Abstract
Severe malaria caused by Plasmodium falciparum is difficult to diagnose accurately in children in high-transmission settings. Using data from 2649 pediatric and adult patients enrolled in four studies of severe illness in three countries (Bangladesh, Kenya, and Uganda), we fitted Bayesian latent class models using two diagnostic markers: the platelet count and the plasma concentration of P. falciparum histidine-rich protein 2 (PfHRP2). In severely ill patients with clinical features consistent with severe malaria, the combination of a platelet count of ≤150,000/μl and a plasma PfHRP2 concentration of ≥1000 ng/ml had an estimated sensitivity of 74% and specificity of 93% in identifying severe falciparum malaria. Compared with misdiagnosed children, pediatric patients with true severe malaria had higher parasite densities, lower hematocrits, lower rates of invasive bacterial disease, and a lower prevalence of both sickle cell trait and sickle cell anemia. We estimate that one-third of the children enrolled into clinical studies of severe malaria in high-transmission settings in Africa had another cause of their severe illness.
Introduction
Severe falciparum malaria is clinically defined as vital organ dysfunction in the presence of circulating Plasmodium falciparum parasites (1). The primary objective of this definition is to identify severely ill patients rapidly and provide lifesaving clinical management, notably parenteral artesunate treatment. This definition prioritizes sensitivity over specificity. In areas of moderate and high P. falciparum transmission, many apparently healthy children have malaria parasitemia upon examination of their blood by light microscopy or a rapid diagnostic test. Many hospitalized children in high-transmission settings will also be parasitemic but, because major clinical features of severe malaria are not specific, it is difficult to differentiate clinically between severe falciparum malaria caused by extensive sequestration of malaria parasites in the microvasculature (i.e., true severe malaria) (2) and other causes of severe febrile illness accompanied by either coincidental asymptomatic parasitemia or uncomplicated malaria (3, 4). We have shown previously that complete blood counts (platelet counts and total white blood cell counts) provide critical discriminating values in distinguishing true severe malaria from other causes of severe illness (5). To this end, about one-third of a cohort of 2220 Kenyan children diagnosed with severe malaria in a moderate-transmission area were estimated to have had another cause of their severe illness (5). The diagnostic value of complete blood counts was validated using genetic polymorphism data in two different ways: First, the distribution of sickle cell trait (HbAS) correlated very strongly with the estimated probability of severe malaria. The prevalence of HbAS was about five times higher in the subgroup of patients likely to have been misdiagnosed compared with the subgroup that had a likely correct diagnosis (5). Second, we showed that genome-wide false discovery rates could be reduced substantially in a case-control whole-genome association study, in which the output probability weights were used in a “data-tilting” framework to adjust for patient misclassification (5).
The diagnostic value of complete blood counts in severe malaria has great operational utility, because blood counts are widely measured in routine practice at low cost. Moderate thrombocytopenia is a consistent feature of all human malaria infections (6–8), although the diagnostic utility of the platelet count has been debated (9, 10). Platelets are activated in malaria infections and have increased turnover. In severe falciparum malaria, there is endothelial activation with the release of platelet aggregating activated high-multimeric von Willebrand factor from specialized secretory vesicles in endothelial cells (the Weibel-Palade bodies). Platelets may also contribute to and be consumed during parasitized erythrocyte cytoadherence and autoagglutination (11, 12). In addition, severe thrombocytopenia is associated with increased mortality in severe malaria (10, 13, 14). The total white blood cell count is informative, although much less so than thrombocytopenia, with a greater prognostic than diagnostic value, because either very high or very low white cell counts in severe disease are associated with high case fatality ratios (8, 5). Total white counts also vary according to age and ethnicity (15), further confounding cross-study assessments.
The main pathophysiological process in severe falciparum malaria is the extensive sequestration of parasitized erythrocytes in the vascular beds of vital organs (1, 2). These parasites, which cause potentially lethal pathology, have stopped circulating and are not represented in the peripheral blood smear. In African children, the peripheral parasite density is a poor indicator of disease severity and an unreliable diagnostic marker (16). The parasite protein P. falciparum histidine-rich protein 2 (PfHRP2), the basis for most rapid diagnostic tests, is liberated mainly at schizont rupture, such that the amount of PfHRP2 released is proportional to the extent of recent schizogony. Plasma PfHRP2 is a much better discriminant of severe falciparum malaria than the peripheral blood parasite count (17, 18). An example of how PfHRP2 can help the interpretation of clinical trial data comes from the large multicenter African Quinine Artesunate Malaria Trial (AQUAMAT), which was a randomized comparison of parenteral artesunate versus parenteral quinine in African children clinically diagnosed as having severe falciparum malaria. Overall, artesunate reduced the mortality by about 22% (19). However, there was evidence for treatment effect heterogeneity across PfHRP2 strata. In the subgroup of children in the highest tertile of PfHRP2, artesunate reduced the mortality by one-third, which is a similar proportion to that observed earlier in the South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT), a randomized comparison conducted in Southeast Asia (20). In contrast, there was no substantial difference in mortality in the subgroup of children in the lowest tertile of PfHRP2, which suggests that this group was likely to have other causes of severe illness (18).
Defining optimal cutoff values for diagnostic markers in severe malaria is difficult, because there is no gold standard for diagnosis against which to calibrate thresholds in high-transmission areas. In the absence of a gold standard, latent class models can be used to assess the sensitivity and specificity of threshold values for diagnostic indices (21). Latent class models typically rely on having multiple markers measured in the same individuals across different populations with varying disease prevalences, thus allowing for triangulation (21, 22). We applied Bayesian parametric latent class models to admission platelet counts and plasma PfHRP2 concentrations in combination to estimate their diagnostic operating characteristics and to estimate the proportion of misdiagnosed patients in studies of severe malaria. We used data from four prospective studies of severe malaria or severe illness from Uganda, Kenya, and Bangladesh, reflecting a range of P. falciparum transmission intensities from high to low and thus a range of false diagnosis rates. Our results suggest that the high rates of severe falciparum malaria misdiagnosis could be reduced substantially by incorporating measurement of platelet counts and plasma PfHRP2 concentrations in the diagnostic criteria.
Results
Platelet counts and PfHRP2 concentrations in severe febrile illness
We pooled individual patient data from 2649 severely ill African children and Asian adults, for whom platelet counts and measured plasma PfHRP2 concentrations were available. The patients were from four separate studies in three countries: (i) an observational study of severe falciparum malaria in Bangladesh (n = 172; all patients were clinically diagnosed with severe falciparum malaria), (ii) the Ugandan sites of the Fluid Expansion as a Supportive Therapy (FEAST) trial (n = 567; a randomized controlled trial of fluid resuscitation approaches in severe childhood illness not specific to severe malaria) (23), (iii) an observational study of cerebral malaria and severe malarial anemia in Kampala, Uganda (n = 492) (24), and (iv) a large cohort of children diagnosed with severe malaria in Kilifi, Kenya (n = 1418) (25). Malaria transmission intensity is generally low in Bangladesh, moderate in Kilifi and Kampala, and high around the other Ugandan sites.
