Abstract
Chemokines are small secretory chemotactic cytokines that control the directed migration of immune cells. Chemokines are involved in both anti-and pro-tumorigenic immune responses. Accumulating evidence suggests that the balance between these responses is influenced by several factors such as the stage of tumorigenesis, immune cell activation, recruitment of immune activating or immunosuppressive cells in the tumor microenvironment (TME), and chemokine receptor expression on effector and regulatory target cells. Cancer cells engage in a complex network with their TME components via several factors including growth factors, cytokines and chemokines that are critical for the growth of primary tumor and metastasis. However, chemokines show a multifaceted role in tumor progression including maintenance of stem-like properties, tumor cell proliferation/survival/senescence, angiogenesis, and metastasis. The heterogeneity of solid tumors in primary and metastatic cancers presents a challenge to the development of successful cancer therapy. Despite extensive research on how solid tumors escape immune cell-mediated anti-tumor response, finding an effective therapy for metastatic cancer still remains a challenge. This review discusses the multifarious roles of chemokines in solid tumors including various chemokine signaling pathways such as CXCL8-CXCR1/2, CXCL9, 10, 11-CXCR3, CXCR4-CXCL12, CCL(X)-CCR(X) in primary and metastatic cancers. We further discuss the novel therapeutic approaches that have been developed by major breakthroughs in chemokine research to treat cancer patients by the strategic blockade/activation of these signaling axes alone or in combination with immunotherapies.
Keywords: Solid tumor, Chemokine, Proliferation, Metastasis, Cancer stem cell
1. Introduction
Chemokines represent a subset of chemoattractant cytokines that bring about the directed migration of immune cells. These include a group of low molecular weight (8–10 kDa) inducible proteins that are triggered by inflammatory cytokines, growth factors and pathogenic stimuli. Chemokines regulate immune cell trafficking by binding to and activating specific seven-transmembrane G protein–coupled receptors (GPCRs) at their N terminal. This causes the phosphorylation of serine/threonine residues at the C-terminus and conformational changes in the GPCRs that lead to the activation of the heterotrimeric G protein complex attached to the intracellular receptor [1]. Chemokines cause the directed movement of cells expressing a specific chemokine receptor, along a chemical gradient of ligand termed as the ‘chemokine gradient’, facilitating cell movement towards the high local concentration of chemokines. The chemokine proteins are divided into four highly conserved groups viz. C, CC, CXC, and CX3C, depending on the relative position of the first two conserved cysteines that are adjacent to the amino terminus. The corresponding XCR, CCR, CXCR, and CX3CR receptors of the GPCR family effectuate the functions of these chemokines in target cells. More than 50 chemokines have been discovered until now and nearly 18 seven-transmembrane-domain chemokine receptors are known in humans. Over 40 chemokine ligands are known and these are denoted by the letter ‘L’ followed by a number. Generally, the chemokine receptors are known to recognize more than one type of chemokine, but six of these receptors including CCR6, CCR9, CXCR4, CXCR5, CXCR6, and CX3CR1 respond to only one chemokine.
Chemokine signaling activates the expression of the genes that are involved in cell motility, invasion, survival, and interactions with the extracellular matrix (ECM). Although chemokines emerged as key regulators of leukocyte migration and chemokine receptors were originally identified on the leukocytes, functional chemokine receptors were also found in endothelial cells and epithelial cells showing malignant transformation [2]. Chemokines are involved in many biological processes, including embryonic development, wound healing, angiogenesis, T-helper (Th)1/Th2 development, leukocyte homeostasis, lymphatic organ development, and immune system homeostasis. The aberrant expression of chemokines/chemokine receptors has been linked to various human diseases such as autoimmune, inflammatory disorders and solid tumors [3,4].
Cancer is one of the leading causes of death worldwide, accounting for nearly 10 million deaths in 2020 [5]. Although cancer refers to a heterogeneous group of diseases, one aspect that unites them is the acquirement of the host cell regulatory machinery that executes growth factor synthesis and signaling, to sustain their own growth, proliferation, spread and survival beyond natural limits [6]. Tumors are not simple masses of cancerous cells, most solid tumors of the epithelial, mesothelial or hematopoietic origin contain both cancerous cells as well as the non-malignant stromal cells. These stromal cells predominantly include the lymphocytes, macrophages, as well as endothelial cells and fibroblasts. Eosinophils, B cells, granulocytes, and natural-killer (NK) cells are also present in some tumor types. The stromal population, therefore, contain many leukocytes that constitute the leukocyte infiltrate. The immune cells, endothelial cells as well as the tumor cells express chemokine receptors and are responsive to chemokine gradients. Tumor cells secrete and respond to these chemokines to modulate tumor growth and progression by increasing the recruitment of proinflammatory cells and endothelial cells, disrupting immune surveillance and manipulating the leukocyte infiltrate along with the prevention of apoptosis in cancer cell [7].
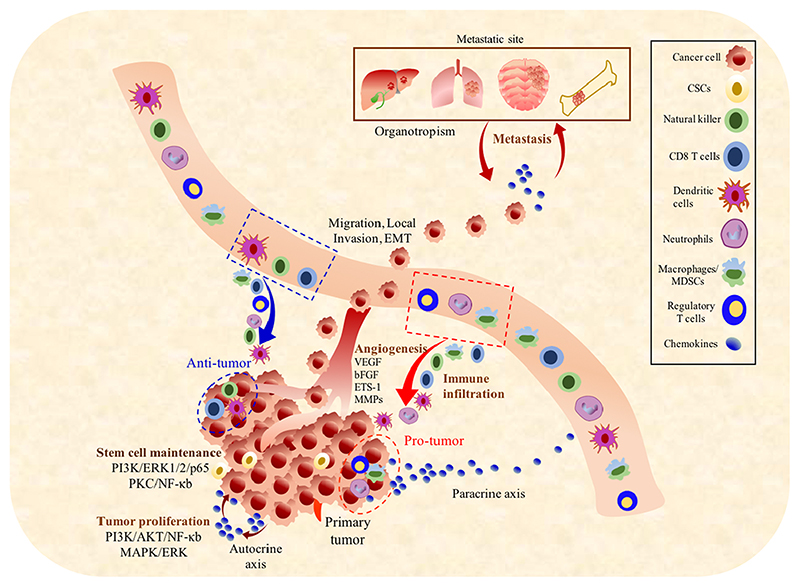
Chemokines regulate immune cell infiltration and shape the tumor microenvironment (TME) to modulate tumor progression via protumoral or anti-tumoral effects and act as a connection between the tumor cells and the TME. In the primary TME, chemokines mediate the stromal to epithelial interactions to control tumor growth and invasion. A plethora of literature demonstrates that the tumor-associated chemokines control leukocyte infiltration into the tumor, modulate immune response of tumor cells (protumorigenic vs antitumorigenic), regulate angiogenesis, act as growth factors (autocrine or paracrine), control the movement of tumor cells and are involved in the maintenance of cancer stem cells (CSCs) and (Fig. 1) [8]. Tumor heterogeneity (intra-tumor heterogeneity and inter-tumor heterogeneity) has been identified as a key factor to regulate tumorigenesis, distant metastasis, tumor recurrence, and resistance to anti-tumor therapy [9]. Recently, the CSC concept has been used to explain heterogeneity in solid tumors [10]. Chemokines are involved in each of these events and affect the metastatic potential as well as the site-specific spread of tumor cells [11]. It is crucial to understand the complex cross-talk between the different cell types within the TME and to study their biological effects that influence immune response, production and expression of markers, and sensitivity to therapeutic modalities.
Fig. 1. Role of chemokines in the progression of solid tumors.
Primary tumor cells secrete chemokines which regulate the infiltration of leukocytes in the TME. Chemokines elicit pro-tumor or anti-tumor immune response such as infiltration of CD8 T cells, dendritic cells and natural killer cells support antitumor immune response while macrophages/MDSCs, Treg cells and neutrophils promote tumor survival/ proliferation. Chemokines are also involved in the process of tumor proliferation, angiogenesis, metastasis at different sites of organ and growth/survival of the metastatic cells.
Most solid tumors display dominant immune infiltration. As compared to the migration of immune cells into lymphoid organs, immune infiltration into solid tumor tissues is more unpredictable as the solid tumors are heterogeneous, ectopic and lack a defined anatomy. It is imperative to understand the role of chemokines in solid tumors to improve the currently available therapeutic interventions. This review aims to explore the multifaceted role of chemokines in solid tumors including their effects on cell proliferation, tumor heterogeneity, stemness, senescence, angiogenesis and tumor metastasis. We further discuss the feasibility of manipulating chemokine networks to treat cancer in combination with other drugs or immunotherapies.
2. Chemokines and chemokine receptors
Chemokine receptors are a superfamily of GPCRs that control immune cell behavior and promote chemotaxis. The four classes (CC, CXC, CX3C, or XC) of the chemokine receptors are based on the chemokine/ ligand interaction motif to which they bind (Table 1). Activated chemokine receptors triggers a variety of effector pathways, including calcium signaling, generating an intracellular calcium ion flux and causing chemotaxis and cell trafficking to desired locations as well as activating phosphoinositide-3-kinase (PI3K), protein kinase C (PKC), phospholipase C (PLC)-b, and src family tyrosine kinases (SFK).
Table 1. Expression levels of chemokines and chemokine receptors in various solid tumors.
| Chemokine family | Subgroups | Ligands(L) | Chemokine receptors (R) | Elevated levels of Ligands/Receptors/L-R axis in solid tumors |
|---|---|---|---|---|
| CXC chemokines |
ELR + chemokines | CXCL1 | CXCR1, CXCR2 | CXCLI↑–Tumor growth: Pancreatic cancer, Breast cancer, Colon cancer and Melanoma; Angiogenesis: Renal Cancer [19–26] |
| CXCL2 | CXCR2 | CXCL2↑ – Tumor growth: Melanoma, and PDA [27,31] | ||
| CXCL3 | CXCR2 | CXCL3↑ – Tumor growth: Melanoma; Angiogenesis: Renal Cancer [12] | ||
| CXCL5 | CXCR2 | CXCL5↑ – Tumor growth: PDA and NSCLC; Angiogenesis: HCC and NSCLC; Metastasis: NSCLC [12,31] | ||
| CXCL6 | CXCR1, CXCR2 | CXCL6↑ – Tumor growth: Melanoma [12] | ||
| CXCL7 | CXCR1, CXCR2 | CXCL7↑ – Tumor growth: Clear cell renal cell carcinoma and breast cancer; Angiogenesis: Breast Cancer | ||
| CXCL8 | CXCR1, CXCR2[12] | CXCL8↑ – Tumor growth: Melanoma, head and neck cancer, PDA and NSCLC, Angiogenesis: Colon, prostate, ovarian, PDA, lung and renal carcinoma; Metastasis: Breast Cancer, prostate cancer [12,20–26, 32] | ||
| CXCL14 | Unknown | CXCR1↑ – Metastasis: Colon cancer [19] | ||
| ELR– chemokines | CXCL15 | Unknown | CXCR2↑ – Tumor growth: Melanoma; Angiogenesis: Renal Cancer; Metastasis: Breast Cancer, Colon cancer [20–26,28–30] CXCR1-CXCL8 axis↑ – Tumor growth: GBM [19] |
|
| CXCL4 | Unknown | CXCR3↑– Metastasis: Melanoma, Breast, Prostate, Renal, ovarian and colon cancer [38–44] | ||
| CXCL9 | CXCR3 | CXCR4↑– Tumor growth: Glioma, lymphoma, ovarian and pancreatic cancer [53–56] | ||
| CXCL10 | CXCR3, KSHV-GPCR | CXCR5↑– Metastasis: Colon cancer | ||
| CXCL11 | CXCR3 | CXCL9↑–Tumor growth: Melanoma [177] | ||
| CXCL12 | CXCR4 | CXCL10↑-Tumor growth: Multiple myeloma [177] | ||
| CXCL13 | CXCR5 | CXCL12-CXCR4 axis↑– Proliferation: Lymphoma, Breast, Ovarian, Pancreatic, and Prostate cancer, multiple myeloma and glioblastoma; Angiogenesis: Breast cancer; Metastasis: Breast, ovarian, prostate, pancreatic, esophageal, colorectal, melanoma, NSCLC, head and neck and bladder cancer, basal cell carcinoma, osteosarcoma, neuroblastoma, ALL, CML and GBM [53–61] | ||
| CXCL16 | CXCR6 | |||
| CC chemokines | CCL1 | CCR8 | CCR1↑– Anti-tumor: HCC; Metastasis: Ovarian, Breast and esophageal cancer [65,66] | |
| CCL2 | CCR2, CCR5, CCR10 | CCR2↑ – Tumor growth: Prostate cancer [79] | ||
| CCL3 | CCR1, CCR5 | CCL2↑ – Tumor growth: Ovarian, Breast and Pancreatic cancer, Metastasis: Breast, Ovarian and esophageal cancers [67,139–141] | ||
| CCL4 | CCR5, CCR10 | CCL2↓– Tumor growth, and CCL2↑ – Anti-tumor effect: Melanoma | ||
| CCL5 | CCR1, CCR3, CCR5, CCR10 | CCL3, CCL4, CCL8, CCL18 and CCL22↑ – Ovarian cancer [129] | ||
| CCL7 | CCR1, CCR2, CCR3, CCR5 | CCL5↑ – Tumor growth: Breast cancer; Antitumor effect: NSCLC, and Breast Cancer, CCR3↑ – Renal cell carcinoma [66,163] | ||
| CCL8 | CCR2, CCR3, CCR5 CCR1 |
CCR4 and CCR10↑– T-cell leukemia/lymphoma [79] | ||
| CCL11 | CCR3 | CCR5↑– Tumor growth & Metastasis: Breast Cancer [64] | ||
| CCL13 | CCR1, CCR2, CCR3, CCR5 | CCR6↑– Tumor growth: Pancreatic cancer and colon cancer [123] | ||
| CCL14 | CCR1 | CCR7↑– Tumor growth: Lung cancer and melanoma [179] | ||
| CCL15 | CCR1, CCR3 | CCR9↑– Tumor growth: Prostate cancer and melanoma [123] | ||
| CCL16 | CCR1 | CCR10 and CCL27↑– Tumor growth: Melanoma [123] | ||
| CCL17 | CCR4 | CCR7-CCL21 axis↑– Metastasis: Breast cancer, NSCLC, colorectal cancer, gastric cancer, murine B16 melanoma, esophageal cell carcinoma and head and neck cancer [17,68–73] | ||
| CCL18 | Unknown | CCL22 and CCL28↑– Tumor growth and angiogenesis: Ovarian Cancer [109] | ||
| CCL19 | CCR7 | CCL22↑– Metastasis: Breast Cancer and Tumor growth: esophageal squamous cell cancer [115,116] | ||
| CCL20 | CCR6 | CCL17↑–Tumor growth: Gastric cancer [117] | ||
| CCL21 | CCR7 | |||
| CCL22 | CCR4 | |||
| CCL23 | CCR1 | |||
| CCL24 | CCR3 | |||
| CCL25 | CCR9 | |||
| CCL26 | CCR3 | |||
| CCL27 | CCR3, CCR2, CCR10 | |||
| CCL28 | CCR10, CCR3 | |||
| C chemokines | XCL1 | XCR1 | XCR1↑– Anti-tumor effect: Breast cancer [64] CXC3CL1↑–Anti-tumor effect: ovarian, gastric, pancreatic and lung cancer [64] CX3CL1mRNA↑– Lung adenocarcinoma patient (survival) and lung squamous cell carcinoma patient (no survival benefit) |
|
| XCL2 | XCR1 | |||
| CX3C chemokines | CX3CL1 | CX3CR1 | CX3CL1-CX3CR1 axis↑– Anti-tumor effect: colon cancer and HCC CX3CL1-CX3CR1 axis↑– Protumor effect: Leukemia, Prostate, Gastric and pancreatic cancer; Angiogenesis: Breast, liver, lung, Melanoma and Multiple myeloma; Metastasis: Renal cell carcinoma CX3CR1↑– Melanoma (anti-tumor effect) |
|
The CXC family of chemokines have a non-conserved amino acid (X) that is present between the first and the second cysteine residue. Functioning of the CXC chemokines is determined by the presence of the Glu-Leu-Arg (ELR motifs) located at the N terminus, preceding the first cysteine amino acid residue [12]. Based on the ELR motifs, the CXC chemokines are further divided into two categories (ELR + and ELR–). The ELR + chemokines (CXCL1–3 and CXCL5–8) are predominantly neutrophil chemotactic and effective angiogenesis promoters whereas the ELR– members (CXCL4 and CXCL9–11) are potent inhibitors of angiogenesis that mainly target T cells and B cells (Table 1). The ELR– chemokine, CXCL12, was initially characterized as a pre-B-cell growth-promoting factor, but it was later found to have angiogenic properties. Currently, six CXC receptors have been identified. CXCR1 binds CXCL1, CXCL6, CXCL7, and CXCL8 with high affinity, whereas CXCR2 may bind to all ELR + chemokines. CXCR2 is another member of the CXCR family and shares 75 % sequence similarity with CXCR1. CXCR3 was found to bind to the ELR– CXC chemokines (CXCL9–11). CXCR4, CXCR5, and CXCR6 bind to CXCL12 (also known as stromal cell-derived factor1 or SDF-1), CXCL13, and CXCL16, respectively. The largest CC chemokine (or β-chemokines) family have cysteine residues adjacent to the N terminus. The members of the CC family include CCL1–5, CCL7, CCL8, CCL11, and CCL13–28 that are the key determinants for the recruitment of T cells, B cells, monocytes, macrophages, eosinophils, basophils, dendritic cells, mast cells, and NK cells. The corresponding receptors for each ligand have been shown in Table 1.
