Abstract
Introduction
Fluid resuscitation is a cornerstone of severe sepsis management, however there are many uncertainties surrounding the type and volume of fluid that is administered. The entire spectrum of coagulopathies can be seen in sepsis, from asymptomatic aberrations to fulminant disseminated intravascular coagulation (DIC). The aim of this study was to determine if fluid resuscitation with saline contributes to the haemostatic derangements in an ovine model of endotoxemic shock.
Materials and Methods
Twenty-one adult female sheep were randomly divided into no endotoxemia (n=5) or endotoxemia groups (n=16) with an escalating dose of lipopolysaccharide (LPS) up to 4 μg/kg/hr administered to achieve a mean arterial pressure below 60mmHg. Endotoxemia sheep received either no bolus fluid resuscitation (n=8) or a 0.9% saline bolus (40 mL/kg over 60 minutes) (n=8). No endotoxemia, saline only animals (n=5) underwent fluid resuscitation with a 0.9% bolus of saline as detailed above. Hemodynamic support with vasopressors was initiated if needed, to maintain a mean arterial pressure (MAP) of 60-65mmHg in all the groups.
Results
Rotational thromboelastometry (ROTEM®) and conventional coagulation biomarker tests demonstrated sepsis induced derangements to secondary haemostasis. This effect was exacerbated by saline fluid resuscitation, with low pH (p = 0.036), delayed clot initiation and formation together with deficiencies in naturally occurring anti-coagulants antithrombin (p = 0.027) and Protein C (p = 0.001).
Conclusions
Endotoxemia impairs secondary haemostasis and induces changes in the intrinsic, extrinsic and anti-coagulant pathways. These changes to haemostasis are exacerbated following resuscitation with 0.9% saline, a commonly used crystalloid in clinical settings.
Keywords: sepsis, thromboelastometry, coagulation, fibrinogen, coagulation factors
Introduction
Sepsis is a global syndrome that remains the leading cause of mortality in critical illness, with an international assessment showing a hospital mortality rate of 35.3% [1]. Recently revised definitions characterize sepsis as life-threatening organ dysfunction due to a dysregulated host response to infection. Septic shock is defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities substantially increase mortality [2]. Hemodynamic support to prevent onset and/or progression of end-organ failure is one of the foundations for the acute management of sepsis however there are many uncertainties surrounding the type and volume of fluid that is administered. Historically, the recommended standard of care was to administer large volumes of fluid to achieve a central venous pressure (CVP) of 8-12 cmH2O [3, 4] yet there is no high quality randomized control trial evidence to support this. This has been superseded by multi-centre trials that demonstrated that aggressive fluid resuscitation does not improve the outcome of patients with severe sepsis and septic shock [5, 6]. In addition, observational studies have shown administration of more than 5 L during the first ICU day [7] and a higher cumulative fluid balance at day three [8] are associated with a significantly increased risk of death.
Coagulopathy is highly prevalent in sepsis and can be complex, ranging from minor changes to fulminant disseminated intravascular coagulation (DIC) characterized by widespread microvascular thrombosis, profuse bleeding and amplification of the inflammatory response through the release of inflammatory cytokines and growth factors [9, 10]. Reliable markers of coagulopathy in sepsis are important; however conventional coagulation tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) may not fully reflect the complex haemostatic disturbances seen in these patients. As a result, viscoelastic whole blood tests using thromboelastography (TEG) and/or thromboelastometry (ROTEM) are gaining in popularity. ROTEM is an ex vivo test that provides information on the dynamics of clot development, stabilization and dissolution. Several studies have used ROTEM and/or TEG to detect hyper- or hypo-coagulability in sepsis with variable results [11, 12]. A systematic review of the utility of point-of-care devices in adults with sepsis concluded they may be useful in diagnosing coagulation alterations, including DIC, but further research was warranted, particularly sequential measurements given the dynamic process of coagulopathy in sepsis [13].
Our study aimed to utilize ROTEM testing alongside conventional methods to discern how fluid resuscitation contributes to the haemostatic changes seen in septic shock. We developed an ovine model of lipopolysaccharide (LPS) induced shock to investigate the impact of bolus fluid resuscitation with 0.9% saline vs no resuscitation. We hypothesized that saline boluses would significantly increase the haemostatic changes seen during ovine endotoxemia.
Materials and methods
Ovine model of endotoxemic shock
This study was approved by the Animal Research and Ethics Committee of the Queensland University of Technology and The University of Queensland (Approval no. 1400000032) and adhered to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes 8th Edition 2013 of the National Health and Medical Research Council (NHMRC). Twenty-one adult female Merino sheep weighing 34-59 kg were randomly divided into three groups (Fig. 1). All sheep had a multilumen central venous catheter and 8 Fr sheath placed in the left internal jugular vein under local anaesthesia and were anaesthetized with midazolam (Pfizer, NSW, Australia) (0.5 mg/kg/i.v), buprenorphine (Reckitt Benckiser Healthcare, Hull, U.K) (300 μg i.v) and alfaxalone (Jurox, NSW, Australia) (3 mg/kg/i.v). Animals were subsequently intubated with an orotracheal tube and mechanically ventilated with central venous, facial artery and pulmonary artery catheters inserted to enable drug administration, cardiovascular monitoring and blood sampling. Sheep were randomly divided into two groups receiving either no endotoxemia (n=5) or endotoxemic shock (n=16) induced by administration of an escalating dose LPS (E coli serotype O55:B5 10μg/ml) (Sigma-Aldrich, St Louis, USA) up to 4 μg/kg/hr (total LPS dose 11.25 μg/kg) as per our previously validated model [14]. Adequate endotoxemia was confirmed by the occurrence of systemic hypotension with a mean arterial pressure (MAP) less than 60 mmHg after 3 hours of the endotoxin infusion. In the final hour endotoxemic sheep received either no bolus fluid resuscitation (endotoxemia only) (n=8) or a 0.9% saline bolus (n=8) (40 mL/kg over 60 minutes) (endotoxemia + saline). No endotoxemia, saline only animals underwent resuscitation with a 0.9% bolus of saline as detailed above. Post-fluid resuscitation, both groups were monitored for 12 hours and received the same protocolised vasopressor and ventilator respiratory support. Vasopressor support consisted of noradrenaline started 60 μg/mL in 5% dextrose (Hospira, Lake Forest, IL, USA) to maintain a MAP between 60-65 mmHg. If noradrenaline reached a predetermined 20 μg/min, vasopressin (PPC, Richmond Hill, ON, Canada) was commenced at 0.8 units/hr and increased to a maximum of 1.6 units/hr if hypotension persisted. The administration protocol was the same for both groups. All animals were euthanized after 12 hours.
