Abstract
Cancer-associated fibroblasts (CAFs) are central players in the microenvironment of solid tumors, affecting cancer progression and metastasis. CAFs have diverse phenotypes, origins and functions, and consist of distinct subpopulations. Recent progress in single-cell RNA-sequencing technologies has enabled detailed characterization of the complexity and heterogeneity of CAF subpopulations in multiple tumor types. In this Review, we discuss the current understanding of CAF subsets and functions as elucidated by single-cell technologies, their functional plasticity, and their emergent shared and organ-specific features that could potentially be harnessed to design better therapeutic strategies for cancer.
Keywords: Tumor microenvironment, CAFs, scRNA-seq
Introduction
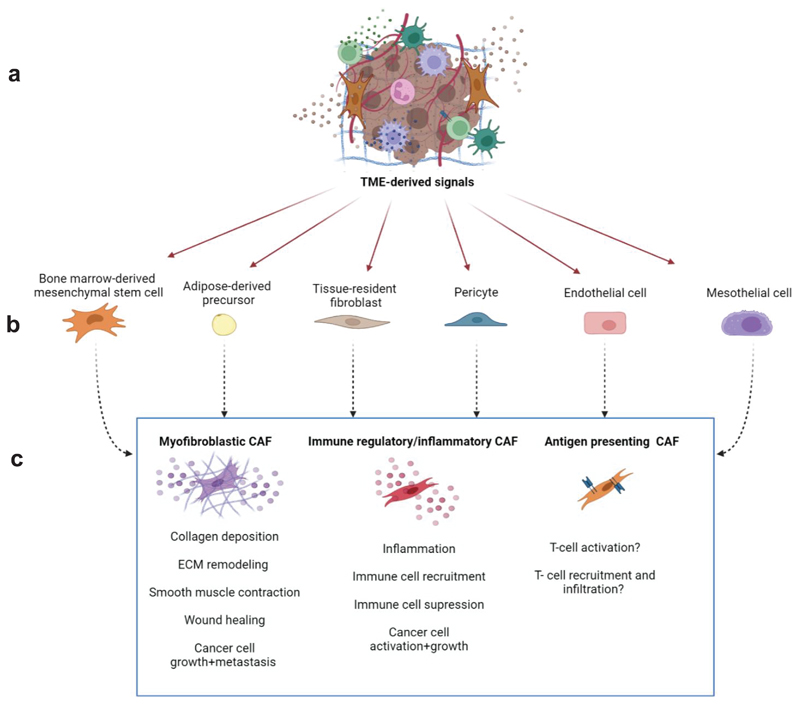
CAFs are a central component of the tumor microenvironment (TME) in solid tumors. In some cancer types, such as breast and pancreatic carcinomas, CAFs are the most prominent stromal cell type and their presence is associated with worse prognosis 1. CAFs are highly heterogeneous in their phenotypes, origins and functions2. They can originate from resident tissue fibroblasts reprogrammed by cancer cell-derived factors 3,4, from mesenchymal cells recruited to the TME from the bone marrow 5,6, or from adipocyte-derived precursor cells 7, endothelial cells 8, mesothelial cells 9–11, or pericytes 12 (Fig. 1). This heterogeneity is evident in the vast array of tasks fibroblasts perform in tumor progression and metastasis 2,13, including promoting cancer cell growth, angiogenesis, and extracellular matrix (ECM) remodeling 14–16. Moreover, CAFs orchestrate tumor-promoting inflammation and modulate the immune microenvironment towards immunosuppression 17. These functions are mediated by intricate reciprocal signaling interactions with cancer cells, matrix components and infiltrating immune cells. In some cancer types, such as pancreatic ductal adenocarcinoma (PDAC), CAFs were also suggested to have tumor inhibitory functions 18,19.
Figure 1. Primary CAF subsets and their potential origins.
TME-derived signals (a) can reprogram a variety of proximal and distal healthy cells, including bone marrow-derived mesenchymal cells, adipocytes, resident fibroblasts, pericytes, endothelial cells, and mesothelial cells (b) into CAFs. The major underlying CAF subgroups can be segregated into myofibroblastic CAFs, immune regulatory/inflammatory CAFs, and antigen presenting CAFs (c) based on the tasks they undertake in the TME, namely reorganization of the ECM, inflammation and modulation of the immune system, and direct effects on cancer cell proliferation and metastatic spread. The figure was created with BioRender.com.
The diversity of CAF functions, origins and markers has led to the notion that CAFs are composed of multiple subpopulations that only partially overlap (Fig. 2). For example, studies in the Rip1Tag2 mouse model of progressive pancreatic cancer and the orthotopic 4T1 breast cancer model, revealed limited overlap between various fibroblast markers, suggesting unique subpopulations 20. Analyses of human cancers and mouse models using immunostaining 6,21–23, in situ hybridization 24,25, flow cytometry and fluorescence activated cell sorting (FACS) of CAF subsets 26,27 and mRNA microarrays confirmed the existence of unique CAF subsets 6,20,22,25,26,28. Whilst these studies delivered crucial initial information regarding CAF diversity, the advent of single-cell RNA-sequencing (scRNA-seq) technologies has revolutionized the CAF field and revealed additional layers of complexity.
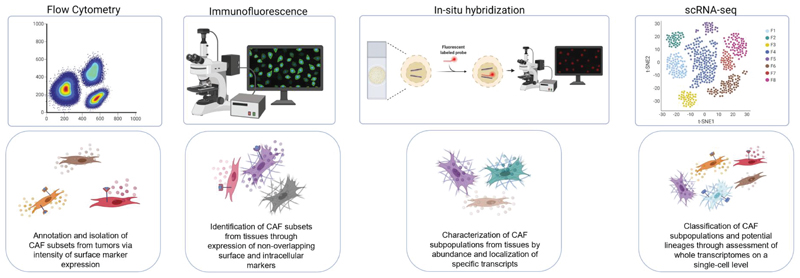
Figure 2. Identification of discrete CAF subsets achieved through different experimental systems.
From left to right: Flow cytometry has been employed to annotate and sort different CAF subgroups from tumors, also enabling CAF subset isolation for further experimentation into CAF phenotypes and tasks. Immunofluorescence and RNA in-situ hybridization experiments revealed CAF subsets from tissue samples, based on discrete expression of surface and intracellular protein markers and transcripts; these methods also reveal important spatial information regarding CAF subtype localization in the TME. Finally, scRNA sequencing has provided a breakthrough in stratification of a multitude of novel CAF subtypes through high resolution characterization of whole transcriptomes on a single cell level, providing information on rare CAF subsets and potential information regarding CAF lineages. The figure was created with BioRender.com.
In this Review, we examine our current understanding of CAF heterogeneity and function considering recent scRNA-seq studies in various cancer types. We portray shared and organ-specific features of CAF subpopulations and discuss promising areas for future research, in particular the potential use of CAFs as a treasure trove of much-needed therapeutic targets.
Classification of CAF subsets through single-cell technologies
Although the heterogeneity of CAFs was previously recognized, dissecting their complexity and plasticity in an unbiased manner was not feasible before the development of scRNA-seq technologies. Whilst this approach was first described more than a decade ago 29, it was scarcely utilized to explore CAF heterogeneity until recently 13,30. As all scientific tools, scRNA-seq has strengths and weaknesses, which greatly depend on the chosen technology 31. Two such limitations are the loss of spatial information and under-representation of some cell types (e.g. fibroblasts), due to the difficulty in isolating them from tissue 32,33. Indeed, the relatively small number of CAFs ultimately analyzed in some studies (Table 1) may have hampered detection of the full spectrum of CAFs subpopulations.
Table 1. Summary of the main CAF subsets and features in each organ.
