Abstract
Rift Valley fever (RVF) is a mosquito-borne viral zoonosis that was first discovered in Kenya in 1930 and is now endemic throughout multiple African countries and the Arabian Peninsula. RVF virus primarily infects domestic livestock (sheep, goats, cattle) causing high rates of neonatal mortality and abortion, with human infection resulting in a wide variety of clinical outcomes, ranging from self-limiting febrile illness to life-threatening haemorrhagic diatheses, and miscarriage in pregnant women. Since its discovery, RVF has caused many outbreaks in Africa and the Arabian Peninsula with major impacts on human and animal health. However, options for the control of RVF outbreaks are limited by the lack of licensed human vaccines or therapeutics. For this reason, RVF is prioritized by the World Health Organization for urgent research and development of countermeasures for the prevention and control of future outbreaks. In this review, we highlight the current understanding of RVF, including its epidemiology, pathogenesis, clinical manifestations and status of vaccine development.
Keywords: Rift Valley fever, epidemiology, pathogenesis, transmission, vaccine, one health
Discovery and Historical Background
Rift Valley fever virus (RVFV) was originally discovered as the causative agent of an outbreak of ‘enzootic hepatitis’ in 1930 near Lake Naivasha in the Rift Valley of Kenya [1]. The outbreak manifested with numerous mortalities among newborn lambs, together with increased instances of mortality and abortions among adult sheep [1]. Within a few weeks, thousands of sheep had died on the farm. To investigate if the disease was caused by a bacterium or a virus, blood from a diseased lamb was passed through a Chamberland porcelain filter and injected into an unaffected lamb, which reproduced the disease. The outbreak occurred in a period of heavy mosquito activity, leading the investigators to suggest the involvement of mosquitoes in disease transmission. In an attempt to limit the outbreak, healthy sheep were moved to a higher altitude, where mosquitoes were absent or were placed under mosquito netting. Both countermeasures were effective, and together with the apparent lack of direct animal-to-animal transmission, corroborated the suggestive involvement of mosquitoes in disease transmission [1, 2]. The involvement of mosquitoes as vectors was later confirmed when RVFV was isolated from several naturally infected species of Aedes and Culex mosquitoes [3, 4].
Enquiries during and after the outbreak revealed that almost all herders had experienced fever and severe pains [1]. Hundreds of human cases were thought to have occurred with no incidence of mortality. Risk of death from infection was therefore considered low and human susceptibility was confirmed by transfusing filtered blood from an infected lamb into a human volunteer [1]. Soon after the outbreak and in the subsequent years, numerous animal infection studies were carried out, documenting the susceptibility of a wide range of animal species [1, 2, 5–7] to inform most of what we know regarding the natural course of RVF in humans and animals. In this review, we highlight recent developments in the understanding of RVF, including its epidemiology, pathogenesis, clinical features and status of vaccine development.
Epidemiology
Transmission
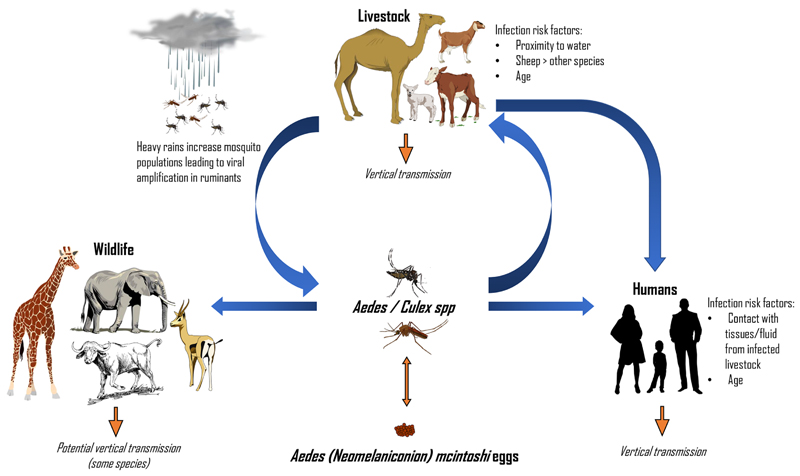
Our understanding of the epidemiology of RVF is incomplete, particularly with regards to viral maintenance during interepizootic periods (IEPs). A single species of Aedes that was misidentified before 1985 as Aedes lineatopennis, later identified as Aedes (Neomelaniconion) mcintoshi [8], has shown the ability to pass the virus to its progeny [9]. It is plausible to assume that RVFV can remain viable in the eggs of this species during the dry season before hatching once the rains return [9, 10]. Whether this is the only species capable of vertical transmission and the extent it allows RVFV to circulate during IEPs requires further investigation. Evidence of seropositivity in sheep and goats that have not lived through an RVF outbreak suggests low-level circulation of the virus can occur in livestock [11, 12]. Mosquitoes can also transmit RVFV to wild ungulates. Indeed, neutralizing antibodies targeting RVFV have been detected in a diverse range of species, such as African buffalo, giraffe, black rhino, impala and African elephants among many others [13, 14]. Some of these species, such as buffalo and giraffe, are susceptible to RVF disease [15], while others such as elephants appear not to be. There is, however, no evidence to suggest these animals develop sufficiently high viraemia to allow transmission to mosquitoes. Low-level viral maintenance in sylvatic cycles during IEPs is plausible, although it is likely that only ruminants and pseudoruminants function as amplifying hosts (Fig. 1).
Fig. 1.
RVFV cycle. Between epidemics RVFV may be maintained through transovarial transmission in Aedes (Neomelaniconion) mcintoshi eggs. Wild ungulates and livestock can also harbour low-level infection. During heavy rains, a surge in mosquito populations leads to increased infection of livestock and viral amplification between numerous vector species and ruminants occurs. As more livestock become infected, the chances of spillover into humans increases. Human infection can occur via mosquito bite, or more commonly, via contact with infected animal tissue and fluid.
During periods of exceptionally heavy rains, subsequent flooding results in large increases in mosquito populations, which can lead to RVF epizootics, whereby large numbers of livestock become infected. Because of the correlative link between RVFV infection and the weather, rainfall data and changes in vegetation have been used to predict RVF outbreaks [16]. However, accurate data on the variables, which feed into models predicting RVF outbreaks, often do not exist. For this reason, the predictive value of these models is variable [16, 17]. Syndromic surveillance of livestock herds during periods when conditions appear to be favourable for RVF outbreaks have also been used as an early warning system in Kenya [18]. These systems may lead to better awareness among farmers towards RVF and increases in vaccination uptake [18].
Numerous mosquito species are capable of transmitting RVFV [10]. Other arthropods, such as midges, ticks and sandflies can become infected with the virus and could potentially act as mechanical vectors [10]. A study of mosquitoes trapped during an epizootic found more than 53 species caught in the field were positive for RVFV [19], while more than 65 species are described as potential vectors, the vast majority from Aedes and Culex spp. [20]. The degree to which some of these potential RVFV vectors can successfully transmit virus varies between species [21].
Unlike animals, most human infections are attributed to contact with infected tissues or fluids, rather than mosquito bite [1, 22]. Numerous cases of human transmission during the 1930–1950s occurred by way of accidental laboratory infection [5]. Indeed, much of our early knowledge of the disease in humans came from these case reports. To date, direct human-to-human transmission has not been documented. Even during epidemics in hospital settings with sub-optimal personal protective equipment, there is no evidence of nosocomial transmission [23]. In one case, viral RNA was isolated from urine and semen in an immunosuppressed patient 4 months after initial onset of symptoms [24]. Whether RVFV can be transmitted sexually, however, is unknown. Vertical transmission, from mothers to foetus, has been documented in human cases [25, 26] and ex vivo experiments have demonstrated that RVFV can directly infect human placental tissue [27].
For livestock, direct transfer between animals may be rare or non-existent as evidenced by early experiments demonstrating that viraemic sheep fail to transfer the virus to naïve animals [1]. More recently, sheep with acute RVFV infections cohoused with highly susceptible immunocompromised naïve lambs failed to transmit any virus [28]. Vertical transmission occurs in all susceptible livestock species; this has been demonstrated even in pregnant sheep with no detectable viremia [29]. In a rodent model of pregnant rats, vertical transmission occurred in both sick and asymptomatic rats, with pregnant animals being more prone to death than their non-pregnant counterparts. The placenta of these animals was shown to be a major site of viral replication [27]. While outbreaks have so far been confined to Africa and the Arabian Peninsula, a wide range of mosquitoes can transmit RVFV and these can be found outside these two geographical settings [19].
Disease burden and impact
After the discovery of RVFV in the 1930s, outbreaks appeared regularly from the 1950s onwards [30]. This began with large outbreaks in Kenya and South Africa in 1950–1951 [14]. In 1974–1975, another outbreak in South Africa resulted in the country’s first human fatalities; 110 human cases were recorded resulting in seven deaths [31]. The largest RVF outbreak on record occurred in Egypt from 1977 to 1979, where 598 deaths were documented from an estimated 200 000 human cases [32]. Considerable human cases were reported in Mauritania in 1987, with 220 deaths [33]. In 1997–1998, after exceptionally heavy rainfall, a large outbreak in East Africa resulted in 478 deaths from an estimated 89 000 human cases [34].
The first outbreak of RVF outside the African continent occurred in the year 2000, where 880 laboratory-confirmed human cases and 123 deaths were recorded in Saudi Arabia over a six-month period [30, 35]. During this time, an additional 1328 cases and 166 deaths were recorded in Yemen. Evidence suggests the strain of RVFV that emerged on the Arabian Peninsula originated from the 1997–1998 East-African outbreak [36]. More recently in 2008, 747 human cases and 230 deaths were reported in Sudan and since 2016, smaller outbreaks in Niger and East Africa have resulted in numerous human deaths [37].