In total, 27 patients (1%: 1 in Bangladesh, 8 in the FEAST sites in Uganda, and 18 from Kenya) had no detectable PfHRP2 in the enzyme-linked immunosorbent assay (ELISA) but had a parasite density >1000/μl by microscopy. These could have been either assay errors or parasites with HRP2/3 gene deletions. They were removed from the analysis, leaving a total of 2622 samples. A summary of the patient characteristics in the analyzed dataset is shown in Table 1.
Table 1. Patient characteristics across four studies.
For age, parasite densities, platelet counts, total white blood cell counts, and PfHRP2 concentrations, we show the median values with IQRs in parentheses. No HbS genotyping was done for Bangladeshi patients, because HbS is absent in this population.
| Kilifi (Kenya) | Kampala (Uganda) | FEAST (Uganda) | Bangladesh | |
|---|---|---|---|---|
| n | 1400 | 492 | 559 | 171 |
| Age (years, IQR) | 2.4 (1.4–3.7) | 3.3 (2.2–4.6) | 2.0 (1.2–3.3) | 30 (23–45) |
| Proportion parasite positive (%) | 100 | 100 | 59.4 | 100 |
| Parasite density * (per μl, IQR) | 69,824 (6099–316,350) | 42,530 (10,635–198,540) | 37,600 (3640–153,680) | 148,874 (23,550–348,540) |
| Platelet count (×103/μl, IQR) | 111 (64–215) | 96 (49–170) | 165 (75–326) | 50 (27–139) |
| PfHRP2 (ng/ml, IQR) | 2207 (419–5072) | 1838 (588–4097) | 175 (0–1953) | 2667 (1083–6128) |
| White blood cell count (/μl, IQR) | 12.6 (8.9–19) | 10.4 (7.5–15.3) | 12.0 (8.4–18.7) | 9.0 (6.9–11.0) |
| Mortality (%) | 11.1 | 6.7 | 11.4 | 26.9 |
| HbAS (n, %) | 41 (2.9) | 4 (0.8) | 46 (8.2) | – |
| HbSS (n, %) | 7 (0.5) | 23 (4.7) | 21 (3.8) | – |
For the FEAST trial, parasite densities refer only to patients with a positive rapid diagnostic test.
Log10-transformed platelet counts and log10-transformed PfHRP2 concentrations were strongly inversely correlated in both studies of severely ill African children (ρ= −0.54) and in Bangladeshi adults with severe malaria (ρ= −0.36). Patients with platelet counts in the normal range (>150,000/μl) generally had low to nonmeasurable plasma PfHRP2 concentrations [median concentration was 269 ng/ml; interquartile range (IQR): 24 to 1043], whereas patients with thromobocytopenia (≤150,000/μl) had a median PfHRP2 concentration of 3031 ng/ml (IQR: 1261 to 6035).
Discriminative value of platelet counts and plasma PfHRP2 in patients diagnosed with severe malaria
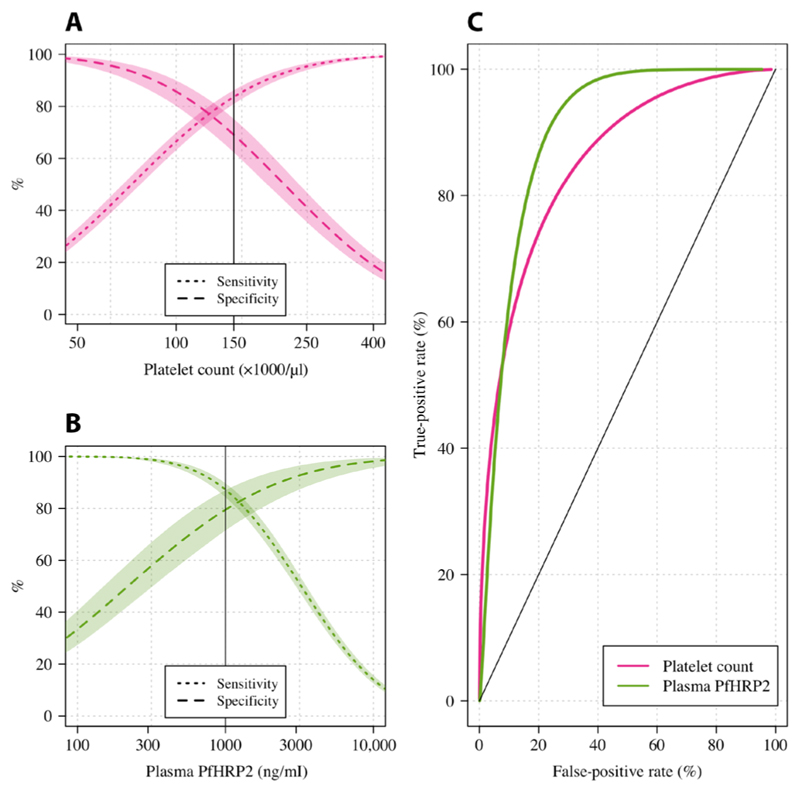
We assessed the diagnostic value of platelet counts and plasma PfHRP2 concentrations in patients who were clinically diagnosed with severe malaria (n = 2063). This analysis excluded patients from the FEAST trial, which explicitly included nonmalarial causes of severe febrile illness. We fitted a two-component parametric Bayesian latent class model, with the two latent classes representing “severe malaria” and “not severe malaria,” using log10-transformed markers (see Materials and Methods for description of the informative priors used and the key assumptions). Figure 1 (A and B) shows the model-estimated sensitivities and specificities for all cutoff values of the platelet count and the PfHRP2 concentration. As expected, the platelet count and the PfHRP2 concentration both had high discriminative value, illustrated by the receiver operating characteristic (ROC) curves (Fig. 1C). Overall, for thresholds giving the same sensitivity, the plasma PfHRP2 concentration had a higher specificity. For example, in these populations, a lower limit of 1000 ng/ml for the PfHRP2 concentration had an estimated sensitivity of 87% and a specificity of 83%. In comparison, an upper limit for the platelet count of 150,000/μl had an estimated sensitivity of 83% but a specificity of 71%. A series of sensitivity analyses (using noninformative priors, using different choices for the parametric model, or using only data from the two studies of African children with severe malaria) showed near-identical results (fig. S1).
Fig. 1. The diagnostic value of platelet counts (pink) and plasma PfHRP2 concentrations (green) estimated using data from 2063 patients diagnosed with severe falciparum malaria in three studies.
(A) and (B) show the mean estimated sensitivity (dotted lines) and specificity (dashed lines) functions for platelet count and plasma PfHRP2, respectively. For the platelet count, thresholds correspond to upper limits, whereas for the PfHRP2 concentration, the thresholds correspond to lower limits. Shaded areas show 95% CI. (C) shows the ROC curves for each marker.