| Organ | Organism | Central Features | Signature/Markers | Total Number of Analyzed Fibroblasts | Ref. | |
|---|---|---|---|---|---|---|
| Inflammatory | Breast | Mouse allograft model of TNBC (BALB/C-derived 4T1 mammary tumors) | Inflammation, immune trafficking, and complement | Ly6c1,Il-6,Il-33, Cxcl1,Cxcl12,Ccl2,Ccl7, C3,C4b, C1s1, C1s2 | 535 | 51 |
| Mouse allograft model of TNBC (BALB/C-derived 4T1 mammary tumors), human TNBC samples | Inflammation | PDPN, subdivided into 2 populations characterized by CXCL1 and IL-6 | 8,033 | 13 | ||
| MMTV-PyMT model, human BC tissue samples | Inflammation and chemotaxis | Cxcl14 | 768 | 30 | ||
| Human BC | Inflammatory response, TNF signaling, cytokine pathway | CXCL12, SOD2. Additional pathways: detoxification (ADH1B and GPX3), response to stimuli (RGMA and SCARA5) | 18,296 | 78 | ||
| PyMT/WT, PyMT/ELF5 | Inflammatory response, complement activation, monocyte recruitment | Involuting CAF signature-CXCL12, Ly6c1, C3, C4b | 2,255 | 80 | ||
| Human TNBC | Inflammation and chemotaxis | IGF1, FIGF, PDGFD,CXCL12 and CXCL13. As opposed to myCAFs, iCAFs characterized by FAPLOW/CD90LOW | 1,409 | 79 | ||
| Pancreas | Human IPMNs and PDAC tissue | Inflammation | VIM, FAP, COL3A1, DES, IL6, and CXCL12 | ≥ 267 | 48 | |
| KPP, PRT, KIC, KPC&KPfCmouse models of PDAC; Human PDAC | Chemoattraction, inflammation, immune trafficking, complement regulation | Cxcl1/2/9/10/12, Cxcl1, Ccl2/7, Il-6/8,IL-1R1,LIF,CFD, C7, C3, C1s,C1r, Pdgfra | 962 (human) & 4,012 (mice) [44]; 8,439 [46]; 5,802 [47]; 2,143 [49]; ~ 10,900 (mice)& 8,931 (human) [50]; 12,239 [71]; 1,753 [72] | 44,46,47,49,50,71,72 | ||
| Lung | Human NSCLC;Murine lung adenocarcinoma model (KrasLA1) | Inflammation, chemotaxis | CXCL12/14, PDGFRA | 3,794 [36];428 [83] | 36,83 | |
| Ovaries | Human ovarian cancer | Inflammation, complement regulation, and chemotaxis | IL-1/6/10, CXCL1/2/1012/14, CCL2, SOCS3,C3/7,C1QA/B/C,CFD,CFB,SERPING1 | 7,760 [56]; 547 [57] | 56,57 | |
| Liver | Human ICC;Murine ICC models (YAP/AKT, KRAS/p19) | Inflammation, chemotaxis, complement, HGF-met signaling | FBLN1, IGFI, CXCL1/12, IGFBP6, SLPI, SAA1, C3, C7, Il-6,HGF,CCL21 | 498 [54]; 12,431 (mice) & 4,463 (human) [88] | 54,89 | |
| Bladder | Human urothelial bladder carcinoma | Inflammation and chemotaxis | CXCL12, IL6, CXCL14, CXCL1, and CXCL2, marked by PDGFRA | N.A. | 58 | |
| Skin | Murine Melanoma | Immune trafficking and inflammation. | Marked by Pdpn, Pdgfra, and Cd34: Cxcl12, Csf1, and Ccl8, Il6ra and Il6st, C3, C2, and C4b | N.A. | 38 | |
| Immune Regulatory | Breast | Mouse allograft model of TNBC (BALB/C-derived 4T1 mammary tumors) | Antigen presentation, MHC-II genes | CD74, H2-Aa, H2-Ab1, H2-Eb, Cd74 | 535 | 51 |
| Mouse allograft model of TNBC (BALB/C-derived 4T1 mammary tumors), human TNBC samples | Immune regulation, chemotaxis | PDPN subpopulation subdivided into Ly6c+, CXCL12, Saa3 subsets | 8,033 | 13 | ||
| Mouse allograft model of TNBC (BALB/C-derived 4T1 mammary tumors), human TNBC samples | MHC-II presentation and immune regulation | S100A4, CD73,H2-Aa, H2-Ab1 and CD74; SLPI and SPP1 | 8,033 | 13 | ||
| Human BC | IFNγ, cytokine signaling, MHCII presentation | CCl19, CCl5, CD74 | 18,296 | 78 | ||
| Human TNBC | Immune regulation | CXCL12 hallmark gene, C5-C5AR1 signaling and TGFB1-TGFBR1 and TGFB2-TGFBR1 axis. CXCL12-CXCR4 and CXCL13-CXCR5 signaling. | 1,409 | 79 | ||
| Pancreas | Human PDAC | Immune regulation via Complement secretion | C3, C7, CFB, CFD, CFH, CFI | 2,958 | 73 | |
| KPP mice& human PDAC | Immuno-regulatory module | Pdgfc, Vegfa, Il33,Il18 | ~ 10,900 (mice)&8,931 (human) | 50 | ||
| Mouse (KPC model) &human PDACs | Antigen presentation, T-cell regulation | MHC-II in mice (H2-Aa and H2-Ab1, CD74) and human (HLA-DRA, HLA-DPA1,HLA-DQA, CD74). Additional immunoregulatory genes: BCAM, F11R, IRF5. | 962 (human) & 4,012 (mice) | 44 | ||
| PRT, KIC, KPC&KPfC mouse models of PDAC;Human PDAC | Antigen presentation, MHC-II genes | CD74, H2-Aa, H2-Dmb1,HLA-DQA1,CD83 | 2,143 [49];12,239 [71];1,753 [72] | 49,71,72 | ||
| Lung | Human lung adenocarcinoma | Immune modulation and antigen presentation | CFD,CXCL14,CXCL12, MHC-II,CD74 | 2,257 | 84 | |
| Prostate | Human prostate cancer | Inflammation, regulation of myeloid cell recruitment | CCL2,CXCL12,CCL11,CXCL1,IL33 | 3,321 | 86 | |
| Liver | Mouse model of liver metastasis (PDAC/CRC tumor cell lines injection)/human CRC liver metastases | Inflammation, IFN response, HGF-Met signaling, antigen expression | HG, H2-Q4, H2-Q7, Ifitm. | N.A. | 55 | |
| Murine ICC model, human ICC | Antigen presentation | CD74, HLADRA, HLA-DRB1, H2-Q4 | 498 [54]; 12,431 (mice) & 4,463 (human) [88] | 54,89 | ||
| Antigen Presentation | Pancreas | Mouse (KPC model) and human PDACs | Antigen presentation (AP); Partial activation of CD4+ T-cells (by TCR ligation) | Mouse: H2-Aa, H2-Ab1 and CD74; SAA3 and SLPI. Human: HLA-DRA, HLA-DPA1, HLA-DQA1, and CD74; SLPI | 962 (human) & 4,012 (mice) | 44 |
| Mouse model of human PDAC (cell line-derived xenograft; CDX) | AP | CD74 and HLA-DRA | 699 | 45 | ||
| Tamoxife-ninducible mouse model of PDAC (PRT) | AP | H2-Aa, CD74 and CD83 | 12,239 | 71 | ||
| KPP mouse model of PDAC | AP | H2-Ab1 and CD74; SAA3 | ~ 10,900 (mice) & 8,931 (human) | 50 | ||
| KIC, KPC&KPfC mouse models of PDAC | AP; Partial activation of CD4+ T-cells (by TCR ligation); Induction of NaïveCD4+ T-cells into regulatory T-cells | H2-Aa, H2-Ab1, H2-Eb1, and CD74; MSLN, UPK3b, LRRN4 and KRT19 (mesothelial genes) | 17,055 (Derived from integrated data of 3 papers: Hosein, Elyada&Domingues) | 11 | ||
| Breast | Mouse allograft model of TNBC (BALB/Cderived 4T1 mammary tumors) | AP | H2-Aa, H2-Ab1 and CD74; SLPI and SPP1 | 8,033 | 13 | |
| Liver | Human ICC | AP | HLA-DRA, HLA-DRB1 and CD74, | 498 | 54 | |
| Lung | Human & mouse (LLC model) lung tumors | AP; Formation of functional spots within tumors that sustain CD4+ T-cells; Priming CD4+ T-cells along with rescuing them from apoptosis | Human: CD74 and SLPI; IL-6, CFD, C1QA, and C1QB. Mouse: CD74 and SLPI | 798 (human), N.A. (mice) | 92 | |
| ECM-remodeling/ myofibroblastic CAFs | Pancreas | Mouse (KPC model) and human PDACs | Smooth muscle contraction, focal adhesion, ECM organization, and collagen formation | Mouse: ACTA2, TAGLN, IGFBP3, THY1,COL12A1 and THBS2 Human: ACTA2, TAGLN, MYL9, TPM1, TPM2, MMP11, POSTN and HOPX |
962 (human) &4,012 (mice) | 44 |
| Mouse model of human PDAC (cell line-derived xenograft; CDX) | ECM organization, cell adhesion, and blood vessel development | ACTA2, TAGLN, BGN, COL8A1, COL15A1, IGFBP7, TPM1 and TPM2 | 699 | 45 | ||
| Human PDAC | Focal adhesion and ECM-receptor interactions | COL10A1 and POSTN | 8,439 | 46 | ||
| Human PDAC | ECM remodeling | ACTA2, MYL9, and POSTN | 5,802 | 47 | ||
| Human IPMNs and PDAC tissue | ECM remodeling | ACTA2 | ≥ 267 | 48 | ||
| Breast | Mouse allograft model of TNBC (BALB/C-derived 4T1 mammary tumors) | ECM organization and wound healing features | ACTA2, THBS2, FBN1, and MFAP5 | 8,033 | 13 | |
| 535 | 51 | |||||
| Transgenic mouse model of breast cancer (MMTV-PyMT) | ECM molecules production | DCN, LUM, VCAN, COL14A1, FBLN1, FBLN2, SMOC, LOX, LOXL1, PDGFRα, and CXCL14 | 768 | 30 | ||
| Lungs | Human NSCLC | Myogenesis, NOTCH pathway, and angiogenesis | ACTA2, MEF2C, MYH11, ITGA7, COL4A1, and COL10A1 | 1,465 | 37 | |
| Human NSCLC | ECM remodeling | COL13A1, COL14A1, ACTA2,TAGLN, MYH11, MYLK, and ACTG2 | 3,794 | 36 | ||
| Liver | Human ICC | ECM and collagen fibril organization | POSTN, FN1, LUM, DCN, VCAN, COL5A1, COL5A2, and COL63A | 498 | 54 | |
| Mouse model of liver metastasis (PDAC/CRC tumor cell lines injection)/human CRC liver metastases | ECM remolding/Opposing effects on tumor growth | Mouse: ACTA2, COL1A1, and COL3A1. Human: COL1A1, COL3A1, and COL63A | N.A. | 55 | ||
| Ovary | Human ovarian cancer | TGβ-induced reactive stroma | ACTA2, POSTN, COMP, COL10A1, COL11A1, MMP11, TAGLN, and FN1 | 7,760 | 56 | |
| Skin | Melanoma mouse model | Desmoplastic reaction/Contraction of actin stress fibers | PDPN, PDGFRα, POSTN, and TNC/ACTA2, ROCK1, MLC2, and MLCK | N.A. | 38 | |
| Bone | Human osteosarcoma | ECM remodeling | COL14A1/ ACTA2, MYL9, and LUM | N.A. | 40 | |
| Colon | Human CRC | Cytoskeleton/ECM remodeling | ACTA2, TAGLN, and PDGFA/ MMP2, DCN, and COL1A2 | 26 | 41 | |
| Urinary bladder | Human urothelial bladder carcinoma | Focal adhesion and contraction | RGS5, MYL9, and MYH11 | N.A. | 58 |
Nevertheless, scRNA-seq has significantly contributed to the expansion of our knowledge of CAFs biological diversity, as will be discussed in detail herein.
ECM-remodeling/myofibroblastic CAFs
One of the crucial elements shaping the TME is the ECM, a complex three-dimensional network of extracellular molecules that form a tissue-supportive physical matrix and affect the structure and function of the stroma in primary tumors and metastases 21,34.
ECM remodeling is a tightly regulated physiological process (e.g. in development and wound healing). However, tumors co-opt this process to create a supportive microenvironment. CAFs are central players in the deposition, modification and degradation of the ECM milieu (Fig. 3a). Dysregulated ECM remodeling by CAFs can lead to the desmoplastic reaction associated with poor outcome in breast, pancreatic and lung cancers 2. Loss of an organized and stable matrix is often considered a hallmark of these tumors, leading to extensive efforts to develop therapies targeting tumor ECM 21,34.
