Summary
Background
Many cancer types display sex and age disparity in incidence and outcome. The mutational load of tumours, including melanoma, varies according to sex and age. However, there are no tools to systematically explore if clinical variables such as age and sex determine the genomic landscape of cancer.
Objectives
To establish a mathematical approach using melanoma mutational data to analyze how sex and age shape the tumour genome.
Methods
We model how age-related (clock-like) somatic mutations that arise during cell division, and extrinsic (environmental ultraviolet light) mutations accumulate in cancer genomes.
Results
Melanoma is primarily driven by cell-intrinsic age-related mutations and extrinsic ultraviolet light-induced mutations, and we show these mutation types differ in magnitude, chronology and by sex in the distinct molecular melanoma subtypes. Our model confirms age and sex are determinants of cellular mutation rate, shaping the final mutation composition. We show mathematically for the first time how, similar to non-cancer tissues, melanoma genomes reflect a decline in cell division during ageing. We find clock-like mutations strongly correlate with the acquisition of ultraviolet light-induced mutations, but critically, males present a higher number and rate of cell division-linked mutations.
Conclusions
These data indicate the contribution of environmental damage to melanoma likely extends beyond genetic damage to affect cell division. Sex and age determine the final mutational composition of melanoma.
Keywords: Melanoma, molecular sex-bias, molecular age bias
Introduction
Sex and age disparity in cancer incidence and outcome are well described and studies reveal age(1,2) and sex differences(3) in genomics(4,5). Somatic mutations are drivers of cancer and mutations arise in cells due to damage following cell-intrinsic processes, as well as due to external environmental damage on the DNA. Recent work describes computational methods to discern the multiple, distinct signatures of DNA damage imprinted on DNA depending on the insult(6), but to date there are no available models to study the relationship between the distinct damaging processes.
We developed a mathematical framework to investigate the dependencies between cell-intrinsic, cell-extrinsic mutational processes, sex and age in cancer. Cutaneous melanoma exemplifies a cancer type primarily presenting cell-intrinsic (cell division) and environmental (ultraviolet light) damaging processes(7), as well as presenting an age and sex bias. Male patients and the aged population have a higher incidence and rate of death, so we studied if the genomic imprint of the major contributors to total autosomal tumour mutation burden (TMB) in melanoma are possible sources for the disparity.
Melanoma presents a broad range of clinical subtypes of disease, categorized by age of onset, history and pattern of UVR exposure(8,9). At one end of the spectrum, we identify elderly patients with melanomas arising at anatomic sites that have been chronically exposed to UVR with a high tumour mutation burden (TMB). In contrast, melanoma in younger patients arises decades after sunburn, over skin that is intermittently exposed to UVR, with a lower TMB(9–11).
The mutually exclusive oncogenic drivers V600BRAF and NRAS underpin the majority of cutaneous melanomas(12). Loss-of-function mutations in the tumour suppressor NF1 drive an additional subset of cases, and a further subgroup is defined by the absence of V600BRAF, NRAS or NF1 mutations (triple wild type; W3)(13). These genetically distinct categories overlap to some extent with clinical characteristics, with V600BRAF being more prevalent in younger patients(12).
Here we examine the relationship between mutational processes and their contribution to the melanoma somatic mutation load, their variation over time and across sexes. We provide a novel approach to model how the specific damage patterns in DNA arise over time and across the sexes. Analysing the strong bias in the mutational landscape could point to key biological differences in how tumours develop and evolve during ageing and across sexes.
Methods
Mutation data
The primary data are the somatic mutation calls from The Cancer Genome Atlas (TCGA) MAF of the whole-exome sequences of the skin cutaneous melanoma (SKCM) cohort9. We classified samples by their mutations in V600BRAF, NRAS, NF1 or none of these genes. Samples with V600BRAFor NRAS and an additional NF1mutation were classified as eitherV600BRAF or NRAS. We inferred the mutational processes by categorizing the single nucleotide substitutions in the trinucleotide context and used mathematical models to infer their contribution to the mutational landscape across biological sex and age (Supplementary material).
Results
Clock-like and UVR-driven mutations accumulate with age at distinct rates in the molecular subtypes of melanoma
We catalogued the base substitutions in 396 whole exome cutaneous melanoma samples from TCGA (TCGA-SKM)(12) according to 96 categories defined by the base substitution, the preceding and following bases(7). 172 had a V600BRAF (BRAF) mutation, 96 NRAS, 44 NF1 and 84 samples were non-BRAF/non-NRAS/non-NF1 (W3; Supplementary Table 1).
