Abstract
The classification of epilepsy is essential for people with epilepsy and their families, healthcare providers, physicians and researchers. The International League Against Epilepsy proposed updated seizure and epilepsy classifications in 2017, while another four-dimensional epilepsy classification was updated in 2019. An Integrated Epilepsy Classification system was proposed in 2020. Existing classifications, however, lack consideration of important pragmatic factors relevant to the day-to-day life of people with epilepsy and stakeholders. Despite promising developments, consideration of comorbidities in brain development, genetic causes, and environmental triggers of epilepsy remains largely user-dependent in existing classifications. Demographics of epilepsy have changed over time, while existing classification schemes exhibit caveats. A pragmatic classification scheme should incorporate these factors to provide a nuanced classification. Validation across disparate contexts will ensure widespread applicability and ease of use. A team-based approach may simplify communication between healthcare personnel, while an individual-centred perspective may empower people with epilepsy. Together, incorporating these elements into a modern but pragmatic classification scheme may ensure optimal care for people with epilepsy by emphasising cohesiveness among its myriad users. Technological advancements such as 7T MRI, next-generation sequencing, and artificial intelligence may affect future classification efforts.
Keywords: Integrated epilepsy classification, 2017 ILAE classification, Four-dimensional epilepsy classification, Epilepsy
1. Introduction
Epilepsy classification is essential for people with epilepsy, caregivers, healthcare personnel, researchers, policymakers, and insurers [1,2]. Classification allows people with epilepsy to identify with a well-defined condition, empower them, and provide a direction to engage with others. Classification schemes also clarify communication, enhancing care and augmenting education and training [1–3].
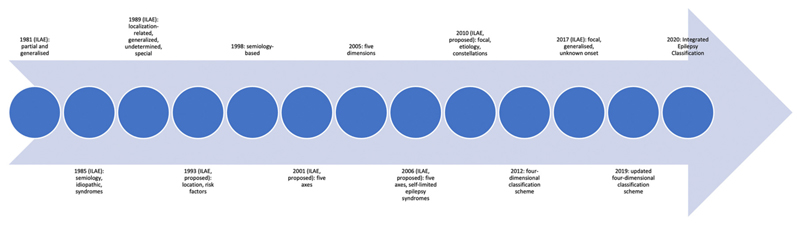
The first classification of seizures and epilepsies was conceived in 1964 and popularised in the 1970’s [2,4,5]. Classifications have been published under the aegis of the International league Against Epilepsy (ILAE) with the most recent updates in 2017 [2,6,7]. In 2001, a multidimensional classification scheme with five axes [8] and in 2012, a four-dimensional classification scheme was proposed [9,10]. An Integrated Epilepsy Classification (IEC) scheme based on commonalities between the ILAE and four-dimensional classification schemes was proposed [11]. Classification continues to evolve while enduring lively debates and having been criticised for its focus on detail and at the same time, lack of inclusiveness and pragmatism [12].
Given the importance of classification, it is crucial to consider the need for further refinement and adaptability. We revisit past and present milestones in epilepsy classification, review past and existing classifications, propose future considerations, and examine rising technologies’ influence. This review provides consideration of previously undervalued factors influencing the debate on the ever-evolving epilepsy classification.
2. Past and current classifications
ILAE published in 1981 its first seizure classification after the development of video-EEG monitoring (Table 1) [13,14]. Seizures were divided into partial and generalised according to onset. The 1985 epilepsies classification scheme was based on semiology with age dependency and etiology in addition to EEG features [14,15]. ILAE revised its proposal in 1989 with categories including localisation-related epilepsies and syndromes, generalised epilepsies and syndromes, undetermined syndromes and special syndromes [14,16]. Contemporaneously, an ILAE expert group also proposed an epidemiologic classification of epilepsies [17]. A diagnostic scheme proposed in 2001 involved five axes mirroring those of the DSM IV: ictal phenomenology, seizure type, syndrome, etiology, and impairment [8,14]. The 2006 update of this scheme further delineated self-limited epilepsy syndromes [1,14,18]. Another proposal (2010) incorporated the concept of brain networks between subcortical and cortical structures and between cortical areas [14,19]. Non-mutually exclusive etiological classifications such as genetic, structural, metabolic, and unknown were created [14,19]. ILAE Commissions generated new classifications for seizures and epilepsies in 2017 (ILAE-EC) involving seizure type, epilepsy types, and etiologies [6,7,14,20].
Table 1. Existing epilepsy classification schemes.
| Organization | Name | Year | Salient features |
|---|---|---|---|
| ILAE | ILAE | 1981 |
|
| 1985 |
|
||
| 1989 |
|
||
| 1993 (proposed) |
|
||
| 2001 (proposed) |
|
||
| 2006 (proposed) |
|
||
| 2010 (proposed) |
|
||
| 2017 |
|
||
| Other | Semiology | 1998 |
|
| Five dimensional | 2005 |
|
|
| 4D-CS | 2012 |
|
|
| 2019 |
|
||
| IEC | 2020 |
|
Integrated Epilepsy Classification (IEC), International League Against Epilepsy (ILAE), four-dimensional classification scheme (4D-CS).
A group of experts proposed a classification entirely based on seizure semiology in 1998 [21–23]. They offered a five-dimensional individual-oriented classification scheme consisting epileptogenic zone location, seizure semiology, etiology, seizure frequency, and related conditions in 2005 [24]. A four-dimensional classification (4D-EC) system consisting of semiology of the seizures, epileptogenic zone location, etiology, and associated comorbidities was proposed in 2012 [9,10]. The updated four-dimensional classification scheme (4D-CS) comprises a sequential approach to categorising non-specific paroxysmal events [10]. The current ILAE classification and 4D-CS were merged into the IEC after considering similarities between the two [11]. The IEC contains five subcategories: header, seizure type, epilepsy type, etiology, and comorbidities and relevant individual preferences [11]. Fig. 1 demonstrates a timeline of past and current classifications.
Fig. 1. Timeline of past and current classifications.
3. The past and present classifications
The intense debate on epilepsy classification is reassuring. Numerous proposals and refutations notwithstanding, the discussions bear out experts’ engagement in the evolution of classification. Limitations, however, have arisen at each step. Some stem from the semantics, syntax and semiotics of seizures. For instance, the term dialeptic seizures has been used in the past to emphasise the phenomenon of behavioural arrest observed in absence seizures and mesial temporal seizures [25]. The term dyscognitive seizures has also been used with diverse connotations by experts, only to be removed in a later version of the classification [6,7,14,20]. Descriptive terminology for mesial temporal seizures has evolved from complex partial seizures to dialeptic seizures, dyscognitive seizures, and focal seizures with or without impaired awareness [6]. The variety of expressions can confuse beginners and prove challenging for the experienced to relearn [25,26]. To the credit of the creators, it is now accepted that any classification should be flexible to meet the needs of different users. There is also a perceived need to explore beyond the boundaries of current classifications to encompass horizons yet not covered.
3.1. Caveats in classifications
Mutually exclusive etiological categories lead to confusion. For example, GLUT1 transporter deficiency may be denoted as genetic or metabolic [11], while neurocysticercosis may be classified as infective or structural. These ambiguities could be addressed by specifying etiological conditions precisely [11]. The classification of a specific condition may, however, have implications for the estimation of the burden of disease. Additionally, classifications have removed anatomical origins due to the imperfect relationship between location and semiology and the electro-clinical similarity between seizures arising from different lobes [1]. The character of seizures, however, bear a relationship to a lobe [27,28]. The ILAE, 4D-EC, and IEC classifications also include syndromes with differing importance [6,7,10,11]. Several population and facility-based studies have examined the yield of the syndromic classification. In these studies (Table 2), syndromes were unambiguously assigned in 4–97% of cases [29–35]. The variation represents the differences between samples assessed, methodologies used and experts’ experience [36–38].
Table 2. Studies describing the yield of ILAE syndromic classifications.
| Type of Study | Study | Country | Classifier(s) | Findings |
|---|---|---|---|---|
| Population-based | Oka et al., 2006 | Japan | Neurologist | • 15.2% of people with epilepsy were classified into syndromic categories |
| Olafsson et al., 2005 | Iceland | Neurologist | • 58% of cases fell into non-informative categories | |
| Wang et al., 2019 | China | Neurologist | • Unknown epilepsy increased from 1.2% with 1985 ILAE classification to 2.8% with 2017 ILAE classification | |
| Primary care-based | Murthy et al., 1998 | India | Neurologists | • 48% of people with epilepsy fell into ILAE categories |
| Tertiary care centrebased | Manford et al., 1992 | United Kingdom | Epileptologist | • 33.6% of people with epilepsy were in diagnostic ILAE categories |
| Kellinghaus et al., 2004 | United States | Epileptologist | • 4% of adults and 21% of children were diagnosed with specific epilepsy syndrome | |
| Gao et al., 2018 | China | Neurologist | • 44.5% of cases were not classified with 1981 ILAE classification, while 7% of cases were not classified with the 2017 ILAE classification |
3.2. Comorbidities
People with epilepsy are up to eight times more likely to have conditions such as depression, anxiety, migraine, heart disease, peptic ulcers, and arthritis relative to the general population [39], and more likely to have other neuropsychiatric disorders, pain disorders, autoimmune diseases and asthma [40,41]. The presence of comorbidities, however, must be assessed across different populations to gain acceptance [41,42]. There seems to be a biological basis for the association of epilepsy with psychosomatic comorbidities [43–47]. Similarly, existing methods of quantifying comorbidities such as the Charlson Comorbidity Index and Elixhauser Comorbidity Index are generally unsuitable for epilepsy [42]. An epilepsy-specific risk adjustment index may be necessary to account for comorbidities properly [42].
