Abstract
Decisions that favor one’s own interest versus the interest of another individual depend on context and the relationships between individuals. The neurobiology underlying selfish choices or choices that benefit others is not understood. We developed a two-choice social decision-making task in which mice can decide whether or not to share a reward with their conspecifics. Preference for altruistic choices was modulated by familiarity, sex, social contact, hunger, hierarchical status, and emotional state matching. Fiber photometry recordings and chemogenetic manipulations demonstrated that BLA neurons are involved in the establishment of prosocial decisions. In particular, BLA neurons projecting to the prelimbic region (PL) of the PFC mediated the development of a preference for altruistic choices, whereas PL projections to the BLA modulated self-interest motives on decision-making. This provides a neurobiological model of altruistic and selfish choices with relevance to pathologies associated with dysfunctions in social decision-making.
Introduction
Many decisions are made in the context of social interactions. These decisions require the integration of different cognitive processes and behaviors, which allow an individual to understand and interact with others.1 The psychological conflict between self-interest and the interest of others (especially when this involves a personal cost) is a key element of social decisions.2 From an evolutionary perspective, altruism likely evolved to promote survival through actions associated with kin selection, parental care, and reciprocity.3–5 Increasing evidence suggests that non-human animals, including rodents, engage in prosocial behaviors that resemble altruism; rats help conspecifics that are in need,6 have been harmed,7 or are seeking food8 and reciprocate previously received help9 and rodents display consolatory10 and collaborative11 behaviors. Current research paradigms can detect animals’ cooperative propensity to help or to prevent pain in others.4,12 However, the social factors and neurobiological determinants of whether an animal engages in altruism or self-interest are unclear.
Mammals live in social groups with dominant and subordinate members, which determine a hierarchy that can affect multiple behaviors13 and represent an important variable in social relationships and prosocial behaviors.14 Moreover, socially close individuals share more easily subjective affective states of another through emotional contagion.14 How social interaction and relationships might change decisions affecting selves and others among members of a group has been overlooked. In non-human primates, activity in a prefrontal-amygdala network contributes to social decision-making.15 Further, the same structures are involved in social interactions16 and social transmission in rodents.17 In particular, the BLA has a central position in the neurobiological circuit for our abilities of choosing among options that differ in rewards and costs.18 Yet, our understanding of its role in decisions involving altruism is still limited.
Here, we devised a social decision-making task (SDM) for mice, modeled on the human game-theoretical paradigm known as the ‘dictator game’19, in which a “dictator” (i.e., the actor) decides whether to share food with a “recipient” (i.e., the observer), who is a passive player. We found that the majority of male mice, but not females, displayed a preference for sharing food with familiar, but not unfamiliar, conspecifics. Substantial individual differences in altruistic choices originated from the hierarchy status of each individual. Chemogenetic silencing of either the BLA or its projections to the PFC abolished the development of altruistic choices, whereas PFC inputs to the BLA modulated self-interest motives on decision making.
Results
Mice choose altruistic actions over selfish decisions
To develop a social decision-making task (SDM) for mice, we expanded a standard operant cage with an adjacent compartment, separated by a metal mesh. This compartment hosted a “recipient” that would receive food rewards depending on the “dictator’s” choice (hereafter referred to as the “actor”). The recipient was a passive player with a chance to receive a food reward from a magazine depending on the actor’s choice. The actor was presented with a two-choice decision-making paradigm in which nose poking resulted in either food rewards for themselves only (selfish choice) or for both themselves and the recipient (altruistic choice; Fig. 1a). We compared this condition against a control group of actor mice without recipients. The structure of the task was identical between these two conditions (“with recipient” and “no recipient”). Thus, any differences in the response could be attributed to the influence of the recipient.
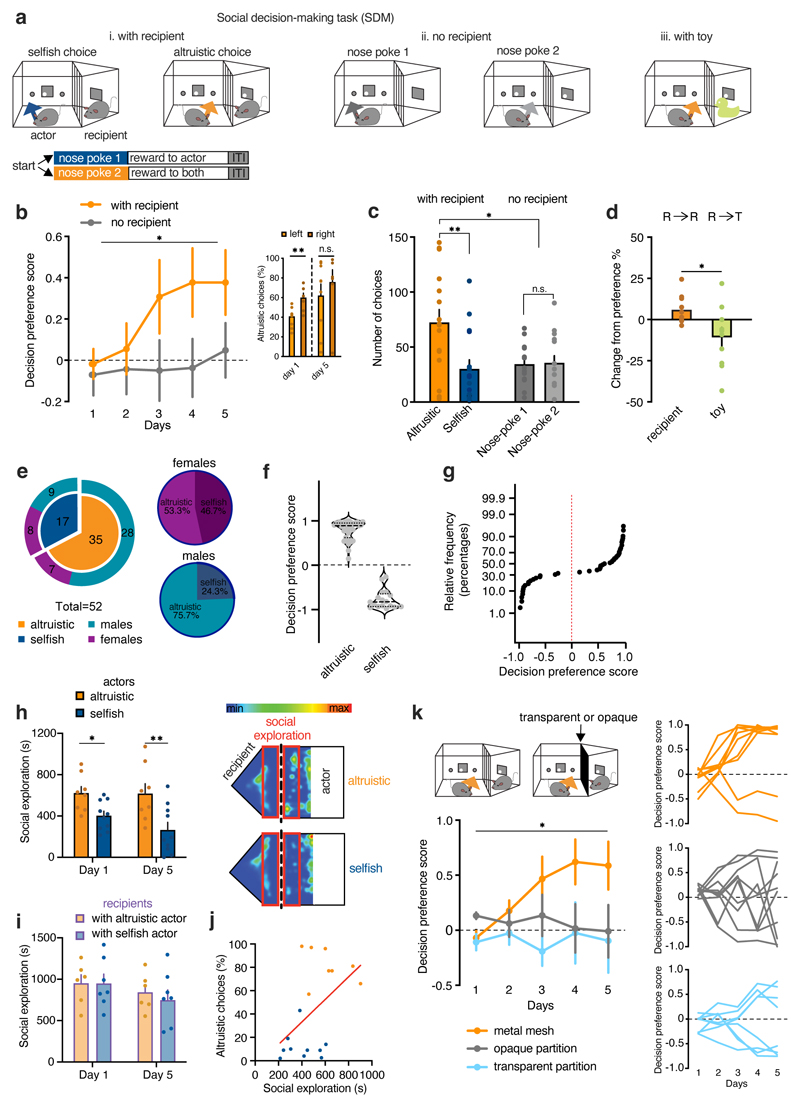
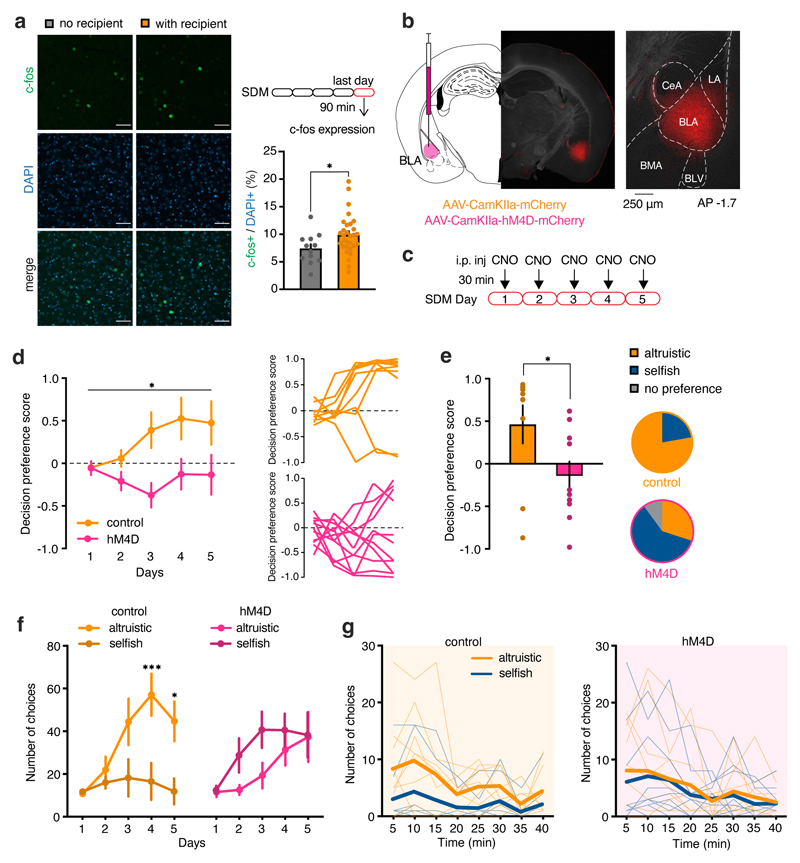
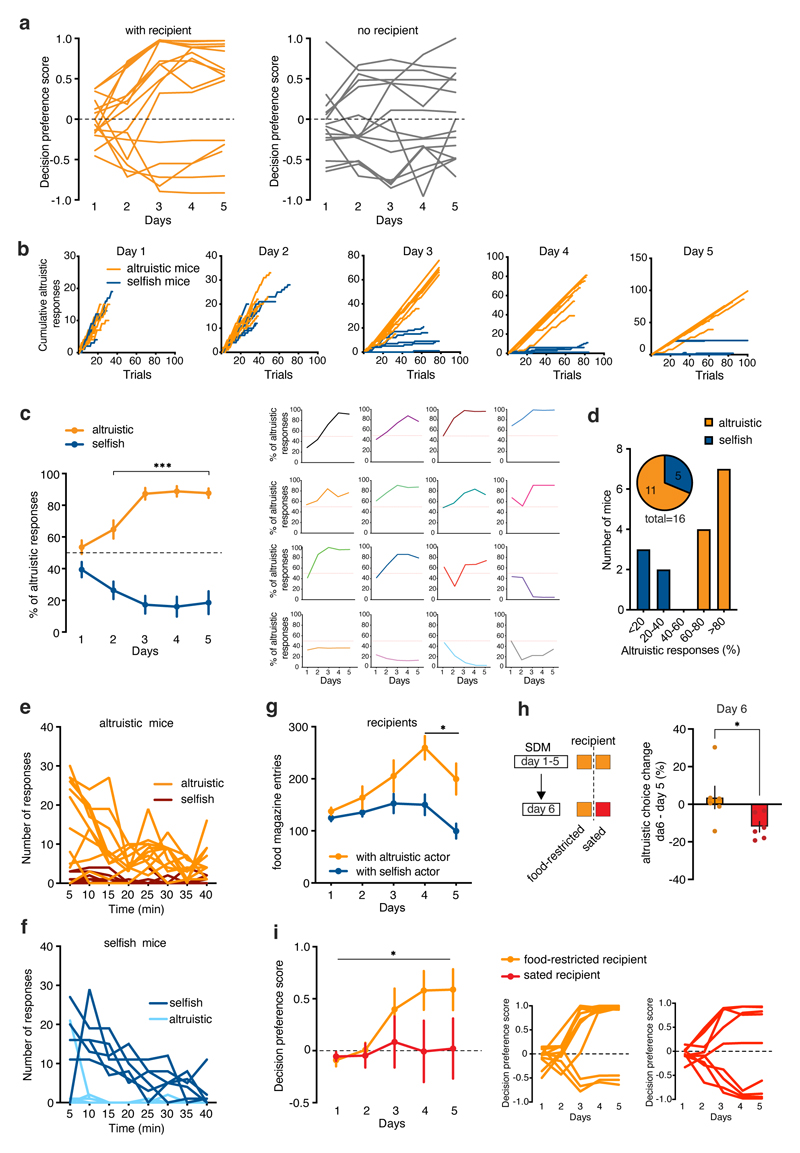
Figure 1. Mice prefer altruistic over selfish decisions.
a, Experimental design of the SDM. b, Decision preference score in mice tested in the SDM with recipient (orange) or no recipient (grey) (two-way RM ANOVA, group (with recipient, no recipient) x time (days 1-5), F(4, 116)=2.771, p=0.0305; the decision preference scores were found to fit a normal distribution across 5 days of testing, D’Agostino and Pearson normality test, ‘with recipient’, min K2=3.122, p=0.225, n=16; ‘no recipient’ min K2=0.944, p=0.623, n=15). Inset, altruistic responses on left (n=9) and right (n=7) nose pokes on day 1 (two-tailed unpaired t-test, t=3.37, d.f.=14, p=0.0046) and day 5 (t=0.79, d.f.=14, p=0.4419). c, Number of nose poke with recipient (n=16) and no recipient (n=15; two-way RM ANOVA, group (with recipient, no recipient) x response (nose poke 1, nose poke 2), F(1, 58)=6.877, p=0.0111). d, Change of preference in an additional session with recipient (R→R, n=10) or with a toy (R→T, n=10) (two-tailed unpaired t-test, t=2.24, d.f.=18, p=0.0374). e, Left, total number of mice grouped by preference and sex. Right, altruistic and selfish preferences in males and females. f, Data distribution of decision preference score in altruistic and selfish mice. g, Cumulative frequency distribution of decision preference scores (n=52). h, Left, social exploration of altruistic (orange, n=8) and selfish (blue, n=10) actors towards their recipients during SDM day 1 and 5 (two-way ANOVA, group (altruistic, selfish), F(1, 32)=16.29, p=0.0003). Right, Schematic of the testing chambers. i, Social exploration of recipients towards altruistic (orange, n=6) or selfish (blue, n=7) actors during SDM day 1 and 5 (two-way ANOVA, group (altruistic, selfish), F(1, 11)=0.16, p=0.6902;). j, Correlation between social exploration on day 1 and preference for altruistic choices on day 5 (linear regression, r=0.4890, p=0.039, n=18 pairs). k, Left, decision preference scores in mice tested with a metal mesh (orange, n=10), a transparent (light blue, n=8) or an opaque partition (grey, n=8) (two-way RM ANOVA, group (metal mesh, transparent partition, opaque partition) x time (days 1-5), F(8, 100)=2.037, p=0.0494). Right, individual curves representing decision preference score. *p<0.05, **p<0.01. n.s. not significant. Values are expressed as mean ± s.e.m.
Male and female 3-6 month-old littermates were housed in same-sex pairs for at least two weeks before the start of testing. The mice were tested for five days until they reached a stable performance for three consecutive days. At the group level, actor mice with recipients preferred to share food rewards (altruistic choices) more frequently than not (selfish choices). They exhibited a positive decision preference score compared with that of mice in the “no recipient” condition, which did not display any choice preference (Fig. 1b and Extended Data Fig. 1a). The location of the nose poke associated with altruistic or selfish responses did not modify the preference for altruistic choices (Fig. 1b). At the end of training, the mice showed an increased number of altruistic over selfish responses when a recipient was present (Fig. 1c and Extended Data Fig. 1b), whereas the mice in the “no recipient” condition chose similarly between the two nose pokes (Fig. 1c). Following the last session (Day 5), we replaced the recipient mice with an inanimate object (“with toy”, Fig. 1a) and tested whether any changes in the actors’ preference could be detected in the absence of social motivation. With an inanimate object, the actors decreased their preference (both altruistic and selfish) compared to their behavior in the presence of the recipient (Fig. 1d). These results confirmed that the preference for altruistic or selfish choices was contingent on the presence of a conspecific.
We observed marked individual differences in the responses of the mice over days. We found that 11 of the 16 mice showed an increase in altruistic responses, more frequently than could be explained by chance (Extended Data Fig. 1c,d). The remaining five mice showed a decrease in altruistic responses (Extended Data Fig. 1c,d). Over the course of training, mice developed a strong preference for one of the two choices. Differences between altruistic and non-altruistic mice appeared from day 2 of testing, even though the mice exhibited both choices at the beginning of the training (Extended Data Fig. 1b). The animals displayed clear preferences starting from day 3 of testing (Extended Data Fig. 1b,c). Indeed, trial-by-trial analyses of responses on day 5 showed that both altruistic and selfish mice displayed a negligible number of non-preferred choices (Extended Data Fig. 1e,f).