Fig. 1. Schematic overview of the experimental plan.
Blood sampling
Arterial blood samples were collected at baseline, pre LPS infusion (or saline only) (T1), post endotoxemia/pre saline resuscitation (T2) and post saline resuscitation at 0, 1, 3, 6, 9 and 12 hrs. Blood samples for ROTEM® analysis were collected in 3.2% sodium citrate tubes (Greiner Bio-One, Amata Nakorn, Chonburi, Thailand) and full blood examination in EDTA tubes (Greiner Bio-One, Kremsmünster, Austria). Citrated blood samples for routine and specialized coagulation tests were centrifuged twice for 15 minutes at 3000 x g to obtain platelet poor plasma and subsequently stored at -80 °C until analysis.
Hemogram and routine coagulation tests
A hemogram using the veterinary mode of the Act diff™ haematology analyser (Beckman Coulter Australia Pty Ltd, NSW, Australia) was used to assess the white cell count (WCC), haemoglobin (Hb) and platelet count (Plt). Prothrombin time (PT), activated partial thromboplastin time (aPTT), antithrombin (AT), Clauss fibrinogen [Fib(C)], protein C, protein S (free) and coagulation factors V, VII, VIII and X were assessed on the Stago STAR Evolution analyser (Diagnostica Stago, Doncaster, VIC, Australia) following the manufacturer’s instructions.
Rotational thromboelastometry (ROTEM®)
Whole blood clot formation profiles were recorded by ROTEM® Thromboelastometry (ROTEM®, Haemoview Diagnostics, Brisbane, Australia) with the EXTEM (thromboplastin-initiated coagulation), INTEM (contact factor-initiated coagulation) and FIBTEM (thromboplastin-initiated coagulation with the platelet inhibitor cytochalasin D) activating reagents in accordance with the manufacturer’s instructions. Parameters evaluated included clotting time (CT), clot formation time (CFT) and maximum clot firmness (MCF).
Statistical analysis
Data are presented as mean ± standard error of mean (SEM). A multivariable mixed effects linear regression model was fitted to determine the effect of the experimental group on each outcome, including random effects for sheep and time. All analyses took into account potential confounders including weight of sheep at baseline and fluid balance. A p value ≤ 0.05 was considered significant. Statistical analyses were performed using STATA™ (StataCorp, TX, USA) statistical software package (version 13).
Results
Physiological, haematological and coagulation parameters
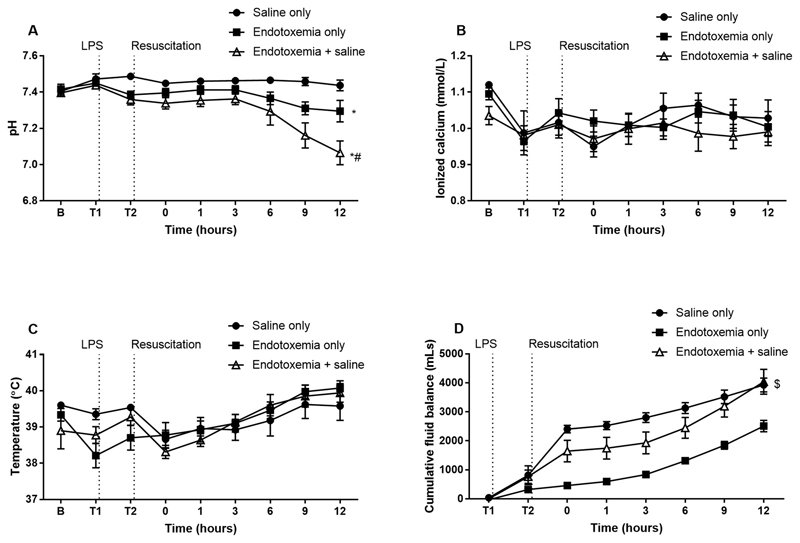
Significant decreases in pH (Fig. 2A) below the normal range for ovine pH (7.32-7.5) were seen with endotoxemia (p = 0.004) compared to saline only with a further reduction in saline-treated endotoxemia (p = 0.036). There was no change in ionized calcium (Fig. 2B) or body temperature (Fig. 2C) in any of the groups. There was a significant positive fluid balance for saline only and endotoxemia + saline animals post saline resuscitation (Fig. 2D). The mean ± SEM of all haematological and plasma based coagulation parameters are described in Table 1 at selected time points. Baseline measurements for full blood examination, routine and specialized coagulation tests did not differ significantly across the experimental groups. WCC decreased in all groups but did not differ between groups while Hb was significantly decreased up to 1hr post resuscitation in animals that received saline compared to no resuscitation. Administration of LPS resulted in a decrease in platelet counts in endotoxemia animals. Standard coagulation tests revealed a prolonged PT and aPTT in the endotoxemia + saline group compared to endotoxemia only. The levels of fibrinogen and protein C were also lower with endotoxemia while AT and protein C were further decreased in endotoxemia + saline animals. Although the levels of factor VII decreased there was no significant difference between the groups while coagulation factors V, VIII and X were decreased in all endotoxemia groups.