We inferred the mutational signatures that account for the somatic mutations from the TCGA data. We extracted the DNA mutational signature linked to UVR (Signature 7, COSMIC database), which is present to varying degrees across melanomas. Next, we identified the intrinsic, age-related signature observed in normal cells and cancers with high cell turnover, which corresponds to spontaneous deamination of methylated cytosine residues into thymine at CpG sites that remain unrepaired due to rapid DNA replication (Signature 1, COSMIC database(7,14,15)). This clock-like mutational process allows estimation of the number of divisions a cell has undergone since its inception. Previous studies have modelled the potential disruption to the linear acquisition of somatic mutations during aging that occurs when the neoplastic phase alters the rate of mutation acquisition and found across multiple cancer types that clock-driven mutations are linked to intrinsic cellular division despite neoplastic and oncogenic driver ontogenesis(14,16). We confirmed a positive correlation between the median number of Signature 1 mutations per year and age for all samples (Spearman ρ 0.41, P<0.0003, Figure 1A), indicating that these mutations accumulate with age. NRAS, NF1 and W3 melanomas presented an increase in the mean Signature 1 mutations with age, but this relationship was less significant in NF1 and W3 melanomas, likely due to the lower sample size (NRAS Spearman ρ 0.35, P<0.01; Figure 1B). Strikingly, there was no significant rank correlation between Signature 1 and age in BRAF samples (Spearman ρ 0.03, P=0.41). To examine the difference in the rates of Signature 1 mutation accumulation between BRAF, NRAS, NF1 and W3 melanomas, we determined the ratio between the number of Signature 1 mutations to age, and found significant differences in the ratios of BRAF and W3 to NF1 melanomas (pairwise Wilcoxon rank-sum test with Bonferroni correction, P<0.0027; Figure 1C), but less pronounced for BRAF and NRAS samples. Next, we examined the relationship between Signature 1 mutations and melanoma cell proliferation by investigating the gene expression of cell cycle genes(17). We found there is a weak, but significant correlation between Signature 1 and both cell cycle checkpoints G1/S (p=0.03 R=0.107) and G2/M (p=0.016, R = 0.121) expression genes. Furthermore, when dividing the melanomas by high versus low Signature 1 mutations based on the median, we observed a significantly higher expression of both G1/S and G2/M genes in the high Signature 1 group, which is more significant for the G2/M expressed genes. Finally, in a multiple regression with the other factors in the data set; age, gender, and molecular subtype, Signature 1 is the only factor associated with G1/S and G2/M gene expression.
Figure 1. The molecular subtypes of melanoma present distinct ratios of clock-like and UVR mutations per unit of time.
(A) Correlation analysis between somatic mutations due to clock-like Signature 1 mutations in cutaneous melanomas and age. Dots represent the median number of mutations for each age.
(B) Correlation analysis between clock-like Signature 1 mutations in the molecular subtypes of cutaneous melanomas (BRAF: red; NRAS: blue) and age. Dots represent the median number of mutations for each age.
(C) Ratio of number of signature 1 mutations per year across the molecular subtypes of cutaneous melanoma.
(D) Correlation analysis between somatic mutations due to UVR Signature 7 mutations in cutaneous melanomas and age. Dots represent the median number of mutations for each age.
(E) Correlation analysis between UVR Signature 7 mutations in the molecular subtypes of cutaneous melanomas (BRAF: red; NRAS: blue) and age. Dots represent the median number of mutations for each age.
(F) Ratio of number of Signature 7 mutations per year across the molecular subtypes of cutaneous melanoma.
We next examined the contribution of Signature 7 mutations and found a progressive increase as patients aged, in accordance with progressive accumulation of UVR damage during the course of life (Spearman ρ = 0.37, P<0.006; Figure 1D). However, the rate of mutations varied depending on the molecular subtype, with no significant correlation found in BRAF melanomas between Signature 7 and increasing age (Spearman ρ = -0.15, P=0.87, Figure 1E), and NRAS samples showing a steady increase of Signature 7 with age (Spearman ρ = 0.37, P<0.006). Similar to Signature 1, there was a significant difference in the ratio of Signature 7 mutations to age across the subtypes (P<0.03, Mann-Whitney U test with Bonferroni correction; Figure 1F).