Functionally, chemokines may be classified as homeostatic or inflammatory. Chemokines are redundant in their activity with the exceptional ability to activate multiple GPCRs, and a single GPCR may bind to more than one chemokine displaying multiple biological outcomes [13]. This promiscuousness of the chemokine/receptors allows them to mimic other ligands during complex interactions. Chemokines and their receptors may trigger distinct migratory responses in cells including directed/undirected motility, haptotaxis, haptokinesis, chemokinesis, and induction of cell adhesion and cell arrest. Accordingly, chemokines are emerging as a critical mediator in the progression of solid tumors and recruitment of a variety of cell types to the TME.
3. Chemokines and chemokine receptors in solid tumors
Solid tumors develop as a result forced consonance between the proliferating cancer cells and the host. Chemokines are involved in cellular transformation, development of a pro-angiogenic environment, invasion through ECM and vascular basement membrane, and tumor cell metastasis. They may directly promote tumor growth, aid in development of blood vessels and control metastatic cell migration. Chemokine receptor ligation with chemokines on tumor cells activates the MAPK/ Erk signaling and induces the expression of crucial growth-promoting genes including cyclin D1, Fos, and the heparin-binding epidermal growth factor. Chemokines shift the balance between pro-apoptotic and anti-apoptotic proteins in the tumor cells by upregulating Mdm2 expression and downregulating the expression of Bcl-2 or by inhibiting caspase-3 and caspase-9 activation. The different cells constituting the tumor milieu determine the chemokine and chemokine receptor expression in the tissue.
Cancer cells engage in a complex network with the TME components including mesenchymal stem cells, adipocytes, tumor-associated fibroblasts (TAFs), endothelial cells, pericytes, proteins of the ECM, and immune cells such as the macrophages, myeloid-derived suppressor cells (MDSCs), NK cells, DCs, B cells and T cells via chemokines, cytokines, and growth factors [7,8]. The number and type of cells present in leukocyte infiltrate of solid tumors are influenced by chemokines locally produced by tumor cells and the stromal cells. For example, the CC chemokines influence macrophage and lymphocyte infiltration in cancers of the pancreas, breast, and cervix, as well as in sarcomas and gliomas [14–17]. Other than infiltrating leukocytes, the cancer cells also express different chemokine receptors and are responsive to chemokine gradients. Cells from various cancer types are known to express different profiles of the CC, CXC, XC, and CX3C chemokine receptors (Table 1). Subsequently, we discuss the importance of the CXCL8-CXCR1/2, CXCL9, 10, 11-CXCR3, CXCL12-CXCR4, and CCL(X)-CCR(X) (X represents different number of ligands and receptors) signaling axes in solid tumors that are commonly involved in the progression of most tumors.
3.1. CXCL8-CXCR1/2 axis in solid tumors
CXCR1/2 chemokines, such as CXCL1, 5, 7, and 8 are produced by various cancer cells and act in an autocrine manner to regulate cancer progression. The CXCL8-CXCR1/2 axis plays an important role in tumor growth, angiogenesis, metastasis, stemness, and recruitment of immune cells into the TME, by activation of downstream signaling cascades such as the PLC-PKC, PI3K-AKT, FAK/Src, MAPK-ERK and RhoGTPase [18, 19]. The CXCL8-CXCR1/2 axis has been associated with certain types of tumors including ovarian, lung, breast, melanoma, prostate, human hepatocellular carcinoma (HCC), colon and pancreatic cancers [19,20] (Table 1 and Fig. 2A). Therefore, targeted treatments against the CXCL8-CXCR1/2 axis are likely to offer substantial therapeutic utility in tumor treatment.
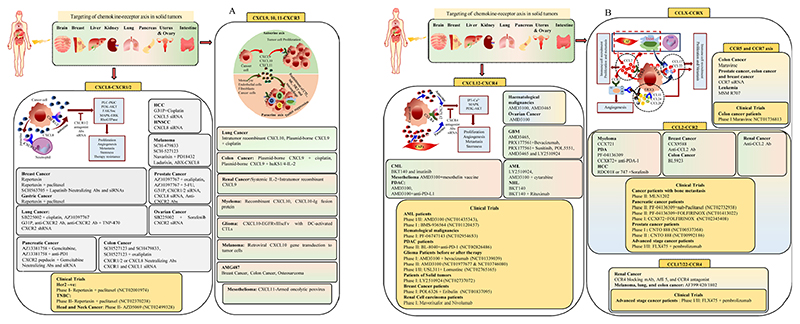
Fig. 2. Chemokines/chemokine receptors signalling axis in cancer therapy.
Various chemokines signalling axis including (A) CXCL8-CXCR1/2, CXCL9, 10, 11-CXCR3, (B) CXCL12-CXCR4 and CCL(X)-CCR(X) are involved in tumor progression of solid cancers. The therapeutic targeting of these axes using inhibitors, Abs and siRNA could provide benefits in cancer treatment.
CXCL8, a pro-inflammatory chemokine produced by the endothelial cells, macrophages, epithelial cells, monocytes, and fibroblasts, has been studied extensively in various diseases including cancer. In unstimulated cells, CXCL8 levels are almost undetectable, which increases by 10–100 folds, by a variety of factors including cytokines (interleukin (IL)-1, IL-6, CXCL12, and tumor necrosis factor (TNF)-α), hypoxia, reactive oxygen species (ROS), pathogen-associated molecular patterns (PAMPs), and other environmental stressors. High expression levels of CXCL8 have been observed in various cancers (Table 1). CXCL1 and CXCL8 have a high affinity for CXCR2 and were found to upregulate the proliferation of diff ;erent melanoma cells, breast cancer cells and pancreatic tumor cell lines [20–26]. CXCL1, CXCL2 or CXCL3 overexpression enhanced the ability of different melanoma cell lines to form colonies in soft agar, and increased tumorigenicity in nude mice [27]. A similar effect has been reported for CXCR2 chemokines in breast, head and neck, pancreatic, and non-small-cell lung carcinoma [26,28–30]. In a mouse model of PDA, tumor cells and tumor associated stroma showed high levels of CXCL2 and CXCL5 [31]. Studies show autophagy dependent secretion of CXCL8 from cancer-associated fibroblast as a contributing factor for increased head and neck cancer metastasis [32].
The CXC chemokine receptor, CXCR2, shows similarity to human GPCRs in the Kaposi’s sarcoma herpes virus (known as KSHV–GPCR) suggesting the direct involvement of chemokines and chemokine receptors in transformation. The KSHV–GPCR generates a signal that is enhanced by the CXCL8 (IL-8) and CXCL1 (Groα) ligands [33]. KSHV–GPCR overexpression leads to formation of a lesion similar to Kaposi’s sarcoma [34]. A point mutation in CXCR2 activates constitutive signaling of the receptor and leads to cellular transformation of transfected cells as seen in KSHV–GPCR [35]. These reports suggest that CXCR2 expression may promote pre-neoplastic to neoplastic cellular transformation in cells under persistent autocrine and paracrine stimulation with the specific CXC chemokine ligands. In 1987, Yoshimura et al. published the first report on CXCL8, a chemokine that modulates neutrophil trafficking [36]. Since then, researchers have focused their efforts on deciphering the activities and roles of chemokines in immune response.
3.2. CXCL9-11-CXCR3 axis in solid tumors
CXCL9-11-CXCR3 axis has been a prominent focus of research in cancer as it governs differentiation, migration, and activation of immune cells (T cells, NK cells, NKT cells, and macrophages) [37]. ELR-negative CXC chemokines, CXCL9-11 are CXCR3 selective ligands that are generally expressed at low levels under homeostatic settings but are increased by cytokine stimulation. CXCR3 is expressed on the surface of monocytes, T cells, NK cells, DCs, and cancer cells, however, their ligands are primarily secreted from monocytes, endothelial cells, fibroblasts, and cancer cells in response to IFN-γ, which is amplified synergistically by TNF-α. Immune cells, primarily Th1, CTLs, NK cells, and NKT cells have anti-tumor effect in tumor models via the paracrine CXCL9-11-CXCR3 axis. However, several studies have found that the autocrine axis is implicated in tumor proliferation, angiogenesis and metastasis, which may be attributed to the different effects of the ligands on CXCR3 variants (CXCR3A, CXCR3B and CXCR3-alt). In vitro and in vivo studies have demonstrated that cancer cells expressing CXCR3 have a higher proclivity to metastasis as a result of the autocrine signaling from the pre-metastatic environment [38]. CXCR3 has recently been discovered to play a significant role in macrophage polarization. CXCR3 loss causes macrophages to polarize towards an M2 phenotype that promotes tumor growth in a mouse model of breast cancer [39]. Upregulated CXCR3 expression levels promote metastasis in various cancers including prostate, colon, melanoma, gastric and ovarian cancer (Table 1) [40–44]. The targeting of this axis may provide therapeutic advantage in metastatic cancer.
3.3. CXCL12-CXCR4/ACKR3 axis in solid tumors
Both CXCR4 and ACKR3 (atypical chemokine receptor 3) are GPCRs with CXCL12 as a physiological ligand, although they display distinct characteristics. ACKR3 gene was first identified as an orphan GPCR, also known as RDC1 and CXCR7. ACKR3 is a chemokine scavenger that lacks the common DRY-LAIV domain involved in G protein activation and selectively signals via β-arrestins [45]. The only chemokine that interacts with CXCR4 is CXCL12 while ACKR3 also binds CXCL11.
The interaction between CXCR4 and CXCL12 regulates the migration and patterning of embryonic cells mobilization of HSCs and lymphocyte transport [46–50]. Different tissues such as the liver, lung, adrenal glands, bone marrow, and lymph nodes constitutively express CXCL12/SDF-1. The CXCR4-CXCL12 axis regulates the migration of an embryonic cell subset that plays a role in the development of bone marrow, heart and the central nervous system (CNS) and has been linked to tumor metastasis and angiogenesis [51,52]. CXCR4 is expressed in nearly 23 types of cancers of the epithelial, mesenchymal or hematopoietic origin [53]. The interactions between CXCL12 and CXCR4 are also known to stimulate proliferation in prostate cancer, glioblastoma and multiple myeloma [54–56]. CXCR4-CXCL12 interaction and downstream signaling is partially regulated by the phosphorylation of Akt, promotes the growth and survival of tumor cells, and facilitates cell growth in distant and less favorable sites [57]. Some cancerous cells (not all) in the primary tumor may express CXCR4. For instance, CXCR4 expression is found in primary tumor sites in glioma, lymphoma, ovarian, non-small-cell lung cancer (NSCLC) and pancreatic cancer, and at the sites of metastasis in breast and thyroid cancer, neuroblastoma and hematological malignancies [58,59]. CXCR4-CXCL12 axis plays a crucial role in the migration of tumor cells to metastatic locations in ovarian, breast, prostate, pancreatic, esophageal, colorectal cancer and melanoma, NSCLC, head and neck cancer, glioblastoma, basal cell carcinoma, neuroblastoma, bladder carcinoma, osteosarcoma, acute lymphoblastic leukemia and chronic myelogenous leukemia (CML) [60, 61]. SDF-1 may bind to CXCR7 that is expressed in B-cells, T cells, endothelial cells, DCs, chondrocytes, and endometrial stromal cells. SDF-1 modulates the migration ability of cells and CXCR7 mediates cell proliferation [62].
