Abstract
Communication between the periphery and the brain is key for maintaining energy homeostasis. To do so, peripheral signals from the circulation reach the brain via the circumventricular organs (CVOs), which are characterized by fenestrated vessels lacking the protective blood-brain barrier (BBB). Glial cells, by virtue of their plasticity and their ideal location at the interface of blood vessels and neurons, participate in the integration and transmission of peripheral information to neuronal networks within the brain for the neuroendocrine control of whole-body metabolism. Metabolic diseases, such as obesity and type 2 diabetes, can disrupt the brain-to-periphery communication mediated by glial cells, highlighting the relevance of these cell types in the pathophysiology of such complications. An improved understanding of how glial cells integrate and respond to metabolic and humoral signals has become a priority for the discovery of promising therapeutic strategies to treat metabolic disorders. This review highlights the role of glial cells in the exchange of metabolic signals between the periphery and the brain that are relevant for the regulation of whole-body energy homeostasis.
Keywords: astrocytes, tanycytes, oligodendrocytes, microglia, hypothalamus, glia, sexual dimorphism, glucose, leptin, insulin, incretins
1. Hypothalamic Glia in Energy Homeostasis
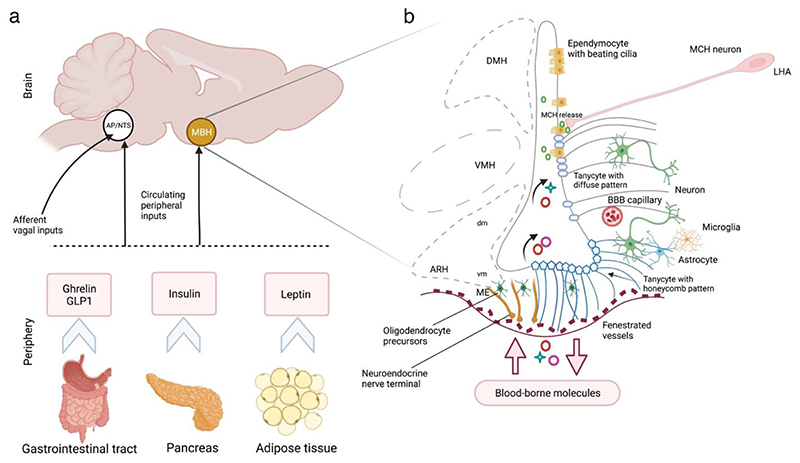
Glial cells—broadly classified into astrocytes, tanycytes, ependymocytes, oligodendrocytes, NG-2 glia and microglia—actively integrate peripheral information into neuronal networks initiating a cascade of central signalling pathways in response to systemic metabolic states1–3. Peripheral signals from the viscera to the CNS can either exert short-term effects, such as the gastric hormone ghrelin, which triggers hunger-related signals4, or have long-term implications, such as leptin secreted by the adipose tissue5, and insulin released from the pancreas6, both of which are hormones involved in the regulation of adiposity and glucose levels, respectively5,6 (Figure 1a). These homeostatic peripheral signals converge on the mediobasal hypothalamus (MBH), a key brain region influencing food intake and energy homeostasis7 composed of the dorsomedial (DMH), ventromedial (VMH) and arcuate (ARH) nuclei of the hypothalamus as well as one of the circumventricular organs (CVOs) called median eminence (ME) adjacent to the ARH at the base of the third ventricle (3V) (Figure 1b). CVOs are situated along the midline of the brain characterized by fenestrated capillaries that allow relatively free passage of molecules between the periphery and the CNS, in contrast to the vasculature in other brain regions secured by highly selective blood-brain barrier (BBB)7,8. Glial cells in the hypothalamic ARH-ME region can sense peripheral signals that reach the brain from the circulation and transmit it to distinct ARH neuronal populations, including the orexigenic neuropeptide Y (NPY) and agouti-related peptide (AgRP) expressing neurons, as well the anorexigenic proopiomelanocortin (POMC), and cocaine- and amphetamine-regulated transcript expressing neurons7,8. POMC and AgRP expressing neurons constitute the central melanocortin system. POMC neurons release ɑ-melanocyte–stimulating hormone and activate melanocortin-4 receptors (MC4R)9, while AgRP acts as an endogenous antagonist of MC4R9. Mutations in the genes encoding POMC and MC4R are known to be the most common cause of monogenic human obesity10.
Figure 1. The cross-talk between the brain and the peripheral organs via metabolic signals for energy homeostasis.
(a) The peripheral signals from the visceral organs (such as ghrelin and GLP1 from the gastrointestinal tract, insulin from the pancreas and leptin from the adipose tissue shown here or other metabolites and regulators discussed in section 3.2.2) reach two key regions of the brain: the MBH via pituitary-portal circulation, and/or the DVC constituting the AP and NTS via the circulation or through the sensory vagus nerve. (b) Schematic representation of the transport of blood-borne molecules to the MBH via the fenestrated capillaries of the ME. Tanycytes at the floor of the third ventricle (ME tanycytes) adapt their plasticity to permit the passage of molecules. Tanycytes at the ARH and VMH sense the uptake of peripheral signals and transmit them to the neighbouring neurons. Astrocytes at the interface of BBB capillaries also sense and transmit the systemic metabolic information to neurons. MCH neuronal projections from the LHA release MCH at the base or the pole of ependymal cells controlling ciliary beat frequency. AP, area postrema; ARH, arcuate nucleus of the hypothalamus; BBB, blood-brain barrier; CVO, circumventricular organ; DVC, dorsal vagus complex; LHA, lateral hypothalamic area; MBH, mediobasal hypothalamus; MCH, melanin-concentrating hormone; ME, median eminence; NTS, nucleus of the solitary tract; VMH, ventromedial nucleus of the hypothalamus.
Here we review recent research on the function of hypothalamic glia, their interaction with neurons and vascular cells, and their sexually-dimorphic implications in energy homeostasis. Furthermore, we will discuss how glial cell mediated brain-to-periphery communication is disrupted in metabolic perturbations such as obesity and type 2 diabetes.
2. Astrocytes in the Neuroendocrine Control of Metabolism
Astrocytes are the most abundant glial cells in the hypothalamus, which are ideally located at the interface of blood vessels within the BBB and adjacent to neurons for participating in neuroendocrine functions2,3,11. Hypothalamic astrocytes sense metabolic signals from the periphery in the form of hormones and nutrients (such as leptin12, ghrelin13, insulin14, glucose14, fatty acids15,16 and amino acids17), and work in concert with neurons to generate distinct metabolic responses, of which the key processes and associated mechanisms are described below.
2.1. Astrocytic response to appetite-regulatory signals
Astrocytes maintain a constant and dynamic interaction with neurons, as well as release neuroactive molecules, i.e., gliotransmitters modulating synaptic transmission and neuronal excitability3,11,18. Leptin, an anorexigenic hormone produced and secreted by the adipose tissue, signals satiety in the brain5,19, and generates rapid synaptic modulatory effects in the ARH20. This leptin-mediated synaptic modulation seems partially under the control of astrocytes that ensheath neurons of the hypothalamic feeding center12,21,22. Along these lines, high circulating leptin levels have been found to alter the expression of structural glial proteins such as glial fibrillary acidic protein (GFAP) and vimentin, with these changes being inversely linked to modifications in synaptic protein densities in adult male rats21. In addition, astrocytes express leptin receptors (LepRs, mainly the long isoform LepRb)12 necessary for the integration of peripheral leptin signals in the brain5,23. LepR deletion in GFAP-expressing astroglial cells can alter glial morphology, reduce glial coverage, modulate synaptic input to POMC as well as AgRP neurons and blunt the anorexic effects of leptin12. However, emerging research implies that blood-borne leptin may sensitize other LepR expressing cells, such as tanycytes24,25 (section 3.2.1), pericytes26, the cells embedded in the basement membrane of blood microvessels or ARH neuronal dendrites in the ME contacting NG-2 glia27, in the MBH and reach the hypothalamic feeding centres via distinct and parallel mechanisms24–27.
Contrary to leptin, ghrelin is an orexigenic hormone synthesized and released from the digestive tract in response to fasting, which stimulates appetite and induces adiposity via a highly conserved G protein-coupled receptor known as the growth hormone secretagogue receptor subtype 1a (GHS-R1a)28. The direct action of ghrelin on ARH neurons stimulates food intake and regulates glucose levels, which is partially mediated by astrocytes12,28. Because GHS-R1a is coupled to Gɑq/11 proteins, the activation of Gɑq/11-mediated signalling in the ARH astrocytes and the associated increase in food intake suggests a role for astrocytes in ghrelin’s central action29. In line with the opposing functions of leptin and ghrelin, specific deletion of LepR signalling in astrocytes can increase rebound feeding upon fasting or ghrelin administration12. Selective activation of AgRP neurons by fasting or ghrelin administration can induce mitochondrial adaptations in neighbouring astrocytes13 and γ-aminobutyric acid (GABA, an inhibitory neurotransmitter), released by AgRP neurons, acts as an effector of ghrelin-induced astrocyte depolarization, and increase the glial coverage of AgRP perikarya with a consequent reduction in the number of inhibitory synaptic connections on AgRP plasma membrane13. Thus, hunger-related signals can trigger an astrocyte-mediated feed-forward auto-activation loop for the control of orexigenic AgRP neurons13.
