Abstract
Objective
To evaluate novel sealing techniques for their biocompatibility and sealing capacity of iatrogenic fetal membrane defects in a pregnant rabbit model.
Method
At day 23 of gestation (term = d31), a standardized fetoscopy was performed through a 14G cannula. The resulting fetal membrane defect was closed with condensed collagen, collagen with fibrinogen, Tissuepatch, Duraseal, or a conventional collagen plug (Lyostypt) as reference. At d30, the fetuses were harvested and full thickness fetal membrane samples were analyzed. The study consisted of 2 consecutive parts: (1) biocompatibility testing by fetal survival, apoptosis, and infiltration of polymorphonuclear cells in the membranes and (2) the efficacy to seal fetal membrane defects.
Results
Three sealants (collagen with fibrinogen, Duraseal, or Lyostypt) were associated with a higher fetal mortality compared to control unmanipulated littermates and hence were excluded from further analysis. Tissuepatch was biocompatible, and amniotic fluid levels were comparable to those of control untouched littermates. Compared to the condensed collagen, Tissuepatch was also easier in surgical handling and induced limited cell proliferation.
Conclusion
Tissuepatch had the best biocompatibility and efficacy in sealing an iatrogenic fetal membrane defect in the pregnant rabbit compared to other readily available sealants.
1. Introduction
Fetal membrane rupture remains the Achilles heel of fetal surgery, occurring in around 28% of laser interventions for twin-twin transfusion syndrome and around 47% of fetoscopic endoluminal tracheal occlusion for congenital diaphragmatic hernia.1,2 Therefore, several strategies to either treat or prevent membrane rupture have been proposed.3–6
Primary prevention of preterm premature rupture of membranes at the time of fetal surgery would be a useful approach. To our knowledge, only collagen and gelatin plugs have been used clinically for this purpose, however with contradicting results.7–9 Clinical studies on potential new solutions are difficult to perform as “first-in-man”studies in pregnancy are challenging, using products that are or would be used off label. It is important to analyze the healing site histologically and to set up a controlled study that is adequately powered. In vivo animal studies are hence vital to allow researchers to explore new solutions. The optimal candidate surgical solution to us should ideally have the following features: (1) The device or material should be readily available; (2) the device or material should be clinically applicable through a 10 Fr, preferentially also an 8 Fr cannula, either as is or after modification; (3) the material should have the ability to be bioactivated; and (4) the material should have the ability to function as a transport of molecular therapeutics to interfere with, apart from the defect, other potential biomolecular mechanism behind iatrogenic preterm premature rupture of membranes.10
Herein, we report on 4 novel sealant techniques, which we compared to the conventional collagen plugs (Lyostypt) as a reference sealant in the study. One sealant is a condensed collagen, which has been tissue engineered from human fetal membranes and which demonstrated good growth support of amnion epithelial cells.11 The second sealant is an off-the-shelf collagen (Lyostypt) imbued with fibrinogen. This was tested before in vitro in human amniotic membranes and showed improved sealing capabilities.12 The third sealant is Tissuepatch, which is a thin film and clinically is used to obtain an airtight sealant in thoracoscopic surgeries. The fourth sealant is Duraseal, which is used in neurosurgery for sealing dura mater defects and preventing cerebrospinal fluid leakage.13 We tested those sealants in vivo for their biocompatibility (safety) and their efficacy to seal a fetal membrane defect in a pregnant rabbit model for fetoscopy.
2. Material and Methods
2.1. Study design
The study design is shown in Figure S1. Pregnant rabbits underwent a laparotomy at 23 days of gestation under general anesthesia, and 3 fetuses out of the total litter were operated on and assigned to one of 5 types of fetal membrane sealant or positive control (no sealant); the remaining fetuses acted as negative unoperated controls. One week later, pregnancies were evaluated for fetal survival, the primary outcome variable of the biocompatibility testing. Secondary outcomes were inflammation and apoptosis. Sealants performing best in the primary outcome measure were further tested for efficacy, ie, by the deepest vertical pocket (DVP) on ultrasound at term. Further secondary outcomes were ease of surgical handling at the time of sealant insertion, and what were considered as additional efficacy outcome measures such as amniotic fluid leakage, persistence of the plug, Ki67 positive cells, and the ratio of vimentin and cytokeratin as a marker for epithelial-mesenchymal transition (EMT). The lung-to-bodyweight ratio (LBWR) of the fetus was quantified as a further efficacy measure to assess pulmonary hypoplasia.
