Abstract
The development of an effective vaccine against respiratory syncytial virus (RSV) has been hampered by major difficulties that occurred in the 1960s when a formalin-inactivated vaccine led to increased severity of RSV disease after acquisition of the virus in the RSV season after vaccination. Recent renewed efforts to develop a vaccine have resulted in about 38 candidate vaccines and monoclonal antibodies now in clinical development. The target populations for effective vaccination are varied and include neonates, young children, pregnant women, and older adults. The reasons for susceptibility to infection in each of these groups may be different and, therefore, could require different vaccine types for induction of protective immune responses, adding a further challenge for vaccine development. Here, we review the current knowledge of RSV vaccine development for these target populations and propose a view and rationale for prioritizing RSV vaccine development.
The Challenges Of Developing An Effective Rsv Vaccine
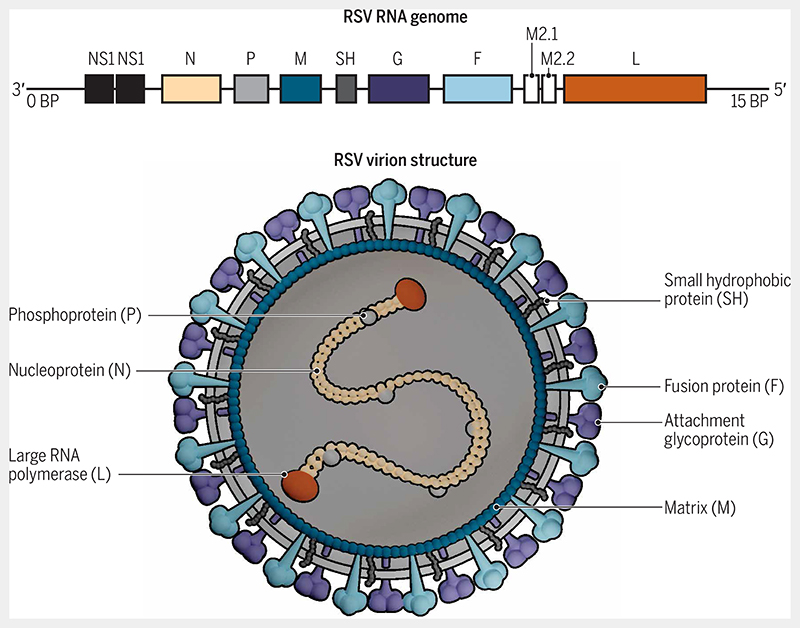
Respiratory syncytial virus (RSV) is a major cause of severe respiratory tract infection worldwide and a major pathogen for which there is no vaccine or clinically effective treatment. RSV belongs to the order Mononegavirales, family Pneumoviridae, and genus Orthopneumovirus. It is an RNA virus containing 10 genes that encode 11 proteins (Fig. 1). These proteins include two nonstructural proteins (NS1 and NS2); four envelope proteins: attachment glycoprotein (G), fusion protein (F), matrix protein (M), and small hydrophobic protein (SH); and five ribonucleocapsid proteins: nucleoprotein (N), phosphoprotein (P), large RNA polymerase (L), M2-1 (a zinc-binding transcription antiterminator), and M2-2 (a regulatory factor involved in the balance between RNA replication and transcription). Transcriptional mapping studies have demonstrated that gene transcription of RSV occurs in a sequential manner in the following order: NS1, NS2, N, P, M, SH, G, F, M2, and L.
Fig. 1. Structure of RSV.
The RNA genome of RSV consists of 10 genes encoding 11 proteins. These proteins include two nonstructural proteins (NS1 and NS2); four envelope proteins: attachment glycoprotein (G), fusion protein (F), matrix protein (M), and small hydrophobic protein (SH); and five ribonucleocapsid proteins: nucleoprotein (N), phosphoprotein (P), large RNA polymerase (L), M2-1 (a zinc-binding transcription antiterminator), and M2-2 (a regulatory factor involved in the balance between RNA replication and transcription). In terms of vaccine development, the most important protein is the F protein. The F protein in the outer envelope of the RSV virion is highly conserved among RSV strains, making it an excellent potential vaccine target. The F protein has two forms, prefusion and postfusion, with the prefusion form being less stable but more immunogenic than the postfusion form [adapted from (132)].
RSV infection results in the hospitalization of large numbers of children under 5 years of age worldwide. A large systematic review estimated that RSV caused 33.1 million episodes of RSV acute lower respiratory tract infection, 3.2 million hospital admissions, and 59,600 in-hospital deaths in 2015 globally. Ninety-nine percent of deaths occur in low- and middle-income countries (1). RSV infection in infancy is also associated with the subsequent development of chronic respiratory morbidity (e.g., asthma). Epidemiological data on RSV infections are more sparse in adults, but it is estimated to cause up to 5% of community-acquired pneumonia, mainly in older adults and those with comorbidities in whom there is a 9 to 12% case fatality rate (2). Recently, it has been shown that more primary care doctor visits, hospitalizations, and deaths are attributable to RSV in older adults than to influenza (3). Because of major advances in new bio-logical platforms for antigen delivery and advances in structural biology for improved epitope presentation, there is now the real prospect of RSV disease control through vaccination. As of January 2020, there are 38 vaccine and monoclonal antibody candidates in clinical development (4), with new vaccine designs under investigation (5). The pipeline of promising vaccine candidates for RSV includes vaccines targeted at both pediatric and adult populations. The global distribution of different RSV clinical trials for vaccines (and antiviral drugs) is shown in Figs. 2, 3, and 4 according to the type of intervention tested (Fig. 2), the phase of the clinical trial (Fig. 3), and the clinical trial completion status as of December 2019 (Fig. 4).
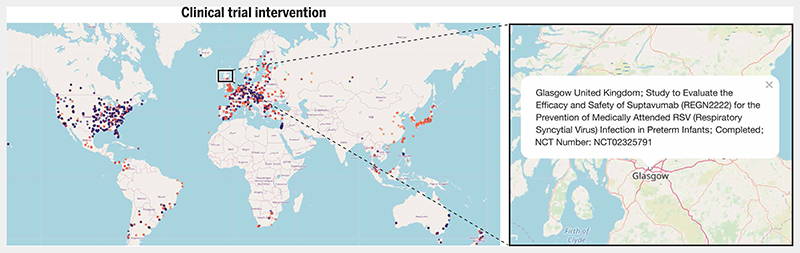
Fig. 2. Different interventions tested in RSV clinical trials worldwide.
The global map shows the distribution of all RSV vaccine and drug trials worldwide. The inset shows an expanded view of clinical trial sites in Scotland. Each dot on the global map represents a single clinical trial site and is color-coded according to the type of intervention tested. Dots are in translucent colors to prevent overlapping dots (depicting proximal trial sites) from obscuring each other. Blue dots represent biologicals (vaccines and monoclonal antibodies), red dots represent antiviral drugs, and yellow dots represent other study designs including observational studies. Clicking on the blue box connects to an interactive map (https://rsvclinicaltrials.org/interventions.html) where each clickable dot produces a popup box containing detailed information about a particular study site. Here, the popup box provides details of a clinical trial at a study site in Glasgow, UK, for evaluating the safety and efficacy of the antiviral drug suptavumab in preterm infants. Source data for this interactive map were downloaded from clinicaltrials.gov (in December 2019). The search term used in the “condition or disease” field was “Respiratory Syncytial Virus”. All clinical trials that fulfilled this search criterion were added to the source data file that was used to generate the maps.
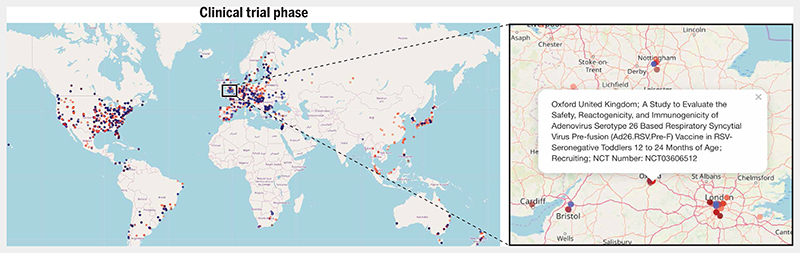
Fig. 3. Global distribution of RSV clinical trials at different phases.
Dots on the global map are color-coded according to the clinical trial phase: Phase 1 trials are shown in maroon, combined phase 1/2 trials are shown in dark red, and phase 2 trials are shown in light red. Combined phase 2/3 trials are shown in light blue and phase 3 trials are shown in dark blue. Purple dots indicate phase 4 trials. Dots are in translucent colors to prevent overlapping dots (depicting proximal trial sites) from obscuring each other. Clicking on the blue box connects to an interactive map (https://rsvclinicaltrials.org/phase.html) where each clickable dot produces a popup box containing detailed information about a particular study site. Here, the popup box provides details of a phase 1/2 trial of an adenovirus vector RSV vaccine in seronegative toddlers aged 12 to 24 months conducted at a trial site in Oxford, UK. Source data for this interactive map were downloaded from clinicaltrials.gov (in December 2019). The search term used in the “condition or disease” field was “Respiratory Syncytial Virus”. All clinical trials that fulfilled this search criterion were added to the source data file that was used to generate the maps.
