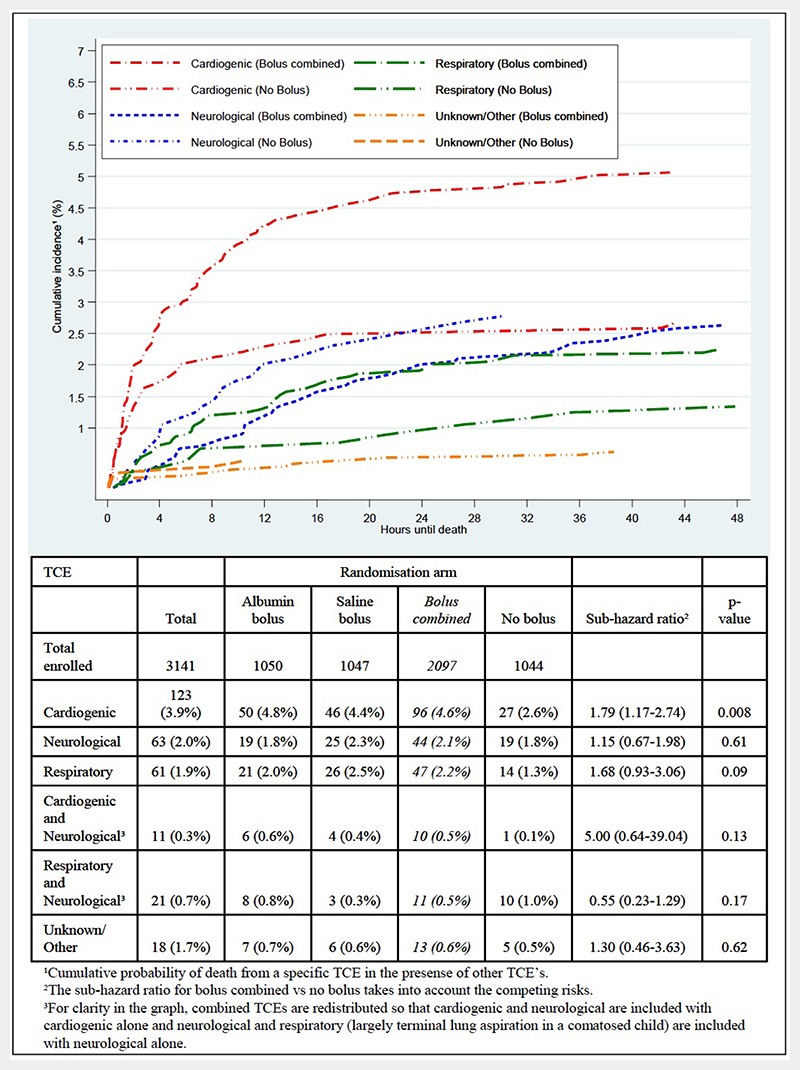
Nobody could have been more surprised than the Fluid Expansion As Supportive Therapy (FEAST) trial clinicians when they heard the results of our 2011 phase III randomised controlled study in six East African clinical centres in Kenya, Tanzania and Uganda.1 Based on what they had witnessed at the bedside in children with severe febrile illness and impaired perfusion, they had all expected fluid bolus therapy (FBT) as compared with no bolus (but solely maintenance fluids at 4 mL/kg/hour) to have a better outcome. Even though FBT leads to substantially better early shock reversal, subsequently it results in excess 48-hour and 28-day mortality. The chief mode of excess mortality was cardiovascular collapse and not fluid overload (figure 1).2 Notable is that the vast majority of children only received a 20 mL/kg bolus of either 5% albumin or 0.9% saline, yet this intervention caused excess mortality in all subgroups (including a large subgroup with sepsis), across all ages, for all definitions of shock, and at each centre. This surprising finding is precisely why we need to do clinical trials!
Figure 1. Cumulative incidence of mortality for bolus combined and no bolus arms by terminal clinical events for 297 children who died within 48 hours (from figure 7 from ref2).
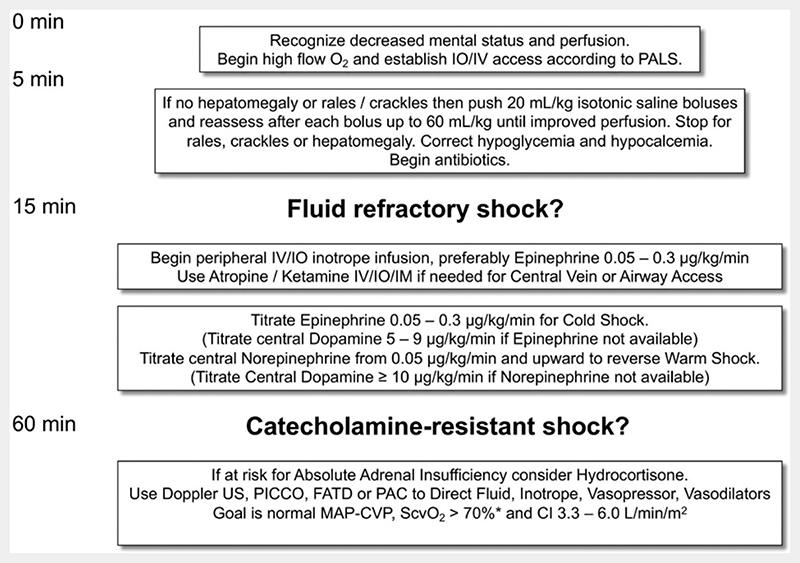
To date, the FEAST trial is the only completed phase III randomised controlled study of FBT in paediatrics. Yet, it has had limited or no impact on clinical guidelines. First, there have been some refinements in the 2013 WHO guideline for those managed in resource-limited hospitals with all four signs of ‘strict shock’, that is, capillary refill time (CRT) more than 3 s, cold peripheries, a weak pulse and a fast pulse. In such paediatric patients, a conservative 10 mL/kg FBT (and not 20 mL/kg) can be used and repeated up to three times.3 However, in our post hoc analysis of the FEAST trial data, we found a general 3% increase in mortality from FBT across all FEAST trial subgroups, including the 65 cases meeting the WHO ‘strict shock’ criteria FBT.4 Second, in the 2014 American College of Critical Care Medicine (ACCM) clinical guidelines for haemodynamic support of neonates and children with septic shock,5 there have been no changes since the 2007 guidelines.6 The recommendation for FBT in initial management remains as 60 mL/kg, given in up to three 20 mL/kg boluses, over 15 min (figure 2).5 So, taking all of the above together, the question remains whether FBT needs to be more conservative.