3.4. CCL(X)-CCR(X) axis in solid tumors
CC chemokines act as ligands for ten classical receptors that serve as a key component of the TME [63,64]. The chemokine receptor CCR1, is expressed on leukocytes in solid ovarian tumors and the infiltrating cells predominantly include macrophages and CD8 + T lymphocytes [65,66]. CCR2 is another member of CC chemokine receptor family which functions through binding to CCL2 (MCP-1). Additionally, CCR2 binds with other ligands including CCL7 (MCP-2), CCL8 (MCP-3), CCL13 (MCP-4) and CCL12 (MCP-5). CCL2-CCR2 axis is associated with various cancers. For instance, CCL2 localizes to the epithelial areas of the ovarian tumor, and its expression correlates with the number macrophages and lymphocytes in that area [67].
CCL17/22-CCR4 signaling axis regulates the infiltration of regulatory T cells (Tregs) in The TME and linked with tumor progression as described below in this review. CCL5 localizes with the infiltrating leukocytes and its expression levels correlate with the CD8 + T-lymphocyte infiltration [66]. The CCL21–CCR7 chemokine ligand-receptor pair is involved in the migration of tumor cells into the sentinel lymph nodes. This has been seen in breast cancer, NSCLC, colorectal cancer, gastric cancer, murine B16 melanoma, esophageal cell carcinoma and head and neck cancer [17,68–73]. In the lymph nodes, CCL21 chemoattracts the tumor cells bearing the chemokine receptor, CCR7. Intriguingly, murine CCL21 (but not human CCL21) causes transduction through CXCR3. In the early detection of primary tumors, the disruption of CCL21–CCR7 may help to prevent lymph node metastasis. The expression levels of chemokines and their receptors in various solid tumors are given in Table 1.
The chemokine signaling axes are crucial in cancer progression and metastasis. Chemokines have diverse functionality in the TME and regulate site-specific tumor growth. The aberrant expression of chemokine receptors, which is not random but selective, develops variable responses in the TME and may contribute to tumor heterogeneity. In the following section, we discuss the role of chemokines in tumor heterogeneity may help in therapeutic intervention to overcome the heterogeneity within primary tumors and among metastases.
4. Role of chemokines in tumor heterogeneity
As cancer progresses, the TME develops diverse cell populations with striking variability, having distinct molecular signatures and variable sensitivity to treatment, which accounts for some of the tumor heterogeneity [9,74,75]. In the TME, interactions between macrophages or mesenchymal stem cells (MSCs) and cancer cells, in particular, play an essential role in regulating tumor activities. For example, M2-type macrophages and MSCs can be converted into tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs) which have largely tumor-supportive features. MSCs interact with cancer cells both indirectly and directly by altering their functions and contributing to cancer cell plasticity [76]. The successive clonal expansion of tumor cell populations is affected by the selection of cells having a growth and/or survival advantage. These cells vary in their size, organization, morphology, antigen expression, membrane composition, proliferation rate, cell-cell interaction, metastatic tendency, and response to chemotherapy [77]. Tumor heterogeneity may be classified as ‘inter-tumor’ heterogeneity that is evident between cancers from different patients and ‘intra-tumor’ heterogeneity, which is seen within a single tumor [75].
Chemokines regulate various processes such as immune infiltration, heterogeneous primary tumor growth, angiogenesis, epithelial and mesenchymal plasticity, organotropism, heterogeneous metastases, cancer stem cells and therapy resistance that contribute to tumor heterogeneity as well as metastatic heterogeneity. Organ specific metastasis or organotropism involves a non-random spread of the malignant cells to distant organs [78]. Various factors such as the pattern of blood circulation, anatomical location of the organs, organ-specific niche/microenvironment of the metastatic sites and its interaction with the tumor cells regulate organotropism. The chemo-attractant properties of chemokines regulate immune trafficking to sites in distant organs. Specific chemokine receptor bearing cancer cells and endothelial cells move towards their coupled chemokine gradient at their non-random, organ-specific locations [79,80]. Chemokines, therefore, control the wheel of distant organ metastasis. In breast cancer, the expression of CCR7 and CXCR4 regulate invasion and organ specific metastasis to the CCL21 (a ligand to CCR7) and CXCL12 positive sites for instance the liver, lung and bone [17,81]. Currently, two concepts are used to describe tumor heterogeneity, the clonal evolution model and the CSC hypothesis.
4.1. Clonal evolution model
The clonal evolution model states that over time, the cancer cells acquire various stochastic mutations within a tumor, this genetic drift and natural selection of cells with a growth advantage, drives aggressive tumor progression. This model describes intra-tumor heterogeneity as a result of natural selection and suggests that tumor initiation occurs following many mutations in any random single cell giving growth advantage over other cells. As a result of genetic instability and uncontrolled proliferation, this produces cells with added mutations and newer characteristics. These new mutations may provide growth advantages such as resistance to apoptosis. The generation of subpopulations of variant cells results in tumor heterogeneity. These cells may turn invasive and metastatic or develop resistance to therapy leading to aggressive disease and recurrence [82,83]. The most concrete evidence for clonal evolution is provided by several studies which reveal that all major human cancers and histological subtypes have various cell subpopulations with distinct heritable abnormalities.
4.2. Cancer stem cell hypothesis
In contrast to clonal evolution model, the CSC hypothesis suggests that a certain subset of tumor cells, referred to as CSCs, possess stem cell like properties (self-renewal and differentiation) that can generate heterogeneous tumors and play an active role in tumor initiation, progression, metastasis, recurrence of the tumor and therapy resistance [84]. The other cells in a tumor may not have unlimited capacity for self-renewal and do not differentiate to generate all types of tumor cells. The normal stem or progenitor cells of an organ give rise to CSCs that remain as a subset of the tumor cells. The CSC hypothesis suggests that the metastatic spread of these cells results in tumor progression and their resistance to therapy leads to cancer recurrence [85]. This concept developed from extensive research in acute myeloid leukemia (AML) which discovered the less-differentiated CD34 +/CD38− cell population with self-renewal capacity like the stem cells and strong tumor-initiating potential in mice [86,87]. CSCs have been identified in various solid tumors such as melanoma, brain, breast, pancreatic, prostate, liver, colon, thyroid, lung, ovary, head and neck, and stomach cancer [88,89]. Some of the cell surface markers could be utilized to detect subpopulations of CSCs in solid tumors including Sox2 in glioblastoma, CD44 and ALDH1 in breast cancer, LGR5 in colon cancer, and CD133 in various malignancies [90]. The CSCs show plasticity, which entails a reversible change from epithelial to mesenchymal states, and forms a primary source of heterogeneity in the phenotypically distinct population of CSCs. As an example, the subpopulation of breast cancer stem cells (BCSCs) such as ALDH+ BCSCs shows epithelial phenotype (epithelial markers; CDH1, OCLN, and CLDN) with proliferative potential, and CD44+ /CD24− BCSCs show mesenchymal phenotype (mesenchymal markers; VIM, ZEB1, and ZEB2) indicating metastatic ability [91,92]. However, ALDH+/CD44 +/CD24− BCSC is highly tumorigenic and invasive implying that tumorigenicity may be enhanced by a combination of markers. Thus, the ability of these populations of BCSCs to transition between different states demonstrates the plasticity and variability of BCSCs. Interestingly, the phenotypic plasticity of CSCs allows them to transdifferentiate into pericytes, endothelial cells, and fibroblasts, contributing to tumor angiogenesis, stem cell niche creation, and inflammation [93]. According to recent research, the lymphoid chemokine CCL19 increased the heterogeneity of breast tumor cell motility within a 3D microenvironment as demonstrated by a Le’vy distribution analysis [94].
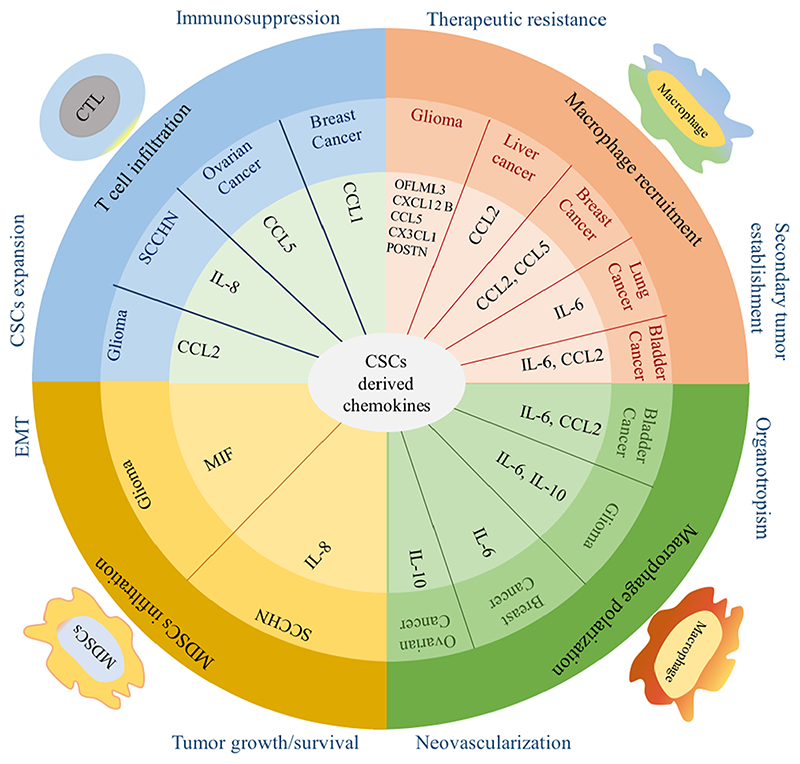
CSCs have immune evasive and immunosuppressive capacity in the TME which is mediated by the chemokines network, cytokines, and growth factors in solid tumors [84,95,96]. CSCs secrete several factors including chemokines which recruit stromal cells and control generation and activation of a tumor niche (Fig. 3). Therefore, targeting the CSCs is an emerging concept in cancer treatment. One of the effective therapeutic strategies is inhibition of the self-renewal or survival pathways (NOTCH, Hedgehog, and WNT) in CSCs [88].
Fig. 3. CSCs derived chemokines maintain immunosuppressive microenvironment and tumor progression.
Different chemokines are secreted from various cancers as depicted here which are essential for the recruitment and polarization of macrophages, infiltration of T cells and MDSCs. Secreted chemokines regulate multiple steps of cancer development such as tumor growth/survival, neovascularization, organotropism, secondary tumor establishment, EMT, CSCs expansion, immunosuppression and therapeutic resistance.
5. Chemokines/receptors axis in CSCs
Different chemokine signaling axes have a key role in regulating the CSC subpopulations. The CXCL-8-CXCR1/2 chemokine axis, for example, has been linked with CSC populations and correlates with clinical outcomes in several cancers including breast, pancreatic, renal cancers and glioblastoma multiforme (GBM) [19,97,98]. CXCR1 and CXCR2 are highly expressed on the surface of BCSCs, where they interact with CXCL-8 to cause self-renewal. CXCL-8 is functionally related to poor prognosis in breast cancer and exogenous CXCL8 treatment increased mammosphere formation and ALDH1 population in breast cancer cell lines and patient-derived breast cancer samples [99–101]. Apart from the CXCL8-CXCR1/2 axis, the significance of other chemokines and chemokine receptors such as CXCR3B (an isoform of CXCR3), CXCR4, CXCR7, CXCL3, CXCL5, CXCL7, CXCL10, CXCL11, and CXCL12 (SDF-1, secreted from CAFs) in the regulation of BCSC population, has been well recognized.
The CXCR4-CXCL12 chemokine/receptor axis plays a critical role in the maintenance of prostatic CSCs. CXCR4 expression is higher in the CD44+/CD133+ population of prostate cancer [88,102,103]. In a CXCL12-dependent manner, CSCs increase proliferation adherence to the extracellular protein fibronectin, via activating the PI3K pathway. Interestingly, the CXCR4 antagonist (AMD3100) or anti-CXCR4 antibody could specifically reduce prostate progenitor growth and tumor size [104]. CXCL12 also increased prostate tumor development, metastasis, and chemoresistance in vivo by inducing CSC and neuroendocrine phenotypes in prostate cancer cells via CXCR4-mediated PKC/NFkB signaling [105]. Similarly, the CXCR4-CXCL12 axis plays an important role in the survival and self-renewal of CSCs in GBM [103]. Members of CC family of chemokines including CCR2, CCR5, CCL2, CCL5, and CCL16 have a critical role in BCSCs population. Moreover, CCL21 binds with CCR7 in CD133+ pancreatic cancer stem-like cells, which prevents apoptosis and enhances survival and migration through the ERK1/2 and p65 pathways [106]. In lung cancer, CCR9/CCL25 axis increased CSC migration and invasion, which may lead to distant metastases and poor overall survival.
Similar to the CSC microenvironment, the chemokines are strongly involved in the regulation of immune activity in the TME. The immune cells are capable of spontaneous movement and migrate to different organs in a context-specific way to determine immune outcome. Immune infiltration into the TME is critical in cancer progression. Chemokines modulate immune checkpoints to affect cancer growth and progression by controlling the migration of immune activating and suppressive immune cells, thereby driving the fate of the developing tumor and metastases. In the following section, we discuss the role of chemokines/receptors in the regulation of immune cell entry in the chemotactic solid tumor microenvironment, which may help to improve the currently available therapeutic interventions.
6. Chemokines/chemokine receptors and leukocyte infiltrate in cancer
Chemokines recruit immunosuppressive cells and activated immune cells in to the TME. A bidirectional crosstalk is mediated by chemokines between tumor cells and infiltrating immune cells to regulate tumor growth and metastasis.
6.1. Regulatory T cells
A specific subset of CD4+, CD25 + T cells, termed as Tregs, are immunosuppressive cells and support immune tolerance during homeostasis [107]. Tregs suppress the anti-tumor immune response and promote tumor growth in cancer [108]. Tregs are frequently present in tumors and act as potent suppressors of innate and adaptive immunity through their production of IL10 and transforming growth factor (TGF-β). The presence of Tregs is associated with poor prognosis in cancer patients [109]. In various experimental and spontaneous tumor models, the selective depletion of Tregs showed potent induction of NK cell and T-cell dependent antitumor effect [110]. The intra-tumoral expression of CCL22 and CCL28 is increased in ovarian cancer and attracts CCR4þ or CCR10þ Tregs, respectively, leading to the suppression of antitumor responses and increased angiogenesis by secreting vascular endothelial growth factor (VEGF) [109,111]. In a breast cancer model of metastasis, CCR4þ Tregs were reported to promote the metastasis by inducing NK cell apoptosis [112].