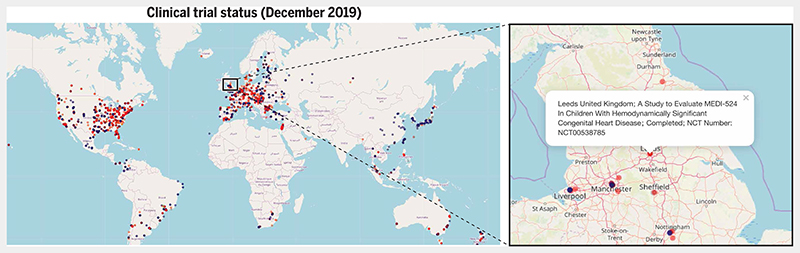
Fig. 4. Status of RSV clinical trials worldwide.
The recruitment status of different RSV vaccine and drug clinical trials as of December 2019 is shown. Yellow dots indicate trials that were active but had not yet started recruitment as of December 2019; red dots indicate completed trials. Green dots indicate inactive trials that had not yet begun recruitment as of December 2019; blue dots indicate trials that were actively recruiting in December 2019. Gray, purple, and black dots indicate trials that were terminated, of unknown status, or were halted, respectively. Dots are plotted in translucent colors to prevent overlapping dots (depicting proximal trial sites) from obscuring each other. Clicking on the blue box connects to an interactive map (https://rsvclinicaltrials.org/status.html) where each clickable dot produces a popup box containing detailed information about a particular study site. Here, the popup box provides details of a trial that evaluated an anti-RSV monoclonal antibody (MEDI-524) in children with congenital heart disease conducted at a trial site in Leeds, UK. Source data for this interactive map were downloaded from clinicaltrials.gov (in December 2019). The search term used in the “condition or disease”field was “Respiratory Syncytial Virus”. All clinical trials that fulfilled this search criterion were added to the source data file that was used to generate the maps.
Severe RSV disease occurs very early in life, typically between the second and third months of life (6), providing limited opportunity for intervention through national immunization programs. This means that a single-dose vaccine would have to be given, or several doses given at very short intervals, to provide protection within the first month of life. Antibody responses are typically of lower magnitude in early infancy (7), and the presence of high titers of maternally derived antibodies (8) is likely to blunt the infant response to vaccination, making induction of protective responses more challenging at this age (9). The risk of severe disease is also elevated in immuno-compromised or immunosuppressed (10) individuals and older adults (11), in whom immunosenescence and underlying comorbidities compromise vaccine responses.
The demographic and immunological risk factors for developing severe RSV disease are different in infants and adults, although any major cardiac, respiratory, or immunological comorbidity increases the risk at any age. It is, therefore, likely that vaccine-induced immune responses required to provide protection against RSV will be different in each population, and an RSV vaccine may not result in sterilizing immunity but rather may prevent severe disease. The argument that future RSV vaccines are unlikely to achieve sterilizing immunity is supported by the fact that neither natural (12) nor experimental human infection (13) induces robust immunity against reinfection. In addition, regulators will probably require large safety databases to ensure that there is no increased risk of severe disease or death upon subsequent natural infection as happened with historical RSV vaccines (14). In this Review, we explore the past and present RSV vaccine landscape and examine the different vaccines and monoclonal anti-bodies currently in development.
The History Of Pediatric Rsv Vaccines
After the successful development of formalin-inactivated vaccines for poliovirus, measles, and parainfluenza in the 1950s (15, 16), studies of formalin-inactivated RSV (FI-RSV) vaccines were conducted in the United States in the mid- to late 1960s, within 10 years of the first description of RSV. A preliminary study of an FI-RSV vaccine showed that children and adults inoculated intramuscularly developed modest serum neutralizing antibodies and did not exhibit any severe vaccine-related adverse effects for up to 10 days after vaccination (17). This vaccine was made from a crude extract of RSV-infected Vervet monkey kidney cells, clarified by centrifugation, formalin-inactivated and alum-precipitated, and concentrated 100-fold (18). A series of large-scale clinical trials of that FI-RSV vaccine were subsequently carried out in infants and young children in the 1960s. In one study, infants and children between 4 months and 10 years old (n = 191) were given two intramuscular doses of the FI-RSV vaccine, whereas children in an active control arm (n = 194) received a trivalent parainfluenza vaccine (18). In concordance with previous results, 68% of the FI-RSV vaccinees had a fourfold or greater rise in RSV antibodies in their postvaccination sera, compared with only 0.9% of controls (18). However, in the subsequent RSV season, the incidence of severe disease in the FI-RSV vaccine group (7.9%) was almost double that in the control group (4.7%) (18). Enhanced respiratory disease was, however, only detected in FI-RSV vaccinees younger than 2 years of age and not older children (18). Sixty percent of the FI-RSV vaccinees infected with natural RSV were hospitalized compared with 22% of controls (18).
In another study, infants between 2 and 7 months of age were vaccinated with an FI-RSV vaccine and postvaccination serum RSV neutralizing antibody titers were found to be sixfold greater in the FI-RSV vaccine group compared with the parainfluenza vaccine control group (14). However, despite serological evidence of comparable exposure between the two groups in the subsequent RSV season, 80% of FI-RSV vaccinees in this study required hospitalization after natural infection compared with only 5% of the control group (14). Tragically, two toddlers who had received the FI-RSV vaccine died upon natural exposure to RSV. Postmortem examinations found evidence of extensive bronchopneumonia, pneumothorax, and eosinophilia (14). The outcome from these studies was that while the FI-RSV vaccine appeared safe, immunogenic, and well tolerated by conventional measures in the postvaccination period, it had induced an aberrant immune response to natural virus. This resulted in a more severe, potentially life-threatening, pulmonary immunopathology. These disastrous trials mandated extensive investigation into understanding the mechanisms underlying the enhanced respiratory disease associated with the FI-RSV vaccine.
An entirely different formulation of FI-RSV was tested in children in the mid-1960s. In one trial conducted in Pennsylvania, an alum-adjuvanted, FI-RSV formulation was concentrated 22-fold and administered intramuscularly to children between the ages of 3 and 5 years, in parallel with formalin-inactivated parainfluenza and Mycoplasma pneumoniae vaccines. A priming dose of each vaccine was given between late October and early November 1965, and booster doses of each formulation were administered 3 to 4 weeks later. About 45% of children who had initially been classified as RSV seronegative developed a greater than fourfold increase in antibody after the boosting dose, whereas only about 11% of previously seropositive children exhibited a similar fold increase in antibody. In the postvaccination surveillance period that ran until May 1966, active clinical assessment visits were undertaken, and it was determined that the vaccines were generally safe, with only a few children reporting respiratory symptoms that were classified as severe. Unlike the trials described above, there did not appear to be enhanced respiratory disease attributable to vaccination. Despite this, compared with an unvaccinated control group, the vaccinated group was not protected against RSV disease after natural exposure (19). In a separate trial carried out in the same location between October and December 1966, these vaccines—FI-RSV; FI-parainfluenza virus types 1, 2, and 3; and FI-M. pneumoniae—were combined into a single vaccine formulation and administered to toddlers between the ages of 3 and 5 years. In the 5-month postvaccination follow-up period, there appeared to be a protective effect against severe respiratory disease, although this effect was only apparent in the first 2 months of follow-up (20).