Fig. 2.
The effect of saline resuscitation on physiological parameters. Endotoxemia + saline altered pH (A) compared to endotoxemia only while there was no change in ionized calcium (B) or body temperature (C). There was a significant increase in the volume of fluid administered during resuscitation for saline only and endotoxemia + saline animals (D). Data are presented as mean ± SEM. *p < 0.01 versus saline only; #p < 0.05 versus endotoxemia only; $p < 0.05 versus endotoxemia only. n = 5 saline only, n = 8 endotoxemia only, n = 8 endotoxemia + saline. LPS = lipopolysaccharide; B = baseline.
Table 1. Routine and specialized haemostatic parameters by experimental group at selected time points.
| Saline only | Endotoxemia only | Endotoxemia + resuscitation | |||||
|---|---|---|---|---|---|---|---|
| Mean (±SEM) | Baseline | 6HR | 12HR | 6HR | 12HR | 6HR | 12HR |
| Full blood examination | |||||||
| WCC (x109/L) | 7.5 (0.36) | 3.0 (0.3) | 3.0 (0.7) | 3.7 (0.4) | 4.6 (0.8) | 3.5 (0.7) | 3.7 (0.8) |
| Hb (g/L) | 95 (3) | 77 (5) | 85 (6) | 89 (7) | 86 (6)a | 97 (7) | 90 (8)a |
| Plt (x109/L) | 351 (38) | 282 (36) | 312 (42) | 162 (27) | 125 (26)a | 120 (20) | 91 (12)a |
| Routine coagulation tests | |||||||
| PT (s) | 13 (0.3) | 21.6 (1.1) | 25.4 (1.5) | 24.9 (1.2) | 34.3 (1.7) | 31.5 (1.7) | 52 (4.6)b |
| aPTT (s) | 29 (1.5) | 38 (3) | 42 (3) | 42 (3) | 52 (5) | 51 (5) | 75 (11)b |
| Fibrinogen (g/L) | 1.7 (0.1) | 1.3 (0.02) | 1.4 (0.04) | 0.6 (0.1) | 0.4 (0.1)a | 0.5 (0.03) | 0.4 (0.01)a |
| Specialized coagulation tests | |||||||
| Protein C (U/mL) | 0.55 (0.02) | 0.42 (0.05) | 0.36 (0.04) | 0.25 (0.02) | 0.20 (0.01)a | 0.18 (0.03) | 0.11 (0.02)a,b |
| Protein S (free) (U/mL) | 1.85 (0.03) | 1.15 (0.06) | 1.07 (0.08) | 1 (0.03) | 0.81 (0.05) | 0.86 (0.09) | 0.56 (0.08) |
| AT (U/mL) | 0.91 (0.03) | 0.55 (0.03) | 0.5 (0.04) | 0.5 (0.03) | 0.42 (0.02) | 0.4 (0.02) | 0.24 (0.04)b |
| FV (U/mL) | 5.01 (0.27) | 4.59 (0.25) | 4.92 (0.2) | 3.14 (0.24) | 2.42 (0.26)a | 2.77 (0.31) | 1.6 (0.25)a |
| FVII (U/mL) | 0.13 (0.01) | 0.06 (0.004) | 0.05 (0.004) | 0.05 (0.004) | 0.03 (0.003) | 0.05 (0.01) | 0.03 (0.004) |
| FVIII (U/mL) | 6.70 (0.28) | 5.08 (0.41) | 5.02 (0.29) | 2.41 (0.41) | 2.39 (0.42)a | 2.17 (0.33) | 1.83 (0.32)a |
| FX (U/mL) | 0.12 (0.02) | 0.06 (0.004) | 0.05 (0.003) | 0.05 (0.003) | 0.04 (0.003)a | 0.02 (0.002) | 0.02 (0.01)a |
ap < 0.05 compared with saline only
bp < 0.05 compared with endotoxemia only. aPTT indicates activated partial thromboplastin time; AT, antithrombin; Hb, haemoglobin; Plt, Platelet; PT, prothrombin time; WCC, white cell count.