Astrocyte-released prostaglandin E2 (PGE2), a potent hypothalamic gliotransmitter30, can promote the activation of AgRP neurons specifically via the PGE2 EP2 receptor13. Deletion of acyl-CoA–binding protein (ACBP, also known as diazepam binding inhibitor and the precursor of the anorectic peptide octadecaneuropeptide (ODN)31), another astrocytic gliotransmitter, from GFAP+ astroglial cells induced hyperphagia and obesity while the selective genetic rescue of ACBP in the astrocytes of the ARH in AcbpGfapKO mice reduced feeding and prevented weight gain32. The anorectic effects of central ODN was diminished in obese MC4R-KO mice suggesting the involvement of the melanocortin system32. Thus, appetite-regulatory signals induce morphological changes in hypothalamic astrocytes, particularly in the ARH, and control the release of gliotransmitters to generate an appropriate neuronal response to hormones that can either signal satiety or hunger.
2.2. Astrocytes in glucose sensing and homeostasis
Hypothalamic astrocytes sense circulating glucose and participate in glucose uptake to meet the high energy demands of neighbouring neurons18. Glucose uptake by astrocytes is stoichiometrically proportional to astrocytic glutamate uptake, which in turn depends on the glutamatergic activity at the synapse18. One of the mechanisms describing the glucose sensing role of astrocytes involves facilitated diffusion of glucose via glucose transporters (GLUTs), particularly GLUT-1 (45 kDa isoform) in the astrocytic end-feet in close apposition with the vasculature18. Glucose taken up by astrocytes enters the glycolytic pathway to generate and release lactate. Lactate is then transferred to neurons via monocarboxylate transporters (MCTs), where it is converted to pyruvate for aerobic energy production in the mitochondria33,34. MCT1 and MCT4 specifically expressed in astrocytes35 (and tanycytes36) serve as lactate importers or exporters based on the cell’s metabolic state, whereas MCT2, mainly expressed in neurons, mediates lactate uptake into neurons35. Moreover, astrocytes express a lactate receptor, hydroxycarboxylic acid receptor 1 (HCAR1), and POMC neurons exhibit an excitatory response to lactate via astrocytic HCAR137.
Metabolic factors such as leptin can alter astrocytic glutamate and glucose uptake through modifications in astrocyte-specific glutamate and glucose transporters, which in turn seem to modulate electrical activity of POMC neurons22. Insulin receptor expression in astrocytes may also co-regulate glucose sensing and uptake for the activation of POMC neurons14. Interestingly, selective deletion of sonic hedgehog (Shh) receptor Patched (Ptc) in glutamate transporter GLAST-expressing astrocytes, present a novel mechanism by which astrocytes enhance glucose metabolism in mice and promote a lean phenotype with reduced adiposity and increased whole-body fatty acid oxidation38. Moreover, postprandial hyperglycemia triggers the retraction of glial coverage making anorexigenic POMC neurons hyperactive39. Another example of astrocyte-neuron interaction under normal physiological state being the response of astrocytes in the ventrolateral preoptic nucleus to an increase in extracellular glucose concentration for the sleep-wake regulation, inducing astrocytic-derived adenosine release, and thus activating sleep-promoting neurons via excitatory G protein-coupled adenosine A2A receptor (A2AR)40. Thus, modulation of hypothalamic glia-neuronal circuits is not just an adaptive mechanism under extreme dietary conditions (such as overfeeding41 and fasting13), but may also develop in response to subtle metabolic changes39,40.
Astrocytes form interconnected and coordinated networks through connexins, a family of proteins that form intercellular membrane channels, for the intercellular transfer of small molecules, such as glucose42. Connexin channels can either be in the form of gap junctions creating a direct conduit between the cytoplasm of two cells, or hemichannels allowing the contact between the extracellular space and the cytosol of astrocytes. The switch from one configuration to another appears to be dynamically regulated by growth factors and cytokines43. Connexin hemichannels, but also the channels formed by pannexins, could be involved in the release of ATP. Hemichannels are also permeable to signalling molecules like NAD+, glutamate, glutathione and prostaglandin E2, known to be important for synaptic activity44. Astroglial connexins (Cx) 30 and 43 apposed in gap junctions allow glucose or lactate from a single astrocyte to propagate within the astroglial network, making it available to the hyperactive neurons42. Cx43 expression was found to increase under hyperglycemia, while its transient inhibition in the rat MBH using RNA interference decreased central glucose sensing, thereby reducing the brain’s glucose-induced insulin secretion42. Absence of Cx43 in astrocytes silences wake-promoting orexin neurons in the lateral hypothalamic area by attenuating glucose and lactate trafficking through astrocytic networks, causing excessive sleepiness and fragmented wakefulness during the nocturnal active phase of mice45.
However, astrocyte-mediated lactate shuttle seems only one among the few other mechanisms by which lactate is transported or taken up by the neurons. For instance, neighbouring tanycytes have the capacity to produce and transport lactate to POMC neurons36 (section 3.2.2). In this context, it is interesting to note that the neurons in the VMH and the ARH can generate an intrinsic response to extracellular glucose concentrations46–48. Thus, high-throughput strategies at single-cell resolution could help in delineating the precise role of astrocytes and other glial cells in the fine-tuning of neuronal function as part of their glucose regulatory functions.
2.3. Astrocytes in hypothalamic inflammation
Obesity and excessive consumption of fat- and sugar-rich diets trigger hypothalamic inflammation and reactive gliosis, a hallmark response to neuronal injury involving the activation and proliferation of both microglia and astrocytes49–51. While a plethora of studies have linked microglia to diet-induced inflammatory signals and metabolic dysfunction (section 6), a similar role for astrocytes has also been explored50. Markers of reactive gliosis become evident during early high fat diet (HFD) feeding49. Targeting the master inflammatory NF-KB pathway, rapidly upregulated in response to inflammation, by deletion or inhibition of its intermediates (such as IKKβ and MyD88) can restore hypothalamic energy balance52–54. The upregulation of astrocytic IKKβ/NF-KB can impair astrocytic plasticity with a sustained shortening of high-order processes, and cause physiological changes such as glucose intolerance, increased blood pressure, as well as fat mass and body weight gain55. On a similar front, IKKβ/NF-KB inactivation silences astrocyte inflammation in the MBH accompanied by reduced glucose intolerance and resistance to diet-induced obesity in mice50. Another potential hypothalamic inflammation target named pyruvate dehydrogenase (PDH) kinase (PDK), a mitochondrial gate-keeping enzyme, regulates glycolytic metabolism and mediates a metabolic shift from oxidative phosphorylation to glycolysis in astrocytes, contributing to inflammatory responses. Astrocyte-specific PDK2 deficiency reverses diabetes-induced increase in food intake, and attenuates inflammation as well as the lactate surge, implying a link between hypothalamic astrocytes and the diabetic phenotype56. Thus, modulating astrocytic inflammatory targets seems promising for improving pathological hypothalamic inflammation related to altered feeding behaviour.
3. Tanycytes as the gatekeepers of brain-to-periphery communication
Tanycytes, specialized ependymoglial cells, modulate brain-to-periphery communication by adapting their physiology in terms of functional and structural organization of the fenestrated endothelium of the ME depending on the metabolic state7. Recently, the plasticity and the diversity of tanycytes has gained attention at both morphological and transcriptomic levels. Although tanycytes have been traditionally categorized into four subtypes (ɑ1, ɑ2, β1, and β2), the classification is evolving57 based on their dorsoventral position, their end-feet projections and function along the 3V7. The cell bodies of β2 tanycytes lining the floor of the 3V project into the ME and come in close contact with the blood pituitary-portal system. This morphological link between tanycytes, ME vasculature and cerebrospinal fluid (CSF) facilitates the shuttling of circulating factors through tanycytes, which are then transmitted to ARH neurons7,58,59. Besides, tanycytic transport is essentially a two-way route. Toxic peptides or proteins (such as aggregates in neurodegenerative disorders) can be cleared from the CSF and shuttled into the blood circulation for disposal (reviewed in7). Tanycytes also seem to trigger hypothalamic neurogenesis in response to dietary or reproductive cues60–62. In this section, we discuss the recent advances in the understanding of tanycytic plasticity and function in the hypothalamus.
3.1. Tanycytes form a physical barrier between the brain and the periphery
The differential distribution of specialized tight junction complexes around tanycytic cell bodies define the physical barrier between tanycytes and the CSF at the ventricular wall7,23,25,58,63,64. The tanycytic tight junction barrier prevents the free movement of molecules that enter the ME via the fenestrated endothelium expressing plasmalemmal vesicle-associated protein 1 (Plvap). The fenestrated microvessels are highly permeable and allow the extravasation of circulating molecules of ~35 kDa, which are sequestered within the ME by the tight junction barrier preventing their diffusion into the CSF, and then to other brain regions. The tight junction protein complex, which consists of transmembrane and membrane-associated constitutive tight junction proteins, zonula occludens 1 (ZO1), occludin, and claudins, also make up for the tanycytic polarity along the basal end-feet and apical cell body influencing the direction of transported molecules. The structural organization of tanycytes and the connected tight junction complex is, however, largely dependent on the nutritional status of an individual. For instance, low blood glucose levels during fasting increase the organization of tanycytic tight junction complexes in both the ME and the ventromedial (vm) ARH, which is reversed upon refeeding via a vascular endothelial growth factor A (VEGFA)-dependent mechanism. In addition, exogenous VEGFA treatment in mice fed ad libitum triggers ME permeability, increasing food intake and sensitivity to the anorectic hormone leptin63. Fasting-induced fenestration of vmARH vessels also facilitates the immediate access of ghrelin, the gut-derived hormone for hunger, to the neighbouring orexigenic NPY/AgRP and anorexigenic POMC neurons65. Interestingly, similar VEGFA-mediated ME permeability mechanism has been put to action by the melanin-concentrating hormone (MCH)-expressing neurons, sending projections to the ARH-ME, independent of tanycytic action66. Nonetheless, nutritional status impacts the organization of tight junction complexes around tanycytic cell bodies, forming a physical barrier between the brain and the periphery and thus impairing free diffusion of peripheral signals.