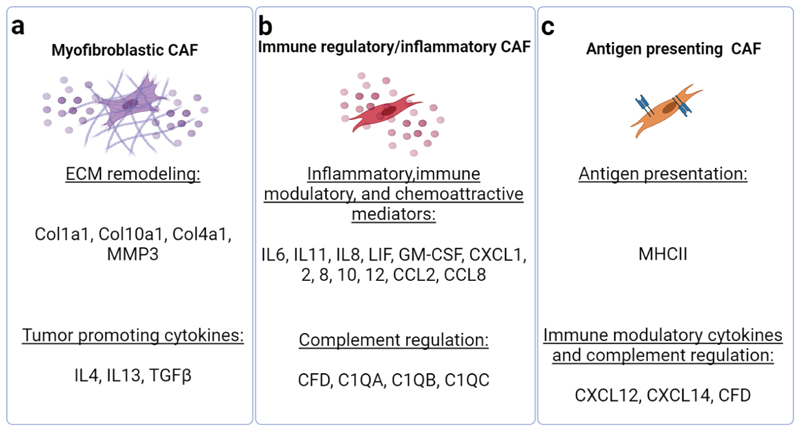
Figure 3. scRNA-seq reveals multiple tasks undertaken by discrete CAF subtypes.
The major tasks performed by the three central CAF subtypes-ECM reorganization, immune regulation, and antigen presentation, are mediated through expression of various cell surface receptors and secreted factors that influence tumor progression. The secreted factors and receptors listed in the figure generally vary according to disease type and organ, and are designated accordingly in Figure 4. The figure was created with BioRender.com.
CAFs were known to be related to myofibroblasts even before they could be characterized by RNA-seq, due to their activated state in which they acquire specialized contractile features (identified by elevated expression of αSMA), similar to the phenotype of fibroblasts in wound healing processes 35. In this section, we discuss CAF subpopulations determined by scRNA-seq studies as ECM-remodeling/myofibroblastic CAFs in multiple organs. Some studies classified ECM remodeling and wound healing-associated features (e.g. contraction) as distinct CAF functional states 13,36–41. However, other studies show that the same subpopulation of fibroblasts is highly enriched for both ECM-associated genes (such as collagens, DCN and FBLN2) and contractile proteins (such as MYL9, TAGLN and ACTA2 - encoding for αSMA), typical of wound fibroblasts 42.
Pancreas
PDAC is characterized by a desmoplastic TME 43. Cross-species analysis of human and mouse (KPC model) PDAC tumors by immunostaining 25 and scRNA-seq analysis 44 identified a subpopulation of myofibroblastic CAFs which was termed myCAFs (Fig 4a). These cells express genes associated with smooth muscle contraction, focal adhesion, ECM organization, and collagen formation, and are located adjacent to neoplastic cells. myCAFs are characterized by high αSMA expression, however scRNA-seq analysis identified various other marker genes encoding contractile proteins (e.g. TAGLN, MYL9, TPM1/2, MMP11, POSTN and HOPX) 44. In a xenograft model of human PDAC one of these marker genes – TPM1, was used in addition to αSMA, to identify a myCAF subpopulation that differentiated from adipose-derived mesenchymal stem cells, demonstrating conservation of this marker between human and murine myCAFs45. Furthermore, POSTN+ myCAFs were found to be the dominant subset specifically in dense-type PDAC tumors from human patients 46, and another scRNA-seq study in human PDAC identified a distinct CAF subpopulation marked by POSTN, in addition to a myofibroblastic CAF subpopulation marked by ACTA2+ and MYL9+ 47. Myofibroblastic CAFs play a role from the early stages of tumorigenesis: scRNA-seq found that myofibroblastic CAFs were present in both samples derived from human intraductal papillary mucinous neoplasia (IPMN) – a common cystic precursor lesion of PDAC— and PDAC samples48. Myofibroblastic CAF heterogeneity can also be temporal, as CAFs were shown to acquire different phenotypes at different stages of PDAC mouse models: during late PDAC progression one CAF subpopulation was lost to the dominance of two other major CAF subsets expressing genes linked to growth factor signaling, inflammation, and the myofibroblast markers Acta2 and Tagln49. Another study demonstrated that CAF subpopulations increase fibrillar collagens and cytokine secretion 50.
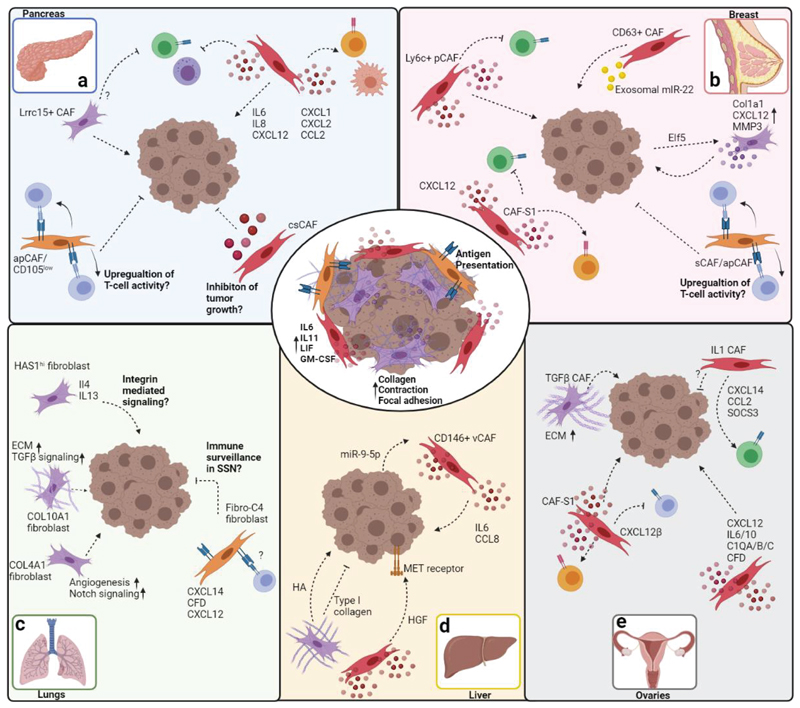
Figure 4. scRNA-seq analyses reveal universal, as well as organ-specific CAF subsets.
The main CAF subsets identified in the TME of most organs are inflammatory CAFs, antigen presenting CAFs, and myfibroblastic CAFs (center circle). Immune regulatory (cytokine and chemokine secretion, crosstalk with immune cells) and myofibroblastic CAF activities (ECM modulation, collagen deposition, contraction and adhesion) are prevalent in all organs and across cancer types. Antigen presenting activities of CAFs were predominantly reported in pancreatic and breast cancers. Organ-specific tasks and pathways identified in CAFs are depicted for pancreas (top left), breast (top right), lungs (bottom left), ovaries (bottom center), and liver (bottom right). Arrows indicate promotion of cancer progression and immune cell recruitment/activation. Inhibitory arrows indicate suppression of cancer progression or immune cell activity. Speculative/unverified pathways are labeled with question marks. The figure was created with BioRender.com.
Breast
Myofibroblastic subsets of CAFs were identified in most scRNA-seq studies performed in breast cancer (Fig 4b). In a syngeneic mouse model of transplantable triple-negative breast cancer (TNBC), ECM remodeling or wound healing signatures were identified in two distinct CAF states, marked by Fbn1 and Mfap5 or Acta2 and Thbs2, respectively 13. Both functional states were derived from a larger population of CAFs termed pCAFs (PDPN+), which consisted of four more subsets. At advanced tumor stages, the two myofibroblastic subsets dominated the pCAF population 13. In two other studies – one utilizing the same mouse model and the other utilizing transgenic MMTV-PyMT mice, the relevant cells were collectively designated myCAFs 51 or mCAFs 30 in the absence of longitudinal analysis. The spatial location of mCAFs was linked to their function as they were most abundant in the invasive part of the tumors, particularly within collagen-rich streaks. Despite differences that may result from the different model systems, these studies highlight the generality of ECM-remodeling/contractile CAF subpopulations.
One of the strongest risk factors of developing breast cancer is advanced age. scRNA-seq of cells isolated from human postmenopausal breast tissue identified two fibroblast subsets expressing various types of collagens. A comparison between the gene signatures of postmenopausal fibroblast subsets and the gene expression profiles of 1,100 breast tumors in The Cancer Genome Atlas (TCGA) dataset revealed significant overlap with a gene profile exclusively associated with luminal breast tumors, suggesting that this fibroblast subpopulation may contribute to breast carcinogenesis in the elderly 52.
Lungs
scRNA-seq of human non-small cell lung cancer (NSCLC; squamous cell and adenocarcinoma) revealed five distinct CAFs types based on the expression of a unique repertoire of collagens and other ECM molecules. Further characterization showed high expression of genes associated with myogenesis (including ACTA2) in one subpopulation, whereas another showed a strong expression of genes linked to epithelial-mesenchymal transition (EMT) and the ECM37. scRNA-seq of tissues obtained from patients with lung adenocarcinoma at different stages of the disease identified seven subpopulations of fibroblasts, three of which were functionally related to ECM modulation (Fig.4c): COL13A1+ matrix fibroblasts, COL14A1+ matrix fibroblasts and myofibroblasts (ACTA2+/TAGLN+). The matrix-related fibroblasts (COL13A1+ and COL14A1+) were the main subpopulations present in normal lungs and early-stage tumors. In contrast, myofibroblasts were dominant in advanced stage tumors (including in metastatic lymph nodes), reflecting a gradual change of fibroblast states associated with tumor progression 36. Intriguingly, a scRNA-seq study which compared ground glass nodule and solid lung adenocarcinoma showed that the fibroblasts of the former, which has low malignant potential and a better survival rate, expressed lower levels of collagens, emphasizing the impact of excessive ECM deposition on patient prognosis 53.