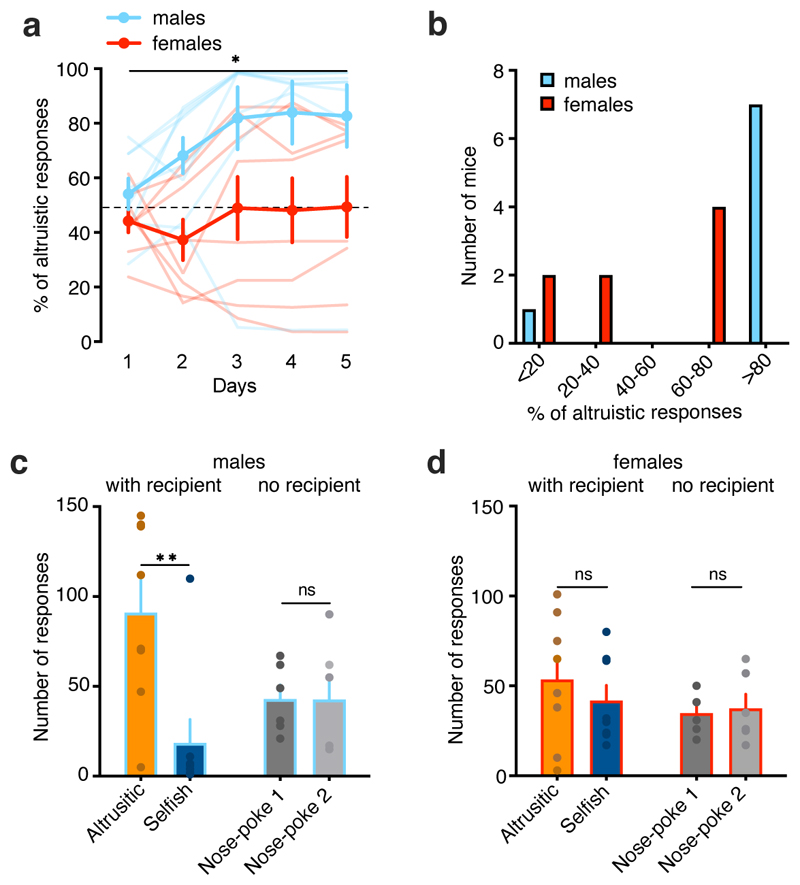
We next analyzed the preference for altruistic or selfish choices in a larger group of animals (n= 52 actor–recipient pairs). We replicated the SDM task several times in naïve and virus-injected mice (for later chemogenetic experiments) and confirmed similar results to our initial findings (Fig. 1e,f), with the majority of mice displaying a preference for altruistic choices (Fig. 1e–g). Overall, the relative frequency of mice not showing an altruistic preference was about 33% (Fig. 1e–g), but this percentage was higher in females. Thus, we analyzed pairs of males and females separately. At the group level, males displayed a preference to allocate food rewards to their recipient (Extended Data Fig. 2a) and only 1 of 8 males preferred selfish over altruistic responses (Extended Data Fig. 2b). By contrast, the females did not show an overall choice preference (Extended Data Fig. 2a), with half of the females displaying a preference for altruistic choices, and half preferring selfish choices (Extended Data Fig. 2b). Therefore, compared to the performance of sex-matched actors that performed the task in the absence of a recipient, only the group of males showed a preference for altruistic responses (Extended Data Fig. 2c, d).
We measured the time spent on social exploration in both groups of mice and found that altruistic actors spent more time than selfish actors exploring their recipients (Fig. 1h). This was evident from the first session onwards. By contrast, we did not observe any differences in social exploration by the recipients (Fig. 1i). Importantly, social exploration of the actor mice during the first day of testing was positively correlated with altruistic responses on the last day, at which point the actors display a consistent preference (Fig. 1j). We replaced the metal mesh with a transparent partition that prevented social contact but allowed the passage of visual, auditory, and olfactory stimuli, or an opaque Plexiglas partition that allowed only auditory and olfactory stimuli (Fig. 1k). Actor mice tested with either partition showed fewer altruistic responses compared to mice who were tested with a metal mesh that allowed social contact. However, an analysis of individual performances revealed that mice tested with the transparent partition established a clear preference for one of the two options (Fig. 1k), whereas mice tested with the opaque partition performed randomly (Fig. 1k). These findings suggest that although mice can use social visual cues to establish their decision preferences, social contact is a determinant of developing an altruistic bias.
We then asked whether the recipients’ food-seeking behavior could modulate decision processes by quantifying the number of recipients’ head entries into the food magazine. Recipient mice that received rewards from altruistic mice showed more head entries after training than before training (Extended Data Fig. 1g). However, at the beginning of the training, food-seeking behavior did not differ between recipients tested with altruistic or selfish mice. To understand how the recipients’ hunger state could motivate altruistic behavior, we tested actor mice with sated or food-restricted recipients following the training in the SDM task (Extended Data Fig. 1h). Actor mice tested with sated recipients decreased their altruistic choices compared to the previous session with food-restricted recipients. In a separate cohort of mice, actors that had been trained in the SDM task with sated recipients (Extended Data Fig. 1i) had a reduced altruistic preference compared to actors trained with food-restricted recipients. These results suggest that the hunger state of the recipients is an important factor in the actors’ decision to share a food reinforcement.
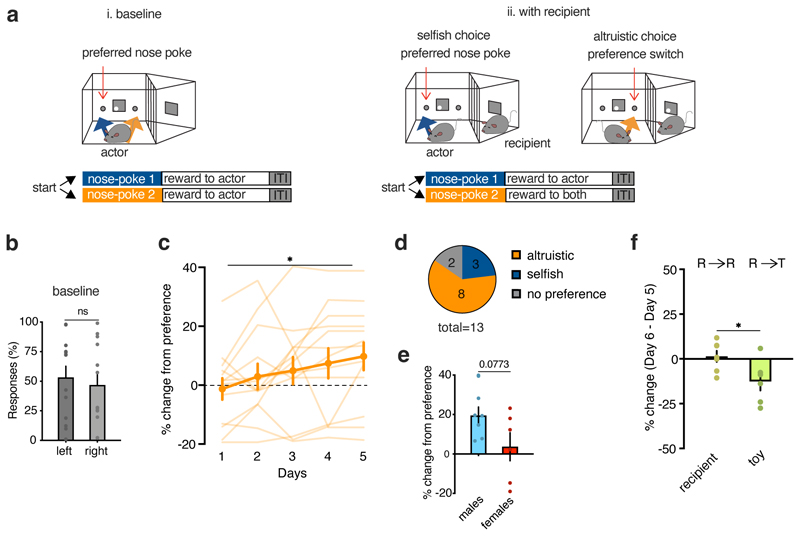
Finally, we tested whether sharing food with recipients could motivate a change in decision preference. The actors were trained to trigger one of the two nose pokes, which both delivered the same food reward to themselves. After the mice displayed a stable preference for one nose poke, a recipient mouse was introduced, and nose poking into the non-preferred hole resulted in the delivery of rewards to both mice, whereas nose poking into the preferred hole delivered rewards only to the actor (Extended Data Fig. 3a). The location of the recipient compartment did not bias actor mice preferences during baseline training (Extended Data Fig. 3c). At the group level, actor mice displayed a positive change from their baseline preference across days, which suggested that some mice shifted their responses to share food rewards with their recipients (Extended Data Fig. 3c). Although there were individual differences, the majority of mice switched their preference (“altruistic” 8/13 mice, Extended Data Fig. 3c-e). On the day following the last session, we replaced the recipient mouse with an inanimate object and found a decrease in preference compared with that expressed when the recipient was present (Extended Data Fig. 3f). These results suggest that mice learned or were willing to change their behaviors to share a food reward with their conspecifics.
Actor mice take altruistic actions under costly conditions
Next, we increased the cost of altruistic decisions by reinforcing the responses at a fixed ratio of 2 (FR2, Fig. 2a). Under this condition, two nose pokes were required to receive food together with the recipient, whereas only one poke was necessary for selfish responses (FR1, Fig. 2a). We tested only those mice that had previously demonstrated a preference for altruistic responses after five days (Extended Data Fig. 2). We similarly tested mice in the ‘no recipient’ condition, in which their natural preference was set to FR2, whereas the other nose poke option was maintained at FR1 (Fig. 2a).
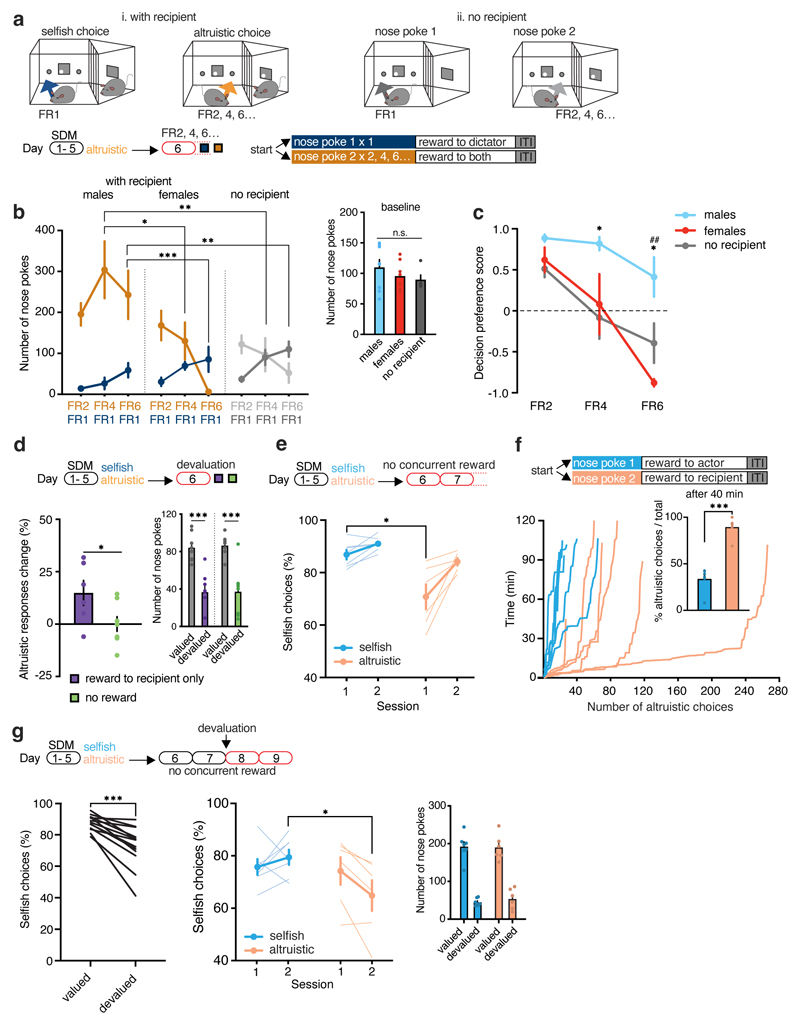
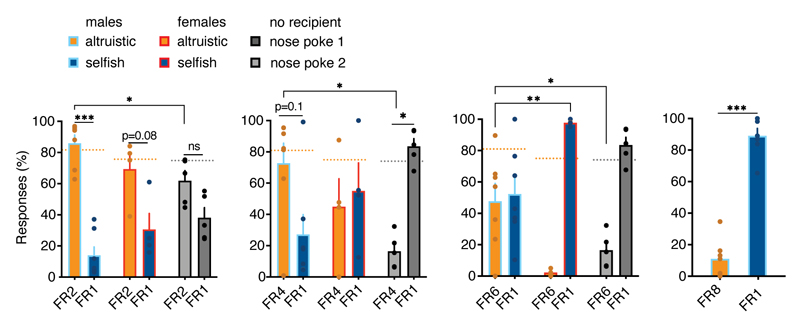
Figure 2. Mice are willing to take altruistic decisions under costly situations.
a, Experimental design of the SDM with different fixed ratio (FR) schedule. b, Left, Number of nose poke on FR1 versus FR2, FR4 and FR6 in males (n=7) and females (n=4) actors and actors tested without recipient (n=5) (between groups: two-way RM ANOVA, group (with recipient males, with recipient females, no recipient) x response (FR2, FR4, FR6), F(10, 52)=4.25, p=0.0002; within groups: two-way RM ANOVA, group (with recipient males, with recipient females, no recipient) x response (FR2, FR4, FR6), F(4, 26)=4.48, p=0.0069). Right, number of nose pokes on SDM day 5 (one-way ANOVA, F(2, 13)=0.67, p=0.5270). c, Decision preference scores with FR2, FR4 and FR6, compared to FR1, in mice tested with recipient (male, n=7 and female, n=4) and without recipient (n=5) (two-way ANOVA, group (with recipient males, with recipient females, no recipient) x response (FR2, FR4, FR6), F(4, 26)=3.55, p=0.0193). males: *p=0.0265 (FR4) and p=0.0678 (FR6) vs. no recipient, ##p=0.0010 vs females. d, Actors’ change in altruistic choices during devaluation test in the condition rewards to recipients only (n=6) and no reward (n=8, two-tailed paired t-test: t=2.28, d.f.=12, p=0.0410), and number of nose pokes during valued and devalued sessions (two-way RM ANOVA, session type (valued, devalued), F(1, 14)=43.07, p<0.0001). (e-f), Following SDM training altruistic choices did not result in concurrent reward for the actor. Percentage of selfish choices (e, two-way RM ANOVA, group (selfish, altruistic) x time (session 1-2), F(1, 11)=4.90, p=0.0488) and number of altruistic choices (f) over 120 minutes of SDM in mice grouped by selfish (n=7) or altruistic (n=6) preference (inset, percentage of altruistic choices in the first 40 minutes / total number of altruistic choices; two-tailed unpaired t-test, t=8.17, d.f.=10, p=0.0001). g, Selfish choices in the SDM (n=13), without concurrent reward (as in e-f), following satiety-induced reward devaluation compared to valued session (two-tailed paired t-test, t=5.41, d.f.=12, p=0.0002) and differences between altruistic (n=6) and selfish mice (n=7) (two-way RM ANOVA, group (selfish, altruistic) x time (session 1-2), F(1, 11)=6.37, p=0.0282). *p<0.05, **p<0.01, ***p<0.001. n.s. not significant. Values are expressed as mean ± s.e.m.
Both males and females showed more altruistic responses over selfish responses, even when additional effort was required (Fig. 2b, c and Extended Data Fig. 4). Moreover, male FR2 responses were higher than those performed by mice tested without a recipient (Extended Data Fig. 4). This difference was not confounded by the baseline number of nose poke responses (Fig. 2b). When the effort necessary to perform an altruistic action was further increased to FR4, males showed more altruistic responses than females or mice without recipients (Fig. 2b, c). Here, females did not show a preference between the two responses, and mice without a recipient switched their preference to nose poke reinforced at FR1. When the altruistic responses were reinforced to FR6, the females switched their preference to the nose poke that delivered food rewards more easily (FR1), whereas males continued to prefer altruistic responses (Fig. 2b, c and Extended Data Fig. 4). Male mice only switched their preference to selfish responses at FR8 (Extended Data Fig. 4). These results suggest that male mice are more willing to share food rewards under more costly conditions.
To dissect the social motivation to make an altruistic decision from the motivation to collect a food reward, we introduced a satiety-induced reward devaluation. After training in the SDM, mice were pre-fed to satiety using reward pellets and tested in a session that did not provide reward pellets for the actors. We tested one condition in which neither the actor nor the recipient received rewards (‘no reward’), whereas another group of actor mice was tested under conditions in which the actors did not receive any reinforcements but were able to allocate rewards to the recipient (‘reward to recipient only’). Both groups of mice displayed reward devaluation, as indicated by a decrease in nose poke responses (Fig 2d) compared with previous session without pre-feeding. However, the mice increased their preference for altruistic responses when allocation to a recipient was possible, whereas mice that did not receive rewards and could not allocate rewards to the recipient did not modify their preference (Fig 2d).
