is a high metabolic load superimposed on a depleted capacity, provoking non-communicable diseases at relatively low thresholds of age and overweight.
In food-secure and low-pathogen settings, conversely, lower infant mortality means that mothers can maximise fitness by producing fewer offspring, and investing more in each during early life. This strategy allows each offspring to divert more energy to maintenance and early growth, promoting life-long homoeostasis and health, and probably a lengthier reproductive period.
This perspective helps explain why in high-income countries with long-term efforts to reduce infectious disease, economic development has induced prolonged secular increases in height and lifespan,135 whereas in many LMICs where poor quality diets and high infectious burdens persist, economic development is more strongly associated with trends in BMI,88 and an escalating burden of non-communicable disease. Secular trends in stature in LMICs remain weak;136,137 instead, a secular decline in menarchal age has occurred, especially in urban settings (appendix pp 43−44).57 The mechanisms of developmental plasticity described in panel 1 might play a key role in orchestrating such life history trade-offs in association with ecological conditions.
Trade-offs between biological functions might prove especially rewarding targets for interventions aiming to reduce malnutrition and associated adverse health effects. Whether efforts to reduce the risk of non-communicable disease in adults in LMICs are successful might be contingent on reducing both early undernutrition and the burden of infection. This proposition is supported by evidence that, independent of nutritional supply, childhood vaccination benefits linear growth.138
Conclusion
Examining the DBM from the perspective of individual health is very different from approaching it at the population level. Beyond the common driving factors,4 undernutrition and overweight show multiple physiological connections and interactions. As LMICs undergo economic development and nutrition transition, the resulting DBM is exposing growing numbers of individuals to various forms of ill health, including growth retardation, dysbiosis, inflammation, obesity, noncommunicable diseases, and childbirth complications. Recognising these interconnections might reveal new, shared opportunities to improve metabolic health.
The evolutionary perspective helps explain why the DBM is so harmful to health. Exposure to malnutrition during early critical periods results in the body reducing its valuation of the future, diverting energy from growth and health to survival and potential reproduction. If the only substantial environmental change through the life course is increased dietary energy, these trade-offs might simply intensify. This scenario could explain why some programmes aiming to prevent undernutrition have inadvertently increased obesity and the risk of non-communicable disease.79 High-energy diets that are low in protein, fibre, and micronutrients might drive over-consumption of fat and carbohydrate.139 High burdens of infectious disease also constrain linear growth and favour inflammation, which is further exacerbated by overweight.
The programme of treatment for childhood malnutrition in Brazil (panel 2) highlights where efforts should be directed to break this cycle.39 The programme improves dietary quality while also cutting the burden of infection. This composite improvement makes the long-term future more attainable, and the body responds by promoting linear growth rather than adiposity, while restoring metabolism to lower the risk of non-communicable disease.
To be effective, the double-duty actions proposed in the third paper in this Series5 should achieve two goals. First, the actions need to affect each generation early in life, so that the trajectory of development can be shifted beneficially through the physiological mechanisms listed in panel 1. This approach highlights the importance of optimising nutrition among adolescents, whose metabolism constitutes the developmental niche for the next generation. Secondly, interventions need to be sufficiently comprehensive to affect the functional trade-offs that we have outlined. Successful interventions might need to begin before conception and continue through pregnancy and lactation. A cautionary note is that these interventions still need to balance health benefits and costs. For example, research is required to optimise the balance between promoting fetal growth and maintaining maternal metabolic health during pregnancy. Such interventions should be supported by the sustained provision of healthy complementary foods, and effective reductions in the burden of infection during childhood.
No single intervention can solve the DBM, and efforts must also be sustained over decades to realise their full benefits. Even if stunting is reduced, adults already overweight will bear additional health consequences if they were undernourished during development. On the positive side, effective double-duty actions5,6 might benefit health across the lifespan and into the next generation. To achieve these goals, major societal shifts are required regarding nutrition and public health. Ultimately, the global DBM reflects many adverse trends through which individuals are disempowered and their nutritional status and health undermined. The fourth paper in this Series6 shows that specific interventions to reduce the DBM can be cost-effective, but substantial progress requires that this approach be scaled up into the entire global food system, while also meeting the need for human food systems to maintain planetary health.140
Supplementary Material
Key messages.
Malnutrition has historically been researched and addressed in two distinct silos, focusing either on chronic or acute undernutrition, energy inadequacy, and micronutrient deficiencies, or on overweight, obesity, and dietary excess. Through rapid changes in food environments and living conditions, global nutrition transition is generating a new double burden of malnutrition (DBM) in which undernutrition and overweight are impossible to separate; however, the recognition of this double burden should reveal opportunities to address both issues simultaneously.
Malnutrition harms health throughout the life course, but the emergence of malnutrition early in life is particularly harmful. A variety of physiological mechanisms propagate effects of early-life malnutrition across the life course, and adolescent and adult malnutrition can transmit effects to the next generation.