Liver
scRNA-seq analysis of human intrahepatic cholangiocarcinoma (ICC), an aggressive desmoplastic carcinoma with poor prognosis, identified a subpopulation designated as matrix CAFs (mCAFs), expressing low levels of ACTA2 but high levels of ECM-associated genes including various collagens, POSTN, FN1, LUM, DCN and VCAN. In agreement with findings from breast cancer 30, these POSTN+ mCAFs were found in the invasive front of the tumor, predominantly within collagen-rich streaks, suggesting a close association with tumor invasiveness 54. A high proportion of ECM-modulating CAFs may be characteristic of highly desmoplastic TMEs. In a mouse model of desmoplastic liver metastasis induced by injection of PDAC and colorectal cancer (CRC) cell lines into the hemispleen, scRNA-seq revealed that myofibroblastic CAFs comprised more than half of the total analyzed CAFs 55. The presence of such ECM-modulating CAFs was also confirmed in human CRC liver metastases, although compared to mice, human CAFs appeared to largely originate from hepatic stellate cells, and lacked a portal fibroblast and mesothelial cell signature which may have distinct functions 55. This was in agreement with functional studies suggesting that CAFs may also have both tumor-promoting and inhibitory functions: in a 3D culture model, collagen type I acted as a mechanical barrier restricting tumor expansion and invasiveness, whereas hyaluronic acid promoted tumor growth and spread55 (Fig.4d).
Ovary
High-resolution scRNA-seq dissection of human ovarian tumors revealed a subset of CAFs, annotated as TGFβ-CAFs, which resembled a combination of the myCAF and TGFβ-driven CAF subsets described in PDAC 44,56 (Fig.4e). The main markers of the TGFβ-CAFs included genes associated with TGFβ-induced reactive stroma, as well as POSTN, ACTA2, MMP11, TAGLN, and FN1 56. In another scRNA-seq study of human serous ovarian cancer derived from primary tumors and metastatic omentum, as well as normal ovary, the fibroblasts were sub-grouped into normal, primary and metastatic subsets. Collagens and MMP-associated genes were upregulated in the primary and metastatic tumor fibroblasts when compared to normal, suggesting a role for ECM modulations in tumor progression 57.
Skin
scRNA-seq profiling of the stromal compartment in a transplantable model of murine melanoma at distinct time points of tumor development identified three functionally and temporally distinct subpopulations, referred to as S1 ("immune"), S2 ("desmoplastic") and S3 ("contractile"), which were also validated in human melanoma. The S2 cells (PDPNHigh/PDGFRαHigh/CD34Low) upregulated genes encoding ECM components, including various collagens, whereas the S3 cells (αSMAHigh) expressed genes involved in the regulation and rearrangement of the actin cytoskeleton, indicating a contractile stromal subset. S3 cells also expressed some pericyte-associated markers, suggesting that this may be their cell of origin. Temporal analysis revealed dynamic changes whereby early tumors primarily comprised S1 ("Immune") and S2 ("desmoplastic") cells, whereas mid and late tumors exhibited S2 ("desmoplastic") and S3 ("contractile") enrichment 38. These detailed definitions further emphasize the question of whether CAF subpopulations are truly distinct, or a “snapshot” of plastic functional states.
ECM remodeling/myofibroblastic CAFs in other organs
Single-cell studies in other organs confirmed the presence of ECM-remodeling/myofibroblastic CAF subtypes that may be defined as two separate states. Analysis of human prostate tumors unveiled two distinct clusters representing either myofibroblastic or ECM-associated phenotypes, which was supported by enrichment in the ECM-associated subpopulation of CREB3L1 and PLAGL1, transcription factors known to control ECM production and composition, compared to increase of HOXB2 and MAFB in the myofibroblastic subpopulation 39. A study of human urothelial bladder carcinoma 58 identified a subset of CAFs termed mCAFs, with similar characteristics to the myCAFs described in PDAC 25,44 and marked by RGS5, suggesting a potential pericyte origin, as shown in breast cancer and melanoma studies 26,30. Accumulation of mCAFs in the tumor correlated with poor patient survival 58. To explore the intratumoral heterogeneity of osteosarcoma, the most frequent primary bone tumor, scRNA-seq was performed on human primary, recurrent and lung metastatic lesions. Three CAF subclusters were identified: (1) COL14A1+ matrix fibroblasts, (2) DES+/ACTA2Low/COL14A1Low fibroblasts (indicative of smooth muscle-like cells), and (3) ACTA2+/MYL9High/LUMHigh fibroblasts (lacking expression of COL14A1 and DES). Although subcluster 3 resembled myofibroblasts, it also showed high expression of osteoblast markers (IBSP and SPP1), once again highlighting the plasticity of mesenchymal cell populations. Moreover, this subcluster was the major source of CAFs both in primary and recurrent lesions, whereas subcluster 2 was the main component in lung metastases 40. A scRNA-seq study in human primary colorectal tumors and matched normal mucosa used a method termed reference component analysis to identify two distinct subtypes of CAFs (CAF-A and CAF-B). CAF-B cells expressed markers of myofibroblasts such as ACTA2, TAGLN and PDGFA, and these markers were downregulated in CAF-A cells, which expressed ECM-related genes such as MMP2, DCN and COL1A2. Activation of the upstream regulators TGFβ1 and SMAD3 in both CAF subtypes suggests common regulation of these two functional states 41. In gastric cancer, a stage dependent increase in three CAF subsets belonging to a stromal meta-cluster with endothelial cells and pericytes was demonstrated: two subsets exhibited expression of myofibroblastic genes such as ACTA2 and TAGLN, whereas a specific cluster induced expression of collagen associated genes that were correlated with INHBA signaling 59, confirming previous findings regarding CAF-mediated INHBA signaling in gastric cancer 60.
Tissue damage myofibroblasts versus ECM remodeling/myofibroblastic CAFs
Comparing the transcriptional landscape of fibroblasts that function in physiological wound healing with ECM-remodeling/myofibroblastic CAFs yields interesting conclusions. For example, scRNA-seq of stromal cells in healthy and myocardial infarct-injured mouse hearts revealed multiple different fibroblast subpopulations 61. Normal fibroblasts were characterized by PDGFRα and Ly6a (Sca1) expression, whereas activated fibroblasts in injured heart tissue upregulated fibrogenic (e.g. POSTN, collagens) and/or contractile proteins (e.g. aSMA), similarly to myofibroblastic CAFs. This analysis also identified a subpopulation of fibroblasts termed F-Wntx in both normal and myocardial infarction-associated tissue, which expressed Wnt signaling inhibitors and had an anti-fibrotic phenotype. Single-cell analysis of fibroblasts isolated from murine skin wound healing 42,62 or from human fibrotic skin 63 identified multiple heterogenous fibroblast states expressing PDGFRα and pro-fibrogenic gene signatures (POSTN, collagens, TGFβ pathway) reminiscent of myofibroblastic CAFs. Similar analysis of fibroblasts isolated from murine lung64 and liver fibrosis65 identified subpopulations of activated myofibroblasts with collagens and matrix remodeling gene signatures, and a population of lipofibroblasts expressing lipid synthesis and transport genes, in addition to common fibroblast genes 64. Contrary to CAFs, these wound healing and fibrosis studies did not identify distinct subpopulations of inflammatory/immune-regulatory fibroblasts. However, single cell analysis of fibroblasts isolated from the colon mucosa of patients with ulcerative colitis (UC) 66 or from the ileal biopsies of patients with Crohn’s disease67 identified both inflammatory fibroblasts and myofibroblasts. Additionally, UC samples contained several subpopulations of Wnt ligand-expressing fibroblasts that were also expressed in normal colon, in a spatial-specific manner. Inflammatory fibroblasts isolated from UC patient samples also expressed CAF-like markers 66 and stromal genes shown to be associated with poor prognosis in colorectal cancer 68. Thus, although CAF-like subpopulations are also found in wound healing contexts, their heterogeneity may be more reminiscent of chronic inflammatory conditions than of fibrotic tissue repair.
Pro-inflammatory and immune regulatory CAFs
Under normal homeostatic conditions, resident tissue fibroblasts play important roles in maintaining tissue integrity. Fibroblasts can sense mechanical changes and tissue damage signals and react by orchestrating tissue repair, mediated by ECM synthesis and remodeling, but also by regulating immune cell responses. This orchestration of immune cell activity appears to be both organ- and context-dependent, and is co-opted in tumors. The role of fibroblasts as central mediators of tumor-promoting inflammation was first suggested over a decade ago 69. CAFs promote cancer growth, immune escape, and metastatic dissemination by modulating the immune landscape in the TME. This is achieved through secretion of cytokines and chemokines, recruitment of suppressive myeloid and regulatory T cells (Tregs), suppression and exclusion of cytotoxic lymphocytes and dendritic cells, and promotion of M2 and Th2 polarization of macrophages and T cells, respectively 17 (Fig. 3b). Until recently it was not clear whether diverse immune-regulatory activities of CAFs, such as promoting inflammation and immune suppression, are performed by a homogenous group, and whether these tasks represent a global feature shared by different cancer types and organs. A surge of studies employing scRNA-seq or utilizing different markers to segregate CAF subpopulations have addressed the heterogeneity of immune-regulatory CAFs and the disease- and organ-specific nature of their activities. Most of these studies were conducted in highly desmoplastic carcinomas of the breast and pancreas.
Pancreas
Inflammatory CAF subtypes were identified in PDAC prior to single-cell studies in human and murine pancreatic cancer. A subset termed iCAF was distinguish from another subset, myCAFs, based on its low expression of the myofibroblastic αSMA marker 25. iCAFs were shown to be spatially separated from myCAFs and from the cancer cells, secreted inflammatory mediators such as IL-6, IL-11, and LIF, and had a unique transcriptomic profile driven by IL-1 signaling 70. The two subsets could interconvert, at least in vitro, depending on growth conditions, further raising the question of whether CAF subpopulations are transient functional states. A later study which included scRNA-seq in mice and in human PDAC samples 44 provided additional iCAF markers such as IL-6, IL-8, CXCL1, CXCL2, CXCL12, and CCL2 (Fig.4a).