We then tested whether actors would give food rewards to the recipients even if they did not receive concurrent reinforcement themselves. We first trained mice in the SDM and then modified the paradigm such that one nose poke resulted in food rewards for themselves only (selfish choice) and the other to the recipient only (altruistic choice; Fig. 2e). We tested mice in longer session (120 minutes) to observe effects of satiety on their choices. Albeit both groups of mice displayed high percentage of selfish choices, this preference was reduced in altruistic mice (Fig. 2e). Moreover, while altruistic mice completed most of the altruistic choices in the first part of the session, selfish mice decided to give rewards later in the session (Fig. 2f), likely due to satiety. Consistent with our previous experiment (Fig. 2d), after satiety-induced reward devaluation both groups of mice decreased their selfish choices (Fig. 2g); however, over sessions, the decrease was greater in altruistic than in selfish mice (Fig. 2g). Altogether, these results suggest that mice were willing to help their conspecifics, even in the absence of a food reward for themselves.
Familiarity facilitates altruistic choices
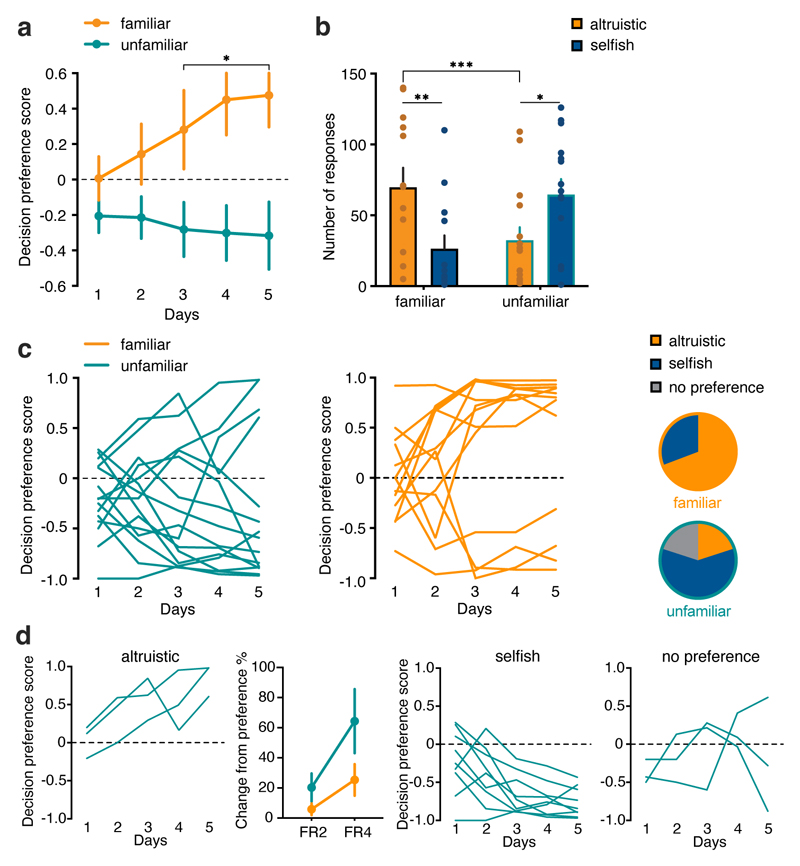
Familiarity between individuals is known to amplify prosocial behaviors.6,10 In a new cohort of mice we therefore tested the actions of actors in response to unfamiliar recipients that were housed in different cages. Actors tested with unfamiliar recipients showed an opposite pattern of choices compared with actor mice tested with familiar recipients (Fig. 3a). Both males and females made fewer altruistic responses in the presence of an unfamiliar compared to mice tested in the presence of cage mates (Fig. 3b). Analyses of individual responses showed that in the unfamiliar-recipient condition, 9 of 15 mice displayed a preference for selfish responses (Fig. 3c, d), whereas only three mice acted altruistically. Three mice did not show any preference (Fig. 3c, d). Moreover, altruistic mice tested with unfamiliar recipient, when challenged with increased FR for the altruistic choices, showed a rapid change in their preference (Fig. 3d). Thus, actor mice paired with non-cage mates acted more selfishly than actors paired with cage mates.
Figure 3. Mice display more selfish choices with unfamiliar recipients.
a, Decision preference score in the five days of SDM in mice tested with familiar (orange, n=13, males/females 7/6) or unfamiliar (green, n=15, males/females 10/5) recipients (two-way RM ANOVA, group (familiar recipient, unfamiliar recipient) x time (days 1-5), F(4, 104)=2.707, p=0.0342). b, Number of nose poke responses in the condition with familiar (black border, n=13) and unfamiliar recipient (green border, n=15; two-way RM ANOVA, group (familiar, unfamiliar) x response (altruistic, selfish), F(1, 52)=12.03, p=0.0011). c, Individual decision preference score in the SDM in mice tested with familiar or unfamiliar recipients and number of mice tested with an unfamiliar recipient (chi-square test, χ2=5.99, p=0.0143). Mice were assigned to altruistic (orange), selfish (blue) or no preference (grey) using one sample t-test to chance (50%, red line). d, Individual decision preference score in mice tested with unfamiliar recipients (n=15) grouped by preference and change from preference (expressed in %, (n=12) in the SDM with altruistic choices reinforced on FR2 and FR4. *p<0.05, **p<0.01, ***p<0.001. n.s. not significant. Values are expressed as mean ± s.e.m.
Social dominance differentiates altruistic preference
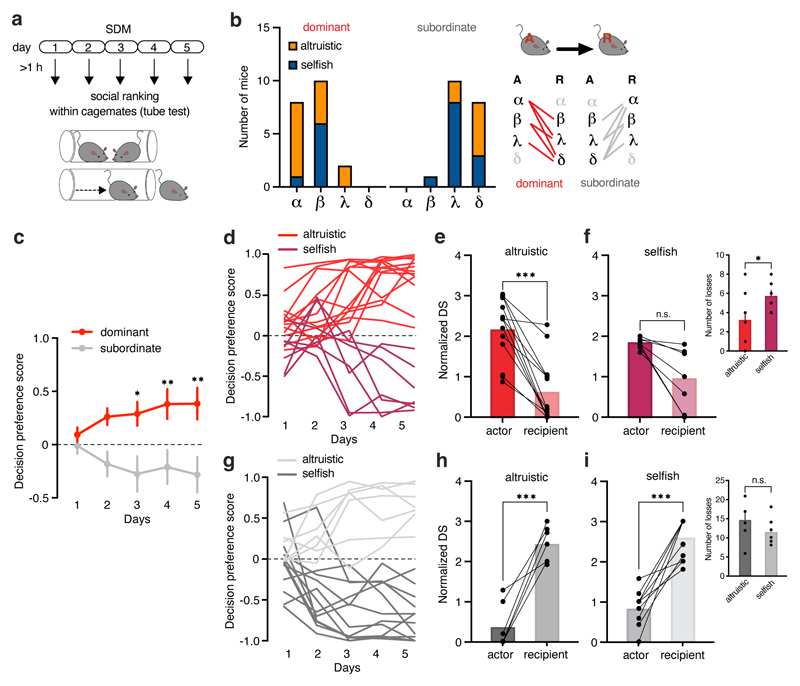
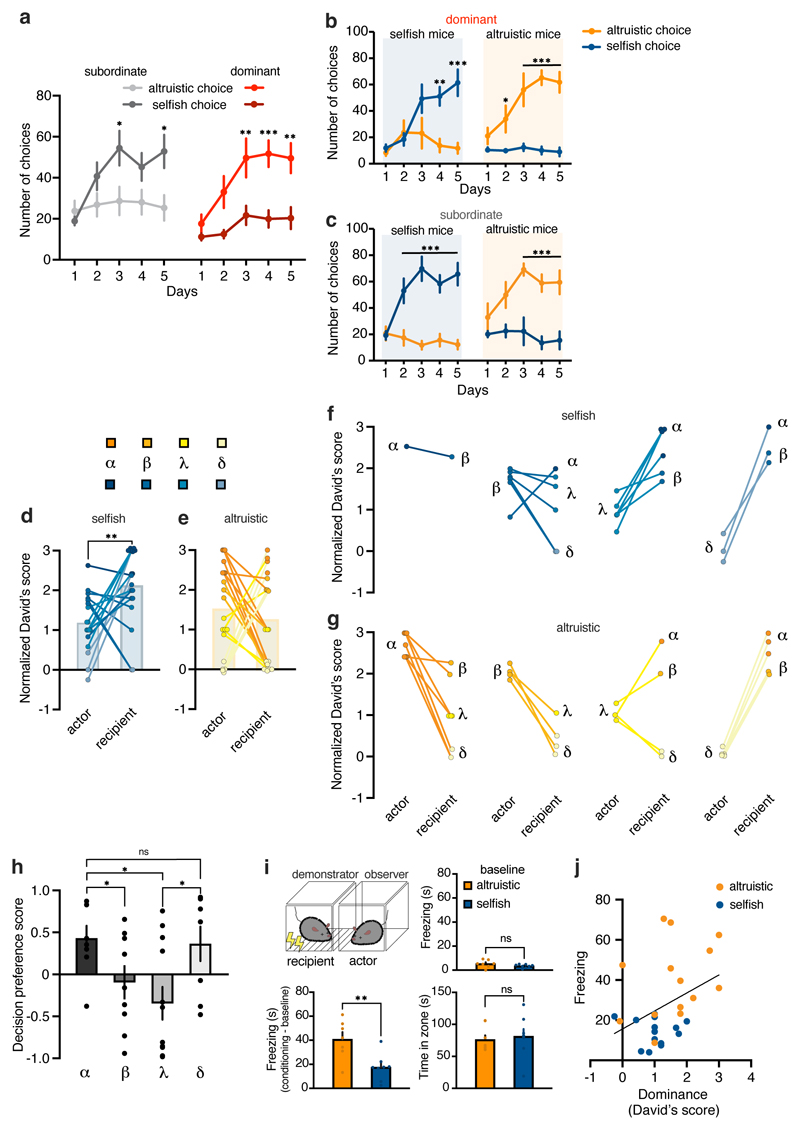
Social hierarchies influence social relationships.14 To determine whether the hierarchical relation between animals within the same cage could influence altruistic propensity, we assessed the social rank of each mouse using the tube test20 (Fig. 4a), and then analyzed the relationships of 39 actor–recipient pairs. In all cages the relationships between mice were transitive and linear (α is dominant over β, β is dominant over γ, and γ is dominant over δ, then α is dominant over all the others). Of mice that performed the SDM task as actors and were dominant in the tube test, the majority preferred altruistic choices (13/20; Fig. 4b); in the group of selfish mice, only 7/19 were dominant. At the group level, dominant actor mice displayed a higher decision preference score, suggesting more altruistic choices, compared to subordinate actor mice (Fig. 4c and Extended Data Fig. 5a–c). We quantified this difference by calculating the David’s score (DS) for each mouse, which is a ranking method based on the outcomes of agonistic interactions between group members (higher values show higher dominance). Actor mice that preferred selfish choices had a lower DS—and thus lower social rank—compared to their recipient conspecifics (Extended Data Fig. 5d, f). By contrast, DS did not differ between actor mice preferring altruistic choices and their recipients (Extended Data Fig. 5e, g).
Figure 4. Social dominance hierarchy modulates preference for altruistic choices.
a, After SDM daily session mice were tested on the tube test (at least 1h after SDM), to measure the hierarchical relationship of animals within the same cage. Actor and recipient mice were tested pairwise and using a round robin design. b, Number of altruistic or selfish actor mice (A) that were dominant (red) or subordinate (grey) compared to the recipient (R) in the tube test (n=39). c, Decision preference score of actor mice that were dominant or subordinate in the tube test compared to their recipient (two-way ANOVA, F(4, 148)=3.46, p=0.097; dominant n=20, subordinate n=19). d, Individual decision preference score in the SDM of dominant actor mice grouped by altruistic or selfish preference (n=20). e-f, Social dominance (normalized David’s score) quantified based on the number and directionality of interactions in the tube test in actor mice that were dominant compared to their recipient, grouped by (e) altruistic (two-tailed paired t-test, t=5.01, d.f.=23,97, p<0.0001; n=13 pairs) and (f) selfish preference (two-tailed paired t-test t=2.27, d.f.=6,87, p=0.0576; n=7 pairs). Inset, number of losses by dominant altruistic and selfish actor mice in the tube test (two-tailed paired t-test, t=2.45, d.f.=18, p=0.0244). g, Individual decision preference score in the SDM of dominant actor mice grouped by altruistic or selfish preference (n=19). h-i, Normalized David’s score in actor mice that were subordinate compared to their recipient, grouped by (h) altruistic (two-tailed paired t-test, t=7.66, d.f.=11,39, p<0.0001; n=7 pairs) and (i) selfish preference (two-tailed paired t-test, t=8.6, d.f.=21,67, p<0.0001; n=12 pairs). Inset, number of losses by subordinate altruistic and selfish actor mice in the tube test (two-tailed paired t-test, t=1.65, d.f.=17, p=0.1154). *p<0.05, **p<0.01, ***p<0.001. n.s. not significant. Values are expressed as mean ± s.e.m.
To explore this variability, we then considered the individual differences of dominant and subordinate actors’ choices (Fig. 4d, f) and analyzed the animals’ dominance in the social hierarchy in relation to their altruistic or selfish preferences (Fig. 4e, g). Dominant actors with altruistic preferences had a higher DS than their recipients (Fig. 4e). By contrast, although dominant actors expressing selfish preferences had a higher rank, they did not display a significant increase in DS compared to their recipients (Fig. 4f). Furthermore, selfish mice suffered more losses in the tube test than altruistic mice (Fig. 4f). These results suggest that dominant mice that developed selfish preference were in competition with their recipient for the same rank. We did not find any differences in DS score among subordinate actors that could differentiate mice with altruistic vs selfish preferences (Fig. 4h, i).
Finally, we analyzed the performance in the SDM task by grouping mice based on their social rank. Mice in the α rank made more altruistic choices compared to mice in the β and γ ranks (Extended Data Fig. 5h) but not compared to mice in the lowest rank (δ), which showed similar altruistic preference (Extended Data Fig. 5h). These results suggest that a mouse’s willingness to share food rewards with their conspecifics is motivated by in-group dynamics involving the social status of the members.
Altruistic choices are linked to emotional-state matching
The motivation to help others can be related to an individual’s sensitivity to others’ emotional states.21 Thus, we assessed whether increased altruistic choices in familiar dominant mice could relate to increased affective-state matching between individuals. We used an observational fear conditioning paradigm (Extended Data Fig. 5i)22 in which the actor mouse was allowed to observe the recipient mouse (“demonstrator”) receiving repetitive foot shocks. Freezing behavior, reflecting the observational fear induced by social transmission, was higher in altruistic mice than in selfish mice (Extended Data Fig. 5i), and observational fear learning scores correlated with social dominance (Extended Data Fig. 5j). Both groups of mice spent similar amount of time in exploration of their conspecific demonstrator (Extended Data Fig. 5i). These results indicate that dominant mice show more emotional contagion, and this may influence social decision-making.
BLA silencing abolishes preference for altruistic choices
We found that emotional contagion and social dominance influences altruistic choices. The encoding of information needed for observational learning depends on the BLA,17,22 and in non-human primates the activity of BLA neurons mirrors the value of reward for self and others.23 We found that SDM training activated the BLA: following SDM training, mice tested with a recipient mouse had more c-fos-positive neurons in the BLA than control mice tested without a recipient (Fig. 5a).