3.2. Tanycytes as sensors and transporters of peripheral homeostatic signals
Tanycytes of the vmARH-ME transcytose blood-borne factors, including peptide-related drugs, into the CSF, and then transmit them to other hypothalamic regions. Here we describe the mechanisms by which tanycytes sense, process and transport these metabolic signals for maintaining energy homeostasis.
3.2.1. Transport of leptin and other peripheral signals
ME tanycytes are indeed the first hypothalamic cells that respond to peripheral leptin25. Under normal metabolic conditions, peripheral leptin crosses the fenestrated capillary plexus and is internalized by clathrin-coated vesicles in the end-feet of ME tanycytes expressing functional LepR via an extracellular signal-regulated kinase (ERK)-dependent mechanism in rodents25. The transcytosed leptin reaches leptin-responsive neurons not only in the neighbouring MBH regions (dorsomedial (dm)ARH, VMH and DMH) but also other brain areas via the CSF25,67 (Figure 2).
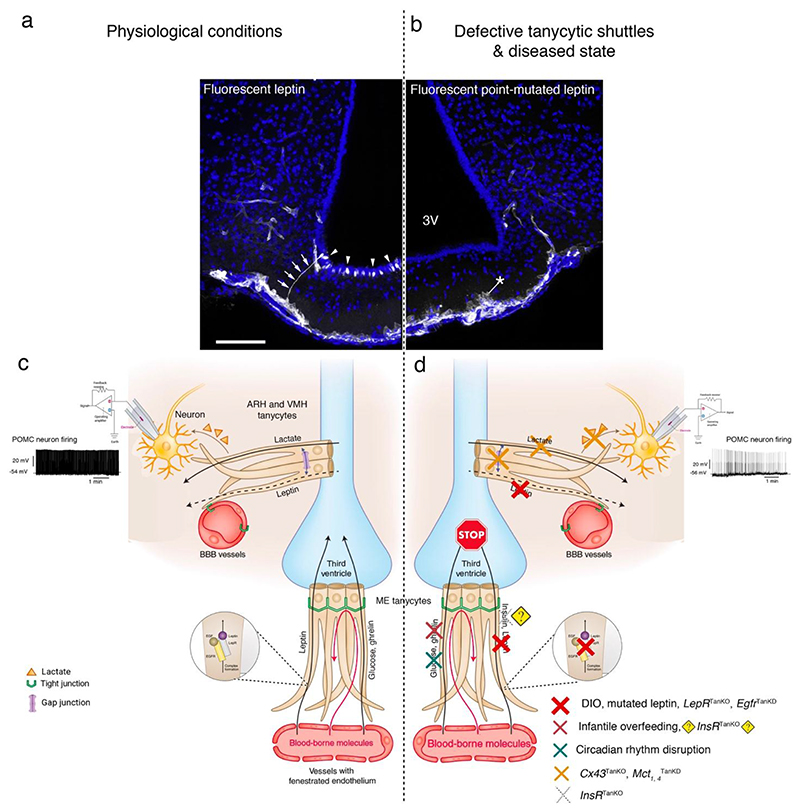
Figure 2. Leptin and other circulating signals are transported from the periphery to hypothalamic and extra-hypothalamic areas around the 3V by tanycytes.
(a) Representative photomicrographs with fluorescent leptin (25 nmol/mouse) labelled tanycytic processes (arrows) and cell bodies (arrowheads; white labeling), where fluorescent bioactive leptin was administered intravenously in wild-type mice. (b) Similar experiment using fluorescent point-mutated leptin and showing that the mutated leptin is taken up by tanycytes, but remains blocked at the tanycytic end-feet (asterisk) due to its inability to stimulate LepR-mediated EGFR activation24, as detailed in the schematics (c,d). The cell nuclei are counterstained with Hoechst solution. Adapted with permission from25. (c) Schematic showing tanycytes as the gateway for the passage of leptin and other blood-borne metabolic signals from fenestrated blood vessels to the CSF of the 3V. Tanycytes of the ARH sense CSF-borne molecules transported by tanycytes from the periphery, translate and transmit the information to neurons under the influence of the tanycytic network. (d) Diseased state or the experimental conditions in which mechanisms related to leptin transport24,25, insulin77, ghrelin transport78,79 or action77 are altered in tanycytes. Cx43 gap junction–mediated tanycytic metabolic networks are required for the transport of lactate, produced and released by tanycytes, to glucose-insensitive POMC neurons36. Tanycytes also play a role in the control of daily circadian changes96. 3V, third ventricle; ARH, arcuate nucleus of the hypothalamus; Cx43, connexin 43; DIO, diet-induced obesity; Egfr, epidermal growth factor receptor; Insr, insulin receptor; LepR, leptin receptor; Mct, monocarboxylate transporters; POMC, pro-opiomelanocortin; SCN, suprachiasmatic nucleus; TanKO, gene selectively knocked out in tanycytes; TanKD, gene selectively knocked down in tanycytes.
However, in individuals with obesity68,69 as well as in mini-pigs and mice with diet-induced obesity25,70, leptin transport into the brain seem disrupted despite high leptin content in the circulation68–71. In leptin-resistant HFD fed animals, blood-borne leptin taken up by tanycytes accumulates in the ME and fails to reach the MBH25. The pharmacological activation of ERK by epidermal growth factor (EGF) in tanycytes can restore leptin transport and rescue the normal phenotype in obese mice25. Recently Duquenne et al.24 explored the mechanisms involved in tanycytic-mediated leptin transport into the brain. The study confirms that ME tanycytes indeed express functional LepR using an array of methods sensitive for the detection of tanycytic LepR24. Tanycytes trigger Ca2+ waves in response to leptin, target STAT3 phosphorylation in the ARH neurons and require sequential activation of the LepR-EGFR complex by leptin and EGF for the transcytotic transport of leptin24. Furthermore, selective deletion of the LepR in tanycytes blocks leptin entry into the brain, which induces increased food intake, lipogenesis as well as glucose intolerance through impaired insulin secretion by the pancreas24. Intriguingly, this animal model with LepR-deleted tanycytes appears to recapitulate at least in part the changes occurring with age in the db mouse model72,73 (mice with mutated long form of the LepR74), i.e., elevated fasting insulin levels and normal glucose management at four weeks after LepR deletion in tanycytes as well as glucose intolerance and impaired glucose-stimulated insulin at twelve weeks24. In addition, lack of tanycytic LepR seems to prevent the adaptive neuroendocrine response to fasting, such as the corticotropin-releasing hormone (CRH)-mediated rise in glucocorticoids essential for the release of glucose from the liver to maintain glycemia24. Compliant with these observations24,25, endozepines—endogenous analogs of benzodiazepine derived from diazepam-binding inhibitor expressed by glial cells in brain structures related to food intake31—induce STAT3 phosphorylation in hypothalamic neurons in wild-type mice, but not in ob leptin-deficient mice, nor in mice in which LepR is selectively knocked out in tanycytes, thus suggesting that endozepines mediate their anti-obesity effects by activating tanycytic leptin shuttles75. Nevertheless, some authors failed to detect LepR expression in tanycytes76, which seems to have stemmed from strong methodological differences between studies, including the use of less sensitive methods.
The action of insulin on neurons and tanycytes can actively govern glucose metabolism and feeding. Recent research on insulin receptor-dependent signalling in different compartments of the BBB has shown that insulin receptors (IR) in tanycytes, as opposed to brain vascular endothelial cells, regulate insulin access to the ARH in mice77. Lack of the IR in tanycytes results in reduced insulin uptake in tanycytes and the MBH, systemic insulin resistance with normal food intake and energy expenditure, increased AgRP neuronal activity but blunted adaptations to feeding-related stimuli, and no notable differences in leptin sensitivity but seemingly revoke the orexigenic action of peripherally applied ghrelin77, which is also transported by tanycytes via a mechanism that is yet to be fully understood78,79
Hepatic FGF21, a central metabolic regulator possibly linking metabolism and reproduction80,81,82 can be taken up from the pituitary-portal blood circulation by tanycytes in mice, as well as in rats fed a HFD when they had benefited from prolonged breastfeeding during infancy, increasing the circulating FGF21 levels83. Tanycytic FGF21 shuttles are likely to involve FGFR183, abundantly expressed in these cells84.
In addition to metabolic hormones and regulators, tanycytes act as conduit for peripheral immune signals and mediate the central effects of systemic inflammation in mice85. Anorexia during systemic inflammation, marked by a prominent increase in the release of proinflammatory cytokines such as IL-1β, stimulate a cascade of proinflammatory events including canonical NF-kB signaling (NEMO). Systemic IL-1β stimulates NFkB activity in the VMH and vmARH tanycytes, inducing the expression of Cox-2 and the release of anorexigenic prostaglandins from tanycytes. Selective deletion of protein kinase IKK subunit NEMO in tanycytes alleviates IL-1β-induced anorexia, suggesting that tanycytes are important transporters of peripheral immune signals into the CNS85.