Tregs have been demonstrated to co-express Th-specific transcription factors and chemokine receptors that control pro-inflammatory effector Th-cell responses. To escape protective immunity of the host that is mainly Th1-dependent, the tumor cells may direct the Tregs to express CXCR3 and T-bet and suppress Th-1 immune response [113]. Notably, the majority of Tregs express CXCR3 in ovarian carcinoma [114]. CCR4 expression mediates the migration of Tregs in various models of cancer. CCL22, a ligand for CCR4 is produced by the primary tumor cells and TAMs in ovarian tumors and caused migration of Tregs [109]. CCL22 mediated recruitment of Tregs has been demonstrated in breast cancer [115]. The increased expression of CCL22 and CCL17 (alternative ligand for CCR4) correlated with higher infiltration of Tregs in esophageal carcinoma and gastric cancer [116,117].
6.2. Tumor associated macrophages (TAMs)
The specific chemokines that are secreted by the tumor cells recruit macrophages to the neoplastic sites. Macrophages may exhibit protumorigenic or tumoricidal activity. TAMs release growth factors that promote the tumor cell proliferation such as epidermal growth factor (EGF) and TGFβ [118]. Macrophages protect the cancer cells from the antitumor response by the suppression of T cell response and the NK cell cytotoxicity, expressing regulatory molecules including arginase-1 (ARG1), TGF-β, and IL-10, and have a role in tissue remodeling and repair, angiogenesis, and metastasis [119]. TAMs are abundantly found in stromal cells in solid tumors. Several TAMs are associated with low survival in cancer patients. Other than the TAMs that are recruited to primary tumors, the TAMs driven to the metastatic sites are called metastasis-associated macrophages (MAMs). Strategies aiming to block TAMs and MAMs recruitment may help to improve metastatic disease outcomes [120,121].
The CC chemokines are well known to preferentially recruit macrophages and T lymphocytes into the tumors. CCL2 is produced by the TAMs and expressed in a number of human cancers. CCL2 production is related to recruitment of macrophages [122,123].
CCL2 expression has been correlated with high inflow of TAMs, metastasis to lymph node and poor prognosis in breast and esophageal cancers [124,125]. However, some studies also report that the effect of CCL2 on neoplastic cells varies with its amount in the TME. Low CCL2 expression promotes tumor growth with moderate macrophage infiltration, in melanoma. The high CCL2 expression leads to the recruitment of macrophages in large numbers and may have anti-tumor effects as seen in pancreatic cancer [126,127]. CCL5 has a similar antitumor effect and is associated with active lymphocyte response in NSCLC. However, CCL5 may be involved in tumor progression and it is associated with advanced stages in breast cancer [128]. Pico to nanomolar quantities of CCL3, CCL4, CCL22 and monocyte chemoattractant protein, MCP-2/CCL8 that correlate with macrophage as well as T cell infiltration, are found in the ascites and ascitic cells in ovarian cancer [129]. High expression of CCL18 by TAMs is also observed in the ascites from ovarian cancer [130]. Kleef et al. reported that TAMs abundantly express macrophage inflammatory protein-3 alpha (MIP3-a)/CCL20 and promoted growth and migration in two among the four CCR6 positive pancreatic cancer cell lines tested [131]. CCL2, 4 and 5 stimulate the production of matrix metallopeptidase (MMP9) by the macrophages, promoting the degradation of ECM and migration of tumor cells away from primary tumor site [132].
The chemokine/chemokine receptor axis CCL2-CCR2 and CCL3-CCR1/CCR5 primarily regulate MAM expression, recruitment and retention. In the metastatic lung, the MAMs express CCL8 and recruit Treg cells through CCR5 receptor [133]. CCL3 was found to be the key mediator that facilitates cross-talk between the neoplastic epithelium and the peripheral tissues including brain and lung in the breast cancer-bearing mice models [119].
6.3. Myeloid-derived suppressor cells (MDSCs)
MDSCs refer to a heterogeneous myeloid progenitor cells present normally in the bone marrow and spleen. The MDSCs are characterized by morphological, phenotypic, and functional heterogeneity. MDSCs display plasticity and undergo continuous changes in response to chemokines and cytokines produced in the TME. Similar to the TAMs, the MDSCs link chronic inflammation to cancer. They remain in an active state in the TME and produce ROS, RNS, and ARG-1 [134]. MDSCs potently suppress T-cells with anti-oncogenic activity. MDSCs are known to downregulate the T cell receptor (TCR) associated ζ-chain that is commonly seen in cancer subjects. The suppression of the TCR-associated ζ-chain impairs the ability of the CD4+ and CD8 + T cells causing immune cell activation [3], and enables the silencing of immune surveillance. Type I NK (invariant or iNKT) cells are known to foster tumor rejection and type II NKT cells support tumor progression by the recruitment of MDSCs mediated by the secretion of IL-13 [135]. MDSCs are also reported to promote CD4+FOXP3+ Tregs (induced Tregs) formation [136]. The chemokine-receptor axes, CXCL5/CXCR2 and CXCL12/CXCR4 cause the increased influx of MDSCs into the tumors in mouse model lacking the TGFβ receptor 2 [137]. The tumor-derived MDSCs migrate to pre-metastatic lungs and enhance pro-inflammatory cytokines, reduce IFN-γ, and stimulate vascular remodeling much before the arrival of tumor cells [138].
Under homeostatic conditions, the myeloid cells, particularly the monocytes, exclusively express CCR2 and direct migration from basement membrane to the peripheral sites under homeostatic/inflammatory situations. CCR2 is crucial for the movement of monocytes and MDSCs into the tumors. Upon infiltration into the tumor, the myeloid cells produce CCL2 and augment monocyte migration into the tumors. Increased CCL2 expression recruits large numbers of monocytes or monocyte-derived cells as seen in mouse models of renal tumors, glioma, lung cancer, prostate cancer, and melanoma. The high expression of CCL2, associated with increased recruitment of monocytes/macrophages, indicated adverse prognosis in breast, ovarian, esophageal and gastric carcinomas [139–141].
6.4. Tumor-associated neutrophils (TANs)
Neutrophils or the polymorphonuclear leukocytes (PMNs) are the immune cells of myeloid origin and play a role in primary response to inflammation and defense against pathogens. Increased neutrophil infiltration has been reported in bronchioalvelolar carcinoma, melanoma, myxofibrosarcoma, gastric carcinoma, and colon cancer [142, 143]. In tumor biopsies, the presence of neutrophils is associated with poor disease prognosis. TANs are classified as “N1” or “N2” based on their anti-tumor or pro-tumor effect [143]. The TANs or N2 neutrophils promote tumor development and show increased ARG I levels. Evidence suggests that neutrophils play a role in neovascularization of tumors [142,144]. The cancer cells regulate neutrophil recruitment to tumor sites through the expression of various chemokine ligands (CXCL8, CXCL1, CXCL2, CXCL5, CXCL6) for neutrophil receptors CXCR1 and CXCR2 [145,146]. The genetic ablation of CXCR2 caused lowered neutrophil infiltration and suppressed tumor growth [31]. In a zebrafish model of glioblastoma, the CXCR1-CXCL8 axis played an essential role in the recruitment of neutrophils to tumor site [147,148]. CXCL1 and CXCL2 chemokines enhanced neutrophil recruitment and induced angiogenesis in melanoma-bearing mice [149]. A high expression of CXCL5 in HCC was linked to neutrophil infiltration and indicated poor prognosis [150]. The overexpression of CXCL6 resulted in increased neutrophil influx and promoted angiogenesis in melanoma [142,151].
6.5. Dendritic cells (DCs)
Antigen-presenting cells (APCs) are crucial role to anti-tumor response. The DCs play an important role in antigen presentation and the cDC1s (subset of DCs) elicit anti-tumor immune response by crosspriming (cross-presenting exogenous antigens) CD8 + T cells. DCs capture the antigens and stimulate T cells in the lymph nodes following maturation. DC infiltration has been reported in thyroid, breast, stomach, lung, prostate, kidney, nasopharynx tumors and in melanoma [152]. The cancer cells in renal, breast, pancreatic, and papillary thyroid carcinoma produce CCL20 that attracts the CCR6-expressing DCs [153]. CCL20 overexpression is reported to attract a large number of DCs which in turn activate the tumor-specific cytotoxic T cells. CCL5 and CXCL12 attract immature DCs to tumor sites through CXCR4 and CCR5 [119, 122,154]. In a mouse melanoma model, defective CCL4 expression, reduced infiltration of DCs to the tumor [9]. In a similar model, another study reported that CCL5 (RANTES) (alternative ligand for CCR5), played critical role in DC infiltration into the tumor [10]. Following recruitment to tumor sites, tumor antigens are engulfed by the DCs. DCs become activated and present them on MHC molecules. The activated DCs stimulate CCR7 and turn responsive to MIP-3b/ CCL19 as well as CCL2. These chemokines/chemokine receptors enable the migration of the DCs to secondary lymphoid organs and stimulate the T cells to evoke immune response [64,155]. Following activation by the tumor or foreign antigens, DCs alter their chemokine receptor expression in order to travel to secondary lymphoid organs. The DCs expressing CCR7 displayed increased ability to move towards the draining nodes when injected to the tumor periphery. Activation-induced expression of CCR7 on the DCs is important for them to migrate from the peripheral tissues to lymph nodes following the gradients of CCL19/21 ligands.
Increased DC infiltration in tumors is generally associated with increased immune response. DCs recruited to the tumor periphery by MCP-3/CCL7 have been associated with good prognosis in cancer. The plasmacytoid DCs (pDCs) cause the largely increased production of type I interferons having anti-tumor effects and promote immune response. Yet, it remains unreasonable that tumor cells would produce anti-tumor chemokines against themselves. Cancer progression is normally believed to follow Darwinian selection that favors the mediators which foster tumor growth, survival and progression.
Chemokines are known to regulate cell trafficking and participate in host response during infection. However, the biological effects of chemokines are much more complex and affect virtually all cells including tumor cells. They may directly or indirectly regulate tumor growth by the recruitment of TAMs, affect activities in the developing blood vessels, and control metastatic cell navigation. The release of growth and chemotactic factors by TAMs helps to sustain tumor development. All these mechanisms are operative in cancer. In the following section, we discuss how tumors divert chemokines to favor their own growth and development through several mechanisms such as tumor proliferation, angiogenesis, metastasis and senescence.
7. Chemokines and chemokine receptors mediate tumor proliferation and senescence
Chemokines released by the tumor cells, CAFs and infiltrating immune cells may directly promote proliferation of cancer cells by the autocrine/paracrine binding to the chemokine receptors expressed on tumor cells via activation of the P13 K/AKT/NF-κB and MAPK/ERK pathway [7,132,156]. Early studies based on melanoma showed that ELR + CXC chemokines increase proliferation in tumor cells. The ELR + CXC chemokines obtained either from the autocrine or paracrine pathways, increased the tumor cell viability, proliferation, anchorage independent growth and decreased tumor cell apoptosis via induction of signaling through CXCR1/CXCR2 [157,158]. Some studies reported that chemokines may also indirectly regulate tumor growth through intensifying the effects of other cell growth regulators like IL-6 and chemotherapy [80]. CCL2 was found to increase breast tumor cell division by activating the estrogen receptor alpha via PI3K/Akt/mTOR signaling and increased cancer cell survival partially by the activation of Rho pathway [124,159,160]. CCL2 also induced cell cycle arrest in triple negative breast cancer (TNBC) via activation of SRC and PKC. Studies based on estrogen responsive luminal-A breast cancer revealed the hormone stimulated expression of CXCL12-CXCR4 that leads to increased tumor cell growth and proliferation along with the transactivation of EGFR and increased DNA synthesis [161,162]. CCL5 enhanced breast cancer cell proliferation via CCR5-dependent mTOR activation [163].
Chemokines are also play a part in the complementary processes involved in tumor cell survival. Cellular senescence is the permanent cessation of cell division. Senescence is important in regulating cancer progression by the permanently stopping cell proliferation as senescent cancer cells cannot re-enter the cell cycle [164]. Chemokines have been reported to play a role in the senescence of several tumors while elevating the pro-metastatic potential by regulating infiltration of leukocytes into the tumor site [165].
8. Chemokines and chemokine receptors mediate tumor angiogenesis
Angiogenesis, also known as neovascularization, is a biological process that involves the development of new blood vessels from the preexisting blood vessels. Angiogenesis occurs during normal growth processes, as well as in disease advancement. Angiogenesis helps to provide a supportive TME rich in oxygen and nutrients that sustains optimal tumor growth, invasion and metastasis. The blood vessels found within the tumor deliver nutrients and take away waste products from the rapidly-proliferating cancerous cells and also act as the points for the tumor cells to move out of the TME, enabling the growth of secondary metastatic tumors. Tumor blood vessel density correlates with increased metastatic spread of tumors [166]. Several chemokines and their receptors are involved in the regulation of angiogenesis [167,168]. The endogenous expression of specific chemokine receptors on endothelial cells is known at both mRNA and protein level [169]. Concurrently, many tumors express increased levels of pro-angiogenic chemokines [11]. Chemokine networks play a critical role in tumor angiogenesis by promoting or suppressing angiogenic factors such as VEGF and basic fibroblast growth factor (bFGF).
Among the four chemokine families, the CXC chemokines play a major role in angiogenesis by suppressing or promoting angiogenesis. Early studies suggest that CXC chemokines with ELR motif (ELR+) promote the movement and proliferation of endothelial cells, and in vivo angiogenesis, while the chemokines without ELR motif (ELR-) inhibit endothelial cell movement, proliferation, and in vivo angiogenesis. However, the CXCL12/CXCR4 (ELR-chemokine) was found to promote angiogenesis in a mechanism different from the ELR + chemokines [12].