Inclusion of comorbidities into classification should be expanded. The 2017 ILAE classification includes comorbidities for the first time [48]. The 4D-CS and IEC included comorbidities, and the IEC asks users to list comorbidities relevant to the individual [9–11]. The incorporation of comorbidities merits further consideration given the occurrence and treatment of comorbidities influences the expression, treatment, and outcome, and vice-versa. Systematic classification of comorbidities should involve creating a master list of known comorbidities while suiting the differing needs of the treatment providers of epilepsy and the comorbidities. Uniform documentation of associated comorbidities diagnoses will permit the identification of appropriate treatments, increase consideration of the impact of comorbidities in epilepsy presentation and its treatment, and improve communication between different specialists.
3.3. Changes in the demography of epilepsy
While current classifications presume a static nature to populations, epilepsy demography has changed. An appropriate representation of these trends may provide a clearer categorisation of individuals with epilepsy.
First, temporal trends are important to examine. Epilepsy incidence is high in the first year of life, perhaps due to to the high proportion of symptomatic cases presenting early in life [49]. Many of these children have epileptic encephalopathies, often with psychomotor arrest that predisposes developmental slowing and seizures later in life [49]. Other children have generalised or focal seizures of the neonatal or infantile period [49]. Medical technology has also contributed to the high first-year incidence as premature children and children with congenital anomalies or severe early life insults survive longer with a higher likelihood of developing epilepsy. Incidence declines by the end of the first decade [50,51]. Decreased exposure to teratogens including some antiseizure medications (ASMs) and environmental risk factors may have enhanced the decrease [52,53]. The incidence increases in the elderly due to cerebrovascular diseases, neurodegenerative disorders, intracerebral tumors, and traumatic brain injury [54–56], although standardisation account for this [55–57]. Second, consideration of changes in the etiology of epilepsy is required. The concept of etiology of epilepsy has changed over time as conceptual models of causality in epilepsy continue to be developed and risk factors continue to be elucidated [58]. Third, the state of epilepsy is relevant, Whether a patient has active epilepsy or epilepsy in remission, controlled or uncontrolled epilepsy, and drug-resistant epilepsy influences the prognosis [59–61]. An adequate epilepsy classification must capture these distinctions to add relevance to clinical practice.
Incorporating the changing demographics of epilepsy will improve communication between specialists, allowing for the greater utility of classification schemes by emphasising the most affected groups.
3.4. Brain age
Brain development stages denoted as “brain age”, measured often as brain-predicted age difference (PAD) [62,63], is probably more relevant than chronological age to epilepsy diagnosis and classification, influencing susceptibility to, presentation, and consequences of seizures. The onset or offset of seizures marks a turning point in brain development, demonstrated by regression of speech with seizure onset in Kleffner-Landau syndrome or resumption of brain maturation with successful seizure control in West syndrome [64,65]. In epileptic encephalopathies, brain maturation arrests at a given time point and might also regress. Therefore, consideration of how brain age or development is reflected in classification is important.
Developing, aging, or degenerating brains are highly susceptible to seizures [66,67]. Brain maturation likely influences seizure semiology. Seizures are featureless, denoted as “hypomotor”, during infancy, plausibly reflecting limited neuronal connectivity, while automatisms and hypermotor seizures occur in older children with presumably mature brains [68]. Lateralizing signs increase with age in people with temporal epilepsy [69]. Epileptic spasms typically occur during infancy, while absence seizures, myoclonic-astatic, and generalised tonic-clonic seizures occur in later childhood [70,71]. Lastly, there is evidence for an interaction between age and pharmacoresistance to ASMs, adversely impacting cognitive development and function in children with epilepsy. Cognitive impairments associated with uncontrolled seizures are particularly severe during infancy and decrease thereafter [72,73]. Individuals with onset of temporal epilepsy in childhood exhibit greater reduction of brain tissue volumes, namely white matter in extratemporal regions, and more marked memory deficits [74].
While the quest for robust markers for brain age continues, it is conceivable that applications of artificial intelligence and machine learning will yield important insights to admit brain age to epilepsy classifications in the future.
3.5. Genetic etiologies
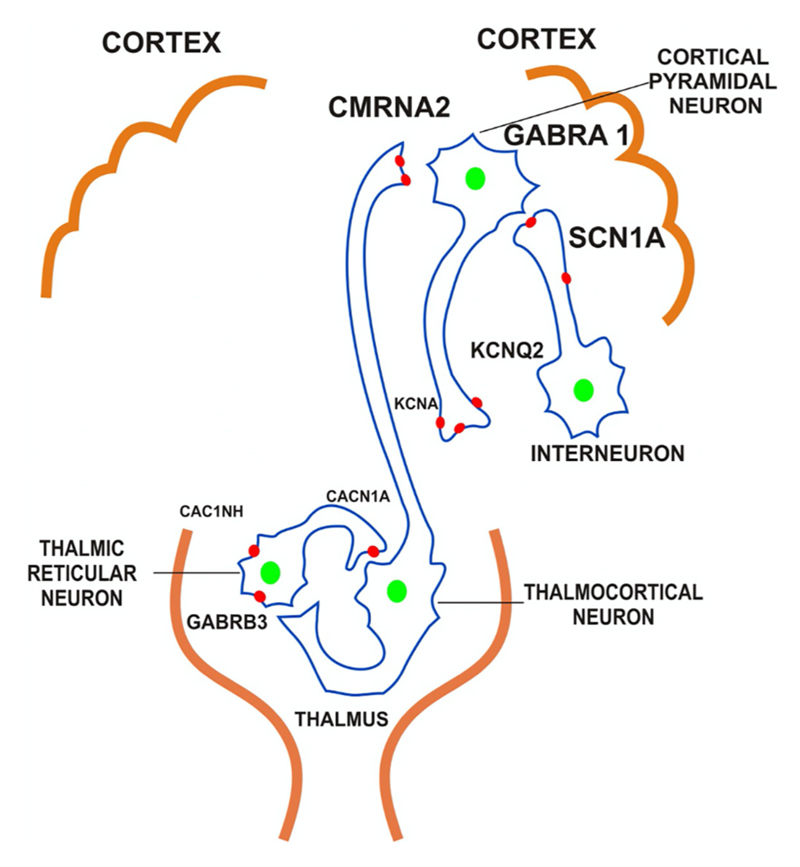
Current classification paradigms incorporate genetic etiologies, but there exists little description of the specific genetic characteristics associated with the diagnosis. Polygenic theory suggests that an accumulation of single nucleotide polymorphisms associated with epilepsy may explain the propensity of certain individuals to develop epilepsy [75]. Conversely, pathogenic variants lead to epilepsy development through several mechanisms. Discovery of genes contributing to epilepsy is rapidly growing. Myriad single gene disorders have been implicated in epilepsy (Table 3) [47,76,77]. Common mechanisms include voltage-gated channelopathies [78–81], ligand-gated channe-lopathies [79,80,82–86], neurotransmitter release machinery [79,87,88], and structural alterations (Table 4) [79,89]. Fig. 2 demonstrates commonly affected channels. Delineation of genetic attributes promotes research to elucidate the natural history of the condition, leads to the development of precision medications, guides treatment paradigms, facilitates preventative measures, and helps individuals with a genetic disorder connect with each other.
Table 3. Single gene disorders implicated in epilepsy.
| Condition | Genes |
|---|---|
| Angelman syndrome | UBE3A |
| Aristaless-relaxed homeobox gene (ARX) disorders | ARX |
| Autosomal dominant epilepsy with auditory features | LG11 |
| Autosomal dominant juvenile myoclonic epilepsy | GABRA1, CACNB4, CLCN2 |
| Autosomal dominant nocturnal frontal lobe epilepsy | CHNRA4, CHNRNB2 |
| Benign familial neonatal convulsion | KCNQ2, KCNQ3 |
| Benign familial neonatal-infantile seizures | SCN2A |
| Dravet syndrome | SCN1A |
| Early onset absence epilepsy | SLC2A1 |
| Generalised epilepsy with febrile seizures plus | SCN1A, SCN2A, SCN2B, GABRG2 |
| Hot water reflex epilepsy | SLC1A1 |
| Juvenile myoclonic epilepsy type 1 | EFHC1 |
| Lafora body disease | EMP2A, NHLRC1 |
| Myoclonic epilepsy with ragged-red fibers (MERRF) | TK, TL1, TH, TS1 |
| Neurofibromatosis | NF1, NF2 |
| Neuronal ceroid-lipofuscinoses / Batten disease | CLN3, CLN5, TPP1 |
| Protocadherin-19 (PCDH 19) related epilepsy | PCDH-19 |
| Rett syndrome | MECP2 |
| Severe myoclonic epilepsy of infancy | SCN1A |
| Sialidosis | NEU1, PCDH19 |
| Tuberous sclerosis | TSC1, TSC2 |
| Unverricht-Lundborg myoclonus epilepsy | CTSB |
Table 4. Common genetic mechanisms for the development of epilepsy.