Thromboelastometry
INTEM
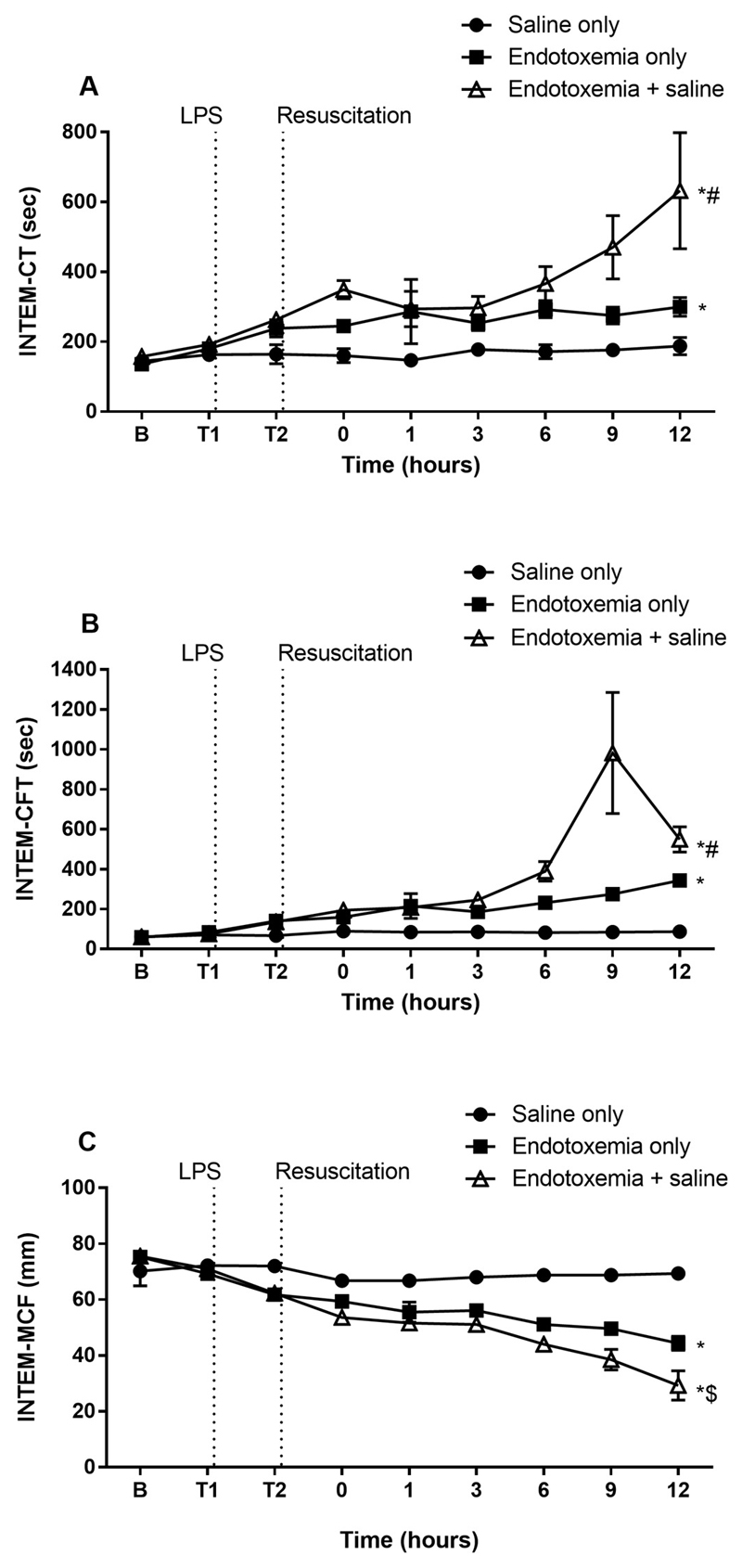
Endotoxemic animals had a prolonged INTEM-CT, and –CFT compared to saline only (Fig. 3). These parameters were further protracted in the endotoxemia + saline group. MCF was correspondingly decreased in endotoxemia groups compared to saline only and was additionally prolonged in endotoxemia + saline animals compared to endotoxemia only.
Fig. 3.
The effect of saline resuscitation on INTEM parameters. INTEM-CT (A) and –CFT (B) were prolonged in endotoxemia + saline compared to endotoxemia only. INTEM-MCF (C) was subsequently lower in endotoxemic animals compared to saline only and lower in the endotoxemia + saline group compared to endotoxemia only. Data are presented as mean ± SEM. *p < 0.01 versus saline only; #p < 0.01 versus endotoxemia only; $p = 0.05 versus endotoxemia only. n = 5 saline only, n = 8 endotoxemia only, n = 8 endotoxemia + saline. LPS = lipopolysaccharide; B = baseline.