Further clinical trials of new RSV vaccine candidates, except for live-attenuated vaccines, needed to wait until animal models of enhanced respiratory disease were sufficiently well developed and capable of reproducing FI-RSV vaccine−like−associated immunopathology after experimental challenge with RSV. A number of animal models of RSV infection have been developed using the cotton rat Sigmodon hispidus, mice, African green monkeys, colostrum-deprived calves (challenged with bovine RSV as a translational model for seronegative infants), and lambs (21). Animal challenge studies and the postmortem findings from the infant fatalities have been used to extensively investigate FI-RSV vaccine−associated enhanced respiratory disease. Early investigations found that children vaccinated with the FI-RSV vaccine failed to develop neutralizing antibody titers comparable to those of age-matched individuals who had undergone natural infection with RSV. These studies postulated that these nonneutralizing anti-bodies could have potentiated disease either through the formation of immune complexes in the lung or through the stimulation of a suboptimal response to the virus attachment glycoprotein (G) in young infants. It was also proposed that severe disease was the result of poorly neutralizing antibodies that delayed the development of effective immune responses needed to clear the virus (22). Subsequent studies found that in addition to the poorly neutralizing antibody response, antibodies that were specific for the virus fusion (F) protein, which mediates fusion of the virus envelope and the host cell plasma membrane, were deficient in fusion-inhibiting activity, promoting the spread of the virus in the respiratory tract upon natural infection (23). Later work suggested that the failure to develop an effective neutralizing response after FI-RSV vaccination was not due to formalin disruption of neutralizing epitopes but rather was due to the development of low-avidity anti-RSV antibodies resulting from the lack of affinity maturation (24). This view, however, has been disputed (25). Later studies showed that treatment of RSV antigens with formalin promoted the development of T cell helper type 2 (TH2) responses in children (26) and that disease exacerbation was the result of an over-exuberant inflammatory response to RSV infection. More recent analyses have demonstrated that formalin and heat inactivation of RSV promote a fast and irreversible transition from the prefusion to the postfusion conformation of the F protein and, in its wake, an almost complete loss of epitopes that are sensitive to antibody neutralization (27). This history continues to cast a long shadow over further RSV vaccine development.
Development Of Vaccines For Active Infant Immunization
Current and future RSV vaccine candidates require careful preclinical evaluation in animal challenge models and, provided no FI-RSV vaccine immunopathology is observed, can then progress from phase 1 clinical trials in healthy adults through a series of age de-escalation trials toward seronegative infants. Studies should include the response in infants over the subsequent RSV transmission season and a longer period of safety observation (28). Although many animal-based studies have been used to postulate the mechanisms by which FI-RSV vaccines potentiated natural infection (22–24, 26, 29, 30), there are uncertainties as to which, if any, of these mechanisms can be feasibly extrapolated to human infants. The FI-RSV vaccine also raised concerns regarding the use of nonreplicating RSV vaccines in seronegative infants. To date, the only RSV vaccine type that has been safely used in seronegative infants is a live-attenuated vaccine (Table 1). Live-attenuated vaccines have a number of features that make them particularly attractive as a platform for delivering virus antigens to the seronegative infant. The intranasal delivery of the vaccines provides an opportunity to directly stimulate mucosal immunity, resulting in the development of functional immunity at the point of contact between the virus and the host (31) and reducing the risk of immune suppression mediated by passively acquired maternal antibodies (9). In adults, the quantity of RSV-specific nasal immunoglobulin A (IgA) antibody has been identified as a major factor in the risk of RSV infection despite the background of robust immune responses in blood (32). Live-attenuated RSV vaccines also have the advantage of a strong safety track record in seronegative infants. A consistent feature of these vaccines has been the lack of enhanced respiratory disease upon subsequent infection with wild-type virus. Notwithstanding this safety record, these vaccines have historically struggled to strike the right balance between achieving enough attenuation for safety and sufficient virulence to induce and maintain protective immunity (33). Despite this, encouraging developments have emerged in this field. By leveraging powerful reverse genetics approaches, recent studies have investigated vaccines containing attenuating mutations on the virus backbone that yield a high degree of attenuation while retaining immunogenicity in animal models (34). These developments raise the prospect of licensure of a replicating RSV vaccine for the seronegative pediatric population in the years ahead. However, this prospect must be tempered by potential concerns about reversion to the wild-type virus, transmission of vaccine virus between household and other contacts, and nasal congestion, which is a big concern in the youngest infants who are obligate nasal breathers (33). Previous clinical trials of live-attenuated RSV vaccines have demonstrated a considerable transmission risk, with one study reporting a transmission rate of 20 to 25% of the vaccine virus to placebo recipients. The same study also reported a case of postvaccination wheezing in a child who had received the vaccine (35).
Table 1. Published studies of recent RSV vaccine candidates tested in clinical trials in different population groups.
Shaded cells signify that the study was carried out in that population. References are in parentheses.
| Vaccine | Class | Adults | Seropositive children | Seronegative children | Pregnant women | Older adults |
|---|---|---|---|---|---|---|
| FI-RSV (14, 17) | FIV* | |||||
| F, G, M subunit (89, 90] | Subunit | |||||
| F-nanoparticle (55, 81] | Nanoparticle | |||||
| Chimpanzee adenovirus RSV vaccine (39) | VVV† | |||||
| BBG2Na (91, 92) | Subunit | |||||
| ΔM2-2 (93) | LAV‡ | |||||
| rA2cp248/404/1030ΔSH (94) | LAV | |||||
| cpts530/1009 (35) | LAV | |||||
| RSV ts-2 (95) | LAV | |||||
| MEDI-559 (96) | LAV | |||||
| MEDI-534 (97–99) | LAV | |||||
| RSV ts-1 A, B, and C (100, 101) | LAV | |||||
| cpts248/955 (35) | LAV | |||||
| cp-52B (102) | LAV | |||||
| rA2cpΔNS2 (103) | LAV | |||||
| cpRSV (104, 105) | LAV | |||||
| RSV ts-1 (106–108) | LAV | |||||
| Cpts248/404 (109,110) | LAV | |||||
| rA2cp248/404ΔSH (94) | LAV | |||||
| rA2cp248/404/1030ΔNS2 (103) | LAV | |||||
| rA2cp530/1009ΔNS2 (103) | LAV | |||||
| PFP1 (111–116) | Subunit | |||||
| PFP2 (110, 117–121) | Subunit | |||||
| PFP3 (122) | Subunit | |||||
| MEDI-7510 (123) | Subunit | |||||
| F nanoparticle (124, 125) | Nanoparticle | |||||
| Pre-F (126) | Subunit | |||||
| Soluble post-F (127) | Subunit | |||||
| Small hydrophobic protein ectodomain (128) | Subunit | |||||
| RSVcps2 (129) | LAV | |||||
| LIDΔM2-2 (130) | LAV | |||||
| RSV Pre-F (131) | Recombinant |
FIV, formalin-inactivated vaccine.
VVV, viral vector vaccine.
LAV, live-attenuated vaccine.
In addition to live-attenuated vaccines, one platform that is likely to be appropriate for delivering RSV antigens to seronegative infants is genetically modified viral vectors such as adeno-associated virus. Viral vectors can be genetically engineered to limit or abolish their replication (36), a safety feature that reduces the risk of unchecked viral replication within the host and potential transmission to others. Viral vector vaccines have been shown to induce immune responses against pathogens causing tuberculosis (37) and malaria (38), RSV (39), and influenza virus (40). They have been tested in different target populations including 10-week-old infants (41), where they have been reported to be safe. Coupled with the relative ease with which transgenes can be inserted into the viral vector backbone, viral vectors appear to be an ideal platform for the delivery of RSV antigens to seronegative infants. The biggest hurdle to overcome with viral vector vaccines is the host immune response to the viral vector, which might reduce the immune response to the antigenic target. This can potentially be surmounted by using higher doses and heterologous prime-boost vaccine regimens (42). A further potential disadvantage of this viral vector−specific immunity is the possibility that the buildup of host immunity against the vector might increasingly preclude its sequential use as a delivery platform for alternative vaccine antigens. Two clinical trials of RSV viral vector vaccines are ongoing in infants and toddlers (ClinicalTrials.gov Identifiers: NCT03303625, using an adenovirus serotype 26 RSV prefusion conformation-stabilized F protein vaccine, and NCT03636906, using a recombinant chimpanzee adenovirus type 155−vector RSV vaccine).
Monoclonal Antibodies For Protecting The Neonatal Population
Because of the difficulties in developing a vaccine against RSV for neonates, another approach is passive immunization with a monoclonal antibody. Palivizumab, a humanized mouse monoclonal antibody that is directed against the RSV F protein, was developed in the 1990s and has been shown to be up to 80% effective in preventing severe RSV infection in selected groups of neonates (43). It has a relatively short half-life (about 20 days), and thus, monthly intramuscular injections are required during the RSV season to provide protection. It is also expensive, thus limiting its use to very high risk individuals (e.g., those born extremely prematurely with chronic lung disease of infancy or infants with major congenital cardiac disease) in high-income countries (44). Motavizumab, a similar but more potent RSV monoclonal antibody, was found to be noninferior to palivizumab in a large multicenter clinical trial (45). However, after the U.S. Food and Drug Administration (FDA) declined a licensing request, partly due to the lack of evidence of superiority to palivizumab, motavizumab’s development was discontinued (46). The phase 3 NURSERY clinical trial recently investigated suptavumab, an anti-RSV monoclonal antibody requiring only one or two doses over the RSV season. More than 1110 healthy preterm infants were recruited, but unfortunately, the study failed to meet its primary end point of preventing RSV infection requiring a medical attendance, and its development has been discontinued (47). The results of this trial are yet to be formally published but were presented at the 11th International RSV symposium in 2018 (https://rsvsymposium.com). It was highlighted that the reason for the failure of the NURSERY study was the development of a dominant mutation in the F protein of RSV-B isolates, which is the antibody’s binding site.