| Category | Component | Mechanism |
|---|---|---|
| Voltage-gated channelopathies | Na+ channel | Inappropriate activation of current Prolonging activation Incomplete activation of channels Acceleration of recovery from inactivation |
| K+ channel | Prolong neuronal depolarization through slow deactivation, loss of high-frequency bursting, or prolongation of membrane repolarization | |
| Ca2+ channel | Promote neuron synchrony by lowering thresholds for electrogenesis | |
| Ligand-gated channelopathies | GABA channel | Reduction of GABA-activated Cl-current Increase in rate of desensitization |
| Nicotinic ACh receptor | Slowed desensitization | |
| NMDA glutamate receptor | Increased duration of excitation | |
| AMPA glutamate receptor | Initiating excitation | |
| Metabotrobic glutamate receptor | Blockade of accommodation to a steady current Potentiation of effects of NMDA, AMPA, and depolarization |
|
| Serotonin receptor | Loss of inhibitory current | |
| Neurotransmitter release machinery | Synapsins 1 and 2 | Decreased size of presynaptic vesicle pool particularly in inhibitory synapses |
| Sv2A | Sustained release of neurotransmitters | |
| Vesicular zinc sequestration | Neuron hypersynchrony | |
| Reduced recycling | Prolonging activation | |
| Structural | Cortical dysplasias | Inhibited postnatal granule cell proliferation in dentate gyrus Hypertrophy of neocortex Cell migration, segmentation, and patterning reduced Inhibitory neurons reduced or inhibited |
As genetic links for epilepsy are increasingly uncovered, conceptualising classification in terms of genetic causation becomes indispensable. The 4D-EC and IEC permit the use of genetic information, given their emphasis on providing as much detail as available [9–11], but existing classification schemes do not intentionally incorporate genetic etiologies. Investigations such as chromosomal microarrays, whole genome and whole exome sequencing, and gene panels are now increasingly available. These investigations secure a genetic diagnosis and aid in the syndromic and etiological classification despite the cost and access issues. Specific syndromes benefit from contemporary genetic testing. These include epilepsies developing before the age of 2, especially epileptic encephalopathies, suspected and imaging-confirmed brain malformations, and certain inborn errors of metabolism and selected syndromes such as West Syndrome and Dravet Syndrome [90–93]. Undoubtedly, the elucidation of a genetic diagnosis is likely to influence classification elements and systems in the future.
3.6. Environmental triggers
Environmental factors might contribute to susceptibility to and development of epilepsy. Febrile infections herald fever-related syndromes [94,95]. Malnutrition lowers seizure threshold perhaps through hyponatremia, or hypocalcemia [96,97]. Traumatic brain injury may trigger seizures through GABA signaling disinhibition [98,99]. Photosensitivity and altered circadian rhythms may lead to seizures through altered sensory integration [100,101]. Other environmental triggers include various prenatal and postnatal factors [102], though these must be elucidated in further human studies.
Environmental and genetic factors possibly interact to trigger epilepsy [102]. Environmental stimuli may be required to express genes involved in epilepsy or enhance the effect of the susceptibility genotype [102]. This effect appears to differ between acquired and the so-called “genetic epilepsies”. Genetic events influence acquired epilepsies, and the genetic epilepsies are modified by acquired factors [103]. Given their epilepsies role, ion channels may be a mechanism involved in the gene-environment interaction [103]. Environmental and genetic factors may synergistically alter the density, stoichiometry, and post-translational modification of the same ion channels [103]. Acknowledging ecological factors and gene-environment interactions in future classification schemes will allow for greater representation of the etiology and targeted management of people with epilepsy.
4. Future epilepsy classifications
Current classifications fulfill clinical needs through their applicability and adaptability in allowing certain epilepsies to be labelled as unknown. Dimensions that may improve the precision of discussions between and address various users of classification are lacking. Cross-contextual validation of the classification and emphasis on a teambased and individual-centred care are required to develop a comprehensive conceptualisation of care.
4.1. Validation of the classification cross-contextually
Any classification scheme must be applicable to contexts differing in socioeconomic factors, cultures, and practice settings. This is the case in epilepsy as approximately 80% of people with epilepsy live in low-and-middle-income countries (LMICs) [104]. Three-fourths of them do not receive appropriate treatment [105,106]. There is a dearth of epilepsy specialists in LMICs [105–107], so people receive care mainly through primary care, if any. They experience markedly higher premature mortality [108]. Hierarchies of importance placing epilepsy below other chronic conditions also contribute to a greater burden of epilepsy in LMICs.
Epilepsy classification is central in tackling the treatment gap and mortality burden in LMICs. Infectious diseases such as neurocysticercosis, malaria, and encephalitis are common in LMICs, and hence, valid case definitions linking these to epilepsies must be applied [109,110]. Local variations in culturally-specific conceptualisations, manifestations, and epilepsy effects must be incorporated into classification schemes [111]. A study in rural China determined a substantial portion of generalised epilepsy previously characterised were labelled unknown upon the release of the 2017 ILAE-EC [35]. Forms of epilepsy common in LMICs differ from those in high income countries due to unique but often multiple risk factors [112]. Classification systems must be adaptable to different settings by all care providers to communicate effectively [105–107]. Clinicians must prioritise the needs of their population in classifying epilepsy to guide resource allocation [110]. ILAE has provided basic and advanced versions of classifications, but a singular classification scheme with flexibility to address local needs will enable public health efforts and policy [110]. It is also essential to allow classification with minimal or no use of technology [110]. Characteristics such as age of onset, semiology, family history, risk factors, treatment response, and relevant comorbidities can be assessed in clinics [110]. Even in LMICs, classifications should enable flexibility to identify locally relevant factors and resource constraints. These efforts can be coupled with capacity-building with field workers, increased availability of low-cost technology such as telemedicine, and public education campaigns to promote and provide appropriate treatment [113–116]. Ensuring broad applicability of the classification will include more people with epilepsy globally, thereby providing them with proper treatment and help close the treatment gap [105,106].
4.2. Comprehensive team-based approach to epilepsy
A classification system must be coupled with a comprehensive team-based approach to care, research, and policymaking. Specialists create current classifications. Involving primary health care workers such as physicians, nurses, and ancillary staff, as well as researchers, policymakers, and other parties, into discussions will prove productive [117–119]. This will allow for appropriate refinement of specific terms used to describe seizures, create a glossary of key terms with associated definitions, and resolve discrepancies and ambiguities [11]. The classification system must then be clarified to all personnel to increase their understanding [120,121].
Given the need for team-based approach, an elucidation of users’ needs of classification is warranted. Table 5 shows a list of potential users of an epilepsy classification and different levels of informational needs to accommodate all parties involved. Incorporating levels of descriptiveness into the classification will ensure that the communication-related needs of all individuals involved are met. The headline portion should be emphasised as the “lingua franca” among health-care personnel with varying experience, care centers, and across socio-cultural contexts [11]. A linkage to the previous classification systems to preserve continuity and allow for monitoring of trends is also necessary [120]. Professional organisations and educational bodies within existing health-care structures can have primary responsibility in encouraging adoption of the classification [122,123].
Table 5. Potential Users of Epilepsy Classification.
| Informational Need | User | Role |
|---|---|---|
| Sufficient knowledge conveyed in a comprehensible manner | People with epilepsy | Understand condition, treatments, and prognosis; care for oneself; connect with other people with a similar condition |
| Family members / caregivers | Understand condition, treatments, and prognosis; care for their family member; join support groups for family members / caregivers of people with epilepsy | |
| Technical information to provide monitoring and basic care | Electroneurodiagnostic technicians Nurses | Acquiring context for EEG outputs Acquire relevant information and convey accurate information to physicians |
| Specialized descriptions to determine management and provide referrals when appropriate | Primary care physician | Manage the everyday care of people with epilepsy and know when to refer to an epilepsy specialist |
| Further technical information for complex epilepsy care | Neurologist | Diagnose and manage the epilepsy-specific care of people with epilepsy; know when to refer to epilepsy specialist |
| Precise classification language to localize and manage epilepsy | Epilepsy specialist | Diagnose and manage the epilepsy-specific care of people with epilepsy |
| medically or surgically | Neurosurgeon | Decide whether surgical management is warranted, select the surgical technique, and perform surgery |
| Sufficient knowledge to conduct studies | Genetics researcher | Understand the genetics, phenotypic expressions, and variations in both with regard to epilepsy |
| Clear descriptions to guide research | Public health researcher | Understanding the epidemiology and outcomes of types of epilepsy |
| Pharmaceutical manufacturer | Understanding which types of epilepsy require development or refinement of antiseizure medications | |
| Precise delineation of conditions to guide financing and policy | Insurer | Understand how to determine reimbursement for epilepsy care |
| Funding authority | Determine funding priorities for epilepsy research | |
| Policymaker | Understanding the burden and economic consequences of epilepsy |
4.3. Person-centered care
It is important to recognise the centrality of people with epilepsy in efforts to refine classifications. Any classification system must be explained to people and family members or caregivers and incorporate mechanisms to obtain feedback during development. This will increase understanding of the condition, connect with the care team through greater trust and self-involvement in care through acquiring additional information and engaging in self-advocacy [117–119,121]. A classification system must involve consideration of person-centred outcomes and the needs of individuals in addition to traditional measures of disease status [124,125]. Measures of health-related quality of life and personal impact should be acquired to assess how epilepsy or ASMs are impacting individuals [125]. This will guide clinicians regarding possible changes to the frequency of clinic visits, event monitoring or medication regimen or alert them of the need for referral to other physicians. Lastly, the classification scheme should emphasise a holistic approach to care [126]. Epilepsy poses a large logistical and psychological burden to individuals [126–128]. Younger people may experience feelings of apprehension regarding revealing their diagnosis, while age-related metabolic changes often burden adults, cognitive decline, increased risk for seizure-related injuries, extensive comorbidities, and polypharmacy [126,129]. Depression, anxiety, and a lack of social connection with other individuals are common [126,129]. Provision of care appropriate to the specific concerns of people with epilepsy, psychosocial interventions to increase self-efficacy and locus of control, and measures to enhance social support may empower people with epilepsy [127,128].