Differences between mouse models and humans can affect interpretation of CAF activity in the context of immune cell regulation. For example, the murine KPC model is extremely sparse in T and B cells compared to human samples44, which can affect conclusions regarding CAF effects on immune exclusion. Nevertheless, similar inflammatory CAF subtypes were found (and given various designations) in multiple scRNA-seq studies in human and murine PDAC 47,49,71,72. These studies support the inflammatory and immune regulatory modalities amongst CAF subtypes, potentially also segregating iCAFs based on expression of inflammatory and chemotactic mediators such as CXCL14, IL-6, CXCL1/12, CCL7/11, and different immune modulatory hubs such as Ly6c1 and Scara3, in addition to antigen presenting modalities 49,71. Another immune regulatory function identified through scRNA-seq of human pancreatic CAFs is complement regulation 73 by csCAFs, a specialized subpopulation confirmed by weighted gene co-expression network analysis. CAFs expressing complement regulatory factors (C3, C7, CFB, CFD, CFH, and CFI) which can promote inflammation and immune activity in the TME appear in close proximity to pancreatic cancer cells in stage I PDAC, and decrease during tumor progression73. The early expression may suggest a tumor repressive role, but it is also possible that their early inflammatory complement-mediated activities pave the way for PDAC development, given that complement activation is positively associated with cancer progression 74. Only some of the PDAC CAF subsets designated "immune regulatory" based on gene expression were mechanistically connected with immune-modulating tumor-regulatory functions. For example, inflammatory mediators secreted from iCAFs, such as IL-6, IL-11, GM-CSF, and LIF promoted activation of cancer cells to enhance tumor growth and survival 25,75. In addition, CyTOF analysis of murine pancreatic tumors demonstrated that Hedgehog pathway inhibition increases the proportion of iCAFs versus myCAFs, sequesters repressive myeloid immune cells and inhibits CD8+ T cell tumor infiltration, while promoting FOXP3+ Treg accumulation, thus generating an immunosuppressive TME 76.
Single-cell analysis using mass cytometry on murine and human PDAC samples unveiled fibroblast subsets with high versus low CD105 (ENG) expression that had opposing effects on immune activity and tumor growth 77. While CD105Low CAFs were characterized by antigen presentation modules (MHC-II and CD74) and correlated with proliferation of T cell subsets, CD105High CAFs were inversely correlated with certain CD4 and CD8 T cell subsets. The suppressive effects of CD105Low CAFs depended on proper immune activity that was abrogated in immune deficient animals, but did not rely on their antigen presenting capabilities 77.
The iCAF phenotype was shown to be driven by IL1R1/JAK/STAT and NF-κB signaling in human PDAC organoids and murine models 75. However, scRNA-seq from mouse and human PDAC revealed that iCAFs also express highly activated TGFβR2 and TGFβR3 44, which can induce the myCAF phenotype, suggesting that the two CAF archetypes may affect each other. The dichotomy of IL-1 and TGFβ signaling in driving iCAF and myCAF subpopulations, respectively, was questioned in another scRNA-seq study of murine pancreatic cancer models 50: PDPNHigh CAFs were shown to play an immune regulatory role in PDAC, where they diverged into two subpopulations through IL-1 and TGFβ signaling. The TGFβ-driven CAFs which accumulated in PDAC were identified by LRRC15 expression and correlated with poor response to anti-PD-L1 therapy (Fig.4a). Thus, although LRRC15+ CAFs are mainly characterized by a myCAF-like signature, they may also play an active immunosuppressive role 50.
Breast
Inflammatory and immune regulatory CAFs in breast cancer can be clustered as one population marked by both inflammatory and immune regulatory genes (e.g. IL-6/8, CXCL1/2, and CXCL12 44) similarly to annotations in PDAC, or subclassified further based on the inflammatory cytokine gene signatures that they express (such as IL-6 secreting inflammatory CAFs), or by the immune cell activity they modulate (for example: immune regulatory CAFs may regulate leukocyte homing via CXCL12) 13. Immune regulatory CAFs may also be subcategorized according to unique tasks such as detoxification (ADH1B and GPX3) or IFNγ and cytokine response (expression of genes such as CCL19 and CCL5) as was shown in human breast cancer 78. Multiple studies revealed compartmentalization of immunomodulatory activities by CAF subsets in breast cancer. scRNA-seq in a mouse model of TNBC revealed two proinflammatory and two immunomodulatory CAF subpopulations within a subset of PDPN+ CAFs (pCAFs) compared to S100a4+ CAFs (sCAFs). Both pCAFs and sCAFs were also distinguishable in human breast cancer patient cohorts 13. The immunomodulatory pCAFs in TNBC diverged into Ly6cLow and Ly6cHigh subpopulations, with the latter shown to inhibit T cell proliferation and activation in vitro and their relative abundance drastically reduced during tumor development 13. Single-cell analysis of the stromal compartment in human TNBC patients revealed that high-inflammatory CAF signatures are associated with T cell dysfunction and poor survival in TNBC 79 (Fig.4b). In addition to experimental mouse models and interspecies differences, cancer type and mutational landscape may also influence CAF activity. For example, PDPNHigh CAFs identified in a TNBC mouse model were significantly less abundant in BRCA-mutated compared to BRCA WT human breast cancer 13. Moreover, a shift of CAF function towards an immunosuppressive phenotype in response to genetic changes in the cancer cells was also observed in the MMTV-PyMT mouse model following mammary-restricted expression of the transcription factor ELF5. This transcription factor drives lactation during pregnancy and is linked to a more aggressive phenotype in pregnancy-associated breast cancer. Work in WT or Elf5-induced MMTV-PyMT mice suggested a shift towards an inflammatory/immunoregulatory CAF profile resembling their role in mammary gland involution 80.
A separate study identified four CAF subsets (CAF-S1-S4, based on expression of CD29, FAP, FSP1, αSMA, PDGFRβ, and CAV1) in human breast cancer, of which CAF-S1 demonstrated immunomodulatory activities including recruitment of CD4+CD25+ T cells through CXCL12 and promotion of Treg differentiation 26. Subsequent scRNA-seq analysis conducted on the CAF-S1 subset highlighted eight subpopulations, three of which resembled the iCAFs discovered in PDAC, with the rest resembling the myCAF subset 78 (Fig.4b). The three iCAF-like subsets were enriched in patients with TNBC compared to luminal breast cancer, which were more enriched in myCAFs, highlighting the specificity of CAF heterogeneity with disease subtype.
Ovary
Most single-cell studies in ovarian cancer described subsets of CAFs with immune regulatory and/or inflammatory phenotypes. Fibroblasts isolated from patients with ovarian tumors expressed inflammatory factors such as CXCL12/14, IL-6, IL-1, CCL2 56, and complement factors such as C3, CFB, and SERPING1, suggesting that this subtype promotes cancer progression through inflammation, immune and complement regulation. Such inflammatory modules were also shown in a different scRNA-seq study conducted on human-derived ovarian tumors57. However, as emphasized above, the context and stage of disease affect CAF activity. Thus, in this study CAFs in metastatic niches were shown to secrete inflammatory mediators contrary to primary CAFs57. Similar findings and two CAF subtypes, termed CAF-S1 and CAF-S4, were reported in a study based on FACS isolation of CAFs from patients with mesenchymal high-grade serous ovarian cancer (HGSOC)81. The CAF-S1 subset, marked by high expression of CD29, FAP, and FSP1, was associated with immunosuppressive functions by increasing attraction, survival, and differentiation of CD25+FOXP3+ T lymphocytes, via its expression of CXCL12β and was associated with worse prognosis (Fig.4e). Single-cell analysis of ascites samples from patients with HGSOC also identified several inflammatory and immunoregulatory CAF populations marked by expression of complement factors (C1QA/B/C and CFB), chemokines (CXCL1/2/10/12), and cytokines (IL-6 and IL-10) 82. Therefore, there is a conserved immune-regulatory network in HGSOC-associated CAFs that may be targeted for cancer inhibition and immune system reinvigoration.
Immunoregulatory CAF subsets in other organs
Studies employing scRNA-seq in lung malignancies have characterized lung fibroblasts in pre-invasive lesions 53 and NSCLC 36,37,83,84. The identified CAFs were predominantly myofibroblast-like, whereas immunoregulatory subtypes were not as clearly defined as in other organs. Single-cell analysis of subsolid nodules (SSN) from patients with early-stage lung adenocarcinoma revealed enrichment of immunomodulatory fibroblasts, characterized by TNF and IL-6-JAK/STAT signaling and enriched for CXCL12/14, which decrease in lung adenocarcinoma and metastases 84. Enrichment of specific CAF subpopulations was shown in patients with squamous cell carcinoma of the lung, relative to adenocarcinoma, and particular subclusters were inversely correlated with patient survival depending on disease type38. Whether early immune-modulatory programs in SSN-associated fibroblasts contribute to lung adenocarcinoma progression remains to be determined. The inflammatory state of lung CAFs may also be regulated by EMT of resident epithelial cells: a mesenchymal program in epithelial lung adenocarcinoma cells from a transgenic mouse model was shown to favor enrichment of inflammatory CAFs in single-cell analysis 83, confirming the effects of carcinoma cell states on stromal heterogeneity.
In prostate cancer, although most scRNA-seq studies of human or mouse prostate tumor samples demonstrated CAF heterogeneity, one study identified mostly myofibroblastic-like signatures (human) 39, whereas others also identified immune-associated subsets (human and mouse)85,86, possibly reflecting species-dependent signatures. A study of mouse prostate stromal cells found that Sca-1+CD90Low fibroblasts express ECM-related genes such as Fn1 but also cytokines, chemokines and complement components (Ccl2, Ccl7, Ccl11, Cxcl1, Cxcl2, and C3) 85, whereas a study of cultured CAFs from human prostate cancer tissues showed a role for inflammatory prostate CAF subsets in recruiting macrophages 86.
scRNA-seq studies in liver metastases and ICC revealed fibroblast inflammatory and immune regulatory activities including M2 polarization of macrophages, activation of Tregs, and reduced activity of CD8+ T cells, natural killer (NK) and dendritic cells 87. 6 CAF subpopulations were reported in human ICC54. The major subset among them were CD146+ vascular CAFs (vCAFs), expressing inflammatory mediators such as IL-6 and CCL8, with other subsets expressing high levels of CXCL1 and complement factors C3 and C7. Ligand-receptor interaction analysis indicated that vCAFs may interact with carcinoma cells through IL-6/IL-6R, promoting tumor growth and stemness. An HGF signaling hub characterized by expression of HGF in inflammatory CAFs and its receptor MET in cancer cells, was identified in multiple liver cancer studies 55,88,89 and may be conserved in mice and humans: scRNA-seq combined with ligand-receptor analysis in a mouse model of ICC demonstrated that the HGF-MET signaling axis promotes tumor growth and may also be relevant in patients 89. This signaling module may be part of a cooperative axis mediated through CAFs and scar-associated macrophages (SAMs) in ICC; single-cell combined with spatial analysis of patient samples with ICC and liver metastases demonstrated that HGF is expressed in both CAFs and SAMs and may interact with cancer cells expressing MET 88 (Fig. 4d). Thus, it is interesting to consider targeting specific inflammatory CAFs and their signaling hubs in ICC, including the IL-6/IL-6R and HGF-MET axes.