We investigated the association between global UV damage Signature 7 and the novel probabilistic UV damage signatures that were more recently defined(13). We calculated the likely UV-associated mutational signatures with the version 3 signatures, using deconstructSigs for consistency. The correlation between the sum of the four new signatures (SBS7a/SBS7b/SBS7c/SBS7d) and the version 2 signature 7 is >0.95 (p-value < 2.2e-16). The vast majority of mutations contributing to these signatures are SBS7a (median proportion of total signature 7 per sample = 0.4742) and SBS7b (median proportion per sample = 0.4954). As both signature mutagens encompass the canonical CC-TT and atypical frequency of C to T substitutions at a dipyrimidine site that is attributable to UV mutagenesis, we retained the original, comprehensive Signature 7 to strengthen our power to detect associations.
Ageing affects the dynamics of the mutational landscape
Common models of cancer have assumed that mutations accumulate at a linear rate over time(18). Genetic changes accumulate from early life(14,16) and a decline in replicative function with age is visible in many tissues(19). We used our cohorts to test the relationship between ageing and clock-like mutation rate in melanoma, and found the ratio of mutations per year decreases with age (Spearman ρ = -0.34, P<0.005; Figure 2A). Specifically, the decline in mutations per year is pronounced in BRAF (Spearman –0.44, P<0.0004) but not statistically significant in NRAS or W3 melanomas. We did not include NF1 samples in the analysis, as this subtype is almost exclusive to the elderly.
Figure 2. Ageing affects the intrinsic mutation rate of the molecular subtypes.
(A) Correlation analysis between clock-like Signature 1 mutations per year and age in cutaneous melanomas. Dots represent the median number of mutations for each age.
(B) Distribution curves displaying Signature 1 mutation frequency across age ranges (red: 50-60 year-old range; green: 60-70 year-old range; blue: 70-80 year-old range; purple: 80-90 year-old range).
(C) Exponential model for the accumulation of Signature 1 mutations in all melanomas. This curve models the Poisson mean distribution of mutations at each age, with age-dependent rate.
(D) Exponential model for the accumulation of Signature 1 mutations in the molecular subtypes of cutaneous melanoma (BRAF: red; NF1: green; NRAS: blue; W3: purple). This curve models the Poisson mean distribution of mutations at each age, with age-dependent rate.
(E) Change in Signature 1 mutations per year with age across BRAF, NRAS and W3 subtypes.
To analyse the differences in ageing dynamics, we considered the mean number of mutations at each age, modelled by a Poisson distribution with age-dependent rate, and show the ratio of mutations by age decreases in older age groups (Figure 2B). We used an overdispersed Poisson (negative binomial) regression to estimate the parameters of the exponential model for each subtype BRAF, NRAS and W3; and found that overall, the amount of Signature 1 mutations increases by a multiplicative factor of eα = 1.0124 per year (Figure 2C). In contrast, the increase factor is only 1.005 for BRAF, 1.0156 for NRAS and 1.0235 for W3 melanomas (Figure 2D, Supplementary Table 2, 3). Thus, whilst the ratio of Signature 1 damage acquisition to age in BRAF and NRAS melanomas decreases during ageing, likely reflecting a deceleration of the cell proliferation rate during maturity, the ratio per year of clock mutations in W3 melanomas slightly increases during the human lifespan, reflecting a distinct behaviour (Figure 2E).
The rate of clock-like mutations is linked to UVR damage
Previous experiments show acute UVR drives melanocyte proliferation(20), but the long-lasting effects of UVR on cell division have not been explored. We investigated the proportion of Signature 1 and Signature 7 across the melanoma subtypes and found that UVR underpins approximately 75% of all mutations in BRAF, NRAS and NF1 samples, while only half of the mutations in W3 samples are accounted for by UVR (Figure 3A). Furthermore, we find a greater proportion of the ageing signature that is uncoupled from cellular division (Signature 5, characterised by T:A to C:G transitions) contributing to the overall mutational burden of W3 melanomas. The underlying biological process driving Signature 5 mutations is unknown, but is linked to ageing independently of cellular division(16).
Figure 3. The Signature 7 UVR imprint predominates in melanoma and is tightly correlated to cell division Signature 1 mutations.
(A) Mutation signature spectra and proportions in BRAF, NF1, NRAS, and W3 cutaneous melanomas.