There are two anti-RSV monoclonal antibodies currently undergoing clinical evaluation, MEDI8897 (48) and MK-1654 (49). MEDI8897 is being investigated in a phase 2 clinical trial (ClinicalTrials.gov Identifier: NCT02878330). In vitro, it has been shown that MEDI8897 targets the prefusion conformation of the RSV F protein and neutralizes both RSV A and B strains with more than 50-fold greater activity than palivizumab (50). A phase 1b/2a dose-escalation study including healthy prematurely born infants (gestational age, 32 to 35 weeks) demonstrated that 5 months after a single intramuscular dose of MEDI8897, 90% of the infants still had a ≥4-fold rise from baseline in serum RSV-neutralizing antibodies, and 87% had serum concentrations above the 90% effective concentration target (48). Those data suggested that a single dose of MEDI8897 would provide protection throughout a typical RSV infection season, except perhaps in regions where RSV circulates throughout the year. One potential concern with any immunization is the induction of mutations in the virus leading to viral escape. An in vitro study investigating viral escape for MEDI8897 found that natural resistance-associated mutations were rare and that escape variants and their parental virus replicated at similar rates, suggesting that resistance-associated substitutions may not develop a replication advantage over naturally circulating strains (51). A phase 1 clinical trial investigating MK-1654 (ClinicalTrials.gov Identifier: NCT03524118) in preterm and full-term infants commenced in September 2018 and is due to be completed in August 2020 (49). The development of a cheap, single-dose monoclonal antibody to protect infants over a whole RSV season could substantially reduce the burden of disease in this cohort, and thus, the results of these studies are eagerly awaited.
Maternal Vaccination And Other Vaccination Strategies
The unfortunate legacy of the FI-RSV vaccine experience and the narrow epidemiological window available for intervention has caused some reluctance by pharmaceutical companies to develop products for the seronegative infant population. This has raised the question of whether alternative population groups can be vaccinated to provide both direct and indirect protection to the infant. In children, older age even within the first year of life is an independent protective factor against the development of severe disease. Therefore, even a modest extension to the period of protection afforded by maternal antibodies could translate into a disproportionate reduction in the burden of severe disease. We next consider the most practical vaccination strategies as well as the barriers that stand in the way of their successful implementation and assess their potential in alleviating the considerable disease burden caused by RSV.
In infants, the peak of severe RSV disease risk occurs in the first 2 months of life (6, 52). Maternal vaccines could protect infants during this window of elevated risk. The last few years have seen an increase in the number of RSV vaccine candidates that are targeted at pregnant women with the aim of boosting RSV-specific antibody that is available for transplacental transfer. Transplacental IgG transfer is an active and efficient physiological process that results in the transport of high titers of protective antibodies from maternal to fetal circulation (53). That passive immunoprophylaxis with palivizumab can reduce hospitalization in infants with risk factors for severe disease by up to 80% has been a powerful demonstration that serum antibodies specific for the F protein alone can be protective in infants (43). Maternal vaccination has the potential to deliver enormous health benefits and substantially reduce infant morbidity and mortality as illustrated by the sharp reduction and near elimination of neonatal tetanus, which is largely attributable to maternal vaccination (54). In addition to the infant, there are limited data on the potential benefit of maternal vaccination to pregnant women. A previous phase 2 clinical trial of a maternal RSV nanoparticle vaccine tested in healthy women of childbearing age (n = 330) showed that 11% of vaccinees had serological evidence of new RSV infection compared with 21% of unvaccinated controls (55). These data suggest that besides the benefit to the infant, a maternal RSV vaccine would also give some protection to the mother. Available data suggest that maternal vaccination is safe and not associated with adverse maternal or neonatal outcomes. Analysis of data from the Vaccine Adverse Events Reporting System (VAERS) in the United States shows that there is no increase in the rate of spontaneous abortion in vaccinated women compared with the rate of this outcome in the general population (56).
The potential global impact of maternal RSV vaccines depends on access to antenatal care. Recent estimates suggest that about 81% of pregnant women across the world attend at least one antenatal care visit although specific estimates vary between countries (57). Women from low-income backgrounds have the poorest coverage, with about 72% attending at least one antenatal care visit compared with 99% of women from higher- and middle-income backgrounds (57). Overall, about 55% of pregnant women across the globe attend at least four antenatal clinic visits over the course of their pregnancy (57). Although these relatively high access rates provide some reassurance of the global potential of maternal RSV vaccination programs, the timing of these visits is a critical factor for the success of these programs, as is having trained immunizers in antenatal clinics.
The most advanced maternal vaccine candidate is a nanoparticle vaccine, which is a recombinant near−full-length RSV F protein produced in Spodoptera frugiperda insect cells with a recombinant baculovirus (58). The vaccine targets the RSV F protein and contains a highly conserved antibody epitope (site II), which is the target of palivizumab. Earlier-phase clinical trials have shown that antibodies induced by vaccination appear to provide protection to vaccinated women against reinfection (55). However, top-line data from the recently completed phase 3 clinical trial (ClinicalTrials.gov identifier NCT02624947) showed that the vaccine just failed to reach its primary end point of prevention of medically notable RSV lower respiratory tract infection. The study did show 44% efficacy of the vaccine against RSV lower respiratory tract infection hospitalizations and 48% efficacy against RSV lower respiratory tract infection with severe hypoxemia (59). There are now ongoing discussions about possible licensure pathways.
Maternal RSV vaccination faces a number of important hurdles. A major concern for global rollout is that maternal diseases such as placental malaria, HIV, and hypergammaglobulinemia can potentially reduce the efficiency of transplacental antibody transfer (60, 61), and the prevalence of these diseases is geographically variable. It is conceivable that in parts of the world where diseases such as malaria are endemic, the effectiveness of maternal vaccination might be substantially reduced relative to regions with a lower disease burden. Another concern relates to the likelihood of achieving adequate protection for newborn infants. Naturally acquired maternal RSV antibodies confer limited protection to the infant (52), suggesting that vaccine-induced antibodies will need to substantially exceed the protective efficacy of maternally derived antibodies. In addition, prematurity is a major risk factor for RSV infection, because of the reduced opportunity for transplacental antibody transfer, which may be entirely absent among those born extremely prematurely. Thus, any vaccine given late in pregnancy will not affect this vulnerable population.
A further complication for maternal vaccination programs is the variable epidemiology of RSV across the globe. In temperate regions, an annual pattern is usually limited to 3 to 5 months during the autumn and winter seasons, whereas in tropical climates, RSV transmission is sustained all year round. Thus, the duration of protection from a maternal vaccine needed to make an impact on hospitalizations due to RSV infections will be different according to geographic location (62, 63). National vaccine programs may also need to vary to be cost-effective, with analyses of the timing of vaccination needing to take into account seasonal vaccination in temperate climates versus year-round vaccination in tropical climates (64, 65). The best time to vaccinate during pregnancy is also unclear. Most maternal vaccine trials have vaccinated during the third trimester; however, there is emerging evidence that vaccinating earlier in pregnancy, from 16 weeks of gestation, may result in higher vaccine-induced neonatal antibodies for maternal influenza vaccines (65). The impact of other maternal vaccines (e.g., those for influenza and pertussis) on transfer of RSV antibody to infants after maternal RSV vaccination is also currently unknown. For infants, combining approaches, i.e., maternal vaccination and subsequent infant immunization, may also be possible, although this would need to be cost-effective.
Vaccination Of Toddlers, Older Children, And Older Adults
Although the highest burden of RSV disease is in infants and older adults, there are still major health care costs associated with RSV infection in older children, particularly in the primary care setting (66). In addition, reducing the circulation of RSV by vaccinating older children may reduce the impact on infants and older adults indirectly, by reducing shedding, as is the case with influenza vaccination (67). However, herd immunity can only be demonstrated in phase 4 postlicensure studies. Efforts, therefore, have been made to develop vaccines for older children (Table 1). There are currently 10 RSV vaccines targeting toddlers as well as older adults that are undergoing early-stage clinical trials (4). These include an adenovirus-vector RSV vaccine (replication deficient) in a phase 2 clinical trial (ClinicalTrials.gov Identifier: NCT03303625) that is recruiting adults and RSV-seropositive toddlers 12 to 24 months old, with results expected in 2020.