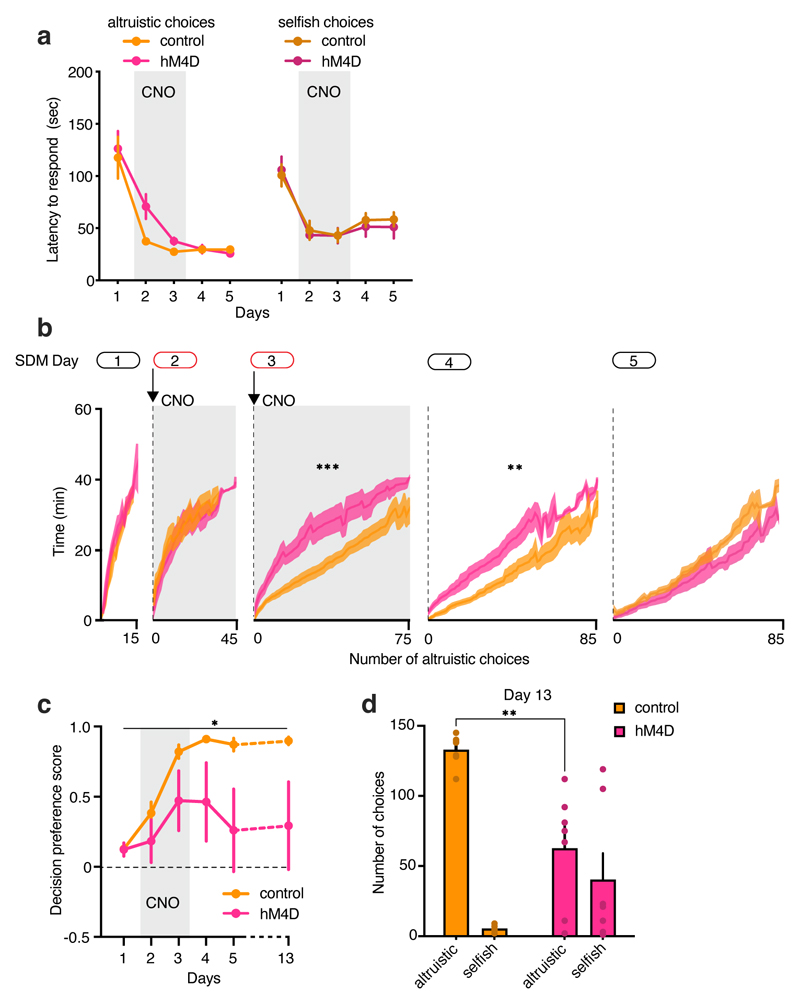
Figure 5. BLA neuronal silencing abolishes the preference for altruistic choices.
a, Representative images and bar graph quantification of c-fos expression in mice following the last day of SDM task and number of c-fos-positive cells in mice tested with or without a recipient (n=42 sections from 7 animals, 3 independent experiments; two-tailed unpaired t-test: t=2.13, d.f.=40, p=0.0394). Scale bar (applicable to all micrographs), 50 μm. b, Male mice were bilaterally injected in the BLA with AAV-CamKIIa-mCherry (control, orange) or AAV-CamKIIa-hM4D-mCherry (hM4D, fuchsia). Representative image of a coronal section of BLA. c, 30 minutes before daily SDM session control and BLA hM4D mice received i.p. injection of CNO. As control, we also tested hM4D animals that received vehicle. Since we did not observe differences, we pooled the control animals together (two-tailed unpaired t-test: t=0.927, d.f.=8, p=0.3810). d, Left, decision preference score in the five days of SDM in control (n=9) and hM4D (n=10) mice (two-way RM ANOVA, group (control, hM4D) x time (days 1-5), F(12, 140)=1.981, p=0.0301; one sample t-test to chance (0.0), control: t=3.146, df=44, p=0.0030; hM4D: t=1.730, df=49, p=0.0899). Right, individual decision preference score in the SDM of control and hM4D. e, Average decision preference score across five day of SDM (two-tailed paired t-test: t=2.175, d.f.=17, p=0.0440) and number of control (n=9) and hM4D (n=10) mice displaying preference for altruistic or selfish choices. f, Number of altruistic and selfish choices in control (two-way ANOVA RM, choice (altruistic, selfish) x time (days 1-5), F(4, 64)=5.0, p=0.0013, n=9) and hM4D mice (choice (altruistic, selfish) x time (days 1-5), F(4, 80)=1.5, p=0.2024, n=10) over five days of SDM task. g, Representation of altruistic and selfish choices at the end of the training in the SDM task (day 5) in control (left) and hM4D (right) mice. *p<0.05, ***p<0.001. Values are expressed as mean ± s.e.m.
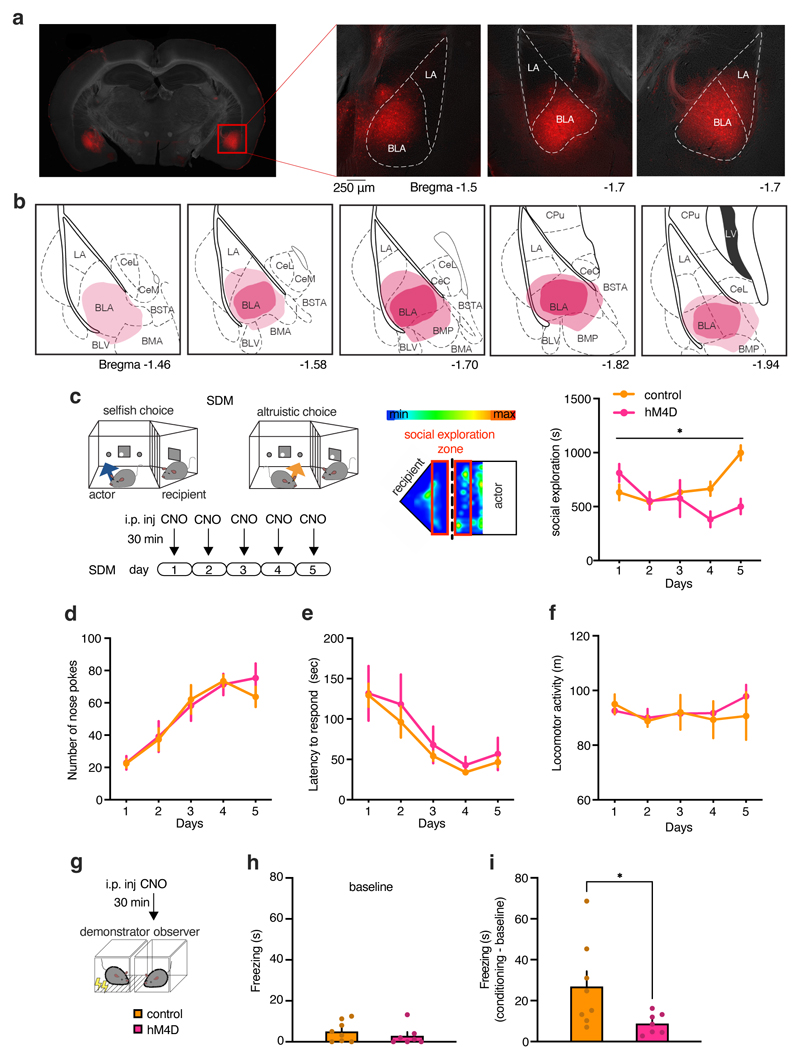
To determine whether BLA activation plays a causal role in the establishment of altruistic preferences, we evaluated the effects of silencing BLA glutamatergic neurons (the majority of BLA neurons24) during the SDM task using a chemogenetic approach. We injected a virus carrying the inhibitory designer receptor exclusively activated by designer drugs (DREADD) receptor hM4Di (AAV-CaMKIIa-hM4Di-mCherry) or a control virus (AAV-CaMKIIa-mCherry into the BLA (Fig. 5b and Extended Data Fig. 6a, b). Both hM4Di mice and control mice injected with AAV-CaMKIIa-mCherry received clozapine-N-oxide (CNO) 30 minutes before testing (Fig. 5c).
Control mice displayed a preference for altruistic choices (as expected), but BLA-silenced mice did not (Fig. 5d, e). Indeed, control mice showed more altruistic choices than selfish choices, whereas BLA-silenced showed no differences in altruistic vs selfish choices (Fig. 5f, g). Individual performance showed that six of the 10 BLA-silenced mice preferred selfish choices, while one did not exhibit a preference (Fig. 5e). In contrast, the majority of control mice (7/9) preferred altruistic choices (Fig. 5e), similar to naïve animals (Fig. 1b). Similarly, BLA-silenced mice showed reduced interest in social exploration over testing days (Extended Data Fig. 6c). BLA silencing did not affect the number of responses or the latency to make a choice and did not produce motor impairments (Extended Data Fig. 6d–f).
Emotional contagion and social hierarchy influenced the preference for altruistic or selfish choices (Fig. 4 and Extended Data Fig. 5i). Therefore, we explored the involvement of these factors in the reduced preference for altruistic choice induced by BLA silencing. BLA-silenced mice froze less during observational fear conditioning than control mice (Extended Data Fig. 6g–i), suggesting reduced social transmission of fear to BLA-silenced mice, consistent with data from a previous study22.
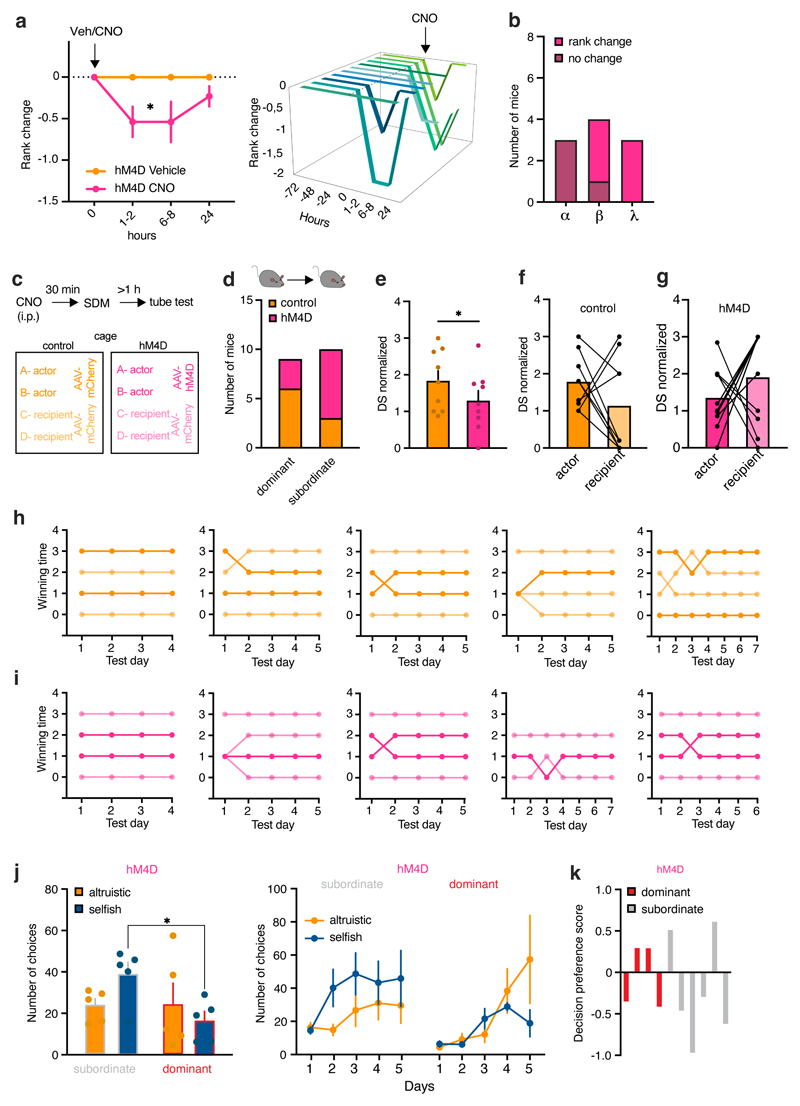
In primates, amygdala lesions produce divergent effects on social dominance.25 To better understand whether BLA activity can be linked to the representation of social ranks, we chemogenetically silenced the BLA before the tube test. BLA silencing reduced ranks in the tube test starting from 1–2 hours following CNO injection (Extended Data Fig. 7a), although this effect was not observed in highest-rank mice (Extended Data Fig. 7b). In a different cohort of mice, a higher number of BLA-silenced actor mice compared to control mice were subordinate to their recipient mouse (Extended Data Fig. 7c, d). Indeed, BLA-silenced actor mice had a lower DS than control actor mice, suggesting reduced dominance (Extended Data Fig. 7e–i). Furthermore, BLA-silenced mice that had a lower rank than their recipient made more selfish choices than dominant BLA-silenced mice (Extended Data Fig. 7j), in agreement with our previous results showing selfish preferences among subordinate actors (Fig. 4d). Altogether, consistent with our findings linking altruistic decision preference with social dominance, these experiments provide initial evidence of the BLA as an information crossroads of social dominance, emotional contagion, and social decision-making.
