Summary
Background
Respiratory syncytial virus (RSV) is the most common cause of acute lower respiratory infection in young children. We previously estimated that in 2015, 33·1 million episodes of RSV-associated acute lower respiratory infection occurred in children aged 0–60 months, resulting in a total of 118 200 deaths worldwide. Since then, several community surveillance studies have been done to obtain a more precise estimation of RSV associated community deaths. We aimed to update RSV-associated acute lower respiratory infection morbidity and mortality at global, regional, and national levels in children aged 0–60 months for 2019, with focus on overall mortality and narrower infant age groups that are targeted by RSV prophylactics in development.
Methods
In this systematic analysis, we expanded our global RSV disease burden dataset by obtaining new data from an updated search for papers published between Jan 1, 2017, and Dec 31, 2020, from MEDLINE, Embase, Global Health, CINAHL, Web of Science, LILACS, OpenGrey, CNKI, Wanfang, and ChongqingVIP. We also included unpublished data from RSV GEN collaborators. Eligible studies reported data for children aged 0–60 months with RSV as primary infection with acute lower respiratory infection in community settings, or acute lower respiratory infection necessitating hospital admission; reported data for at least 12 consecutive months, except for in-hospital case fatality ratio (CFR) or for where RSV seasonality is well-defined; and reported incidence rate, hospital admission rate, RSV positive proportion in acute lower respiratory infection hospital admission, or in-hospital CFR. Studies were excluded if case definition was not clearly defined or not consistently applied, RSV infection was not laboratory confirmed or based on serology alone, or if the report included fewer than 50 cases of acute lower respiratory infection. We applied a generalised linear mixed-effects model (GLMM) to estimate RSV-associated acute lower respiratory infection incidence, hospital admission, and in-hospital mortality both globally and regionally (by country development status and by World Bank Income Classification) in 2019. We estimated country-level RSV-associated acute lower respiratory infection incidence through a risk-factor based model. We developed new models (through GLMM) that incorporated the latest RSV community mortality data for estimating overall RSV mortality. This review was registered in PROSPERO (CRD42021252400).
Findings
In addition to 317 studies included in our previous review, we identified and included 113 new eligible studies and unpublished data from 51 studies, for a total of 481 studies. We estimated that globally in 2019, there were 33·0 million RSV-associated acute lower respiratory infection episodes (uncertainty range [UR] 25·4–44·6 million), 3·6 million RSV-associated acute lower respiratory infection hospital admissions (2·9–4·6 million), 26 300 RSV-associated acute lower respiratory infection in-hospital deaths (15 100–49 100), and 101 400 RSV-attributable overall deaths (84 500–125 200) in children aged 0–60 months. In infants aged 0–6 months, we estimated that there were 6·6 million RSV-associated acute lower respiratory infection episodes (4·6–9·7 million), 1·4 million RSV-associated acute lower respiratory infection hospital admissions (1·0–2·0 million), 13 300 RSV-associated acute lower respiratory infection inhospital deaths (6800–28 100), and 45700 RSV-attributable overall deaths (38 400–55 900). 2·0% of deaths in children aged 0–60 months (UR 1·6–2·4) and 3·6% of deaths in children aged 28 days to 6 months (3·0–4·4) were attributable to RSV. More than 95% of RSV-associated acute lower respiratory infection episodes and more than 97% of RSV-attributable deaths across all age bands were in low-income and middle-income countries (LMICs).
Interpretation
RSV contributes substantially to morbidity and mortality burden globally in children aged 0–60 months, especially during the first 6 months of life and in LMICs. We highlight the striking overall mortality burden of RSV disease worldwide, with one in every 50 deaths in children aged 0–60 months and one in every 28 deaths in children aged 28 days to 6 months attributable to RSV. For every RSV-associated acute lower respiratory infection in-hospital death, we estimate approximately three more deaths attributable to RSV in the community. RSV passive immunisation programmes targeting protection during the first 6 months of life could have a substantial effect on reducing RSV disease burden, although more data are needed to understand the implications of the potential age-shifts in peak RSV burden to older age when these are implemented.
Funding
EU Innovative Medicines Initiative Respiratory Syncytial Virus Consortium in Europe (RESCEU).
Introduction
Human respiratory syncytial virus (RSV) is the most common pathogen identified in infants and young children with acute lower respiratory infection.1,2 We previously estimated3 that in 2015, there were 33·1 million episodes of RSV-associated acute lower respiratory infection, 3·2 million hospital admissions for RSV-associated acute lower respiratory infection, and 59 600 in-hospital RSV-associated acute lower respiratory infection deaths in children younger than 5 years.3 We also estimated that overall RSV-associated deaths from acute lower respiratory infection could be as high as 118 200, based on an indirect approach that inflated in-hospital mortality estimates due to the absence of RSV mortality data in community settings at the time of the analysis.3 Since 2017, new data on RSV burden in young children have become available, including from several new RSV community surveillance studies initiated to measure RSV mortality in the community (RSV community mortality surveillance studies4 and child health and mortality prevention surveillance [CHAMPS]5 supported by the Bill & Melinda Gates Foundation). Meanwhile, there have been substantial advances in the development of RSV prophylactic products, with several prophylactic candidates in late-phase clinical development.6 A monoclonal antibody with extended half-life, nirsevimab (AstraZeneca and Sanofi), which demonstrated high efficacy among healthy preterm infants in a phase 2b trial,7 reduced medically-attended RSV lower respiratory tract infections in healthy late-preterm and term infants in its phase 3 trial (NCT03979313).8 Moreover, two maternal vaccine candidates aimed to protect infants through transplacental transfer of vaccine-induced maternal antibodies (RSV MAT [GlaxoSmithKline; NCT04605159] and RSVpreF [Pfizer; NCT04424316]), and one monoclonal antibody (MK-1654 [MSD; NCT04767373]) have initiated recruitment for the phase 3 clinical trials, and are expected to complete in the next 3–5 years.
In this study, we aim to estimate RSV-associated acute lower respiratory infection morbidity and mortality in 2019 at global, regional, and national levels in children aged 0–60 months, with a primary focus on narrower infant age groups that are targeted by RSV prophylactics under development, and on overall mortality.
Methods
Definitions
As previously described,3 acute lower respiratory infection was defined by setting. For community-level setting (eg, primary care), we used WHO Integrated Management of Childhood Illnesses pneumonia case definitions and replaced the terms “clinical pneumonia” with “ALRI”; for hospital setting, we used physician-confirmed diagnosis of acute lower respiratory infection (pneumonia or bronchiolitis). RSV-associated acute lower respiratory infection was defined as acute lower respiratory infection with laboratory-confirmed RSV infection. RSV-attributable acute lower respiratory infection was defined as acute lower respiratory infection that could be causally attributable to laboratory-confirmed RSV infection. Hypoxaemia was defined as SpO2 less than 90% (or <87% if at altitude >2500 metres) in children aged 1–60 months and less than 88% (or <85% if at altitude >2500 metres) for children younger than 1 month. For more detailed definitions see the appendix (p 8).
Search strategy and selection criteria
We conducted a systematic literature review, updating our previous review.3 We searched MEDLINE, Embase, Global Health, CINAHL, Web of Science, LILACS, OpenGrey, CNKI, Wanfang, and ChongqingVIP for studies published between Jan 1, 2017, and Dec 31, 2020, that reported RSV-associated acute lower respiratory infection morbidity and mortality estimates in children aged 0–60 months in 2019 or before, (ie, before the onset of the COVID-19 pandemic). The literature search used the terms (with synonyms and closely related words) “respiratory syncytial virus”, “pneumonia”, “bronchiolitis”, “respiratory tract infections”, “incidence”, “morbidity”, “mortality”, “burden”, and “epidemiology”. For a detailed search strategy, see the appendix (pp 4–7). References cited in retrieved articles were also examined for eligibility. No language restrictions were applied. Two authors (YL and ABP) searched and screened the literature independently. Eligible studies reported data for children aged 0–60 months with RSV as primary infection with acute lower respiratory infection in community settings or acute lower respiratory infection necessitating hospital admission; reported data for at least 12 consecutive months, except for in-hospital case fatality ratio (CFR) or where RSV seasonality is well defined (eg, in temperate regions); and reported incidence rate, hospital admission rate, RSV positive proportion in acute lower respiratory infection hospital admission, or in-hospital CFR.
Studies were excluded if case definition was not clearly defined or not consistently applied, RSV infection was not laboratory confirmed or based on serology alone, or if the report included fewer than 50 cases of acute lower respiratory infection.
Data extraction was done independently by two authors (XW and ABP) using a tailored spreadsheet, with any disagreements arbitrated by YL. The data collection spreadsheet collected study-level information, such as location or country, study period, eligibility criteria, case definition, clinical specimen and diagnostic tests, and the reported morbidity and mortality estimates.
Unpublished RSV data
Previously, we established the Respiratory Virus Global Epidemiology Network (RSV GEN) to collect unpublished data (including re-analysis of published data), applying common case definitions and approaches to data analysis, yielding unpublished data from over 70 individual study sites.3 For this study, we continued to encourage existing members of RSV GEN to contribute new RSV data while inviting other investigators with eligible RSV data to join this collaborative network. This resulted in novel unpublished data from 51 study sites to supplement published data and data included in our previous estimates (appendix pp 9–11).
Quality assessment
For both published and unpublished RSV data, two authors (YL and XW) conducted quality assessment at the study level independently using a self-designed quality scoring form (appendix p 18).9–11 The quality scoring form assessed the study quality and risk of bias on study design, subjects, case definition, sampling strategy (for RSV testing), and diagnostic tests; for hospital-based studies, adjustment for health-care utilisation and ascertainment for hypoxaemia were also assessed where applicable. Based on the individual assessment questions above, an overall quality score was calculated for each study, ranging between 0 (lowest quality) and 1 (highest quality). Regardless of the scores, all studies were included in main analysis; studies with quality scores <0·6 were excluded in sensitivity analysis.
Data analysis
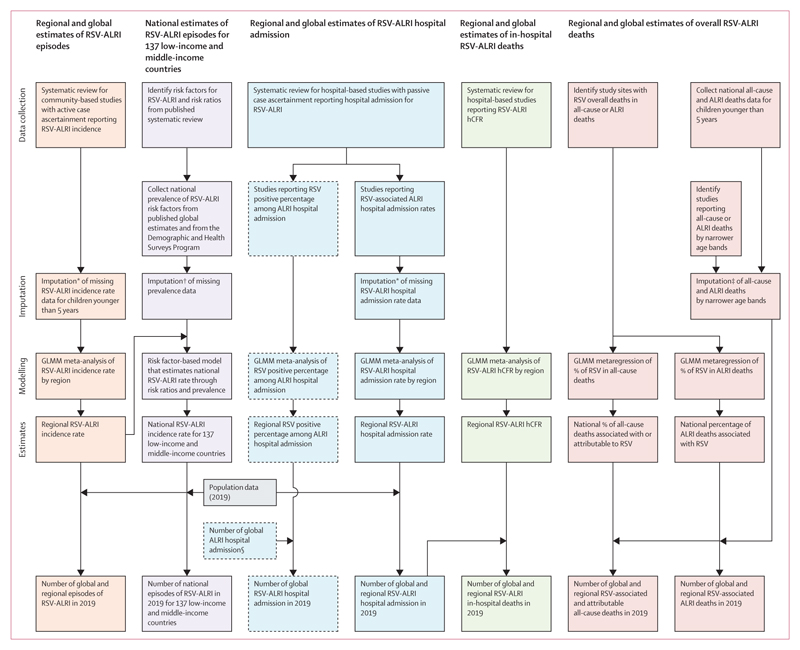
We estimated RSV-associated acute lower respiratory infection incidence, RSV-associated acute lower respiratory infection hospital admission, RSV-associated acute lower respiratory infection in-hospital deaths, and RSV-associated and RSV-attributable overall deaths for children aged 0–60 months and, where data were available, for narrower age bands (figure 1).
Figure 1. Approaches for estimation of global RSV morbidity and mortality in children aged 0–60 months.