3.2.2. Metabolite sensing and processing
Tanycytes are directly exposed to nutritional signals, such as glucose86, amino acids87 and fatty acids88,89 that reach the CSF through the fenestrated capillaries of the CVOs, sense changes in the composition of the CSF, and respond to metabolic challenges via physiological adaptations63 as well as mechanisms such as ATP-mediated calcium signaling86,90. One of the first studies suggesting a role for tanycytes in metabolic sensing showed that destruction of the walls of the 3V containing tanycytes impaired the normal feeding response to hypoglycemia, which was then reversed with the regeneration of these cells91. Many studies further showed that tanycytes expressed glucose sensing-related proteins, including GLUT2, glucokinase (GCK) and ATP-sensitive K+ (KATP) channels, thus establishing a role for tanycytes as glucosensors that are key for maintaining glucose homeostasis92, partly by modulating POMC36 and NPY93 neuronal activity.
Tanycytes can detect glucose levels in the CSF and release ATP, potentially influencing the activity of neighbouring neurons86. This was first studied via the selective stimulation of tanycytic cell bodies by glucose or non-metabolisable analogues of glucose in acute brain slice preparations, generating a robust ATP-mediated Ca2+ response and initiating an intercellular calcium wave propagation via purinergic P2Y1 receptors86. Similarly, the sweet taste receptor, Tas1r2 (a G protein–coupled receptor), also mediates the glucosensitivity of tanycytes90. Tas1r2 can bind to glucose as well as a number of non-nutritive sweeteners such as sucralose, acesulfame K, and RebA. The focal application of these sweet substances to the brain slices evokes a response in tanycytes by an increase in intracellular calcium that propagates between tanycytes in the form of calcium waves90 via mechanisms similar to what has been previously described86,90.
In addition to glucosensing, tanycytes can convert brain glucose supplies into lactate and transport it to neighbouring neurons via MCT1 and MCT4, thereby stimulating ATP synthesis necessary for neuronal action36,94. The transport of lactate, produced and released by tanycytes, to glucose-insensitive POMC neurons requires Cx43 gap junction–mediated tanycytic metabolic networks, resulting in altered feeding behaviour and energy metabolism in mice36. Though in its infancy, these results indicate that tanycytes not only shuttle, but also actively process peripheral information (such as glucose to lactate translation), and transmit it to the neuronal circuitry depending on fluctuating systemic glucose levels. Moreover, control of tanycytic action via tanycyte-neuron synaptoid contacts remains a plausible hypothesis, similar to synaptoid contacts between MCH neurons and ependymal cells95 (section 4). In this light, Rodriguez-Cortes et al. recently verified that neuronal inputs from the suprachiasmatic nucleus (SCN) —a bilateral structure in the anterior hypothalamus regulating circadian rhythms— to tanycytes trigger direct access of glucose from the circulation to glucose-sensing ARH neurons, thereby controlling daily circadian changes in hypothalamic glucosensing in the rat96,97.
Hypothalamic ERK signalling plays a crucial role in glucose homeostasis in response to metabolic factors such as leptin25, FGF1998, and FGF199,100. Tanycytes respond to centrally administered FGF1100, reported to induce sustained remission of type 2 diabetes in leptin-deficient mice101,102 and cause robust transcriptional changes related to the ERK pathway100,102. The sustained anti-diabetic effect of FGF1 is indeed due to the prolonged activation of ERK(1/2), as shown by two complementary strategies of pharmacologically targeting MAPK/ERK signalling and FGF1 receptors. Transcriptomic evidence further pointed to a role for astrocytes and possibly tanycytes in the hypothalamic response to anti-diabetic FGF199. The central or peripheral administration of the monoclonal antibody IMC-H7 that selectively antagonizes FGFR1c isoform can induce a catabolic lean state in Siberian hamsters103. FGFR1c is expressed in tanycytes, and FGFR1c blockade induces reduction in food intake and body weight, which is in turn associated with reduced deiodinase 2 (Dio2) gene expression in tanycytes103. Of note, FGFs have been shown to exert proliferative and neurogenic properties (section 3.4), and have been characterized in tanycytes with potential metabolic endocrine functions84.
Besides acting as glucose sensors, tanycytes can detect amino acids such as Arg, Lys and Ala in the CSF via Tas1r1/Tas1r3 heterodimers, the taste receptors for the perception of umami taste87. Tanycytes also function as gatekeepers for lipid uptake into the hypothalamus and mediate lipid processing, as shown by their increased lipid droplet content after a prolonged HFD and differential fatty acid metabolism88. Tanycytic ablation of translocator protein (TSPO, an outer mitochondrial membrane protein) elicits AMPK-dependent lipophagy and protects mice from diet-induced obesity by reducing food intake and enhancing energy expenditure, suggesting tanycytic TSPO is involved in hypothalamic lipid sensing in response to overnutrition in mice104. Although not fully understood, tanycytes may also secrete certain molecules such as FGF21, in response to circulating fatty acids. Depletion of FGF21 from tanycytes results in increased energy expenditure and reduced fat mass gain89. Recent findings have shown that tanycytes can transport blood-borne FGF21 from the liver into the hypothalamus83.
3.2.3. Brain shuttles for peptide-related drugs
Blood-borne liraglutide, a glucagon-like peptide 1 receptor (GLP1R) agonist with an amino acid sequence 97% identical to that of human GLP1 and used for the treatment of obesity and type 2 diabetes105, can be shuttled into the brain by tanycytes106. The selective blockade of vesicular trafficking in tanycytes using transgenic expression of botulinum toxin in these cells blunted liraglutide-mediated activation of both cFos expression in hypothalamic neurons and fatty acid oxidation in male mice. This latter phenomenon was also blunted by the selective knockdown of GLP1R in tanycytes, which dramatically impaired liraglutide transport into the hypothalamus. It is likely that a similar route and mode of action is taken by other glucagon-like peptide 1 (GLP1) analogues, such as semaglutide107, highlighting the therapeutic potential of targeting tanycytes for the treatment of metabolic diseases.
3.2.4. Effects of targeted tanycyte ablation
Recent reports on targeted ablation of tanycytes further suggest that these cells are indeed necessary for maintaining energy balance and the regulation of adiposity104,108,109. Specific deletion of Gck, a glycolytic enzyme, along the 3V induces cell death in Gck-expressing vmARH tanycytes via apoptotic BAD pathway108. Ablation of Gck-expressing tanycytes increased adiposity phenotypically analogous to defective NPY neuronal activity108. These observations from mouse studies are in line with a previous study, where treatment with the Gck inhibitor alloxan destroyed tanycytes in the 3V affecting arcuate NPY mRNA levels in rats91.
Yoo et al. achieved conditional genetic ablation of tanycytes in the mouse ARH and ME, which increased visceral fat distribution, rebound feeding after fasting and systemic insulin resistance, and enhanced fat accumulation at thermoneutral condition, but without body weight changes in male mice109. Tanycyte deficiency seemed to accelerate the gain in body weight under HFD, but visceral adiposity and insulin sensitivity was found comparable to wild-type mice109. It appears that tanycyte ablation109 phenocopies mice lacking IR in tanycytes, which exhibit reduced insulin sensitivity, increased food intake after fasting, but with no changes in ad libitum food intake, leptin sensitivity and only a mild increase in body weight77. However, further studies are warranted to understand the precise mechanisms behind these pleiotropic effects of tanycyte ablation.
3.3. Tanycytes as regulators of the hypothalamic-pituitary axes
The ME not only provides a port of entry for metabolic signals and molecules, but also an exit route for hormones produced by neurosecretory neurons that come in close contact with the pituitary-portal circulation forming three main axes: the hypothalamic–pituitary –thyroid (HPT), –gonadal (HPG), and –adrenal (HPA) axes7. While the role of tanycytes in the HPT and HPG (section 7) axes have been studied extensively, neurons of the HPA axis producing CRH and their interaction with tanycytes has been less explored.
The axon terminals of thyrotropin-releasing hormone (TRH)-secreting neurons of the HPT axis are surrounded by the end-feet of ME (β2) tanycytes to control the release of TRH into the pituitary-portal circulation110. TRH stimulates the secretion of thyroid stimulating hormone (TSH), which then acts on the thyroid gland to produce L-thyroxine (T4). Tanycytes express MCT8 and OATP1C1, the thyroid hormone transporters, allowing the uptake of T4 from the circulation or CSF. Dio2 enzyme, expressed in tanycytes, converts T4 to active T3, which is necessary for a negative feedback effect on neural TRH production110. TRH-degrading ectoenzyme (Trhde) expression in tanycytes affects TRH availability before being released into the blood circulation via Gɑq/11-coupled pathway. Activation of Gɑq/11 pathways promotes the outgrowth of ME tanycyte end-feet ensheathing TRH neuroendocrine terminals of the pituitary vessels inducing Trhde activity111. Modulation of endocannabinoid production in ME explants for TRH secretion together with the action of glutamate (secreted by TRH neurons) on tanycytes via ɑ-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors, suggest the presence of a microcircuit between ME (β2) tanycytes and hypophysiotropic cannabinoid receptor 1-expressing TRH neurons112. Thus, tanycytes can sense, control the transport, and modify the release of HPT axis hormones and engage in its feedback regulation.