Different forms of malnutrition can interact through the life course and across generations. In some settings, early stunting might predispose an individual to a more central distribution of adiposity at later ages, and the extent to which maternal obesity adversely affects early growth and development of the offspring might be exacerbated if the mother was undernourished in early life.
Life-course exposure to the DBM (early undernutrition followed by later overweight) increases the risk of non-communicable disease, by imposing a high metabolic load on a depleted capacity for homoeostasis. The health costs of adult obesity are therefore exacerbated among individuals who have previously had undernutrition. In women, life-course exposure to the DBM increases the risk of childbirth complications.
Exclusive and appropriate breastfeeding protects infants against all forms of malnutrition, and protects mothers against diabetes and breast cancer, in part through healthy-weight benefits. However, maternal obesity, diabetes, and micronutrient deficiencies alter the biology of lactation, and should be addressed to maximise the success of breastfeeding.
Exposure to the DBM can only be fully understood in the context of broader societal drivers acting across culture, behaviour, and technology. Various groups are at high risk of the double burden through elevated exposure to these drivers, often exacerbated by biological susceptibility.
Developmental responses to malnutrition in early life are shaped by ecological factors, such as pathogen burden and extrinsic mortality risk. An evolutionary perspective, focusing on how our biological plasticity was shaped in ancestral environments to promote survival and reproduction, might help design interventions that promote linear growth and lean tissue accretion rather than excess adiposity.
Intergenerational cycles of malnutrition have proven difficult to disrupt through public health interventions. Major societal shifts are required regarding nutrition and public health to implement comprehensive change that is sustained over decades, and scaled up into the entire global food system.
Panel 1. Physiological mechanisms through which exposure to undernutrition or nutritional excess during early life is associated with long-term phenotypic variability.
Early nutrition generates long-term effects on organ size, structure, and function. Mammalian growth in fetal life and early infancy comprises hyperplasia (cell proliferation), crucial for the development of organ structure, whereas from late infancy, growth comprises hypertrophy (increases in cell size).12 Early growth variability has long-term effects—eg, infants with a low birthweight have altered cardiac structure and small liver, kidneys, and spleen,13,14 whereas macrosomic infants might have organomegaly.
-
Early nutrition affects hormonal axes regulating growth and appetite.
Both undernutrition and excess nutrition in the perinatal period affect insulin metabolism and hypothalamic circuits regulating food intake.15 Infants with low birthweight might be insulin-sensitive at birth, but are susceptible to insulin resistance in association with faster weight gain in childhood.
Both interuterine growth retardation and maternal diabetes expose the fetus to oxidative stress, affecting cardiac and vascular structure, haemodynamics, and endothelial function.
Gene expression in the offspring is shaped by maternal nutrition in pregnancy and by nutritional experience after birth. For example, periconceptional exposure to maternal famine has been associated with epigenetic changes in IGF1 expression that persisted into early old-age,16 while when the offspring was conceived in rural Africa was associated with diverse epigenetic effects in infancy.17 Gestational diabetes is associated with epigenetic effects on genes associated with metabolic disease. Some epigenetic changes might have adverse long-term health effects.
Early exposure or lack of exposure to different food tastes might shape food preferences and diet choices at later ages.
Telomere length provides a marker of cellular ageing that is sensitive to early nutritional experience. For example, placental and neonatal telomere length are both associated with some components of maternal nutritional status, and predict postnatal body composition,18 and exclusive breastfeeding might reduce telomere attrition.19
The gut microbiome rapidly matures in early life, and early undernutrition can disrupt this process. For example, among twins discordant for kwashiorkor the affected sibling developed narrower gut microbiome diversity, and transplanting this biota to germ-free mice induced weight loss.20
Collectively, these mechanisms contribute to a profound imprint of early malnutrition on later phenotype, affecting both the risk and the metabolic effects of subsequent overweight.
An expanded, fully referenced version of this panel is available in the appendix (pp 10−12).
Panel 2. Developmental links between stunting, obesity, and cardiometabolic risk in Brazil.
Undernutrition in early life promotes survival by energy sparing, selectively preserving some tissues and organs over others.37,38 This adaptation is achieved by endocrine changes affecting growth, energy expenditure, and body composition, which then interact with the composition and energy content of the diet.
Among children from shanty towns in Brazil, stunting is associated with reduced lean mass but greater adiposity, especially central abdominal fat. These physical traits are associated with increased insulin sensitivity, reduced insulin production, higher cortisol, and a reduced capacity for fat oxidation.39
By adulthood, the adverse effects of overweight on cardiometabolic traits are exacerbated among individuals who are also stunted. Among adults who are overweight, stunting is associated with lower tri-iodothyronine, higher insulin resistance, and higher glycated haemoglobin. In women who are overweight, stunting is also associated with dyslipidaemia and higher blood pressure.40
Adequate treatment of undernutrition during childhood with recovery in height and weight might lead to normalisation of insulin activity, leptin, cortisol stress response, body composition, and bone mineral density.39
An expanded, fully referenced version of this panel is available in the appendix (pp 19−20).