(B) Correlation analysis between somatic mutations due to extrinsic, UVR-driven Signature 7 mutations in cutaneous melanomas and intrinsic, clock-like Signature 1 mutations.
(C) Correlation analysis between somatic mutations due to extrinsic, UVR-driven Signature 7 mutations in cutaneous melanomas subtypes and intrinsic, clock-like Signature 1 mutations (BRAF: red; NRAS: blue).
We then used Signature 1 and 7 to investigate the relationship between UVR damage and cell cycle, and show that cell division rate, predicted from Signature 1, tightly correlates with the total UVR-induced mutations in melanoma (Spearman ρ =0.82, P<10-36, Figure 3B). The correlation between Signatures 1 and 7 remains significant across all the subtypes (BRAF: Spearman ρ 0.70, P<10-13; NRAS: ρ 0.72, P<10-12; NF1: ρ 0.8, P<10-8; W3: ρ 0.64, P<10-6; Figure 3C). There is a marked difference in the increase of Signature 1 that is dependent on Signature 7 in both BRAF and NRAS melanomas (BRAF: robust regression with slope 0.033; P<10-16); NRAS: robust regression with slope 0.046; P<10-12). These data suggest UVR increases the total mutation burden by damaging DNA directly, but may also modify the TMB by affecting the dynamics of cell division. Importantly, these data show cell proliferation is coupled to UVR, not age, in BRAF melanomas.
Ageing-associated mutations accumulate at different rates in males and females
Melanomas from male patients present an overall higher number of missense mutations than women, adjusted for age and relevant clinical covariates(3). We investigated the relationship between Signature 1 and sex, and critically, we observed that males present a higher number of Signature 1 mutations per age (P<0.01, Mann-Whitney U test with Bonferroni correction; median male-to-female ratio 1.24; Figure 4A). The difference is visible in the BRAF and NRAS subtypes (Figure 4B), although it is less statistically significant (P=0.1 for BRAF, P=0.6 for NRAS; Mann-Whitney U test with Bonferroni correction). For males, we found a significant rank-correlation with age (Spearman ρ 0.45, P<0.00023) but not for female samples (Spearman ρ 0.124, P=0.35; Figure 4A). Using multivariate negative binomial regression we found that sex affects the rate of mutation accumulation (P<10-16) and estimated the factor by which mutations increase per year in male and female samples (Supplementary Table 2; Figure 4C-4D). We observed only males presented a factor of mutation increase per year greater than one (Supplementary Table 2). The results remain robust when restricting the analysis to the molecular subtypes (except NF1 due to sample size), showing an increase in Signature 1 mutations with age in males (Supplementary Table 2).
Figure 4. Melanoma in males accumulate more Signature 1 mutations.
(A) Difference in Signature 1 mutations per age between males and females.
(B) Difference in Signature 1 mutations per age between males and females by subtype (BRAF and NRAS).
(C) Exponential model for accumulation of Signature 1 mutations by sex. This curve models the Poisson mean distribution of mutations at each age, with age-dependent rate.
(D) Change of Signature 1 mutations per age over time, according to sex.
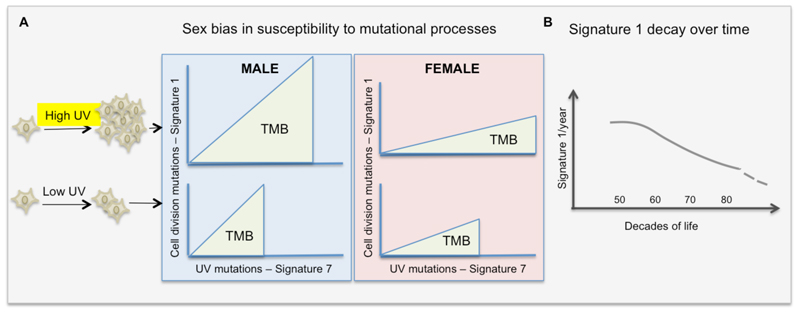
We next investigated whether the difference in the rate of mutation accumulation persists when accounting for the effect of UVR-driven Signature 7 mutations. Assuming that the number of Signature 1 mutations is proportional to the number of Signature 7 (see previous section Figure 3B, 3C), we investigated the ratios of Signature 1 to Signature 7 across melanomas, adjusted for the effect of Signature 7 on cell division, and found that there is little to no increase in Signature 1 mutations per year in either sex. Moreover, the multiplicative rate of increase of the ratio of Signature 1 to Signature 7 mutations per year turns out to be slightly smaller (by 0.01) in males (Supplementary Table 4) in all samples, across BRAF and NF1 subtypes, and is not detected in NRAS and W3. These data imply that the rate at which clock-like mutations accumulate per year depends on UVR, and this dependence is stronger in males than in females. Thus, men and women exposed to equal doses of UV will adjust their cell cycles differently, with men increasing the rate of cell division. In summary, our results suggest that the mutation rate due to cell division is determined by UVR exposure, and males are more susceptible to UVR-induced cell proliferation. In contrast, females accumulate fewer Signature 1 mutations, at a slower rate, despite UVR exposure (Figure 5).