Joint diagnostic thresholds for platelet counts and plasma PfHRP2
For clinical and epidemiological studies, and in contrast to clinical practice, specificity is usually more important than sensitivity. For example, interpretation of effect sizes in randomized trials will depend on the included population and, thus, the specificity of the inclusion criteria. We used the two markers in combination to improve the precision of the definition of severe malaria to achieve low false-positive rates. Under the model, multiple combinations of the platelet count and PfHRP2 concentration have approximately the same operating characteristics for diagnosis, so the optimal choice is subjective and can be made based on operational simplicity (fig. S2). In severe illness with clinical features consistent with severe malaria, the combination of platelet counts of ≤150,000/μl and plasma PfHRP2 concentrations of ≥1000 ng/ml had an estimated diagnostic specificity of 93% and a sensitivity of 74%. If this definition was applied to a hospital cohort with a prevalence of severe malaria of 60%, which is the prevalence estimated for the Kenyan cohort of children with clinically diagnosed severe malaria, we would expect >94% of the resulting population identified by these two markers to have true severe malaria (positive predictive value).
Estimating the probability of severe malaria
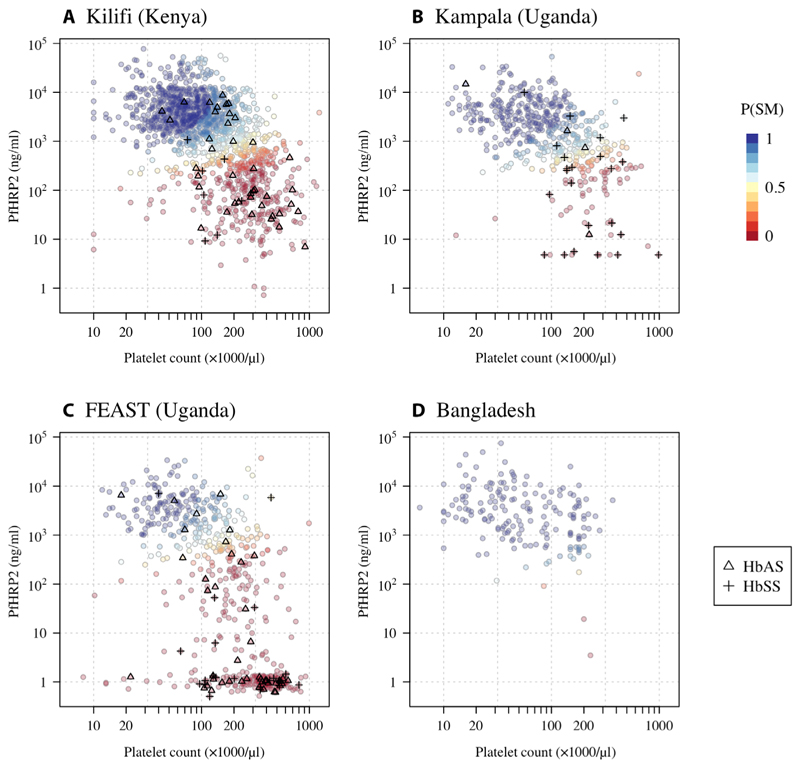
We fitted a three-component parametric Bayesian latent class model to all available platelet count and PfHRP2 concentration data from patients from the four studies of severe febrile illness (n = 2622 patients) to estimate the individual patient probabilities that severe malaria was the true cause of their severe illness and to calculate the prevalences of true severe malaria among those diagnosed. The additional third component captured a cluster of patients in the FEAST trial who had no detectable plasma PfHRP2 and normal platelet counts (Fig. 2). Under this model, we estimated that the prevalence of true severe malaria was 96% [95% credible interval (CI): 91 to 99] in Bangladeshi adults, 37% (95% CI: 31 to 42) in the Ugandan children enrolled in the FEAST trial, 74% (95% CI: 67 to 79) in the children enrolled in Kampala, Uganda (24), and 66% (95% CI: 61 to 70) in the children diagnosed with severe malaria in Kilifi, Kenya. We note that the FEAST trial intentionally enrolled severely ill children with and without malaria, although 66% of all patients had a diagnosis of severe malaria (23).
Fig. 2. Probabilistic model of severe falciparum malaria using platelet counts and plasma PfHRP2 concentrations in 2622 severely ill patients based on a Bayesian parametric latent class model with three latent classes (a severe malaria class and two not severe malaria classes).
(A) to (D) show the individual data for each study [(A) Kilifi cohort, (B) Kampala cohort, (C) FEAST trial, and (D) Bangladesh cohort]. The colors correspond to the probability of severe malaria under the model (dark blue: high probability; dark red: low probability). Triangles show the individuals with HbAS; crosses show the individuals with HbSS. To show data points with nonmeasurable plasma PfHRP2, nonmeasurable concentrations were set to 1 ng/ml ± random jitter on the log10 scale (about half the lower limit of quantification of the assay). P(SM), Probability(Severe Malaria).
Figure 2 shows scatterplots of the platelet counts versus the plasma PfHRP2 concentrations that are colored by the probability of a patient having severe malaria and grouped by study. Dark blue represents a high probability of severe malaria, and dark red represents a low probability of severe malaria. The model estimated a geometric mean platelet count in severe malaria across the four studies of 74,000/μl (95% of patients are predicted to have platelet counts between 17,000 and 312,000) and a geometric mean PfHRP2 of 3135 ng/ml (95% prediction interval: 402 to 24,452). The model-based probabilities of severe malaria were highly concordant with our previously published model that used platelet counts and total white blood cell counts (ρ = 0.64; fig. S3) (5).
We compared the estimated false diagnosis rates for cerebral malaria versus severe malaria without coma and for severe malarial anemia versus severe malaria without severe anemia, where severe anemia is defined as a hematocrit ≤15% in the two cohorts of children clinically diagnosed with severe malaria (table S1). In the Kenyan cohort, the estimated false diagnosis rate was slightly higher in the cerebral malaria group relative to the noncerebral malaria groups: 36% of patients with coma and 26% of patients without coma were classified as “falsely diagnosed” with severe malaria (P = 0.001). For the subgroup with severe anemia, the false diagnosis rate was 15%, compared to 36% in the subgroup without severe anemia. In the Ugandan cohort, both of these trends were reversed. A false diagnosis of severe malaria was estimated for only 12% of patients with coma but 40% of patients without coma. For severe anemia, these proportions were 36 and 15%, respectively (the Ugandan cohort only recruited patients with either severe malarial anemia or patients with cerebral malaria).
Mortality rates varied substantially as a function of the estimated probability of severe malaria and across the studies (Fig. 3). In African children with a high probability of having severe malaria, the mean mortality was consistently ~10% for the three studies included (Fig. 3, A to C). Apart from the Bangladeshi adults (Fig. 3D), all patients were initially treated with intravenous quinine. The severe malaria mortality in adults from Bangladesh was substantially higher (~30%), consistent with previous studies (20). In Kenyan children (Fig. 3A), the mortality in the misclassified group of patients was higher than in the correctly classified patients (14 versus 10%), whereas the trend was reversed in the Ugandan study (1 versus 10%; Fig. 3B). This is largely explained by the study populations, such that the group with a low probability of having severe malaria in the Ugandan study was predominantly composed of patients with severe anemia without other features of severity.
Fig. 3. Mortality as a function of the probability of having severe malaria under the Bayesian latent class model (based on platelet counts and PfHRP2 concentrations).