The BLA is required to develop altruistic preference
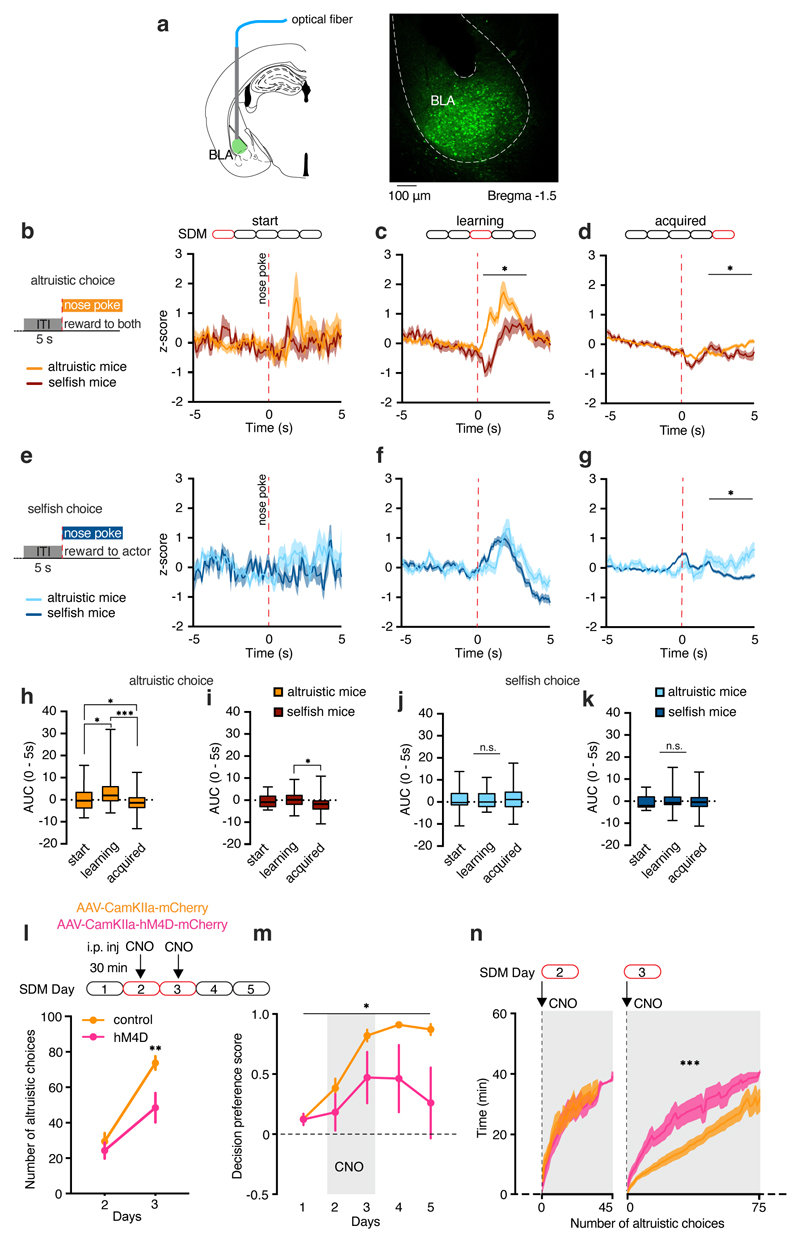
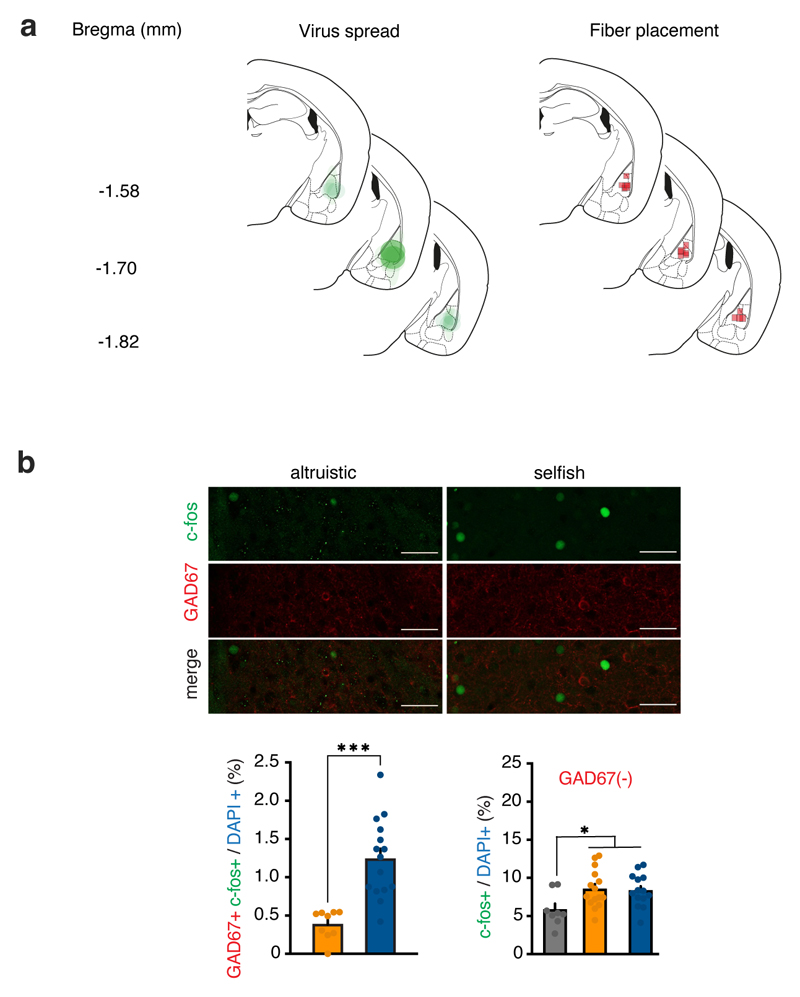
To monitor BLA neural activity during all decisional processes leading to the development of altruistic versus selfish choices, we performed fiber photometry recordings during the SDM task (Fig. 6a and Extended Data Fig. 8a). We injected the genetically encoded fluorescent calcium indicator AAV-CaMKIIa-GCaMP6f into the BLA. Beginning on the third day of testing (learning phase), we found increased neural activity time-locked to the nose poke response after both altruistic and selfish choices (Fig. 6c, f) compared to the baseline. Moreover, in this phase, BLA neural activity was higher in altruistic mice than in selfish mice after altruistic choices (Fig. 6c), but not after selfish choices (Fig. 6f). There was no such difference in BLA neural activity on the first day of the SDM task (Fig. 6b, e). Moreover, BLA neural activity in altruistic mice was higher in the learning phase than on the first day of testing after altruistic (Fig. 6h) but not selfish choices (Fig. 6j). When the task was fully acquired, neural activity after nose poke responses was lower than baseline (Fig. 6d, g). In particular, neural activity after altruistic choices was lower in selfish than altruistic mice (Fig. 6d, g). Accordingly, neural activity was decreased during the last day of SDM compared to the learning phase after altruistic choices (Fig. 6h, i) but not selfish choices (Fig. 6j, k). To examine this difference, we analyzed c-fos expression following the last day of SDM. The percentage of c-fos-positive GABAergic interneurons (GAD67-positive cells) was small; nevertheless, selfish mice had more c-fos-positive GABAergic cells than altruistic mice (Extended Data Fig. 8b). By contrast, the number of c-fos-positive GAD67-negative cells did not differ between altruistic and selfish mice and was higher than in mice tested without recipient (Extended Data Fig. 8b). These data, combined with the results obtained using fiber photometry, suggest that inhibition of neural activity by activation of GABAergic cells following altruistic choices may be stronger in selfish mice than in altruistic mice.
Figure 6. The BLA is required for learning of altruistic choices.
a, Virus encoding GCaMP6f (AAV-CaMKIIa-GCaMP6f) in the BLA for fiber photometry. Scale bar, 100 μm. b-d, GCaMP6f fluorescent changes in the BLA of altruistic and selfish actor mice in response to altruistic nose poke during (b) the first day of testing (‘start’, two-way RM ANOVA, group (altruistic, selfish) x time (minutes), F(1, 101)=0.86, p=0.8248; n=42 trials from 6 mice), (c) the ‘learning’ phase (two-way RM ANOVA, group x time, F(101, 14948)=3.01, p<0.00001; group, F(1, 148)=5.45, p=0.0208; n=151 trials) and (d) the last day of testing in the SDM (‘acquired’, two-way RM ANOVA, group x time, F(101, 68882)=2.46, p<0.00001; n=684 trials). e-g, GcaMP6f fluorescent changes in the BLA of altruistic and selfish actor mice in response to selfish nose poke during (e) the first day of testing (two-way RM ANOVA, group (altruistic, selfish) x time (minutes), F(101, 3939)=0.97, p=0.5541; n=42 trials from 6 mice), (f) the learning phase (two-way RM ANOVA, group x time, F(101, 56964)=2.87, p<0.00001; n=144 trials) and (g) the last day of testing in the SDM (two-way RM ANOVA, group x time, F(101, 68882)=4.25, p<0.00001; group, F(1, 564)=5.63, p=0.0179; n=566 trials). h-i, Area under the curve (AUC) after altruistic choices (0 to 5 seconds) at different periods of the SDM task in altruistic (h, one-way ANOVA, F(2, 680)=51.17, p<0.0001, first day n=25, learning phase n=117, last day n=551) and selfish (i, one-way ANOVA, F(2, 179)=5.62, p=0.0043, first day n=16, learning phase n=33, last day n=133). j-k, AUC after selfish choices in altruistic (j, one-way ANOVA, F(2, 137)=0.07, p=0.9434, first day n=28, learning phase n=48, last day n=64) and selfish (k, one-way ANOVA, F(2, 607)=0.55, p=0.5741, first day n=13, learning phase n=95, last day n=502) actor mice. l, Male mice were bilaterally injected in the BLA with AAV-CamKIIa-mCherry (control, n=7) or AAV-CamKIIa-hM4D-mCherry (hM4D, n=7). Both groups received CNO on testing day 2 and 3, 30 minutes before SDM session with familiar recipients. Number of altruistic choices in control and hM4D (two-way ANOVA RM, group (control, hM4D) x time (days 2-3), F(1, 12)=5.44, p=0.0378). m, Decision preference score in the 5 days of SDM in control (n=7) and hM4D (n=7) mice (two-way RM ANOVA, group (control, hM4D) x time (days 1-5), F(4, 48)=2.719, p=0.0404; one sample t-test to chance (0.0), control: t=10.81, df=34, p<0.0001; hM4D: t=3.17, df=34, p=0.0032). n, Number of altruistic choices on day 2-3 of the SDM in control and hM4D mice (mixed model analysis, day 2: group (control, hM4D) x time (min), F(42, 267)=0.42, p=0.9994; day 3: F(73, 685)=2.85, p<0.0001). h-k, box plots: center = median, box = quartiles, whiskers = min and max. All other values are expressed as mean ± s.e.m
Finally, to test whether the increased BLA activity in the learning phase underlies the establishment of altruistic preferences (Fig. 6c), we silenced BLA neuronal activity only during the learning phase (days 2-3, Fig. 6l) of the SDM test. This reduced the number of altruistic choices compared to control mice (Fig. 6l) on test day 3, but not day 2. This effect was long-lasting as these mice displayed reduced decision preference scores also in the absence of CNO administration in the following two days (Fig. 6m). To investigate the effects of BLA silencing on decision processing we analysed the latency to respond, (i.e. the time between one choice and the following). BLA silencing increased the latency to make altruistic, but not selfish, choices (Extended Data Fig. 9a). We then monitored the number of altruistic choices over time, and we found that BLA-silenced mice displayed increased time to make altruistic choices on test day 3, but not day 2 (Fig. 6n and Extended Data Fig. 9b). This suggests that BLA-silenced mice shared food reward only later in the session, unlike controls. This effect disappeared with additional training (Extended Data Fig. 9b). When we tested mice one week later, BLA-silenced mice still made fewer altruistic choices than control mice (Extended Data Fig. 9c, d). Collectively, these results indicate that the BLA is differently activated by decisions to share or not share a positive reinforcement in mice that prefer altruistic choice vs mice that prefer selfish choices. In particular, our data point to a crucial role of the BLA in the establishment of altruistic preference.
Distinct roles of BLA–PFC connections in altruistic choices
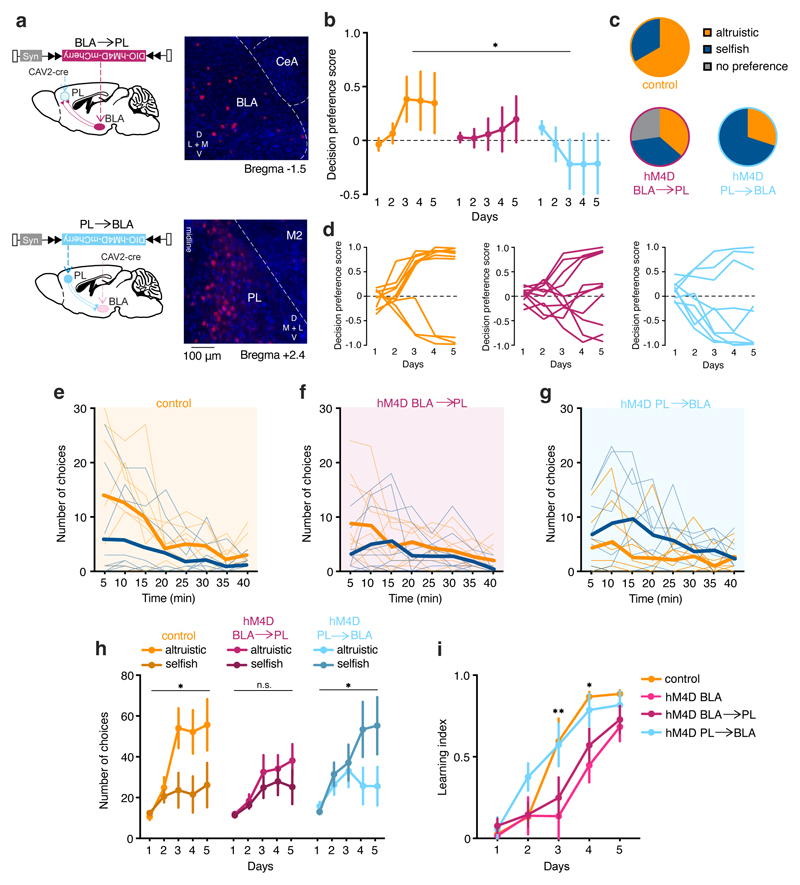
The BLA inputs and outputs mediate different types of learning26 and support circuits involved in the valence processing of environmental stimuli.27 PFC subregions are both major targets of the BLA and a major source of inputs.26 We targeted the prelimbic (PL) region of the PFC, corresponding to primate Brodmann area A32,28 which supports goal-directed behavior.29 We injected a retrogradely transported canine-adenovirus-2-expressing Cre recombinase (CAV2-Cre) into the PL and injected the BLA with an rAAV carrying a Cre-dependent hM4D(Gi)DREADD receptor and mCherry (hM4D BLA →PL, Fig. 7a). With this combination, we achieved DREADD(Gi)-mCherry expression exclusively in BLA neurons projecting to the PL. We used the same approach to study PL neurons projecting to the BLA (hM4D PL→BLA, Fig. 7a). CNO was injected 30 minutes before each session.
Figure 7. Chemogenetic silencing of BLA-PFC reciprocal connection have different impact on altruistic choices.
a, Schematic showing viral injection and projection areas and example images of coronal section of BLA and PL. Mice received hM4D cre-dependent receptors in the BLA and CAV2-Cre in the PL or hM4D cre-dependent receptors in the PL and CAV2-Cre in the BLA. With this combination, we achieved DREADD expression exclusively in BLA neurons projecting to the PL (hM4D BLA→PL) and viceversa (hM4D PL→BLA). CeA=Central Amygdala. M2=secondary motor cortex. b, Decision preference score in the five days of SDM in control CNO (orange, n=10) and hM4D BLA→PL (purple, n=11) and hM4D PL→BLA (light blue, n=9) mice (two-way RM ANOVA, group (control CNO, hM4D BLA→PL, hM4D PL→BLA) x time (days 1-5), F(8, 108)=2.03, p=0.0493). c, Number of mice displaying preference for altruistic or selfish choices. Mice were assigned to altruistic (orange) or selfish (blue) or no preference (grey) analyzing decision preference scores using one sample t-test to chance. d, Individual decision preference score in the SDM of control CNO and hM4D BLA CNO. e-g, Representation of altruistic and selfish choices at the end of the training in the SDM task (day 5). h, Number of altruistic and selfish choices in control CNO (two-way ANOVA RM, choice (altruistic, selfish) x time (days 1-5), F(4, 72)=3.6, p=0.0088, n=10) hM4D BLA→PL (choice (altruistic, selfish) x time (days 1-5), F(4, 64)=2.6, p=0.0401, n=11) and hM4D PL→BLA (choice (altruistic, selfish) x time (days 1-5), F(4, 80)=0.69, p=0.5981, n=9) over five days of SDM task. i, Learning index representing the preference development in control CNO (n=10), hM4D BLA (n=10), hM4D BLA→PL (n=11), and hM4D PL→BLA (n=9) mice (two-way ANOVA RM, group x time, F(12, 140)=1.91, p=0.0376). *p<0.05, **p<0.01. n.s. not significant. Values are expressed as mean ± s.e.m.
Silencing BLA→PL projections abolished the preference for altruistic choices (Fig. 7b), similar to the effect of silencing the entire BLA (Fig. 6). While the majority of control mice (7/10) showed a preference for altruistic choices (Fig. 7c), after silencing BLA→PL projections, the preference was equally distributed between selfish and altruistic choices, and three of these mice did not display any preference (Fig. 7c, d). In line with these results, control mice made more altruistic than selfish choices, which was not observed after silencing of BLA→PL projections (Fig. 7e, f, h). By contrast, silencing PL→BLA projections produced a negative decision preference score (Fig. 7b); the majority of mice expressed a selfish preference (6/9; Fig. 7c), and made more selfish than altruistic choices (Fig. 7e,g,h).
Finally, to quantify the efficiency of preference development regardless of its value (positive or negative decision score), we calculated a learning index. We found that, similarly to BLA silencing, silencing BLA→PL projections resulted in a lower learning index compared to that seen in control mice or after silencing PL→BLA projections (Fig. 7i). This suggests that silencing BLA→PL projections slows the development of choice preferences, consistent with the finding of increased BLA neural activity during the learning phase of the task. Collectively, these data suggest that reciprocal PFC–BLA connections have distinct roles in the establishment of altruistic or selfish choices (Fig. 8a).