Although there are few data on the global burden of RSV disease in older adults, a consistent feature of the available information suggests that the morbidity and mortality burden due to RSV in older adults is similar to that caused by seasonal influenza (66, 68–72). One of the few prospective studies that investigated the relative incidence of RSV and influenza infections over four winter seasons showed a mean incidence of 5.5 RSV infections per 100 individuals per season compared with an estimate of about 2.2 influenza infections per 100 individuals per season (11). The seasonal infection rates for RSV for older adults appear to be the same as those measured in young healthy adults but greater rates of progression to lower respiratory tract infection and severe disease are notable with increasing age after 65 years. It should be noted that these studies were performed in populations with influenza vaccination available for older adults, thus potentially affecting the influenza epidemiology of this group. Most older adults who are hospitalized with RSV infection have comorbid conditions. For example, 14 to 68% of elderly adults hospitalized with severe RSV infection have underlying lung disease and 14 to 63% have underlying heart disease (2, 73–75). Overall, more than 70% of hospitalized older adults will have one or both of these conditions (2).
Development of RSV vaccines targeted at older adults faces several hurdles. These include the lack of sufficiently sensitive clinical end points for detecting disease in older adults, the absence of a population-specific immune correlate of protection, the high prevalence of comorbid conditions, which are likely to confound the assessment of clinical end points of vaccine efficacy, and the low and variable rates of infection necessitating very large and expensive studies to demonstrate protective efficacy. There remains uncertainty about whether the increased risk of severe disease in this population is associated with age-related changes in cellular or humoral immunity or both (76). A widely held view is that the goal of older adult vaccination should be the augmentation of T cell immunity as there is evidence that the amount of serum neutralizing antibody in older adults appears to be no different from that of younger adults (77), whereas the RSV-specific T cell responses of older adults appear to become attenuated with age (78).
Recent years have seen an expansion of vaccine candidates targeted at older adults. The most advanced of these programs to date is the previously highlighted nanoparticle vaccine for which a phase 3 clinical trial has recently been concluded. Unfortunately, the results of the trial showed no evidence of protection against lower respiratory tract disease (79). Although the results of this trial are disappointing, the pipeline of promising vaccine candidates and antigen delivery platforms that could be suitable for this population continues to expand. Prefusion-stabilized F protein subunit vaccines are undergoing clinical trials, including in older adults (ClinicalTrials.gov Identifier: NCT03572062). Trials of viral vector vaccines expressing viral targets of both T and B cell immunity are being tested in older adults and carry the potential to overcome age-related immunosenescence by augmenting these critical arms of adaptive immunity against RSV. Recent developments in the structural design of nonreplicating vaccines have opened up new prospects for development of effective vaccines for different adult population target groups, including older adults. A recent study has reported the successful development of self-assembling nanoparticle formulations presenting prefusion-stabilized F proteins in a polymeric array on a nanoparticle scaffold. Preclinical analyses have shown that, in this configuration, the prefusion-stabilized F protein nanoparticles induced >10-fold higher neutralizing antibodies than did previous trimeric formulations of prefusion-stabilized F protein (80). These encouraging developments continue to provide reassurance that a vaccine against RSV in older adults may be achievable.
Animal Models In Rsv Vaccine Research
Well-conducted animal studies can provide powerful data to support the advancement of vaccine candidates to the clinical evaluation stage (38). Although many immunological responses to vaccination in preclinical animal models correlate reasonably well with human immune responses (81), the central role of animal models in RSV vaccine research is as predictors of potential vaccine-induced pathology. Animals can, therefore, be used as in vivo models for assessing the complex immune and physiological mechanisms that underlie vaccine-related pathologies.
Animal models of RSV infection, such as the mouse and cotton rat, have been used to replicate the complex immunopathological mechanisms of the FI-RSV vaccine (82, 83). Although invaluable for such mechanistic research, these models have shortcomings that limit their potential extrapolative value in the forecast of infant responses to vaccination (84). Early murine RSV studies showed that there was up to a 100-fold difference in the infectivity of mice with different genetic backgrounds (85), suggesting that the genetic background of the animal and not the intrinsic pathogenicity of the virus may be the main determinant of disease severity. The effect of animal genetics on pathological outcome can have profound implications on the interpretation of preclinical data. For example, postvaccination lung eosinophilia, which was one of the key features of FI-RSV vaccine pathology in children (18), can be induced in the BALB/c mouse by presensitization with the RSV G protein (86) but can be effectively annulled when alternative strains of mice are used (87). The modification of pathology by a change in the genetic background of the animal adds an enormous amount of complexity to the interpretation of animal-based safety data, with potential implications for interpreting small human studies and a reduction in the value of such data as a preclinical safety checkpoint.
The predictive utility of the mouse model in studies of vaccine-induced immunopathology is further limited by the fact that pathology can be abrogated by the depletion of certain mediators (82) or adjusted by changing experimental parameters such as the delivery route and type of sensitizing antigen (88). For example, poor-quality antibodies passively administered or transferred from rodents vaccinated with an FI-RSV vaccine have not caused immunopathology or enhanced respiratory disease in rodent recipients (28). In addition, there is concern about using the rodent model to screen for enhanced respiratory disease when nonviral components of the FI-RSV vaccine have been shown to cause enhanced immunopathology consistent with enhanced respiratory disease (84). The formalin inactivation procedure has been shown to result in an abundance of carbonyl groups that appeared to induce a TH2-mediated enhanced respiratory disease response in mice, an effect that could be almost completely reversed by chemical reduction of these carbonyl groups (26). Together, these observations suggest that the patterns of pathology induced by vaccinating small rodents are, in part, subject to the nuances of experimental design and may deviate substantially from human responses to the same antigens. The mouse, in particular, appears to have a tendency to emphasize the immunopathogenic potential of vaccine candidates, which may not be reflected in humans. In assessing potential vaccine safety issues using animal models, indicators such as lung eosinophilic infiltration should not be rigidly applied as preclinical stop signals that preclude products from further development. Rather, these indicators should be used as a basis for continued investigation in other animal models to demonstrate safety before advancing to properly controlled phase 1 safety studies in humans. At present, there is no consensus on how this is regulated, i.e., which animal models should be used in preclinical studies (28).
Conclusions
RSV disease is a major burden on pediatric and older adult health care services around the world, causing marked morbidity and mortality. Multiple RSV vaccines are in development to try to counter this challenge using a variety of traditional and new technologies. The approaches used need to be tailored to each population owing to differences in risk factors for severe disease and immunological factors that vary among populations. Although the road has been long, we are now entering an era where an RSV vaccine is likely to become available that could revolutionize pediatric and older adult medicine.
Acknowledgments
Funding
We were supported by the NIHR Oxford Biomedical Research Centre. S.B.D., A.J.P., and C.J.S. are members of the REspiratory Syncytial virus Consortium in Europe (RESCEU). RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 116019. This Joint Undertaking received support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). C.J.S. is supported by fellowship funding from the Wellcome Trust (WT105882MA).
Footnotes
Competing interests: A.J.P. chairs the UK Department of Health and Social Care’s Joint Committee on Vaccination and Immunisation (JCVI) and the European Medicines Agency Scientific Advisory Group on Vaccines and is a member of the World Health Organization’s Strategic Group of Experts. The views expressed in this manuscript do not necessarily reflect the views of these agencies. A.J.P. and C.S.R. are Jenner Institute investigators. S.B.D. has been an investigator on studies funded by Janssen and MedImmune; all funds have been paid to S.B.D.’s institution, and S.B.D. has received no personal payments. The other authors declare that they have no competing interests.
References and Notes
- 1.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, et al. RSV Global Epidemiology Network, Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee N, Lui GCY, Wong KT, Li TCM, Tse ECM, Chan JYC, Yu J, Wong SSM, Choi KW, Wong RYK, Ngai KLK, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57:1069–1077. doi: 10.1093/cid/cit471. [DOI] [PubMed] [Google Scholar]
- 3.Ackerson B, Tseng HF, Sy LS, Solano Z, Slezak J, Luo Y, Fischetti CA, Shinde V. Severe morbidity and mortality associated with respiratory syncytial virus versus Influenza infection in hospitalized older adults. Clin Infect Dis. 2019;69:197–203. doi: 10.1093/cid/ciy991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PATH RSV vaccine snapshot. [accessed 15 May 2019]. https://vaccineresources.org/files/RSV-snapshot-2019_04_05_April_High%20Resolution.pdf .