The lines (shaded areas) show mean (95% CIs) mortality estimates from logistic regression fits. (A) to (D) show the individual data for each study [(A) Kilifi cohort, (B) Kampala cohort, (C) FEAST trial, and (D) Bangladesh cohort].
Relationship with other admission variables
We explored the relationship between the model-estimated probability of severe malaria and the admission parasite densities, admission hematocrit, the total white blood cell counts, and the blood culture positivity rates (i.e., cultures growing a likely pathogen). In the three African studies, parasite densities were between 12 and 16 times higher in patients with a high probability of severe malaria versus those with a low probability of severe malaria (Fig. 4, A to C). After adjusting for study differences, the parasite densities were estimated to be 12.8-fold higher (95% CI: 9.9 to 16.5) in severe malaria versus nonsevere malaria.
Fig. 4. Admission parasite densities as a function of the probability of severe malaria under the Bayesian latent class model.
Data from the FEAST trial include only the patients with a positive malaria rapid diagnostic test. The thick lines show the additive linear model fit (spline-based). (A) to (D) show the individual data for each study [(A) Kilifi cohort, (B) Kampala cohort, (C) FEAST trial, and (D) Bangladesh cohort].
The admission hematocrits also highly correlated with the model-estimated probability of severe malaria (fig. S4, A to D). In the three African studies, children with a high probability of having severe malaria had median admission hematocrit values between 16 and 20% (FEAST: 19%, Kampala: 16%, and Kilifi: 20%). The hematocrit distributions in this group were unimodal (fig. S5, A to C). In contrast, the hematocrit distributions in patients with low probabilities of having severe malaria (<0.2) were strongly bimodal, with the majority of patients having higher hematocrits (the median hematocrit in this group were as follows: FEAST: 30%, Kampala: 12%, and Kilifi: 28%), but a substantial minority had low hematocrits of ~10% (fig. S5, D to F). The few Bangladeshi adults with a low probability of severe malaria also had higher hematocrits (fig. S4D).
Blood cultures were done for all 1400 Kenyan children and for 298 of the 332 (90%) Ugandan children in the FEAST study who had malaria parasitemia on admission. Overall, 51 and 35 patients, respectively, had positive blood cultures after removing likely contaminants. The probability of having severe malaria was highly predictive of the blood culture result, with an adjusted odds ratio of 0.43 (95% CI: 0.27 to 0.66, P = 0.0002) for a positive culture in patients likely to have severe malaria versus those unlikely to have severe malaria. This difference in blood culture positivity rates was also reflected in the total white blood cell counts (fig. S6). Across the four studies, after adjustment for age, in patients likely to have severe malaria (probability >0.5) compared to those unlikely to have severe malaria (probability <0.5), the odds ratio for having a total white count >15,000/μl was 0.50 (95% CI: 0.46 to 0.56, P = 10−12).
Gene dose relationship for hemoglobin S and severe malaria
In the three studies in African children, the prevalence of both HbAS and HbSS (homozygous sickle cell anemia) was strongly inversely correlated with the model-estimated probability of severe malaria (Fig. 2, A to C). Pooling the three African studies and adjusting for differences in the estimated prevalence of severe malaria, the odds ratio for being classified as severe malaria (probability >0.5) for patients with HbAS relative to patients with HbAA was 0.25 (95% CI: 0.15 to 0.42, P = 10−8), and for patients with HbSS relative to patients with HbAA, the odds ratio was 0.08 (95% CI: 0.04 to 0.19, P = 10−9). Under an additive model of association, each additional hemoglobin S (HbS) allele was associated with an odds ratio for severe malaria of 0.27 (95% CI: 0.20 to 0.38, P = 10−15). This association between HbS genotypes and the marker-based severe malaria classification was highly concordant across the three studies.
Discussion
The diagnosis of severe malaria in African children is imprecise (3, 4, 26). This is because it is difficult to distinguish clinically between severe illness caused by malaria from severe illness with incidental asymptomatic or uncomplicated malaria, because they share many clinical characteristics. This is a substantial problem for studies of one of the most important life-threatening infections in childhood. It dilutes and distorts the results of epidemiology (27), pathophysiology (3), genetic association (5), and therapeutic investigations (18). In areas of moderate and high levels of malaria transmission, asymptomatic parasitemia is very common. At any given time, a high proportion of children have detectable malaria parasitemia. Malaria parasitemia is therefore a sensitive but not specific indicator that malaria is the cause of illness (26). Other simple laboratory tests provide valuable diagnostic information. Thrombocytopenia is a common feature of all symptomatic malarias (6–8). A low platelet count therefore supports but does not prove that P. falciparum infection is the cause of the severe illness. We estimate that fewer than one in five patients with severe malaria will have platelet counts in the normal range (>150,000/μl). This analysis of data from large prospective studies of severe illness in African children shows that the combined measurement of platelet counts and the plasma concentration of the parasite protein PfHRP2 substantially improve the specificity of the diagnosis of severe falciparum malaria.
A key strength of this work is that we can validate the discriminant power of the platelet count and the plasma PfHRP2 concentration by comparing the prevalence of HbS genotypes (HbAS and HbSS) and the blood culture positivity rates across in the inferred subgroups. HbAS is the genotype that provides the strongest known protection against severe falciparum malaria (28, 29). The prevalence of HbAS was four times lower in children considered likely to have severe malaria compared with those considered less likely to have severe malaria. Note that HbAS also protects against complications of malaria, such as an increased risk of bacterial infections; thus, the HbAS prevalence in children with presumed bacterial infections isstill expected to be lower than that in the healthy population (30). Recent work suggests that the protective effect of HbAS may vary according to parasite genotype (31). It may be that the few HbAS individuals who have true severe malaria have parasite genotypes that can evade the HbAS defense mechanisms. Future work will assess the relationship between these probabilistic classifications and the parasite genotypes. Whereas people with the AS genotype are essentially hematologically normal, those with homozygous SS suffer from sickle cell anemia. This causes anemia and leukocytosis and may also cause thrombocytopenia. This could confound the use of full blood count data in the probabilistic assessment of severe malaria. However, the interpretation of the plasma PfHRP2 concentration or other parasite biomass indicators should not be affected by sickle cell anemia. In this study, there was strong evidence for an additive effect between the number of HbS alleles and a decreased probability of severe malaria under the platelet/HRP2 model. This suggests that HbSS is strongly protective against a high parasite biomass (32). However, because sickle cell anemia crises may be severe and are often triggered by infections, it is possible that a low parasite biomass could trigger severe events. Whether or not this should be described as severe malaria is a semantic question (33). A recent in-depth analysis of the Ugandan cohort from Kampala showed that the children with HbSS considered to have severe malaria had a lower parasite biomass but higher levels of endothelial dysregulation (32). The main differential diagnosis in suspected severe malaria is bacterial sepsis. The relationship is complicated as severe malaria does predispose to bacterial sepsis (34). Blood culture has low sensitivity; however, we observed a threefold increase in positivity rates in the patients likely to have been misdiagnosed compared to those with a likely correct diagnosis.