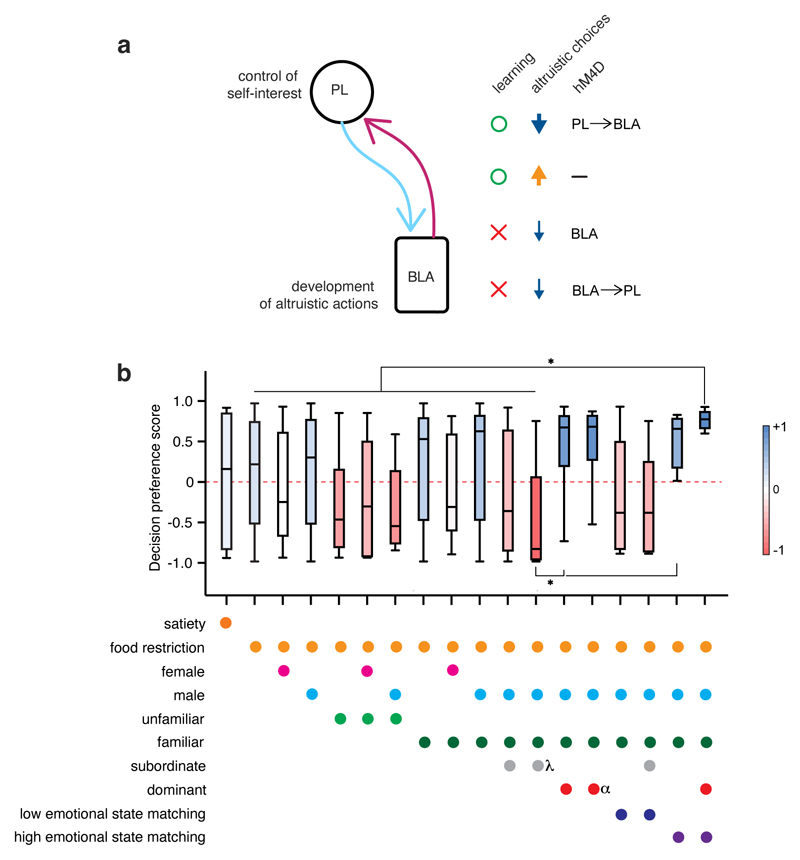
Figure 8. A brain circuit and factors involved in altruistic choices.
a, Schematic model of the involvement of the BLA-PFC reciprocal connections in social decision making. Green circle = normal, red cross = impaired. b, Influence of multiple factors on the SDM task (n=146 mice). Average decision preference score (stable performance for the last three days) considering the impact of multiple factors (from 1 to 5 factors simultaneously, One-way ANOVA, Welch’s test, F(17, 67.28)=9.03, p<0.0001). *p<0.05. Right bar = mean (with blue as highest preference for altruistic choices, red as highest preference for selfish choices). Box plots: center = median, box = quartiles, whiskers = min and max.
Discussion
In this study, we showed that mice intentionally engage in choices that will favor another conspecific or only themselves. Divergence in social decision-making originates from different inter-individual factors, including familiarity, sex, social contact, physiological need (i.e., hunger), hierarchical status, and emotional state matching. Notably, prosocial actions, even if they required more effort or had no direct benefit to the actor mouse, were more generally observed toward familiar hungry males with the highest hierarchical distance to the actor. We showed that BLA neuronal activity is needed to develop a preference for altruistic behaviors in the SDM task. More specifically, BLA→PFC projections guide the establishment of altruistic actions, whereas PFC→BLA projections exert control over selfish decisions.
We developed an operant task to explore how basic decision-making systems operate within a socially interactive environment. Most experimental studies of decision-making have examined behaviors with clearly defined probabilities and outcomes, such as choosing between food rewards. In our task, mice chose between two actions that yielded either a reward only to themselves or to both themselves and a partner placed in an adjacent compartment. No previous studies in mice included such complexity of social interactions30, even though many important decisions are made in the context of social interactions with others and change based on social feedback. Thus, our social decision-making paradigm provides an approach to examine distinct and complex social behaviors in mice.
Our results are consistent with recent studies showing complex prosocial behaviors in rats, such as preference for mutual rewards,8,31 helping behaviors,6 and the avoidance of harming others.7 Rodents show social behaviors that suggest they can act to increase mutual benefits. 6,10,11 Few studies have demonstrated prosocial behaviors reminiscent of altruism constructs in mice. In the SDM task, mice learned to make altruistic choices through conditioned responses that were reinforced by a positive outcome for the actor as well as the recipient; however, they displayed an interest in sharing food with their hungered conspecifics even in the absence of concurrent positive reinforcement or any explicit return favors from their actions (i.e., without evident self-benefit), a critical factor that defines “altruistic” choices.32 Moreover, when mice were presented with the opportunity to stop making altruistic choices by exerting less effort to obtain a food reward only for themselves, they continued to display a preference for sharing food with their companions. Altogether, these findings identify social values that determine the development of choices that will favor another conspecific.
The establishment of social choices is complex and heterogeneous, depending on several factors. Indeed, our results reveal that when considering components such as familiarity, sex, dominance, hunger state, social contacts, and emotional state matching individually, their effects on selfish vs altruistic choices were considerably heterogeneous. By contrast, the combination of all these factors revealed cumulative effects that reduced the heterogeneity and better separated subpopulations of mice that showed altruistic choices (Fig. 8b).
Dominance hierarchy contributed to the preference for altruistic or selfish choice. Social status can guide behavior and motivation in a social group, including in humans.33 Our finding that most mice that displayed a preference for selfish over altruistic choices were subordinate to their recipient and belonged to an intermediate rank could reflect competition for food, as dominant members might benefit from easier access to food and dictate priorities to access resources.20 Similarly, in non-human primates14 and rats34, prosocial responses are more often directed from dominant towards subordinate members. Thus, dominant individuals may behave in ways that benefit others to advertise their dominance. On the contrary animals acted selfishly because they were in direct competition for both upward and downward ranks. In summary, our task generated distinct behavioral responses that could address several complex aspects of social decision-making triggered by interpersonal interactions.
Studies in rodents under several conditions, such as risk-taking18, punishments35, and threats36 have revealed critical role of the BLA in integrating reward-related information and costs to guide decision-making. Our results expanded this role to the social domain., The BLA integrates cue–response associations with motivational and emotional inputs, updating the value of these associations through connections with the PFC.37 In non-human primates, the synchronization of neural activity between the BLA and PFC is important for the establishment of other-regarding preferences.15 Accordingly, silencing of PFC→BLA neuronal projections produced a bias in the development of a preference toward selfish choices. This result supports the hypothesis that the dorsolateral part of the PFC in humans is involved in the modulation of the relative impact of self-interest impulses on decision-making.38 The preferential connection between the BLA and cortical structures, such as the PFC, also has an important modulatory effect on social behavior and the transmission of social cues.16,17 Indeed, similarly to a previous study,22 the BLA silencing reduces social fear learning, which was correlated with preferences in the SDM. Thus, the establishment of a preference toward altruistic or selfish choices could be at least partially related to empathy-like capacity in mice. Altogether, the effects observed following neuronal silencing of the BLA inputs and outputs indicate its involvement in the social value of reward for self and for others.
Previous studies have provided evidence pointing to the involvement of the PFC in the plastic modulation of social hierarchy.20 However, it has been unclear whether PFC–BLA reciprocal connections are involved in social hierarchy. We found that the effects of BLA→PFC projections on altruistic choices were more similar to overall BLA silencing than the effects of PFC→BLA projections, which were more relevant to selfish choices. Hierarchy was correlated to altruistic but not selfish preference, and silencing of the BLA was associated with rank changes down the hierarchy, suggesting that BLA→PFC projections could also be more relevant for regulating hierarchy. In agreement with this, PFC→BLA projections are not involved in the modulation of hierarchical dominance.39 Thus, if the PFC and BLA distinctly modulates social status or bottom-up BLA→PFC connections are a key circuit involved in social hierarchy is an intriguing topic for future studies.
Altruistic behaviors were learned in our task through positive reinforcements. Nevertheless, we cannot rule out the possibility that some degree of innate or impulsive altruism may have assisted during the initial learning process.12 In support of this possibility, mice displaying a preference for altruistic choices were more interested in social exploration than mice that preferred selfish choices. Kin selection has been suggested as a biological explanation of the motivation to act in an altruistic manner.12 However, in a laboratory setting, animals do not face such selection pressures. Therefore, other explanations should be considered, such as the emotional engagement between the actor and recipient. Mice can discriminate40 and share41 the affective state of their conspecifics. Indeed, mice that expressed a preference for altruistic choices displayed more empathy-like behaviors. The food-seeking behavior of the recipient could trigger an emotional transfer between mice, which may motivate altruism. Moreover, familiarity can amplify the empathic response.42 Consistently, emotional contagion was linked with the preference for altruistic choices, suggesting that affective state matching could motivate altruism in mice. Together with empathy, social motivation could represent another explanation for altruism. Social value can guide how social animals interact with others and adapt behavior and actions in response to them, influencing social-value-based decisions43. Rodents are social animals that display preferences for social closeness and avoid social isolation,44 which can have rewarding properties.45 Thus, well-being conferred by sharing a positive experience may also have a social value for the actor. Altogether our results suggest that altruistic choices in mice are motivated by a positive connection favoring prosocial behavior.
In summary, we developed a task enabling the detection of inter-individual propensities in mice for altruistic or selfish choices as well as the factors modulating them (i.e., hierarchy, familiarity, sex, hunger state, and social contact). We started to elucidate the neurobiology of such social decisions, reveling the BLA and BLA-PFC reciprocal connections as critical substrates for the establishment of altruistic choices. These results could have important implications for psychiatric, psychological, and neurodevelopmental conditions associated with disruptions in social decision-making.
Methods
Mice
All procedures were approved by the Italian Ministry of Health (permits n. 107/2015-PR and 749/2017-PR and 191/2020-PR) and local Animal Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the European Community Council Directives. Routine veterinary care and animals’ maintenance was provided by dedicated and trained personnel. Two to six-month-old males and females C57BL/6J animals were used. Distinct cohorts of naïve mice were used for each experiment. Animals were housed two to four per cage in a climate-controlled facility (temperature 22±2 °C, humidity 45-65%), with ad libitum access to food and water throughout, and with a 12-hour light/dark cycle (7pm/7am schedule). Experiments were run during the light phase (within 10am-5pm). All mice were handled on alternate days during the week preceding the first behavioral testing.
Behavioral paradigm
Social decision-making task. Experimental setup
Experiments were conducted in a standard operant chamber (actor’s compartment, L: 24 cm x W: 20 cm x H: 18,5 cm; ENV-307W-CT; Med Associates, Inc.) fused with a custom-made small triangle-shaped chamber that hosted the recipient (L: 18 x W: 14 cm x H: 18,5). The separation wall between the compartments (operant chamber and recipient chamber) was replaced by a metal mesh with 1cm holes that allowed social exploration and nose-to-nose interaction. The actor’s compartment was equipped with two nose poke holes and a food magazine between them, for delivery of food rewards (14 mg; Test Diet, 5-TUL). The recipient’s compartment presented only a food magazine connected to a food dispenser. The setup was placed inside a sound attenuating cubicle (ENV-022V, Med Associates, Inc) homogeneously and dimly lit (6 ± 1 lux) to minimize gradients in light, temperature, sound, and other environmental conditions that could produce a side preference. All tasks were controlled by custom scripts written in MED-PC IV (Med Associates, Inc.). A digital camera (Imaging Source, DMK 22AUC03 monochrome) was placed on top of the setup to record the test using a behavioral tracking system (Anymaze 6.0, Stoelting).
Task design
The testing subjects, the “actors”, were tested in three different conditions: i) with recipient, in which a cage mate was placed in the adjacent compartment, and acted as recipient; ii) no recipient, the compartment of the recipient was empty; iii) with toy, the recipient was replaced with an inanimate object. The actor (Fig. 1a) determined to receive a food reward for himself (selfish choice) or to allocate the reward also to his companion (altruistic choice), “the recipient”. Both choices were reinforced on fixed ratio 1, such that poking into the left or right nose poke resulted in one food reward delivery. Altruistic and selfish responses were counterbalanced between left and right nose pokes across mice. After one nose poke, an intertrial interval of 5 seconds occurred. The recipient was a passive player and only received food rewards upon actor choices. In the no recipient condition, the adjacent compartment was empty, while in the toy condition, an inanimate black object was placed in the recipient compartment. The task design was identical across the condition with recipient and served as a control for pellet delivery sounds and for potential secondary effects of reinforcement.
Actor and recipient were mildly food-restricted to 90% of their free-feeding body weights to promote food-seeking behavior and were housed together for at least two weeks before the experiment. In the condition with unfamiliar recipient, actor and recipient were never housed together. The actors were tested for five days, in 40 minutes sessions, with a partner (with recipient) or without (no recipient), with an inanimate object, or with an opaque or transparent partition dividing recipient and actor, depending on the testing condition. Actors were always paired with the same recipient throughout the same experiment. In the toy condition, actors were tested for five days with a partner and the day following the last session (day 5), the recipient was replaced by an inanimate object (day 6). In condition with the opaque or transparent partition between actor and recipient actor mice were tested for five days. The opaque partition did not allow visual cues and social exploration/interaction, while the transparent partition allowed visual cues but not social exploration/interaction.
Fixed ratio schedules
To test whether mice made voluntary choices to benefit others under costly conditions, we tested mice using increasing fixed ratio (FR) schedule for altruistic decisions from FR2 to FR8. In this condition, the number of operant responses required to dispense food to the recipient is increased on each day (from 2 to 8). Selfish responses remained on FR1 throughout the experiment. In the ‘no recipient’ condition, for each actor the preferred nose poke was reinforced using the increasing FR schedule and the other nose poke was kept on FR1.
SDM task without concurrent reward
Mice were trained in the SDM and then the paradigm have been modified such that one nose poke resulted in food rewards for themselves only (selfish choice) and the other to the recipient only (altruistic choice). Mice were tested in longer session (120 minutes) to observe possible effects of satiety on their choices.
Satiety-induced reward devaluation
Mice were tested for five days in the SDM and following the last session, actors were singly housed for two hours, and reward outcome was devalued by pre-feeding them to satiety giving free access to reward pellets in their cage. Then, mice were transferred to the operant chamber and test in a non-reinforced session. Sated actor mice were tested in two different experiments. Experiment 1. Two groups of mice were tested: in one condition, both actor and recipient did not receive rewards (‘no reward’) and in the other, actor mice did not receive any reinforcement, but they could still allocate food rewards to their recipients (‘reward to recipient only’). Experiment 2. Mice were tested in the ‘SDM without concurrent reward’ after altruistic choices.
SDM task with sated recipients
Both actors and recipients mice were food-restricted as described above. Two hours before each session of the SDM task recipients mice were separated from their cage mates actors and reward outcome was devalued by pre-feeding them to satiety giving free access to reward pellets in their cage. Then, both actors and recipients mice were transferred to the operant chamber and test in a reinforced session. In a different cohort of mice, we tested satiety-induced reward devaluation in the recipients mice after standard training in the SDM task for five days. In this condition one group of actor mice was tested with food-restricted recipients and one group with sated recipients following satiety-induced reward devaluation.
Analyses
The number of nose poke responses was counted by a software (MED-PC V, Med Associates, Inc). To quantify individual preferences of altruistic over selfish responses or left and right nose pokes we calculated a decision preference score, as following: (number of altruistic responses - number of selfish responses) / total number of responses. Video images were analyzed a posteriori for scoring of exploratory behavior using Anymaze 6.2 (Stoelting, UK) and Boris.46 We measured the time spent by the recipient and the actor in social exploration in the area (highlighted in red, Fig. 1h) in the proximity of the adjacent compartment, where they could explore each other.
Tube test
The tube test was performed as described in a previous study.47 We used a transparent Plexiglas tube (L: 30 cm, inside diameter 3 cm). For habituation, the tube was placed inside the cage for three consecutive days. After habituation, mice were trained to run inside the tube. Each mouse was released at alternating ends of the tube and was allowed to run through the tube. We used a plastic stick to guide the mouse to the end of the tube if needed. Each animal was given ten training trials on two consecutive days. For the test, two mice were simultaneously released into the opposite ends of the tube and care was taken to ensure that they met in the middle of the tube. The first mouse that retreated and placed its two rear paws outside the tube was recorded as the “loser” of the trial and the other mouse the “winner”. Between each trial, tube was cleaned with 75% ethanol. Mice were tested pairwise using a round robin tournament, on daily sessions. Each pair of cage-mates was tested in consecutive trials, alternating the starting side of the tube. The test was performed until all the ranks were stable for at least 4 continuous daily trials. To assign each animal social rank we used the normalized David’s score (DS) for dominance. The score was calculated from the individual proportion of wins and losses in all the trials, in relation to the wins and losses of its opponents, as reported in a previous study.48 We then normalized the score to be between 0 and N-1 (where N is the number of subjects in each cage), using the following formula:
In hM4D-expressing animals, the tube test was performed in different cohort of mice with or without the SDM task. The training in the tube for habituation was performed before the SDM task, then the tube test started and the SDM task started on the same day. The tube test was performed at least one hour after the SDM task (with CNO injection). In hM4D-expressing animals that did not perform the SDM task, after reaching stable ranking, mice received CNO or vehicle and were tested at different time points following injection (1-2 hours, 6-8 hours, 24 hours). For BLA silencing, one mouse received CNO, and the other cage mates received vehicle. In control cages, all the animals received vehicle.