EXTEM
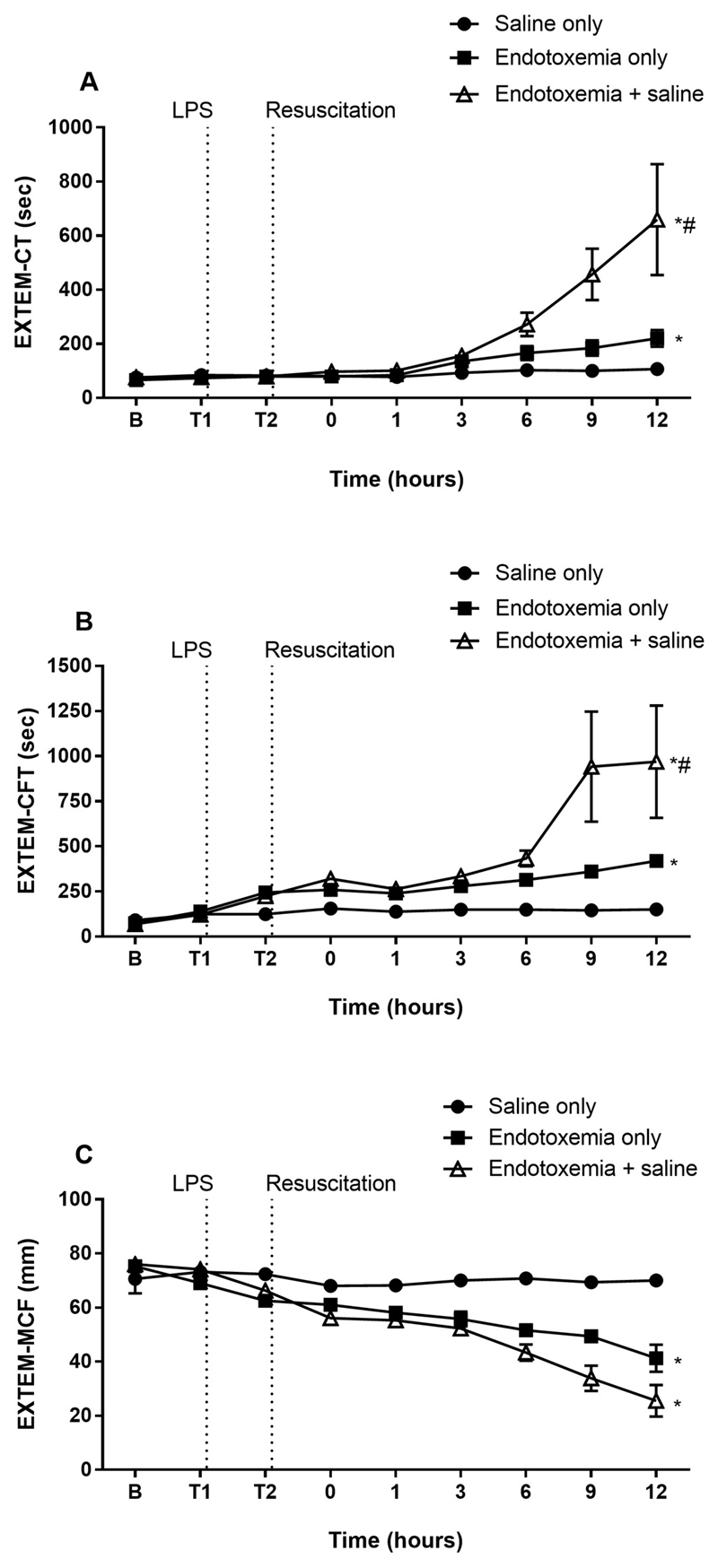
EXTEM-CT and -CFT was prolonged in the endotoxemia groups and further prolonged in the endotoxemia + saline group compared to endotoxemia only (Fig. 4). MCF was decreased in both endotoxemia groups compared to saline only.
Fig. 4.
The effect of saline resuscitation on EXTEM parameters. EXTEM-CT (A) and –CFT (B) were prolonged in endotoxemia + saline compared to endotoxemia only. EXTEM-MCF (C) decreased in endotoxemic animals compared to saline only. Data are presented as mean ± SEM. *p < 0.05 versus saline only; #p < 0.01 versus endotoxemia only. n = 5 saline only, n = 8 endotoxemia only, n = 8 endotoxemia + saline. LPS = lipopolysaccharide; B = baseline.
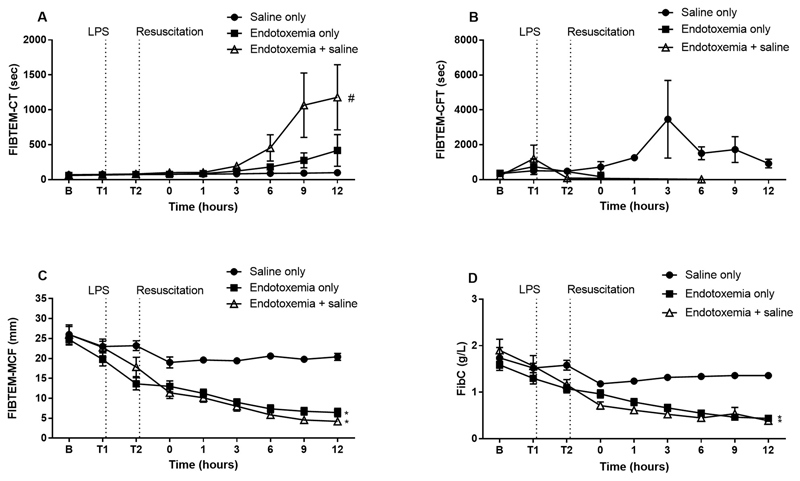
FIBTEM
FIBTEM-CT was prolonged in the endotoxemia + saline group compared to endotoxemia only (Fig. 5). CFT was unchanged across all groups while -MCF was decreased after endotoxin administration in both endotoxemia groups compared to saline only.
Fig. 5.
The effect of saline resuscitation on FIBTEM parameters. FIBTEM-CT (A) was prolonged in endotoxemia + saline animals compared to endotoxemia only while FIBTEM-CFT (B) was unchanged. FIBTEM-MCF (C) was decreased after LPS was established in both endotoxemic groups compared to saline only. Clauss fibrinogen (D) strongly correlated with FIBTEM-MCF. Data are presented as mean ± SEM. *p < 0.001 versus saline only; #p < 0.01 versus endotoxemia only. n = 5 saline only, n = 8 endotoxemia only, n = 8 endotoxemia + saline. LPS = lipopolysaccharide; B = baseline.
Discussion
This ovine model confirms that while endotoxemia disrupts haemostasis, saline resuscitation augments this disruption by delaying clot initiation and formation. Poor fibrin clot quality, as a result of inadequate fibrinogen availability or fibrin was not affected further by saline resuscitation but was due to endotoxemic shock alone.