Panel 3. The pivotal role of breastfeeding in mitigating the double burden of malnutrition.
Breastfeeding has the potential to reduce the risk of both components of malnutrition in the offspring, and to promote maternal health. First, breastfeeding is strongly protective against diarrhoea and infections in the offspring, and therefore reduces the risk of mortality, stunting, and wasting in early life.80 Second, breastfeeding constrains excess body-mass index in the offspring during early sensitive periods, and is suggested to protect against later obesity, as well as non-communicable diseases such as diabetes, although it is not associated with all non-communicable disease risk markers.80 Third, breastfeeding might be considered to mitigate some of the maternal metabolic stresses induced by pregnancy. For example, prolonged breastfeeding is associated with increased maternal insulin sensitivity that persists for at least 2 years after weaning, and reduces long-term risk of diabetes in mothers and the risk of breast cancer.81 Given these beneficial effects, breastfeeding is an ideal target for interventions, as explored in the third paper of this Series.5
Successful breastfeeding is challenged not only by societal constraints on women’s autonomy and employment, but also by both forms of maternal malnutrition. Among mothers who are severely undernourished, low breastmilk volume and micronutrient status might affect growth and micronutrient status of the offspring.82 Poor maternal diet reduces the diversity of the maternal microbiome, which is then transmitted to the offspring and is associated with increased risk of severe malnutrition. Human milk oligosaccharides, unique to our species, play a key role in the establishment of a healthy gut microbiome. Animal studies have shown that promoting specific types of oligosaccharides increases lean tissue accretion in early life.83
At the other extreme, maternal obesity is associated with lower likelihood of breastfeeding and reduced duration of any or exclusive breastfeeding. At the level of physiology, glucose intolerance during pregnancy might impede milk synthesis and contribute to delayed lactogenesis, and excessive gestational weight gain is associated with raised inflammatory markers in breastmilk.84 Studies of women with diabetes show that a longer duration of breastfeeding than in women without diabetes is necessary to achieve the beneficial protective effects against childhood obesity (appendix pp 37−38).85
Both social support and metabolic health of the mother are therefore crucial to maximising the success of breastfeeding and capturing the health benefits to both mother and offspring. Maternal dietary intake, non-communicable disease status, and the composition of the maternal microbiome are all potential targets for interventions, but further work is required to better understand the mechanisms and to develop effective solutions.
Acknowledgments
Funding for the preparation of the Series was provided by WHO, through a grant from the Bill & Melinda Gates Foundation. The funder had no role in the analysis and interpretation of the evidence or in writing the paper and the decision to submit for publication.
Footnotes
Contributors
This paper was conceptualised by AD, JCW, and CSY, and its development steered by JCW, CSY, ALS, and AD. Literature reviews were done by JCW, RW, and MM. Data extraction and coding was done by MSP. Statistical analysis was done by RW and JCW. Summaries of research in India and Brazil were prepared by CSY and ALS, respectively. The conceptual diagram was developed by JCW and RW. JCW wrote the first draft of the manuscript, and all authors contributed to revising it and approved the final version.
Declaration of interests
We declare no competing interests.
Contributor Information
Jonathan C Wells, Childhood Nutrition Research Centre, UCL Great Ormond Street Institute of Child Health, London, UK.
Ana Lydia Sawaya, Department of Physiology, Federal University of São Paulo, São Paulo, Brazil.
Rasmus Wibaek, Department of Nutrition, Exercise and Sports and School of Global Health, University of Copenhagen, Copenhagen, Denmark; Clinical Epidemiology, Steno Diabetes Center Copenhagen, Copenhagen, Denmark.
Martha Mwangome, Kenya Medical Research Institute Wellcome Trust Research Program, Kilifi, Kenya.
Marios S Poullas, Childhood Nutrition Research Centre, UCL Great Ormond Street Institute of Child Health, London, UK.
Chittaranjan S Yajnik, Diabetes Unit, KEM Hospital and Research Centre, Pune, India.
Alessandro Demaio, Department of Public Health University of Copenhagen, Copenhagen, Denmark; EAT Foundation, Oslo, Norway; Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia; VicHealth, Melbourne, VIC, Australia.