Observational fear conditioning
The apparatus consisted of two identical and adjacent fear conditioning chambers (Ugo Basile, 24×20×30 cm) separated by a transparent Plexiglas partition. Olfactory and auditory cues could be transmitted between the chambers. A demonstrator mouse (previously recipient in the SDM task) and an observer (previously actor in the SDM task) were individually placed in the two chambers and allowed to explore the chambers for 5 min (baseline). Then, a 2-s foot shock (0.7 mA) was delivered every 10 s for 4 min to the demonstrator mouse using a behavior tracking software (Anymaze 6.0, Stoelting). The same pairs of mice tested in the SDM were used. Based on previous studies,28 we used 10-s intervals for foot shocks and a 4-min training. At the end of the procedure mice returned to their home-cage.
Stereotaxic surgeries
Viral vectors
AAV5-CamKIIa-mCherry (114469, titer ≥ 7×1012 vg/mL), AAV5-CamKIIa-hM4D(Gi)-mCherry (50477, titer ≥ 3×1012 vg/mL), AAV5-Syn-DIO-hM4D(Gi)-mCherry (44362, titer ≥ 7×1012 vg/mL) and AAV1.CamKII.GCaMP6f.WPRE.SV40, (100834, titer ≥ 1×1013 vg/mL) were purchased from Addgene. CAV2 equipped with Cre recombinase (titer ≥ 2.5×1011 vg/mL) was purchased from the Institute of Molecular Genetics in Montpellier CNRS, France.
Surgical procedures
C57BL/6J mice were naïve and 2 months old at the time of surgery. All mice were anesthetized with a mix of isoflurane/oxygen 2%/1.5% by inhalation and mounted into a stereotaxic frame (Stoelting) linked to a digital micromanipulator. Brain coordinates of viral injection were chosen in accordance with the mouse brain atlas49: 1) BLA, AP: -1.7 mm; ML: ± 3 mm; DV: -4.5 mm; 2) PL, AP: 2.0 mm; ML ± 0.25 mm; DV - 2.4mm. The volume of AAVs injection was 300 nL for hM4D and 150 nL for CAV2-cre, per hemisphere. We infused virus through a 10-μL Hamilton syringe. After infusion, the pipette was kept in place for 5 min. After virus injection mice were allowed 4 weeks to recover and for the viral transgenes to adequately express before behavioral experiments.
For fiber photometry, a glass micropipette connected to a 10-μL Hamilton syringe was lowered into the BLA (DV -4.75 from skull) and 300 nL of virus (AAV1.CamKII.GCaMP6f.WPRE.SV40) was injected (0.1 μl/min) using a syringe pump (Harvard apparatus, CA). After infusion, the pipette was kept in place for additional 10 minutes and then slowly withdrawn. A multimode fiber optic cannula (200 μm core, 0.5 NA, ~ 6 mm, Thorlabs) was implanted 0.15 mm above the injection site (DV -4.60 from skull). Implant was secured to the skull with MetaBond and dental cement. Mice were housed in pairs immediately after surgery and at least 4 weeks post-surgery were allowed for virus expression before the experiment began.
Fibre photometry recordings
To assess the activity of BLA neurons during the SDM task, the fluorescence signal emitted by GCaMP6f expressing neurons was recorded using fiber photometry.50 A signal processor (RZP5; Tucker Davis Technologies) was used to control two light sources (465 nm LED, CLED_465; 405 nm LED, CLED_405, Doric Lenses), which were modulated at 211 Hz and 539 Hz respectively. The two wavelengths were combined by a fluorescence minicube (Doric Lenses) and transmitted through an optical patch cable (Doric Lenses) to the mice head implant. Emitted fluorescence was collected by the same patch cable, delivered back to the same minicube through a 525 nm filter and sent to a photoreceiver (Femtowatt Silicon Photoreceiver, DC-750 Hz; Newport). Real time signals were acquired, lowpass filtered (6 Hz) and demodulated with Synapse Essentials software (Tucker Davis Technologies). Med-PC system (Med-PC V Software Suite, Med Associates) generated TTLs to time-stamp specific events (i.e., nose pokes, food receptacle entries and pellet deliveries).
Data was extracted from TDT files and analyzed using custom MatLab scripts. Demodulated data streams were filtered at 1017 Hz, divided into discrete trials by aligning with TTL representing a trial (i.e., a nose poke resulting in a pellet delivery) and binned into 100 ms bins. Z-scores were calculated for each nose poke-related signal by taking the mean divided by the SD of a 5 s baseline period preceding each nose poke. Area under the curve was calculated for 5 s following the nose poke. Pre-learning phase was defined as the first experimental day, when mice underwent the task for the first time. If no nose pokes were observed during the session, pre-learning was considered as the first day in which mice were performing at least one nose poke for each side. Learning phase was defined as the day in which a mouse performed more than 20 pokes throughout the session. Post-learning included the last two experimental days, in which altruistic or selfish behavior was consistently observed. For the post-learning phase, data was averaged across the two days.
Quantification of c-fos+ cells
Actor mice were tested in the SDM task with or without recipient mice for 5 days. On the last day of training, actor mice were euthanized 90 min after the session and brains were collected and processed for immunohistochemical detection of fos protein. All cells were counted bilaterally from two to six coronal sections of BLA for each mouse.
Drugs
For hM4D activation we used i.p. administration of Clozapine N-Oxide dihydrochloride (CNO, HB6149 Hello Bio) dissolved in physiological saline (0.9% NaCl) at a dose of 3mg/kg in a volume of 10 ml/kg, 30 minutes before the behavioral experiments. All mice (control and hM4D) received i.p. CNO injection.
Tissue-slice preparation and immunohistochemistry
Mice were transcardially perfused with 40 ml of 0.1 M phosphate buffered saline (PBS) and then with cold paraformaldehyde (PFA, 4% in PBS). The brain was removed from the skull and post-fixed in 4% PFA in PBS for 1h at 4°C. The brain was sliced in 40 μm coronal sections using a vibratome 1000 Plus Sectioning System (3 M). Brain slices were permeabilized in 0.3% Triton X-100 in PBS (0.3% T-PBS) for 1 h at RT, shaking. After permeabilization, brain slices were blocked with 0.1% Triton X-100 in PBS (0.1% T-PBS) supplemented with 10% normal goat serum (NGS) for 2 h at RT, shaking. After permeabilization and blocking, to examine c-fos expression slices were incubated with anti-cfos and anti-GAD67 antibodies in 0.1% T-PBS supplemented with 3% NGS for 3 o/n at 4 °C, shaking. The appropriate Alexa Fluor-conjugated secondary antibodies in 0.1% T-PBS with 3% NGS were applied for 2 h at RT followed by nuclei staining with the fluorescent dye4′,6-diamidino-2-phenylindole (DAPI; 1:50,000 in PBS; Thermo Fisher Scientific). Labelling in the BLA and in the PFC was visualized with confocal microscope (Zeiss) with 20x objective. Specifically, Z-stack of 1 μm steps were taken and then analysed using Fiji (ImageJ) software. To detect viral expression of hM4D in BLA- and PFC-projecting neurons brain slices were stained with rabbit anti-DsRed. To visualize viral expression of hM4D and GCaMP6f and fiber placement, BLA- and PFC-containing brain slices were acquired with Nanozoomer S60 (Hamamatsu), using constant settings, or Axiovert 200M microscope (Zeiss)
Antibodies
For immunohistochemistry analyses, the following primary antibodies were used: rabbit anti-cfos (sc-7202, Santa Cruz Biotechnology; dilution: 1:1000), mouse anti-GAD67 (MAB5406, Sigma-Aldrich; dilution: 1:800), rabbit anti-DsRed (632496, Takara, 1:1000). The following secondary antibodies were used: goat anti-rabbit-Alexa488 (A-11034, Invitrogen, 1:1000); goat anti-mouse-Alexa647 (A-21235, Invitrogen, dilution: 1:1000).
Statistics and Reproducibility
No statistical methods were used to predetermine sample sizes but were selected based on previous experience and on estimation from related studies.6–8,17 Animals were randomly assigned to control and manipulation groups. Experimenters were not blinded during data acquisition, but all analyses were performed with blinding of the experimental conditions as stated in the methods section. One mouse was excluded from data collection because showed little motivation to engage in nose-pokes responses (fewer than ten total pokes), two were excluded because viral expression patterns were not appropriate (outside the target region) and two were excluded due to fiber misplacement. Statistical analyses and figures plotting were performed using Prism version 9 (GraphPad). Data are reported as mean ± s.e.m. plots or box plots. In box plots, the central mark indicates the median; the bottom and top edges of the box indicate the maximum and the minimum. Statistical methods used in this study include two-way repeated measures (RM) ANOVA and one-way ANOVA followed by Bonferroni correction, Wilcoxon matched-pairs signed-rank test. Single variable comparisons were made using two-tailed paired and unpaired t-test. Mice were assigned to altruistic or selfish groups using one sample t-test to chance (50%). The accepted value for significance was p<0.05. Sample sizes and statistical tests are reported in the figure legends. Data distribution was tested using D’Agostino and Pearson normality test. The experiments reported in this work were repeated independently at least two to four time.
Extended Data
Extended Data Figure 1. Characterization of behaviors in the SDM task.
a, Individual decision preference scores in mice tested with (orange) or without (grey) recipient mouse over the five days of SDM task. b, Cumulative number of altruistic choices for each mouse (altruistic, orange; selfish, blue) during each daily session in the SDM task. c, Altruistic responses (in %) in altruistic (n=11) and selfish (n=5) mice (two-way RM ANOVA, group (altruistic, selfish) x time (days 1-5), F(4, 56)=21.55, p<0.0001) and individual scores of altruistic responses across five days of SDM. d, Number of tested mice grouped by percentage of altruistic responses. e, Number of choices (orange, altruistic; maroon, selfish) of altruistic mice on the last session of training in the SDM task (two-way RM ANOVA, choice (altruistic, selfish) x time (minutes), F(7, 70)=5.67, p<0.0001). f, Number of choices (light blue, altruistic; blue, selfish) of selfish mice on the last session of training in the SDM task (two-way RM ANOVA, choice (altruistic, selfish) x time (minutes), F(7, 35)=2.61, p=0.0276). g, Number of head entries in the food magazine of recipient mice tested with altruistic (orange, n=14) or selfish (blue, n=14) actors (two-way RM ANOVA, group (altruistic, selfish) x time (days 1-5), F(4, 103)=3.04, p=0.0203). h, Following training in the SDM task actor mice were tested in an additional session with sated (red, n=6) or food-restricted (orange, n=6) recipient mice (two-tailed unpaired t-test: t=2.37, d.f.=10, p=0.0387). i, Left, decision preference score in mice tested with food-restricted (orange, n=12) or sated (red, n=9) recipient mice over the five days of SDM task (two-way RM ANOVA, group (sated, food-restricted) x time (days 1-5), F(4, 76)=2.62, p=0.0409). Right, individual curves representing decision preference scores. *p<0.05, ***p<0.001. Values are expressed as mean ± s.e.m.
Extended Data Figure 2. Male mice make more altruistic responses than females.
a, Altruistic responses in males (n=8) and females (n=8) across five days of testing in the SDM (two-way RM ANOVA, gender, F(1, 14)=5.90, p=0.0292; time (days 1-5), F(4, 56)=4.59, p=0.0028). b, Number of tested mice grouped by gender and by percentage of altruistic responses. c, Number of nose pokes responses in male mice tested in the conditions with recipient (n=8) and no recipient (n=6) on day five of the SDM (two-way ANOVA, group (with recipient, no recipient) x response (nose-poke 1, nose-poke 2), F(1, 24)=6.2, p=0.0199). d, Number of nose pokes responses in female mice tested in the conditions with recipient (n=8) and no recipient (n=6) (two-way ANOVA, group (with recipient, no recipient), F(1, 12)=4.1, p=0.0630). *p<0.05, **p<0.01, n.s., not significant. Values are expressed as mean ± s.e.m.
Extended Data Figure 3. Mice change their responses to share food rewards with their conspecifics.
a, Experimental design of the SDM. Actor mice were trained on a two-choice decision paradigm where nose pokes resulted in food rewards. In the condition i.”with recipient” (orange) one nose poke resulted in food reward to actor (selfish choice) and the other nose poke in food reward both to the actor and to the recipient, in the adjacent compartment (altruistic choice). After an inter-trial interval of 5 seconds (ITI), a new trial started, and actor could make their choice. The location of the two responses were counterbalanced between left and right nose-pokes. In the condition ii. “no recipient” (grey) the structure of the task was identical, but the adjacent compartment was empty. iii. In the condition “with toy”, the recipient was replaced. b, Nose poke responses (in percentage) during baseline training in the right and left nose poke holes were not different at the group level (two-tailed paired t-test: t=0.47, d.f.=24, p=0.6423, n=13 mice). c, Change of preference (in percentage) to altruistic responses during the SDM with recipient compared to the baseline (one-sample t-test, t=2.36, d.f.=64, p=0.0211, n=13 mice). d, Number of tested mice grouped by preference. e, Change of preference (in percentage) to altruistic responses during the last session of SDM in males (n=7) compared to females (n=6) mice (two-tailed paired t-test: t=1.94, d.f.=11, p=0.0773). f, Change of preference when animals were tested one additional day with their recipient (R→R, n=7) or with an inanimate object (toy, R→T, n=6) (two-tailed unpaired t-test, t=2.49, d.f.=11, p=0.0296). *p<0.05. Values are expressed as mean ± s.e.m.
Extended Data Figure 4. Mice are willing to take altruistic decisions under costly situations.
Altruistic responses (orange) reinforced on FR2, FR4 and FR6 and selfish responses (blue) reinforced on FR1 expressed as percentage of the total in males (light blue, (n=7) and females (red, n=4) mice and responses on the preferred nose poke (NP1, dark grey) reinforced on FR2, FR4 and FR6 and responses on the non-preferred nose poke (NP2, light grey) reinforced on FR1 in mice tested without recipient (n=6) (FR2: two-way RM ANOVA, group (with recipient males, with recipient females, no recipient) x response (FR1, FR2), F(2, 13)=3.5, p=0.05. FR4: two-way RM ANOVA, group (with recipient males, with recipient females, no recipient) x response (FR1, FR2), F(2, 13)=5.1, p=0.0192; FR6. two-way RM ANOVA, group (with recipient males, with recipient females, no recipient) x response (FR1, FR2), F(2, 13)=6.6, p=0.0103. FR8: two-tailed unpaired t-test, t=8.32, d.f.=6, p=0.0002). *p<0.05, **p<0.01, ***p<0.005 n.s. not significant. Values are expressed as mean ± s.e.m.