Estimating total animal losses during outbreaks is difficult as these numbers are generally poorly documented. The first recorded outbreak in Kenya resulted in an estimated 5000 animal deaths [1], while the much larger outbreaks in Kenya and South Africa in 1950–1951 resulted in over 100 000 sheep dying and approximately half a million abortions [14]. Even more were estimated to have died in the South African 1975 outbreak [31]. In Saudi Arabia, the outbreak resulted in wide-spread livestock deaths and up to 10 000 abortions in cattle, sheep, goats and camels [38]. In the 2006–2007 outbreak in Tanzania, livestock mortality was estimated at 50 000 [30].
RVF outbreaks can have dire socio-economic consequences at both local and national levels [39, 40]. In addition to the direct cost of human morbidity and death, livestock deaths and subsequent reduction in production has cost producers millions of dollars. These animal losses can have knock-on effects altering herd dynamics. For example, when significant proportions of young animals are killed, there are fewer to breed the following generation resulting in further, potentially long-term, production losses [39]. In addition to the direct losses of livestock, embargoes are placed on the exportation of livestock during outbreaks, which can have crippling impacts on economies heavily dependent on animal trade. In Somalia, for example, livestock exporters lost approximately US$330M between 1998–2003 as a result of the ban on livestock imports, with further losses from producers and the subsequent reduction in tax intake for the government [41]. Overall, losses in Somalia due to the 2006–2007 outbreak have been estimated at US$471M, representing 5 % of the total GDP. The same outbreak caused US$66M losses in Kenya [39]. Saudi Arabia and Yemen suffered economic losses of US$10M and US$107M, respectively.
Risk factors
Numerous seroprevalence studies in human populations have been carried out, which shed light onto those most at risk of infection [42]. The most significant risk factor is having contact with susceptible animals and being involved in slaughter [43, 44]. Adults are more likely to be seropositive than children, either as a function of their age providing them more time to come into contact with RVFV, or due to the increased occupational risk [45]. Heavy rainfall often precedes RVF outbreaks, where an increase in vector population may increase transmission potential. While it is not considered the primary cause of human infection, any behavioural factors that increase the frequency of mosquito bites, such as sleeping outdoors in RVFV-endemic areas, may also increase the chance of becoming infected [46]. Larger outbreaks, however, such as the Egyptian outbreak of 1977, are unlikely to have all resulted from direct animal contact. Here, Culex pipiens was implicated for the first time [47]. There are documented cases of human infections with no direct link to livestock, which were consequently attributed to mosquito bites [48].
It is currently poorly understood why some people remain asymptomatic or have only mild symptoms before recovering while others develop more severe disease with lasting problems. There is some evidence that coinfection with other pathogens may increase susceptibility. For example, HIV-1 coinfection was found to increase the likelihood of severe disease and death due to RVF, with case fatality estimated at 75 % [49]. Other studies have shown a role of host genetic factors on susceptibility, identifying an association between single nucleotide polymorphisms in genes involved in immunological pathways and severe disease [50].
Risk factors for livestock infections are less well understood and often contradictory [42]. For instance, numerous studies have associated either males or females with increased chance of seropositivity [42]. The relationship between animal type (sheep or goats) and risk of infection is also not always clear. While no association was found in certain herds, in others sheep seemed significantly more at risk [11, 12, 51]. Differences in host-genetic factors, environmental factors such as feeding preferences of vectors or differences in animal husbandry may explain why sheep could have a higher chance of infection. Proximity to water has also been identified as a risk factor, most likely linked to the number of mosquitoes present [52]. As with humans, older animals are more likely to be seropositive [42].
Disease Manifestations
Humans
Human infections can result in a wide spectrum of clinical outcomes [32]. While most cases induce a self-limiting febrile illness, an estimated 1–2 % of infections result in much more severe disease, often with high levels of mortality (Fig. 2). Following an incubation period of 2–6 days, clinical symptoms of RVF include fever, headache, backache, vertigo, anorexia and photophobia [53, 54]. The fever may last several days with a convalescent period ranging from a few days to a month. Some patients experience a biphasic febrile illness, where a reduction of symptoms occurs around the third day before recrudescence 1–3 days later [53]. Severe RVF disease can encompass a wide range of manifestations, summarized below.
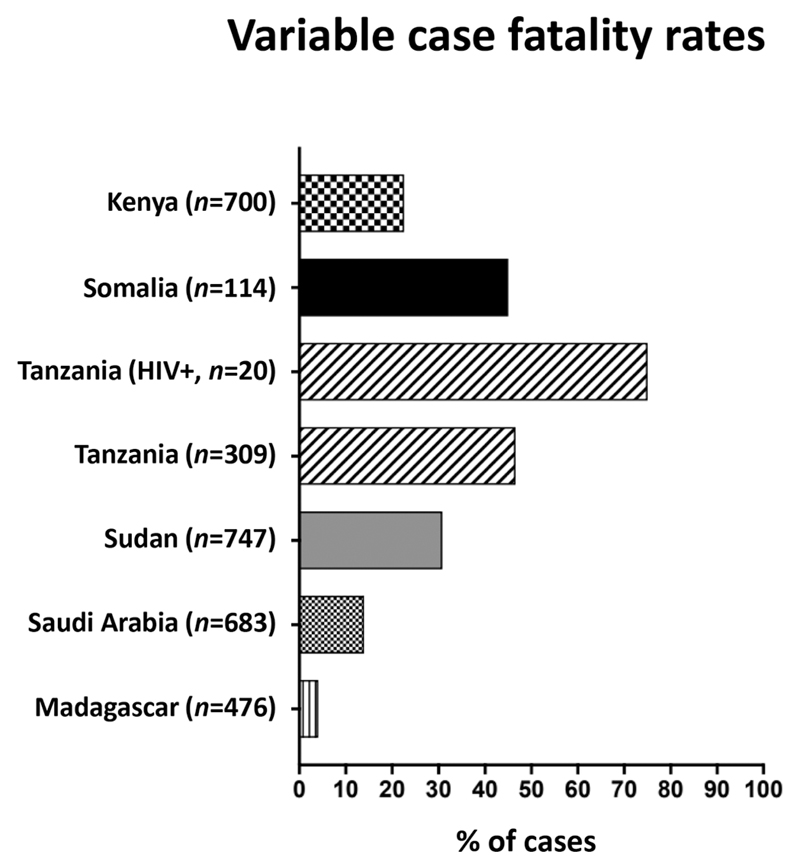
Fig. 2.
Case fatality rates among patients hospitalized with RVF disease. Data sourced from [35, 37, 49, 55].
Hepatitis, jaundice and haemorrhagic disease
As the major site of RVFV replication, the liver becomes damaged, which can lead to jaundice and haemorrhagic disease. Elevation of liver enzymes aspartate transaminase (AST) and alanine transaminase (ALT) occurs, with highest levels occurring in fatal cases [56]. Haemoglobin and platelet counts are reduced with clotting time increased. Other manifestations may include thrombosis [54]. Patients with haemorrhagic fever have a very high fatality rate and usually succumb within a week or two after the onset of symptoms [54, 57].
Ocular disease
Ocular disease is estimated to occur in 2–5 % of RVFV infections, while prevalence among those experiencing severe disease can be >10 % [58, 59]. It usually develops 1–3 weeks after initial onset of symptoms and most commonly involves macular and paramacular oedema [58–60]. Other symptoms may include photophobia, reduced vision, blind spots, uveitis, retinitis and retinal haemorrhage [30, 54]. Disease duration varies from being permanent to resolving in a matter of weeks [30].
Encephalitis and neurological disease
In addition to the symptoms of mild disease, severe and lasting problems can occur shortly after initial symptoms subside, including reduced consciousness, hallucinations, confusion, vertigo, excessive salivation, weakness, paralysis, decerebrate posturing, hemiparesis and pleocytosis [30, 54].
Abortions/miscarriage
The link between RVFV infection and human abortion is less clear than in ruminants. A study looking into the incidence of abortion during the 1977 outbreak in Egypt found no increase above the normal frequency of abortions [61]. However, a study in 2016 showed for the first time a significantly increased risk of miscarriage after laboratory-confirmed RVFV infection during pregnancy [62]. The risk of abortion in humans, however, appears to be lower than that in livestock. Further studies on the mechanisms underlying pregnancy loss due to RVF are warranted.
Animals
RVFV affects a wide range of animals in an age-dependent manner, where young animals are significantly more likely to succumb than adults. However, the various species are not equally susceptible to disease [54]. As in human disease, the major site of RVFV replication is the liver and it is this organ which bears the brunt of disease in all species. Liver lesions generally result in elevated expression of liver enzymes. Animals with severe RVF disease exhibit leukopenia [21] and while tropism is mostly limited to hepatocytes and monocytes, virus is disseminated to other tissues through circulation in blood [63]. Severe disease can also occur suddenly, causing death in the absence of any other symptoms [64].
Sheep are the most susceptible of livestock species. The incubation period of the disease is 24–36 h with signs including fever, listlessness, loss of appetite, disinclination to move, abdominal pain and bloody diarrhea [54]. Post-mortem analysis reveals multi-focal liver necrosis and occasional mild splenomegaly [65]. The breed of sheep can influence the severity of clinical manifestations [65] Adult sheep mortality after experimental infection is between 20–30 % [5]. Mortality in newborn lambs is much greater at 95–100 % [5] and acutely infected pregnant ewes have almost 100 % chance of abortion [54].
Goats are also highly susceptible with similar symptoms to sheep. However, the course of disease can be somewhat more variable, with viremia and symptoms inconsistent after infection [5]. Peak viremia in the blood of goats is also significantly lower than in lambs of identical age after experimental infection [5]. Also, unlike lambs, goats do not always experience febrile illness [5]. When disease does occur, the formation of hepatic lesions is followed by necrotic hepatitis. In adults, hepatic lesions are more focal.