- 5.Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, Holman LSA, Hickman SP, Berkowitz NM, Gordon IJ, Yamshchikov GV, et al. VRC 317 Study Team, A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365:505–509. doi: 10.1126/science.aav9033. [DOI] [PubMed] [Google Scholar]
- 6.Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, Saxena S. Medicines for Neonates Investigator Group, Risk factors for hospital admission with RSV bronchiolitis in England: A population-based birth cohort study. PLOS ONE. 2014;9:e89186. doi: 10.1371/journal.pone.0089186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton CA, Upham JW, Wikström ME, Holt BJ, White GP, Sharp MJ, Sly PD, Holt PG. Functional maturation of CD4+ CD25+ CTLA4+ CD45RA+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J Immunol. 2004;173:3084–3092. doi: 10.4049/jimmunol.173.5.3084. [DOI] [PubMed] [Google Scholar]
- 8.Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, Nokes DJ. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLOS ONE. 2009;4:e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe JE., Jr Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin Infect Dis. 2001;33:1720–1727. doi: 10.1086/322971. [DOI] [PubMed] [Google Scholar]
- 10.Moyes J, Cohen C, Pretorius M, Groome M, von Gottberg A, Wolter N, Walaza S, Haffejee S, Chhagan M, Naby F, Cohen AL, et al. South African Severe Acute Respiratory Illness Surveillance Group, Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010-2011. J Infect Dis. 2013;208:S217–S226. doi: 10.1093/infdis/jit479. [DOI] [PubMed] [Google Scholar]
- 11.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 12.Henderson FW, Collier AM, Clyde WA, Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity—A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 13.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 14.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 15.Salk JE. Studies in human subjects on active immunization against poliomyelitis. I. A preliminary report of experiments in progress. J Am Med Assoc. 1953;151:1081–1098. [PubMed] [Google Scholar]
- 16.Strebel PM, Sutter RW, Cochi SL, Biellik RJ, Brink EW, Kew OM, Pallansch MA, Orenstein WA, Hinman AR. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin Infect Dis. 1992;14:568–579. doi: 10.1093/clinids/14.2.568. [DOI] [PubMed] [Google Scholar]
- 17.Potash L, Tytell AA, Sweet BH, Machlowitz RA, Stokes J, Jr, Weibel RE, Woodhour AF, Hilleman MR. Respiratory virus vaccines. I. Respiratory syncytial and parainfluenza virus vaccines. Am Rev Respir Dis. 1966;93:536–548. doi: 10.1164/arrd.1966.93.4.536. [DOI] [PubMed] [Google Scholar]
- 18.Chin J, Magoffin RL, Shearer LA. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 19.Weibel RE, Stokes J, Jr, Leagus MB, Mascoli CC, Tytell AA, Woodhour AF, Vella PP, Hilleman MR. Respiratory virus vaccines. VII. Field evaluation of respiratory syncytial, parainfluenza 1,2, 3, and Mycoplasma pneumoniae vaccines, 1965 to 1966. Am Rev Respir Dis. 1967;96:724–739. doi: 10.1164/arrd.1967.96.4.724. [DOI] [PubMed] [Google Scholar]
- 20.Weibel RE, Stokes J, Jr, Leagus MB, Mascoli CC, Hilleman MR. Respiratory virus vaccines. V. Field evaluation for efficacy of heptavalent vaccine. Am Rev Respir Dis. 1966;94:362–379. doi: 10.1164/arrd.1966.94.3.362. [DOI] [PubMed] [Google Scholar]
- 21.Taylor G. Animal models of respiratory syncytial virus infection. Vaccine. 2017;35:469–480. doi: 10.1016/j.vaccine.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy BR, Prince GA, Walsh EE, Kim HW, Parrott RH, Hemming VG, Rodriguez WJ, Chanock RM. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26:1595–1597. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang H-Y, Mitzner W, Ravetch J, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw CA, Otten G, Wack A, Palmer GA, Mandl CW, Mbow ML, Valiante N, Dormitzer PR. Antibody affinity maturation and respiratory syncytial virus disease. Nat Med. 2009;15:725. doi: 10.1038/nm0709-725a. [DOI] [PubMed] [Google Scholar]
- 26.Moghaddam A, Olszewska W, Wang B, Tregoning JS, Helson R, Sattentau QJ, Openshaw PJM. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 27.Killikelly AM, Kanekiyo M, Graham BS. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci Rep. 2016;6:34108. doi: 10.1038/srep34108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Medicines Agency. Guideline on the clinical evaluation of medicinal products indicated for the prophylaxis or treatment of respiratory syncytial virus (RSV) disease. [accessed 15 May 2019]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-evaluation-medicinal-products-indicated-prophylaxis-treatment-respiratory_en.pdf .
- 29.Connors M, Collins PL, Firestone C-Y, Sotnikov AV, Waitze A, Davis AR, Hung PP, Chanock RM, Murphy BR. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia—RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 30.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambrose CS, Wu X, Belshe RB. The efficacy of live attenuated and inactivated influenza vaccines in children as a function of time postvaccination. Pediatr Infect Dis J. 2010;29:806–811. doi: 10.1097/INF.0b013e3181e2872f. [DOI] [PubMed] [Google Scholar]
- 32.Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 33.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rostad CA, Stobart CC, Gilbert BE, Pickles RJ, Hotard AL, Meng J, Blanco JCG, Moin SM, Graham BS, Piedra PA, Moore ML. A recombinant respiratory syncytial virus vaccine candidate attenuated by a low-fusion F protein Is immunogenic and protective against challenge in cotton rats. J Virol. 2016;90:7508–7518. doi: 10.1128/JVI.00012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karron RA, Wright PF, Crowe JE, Jr, Clements-Mann ML, Thompson J, Makhene M, Casey R, Murphy BR. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J Infect Dis. 1997;176:1428–1436. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 36.Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344:230–239. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Xing Z, Lichty BD. Use of recombinant virus-vectored tuberculosis vaccines for respiratory mucosal immunization. Tuberculosis. 2006;86:211–217. doi: 10.1016/j.tube.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Ewer KJ, Sierra-Davidson K, Salman AM, Illingworth JJ, Draper SJ, Biswas S, Hill AVS. Progress with viral vectored malaria vaccines: A multi-stage approach involving “unnatural immunity”. Vaccine. 2015;33:7444–7451. doi: 10.1016/j.vaccine.2015.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green CA, Scarselli E, Sande CJ, Thompson AJ, de Lara CM, Taylor KS, Haworth K, Del Sorbo M, Angus B, Siani L, Di Marco S, et al. Chimpanzee adenovirus− and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci Transl Med. 2015;7:300ra126. doi: 10.1126/scitranslmed.aac5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripp RA, Tompkins SM. Virus-vectored influenza virus vaccines. Viruses. 2014;6:3055–3079. doi: 10.3390/v6083055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afolabi MO, Tiono AB, Adetifa UJ, Yaro JB, Drammeh A, Nébié I, Bliss C, Hodgson SH, Anagnostou NA, Sanou GS, Jagne YJ, et al. Safety and immunogenicity of ChAd63 and MVA ME-TRAP in West African children and infants. Mol Ther. 2016;24:1470–1477. doi: 10.1038/mt.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AVS, Dorrell L. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr Opin Immunol. 2016;41:47–54. doi: 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 43.IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 44.Public Health England. Immunisation against infectious disease (2015): Green Book, Chapter 27a Respiratory syncytial virus. [accessed 15 May 2019]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/458469/Green_Book_Chapter_27a_v2_0W.PDF .
- 45.Carbonell-Estrany X, Simões EAF, Dagan R, Hall CB, Harris B, Hultquist M, Connor EM, Losonsky GA. Motavizumab Study Group, Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: A noninferiority trial. Pediatrics. 2010;125:e35–e51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 46.AstraZeneca withdraws BLA for motavizumab for serious respiratory syncytial virus (RSV) - MPR. [accessed 5 March 2019]. https://www.empr.com/home/news/drugs-in-the-pipeline/astrazeneca-withdraws-bla-for-motavizumab-for-serious-respiratory-syncytial-virus-rsv/
- 47.Regeneron Pharmaceuticals I. Regeneron to discontinue development of Suptavumab for Respiratory Syncytial Virus—Regeneron Pharmaceuticals Inc. 2017. [accessed 4 January 2019]. https://investor.regeneron.com/news-releases/news-release-details/regeneron-discontinue-development-suptavumab-respiratory?releaseid=1037184 .
- 48.Domachowske JB, Khan AA, Esser MT, Jensen K, Takas T, Villafana T, Dubovsky F, Griffin MP. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single-dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr Infect Dis J. 2018;37:886–892. doi: 10.1097/INF.0000000000001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Safety, tolerability, and pharmacokinetics of MK-1654 in infants (MK-1654-002) [accessed 5 March 2019]. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03524118?term=merck&cond=RSV+Infection&rank=1.