2.2. Animal and surgical protocols
This experiment was approved by the local ethics committee of KU Leuven (P250/2014), and animals were handled according to the current guidelines on animal welfare. Time-mated pregnant does (hybrid of New Zealand and Dendermonde) were housed at the animalium of the group Biomedical Sciences in separate cages on a 12-hour light cycle with free access to food and water. At 23 days of gestational age (GA; term = 31 d), the doe was sedated with intramuscular ketamine 35 mg/kg bodyweight (Ketamine 1000 CEVA; CEVA Santé Animal, Brussels, Belgium) and xylazin 6 mg/kg bodyweight (Vexylan; CEVA Santé Animal). Buprenorphine 0.03 mg/kg (Vetergesic; Reckitt Benckiser Healthcare, Brussels, Belgium), medroxyprogesterone acetate (Depo-Provera, Pfizer, Puurs, Belgium), and penicillin G (Kela Pharma, Hoogstraten, Belgium) were injected subcutaneously. General anesthesia was maintained using isoflurane 1.5% (Isoba Vet; Abbott Laboratories Ltd, Queenborough, Kent, UK) in oxygen at 2 L/min via a facemask. Maternal vital parameters were monitored with a pulse oximeter (Nellcor N-20P; Nellcor Inc, Haasrode, Belgium). Physiologic body temperature was maintained by a heating pad.
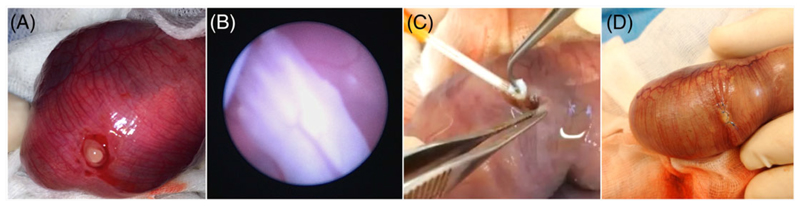
The doe was placed in the supine position; the abdomen was shaved and disinfected with povidone iodine (Isobetadine; Asta Medica, Brussels, Belgium) and covered with sterile drapes. After midline laparotomy, the uterus was exposed and the fetal sacs counted. We operated on 3 fetuses in each litter, ie, both ovarian end fetuses and one more in the horn with the highest number of fetuses, leaving one unoperated fetus in between. In case of equal numbers of fetuses in both horns, a random side was chosen. First, a small myometrial incision of 3 to 4 mm was made on the antimesometrial side. Blunt dissection of the chorion was performed to gain access to the amnion and to avoid damage to the vitelline vessels (Figure 1A). After exposure of the amniotic membrane, it was punctured with a 14G cannula (Braun Medical N.V., Diegem, Belgium) at a perpendicular angle, and a standardized diagnostic fetoscopy of fixed duration (2 min) was performed with a 1.0-mm embryoscope (11510A, Karl Storz, Tutlingen, Germany) within a 1.3-mm examination sheath (11510KA, Karl Storz) (Video S1; Figure 1B), as previously described.14 By using this type of diameter instruments, the defect was large enough (around 1.3 mm) to surgically introduce the sealant.
Figure 1. Photographs of the surgical procedure.
A, Opening of the myometrium and chorion. View on amnion before puncturing. B, Fetoscopic view of fetal front limb. C, Insertion of a collagen plug while withdrawing the cannula. D, Closure of the myometrium
The gestational sacs were randomly assigned to one of the following treatment groups: (1) Lyostypt (B. Braun Medical N.V.), (2) Lyostypt soaked in fibrinogen concentrate (Haemocomplettan, CSL Behring, Breda, The Netherlands), (3) condensed collagen, (4) Tissuepatch (Tissuemed Ltd, Leeds, UK), (5) Duraseal (Integra LS N.V., Zaventem, Belgium), or (6) positive controls (SHAM surgery without sealant). The sealant was positioned in or over the amnion defect. The collagen sealants were fixed within the defect by a single stitch 6-0 Prolene to the fetal membranes and the myometrium (Ethicon, Johnson & Johnson Medical N.V., Diegem, Belgium). Tissuepatch was fixed on the inside of the amniotic cavity with its self-adhesive side to the amnion epithelium with two 6-0 Prolene sutures to the fetal membranes and the myometrium. Duraseal was sprayed upon the amniotic defect. Any problem with proper placement of the plug or any discordant opinion on appropriate plug positioning by the surgeons (ACE and LJ) was categorized as problematic. The unoperated gestation sacs were used as negative controls. The myometrium was closed with 3 single stitches with 6-0 Prolene suture (Figure 1D). The doe’s abdominal wall was closed in 2 layers with 2-0 Vicryl (Ethicon) for the muscular layer and 3-0 Ethilon (Ethicon) for the intracutaneous skin suture.