Cattle are less susceptible to disease than sheep and goats. RVFV infections in adults are usually asymptomatic but can also manifest as acute disease with mortality between 0–5 % [54]. As with the other livestock species, younger animals are more susceptible and calves usually develop acute disease with mortality of approximately 10 % [54, 66], although experimental infections have resulted in significantly higher rates [5]. A more recent study has demonstrated that the onset and duration of fever, in addition to other aspects of disease, including viraemia and liver pathology, are less consistent when compared with sheep [66].
There are few examples describing clinical disease in camels suggesting that these pseudoruminants are less susceptible than cattle, with the vast majority experiencing asymptomatic infection. However, acute RVFV infection may still result in severe disease and death. Symptoms can include ocular discharge, haemorrhages and foot lesions [67]. The first human deaths from RVF in an outbreak in Mauritania, 2010, were associated with contact with an infected dromedary camel [67]. Abortions of pregnant camels during outbreaks have also been documented [67].
Virology
Structure and host cell entry
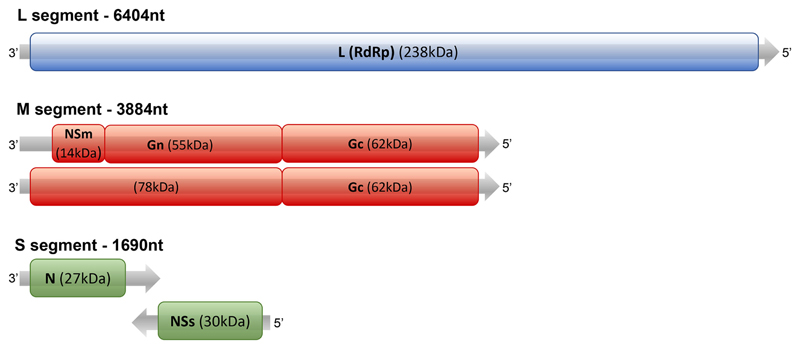
RVFV is a Phlebovirus in the Phenuiviridae family (formerly Bunyaviridae). The Phenuviridae family includes other viruses of biomedical significance (e.g. Severe fever with thrombocytopenia syndrome virus [SFTSV] and Sandfly fever virus [SFV], which encompasses sandfly Naples virus, sandfly Sicilian virus and Toscana virus). RVFV contains a predominantly negative-sense tripartite RNA genome consisting of a small (S), medium (M) and large (L) segment [20] (Fig. 3). The S segment encodes the nucleoprotein (N) and a non-structural protein (NSs), and is the only segment that employs an ambi-sense strategy (Fig. 3). The L segment encodes the viral polymerase. The M segment encodes another non-structural protein (NSm) and a 78 kDa glycoprotein in addition to the two structural glycoproteins, Gn and Gc. The function of the 78 kDa protein is not fully understood but appears to play a structural role in mosquito-cell-derived virions but not those expressed in mammalian cells [68, 69].
Fig. 3.
Schematic of the RVFV genome. The L segment encodes the RNA-dependent RNA polymerase (RdRp) gene. The M segment encodes the precursor protein, which is cleaved into NSm, Gn and Gc. By using different AUG initiation sites, the M segment also codes for a precursor protein containing the 78 kD protein (which includes Gn) together with Gc. The S segment encodes for the N and NSs proteins in an ambisense manner.
RVFV Gn and Gc form heterodimers, which further assemble as pentamers and hexamers with T=12 icosahedral symmetry on the envelope surface of the mature virus, and work in concert to facilitate host-cell entry [70–73]. RVFV Gc facilitates the pH-dependent merger of the host and virion membrane and adopts a class-II fusion protein fold, resembling fusion proteins of other phleboviruses, hantaviruses, alphaviruses and flaviviruses [74–79]. The N-terminal ectodomain region of RVFV Gn shares the same fold architecture with the Gn of another Phlebovirus, SFTSV, and has been shown to shield hydrophobic fusion loops on the cognate Gc protein [70, 80]. Prior to endocytosis of the virus, virions can attach to cells via the interaction between Gn- and Gc-displayed oligomannose-type glycans and the C-type lectins, DC-SIGN and L-SIGN [81–83]. DC-SIGN is highly expressed on dermal dendritic cells, where RVFV can replicate productively [84]. Heparan sulfate proteoglycans have also been identified as an important host-cell attachment factor (Fig. 4) [85, 86].
Fig. 4.
Replication cycle of RVFV. (1) Viral attachment to host membrane. (2) GnGc-mediated endocytosis. (3) Uncoating by acidification of endocytic vesicles, fusion of viral and endosomal membranes. (4) Primary transcription of mRNA by viral RNA polymerase. (5) Translation of viral proteins, cleavage of M-segment polyprotein and dimerization of GnGc in the endoplasmic reticulum (ER). (6) Transportation of GnGc hetrerodimers to the Golgi, glycosylation of Gn and Gc and budding into the Golgi cisternae. (7) RNA replication into positive-sense complimentary RNA (cRNA), which serves as a template for negative-sense viral RNA (or in the case of the ambisense S segment, templates for sub-genomic mRNA). (8) Migration of Golgi vesicles containing viruses to cell surface, fusion of vesicular membranes with plasma membrane, release of infectious virions. Adapted from [87]. Permission was obtained to adapt the figure from the authors and the copyright holder.
Phylogeny
It is estimated that RVFV diverged from a recent common ancestor during the 1880–90s [88, 89]. The virus circulates throughout East Africa, with outbreaks in other regions often the result of a single introduction [36]. Several studies conducting sequence analysis of RVFV isolates throughout Africa and Saudi Arabia have shown limited genetic diversity of ~5 % at the nucleotide level and ~2 % at the amino-acid level [88–90]. A large study of 198 isolates obtained over a 67 year period from numerous countries categorized sequences into 15 lineages (A–O) [88]. These individual RVFV strains are not unique to specific regions and many have been isolated from multiple geographical settings [91]. Sequence analysis has demonstrated that numerous strains can circulate concurrently during outbreaks [91] and there is evidence of previous genetic reassortment between them [88, 90]. Data regarding the virulence of different RVFV strains is limited. While all isolates belong to a single serotype [92], importantly, and despite the limited diversity, individual strains of RVFV can cause differential disease severity in natural host species [65, 66].
Domain-specific analysis of the glycoproteins demonstrated that the most membrane distal region of Gn undergoes the greatest level of non-synonymous mutations, suggestive that this region of the molecule is under comparatively strong selection pressure and is subject to fewer structural constraints [93]. The relatively conserved nature of the antigenic RVFV Gn-Gc surface bodes well for efforts to develop broadly efficacious vaccines capable of protecting against any strain.
Immunology
Naturally acquired immunity
Protection against RVFV in all species is conferred by long-lived neutralizing antibodies (nAbs), which can be detected within the first week post-infection [21, 94]. Sheep and cattle exposed to RVFV have been shown to be completely resistant to disease upon re-infection [1]. Passive serum transfer experiments in non-human primate and rodent models have confirmed the role of antibodies in protection against RVFV infection [95, 96]. Neutralizing antibodies were detected in two persons 12 and 25 years post-infection, respectively, in the absence of subsequent exposure [53, 97].
The N protein is the immunodominant protein of many bunyaviruses, where IgG and IgM specific to N are abundant after natural infection in all species evaluated [21, 98, 99]. However, there is no evidence that anti-N antibodies exhibit virus neutralizing activity. Instead, the only identified targets of nAbs appear to be the surface glycoproteins, Gn and Gc [100–105]. Experimental challenge in animals has revealed measurable IgM levels in the first few days after infection. This drops to <10 % prevalence in animals after 50 days. Positivity for RVFV-specific IgG begins to appear shortly after IgM with animals generally becoming IgG positive within 3 weeks [21].
The production of anti-RVFV neutralizing antibodies is likely the primary mechanism of protection in all species. It is possible that the diversity of known IgG structures amongst species, such as the ultralong loop in the third heavy-chain complementarity-determining-region (CDR H3) of cattle antibodies and the heavy-chain only antibodies of camels may allow the recognition of species-specific epitopes [106, 107]. The single domain antibodies of camelids, for example, are only ~90 kDa in size, significantly smaller than conventional IgG and may have access to epitopes that larger antibodies do not. A more detailed understanding of the humoral immune response to RVFV could help to guide future vaccine strategies. Identification of neutralizing epitopes may allow the omission of parts of the RVFV proteins, which do not contribute to protection, leaving the immune response to focus on those that do [108]. The possibility of reducing the antigen size while maintaining or increasing immunity could potentially be exploited by allowing the combination of additional antigens in vaccines. Furthermore, vaccine development can be guided by a detailed characterization of naturally acquired immunity, known to provide long-lived protection. This may also highlight immunological differences within or between species which affect susceptibility. The degree to which virus–host interactions differ between species is an area in need of future research.
In comparison to antibodies, relatively little is known about T-cell responses against RVFV, particularly after natural exposure. Dendritic cells and macrophages are among the first immune cells encountered after infection and replication of RVFV has been demonstrated in both [84, 109]. African green monkeys exposed to RVFV via aerosolized droplets develop neurological disease similar to that seen in humans [110]. In these animals challenged with RVFV, early proliferation of CD4+ and CD8+ T cells and expression of Th1 cytokines was associated with non-lethal outcomes [110]. This is in line with findings that higher concentrations of IL-10, a cytokine that suppresses Th1 response, is associated with fatal cases compared to non-fatal cases in humans [56]. The same study also found that B cell and neutrophil activators were elevated in non-fatal compared to fatal cases [56].