References
- 1.Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. IntJ Obes (Lond) 2005;29:129–36. doi: 10.1038/sj.ijo.0802824. [DOI] [PubMed] [Google Scholar]
- 2.Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2019 doi: 10.1016/S0140-6736(19)32497-3. published online Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells JCK, Wibaek R, Poullas M. The dual burden of malnutrition increases the risk of cesarean delivery: evidence from India. Front Public Health. 2018;6:292. doi: 10.3389/fpubh.2018.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swinburn BA, Kraak VI, Allender S, et al. The global syndemic of obesity, undernutrition, and climate change: the Lancet Commission report. Lancet. 2019;393:791–846. doi: 10.1016/S0140-6736(18)32822-8. [DOI] [PubMed] [Google Scholar]
- 5.Hawkes C, Ruel MT, Salm L, Sinclair B, Branca F. Double-duty actions: seizing programme and policy opportunities to address malnutrition in all its forms. Lancet. 2019 doi: 10.1016/S0140-6736(19)32506-1. published online Dec 15. [DOI] [PubMed] [Google Scholar]
- 6.Nugent R, Levin C, Hale J, Hutchinson B. Economic effects of the double burden of malnutrition. Lancet. 2019 doi: 10.1016/S0140-6736(19)32473-0. published online Dec 15. [DOI] [PubMed] [Google Scholar]
- 7.Harika R, Faber M, Samuel F, Kimiywe J, Mulugeta A, Eilander A. Micronutrient status and dietary intake of iron, vitamin A, iodine, folate and zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: a systematic review of data from 2005 to 2015. Nutrients. 2017;9:e1096. doi: 10.3390/nu9101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuper H, Taylor A, Krishna KV, et al. Is vulnerability to cardiometabolic disease in Indians mediated by abdominal adiposity or higher body adiposity. BMC Public Health. 2014;14:1239. doi: 10.1186/1471-2458-14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 10.Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–38. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 11.Khoruts A. Targeting the microbiome: from probiotics to fecal microbiota transplantation. Genome Med. 2018;10:80. doi: 10.1186/s13073-018-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winick M, Noble A. Cellular response in rats during malnutrition at various ages. J Nutr. 1966;89:300–06. doi: 10.1093/jn/89.3.300. [DOI] [PubMed] [Google Scholar]
- 13.Crispi F, Miranda J, Gratacos E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am J Obstet Gynecol. 2018;218:S869–79. doi: 10.1016/j.ajog.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Latini G, De Mitri B, Del Vecchio A, Chitano G, De Felice C, Zetterstrom R. Foetal growth of kidneys, liver and spleen in intrauterine growth restriction: “programming” causing “metabolic syndrome” in adult age. Acta Paediatr. 2004;93:1635–39. doi: 10.1080/08035250410023106. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Gronert MS, Ozanne SE. Metabolic programming of insulin action and secretion. Diabetes Obes Metab. 2012;14(suppl 3):29–39. doi: 10.1111/j.1463-1326.2012.01653.x. [DOI] [PubMed] [Google Scholar]
- 16.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominguez-Salas P, Moore SE, Baker MS, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Zegher F, Diaz M, Lopez-Bermejo A, Ibanez L. Recognition of a sequence: more growth before birth, longer telomeres at birth, more lean mass after birth. Pediatr Obes. 2017;12:274–79. doi: 10.1111/ijpo.12137. [DOI] [PubMed] [Google Scholar]
- 19.Wojcicki JM, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E. Early exclusive breastfeeding is associated with longer telomeres in Latino preschool children. Am J Clin Nutr. 2016;104:397–405. doi: 10.3945/ajcn.115.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MI, Yatsunenko T, Manary MJ, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–54. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming TP, Watkins AJ, Velazquez MA, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391:1842–52. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei Z, Grummer-Strawn LM, Thompson D, Dietz WH. Shifts in percentiles of growth during early childhood: analysis of longitudinal data from the California Child Health and Development Study. Pediatrics. 2004;113:e617–27. doi: 10.1542/peds.113.6.e617. [DOI] [PubMed] [Google Scholar]
- 25.Wells JC. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22:1–17. doi: 10.1002/ajhb.20994. [DOI] [PubMed] [Google Scholar]
- 26.Yajnik CS. Nutrient-mediated teratogenesis and fuel-mediated teratogenesis: two pathways of intrauterine programming of diabetes. Int J Gynaecol Obstet. 2009;104(suppl 1):S27–31. doi: 10.1016/j.ijgo.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–80. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 28.Schoenbuchner SM, Dolan C, Mwangome M, et al. The relationship between wasting and stunting: a retrospective cohort analysis of longitudinal data in Gambian children from 1976-2016. Am J Clin Nutr. 2019;110:498–507. doi: 10.1093/ajcn/nqy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceesay SM, Prentice AM, Cole TJ, et al. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. BMJ. 1997;315:786–90. doi: 10.1136/bmj.315.7111.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev. 2003;4:CD000032. doi: 10.1002/14651858.CD000032. [DOI] [PubMed] [Google Scholar]