3.4. Tanycytes as a metabolically sensitive neurogenic niche
Tanycytes seem to form a metabolically sensitive neurogenic niche in the brain triggering post-natal neurogenesis in the ARH-ME region of the hypothalamus based on diet, environment and hormonal signals61,113,114. One of the first evidences on the existence of neural stem cells in the adult hypothalamus was that the cells of the hypothalamic ventricular zone could form multipotent neurospheres in vitro, giving rise to neurons and astrocytes115. Since then, a range of in vitro or in vivo strategies using transgenic mouse lines expressing reporter genes under the control of tanycyte promoters provide evidence supporting the capacity of tanycytes to act as neural stem/progenitor cells (NSPCs)61,113,114,116. Tanycytes express NSPC markers such as nestin, vimentin, Sox2, GFAP, GLAST, BLBP, and Musashi-1, they proliferate at very low levels and lineage tracing studies have demonstrated their ability to differentiate into neurons, astrocytes and possibly oligodendrocytes (reviewed in7,61,114). However, heterogeneity of tanycyte subpopulations impact NSPC marker expression, proliferation, and the fate of their progeny. In this regard, many studies have focused on the lineage tracing of adult tanycytes in transgenic mice expressing reporter genes under inducible promoters such as FGF10 for ME (β) tanycytes62 and GLAST for dorsal (ɑ) tanycytes117. These studies suggest that GFAP+ ɑ2 tanycytes are the actual neural stem cells with the ability to generate neurospheres, while β- are committed neural precursor cells. Conditional deletion of FGF10 from β-tanycytes can enhance postnatal neurogenesis118. On the other hand, FGF2 induces tanycyte proliferation via a Cx43 hemichannel/purinergic-dependent pathway119, also involved in tanycyte glucosensing mechanisms86,120. FGF2 was specifically found to increase the proliferation of β-tanycytes that were sensitive to Cx43 hemichannel blockade, with no dramatic effect on parenchymal cells of hypothalamic nuclei, neurons, ME, and ɑ-tanycyte cells119, suggesting that the proliferative potential of tanycytes is subtype specific and may adapt depending on metabolic cues.
Hypothalamic neurogenesis is indeed relevant in the context of altered metabolism such as HFD exposure60,116,121. Short-term HFD exposure may activate neurogenesis in the ME of female mice, with β2-tanycytes giving rise to new neurons60,121. However, the enhanced neurogenesis during short-term HFD feeding was deemed a compensatory mechanism by another study demonstrating that chronic HFD feeding depleted hypothalamic neurogenesis, which was in turn linked with IKKβ/NF-KB overactivation and hypothalamic inflammation in obese adult mice116.
Selective deletion of NFI family of transcription factors (Nfia/b/x) in tanycytes can lead to robust tanycyte proliferation and tanycyte-derived neurogenesis. Nfia/b/x-deficient tanycytes can give rise to different neuronal subtypes (GABAergic and glutamatergic neurons) that mature and selectively respond as would neurons in the MBH. In this case, ɑ2 tanycytes with enhanced Shh and Wnt expression give rise to proliferating tanycytes, which later generate neural precursors when they exit the cell cycle122. Intriguingly, neural injury triggers self-renewal and regeneration in retina and anterior neural fold homeobox (Rax)-expressing tanycytes in the ME via insulin-like growth factor 1 (IGF1) receptor signalling, but with limited neurogenesis, suggesting tanycytes themselves may regenerate and aid in tissue repair123. Moreover, Rax+ progenitors were found capable of generating POMC neurons during adulthood that rescued the obese phenotype of the hypothalamic POMC-deficient mice. Mice with Rax+ progenitor-derived POMC neurons exhibited reduced body fat mass and body weight as well as improved glucose tolerance and insulin sensitivity in proportion with the newly generated POMC neurons124. In addition to tanycyte-derived neurons, a newly identified NSPC population expressing Irx3 and Irx5 in the early postnatal hypothalamus regulates hypothalamic neurogenesis giving rise not only to leptin-sensing neurons but also tanycytes125. Irx3 and Irx5 control energy homeostasis in terms of feeding regulation and leptin response125,126. Thus, novel gene expression profiling methods will continue to help us better understand the heterogeneity of tanycytes and the complex hypothalamic neurogenic niche.
4. Ependymal Cells in CSF Homeostasis
Ependymal cells form a continuous sheet of ciliated epithelial cells that line the wall of the cerebral ventricles and separate the CSF from the brain parenchyma. These cuboidal cells with tufts of motile cilia are different from the uniciliated tanycytes on the floor and biciliated tanycytes on the lateral walls of the 3V127. Their coordinated ciliary beating is responsible for maintaining the CSF flow through the ventricular system128–130, while impaired ciliary function may result in hydrocephalus or excess CSF accumulation. Although studies on the biogenesis and orientation of ependymal cilia have gained more attention, only a few have shed light on the cilia beat frequency and its regulation by neuropeptide factors. The changes in ciliary beat frequency is fine-tuned in response to metabolic and neurohormonal molecules. The anabolic peptide MCH positively controls the ciliary beat frequency, thereby modulating the CSF flow95. MCH can act either on the basal pole of ependymal cells when released from synaptic contacts with MCH neuronal fibres or directly at the apex of ciliated cells through non-synaptic mechanisms, both mediated by MCHR1. The lack of MCH receptors results in an enlargement of ventricles with consequent alteration of CSF flow and dysfunction of ependymal cilia in the 3V95,131. Increase in the levels of MCH in the CSF influences feeding behaviour and has been associated with increased food intake upon selective activation of MCH neurons contacting the CSF132. Thus, the large repertoire of physiological and metabolic actions of MCH neurons133 may be facilitated, at least in part, by the MCH-mediated control of ciliary beat frequency in ependymocytes regulating CSF flow95,131 and hence volume transmission of CSF-borne MCH into the brain132.
5. NG2 glia and Oligodendrocytes in Energy Balance
Among the constitutive new-born cells in the adult hypothalamus, NG2 glia (also known as oligodendrocyte precursors) form the large majority of proliferating cells, especially in the ME134. Generally, gliogenesis begins right before E13.5 in the tuberal hypothalamus, and glioblasts and oligodendrocyte precursors localize in a confined domain next to the 3V135. Metabolic hormones and peptides seem to control proliferation, differentiation, maturation and myelination of oligodendrocytes to maintain energy homeostasis27,136. Particularly, the proliferative capacity of the hypothalamic NG2 glia may contribute to the BBB maintenance and preservation of neuronal processes that sense leptin. NG2 glia contacting the dendritic processes of LepR expressing ARH neurons in the ME are important to maintaining the leptin sensitivity of LepR neurons in the ARH27. This was investigated by the selective ablation of NG2 glia in the ME, which attenuated the electrical response of ARH neurons to leptin, leading to leptin resistance and obesity in mice. ME-directed X-irradiation reproduces these effects, partially explaining the risk of obesity after cranial radiation therapy27. On the other hand, conditional embryonic deletion of LepR from nestin-expressing cells, i.e., neural stem cells, can lead to extreme obesity in mice with enhanced neuronal differentiation, as well as oligodendrocyte proliferation in the early postnatal hypothalamus137.
Kohnke et al.discovered that oligodendrocyte proliferation and differentiation in the ME specifically respond to feeding and remodel peri-neuronal nets (PNNs) affecting ME plasticity136. PNNs form a mesh around a cluster of metabolic-sensing neurons tightly packed at the ARH-ME junction in the MBH with physiological implications in food intake and glucose homeostasis138. Fasting reduces oligodendrocyte proliferation and differentiation, reduces PNN formation and maintains a positive energy balance, which is completely reversed upon refeeding. In genetically obese ob/ob mice, ME oligodendrocyte differentiation and PNN remodelling is diminished, and enzymatic digestion of local PNN increases food intake and weight gain136.
Oligodendrocytes may be a source of pyruvate and lactate to the contacting neurons139,140. An increase in oligodendrocyte lactate production has been associated with the lean phenotype of mice deficient of G-protein coupled receptor Gpr17141. Gpr17 regulates appetite, energy expenditure, and metabolism via FoxO1-dependent pathways in AgRP neurons142,143. Accordingly, loss of Gpr17 in oligodendrocytes improved glycolysis and lactate production, which in turn activated AKT and STAT3 signalling in hypothalamic neurons, leading to reciprocal action of AgRP and POMC neurons, increased energy expenditure, and reduced body weight gain upon chronic exposure to HFD141. Though NG2 glia and oligodendrocytes have key roles in energy homeostasis, their role in obesity as well as other nutritional and metabolic states needs more investigation.
6. Microglia and hypothalamic inflammation
Hypothalamic inflammation as a consequence of energy imbalance, either evolves alongside the pathophysiology of the metabolic syndrome, or occurs as a downstream effect of infection-associated anorexia51,144. Microglia, the primary innate immune cells of the brain, act as central coordinators of inflammatory responses in the hypothalamus (Figure 3). Under normal physiological conditions, ‘resting microglia’ vigilantly monitor neuronal synapses, while the infection-induced increase in systemic cytokines or any metabolic dysfunction give rise to morphologically and functionally ‘reactive microglia’ for the control of inflammatory responses and hypothalamic synaptic plasticity. Lipopolysaccharide (LPS, commonly used to model infection-induced anorexia) and saturated fatty acids (SFAs, simulating dietary inflammation) stimulate a pro-inflammatory microglial phenotype by binding toll-like receptor (TLR)4 on the microglial membrane144.