Innate immunity plays a critical role in RVF disease progression [56]. The NSs protein, an IFN antagonist, is the major virulence determinant of RVFV. RVFV lacking NSs induces strong type-I IFN responses, which explains its attenuation in livestock and humans [111–114]. Counteracting the innate immune response by RVFV is achieved through various mechanisms [115]. YY1, a host protein involved in modifying IFN-β transcription, is bound by NSs and another host protein, SAP30. Together this complex serves to suppress IFN-β at the transcriptional level, occurring very early on in the first few hours post-infection [21, 116]. After this, NSs interacts with components of the transcription factor TFIIH, preventing their assembly and thereby causing a rapid general decrease in host cellular RNA synthesis [117]. Alongside transcriptional shutoff, NSs promotes viral replication via degradation of Protein Kinase R (PKR) [115, 118, 119]. PKR plays a key function in antiviral defense by inhibiting protein synthesis and thus its degradation blocks host translational shutoff.
Vaccine-induced immunity
As with natural exposure, protection through vaccination is primarily conferred via induction of nAbs towards the viral glycoproteins. Hence, the aim of RVF vaccine programmes is to elicit high titre RVFV nAbs. Vaccination with attenuated RVFV in humans, depending on the particular vaccine and schedule, can induce nAbs which are long-lived [120, 121].
In livestock, immunization can induce detectable nAbs in as early as 4–5 days in some animals and in all animals by 2–4 weeks [104, 122, 123]; nAb titres can be further boosted following challenge [104, 122]. In some areas there have been questions about the efficacy of live-attenuated livestock vaccines. In Egypt, for example, despite 70 % of cattle being vaccinated some studies have found significantly lower seropositivity [124, 125]. Whether this is directly related to the vaccine rather than issues with implementing the vaccination programme requires further investigation. Some widely used livestock vaccines, such as the inactivated RVF ZH501 and RVF Menya require multiple doses in order to generate protective immunity [126], increasing the costs and logistics involved in vaccination.
There are examples of challenge studies carried out in mice showing partial protection in the absence of detectable nAbs [105, 127]. This is the case when vaccinating mice with the N protein [105, 127]. The mechanism of this protection is poorly understood but cellular immunity has been implicated [105]. Indeed, there is evidence that the N protein is a potent human CD8+ T-cell antigen [128]. This protection may also come from non-neutralizing antibodies via cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) [127].
Immune epitopes
Wang et al. recently isolated RVFV monoclonal antibodies (mAbs) from a convalescent human returning from Angola [129], and found that Gn-specific mAbs were protective and function by blocking virus binding to cells. Another study in which rabbits were vaccinated with the ectodomain of the Gn identified a class of mAbs, which protects against RVFV infection in a mouse model by targeting the membrane-distal domain of Gn [93]. This class of antibodies recognizes a region that is spatially distinct from that targeted by the characterized human-derived mAbs, supportive of the possibility of a potent mAb cocktail targeting multiple epitopes for therapeutic use. No epitopes have yet been characterized in target livestock species.
In mice, both B- and T-cell epitopes have been identified in the viral glycoproteins Gn and Gc. A widely used commercially available mouse mAb recognizes a linear B-cell epitope on Gn [130], while CD8+ T-cell epitopes have been identified on both Gn and Gc [105]. More broadly, a study in the 1990s investigated mAbs raised in vaccinated mice and found examples targeting either Gn or Gc that were capable of neutralizing RVFV [101]. A later study in mice vaccinated with cDNA containing Gn or Gc, showed only Gn-vaccinated mice developed neutralizing antibodies, while Gc-vaccinated mice did not [102]. A limitation of identifying epitopes using individual glycoproteins, however, is that the conformation of Gc or Gn alone will be different than they appear when presented as pentamers and hexamers of Gn/Gc heterodimers on the mature virion surface. Important epitopes may therefore be missed, particularly at the interface between Gn and Gc. While a low-resolution reconstruction of the RVFV envelope has allowed the positions of the Gn and Gc glycoproteins to be revealed [70], higher resolution structural information of the Gn/Gc assembly is needed to guide our understanding of the mature, antigenic surface of the virus.
Disease Control and Treatment
Diagnosis
As most human infections are asymptomatic or cause flu-like illness, often the first sign of an RVF outbreak is the near simultaneous abortions in herds of pregnant sheep termed ‘abortion storms’. There are several methods to diagnose acute RVFV infection in livestock and humans, but all must be carried out in laboratory settings [20]. One method of diagnosing acute or very recent infection is to use ELISA detecting IgM towards RVFV antigens [131]. Molecular methods using real-time reverse transcriptase (RT)-PCR to detect viral RNA can also be used and are highly sensitive and specific. Virus can also be isolated by cell culture from blood samples taken during the febrile stage (or from organ samples collected post-mortem). Lastly, histopathological methods can be used; i.e. post-mortem examination of liver for hepatic lesions.
Viral neutralization assays are the gold standard in detecting previous exposure to RVFV due to their high sensitivity and specificity. However, these require specialist laboratory facilities capable of working safely with live RVFV. Commercial ELISA kits that detect antibody against the N protein are also available (ID-VET, Montpellier, France).
Treatment
Most human cases of RVF do not require treatment. For those with severe RVF disease there is no specific treatment other than general supportive therapy. Several groups are investigating potential RVF therapeutics, and some, such as Ribavirin and Favipiravir have shown efficacy in rodent models (reviewed in [132]). During the 2000 outbreak in Saudi Arabia, intravenous administration of Ribavirin to RVF patients was stopped due to the apparent increase of neurological disease in these patients [64]. The development of an effective treatment for RVF disease is of considerable importance and continued research is warranted.
Vaccines
At present, vaccination is regarded as the only method to prevent RVFV infections in livestock. However, there is significant room for improvement of existing livestock vaccines. In addition, tools to limit spillover into humans are hampered by the absence of any licensed human vaccines [133]. Replicating the long-lived protection resulting from natural exposure is an attractive goal. For humans, there are two vaccines currently defined as Investigational New Drugs in the USA; MP-12 and TSI-GSD-200 [134]. MP-12 was created in the 1980s, the strain contains 23 nucleotide mutations distributed over all three genome segments [135]. It has been conditionally licensed for animal vaccination [134] and is safe and immunogenic in both animals and humans although some teratogenic effects have occurred in pregnant ruminants [20, 120]. TSI-GSD-200 is a formalin-inactivated vaccine created by the US army to protect those whose work may put them at risk of infection. It has an excellent safety profile but requires multiple boosters to achieve efficacy, and even then almost 10 % of vaccinees tend to have low nAb titres or fail to seroconvert [121].
For livestock, RVF vaccine use is inconsistent across Africa and the Arabian Peninsula with most countries having no implementation due to the sporadic nature of outbreaks or lack of reported cases [134]. The frequency of vaccination in countries that do vaccinate, such as Kenya, South Africa and Egypt among others, using a handful of licensed vaccines, varies based on the ecological setting [134]. For livestock, the most widely used commercial vaccine, named after its developer Smithburn, is a live-attenuated RVFV that induces long-lasting protection after a single dose [133]. However, the Smithburn vaccine cannot be administered to pregnant animals as residual virulence results in a high risk of abortion [136]. In addition, there is a possibility of reversion to virulence as may have occurred previously with this vaccine [137]. There is also the prospect of genetic reassortment with wild-type RVFV, however, this is unlikely to result in an increase in pathogenicity beyond that of the wild-type virus. A bonus of a novel RVFV vaccine would be the ability to differentiate between infected and vaccinated animals (DIVA). When using live-attenuated RVFV as a vaccine, such as Smithburn, the antibody profile generated is similar to natural infection, rendering the mapping of outbreaks difficult in the face of vaccination. Subunit vaccines have the advantage of not including all the RVFV antigens. By measuring responses to the N protein, if not present in the vaccine, it is possible to differentiate animals that have been naturally exposed from those that have been vaccinated.
Another livestock vaccine, clone 13, was one of a number of viral clones isolated from a human patient infected with the 74HB59 strain in the Central African Republic. It was found to be naturally attenuated due to a large deletion in the NSs gene, the primary virulence factor, with subsequent infection in mice showing it did not cause disease [138]. Clone 13 has proven safe and immunogenic after a single dose in cattle, sheep and goats [111, 112, 114]. Overdose studies in pregnant ewes, however, have found clone 13 able to cross the placental barrier and cause teratogenic effects [139]. A thermostabilized version, selected from viable clones after incubation at 56 °C, has been used to vaccinate livestock in Senegal and Mali [134].
There are several promising vaccine candidates currently under development, which aim to address the shortfalls of current vaccines, namely single dose-efficacy and safety concerns. A subunit vaccine using the GnGc glycoproteins has shown 100 % efficacy in sheep [140]. Another, RVFV-4s, where the M segment is split into two parts separately encoding Gn and Gc, has conferred sterile immunity in lambs after a single vaccination [141]. In addition, vaccinated pregnant sheep showed no teratogenic affects with no presence of RVFV-4s virus in the blood or organs of their foetuses [142]. Another candidate is ChAdOx1 RVF, a replication-deficient chimpanzee adenovirus vectored vaccine encoding the Gn and Gc glycoproteins. ChAdOx1 RVF has shown 100 % efficacy against RVF viral challenge in sheep, goats and cattle [104]. ChAdOx1 RVF is also planned for use in humans where the ChAdOx1 vector expressing other antigens has demonstrated an excellent safety profile [143, 144]. A first-in-human trial of ChAdOx1 RVF is due in 2020.