At 30 days GA, the does were premedicated and anesthetized as above. Micro-ultrasound (Vevo 2100 System, Visual Sonics, Toronto) was used to assess the DVP and determine fetal survival. After that, macroscopic amniotic fluid leakage at the operated area was evaluated with blotting paper (3 × 1 cm, 125 g/m2, Aurora Productions SA, Turnhout, Belgium) for 10 seconds after drying the area with surgical gauze. Thereafter, the does and fetuses were euthanized with an intravenous bolus of a mixture of embutramide 200 mg, mebezonium 50 mg, and tetracaine hydrochloride 5 mg (0.3 mL/kg T61; Marion Roussel Hoechst, Brussels, Belgium), and samples were obtained.
2.3. Harvesting and sample preparation
We identified the entry point to the fetal sac by the marking suture and pre-elevated it “en bloc” (amnion, chorion, and myometrium; further referred to as FM sample). Persistence or absence of the plug in the myometrium and/or the fetal membranes was macroscopically assessed from the inside of the amniotic cavity. FM samples of surviving fetuses were stored in tissue embedding cassettes (Simport, Beloeil, Quebec, Canada) on foam biopsy pads (Leica Biosystems N. V., Maarn, The Netherlands) and submerged in 4% buffered paraformaldehyde (Klinipath BV, Duiven, The Netherlands) for 24 hours. They were then transferred to 70% ethanol and embedded in paraffin. At necropsy, the fetus and its lungs were weighed and the LBWR was calculated.
2.4. Histology and semiautomatic quantification
Paraffin blocks were cut in 4-μm sections displaying the area of the defect. Staining with hematoxylin and eosin was performed, and an average score from 3 high power fields per slide (×40 magnification) around the defect was calculated for polymorphonuclear cell (PMN) infiltration as previously described.15 Immunohistochemistry (IHC) for Ki67, vimentin, and cytokeratin was performed according to standard IHC protocols as described before.16 TUNEL staining (In Situ Cell Death Detection Kit, Fluorescein; Roche Diagnostics GmbH, Mannheim, Germany), detecting apoptotic cells, was performed according to the producer’s recommendations with DAPI as a background staining. Ki67 was quantified to assess cell proliferation. The presence of EMT was investigated by an IHC vimentin/cytokeratin ratio.
Immunohistochemistry and TUNEL positive cell density measurements were performed semiautomatically, using ImageJ software (v1.48, NIH, USA). From each slide, 3 random nonoverlapping images were recorded (Axioskop; Carl Zeiss) at ×40 magnification at the contact area between sealant and tissue. An average density was calculated for each slide and presented as mean ± standard deviation. The digital color images were segmented, and Ki67, vimentin, and cytokeratin staining was quantified. The total number of cells as well as the amount of positively stained cells was determined, and the density was expressed as a percentage (positive cell area/total area of cells). TUNEL staining was expressed as a percentage of TUNEL positive cells/DAPI positive cells.
2.5. Statistics
Statistical analysis was performed with SPSS Statistics software v21.0. (IBM, Company). A P value of <.05 was considered significant. Continuous variables were tested for normal distribution by Kolmogorov-Smirnov test and expressed as mean and standard deviation. Binomial and ordinal variables were expressed as percentage and score, respectively. One-way analysis of variance with Tukey post hoc analysis was used to compare normally distributed continuous variables and Kruskal-Wallis test for nonnormally distributed continuous variables. Chi-square test was used for binomial and ordinal variables. The investigational groups were compared to the negative controls. Positive controls were used as reference, if no negative control group was included in the outcome. Lyostypt was used as reference for surgical handling readouts (Table S1). Power calculation was performed with StatMate v2.0 (GraphPad, San Diego, California, USA) according to the published literature4; we calculated that a sample size of 9 fetuses/group would provide a power of ≥85% with a 5% 2-sided type I error to detect a 20% difference in fetal survival.
3. Results
A total of 35 does and 332 fetuses were included in the study. There were 231 unmanipulated control fetuses, 18 in the Lyostypt group, 14 in the condensed collagen group, 25 in the collagen + fibrinogen group, 13 in the Tissuepatch group, 15 in the Duraseal group, and 16 in the unsealed positive control group.