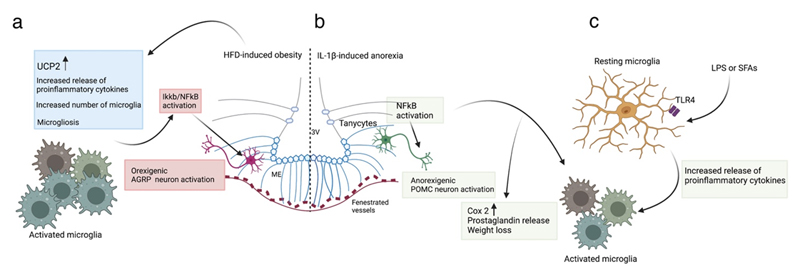
Figure 3. Microglia in HFD-induced obesity and IL-1β/LPS-induced activation.
(a) In HFD-induced obesity, ARH microglia are activated, leading to hypothalamic inflammation with increased release of proinflammatory cytokines, increased expression of microglial UCP2, increased number of microglia and altered microglial morphology (microgliosis)151,155,157. Of note, invalidating IKKB/ NF-KB signalling selectively in AgRP neurons results in an anti-obesity and anti-diabetic phenotype in mice fed a HFD58. (b) Anorexia induced by systemic administration of IL-1β prominently activates NF-KB in tanycytes and microglia. This increases Cox2 expression and prostaglandin release mediating weight loss85. NF-KB activation also directly stimulates POMC transcription in LPS-induced illness146. (c) Microglia of the ARH are sensitive to LPS (used to model sickness-induced anorexia) and saturated fatty acids (SFAs, which stimulate dietary inflammation), activating TLR4 to stimulate the release of proinflammatory cytokines and microglial activation144. ARH, arcuate nucleus of hypothalamus; HFD, high fat diet; LPS, lipopolysaccharide; POMC, pro-opiomelanocortin; SFA, saturated fatty acids; TLR4, toll-like receptor; UCP2, uncoupled protein 2.
Studies implementing infection-induced anorexia models have linked the loss of appetite to the hypothalamic melanocortin system and central inflammatory pathways145–147. LPS-induced sickness can activate neuronal NFkB in the hypothalamus, which in turn triggers NFkB-dependent cytokine release and anorexigenic hypothalamic POMC activity146. To pin down specific signalling modalities for sickness-induced anorexia, Jin et al. centrally administered a TLR2 agonist instead of LPS (that stimulates both TLR2 and TLR4)147. TLR2 activation alone triggers sickness-like behaviours such as anorexia, hypoactivity and hypothermia147. Mechanistically, TLR2 signalling contributes to strong microglial activation accompanied by increased physical contacts between microglia and POMC neurons, resulting in anorexia and body weight loss147. TLR2-induced sickness appears to be at least in part mediated by the activation of the inflammatory NFKB and COX pathways in the hypothalamus147. MCH has been identified as the central target for the chemokine ligand CCL2 eliciting LPS-like effects. CCL2 promotes neuroinflammation and can either directly act on MCH neurons to decrease MCH expression and food intake148 or bind to their receptors expressed by glial cells such as microglia, which in turn produce more chemokine and cytokines144,148. However, the question remains how the circulating cytokines or peripheral inflammatory mediators reach or send signals to the brain for its central action, and has been construed to occur by binding to their receptors on the endothelial cell membrane149, via fenestrated capillaries of the CVOs85 or directly through the BBB that is rendered increasingly permeable by inflammation150.
Diets rich in saturated fats promote inflammation, gliosis, and neuronal stress in the MBH49,151. While inflammation in peripheral tissues may develop over weeks 152, hypothalamic inflammation may begin as early as 24 to 72 hours of HFD feeding, much before substantial weight gain is observed49. Subsequently, reactive gliosis indicative of neuronal injury in the ARH transpires within the first week of HFD exposure49. The number of microglia in the MBH has been positively correlated with the intensity of hypothalamic inflammation induced by saturated fats, while microglial depletion from the MBH alleviates inflammation and improves leptin sensitivity during HFD feeding151.
Targeting specific microglia-related hypothalamic inflammatory mechanisms could help restore energy balance. Blocking IKKβ (inhibitor of NFkB kinase subunit beta) in hypothalamic microglia prevents the increase of microglial cells and controls TNF-ɑ expression in microglia as well as the neighbouring cells153. Depletion of gut microbiota (which regulates microglial maturation and function) protects against diet-induced hypothalamic inflammation and promotes leptin sensitivity via GLP1/GLP1R dependent mechanisms154. Uncoupling protein 2 (UCP2), a mitochondrial protein highly expressed in microglia, is transiently upregulated under HFD feeding, and the selective deletion of UCP2 from microglia protects against diet-induced obesity, prevents mitochondrial changes, increases energy expenditure and alters synaptic input organization of anorexigenic POMC neurons155. HFD-induced hypothalamic microglial activation via the CX3CL1-CX3CR1 pathway seems sex-specific, where females on a HFD maintain CX3CL1-CX3CR1 levels as opposed to reduced levels in male mice, suggesting a potential mechanism for the lower susceptibility of females to HFD-induced obesity156. Morphological modifications of microglia reflecting hypothalamic inflammation can also occur postprandially157. Although postprandial inflammation aids in regulating energy balance, continuous and excessive lipid exposure may eventually lead to obesity. Given the critical role of microglia in hypothalamic inflammation, studying microglial chronic activation, loss-of-function and cross-talk with other glial cells under specific metabolic states may provide clues for better understanding neuroendocrine disorders.
7. Sexually-Dimorphic Responses of Hypothalamic Glial Cells
Since circulating and local levels of gonadal hormones and their metabolites are different in adult males and females, it is only logical to reckon that glial cells with sex-specific differential access to circulating factors may generate sexually-dimorphic responses in energy homeostasis. The astrocytic response to stimuli such as HFD and gonadal steroids can be highly sex-dependent, including morphological differences (higher GFAP expression density in male compared to female astrocytes)158. Tanycytes in the ME exhibit structural plasticity based on the estrous cycle of rodents in association with the changes in the HPG axis. Estrous cycle, the 4- or 5-day ovarian cycle in female rodents (similar to the human reproductive cycle called the menstrual cycle) is divided into four phases: proestrus, oestrus, metestrus and dioestrus159. Fluctuating ovarian hormones during the estrous cycle drives ME structural plasticity and cellular interactions, thereby controlling the juxtaposition of neurosecretory terminals within the portal vasculature7. In the dioestrus stage characterized by low gonadal steroid levels, tanycytes tightly ensheath GnRH hormone secreting nerve terminals, creating a diffusion barrier and preventing the access and release of GnRH to the blood vessels. In the preovulatory stage of proestrus, tanycytes undergo a structural remodelling and retract from the GnRH axons allowing the direct contact between GnRH neurosecretory terminals and pituitary-portal vessels. Release of the chemotrophic factor semaphorin 3A (Sema3A) and its action on neuropilin-1 receptor mediate the sprouting of GnRH axon terminals160. Simultaneously, TGF-ɑ-PGE2-induced release of TGF-β1 promotes retraction of tanycytic processes161 wrapping GnRH nerve terminals7. It is, however, interesting that TGFɑ-mediated activation of avian erythroblastic leukemia Viral (V-Erb-B) oncogene homolog (erbB1) or EGFR) involved in tanycytic plasticity during estrous cycle coincides with the mechanism facilitating transcytotic transport of leptin via the Leprb-EGFR complex in tanycytes24. It seems conspicuous yet unidentified whether the tanycytic plasticity due to TGFɑ/erbB1161 causes peri-ovulatory reduction in the food intake at proestrus stage162. However, these cyclic ultrastructural changes in the ME defining GnRH release might not be confined to GnRH neurons and tanycytes. Astrocytes in the ME also release PGE2 resulting from the activation of astrocytic erbB1 and erbB4 ligands, such as TGF-ɑ and the neuregulins, respectively163. Deletion of ErbB4 in mice, recently found important for the anchoring of hypothalamic astrocytes to the neuroendocrine neurons controlling reproduction during early postnatal development164, causes metabolic syndrome after chronic exposure of fat-enriched diet165. ERBB4 is one of the strongest gene loci associated with polycystic ovary syndrome166, which is linked to fertility alterations and metabolic disorders in women of childbearing age167.
Oligodendrocytes are also sensitive to neuroactive steroids and exhibit sexual dimorphism in how they respond to metabolic signals168. Significant sexual dimorphism has been reported in the proliferation, migration, differentiation, energy metabolism, barrier functions and transcriptomic profiles of oligodendrocyte progenitor cells (OPCs), which may confer some advantages in the remyelination process in females compared to males158,169,170. G protein-coupled oestrogen receptor (GPER), involved in the oestrogenic control of food intake and sexual receptivity, was identified in hypothalamic astrocytes, neurons and oligodendrocytes, where a proportion of GPER-positive cells were dependent on the hypothalamic region, sex and estrous cycle171.
Finally, microglia may exert sexually dimorphic neuroprotective functions as identified by differences in the gene expression profile of male and female microglia172,173. Despite these notable facts, most studies either focus only on males or in some cases combine experimental outcomes from both males and females, without considering sex as an experimental parameter. Sexually-dimorphic hypothalamic glial cell responses are crucial, especially in the context of metabolism-related research, which might explain the varying propensities to develop obesity and related comorbidities in males, as well as in pre- and post-menopausal women174.