Antigen presenting CAFs
The ability to stimulate T cell activation is associated with MHC class II-expressing cells termed antigen-presenting cells (APCs). The classical/professional APCs are dendritic cells, macrophages and B cells. However, recent studies have identified additional cell types expressing MHC class II molecules which may therefore be capable of antigen presentation. These atypical APCs include mast cells, granulocytes, endothelial cells, epithelial cells and lymph node stromal cells90. Whether these cells can supply the three signals required for full activation of naïve T cells, namely antigen presentation, co-stimulation and regulatory cytokines, remains unclear 90. The identification and characterization of diverse APC types in the TME (Fig. 3c) may improve immunotherapeutic strategies for cancer treatment.
Pancreas
A subpopulation of CAFs expressing MHC class II related genes, was first described in the mouse KPC model and human PDAC tumors using scRNA-seq, RNA in situ hybridization, immunohistochemistry (IHC) and imaging mass cytometry 44 (Fig. 4a). These cells, termed apCAFs, were capable of partially activating CD4+ T cells in vitro by TCR ligation in an antigen-dependent manner. However, they expressed low levels of the CD80, CD86 and CD40 co-stimulatory molecules required for full T cell activation. Injection of a human PDAC cell line and human adipose-derived mesenchymal stem cells to immunodeficient mice also led to the differentiation of the latter into CD74+ and HLA-DRA+ apCAFs45. These cells were also found at late and invasive tumor stages in a tamoxifen-inducible mouse model of PDAC 71. In contrast, scRNA-seq on PDAC patient samples in another study did not identify a distinct apCAF subset 46,72. These discrepancies may be caused by technical differences in cell sorting markers chosen, single-cell preparation methods, the possible scarcity of this population, and CAF plasticity. A recent study reanalyzing published scRNA-seq datasets indicated that apCAFs may originate from mesothelial cells through the IL-1- and TGFβ-induced down-regulation of mesothelial features and upregulation of fibroblastic ones 11. A separate scRNA-seq analysis also reported transcriptional similarities between and clustering of apCAFs and mesothelial cells 50. Although in mice apCAFs formed a distinct transcriptional subset, in human tumors they may be admixed with other subpopulations such as iCAFs 44,50 and share the plasticity shown for other CAF subpopulations, as they were able to convert into myCAFs under certain culture conditions 44.
Breast
apCAFs were described in a scRNA-seq study of a mouse model of triple-negative breast cancer, as a subset within a larger S100A4+ sCAF population13 (Fig.4b). A high ratio of sCAFs to the other dominant CAF population, PDPN+ pCAFs, correlated with BRCA mutations in the cancer cells and with improved disease outcome in patients with breast cancer, indicating that apCAFs may be tumor-repressive 13. Temporal analysis of CAFs during different stages of tumor progression demonstrated that apCAFs appeared predominantly at advanced stages of tumor progression and metastases 13. A different study identified apCAFs also in healthy mammary and pancreatic murine tissues, suggesting a role for them in tissue homeostasis 51.
apCAFs in other organs
scRNA-seq of samples from patients with ICC revealed a subpopulation expressing MHC class II-related genes such as CD74, HLA-DRA and HLA-DRB1 54. A recent study suggested that dense apCAF regions in human lung tumors define immunologically active regions with increased CD4+ T cell infiltration 91. Leveraging published scRNA-seq data 37 and a defined metric for assessing physiologically relevant MHC class II gene expression, this study defined a population of potential apCAFs in human lung cancer and in mice, and proposed alveolar epithelial cells as their potential origin.
Although a functional antigen presenting role has not described in all these cases, the multiple studies describing apCAFs in different tumor types and models suggest that this is a robust CAF subtype in carcinomas. More work is required to establish apCAFs as an independent CAF subpopulation, understand their origin, and define similarities and differences with professional APCs regarding their ability to mediate full activation of T cells.
Additional CAF subpopulations identified by scRNA-seq
Although the most prevalent CAF subpopulations identified are ECM-remodeling/myofibroblastic and immune regulatory ones, CAFs appear to be much more diverse, based on their origins and functions 92. In this section, we discuss unique and rare CAF subpopulations identified in various tissues by scRNA-seq approaches, and associated with less known fibroblast functions.
Vascular CAFs
scRNA-seq of CAFs from the MMTV-PyMT transgenic mouse model of breast cancer revealed a subpopulation enriched for expression of angiogenic genes, which was associated with blood vessels and designated vascular CAFs (vCAFs) 30. A study in human ICC classified the majority of CAFs as vCAFs, which highly expressed microvasculature-associated genes (e.g. CD146), as well as inflammatory chemokines, such as CCL8 and IL-6 (Fig 4d). IHC and multiplex immunofluorescence staining revealed that CD146+ vCAFs mainly localized to the tumor core and microvasculature, suggesting extensive interactions with cancer cells presumably via the IL-6/IL-6R axis 54. A strong connection between CAFs and the vasculature was also demonstrated through scRNA-seq on tumor samples from an orthotopic ICC mouse model cultured in vitro with a neutralizing antibody against placental growth factor (PIGF), a member of the vascular endothelial growth factor (VEGF) family, which indicated that CAFs were the major cell population affected93.
Metabolic CAFs
scRNA-seq in tissues from PDAC patients with different degrees of desmoplasia identified a novel subtype of CAFs with a highly activated metabolic state, termed metabolic CAFs (meCAFs), which were found predominantly in loose-type (low desmoplasia) PDAC and utilized glycolysis as a major metabolic mode. Multiplex immunofluorescence staining of PLA2G2A+ meCAFs on matched samples confirmed their distinct identity. meCAFs were strongly correlated with the presence of immune cells, and thought to communicate with T cells and macrophages. Although PDAC patients with abundant meCAFs had a higher risk of metastasis, they had a better immunotherapy response when treated with PD-1 blockade46.
CD63+ CAFs
scRNA-seq in the MMTV-PyMT mouse model identified CD63+ CAFs predominantly in advanced stages of breast carcinoma (Fig. 4b) 94. This subset promoted breast cancer resistance to tamoxifen by secreting exosomal miR-22 thereby downregulating estrogen receptor-α (ERα) and PTEN in cancer cells. These CAFs were also found in ERα low/negative human primary breast cancer. Co-culture of human primary breast CD63+ CAFs with ERα+ human breast cancer cells endowed tamoxifen resistance, and treatment with an anti-CD63 neutralizing antibody enhanced tamoxifen sensitivity in breast tumor-bearing mice 94. CD63+ CAFs were also identified in scRNA-seq of cultured CAFs derived from human prostate cancer tissue, suggesting that they are not limited to breast cancer 86.
Rare pericryptal Ptgs2-expressing fibroblasts
scRNA-seq analysis of the mouse intestinal mesenchyme unveiled a subset of PdgfrαLow fibroblasts expressing high levels of Ptgs2 (COX2) 95. These cells, termed rare pericryptal Ptgs2-expressing fibroblasts (RPPFs), were found also in healthy human colons, near the stem cell zone at the bottom of the crypts, where intestinal tumors are primarily initiated 95. Genetic ablation of Ptgs2 in fibroblasts was sufficient to prevent tumor initiation in the ApcMin/+ and azoxymethane models of intestinal cancer. RPPFs were suggested to promote tumorigenesis by prostaglandin E2 (PGE2)-mediated expansion of colon stem-like cells 95. An additional scRNA-seq study on normal murine colon also revealed a subset of crypt-bottom fibroblasts (CBFs) defined by low PDGFRα and high CD34 and CD90 expression 96. CBFs maintained intestinal stem cell proliferation through expression of canonical Wnt ligands (Wnt2 and Wnt2b), Wnt signaling potentiators (Rspo3), and Bmp antagonists (Grem1) 96. It would be interesting to study whether RPPFs and CBFs share developmental trajectories in the colonic crypts.
Are the different CAF subtypes universal?
The wealth of scRNA-seq profiling of CAFs in diverse tumor types, mouse models and human patients raises the question of whether CAF subtypes are universal. The studies discussed herein and summarized in Table 1 suggest that central features of different CAF subsets are conserved across organs, cancer subtypes, and species. Similar hallmark genes were identified in multiple cancer types, with IL-6, Ly6C and PDGFRα marking inflammatory CAFs; Cxcl12 marking immune-regulatory CAFs; MHC-II (H2-Aa, H2-Ab1, and CD74 in mice; HLA-DRA, HLA-DPA1, HLA-DQA, and CD74 in human) marking antigen-presenting CAFs; and ACTA2, TAGLN and POSTN marking myCAFs. Nevertheless, CAFs also possess organ-specific phenotypes, such as the HGF-MET signaling axis in the liver 89. The colon, in which a unique segregation of the mesenchyme supports epithelial differentiation, also has specialized CAF subpopulations, such as the RPPFs and CBFs that promote epithelial stemness 95. CAFs originating from bone marrow-derived mesenchymal cells appear to display similar phenotypes in organs such as the breast and pancreas, that can be recapitulated in ex-vivo co-culture experiments 6,23. These bone marrow-derived CAFs display inflammatory and specialized programs that promote tumor progression and metastatic dissemination through increased vascularization, growth, and migration. Such subsets can be identified in the TME through classical MSC markers (e.g. CD44 and CD73) and additional markers that are upregulated in the TME, such as clusterin 6,13,23.
CAF composition may be heterogeneous even in different cancer subtypes within the same organ. Breast cancer luminal A tumors were found to contain a larger CAF-S2 subpopulation, HER2+ tumors promoted accumulation of CAF-S4, and TNBC tumors were enriched in either CAF-S1 or S426. Similarly, in NSCLC, distinct enrichment of CAF subpopulations was observed in patients with squamous cell carcinoma compared to adenocarcinoma, which may have varying effects on patient outcome38. A recent cross-tissue analysis of fibroblasts from normal and perturbed disease states in human and mouse suggested that universal fibroblast states exist in normal tissues, serving as reservoirs that yield specialized and activated fibroblasts in disease 97. Both the universal and the specialized CAF subtypes were similar between human and mouse.