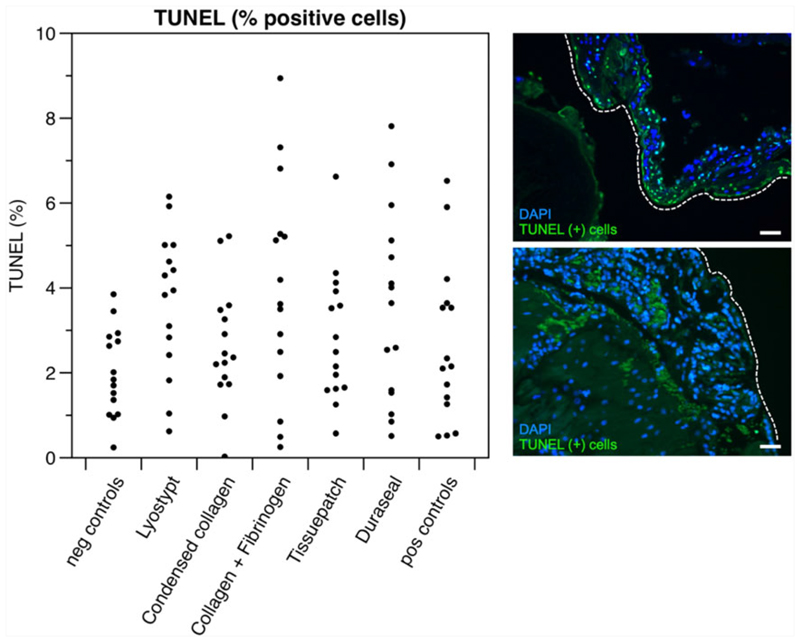
Overall, fetal survival in unsealed sacs tended to be lower (75%) than in unmanipulated control sacs (91%), yet that did not reach statistical significance ( = 3.478; P = .082, Table 1). Two sealants had no effect on fetal survival when compared to unmanipulated control sacs: condensed collagen (11/14, 78%; = 1.831; P = .175) and Tissuepatch (10/13, 77%; = 2.225; P = .149). Sealing with 3 of the used strategies was associated with a reduced fetal survival compared to negative controls, ie, Lyostypt (13/18, 72%; = 5.314; P = .038), collagen + fibrinogen (12/25, 48%; = 32.996; P < .001), and Duraseal (9/15, 60%; = 12.226; P = .004, Table 1). However, in sacs of surviving fetuses from these 3 sealant groups, there was no difference with positive controls in terms of inflammation (number of PMN) ( = 13.067; P = .364) or TUNEL positive cells (F = 2.135; P = .056) (Figure 2 and Table 1).
Table 1. Results for each variable and each sealant are given including the significance level.
| Readout | Neg Controls | Lyostypt | Condensed Collagen | Coll + Fibr | Tissuepatch | Duraseal | Pos Controls | P Value |
|---|---|---|---|---|---|---|---|---|
| Fetal survival (%) | 208/231 (91%) | 13/18 (72%)* | 11/14 (78%) | 12/25 (48%)* | 10/13 (77%) | 9/15 (60%)* | 12/16 (75%) | <.001 |
| PMNs (mean score) | 0.73 (±0.458) | 1.00 (±0.378) | 0.80 (±0.414) | 1.00 (±0.535) | 0.80 (±0.414) | 1.13 (±0.352) | 0.87 (±0.352) | .364 |
| TUNEL (%) | 2.01 (±1.03) | 3.67 (±1.67) | 2.61 (±1.39) | 3.93 (±2.56) | 2.81 (±1.56) | 3.53 (±2.27) | 2.66 (±1.87) | .056 |
| Part 2: Efficacy | ||||||||
| Readout | Neg Controls | Condensed Collagen | Tissuepatch | Pos Controls | P Value | |||
| DVP (mm) | 6.93 (±3.80) | 1.89 (±1.30)* | 4.68 (±2.24) | 1.60 (±1.73)* | <.001 | |||
| Fluid leakage (%) | - | 6/11 (55%) | 1/10 (10%)* | 6/12 (50%) | <.001 | |||
| Problems at plug insertion (%) | - | 5/11 (45%)* | 1/10 (10%) | - | <.001 | |||
| Persistence of plug (%) | - | 7/11 (64%) | 9/10 (90%) | - | .449 | |||
| LBWR (ratio) | 0.025 ± 0.003 | 0.022 ± 0.002 | 0.023 ± 0.002 | 0.023 ± 0.003 | .213 | |||
| Ki67 (%) | 1.23 (±0.722) | 2.00 (±1.274) | 3.03 (±1.805)* | 2.70 (±1.609) | <.001 | |||
| Vim/Cyt (ratio) | 1.00 (±0.536) | 0.97 (±0.512) | 1.33 (±0.568) | 1.38 (±0.579) | .106 | |||
Abbreviations: DVP, deepest vertical pocket; LBWR, lung-to-bodyweight ratio; PMN, polymorphonuclear cell infiltration.