5. Technologies likely to impact future classifications
Consideration of the effect of seven Tesla (7 T) magnetic resonance (MRI), next-generation sequencing (NGS), and artificial intelligence and machine learning on classification is required to reduce the fraction of unknown epilepsies and enhance the versatility of classification as future technological developments change clinical practice.
5.1. 7T MRI
The 2017 ILAE classification scheme reclassified 27% of generalised and 7% of focal cryptogenic epilepsies into epilepsies of unknown type in one study [130]. There is a need to reduce the proportion of unknown epilepsies further. Seven Tesla MRI with increased spatial resolution allowing visualisation of internal structures and differentiation of pathological tissue from normal tissue might conceivably help in reducing this proportion [131–134]. 7 T provides for detection of lesions previously undiscovered [135–137]. Unaided review of 7 T images reveals previously unseen lesions in 22% of cases, while utilising a morphometric analysis program raises this proportion to 43% [135]. 7 T-morphometric analysis uncovers a quarter more lesions than 3 T morphometrics [135].
Additionally, 7 T allows characterisation of focal cortical dysplasia and hippocampal sclerosis and volumetric analysis of epilepsy-related brain regions [131,132,138–141]. The efficacy of 7T MRI in epilepsy classification relative to 3T MRI is yet to be fully assessed. The 4D-EC and IEC allow for incorporating imaging findings [9–11], but there is no concerted effort. However, the increased utilisation of 7 T may enable precise classification by distinguishing epilepsy types and etiologies, thereby reducing the proportion of unknown epilepsies.
5.2. Genome sequencing
NGS has markedly increased the speed of genome sequencing [142–144]. The ability of NGS to find causal mutations, including de novo mutations, associated with epilepsy syndromes enhances molecular diagnosis [145]. NGS is beneficial to identify genetic causes in people with earlier seizure onset, and a family history [146,147]. Currently, available gene panels exhibit substantial variability, ascertaining up to 265 genes with reported diagnostic yields up to 48.5% [148]. NGS may be unable to determine the precise genetic etiology for epilepsies with polygenic inheritance [149] but its utility extends beyond genetic factors. NGS uncovers inherited metabolic disorders in 13% of people with normal metabolic investigations [150]. NGS has the potential to refine metabolic, infective, and autoimmune causes by identifying genetic alterations associated with these etiological categories. Similarly, NGS enhances understanding of pathogenesis through genotype-phenotype correlations, allowing for refined diagnosis [151]. Through the inclusion of genes associated with epilepsy and the possibility of discovering novel mutations, greater adoption of NGS may improve classification by comprehensively characterising genetic factors, catalysing reclassification of unknown epilepsies into well-delineated categories.
5.3. Artificial intelligence and machine learning
The use of artificial intelligence and machine learning in epilepsy has grown substantially [152,153]. Artificial neural networks have been utilised in tandem with multiwavelet transform techniques to diagnose epilepsy with high accuracy, sensitivity, and specificity based on EEG data [154–159]. Artificial intelligence and machine learning have also been used to localize seizure onset zones from EEG data [160–164]. For example, an unsupervised algorithm can collate the localisation of epileptiform discharges over a day into a single map [164]. Recording periods of less than two hours may enable clinically meaningful characterisation of seizure onset zone [162]. Recently, artificial intelligence and machine learning approaches have examined seizure classification [165–169]. A text mining approach based on ICD-9 yielded good performance in detecting complex focal seizure, simple focal seizure, and convulsive epilepsy based on data from the electronic medical record [166]. It is possible to distinguish temporal from extratemporal seizure by extracting spatiotemporal features from facial and pose semiology from EEG-records [167]. Studies analysing EEG data with multiple extraction methods have found high accuracy, sensitivity, and specificity [168,169]. Development of capabilities to differentiate a more significant number of seizure types, identify associated pathology and probable etiology, and characterise epilepsies based on multimodal inputs may enable delineation of previously unrecognised factors to clarify ambiguities in classification, create additional classes, and reduce the proportion of unknowns.
6. Conclusions
Epilepsy classification is evolving with promising recent developments. Incorporating stages of brain development, genetic and environmental triggers, and changes in the demography into a modernised classification is necessary. Validation of this classification in different socioeconomic status contexts and coupling with a team-based approach and person-centred perspective is also required. These factors may ensure optimal care by addressing increasing the ease and precision of communication between the myriad of individuals who utilise the epilepsy classification. Technological advances, including 7T MRI, genome sequencing, and artificial intelligence, may prove helpful in improving future epilepsy classification.
Fig. 2. Channels commonly affected in epilepsy.
Acknowledgements
We acknowledge Naveen Bhatia for assistance with the illustration. JWS is based at University College London Hospital Comprehensive Biomedical Research Centre, which receives a proportion of funding from the UK Department of Health’s National Institute for Health Research centres funding scheme; he also receives research support from the National Dr. Marvin Weil Epilepsy Research Fund, UK Epilepsy Society, and Christelijke Vereniging voor de Verplegingvan Lijders aan Epilepsie (Netherlands).
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of Competing Interest
NAS and CRN having no disclosure to make. GS reports personal fees from Sanofi India and the Department of Biotechnology outside the submitted work. JWS reports personal fees from Eisai, UCB Pharma, Arvelle and Zogenix Pharma; and grants from Eisai, UCB Pharma, National Epilepsy Funds (Netherlands), National Institute for Health Research and GW Pharma, outside the submitted work.
References
- [1].Engel J., Jr ILAE classification of epilepsy syndromes. Epilepsy Res. 2006;70:5–10. doi: 10.1016/j.eplepsyres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- [2].Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018;139:73–79. doi: 10.1016/j.eplepsyres.2017.11.015. [DOI] [PubMed] [Google Scholar]
- [3].Zhang GQ, Sahoo SS, Lhatoo SD. From classification to epilepsy ontology and informatics. Epilepsia. 2012;53:28–32. doi: 10.1111/j.1528-1167.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caveness W, Lorentz Hjm Am, Radermecker J. A proposed international classification of epileptic seizures. Epilepsia. 1964;5:297–306. doi: 10.1111/j.1528-1157.1964.tb03337.x. [DOI] [PubMed] [Google Scholar]
- [5].Gastaut H. Clinical and electroencephalographical classification of epileptic seizures. Epilepsia. 1970;11:102–112. doi: 10.1111/j.1528-1157.1970.tb03871.x. [DOI] [PubMed] [Google Scholar]
- [6].Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- [7].Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshé SL. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- [9].Lüders HO, Amina S, Baumgartner C, Benbadis S, Bermeo-Ovalle A, Devereaux M, Diehl B, Edwards J, Baca-Vaca GF, Hamer H. Modern technology calls for a modern approach to classification of epileptic seizures and the epilepsies. Epilepsia. 2012;53:405–411. doi: 10.1111/j.1528-1167.2011.03376.x. [DOI] [PubMed] [Google Scholar]
- [10].Lüders H, Vaca GFB, Akamatsu N, Amina S, Arzimanoglou A, Baumgartner C, Benbadis SR, Bleasel A, Bermeo-Ovalle A, Bozorgi A. Classification of paroxysmal events and the four-dimensional epilepsy classification system. Epileptic Disorders. 2019;21:1–29. doi: 10.1684/epd.2019.1033. [DOI] [PubMed] [Google Scholar]
- [11].Rosenow F, Akamatsu N, Bast T, Bauer S, Baumgartner C, Benbadis S, Bermeo-Ovalle A, Beyenburg S, Bleasel A, Bozorgi A. Could the 2017 ILAE and the Four-Dimensional Epilepsy Classifications Be Merged to a New “Integrated Epilepsy Classification”? Seizure. 2020 doi: 10.1016/j.seizure.2020.02.018. [DOI] [PubMed] [Google Scholar]
- [12].Scheffer IE. Epilepsy: a classification for all seasons? Epilepsia. 2012;53:6–9. doi: 10.1111/j.1528-1167.2012.03551.x. [DOI] [PubMed] [Google Scholar]