Extended Data Figure 5. Characterization of the role of social dominance and affective sensitivity in social decision-making.
a, Number of altruistic and selfish choices in subordinate (two-way RM ANOVA, choice (altruistic, selfish) x time (days), F(4, 152)=4.92, p=0.0009, n=19), and dominant actor mice (F(4, 144)=3.26, p=0.0122, n=20). b, Dominant actor mice grouped by altruistic (two-way RM ANOVA, choice (altruistic, selfish) x time (days), F(4, 96)=8.83, p<0.0001, n=13) and selfish preference (two-way RM ANOVA, choice (altruistic, selfish) x time (days), F(4, 48)=10.45, p<0.0001, n=7) on the SDM task. c, Subordinate actor mice grouped by altruistic (two-way RM ANOVA, choice (altruistic, selfish) x time (days), F(4, 48)=3.09, p<0.0001, n=7) and selfish preference (two-way RM ANOVA, choice (altruistic, selfish) x time (days), F(4, 88)=12.52, p<0.0001, n=12) on the SDM task. d-e, Social dominance (normalized David’s Score) quantified based on the number and directionality of interactions in the tube test in actor and recipient mice grouped by selfish (d, two-tailed paired t-test: t=3.17, d.f.=34, p=0.0032; n=18) and altruistic (e, two-tailed paired t-test: t=0.79, d.f.=38, p=0.4324; n=20) actors and respective recipient conspecific. f-g, Normalized David’s Score in actor mice grouped by altruistic (f) or selfish (g) preference and by their social rank in relation to the normalized David’s Score of their respective recipient. h, Decision preference score in actor mice grouped by social rank (α: n=8, β: n=9, γ: n=12, δ: n=8; one-way ANOVA, F(3, 33)=3.90, p=0.0172; two-tailed unpaired t-test, α vs. β: t=2.12, d.f.=15, p=0.050; α vs. γ: t=2.87, d.f.=18, p=0.0100; α vs. δ: t=2.26, d.f.=14, p=0.7982; δ vs. γ: t=2.42, d.f.=18, p=0.0261). i, Top, Schematic representation of the observational fear learning and freezing behavior in actor mice, grouped by altruistic (n=6) or selfish (n=7) preference during baseline (two-tailed unpaired t-test: t=15.31, d.f.=13, p=0.1497). Bottom, freezing behavior (conditioning-baseline) in altruistic and selfish actors (two-tailed unpaired t-test: t=3.30, d.f.=13, p=0.0057) and total time spent in the proximity of the divider between the actor and recipient compartment (two-tailed paired t-test: t=0.39, d.f.=13, p=0.7021). j, Social dominance (normalized David’s score) predicts affective sensitivity (freezing behavior during observational fear learning) (linear regression, n=27 mice, y=8.971x+15.61, F(1, 25)=4.47, p=0.0446). *p<0.05, **p<0.01, ***p<0.001. n.s. not significant. Values are expressed as mean ± s.e.m.
Extended Data Figure 6. Behavioral effects of BLA neuronal silencing.
a, Representative images of viral expression in the BLA after injection with AAV-CamKIIa-hM4D-mCherry (data from 3 independent experiments). b, Reconstruction of viral expression. Red areas represent viral expression (higher expression = darker color). c, Left, schematic illustration of the actor-recipient testing chambers with graphical representation of the amount of time mice spent in different parts of the chambers (with blue as the shortest and red as the longest time). Right, social exploration, was measured in the area highlighted in red, in control (n=10) and hM4D (n=7) mice towards their recipients during the SDM task (two-way ANOVA, group (control, hM4D), F(4, 60)=5.0, p=0.0013). d, Number of nose pokes in control (n=9) and hM4D (n=10) mice (two-way ANOVA, group (control, hM4D), F(1, 13)=0.54, p=0.4721), e, Latency to respond in control (n=8) and hM4D (n=9) mice (two-way ANOVA, group (control, hM4D), F(1, 11)=0.02, p=0.877), and f, Locomotor activity (two-way ANOVA, group (control, hM4D), F(1, 11)=0.10, p=0.7566), during the five days of testing in the SDM task in control (n=6) and hM4D (n=7) mice. g, Observers mice received intraperitoneal (i.p.) injection of CNO (3 mg/kg) and after 30 minutes were tested with their respective demonstrators on the observational fear learning paradigm. h-i, Freezing behavior displayed by actor mice, control (n=8) and hM4D (n=7), during baseline (h, two-tailed unpaired t-test: t=0.83, d.f.=13, p=0.4170) and conditioning phases of the test (i, two-tailed unpaired t-test: t=2.22, d.f.=13, p=0.0447). *p<0.05. Values are expressed as mean ± s.e.m.
Extended Data Figure 7. BLA neuronal silencing reduces dominance and altruistic choices.
a, Left, average rank change after CNO or vehicle injection in mice that received hM4D in the BLA (two-sided Wilcoxon matched-pairs signed-rank test, p=0.0002; vehicle n=10, CNO n=13). Right, rank changes in each hM4D-expressing mice after i.p. injection of CNO. b, Number of hM4D-expressing mice, grouped by social rank, that showed rank change following CNO injection. c, Left, control and mice that received hM4D for BLA silencing were injected with CNO (3mg/kg) 30 minutes before the SDM task. At least 1 hour after daily session, mice were tested in the tube test for assessment of social ranking within cage mates. Right, cage composition. Each cage hosted 2 actor-recipient pairs. Actor mice received hM4D or control virus in the BLA. All the recipients received the control virus. d, Number of dominant or subordinate actor mice compared to their recipient conspecific (n=19; two-sided Fisher’s exact test p=0.1789). e, Social dominance (normalized David’s Score) quantified based on the number and directionality of interactions in the tube test in actor grouped by control (n=9) and hM4D (n=9) mice (two-tailed paired t-test: t=2.15, d.f.=14, p=0.0493). f-g, Social dominance (normalized David’s Score) quantified based on the number and directionality of interactions in the tube test in (f) control (two-tailed paired t-test: t=1.30, d.f.=16, p=0.2120) and (g) hM4D actor mice (two-tailed paired t-test: t=1.331, d.f.=14, p=0.2045). h-i, Rank positions of (h) control and (i) hM4D mice in each cage over testing days. j, Number of altruistic and selfish choices in subordinate (grey, n=6) and dominant (red, n=4) mice that received hM4D in the BLA and CNO at the end of training and during the five days of SDM task (two-way ANOVA, group (dominant, subordinate), F(3, 80)=3.9, p=0.0107). k, Decision preference scores in the SDM task of hM4D-injected animal, grouped by dominant and subordinate. *p<0.05. Values are expressed as mean ± s.e.m.
Extended Data Figure 8. Fiber photometry and c-fos expression in the BLA.
a, Targeting maps of GCaMP6f expression and location of optic fibers in the BLA for fiber photometry recordings. b, Top, representative images of c-fos (green) and GAD67 (red) expression. Scale bar (applicable to all micrographs), 50 μm. Bottom, bar graph quantification of c-fos GAD67 double positive cells in altruistic and selfish mice (n=21 sections from 5 animals; two-tailed unpaired t-test: t=3.33, d.f.=22, p=0.0030) and bar graph quantification of cells that were c-fos positive and GAD67 negative in altruistic and selfish mice and mice without recipient (n=37 sections from 7 animals; one-way ANOVA, F(2, 34)=3.97, p=0.0282). *p<0.05, ***p<0.001. Values are expressed as mean ± s.e.m.
Extended Data Figure 9. BLA is required to develop altruistic preference.
a, Latency to respond (in sec) grouped by altruistic and selfish choices in control (light and dark orange, n=7) and hM4D mice (light and dark fuchsia, n=7) during the five days of SDM task (two-way ANOVA, group (control, hM4D, altruistic, selfish), x time (days 1-5), F(12, 96)=2.39, p=0.0093). b, Number of altruistic choices over 40 minutes of testing during the five days of the SDM in control and hM4D mice (mixed model analysis for each day of testing, day1: (control, hM4D) x time (min), F(16, 132)=0.48, p=0.9915; day 2: group F(42, 267)=0.42, p=0.9994; day 3: F(73, 685)=2.85, p<0.0001; day 4: F(80, 716)=2.13, p<0.0001; day 5: F(77, 696)=0.85, p=0.8057). c, Decision preference score in the five days of SDM and following one additional week of testing (day 13) in control (n=7) and hM4D (n=7) mice (two-way RM ANOVA, group (control, hM4D) x time (days), F(5, 60)=2.875, p=0.0416). d, Number of altruistic and selfish choices on test day 13 of the SDM in control (n=7) and hM4D (n=7) mice (two-way RM ANOVA, group (control, hM4D) x choice (altruistic, selfish), F(1, 12)=9.661, p=0.0091). *p<0.05, **p<0.01, ***p<0.001. Values are expressed as mean ± s.e.m.
Acknowledgments
We are grateful to G. Contarini, J. Stanic, M. Morini, D. Cantatore, and C. Esposto for their excellent technical support. We thank NOLIMITS, an advanced imaging facility established by the Università degli Studi di Milano. This work was supported by funding from Fondazione Istituto Italiano di Tecnologia, Ministero della Salute (project GR-2016-02362413), and Fondazione Telethon Italia (project GGP19103) to FP, and Fondazione Cariplo to DS (2019-1747).
Footnotes
Author contributions
DS conceptualized and designed the study. DS, FLG, FM, GCo, CM and EZ performed chemogenetics and behavioral experiments. FM and GCh performed fiber photometry. GCh, CM and FB analyzed fiber photometry data. MI performed c-fos experiments. FG provide suggestions on c-fos experiments and imaging. FLG, FM, GCo, MI and NC performed histology. DS, FLG, MD, FP wrote the manuscript. DS supervised the study. DS, FG, MD and FP provided resources and acquired funding. All the authors revised the manuscript
Competing interests
The authors declare that they have no competing interests.
Code availability
Custom-written analysis code is available at: https://doi.org/10.5061/dryad.bnzs7h4dv
Data availability
All source data used to generate the figures are available at: https://doi.org/10.5061/dryad.bnzs7h4dv
References
- 1.Frith CD. Social cognition. Philos Trans R Soc B Biol Sci. 2008;363:2033–2039. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Curr Opin Neurobiol. 2008;18:159–165. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Batson CD. The naked emperor: Seeking a more plausible genetic basis for psychological altruism. Econ Philos. 2010 doi: 10.1017/S0266267110000179. [DOI] [Google Scholar]
- 4.Preston SD. The origins of altruism in offspring care. Psychol Bull. 2013 doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- 5.Marsh AA. Neural, cognitive, and evolutionary foundations of human altruism. Wiley Interdiscip Rev Cogn Sci. 2016;7:59–71. doi: 10.1002/wcs.1377. [DOI] [PubMed] [Google Scholar]
- 6.Bartal IB-A, Decety J, Mason P. Empathy and Pro-Social Behavior in Rats. Science (80-) 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Lallement J, et al. Harm to others acts as a negative reinforcer in rats. Curr Biol. 2020:1–13. doi: 10.1016/j.cub.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Márquez C, Rennie SM, Costa DF, Moita MA. Prosocial Choice in Rats Depends on Food-Seeking Behavior Displayed by Recipients. Curr Biol. 2015;25:1736–1745. doi: 10.1016/j.cub.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Dolivo V, Taborsky M. Norway rats reciprocate help according to the quality of help they received. Biol Lett. 2015;11 doi: 10.1098/rsbl.2014.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkett JP, et al. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–8. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe IH, et al. Mice in social conflict show rule-observance behavior enhancing long-term benefit. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Waal FBM. Putting the Altruism Back into Altruism: The Evolution of Empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 13.Qu C, Ligneul R, Van der Henst JB, Dreher JC. An Integrative Interdisciplinary Perspective on Social Dominance Hierarchies. Trends Cogn Sci. 2017;21:893–908. doi: 10.1016/j.tics.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Cronin KA. Prosocial behaviour in animals: The influence of social relationships, communication and rewards. Anim Behav. 2012;84:1085–1093. [Google Scholar]
- 15.Dal Monte O, Chu CCJ, Fagan NA, Chang SWC. Specialized medial prefrontal–amygdala coordination in other-regarding decision preference. Nat Neurosci. 2020;23:565–574. doi: 10.1038/s41593-020-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allsop SA, et al. Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell. 2018;173:1329–1342.:e18. doi: 10.1016/j.cell.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camerer CF, Fehr E. Foundations of Human Sociality. Oxford University Press; 2004. Measuring Social Norms and Preferences Using Experimental Games: A Guide for Social Scientists; pp. 55–95. [DOI] [Google Scholar]
- 20.Zhou T, Sandi C, Hu H. Advances in understanding neural mechanisms of social dominance. Curr Opin Neurobiol. 2018;49:99–107. doi: 10.1016/j.conb.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Paradiso E, Gazzola V, Keysers C. Neural mechanisms necessary for empathy-related phenomena across species. Curr Opin Neurobiol. 2021;68:107–115. doi: 10.1016/j.conb.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Jeon D, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang SWC, et al. Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci. 2015;112:16012–16017. doi: 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Kessels HW, Hu H. The mouse that roared: Neural mechanisms of social hierarchy. Trends Neurosci. 2014;37:674–682. doi: 10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Yizhar O, Klavir O. Reciprocal amygdala-prefrontal interactions in learning. Curr Opin Neurobiol. 2018;52:149–155. doi: 10.1016/j.conb.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Tye KM. Neural Circuit Motifs in Valence Processing. Neuron. 2018;100:436–452. doi: 10.1016/j.neuron.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogt BA, Paxinos G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct Funct. 2014;219:185–192. doi: 10.1007/s00429-012-0493-3. [DOI] [PubMed] [Google Scholar]
- 29.Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci. 2005;25:7763–70. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juavinett AL, Erlich JC, Churchland AK. Decision-making behaviors: weighing ethology, complexity, and sensorimotor compatibility. Curr Opin Neurobiol. 2018;49:42–50. doi: 10.1016/j.conb.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez-Lallement J, Van Wingerden M, Marx C, Srejic M, Kalenscher T. Rats prefer mutual rewards in a prosocial choice task. Front Neurosci. 2015;9:1–9. doi: 10.3389/fnins.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivers RL. The Evolution of Reciprocal Altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 33.Zink CF, et al. Know Your Place: Neural Processing of Social Hierarchy in Humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gachomba MJM, et al. Multimodal cues displayed by submissive rats promote prosocial choices by dominants. Curr Biol. 2022;32:3288–3301.:e8. doi: 10.1016/j.cub.2022.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Killcross S, Robbins TW, Everitt BJ. Different Types of Fear Conditioned Behavior Mediated by Separate Nuclei Within Amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 36.Terburg D, et al. The Basolateral Amygdala Is Essential for Rapid Escape: A Human and Rodent Study. Cell. 2018;175:723–735.:e16. doi: 10.1016/j.cell.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Knoch D, Fehr E. Resisting the power of temptations: The right prefrontal cortex and self-control. Ann N Y Acad Sci. 2007;1104:123–134. doi: 10.1196/annals.1390.004. [DOI] [PubMed] [Google Scholar]
- 39.Padilla-Coreano N, et al. Cortical ensembles orchestrate social competition through hypothalamic outputs. Nature. 2022;603:667–671. doi: 10.1038/s41586-022-04507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheggia D, et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat Neurosci. 2020;23:47–60. doi: 10.1038/s41593-019-0551-8. [DOI] [PubMed] [Google Scholar]
- 41.Keysers C, Knapska E, Moita MA, Gazzola V. Emotional contagion and prosocial behavior in rodents. Trends Cogn Sci. 2022;26:688–706. doi: 10.1016/j.tics.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Preston SD, de Waal FBM. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, et al. Oxytocin modulates social value representations in the amygdala. Nat Neurosci. 2019;22:633–641. doi: 10.1038/s41593-019-0351-1. [DOI] [PubMed] [Google Scholar]
- 44.Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes, Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu RK, et al. An amygdala-to-hypothalamus circuit for social reward. Nat Neurosci. 2021 doi: 10.1038/s41593-021-00828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol. 2016;7:1325–1330. [Google Scholar]
- 47.Wang F, et al. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science (80-) 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- 48.De Vries H, Stevens JMG, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Anim Behav. 2006;71:585–592. [Google Scholar]
- 49.Konsman J-P. The mouse brain in stereotaxic coordinates. Psychoneuroendocrinology. 2003;28:827–828. [Google Scholar]
- 50.Gunaydin LA, et al. Natural Neural Projection Dynamics Underlying Social Behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Custom-written analysis code is available at: https://doi.org/10.5061/dryad.bnzs7h4dv
All source data used to generate the figures are available at: https://doi.org/10.5061/dryad.bnzs7h4dv