The pro-angiogenic, ELR + chemokines including CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8 may directly act on the endothelial cells through their receptors and promote their proliferation, migration, and invasion to foster angiogenesis [12]. The VEGF mediated activation of endothelial cells may upregulate Bcl-2 (anti--apoptotic protein) that increases CXCL8 expression [170]. CXCL8 is involved in a paracrine feed-forward loop where the fMLP-activated neutrophils produce VEGF and CXCL8, that in turn promote the release of CXCL8 by the surrounding endothelial cells resulting in capillary sprouting [171]. CXCL8/IL8, which is largely produced by endothelial cells, has been identified as a critical modulator of tumor angiogenesis. CXCL8-dependent signaling can also promote angiogenesis by upregulating metalloproteases (MMP-2 and MMP-9) in endothelial cells and tumor cells which can remodel the extracellular matrix, allowing endothelial cells to migrate, reorganize, and promote angiogenesis [172]. Increased expression of CXCL5 and CXCL8 are associated with the vascularity in NSCLC, however, depletion of CXCL5 inhibited tumor development, angiogenesis, and spontaneous metastasis in a mice model of NSCLC [157]. CXCL7/NAP-2 can promote cancer cell proliferation and the growth of the tumor-associated lymphatic network in breast cancer by directly influencing the production of VEGF-C/D and heparinase. In addition to CXC chemokines, CC chemokines (secreted by endothelial cells, smooth muscle cells, as well as inflammatory cells) are also angiogenic modulators of inflammation-driven angiogenesis. Recent findings suggest that CCL2 accumulation in tumors induces a neovascularization process acting directly by promoting endothelial cell chemotaxis and tube formation through the increased expression of ETS-1 (transcription factor), MMP14, MCPIP, and VEGF-A. Additionally, CCL1, CCL11, CCL15, and CCL16 may initiate endothelial tube formation in vitro. CX3CL1 regulates angiogenesis in various solid tumors (breast, lung, liver, melanoma, and multiple myeloma) in two ways by (1) recruitment of TAMs and (2) acting on endothelial cells directly causing proliferation, migration, and tube formation. Apart from cancer, the CX3CL1-CX3CR1 axis contributes to angiogenesis in inflammatory disease, rheumatoid arthritis, and atherosclerosis.
9. Chemokines and chemokine receptors mediate tumor metastasis
Tumor metastasis is the spread of malignant tumor cells in which the selected subpopulations of tumor cells detach from the primary tumor foci, enter the blood and/or lymph vasculature and travel to a secondary growth site to re-establish a secondary tumor. These cancerous cells that migrate through the circulation following detachment from the primary tumor are referred to as circulating tumor cells (CTCs). CTCs disperse as single cells or as clusters. These cells may remain dormant and retain stem cell capabilities, which could lead to subsequent tumor growth at secondary sites [78]. The classical ‘seed and soil’ hypothesis for organotropism by Steven Paget, suggests that the tumor cells metastasize to the sites with a favorable local microenvironment in a distant organ that acts in a way similar to a fertile soil that supports the growth of seeds [173]. For instance, breast cancer preferably metastasizes to bone, lung, liver, and brain while prostate cancer colonizes in bone [78]. Several chemokine receptors are present in different cancers, and their ligands are expressed in the metastatic location [174]. Metastasis involves a complex cascade of events including remodeling of the ECM, EMT, angiogenesis, local invasion of the basement membrane, movement of the CTCs through the blood, extravasation, and development of a pre-metastatic niche to enable the re-establishment of tumor cells at the distant metastatic site [78].
Chemokines are thought to regulate organotropism (organ-specific metastasis) [174]. CXCR1, CXCR2 expression correlates with tumor growth and metastasis in melanoma [19,175]. CXCR3 was found to be expressed by various solid tumors, but its significance in the metastasis of melanoma and colon cancer to lymph nodes has recently been discovered [176]. ELR – chemokines, CXCL9, and CXL10 are also involved in the growth and metastasis of melanoma and multiple myeloma cells [177]. CCR7 and CXCR4 expression in breast cancer cells promote invasion and metastasis of cells towards their chemotactic ligands CCL21 and CXCL12 expressing organs including liver, lung, and bone [17,81]. CCR7 and CXCR4 expression in a B16 melanoma cell showed lymph node and lung metastasis, respectively.
Interestingly, the role of CXCL12/CXCR4 axis in metastasis has been demonstrated in various solid tumors, including breast, pancreatic, ovarian, lung, renal, colorectal, prostate cancers and nasopharyngeal carcinoma, and osteosarcoma. CXCR4 expression was associated with increased tumor metastasis in human patients [57,178]. The entry of the tumor cells into the lymph vasculature is crucial for metastasis. The expression of CXCR4/CXCL12 by TAFs promotes evasion in pancreatic cancer. CXCR4 and CXCR5 mediate metastasis in prostate cancer stem-like cells (PCSLC). CXCR4 inhibition alters the targeting of stem-like prostate cancer cells to bone. Moreover, colon cancer cells express CXCR5, which increases tumor development and liver metastasis. CCR2 and CCR3 expression are associated with the progression of prostate cancer and renal cell carcinoma, respectively. CCR4, CCR6, CCR9, CCR10, and CCL27 involves in the growth and metastasis of different cancers including adult T-cell leukemia/lymphoma (ATLL), colon cancer, prostate, and melanoma cells (Table 1) [79]. CCR5 also plays a role in breast cancer growth and metastasis. CCR7 expression in a metastatic lung cancer cell line showed lymph node metastasis [179].
Chemokines are also involved in the establishment of a premetastatic niche. The inhibition of CXCR2 and CXCR4 suppressed the recruitment of MDSCs to the pre-metastatic lung leading to reduction of lung metastasis in breast cancer [180]. The tumor-entrained neutrophils (TENs) were reported to prevent metastatic seeding in pre-metastatic lung through the production of H2O2 [181]. Some contradictory findings also suggest the pro-tumorigenic role of CCL2 and CCL3 as they recruit pro-tumorigenic macrophages in the TME [64,182]. The complexity of the chemokine network in cancer suggests a context dependent contribution of various chemokines in tumor growth and metastasis.
The altered expression of various chemokines in tumor cell proliferation, angiogenesis and metastasis illustrates their critical role in various stages of cancer development and highlights them as ideal targets for cancer immunotherapy. We further discuss the role of the chemokine signaling axes as therapeutic targets in cancer as a monotherapy or combination therapy.
10. Chemokines and chemokine receptors as a therapeutic target in cancer
Given the crucial role of chemokines/receptors in cancer development and progression, chemokine networks may be potential therapeutic targets in managing solid tumors.
10.1. Therapeutic targeting of CXCL8-CXCR1/2 axis in solid tumors
Repertaxin is a small molecule inhibitor of the CXCL8-CXCR1/2 axis that is under clinical development, owing to its promising effects in preclinical investigations. Repertaxin and blocking anti-CXCR1 monoclonal antibodies (but not anti-CXCR2 antibody) caused a considerable reduction in BCSC population, and FASL production in vitro (mediated by FAK/AKT/FOXO3A pathway), and tumor growth in a xenograft model of nude mice [99]. Furthermore, repertaxin indicates the possibility of chemotherapy-induced synergy in breast cancer; for example, a combination of repertaxin with paclitaxel reduced brain metastasis as well as the CSC population [183]. Higher expression of CXCL1/2 in breast tumors leads to metastasis and resistance to chemotherapy in a paracrine manner involving the TME and cancer cells. However, the blockade of CXCR2 inhibits the vicious cycle, increasing the efficacy of chemotherapy against breast cancer [184]. Thus, the use of another CXCR1/2 inhibitor, SCH563705, in combination with Lapatinib (HER2 targeted drug), was recently found to reduce CSC activity compared with either treatment alone in HER2-positive breast cancer via a novel SRC and EGFR/HER2-dependent mechanism [185]. Repertaxin also improved the efficacy of 5-fluorouracil (5-FU) in gastric cancer [186]. A phase I clinical trial of Repertaxin in combination with paclitaxel was found to be safe and well-tolerated in Her2 negative breast cancer with no significant side effects (NCT02001974) and phase II clinical trial of the same combination in metastatic TNBC aimed to determine median PFS, overall survival (OS), and objective response rates (NCT02370238) [183,187–189]. In head and neck cancer, AZD5069 (a CXCR2 inhibitor) is being tested in a phase II clinical trial (NCT02499328) [190].
CXCR2 inhibitors, AZ13381758 (referred to as CXCR2 SM) and CXCR2 pepducin (a short peptide) in combination with additional treatment (mainly anti-PD1 therapy and gemcitabine) were found to be more effective than any of the single drugs against pancreatic cancer [191]. A small molecule antagonist of CXCR1/2 (SCH-479833 or SCH-527123) reduced tumor cell proliferation, survival, invasive potential, and angiogenesis in mouse models of melanoma [192]. A CXCR2 inhibitor SB225002 synergized with Sorafenib (VEGFR inhibitor) in ovarian cancer [193]. Furthermore, in mice models of colon cancer, the CXCR1/2 antagonists, SCH479833 and SCH527123 decreased liver metastasis of colon cancer, and SCH527123 sensitize cells to oxaliplatin [194,195]. Another CXCR2 antagonist, AZ10397767 increased oxaliplatin and 5-FU-induced apoptosis in prostate cancer [196,197]. Importantly, CXCR2 inhibition using SB225002 decreased lung cancer progression and enhanced the therapeutic efficacy of cisplatin [30]. AZ10397767 decreased neutrophil infiltration in lung cancer [198]. In addition to that, G31 P, an antagonist of CXCR1/2, inhibited cell proliferation, adhesion, and migration of prostate cancer, hepatocellular carcinoma, and lung cancer [18,199]. Ladarixin, a small molecule inhibitor of CXCR1/2 had a multifactorial antitumoral effect on melanoma cells and blocked ALDH+ cell-dependent melanoma self-renewal mechanism [200]. Furthermore, neutralizing antibodies and siRNAs were utilized to treat various cancers by blocking the CXCL8-CXCR1/2 axis (as shown in Fig. 2A).
10.2. Targeting of CXCL9-11-CXCR3 axis in cancer
The CXCL9-11/CXCR3 axis is a promising target for drug development. Indeed, agents that increase paracrine CXCL9-11 expressions and decrease CXCR3 expression on cancer cells have shown an anti-tumor effect in various tumor models [37]. For the first time, it was demonstrated that the combination of gene therapy with CXCL9, also termed as monokine induced by gamma interferon (MIG) and recombinant antibody-IL-2 fusion protein (huKS1/4-IL-2), reduced lung metastases of CT26-KSA colon carcinoma in syngeneic BALB/c mice [201]. Further, the combination of plasmid-borne MIG (CXCL9) with cisplatin increased therapeutic efficacy and CTLs activation in lung and colon cancer [202]. In comparison to either IL-2 or CXCL9 alone, combining systemic IL-2 with an intratumor CXCL9 resulted in higher reduction in tumor growth and angiogenesis, enhanced tumor necrosis, and increased intratumor infiltration of CXCR3+ mononuclear cells in renal cancer [203]. Overexpression of CXCL10 inhibits tumor growth in the xenograft models of renal, breast, cervical, melanoma, sarcoma, lymphoma, and lung cancer [204]. The intratumor injection of CXCL10 inhibited tumor growth in a SCID mouse model of NSCLC and prolonged the survival of mice [205]. Indeed, CXCL10-Ig fusion protein dramatically increased the amount of NK and T cytotoxic cells in myeloma, resulting in decreased tumor size [206]. Furthermore, combining CXCL10-EGFRvIIIscFv with DC-activated CTLs, increased lymphocyte infiltration and improved treatment efficacy in glioma [37,204]. CXCL11-dependent therapy could be problematic as a target for cancer therapy as it triggers Treg migration and/or increases Tr1 and Th2 cell polarization. CXCL10, contrary to its tumor suppressor qualities, has a tumor-promoting activity in other cancers, which is thought to be mediated by increased expression of CXCR3 [207]. The inhibition of CXCR3 suppressed tumor growth and metastasis in mouse models. Interestingly, AMG487, a CXCR3 antagonist, showed promising results in various cancers including colon cancer, osteosarcoma, breast and liver cancer. AMG487 targets all CXCR3 variants and AMG487 mediated suppression of the paracrine axis may have a pro-tumor effect. Thus, a combination of immune activation ligands and pharmacological suppression of CXCR3A could be a novel approach to prevent metastasis [37].
Anti-PD-1 therapy may prevent “immune escape” and improve immunological trigger by increasing the expression of programmed cell death-1 (PD-1) in T cells at tumor site, which is higher than the T cells in peripheral circulation. Anti-PD-1 improved T cell-mediated tumor regression and increased the expression of CXCL10 and IFN-γ and CXCL9 or CXCL11 expression was unaffected. In cancer immunotherapy, increased secretion of Th1-type chemokines and effector T cells in TME are linked to enhanced therapeutic response [37]. Cancer epigenetic reprogramming has also been recognized as a master regulator of immunotherapy that involves the removal of epigenetic suppression of genes that encode TH1 chemokines, and promotion of the effector T cell recruitment into the TME [208]. Consequently, treatment with EZH2 inhibitors (DZNep199), a specific inhibitor of EZH2 methyltransferase activity (GSK126)200, or a DNMT inhibitor (5-aza-2′ -deoxycytidine) increases the anti-tumor response and enhances the therapeutic consequence of PDL1 blockade and T cell mediated therapy. Thus, targeted epigenetic reprogramming alters the immune microenvironment of tumors and may improve the clinical efficacy of cancer therapy.
10.3. Targeting of CXCL12-CXCR4 chemokine/receptor axis in solid tumors
CXCL12 and CXCR4 signaling is associated with trafficking of plasmacytoid DC and Treg cells into tumor sites, which is critical for tumor cell proliferation, metastasis, and vascularization [122]. Although AMD3100 (Plerixafor), a CXCR4 antagonist, has received clinical approval for HIV infection and inflammation, some other inhibitors still remain in the early developmental stages. Blocking CXCR4-CXCL12 signaling may inhibit tumor angiogenesis, invasiveness, and immunosuppression in TME [64,122,209]. Indeed, a CXCR4 antagonist, AMD3100 and AMD3465 exhibited anticancer and anti-metastatic properties in solid tumors, enhancing the efficacy of standard treatment in haematological malignancies. As a result, it is tempting to speculate that using CXCR4-CXCL12 signaling antagonists in combination with current conventional and immunotherapies could be therapeutically advantageous. For example, AMD3100 improved the efficacy of cytarabine (Ara-C) treatment in AML mouse models. LY2510924, another CXCR4 antagonist used as a monotherapy, reduced AML proliferation and progression.