Figure 5. Summary findings.
(A) UV-light somatic mutations are strongly associated to the rate of cell-division mutations, suggesting that an extrinsic mutational process (UV) influences the intrinsic mutational process due to cell division. The rate of cell division in male melanoma is more strongly correlated to UV damage than in females.
(B) Cancer cells bear the genomic imprint of decreasing rate of cell division during ageing.
Discussion
Sex and age differences have been observed in cancers(3,21). We provide a novel mathematical framework to analyse the relationship between different damaging processes shaping the mutational landscape of cancer; and how the mutational processes can reveal the effect of age and sex on carcinogenesis. We use the predominant mutational processes of the exomes of cutaneous melanomas. Melanoma DNA is imprinted primarily by (1) the clock-like changes due to cell division and (2) UVR-driven mutations. We reveal both processes increase during ageing and are tightly correlated, which poses the intriguing possibility that UVR exposure not only drives melanoma by damaging DNA directly, but also likely influences the intrinsic biological process of cell division and damage repair.
We observe the correlation of mutations to age is absent in BRAF melanomas, in sharp contrast to NRAS and NF1 melanomas, where we observe a gradual, long-term UVR exposure and cell division mutation burden increase as patients’ age. These data support melanoma arises through different pathogenic pathways in the distinct molecular subtypes. Although our correlation studies do not imply causation, the punctuated rate of mutation accumulation in BRAF melanomas could be driven by episodic acute sunburn. Our model is in keeping with the clinical subtypes of disease, where BRAF melanomas are more prevalent in younger patients, over intermittently sun exposed skin with low levels of sun damage in the dermis (22–24). Furthermore, mouse studies lend further support to the association between episodic UV and BRAF melanoma, showing episodic UV is more efficient at driving BRAF melanoma than cumulative UV (20,25). However, relying solely on the UV-damage mutation pattern to infer the contribution of extrinsic damage is a limited approach, as previous animal studies show that neonatal UV damage can lead to melanoma in the absence of accumulated DNA mutations by triggering inflammation(26).
Recent models examining the correlation between lifetime risk of a cancer and cell division show that tissues with higher cell turnover present an increased cancer incidence in the population(27,28), which suggests tissues with high cell turnover require less environmental damage to drive tumourogenesis. Our results challenge this assumption in melanoma and suggest extrinsic processes such as UVR can modulate the contribution of cell division to mutation burden.
We show the decline in cell division during ageing present in healthy tissues(19) is discernible in the genomic imprint of cancer cells. Recent work shows the stem cell division rate declines with age(29), and we replicate these findings mathematically in melanoma. The rate of proliferative decline, moreover, is not uniform across all melanoma subtypes, and our framework could test if the decline in cell division with age varies according to the tissue of origin, and whether cell division decline due to age mirrors a decrease in cancer incidence observed in the super-aged population.
Finally, we reveal an increase in cell division-linked mutations in males, which could be due to an increase in the inherent proliferation rate of male melanocytes, or to a decrease in the mutational repair of extrinsic UVR-related mutations. A recent pan-cancer analysis has shown sex biases in mutational load, tumour evolution, mutational processes and at the gene level(4,5,30); and our study suggests that sex differences in melanoma cannot be explained by lifestyle or age alone, and likely reflect sex-specific biology. Intriguingly, Signature 1 is increased in females in an age-adjusted, pan-cancer whole genome analysis(30), whilst our results reveal a contrary sex bias in Signature 1 in melanoma.