- 50.Zhu Q, McLellan JS, Kallewaard NL, Ulbrandt ND, Palaszynski S, Zhang J, Moldt B, Khan A, Svabek C, McAuliffe JM, Wrapp D, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci TranslMed. 2017;9:eaaj1928. doi: 10.1126/scitranslmed.aaj1928. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Q, Lu B, McTamney P, Palaszynski S, Diallo S, Ren K, Ulbrandt ND, Kallewaard N, Wang W, Fernandes F, Wong S, et al. Prevalence and significance of substitutions in the fusion protein of respiratory syncytial virus resulting in neutralization escape from antibody MEDI8897. J Infect Dis. 2018;218:572–580. doi: 10.1093/infdis/jiy189. [DOI] [PubMed] [Google Scholar]
- 52.Sande CJ, Cane PA, Nokes DJ. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine. 2014;32:4726–4729. doi: 10.1016/j.vaccine.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashira S, Okitsu-Negishi S, Yoshino K. Placental transfer of IgG subclasses in a Japanese population. Pediatr Int. 2000;42:337–342. doi: 10.1046/j.1442-200x.2000.01245.x. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 55.Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, Flyer D, Jani D, Hickman SP, Piedra PA. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis. 2016;213:411–422. doi: 10.1093/infdis/jiv406. [DOI] [PubMed] [Google Scholar]
- 56.MacDorman MF, Gregory ECW. Fetal and perinatal mortality: United States, 2013. Natl Vital Stat Rep. 2015;64:1–24. [PubMed] [Google Scholar]
- 57.World Health Organization. World Health Statistics. 2013. [accessed 19 February 2019]. https://who.int/gho/publications/world_health_statistics/EN_WHS2013_Full.pdf .
- 58.Raghunandan R, Lu H, Zhou B, Xabier MG, Massare MJ, Flyer DC, Fries LF, Smith GE, Glenn GM. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine. 2014;32:6485–6492. doi: 10.1016/j.vaccine.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novavax announces topline results from Phase 3 PrepareTM trial of ResVaxTM for prevention of RSV disease in infants via maternal immunization—Novavax Inc.—IR Site. [accessed 5 March 2019]. http://ir.novavax.com/news-releases/news-release-details/novavax-announces-topline-results-phase-3-preparetm-trial .
- 60.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atwell JE, Thumar B, Robinson LJ, Tobby R, Yambo P, Ome-Kaius M, Siba PM, Unger HW, Rogerson SJ, King CL, Karron RA. Impact of placental malaria and bol on transplacental transfer of respiratory syncytial virus antibody in Papua New Guinea. J Infect Dis. 2016;213:423–431. doi: 10.1093/infdis/jiv401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hogan AB, Campbell PT, Blyth CC, Lim FJ, Fathima P, Davis S, Moore HC, Glass K. Potential impact of a maternal vaccine for RSV: A mathematical modelling study. Vaccine. 2017;35:6172–6179. doi: 10.1016/j.vaccine.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 63.Nyiro JU, Kombe IK, Sande CJ, Kipkoech J, Kiyuka PK, Onyango CO, Munywoki PK, Kinyanjui TM, Nokes DJ. Defining the vaccination window for respiratory syncytial virus (RSV) using age-seroprevalence data for children in Kilifi, Kenya. PLOS ONE. 2017;12:e0177803. doi: 10.1371/journal.pone.0177803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cromer D, van Hoek AJ, Newall AT, Jit M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: A modelling and cost-effectiveness analysis for England. Lancet Public Health. 2017;2:e367–e374. doi: 10.1016/S2468-2667(17)30103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janet S, Broad J, Snape MD. Respiratory syncytial virus seasonality and its implications on prevention strategies. Hum Vaccin Immunother. 2018;14:234–244. doi: 10.1080/21645515.2017.1403707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor S, Taylor RJ, Lustig RL, Schuck-Paim C, Haguinet F, Webb DJ, Logie J, Matias G, Fleming DM. Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK. BMJ Open. 2016;6:e009337. doi: 10.1136/bmjopen-2015-009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2018;2:CD004879. doi: 10.1002/14651858.CD004879.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mullooly JP, Bridges CB, Thompson WW, Chen J, Weintraub E, Jackson LA, Black S, Shay DK. Vaccine Safety Datalink Adult Working Group, Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25:846–855. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 69.Jansen AGSC, Sanders EAM, Hoes AW, van Loon AM, Hak E. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur Respir J. 2007;30:1158–1166. doi: 10.1183/09031936.00034407. [DOI] [PubMed] [Google Scholar]
- 70.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54:1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Asten L, van den Wijngaard C, van Pelt W, van de Kassteele J, Meijer A, van der Hoek W, Kretzschmar M, Koopmans M. Mortality attributable to 9 common infections: Significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206:628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 72.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 73.Volling C, Hassan K, Mazzulli T, Green K, Al-den A, Hunter P, Mangat R, Ng J, McGeer A. Respiratory syncytial virus infection-associated hospitalization in adults: A retrospective cohort study. BMC Infect Dis. 2014;14:665. doi: 10.1186/s12879-014-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dowell SF, Anderson LJ, Gary HE, Erdman DD, Plouffe JF, File TM, Jr, Marston BJ, Breiman RF. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 76.Openshaw PJM, Chiu C, Culley FJ, Johansson C. Protective and harmful immunity to RSV infection. Annu Rev Immunol. 2017;35:501–532. doi: 10.1146/annurev-immunol-051116-052206. [DOI] [PubMed] [Google Scholar]
- 77.Walsh EE, Falsey AR. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004;73:295–299. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- 78.Cherukuri A, Patton K, Gasser RA, Jr, Zuo F, Woo J, Esser MT, Tang RS. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novavax. Novavax announces topline RSV F vaccine data from two clinical trials in older adults—Novavax Inc.—IR Site. 2016. [accessed 5 January 2019]. http://ir.novavax.com/news-releases/news-release-details/novavax-announces-topline-rsv-f-vaccine-data-two-clinical-trials .
- 80.Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, Hodge E, Benhaim M, Ravichandran R, Carter L, Sheffler W, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. 2019;176:1420–1431.:e17. doi: 10.1016/j.cell.2019.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, Thomas DN, Hickman SP, Kpamegan E, Boddapati S, Piedra PA. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31:524–532. doi: 10.1016/j.vaccine.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 82.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC, III, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 84.Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301:L148–L156. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prince GA, Horswood RL, Berndt J, Suffin SC, Chanock RM. Respiratory syncytial virus infection in inbred mice. Infect Immun. 1979;26:764–766. doi: 10.1128/iai.26.2.764-766.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alwan WH, Kozlowska WJ, Openshaw PJM. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hussell T, Georgiou A, Sparer T, Matthews S, Pala P, Openshaw PJ. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol. 1998;161:6215–6222. [PubMed] [Google Scholar]
- 88.Johnson TR, Teng MN, Collins PL, Graham BS. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J Virol. 2004;78:6024–6032. doi: 10.1128/JVI.78.11.6024-6032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, Gorse GJ, Edelman R, Hayden FG, McElhaney JE, Neuzil KM, et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines—Nonadjuvanted vaccine or vaccine adjuvanted with alum—Given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis. 2008;198:1317–1326. doi: 10.1086/592168. [DOI] [PubMed] [Google Scholar]
- 90.Langley JM, Sales V, McGeer A, Guasparini R, Predy G, Meekison W, Li M, Capellan J, Wang E. A dose-ranging study of a subunit respiratory syncytial virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults ≥65 years of age. Vaccine. 2009;27:5913–5919. doi: 10.1016/j.vaccine.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 91.Power UF, Nguyen TN, Rietveld E, de Swart RL, Groen J, Osterhaus ADME, de Groot R, Corvaia N, Beck A, Bouveret-le-Cam N, Bonnefoy J-Y. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J Infect Dis. 2001;184:1456–1460. doi: 10.1086/324426. [DOI] [PubMed] [Google Scholar]
- 92.Murata Y. Respiratory syncytial virus vaccine development. Clin Lab Med. 2009;29:725–739. doi: 10.1016/j.cll.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, Buchholz UJ. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med. 2015;7:312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, Gruber W, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 95.Wright PF, Belshe RB, Kim HW, Van Voris LP, Chanock RM. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect Immun. 1982;37:397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malkin E, Yogev R, Abughali N, Sliman J, Wang CK, Zuo F, Yang CF, Eickhoff M, Esser MT, Tang RS, Dubovsky F. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLOS ONE. 2013;8:e77104. doi: 10.1371/journal.pone.0077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bernstein DI, Malkin E, Abughali N, Falloon J, Yi T, Dubovsky F. MI-CP149 Investigators, Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr Infect Dis J. 2012;31:109–114. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 98.Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase-I study medi-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr Infect Dis J. 2009;28:655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]