The main limitation of this study is the absence of a gold standard diagnosis, and thus the reliance on parametric latent class models. The application of latent class models to multiple populations with varying disease prevalence provides a powerful framework for estimating the diagnostic accuracy of imperfect tests, but the model outputs rely on the validity of key assumptions, notably the underlying parametric models used for the marker distributions. We used log-normal distributions for the platelet count and plasma PfHRP2 concentration in both true severe malaria and nonsevere malaria. Visual assessment of data plots suggest that this is a good approximation of the true underlying distributions. Each individual marker has its own limitations. Thrombocytopenia can be caused by other infections (e.g., severe arbovirus infection) and may occur in sepsis, although it is much less prevalent in sepsis than malaria (8). The main limitation of plasma PfHRP2 as a marker of total parasite biomass is that it requires specialist measurement [although rapid point-of-care tests can be applied to plasma (35) and modifications are under development]. As plasma PfHRP2 accumulates each asexual parasite cycle, an individual with a sustained low parasite multiplication rate at high parasite densities can have the same PfHRP2 concentration as an individual with a fulminant infection and a high sequestered biomass (17). In addition, mutations in the PfHRP2 gene causing changes in antigenicity may affect immunoassays, although these are currently rare outside of the Horn of Africa (36, 37).
In these large prospective series of patients hospitalized with a diagnosis of severe malaria, combining platelet counts and plasma PfHRP2 concentrations provided good discrimination between true severe falciparum malaria and other severe illnesses, which are likely to be bacteremia in many of the cases, with concomitant incidental malaria. A major strength of this study is the combination of two measures that are mechanistically distinct: Platelet counts measure the host response to acute malaria illness, and PfHRP2 measures the parasite biomass. We show that the combination of these markers provides a high level of discrimination between patients who were likely to have had true severe malaria and those with a different illness etiology. This proportion ranged from one-third in the FEAST trial, which intentionally studied children with severe malaria and other severe illnesses requiring fluid resuscitation, to >90% in Bangladesh, which is a low-transmission area where the diagnosis of falciparum malaria as the cause of illness is highly specific. These data suggest that about one-third of African children diagnosed with severe malaria have another cause of severe illness (5). Our results suggest that severe malaria with a normal platelet count is unusual, and a platelet count >150,000/μl in a child with suspected severe malaria should motivate further examination for a potential alternative cause of illness concomitant with prompt antimalarial treatment. The high diagnostic accuracy of the plasma PfHRP2 concentration should motivate future work on simple modifications to the currently used PfHRP2 rapid tests to estimate plasma PfHRP2 at the bedside. Last, we recommend that future studies of severe falciparum malaria should include only children with platelet counts of ≤150,000/μl and plasma PfHRP2 concentrations of ≥1000 ng/ml.
Materials And Methods
Study design
This study is a retrospective analysis of platelet counts and plasma PfHRP2 concentrations in patients with severe febrile illness. We merged data from four separate studies, three of which were studies of severe malaria, and one was a study of fluid resuscitation in severe febrile illness (FEAST trial). The main goal of the analysis was to use Bayesian latent class modeling to estimate the proportion of misdiagnosed patients in the three severe malaria studies and to assess the operating characteristics of different cutoff values for the platelet count and PfHRP2 concentrations for future clinical studies. For the analysis and reporting, we followed the Enhancing the Quality and Transparency of health Research (EQUATOR) guidelines for the reporting of diagnostic accuracy studies that use Bayesian latent class models [Standards for the Reporting of Diagnostic accuracy studies that use Bayesian Latent Class Models (STARD-BLCM); see the Supplementary Materials].
Data
All the clinical studies were prospective studies of severe illness and had appropriate ethical approval. For the Kilifi cohort (Kenya), ethical approval was granted by the Kenya Medical Research Institute/National Ethical Review Committee in Nairobi, Kenya (SCC1192) and the Oxford Tropical Research Ethics Committee (OxTREC) in Oxford, UK (020-06). For the Kampala cohort (Uganda), institutional review boards for human studies at Makerere University and the University of Minnesota granted ethical approval for the study. Additional regulatory approval was obtained from the Uganda National Council for Science and Technology. The FEAST trial protocol was approved by ethics committees at Imperial College, London; Makerere University, Uganda; Medical Research Institute, Kenya; and National Medical Research Institute, Tanzania. For the Bangladesh cohort, ethical approval was given by the OxTREC and the Chittagong Medical College Hospital Ethics Committee. Reuse of existing, appropriately anonymized, human data does not require ethical approval under the OxTREC regulations.
Bangladesh
We included data from observational studies in severe falciparum malaria conducted by the Mahidol Oxford Tropical Medicine Research Unit in Bangladesh between 2003 and 2019. These pooled data have been described previously (38). Malaria transmission is seasonal and of low intensity in this location. In brief, adults and children with asexual stage malaria parasites found by microscopy in thick and thin blood smears who met the WHO definition for severe malaria were enrolled in the study after written informed consent was obtained from the patient or an attending relative.
Criteria for severe malaria included coma (Glasgow Coma Scale Score <11 or Blantyre Coma Score <3), pulmonary edema, repeated convulsions (≥2 in 24 hours), severe anemia (hematocrit level <20% plus a parasite count >100,000 parasites/μl) or jaundice (bilirubin level >3.0 mg/dl in addition to a parasite count >100,000 parasites/μl), renal failure (serum creatinine level >3 mg/dl), hypoglycemia (blood glucose level <40 mg/dl), shock (systolic blood pressure <80 mm Hg with cool extremities), hyperparasitemia (peripheral asexual stage parasitemia >10%), hyperlactemia (venous plasma lactate >4 mM), and/or acidemia (venous plasma bicarbonate level <15 mM). Platelet counts and PfHRP2 concentrations were jointly measured in a total of 172 patients. The majority of these patients received intravenous artesunate.
Kilifi (Kenya)
The Kenyan case-control cohort has been described in detail previously (25). Severe malaria cases consisted of all children aged <14 years who were admitted with clinical features of severe falciparum malaria to the high dependency ward of Kilifi County Hospital between 11 June 1999 and 12 June 2008. Severe malaria was defined as a positive blood smear for P. falciparum along with the following: prostration (Blantyre Coma Score of 3 or 4), cerebral malaria (Blantyre Coma Score <3), respiratory distress (abnormally deep breathing), and severe anemia (hemoglobin <5 g/dl). The standard-of-care antimalarial treatment during this period was intravenous quinine.
FEAST (Uganda)
FEAST was a multicenter randomized controlled trial comparing fluid boluses for severely ill children with shock (n = 3161) that was not specific to severe malaria (23). Children between 2 months and 12 years of age were eligible for the trial if they presented with a severe febrile illness complicated by impaired consciousness (prostration or coma), respiratory distress (increased labored breathing), or both, and with impaired perfusion, as evidenced by one or more of the following: a capillary refill time of 3 s or more, lower limb temperature gradient, weak radial pulse volume, or severe tachycardia (>180 beats per minute in children younger than 12 months of age, >160 beats per minute in children 1 to 5 years of age, or >140 beats per minute in children older than 5 years of age). Exclusion criteria were severe malnutrition, gastroenteritis, and noninfectious causes of shock (e.g., trauma, surgery, or burns).