Boxes with solid borders are main analyses. Boxes with dashed borders are sensitivity analyses. RSV=respiratory syncytial virus. ALRI=acute lower respiratory infection. GLMM=generalised linear mixed-effects model. hCFR= in-hospital case fatality ratio. *Details in the appendix (p 12). †Details in the appendix (p 13). ‡Details in the appendix (p 17). §Details in the appendix (p 19).
We conducted meta-analysis of RSV-associated acute lower respiratory infection incidence rate and hospital admission rate by regions (UNICEF country development status and World Bank income classification),12 age band, and severity (ie, acute lower respiratory infection with or without chest wall indrawing, and with or without hypoxaemia), through a generalised linear mixed-effects model (GLMM) of two levels (within-study and between-study).13 As we prioritised the geographical representativeness of our study sites, we were flexible in terms of study years considered in the meta-analysis. We adjusted for proportion of acute lower respiratory infection cases not tested for RSV by applying the RSV positive proportion among acute lower respiratory infection cases tested for RSV to the total tested and untested acute lower respiratory infection cases, if it was not done already in the individual studies. Data imputation was done for studies not reporting RSV-associated acute lower respiratory infection incidence rate or hospital admission rate for children aged 0–60 months (appendix p 12). The incidence rate and hospital admission rate meta-estimates were applied to regional population estimates for 201912 to yield the number of RSV-associated acute lower respiratory infection episodes and hospital admission for RSV-associated acute lower respiratory infection or RSV-associated acute lower respiratory infection with hypoxaemia.
For RSV-associated acute lower respiratory infection incidence, we also estimated country-specific RSV-associated acute lower respiratory infection incidence rate and episodes in children aged 0–60 months in 137 LMICs using a risk-factor based model similar to previous work (appendix pp 13–14).3 For RSV-associated acute lower respiratory infection hospital admission, we validated the estimates with independent data by applying the meta-estimates of RSV positive proportion among acute lower respiratory infection hospital admissions (through GLMM) in our study to the external sources of acute lower respiratory infection hospital admission estimates (appendix p 19 and p 42).14,15
We obtained meta-estimates of in-hospital CFR for RSV-associated acute lower respiratory infection hospital admission by region (UNICEF country development status and World Bank Income classification) and age band using GLMM. Then we applied the in-hospital CFR to the RSV-associated acute lower respiratory infection hospital admission estimates, by region and age band, to calculate RSV-associated acute lower respiratory infection in-hospital deaths.
With RSV community mortality data from 2017–19,16–20 we developed a new suite of models for estimating RSV-associated and attributable overall deaths (ie, in-hospital and out-of-hospital or community deaths) for children aged 0–60 months and for narrower age bands, both regionally and globally. Rather than inflating indirectly from in-hospital estimates as previously, we aimed to model the RSV proportion among overall all-cause deaths (as the main model) and among overall acute lower respiratory infection deaths (as secondary model). Both models were based on a GLMM framework that accounted for study setting (ie, community only vs community and in-hospital), method for RSV confirmation, age band (0–27 days, 28 days–6 months, 6–12 months, and 12–60 months), country’s under-5-years all-cause mortality rate, and method for assigning cause of death (ie, whether only verbal autopsy was available for the secondary acute lower respiratory infection model) as model covariates.
The three recorded outcomes were RSV-attributable deaths (primary outcome, defined as RSV being in the causal chain based on CHAMPS, including RSV being the underlying, intermediate [comorbid or antecedent causes], and immediate causes of death5,16), RSV-associated all-cause, and acute lower respiratory infection deaths (secondary outcomes, defined as RSV being tested positive in upper respiratory samples). Detailed methods are presented in the appendix (pp 16–17).
For estimates that were generated from single metaanalysis, the uncertainty range (UR) was derived from the coefficient and its standard error of that metaanalysis. For estimates that were generated through results from multiple meta-analyses, the UR of the estimates were generated using the Monte Carlo simulation to avoid inflation of the UR, based on 1000 samples of each of the meta-estimates from log-normal distributions, with 2·5th percentile and 97·5th percentile defining the lower and upper bounds.3
All data analyses were done using R software (version 4.0.5). The study data and R codes for the analysis are available in Edinburgh DataShare (https://doi.org/10.7488/ds/3138). This study was conducted and reported in accordance with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) recommendations (appendix p 51). The systematic literature review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist 2020 (appendix pp 52–54). This review was registered in PROSPERO (CRD42021252400).
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit.
Results
In addition to the 317 studies included in the previous review,3 we identified and included 113 new eligible studies from the literature review update and included new unpublished data from 51 studies from RSV GEN collaborators. This brought the total number of studies included in the present analysis to 481. Among the 481 studies, 140 contributed to the estimate of RSV-associated acute lower respiratory infection incidence or hospitalisation rate; 339 contributed to the estimate of RSV-positive proportion among acute lower respiratory infection hospital admission; 147 contributed to the estimate of RSV-associated in-hospital deaths; and 15 studies contributed to the estimate of RSV overall deaths (appendix pp 20–23). Study-level characteristics are presented in the appendix (pp 24–31). Through regional-level meta-analyses, we estimated that 33·0 million (UR 25·4–44·6 million) RSV-associated acute lower respiratory infection episodes occurred globally in children aged 0–60 months in 2019, with one in five episodes occurring in infants aged 0–6 months (6·6 million, 4·6–9·7 million). In low-income and lower-middle-income countries, the RSV-associated acute lower respiratory infection incidence rate peaked in children aged 0–3 months whereas the rate peaked in children aged 3–6 months in upper-middle-income and high-income countries. More than 95% of RSV-associated acute lower respiratory infection episodes occurred in low-income and middle-income countries (LMICs) across all age groups; table 1) Country-level estimates from the risk-factor-based model showed substantial variations in RSV-associated acute lower respiratory infection rate among LMICs, ranging from 40·3 per 1000 children per year in China (95% UR 29·7-54·6) to 83·4 per 1000 children per year in Eswatini (61·6–113·1; appendix pp 32–34). Approximately 5·5 million (17%) of 33·0 million RSV-associated acute lower respiratory infection episodes had chest wall indrawing (2·2–15·7 million). Infants aged 0–6 months had the highest incidence of RSV-associated acute lower respiratory infection with chest wall indrawing, with an estimated 2·3 million episodes (1·4–4·3 million), which accounted for 36% of all 6·6 million acute lower respiratory infection episodes in this age group (4·6–9·7 million; table 1).
Table 1. Incidence and number of episodes of RSV-associated acute lower respiratory infection in children younger than 5 years in 2019, by World Bank income regions and development status.
| Low income | Lower-middle income | Upper-middle income | High income | Developing countries | Industrialised countries | Global* | |
|---|---|---|---|---|---|---|---|
| RSV-associated acute lower respiratory infection | |||||||
| 0–3 months | |||||||
| Studies | 2 | 7 | 5 | 3 | 14 | 3 | 17 |
| Incidence rate | 8·0 (4·8-13·6) | 57·1 (22·6-144·4) | 121·5 (55·9–264·1) | 19·6 (6·5–597) | 55–3 (27·1–113·0) | 19·6 (6·5–59·7) | 51·8 (277–105·6) |
| Number of episodes | 49 000 (29000–82 000) | 895000 (354000-2 263 000) | 1085000 (499 000–2 357000) | 66000 (22 000–200 000) | 1699000 (832000–3469000) | 65000 (21000-198000) | 1763000 (941000–3592000) |
| 3–6 months | |||||||
| Studies | 2 | 7 | 5 | 3 | 14 | 3 | 17 |
| Incidence rate | 82·9 (44·5-154·7) | 142·2 (95·8–211·2) | 91·6 (28·8–291·6) | 17·9 (4·8–66·7) | 116·1 (73·8–182·6) | 17·9 (4·8–66·7) | 106·4 (70·6–168·3) |
| Number of episodes | 504000 (270000-939000) | 2228000 (1501000–3 309 000) | 817000 (257000–2 602 000) | 60000 (16000–223000) | 3564000 (2 266000–5607000) | 59000 (16000–221000) | 3620000 (2403 000–5726000) |
| 0–6 months† | |||||||
| Studies | 5 | 7 | 6 | 4 | 18 | 4 | 22 |
| Incidence rate | 75·9 (42·7-134·7) | 106·0 (63·5–177·0) | 130·8 (56·8–300·8) | 29·0 (12·9–65–0) | 103·7 (70·0-153·6) | 29·0 (12·9–65·0) | 96·3 (67·9–142·6) |
| Number of episodes | 921000 (518000–1636 000) | 3323000 (1991000–5 547 000) | 2334000 (1014000–5369000) | 194000 (86 000–435 000) | 6371000 (4302 000–9436000) | 192000 (86 000–431000) | 6554000 (4620000–9702000) |
| 6–12 months | |||||||
| Studies | 5 | 8 | 6 | 4 | 19 | 4 | 23 |
| Incidence rate | 68·7 (31·5-15O·0) | 105·4 (73·1–152·1) | 84·3 (39·5–180·1) | 32·5 (19·9–53·0) | 88·2 (62·2–125·2) | 32·5 (19·9–53·0) | 82·6 (60·8–116·9) |
| Number of episodes | 835000 (383000–1821000) | 3303000 (2289000–4766000) | 1505000 (705 000–3 215 000) | 217000 (133000–354000) | 5419000 (3820000–7689000) | 215000 (132000–351000) | 5 619000 (4135000–7953000) |
| 0–12 months† | |||||||
| Studies | 5 | 8 | 6 | 5 | 19 | 5 | 24 |
| Incidence rate | 78·3 (43·2–142·2) | 111·2 (81✓-151·2) | 108·8 (48·6–243·7) | 38·5 (21·6–68·8) | 1010 (72·5-140·6) | 38·5 (21·6–68·8) | 94·6 (70·8–131·6) |
| Number of episodes | 1902000 (1048 000-3453000) | 6969000 (5123000–9480000) | 3 885000 (1735000–8 698000) | 515000 (288000–920000) | 12 401000 (8 907000–17267000) | 510000 (286000–911000) | 12 875 000 (9635000–17909000) |
| 12–60 months | |||||||
| Studies | 2 | 4 | 0 | 0 | 6 | 0 | 0 |
| Incidence rate | 35·9 (8·4–154·2) | 25·4 (17·3–37·3) | .. | .. | 27·7 (15·3–50·2) | . | . |
| Number of episodes | 3307000 (770 000–14 208 000) | 6274000 (4 273 000–9 212 000) | .. | .. | 13500000 (7465 000–24413 000) | . | . |
| 0–60 months† | |||||||
| Studies‡ | 7(4) | 9(4) | 6(6) | 7(5) | 22(14) | 7(5) | 29(19) |
| Incidence rate | 49·3 (29·4–82·8) | 51·4 (37·8–69·8) | 55·2 (25·4–119·9) | 24·3 (13·8–427) | 51·6 (38·1–69·9) | 24·3 (13·8–42·7) | 48·8 (37·4–65·9) |
| Number of episodes | 5738000 (3418000–9633000) | 15913 000 (11715 000–21616 000) | 10079000 (4639000–21895000) | 1657000 (943 000–2 914000) | 31434000 (23194000–42601000) | 1646 000 (936000–2 894000) | 33028000 (25353000–44638000) |
| RSV-associated acute lower respiratory infection with chest wall indrawing | |||||||
| 0–3 months | |||||||
| Studies | 2 | 6 | 5 | 3 | 13 | 3 | 16 |
| Incidence rate | 2–0 (03–12·1) | 45·6 (24·2–85·9) | 75–6 (28·8–198·5) | 4–0 (0·6–28·2) | 30–9 (13·0–73·5) | 4–0 (0·6–28·2) | 28–3 (12·9–68·2) |
| Number of episodes | 12 000 (2000–73 000) | 714000 (379000–1345000) | 675000 (257000–1771000) | 13 000 (2000–94000) | 949000 (399000–2256000) | 13 000 (2000–93000) | 963000 (439000–2321000) |