- 99.Tang RS, Spaete RR, Thompson MW, MacPhail M, Guzzetta JM, Ryan PC, Reisinger K, Chandler P, Hilty M, Walker RE, Gomez MM, et al. Development of a PIV-vectored RSV vaccine: Preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine. 2008;26:6373–6382. doi: 10.1016/j.vaccine.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 100.Watt PJ, Robinson BS, Pringle CR, Tyrrel DAJ. Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine. 1990;8:231–236. doi: 10.1016/0264-410x(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 101.Pringle CR, Filipiuk AH, Robinson BS, Watt PJ, Higgins P, Tyrrell DAJ. Immunogenicity and pathogenicity of a triple temperature-sensitive modified respiratory syncytial virus in adult volunteers. Vaccine. 1993;11:473–478. doi: 10.1016/0264-410x(93)90290-e. [DOI] [PubMed] [Google Scholar]
- 102.Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, Clements-Mann ML, Harris DO, Randolph VB, Udem SA, Murphy BR, Sidhu MS. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: Clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O’Shea A, Ikizler MR, Zhu Y, Collins PL, Cutland C, Randolph VB, et al. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis. 2006;193:573–581. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- 104.Friedewald WT, Forsyth BR, Smith CB, Gharpure MA, Chanock RM. Low-temperature-grown RS virus in adult volunteers. JAMA. 1968;204:690–694. [PubMed] [Google Scholar]
- 105.Kim HW, Arrobio JO, Pyles G, Brandt CD, Camargo E, Chanock RM, Parrott RH. Clinical and immunological response of infants and children to administration of low-temperature adapted respiratory syncytial virus. Pediatrics. 1971;48:745–755. [PubMed] [Google Scholar]
- 106.Wright PF, Mills V, Chanock RMJ. Evaluation of a temperature-sensitive mutant of respiratory syncytial virus in adults. J Infect Dis. 1971;124:505–511. doi: 10.1093/infdis/124.5.505. [DOI] [PubMed] [Google Scholar]
- 107.Kim HW, Arrobio JO, Brandt CD, Wright P, Hodes D, Chanock RM, Parrott RH. Safety and antigenicity of temperature sensitive (TS) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973;52:56–63. [PubMed] [Google Scholar]
- 108.Wright PF, Shinozaki T, Fleet W, Sell SH, Thompson J, Karzon DT. Evaluation of a live, attenuated respiratory syncytial virus vaccine in infants. J Pediatr. 1976;88:931–936. doi: 10.1016/s0022-3476(76)81044-x. [DOI] [PubMed] [Google Scholar]
- 109.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, Halburnt LL, Reed GW, Whitehead SS, Anderson EL, Wittek AE, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. Respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez IM, Karron RA, Eichelberger M, Walsh EE, Delagarza VW, Bennett R, Chanock RM, Murphy BR, Clements-Mann ML, Falsey AR. Evaluation of the live attenuated cpts 248/404 RSV vaccine in combination with a subunit RSV vaccine (PFP-2) in healthy young and older adults. Vaccine. 2000;18:1763–1772. doi: 10.1016/s0264-410x(99)00527-7. [DOI] [PubMed] [Google Scholar]
- 111.Belshe RB, Anderson EL, Walsh EE. Immunogenicity of purified F glycoprotein of respiratory syncytial virus: Clinical and immune responses to subsequent natural infection in children. J Infect Dis. 1993;168:1024–1029. doi: 10.1093/infdis/168.4.1024. [DOI] [PubMed] [Google Scholar]
- 112.Paradiso PR, Hildreth SW, Hogerman DA, Speelman DJ, Lewin EB, Oren J, Smith DH. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24 to 48 months old. Pediatr Infect Dis J. 1994;13:792–798. doi: 10.1097/00006454-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 113.Piedra PA, Glezen WP, Kasel JA, Welliver RC, Jewel AM, Rayford Y, Hogerman DA, Hildreth SW, Paradiso PR. Safety and immunogenicity of the PFP vaccine against respiratory syncytial virus (RSV): The Western blot assay aids in distinguishing immune responses of the PFP vaccine from RSV infection. Vaccine. 1995;13:1095–1101. doi: 10.1016/0264-410x(95)00034-x. [DOI] [PubMed] [Google Scholar]
- 114.Tristram DA, Welliver RC, Hogerman DA, Hildreth SW, Paradiso P. Second-year surveillance of recipients of a respiratory syncytial virus (RSV) F protein subunit vaccine, PFP-1: Evaluation of antibody persistence and possible disease enhancement. Vaccine. 1994;12:551–556. doi: 10.1016/0264-410x(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 115.Tristram DA, Welliver RC, Mohar CK, Hogerman DA, Hildreth SW, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18-36 months old. J Infect Dis. 1993;167:191–195. doi: 10.1093/infdis/167.1.191. [DOI] [PubMed] [Google Scholar]
- 116.Walsh EE, Hall CB, Schlesinger JJ, Brandriss MW, Hildreth S, Paradiso P. Comparison of antigenic sites of subtype-specific respiratory syncytial virus attachment proteins. J Gen Virol. 1989;70:2953–2961. doi: 10.1099/0022-1317-70-11-2953. [DOI] [PubMed] [Google Scholar]
- 117.Muñoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465–3467. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 118.Falsey AR, Walsh EE. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine. 1996;14:1214–1218. doi: 10.1016/s0264-410x(96)00030-8. [DOI] [PubMed] [Google Scholar]
- 119.Falsey AR, Walsh EE. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine. 1997;15:1130–1132. doi: 10.1016/s0264-410x(97)00002-9. [DOI] [PubMed] [Google Scholar]
- 120.Groothuis JR, King SJ, Hogerman DA, Paradiso PR, Simoes EAF. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177:467–469. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- 121.Welliver RC, Tristram DA, Batt K, Sun M, Hogerman D, Hildreth S. Respiratory syncytial virus-specific cell-mediated immune responses after vaccination with a purified fusion protein subunit vaccine. J Infect Dis. 1994;170:425–428. doi: 10.1093/infdis/170.2.425. [DOI] [PubMed] [Google Scholar]
- 122.Piedra PA, Cron SG, Jewell A, Hamblett N, McBride R, Palacio MA, Ginsberg R, Oermann CM, Hiatt PW. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: A multi-center trial in children with cystic fibrosis. Vaccine. 2003;21:2448–2460. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 123.Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, Takas T, Levin MJ, Falsey AR. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis. 2017;216:1362–1370. doi: 10.1093/infdis/jix503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.August A, Glenn GM, Kpamegan E, Hickman SP, Jani D, Lu H, Thomas DN, Wen J, Piedra PA, Fries LF. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine. 2017;35:3749–3759. doi: 10.1016/j.vaccine.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 125.Fries L, Shinde V, Stoddard JJ, Thomas DN, Kpamegan E, Lu H, Smith G, Hickman SP, Piedra P, Glenn GM. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing. 2017;14:8. doi: 10.1186/s12979-017-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Langley JM, Aggarwal N, Toma A, Halperin SA, McNeil SA, Fissette L, Dewé W, Leyssen M, Toussaint JF, Dieussaert I. A randomized, controlled, observer-blinded phase 1 study of the safety and immunogenicity of a respiratory syncytial virus vaccine with or without alum adjuvant. J Infect Dis. 2017;215:24–33. doi: 10.1093/infdis/jiw453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Falloon J, Ji F, Curtis C, Bart S, Sheldon E, Krieger D, Dubovsky F, Lambert S, Takas T, Villafana T, Esser MT. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine. 2016;34:2847–2854. doi: 10.1016/j.vaccine.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 128.Langley JM, MacDonald LD, Weir GM, MacKinnon-Cameron D, Ye L, McNeil S, Schepens B, Saelens X, Stanford MM, Halperin SA. A respiratory syncytial virus vaccine based on the small hydrophobic protein ectodomain presented with a novel lipid-based formulation is highly immunogenic and safe in adults: A first-in-humans study. J Infect Dis. 2018;218:378–387. doi: 10.1093/infdis/jiy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buchholz UJ, Cunningham CK, Muresan P, Gnanashanmugam D, Sato P, Siberry GK, Rexroad V, Valentine M, Perlowski C, Schappell E, Thumar B, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1114 Study Team, Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis. 2018;217:1338–1346. doi: 10.1093/infdis/jiy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McFarland EJ, Karron RA, Muresan P, Cunningham CK, Valentine ME, Perlowski C, Thumar B, Gnanashanmugam D, Siberry GK, Schappell E, Barr E, et al. International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 2000 Study Team, Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J Infect Dis. 2018;217:1347–1355. doi: 10.1093/infdis/jiy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beran J, Lickliter JD, Schwarz TF, Johnson C, Chu L, Domachowske JB, van Damme P, Withanage K, Fissette LA, David MP, Maleux K, et al. Safety and immunogenicity of 3 formulations of an investigational respiratory syncytial virus vaccine in nonpregnant women: Results from 2 phase 2 trials. J Infect Dis. 2018;217:1616–1625. doi: 10.1093/infdis/jiy065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ. 2019;366:15021. doi: 10.1136/bmj.l5021. [DOI] [PubMed] [Google Scholar]