Platelet counts and plasma PfHRP2 concentrations were measured in 502 children in the Ugandan sites (Mulago National Referral Hospital, Mbale and Soroti Regional Referral Hospitals, and St. Mary’s Hospital, Lacor). The standard-of-care antimalarial treatment during this period was intravenous quinine.
Kampala (Uganda)
The trial by Brand et al. (24) was an observational study of cerebral malaria and severe malarial anemia in Mulago Hospital, Kampala, Uganda. Children were enrolled if they were between 18 months and 12 years of age. Cerebral malaria was defined as coma (Blantyre Coma Score <3 or Glasgow Coma Scale Score <8) in the presence of asexual parasites by blood smear. Severe malarial anemia was defined as the presence of P. falciparum parasites in a blood smear in children with a hemoglobin level ≤5 g/dl. The standard-of-care antimalarial treatment during this study was intravenous quinine.
Procedures
For the Kilifi cohort and the Ugandan sites in the FEAST trial, blood samples for bacterial cultures were collected in BD BACTEC Peds Plus bottles and processed with a BD BACTEC automated blood culture instrument (Becton Dickinson) for initial detection of bacteria in the blood. BD BACTEC-positive samples were subcultured on standard media by routine microbiological techniques. Either biochemical test kits (API, bioMérieux), serological tests, or both were used to confirm suspected pathogens. Good Clinical Laboratory Practice was audited by Qualogy, and external quality assurance was provided by the UK National External Quality Assessment service. The following organisms were considered as contaminants: Bacillus species, coryneforms, Micrococcus species, coagulase-negative Staphylococcus, and citrobacter.
In all four studies, plasma PfHRP2 levels were quantitated using the previously published methodology (18). The lower limit of detection of the ELISA plasma assay is about 2 ng/ml. Patients in the Kilifi study and the FEAST trial were genotyped for the rs334 single-nucleotide polymorphism (HbS) using DNA extracted from fresh or frozen samples of whole blood as described in detail previously (25, 39).
Statistical analysis
We fitted a series of Bayesian parametric latent class models to the available marker data (21). The key assumptions of the main models are summarized as follows:
-
1)
The marginal distribution of each marker in each latent class is log-normal.
-
2)
The marginal distribution of each marker in each latent class is the same across the different studies and countries.
-
3)
Informative Bayesian priors on all parameters.
Sensitivity analyses relaxed assumptions 1 to 3 (using bivariate t distributions, only using data from African children, and using weakly informative priors). We compared models with and without correlation between the markers within each latent class. The models with correlation performed better and were more conservative.
In an initial exploratory analysis, we used the R package mclust (40) (Gaussian Mixture Modelling for Model-Based Clustering; this uses the expectation-maximization algorithm for parameter estimation) to estimate the number of latent components in the data (fig. S7). This suggested three major underlying clusters, one of which was only present in the FEAST trial (patients with no measurable plasma PfHRP2 and normal platelet counts). We then used full Bayesian inference with informative priors to determine the distributions of the latent clusters. In the initial exploratory analysis, we also looked at the relationship between the peripheral parasite density and the two markers (fig. S8). This preliminary analysis showed that the malaria parasite density is a poor marker for discriminating between true severe malaria and other causes of illness.
It was not possible to obtain robust model fits when using the parasite count in combination with either the platelet count or the plasma PfHRP2.
We used the posterior predictive distribution over each class in the two latent class model to estimate the sensitivity and specificity for any given threshold for the platelet count and the plasma PfHRP2. Thus, for example, for a given platelet count threshold x (where x is an upper threshold value), the sensitivity is defined as 1 – FSM(x), and the specificity is defined as FnotSM(x), where FSM and FnotSM are the cumulative distribution functions for the platelet count in the severe malaria and not the severe malaria components, respectively.
The first set of models used data from the three severe malaria studies only (Kenyan children from Kilifi, Ugandan children from Kampala, and Bangladeshi adults). For each marker, the marginal distribution in each latent class (severe malaria versus not severe malaria) was assumed to be log-normal with the following informative priors (weakly informative): Means and standard deviations (SD) are given on the log 10 scale. For the mean platelet counts in the two latent classes, we used N(log10100,0.1) [weakly informative: N(log10100,0.5)] for the severe malaria class and N(log10200,0.1) [weakly informative: N(log10200,0.5)] for the not severe malaria class. The platelet count geometric mean of 100,000/μl versus 200,000/μl in severe malaria versus not severe malaria is informed by the results in (3) (an autopsy study in patients who had died with a diagnosis of cerebral malaria: The patients with evidence of parasite sequestration all had very low platelet counts compared to those who did not; we expect slightly lower platelet counts in all patients as the platelet count is a prognostic factor and has a diagnostic value).
For the mean PfHRP2 in the two latent classes, we used N(log102500,0.2) for severe malaria and N(log10500,0.2) for not severe malaria. The weakly informative prior used an SD of 0.5 instead of 0.2. The mean plasma PfHRP2 values are informed from previous modeling of data from the AQUAMAT trial (18).
The SD on the log 10 scale for each log-normal distribution was given an exponential prior, with the rate parameter set to 2 (i.e., a mean SD of 0.5 on the log 10 scale). We used informative beta priors for the prevalence of severe malaria in the three studies: Beta(19,1) for Bangladesh, beta(14,6) for Kilifi, and beta(14,6) for Kampala [the weakly informative beta priors: beta(4,1), beta(2,1), and beta(2,1), respectively].
To assess the robustness of the model outputs, we performed three sensitivity analyses: (i) by changing the parametric form to a bivariate student-t distribution with 10 degrees of freedom (robustness to assumption 1), (ii) by fitting the model to data only from the studies in African children with severe malaria (Kampala and Kilifi; robustness to assumption 2 as the Bangladeshi adults could plausibly be different from African children in terms of parasite biomass and host response), and (iii) by using weakly informative priors (larger SDs around the prior mean values and weaker beta priors on the prevalence parameters).
For the second set of models, we included data from the FEAST trial (23). FEAST intentionally enrolled severely ill patients with and without malaria; thus, there is a subset of patients who are clearly distinct from all patients in the studies of severe malaria (this subset is characterized, on average, by a negative malaria blood slide, no measurable plasma PfHPR2, and a normal platelet count). Thus, the latent class model needed to include a third component to fit the data. Under this model, the marker distributions were also assumed to be log-normal in the severe malaria class but were a mixture of two log-normal distributions for the not severe malaria class. We used the same priors for the second set of models with the addition of the following priors for the third additional component (which can be summarized as “severe illness with no evidence of acute malarial infection”): For the mean platelet count, we used N(log10300,0.1) for the second class of not severe malaria; for the mean PfHRP2, we used N(log101,0.2).
For the three-component model, Dirichlet priors were used for the prevalence parameters for each latent class: Dirichlet(19,1,0.1) for the Bangladesh study, Dirichlet(3,3,3) for the FEAST trial, and Dirichlet(14,7,1) for the Kampala and Kilifi studies. For all Dirichlet priors, the hyperparameters correspond to the severe malaria and the two not severe malaria classes in that order.