In a mouse model of Non-Hodgkin Lymphoma (NHL), another CXCR4 antagonist, BKT140, demonstrated anti-leukemic activity, and its action was synergistic with Rituximab (anti-CD20 antibody). Furthermore, BKT140 and imatinib (a tyrosine kinase inhibitor) had a synergistic impact in chronic myelogenous leukemia (CML) in both in vitro and in vivo studies. AMD3465, a CXCR4 antagonist has a strong antitumor effect in GBM and medulloblastoma cell lines. Moreover, PRX177561 (CXCR4 antagonist), improved the anticancer effects of Bevacizumab and Sunitinib in GBM models. POL5551 obstructed GBM growth and dissemination following anti-VEGF therapy. Finally, AMD3465 and LY2510924 were demonstrated to suppress tumor growth and metastasis in various tumors.
In PDAC, CXCR4 inhibitors like AMD3100 increase anti-tumor T cell responses by interfering with the CXCL12-CXCR4 axis, causing T cell accumulation and functioning synergistically with anti-PD-L1, whereas AMD3100 also inhibits Treg infiltration and promotes antitumoral T cell responses in ovarian cancer. Several clinical trials have been conducted to examine the efficacy and effectiveness of CXCR4 antagonists alone or in combination against a solid tumor (Table 1).
A phase I/II clinical trial in patients with AML relapse showed that AMD3100 increased the efficacy of idarubicin, cytarabine, fludarabine, and G-CSF (PLERIFLAG regimen) treatment (NCT01435343) [210]. The anti-CXCR4 mAbs BMS-936564 in AML patients (NCT01120457) and PF-06747143 in hematological malignancies (NCT02954653) are now undergoing phase I clinical trials to assess their safety and tolerability [211,212]. In phase II clinical trial, CXCR4 inhibitors, such as BL-8040, along with a PD-1 antagonist (pembrolizumab) promoted anti-tumor response via infiltration of effector T cells and decreased the number of immune suppressor cells, indicating that effector T cells potentiate chemotherapy advantage (NCT02826486) and could be used as a second or third-line therapy for in metastatic PDAC patients [213]. AMD3100 + bevacizumab (VEGFR inhibitor) was tested in recurrent high-grade glioma patients in phase I clinical trial, and it was well tolerated (NCT01339039) [214]. Another phase II clinical trial of AMD3100 after radiation therapy and temozolomide in patients with newly diagnosed high-grade glioma was completed (NCT01977677), and a phase II clinical trial of whole-brain radiation therapy with standard temozolomide and AMD3100 in patients with glioblastoma is currently underway (NCT03746080) [215,216]. Another phase I/II study based on USL311 (CXCR4 antagonist) alone or in combination with Lomustine has been completed in cases ofadvanced solid tumors or with relapsed/recurrent GBM (NCT02765165) to explore the safety, efficacy, tolerability, and pharmacokinetics [217]. In phase, I clinical trial (NCT02737072), a CXCR4 peptide antagonist, LY2510924 was found to be safe and well-tolerated as seen in cases of advanced solid tumors (colorectum, lung, breast, and prostate) [218]. POL6326 (Balixafortide), a CXCR4 peptide antagonist, is effective in patients with metastatic breast cancer in phase I clinical trial in combination with Eribulin (NCT01837095) [219]. A phase I clinical trial of CXCR4 inhibitor mavorixafor and nivolumab has been conducted in renal cell carcinoma patients with no prior response to nivolumab monotherapy.
10.4. Targeting of CCL(X)-CCR(X) axis in solid tumors
In preclinical cancer models, CCR1 and CCR2-targeted therapies predominantly affect macrophage infiltration and repress tumor growth and metastasis [133]. CCR1 antagonists, such as CCX721, BL5923, and CCX9588, have been shown to inhibit tumor growth in myeloma, liver and lung metastases of colon cancer, and breast cancer, in preclinical studies whether taken alone or in combination with immunotherapy. In a mouse model, the anti-CCL2 antibodies showed therapeutic benefit in renal cell carcinoma. CCR2/CCL2 inhibition prohibited monocyte infiltration and seeding of metastatic breast cancer cells and enhanced response to radiotherapy in breast cancer [220]. TAM infiltration and tumor growth was reduced by the CCR2 inhibitor (PF-04136309) in a syngeneic PDA tumor model whereas combination of PF-04136309 (CCR2 antagonists) and Gemcitabine (chemotherapeutic drug) had a synergistic effect on tumor growth in pancreatic tumors. Another, CCR2 inhibitor, CCX872 also improved the efficacy of anti-PDA-1 treatment in pancreatic tumors. CCR2 antagonists, RDC018 or 747 plus Sorafenib were seen to repress tumor growth and metastasis in mouse models of HCC [220]. In a phase II clinical based on cancer bone metastasis patients, anti-CCR2 antibody (MLN1202) reduced the N-telopeptide (uNTX) following 43 days of treatment in 14 out of the 43 cancer patients. In a phase I/II clinical, PF-04136309 along with nab-Paclitaxel (PTX) and FOLFIRINOX (FX) in pancreatic cancer patients (NCT02732938 and NCT01413022) showed that combination treatment could increase the percentage of objective response [221,222]. In pancreatic cancer patients, the CCR2 antagonist, CCX872, when used along with FOLFIRINOX was reported as safe and enhanced the overall survival than FOLFIRINOX alone (29 % versus 18.6 % at 18 months).
The blocking of the CCR4–CCL22/17 axis through siRNAs, use of blocking antibodies and antagonists successfully abrogated Tregs recruitment in preclinical cancer models [223]. This axis is currently being explored as a therapeutic target in clinical trials. CCR4 blocking mAb, Affi 5, and CCR4 antagonist inhibited tumor growth in renal cancer and is considered as a novel target. Moreover, the CCR4 antagonist, AF399/420/1802, enhanced the efficacy in cancer vaccines by Tregs inhibition in melanoma, lung, and colon cancer. Mogamulizumab (KW-0761), a glycol-engineered, humanized IgG1κ monoclonal antibody targets CCR4-positive cells through cell mediated cytotoxicity (ADCC). Pharmaceutical companies such as GlaxoSmithKline (GSK2239633) and AstraZeneca (AZD-1678, AZD-2098) have developed allosteric antagonists which bind to intracellular domain of CCR4. Small-molecule inhibitors of CCR4, termed FLX475 and RPT193, have been developed by RAPT pharmaceuticals. Phase 1/2 clinical trials of FLX475 in combination with pembrolizumab have recently been completed (NCT03674567) [224]. However, the exact therapeutic potential of Treg depletion through CCR4 inhibition is yet to be explored.
Targeting CCR5 with Maraviroc (negative allosteric inhibitor) induced anti-tumor immunity with the polarization of macrophages in colon cancer [225]. A phase I clinical trial of CCR5-antagonist Maraviroc has been done in advanced colorectal cancer patients with hepatic liver metastases which inhibited non-toxic tumor growth (NCT01736813)[226]. CCR7 silencing using siRNA inhibited tumor growth of prostate cancer and metastasis of colon cancer and breast cancer [81]. Recent studies show that CCR7 is expressed on BCSCs and inhibition of CCR7 reduces self-renewal and mammosphere formation in mice. Finally, T-cell acute lymphoblastic leukaemia brain metastasis was reported to be inhibited by single-chain antibodies targeting CCR7 (MSM R707)[227].
11. Conclusion and future directions
Chemokines have multifaceted role in tumor progression and are involved leukocyte infiltration, proliferation/survival, angiogenesis maintenance of cancer stem cells and metastasis. Despite the availability of a large body of literature on the complex nature of chemokine network in tumors, our understanding of the multifarious roles of chemokines and their value as prognostic indicators in various cancers, particularly in response to anticancer therapies, remains limited. The chemokine network in human cancers is still partially understood. Chemokines could be tumor suppressors or promoters and measurement of specific chemokine levels and their receptor expression in cancer cells may have differential diagnostic value. If upregulation of a chemokine/ chemokine receptor positively correlated with the progression of any solid tumor then its therapeutic potential would necessitate preclinical demonstration of anti-cancer efficacy prior to clinical development. Conversely, if downregulation of a chemokine/chemokine receptor is correlated with the progression of any solid tumor then increasing its expression specifically at the tumor site is required which is arduous and avoided due to lack of feasibility. However, the chemokines system may pose a major challenge due to their promiscuity and expression in a wide variety of tissues. Hence, inhibiting chemokine signaling either by receptor blockade or ligand neutralization is expected to exert generalized systemic impact that could result in undesirable side effects. Therefore, targeting the cancer cells with the drug candidate (receptor antagonist or ligand neutralizing molecule) would afford a safer option to treat the cancer types regulated by a chemokine.
Acknowledgements
This work was supported by grants from the Department of Health Research awarded to BC (12013/21/2020-HR), SR (YSS/2020/000009/ PRCYSS), and Wellcome Trust/DBT India Alliance Fellowship to RAS (IA/I/16/2/502691).
Abbreviations
- TME
Tumor microenvironment
- GPCRs
G protein-coupled receptors
- ECM
Extracellular matrix
- NK
Natural-killer
- CSCs
Cancer stem cells
- PI3K
Phosphoinositide-3-kinase
- PKC
Protein kinase C
- PLC
Phospholipase C
- SFK
Src family tyrosine kinases
- SDF-1
Stromal cell-derived factor1
- TAFs
Tumor-associated fibroblasts
- MDSCs
Myeloid-derived suppressor cells
- Tregs
Regulatory T cells
- CAFs
Cancer-associated fibroblasts
- TAMs
Tumor-associated macrophages
- DCs
Dendritic Cells
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- [1].Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- [2].Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9(9):949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- [3].Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28(5):492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- [4].Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- [5].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- [6].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [7].Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- [8].Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54(5):859–874. doi: 10.1016/j.immuni.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- [10].Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16(1):41. doi: 10.1186/s12943-017-0600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis–tracing the accessory. Oncogene. 2014;33(25):3217–3224. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- [12].Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270(45):27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- [13].Chen K, Bao Z, Tang P, Gong W, Yoshimura T, Wang JM. Chemokines in homeostasis and diseases. Cell Mol Immunol. 2018;15(4):324–334. doi: 10.1038/cmi.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England) 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- [15].Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220(4593):210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- [16].Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345(11):833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- [17].Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- [18].Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71. doi: 10.1016/j.cytogfr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7(6):1543–1588. doi: 10.7150/thno.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mishra A, Suman KH, Nair N, Majeed J, Tripathi V. An updated review on the role of the CXCL8-CXCR1/2 axis in the progression and metastasis of breast cancer. Mol Biol Rep. 2021;48(9):6551–6561. doi: 10.1007/s11033-021-06648-8. [DOI] [PubMed] [Google Scholar]
- [21].Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72(1):9–18. [PMC free article] [PubMed] [Google Scholar]
- [22].Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol. 1996;156(3):1132–1137. [PubMed] [Google Scholar]
- [23].Todorovic-Rakovic N, Milovanovic J. Interleukin-8 in breast cancer progression. J Interferon Cytokine Res. 2013;33(10):563–570. doi: 10.1089/jir.2013.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lesina M, Wormann SM, Morton J, Diakopoulos KN, Korneeva O, Wimmer M, Einwachter H, Sperveslage J, Demir IE, Kehl T, Saur D, et al. RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J Clin Invest. 2016;126(8):2919–2932. doi: 10.1172/JCI86477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Geismann C, Schafer H, Gundlach JP, Hauser C, Egberts JH, Schneider G, Arlt A. NF-kappaB dependent chemokine signaling in pancreatic cancer. Cancers (Basel) 2019;11(10) doi: 10.3390/cancers11101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line. Capan-1, Pancreas. 2000;21(1):52–56. doi: 10.1097/00006676-200007000-00051. [DOI] [PubMed] [Google Scholar]
- [27].Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer. 1997;73(1):94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- [28].Erin N, Tavsan E, Akdeniz O, Isca VMS, Rijo P. Rebound increases in chemokines by CXCR2 antagonist in breast cancer can be prevented by PKCdelta and PKCepsilon activators. Cytokine. 2021;142:155498. doi: 10.1016/j.cyto.2021.155498. [DOI] [PubMed] [Google Scholar]
- [29].Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL. Coexpression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg. 1997;174(5):507–512. doi: 10.1016/s0002-9610(97)00165-7. [DOI] [PubMed] [Google Scholar]
- [30].Cheng Y, Mo F, Li Q, Han X, Shi H, Chen S, Wei Y, Wei X. Targeting CXCR2 inhibits the progression of lung cancer and promotes therapeutic effect of cisplatin. Mol Cancer. 2021;20(1):62. doi: 10.1186/s12943-021-01355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chao T, Furth EE, Vonderheide RH. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2016;4(11):968–982. doi: 10.1158/2326-6066.CIR-16-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].New J, Arnold L, Ananth M, Alvi S, Thornton M, Werner L, Tawfik O, Dai H, Shnayder Y, Kakarala K, Tsue TT, et al. Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target. Cancer Res. 2017;77(23):6679–6691. doi: 10.1158/0008-5472.CAN-17-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391(6662):86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- [34].Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M, Sullivan L, Jenh CH, Narula SK, Chensue SW, Lira SA. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi’s sarcoma. J Exp Med. 2000;191(3):445–454. doi: 10.1084/jem.191.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Burger M, Burger JA, Hoch RC, Oades Z, Takamori H, Schraufstatter IU. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi’s sarcoma herpesvirus-G protein-coupled receptor. J Immunol. 1999;163(4):2017–2022. [PubMed] [Google Scholar]
- [36].Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, McSkane M, Baba H, Lenz HJ. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - a target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–47. doi: 10.1016/j.ctrv.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cannon A, Thompson CM, Bhatia R, Kandy RRK, Solheim JC, Batra SK, Kumar S. Contribution of CXCR3-mediated signaling in the metastatic cascade of solid malignancies. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188628. doi: 10.1016/j.bbcan.2021.188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oghumu S, Varikuti S, Terrazas C, Kotov D, Nasser MW, Powell CA, Ganju RK, Satoskar AR. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology. 2014;143(1):109–119. doi: 10.1111/imm.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wu Q, Dhir R, Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol Cancer. 2012;11:3. doi: 10.1186/1476-4598-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M, Taketo MM. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26(32):4679–4688. doi: 10.1038/sj.onc.1210267. [DOI] [PubMed] [Google Scholar]