Results are presented as percentages or as mean with standard deviation (SD) where appropriate. P values were calculated by cross-tables including all groups. Results that are significantly different from the reference group are marked with an asterisk.
Figure 2.
Results of TUNEL staining displayed along with 2 microscopic images of TUNEL stainings at ×40 magnification. DAPI was used as background staining (blue)
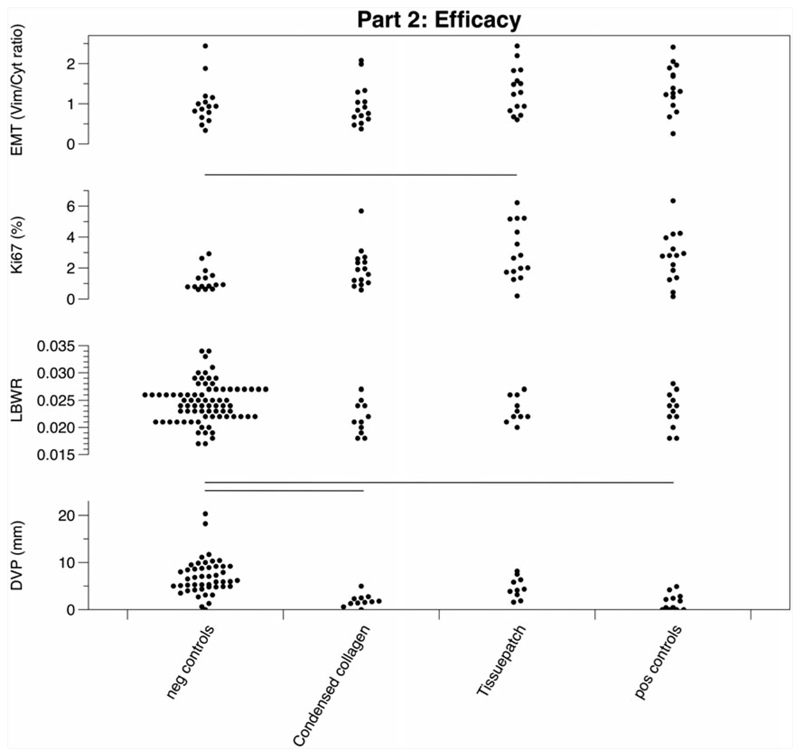
Pregnancies sealed using condensed collagen and Tissuepatch underwent further efficacy testing. Tissuepatch sealed sacs had a DVP comparable to that of normal controls (4.64 ± 2.24 vs 6.93 ± 3.80), but condensed collagen sealed sacs had a significantly lower DVP at harvesting (1.89 ± 1.30; F = 8.638; P < .001). There was a 10% amniotic fluid leakage rate (n = 1/10) in the Tissuepatch group, which was significantly less than the condensed collagen group (n = 6/11) and the positive controls (n = 6/12; = 4.023; P = .045). It was also easier to insert Tissuepatch than the condensed collagen plug with less problems at plug insertion ( = 179.535; P < .001). There was no difference in macroscopic persistence of the sealing materials used ( = 11.399; P = .449). Remarkably, there were no differences in LBWR between any of the 4 groups (F = 1.417; P = .213). The degree of cell proliferation (Ki67) was higher in the Tissuepatch group than in negative controls (3.03 ± 1.81 versus 1.23 ± 0.72; F = 4.575; P < .001). There was no difference in the proportion of cells staining for vimentin or cytokeratin (EMT) between the treatment groups and positive or negative controls (F = 1.804; P = .106) (Figure 3 and Table 1). Single electron microscopy images were taken from the Lyostypt and condensed collagen plugs to compare the structure of the 2 sealants and revealed a smaller diameter, higher density, and better alignment of collagen fibers in the condensed collagen (Figure S2).