For patients with nonmeasurable plasma PfHRP2 concentrations to be included in this analysis, we set all nonmeasurable concentrations to 1 ng/ml (about half the lower limit of detection of the ELISA assay). Patients who had nonmeasurable plasma PfHRP2 concentrations but had parasite densities above 1000/μl were excluded from the analysis as these could represent P. falciparum parasites with HRP2/3 gene deletions. The median platelet count in these samples was 142,000/μl (range: 28,000 to 808,000/μl).
All posterior distributions were estimated using Monte Carlo methods. All models were implemented in the Stan language (41). For each model, we ran four independent chains for 5000 iterations, discarding half for burn-in. Convergence was checked by visually assessing traceplots. Convergence problems due to “label switching” were avoided by using parameter constraints: The mean value of each likelihood distribution was set as increasing (for the platelet count) or decreasing (for the PfHRP2 and parasite density). This uses the ordered parameter class in Stan. The Stan language does not support discrete parameters; however, posterior distributions of latent class models can be sampled by using the LogSumExp trick, computing the marginal likelihood over the different class combinations.
Supplementary Material
Severe malaria insights
Diagnosis of children with severe malaria caused by infection with Plasmodium falciparum has been difficult in hightransmission settings because of the high coincidence of malaria with other febrile illnesses. Watson et al. analyzed data from >2000 severely ill children and adults in low-transmission (Bangladesh) and high-transmission (Kenya and Uganda) settings. By fitting Bayesian latent class models using a combination of platelet counts and plasma concentrations of P. falciparum histidine-rich protein 2 (PfHRP2), they estimated that detection of #150,000 platelets/ μl and a plasma PfHRP2 concentration of #1000 ng/ml had a sensitivity of about 74% and a specificity of about 93% in identifying severe malaria. These findings revealed that a proportion of children enrolled in several malaria clinical studies in high-transmission settings have severe febrile illness caused by other types of pathogens.
Funding
This research was funded by Wellcome. A CC BY or equivalent license is applied to the author-accepted manuscript arising from this submission, in accordance with the grant’s open access conditions. This work was done as part of SMAART (Severe Malaria Africa—A consortium for Research and Trials) funded by a Wellcome Collaborative Award in Science grant (209265/Z/17/Z) held in part by K.M., N.P.J.D., and A.M.D. J.A.W. is a Sir Henry Dale Fellow jointly funded by the Wellcome Trust and the Royal Society (223253/Z/21/Z). T.N.W. and N.J.W. are senior and principal research fellows, respectively, funded by the Wellcome Trust (202800/Z/16/Z and 093956/Z/10/C, respectively). S.U. is funded by a Wellcome Intermediate Fellowship (221998/Z/20/Z). E.C.G. acknowledges funding from a core grant to the Medical Research Council (MRC) CTU at UCL from the MRC (MC_UU_12023/26). C.C.J. and R.O.O. acknowledge grant R01 NS055349 from the National Institute for Neurological Disorders and Stroke. The FEAST trial was supported by a grant (G0801439) from the MRC, UK provided through the (MRC) DFID concordat. K.M. and E.C.G. were supported by this grant. This paper is published with permission from the Director of the Kenya Medical Research Institute (KEMRI).
Footnotes
Author contributions: J.A.W. conceived the study, designed the experiments, analyzed the data, and wrote the first draft of the paper. N.J.W., T.N.W., and K.M. supervised the work. S.U., P.W., J.M., G.M.N., N.M., E.C.G., C.J.W., N.P.J.D., P.B., R.O.O., A.M.D., C.C.J., K.M., T.N.W., and N.J.W. contributed data from the clinical studies. All authors read and revised the paper.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability
All data associated with this study are present in the paper or the Supplementary Materials. All data and code used in the analysis are fully available on a GitHub repository at https://github.com/jwatowatson/SevereMalariaDiagnosis (42). The latest version has been archived on Zenodo at https://zenodo.org/badge/latestdoi/412716066. All figures can be reproduced via the R Markdown script provided on the GitHub repository.
References
- 1.World Health Organization. Severe malaria. Trop Med Int Health. 2014;19:7–131. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- 2.White NJ, Turner GD, Day NP, Dondorp AM. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis. 2013;208:192–198. doi: 10.1093/infdis/jit116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JG, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 4.Anstey NM, Price RN. Improving case definitions for severe malaria. PLOS Med. 2007;4:e267. doi: 10.1371/journal.pmed.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson JA, Ndila CM, Uyoga S, Macharia A, Nyutu G, Mohammed S, Ngetsa C, Mturi N, Peshu N, Tsofa B, Rockett K, et al. Improving statistical power in severe malaria genetic association studies by augmenting phenotypic precision. eLife. 2021;10:e69698. doi: 10.7554/eLife.69698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naing C, Whittaker MA. Severe thrombocytopenia in patients with vivax malaria compared to falciparum malaria: A systematic review and meta-analysis. Infect Dis Poverty. 2018;7:10. doi: 10.1186/s40249-018-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kho S, Barber BE, Johar E, Andries B, Poespoprodjo JR, Kenangalem E, Piera KA, Ehmann A, Price RN, William T, Woodberry T, et al. Platelets kill circulating parasites of all major Plasmodium species in human malaria. Blood. 2018;132:1332–1344. doi: 10.1182/blood-2018-05-849307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CRJC. Changes in white blood cells and platelets in children with falciparum malaria: Relationship to disease outcome. Br J Haematol. 2002;119:839–847. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 9.Chimalizeni Y, Kawaza K, Taylor T, Molyneux M. The platelet count in cerebral malaria, is it useful to the clinician? Am J Trop Med Hyg. 2010;83:48–50. doi: 10.4269/ajtmh.2010.09-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson J, Phu NH, Hasan MU, Charunwatthana P, Plewes K, Maude RJ, Prapansilp P, Kingston HW, Mishra SK, Mohanty S, Price RN, et al. The clinical implications of thrombocytopenia in adults with severe falciparum malaria: A retrospective analysis. BMC Med. 2015;13:97. doi: 10.1186/s12916-015-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin D, de Laat B, Jenkins PV, Bunn J, Craig AG, Terraube V, Preston RJS, Donkor C, Grau GE, van Mourik JA, O’Donnell JS. Severe plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLOS Pathog. 2009;5:e1000349. doi: 10.1371/journal.ppat.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Löwenberg EC, Charunwatthana P, Cohen S, van den Born B-J, Meijers JCM, Yunus EB, Hassan MB, Hoque G, Maude RJ, Nuchsongsin F, Levi M, et al. Severe malaria is associated with a deficiency of von Willebrand factor cleaving protease, ADAMTS13. Thromb Haemost. 2017;103:181–187. doi: 10.1160/TH09-04-0223. [DOI] [PubMed] [Google Scholar]