| 3–6 months | |||||||
| Studies | 2 | 6 | 5 | 3 | 13 | 3 | 16 |
| Incidence rate | 18–1 (7·5–43·2) | 40–6 (14·6–113·0) | 14–1 (1·1–182·7) | 4–0 (0·6–28·2) | 27–6 (11·1–68·4) | 4–0 (0·6–28·2) | 25–3 (11·1–63·6) |
| Number of episodes | 110000 (46 000–262 000) | 636000 (229000–1770000) | 126000 (10000–1630000) | 13 000 (2000–94000) | 848000 (342000–2101000) | 13 000 (2000–93000) | 862000 (379 000–2165 000) |
| 0–6 months† | |||||||
| Studies | 4 | 6 | 5 | 3 | 15 | 3 | 18 |
| Incidence rate | 20–7 (6·8–62·9) | 45·8 (23·7–88·4) | 50–8 (15·5–166·2) | 1–4 (1-3-1-5) | 38–2 (21·1–69·1) | 1–4 (1-3-1-5) | 34–4 (19·9–62·7) |
| Number of episodes | 251000 (82000–764000) | 143 6 000 (744 000–2 769 000) | 907000 (277000–2 966000) | 9000 (9000–10000) | 2348000 (1299000–4 247 000) | 9000 (9000–10000) | 2 343 000 (1352000–4267000) |
| 6–12 months | |||||||
| Studies | 4 | 7 | 5 | 3 | 16 | 3 | 19 |
| Incidence rate | 13–1 (2·6–65·5) | 27–8 (20·9–36·9) | 9–7 (2·2–43·0) | 12–6 (5·6–28·4) | 17–4 (9·6–31·6) | 12–6 (5·6–28·4) | 16–9 (10·3–30·2) |
| Number of episodes | 160000 (32000–796000) | 871000 (656000-1i55000) | 174 000 (39000–768000) | 84000 (37000–190000) | 1070000 (589000–1943000) | 83000 (37000–188000) | 1152 000 (698000–2 057000) |
| 0–12 months† | |||||||
| Studies | 4 | 7 | 5 | 4 | 16 | 4 | 20 |
| Incidence rate | 19·5 (5·9–64·5) | 36–7 (24·1–55·8) | 31–0 (9·4–102·5) | 9–0 (4·8–17·0) | 30–0 (17·8–50·5) | 9–0 (4·8–17·0) | 27–9 (17·6–47·1) |
| Number of episodes | 474000 (143000–1567000) | 2300000 (1513 000–3 499 000) | 1107000 (335000–3 657000) | 121000 (64000–228000) | 3 684000 (2187 000–6 206 000) | 120000 (63000–225000) | 3794000 (2394000–6405000) |
| 12–60 months | |||||||
| Studies | 2 | 3 | 0 | 0 | 5 | 0 | 0 |
| Incidence rate | 1·4 (<0·05–38·5) | 8–1 (3·0–22·2) | 3–7 (0·7–19·0) | ||||
| Number of episodes | 124000 (4000–3550000) | 2007000 (733000–5495000) | 1809000 (355000–9 227000) | ||||
| 0–60 months† | |||||||
| Studies‡ | 2 | 3 | 0 | 1 | 5 | 1 | 6 |
| Incidence rate | 4·8 (0·5–45·2) | 14–0 (8·0–24·2) | 3–1 (1·7–5-5) | 8–8 (3·0–25·3) | 3–1 (1·7–5-5) | 8–1 (3·2–23·2) | |
| Number of episodes | 560000 (59 000–5 262 000) | 4325000 (2492000–7507000) | 208000 (116000–376000) | 5341000 (1850 000–15 419 000) | 207000 (115 000–374000) | 5488000 (2192000–15744000) | |
Data are n, incidence rate per 1000 children per year (UR), or n (UR). RSV=respiratory syncytial virus. UR=uncertainty range. *Global estimates were obtained by summing the numbers of developing and industrialised countries for each of the 1000 samples in the Monte Carlo simulation. †The point estimates and uncertainty range estimates are not necessarily equal to the sum of the estimates by finer age bands; this is because the studies that contributed to different age-group-specific estimates were different. ‡Data in parentheses indicate the number of studies with imputed data; comparisons between estimates using imputed data and not using imputed data are presented in the appendix (p 44).
We estimated that there were 3·6 million RSV-associated acute lower respiratory infection hospital admissions globally in children aged 0–60 months in 2019 (UR 2·9–4·6 million), approximately 1·4 million (39%) of which occurred in infants aged 0–6 months (1·0–2·0 million). There was little variation in admission to hospital for RSV-associated acute lower respiratory infection among different income regions, although this rate was lower in low-income countries. The rate of hospital admission for RSV-associated acute lower respiratory infection peaked in children aged 0–3 months across all regions (more specifically at 28 days to 3 months; appendix pp 36–37). Approximately 0·9 million (26%) of 3·6 million hospital admissions for RSV-associated acute lower respiratory infection had hypoxaemia (UR 0·5–1·9 million). In infants aged 0–6 months, we estimated that there were 0·4 million hospital admissions for RSV-associated acute lower respiratory infection with hypoxaemia (0·2–0·8 million). Within the first six months of life, more than 60% of the RSV-associated acute lower respiratory infection hospital admissions were during the first three months of life (table 2).
Table 2. Estimates of RSV-associated acute lower respiratory infection hospital admissions in children younger than 5 years by World Bank income regions and development status, 2019.
| Low income | Lower-middle income | Upper-middle income | High income | Developing countries | Industrialised countries | Global* | |
|---|---|---|---|---|---|---|---|
| RSV-associated acute lower respiratory infection hospital admission | |||||||
| 0–3 months | |||||||
| Studies | 6 | 11 | 16 | 19 | 36 | 16 | 52 |
| Hospital admission rate | 10·6 (3·3–33·5) | 31·0 (17·0–56·4) | 26·4 (12·8–54·5) | 34·7 (21·5–56·2) | 23·5 (15·2–36·3) | 36·9 (20·9–65·0) | 24·7 (17·5–37·1) |
| Number of episodes | 64 000 (20 000–204 000) | 485 000 (267 000–884 000) | 236 000 (114 000–486 000) | 116 000 (72 000–188 000) | 721 000 (466 000–1 115 000) | 122 000 (69 000–215 000) | 841 000 (597 000–1 261 000) |
| 3–6 months | |||||||
| Studies | 6 | 13 | 16 | 21 | 38 | 18 | 56 |
| Hospital admission rate | 6·0 (0·9–39·9) | 19·2 (11·5–32·1) | 20·6 (11·8–36·0) | 20·7 (13·5–31·6) | 16·7 (11·2–24·9) | 20·6 (12·4–34·1) | 17·0 (12·4–24·9) |
| Number of episodes | 36 000 (5000–242 000) | 301 000 (180 000–503 000) | 184 000 (106 000–321 000) | 69 000 (45 000–106 000) | 513 000 (345 000–765 000) | 68 000 (41 000–113 000) | 579 000 (422 000–846 000) |
| 0–6 months† | |||||||
| Studies | 10 | 12 | 16 | 27 | 41 | 24 | 65 |
| Hospital admission rate | 7·9 (2·8–21·9) | 27·9 (16·7–46·6) | 24·3 (13·2–44·7) | 28·4 (20·2–40·0) | 19·3 (13·1–28·6) | 29·3 (20·0–42·8) | 20·2 (14·9–29·1) |
| Number of episodes | 96 000 (34 000–266 000) | 873 000 (523 000–1 460 000) | 434 000 (236 000–798 000) | 190 000 (135 000–267 000) | 1 188 000 (802 000–1 759 000) | 194 000 (133 000–283 000) | 1 376 000 (1 017 000–1 982 000) |
| 6–12 months | |||||||
| Studies | 10 | 13 | 15 | 27 | 41 | 24 | 65 |
| Hospital admission rate | 5·7 (2·6–12·3) | 12·1 (6·5–22·8) | 12·1 (6·6–22·1) | 11·2 (7·5–16·7) | 10·0 (6·9–14·4) | 11·1 (7·1–17·4) | 10·0 (7·4–14·3) |
| Number of episodes | 69 000 (32 000–150 000) | 381 000 (203 000–715 000) | 215 000 (117 000–394 000) | 75 000 (50 000–112 000) | 612 000 (422 000–886 000) | 74 000 (47 000–116 000) | 683 000 (507 000–973 000) |
| 0–12 months† | |||||||
| Studies | 13 | 20 | 15 | 41 | 51 | 38 | 89 |
| Incidence rate | 9·6 (5·0–18·7) | 17·5 (11·5–26·5) | 18·7 (10·2–34·5) | 22·0 (17·1–28·4) | 15·3 (11·3–20·8) | 22·5 (17·1–29·5) | 15·9 (12·6–21·2) |
| Number of episodes | 234 000 (120 000–455 000) | 1 095 000 (722 000–1 661 000) | 669 000 (363 000–1 232 000) | 294 000 (228 000–380 000) | 1 881 000 (1 386 000–2 552 000) | 298 000 (227 000–391 000) | 2 170 000 (1 713 000–2 882 000) |
| 12–60 months | |||||||
| Studies | 9 | 12 | 8 | 17 | 31 | 15 | 46 |
| Hospital admission rate | 1·6 (0·5–4·6) | 1·6 (1·0–2·7) | 1·5 (0·8–2·8) | 1·6 (1·2–2·1) | 1·5 (1·0–2·3) | 1·7 (1·3–2·3) | 1·5 (1·1–2·2) |
| Number of episodes | 145 000 (50 000–421 000) | 396 000 (235 000–667 000) | 220 000 (117 000–415 000) | 88 000 (67 000–116 000) | 735 000 (491 000–1 101 000) | 95 000 (72 000–125 000) | 827 000 (600 000–1 207 000) |
| 0–60 months† | |||||||
| Studies‡ | 16 (4) | 22 (6) | 16 (8) | 51 (28) | 57 (19) | 48 (27) | 105 (46) |
| Hospital admission rate | 3·5 (2·0–6·3) | 6·2 (4·0–9·4) | 6·2 (3·8–10·3) | 6·0 (4·7–7·7) | 5·2 (3·9–6·9) | 6·1 (4·7–7·9) | 5·3 (4·2–6·8) |
| Number of episodes | 411 000 (231 000–731 000) | 1 908 000 (1 251 000–2 909 000) | 1 139 000 (693 000–1 872 000) | 409 000 (319 000–524 000) | 3 163 000 (2 395 000–4 179 000) | 413 000 (318 000–537 000) | 3 567 000 (2 856 000–4 634 000) |
| RSV-associated acute lower respiratory infection hospital admission with hypoxaemia | |||||||
| 0–3 months | |||||||
| Studies | 9 | 7 | 15 | 9 | 32 | 8 | 40 |
| Hospital admission rate | 2·5 (0·4–14·9) | 5·7 (1·5–18·0) | 9·2 (2·4–29·9) | 12·4 (2·7–40·7) | 6·9 (3·1–15·4) | 9·4 (1·4–41·3) | 7·4 (3·6–15·5) |
| Number of episodes | 15 000 (2000–90 000) | 90 000 (24 000–282 000) | 82 000 (21 000–267 000) | 42 000 (9000–136 000) | 211 000 (95 000–473 000) | 31 000 (5000–137 000) | 252 000 (121 000–527 000) |
| 3–6 months | |||||||
| Studies | 9 | 10 | 15 | 10 | 35 | 9 | 44 |
| Hospital admission rate | 1·0 (0·1–12·4) | 3·2 (1·1–7·9) | 6·3 (2·0–17·4) | 7·9 (1·9–22·9) | 4·0 (1·9–8·6) | 5·5 (1·0–21·3) | 4·2 (2·1–8·6) |
| Number of episodes | 6000 (0–76 000) | 50 000 (18 000–123 000) | 56 000 (17 000–155 000) | 26 000 (6000–77 000) | 122 000 (57 000–263 000) | 18 000 (3000–70 000) | 144 000 (73 000–293 000) |
| 0–6 months† | |||||||
| Studies | 9 | 7 | 15 | 10 | 32 | 9 | 41 |
| Hospital admission rate | 1·5 (0·3–7·8) | 4·5 (1·5–11·9) | 8·3 (2·5–23·7) | 10·9 (3·0–28·6) | 5·2 (2·5–11·2) | 8·2 (1·7–26·6) | 5·7 (2·9–11·3) |
| Number of episodes | 19 000 (3000–95 000) | 142 000 (47 000–372 000) | 149 000 (44 000–423 000) | 73 000 (20 000–191 000) | 322 000 (153 000–688 000) | 54 000 (11 000–176 000) | 385 000 (198 000–768 000) |
| 6–12 months | |||||||
| Studies | 9 | 10 | 15 | 10 | 35 | 9 | 44 |
| Hospital admission rate | 0·9 (0·2–3·7) | 2·0 (0·7–5·3) | 3·0 (0·6–10·8) | 5·0 (1·4–12·8) | 2·2 (1·0–4·9) | 3·5 (0·8–11·1) | 2·3 (1·2–4·9) |
| Number of episodes | 11 000 (2000–45 000) | 62 000 (21 000–167 000) | 53 000 (11 000–193 000) | 33 000 (10 000–85 000) | 133 000 (59 000–301 000) | 23 000 (5000–74 000) | 159 000 (79 000–336 000) |
| 0–12 months† | |||||||
| Studies | 9 | 11 | 15 | 10 | 36 | 9 | 45 |
| Hospital admision rate | 1·7 (0·4–6·2) | 3·9 (1·6–8·7) | 6·0 (1·7–17·9) | 8·9 (2·8–20·8) | 4·1 (2·2–7·9) | 6·6 (1·7–18·5) | 4·5 (2·5–8·0) |
| Number of episodes | 42 000 (11 000–151 000) | 245 000 (98 000–543 000) | 214 000 (60 000–639 000) | 120 000 (37 000–279 000) | 509 000 (268 000–976 000) | 88 000 (22 000–245 000) | 606 000 (342 000–1 095 000) |
| 12–60 months | |||||||
| Studies | 8 | 8 | 9 | 8 | 26 | 7 | 33 |
| Hospital admission rate | 0·1 (<0·05–1·0) | 0·2 (<0·05–0·8) | 0·3 (<0·05–1·9) | 0·6 (0·1–1·6) | 0·2 (0·1–0·7) | 0·4 (0·1–1·4) | 0·2 (0·1–0·7) |
| Number of episodes | 13 000 (2000–92 000) | 52 000 (10 000–209 000) | 50 000 (5000–272 000) | 34 000 (8000–89 000) | 106 000 (32 000–346 000) | 24 000 (5000–76 000) | 134 000 (51 000–383 000) |
| 0–60 months† | |||||||
| Studies‡ | 8 | 8 | 9 | 8 | 26 | 7 | 33 |
| Hospital admission rate | 0·5 (0·1–1·7) | 1·3 (0·4–3·3) | 2·1 (0·4–7·2) | 2·3 (0·6–5·9) | 1·3 (0·6–2·8) | 1·5 (0·3–4·8) | 1·3 (0·7–2·8) |
| Number of episodes | 56 000 (15 000–198 000) | 406 000 (138 000–1 009 000) | 385 000 (67 000–1 313 000) | 159000 (41 000–401 000) | 795 000 (361 000–1 710 000) | 102 000 (21 000–328 000) | 911 000 (459 000–1 866 000) |
Data are n, hospital admission rate per 1000 children per year (UR), or n (UR). Number of episodes were were rounded to the nearest 1000. RSV=respiratory syncytial virus. UR=uncertainty range. *Global estimates were obtained by summing the numbers of developing and industrialised countries for each of the 1000 samples in the Monte Carlo simulation. †The point estimates and uncertainty range estimates are not necessarily equal to the sum of the estimates by finer age bands; this is because the studies that contributed to different age-group-specific estimates were different. ‡Data in parentheses indicate the number of studies with imputed data; comparisons between estimates using imputed data and not using imputed data are presented in the appendix (p 44).