Future Outlook and Research Priorities
In recent years, more research has focused on emerging pathogens with the aim of increasing our preparedness for future outbreaks [145, 146]. RVFV has a complicated ecological cycle involving a wide range of vectors, livestock and wildlife species. Due to the large geographic area potentially vulnerable to RVF outbreaks, adequate surveillance is key to coordinating efforts to limit its spread. In addition, as human outbreaks appear to occur only after initial amplification in livestock, by identifying livestock outbreaks early, human disease can be minimized by implementing appropriate control measures (e.g. vaccination) [147]. Unfortunately, current surveillance is sub-optimal in numerous at-risk countries with many outbreaks remaining unreported [148]. Ideally, proper surveillance measures will enable farmers to report unexplained disease in their livestock, which can be investigated and allow suitable measures to be implemented to reduce spread [18].
The development of specific therapeutics and vaccines is also of major importance. Disadvantages of current livestock vaccines and the absence of a licensed human vaccine have limited our ability to effectively respond to outbreaks. Further research is required for a better understanding of viral maintenance during IEPs; the role of vertical transmission in mosquitoes and circulation in wildlife in various ecological settings. Knowledge of how different routes of human exposure, such as through mosquito bite or contact with infected animal products (via percutaneous route or through aerosols), affect the immune response and disease outcomes is still lacking. Additionally, the role cellular immunity plays in livestock and humans is still unclear. Finally, a better understanding of the causes of the various manifestations of RVF disease may help to rationalize why an infection is asymptomatic or associated with clinical, sometimes fatal, illness. As a single serotype of RVFV causes disease in multiple species, opportunities exist to look more broadly at immunological differences between species and how they influence disease outcome.
Five reasons to publish your next article with a Microbiology Society journal.
The Microbiology Society is a not-for-profit organization.
We offer fast and rigorous peer review – average time to first decision is 4–6 weeks.
Our journals have a global readership with subscriptions held in research institutions around the world.
80% of our authors rate our submission process as ‘excellent’ or ‘very good’.
Your article will be published on an interactive journal platform with advanced metrics.
Find out more and submit your article at microbiologyresearch.org.
Funding information
G.M.W. is supported by an OAK foundation fellowship. T.A.B. is supported by the Medical Research Council (grant number MR/L009528/1). This paper is published with the permission of the Director of the Kenya Medical Research Institute.
Abbreviations
- ADCC
Antibody-dependant cell-mediated cytotoxicity
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- CDC
Complementdependent cytotoxicity
- ELISA
Enzyme-linked immunosorbent assay
- ER
Endoplasmic reticulum
- GDP
Gross domestic product
- HIV-1
Human immunodeficiency virus - 1
- IEP
Inter-epizootic period
- IFN
Interferon
- mAbs
Monoclonal antibodies
- N
Nucleoprotein
- nAbs
Neutralizing antibodies
- NSm
Non-structural protein (M segment)
- NSs
Non-structural protein (S segment)
- PKR
Protein Kinase R
- RdRp
RNA-dependent RNA polymerase
- RVF
Rift Valley fever
- RVFV
Rift Valley fever virus
- SAP30
Sin3A-Associated protein
- SFV
Sandfly fever virus
- SVTSV
Severe fever with thrombocytopenia syndrome virus
- TFIIH
Transcription factor II Human.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or Rift Valley fever. an undescribed virus disease of sheep cattle and man from East Africa. J Pathol Bacteriol. 1931;34:545–579. [Google Scholar]
- 2.Daubney R, Hudson JR. Rift Valley fever. East African Medical Journal. 1933;10:2–19. [Google Scholar]
- 3.Smithburn KC, Haddow AJ, Gillett JD. Rift Valley fever; isolation of the virus from wild mosquitoes. Br J Exp Pathol. 1948;29:107–121. [PMC free article] [PubMed] [Google Scholar]
- 4.Gear J, De Meillon B, Le Roux AF, Kofsky R, Innes RR, et al. Rift Valley fever in South Africa; a study of the 1953 outbreak in the orange free state, with special reference to the vectors and possible reservoir hosts. S Afr Med J. 1955;29:514–518. [PubMed] [Google Scholar]
- 5.Easterday BC. Rift Valley fever. Adv Vet Sci. 1965;10:65–127. [PubMed] [Google Scholar]
- 6.Findlay GM. Rift Valley fever or enzootic hepatitis. Trans R Soc Trop Med Hyg. 1932;25:229-IN11 [Google Scholar]
- 7.Dickson JL. Rift Valley fever in the Union. J S Afr Vet Assoc. 1951;22:110–111. [Google Scholar]
- 8.Huang YM. A new african species of aedes (Diptera: Culicidae) Mosquito systematics. 1985;17:108–120. [Google Scholar]
- 9.Linthicum KJ, Davies FG, Kairo A, Bailey CL. Rift Valley fever virus (family Bunyaviridae, genus phlebovirus). isolations from Diptera collected during an inter-epizootic period in Kenya. J Hyg. 1985;95:197–209. doi: 10.1017/s0022172400062434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumley S, Horton DL, Hernandez-Triana LLM, Johnson N, Fooks AR, et al. Rift Valley fever virus: strategies for maintenance, survival and vertical transmission in mosquitoes. J Gen Virol. 2017;98:875–887. doi: 10.1099/jgv.0.000765. [DOI] [PubMed] [Google Scholar]
- 11.Rostal MK, Evans AL, Sang R, Gikundi S, Wakhule L, et al. Identification of potential vectors of and detection of antibodies against Rift Valley fever virus in livestock during interepizootic periods. Am J Vet Res. 2010;71:522–526. doi: 10.2460/ajvr.71.5.522. [DOI] [PubMed] [Google Scholar]
- 12.Blomström AL, Scharin I, Stenberg H, Figueiredo J, Nhambirre O, et al. Seroprevalence of Rift Valley fever virus in sheep and goats in Zambézia, Mozambique. Infect Ecol Epidemiol. 2016;6:31343. doi: 10.3402/iee.v6.31343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans A, Gakuya F, Paweska JT, Rostal M, Akoolo L, et al. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol Infect. 2008;136:1261–1269. doi: 10.1017/S0950268807009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murithi RM, Munyua P, Ithondeka PM, Macharia JM, Hightower A, et al. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol Infect. 2011;139:372–380. doi: 10.1017/S0950268810001020. [DOI] [PubMed] [Google Scholar]
- 15.Rostal MK, Liang JE, Zimmermann D, Bengis R, Paweska J, et al. Rift Valley fever: does wildlife play a role? Ilar J. 2017;58:359–370. doi: 10.1093/ilar/ilx023. [DOI] [PubMed] [Google Scholar]
- 16.Anyamba A, Linthicum KJ, Small J, Britch SC, Pak E, et al. Prediction, assessment of the Rift Valley fever activity in East and southern Africa 2006-2008 and possible vector control strategies. Am J Trop Med Hyg. 2010;83:43–51. doi: 10.4269/ajtmh.2010.09-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anyamba A, Chretien JP, Small J, Tucker CJ, Linthicum KJ. Developing global climate anomalies suggest potential disease risks for 2006-2007. Int J Health Geogr. 2006;5:60. doi: 10.1186/1476-072X-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyas H, Holmstrom L, Kemunto NP, Muturi M, Mwatondo A, et al. Enhanced surveillance for Rift Valley fever in livestock during El Niño rains and threat of RVF outbreak, Kenya, 201 5-2016. PLoS Negl Trop Dis. 2018;12:e0006353-e:e0006353. doi: 10.1371/journal.pntd.0006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linthicum KJ, Britch SC, Anyamba A. Rift Valley fever: an emerging mosquito-borne disease. Annu Rev Entomol. 2016;61:395–415. doi: 10.1146/annurev-ento-010715-023819. [DOI] [PubMed] [Google Scholar]
- 20.Mansfield KL, Banyard AC, McElhinney L, Johnson N, Horton DL, et al. Rift Valley fever virus: a review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33:5520–5531. doi: 10.1016/j.vaccine.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus(Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas DE, Jacobsen KH, Waters NM. Risk factors associated with human Rift Valley fever infection: systematic review and meta-analysis. Trop Med Int Health. 2014;19:1420–1429. doi: 10.1111/tmi.12385. [DOI] [PubMed] [Google Scholar]
- 23.Al-Hamdan NA, Panackal AA, Al Bassam TH, Alrabea A, Al Hazmi M, et al. The risk of nosocomial transmission of Rift Valley fever. PLoS Negl Trop Dis. 2015;9:e0004314. doi: 10.1371/journal.pntd.0004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haneche F, Leparc-Goffart I, Simon F, Hentzien M, Martinez-Pourcher V, et al. Rift Valley fever in kidney transplant recipient returning from Mali with viral RNA detected in semen up to four months from symptom onset, France, autumn 2015. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.18.30222. [DOI] [PubMed] [Google Scholar]