An important element in addressing the universality of CAF subsets is their origin, which also presents a technical challenge when comparing different studies (Fig.1). scRNA-seq has provided a wealth of data, revealing CAF clusters expressing genes that are classically used as markers of immune cells such as MHC-II, or pericytes such as RGS5. Another confounding issue is the possibility of co-clustering of fibroblasts with cancer cells that have undergone EMT and therefore share mesenchymal markers. Several validation approaches can be conducted to avoid such misclassification, including injection of unlabeled cancer cells to reporter mouse models 39 (or labeled cancer cells into unlabeled mice), restricted expression of known oncogene mutations to tumor clusters 55, detection of large-scale copy number variations 80, and determination of cellular proliferation status 70. The overlap of CAF features with those other cell types raises the question of how CAFs should be designated and whether they should be defined by origin or function. CAF plasticity also bears implications for classification and therapy. In vitro, iCAFs, myCAFs and apCAFs were shown to interconvert depending on culture conditions. As RNA-seq provides a snapshot of the transcriptome, it is difficult to ascertain whether such data provide information about a continuous state transition of cells, or a fixed cell type identity that undertakes a specific task. Time-resolved experiments, employing lineage tracing and pseudotime inference, and careful assessment of the potential effects of in vitro growth conditions can provide critical information about CAF subtype trajectories in the TME. For example, scRNA-seq in conjunction with pseudotime inference was used to identify two possible universal fibroblast populations marked by Pi16+ and Col15a1+ 97. A similar approach in a mouse model of TNBC inferred lineage trajectories during cancer development 13. Both the potential origins and the plasticity of CAF subsets should be taken into consideration when categorizing CAFs from single cell data, and when developing potential treatments aimed at a specific subtype.
Establishing comprehensive nomenclature and annotation of CAF subpopulations is critical. The single-cell studies highlighted herein support the notion of 3 major CAF subsets that can be parsed by myofibroblastic, inflammatory/immune regulatory, and antigen-presenting activities. These then further diverge into subpopulations with distinct markers that may differ between tumor types, organs and species. We therefore propose to hierarchically classify CAFs into one of these broad populations, and annotate specialized functions through specific markers. For example, PDPN+Ly6c+ immune regulatory CAFs or PDPN+LRRC15+ myofibroblastic CAFs are annotations that provide both a general understanding of CAF tasks, as well as a precise and potentially targetable moiety.
Therapeutic approaches and future perspectives
Much work is still needed to map the full landscape of CAF subpopulations across human cancers. Better understanding of CAF plasticity, origin, and interactions is required, especially in carcinomas other than pancreas and breast. Nevertheless, with the wealth of data already accumulated, the field is ready to move to translating these emerging CAF atlases into therapeutic targets. Based on the insights detailed here, targeting specific signaling molecules and pathways, rather than a specific CAF subtype or cell of origin, may be a more viable therapeutic strategy, considering the plasticity and heterogeneity of the mesenchymal tumor-supporting milieu (Fig. 5). For example, TGFβ-driven LRRC15+ CAFs correlate with poor response to anti–PD-L1 therapy in PDAC 50. Reverting the LRRC15+phenotype 98 by combining anti-TGFβ therapies with anti-PD-1/PD-L1 treatment may have beneficial and additive effects. Such treatments were shown to revert the matrix remodeling transcriptional profile of CAFs in breast cancer 99. However, reversion of one pathway prevalent in a given CAF population (such as myCAFs) may promote dominance of a different CAF subtype that can hamper therapy. Indeed, inhibition of Hedgehog signaling was shown to impair myCAF activity and tumor growth, while promoting inflammatory CAF accumulation and an immune-suppressive TME 76. Recent findings suggest, however, a limited plasticity in some CAF lineages which may serve as stable therapeutic targets, such as CD105+ pancreatic CAFs 77 and pancreatic stellate cell-derived CAFs which may be identified by a combination of αSMA and TIE1 100. A common hallmark of inflammatory CAFs across tumor types is upregulation of IL6/IL6R signaling. Anti-IL6 therapies such as Siltuximab and Tocilizumab are expected to target these CAFs 101,102. Given that immunoregulatory CAFs can also drive JAK/STAT signaling in cancer cells 25, inhibition of JAK/STAT signaling may prove to be a promising arm of a combined therapeutic approach 82. Another interesting therapeutic approach is targeting of CAF-derived ECM modifications. Immunotherapies have shown limited efficacy in highly stromal tumors, to which immune cells cannot infiltrate 103–105. ECM normalization by modulating CAF activity, may help restrict tumor growth and enhance the response to immunotherapy.
Figure 5. Examples of potential CAF targeting.
a targeting LRCC15+ CAFs with antibodies/antibody drug conjugates such as ABBV-085, in conjunction with conventional checkpoint inhibitor therapy (anti-PD-L1) may synergize to abolish their suppressive effect during immunotherapy 50,98. b Targeting hedgehog signaling pathways via administration of SMO antagonists such as LDE225 can prevent their pro-tumorigenic activities 76. Since such treatment promotes accumulation of immune suppressive inflammatory CAFs, dual treatment with checkpoint inhibitors may provide important additive effects. c Enhancing the activity of antigen presenting CAFs and their ability to recruit CD4+ T cells 91 could promote anti-tumor immune activity. d Targeting inflammatory CAFs with IL1R antagonists (e.g. anakinra) and anti-TNFα antibodies could inhibit their immune suppressive effects in the TME 75. The figure was created with BioRender.com.
Deeper understanding of CAF plasticity and complexities in the coming years will reveal more therapeutic options that will instruct novel CAF targeting strategies to formulate better cancer treatments.
Acknowledgements
ABS is supported by the Israel Cancer Research Fund (ICRF). NE is supported by the Department of Defense (DoD), Worldwide Cancer Research, Israel Science Foundation (ISF), and ICRF. RSS is supported by the ISF, ERC starting grant 754320, the ICRF, the Laura Gurwin Flug Family Fund, and the estate of David Levinson. RSS is the incumbent of the Ernst and Kaethe Ascher Career Development Chair in Life Sciences.
Footnotes
Competing interest statement
The authors declare no competing interests.
Data availability statement
All data discussed in the manuscript is available through the referenced articles.
References
- 1.Liu L, et al. Stromal Myofibroblasts Are Associated with Poor Prognosis in Solid Cancers: A Meta-Analysis of Published Studies. Plos One. 2016;11:e0159947. doi: 10.1371/journal.pone.0159947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharon Y, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–973. doi: 10.1158/0008-5472.CAN-14-1990. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 5.Quante M, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272.:S1535-6108(11)00042-0 [pii] doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raz Y, et al. Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med. 2018;215:3075–3093. doi: 10.1084/jem.20180818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidd S, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 9.Rynne-Vidal A, Jimenez-Heffernan JA, Fernandez-Chacon C, Lopez-Cabrera M, Sandoval P. The Mesothelial Origin of Carcinoma Associated-Fibroblasts in Peritoneal Metastasis. Cancers (Basel) 2015;7:1994–2011. doi: 10.3390/cancers7040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandoval P, et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J Pathol. 2013;231:517–531. doi: 10.1002/path.4281. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Wang Z, Zhang Y, Brekken RA. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. bioRxiv. 2021:2021.2002.2004.429827. doi: 10.1101/2021.02.04.429827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murgai M, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med. 2017;23:1176–1190. doi: 10.1038/nm.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman G, et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4+ and PDPN+ CAFs to clinical outcome. Nature Cancer. 2020;1:692–708. doi: 10.1038/s43018-020-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30:1002–1019. doi: 10.1101/gad.279737.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi-Galibov O, et al. Heat Shock Factor 1-dependent extracellular matrix remodeling mediates the transition from chronic intestinal inflammation to colon cancer. Nature Communications. 2020;11:6245. doi: 10.1038/s41467-020-20054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander J, Cukierman E. Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Curr Opin Cell Biol. 2016;42:80–93. doi: 10.1016/j.ceb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteran L, Erez N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front Immunol. 2019;10:1835. doi: 10.3389/fimmu.2019.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozdemir BC, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhim AD, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 21.Cox TR. The matrix in cancer. Nat Rev Cancer. 2021;21:217–238. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 22.Neuzillet C, et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248:51–65. doi: 10.1002/path.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waghray M, et al. GM-CSF Mediates Mesenchymal-Epithelial Cross-talk in Pancreatic Cancer. Cancer Discov. 2016;6:886–899. doi: 10.1158/2159-8290.CD-15-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonneau C, et al. A subset of activated fibroblasts is associated with distant relapse in early luminal breast cancer. Breast Cancer Res. 2020;22:76. doi: 10.1186/s13058-020-01311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohlund D, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa A, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell. 2018;33:463–479.:e410. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Pelon F, et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat Commun. 2020;11:404. doi: 10.1038/s41467-019-14134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su S, et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell. 2018;172:841–856.:e816. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Tang F, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 30.Bartoschek M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformaticspipelines. Experimental & Molecular Medicine. 2018;50:1–14. doi: 10.1038/s12276-018-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moncada R, et al. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nature Biotechnology. 2020;38:333–342. doi: 10.1038/s41587-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 33.Waise S, et al. An optimised tissue disaggregation and data processing pipeline for characterising fibroblast phenotypes using single-cell RNA sequencing. Scientific Reports. 2019;9:9580. doi: 10.1038/s41598-019-45842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deasy SK, Erez N. A glitch in the matrix: organ-specific matrisomes in metastatic niches. Trends Cell Biol. 2021 doi: 10.1016/j.tcb.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Darby IA, Zakuan N, Billet F, Desmouliere A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci. 2016;73:1145–1157. doi: 10.1007/s00018-015-2110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim N, et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat Commun. 2020;11:2285. doi: 10.1038/s41467-020-16164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambrechts D, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 38.Davidson S, et al. Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. Cell Rep. 2020;31:107628. doi: 10.1016/j.celrep.2020.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S, et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat Cell Biol. 2021;23:87–98. doi: 10.1038/s41556-020-00613-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, et al. Author Correction: Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat Commun. 2021;12:2567. doi: 10.1038/s41467-021-23119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet. 2017;49:708–718. doi: 10.1038/ng.3818. [DOI] [PubMed] [Google Scholar]