- [42].Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64(11):4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- [43].Yang C, Zheng W, Du W. CXCR3A contributes to the invasion and metastasis of gastric cancer cells. Oncol Rep. 2016;36(3):1686–1692. doi: 10.3892/or.2016.4953. [DOI] [PubMed] [Google Scholar]
- [44].Windmuller C, Zech D, Avril S, Boxberg M, Dawidek T, Schmalfeldt B, Schmitt M, Kiechle M, Bronger H. CXCR3 mediates ascites-directed tumor cell migration and predicts poor outcome in ovarian cancer patients. Oncogenesis. 2017;6(5):e331. doi: 10.1038/oncsis.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Quinn KE, Mackie DI, Caron KM. Emerging roles of atypical chemokine receptor 3 (ACKR3) in normal development and physiology. Cytokine. 2018;109:17–23. doi: 10.1016/j.cyto.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- [47].Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- [48].Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16(10):1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- [49].Ara T, Itoi M, Kawabata K, Egawa T, Tokoyoda K, Sugiyama T, Fujii N, Amagai T, Nagasawa T. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170(9):4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- [50].Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15(2):323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- [51].Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- [52].Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- [55].Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T, Schettini G. Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003;63(8):1969–1974. [PubMed] [Google Scholar]
- [56].Hideshima T, Chauhan D, Hayashi T, Podar K, Akiyama M, Gupta D, Richardson P, Munshi N, Anderson KC. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002;1(7):539–544. [PubMed] [Google Scholar]
- [57].Shi Y, Riese DJ, 2nd, Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol. 2020;11:574667. doi: 10.3389/fphar.2020.574667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Guo Q, Gao BL, Zhang XJ, Liu GC, Xu F, Fan QY, Zhang SJ, Yang B, Wu XH. CXCL12-CXCR4 axis promotes proliferation, migration, invasion, and metastasis of ovarian cancer. Oncol Res. 2014;22(5–6):247–258. doi: 10.3727/096504015X14343704124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fung AS, Kopciuk K, Dean ML, D’Silva A, Otsuka S, Klimowicz A, Hao D, Morris D, Bebb DG. CXCR4 expression in lung carcinogenesis: evaluating gender-specific differences in survival outcomes based on CXCR4 expression in early stage non-small cell lung cancer patients. PLoS One. 2021;16(1):e0241240. doi: 10.1371/journal.pone.0241240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B. CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget. 2015;6(7):5022–5040. doi: 10.18632/oncotarget.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].do Carmo A, Patricio I, Cruz MT, Carvalheiro H, Oliveira CR, Lopes MC. CXCL12/CXCR4 promotes motility and proliferation of glioma cells. Cancer Biol Ther. 2010;9(1):56–65. doi: 10.4161/cbt.9.1.10342. [DOI] [PubMed] [Google Scholar]
- [62].Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- [63].Korbecki J, Kojder K, Siminska D, Bohatyrewicz R, Gutowska I, Chlubek D, Baranowska-Bosiacka I. CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of the ligands of receptors CCR1, CCR2, CCR3, and CCR4. Int J Mol Sci. 2020;21(21) doi: 10.3390/ijms21218412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bule P, Aguiar SI, Aires-Da-Silva F, Dias JNR. Chemokine-directed tumor microenvironment modulation in cancer immunotherapy. Int J Mol Sci. 2021;22(18) doi: 10.3390/ijms22189804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Scotton C, Milliken D, Wilson J, Raju S, Balkwill F. Analysis of CC chemokine and chemokine receptor expression in solid ovarian tumours. Br J Cancer. 2001;85(6):891–897. doi: 10.1054/bjoc.2001.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997;150(5):1723–1734. [PMC free article] [PubMed] [Google Scholar]
- [67].Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, Allavena P, Sozzani S, Mantovani A, Balkwill FR. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95(5):2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167(12):1676–1686. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- [69].Gunther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Forster R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer. 2005;116(5):726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- [70].Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62(10):2937–2941. [PubMed] [Google Scholar]
- [71].Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93(21):1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- [72].Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H, Imamura M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9(9):3406–3412. [PubMed] [Google Scholar]
- [73].Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119(9):2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- [74].C. Group Young Researchers In Inflammatory. Wandmacher AM, Mehdorn AS, Sebens S. The heterogeneity of the tumor microenvironment as essential determinant of development, progression and therapy response of pancreatic cancer. Cancers (Basel) 2021;13(19) doi: 10.3390/cancers13194932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].De Sousa EMF, Vermeulen L, Fessler E, Medema JP. Cancer heterogeneity–a multifaceted view. EMBO Rep. 2013;14(8):686–695. doi: 10.1038/embor.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hass R. Role of MSC in the tumor microenvironment. Cancers (Basel) 2020;12(8) doi: 10.3390/cancers12082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59. doi: 10.1186/s12964-020-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gao Y, Bado I, Wang H, Zhang W, Rosen JM, Zhang XH. Metastasis organotropism: redefining the congenial soil. Dev Cell. 2019;49(3):375–391. doi: 10.1016/j.devcel.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jacquelot N, Duong CPM, Belz GT, Zitvogel L. Targeting chemokines and chemokine receptors in melanoma and other cancers. Front Immunol. 2018;9:2480. doi: 10.3389/fimmu.2018.02480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Vilgelm AE, Richmond A. Chemokines modulate immune surveillance in Tumorigenesis, metastasis, and response to immunotherapy. Front Immunol. 2019;10:333. doi: 10.3389/fimmu.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rizeq B, Malki MI. The role of CCL21/CCR7 chemokine axis in breast cancer progression. Cancers (Basel) 2020;12(4) doi: 10.3390/cancers12041036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].McGranahan N, Swanton C. Clonal Heterogeneity, Tumor evolution: past, present, and the future. Cell. 2017;168(4):613–628. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- [83].Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Prager BC, Xie Q, Bao S, Rich JN. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell. 2019;24(1):41–53. doi: 10.1016/j.stem.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gerber JM, Smith BD, Ngwang B, Zhang H, Vala MS, Morsberger L, Galkin S, Collector MI, Perkins B, Levis MJ, Griffin CA, et al. A clinically relevant population of leukemic CD34(+)CD38(-) cells in acute myeloid leukemia. Blood. 2012;119(15):3571–3577. doi: 10.1182/blood-2011-06-364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- [88].Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chen P, Hsu WH, Han J, Xia Y, DePinho RA. Cancer stemness meets immunity: from mechanism to therapy. Cell Rep. 2021;34(1):108597. doi: 10.1016/j.celrep.2020.108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017;50(6):285–298. doi: 10.5483/BMBRep.2017.50.6.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Luond F, Tiede S, Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br J Cancer. 2021;125(2):164–175. doi: 10.1038/s41416-021-01328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Turner KM, Yeo SK, Holm TM, Shaughnessy E, Guan JL. Heterogeneity within molecular subtypes of breast cancer. Am J Physiol Cell Physiol. 2021;321(2):C343–C354. doi: 10.1152/ajpcell.00109.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fico F, Santamaria-Martinez A. The tumor microenvironment as a driving force of breast cancer stem cell plasticity. Cancers (Basel) 2020;12(12) doi: 10.3390/cancers12123863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kim BJ, Hannanta-Anan P, Ryd A, Swartz MA, Wu M. Lymphoidal chemokine CCL19 promoted the heterogeneity of the breast tumor cell motility within a 3D microenvironment revealed by a Levy distribution analysis. Integr Biol (Camb) 2020;12(1):12–20. doi: 10.1093/intbio/zyaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lopez de Andres J, Grinan-Lison C, Jimenez G, Marchal JA. Cancer stem cell secretome in the tumor microenvironment: a key point for an effective personalized cancer treatment. J Hematol Oncol. 2020;13(1):136. doi: 10.1186/s13045-020-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lei MML, Lee TKW. Cancer stem cells: emerging key players in immune evasion of cancers. Front Cell Dev Biol. 2021;9:692940. doi: 10.3389/fcell.2021.692940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ruffini PA. The CXCL8-CXCR1/2 axis as a therapeutic target in breast cancer stem-like cells. Front Oncol. 2019;9:40. doi: 10.3389/fonc.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhou J, Yi L, Ouyang Q, Xu L, Cui H, Xu M. Neurotensin signaling regulates stem-like traits of glioblastoma stem cells through activation of IL-8/CXCR1/ STAT3 pathway. Cell Signal. 2014;26(12):2896–2902. doi: 10.1016/j.cellsig.2014.08.027. [DOI] [PubMed] [Google Scholar]
- [99].Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120(2):485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15(4):210. doi: 10.1186/bcr3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, Yan F, Wang Y, Bouchez LC, Kularatne SA, Watson J, et al. CXCR4 expression in prostate cancer progenitor cells. PLoS One. 2012;7(2):e31226. doi: 10.1371/journal.pone.0031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lopez-Gil JC, Martin-Hijano L, Hermann PC, Sainz B., Jr The CXCL12 crossroads in cancer stem cells and their niche. Cancers (Basel) 2021;13(3) doi: 10.3390/cancers13030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G, De Vries EG, de Jong IJ, Walenkamp AM. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia. 2012;14(8):709–718. doi: 10.1593/neo.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jung Y, Cackowski FC, Yumoto K, Decker AM, Wang J, Kim JK, Lee E, Wang Y, Chung JS, Gursky AM, Krebsbach PH, et al. CXCL12gamma promotes metastatic castration-resistant prostate cancer by inducing cancer stem cell and neuroendocrine phenotypes. Cancer Res. 2018;78(8):2026–2039. doi: 10.1158/0008-5472.CAN-17-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang L, Wang D, Li Y, Liu Y, Xie X, Wu Y, Zhou Y, Ren J, Zhang J, Zhu H, Su Z. CCL21/CCR7 axis contributed to CD133+ pancreatic cancer stem-like cell metastasis via EMT and Erk/NF-kappaB pathway. PLoS One. 2016;11(8):e0158529. doi: 10.1371/journal.pone.0158529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Khor B. Regulatory T cells: central concepts from ontogeny to therapy. Transfus Med Rev. 2017;31(1):36–44. doi: 10.1016/j.tmrv.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- [109].Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- [110].Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70(20):7800–7809. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- [111].Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10:2453. doi: 10.3389/fimmu.2019.02453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ben-Shmuel A, Biber G, Barda-Saad M. Unleashing natural killer cells in the tumor microenvironment-the next generation of immunotherapy? Front Immunol. 2020;11:275. doi: 10.3389/fimmu.2020.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116. doi: 10.1186/s12943-020-01234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M. CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res. 2012;72(17):4351–4360. doi: 10.1158/0008-5472.CAN-12-0579. [DOI] [PubMed] [Google Scholar]
- [115].Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- [116].Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122(10):2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- [117].Maruyama T, Kono K, Izawa S, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis Esophagus. 2010;23(5):422–429. doi: 10.1111/j.1442-2050.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- [118].Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26(1):78. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. doi: 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021;6(1):127. doi: 10.1038/s41392-021-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Korbecki J, Grochans S, Gutowska I, Barczak K, Baranowska-Bosiacka I. CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. 2020;21(20) doi: 10.3390/ijms21207619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Li D, Ji H, Niu X, Yin L, Wang Y, Gu Y, Wang J, Zhou X, Zhang H, Zhang Q. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. 2020;111(1):47–58. doi: 10.1111/cas.14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Jin J, Lin J, Xu A, Lou J, Qian C, Li X, Wang Y, Yu W, Tao H. CCL2: an important mediator between tumor cells and host cells in tumor microenvironment. Front Oncol. 2021;11:722916. doi: 10.3389/fonc.2021.722916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol. 2001;166(11):6483–6490. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- [127].Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, Di Carlo V, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63(21):7451–7461. [PubMed] [Google Scholar]
- [128].Aldinucci D, Borghese C, Casagrande N. The CCL5/CCR5 axis in cancer progression. Cancers (Basel) 2020;12(7) doi: 10.3390/cancers12071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Milliken D, Scotton C, Raju S, Balkwill F, Wilson J. Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin Cancer Res. 2002;8(4):1108–1114. [PubMed] [Google Scholar]
- [130].Lane D, Matte I, Laplante C, Garde-Granger P, Carignan A, Bessette P, Rancourt C, Piche A. CCL18 from ascites promotes ovarian cancer cell migration through proline-rich tyrosine kinase 2 signaling. Mol Cancer. 2016;15(1):58. doi: 10.1186/s12943-016-0542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kleeff J, Kusama T, Rossi DL, Ishiwata T, Maruyama H, Friess H, Buchler MW, Zlotnik A, Korc M. Detection and localization of Mip-3alpha/LARC/ Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int J Cancer. 1999;81(4):650–657. doi: 10.1002/(sici)1097-0215(19990517)81:4<650::aid-ijc23>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- [132].Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256(2):137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Argyle D, Kitamura T. Targeting macrophage-recruiting chemokines as a novel therapeutic strategy to prevent the progression of solid tumors. Front Immunol. 2018;9:2629. doi: 10.3389/fimmu.2018.02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hatziioannou A, Alissafi T, Verginis P. Myeloid-derived suppressor cells and T regulatory cells in tumors: unraveling the dark side of the force. J Leukoc Biol. 2017;102(2):407–421. doi: 10.1189/jlb.5VMR1116-493R. [DOI] [PubMed] [Google Scholar]
- [137].Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70(15):6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015;7(5) doi: 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322. doi: 10.1002/JLB.4RI0818-311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in Tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019;9:1146. doi: 10.3389/fonc.2019.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601–620. doi: 10.1038/s41571-019-0222-4. [DOI] [PubMed] [Google Scholar]