One limitation of applying mutational signatures to infer disease evolution is that different mutational stresses occur at different time points of disease progression. In melanoma, UV damage is acquired when melanoma is located to the skin. In contrast, Signature 1 summarises cell divisions throughout the lifespan of the cell; from pre-malignancy to advanced stages. However, recent work found most melanoma mutations to be primarily early truncal and monoclonal, and early and late stage TMB homogenous (31).
Our study provides new tools to examine the rate of mutation accumulation in cancer, and to analyse how sex and age contribute to tumour development. Tumour burden, age and sex are known to influence the response to immunotherapies(32,33) and future studies should address how these data can be leveraged to predict response to therapy and design strategies to improve survival. Although limited to a single melanoma cohort, this work supports the rationale for using the mutational processes, together with age and sex, to stratify patients for novel immunotherapy trials as well as to inform public health prevention and diagnostic campaigns.
Supplementary Material
What’s already known about this topic?
Cancer incidence and mortality are influenced by sex and age. Melanoma is more frequent in men, and the incidence and mortality rise with increasing age. The main mutations driving melanoma are predominantly linked to UV light damage and to errors accumulated in the DNA after each cell division, which are unrepaired. These clock-like mutations linked to cell division accumulate steadily over time in healthy tissue and cancers.
What does this study add?
Clock and UV mutations are tightly correlated and arise in melanoma as a function of age and sex. The molecular subtypes have a distinct pattern and rate of UV and clock mutations, and clock mutations depend on the amount of UV damage. The rate of clock mutations decreases as individuals’ age, reflecting a decrease in tissue proliferation during ageing. Men have more clock mutations, which reflect a distinct proliferation rate.
What is the translational message?
This study indicates age and sex shape the rate of mutations observed in melanoma. The mutational burden affects response to novel immunotherapies, so this work supports the rationale to stratify patients by their mutational landscape, age and sex to best discriminate possible responders.
What are the clinical implications of this work?
The decline of cell division-linked mutations in super-aged melanoma cells may be linked to the decrease in cancer incidence observed in the “super-aged” population. UV light not only damages DNA directly leading to mutations, but also influences the rate of cell proliferation. Additionally, men present a higher rate of mutations independently of UV. These data can better inform public health prevention campaigns.
Funding and acknowledgements
AV is a Wellcome Beit Fellow and personally funded by a Wellcome Trust Intermediate Fellowship (110078/Z/15/Z) and Cancer Research UK (A27412). SJF acknowledges support from the European Commission (FP7-PEOPLE-2013-IEF – 6270270) and the Royal College of Surgeons in Ireland StAR programme.
Footnotes
Ethics
Sequencing data was obtained from TCGA, in accordance with ethical guidelines
Consent for publication
All authors consent to the data publication
Authors’ contributions
AV conceived the project. ML led the mathematical models and SF led the bioinformatics. AV, ML and SF interpreted the data and wrote the manuscript.
Competing interests
The authors declare that they have no competing interests
Availability of data and materials
Patient data is available from cBioportal
References
- 1.Balch CM, Soong S, Gershenwald JE, Thompson JF, Coit DG, Atkins MB, et al. Age as a Prognostic Factor in Patients with Localized Melanoma and Regional Metastases. [cited 2018 Nov 14];Ann Surg Oncol. 2013 Nov 10;20(12):3961–8. doi: 10.1245/s10434-013-3100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanaugh-Hussey MW, Mu EW, Kang S, Balch CM, Wang T. Older Age is Associated with a Higher Incidence of Melanoma Death but a Lower Incidence of Sentinel Lymph Node Metastasis in the SEER Databases (2003–2011) [cited 2018 Nov 14];Ann Surg Oncol. 2015 Jul 5;22(7):2120–6. doi: 10.1245/s10434-015-4538-8. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Artomov M, Goggins W, Daly M, Tsao H. Gender Disparity and Mutation Burden in Metastatic Melanoma. [cited 2018 Nov 16];J Natl Cancer Inst. 2015 Nov 20;107(11):djv221. doi: 10.1093/jnci/djv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CH, Haider S, Shiah YJ, Thai K, Boutros PC. Sex differences in cancer driver genes and biomarkers. Cancer Res. 2018;78(19):5527–37. doi: 10.1158/0008-5472.CAN-18-0362. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Y, Liu L, Chen H, Wang Y, Xu Y, Mao H, et al. Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell. 2016;29(5):711–22. doi: 10.1016/j.ccell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucab JE, Zou X, Morganella S, Joel M, Nanda AS, Nagy E, et al. A Compendium of Mutational Signatures of Environmental Agents. Cell. 2019;177(4):821–836.:e16. doi: 10.1016/j.cell.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. [cited 2018 Nov 15];Lancet. 2018 Sep 15;392(10151):971–84. doi: 10.1016/S0140-6736(18)31559-9. [DOI] [PubMed] [Google Scholar]