- 31.Devakumar D, Fall CH, Sachdev HS, et al. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis. BMC Med. 2016;14:90. doi: 10.1186/s12916-016-0633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barquera S, Peterson KE, Must A, et al. Coexistence of maternal central adiposity and child stunting in Mexico. Int J Obes (Lond) 2007;31:601–07. doi: 10.1038/sj.ijo.0803529. [DOI] [PubMed] [Google Scholar]
- 33.Hemachandra AH, Klebanoff MA. Use of serial ultrasound to identify periods of fetal growth restriction in relation to neonatal anthropometry. Am J Hum Biol. 2006;18:791–97. doi: 10.1002/ajhb.20552. [DOI] [PubMed] [Google Scholar]
- 34.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuzawa CW, Hallal PC, Adair L, et al. Birth weight, postnatal weight gain, and adult body composition in five low and middle income countries. Am J Hum Biol. 2012;24:5–13. doi: 10.1002/ajhb.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dulloo AG. Regulation of body composition during weight recovery: integrating the control of energy partitioning and thermogenesis. ClinNutr. 1997;16(suppl 1):25–35. doi: 10.1016/s0261-5614(97)80046-5. [DOI] [PubMed] [Google Scholar]
- 38.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Martins VJ, de Albuquerque MP, Sawaya AL. In: Handbook of famine, starvation, and nutritional deprivation. Preedy VR, Patel VB, editors. Springer International Publishing AG; Switzerland: 2017. Endocrine changes in undernutrition, metabolic programming, and nutritional recovery; pp. 1–21. [Google Scholar]
- 40.Florencio TT, Ferreira HS, Cavalcante JC, Stux GR, Sawaya AL. Short stature, abdominal obesity, insulin resistance and alterations in lipid profile in very low-income women living in Maceio, north-eastern Brazil. Eur J Cardiovasc Prev Rehabil. 2007;14:346–48. doi: 10.1097/hjr.0b013e328010f24d. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am J Clin Nutr. 2000;72:702–07. doi: 10.1093/ajcn/72.3.702. [DOI] [PubMed] [Google Scholar]
- 42.Said-Mohamed R, Bernard JY, Ndzana AC, Pasquet P. Is overweight in stunted preschool children in Cameroon related to reductions in fat oxidation, resting energy expenditure and physical activity? PLoS One. 2012;7:e39007. doi: 10.1371/journal.pone.0039007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pomeroy E, Stock JT, Stanojevic S, Miranda JJ, Cole TJ, Wells JC. Stunting, adiposity, and the individual-level “dual burden” among urban lowland and rural highland Peruvian children. Am J Hum Biol. 2014;26:481–90. doi: 10.1002/ajhb.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fabiansen C, Yameogo CW, Iuel-Brockdorf AS, et al. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: a randomised 2 × 2 × 3 factorial trial in Burkina Faso. PLoS Med. 2017;14:e1002387. doi: 10.1371/journal.pmed.1002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–34. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 46.Dodds R, Denison HJ, Ntani G, et al. Birth weight and muscle strength: a systematic review and meta-analysis. J Nutr Health Aging. 2012;16:609–15. doi: 10.1007/s12603-012-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lelijveld N, Seal A, Wells JC, et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. Lancet Glob Health. 2016;4:e654–62. doi: 10.1016/S2214-109X(16)30133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Dadelszen P, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. J Obstet Gynaecol Can. 2002;24:941–45. doi: 10.1016/s1701-2163(16)30592-8. [DOI] [PubMed] [Google Scholar]
- 49.Garcia OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. 2009;67:559–72. doi: 10.1111/j.1753-4887.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- 50.Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One. 2014;9:e113026. doi: 10.1371/journal.pone.0113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cnattingius S, Villamor E, Lagerros YT, Wikstrom AK, Granath F. High birth weight and obesity-a vicious circle across generations. Int J Obes (Lond) 2012;36:1320–24. doi: 10.1038/ijo.2011.248. [DOI] [PubMed] [Google Scholar]
- 52.Carslake D, Pinger PR, Romundstad P, Davey Smith G. Early-onset paternal smoking and offspring adiposity: further investigation of a potential intergenerational effect using the HUNT study. PLoS One. 2016;11:e0166952. doi: 10.1371/journal.pone.0166952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isganaitis E, Suehiro H, Cardona C. Who’s your daddy?: paternal inheritance of metabolic disease risk. Curr Opin Endocrinol Diabetes Obes. 2017;24:47–55. doi: 10.1097/MED.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 54.Rachmi CN, Agho KE, Li M, Baur LA. Stunting, underweight and overweight in children aged 2.0-4.9 years in Indonesia: prevalence trends and associated risk factors. PLoS One. 2016;11:e0154756. doi: 10.1371/journal.pone.0154756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634–47. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells JC. The thrifty phenotype: an adaptation in growth or metabolism? Am J Hum Biol. 2011;23:65–75. doi: 10.1002/ajhb.21100. [DOI] [PubMed] [Google Scholar]
- 57.Wells JC. The metabolic ghetto: an evolutionary perspective on nutrition, power relations and chronic disease. Cambridge University Press; Cambridge: 2016. [Google Scholar]