Resuscitation with saline results in hemodilution, as evidenced by decreased haemoglobin levels up to one hour post infusion. This may account for the initial haemostatic changes post-resuscitation, however, these persist up to 12 hours following saline bolus suggesting that fluid resuscitation itself is exacerbating these changes. Saline has been shown to be associated with deleterious effects including normal anion gap metabolic acidosis, immune suppression [15] and decreased renal perfusion [16, 17]. Moreover, animal models of trauma and haemorrhage have shown saline is associated with dilutional acidosis linked to impaired thrombin generation and fibrin polymerization [18, 19] suggesting a potential mechanism for these changes.
Platelets have a central role in the development of coagulation abnormalities in sepsis. Thrombocytopenia has been shown to be associated with increased mortality in patients with sepsis [20] potentially affected by decreased platelet production, enhanced consumption, obliteration, or sequestration in the spleen [21]. Consistent with the literature, this model showed that LPS-induced sepsis caused a significant decrease in platelet numbers, which was not exacerbated by saline resuscitation.
In this animal model, saline resuscitation did prolong aPTT tests, which are sensitive to the initiation rather than propagation phase of hemostasis [11]. Thromboelastometry measurements using ROTEM verify this result with prolonged INTEM-CT’s, potentially reflecting a reduction in coagulation factors or impaired fibrin polymerization as a result of acidosis [18]. The serial measurements clearly show that bolus saline resuscitation caused deterioration of all the INTEM parameters over time. This shows that the hemostatic disruption induced by saline resuscitation is not immediate but delayed. CFT was also lengthened with saline resuscitation, together with a corresponding reduction in MCF amplitude, consistent with thrombocytopenia/platelet dysfunction or a fibrinogen deficiency/fibrin polymerization disorder. These results corroborate previous studies showing endotoxemia prolongs CFT and leads to a reduced MCF [22]. Factor VIII is an acute-phase reactant and is expected to increase in response to inflammation. Thus, the reduced factor VIII levels seen with endotoxemia likely indicate acute concomitant consumption of this factor. As SAMM/Border Leicester Cross sheep have significantly higher baseline levels of factor VIII [23] this change may be more readily detected in sheep. Results from our study demonstrate that while endotoxemic shock is associated with a decrease in circulating factor VIII, this is unaffected by saline resuscitation. Normal saline contains no cells or plasma and it appears to have little active interference on factor VIII activity.
Although there are alterations to the intrinsic pathway with sepsis, studies indicate that the extrinsic pathway is the main pathway through which sepsis activates the coagulation system, via tissue factor/factor VII activation [24, 25]. Results from our study confirm a reduction in factor VII levels although surprisingly there was no difference between the groups. A decrease indicates conversion of factor VII to its active form and thus activation of this factor and of the extrinsic pathway of blood coagulation. This reduction of factor VII may have been due to a combination of consumption and activation as a result of instrumentation and surgery. Endotoxemia + saline animals had a prolonged PT compared to endotoxemia only. EXTEM measurements confirm these results with a prolonged CT and CFT. This coagulation derangement may be a dilutional effect at the time of fluid resuscitation [26] or alternatively it could be linked with the impairment of thrombin formation and fibrin polymerization by the administration of a large crystalloid volume [27]. The fact that these changes persist up to 12 hours post infusion however suggests saline resuscitation has a longer-term impact on hemostasis affecting pro- and anti- coagulant factors of the extrinsic pathway.
Measurement of the common pathway show factor V and X decreased in endotoxemic shock, thus providing evidence for activation and protein consumption of the final pathway of blood coagulation. In the endotoxemia group of animals it is likely that the reduced levels of factors VII and X led to a delay in thrombin generation, thereby leading to decreased levels of factor V and VIII and platelet phospholipids. Our study also confirms that ovine fibrinogen levels correlate well with whole blood fibrinogen function as measured by ROTEM with a decrease in fibrinogen and clot firmness with endotoxemic shock. As the FIBTEM test removes the platelet contribution to clot amplitude, the FIBTEM-MCF provides a direct measure of the quality of the fibrinogen/fibrin based clot, confirming a fibrinogen deficiency or fibrin polymerization disorder in the endotoxemia groups. The results indicate that endotoxemia compromises the host’s ability to recover fibrinogen function. Saline resuscitation did not affect clot firmness, however lead to a prolongation in clot initiation. Whether the host is able to recover baseline levels of fibrinogen level and function after 12 hours remains to be investigated. Fibrinogen is an acute-phase protein, often with normal or even increased plasma levels during the early stages of sepsis and DIC development. Some studies have suggested low levels of fibrinogen can be used to diagnose the late severe consumptive stage of DIC [22].
Levels of the naturally occurring anticoagulant protein C were lower in endotoxemic animals. This impaired function of the anticoagulant system was further aggravated with saline resuscitation with decreases in protein C and antithrombin levels. Reports in the literature have shown low plasma levels of both protein C and antithrombin in septic patients often relates to outcome [28, 29]. The dysfunction in anti-coagulant mechanisms in endotoxemia, in particular antithrombin, may be due to consumption and impaired hepatic synthesis and degradation by elastase from activated neutrophils [30]. Levels of protein C and its cofactor protein S are often reduced in sepsis [31] possibly due to impaired synthesis and degradation by neutrophil elastase with downregulation of thrombomodulin at the endothelial surface, mediated by pro-inflammatory cytokines [30]. There is extensive cross-talk between inflammatory cytokines and coagulation products and previous work by our group show endotoxemia induced increases in TNF-α, IL-1β, IL-8, IL-6 and IL-10 in plasma [32]. Our group has also demonstrated bolus saline resuscitation results in increased vasopressor requirements and may be associated with endothelial glycocalyx degradation, a carbohydrate-rich layer coating the endothelial cell surface [32] suggesting a potential mechanism for these changes. Antithrombin, protein C and tissue pathway factor inhibitor are present in the glycocalyx so its degradation may also compromise anti-coagulant properties [33].