- 9.Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16(6):345–58. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Network. Electronic address irwatson mdanderson org, Cancer Genome Atlas N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–96. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95(11):806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 12.Akbani R, Akdemir KC, Aksoy BA, Albert M, Ally A, Amin SB, et al. Genomic Classification of Cutaneous Melanoma. [cited 2018 Nov 15];Cell. 2015 Jun 18;161(7):1681–96. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nat Publ Gr. 2017;545(7653):175–80. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 14.Alexandrov LB, Jones PH, Wedge DC, Sale JE, Campbell PJ, Nik-Zainal S, et al. Clock-like mutational processes in human somatic cells. [cited 2019 Feb 21];Nat Genet. 2015 Dec 9;47(12):1402–7. doi: 10.1038/ng.3441. [Internet] Available from: http://www.nature.com/articles/ng.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, et al. The Life History of 21 Breast Cancers. [cited 2018 Dec 13];Cell. 2012 May 25;149(5):994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blokzijl F, De Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538(7624):260–4. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science (80- .) 2016;352(6282):189–96. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458(7239):719–24. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Hekimi S. Stem cells and healthy aging. Science (80- .) 2015;350(6265):1199–204. doi: 10.1126/science.aab3388. [DOI] [PubMed] [Google Scholar]
- 20.Viros A, Sanchez-Laorden B, Pedersen M, Furney SJ, Rae J, Hogan K, et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nature. 2014;511(7510):478–82. doi: 10.1038/nature13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. [cited 2018 Nov 15];J Clin Oncol. 2001 Aug 15;19(16):3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 22.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 23.Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med. 2008;5(6):e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landi MT, Bauer J, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science (80- .) 2006;313(5786):521–2. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 25.Trucco LD, Mundra PA, Hogan K, Garcia-Martinez P, Viros A, Mandal AK, et al. Ultraviolet radiation–induced DNA damage is prognostic for outcome in melanoma. [cited 2018 Dec 11];Nat Med. 2018 Dec 3; doi: 10.1038/s41591-018-0265-6. [DOI] [PubMed] [Google Scholar]
- 26.Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469(7331):548–53. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomasetti C, Vogelstein B, Cancer SK, Hopkins J, Cancer K. Variation in cancer risk is explained by number of stem cell divisions. 2016;347(6217):78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529(7584):43–7. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasetti C, Poling J, Roberts NJ, London NR, Pittman ME, Haffner MC, et al. Cell division rates decrease with age, providing a potential explanation for the age-dependent deceleration in cancer incidence. Proc Natl Acad Sci U S A. 2019 doi: 10.1073/pnas.1905722116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li CH, Prokopec SD, Sun RX, Yousif F, Schmitz N, Boutros PC. Sex Differences in Oncogenic Mutational Processes. bioRxiv. 2019 Jan 1;:528968. doi: 10.1038/s41467-020-17359-2. [Internet] Available from: http://biorxiv.org/content/early/2019/01/28/528968.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birkeland E, Zhang S, Poduval D, Geisler J, Nakken S, Vodak D, et al. Patterns of genomic evolution in advanced melanoma. Nat Commun. 2018;9(1):1–12. doi: 10.1038/s41467-018-05063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kugel CH, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. [cited 2019 Feb 6];Clin Cancer Res. 2018 Nov 1;24(21):5347–56. doi: 10.1158/1078-0432.CCR-18-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. [cited 2019 Feb 6];Nat Genet. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal R, Mcgranahan N, Herrero J, Taylor BS, Swanton C. deconstructSigs : delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. 2016:1–11. doi: 10.1186/s13059-016-0893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podolsky MD, Barchuk AA, Kuznetcov VI, Gusarova NF, Gaidukov VS, Tarakanov SA. Evaluation of Machine Learning Algorithm Utilization for Lung Cancer Classification Based on Gene Expression Levels. Asian Pacific J Cancer Prev. 2016;17(2):835–8. doi: 10.7314/apjcp.2016.17.2.835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient data is available from cBioportal