- 13.Lampah DA, Yeo TW, Malloy M, Kenangalem E, Douglas NM, Ronaldo D, Sugiarto P, Simpson JA, Poespoprodjo JR, Anstey NM, Price RN. Severe malarial thrombocytopenia: A risk factor for mortality in Papua, Indonesia. J Infect Dis. 2015;211:623–634. doi: 10.1093/infdis/jiu487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gérardin P, Rogier C, Amadou SK, Jouvencel P, Brousse V, Imbert P. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–691. doi: 10.4269/ajtmh.2002.66.686. [DOI] [PubMed] [Google Scholar]
- 15.Reich D, Nalls MA, Kao WHL, Akylbekova EL, Tandon A, Patterson N, Mullikin J, Hsueh W-C, Cheng C-Y, Coresh J, Boerwinkle E, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLOS Genet. 2009;5:e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Seidlein L, Olaosebikan R, Hendriksen ICE, Lee SJ, Adedoyin OT, Agbenyega T, Nguah SB, Bojang K, Deen JL, Evans J, Fanello CI, et al. Predicting the clinical outcome of severe falciparum malaria in African children: Findings from a large randomized trial. Clin Infect Dis. 2012;54:1080–1090. doi: 10.1093/cid/cis034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, Day NPJ. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLOS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriksen ICE, Mwanga-Amumpaire J, von Seidlein L, Mtove G, White LJ, Olaosebikan R, Lee SJ, Tshefu AK, Woodrow C, Amos B, Karema C, et al. Diagnosing severe falciparum malaria in parasitaemic African children: A prospective evaluation of plasma PfHRP2 measurement. PLOS Med. 2012;9:e1001297. doi: 10.1371/journal.pmed.1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AQUAMAT group. Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): An open-label, randomised trial. Lancet. 2010;376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Dondorp AM, Nosten F, Stepniewska K, Day N, White N. Artesunate versus quinine for treatment of severe falciparum malaria: A randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 21.Collins J, Huynh M. Estimation of diagnostic test accuracy without full verification: A review of latent class methods. Stat Med. 2014;33:4141–4169. doi: 10.1002/sim.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui SL, Walter SD. Estimating the error rates of diagnostic tests. Biometrics. 1980;36:167–171. [PubMed] [Google Scholar]
- 23.FEAST Trial Group. Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 24.Brand NR, Opoka RO, Hamre KE, John CC. Differing causes of lactic acidosis and deep breathing in cerebral malaria and severe malarial anemia may explain differences in acidosis-related mortality. PLOS ONE. 2016;11:e0163728. doi: 10.1371/journal.pone.0163728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MalariaGEN Consortium. Ndila CM, Uyoga S, Macharia AW, Nyutu G, Peshu N, Ojal J, Shebe M, Awuondo KO, Mturi N, Tsofa B, Sepúlveda N, et al. Human candidate gene polymorphisms and risk of severe malaria in children in Kilifi, Kenya: A case-control association study. Lancet Haematol. 2018;5:e333–e345. doi: 10.1016/S2352-3026(18)30107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bejon P, Berkley JA, Mwangi T, Ogada E, Mwangi I, Maitland K, Williams T, Scott JAG, English M, Lowe BS, Peshu N, et al. Defining childhood severe falciparum malaria for intervention studies. PLOS Med. 2007;4:e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Greenwood BM, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: A prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12:457–468. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malaria Genomic Epidemiology Network. Insights into malaria susceptibility using genome-wide data on 17,000 individuals from Africa, Asia and Oceania. Nat Commun. 2019;10:5732. doi: 10.1038/s41467-019-13480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyoga S, Macharia AW, Ndila CM, Nyutu G, Shebe M, Awuondo KO, Mturi N, Peshu N, Tsofa B, Scott JAG, Maitland K, et al. The indirect health effects of malaria estimated from health advantages of the sickle cell trait. Nat Commun. 2019;10:856. doi: 10.1038/s41467-019-08775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Band G, Leffler EM, Jallow M, Sisay-Joof F, Ndila CM, Macharia AW, Hubbart C, Jeffreys AE, Rowlands K, Nguyen T, Goncalves SM, et al. Malaria protection due to sickle haemoglobin depends on parasite genotype. Nature. 2022;602:106–111. doi: 10.1038/s41586-021-04288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henrici RC, Sautter CL, Bond C, Opoka RO, Namazzi R, Datta D, Ware RE, Conroy AL, John CC. Decreased parasite burden and altered host response in children with sickle cell anemia and severe anemia with malaria. Blood Adv. 2021;5:4710–4720. doi: 10.1182/bloodadvances.2021004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams TN, Obaro SK. Sickle cell disease and malaria morbidity: A tale with two tails. Trends Parasitol. 2011;27:315–320. doi: 10.1016/j.pt.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: A systematic review. BMC Med. 2014;12:31. doi: 10.1186/1741-7015-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha I, Ekapirat N, Dondorp AM, Woodrow CJ. Use of a rapid test to assess plasma Plasmodium falciparum HRP2 and guide management of severe febrile illness. Malar J. 2015;14:362. doi: 10.1186/s12936-015-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feleke SM, Reichert EN, Mohammed H, Brhane BG, Mekete K, Mamo H, Petros B, Solomon H, Abate E, Hennelly C, Denton M, et al. Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat Microbiol. 2021;6:1289–1299. doi: 10.1038/s41564-021-00962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosco AB, Nankabirwa JI, Yeka A, Nsobya S, Gresty K, Anderson K, Mbaka P, Prosser C, Smith D, Opigo J, Namubiru R, et al. Limitations of rapid diagnostic tests in malaria surveys in areas with varied transmission intensity in Uganda 2017-2019: Implications for selection and use of HRP2 RDTs. PLOS ONE. 2021;15:e0244457. doi: 10.1371/journal.pone.0244457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leopold SJ, Watson JA, Jeeyapant A, Simpson JA, Phu NH, Hien TT, Day NP, Dondorp AM, White NJ. Investigating causal pathways in severe falciparum malaria: A pooled retrospective analysis of clinical studies. PLOS Med. 2019;16:e1002858. doi: 10.1371/journal.pmed.1002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wambua S, Mwangi TW, Kortok M, Uyoga SM, Macharia AW, Mwacharo JK, Weatherall DJ, Snow RW, Marsh K, Williams TN. The effect of α+-thalassaemia on the incidence of malaria and other diseases in children living on the coast of Kenya. PLOS Med. 2006;3:e158. doi: 10.1371/journal.pmed.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scrucca L, Fop M, Murphy TB, Raftery AE. mclust: Clustering, classification and density estimation using Gaussian finite mixture models. R J. 2016;8:289–317. [PMC free article] [PubMed] [Google Scholar]
- 41.Stan Development Team. RStan: The R interface to Stan. Stan; 2020. http://mc-stan.org/ [Google Scholar]
- 42.Watson J. jwatowatson/SevereMalariaDiagnosis: Preprint code version. Zenodo; 2021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. All data and code used in the analysis are fully available on a GitHub repository at https://github.com/jwatowatson/SevereMalariaDiagnosis (42). The latest version has been archived on Zenodo at https://zenodo.org/badge/latestdoi/412716066. All figures can be reproduced via the R Markdown script provided on the GitHub repository.