- [13].Angeles D. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- [14].Chang RS-K, Leung CYW, Ho CCA, Yung A. Classifications of seizures and epilepsies, where are we?–a brief historical review and update. J Formos Med Assoc. 2017;116:736–741. doi: 10.1016/j.jfma.2017.06.001. [DOI] [PubMed] [Google Scholar]
- [15].Dreifuss F, Martinez-Lage M, Johns RA. Proposal for classification of epilepsies and epileptic syndromes. Epilepsia. 1985;26:268–278. [PubMed] [Google Scholar]
- [16].Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- [17].Epidemiology Co, Prognosis ILAE. Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- [18].Engel J., Jr Report of the ILAE classification core group. Epilepsia. 2006;47:1558–1568. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- [19].Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW. Revised terminology and concepts for organisation of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005-2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- [20].Fisher RS, Cross JH, D’souza C, French JA, Haut SR, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531–542. doi: 10.1111/epi.13671. [DOI] [PubMed] [Google Scholar]
- [21].Lüders HO, Burgess R, Noachtar S. Expanding the international classification of seizures to provide localisation information. Neurology. 1993;43:1650–1655. doi: 10.1212/wnl.43.9.1650. [DOI] [PubMed] [Google Scholar]
- [22].Lüders H, Acharya J, Baumgartner C, Benbadis S, Bleasel A, Burgess R, Dinner D, Ebner A, Foldvary N, Geller E. Semiological seizure classification. Epilepsia. 1998;39:1006–1013. doi: 10.1111/j.1528-1157.1998.tb01452.x. [DOI] [PubMed] [Google Scholar]
- [23].Lüders H, Acharya J, Baumgartner C, Benbadis S, Bleasel A, Burgess R, Dinner D, Ebner A, Foldvary N, Geller E. A new epileptic seizure classification based exclusively on ictal semiology. Acta Neurol Scand. 1999;99:137–141. doi: 10.1111/j.1600-0404.1999.tb07334.x. [DOI] [PubMed] [Google Scholar]
- [24].Loddenkemper T, Kellinghaus C, Wyllie E, Najm IM, Gupta A, Rosenow F, Lüders HO. A proposal for a five-dimensional patient-oriented epilepsy classification. Epileptic Disorders. 2005;7:308–316. [PubMed] [Google Scholar]
- [25].Lüders H, Amina S, Bailey C, Baumgartner C, Benbadis S, Bermeo A, Carreño M, Devereaux M, Diehl B, Eccher M. Proposal: different types of alteration and loss of consciousness in epilepsy. Epilepsia. 2014;55:1140–1144. doi: 10.1111/epi.12595. [DOI] [PubMed] [Google Scholar]
- [26].Blumenfeld H, Meador KJ. Consciousness as a useful concept in epilepsy classification. Epilepsia. 2014;55:1145–1150. doi: 10.1111/epi.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ramantani G, Dümpelmann M, Koessler L, Brandt A, Cosandier-Rimélé D, Zentner J, Schulze-Bonhage A, Maillard LG. Simultaneous subdural and scalp EEG correlates of frontal lobe epileptic sources. Epilepsia. 2014;55:278–288. doi: 10.1111/epi.12512. [DOI] [PubMed] [Google Scholar]
- [28].Bartolomei F, Cosandier-Rimele D, McGonigal A, Aubert S, Régis J, Gavaret M, Wendling F, Chauvel P. From mesial temporal lobe to temporoperisylvian seizures: a quantified study of temporal lobe seizure networks. Epilepsia. 2010;51:2147–2158. doi: 10.1111/j.1528-1167.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- [29].Manford M, Hart YM, Sander JW, Shorvon SD. The National General Practice Study of Epilepsy: the syndromic classification of the international league against Epilepsy applied to epilepsy in a general population. Arch Neurol. 1992;49:801–808. doi: 10.1001/archneur.1992.00530320025008. [DOI] [PubMed] [Google Scholar]
- [30].Murthy J, Yangala R, Srinivas M. The syndromic classification of the international league against Epilepsy: a hospital-based study from South India. Epilepsia. 1998;39:48–54. doi: 10.1111/j.1528-1157.1998.tb01273.x. [DOI] [PubMed] [Google Scholar]
- [31].Oka E, Ohtsuka Y, Yoshinaga H, Murakami N, Kobayashi K, Ogino T. Prevalence of childhood epilepsy and distribution of epileptic syndromes: a population-based survey in Okayama, Japan. Epilepsia. 2006;47:626–630. doi: 10.1111/j.1528-1167.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- [32].Kellinghaus C, Loddenkemper T, Najm IM, Wyllie E, Lineweaver T, Nair DR, Lüders HO. Specific epileptic syndromes are rare even in tertiary epilepsy centers: a patient-oriented approach to epilepsy classification. Epilepsia. 2004;45:268–275. doi: 10.1111/j.0013-9580.2004.36703.x. [DOI] [PubMed] [Google Scholar]
- [33].Olafsson E, Ludvigsson P, Hesdorffer D, Kjartansson O, Hauser WA, Gudmundsson G. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurol. 2005;4:627–634. doi: 10.1016/S1474-4422(05)70172-1. [DOI] [PubMed] [Google Scholar]
- [34].Gao H, Sander JW, Xiao Y, Zhang Y, Zhou D. A comparison between the 1981 and 2017 international league against Epilepsy classification of seizure types based on an outpatient setting. Epileptic Disorders. 2018;20:257–264. doi: 10.1684/epd.2018.0982. [DOI] [PubMed] [Google Scholar]
- [35].Wang F, Chen Z, Davagnanam I, Hoskote C, Ding D, Wang W, Yang B, Wang Y, Wang T, Li W. Comparing two classification schemes for seizures and epilepsy in rural China. Eur J Neurol. 2019;26:422–427. doi: 10.1111/ene.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Berg AT, Levy SR, Testa FM, Shinnar S. Classification of childhood epilepsy syndromes in newly diagnosed epilepsy: interrater agreement and reasons for disagreement. Epilepsia. 1999;40:439–444. doi: 10.1111/j.1528-1157.1999.tb00738.x. [DOI] [PubMed] [Google Scholar]
- [37].Aicardi J. Syndromic classification in the management of childhood epilepsy. J Child Neurol. 1994;9:2S14-2S18. [PubMed] [Google Scholar]
- [38].Eriksson K, Koivikko M. Prevalence, classification, and severity of epilepsy and epileptic syndromes in children. Epilepsia. 1997;38:1275–1282. doi: 10.1111/j.1528-1157.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- [39].Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15:106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- [40].Ottman R, Lipton RB, Ettinger AB, Cramer JA, Reed ML, Morrison A, Wan GJ. Comorbidities of epilepsy: results from the Epilepsy comorbidities and health (EPIC) survey. Epilepsia. 2011;52:308–315. doi: 10.1111/j.1528-1167.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- [41].Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- [42].St Germaine-Smith C, Liu M, Quan H, Wiebe S, Jette N. development of an epilepsy-specific risk adjustment comorbidity index. Epilepsia. 2011;52:2161–2167. doi: 10.1111/j.1528-1167.2011.03292.x. [DOI] [PubMed] [Google Scholar]
- [43].Gaitatzis A, Sisodiya SM, Sander JW. The somatic comorbidity of epilepsy: a weighty but often unrecognised burden. Epilepsia. 2012;53:1282–1293. doi: 10.1111/j.1528-1167.2012.03528.x. [DOI] [PubMed] [Google Scholar]
- [44].Shlobin NA, Sander JW. Drivers for the comorbidity of type 2 diabetes mellitus and epilepsy: a scoping review. Epilepsy Behav. 2020;106:107043. doi: 10.1016/j.yebeh.2020.107043. [DOI] [PubMed] [Google Scholar]
- [45].Shlobin NA, Sander JW. Metabolic issues in people with epilepsy. Epilepsy Behavior. 2020;108:107081. doi: 10.1016/j.yebeh.2020.107081. [DOI] [PubMed] [Google Scholar]
- [46].Schenkel LC, Bragatti JA, Becker JA, Torres CM, Martin KC, de Souza AC, Manfro GG, Leistner-Segal S, Bianchin MM. Serotonin gene polymorphisms and psychiatry comorbidities in temporal lobe epilepsy. Epilepsy Res. 2012;99:260–266. doi: 10.1016/j.eplepsyres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- [47].Noebels JL. Single-gene determinants of epilepsy comorbidity. Cold Spring Harbor Perspect Med. 2015;5:a022756. doi: 10.1101/cshperspect.a022756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Brodie MJ, Zuberi SM, Scheffer IE, Fisher RS. The 2017 ILAE classification of seizure types and the epilepsies: what do people with epilepsy and their caregivers need to know? Epileptic Disorders. 2018;20:77–87. doi: 10.1684/epd.2018.0957. [DOI] [PubMed] [Google Scholar]
- [49].Kramer U. Topical review: Epilepsy in the first year of life: a review. J Child Neurol. 1999;14:485–489. doi: 10.1177/088307389901400801. [DOI] [PubMed] [Google Scholar]
- [50].Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disorders. 2015;17:117–123. doi: 10.1684/epd.2015.0736. [DOI] [PubMed] [Google Scholar]
- [51].Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon C-S, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88:296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tomson T, Xue H, Battino D. Major congenital malformations in children of women with epilepsy. Seizure. 2015;28:46–50. doi: 10.1016/j.seizure.2015.02.019. [DOI] [PubMed] [Google Scholar]