Limitations
The study design allowed us to test the independent effects of endotoxemia and saline resuscitation on haemostasis in a controlled fashion. As with any animal model, species-associated limitations were present in this study as plasma assays (FV, FVII, FVIII, FX, AT, protein C, protein S) were referenced against human calibration plasma with assigned values traceable to National Institute for Biological Standards and Control standards. Secondly, there are differences in ovine secondary haemostasis with sheep having higher levels of FVIII and lower levels of protein C [23]. Thirdly, we only assessed a single fluid with one dose. This limits the conclusions that can be drawn and future studies will be required to establish additional fluid and dose interactions. Finally, this study was limited by lack of data on platelet function, however previous studies show significantly reduced platelet aggregation in patients with sepsis compared with the saline controls [34–36].
Conclusion
In this model of endotoxemic shock, saline resuscitation produced secondary derangements in coagulation compounding the changes seen with endotoxemia alone. Saline resuscitation resulted in delayed initiation of haemostasis with reduced clot firmness, reflecting low platelets and fibrin activity. Moreover, it also reduced levels of naturally occurring anti-coagulants. Both these effects persisted 12 hours after resuscitation suggesting saline resuscitation may produce delayed temporally distant changes in haemostasis, confirming the potential detrimental effect of using saline as a resuscitation fluid. Longer duration studies are required to characterise the haemostatic changes beyond 12 hours. The potential of point-of-care coagulation tests for guiding haemostatic management during sepsis warrants further exploration.
Highlight.
Fluid resuscitation results in prolonged prothrombin time and aPTT
Fluid resuscitation results in decreased levels of antithrombin and Protein C
ROTEM clotting time and clot formation time are prolonged with fluid resuscitation
Funding
This work was supported by the National Health and Medical Research Council (APP1061382) and the Queensland Emergency Medicine Research Foundation (EMPJ-358R25-2016).
Abbreviations
- aPTT
activated partial thromboplastin time
- AT
antithrombin
- CFT
clot formation time
- CT
clotting time
- CVP
central venous pressure
- DIC
disseminated intravascular coagulation
- Hb
haemoglobin
- Hct
haematocrit
- LPS
lipopolysaccharide
- MAP
mean arterial pressure
- MCF
maximum clot firmness
- PLT
platelets
- PT
prothrombin time
- RCC
red cell count
- ROTEM
rotational thromboelastometry
- TEG
thromboelastography
- WCC
white cell count.
Footnotes
Conflict of interest
JFF received funding from the Office of Health and Medical Research, Queensland Health. The remaining authors declare no competing financial interests
Authors’ contributions
MRP, NGO, LB, YLF, ACB, MMS, JPT performed analysis and interpretation; JFF, KM, KS, JPT, YLF conceived and designed the study; LB, NGO, SDD, KRD, MHF, SEP, MRP, ACB, GS performed the animal studies; CMA performed statistical analysis. All authors read and approved the final manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A part of this study was presented at HAA (Haematology Society of Australia and New Zealand, the Australian & New Zealand Society of Blood Transfusion and the Thrombosis and Haemostasis Society of Australia and New Zealand), Sydney, 2017
Contributor Information
Nchafatso G Obonyo, Email: gnchafatso@gmail.com.
Liam Byrne, Email: liambyrne.syd@gmail.com.
Ai-Ching Boon, Email: c.boon0207@gmail.com.
Sara D Diab, Email: sara_diab5@hotmail.com.
Kimble R Dunster, Email: Kimble.Dunster@health.qld.gov.au.
Yoke L Fung, Email: ylfung@usc.edu.au.
Michelle M Spanevello, Email: mmspanevello@gmail.com.
Mohd H Fauzi, Email: hashairi@usm.my.
Sanne E Pedersen, Email: sanne.pedersen.work@gmail.com.
Gabriela Simonova, Email: GSimonova@redcrossblood.org.au.
Chris M Anstey, Email: Chris.Anstey@health.qld.gov.au.
Kiran Shekar, Email: kiran.shekar@health.qld.gov.au.
John-Paul Tung, Email: JTung@redcrossblood.org.au.
Kathryn Maitland, Email: kathryn.maitland@gmail.com.
John F Fraser, Email: j.fraser@uq.edu.au.