- 58.Li Y, Ley SH, Tobias DK, et al. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: prospective cohort study. BMJ. 2015;351:h3672. doi: 10.1136/bmj.h3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Ley SH, VanderWeele TJ, et al. Joint association between birth weight at term and later life adherence to a healthy lifestyle with risk of hypertension: a prospective cohort study. BMC Med. 2015;13:175. doi: 10.1186/s12916-015-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitanchez D, Chavatte-Palmer P. Review shows that maternal obesity induces serious adverse neonatal effects and is associated with childhood obesity in their offspring. Acta Paediatr. 2018;107:1156–65. doi: 10.1111/apa.14269. [DOI] [PubMed] [Google Scholar]
- 61.Diaz M, Garcia C, Sebastiani G, de Zegher F, Lopez-Bermejo A, Ibanez L. Placental and cord blood methylation of genes involved in energy homeostasis: association with fetal growth and neonatal body composition. Diabetes. 2017;66:779–84. doi: 10.2337/db16-0776. [DOI] [PubMed] [Google Scholar]
- 62.El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction. 2014;148:R111–20. doi: 10.1530/REP-14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan Q, Frost M, Heijmans BT, et al. Epigenetic signature of birth weight discordance in adult twins. BMC Genomics. 2014;15:1062. doi: 10.1186/1471-2164-15-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 2001;16:254–60. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- 65.Yang TO, Reeves GK, Green J, et al. Birth weight and adult cancer incidence: large prospective study and meta-analysis. Ann Oncol. 2014;25:1836–43. doi: 10.1093/annonc/mdu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green J, Cairns BJ, Casabonne D, et al. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–94. doi: 10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rao S, Yajnik CS, Kanade A, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J Nutr. 2001;131:1217–24. doi: 10.1093/jn/131.4.1217. [DOI] [PubMed] [Google Scholar]
- 69.Yajnik CS, Fall CH, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 70.Bavdekar A, Yajnik CS, Fall CH, et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes. 1999;48:2422–29. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 71.Yajnik CS, Katre PA, Joshi SM, et al. Higher glucose, insulin and insulin resistance (HOMA-IR) in childhood predict adverse cardiovascular risk in early adulthood: the Pune Children’s Study. Diabetologia. 2015;58:1626–36. doi: 10.1007/s00125-015-3602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kale SD, Kulkarni SR, Lubree HG, et al. Characteristics of gestational diabetic mothers and their babies in an Indian diabetes clinic. J Assoc Physicians India. 2005;53:857–63. [PubMed] [Google Scholar]
- 73.Kale SD, Yajnik CS, Kulkarni SR, et al. High risk of diabetes and metabolic syndrome in Indian women with gestational diabetes mellitus. Diabet Med. 2004;21:1257–58. doi: 10.1111/j.1464-5491.2004.01337.x. [DOI] [PubMed] [Google Scholar]
- 74.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–68. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 75.Leon DA, Koupilova I, Lithell HO, et al. Failure to realise growth potential in utero and adult obesity in relation to blood pressure in 50 year old Swedish men. BMJ. 1996;312:401–06. doi: 10.1136/bmj.312.7028.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith ER, Shankar AH, Wu LS, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health. 2017;5:e1090–100. doi: 10.1016/S2214-109X(17)30371-6. [DOI] [PubMed] [Google Scholar]
- 77.Hawkesworth S, Walker CG, Sawo Y, et al. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in The Gambia. Am J Clin Nutr. 2011;94(suppl):1853–60S. doi: 10.3945/ajcn.110.000877. [DOI] [PubMed] [Google Scholar]
- 78.Stein AD, Wang M, Ramirez-Zea M, et al. Exposure to a nutrition supplementation intervention in early childhood and risk factors for cardiovascular disease in adulthood: evidence from Guatemala. Am J Epidemiol. 2006;164:1160–70. doi: 10.1093/aje/kwj328. [DOI] [PubMed] [Google Scholar]
- 79.Ford ND, Behrman JR, Hoddinott JF, et al. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Glob Health. 2018;6:e875–84. doi: 10.1016/S2214-109X(18)30231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–90. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 81.Bajaj H, Ye C, Hanley AJ, et al. Prior lactation reduces future diabetic risk through sustained postweaning effects on insulin sensitivity. Am Journal Physiol Endocrinol Metab. 2017;312:e215–23. doi: 10.1152/ajpendo.00403.2016. [DOI] [PubMed] [Google Scholar]
- 82.Samuel TM, Thomas T, Thankachan P, Bhat S, Virtanen SM, Kurpad AV. Breast milk zinc transfer and early post-natal growth among urban South Indian term infants using measures of breast milk volume and breast milk zinc concentrations. Matern Child Nutr. 2014;10:398–409. doi: 10.1111/j.1740-8709.2012.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Charbonneau MR, O’Donnell D, Blanton LV, et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 2016;164:859–71. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Whitaker KM, Marino RC, Haapala JL, et al. Associations of maternal weight status before, during, and after pregnancy with inflammatory markers in breast milk. Obesity (Silver Spring) 2017;25:2092–99. doi: 10.1002/oby.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dugas C, Perron J, Kearney M, et al. Postnatal prevention of childhood obesity in offspring prenatally exposed to gestational diabetes mellitus: where are we now? Obes Facts. 2017;10:396–406. doi: 10.1159/000477407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128?9 million children, adolescents, and adults. Lancet. 2017;390:2627–42. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wamani H, Astrom AN, Peterson S, Tumwine JK, Tylleskar T. Boys are more stunted than girls in sub-Saharan Africa: a meta-analysis of 16 demographic and health surveys. BMC Pediatr. 2007;7:17. doi: 10.1186/1471-2431-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19?2 million participants. Lancet. 2016;387:1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Traissac P, El Ati J, Gartner A, Ben Gharbia H, Delpeuch F. Gender inequalities in excess adiposity and anaemia combine in a large double burden of malnutrition gap detrimental to women in an urban area in North Africa. Public Health Nutr. 2016;19:1428–37. doi: 10.1017/S1368980016000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiology (Bethesda) 2014;29:122–32. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wells JC. Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: addressing the full range of ethnicity, including those of mixed ethnicity. Obes Rev. 2012;13(suppl 2):14–29. doi: 10.1111/j.1467-789X.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 92.D’Angelo S, Yajnik CS, Kumaran K, et al. Body size and body composition: a comparison of children in India and the UK through infancy and early childhood. J Epidemiol Community Health. 2015;69:1147–53. doi: 10.1136/jech-2014-204998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chandalia M, Lin P, Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One. 2007;2:e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prendergast AJ, Rukobo S, Chasekwa B, et al. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One. 2014;9:e86928. doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232–44. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 96.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–35. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 97.Merrill RD, Burke RM, Northrop-Clewes CA, et al. Factors associated with inflammation in preschool children and women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(suppl 1):348–58S. doi: 10.3945/ajcn.116.142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McDade TW, Ryan C, Jones MJ, et al. Social and physical environments early in development predict DNA methylation of inflammatory genes in young adulthood. Proc Natl Acad Sci USA. 2017;114:7611–16. doi: 10.1073/pnas.1620661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pedersen JM, Budtz-Jorgensen E, Mortensen EL, et al. Late midlife C-reactive protein and interleukin-6 in middle aged Danish men in relation to body size history within and across generations. Obesity (Silver Spring) 2016;24:461–68. doi: 10.1002/oby.21311. [DOI] [PubMed] [Google Scholar]
- 100.Tzoulaki I, Jarvelin MR, Hartikainen AL, et al. Size at birth, weight gain over the life course, and low-grade inflammation in young adulthood: northern Finland 1966 Birth Cohort study. Eur Heart J. 2008;29:1049–56. doi: 10.1093/eurheartj/ehn105. [DOI] [PubMed] [Google Scholar]
- 101.Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science. 2016;352:1533. doi: 10.1126/science.aad9359. [DOI] [PubMed] [Google Scholar]
- 102.Dinh DM, Ramadass B, Kattula D, et al. Longitudinal analysis of the intestinal microbiota in persistently stunted young children in south India. PLoS One. 2016;11:e0155405. doi: 10.1371/journal.pone.0155405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–46. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 104.Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39:509–21. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- 105.de la Cuesta-Zuluaga J, Corrales-Agudelo V, Carmona JA, Abad JM, Escobar JS. Body size phenotypes comprehensively assess cardiometabolic risk and refine the association between obesity and gut microbiota. Int J Obes (Lond) 2017;42:424–32. doi: 10.1038/ijo.2017.281. [DOI] [PubMed] [Google Scholar]
- 106.Grosicki GJ, Fielding RA, Lustgarten MS. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut-muscle axis. Calcif Tissue Int. 2017;102:433–42. doi: 10.1007/s00223-017-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wells JC. The new “obstetrical dilemma”: stunting, obesity and the risk of obstructed labour. Anat Rec (Hoboken) 2017;300:716–31. doi: 10.1002/ar.23540. [DOI] [PubMed] [Google Scholar]
- 108.Alonso BD, Silva F, Latorre M, Diniz CSG, Bick D. Caesarean birth rates in public and privately funded hospitals: a cross-sectional study. Rev Saude Publica. 2017;51:101. doi: 10.11606/S1518-8787.2017051007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Betran AP, Temmerman M, Kingdon C, et al. Interventions to reduce unnecessary caesarean sections in healthy women and babies. Lancet. 2018;392:1358–68. doi: 10.1016/S0140-6736(18)31927-5. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez-Ramirez S, Munoz-Espinosa A, Rivera JA, Gonzalez-Castell D, Gonzalezde Cosio T. Mexican children under 2 years of age consume food groups high in energy and low in micronutrients. J Nutr. 2016;146:1916–23S. doi: 10.3945/jn.115.220145. [DOI] [PubMed] [Google Scholar]
- 111.Rynders CA, Blanc S, DeJong N, Bessesen DH, Bergouignan A. Sedentary behaviour is a key determinant of metabolic inflexibility. J Physiol. 2018;596:1319–30. doi: 10.1113/JP273282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–94. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 113.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 114.Marphatia AA, Cole TJ, Grijalva-Eternod CS, Wells JC. Associations of gender inequality with child malnutrition and mortality across 96 countries. Glob Health Epidemiol Genom. 2016;1:e6. doi: 10.1017/gheg.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wells JC, Marphatia AA, Cole TJ, McCoy D. Associations of economic and gender inequality with global obesity prevalence: understanding the female excess. Soc Sci Med. 2012;75:482–90. doi: 10.1016/j.socscimed.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 116.Rosset PM. Food is different: why we must get the WTO out of agriculture. Zed Books; London: 2006. [Google Scholar]