The proportion of patients admitted to hospital for acute lower respiratory infection who were positive for RSV was highest in high-income countries for both children aged 0–60 months (29% in high-income countries vs 23–26% in LMICs) and those aged 0–6 months (50% for high-income countries vs 32–33% in LMICs). Among narrower age bands, the proportion of admitted patients with acute lower respiratory infection peaked in children aged 0–3 months (32–51% across different income levels) and then decreased with increasing age (15–24% in children aged 12–60 months across different income levels; appendix p 38). As a sensitivity analysis, we applied these proportion results to external estimates14,15 of hospital admissions for acute lower respiratory infection to cross-validate our estimate for hospital admission for RSV-associated acute lower respiratory infection, which yielded an estimated 1·3–4·1 million hospital admissions for RSV-associated acute lower respiratory infection, consistent with our primary point estimate of 3·6 million (appendix p 39).
We estimated that the in-hospital CFR of RSV-associated acute lower respiratory infection ranged from 0·1% (UR 0·1–0·2) in high-income countries to 1·4% (0·6–2·8) in low-income countries among children aged 0–60 months. The regional variation in in-hospital CFR was even more pronounced in children aged 0–6 months. Although the in-hospital CFR remained consistently low in high-income countries across all age groups, there were substantial variations in in-hospital CFR across age groups in developing countries; for example, CFR was highest in children aged 0–3 months (1·1%, UR 0·7–1·8) and lowest in children aged 12–60 months (0·5%, 0·3–1·0) in developing countries. Our stratified analysis by the median study year (which was 2012) suggests that there was substantial decrease in in-hospital CFR over time among developing countries (0·99%, 0·69-1·45 before 2012 vs 0·54%, 0·31-0·98 after 2012 in children aged 0–60 months) but limited decrease was observed in industrialised countries (0·11%, 0·07–0·16 vs 0·08%, 0·02–0·31 in children aged 0–60 months; appendix p 41). At the global level, in 2019, we estimated that there were 26 300 (UR 15 100–49 100) RSV-associated acute lower respiratory infection in-hospital deaths in children aged 0–60 months, 13 300 (51%, 6800–28 100) of which occurred in children aged 0–6 months. LMICs accounted for more than 97% of RSV-associated acute lower respiratory infection in-hospital deaths across all age groups in children younger than 60 months (table 3).
Table 3. CFR estimates and number of in-hospital deaths in children younger than 5 years with RSV-associated acute lower respiratory infection in 2019, by World Bank income regions and development status.
| Low income | Lower-middle income | Upper-middle income | High income | Developing countries | Industrialised countries | Global* | |
|---|---|---|---|---|---|---|---|
| 0–3 months | |||||||
| Studies | 15 | 15 | 20 | 18 | 52 | 16 | 68 |
| In-hospital CFR (%) | 2·6 (1·8–3·6) | 1·8 (0·8–3·6) | 0·7 (0·4–1·4) | <0·05 (<0·05–0·3) | 1·1 (0·7–1·8) | <0·05 (<0·05–0·2) | 1·0 (0·6–1·6) |
| Number of deaths | 1600 (300–7800) | 8500 (2100–31 300) | 1800 (400–6700) | <50 (0–500) | 8000 (3500–19 800) | <50 (0–500) | 8100 (3600–20 100) |
| 3–6 months | |||||||
| Studies | 15 | 20 | 20 | 17 | 57 | 15 | 72 |
| In-hospital CFR (%) | 2·2 (1·5–3·3) | 1·0 (0·4–2·5) | 0·7 (0·3–1·7) | <0·05 (<0·05–0·1) | 0·9 (0·6–1·6) | <0·05 (<0·05–0·1) | 0·8 (0·5–1·4) |
| Number of deaths | 800 (100–8900) | 3000 (600–12 200) | 1300 (300–5500) | <50 (0–100) | 4700 (2000–12 000) | <50 (0–200) | 4800 (2100–12 100) |
| 0–6 months† | |||||||
| Studies | 16 | 17 | 21 | 17 | 56 | 15 | 71 |
| In-hospital CFR (%) | 2·4 (1·8–3·1) | 1·7 (1·0–2·8) | 0·8 (0·4–1·3) | <0·05 (<0·05–0·2) | 1·1 (0·8–1·6) | <0·05 (<0·05–0·2) | 1·0 (0·7–1·4) |
| Number of deaths | 2300 (600–8800) | 15 100 (5200–40 500) | 3400 (1100–10 300) | 100 (0–500) | 13 200 (6600–27 800) | <50 (0–500) | 13 300 (6800–28 100) |
| 6–12 months | |||||||
| Studies | 17 | 20 | 21 | 17 | 60 | 15 | 75 |
| In-hospital CFR (%) | 1·8 (0·9–3·4) | 0·8 (0·3–2·1) | 0·4 (0·2–1·2) | 0·1 (<0·05–0·3) | 0·8 (0·5–1·3) | <0·05 (<0·05–0·2) | 0·7 (0·4–1·2) |
| Number of deaths | 1200 (300–5400) | 3100 (600–14 500) | 1000 (200–4800) | <50 (0–400) | 4800 (2100–11 600) | <50 (0–200) | 4900 (2200–11 700) |
| 0–12 months† | |||||||
| Studies | 18 | 22 | 27 | 29 | 70 | 26 | 96 |
| In-hospital CFR (%) | 1·5 (0·8–2·8) | 1·5 (0·7–3·2) | 0·8 (0·5–1·3) | 0·1 (0·1–0·3) | 1·1 (0·8–1·5) | 0·1 (0·1–0·3) | 0·9 (0·7–1·4) |
| Number of deaths | 3500 (900–13 100) | 16 500 (4700–52 100) | 5600 (2000–15 600) | 400 (200–1100) | 20 100 (10 900–39 100) | 300 (100–1000) | 20 500 (11 300–39 800) |
| 12–60 months | |||||||
| Studies | 16 | 18 | 20 | 17 | 58 | 13 | 71 |
| In-hospital CFR (%) | 1·6 (0·4–5·7) | 0·8 (0·3–1·9) | 0·1 (<0·05–0·8) | 0·2 (0·1–0·4) | 0·5 (0·3–1·0) | 0·1 (0·1–0·1) | 0·5 (0·3–0·9) |
| Number of deaths | 2300 (200–25 400) | 3200 (700–12 300) | 300 (0–3200) | 200 (100–500) | 3800 (1 500–10 900) | 100 (100–200) | 3900 (1600–11 100) |
| 0–60 months† | |||||||
| Studies | 19 | 26 | 30 | 26 | 77 | 24 | 101 |
| In-hospital CFR (%) | 1·4 (0·6–2·8) | 0·8 (0·4–1·5) | 0·6 (0·3–1·0) | 0·1 (0·1–0·2) | 0·8 (0·6–1·2) | 0·1 (0·1–0·2) | 0·7 (0·5–1·1) |
| Number of deaths | 5600 (1400–21 300) | 14 600 (4400–44 100) | 6800 (2400–19 000) | 500 (300–1300) | 25 900 (14 500–48 600) | 400 (200–900) | 26 300 (15 100–49 100) |
Data are n, CFR (UR), or n (UR). Numbers of deaths were rounded to the nearest hundred, except when the estimate was <50. RSV=respiratory syncytial virus. CFR=case fatality ratio. UR=uncertainty range. *Global estimates were obtained by summing the numbers of developing and industrialised countries for each of the 1000 samples in the Monte Carlo simulation. †The point estimates and uncertainty range estimates are not necessarily equal to the sum of the estimates by finer age bands; this is because the studies that contributed to different age-group-specific estimates were different.
To further supplement these RSV-associated morbidity and in-hospital mortality estimates, we also estimated the acute lower respiratory infection morbidity and in-hospital mortality that could be attributable to RSV (appendix p 15). We estimated that in 2019, there were 29·7 million (22·8–40·2 million) acute lower respiratory infection episodes, 3·2 million (2·6–4·2 million) hospital admissions for acute lower respiratory infection, and 21 100 (12 100–39 300) in-hospital deaths for acute lower respiratory infection that could be attributable to RSV in children aged 0–60 months.