- [144].Tolle F, Umansky V, Utikal J, Kreis S, Brechard S. Neutrophils in tumorigenesis: missing targets for successful next generation cancer therapies? Int J Mol Sci. 2021;22(13) doi: 10.3390/ijms22136744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Capucetti A, Albano F, Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front Immunol. 2020;11:1259. doi: 10.3389/fimmu.2020.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S. How do chemokines navigate neutrophils to the target site: dissecting the structural mechanisms and signaling pathways. Cell Signal. 2019;54:69–80. doi: 10.1016/j.cellsig.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Loveless R, Shay C, Teng Y. Unveiling tumor microenvironment interactions using zebrafish models. Front Mol Biosci. 2020;7:611847. doi: 10.3389/fmolb.2020.611847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Powell D, Lou M, Barros Becker F, Huttenlocher A. Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. Sci Rep. 2018;8(1):13285. doi: 10.1038/s41598-018-31675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Jablonska J, Wu CF, Andzinski L, Leschner S, Weiss S. CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-beta. Int J Cancer. 2014;134(6):1346–1358. doi: 10.1002/ijc.28551. [DOI] [PubMed] [Google Scholar]
- [150].Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56(6):2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- [151].Van Coillie E, Van Aelst I, Wuyts A, Vercauteren R, Devos R, De Wolf-Peeters C, Van Damme J, Opdenakker G. Tumor angiogenesis induced by granulocyte chemotactic protein-2 as a countercurrent principle. Am J Pathol. 2001;159(4):1405–1414. doi: 10.1016/S0002-9440(10)62527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Noubade R, Majri-Morrison S, Tarbell KV. Beyond cDC1: emerging roles of DC crosstalk in cancer immunity. Front Immunol. 2019;10:1014. doi: 10.3389/fimmu.2019.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, Mantovani A. The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol Rev. 2000;177:141–149. doi: 10.1034/j.1600-065x.2000.17714.x. [DOI] [PubMed] [Google Scholar]
- [154].Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940. doi: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kim CW, Kim KD, Lee HK. The role of dendritic cells in tumor microenvironments and their uses as therapeutic targets. BMB Rep. 2021;54(1):31–43. doi: 10.5483/BMBRep.2021.54.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, Deng S, Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6(1):218. doi: 10.1038/s41392-021-00641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42(6):768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [158].Zhou X, Peng M, He Y, Peng J, Zhang X, Wang C, Xia X, Song W. CXC chemokines as therapeutic targets and prognostic biomarkers in skin cutaneous melanoma microenvironment. Front Oncol. 2021;11:619003. doi: 10.3389/fonc.2021.619003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Riverso M, Kortenkamp A, Silva E. Non-tumorigenic epithelial cells secrete MCP-1 and other cytokines that promote cell division in breast cancer cells by activating ERalpha via PI3K/Akt/mTOR signaling. Int J Biochem Cell Biol. 2014;53:281–294. doi: 10.1016/j.biocel.2014.05.023. [DOI] [PubMed] [Google Scholar]
- [160].Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. J Biol Chem. 2012;287(43):36593–36608. doi: 10.1074/jbc.M112.365999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sauve K, Lepage J, Sanchez M, Heveker N, Tremblay A. Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway. Cancer Res. 2009;69(14):5793–5800. doi: 10.1158/0008-5472.CAN-08-4924. [DOI] [PubMed] [Google Scholar]
- [162].Morein D, Erlichman N, Ben-Baruch A. Beyond cell motility: the expanding roles of chemokines and their receptors in malignancy. Front Immunol. 2020;11:952. doi: 10.3389/fimmu.2020.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Gao D, Rahbar R, Fish EN. CCL5 activation of CCR5 regulates cell metabolism to enhance proliferation of breast cancer cells. Open Biol. 2016;6(6) doi: 10.1098/rsob.160122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- [165].Do HTT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers (Basel) 2020;12(2) doi: 10.3390/cancers12020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26(3–4):453–467. doi: 10.1007/s10555-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Rosenkilde MM, Schwartz TW. The chemokine system – a major regulator of angiogenesis in health and disease. APMIS. 2004;112(7–8):481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- [169].Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273(7):4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- [170].Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154(2):375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Lecot P, Sarabi M, Pereira Abrantes M, Mussard J, Koenderman L, Caux C, Bendriss-Vermare N, Michallet MC. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol. 2019;10:2155. doi: 10.3389/fimmu.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Quintero-Fabian S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argaez V, Lara-Riegos J, Ramirez-Camacho MA, Alvarez-Sanchez ME. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- [174].Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79(4):639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- [175].Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4(12):2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Zhang Y, Xu L, Peng M. CXCR3 is a prognostic marker and a potential target for patients with solid tumors: a meta-analysis. OncoTargets Ther. 2018;11:1045–1054. doi: 10.2147/OTT.S157421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol. 2019;10:379. doi: 10.3389/fimmu.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Khare T, Bissonnette M, Khare S. CXCL12-CXCR4/CXCR7 axis in colorectal cancer: therapeutic target in preclinical and clinical studies. Int J Mol Sci. 2021;22(14) doi: 10.3390/ijms22147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Fang L, Lee VC, Cha E, Zhang H, Hwang ST. CCR7 regulates B16 murine melanoma cell tumorigenesis in skin. J Leukoc Biol. 2008;84(4):965–972. doi: 10.1189/jlb.1107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Zhu H, Gu Y, Xue Y, Yuan M, Cao X, Liu Q. CXCR2(+) MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget. 2017;8(70):114554–114567. doi: 10.18632/oncotarget.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Sionov RV. Leveling up the controversial role of neutrophils in cancer: when the complexity becomes entangled. Cells. 2021;10(9) doi: 10.3390/cells10092486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Nasrollahzadeh E, Razi S, Keshavarz-Fathi M, Mazzone M, Rezaei N. Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol Immunother. 2020;69(9):1673–1697. doi: 10.1007/s00262-020-02616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, Perez RP, Kato G, Wicha M. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res. 2017;23(18):5358–5365. doi: 10.1158/1078-0432.CCR-16-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Cheng Y, Ma XL, Wei YQ, Wei XW. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer. 2019;1871(2):289–312. doi: 10.1016/j.bbcan.2019.01.005. [DOI] [PubMed] [Google Scholar]
- [185].Singh JK, Farnie G, Bundred NJ, Simoes BM, Shergill A, Landberg G, Howell SJ, Clarke RB. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19(3):643–656. doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Wang J, Hu W, Wang K, Yu J, Luo B, Luo G, Wang W, Wang H, Li J, Wen J. Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. Int J Oncol. 2016;48(4):1341–1352. doi: 10.3892/ijo.2016.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Goldstein LJ, Perez RP, Yardley D, Han LK, Reuben JM, Gao H, McCanna S, Butler B, Ruffini PA, Liu Y, Rosato RR, et al. Correction to: a window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in operable HER-2-negative breast cancer. Breast Cancer Res. 2020;22(1):52. doi: 10.1186/s13058-020-01294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Pilot Study to Evaluate Reparixin With Weekly Paclitaxel in Patients With H.E.R. 2 Negative Metastatic Breast Cancer (MBC) https://ClinicalTrials.gov/show/NCT02001974 . [DOI] [PMC free article] [PubMed]
- [189].A Double-blind Study of Paclitaxel in Combination With Reparixin or Placebo for Metastatic Triple-Negative Breast Cancer. https://ClinicalTrials.gov/show/NCT02370238 . [DOI] [PMC free article] [PubMed]
- [190].Study to Assess MEDI4736 With Either AZD9150 or AZD5069 in Advanced Solid Tumors & Relapsed Metastatic Squamous Cell Carcinoma of Head & Neck. https://ClinicalTrials.gov/show/NCT02499328 .
- [191].Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, Eberlein C, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016;29(6):832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, Singh RK. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res. 2009;15(7):2380–2386. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Devapatla B, Sharma A, Woo S. CXCR2 inhibition combined with sorafenib improved antitumor and antiangiogenic response in preclinical models of ovarian cancer. PLoS One. 2015;10(9):e0139237. doi: 10.1371/journal.pone.0139237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011;300(2):180–188. doi: 10.1016/j.canlet.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Nagulapalli Venkata KC, Louie SG, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11(6):1353–1364. doi: 10.1158/1535-7163.MCT-11-0915. [DOI] [PubMed] [Google Scholar]
- [196].Wilson C, Maxwell PJ, Longley DB, Wilson RH, Johnston PG, Waugh DJ. Constitutive and treatment-induced CXCL8-signalling selectively modulates the efficacy of anti-metabolite therapeutics in metastatic prostate cancer. PLoS One. 2012;7(5):e36545. doi: 10.1371/journal.pone.0036545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O’Sullivan JM, Wilson RH, Johnston PG, Waugh DJ. Chemotherapy-induced CXC-chemokine/ CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J Pharmacol Exp Ther. 2008;327(3):746–759. doi: 10.1124/jpet.108.143826. [DOI] [PubMed] [Google Scholar]
- [198].Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE, Murdoch C. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129(4):847–858. doi: 10.1002/ijc.25987. [DOI] [PubMed] [Google Scholar]
- [199].Liu X, Peng J, Sun W, Yang S, Deng G, Li F, Cheng JW, Gordon JR. G31P, an antagonist against CXC chemokine receptors 1 and 2, inhibits growth of human prostate cancer cells in nude mice. Tohoku J Exp Med. 2012;228(2):147–156. doi: 10.1620/tjem.228.147. [DOI] [PubMed] [Google Scholar]
- [200].Kemp DM, Pidich A, Larijani M, Jonas R, Lash E, Sato T, Terai M, De Pizzol M, Allegretti M, Igoucheva O, Alexeev V. Ladarixin, a dual CXCR1/2 inhibitor, attenuates experimental melanomas harboring different molecular defects by affecting malignant cells and tumor microenvironment. Oncotarget. 2017;8(9):14428–14442. doi: 10.18632/oncotarget.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ruehlmann JM, Xiang R, Niethammer AG, Ba Y, Pertl U, Dolman CS, Gillies SD, Reisfeld RA. MIG (CXCL9) chemokine gene therapy combines with antibody-cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Res. 2001;61(23):8498–8503. [PubMed] [Google Scholar]
- [202].Zhang R, Tian L, Chen LJ, Xiao F, Hou JM, Zhao X, Li G, Yao B, Wen YJ, Li J, Zhang L, et al. Combination of MIG (CXCL9) chemokine gene therapy with low-dose cisplatin improves therapeutic efficacy against murine carcinoma. Gene Ther. 2006;13(17):1263–1271. doi: 10.1038/sj.gt.3302756. [DOI] [PubMed] [Google Scholar]
- [203].Pan J, Burdick MD, Belperio JA, Xue YY, Gerard C, Sharma S, Dubinett SM, Strieter RM. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J Immunol. 2006;176(3):1456–1464. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- [204].Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer (Review) Oncol Lett. 2011;2(4):583–589. doi: 10.3892/ol.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Arenberg DA, White ES, Burdick MD, Strom SR, Strieter RM. Improved survival in tumor-bearing SCID mice treated with interferon-gamma-inducible protein 10 (IP-10/CXCL10) Cancer Immunol Immunother. 2001;50(10):533–538. doi: 10.1007/s00262-001-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Bonanni V, Antonangeli F, Santoni A, Bernardini G. Targeting of CXCR3 improves anti-myeloma efficacy of adoptively transferred activated natural killer cells. J Immunother Cancer. 2019;7(1):290. doi: 10.1186/s40425-019-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Wightman SC, Uppal A, Pitroda SP, Ganai S, Burnette B, Stack M, Oshima G, Khan S, Huang X, Posner MC, Weichselbaum RR, et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br J Cancer. 2015;113(2):327–335. doi: 10.1038/bjc.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, Kotarski J, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Scala S. Molecular pathways: targeting the CXCR4-CXCL12 axis–untapped potential in the tumor microenvironment. Clin Cancer Res. 2015;21(19):4278–4285. doi: 10.1158/1078-0432.CCR-14-0914. [DOI] [PubMed] [Google Scholar]
- [210].Treatment of Relapsed or Refractory Acute Myeloblastic Leukemia. https://ClinicalTrials.gov/show/NCT01435343 .
- [211].First in Human Study to Determine the Safety, Tolerability and Preliminary Efficacy of an Anti-CXCR4 Antibody in Subjects With Acute Myelogenous Leukemia and Selected B-cell Cancers. https://ClinicalTrials.gov/show/NCT01120457 .
- [212].A Study Of PF-06747143, As Single Agent Or In Combination With Standard Chemotherapy In Adult Patients With Acute Myeloid Leukemia. https://ClinicalTrials.gov/show/NCT02954653 .
- [213].Study Assessing Safety and Efficacy of Combination of BL-8040 and Pembrolizumab in Metastatic Pancreatic Cancer Patients (COMBAT/KEYNOTE-202) https://ClinicalTrials.gov/show/NCT02826486 .
- [214].Plerixafor (AMD3100) and Bevacizumab for Recurrent High-Grade Glioma. https://ClinicalTrials.gov/show/NCT01339039 .
- [215].Plerixafor After Radiation Therapy and Temozolomide in Treating Patients With Newly Diagnosed High Grade Glioma. https://ClinicalTrials.gov/show/NCT01977677 .
- [216].Whole Brain Radiation Therapy With Standard Temozolomide Chemo-Radiotherapy and Plerixafor in Treating Patients With Glioblastoma. https://ClinicalTrials.gov/show/NCT03746080 .
- [217].Phase 1/2 Study of USL311 +/- Lomustine in Advanced Solid Tumors or Relapsed/Recurrent Glioblastoma Multiforme (GBM) https://ClinicalTrials.gov/show/NCT02765165 .
- [218].A Study of LY2510924 and Durvalumab in Participants With Solid Tumors. https://ClinicalTrials.gov/show/NCT02737072 .
- [219].Dose Escalation of POL6326 in Combination With Eribulin in Patients With Metastatic Breast Cancer. https://ClinicalTrials.gov/show/NCT01837095 .
- [220].Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7(19):28697–28710. doi: 10.18632/oncotarget.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Ph1b/2 Study of PF-04136309 in Combination With Gem/Nab-P in First-line Metastatic Pancreatic Patients. https://ClinicalTrials.gov/show/NCT02732938 .
- [222].FOLFIRINOX Plus PF-04136309 in Patients With Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma. https://ClinicalTrials.gov/show/NCT01413022 .
- [223].Zhou J, Xiang Y, Yoshimura T, Chen K, Gong W, Huang J, Zhou Y, Yao X, Bian X, Wang JM. The role of chemoattractant receptors in shaping the tumor microenvironment. Biomed Res Int. 2014;2014:751392. doi: 10.1155/2014/751392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Dose Escalation and Expansion Study of FLX475 Monotherapy and in Combination With Pembrolizumab. https://ClinicalTrials.gov/show/NCT03674567 .
- [225].Jiao X, Nawab O, Patel T, Kossenkov AV, Halama N, Jaeger D, Pestell RG. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Cancer Res. 2019;79(19):4801–4807. doi: 10.1158/0008-5472.CAN-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].CCR5-blockade in Metastatic Colorectal Cancer. https://ClinicalTrials.gov/show/NCT01736813 .
- [227].Lenk L, Alsadeq A, Schewe DM. Involvement of the central nervous system in acute lymphoblastic leukemia: opinions on molecular mechanisms and clinical implications based on recent data. Cancer Metastasis Rev. 2020;39(1):173–187. doi: 10.1007/s10555-020-09848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]