- 25.Adam I, Karsany MS. Case report: Rift Valley fever with vertical transmission in a pregnant Sudanese woman. J Med Virol. 2008;80:929. doi: 10.1002/jmv.21132. [DOI] [PubMed] [Google Scholar]
- 26.Arishi HM, Aqeel AY, Al Hazmi MM. Vertical transmission of fatal Rift Valley fever in a newborn. Ann Trop Paediatr. 2006;26:251–253. doi: 10.1179/146532806X120363. [DOI] [PubMed] [Google Scholar]
- 27.McMillen CM, Arora N, Boyles DA, Albe JR, Kujawa MR, et al. Rift Valley fever virus induces fetal demise in Sprague-Dawley rats through direct placental infection. Sci Adv. 2018;4:eaau9812. doi: 10.1126/sciadv.aau9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichgers Schreur PJ, van Keulen L, Kant J, Oreshkova N, Moor-mann RJM, et al. Co-housing of Rift Valley fever virus infected lambs with immunocompetent or immunosuppressed lambs does not result in virus transmission. Front Microbiol. 2016;7:287. doi: 10.3389/fmicb.2016.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonis AFG, Kortekaas J, Kant J, Vloet RPM, Vogel-Brink A, et al. Vertical transmission of Rift Valley fever virus without detectable maternal viremia. Vector Borne Zoonotic Dis. 2013;13:601–606. doi: 10.1089/vbz.2012.1160. [DOI] [PubMed] [Google Scholar]
- 30.McMillen CM, Hartman AL. Rift Valley fever in animals and humans: current perspectives. Antiviral Res. 2018;156:29–37. doi: 10.1016/j.antiviral.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntosh BM, Russell D, dos Santos I, Gear JH. Rift Valley fever in humans in South Africa. S Afr Med J. 1980;58:803–806. [PubMed] [Google Scholar]
- 32.Laughlin LW, Meegan JM, Strausbaugh LJ, Morens DM, Watten RH. Epidemic Rift Valley fever in Egypt: observations of the spectrum of human illness. Trans R Soc Trop Med Hyg. 1979;73:630–633. doi: 10.1016/0035-9203(79)90006-3. [DOI] [PubMed] [Google Scholar]
- 33.Digoutte JP, Peters CJ. General aspects of the 1987 Rift Valley fever epidemic in Mauritania. Res Virol. 1989;140:27–30. doi: 10.1016/s0923-2516(89)80081-0. [DOI] [PubMed] [Google Scholar]
- 34.Anonymous. An outbreak of Rift Valley Fever, eastern Africa, 1997-1998. EMHJ. 1998;4:379–381. [Google Scholar]
- 35.Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, et al. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37:1084–1092. doi: 10.1086/378747. [DOI] [PubMed] [Google Scholar]
- 36.Samy AM, Peterson AT, Hall M. Phylogeography of Rift Valley fever virus in Africa and the Arabian Peninsula. PLoS Negl Trop Dis. 2017;11:e0005226. doi: 10.1371/journal.pntd.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baba M, Masiga DK, Sang R, Villinger J. Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg Microbes Infect. 2016;5:1–10.:e58. doi: 10.1038/emi.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.FAO. Transboundary animal diseases Bulletin FAO corporate document Repository. EMPRES; 2000. pp. 4–9. [Google Scholar]
- 39.Peyre M, Chevalier V, Abdo-Salem S, Velthuis A, Antoine-Mous-siaux N, et al. A systematic scoping study of the socio-economic impact of Rift Valley fever: research gaps and needs. Zoonoses Public Health. 2015;62:309–325. doi: 10.1111/zph.12153. [DOI] [PubMed] [Google Scholar]
- 40.Orinde AB, Kimani T, Schelling E, Omolo J, Kikuvi GM, et al. Estimation of the Rift Valley Fever Burden of Disease in the 2006/2007 Outbreak in Kenya; ILRI, Nairobi: 61st Conference of the American society of Tropical Medicine and Hygiene; 2012. [Google Scholar]
- 41.Baba S, Eric T, Dirk B, Ahmed HA, Guido VH. Effects of livestock importbans imposed by Saudi Arabia on Somaliland for sanitary reasons related to Rift Valley fever. Outlook on AGRICULTURE. 2006;35:19–24. [Google Scholar]
- 42.Clark MHA, Warimwe GM, Di Nardo A, Lyons NA, Gubbins S. Systematic literature review of Rift Valley fever virus seroprevalence in livestock, wildlife and humans in Africa from 1968 to 2016. PLoS Negl Trop Dis. 2018;12:e0006627-e:e0006627. doi: 10.1371/journal.pntd.0006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaBeaud AD, Pfeil S, Muiruri S, Dahir S, Sutherland LJ, et al. Factors associated with severe human Rift Valley fever in Sangailu, Garissa County, Kenya. PLoS Negl Trop Dis. 2015;9:e0003548. doi: 10.1371/journal.pntd.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woods CW, Karpati AM, Grein T, McCarthy N, Gaturuku P, et al. An outbreak of Rift Valley fever in northeastern Kenya, 1997-98. Emerg Infect Dis. 2002;8:138–144. doi: 10.3201/eid0802.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grossi-Soyster EN, Banda T, Teng CY, Muchiri EM, Mungai PL, et al. Rift Valley fever seroprevalence in Coastal Kenya. Am J Trop Med Hyg. 2017;97:115–120. doi: 10.4269/ajtmh.17-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.CDC. Rift Valley fever (RVF) centers for disease control and prevention. 2018. https://wwwnc.cdc.gov/travel/diseases/rift-river-valley .
- 47.Meegan JM, Khalil GM, Hoogstraal H, Adham FK. Experimental transmission and field isolation studies implicating Culex pipiens as a vector of Rift Valley fever virus in Egypt. Am J Trop Med Hyg. 1980;29:1405–1410. doi: 10.4269/ajtmh.1980.29.1405. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Sun Y, Shi W, Tan S, Pan Y, et al. The first imported case of Rift Valley fever in China reveals a genetic reassortment of different viral lineages. Emerg Microbes Infect. 2017;6:e4. doi: 10.1038/emi.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed M, Mosha F, Mghamba J, Zaki SR, Shieh WJ, et al. Epidemiologic and clinical aspects of a Rift Valley fever outbreak in humans in Tanzania, 2007. Am J Trop Med Hyg. 2010;83:22–27. doi: 10.4269/ajtmh.2010.09-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hise AG, Traylor Z, Hall NB, Sutherland LJ, Dahir S, et al. Association of symptoms and severity of Rift Valley fever with genetic polymorphisms in human innate immune pathways. PLoS Negl Trop Dis. 2015;9:e0003584. doi: 10.1371/journal.pntd.0003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichoti JK, Kihara A, Oriko AA, Okutoyi LA, Wauna JO, et al. Detection of Rift Valley fever virus interepidemic activity in some hotspot areas of Kenya by sentinel animal surveillance, 2009-2012. Vet Med Int. 2014;2014:1–9. doi: 10.1155/2014/379010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lernout T, Cardinale E, Jego M, Desprès P, Collet L, et al. Rift Valley fever in humans and animals in Mayotte, an endemic situation? PLoS One. 2013;8:e74192. doi: 10.1371/journal.pone.0074192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smithburn KC, Mahaffy AF, Haddow AJ, Kitchen SF, Smith JF. Rift Valley fever; accidental infections among laboratory workers. Journal of immunology. 1949;62:213–227. [PubMed] [Google Scholar]
- 54.Ikegami T, Makino S. The pathogenesis of Rift Valley fever. Viruses. 2011;3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguku PM, Sang R, Amwayi S, Sharif SK, Hightower A, et al. An investigation of a major outbreak of Rift Valley fever in Kenya: 2006-2007. Am J Trop Med Hyg. 2010;83:05–13. doi: 10.4269/ajtmh.2010.09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McElroy AK, Nichol ST. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology. 2012;422:6–12. doi: 10.1016/j.virol.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meegan JM, Watten RH, Laughlin LW. Clinical experience with Rift Valley fever in humans during the 1977 Egyptian epizootic. Contrib Epidemiol Biostat. 1981;3:114–123. [Google Scholar]
- 58.Arthur RR, el-Sharkawy MS, Cope SE, Botros BA, Oun S, et al. Recurrence of Rift Valley fever in Egypt. Lancet. 1993;342:1149–1150. doi: 10.1016/0140-6736(93)92128-g. [DOI] [PubMed] [Google Scholar]
- 59.Al-Hazmi A, Al-Rajhi AA, Abboud EB, Ayoola EA, Al-Hazmi M, et al. Ocular complications of Rift Valley fever outbreak in Saudi Arabia. Ophthalmology. 2005;112:313–318. doi: 10.1016/j.ophtha.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Freed I. Rift Valley fever in man, complicated by retinal changes and loss of vision. S Afr Med J. 1951;25:930–932. [PubMed] [Google Scholar]
- 61.Abdel-Aziz AA, Meegan JM, Laughlin LW. Rift Valley fever as a possible cause of human abortions. Trans R Soc Trop Med Hyg. 1980;74:685–686. doi: 10.1016/0035-9203(80)90167-4. [DOI] [PubMed] [Google Scholar]
- 62.Baudin M, Jumaa AM, Jomma HJE, Karsany MS, Bucht G, et al. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health. 2016;4:e864–e871. doi: 10.1016/S2214-109X(16)30176-0. [DOI] [PubMed] [Google Scholar]
- 63.Swartz TA, Klinberg MA, Goldblum N, P CM, editors. The symptomatology and pathology of Rift Valley fever in domestic animals. 1981.