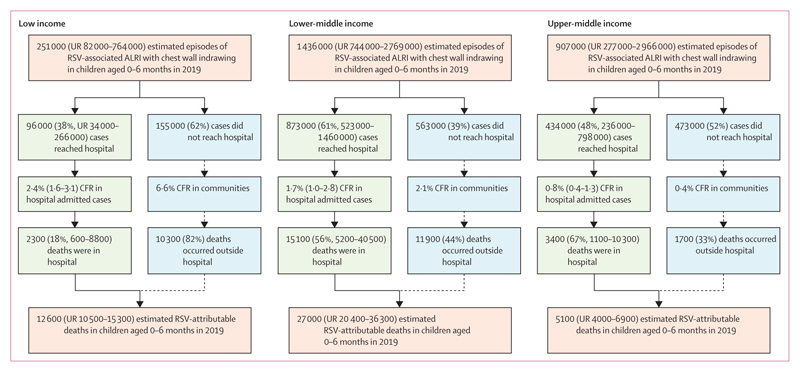
For overall RSV deaths, we estimated that 101 400 (2·0%, UR 84 500-125 200) of 52 million all-cause deaths were attributable to RSV in children aged 0–60 months and this proportion was highest in children aged 28 days-6 months (3·6%, 3·0–4·4). Although only 0·7% of all-cause deaths during the neonatal period (age 0–27 days) were attributable to RSV, RSV deaths inneonates still accounted for 19% of the RSV-attributable deaths in the first six months of life. LMICs accounted for more than 97% of the RSV-attributable deaths across all age groups. As a secondary estimate, we computed that there were 229 000 (UR 196 000–271 200) RSV-associated all-cause deaths and 109 600 (97 200-124 900) RSV-associated acute lower respiratory infection deaths in children aged 0–60 months in 2019 (table 4). Taken with the estimated RSV-associated acute lower respiratory infection in-hospital deaths, only 26 300 (26%) of 101 400 RSV-attributable deaths occurred in hospitals in patients aged 0–60 months and 13 300 (29%) of 45 700 RSV-attributable deaths occurred in hospitals in children aged 0–6 months. Only 2300 (18%) of 12 500 RSV-attributable deaths occurred in hospitals in infants aged 0–6 months in low-income countries, and CFR was as high as 6·6% in communities (figure 2).
Table 4. Estimates (with 95% uncertainty range) of RSV-attributable deaths, and RSV-associated all-cause and acute lower respiratory infection deaths in children younger than 5 years in 2019, by World Bank income regions and development status.
| Low income | Lower-middle income | Upper-middle income | High income | Developing countries | Industrialised countries | Global* | |
|---|---|---|---|---|---|---|---|
| RSV-attributable† deaths (primary measure) | |||||||
| 0–27 days | |||||||
| Proportion (%), in all-cause deaths | 0·8 (0·6–0·9) | 0·7 (0·6–1·0) | 0·6 (0·5–0·9) | 0·6 (0·4–0·9) | 0·7 (0·6–0·9) | 0·6 (0·4–1·0) | 0·7 (0·6–0·9) |
| Number of deaths | 2600 (2100–3200) | 4700 (3500–6400) | 1000 (800–1500) | 200 (200–400) | 8300 (6900–10 100) | 200 (200–400) | 8500 (7100–10 400) |
| 28 days to 6 months | |||||||
| Proportion (%), in all-cause deaths | 3·8 (3·2–4·6) | 3·6 (2·7–4·9) | 3·2 (2·5–4·2) | 2·9 (2·2–4·2) | 3·6 (3·0–4·4) | 2·8 (2·1–4·3) | 3·6 (3·0–4·4) |
| Number of deaths | 10 000 (8400–12 200) | 22 300 (16 900–30 300) | 4100 (3200–5500) | 500 (400–800) | 36 700 (30 800–45 100) | 500 (300–700) | 37 200 (31 200–45 500) |
| 0–6 months | |||||||
| Proportion (%), in all-cause deaths | 2·1 (1·8–2·6) | 2·2 (1·6–2·9) | 1·8 (1·4–2·4) | 1·3 (1·0–2·0) | 2·1 (1·8–2·6) | 1·2 (0·9–2·0) | 2·1 (1·7–2·5) |
| Number of deaths | 12 600 (10 500–15 300) | 27 000 (20 400–36 300) | 5100 (4000–6900) | 700 (500–1100) | 45 000 (37 800–55 300) | 700 (500–1100) | 45 700 (38 400–55 900) |
| 6–12 months | |||||||
| Proportion (%), in all-cause deaths | 2·6 (2·1–3·2) | 2·4 (1·8–3·5) | 2·1 (1·6–2·9) | 2·0 (1·4–2·8) | 2·5 (2·0–3·1) | 1·9 (1·4–2·9) | 2·5 (2·0–3·1) |
| Number of deaths | 6300 (5100–8000) | 12 500 (9100–17 800) | 1600 (1200–2100) | 100 (100–200) | 20 500 (16 700–25 900) | 100 (100–100) | 20 600 (16 800–26 000) |
| 0–12 months | |||||||
| Proportion (%), in all-cause deaths | 2·2 (1·9–2·8) | 2·2 (1·7–3·1) | 1·8 (1·4–2·5) | 1·4 (1·0–2·0) | 2·2 (1·8–2·7) | 1·3 (0·9–2·0) | 2·2 (1·8–2·7) |
| Number of deaths | 18 900 (15 700–23 300) | 39 600 (29 400–54 100) | 6700 (5200–9000) | 900 (600–1300) | 65 500 (54 600–81 200) | 800 (600–1200) | 66 300 (55 200–82 000) |
| 12–60 months | |||||||
| Proportion (%), in all-cause deaths | 1·7 (1·4–2·1) | 1·6 (1·2–2·2) | 1·4 (1·1–1·9) | 1·3 (1·0–1·8) | 1·6 (1·4–2·0) | 1·2 (0·9–1·9) | 1·6 (1·4–2·0) |
| Number of deaths | 11 200 (9200–13 900) | 21 300 (15 900–29 300) | 2400 (1800–3200) | 200 (100–200) | 35 000 (29 000–43 600) | 100 (100–200) | 35 100 (29 100–43 600) |
| 0–60 months | |||||||
| Proportion (%), in all-cause deaths | 2·0 (1·6–2·5) | 2·0 (1·5–2·7) | 1·7 (1·3–2·3) | 1·4 (1·0–2·0) | 2·0 (1·6–2·4) | 1·3 (0·9–2·0) | 2·0 (1·6–2·4) |
| Number of deaths | 30 100 (24 900–37 200) | 60 900 (45 300–84 300) | 9100 (7000–12 100) | 1000 (700–1500) | 100 500 (83 600–124 500) | 900 (600–1400) | 101 400 (84 500–125 200) |
| RSV-associated all-cause deaths (secondary measure) | |||||||
| 0–27 days | |||||||
| Proportion (%), in all-cause deaths | 1·8 (1·5–2·1) | 1·7 (1·3–2·2) | 1·4 (1·1–1·9) | 1·3 (1·0–1·9) | 1·7 (1·5–2·0) | 1·3 (1·0–1·9) | 1·7 (1·5–2·0) |
| Number of deaths | 5900 (5100–7000) | 10 800 (8500–14 000) | 2300 (1800–3100) | 500 (400–700) | 19 100 (16 400–22 300) | 500 (400–800) | 19 600 (16 900–22 900) |
| 28 days to 6 months | |||||||
| Proportion (%), in all-cause deaths | 8·4 (7·3–9·8) | 8·1 (6·4–10·1) | 7·0 (5·8–8·6) | 6·4 (5·1–8·4) | 8·0 (7·0–9·4) | 6·3 (4·9–8·6) | 8·0 (7·0–9·3) |
| Number of deaths | 22 200 (19 300–25 800) | 49 800 (39 800–62 300) | 9200 (7600–11 300) | 1200 (900–1500) | 81 400 (70 800–95 300) | 1000 (800–1400) | 82 500 (71 700–96 300) |
| 0–6 months | |||||||
| Proportion (%), in all-cause deaths | 4·7 (4·1–5·5) | 4·8 (3·9–6·1) | 3·9 (3·3–4·9) | 3·0 (2·3–4·0) | 4·7 (4·1–5·5) | 2·8 (2·1–3·9) | 4·6 (4·0–5·4) |
| Number of deaths | 28 100 (24 400–32 800) | 60 700 (48 300–76 500) | 11 400 (9500–14 300) | 1700 (1300–2200) | 100 500 (87 400–117 400) | 1600 (1200–2200) | 102 000 (88 800–118 800) |
| 6–12 months | |||||||
| Proportion (%), in all-cause deaths | 5·7 (4·8–7·0) | 5·5 (4·2–7·4) | 4·8 (3·8–6·2) | 4·5 (3·5–5·9) | 5·5 (4·6–6·7) | 4·2 (3·2–6·0) | 5·5 (4·6–6·7) |
| Number of deaths | 14 300 (12 000–17 300) | 28 300 (21 500–37 800) | 3500 (2800–4500) | 300 (200–300) | 46 200 (38 800–56 200) | 200 (100–200) | 46 400 (38 900–56 300) |
| 0–12 months | |||||||
| Proportion (%), in all-cause deaths | 5·0 (4·3–5·9) | 5·0 (4·0–6·5) | 4·1 (3·4–5·1) | 3·1 (2·4–4·1) | 4·9 (4·2–5·8) | 2·9 (2·2–4·1) | 4·9 (4·2–5·8) |
| Number of deaths | 42 300 (36 300–50 100) | 89 000 (69 900–114 600) | 15 000 (12 300–18 700) | 1900 (1500–2500) | 146 600 (126 200–173 400) | 1700 (1300–2400) | 148 500 (128 000–174 900) |
| 12–60 months | |||||||
| Proportion (%), in all-cause deaths | 3·9 (3·3–4·7) | 3·7 (2·9–4·9) | 3·2 (2·6–4·1) | 3·0 (2·3–3·9) | 3·7 (3·2–4·5) | 2·8 (2·2–3·9) | 3·7 (3·2–4·5) |
| Number of deaths | 25 600 (21 700–30 800) | 48 800 (37 800–64 600) | 5400 (4400–6800) | 400 (300–500) | 80 300 (67 700–96 400) | 200 (200–300) | 80 600 (68 000–96 600) |
| 0–60 months | |||||||
| Proportion (%), in all-cause deaths | 4·5 (3·8–5·4) | 4·5 (3·5–5·8) | 3·8 (3·1–4·8) | 3·1 (2·4–4·1) | 4·4 (3·8–5·3) | 2·9 (2·2–4·1) | 4·4 (3·8–5·2) |
| Number of deaths | 67 900 (58 000–80 900) | 137 800 (107 600–178 800) | 20 500 (16 700–25 500) | 2300 (1800–3000) | 226 800 (194 000–269 400) | 2000 (1500–2800) | 229000 (196 000–271 200) |
| RSV-associated acute lower respiratory infection deaths (secondary measure) | |||||||
| 0–27 days | |||||||
| Proportion | 7·4 (6·4–8·6) | 7·4 (6·0–9·5) | 8·3 (6·5–11·3) | 8·8 (7·3–10·8) | 7·4 (6·6–8·6) | 8·9 (7·2–11·6) | 7·4 (6·6–8·6) |
| Number of deaths | 1500 (1300–1800) | 2000 (1600–2600) | 200 (200–300) | <50 (<50-<50) | 3800 (3300–4400) | <50 (<50-<50) | 3800 (3400–4400) |
| 28 days to 6 months | |||||||
| Proportion | 16·6 (14·7–18·6) | 17·1 (14·4–20·7) | 18·1 (15·5–21·2) | 18·4 (15·0–22·9) | 17·0 (15·2–19·4) | 19·5 (16·2–23·4) | 17·1 (15·2–19·4) |
| Number of deaths | 15 100 (13 500–17 000) | 36 100 (30 300–43 700) | 6000 (5100–7000) | 300 (300–400) | 57 300 (51 100–65 300) | 200 (100–200) | 57 500 (51 200–65 400) |
| 0–6 months | |||||||
| Proportion | 14·9 (13·2–16·7) | 16·0 (13·5–19·4) | 17·3 (14·8–20·3) | 17·6 (14·3–21·8) | 15·8 (14·1–17·9) | 17·7 (14·6–21·3) | 15·8 (14·1–17·9) |
| Number of deaths | 16 700 (14 800–18 700) | 38 000 (32 000–46 100) | 6200 (5300–7300) | 300 (300–400) | 61 100 (54 500–69 200) | 200 (200–200) | 61 300 (54 700–69 400) |
| 6–12 months | |||||||
| Proportion | 10·5 (9·0–12·2) | 10·8 (8·7–13·5) | 11·6 (9·6–14·2) | 12·0 (9·6–15·7) | 10·7 (9·4–12·5) | 12·5 (10·0–16·2) | 10·7 (9·4–12·5) |
| Number of deaths | 9000 (7800–10 500) | 17 200 (14 000–21 600) | 2300 (1900–2800) | 100 (100–200) | 28 500 (25 000–33 100) | 100 (100–100) | 28 700 (25 100–33 200) |
| 0–12 months | |||||||
| Proportion | 13·0 (11·4–14·7) | 13·9 (11·6–17·0) | 15·3 (12·9–18·2) | 15·4 (12·5–19·2) | 13·7 (12·2–15·7) | 15·4 (12·6–19·1) | 13·7 (12·2–15·6) |
| Number of deaths | 25 700 (22 500–29 200) | 55 300 (46 000–67 500) | 8500 (7200–10 200) | 500 (400–600) | 89 700 (79 600–102 200) | 300 (200–400) | 89 900 (79 800–102 400) |
| 12–60 months | |||||||
| Proportion | 7·3 (6·3–8·5) | 7·5 (6·2–9·2) | 8·2 (6·7–10·3) | 8·7 (7·2–10·8) | 7·5 (6·6–8·6) | 8·8 (7·0–11·8) | 7·5 (6·6–8·6) |
| Number of deaths | 6300 (5400–7200) | 11 200 (9200–13 900) | 2000 (1700–2500) | 200 (200–200) | 19 500 (17 200–22 300) | 200 (200–300) | 19 700 (17 400–22 500) |
| 0–60 months | |||||||
| Proportion | 11·3 (9·9–12·9) | 12·2 (10·1–14·9) | 13·1 (11·0–15·7) | 12·6 (10·3–15·5) | 12·0 (10·6–13·6) | 11·7 (9·5–15·0) | 12·0 (10·6–13·6) |
| Number of deaths | 32 000 (27 900–36 500) | 66 600 (55 300–81 600) | 10 500 (8900–12 700) | 700 (600–800) | 109 100 (96 700–124 400) | 500 (400–600) | 109 600 (97 200–124 900) |
Data are n, proportion (%) in acute lower respiratory infection deaths (UR), or n (UR). Number of deaths were rounded to the nearest 100, except when the estimate was <50. RSV=respiratory syncytial virus. UR=uncertainty range. *Global estimates were obtained by summing the numbers of developing and industrialised countries for each of the 1000 samples in the Monte Carlo simulation. †RSV-attributable death is defined as RSV being anywhere in the causal chain of death.5
Figure 2. Burden of RSV-associated ALRI in infants aged 0–6 months in LMICs by severity and outcome including burden on health-care services.