- [53].Samren E, Van Duijn C, Koch S, Hiilesmaa V, Klepel H, Bardy A, Mannagetta GB, Deichl A, Gaily E, Granström I. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- [54].Liu S, Yu W, Lü Y. The causes of new-onset epilepsy and seizures in the elderly. Neuropsychiatr Dis Treat. 2016;12:1425. doi: 10.2147/NDT.S107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Everitt AD, Sander JW. Incidence of epilepsy is now higher in elderly people than children. BMJ. 1998;316:780. doi: 10.1136/bmj.316.7133.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sander J, Cockerell O, Hart Y, Shorvon S. Is the incidence of epilepsy falling in the UK? Lancet. 1993;342:874. doi: 10.1016/0140-6736(93)92737-e. [DOI] [PubMed] [Google Scholar]
- [57].Leppik IE, Birnbaum AK. Epilepsy in the elderly. Ann N Y Acad Sci. 2010;1184:208. doi: 10.1111/j.1749-6632.2009.05113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shorvon SD. The causes of epilepsy: changing concepts of etiology of epilepsy over the past 150 years. Epilepsia. 2011;52:1033–1044. doi: 10.1111/j.1528-1167.2011.03051.x. [DOI] [PubMed] [Google Scholar]
- [59].Cockerell OC, Johnson AL, Sander JW, Shorvon SD. Prognosis of epilepsy: a review and further analysis of the first nine years of the British National General Practice Study of Epilepsy, a prospective population-based study. Epilepsia. 1997;38:31–46. doi: 10.1111/j.1528-1157.1997.tb01075.x. [DOI] [PubMed] [Google Scholar]
- [60].Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365:919–926. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- [61].Hao X, Goldberg D, Kelly K, Stephen L, Kwan P, Brodie MJ. Uncontrolled epilepsy is not necessarily the same as drug-resistant epilepsy: differences between populations with newly diagnosed epilepsy and chronic epilepsy. Epilepsy Behav. 2013;29:4–6. doi: 10.1016/j.yebeh.2013.06.019. [DOI] [PubMed] [Google Scholar]
- [62].Sone D, Beheshti I, Maikusa N, Ota M, Kimura Y, Sato N, Koepp M, Matsuda H. Neuroimaging-based brain-age prediction in diverse forms of epilepsy: a signature of psychosis and beyond. Mol Psychiatry. 2019:1–10. doi: 10.1038/s41380-019-0446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen C-L, Shih Y-C, Liou H-H, Hsu Y-C, Lin F-H, Tseng W-YI. Premature white matter aging in patients with right mesial temporal lobe epilepsy: a machine learning approach based on diffusion MRI data. NeuroImage Clin. 2019;24:102033. doi: 10.1016/j.nicl.2019.102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Stefanatos GA, Kinsbourne M, Wasserstein J. Acquired epileptiform aphasia: a dimensional view of Landau-Kleffner syndrome and the relation to regressive autistic spectrum disorders. Child Neuropsychol. 2002;8:195–228. doi: 10.1076/chin.8.3.195.13498. [DOI] [PubMed] [Google Scholar]
- [65].Hancock EC, Osborne JP, Edwards SW. Treatment of infantile spasms. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD001770.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- [67].Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy—a review. Epilepsy Res. 2009;85:31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Källén K, Wyllie E, Lüders HO, Lachhwani D, Kotagal P. Hypomotor seizures in infants and children. Epilepsia. 2002;43:882–888. doi: 10.1046/j.1528-1157.2002.16301.x. [DOI] [PubMed] [Google Scholar]
- [69].Fogarasi A, Tuxhorn I, Janszky J, Janszky I, Rásonyi G, Kelemen A, Halász P. Age-dependent seizure semiology in temporal lobe epilepsy. Epilepsia. 2007;48:1697–1702. doi: 10.1111/j.1528-1167.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- [70].Nordli DR. Handbook of Clinical Neurology. Elsevier; 2013. Varying seizure semiology according to age; pp. 455–460. [DOI] [PubMed] [Google Scholar]
- [71].Noachtar S, Peters AS. Semiology of epileptic seizures: a critical review. Epilepsy Behav. 2009;15:2–9. doi: 10.1016/j.yebeh.2009.02.029. [DOI] [PubMed] [Google Scholar]
- [72].Berg AT, Zelko FA, Levy SR, Testa FM. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology. 2012;79:1384–1391. doi: 10.1212/WNL.0b013e31826c1b55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schmid R, Tandon P, Stafstrom CE, Holmes GL. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999;53:1754–1761. doi: 10.1212/wnl.53.8.1754. [DOI] [PubMed] [Google Scholar]
- [74].Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, Wendt G, O’Leary D, Magnotta V. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- [75].Ferraro TN, Buono RJ. Polygenic epilepsy. Adv Neurol. 2006;97:389. [PubMed] [Google Scholar]
- [76].Merwick A, O’Brien M, Delanty N. Complex single gene disorders and epilepsy. Epilepsia. 2012;53:81–91. doi: 10.1111/j.1528-1167.2012.03617.x. [DOI] [PubMed] [Google Scholar]
- [77].Steinlein OK. Genetics and epilepsy. Dialogues Clin Neurosci. 2008;10:29. doi: 10.31887/DCNS.2008.10.1/oksteinlein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Noebels J. Pathway-driven discovery of epilepsy genes. Nat Neurosci. 2015;18:344. doi: 10.1038/nn.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- [80].Scheffer IE, Berkovic SF. The genetics of human epilepsy. Trends Pharmacol Sci. 2003;24:428–433. doi: 10.1016/S0165-6147(03)00194-9. [DOI] [PubMed] [Google Scholar]
- [81].Jouvenceau A, Eunson LH, Spauschus A, Ramesh V, Zuberi SM, Kullmann DM, Hanna MG. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet. 2001;358:801–807. doi: 10.1016/S0140-6736(01)05971-2. [DOI] [PubMed] [Google Scholar]
- [82].Marini C, Guerrini R. The role of the nicotinic acetylcholine receptors in sleep-related epilepsy. Biochem Pharmacol. 2007;74:1308–1314. doi: 10.1016/j.bcp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- [83].Chapman AG. Glutamate and epilepsy. J Nutr. 2000;130:1043S–1045S. doi: 10.1093/jn/130.4.1043S. [DOI] [PubMed] [Google Scholar]
- [84].Rogawski MA. AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol Scand. 2013;127:9–18. doi: 10.1111/ane.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100:857–873. doi: 10.1111/j.1471-4159.2006.04277.x. [DOI] [PubMed] [Google Scholar]
- [86].Ghasemi M, Schachter SC. The NMDA receptor complex as a therapeutic target in epilepsy: a review. Epilepsy Behav. 2011;22:617–640. doi: 10.1016/j.yebeh.2011.07.024. [DOI] [PubMed] [Google Scholar]
- [87].Garcia C, Blair H, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship J. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet. 2004;41:183–186. doi: 10.1136/jmg.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Upreti C, Otero R, Partida C, Skinner F, Thakker R, Pacheco LF, Zhou Z-y, Maglakelidze G, Velísková J, Velísek L. Altered neurotransmitter release, vesicle recycling and presynaptic structure in the pilocarpine model of temporal lobe epilepsy. Brain. 2012;135:869–885. doi: 10.1093/brain/awr341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang VY, Chang EF, Barbaro NM. Focal cortical dysplasia: a review of pathological features, genetics, and surgical outcome. Neurosurg Focus. 2006;20:1–7. doi: 10.3171/foc.2006.20.1.8. [DOI] [PubMed] [Google Scholar]
- [90].McTague A, Howell KB, Cross JH, Kurian MA, Scheffer IE. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 2016;15:304–316. doi: 10.1016/S1474-4422(15)00250-1. [DOI] [PubMed] [Google Scholar]
- [91].Scheffer IE, Wallace R, Mulley JC, Berkovic SF. Clinical and molecular genetics of myoclonic–astatic epilepsy and severe myoclonic epilepsy in infancy (Dravet syndrome) Brain and Development. 2001;23:732–735. doi: 10.1016/s0387-7604(01)00272-8. [DOI] [PubMed] [Google Scholar]
- [92].Burgess R, Wang S, McTague A, Boysen KE, Yang X, Zeng Q, Myers KA, Rochtus A, Trivisano M, Gill D. The genetic landscape of epilepsy of infancy with migrating focal seizures. Ann Neurol. 2019;86:821–831. doi: 10.1002/ana.25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Berg AT, Chakravorty S, Koh S, Grinspan ZM, Shellhaas RA, Saneto RP, Wirrell EC, Coryell J, Chu CJ, Mytinger JR. Why west? Comparisons of clinical, genetic and molecular features of infants with and without spasms. PLoS One. 2018;13:e0193599. doi: 10.1371/journal.pone.0193599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kramer U, Chi CS, Lin KL, Specchio N, Sahin M, Olson H, Nabbout R, Kluger G, Lin JJ, Van Baalen A. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. 2011;52:1956–1965. doi: 10.1111/j.1528-1167.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- [95].Van Baalen A, Häusler M, Boor R, Rohr A, Sperner J, Kurlemann G, Panzer A, Stephani U, Kluger G. Febrile infection–related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia. 2010;51:1323–1328. doi: 10.1111/j.1528-1167.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- [96].Hackett R, Iype T. Malnutrition and childhood epilepsy in developing countries. Seizure. 2001;10:554–558. doi: 10.1053/seiz.2001.0532. [DOI] [PubMed] [Google Scholar]