- 64.Hartman A, Fever RV. Rift Valley fever. Clin Lab Med. 2017;37:285–301. doi: 10.1016/j.cll.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faburay B, Gaudreault NN, Liu Q, Davis AS, Shivanna V, et al. Development of a sheep challenge model for Rift Valley fever. Virology. 2016;489:128–140. doi: 10.1016/j.virol.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Wilson W, Davis A, Gaudreault N, Faburay B, Trujillo J, et al. Experimental infection of calves by two genetically-distinct strains of Rift Valley fever virus. Viruses. 2016;8:145. doi: 10.3390/v8050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El Mamy AB, Baba MO, Barry Y, Isselmou K, Dia ML, et al. Unexpected Rift Valley fever outbreak, Northern Mauritania. Emerg Infect Dis. 2011;17:1894–1896. doi: 10.3201/eid1710.110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weingartl HM, Zhang S, Marszal P, McGreevy A, Burton L, et al. Rift Valley fever virus incorporates the 78 kDa glycoprotein into virions matured in mosquito C6/36 cells. PLoS One. 2014;9:e87385. doi: 10.1371/journal.pone.0087385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kreher F, Tamietti C, Gommet C, Guillemot L, Ermonval M, et al. The Rift Valley fever accessory proteins NSm and P78/NSm-GN are distinct determinants of virus propagation in vertebrate and invertebrate hosts. Emerg Microbes Infect. 2014;3:1–12. doi: 10.1038/emi.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halldorsson S, Li S, Li M, Harlos K, Bowden TA, et al. Shielding and activation of a viral membrane fusion protein. Nat Commun. 2018;9:349. doi: 10.1038/s41467-017-02789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freiberg AN, Sherman MB, Morais MC, Holbrook MR, Watowich SJ. Three-dimensional organization of Rift Valley fever virus revealed by cryoelectron tomography. J Virol. 2008;82:10341–10348. doi: 10.1128/JVI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherman MB, Freiberg AN, Holbrook MR, Watowich SJ. Singleparticle cryo-electron microscopy of Rift Valley fever virus. Virology. 2009;387:11–15. doi: 10.1016/j.virol.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huiskonen JT, Overby AK, Weber F, Grünewald K. Electron cryomicroscopy and single-particle averaging of Rift Valley fever virus: evidence for GN-GC glycoprotein heterodimers. J Virol. 2009;83:3762–3769. doi: 10.1128/JVI.02483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halldorsson S, Behrens AJ, Harlos K, Huiskonen JT, Elliott RM, et al. Structure of a phleboviral envelope glycoprotein reveals a consolidated model of membrane fusion. Proc Natl Acad Sci U S A. 2016;113:7154–7159. doi: 10.1073/pnas.1603827113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Boer SM, Kortekaas J, Spel L, Rottier PJM, Moormann RJM, et al. Acid-activated structural reorganization of the Rift Valley fever virus GC fusion protein. J Virol. 2012;86:13642–13652. doi: 10.1128/JVI.01973-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harmon B, Schudel BR, Maar D, Kozina C, Ikegami T, et al. Rift Valley fever virus strain MP-12 enters mammalian host cells via caveola-mediated endocytosis. J Virol. 2012;86:12954–12970. doi: 10.1128/JVI.02242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guardado-Calvo P, Bignon EA, Stettner E, Jeffers SA, Pérez-Vargas J, et al. Mechanistic insight into Bunyavirus-Induced membrane fusion from structure-function analyses of The Hantavirus envelope glycoprotein gC. PLoS Pathog. 2016;12:e1005813. doi: 10.1371/journal.ppat.1005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willensky S, Bar-Rogovsky H, Bignon EA, Tischler ND, Modis Y, et al. Crystal structure of glycoprotein C from a hantavirus in the Post-fusion conformation. PLoS Pathog. 2016;12:e1005948. doi: 10.1371/journal.ppat.1005948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu Y, Wu Y, Chai Y, Qi J, Peng R, et al. The postfusion structure of the heartland virus GC glycoprotein supports taxonomic separation of the Bunyaviral families Phenuiviridae and Hantaviridae. Journal of virology. 2018;92 doi: 10.1128/JVI.01558-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y, Zhu Y, Gao F, Jiao Y, Oladejo BO, et al. Structures of phle-bovirus glycoprotein GN and identification of a neutralizing antibody epitope. Proc Natl Acad Sci U S A. 2017;114:E7564–E7573. doi: 10.1073/pnas.1705176114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lozach P-Y, Kühbacher A, Meier R, Mancini R, Bitto D, et al. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe. 2011;10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Phoenix I, Nishiyama S, Lokugamage N, Hill TE, Huante MB, et al. N-glycans on the Rift Valley fever virus envelope glycoproteins GN and GC redundantly support viral infection via DC-SIGN. Viruses. 2016;8:149. doi: 10.3390/v8050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Léger P, Tetard M, Youness B, Cordes N, Rouxel RN, et al. Differential use of the C-type lectins L-SIGN and DC-SIGN for phlebo-virus endocytosis. Traffic. 2016;17:639–656. doi: 10.1111/tra.12393. [DOI] [PubMed] [Google Scholar]
- 84.Lozach P-Y, Kühbacher A, Meier R, Mancini R, Bitto D, et al. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe. 2011;10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Riblett AM, Blomen VA, Jae LT, Altamura LA, Doms RW, et al. A haploid genetic screen identifies heparan sulfate proteoglycans supporting Rift Valley fever virus infection. J Virol. 2016;90:1414–1423. doi: 10.1128/JVI.02055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Boer SM, Kortekaas J, de Haan CAM, Rottier PJM, Moor-mann RJM, et al. Heparan sulfate facilitates Rift Valley fever virus entry into the cell. J Virol. 2012;86:13767–13771. doi: 10.1128/JVI.01364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, et al., editors. Fields Virology. 6. Lippincot Williams & Wilkins; 2013. [Google Scholar]
- 88.Grobbelaar AA, Weyer J, Leman PA, Kemp A, Paweska JT, et al. Molecular epidemiology of Rift Valley fever virus. Emerg Infect Dis. 2011;17:2270–2276. doi: 10.3201/eid1712.111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bird BH, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J Virol. 2007;81:2805–2816. doi: 10.1128/JVI.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Battles JK, Dalrymple JM. Genetic variation among geographic isolates of Rift Valley fever virus. Am J Trop Med Hyg. 1988;39:617–631. doi: 10.4269/ajtmh.1988.39.617. [DOI] [PubMed] [Google Scholar]
- 91.Nanyingi MO, Munyua P, Kiama SG, Muchemi GM, Thumbi SM, et al. A systematic review of Rift Valley fever epidemiology 19312014. Infect Ecol Epidemiol. 2015;5:28024. doi: 10.3402/iee.v5.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.FAO. The last hurdles towards Rift Valley Fever control. Report on the Ad hoc workshop on the Current State of Rift Valley Fever Vaccine And Diagnostics Development. Vol. 9 Rome, Italy: FAO Animal Production and Health Report; 2015. [Google Scholar]
- 93.Allen ER, Krumm SA, Raghwani J, Halldorsson S, Elliott A, et al. A protective monoclonal antibody targets a site of vulnerability on the surface of Rift Valley fever virus. Cell Rep. 2018;25:3750–3758. doi: 10.1016/j.celrep.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eddy GA, Peters CJ, Meadors G, Cole FE., Jr . In: Contributions to epidemiology and biostatistics S Karger AG. Swartz TA, Klinberg MA, Goldblum N, Papier CM, editors. Rift Valley fever; Basel: 1981. Rift valley fever vaccine for humans; pp. 124–141. [Google Scholar]
- 95.Peters CJ, Jones D, Trotter R, Donaldson J, White J, et al. Experimental Rift Valley fever in rhesus macaques. Arch Virol. 1988;99:31–44. doi: 10.1007/BF01311021. [DOI] [PubMed] [Google Scholar]
- 96.Labeaud D. Towards a safe, effective vaccine for Rift Valley fever virus. Future Virol. 2010;5:675–678. doi: 10.2217/fvl.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown RD, Scott GR, Dalling T. Persistence of antibodies to Rift Valley fever in man. The Lancet. 1957;270:345. [Google Scholar]
- 98.McElroy AK, Albariño CG, Nichol ST. Development of a RVFV ELISA that can distinguish infected from vaccinated animals. Virol J. 2009;6:125. doi: 10.1186/1743-422X-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fafetine JM, Tijhaar E, Paweska JT, Neves LCBG, Hendriks J, et al. Cloning and expression of Rift Valley fever virus nucleocapsid (N) protein and evaluation of a N-protein based indirect ELISA for the detection of specific IgG and IgM antibodies in domestic ruminants. Vet Microbiol. 2007;121:29–38. doi: 10.1016/j.vetmic.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 100.Besselaar TG, Blackburn NK. Topological mapping of antigenic sites on the Rift Valley fever virus envelope glycoproteins using monoclonal antibodies. Arch Virol. 1991;121:111–124. doi: 10.1007/BF01316748. [DOI] [PubMed] [Google Scholar]
- 101.Besselaar TG, Blackburn NK. The synergistic neutralization of Rift Valley fever virus by monoclonal antibodies to the envelope glycoproteins. Arch Virol. 1992;125:239–250. doi: 10.1007/BF01309641. [DOI] [PubMed] [Google Scholar]