8. Conclusion
The paradigm-shifting research on hypothalamic glia has revealed their distinct functions, such as sensing, processing and transmission of blood-borne metabolic signals to neurons, causing synaptic modulations, while undergoing physiological adaptations based on feeding behaviour. Specifically, we described how astrocytes, tanycytes and oligodendrocytes are sensitive to changes in the nutritional status and exhibit structural plasticity based on the metabolic cues. Ependymal cells with tufts of motile cilia engage in CSF homeostasis, where ciliary beat frequency controlled by the anabolic peptide MCH95,131 can influence feeding behaviour132. Microglia, on the other hand, are coordinators of inflammatory responses in the hypothalamus due to either infection-associated anorexia or HFD-induced obesity51,144. Though beyond the scope of this review focussed on hypothalamic glial cells, the glialneuronal network in the dorsal vagus complex (DVC) of the brainstem, composed of the nucleus of the solitary tract (NTS), the vagus nerve and the sensory CVO called the area postrema (AP), is also capable of integrating peripheral information175–177. The NTS directly receives both humoral and neural information from the gastrointestinal tract via the AP178 or the sensory vagus nerve179–183 (Figure 1a).
Glial cells work in concert with vascular components of the CVOs, forming a conduit for the brain-to-periphery communication, with the brain access of leptin being the prototypical example of such processes12,24–27,184. To understand such distinct but parallel mechanisms, state-of-the-art systems neuroscience strategies must be implemented. For instance, mouse models genetically engineered for promoters such as GFAP12,185, GLAST38, or Cx4345 have been commonly used to study astrocytes. But notably these targets are also expressed by tanycytes36,117. Targeting these genes using tamoxifen-inducible CreERT2 lines can lead to their non-specific deletion in both astrocytes and tanycytes. Though Rax-123,186 and Nes-CreERT2121 have been used to target tanycytes, Nes-CreERT2 may be ineffective for lineage tracing of tanycytes in mice at P28 or older62 and Rax-CreERT2 may cause non-specific recombination in the pituitary gland and the eyes186. Fgf10-62 and Fgf18-CreERT284 can target distinct populations of tanycytes, but come with similar repercussions of non-specificity58. Besides non-specificity of CreERT2 models and the putative tamoxifen-independent activity185, there are also serious concerns involving the interference of tamoxifen with oestrogen receptors leading to altered chromatin structure187,188 and the self-regulation of metabolic activities189.
Alternatively, viral-based strategies for gene deletion is now widely used to manipulate glial cells, without having to generate multiple mouse lines190. For instance, AAV5 pseudotype shows a strong tropism for astrocytes, while AAVs 1, 2, 6, 7, 8, and 9 can transduce both astrocytes and neurons191. Viral-based selective targeting of tanycytes involves a recombinant AAV with a hybrid serotype 1 and 2, which traps the virus in the ependymal layer of the ventricular system. Recombinant AAV 1/2 can be constructed under the Dio2 promoter to specifically drive the expression of Dio2-expressing tanycytes111. Selective expression of transgenes in tanycytes can also be achieved by using adenoviruses and the TSHR promoter for cell specificity93. For oligodendrocytes, only a small number of serotypes with pan-cell markers have been reported with low levels of transduction190. Additionally, the remote and non-invasive nature of chemogenetic technology based on engineered G-protein coupled receptor (GPCR) designer receptors (DREADDs) has attracted much attention for the manipulation of glial cells192,193. The implementation of high-throughput transcriptomic analysis at the single-cell level will open novel prospects in understanding complex metabolic diseases and develop tailor-made therapies.
Acknowledgments
This work was supported by the European Research Council (ERC) Synergy Grant WATCH (Well Aging and the Tanycytic Control of Health), No 810331 to RN, VP and MS.
Footnotes
Authors contribution
S.N. and V.P. designed the structure of the review. S.N. wrote the first draft. R.N., M.S. and V.P. discussed and edited the manuscript.
Competing interest
The authors have no competing interests to declare.
References
- 1.Garcia-Caceres C, et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22:7–14. doi: 10.1038/s41593-018-0286-y. [DOI] [PubMed] [Google Scholar]
- 2.Chowen JA, Frago LM, Fernandez-Alfonso MS. Physiological and pathophysiological roles of hypothalamic astrocytes in metabolism. J Neuroendocrinol. 2019;31:e12671. doi: 10.1111/jne.12671. [DOI] [PubMed] [Google Scholar]
- 3.Clasadonte J, Prevot V. The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol. 2018;14:25–44. doi: 10.1038/nrendo.2017.124. [DOI] [PubMed] [Google Scholar]
- 4.Al Massadi O, Lopez M, Tschop M, Dieguez C, Nogueiras R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017;40:167–180. doi: 10.1016/j.tins.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1:754–764. doi: 10.1038/s42255-019-0095-y. [DOI] [PubMed] [Google Scholar]
- 6.Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol. 2021;22:142–158. doi: 10.1038/s41580-020-00317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prevot V, et al. The Versatile Tanycyte: A Hypothalamic Integrator of Reproduction and Energy Metabolism. Endocr Rev. 2018;39:333–368. doi: 10.1210/er.2017-00235. [DOI] [PubMed] [Google Scholar]
- 8.Yeo GS, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci. 2012;15:1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 9.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 10.Dubern B, et al. Mutational analysis of the pro-opiomelanocortin gene in French obese children led to the identification of a novel deleterious heterozygous mutation located in the alpha-melanocyte stimulating hormone domain. Pediatr Res. 2008;63:211–216. doi: 10.1203/PDR.0b013e31815ed62b. [DOI] [PubMed] [Google Scholar]
- 11.Chowen JA, et al. The role of astrocytes in the hypothalamic response and adaptation to metabolic signals. Prog Neurobiol. 2016;144:68–87. doi: 10.1016/j.pneurobio.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Kim JG, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908–910. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varela L, et al. Hunger-promoting AgRP neurons trigger an astrocyte-mediated feed-forward autoactivation loop in mice. J Clin Invest. 2021;131 doi: 10.1172/JCI144239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Caceres C, et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell. 2016;166:867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y, et al. Disruption of Lipid Uptake in Astroglia Exacerbates Diet-Induced Obesity. Diabetes. 2017;66:2555–2563. doi: 10.2337/db16-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varela L, et al. Astrocytic lipid metabolism determines susceptibility to diet-induced obesity. Sci Adv. 2021;7:eabj2814. doi: 10.1126/sciadv.abj2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreft M, Bak LK, Waagepetersen HS, Schousboe A. Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro. 2012;4 doi: 10.1042/AN20120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonvento G, Bolanos JP. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021;33:1546–1564. doi: 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Obradovic M, et al. Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne) 2021;12:585887. doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaum SR, et al. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Mol Pharmacol. 1996;50:230–235. [PubMed] [Google Scholar]
- 21.Garcia-Caceres C, et al. Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology. 2011;152:1809–1818. doi: 10.1210/en.2010-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuente-Martin E, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest. 2012;122:3900–3913. doi: 10.1172/JCI64102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks WA. The blood-brain barrier as an endocrine tissue. Nat Rev Endocrinol. 2019;15:444–455. doi: 10.1038/s41574-019-0213-7. [DOI] [PubMed] [Google Scholar]
- 24.Duquenne M, et al. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function. Nat Metab. 2021;3:1071–1090. doi: 10.1038/s42255-021-00432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balland E, et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butiaeva LI, et al. Leptin receptor-expressing pericytes mediate access of hypothalamic feeding centers to circulating leptin. Cell Metab. 2021;33:1433–1448.:e1435. doi: 10.1016/j.cmet.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Djogo T, et al. Adult NG2-Glia Are Required for Median Eminence-Mediated Leptin Sensing and Body Weight Control. Cell Metab. 2016;23:797–810. doi: 10.1016/j.cmet.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Muller TD, et al. Ghrelin. Mol Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen N, et al. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife. 2016;5 doi: 10.7554/eLife.18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clasadonte J, et al. Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc Natl Acad Sci U S A. 2011;108:16104–16109. doi: 10.1073/pnas.1107533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebrun B, et al. Glial endozepines and energy balance: Old peptides with new tricks. Glia. 2021;69:1079–1093. doi: 10.1002/glia.23927. [DOI] [PubMed] [Google Scholar]