- [97].Crepin S, Houinato D, Nawana B, Avode GD, Preux PM, Desport JC. Link between epilepsy and malnutrition in a rural area of Benin. Epilepsia. 2007;48:1926–1933. doi: 10.1111/j.1528-1167.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- [98].Jennett B. Trauma as a cause of epilepsy in childhood. Dev Med Child Neurol. 1973;15:56–62. doi: 10.1111/j.1469-8749.1973.tb04866.x. [DOI] [PubMed] [Google Scholar]
- [99].Benardo LS. Prevention of epilepsy after head trauma: do we need new drugs or a new approach? Epilepsia. 2003;44:27–33. doi: 10.1046/j.1528-1157.44.s10.2.x. [DOI] [PubMed] [Google Scholar]
- [100].Harding GF, Jeavons PM. Photosensitive Epilepsy. Cambridge University Press; 1994. [Google Scholar]
- [101].Hofstra WA. The circadian rhythm and its interaction with human epilepsy: a review of literature. Sleep Med Rev. 2009;13:413–420. doi: 10.1016/j.smrv.2009.01.002. [DOI] [PubMed] [Google Scholar]
- [102].Ottman R, Annegers JF, Risch N, Hauser WA, Susser M. Relations of genetic and environmental factors in the etiology of epilepsy. Ann Neurol. 1996;39:442–449. doi: 10.1002/ana.410390406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29:391–397. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- [104].Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49:1491–1503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Meyer A-C, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ. 2010;88:260–266. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Meinardi H, Scott R, Reis R. On Behalf Of The Ilae Commission on the Developing World JS, The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2001;42:136–149. doi: 10.1046/j.1528-1157.2001.32800.x. [DOI] [PubMed] [Google Scholar]
- [108].Levira F, Thurman DJ, Sander JW, Hauser WA, Hesdorffer DC, Masanja H, Odermatt P, Logroscino G, Newton CR. Epilepsy ECotILA, Premature mortality of epilepsy in low-and middle-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58:6–16. doi: 10.1111/epi.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet. 2012;380:1193–1201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- [110].Birbeck GL. Revising and refining the epilepsy classification system: priorities from a developing world perspective. Epilepsia. 2012;53:18–21. doi: 10.1111/j.1528-1167.2012.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kaddumukasa M, Kaddumukasa MN, Buwembo W, Munabi IG, Blixen C, Lhatoo S, Sewankambo N, Katabira E, Sajatovic M. Epilepsy misconceptions and stigma reduction interventions in sub-Saharan Africa, a systematic review. Epilepsy Behav. 2018;85:21–27. doi: 10.1016/j.yebeh.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Everitt AD, Sander J. Classification of the epilepsies: time for a change? Eur Neurol. 1999;42:1–10. doi: 10.1159/000008061. [DOI] [PubMed] [Google Scholar]
- [113].Van Hees VT, Van Diessen E, Sinke MR, Buitenhuis JW, Van Der Maas F, Ridder L, Otte WM. Reliable and automatic epilepsy classification with affordable, consumer-grade electroencephalography in rural sub-Saharan Africa. BioRxiv. 2018:324954 [Google Scholar]
- [114].Rajbhandari H, Joshi S, Malakar S, Paudel P, Jain P, Uppadaya K, Singh M, Patterson V. Epilepsy field workers, a smartphone application and telephone telemedicine: safe and effective epilepsy care in rural Nepal. Seizure. 2019;64:54–58. doi: 10.1016/j.seizure.2018.12.005. [DOI] [PubMed] [Google Scholar]
- [115].Guekht A, Zharkinbekova N, Shpak A, Hauser WA. Epilepsy and treatment gap in urban and rural areas of the southern Kazakhstan in adults. Epilepsy Behav. 2017;67:98–104. doi: 10.1016/j.yebeh.2016.11.028. [DOI] [PubMed] [Google Scholar]
- [116].Hunter E, Rogathi J, Chigudu S, Jusabani A, Jackson M, Whittaker RG, Gray W, McNally RJ, Aris E, Mushi D. The epilepsy treatment gap in rural Tanzania: a community-based study in adults. Seizure. 2016;36:49–56. doi: 10.1016/j.seizure.2016.02.008. [DOI] [PubMed] [Google Scholar]
- [117].Hill CE, Thomas B, Sansalone K, Davis KA, Shea JA, Litt B, Dahodwala N. Improved availability and quality of care with epilepsy nurse practitioners. Neurol Clin Pract. 2017;7:109–117. doi: 10.1212/CPJ.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Higgins A, Downes C, Varley J, Tyrell E, Normand C, Doherty CP, Begley C, Elliott N. Patients with epilepsy care experiences: comparison between services with and without an epilepsy specialist nurse. Epilepsy Behav. 2018;85:85–94. doi: 10.1016/j.yebeh.2018.05.038. [DOI] [PubMed] [Google Scholar]
- [119].Fujimoto A, Ichikawa N, Sato K, Nishimura M, Enoki H, Okanishi T. Inclusion of general physicians in the multidisciplinary treatment team for epilepsy may lead to an economic benefit. Epilepsy Behav. 2019;95:56–60. doi: 10.1016/j.yebeh.2019.03.041. [DOI] [PubMed] [Google Scholar]
- [120].Beghi E, Sander JW. The ILAE classification of seizures and epilepsies: implications for the clinic. Expert Rev Neurother. 2018;18:179–183. doi: 10.1080/14737175.2018.1427066. [DOI] [PubMed] [Google Scholar]
- [121].Higgins A, Downes C, Varley J, Doherty CP, Begley C, Elliott N. Supporting and empowering people with epilepsy: contribution of the Epilepsy specialist nurses (SENsE study) Seizure. 2019;71:42–49. doi: 10.1016/j.seizure.2019.06.008. [DOI] [PubMed] [Google Scholar]
- [122].Noordegraaf M. Remaking professionals? How associations and professional education connect professionalism and organisations. Curr Sociol. 2011;59:465–488. [Google Scholar]
- [123].Fleishon HB, Itri JN, Boland GW, Duszak R., Jr Academic medical centers and community hospitals integration: trends and strategies. J Am Coll Radiol. 2017;14:45–51. doi: 10.1016/j.jacr.2016.07.006. [DOI] [PubMed] [Google Scholar]
- [124].Miller WR. Patient-centered outcomes in older adults with epilepsy. Seizure. 2014;23:592–597. doi: 10.1016/j.seizure.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Choi H, Hamberger MJ, Munger Clary H, Loeb R, Onchiri FM, Baker G, Hauser WA, Wong JB. Seizure frequency and patient-centered outcome assessment in epilepsy. Epilepsia. 2014;55:1205–1212. doi: 10.1111/epi.12672. [DOI] [PubMed] [Google Scholar]
- [126].Miller WR, Bakas T, Buelow JM. Problems, needs, and useful strategies in older adults self-managing epilepsy: implications for patient education and future intervention programs. Epilepsy Behav. 2014;31:25–30. doi: 10.1016/j.yebeh.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Charyton C, Elliott JO, Lu B, Moore JL. The impact of social support on health related quality of life in persons with epilepsy. Epilepsy Behav. 2009;16:640–645. doi: 10.1016/j.yebeh.2009.09.011. [DOI] [PubMed] [Google Scholar]
- [128].Amir M, Roziner I, Knoll A, Neufeld MY. Self-efficacy and social support as mediators in the relation between disease severity and quality of life in patients with epilepsy. Epilepsia. 1999;40:216–224. doi: 10.1111/j.1528-1157.1999.tb02078.x. [DOI] [PubMed] [Google Scholar]
- [129].Wilde M, Haslam C. Living with epilepsy: a qualitative study investigating the experiences of young people attending outpatients clinics in Leicester. Seizure. 1996;5:63–72. doi: 10.1016/s1059-1311(96)80065-3. [DOI] [PubMed] [Google Scholar]
- [130].Legnani M, Bertinat A, Decima R, Demicheli E, Higgie JR, Preve F, Braga P, Bogacz A, Scaramelli A. Applicability and contribution of the new ILAE 2017 classification of epileptic seizures and epilepsies*. Epileptic Disorders. 2019;21:549–554. doi: 10.1684/epd.2019.1108. [DOI] [PubMed] [Google Scholar]
- [131].Breyer T, Wanke I, Maderwald S, Woermann FG, Kraff O, Theysohn JM, Ebner A, Forsting M, Ladd ME, Schlamann M. Imaging of patients with hippocampal sclerosis at 7 tesla: initial results. Acad Radiol. 2010;17:421–426. doi: 10.1016/j.acra.2009.10.013. [DOI] [PubMed] [Google Scholar]
- [132].Zhang Y, Lv Y, You H, Dou W, Hou B, Shi L, Zuo Z, Mao W, Feng F. Study of the hippocampal internal architecture in temporal lobe epilepsy using 7 T and 3 T MRI. Seizure. 2019;71:116–123. doi: 10.1016/j.seizure.2019.06.023. [DOI] [PubMed] [Google Scholar]
- [133].Thapaliya K, Urriola J, Barth M, Reutens DC, Bollmann S, Vegh V. 7T GRE-MRI signal compartments are sensitive to dysplastic tissue in focal epilepsy. Magn Reson Imaging. 2019;61:1–8. doi: 10.1016/j.mri.2019.05.011. [DOI] [PubMed] [Google Scholar]
- [134].Sidhu MK, Duncan JS, Sander JW. Neuroimaging in epilepsy. Curr Opin Neurol. 2018;31:371–378. doi: 10.1097/WCO.0000000000000568. [DOI] [PubMed] [Google Scholar]
- [135].Wang I, Oh S, Blümcke I, Coras R, Krishnan B, Kim S, McBride A, Grinenko O, Lin Y, Overmyer M. Value of 7T MRI and post-processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia. 2020;61(11):2509–2520. doi: 10.1111/epi.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Colon A, van Osch M, Buijs M, Grond J, Hillebrand A, Schijns O, Wagner G, Ossenblok P, Hofman P, Buchem M. MEG-guided analysis of 7T-MRI in patients with epilepsy. Seizure. 2018;60:29–38. doi: 10.1016/j.seizure.2018.05.019. [DOI] [PubMed] [Google Scholar]