ALRI=acute lower respiratory infection. CFR=case fatality ratio. LMICs=low and middle income countries. RSV=respiratory syncytial virus. UR=uncertainty range.
Discussion
In this study, we expanded our existing global RSV disease burden dataset by including 113 new eligible studies from the literature review update and new unpublished data from 51 studies shared through RSV GEN. We estimated that globally in 2019, there were 33·0 million RSV-associated acute lower respiratory infection episodes, 3·6 million RSV-associated acute lower respiratory infection hospital admissions, and 26 300 RSV-associated acute lower respiratory infection in-hospital deaths in children aged 0–60 months. In infants aged 0–6 months, there were 6·6 million RSV-associated acute lower respiratory infection episodes, 1·4 million RSV-associated acute lower respiratoryinfection hospital admissions, and 13 300 RSV-associated acute lower respiratory infection in-hospital deaths. We highlighted the substantial unmeasured burden of RSV mortality, with one in every 50 deaths in children aged 0–60 months and one in every 28 deaths in children aged 28 days–6 months attributable to RSV. These findings suggest that RSV passive immunisation programmes targeting the first 6 months of life could have a substantial effect on reducing RSV morbidity and mortality burden.
Our estimates of RSV morbidity (including disease burden in the community and hospital) were broadly consistent with our previous estimates for the year 2015.3 The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 201615 reported 10·7 million acute lower respiratory infection episodes in children aged 0–60 months in all health-care settings. This was between our estimates of 3·6 million hospital admissions for RSV-associated acute lower respiratory infection (a narrower definition than that used in GBD 2016), and 33·0 million RSV-associated acute lower respiratory infection episodes (attending or not attending health care, a broader definition than that used in GBD 2016). The estimate of 3·6 million hospital admissions for RSV-associated acute lower respiratory infection in the present study is also broadly consistent with extrapolated global estimates (2·5–4·1 million) from the national estimates for 58 countries.21
In our previous estimates for 2015,3 we had to use imputed rates for children aged 0–6 months due to paucity of data. For example, our previous estimates of RSV-associated acute lower respiratory infection incidence in children aged 0–6 months for low-income and high-income countries were exclusively based on imputed rates from older age groups. Because infant age groups are crucial for RSV immunisation strategy, we made extensive efforts to identify and collect unpublished data on these age groups in this study. For children aged 0–6 months, we included 12 more community-based studies with RSV incidence data, more than double the number of studies in the previous study (22 vs ten); each of the income regions had available RSV data so imputation was not required in this study. Because of the expanded dataset, we could observe a larger gap in RSV disease burden between LMICs and high-income countries in children aged 0–6 months than in children aged 0–60 months. The disproportionately high RSV burden in the younger age groups in LMICs warrants more extensive community case management and effective and affordable immunisation programmes. The gap is even larger when it comes to hospital admission, reflecting the fact that access and availability to hospital care is still limited in LMICs. Despite higher incidence in the community, the RSV-associated acute lower respiratory infection hospital admission rate in LMICs was similar to that in high-income countries for children aged 0–60 months, and for children aged 0–6 months, astonishingly, the hospital admission rate in LMICs was consistently lower than that in high-income countries.
Since our previous estimates for 20153, we have improved our methods to develop more robust estimates in our subsequent global disease burden studies for other viral infections.9–11,22 One important change is the use of GLMM framework in place of the conventional random-effects model (REM); compared with REM, GLMM has advantages when handling sparse data (ie, when case or denominator counts are small).23 This helps explain the differences in the RSV-associated acute lower respiratory infection in-hospital deaths between our present and previous estimates, as REM tends to bias CFR estimates upwards, towards 0·5,23 which inflated the CFR estimate for older children in particular. When applying the same model to our present and previous data, there was only moderate difference in the estimates. Nevertheless, the decrease in the in-hospital mortality estimates from previous estimates cannot be attributed entirely to the use of different models. Our stratified analysis (by the study median year, 2012) identified a decrease in in-hospital CFR over time, especially among LMICs, highlighting the overall improvement in quality of care in recent years. We observed some interesting trends for RSV-associated acute lower respiratory infection in-hospital CFR when stratified by age and income regions. Overall, in-hospital CFR of RSV-associated acute lower respiratory infection decreased with increase in income level. This could be due to several reasons. First, the paucity of appropriate care in the form of supportive management (eg, oxygen supplementation and suction of respiratory secretions) in LMICs. Second, differences in health-care accessibility and affordability in LMICs where children are likely to be more severely ill when brought to hospitals. In addition, CFR decreased with age in LMICs but did not change substantially over age in high-income countries; we were unable to observe such trends over age in our previous analysis,3 largely because of REM inflating the CFR estimates in older children. Results from our sensitivity analysis suggest that the gap in in-hospital CFR between LMICs and high-income countries has reduced but is still substantial.
We previously estimated that there were 118 200 RSV-associated deaths from acute lower respiratory infection in children aged 0–60 months in 2015, but this was based on limited data, through an indirect excess mortality approach that relied on the statistical correlation between RSV morbidity and acute lower respiratory infection mortality.3 In the present study, we developed new models that could incorporate all the RSV community mortality data that became available in the last 3 years. This has been a major advancement in understanding the previously unrecognised burden of overall RSV mortality in infants. For the first time, we were able to estimate RSV overall mortality burden by narrower age bands and by region. We decided to report RSV-attributable all-cause deaths as the primary estimate and RSV-associated all-cause and acute lower respiratory infection deaths as secondary estimates in this study, with several considerations. Compared with all-cause death, determination of acute lower respiratory infection as cause of death relied mostly on verbal autopsy; misclassification and recall bias related to verbal autopsy could affect the estimate,24 which was also reflected by our finding that RSV-associated acute lower respiratory infection deaths only accounted for 48% of RSV-associated all-cause deaths. Compared with RSV-associated death where RSV was identified in the upper respiratory tract, but not necessarily in the causal chain, using RSV-attributable death is more relevant for understanding the impact of RSV prophylactics on RSV mortality. As the primary measure, we estimated that there were RSV-attributable deaths accounted for 2% of all-cause deaths in children aged 0–60 months. Approximately 45% of these RSV-attributable deaths occurred in the first 6 months of life, with RSV being in the causal chain in about 4% of all-cause deaths for post-neonatal infants. Although not being a common cause of deaths in the neonatal period, RSV was in the causal chain for about 19% of RSV-attributable deaths in children aged 0–6 months. A study25 based on global case series of RSV mortality reported a greater proportion of RSV neonatal deaths in community than in hospitals. These findings highlight the need for RSV maternal vaccine or a birth-dose of RSV monoclonal antibody.
Based on the estimates for in-hospital and overall mortality above, we further showed that globally, only 26% of RSV-attributable deaths occurred in hospitals in children aged 0–60 months; that is three deaths in the community for every RSV-associated acute lower respiratory infection in-hospital death. This proportion is lower than the 50% that we estimated previously with limited data.3 The gap between in-hospital and community deaths is even more pronounced in low-income countries, with 19% of the RSV-attributable deaths occurring in hospitals (ie, four deaths in community for every in-hospital death). A study conducted in a remote rural area with poor access to care in India reported that for every RSV in-hospital death, there could be as many as 13 RSV community deaths.26 Most of the striking gap between in-hospital and community deaths in low-income settings can be explained by the poor access to care, cost of care, and limited beds in hospitals during an RSV epidemic.27 Another explanation is that some of the RSV deaths might be in children with rapidly progressive illness who, initially, do not appear to be severely ill. An RSV community mortality study in urban slums in Buenos Aires, Argentina, showed that home deaths could occur during sleep, with mild bronchiolitis or even without any apparent lung disease.17 This justifies our reporting of all-cause deaths over acute lower respiratory infection death for overall deaths estimation.
One of the major differences between in-hospital mortality data and community mortality data included in our study is the time of testing. RSV was often tested upon admission in hospital-based studies, whereas in community mortality studies, only post-mortem samples were tested. It is widely acknowledged that RSV could predispose individuals to secondary bacterial infection,28–30 the latter of which could be lethal. Therefore, it is likely that some of the RSV-attributable deaths could not be captured by post-mortem testing alone as RSV would probably be undetectable by the time of testing. An analysis of the Scottish health-care data31 suggests that deaths occurring up to 1 month following the initial RSV diagnosis could be attributable to RSV. This suggests that the estimated overall RSV mortality burden in our study could still be an underestimate of the true burden, which might only be quantified by vaccine probe studies. Moreover, some of the community mortality studies were done in under-resourced settings and therefore might not be fully representative of the country. This could affect our estimates if the proportion of RSV in all-cause and acute lower respiratory infection deaths differed by level of deprivation within that country.
Given the disproportionally high burden of RSV morbidity and mortality in children aged 0–6 months, passive immunisation programmes targeting the first 6 months of life could have a substantial effect on reducing RSV disease burden. For example, assuming that RSV passive immunisation could confer 70% protection to infants aged up to 5 months, then this could directly avert up to 864000 RSV-associated acute lower respiratory infection hospital admissions and 26 800 RSV-attributable deaths globally per year. Within the first 6 months of life, over 60% of the RSV-associated acute lower respiratory infection hospital admissions were during the first 3 months of life. This suggests that RSV immunisation products could still be impactful even with a shorter duration of protection, although they would probably miss the substantial RSV-associated acute lower respiratory infection incidence that only peaked during 3–6 months of age in low-income and lower-middle-income countries. Compared with children aged 0–6 months, the disease burden in children aged 6–12 months was smaller but still substantial, especially for morbidity burden. This suggests that further investment is warranted for RSV prophylactic products targeting this age group.