Figure 2. Abridged figure 2 from ref5.
In this context, Inwald et al7 report the findings of the Fluids In SHock (FISH) phase II trial, which examined the feasibility of a more conservative versus standard FBT strategy (10 mL/kg vs 20 mL/kg). This UK study was carried out in 13 hospitals (July 2016–April 2017) in children presenting to emergency departments who had not received any more than 20 mL/kg as initial FBT before study enrolment. Overall, 75 children were enrolled, with 39 and 34 randomised to 10 mL/kg and 20 mL/kg, respectively (73/75 with two withdrawals in the deferred consent process). The shock entry criteria were based on systolic blood pressure (less than the fifth percentile for age) or CRT (≥3 s) findings. Of note, 60/73 (82%) had CRT criteria only. Over a 4-hour intervention period, there was good separation in the total FBT volumes received by the two groups in the study: 44% lower in the 10 mL/kg versus the 20 mL/kg groups (mean volumes 14.5 vs 27.5 mL/kg, respectively). Most children received only one fluid bolus, 23/39 (59%) in the 10 mL/kg group and 25/34 (74%) in the 20 mL/kg group. For these respective arms, within the first 15 mins, 37/39 (80%) and 30/34 (88%) completed their FBT on time (<15 min). However, clinicians reported that they found difficulty in administering a 20 mL/kg bolus in the immediate 15 min window postrandomisation. A perspectives study, embedded within the phase II trial, also indicated that some clinicians lacked equipoise, favouring the 10 mL/kg over 20 mL/kg FBT strategy. In regard to the clinical endpoints in the study, there were no deaths in the trial and, as expected from a phase II study, none of the secondary patient-centred endpoints were significantly different between the study groups. That is, for 10 mL/kg versus 20 mL/kg FBT groups we have: paediatric intensive care unit (PICU) admission rate 26% versus 32%; median length of PICU stay 45 (IQR 18–143) versus 119 (IQR 52–228) hours; use of mechanical ventilation 4/36 (11%) versus 8/32 (25%); and use of inotropes 1/36 (2.8%) versus 5/32 (15.6%). However, from the perspective of health services usage and costs, the results indicated higher requirement in the standard 20 mL/kg FBT group, despite this approach being far less ‘aggressive’ than the current ACCM recommendations.5
The FISH phase II trial was designed to determine whether it would be feasible to conduct a phase III trial of FBT (10 mL/kg vs 20 mL/kg) in the emergency setting in the UK.7 The investigators have concluded that such a trial cannot be done in the UK. This conclusion was based on their experience of study adherence, and the contemporary views of practising clinicians and the views of parents of children needing emergency FBT. The investigators also found that children in the FISH study were less critically ill than they had expected, which may reflect a change in the epidemiology of sepsis because of better immunity to vaccine-preventable infections. The UK is unlikely to be an outlier in this respect since European and North American countries are undoubtedly witnessing the same trend. So, allowing for the likelihood that there will not be a future phase III paediatric FBT trial what are the next steps?
As stated earlier, the recently updated 2014 ACCM guidance continues to recommend up to 60 mL/kg given as 20 mL/kg boluses in the first 15 min.5 This recommendation was based on a single-centre tertiary hospital experience using such guidance over a 9-year period (January 1993–December 2001) in 91 children (~10/year) managed initially by ‘community physicians’ in whom better outcomes occurred in those achieving shock reversal by 75 min.8 The initial 2007 ACCM guidelines rated the level of this evidence for a recommendation as 2C (weak evidence based on cohort study). The major change in the 2014 ACCM guidelines is that ‘2C’ is now rated as 1C (strong evidence based on cohort studies). Perhaps it is now time to reconsider this guideline given the current epidemiology of sepsis and clinicians reluctance to follow FBT with 20 mL/kg over 15 min, to a more conservative approach with 10 mL/kg over 15 min with apparently no adverse effects.
Funding
None declared.
Footnotes
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.
References
- 1.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 2.Maitland K, George EC, Evans JA, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Pocket book of hospital care for children: Second edition Guidelines for the management of common childhood illnesses. World Health Organization; Geneva: 2013. [PubMed] [Google Scholar]
- 4.Houston KA, George EC, Maitland K. Implications for paediatric shock management in resource-limited settings: a perspective from the FEAST trial. Crit Care. 2018;22:119. doi: 10.1186/s13054-018-1966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis AL, Carcillo JA, Aneja RK, et al. The American college of critical care medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: executive summary. Pediatr Crit Care Med. 2017;18:884–90. doi: 10.1097/PCC.0000000000001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inwald DP, Canter R, Woolfall K, et al. PERUKI (Paediatric Emergency Research in the UK and Ireland) and PICS SG (Paediatric Intensive Care Society Study Group). Restricted fluid bolus volume in early septic shock: results of the Fluids in Shock pilot trial. Arch Dis Child. 2019;104:426–31. doi: 10.1136/archdischild-2018-314924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]