- 117.Paul H, Steinbrecher R. Hungry corporations: transnational biotech companies colonise the food chain. Zed Books; London: 2003. [Google Scholar]
- 118.Ruel MT, Alderman H, Maternal and Child Nutrition Study Group Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382:536–51. doi: 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 120.Kshatriya GK, Acharya SK. Triple burden of obesity, undernutrition, and cardiovascular disease risk among Indian tribes. PLoS One. 2016;11:e0147934. doi: 10.1371/journal.pone.0147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hudda MT, Nightingale CM, Donin AS, et al. Reassessing ethnic differences in mean BMI and changes between 2007 and 2013 in English Children. Obesity (Silver Spring) 2018;26:412–19. doi: 10.1002/oby.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nightingale CM, Rudnicka AR, Owen CG, et al. Influence of adiposity on insulin resistance and glycemia markers among United Kingdom children of South Asian, black African-Caribbean, and white European origin: Child Heart and Health Study in England. Diabetes Care. 2013;36:1712–19. doi: 10.2337/dc12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paciorek CJ, Stevens GA, Finucane MM, Ezzati M, Nutrition Impact Model Study Group (Child Growth) Children’s height and weight in rural and urban populations in low-income and middle-income countries: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e300–09. doi: 10.1016/S2214-109X(13)70109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hernandez AV, Pasupuleti V, Deshpande A, Bernabe-Ortiz A, Miranda JJ. Effect of rural-to-urban within-country migration on cardiovascular risk factors in low-and middle-income countries: a systematic review. Heart. 2012;98:185–94. doi: 10.1136/heartjnl-2011-300599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tyrovolas S, Koyanagi A, Olaya B, et al. The role of muscle mass and body fat on disability among older adults: a cross-national analysis. Exp Gerontol. 2015;69:27–35. doi: 10.1016/j.exger.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 126.McEniry M, McDermott J. Early-life conditions, rapid demographic changes, and older adult health in the developing world. Biodemography Soc Biol. 2015;61:147–66. doi: 10.1080/19485565.2015.1047488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tzioumis E, Adair LS. Childhood dual burden of under-and overnutrition in low-and middle-income countries: a critical review. Food Nutr Bull. 2014;35:230–43. doi: 10.1177/156482651403500210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caleyachetty R, Thomas GN, Kengne AP, et al. The double burden of malnutrition among adolescents: analysis of data from the Global School-Based Student Health and Health Behavior in School-Aged Children surveys in 57 low-and middle-income countries. Am J Clin Nutr. 2018;108:414–24. doi: 10.1093/ajcn/nqy105. [DOI] [PubMed] [Google Scholar]
- 129.Huffman SL, Piwoz EG, Vosti SA, Dewey KG. Babies, soft drinks and snacks: a concern in low-and middle-income countries? Matern Child Nutr. 2014;10:562–74. doi: 10.1111/mcn.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stearns SC. The evolution of life histories. Oxford University Press; Oxford: 1992. [Google Scholar]
- 131.Wells JC, Nesse RM, Sear R, et al. Evolutionary public health: introducing the concept. Lancet. 2017;390:500–09. doi: 10.1016/S0140-6736(17)30572-X. [DOI] [PubMed] [Google Scholar]
- 132.Wells JC, Cole TJ, Cortina-Borja M, et al. Low maternal capital predicts life history trade-offs in daughters: why adverse outcomes cluster in individuals. Front Public Health. 2019;7:206. doi: 10.3389/fpubh.2019.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol. 2015;29:498–506. doi: 10.1016/j.bpobgyn.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 134.Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol. 2012;22:R733–40. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci USA. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.NCD Risk Factor Collaboration. A century of trends in adult human height. Elife. 2016;5:e13410. doi: 10.7554/eLife.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Penuelas J, Janssens IA, Ciais P, et al. Increasing gap in human height between rich and poor countries associated to their different intakes of N and P. Sci Rep. 2017;7:17671. doi: 10.1038/s41598-017-17880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anekwe TD, Kumar S. The effect of a vaccination program on child anthropometry: evidence from India’s Universal Immunization Program. J Public Health (Oxf) 2012;34:489–97. doi: 10.1093/pubmed/fds032. [DOI] [PubMed] [Google Scholar]
- 139.Gosby AK, Conigrave AD, Raubenheimer D, Simpson SJ. Protein leverage and energy intake. Obes Rev. 2014;15:183–91. doi: 10.1111/obr.12131. [DOI] [PubMed] [Google Scholar]
- 140.Willett W, Rockström J, Loken B, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–92. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.