Figure 3.
Graphical results of the efficacy testing. Results of the second part of the study that were retrieved as percentage are displayed in Table 1. DVP, deepest vertical pocket; EMT, epithelial-mesenchymal transition; LBWR, lung-to-bodyweight ratio
4. Discussion
Herein, we compared 4 new techniques to seal iatrogenic fetal membrane defects in a fetoscopy rabbit model in terms of biocompatibility and efficacy with a previously used technique of Lyostypt. Three of the sealants were associated with a higher rate of fetal demise compared to nonoperated control pregnancies. Tissuepatch and condensed collagen were the only sealants that had similar results to nonoperated controls in the primary outcome variable of fetal survival, but Tissuepatch proved easier to manipulate surgically and induced limited cell proliferation.
We first screened candidate sealants for the most direct adverse effect, ie, fetal death. In the group of products initially screened yet later disqualified, the fetal death rate ranged from 28% to 52% versus a death rate of around 25% in positive controls. The latter loss rate is in line with previous studies, confirming reproducibility of the model.4,16 At the same time, it indicates a frailty of the model due to anesthesia and manipulation of the uterus and its contents. The increased death rate in 3 of the sealant treatment groups would therefore suggest an additional factor besides fetoscopy as potential cause of death. Histological examination did not demonstrate any increased apoptosis or infiltration of PMN in the membranes of these 3 sealant groups. With the current design, we were unable to investigate precisely the cause and timing of fetal death, as there was only one time point of fetal viability evaluation, to limit repeated anesthesia in the does. In the collagen + fibrinogen group, there was a higher (>50%) death rate, yet no obvious factor explaining that excess could be identified. Potential explanations are local fibrin formation, when fibrinogen gets in contact with the amniotic fluid. Such increased mortality was already described in earlier experiments, where fibrin glue was used.17 The exact mechanism behind that remains unclear. The fetal survival in the Duraseal group was also lower. Again, we did not find a direct explanation on necropsy or histology in that group. Potentially, it ties to the injectable nature of the product or its expanding nature.18 During injection, components of the glue can freely and excessively enter the amniotic cavity and may have direct effects on the fetus.
We then moved into further experimentation with Tissuepatch and condensed collagen, to study efficacy. Condensed collagen plug created unfortunate problems at surgical plug insertion despite its good biocompatibility. Problems associated with insertion of the condensed collagen plug included structural break down of the plug during surgical manipulation and difficulties at suturing, which influenced further readouts. Tissuepatch sealed sacs had a DVP in the normal range and obvious leakage rate was low, within the range of intact sacs of unmanipulated controls. Macroscopically, the plug seemed well locked to the sealing site by sutures. On histological examination, we did not see direct evidence of integration and/or healing response despite an elevated proportion of Ki67 positive cells in the Tissuepatch group compared to negative controls. During the fixation of the sealing site in paraformaldehyde, Tissuepatch dissolves partly, making it impossible to trace the initial borders and integrity of the plug. Indirectly, however, there was no evidence for much interaction between the plug and the sealing product, as we did not see an increase in proliferation, signs of inflammation, nor apoptotic effect around the sealing site. It is therefore not impossible that the effect of this product, being sutured into a fetoscopic defect, was merely mechanical plugging, yet without causing direct local inflammatory or fetal side effects. Assuming the efficacy of Tissuepatch, one could speculate on how this would be clinically translated. This would obviously be an off-label use, yet it is an FDA-approved product for visceral pleura sealing. The patch has a self-adhesive visceral side, yet we could not take advantage of this in this model and still needed to fix the patch as the adhesive properties in under water conditions are not documented. A more intuitive clinical application would be to use this patch in open fetal surgeries where suturing to the amnion would be feasible.