References
- [1].Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, Jimenez E, et al. Icon investigators, Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- [2].Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- [4].Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, McGuinness S, Mehrtens J, Myburgh J, Psirides A, Reddy S, et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- [5].Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- [6].Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- [7].Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43:625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- [8].Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, Schroeder ME, Marshall JC, Vincent JL, Intensive I. Care Over Nations, Higher Fluid Balance Increases the Risk of Death From Sepsis: Results From a Large International Audit. Crit Care Med. 2017;45:386–394. doi: 10.1097/CCM.0000000000002189. [DOI] [PubMed] [Google Scholar]
- [9].Levi M. The coagulant response in sepsis. Clin Chest Med. 2008;29:627–642. doi: 10.1016/j.ccm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- [10].Levi M, van der Poll T, Schultz M. New insights into pathways that determine the link between infection and thrombosis. Neth J Med. 2012;70:114–120. [PubMed] [Google Scholar]
- [11].Collins PW, Macchiavello LI, Lewis SJ, Macartney NJ, Saayman AG, Luddington R, Baglin T, Findlay GP. Global tests of haemostasis in critically ill patients with severe sepsis syndrome compared to controls. Br J Haematol. 2006;135:220–227. doi: 10.1111/j.1365-2141.2006.06281.x. [DOI] [PubMed] [Google Scholar]
- [12].Ostrowski SR, Windelov NA, Ibsen M, Haase N, Perner A, Johansson PI. Consecutive thrombelastography clot strength profiles in patients with severe sepsis and their association with 28-day mortality: a prospective study. J Crit Care. 2013;28:317 e311–311. doi: 10.1016/j.jcrc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- [13].Muller MC, Meijers JC, Vroom MB, Juffermans NP. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care. 2014;18:R30. doi: 10.1186/cc13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Byrne L, Obonyo NG, Diab S, Dunster K, Passmore M, Boon AC, Hoe LS, Hay K, Van Haren F, Tung JP, Cullen L, et al. An Ovine Model of Hyperdynamic Endotoxemia and Vital Organ Metabolism. Shock. 2017;49:99–107. doi: 10.1097/SHK.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- [16].Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- [17].Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harr JN, Moore EE, Wohlauer MV, Droz N, Fragoso M, Banerjee A, Silliman CC. The acute coagulopathy of trauma is due to impaired initial thrombin generation but not clot formation or clot strength. J Surg Res. 2011;170:319–324. doi: 10.1016/j.jss.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martini WZ, Pusateri AE, Uscilowicz JM, Delgado AV, Holcomb JB. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58:1002–1009. doi: 10.1097/01.ta.0000156246.53383.9f. [DOI] [PubMed] [Google Scholar]
- [20].Sharma B, Sharma M, Majumder M, Steier W, Sangal A, Kalawar M. Thrombocytopenia in septic shock patients--a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesth Intensive Care. 2007;35:874–880. doi: 10.1177/0310057X0703500604. [DOI] [PubMed] [Google Scholar]
- [21].Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- [22].Velik-Salchner C, Streif W, Innerhofer P, Maier S, Knotzer H, Pajk W, Klingler A, Mittermayr M, Haas T. Endotoxinemia-induced changes in coagulation as measured by rotation thrombelastometry technique and conventional laboratory tests: results of a pilot study on pigs. Blood Coagul Fibrinolysis. 2009;20:41–46. doi: 10.1097/MBC.0b013e32831be9ad. [DOI] [PubMed] [Google Scholar]
- [23].Foley SR, Solano C, Simonova G, Spanevello MM, Bird RJ, Semple JW, Jackson DE, Schibler A, Fraser JF, Fung YL. A comprehensive study of ovine haemostasis to assess suitability to model human coagulation. Thromb Res. 2014;134:468–473. doi: 10.1016/j.thromres.2014.05.026. [DOI] [PubMed] [Google Scholar]
- [24].van der Poll T, Buller HR, ten Cate H, Wortel CH, Bauer KA, van Deventer SJ, Hack CE, Sauerwein HP, Rosenberg RD, ten Cate JW. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322:1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- [25].van Deventer SJ, Buller HR, ten Cate JW, Aarden LA, Hack CE, Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- [26].Coats TJ, Brazil E, Heron M. The effects of commonly used resuscitation fluids on whole blood coagulation. Emerg Med J. 2006;23:546–549. doi: 10.1136/emj.2005.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sorensen B, Fries D. Emerging treatment strategies for trauma-induced coagulopathy. Br J Surg. 2012;99(Suppl 1):40–50. doi: 10.1002/bjs.7770. [DOI] [PubMed] [Google Scholar]
- [28].Mavrommatis AC, Theodoridis T, Economou M, Kotanidou A, El Ali M, Christopoulou-Kokkinou V, Zakynthinos SG. Activation of the fibrinolytic system and utilization of the coagulation inhibitors in sepsis: comparison with severe sepsis and septic shock. Intensive Care Med. 2001;27:1853–1859. doi: 10.1007/s00134-001-1139-8. [DOI] [PubMed] [Google Scholar]
- [29].Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcome in severe sepsis. Chest. 2001;120:915–922. doi: 10.1378/chest.120.3.915. [DOI] [PubMed] [Google Scholar]
- [30].Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39:559–566. doi: 10.1055/s-0033-1343894. [DOI] [PubMed] [Google Scholar]
- [31].Saracco P, Vitale P, Scolfaro C, Pollio B, Pagliarino M, Timeus F. The coagulopathy in sepsis: significance and implications for treatment. Pediatr Rep. 2011;3:e30. doi: 10.4081/pr.2011.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon AC, See Hoe L, Pedersen S, Hashairi Fauzi M, Pretti Pimenta L, Van Haren F, et al. Unintended Consequences; Fluid Resuscitation Worsens Shock in an Ovine Model of Endotoxemia. Am J Respir Crit Care Med. 2018 doi: 10.1164/rccm.201801-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adamzik M, Gorlinger K, Peters J, Hartmann M. Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis. Crit Care. 2012;16:R204. doi: 10.1186/cc11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Reddi BA, Iannella SM, O’Connor SN, Deane AM, Willoughby SR, Wilson DP. Attenuated platelet aggregation in patients with septic shock is independent from the activity state of myosin light chain phosphorylation or a reduction in Rho kinase-dependent inhibition of myosin light chain phosphatase. Intensive Care Med Exp. 2015;3:37. doi: 10.1186/s40635-014-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Woth G, Varga A, Ghosh S, Krupp M, Kiss T, Bogar L, Muhl D. Platelet aggregation in severe sepsis. J Thromb Thrombolysis. 2011;31:6–12. doi: 10.1007/s11239-010-0486-0. [DOI] [PubMed] [Google Scholar]