- [137].Feldman RE, Delman BN, Pawha PS, Dyvorne H, Rutland JW, Yoo J, Fields MC, Marcuse LV, Balchandani P. 7T MRI in epilepsy patients with previously normal clinical MRI exams compared against healthy controls. PLoS One. 2019;14:e0213642. doi: 10.1371/journal.pone.0213642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Shah P, Bassett DS, Wisse LE, Detre JA, Stein JM, Yushkevich PA, Shinohara RT, Elliott MA, Das SR, Davis KA. Structural and functional asymmetry of medial temporal subregions in unilateral temporal lobe epilepsy: a 7T MRI study. Hum Brain Mapp. 2019;40:2390–2398. doi: 10.1002/hbm.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Santyr BG, Goubran M, Lau JC, Kwan BY, Salehi F, Lee DH, Mirsattari SM, Burneo JG, Steven DA, Parrent AG. Investigation of hippocampal substructures in focal temporal lobe epilepsy with and without hippocampal sclerosis at 7T. J Magn Reson Imaging. 2017;45:1359–1370. doi: 10.1002/jmri.25447. [DOI] [PubMed] [Google Scholar]
- [140].Voets NL, Hodgetts CJ, Sen A, Adcock JE, Emir U. Hippocampal MRS and subfield volumetry at 7T detects dysfunction not specific to seizure focus. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-16046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Veersema TJ, van Eijsden P, Gosselaar PH, Hendrikse J, Zwanenburg JJ, Spliet WG, Aronica E, Braun KP, Ferrier CH. 7 tesla T2*-weighted MRI as a tool to improve detection of focal cortical dysplasia. Epileptic Disorders. 2016;18:315–323. doi: 10.1684/epd.2016.0838. [DOI] [PubMed] [Google Scholar]
- [142].Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen Y-J, Chen Z. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Shendure J, Porreca GJ, Reppas NB, Lin X, McCutcheon JP, Rosenbaum AM, Wang MD, Zhang K, Mitra RD, Church GM. Accurate multiplex polony sequencing of an evolved bacterial genome. Science. 2005;309:1728–1732. doi: 10.1126/science.1117389. [DOI] [PubMed] [Google Scholar]
- [144].Schuster SC. Next-generation sequencing transforms today’s biology. Nat Methods. 2008;5:16–18. doi: 10.1038/nmeth1156. [DOI] [PubMed] [Google Scholar]
- [145].Dunn P, Albury CL, Maksemous N, Benton MC, Sutherland HG, Smith RA, Haupt LM, Griffiths LR. Next generation sequencing methods for diagnosis of epilepsy syndromes. Front Genet. 2018;9:20. doi: 10.3389/fgene.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ortega-Moreno L, Giráldez BG, Soto-Insuga V, Losada-Del Pozo R, Rodrigo-Moreno M, Alarcón-Morcillo C, Sánchez-Martín G, Díaz-Gómez E, Guerrero-López R, Serratosa JM. Molecular diagnosis of patients with epilepsy and developmental delay using a customized panel of epilepsy genes. PLoS One. 2017;12:e0188978. doi: 10.1371/journal.pone.0188978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lee J, Lee C, Ki CS, Lee J. Determining the best candidates for next-generation sequencing-based gene panel for evaluation of early-onset epilepsy. Mol Genet Genom Medi. 2020;8(9):e1376. doi: 10.1002/mgg3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Mercimek-Mahmutoglu S, Patel J, Cordeiro D, Hewson S, Callen D, Donner EJ, Hahn CD, Kannu P, Kobayashi J, Minassian BA. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia. 2015;56:707–716. doi: 10.1111/epi.12954. [DOI] [PubMed] [Google Scholar]
- [149].Møller RS, Dahl HA, Helbig I. The contribution of next generation sequencing to epilepsy genetics. Expert Rev Mol Diagn. 2015;15:1531–1538. doi: 10.1586/14737159.2015.1113132. [DOI] [PubMed] [Google Scholar]
- [150].Costain G, Cordeiro D, Matviychuk D, Mercimek-Andrews S. Clinical application of targeted next-generation sequencing panels and whole exome sequencing in childhood epilepsy. Neuroscience. 2019;418:291–310. doi: 10.1016/j.neuroscience.2019.08.016. [DOI] [PubMed] [Google Scholar]
- [151].Zhou P, He N, Zhang JW, Lin ZJ, Wang J, Yan LM, Meng H, Tang B, Li BM, Liu XR. Novel mutations and phenotypes of epilepsy-associated genes in epileptic encephalopathies. Genes Brain Behav. 2018;17:e12456. doi: 10.1111/gbb.12456. [DOI] [PubMed] [Google Scholar]
- [152].Abbasi B, Goldenholz DM. Machine learning applications in epilepsy. Epilepsia. 2019;60:2037–2047. doi: 10.1111/epi.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Si Y. Machine learning applications for electroencephalograph signals in epilepsy: a quick review. Acta Epileptol. 2020;2:1–7. [Google Scholar]
- [154].Walczak S, Nowack WJ. An artificial neural network approach to diagnosing epilepsy using lateralised bursts of theta EEGs. J Med Syst. 2001;25:9–20. doi: 10.1023/a:1005680114755. [DOI] [PubMed] [Google Scholar]
- [155].Gómez-Gil P, Juárez-Guerra E, Alarcón-Aquino V, Ramírez-Cortés M, Rangel-Magdaleno J. Recent Advances on Hybrid Approaches for Designing Intelligent Systems. Springer; 2014. Identification of epilepsy seizures using multi-resolution analysis and artificial neural networks; pp. 337–351. [Google Scholar]
- [156].Abbasi R, Esmaeilpour M. Selecting statistical characteristics of brain signals to detect epileptic seizures using discrete wavelet transform and perceptron neural network. Int J Interact Multimedia Artificial Intel. 2017;4 [Google Scholar]
- [157].Fergus P, Hignett D, Hussain A, Al-Jumeily D, Abdel-Aziz K. Automatic epileptic seizure detection using scalp EEG and advanced artificial intelligence techniques. Biomed Res Int. 2015;2015 doi: 10.1155/2015/986736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Guo L, Rivero D, Pazos A. Epileptic seizure detection using multiwavelet transform based approximate entropy and artificial neural networks. J Neurosci Methods. 2010;193:156–163. doi: 10.1016/j.jneumeth.2010.08.030. [DOI] [PubMed] [Google Scholar]
- [159].Kumar SP, Sriraam N, Benakop P, Jinaga B. Entropies based detection of epileptic seizures with artificial neural network classifiers. Expert Syst Appl. 2010;37:3284–3291. [Google Scholar]
- [160].Elahian B, Yeasin M, Mudigoudar B, Wheless JW, Babajani-Feremi A. Identifying seizure onset zone from electrocorticographic recordings: a machine learning approach based on phase locking value. Seizure. 2017;51:35–42. doi: 10.1016/j.seizure.2017.07.010. [DOI] [PubMed] [Google Scholar]
- [161].Murin Y, Kim J, Parvizi J, Goldsmith A. SozRank: a new approach for localising the epileptic seizure onset zone. PLoS Comput Biol. 2018;14:e1005953. doi: 10.1371/journal.pcbi.1005953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Varatharajah Y, Berry B, Cimbalnik J, Kremen V, Van Gompel J, Stead M, Brinkmann B, Iyer R, Worrell G. Integrating artificial intelligence with real-time intracranial EEG monitoring to automate interictal identification of seizure onset zones in focal epilepsy. J Neural Eng. 2018;15:046035. doi: 10.1088/1741-2552/aac960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Nissen IA, Stam CJ, van Straaten EC, Wottschel V, Reijneveld JC, Baayen JC, de Witt Hamer PC, Idema S, Velis DN, Hillebrand A. Localization of the epileptogenic zone using interictal MEG and machine learning in a large cohort of drug-resistant epilepsy patients. Front Neurol. 2018;9:647. doi: 10.3389/fneur.2018.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Baud MO, Kleen JK, Anumanchipalli GK, Hamilton LS, Tan Y-L, Knowlton R, Chang EF. Unsupervised learning of spatiotemporal interictal discharges in focal epilepsy. Neurosurgery. 2018;83:683–691. doi: 10.1093/neuros/nyx480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Kassahun Y, Perrone R, De Momi E, Berghöfer E, Tassi L, Canevini MP, Spreafico R, Ferrigno G, Kirchner F. Automatic classification of epilepsy types using ontology-based and genetics-based machine learning. Artif Intell Med. 2014;61:79–88. doi: 10.1016/j.artmed.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [166].Pereira L, Rijo R, Silva C, Agostinho M. ICD9-based text mining approach to children epilepsy classification. Procedia Technol. 2013;9:1351–1360. [Google Scholar]
- [167].Ahmedt-Aristizabal D, Nguyen K, Denman S, Sridharan S, Dionisio S, Fookes C. Deep motion analysis for epileptic seizure classification; 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE. 2018. pp. 3578–3581. [DOI] [PubMed] [Google Scholar]
- [168].Sriraam N, Temel Y, Rao SV, Kubben PL. A convolutional neural network based framework for classification of seizure types; 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE. 2019. pp. 2547–2550. [DOI] [PubMed] [Google Scholar]
- [169].Saputro IRD, Maryati ND, Solihati SR, Wijayanto I, Hadiyoso S, Patmasari R. J Phys Conf Ser. IOP Publishing; 2019. Seizure type classification on EEG signal using support vector machine; 012065 [Google Scholar]