Our study had several limitations, as discussed previously.3 First, heterogeneities in factors such as study setting, exact case definition for acute lower respiratory infection, health-care access and seeking behaviour, eligibility for RSV testing and proportion of specimens tested, and RSV testing assay could affect our estimates, although sensitivity analyses based on the factors above that removed studies with high risks of bias showed broadly consistent estimates (appendix pp 44–48). Second, for both morbidity and mortality estimates, we were constrained by the data to break down the age bands any further or to model the year-on-year changes in the RSV disease burden; for which more data on multi-year changes in RSV morbidity and mortality are warranted. Third, we did not specifically report the burden of RSV-associated acute lower respiratory infection in primary care, such as general practice and outpatient. Fourth, we applied data imputation for estimating the RSV-associated acute lower respiratory infection incidence and hospital admission rates for children aged 0–60 months among studies that did not report data in specific age bands but reported data either overall for children aged 0–60 months or some of the narrower age bands; nonetheless, sensitivity analyses that excluded imputed rates did not yield substantial differences in the estimates. Fifth, the data for RSV overall mortality estimates were still scarce (15 studies) and mostly represented under-resourced settings. Finally, all of our included data were collected before the coronavirus disease 2019 (COVID-19) pandemic; reports from France,32 Iceland,33 and Australia34 showed that children hospitalised for RSV disease in the first wave of RSV epidemics following the onset of the COVID-19 pandemic were older than those in the pre-pandemic period. It is unknown how the COVID-19 pandemic could affect RSV disease burden in the long term. Our estimates could serve as a reference for understanding RSV epidemiology in the context of the ongoing COVID-19 pandemic.
Despite these limitations, our revised estimates are based on a substantially expanded dataset (including the community mortality data) and improved methodology. For the first time, we managed to break down the population of children aged 0–6 months into narrower age bands for both morbidity and mortality estimates that are essential for estimating the impact of RSV prophylactics. These estimates should provide a comprehensive global overview of RSV morbidity and mortality burden in infants and young children. With the numerous RSV prophylactic products in the pipeline, our estimates provide an important baseline profile of RSV disease burden for evaluating their potential clinical impact and cost-effectiveness of public health programmes.
Supplementary Material
Research in context.
Evidence before this study
Respiratory syncytial virus (RSV) is the main cause of acute lower respiratory infection among young children. We searched PubMed for studies published between Jan 1, 1995, and Oct 31, 2021, that reported global estimates of RSV morbidity and mortality in young children, using the search terms “(“Respiratory syncytial virus*” OR “RSV”) AND (“incidence*” OR “mortality” OR “death*” OR “morbidity” OR “burden”) AND (“child*” OR “pediatric” OR “paediatric” OR “infant*”)”. We previously estimated that in 2015, there were 33·1 million acute lower respiratory infection episodes, 3·2 million hospital admissions for acute lower respiratory infection and 59 600 acute lower respiratory infection in-hospital deaths associated with RSV in children aged 0–60 months; we also reported an estimated 118 200 overall deaths (ie, in-hospital and out-of-hospital or community deaths), derived indirectly based on in-hospital deaths. The Global Burden of Disease study by the Institute of Health Metrics and Evaluation estimated that 10·7 million acute lower respiratory infection episodes and over 41 000 acute lower respiratory infection deaths in children aged 0–60 months in 2016 were attributable to RSV. In a separate study, we estimated the national burden of RSV hospitalisation for 58 countries and reported an extrapolated estimate of 2·5–4·1 million RSV-associated hospitalisations globally in children aged 0–60 months in 2019. No robust estimates of RSV overall deaths were available from existing global studies that incorporated RSV community mortality data.
Added value of this study
We substantially expanded our global RSV burden dataset by including 113 new studies from the literature review update and new unpublished data from 51 studies shared through RSV GEN. With these data and improved methods, we have now been able to estimate RSV morbidity and mortality burden for hospital settings and overall, and focus on narrower infant age groups that are targeted by RSV prophylactics in development. With data on RSV community mortality, we estimated the overall RSV mortality burden in different age groups–one death was attributable to RSV in every 50 deaths in children aged 0–60 months and in every 28 deaths in children aged 28 days to 6 months. For every RSV-associated acute lower respiratory infection in-hospital death, there were approximately three deaths attributable to RSV in the community.
Implications of all the available evidence
By incorporating the latest RSV community mortality data, we provide robust estimates of global and regional burden of RSV-related mortality. Our findings, together with other existing RSV disease burden estimates, provide a comprehensive global overview of RSV morbidity and mortality burden in infants and young children. With many RSV prophylactic products in the pipeline, our estimates by narrower age bands provide important baseline information to the introduction, prioritisation, and evaluation of these products. Further evidence is warranted to understand the implications of the potential age-shifts in peak RSV burden to older age after administration of RSV prophylactics to infants younger than 6 months.
Acknowledgements
This study is funded by RESCEU. RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement 116019. This Joint Undertaking receives support from the EU Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries. This work reflects only the authors’ view and the Joint Undertaking is not responsible for any use that may be made of the information it contains. We acknowledge Veena Kumar, Scott Gallichan, and Eva Molero for their inputs as members of RESCEU. We thank Padmini Srikantiah and Keith P Klugman from the Gates Foundation for their feedback on the RSV mortality estimates. We thank Shuyu Deng for her assistance in preparing the revision of the manuscript. We acknowledge the Ministry of Health in Mongolia for their support in the pneumonia surveillance and community carriage surveys project in Mongolia and the sponsor of the two projects: GAVI and the Gates Foundation. We acknowledge the sponsors for RSV surveillance in Melbourne, WHO funding and Murdoch Children’s Research Institute Theme grant. We thank study staff, laboratory staff and participants in Mongolia, the Royal Children’s Hospital in Melbourne, Australia, and Murdoch Children’s Research Institute in Melbourne, Australia. The RSV data in Vietnam from Nagasaki University were supported by Japan Program for Infectious Diseases Research and Infrastructure, Japan Agency for Medical Research and Development under grant number JP21wm0125006. The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government. The RSV data in Lima and Iquitos were funded by South America Influenza Initiative, work unit number AOA#316, IAA NMR-9619. The study protocol was approved by the Naval Medical Research Unit Number Six Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. Some authors are military service members (or employees of the US Government); this work was prepared as part of their official duties. Title 17 US Code §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 US Code §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
Footnotes
Contributors
YL and HN conceptualised the study. YL led the literature review with contributions from XW and ABP. YL led the data analysis with substantial contributions from XW. YL, HN, and XW led the data interpretation. DMB, MTC, DRF, CJG, SAM, SBO, and EAFS were members of a mortality subgroup that provided critical inputs to the mortality estimates. YL wrote the first draft of the manuscript with inputs from XW and HN. All other authors contributed to collection and analysis of the primary data and data interpretation, and critically reviewed the manuscript. All authors read and approved the final draft of the manuscript. All authors who contributed to primary data analysis accessed and verified the disaggregated data from their local studies, and provided aggregated data for the analysis of this study. YL and HN have full access to and have verified all the aggregated study data provided for the analysis. YL and HN were responsible for the decision to submit the manuscript.
Declaration of interests
YL reports grants from Wellcome Trust and WHO outside the submitted work. MTC reports grants from the Bill & Melinda Gates Foundation related to the submitted work, grants from MITS Surveillance Alliance, and support for attending the RSVVW meeting from ReSViNET outside the submitted work. SAM reports grants from Pfizer, Minervax, GSK, the Gates Foundation, and South African Medical Research Council; honoraria from the Gates Foundation; and participation on data safety monitoring boards for PATH and CAPRISA, outside the submitted work. SBO reports grants from the Gates Foundation outside the submitted work. EAFS reports grants, personal fees, and travel fees from AstraZeneca, Merck, Regeneron, Pfizer, and Roche; consultation, lecture fees, travel support, and data and safety monitoring board fees from AbbVie; data and safety monitoring board fees from GSK; consultation fees from Alere; grants from Johnson & Johnson; and grants and travel support from Novavax outside the submitted work. HC reports grants from the Gates Foundation outside the submitted work. AGo reports grants from the National Institute of Allergy and Infectious Diseases and Centers for Disease Control and Prevention (CDC) related to the submitted work and participation on an advisory board for Janssen outside the submitted work. TH reports personal fees from Janssen and Sanofi Pasteur outside the submitted work. AK reports grants from CDC and honoraria from CDC and WHO outside the submitted work. AM-I reports grants from FISABIO-Public Health, Sanofi Pasteur, and CIBER-ESP (ISCIII) related to the submitted work, honoraria from MSD as a speaker in a vaccine research course, and travel grants for attending meetings sponsored by Sanofi, outside the submitted work. HCM reports grants from National Health and Medical Research Council related to the submitted work and honoraria from MSD for participation on an expert input forum outside the submitted work. DJN reports grants from Wellcome Trust related to the submitted work. EO reports receipt of PhD scholarship from DAAD (German Academic Exchange Service) Government of Ghana scholarship outside the submitted work. CR reports grants from CDC in collaboration with US Naval Medical Research Unit No6 related to the submitted work and grants from South America Influenza Initiative outside the submitted work. AS reports grants from University of Colorado outside the submitted work. RS reports grants from Merck outside the submitted work. SKS reports salaries from GSK for working on the data abstraction, leading the prospective cohort study from which the data were abstracted, and for providing input for the manuscript development related to the submitted work and stock in GSK outside the submitted work. AvG reports grants from CDC outside the submitted work. DW reports grants from Murdoch Children’s Research Institute related to the submitted work and honoraria from MSD for participation on an expert input forum outside the submitted work. L-MY reports grants from Japan Agency for Medical Research and Development related to the submitted work and honoraria for a lecture from MSD KK. HJZ reports grants from the Gates Foundation, South African Medical Research Council, National Institutes for Health, and AstraZeneca and participation on WHO Technical Advisory Group with no payment, outside the submitted work. HN reports grants from the Innovative Medicines Initiative related to the submitted work and consulting fees from the Gates Foundation, Pfizer, and Sanofi; honoraria from AbbVie; support from Sanofi for attending meetings; and participation on advisory boards from Sanofi, Janssen, Novavax, Reviral, Resvinet, and WHO outside the submitted work.
Contributor Information
Prof You Li, School of Public Health, Nanjing Medical University, Nanjing, China.
Xin Wang, School of Public Health, Nanjing Medical University, Nanjing, China.
Dianna M Blau, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Mauricio T Caballero, Fundacion INFANT, Buenos Aires, Argentina.
Daniel R Feikin, Department of Immunizations, Vaccines, and Biologicals, WHO, Geneva, Switzerland.
Christopher J Gill, Boston University School of Public Health, Department of Global Health, Boston, Massachusetts, USA.
Shabir A Madhi, South African Medical Research Council Vaccines and Infectious Diseases Analytics Research Unit.
Prof Saad B Omer, University of the Witwatersrand, Faculty of Health Sciences, Johannesburg, South Africa; Yale Institute for Global Health, New Haven, CT, USA.
Eric A F Simões, Department of Pediatrics, Section of Infectious Diseases, University of Colorado, School of Medicine, Aurora, CO, USA.
Harry Campbell, Centre for Global Health, Usher Institute, University of Edinburgh, Edinburgh, UK.
Ana Bermejo Pariente, Centre for Global Health, Usher Institute, University of Edinburgh, Edinburgh, UK.
Darmaa Bardach, National Center for Communicable Diseases (Mongolia), Ulaanbaatar, Mongolia.
Prof Quique Bassat, ISGlobal, Hospital Clínic – Universitat de Barcelona, Barcelona, Spain.
Giorgi Chakhunashvili, National Center for Disease Control and Public Health, Tbilisi, Georgia.
Nigel Crawford, The Royal Children’s Hospital, Melbourne, Australia.
Daria Danilenko, The University of Melbourne, Melbourne, Australia; Smorodintsev Research Institute of Influenza, Saint Petersburg, Russia.
Lien Anh Ha Do, Department of Paediatrics.
Marcela Echavarria, Clinical Virology Unit, Centro de Educación Médica e Investigaciones Clínicas, Buenos Aires, Argentina.
Prof Angela Gentile, Ricardo Gutiérrez Children Hospital, Buenos Aires, Argentina.
Aubree Gordon, Department of Epidemiology, University of Michigan, Ann Arbor, MI, USA.
Terho Heikkinen, Pediatrics, University of Turku and Turku University Hospital, Turku, Finland.