This study has some limitations, particularly when comparing the rabbit model of fetal membrane sealing with human pregnancy. The myometrial thickness and contractility as well as the resealing capabilities of the rabbit amnion are different from those in women.19 Also, there is the difference of the application method one can use in this animal model and what would be possible in the clinical situation. The size difference of the defect makes surgical applications of sealants more difficult compared to the clinical situation. In clinical fetoscopy, it is also not possible to place a sealant on the chorionic side of the amnion, which is possible in the rabbit model as amnion, chorion, and uterine wall are not attached to each other. Also in this experiment, there was a clear relationship between difficulties handling properties and later efficacy. So it is possible that we underestimate efficacy of certain methods because they are difficult to insert in a rabbit. As the application technique itself was not a focus of our study, one would need to investigate products in another model that is better simulating clinical circumstances. The choice for some readouts may also need to be questioned. For instance, we assessed EMT, because it was earlier named to be relevant in human amnion samples from clinical fetoscopies.20 Epithelial-mesenchymal transition is a key component of wound healing, and we speculated that it would point to local regeneration. Herein, we could not demonstrate any change in EMT between any of the groups, questioning it as an outcome measure in this model. In retrospect, we could have included other readouts such as fetal systemic inflammation to understand adverse effects more effectively or could have followed up on fetuses postoperatively to determine the GA at intrauterine fetal death. This study also involved several subjective outcome measures. No matter how much we tried to make our readouts as objective as possible, they are not free of a certain amount of operator bias. Lastly, some of our observations were not in line with other studies using the same model. For instance, we did not find a decrease of LBWR due to the decreased amniotic fluid.21
On the other hand, our study had strengths; we observed that many other outcomes were similar to what was demonstrated earlier (survival rate, outcomes in positive and negative controls, and the amniotic fluid). This would suggest that findings in the rabbit model are reproducible. Also, we explored several methods, many of them clinically conceivable. The numbers of animals used are high compared to previous studies, and our study was adequately powered. The microscopic readouts were done semiautomatically.
In summary, this study provides experimental evidence that Tissuepatch has the best combination of biocompatibility, surgical ease, and efficacy in sealing an iatrogenic fetal membrane defect in the pregnant rabbit compared to other sealants. However, the current formulation of Tissuepatch is not easy to insert fetoscopically in an effective way. Therefore, our quest into an effective and clinically usable and approved sealing technique is ongoing.
What's already known about this topic?
Iatrogenic PPROM is a major risk factor of fetoscopic procedures.
So far, used sealants are not efficient to prevent iPPROM.
Further research is needed.
What does this study add?
We screened 5 sealants for biocompatibility and efficacy to seal fetal membrane defects.
Tissuepatch was biocompatible and had a good efficacy.
Tissuepatch could be used in open fetal surgery to prevent iPPROM.
Acknowledgements
We are very grateful to Godelieve Verbiest in performing the histology and immunohistochemistry and Ivan Laermans and Rosita Kinnart for handling the animals. We thank Tissuemed Ltd and Integra LS N.V. for donating us samples of their products.
Funding information
Engineering and Physical Sciences Research Council (EPSRC), Grant/Award Number: NS/A000027/1; Wellcome Trust, Grant/Award Number: WT101957
Funding sources
The work was supported by an Innovative Engineering for Health award by Wellcome Trust (WT101957), the Engineering and Physical Sciences Research Council (EPSRC) (NS/A000027/1), and the Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO) (18.01207). A. E. and J. V. D. M. are a grantee of the Erasmus+ Program of the European Union (framework agreement number 2013-0040; contract 1011990). A. L. D. is funded by the NIHR University College London Hospitals Biomedical Research Centre. This project was funded by the RoseTrees Trust (M400, TTC).
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
References
- 1.Yamamoto M, Ville Y. Twin-to-twin transfusion syndrome: management options and outcomes. Clin Obstet Gynecol. 2005;48(4):973–980. doi: 10.1097/01.grf.0000184796.71677.11. [DOI] [PubMed] [Google Scholar]