- 102.Lagerqvist N, Näslund J, Lundkvist A, Bouloy M, Ahlm C, et al. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley fever virus cDNA constructs. Virol J. 2009;6:6. doi: 10.1186/1743-422X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wallace DB, Ellis CE, Espach A, Smith SJ, Greyling RR, et al. Protective immune responses induced by different recombinant vaccine regimes to Rift Valley fever. Vaccine. 2006;24:7181–7189. doi: 10.1016/j.vaccine.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 104.Warimwe GM, Gesharisha J, Carr BV, Otieno S, Otingah K, et al. Chimpanzee adenovirus vaccine provides multispecies protection against Rift Valley fever. Sci Rep. 2016;6:20617. doi: 10.1038/srep20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.López-Gil E, Lorenzo G, Hevia E, Borrego B, Eiden M, et al. A single immunization with MVA expressing GnGc glycoproteins promotes epitope-specific CD8+-T cell activation and protects immune-competent mice against a lethal RVFV infection. PLoS Negl Trop Dis. 2013;7:e2309. doi: 10.1371/journal.pntd.0002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 107.Wang F, Ekiert DC, Ahmad I, Yu W, Zhang Y, et al. Reshaping antibody diversity. Cell. 2013;153:1379–1393. doi: 10.1016/j.cell.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oscherwitz J. The promise and challenge of epitope-focused vaccines. Hum Vaccin Immunother. 2016;12:2113–2116. doi: 10.1080/21645515.2016.1160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terasaki K, Makino S. Interplay between the virus and host in Rift Valley fever pathogenesis. J Innate Immun. 2015;7:450–458. doi: 10.1159/000373924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wonderlich ER, Caroline AL, McMillen CM, Walters AW, Reed DS, et al. Peripheral blood biomarkers of disease outcome in a monkey model of Rift Valley fever encephalitis. J Virol. 2018;92:e01662-17. doi: 10.1128/JVI.01662-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dungu B, Louw I, Lubisi A, Hunter P, von Teichman BF, et al. Evaluation of the efficacy and safety of the Rift Valley fever clone 13 vaccine in sheep. Vaccine. 2010;28:4581–4587. doi: 10.1016/j.vaccine.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 112.von Teichman B, Engelbrecht A, Zulu G, Dungu B, Pardini A, et al. Safety and efficacy of Rift Valley fever Smithburn and clone 13 vaccines in calves. Vaccine. 2011;29:5771–5777. doi: 10.1016/j.vaccine.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 113.Bouloy M, Janzen C, Vialat P, Khun H, Pavlovic J, et al. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSS. J Virol. 2001;75:1371–1377. doi: 10.1128/JVI.75.3.1371-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Njenga MK, Njagi L, Thumbi SM, Kahariri S, Githinji J, et al. Randomized controlled field trial to assess the immunogenicity and safety of Rift Valley fever clone 13 vaccine in livestock. PLoS Negl Trop Dis. 2015;9:e0003550. doi: 10.1371/journal.pntd.0003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ly HJ, Ikegami T. Rift Valley fever virus NSs protein functions and the similarity to other bunyavirus NSS proteins. Virol J. 2016;13:118. doi: 10.1186/s12985-016-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le May N, Mansuroglu Z, Léger P, Josse T, Blot G, et al. A SAP30 complex inhibits IFN-beta expression in Rift Valley fever virus infected cells. PLoS Pathog. 2008;4:e13. doi: 10.1371/journal.ppat.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le May N, Dubaele S, Proietti De Santis L, Billecocq A, Bouloy M, et al. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell. 2004;116:541–550. doi: 10.1016/s0092-8674(04)00132-1. [DOI] [PubMed] [Google Scholar]
- 118.Swanepoel R, Blackburn NK. Demonstration of nuclear immunofluorescence in Rift Valley fever infected cells. J Gen Virol. 1977;34:557–561. doi: 10.1099/0022-1317-34-3-557. [DOI] [PubMed] [Google Scholar]
- 119.Ikegami T, Narayanan K, Won S, Kamitani W, Peters CJ, et al. Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog. 2009;5:e1000287. doi: 10.1371/journal.ppat.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ikegami T. Rift Valley fever vaccines: an overview of the safety and efficacy of the live-attenuated MP-12 vaccine candidate. Expert Rev Vaccines. 2017;16:601–611. doi: 10.1080/14760584.2017.1321482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pittman PR, Liu CT, Cannon TL, Makuch RS, Mangiafico JA, et al. Immunogenicity of an inactivated Rift Valley fever vaccine in humans: a 12-year experience. Vaccine. 1999;18:181–189. doi: 10.1016/s0264-410x(99)00218-2. [DOI] [PubMed] [Google Scholar]
- 122.von Teichman B, Engelbrecht A, Zulu G, Dungu B, Pardini A, et al. Safety and efficacy of Rift Valley fever Smithburn and clone 13 vaccines in calves. Vaccine. 2011;29:5771–5777. doi: 10.1016/j.vaccine.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 123.Wilson WC, Bawa B, Drolet BS, Lehiy C, Faburay B, et al. Evaluation of lamb and calf responses to Rift Valley fever MP-12 vaccination. Vet Microbiol. 2014;172:44–50. doi: 10.1016/j.vetmic.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Mroz C, Gwida M, El-Ashker M, Ziegler U, Homeier-Bachmann T, et al. Rift Valley fever virus infections in Egyptian cattle and their prevention. Transbound Emerg Dis. 2017;64:2049–2058. doi: 10.1111/tbed.12616. [DOI] [PubMed] [Google Scholar]
- 125.Zidan S, Byomi A, Samaha H, Hadad G. Some associated risk factors with occurrence of Rift Valley fever in some animals and man in certain localities of Nile Delta, Egypt. 2015 [Google Scholar]
- 126.Dungu B, Donadeu M, Bouloy M. Vaccination for the control of Rift Valley fever in enzootic and epizootic situations. Dev Biol. 2013;135:61–72. doi: 10.1159/000157178. [DOI] [PubMed] [Google Scholar]
- 127.Jansen van Vuren P, Tiemessen CT, Paweska JT. Anti-nucle-ocapsid protein immune responses counteract pathogenic effects of Rift Valley fever virus infection in mice. PLoS One. 2011;6:e25027. doi: 10.1371/journal.pone.0025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu W, Watts DM, Costanzo MC, Tang X, Venegas LA, et al. The nucleocapsid protein of Rift Valley fever virus is a potent human CD8+ T cell antigen and elicits memory responses. PLoS One. 2013;8:e59210. doi: 10.1371/journal.pone.0059210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Q, Ma T, Wu Y, Chen Z, Zeng H, et al. Neutralization mechanism of human monoclonal antibodies against Rift Valley fever virus. Nat Microbiol. 2019;34 doi: 10.1038/s41564-019-0411-z. [DOI] [PubMed] [Google Scholar]
- 130.Keegan K, Collett MS. Use of bacterial expression cloning to define the amino acid sequences of antigenic determinants on the G2 glycoprotein of Rift Valley fever virus. J Virol. 1986;58:263–270. doi: 10.1128/jvi.58.2.263-270.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams R, Ellis CE, Smith SJ, Potgieter CA, Wallace D, et al. Validation of an IgM antibody capture ELISA based on a recombinant nucleoprotein for identification of domestic ruminants infected with Rift Valley fever virus. J Virol Methods. 2011;177:140–146. doi: 10.1016/j.jviromet.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 132.Atkins C, Freiberg AN. Recent advances in the development of antiviral therapeutics for Rift Valley fever virus infection. Future Virol. 2017;12:651–665. doi: 10.2217/fvl-2017-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Faburay B, LaBeaud AD, McVey DS, Wilson WC, Richt JA. Current status of Rift Valley fever vaccine development. Vaccines. 2017;5:E29. doi: 10.3390/vaccines5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dungu B, Lubisi BA, Ikegami T. Rift Valley fever vaccines: current and future needs. Curr Opin Virol. 2018;29:8–15. doi: 10.1016/j.coviro.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 135.Ikegami T, Hill TE, Smith JK, Zhang L, Juelich TL, et al. Rift Valley fever virus MP-12 vaccine is fully attenuated by a combination of partial Attenuations in the S, M, and L segments. J Virol. 2015;89:7262–7276. doi: 10.1128/JVI.00135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Botros B, Omar A, Elian K, Mohamed G, Soliman A, et al. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J Med Virol. 2006;78:787–791. doi: 10.1002/jmv.20624. [DOI] [PubMed] [Google Scholar]
- 137.Ahmed Kamal S. Observations on Rift Valley fever virus and vaccines in Egypt. Virol J. 2011;8:532. doi: 10.1186/1743-422X-8-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muller R, Saluzzo JF, Lopez N, Dreier T, Turell M, et al. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg. 1995;53:405–411. doi: 10.4269/ajtmh.1995.53.405. [DOI] [PubMed] [Google Scholar]
- 139.Makoschey B, van Kilsdonk E, Hubers WR, Vrijenhoek MP, Smit M, et al. Rift Valley fever vaccine virus clone 13 is able to cross the ovine placental barrier associated with foetal infections, malformations, and stillbirths. PLoS Negl Trop Dis. 2016;10:e0004550. doi: 10.1371/journal.pntd.0004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Faburay B, Wilson WC, Gaudreault NN, Davis AS, Shivanna V, et al. A recombinant Rift Valley fever virus glycoprotein subunit vaccine confers full protection against Rift Valley fever challenge in sheep. Sci Rep. 2016;6:27719. doi: 10.1038/srep27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wichgers Schreur PJ, Kant J, van Keulen L, Moormann RJM, Kortekaas J. Four-segmented Rift Valley fever virus induces sterile immunity in sheep after a single vaccination. Vaccine. 2015;33:1459–1464. doi: 10.1016/j.vaccine.2015.01.077. [DOI] [PubMed] [Google Scholar]
- 142.Wichgers Schreur PJ, van Keulen L, Kant J, Kortekaas J. Four-segmented Rift Valley fever virus-based vaccines can be applied safely in ewes during pregnancy. Vaccine. 2017;35:3123–3128. doi: 10.1016/j.vaccine.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 143.Antrobus RD, Coughlan L, Berthoud TK, Dicks MD, Hill AV, et al. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved influenza A antigens. Mol Ther. 2014;22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stylianou E, Griffiths KL, Poyntz HC, Harrington-Kandt R, Dicks MD, et al. Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine. 2015;33:6800–6808. doi: 10.1016/j.vaccine.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 146.Sands P, Mundaca-Shah C, Dzau VJ. The neglected dimension of global security-A framework for countering infectious-disease crises. N Engl J Med. 2016;374:1281–1287. doi: 10.1056/NEJMsr1600236. [DOI] [PubMed] [Google Scholar]
- 147.Bird BH, Nichol ST. Breaking the chain: Rift Valley fever virus control via livestock vaccination. Curr Opin Virol. 2012;2:315–323. doi: 10.1016/j.coviro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 148.Davies FG. The historical and recent impact of Rift Valley fever in Africa. Am J Trop Med Hyg. 2010;83:73–74. doi: 10.4269/ajtmh.2010.83s2a02. [DOI] [PMC free article] [PubMed] [Google Scholar]