Q Sue Huang, WHO National Influenza Centre, Institute of Environmental Science and Research, Wellington, New Zealand.
Sophie Jullien, Jigme Dorji Wangchuck National Referral Hospital, Gongphel Lam, Thimphu, Bhutan.
Prof Anand Krishnan, Centre for Community Medicine, All India Institute of Medical Sciences, New Delhi, India.
Eduardo Luis Lopez, Hospital de Niños Dr. Ricardo Gutiérrez, Department of Medicine, Pediatric Infectious Diseases Program, Universidad de Buenos Aires, Buenos Aires, Argentina.
Joško Markić, Department of Pediatrics, University Hospital Split, Split, Croatia.
Ainara Mira-Iglesias, Área de Investigación en Vacunas, Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana, Salud Pública, Valencia, Spain.
Hannah C Moore, Wesfarmers Centre for Vaccines and Infectious Diseases, Telethon Kids Institute, University of Western Australia, Perth, Australia.
Jocelyn Moyes, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa.
Lawrence Mwananyanda, Boston University School of Public Health, Department of Global Health, Boston, Massachusetts, USA.
D James Nokes, Kenya Medical Research Institute–Wellcome Trust Research Programme, Kilifi, Kenya.
Faseeha Noordeen, Department of Microbiology, Faculty of Medicine, University of Peradeniya, Peradeniya, Sri Lanka.
Evangeline Obodai, Virology Department, Noguchi Memorial Institute for Medical Research, University of Ghana, Legon, Accra, Ghana.
Nandhini Palani, Department of Microbiology, Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry, India.
Candice Romero, Vysnova Partners, Lima, Perú.
Vahid Salimi, Department of Virology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
Ashish Satav, MAHAN Trust Mahatma Gandhi Tribal Hospital, Karmgram, Utavali, Tahsil, Dharni, India.
Prof Euri Seo, Department of Pediatrics, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, South Korea.
Zakhar Shchomak, Department of Pediatrics, Hospital Santa Maria, Centro Hospitalar Universitário Lisboa Norte, Lisbon, Portugal.
Rosalyn Singleton, Alaska Native Tribal Health Consortium, Anchorage, AK, USA.
Kirill Stolyarov, The University of Melbourne, Melbourne, Australia; Smorodintsev Research Institute of Influenza, Saint Petersburg, Russia.
Sonia KStoszek, Glaxo Smith Kline, Rockville, Maryland, USA.
Prof Anne von Gottberg, Department of Pathology, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Danielle Wurzel, Murdoch Children’s Research Institute, Melbourne, Australia.
Lay-Myint Yoshida, Department of Pediatric Infectious Diseases, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan.
Chee Fu Yung, Infectious Diseases Service, Department of Paediatrics, KK Women’s and Children’s Hospital, Singapore.
Heather J Zar, Department of Paediatrics and Child Health, and South African Medical Research Council Unit on Child & Adolescent Health, University of Cape Town and Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
Respiratory Virus Global Epidemiology Network investigators:
You Li, Xin Wang, Harish Nair, Harry Campbell, Ana Bermejo Pariente, Dianna M Blau, Mauricio T Caballero, Fernando P Polack, Daniel R Feikin, Christopher J Gill, Lawrence Mwananyanda, Shabir A Madhi, Saad B Omer, Eric AF Simões, Rowena Crow, Darmaa Bardach, Tuya Mungun, Naranzul Tsedenbal, Quique Bassat, Sophie Jullien, Jean-Sebastien Casalegno, Yves Gillet, Come Horvat, Etienne Javouhey, Bruno Lina, Dominique Ploin, Giorgi Chakhunashvili, Irakli Karseladze, Nigel Crawford, Danielle Wurzel, Anna-Maria Costa, Andrew Daley, Gregory Walker, Daria Danilenko, Kirill Stolyarov, Lien Anh Ha Do, Annette Alafaci, Alissa McMinn, Kim Mulholland, Claire von Mollendorf, Marcela Echavarria, Noelia Reyes, Angela Gentile, Florencia Lucion, Aubree Gordon, John Kubale, Terho Heikkinen, Q Sue Huang, Claire Newbern, Namrata Prasad, Anand Krishnan, Rakesh Kumar, Eduardo Luis Lopez, Fausto Martín Ferolla, Joško Markić, Ena Batinović, Petra Milić, Ainara Mira-Iglesias, Javier Díez-Domingo, Joan Puig-Barberà, Hannah C Moore, Christopher C Blyth, Amanuel Gebremedhin, Mark P Nicol, Jocelyn Moyes, Anne von Gottberg, Jinal Bhiman, Cheryl Cohen, Mignon du Plessis, Sibongile Walaza, D James Nokes, Nickson Murunga, Faseeha Noordeen, Maduja Divarathna, Evangeline Obodai, John Kofi Odoom, Nandhini Palani, Sujatha Sistla, Candice Romero, Vahid Salimi, Forough Tavakoli, Ashish Satav, Shilpa Satao, Euri Seo, Zakhar Shchomak, Teresa Bandeira, Rosalyn Singleton, Sonia K Stoszek, Rachel Cohen, Lay-Myint Yoshida, Michiko Toizumi, Chee Fu Yung, Heather J Zar, Rae MacGinty, Angel Balmaseda, Ian Barr, Sara S Bressler, Duc-Anh Dang, Christine Desnoyers, Roger Hernandez, Giselle Soto, Adrian Morel, and Janine Reiche
RESCEU investigators:
You Li, Xin Wang, Prof Harish Nair, Harry Campbell, Madeleine Edgoose, Yanran Song, Ting Shi, Philippe Beutels, Louis Bont, Andrew J Pollard, Matthew Snape, Peter Openshaw, Federico Martinon-Torres, Terho Heikkinen, Adam Meijer, Thea K Fischer, Maarten van den Berge, Carlo Giaquinto, Michael Abram, Bishoy Rizkalla, Clarisse Demont, Charlotte Vernhes, and Jeroen Aerssens
Data sharing
The study data and R codes for the analysis are freely available in Edinburgh DataShare (https://doi.org/10.7488/ds/3138).
References
- 1.O’Brien KL, Baggett HC, Brooks WA, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394:757–79. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bénet T, Sánchez Picot V, Messaoudi M, et al. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: the GABRIEL pneumonia multicenter, prospective, case-control study. Clin Infect Dis. 2017;65:604–12. doi: 10.1093/cid/cix378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–58. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srikantiah P, Vora P, Klugman KP. Assessing the full burden of respiratory syncytial virus in young infants in low- and middleincome countries: the importance of community mortality studies. Clin Infect Dis. 2021;73(suppl 3):S177–79. doi: 10.1093/cid/ciab486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor AW, Blau DM, Bassat Q, et al. Initial findings from a novel population-based child mortality surveillance approach: a descriptive study. Lancet Glob Health. 2020;8:e909–19. doi: 10.1016/S2214-109X(20)30205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PATH. RSV Vaccine and mAb Snapshot. 2021. [accessed Aug 17, 2021]. https://path.azureedge.net/media/documents/RSV-snapshot-07APR2021_HighResolution_NonEditable_PDF_3KgK9PB.pdf .
- 7.Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383:415–25. doi: 10.1056/NEJMoa1913556. [DOI] [PubMed] [Google Scholar]
- 8.Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837–46. doi: 10.1056/NEJMoa2110275. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Li Y, Deloria-Knoll M, et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health. 2021;9:e33–43. doi: 10.1016/S2214-109X(20)30393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Li Y, Deloria-Knoll M, et al. Global burden of acute lower respiratory infection associated with human parainfluenza virus in children younger than 5 years for 2018: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e1077–87. doi: 10.1016/S2214-109X(21)00218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Li Y, O’Brien KL, et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health. 2020;8:e497–510. doi: 10.1016/S2214-109X(19)30545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United Nations. World population prospects 2019. 2019. [accessed March 5, 2021]. https://population.un.org/wpp/Download/Standard/Population/
- 13.Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta-analysis of clinical trials with binary outcomes. Stat Med. 2000;19:3417–32. doi: 10.1002/1097-0258(20001230)19:24<3417::aid-sim614>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health. 2019;7:e47–57. doi: 10.1016/S2214-109X(18)30408-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blau DM, Baillie VL, Els T, et al. Deaths attributed to respiratory syncytial virus in young children in high-mortality rate settings: report from child health and mortality prevention surveillance (CHAMPS) Clin Infect Dis. 2021;73(suppl 3):S218–28. doi: 10.1093/cid/ciab509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caballero MT, Bianchi AM, Grigaites SD, et al. Community mortality due to respiratory syncytial virus in Argentina: population-based surveillance study. Clin Infect Dis. 2021;73(suppl 3):S210–17. doi: 10.1093/cid/ciab497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazi AM, Aguolu OG, Mughis W, et al. Respiratory syncytial virus-associated mortality among young infants in Karachi, Pakistan: a prospective postmortem surveillance study. Clin Infect Dis. 2021;73(suppl 3):S203–09. doi: 10.1093/cid/ciab488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy C, MacLeod WB, Forman LS, et al. Risk factors for respiratory syncytial virus-associated community deaths in Zambian infants. Clin Infect Dis. 2021;73(suppl 3):S187–92. doi: 10.1093/cid/ciab453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satav A, Crow R, Potdar V, et al. The burden of respiratory syncytial virus in children under 2 years of age in a rural community in Maharashtra, India. Clin Infect Dis. 2021;73(suppl 3):S238–47. doi: 10.1093/cid/ciab508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Johnson EK, Shi T, et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med. 2021;9:175–85. doi: 10.1016/S2213-2600(20)30322-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Li Y, Mei X, Bushe E, Campbell H, Nair H. Global hospital admissions and in-hospital mortality associated with all-cause and virus-specific acute lower respiratory infections in children and adolescents aged 5-19 years between 1995 and 2019: a systematic review and modelling study. BMJ Glob Health. 2021;6:e006014. doi: 10.1136/bmjgh-2021-006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–67. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 24.Menendez C, Castillo P, Martínez MJ, et al. Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: an observational study. PLoS Med. 2017;14:e1002318. doi: 10.1371/journal.pmed.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazur NI, Löwensteyn YN, Willemsen JE, et al. Global respiratory syncytial virus-related infant community deaths. Clin Infect Dis. 2021;73(suppl 3):S229–37. doi: 10.1093/cid/ciab528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simões EAF, Dani V, Potdar V, et al. Mortality from respiratory syncytial virus in children under 2 years of age: a prospective community cohort study in rural Maharashtra, India. Clin Infect Dis. 2021;73(suppl 3):S193–202. doi: 10.1093/cid/ciab481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha S, Santosham M, Hussain M, Black RE, Saha SK. Rotavirus vaccine will improve child survival by more than just preventing diarrhea: evidence from Bangladesh. Am J Trop Med Hyg. 2018;98:360–63. doi: 10.4269/ajtmh.17-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med. 2015;12:e1001776. doi: 10.1371/journal.pmed.1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Peterson ME, Campbell H, Nair H. Association of seasonal viral acute respiratory infection with pneumococcal disease: a systematic review of population-based studies. BMJ Open. 2018;8:e019743. doi: 10.1136/bmjopen-2017-019743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danino D, Ben-Shimol S, Van Der Beek BA, et al. Decline in pneumococcal disease in young children during the COVID-19 pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort Study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab1014. published online Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Campbell H, Nair H, Investigators R Unveiling the risk period for death after respiratory syncytial virus illness in young children using a self-controlled case series design. J Infect Dis. 2020;222(suppl 7):S634–39. doi: 10.1093/infdis/jiaa309. [DOI] [PubMed] [Google Scholar]
- 32.Fourgeaud J, Toubiana J, Chappuy H, et al. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur J Clin Microbiol Infect Dis. 2021;40:2389–95. doi: 10.1007/s10096-021-04323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Summeren J, Meijer A, Aspelund G, et al. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill. 2021;26:2100639. doi: 10.2807/1560-7917.ES.2021.26.29.2100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley DA, Phuong LK, Peplinski J, et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch Dis Child. 2022;107:e7. doi: 10.1136/archdischild-2021-322507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data and R codes for the analysis are freely available in Edinburgh DataShare (https://doi.org/10.7488/ds/3138).