- 42.Guerrero-Juarez CF, et al. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun. 2019;10:650. doi: 10.1038/s41467-018-08247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannon A, et al. Desmoplasia in pancreatic ductal adenocarcinoma: insight into pathological function and therapeutic potential. Genes Cancer. 2018;9:78–86. doi: 10.18632/genesandcancer.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elyada E, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki Y, et al. Adipose-derived mesenchymal stem cells differentiate into heterogeneous cancer-associated fibroblasts in a stroma-rich xenograft model. Sci Rep. 2021;11:4690. doi: 10.1038/s41598-021-84058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, et al. Single-cell analysis of pancreatic ductal adenocarcinoma identifies a novel fibroblast subtype associated with poor prognosis but better immunotherapy response. Cell Discovery. 2021;7:36. doi: 10.1038/s41421-021-00271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng J, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29:725–738. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernard V, et al. Single-Cell Transcriptomics of Pancreatic Cancer Precursors Demonstrates Epithelial and Microenvironmental Heterogeneity as an Early Event in Neoplastic Progression. Clin Cancer Res. 2019;25:2194–2205. doi: 10.1158/1078-0432.CCR-18-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosein AN, et al. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight. 2019;5 doi: 10.1172/jci.insight.129212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dominguez CX, et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15(+) Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020;10:232–253. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 51.Sebastian A, et al. Single-Cell Transcriptomic Analysis of Tumor-Derived Fibroblasts and Normal Tissue-Resident Fibroblasts Reveals Fibroblast Heterogeneity in Breast Cancer. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng S, Hebert LL, Eschbacher JM, Kim S. Single-Cell RNA Sequencing of a Postmenopausal Normal Breast Tissue Identifies Multiple Cell Types That Contribute to Breast Cancer. Cancers (Basel) 2020;12 doi: 10.3390/cancers12123639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu T, et al. Single-cell transcriptome atlas of lung adenocarcinoma featured with ground glass nodules. Cell Discov. 2020;6:69. doi: 10.1038/s41421-020-00200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M, et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73:1118–1130. doi: 10.1016/j.jhep.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 55.Bhattacharjee S, et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J Clin Invest. 2021;131 doi: 10.1172/JCI146987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hornburg M, et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell. 2021;39:928–944.:e926. doi: 10.1016/j.ccell.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Shih AJ, et al. Identification of grade and origin specific cell populations in serous epithelial ovarian cancer by single cell RNA-seq. Plos One. 2018;13:e0206785. doi: 10.1371/journal.pone.0206785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z, et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat Commun. 2020;11:5077. doi: 10.1038/s41467-020-18916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar V, et al. Single-Cell Atlas of Lineage States, Tumor Microenvironment, and Subtype-Specific Expression Programs in Gastric Cancer. Cancer Discov. 2021 doi: 10.1158/2159-8290.CD-21-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grunberg N, et al. Cancer-Associated Fibroblasts Promote Aggressive Gastric Cancer Phenotypes via Heat Shock Factor 1-Mediated Secretion of Extracellular Vesicles. Cancer Res. 2021;81:1639–1653. doi: 10.1158/0008-5472.CAN-20-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farbehi N, et al. Single-cell expression profiling reveals dynamic flux of cardiac stromal vascular and immune cells in health and injury. Elife. 2019;8 doi: 10.7554/eLife.43882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phan QM, Sinha S, Biernaskie J, Driskell RR. Single-cell transcriptomic analysis of small and large wounds reveals the distinct spatial organization of regenerative fibroblasts. Experimental Dermatology. 2021;30:92–101. doi: 10.1111/exd.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deng C-C, et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nature Communications. 2021;12:3709. doi: 10.1038/s41467-021-24110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie T, et al. Single-Cell Deconvolution of Fibroblast Heterogeneity in Mouse Pulmonary Fibrosis. Cell Rep. 2018;22:3625–3640. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krenkel O, Hundertmark J, Ritz TP, Weiskirchen R, Tacke F. Single Cell RNA Sequencing Identifies Subsets of Hepatic Stellate Cells and Myofibroblasts in Liver Fibrosis. Cells. 2019;8 doi: 10.3390/cells8050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smillie CS, et al. Intra- and Inter-cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell. 2019;178:714–730.:e722. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin JC, et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell. 2019;178:1493–1508.:e1420. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calon A, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320–329. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 69.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147.:S1535-6108(10)00004-8 [pii] doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 70.Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev. 2021;101:147–176. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schlesinger Y, et al. Single-cell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity. Nat Commun. 2020;11:4516. doi: 10.1038/s41467-020-18207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin W, et al. Single-cell transcriptome analysis of tumor and stromal compartments of pancreatic ductal adenocarcinoma primary tumors and metastatic lesions. Genome Med. 2020;12:80. doi: 10.1186/s13073-020-00776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen K, et al. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine. 2021;66:103315. doi: 10.1016/j.ebiom.2021.103315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127:780–789. doi: 10.1172/JCI90962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Biffi G, et al. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9:282–301. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steele NG, et al. Inhibition of Hedgehog Signaling Alters Fibroblast Composition in Pancreatic Cancer. Clin Cancer Res. 2021;27:2023–2037. doi: 10.1158/1078-0432.CCR-20-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hutton C, et al. Single-cell analysis defines a pancreatic fibroblast lineage that supports anti-tumor immunity. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kieffer Y, et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020;10:1330–1351. doi: 10.1158/2159-8290.CD-19-1384. [DOI] [PubMed] [Google Scholar]
- 79.Wu SZ, et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020;39:e104063. doi: 10.15252/embj.2019104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valdes-Mora F, et al. Single-cell transcriptomics reveals involution mimicry during the specification of the basal breast cancer subtype. Cell Rep. 2021;35:108945. doi: 10.1016/j.celrep.2021.108945. [DOI] [PubMed] [Google Scholar]
- 81.Givel AM, et al. miR200-regulated CXCL12beta promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun. 2018;9:1056. doi: 10.1038/s41467-018-03348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Izar B, et al. A single-cell landscape of high-grade serous ovarian cancer. Nature Medicine. 2020;26:1271–1279. doi: 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bota-Rabassedas N, et al. Contextual cues from cancer cells govern cancer-associated fibroblast heterogeneity. Cell Rep. 2021;35:109009. doi: 10.1016/j.celrep.2021.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xing X, et al. Decoding the multicellular ecosystem of lung adenocarcinoma manifested as pulmonary subsolid nodules by single-cell RNA sequencing. Sci Adv. 2021;7 doi: 10.1126/sciadv.abd9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwon OJ, et al. Functional Heterogeneity of Mouse Prostate Stromal Cells Revealed by Single-Cell RNA-Seq. iScience. 2019;13:328–338. doi: 10.1016/j.isci.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vickman RE, et al. Heterogeneity of human prostate carcinoma-associated fibroblasts implicates a role for subpopulations in myeloid cell recruitment. The Prostate. 2020;80:173–185. doi: 10.1002/pros.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banales JM, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Massalha H, et al. A single cell atlas of the human liver tumor microenvironment. Mol Syst Biol. 2020;16:e9682. doi: 10.15252/msb.20209682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Affo S, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866–882.:e811. doi: 10.1016/j.ccell.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14:719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 91.Kerdidani D, et al. Tumor MHCII immunity requires in situ antigen presentation by cancer-associated fibroblasts. bioRxiv. 2020:2020.2003.2024.005355. doi: 10.1101/2020.03.24.005355. [DOI] [Google Scholar]
- 92.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Disease Models & Mechanisms. 2018;11 doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aoki S, et al. Placental growth factor promotes tumour desmoplasia and treatment resistance in intrahepatic cholangiocarcinoma. Gut. 2021:gutjnl-2020-322493. doi: 10.1136/gutjnl-2020-322493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao Y, et al. CD63(+) Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal miR-22. Adv Sci (Weinh) 2020;7:2002518. doi: 10.1002/advs.202002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roulis M, et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 2020;580:524–529. doi: 10.1038/s41586-020-2166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brugger MD, Valenta T, Fazilaty H, Hausmann G, Basler K. Distinct populations of crypt-associated fibroblasts act as signaling hubs to control colon homeostasis. PLoS Biol. 2020;18:e3001032. doi: 10.1371/journal.pbio.3001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buechler MB, et al. Cross-tissue organization of the fibroblast lineage. Nature. 2021;593:575–579. doi: 10.1038/s41586-021-03549-5. [DOI] [PubMed] [Google Scholar]
- 98.Purcell JW, et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody-Drug Conjugates. Cancer Res. 2018;78:4059–4072. doi: 10.1158/0008-5472.CAN-18-0327. [DOI] [PubMed] [Google Scholar]
- 99.Lim YW, et al. Single-cell transcriptomics reveals the effect of PD-L1/TGF-β blockade on the tumor microenvironment. BMC Biology. 2021;19:107. doi: 10.1186/s12915-021-01034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Helms EJ, et al. Mesenchymal Lineage Heterogeneity Underlies Nonredundant Functions of Pancreatic Cancer-Associated Fibroblasts. Cancer Discov. 2022;12:484–501. doi: 10.1158/2159-8290.CD-21-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karakasheva TA, et al. IL-6 Mediates Cross-Talk between Tumor Cells and Activated Fibroblasts in the Tumor Microenvironment. Cancer Research. 2018;78:4957–4970. doi: 10.1158/0008-5472.Can-17-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang W, et al. Stromal induction of BRD4 phosphorylation Results in Chromatin Remodeling and BET inhibitor Resistance in Colorectal Cancer. Nature Communications. 2021;12:4441. doi: 10.1038/s41467-021-24687-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herting CJ, Karpovsky I, Lesinski GB. The tumor microenvironment in pancreatic ductal adenocarcinoma: current perspectives and future directions. Cancer Metastasis Rev. 2021;40:675–689. doi: 10.1007/s10555-021-09988-w. [DOI] [PubMed] [Google Scholar]
- 104.Gorchs L, Kaipe H. Interactions between Cancer-Associated Fibroblasts and T Cells in the Pancreatic Tumor Microenvironment and the Role of Chemokines. Cancers (Basel) 2021;13 doi: 10.3390/cancers13122995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Norton J, Foster D, Chinta M, Titan A, Longaker M. Pancreatic Cancer Associated Fibroblasts (CAF): Under-Explored Target for Pancreatic Cancer Treatment. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data discussed in the manuscript is available through the referenced articles.