- 2.Jani JC, Nicolaides KH, Gratacos E, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34(3):304–310. doi: 10.1002/uog.6450. [DOI] [PubMed] [Google Scholar]
- 3.Mann LK, Papanna R, Moise KJ, Jr, et al. Fetal membrane patch and biomimetic adhesive coacervates as a sealant for fetoscopic defects. Acta Biomater. 2012;8(6):2160–2165. doi: 10.1016/j.actbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Deprest JA, Papadopulos NA, Decaluw H, et al. Closure techniques for fetoscopic access sites in the rabbit at mid-gestation. Hum Reprod. 1999;14(7):1730–1734. doi: 10.1093/humrep/14.7.1730. [DOI] [PubMed] [Google Scholar]
- 5.Bilic G, Brubaker C, Messersmith PB, et al. Injectable candidate sealants for fetal membrane repair: bonding and toxicity in vitro. Am J Obstet Gynecol. 2010;202(1):85 e1–85 e9. doi: 10.1016/j.ajog.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quintero RA, Morales WJ, Allen M, et al. Treatment of iatrogenic previable premature rupture of membranes with intra-amniotic injection of platelets and cryoprecipitate (amniopatch): preliminary experience. Am J Obstet Gynecol. 1999;181(3):744–749. doi: 10.1016/s0002-9378(99)70522-3. [DOI] [PubMed] [Google Scholar]
- 7.Engels AC, Van Calster B, Richter J, et al. Collagen plug sealing of iatrogenic fetal membrane defects after fetoscopic surgery for congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2014;43(1):54–59. doi: 10.1002/uog.12547. [DOI] [PubMed] [Google Scholar]
- 8.Papanna R, Mann LK, Moise KY, et al. Absorbable gelatin plug does not prevent iatrogenic preterm premature rupture of membranes after fetoscopic laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2013;42(4):456–460. doi: 10.1002/uog.12487. [DOI] [PubMed] [Google Scholar]
- 9.Luks FI, Deprest JA, Peers KH, et al. Gelatin sponge plug to seal fetoscopy port sites: technique in ovine and primate models. Am J Obstet Gynecol. 1999;181(4):995–996. doi: 10.1016/s0002-9378(99)70338-8. [DOI] [PubMed] [Google Scholar]
- 10.Barrett DW, David AL, Thrasivoulou C, et al. Connexin 43 is overexpressed in human fetal membrane defects after fetoscopic surgery. Prenat Diagn. 2016;36(10):942–952. doi: 10.1002/pd.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mi S, David AL, Chowdhury B, et al. Tissue engineering a fetal membrane. Tissue Eng Part A. 2012;18(3–4):373–381. doi: 10.1089/ten.TEA.2011.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels AC, Hoylaerts MF, Endo M, et al. In vitro sealing of iatrogenic fetal membrane defects by a collagen plug imbued with fibrinogen and plasma. Prenat Diagn. 2013;33(2):162–167. doi: 10.1002/pd.4032. [DOI] [PubMed] [Google Scholar]
- 13.Chin CJ, Kus L, Rotenberg BW. Use of duraseal in repair of cerebrospinal fluid leaks. J Otolaryngol Head Neck Surg J Oto-Rhino-Laryngol Chir Cervico-Faciale. 2010;39(5):594–599. [PubMed] [Google Scholar]
- 14.Gratacos E, Yamamoto H, Papadopulos NA, et al. The midgestational rabbit as a model for the creation of membrane defects after needle fetoscopy. Am J Obstet Gynecol. 1999;180(5):1263–1267. doi: 10.1016/s0002-9378(99)70626-5. [DOI] [PubMed] [Google Scholar]
- 15.Badylak S, Kokini K, Tullius B, et al. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103(2):190–202. doi: 10.1006/jsre.2001.6349. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood MP, Kampmeijer A, Jimenez J, et al. The effect of transplacental administration of glucagon-like peptide-1 on fetal lung development in the rabbit model of congenital diaphragmatic hernia. Fetal Diagn Ther. 2016;39(2):125–133. doi: 10.1159/000436962. [DOI] [PubMed] [Google Scholar]
- 17.Devlieger R, Ardon H, Verbist L, et al. Increased polymorphonuclear infiltration and iatrogenic amniotic band after closure of fetoscopic access sites with a bioactive membrane in the rabbit at midgestation. Am J Obstet Gynecol. 2003;188(3):844–848. doi: 10.1067/mob.2003.213. [DOI] [PubMed] [Google Scholar]
- 18.Lee S-H, Park C-W, Lee S-G, Kim W-K. Postoperative cervical cord compression induced by hydrogel dural sealant (DuraSeal®) Korean J Spine. 2013;10(1):44–46. doi: 10.14245/kjs.2013.10.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devlieger R, Millar LK, Bryant-Greenwood G, et al. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: a review of current evidence. Am J Obstet Gynecol. 2006;195(6):1512–1520. doi: 10.1016/j.ajog.2006.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papanna R, Mann LK, Moise KJ, Jr, et al. Histologic changes of the fetal membranes after fetoscopic laser surgery for twin-twin transfusion syndrome. Pediatr Res. 2015;78(3):247–255. doi: 10.1038/pr.2015.105. [DOI] [PubMed] [Google Scholar]
- 21.Asabe K, Toki N, Hashimoto S, et al. An immunohistochemical study of the expression of surfactant apoprotein in the hypoplastic lung of rabbit fetuses induced by oligohydramnios. Am J Pathol. 1994;145(3):631–639. [PMC free article] [PubMed] [Google Scholar]