- 32.Bouyakdan K, et al. The gliotransmitter ACBP controls feeding and energy homeostasis via the melanocortin system. J Clin Invest. 2019;129:2417–2430. doi: 10.1172/JCI123454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab. 2015;35:176–185. doi: 10.1038/jcbfm.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 36.Lhomme T, et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J Clin Invest. 2021;131:e140521. doi: 10.1172/JCI140521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ordenes P, et al. Lactate activates hypothalamic POMC neurons by intercellular signaling. Sci Rep. 2021;11:21644. doi: 10.1038/s41598-021-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tirou L, et al. Sonic Hedgehog receptor Patched deficiency in astrocytes enhances glucose metabolism in mice. Mol Metab. 2021;47:101172. doi: 10.1016/j.molmet.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuzzaci D, et al. Postprandial Hyperglycemia Stimulates Neuroglial Plasticity in Hypothalamic POMC Neurons after a Balanced Meal. Cell Rep. 2020;30:3067–3078.:e3065. doi: 10.1016/j.celrep.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Scharbarg E, et al. Astrocyte-derived adenosine is central to the hypnogenic effect of glucose. Sci Rep. 2016;6:19107. doi: 10.1038/srep19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benani A, et al. Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. J Neurosci. 2012;32:11970–11979. doi: 10.1523/JNEUROSCI.0624-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allard C, et al. Hypothalamic astroglial connexins are required for brain glucose sensing-induced insulin secretion. J Cereb Blood Flow Metab. 2014;34:339–346. doi: 10.1038/jcbfm.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giaume C, Leybaert L, Naus CC, Saez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol. 2013;4:88. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung G, Chever O, Rouach N. Connexons and pannexons: newcomers in neurophysiology. Front Cell Neurosci. 2014;8:348. doi: 10.3389/fncel.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clasadonte J, Scemes E, Wang Z, Boison D, Haydon PG. Connexin 43-Mediated Astroglial Metabolic Networks Contribute to the Regulation of the Sleep-Wake Cycle. Neuron. 2017;95:1365–1380.:e1365. doi: 10.1016/j.neuron.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fioramonti X, et al. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- 47.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- 48.Claret M, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP. Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab. 2017;6:366–373. doi: 10.1016/j.molmet.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai D, Khor S. Hypothalamic microinflammation. Handb Clin Neurol. 2021;181:311–322. doi: 10.1016/B978-0-12-820683-6.00023-3. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleinridders A, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benzler J, et al. Central inhibition of IKKbeta/NF-kappaB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes. 2015;64:2015–2027. doi: 10.2337/db14-0093. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Reichel JM, Han C, Zuniga-Hertz JP, Cai D. Astrocytic Process Plasticity and IKKbeta/NF-kappaB in Central Control of Blood Glucose, Blood Pressure, and Body Weight. Cell Metab. 2017;25:1091–1102.:e1094. doi: 10.1016/j.cmet.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahman MH, et al. Astrocytic pyruvate dehydrogenase kinase-2 is involved in hypothalamic inflammation in mouse models of diabetes. Nat Commun. 2020;11:5906. doi: 10.1038/s41467-020-19576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell JN, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20:484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prevot V, Nogueiras R, Schwaninger M. Tanycytes in the infundibular nucleus and median eminence and their role in the blood-brain barrier. Handb Clin Neurol. 2021;180:253–273. doi: 10.1016/B978-0-12-820107-7.00016-1. [DOI] [PubMed] [Google Scholar]
- 59.Nampoothiri S, Duquenne M, Prevot V. In: Glial-Neuronal Signaling in Neuroendocrine Systems. Tasker JeffreyG, Bains JaideepS, Chowen JA., editors. Vol. 11. Cham: Springer; 2021. Unveiling the Importance of Tanycytes in the Control of the Dialogue Between the Brain and the Periphery; pp. 255–284. (Masterclass in Neuroendocrinology). [Google Scholar]
- 60.Lee DA, et al. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front Neurosci. 2014;8:157. doi: 10.3389/fnins.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharif A, Fitzsimons CP, Lucassen PJ. Neurogenesis in the adult hypothalamus: A distinct form of structural plasticity involved in metabolic and circadian regulation, with potential relevance for human pathophysiology. Handb Clin Neurol. 2021;179:125–140. doi: 10.1016/B978-0-12-819975-6.00006-6. [DOI] [PubMed] [Google Scholar]
- 62.Haan N, et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33:6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langlet F, et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17:607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaeffer M, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci U S A. 2013;110:1512–1517. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang H, et al. MCH Neurons Regulate Permeability of the Median Eminence Barrier. Neuron. 2020;107:306–319.:e309. doi: 10.1016/j.neuron.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518:459–476. doi: 10.1002/cne.22219. [DOI] [PubMed] [Google Scholar]
- 68.Caro JF, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 70.Chmielewski A, et al. Preclinical Assessment of Leptin Transport into the Cerebrospinal Fluid in Diet-Induced Obese Minipigs. Obesity (Silver Spring) 2019;27:950–956. doi: 10.1002/oby.22465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 72.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 73.Wyse BM, Dulin WE. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia. 1970;6:268–273. doi: 10.1007/BF01212237. [DOI] [PubMed] [Google Scholar]
- 74.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 75.Guillebaud F, et al. Glial Endozepines Reverse High-Fat Diet-Induced Obesity by Enhancing Hypothalamic Response to Peripheral Leptin. Mol Neurobiol. 2020;57:3307–3333. doi: 10.1007/s12035-020-01944-z. [DOI] [PubMed] [Google Scholar]
- 76.Yoo S, Cha D, Kim DW, Hoang TV, Blackshaw S. Tanycyte-Independent Control of Hypothalamic Leptin Signaling. Front Neurosci. 2019;13:240. doi: 10.3389/fnins.2019.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Porniece Kumar M, et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat Metab. 2021;3:1662–1679. doi: 10.1038/s42255-021-00499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collden G, et al. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol Metab. 2015;4:15–24. doi: 10.1016/j.molmet.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uriarte M, et al. Circulating ghrelin crosses the blood-cerebrospinal fluid barrier via growth hormone secretagogue receptor dependent and independent mechanisms. Mol Cell Endocrinol. 2021;538:111449. doi: 10.1016/j.mce.2021.111449. [DOI] [PubMed] [Google Scholar]
- 80.Liang Q, et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–4075. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- 81.Owen BM, et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med. 2013;19:1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu C, et al. KLB, encoding beta-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol Med. 2017;9:1379–1397. doi: 10.15252/emmm.201607376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pena-León V, et al. Prolonged breastfeeding protects from obesity by hypothalamic action of hepatic FGF21. Nat Metab. 2022 doi: 10.1038/s42255-022-00602-z. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaminskas B, et al. Characterisation of endogenous players in fibroblast growth factor-regulated functions of hypothalamic tanycytes and energy-balance nuclei. J Neuroendocrinol. 2019;31:e12750. doi: 10.1111/jne.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bottcher M, et al. NF-kappaB signaling in tanycytes mediates inflammation-induced anorexia. Mol Metab. 2020;39:101022. doi: 10.1016/j.molmet.2020.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol. 2011;589:2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lazutkaite G, Solda A, Lossow K, Meyerhof W, Dale N. Amino acid sensing in hypothalamic tanycytes via umami taste receptors. Mol Metab. 2017;6:1480–1492. doi: 10.1016/j.molmet.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofmann K, et al. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia. 2017;65:231–249. doi: 10.1002/glia.23088. [DOI] [PubMed] [Google Scholar]
- 89.Geller S, et al. Tanycytes Regulate Lipid Homeostasis by Sensing Free Fatty Acids and Signaling to Key Hypothalamic Neuronal Populations via FGF21 Secretion. Cell Metab. 2019;30:833–844.:e837. doi: 10.1016/j.cmet.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Benford H, et al. A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes. Glia. 2017;65:773–789. doi: 10.1002/glia.23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanders NM, Dunn-Meynell AA, Levin BE. Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes. 2004;53:1230–1236. doi: 10.2337/diabetes.53.5.1230. [DOI] [PubMed] [Google Scholar]
- 92.Haddad-Tovolli R, Claret M. Cooperative tanycytes fuel the neuronal tank. J Clin Invest. 2021;131 doi: 10.1172/JCI153279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bolborea M, Pollatzek E, Benford H, Sotelo-Hitschfeld T, Dale N. Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. Proc Natl Acad Sci U S A. 2020;117:14473–14481. doi: 10.1073/pnas.1919887117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortes-Campos C, et al. MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction. PLoS One. 2011;6:e16411. doi: 10.1371/journal.pone.0016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conductier G, et al. Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat Neurosci. 2013;16:845–847. doi: 10.1038/nn.3401. [DOI] [PubMed] [Google Scholar]
- 96.Rodriguez-Cortes B, et al. Suprachiasmatic nucleus-mediated glucose entry into the arcuate nucleus determines the daily rhythm in blood glycemia. Curr Biol. 2022;32:796–805.:e794. doi: 10.1016/j.cub.2021.12.039. [DOI] [PubMed] [Google Scholar]
- 97.Imbernon M, Dehouck B, Prevot V. Glycemic control: Tanycytes march to the beat of the suprachiasmatic drummer. Curr Biol. 2022;32:R173–R176. doi: 10.1016/j.cub.2022.01.038. [DOI] [PubMed] [Google Scholar]
- 98.Marcelin G, et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab. 2014;3:19–28. doi: 10.1016/j.molmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown JM, et al. Role of hypothalamic MAPK/ERK signaling and central action of FGF1 in diabetes remission. iScience. 2021;24:102944. doi: 10.1016/j.isci.2021.102944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bentsen MA, et al. Transcriptomic analysis links diverse hypothalamic cell types to fibroblast growth factor 1-induced sustained diabetes remission. Nat Commun. 2020;11:4458. doi: 10.1038/s41467-020-17720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scarlett JM, et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med. 2016;22:800–806. doi: 10.1038/nm.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown JM, et al. The Hypothalamic Arcuate Nucleus-Median Eminence Is a Target for Sustained Diabetes Remission Induced by Fibroblast Growth Factor 1. Diabetes. 2019;68:1054–1061. doi: 10.2337/db19-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Samms RJ, et al. Antibody-Mediated Inhibition of the FGFR1c Isoform Induces a Catabolic Lean State in Siberian Hamsters. Curr Biol. 2015;25:2997–3003. doi: 10.1016/j.cub.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 104.Kim S, et al. Tanycytic TSPO inhibition induces lipophagy to regulate lipid metabolism and improve energy balance. Autophagy. 2020;16:1200–1220. doi: 10.1080/15548627.2019.1659616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127:4217–4227. doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Imbernon M, et al. Tanycytes Control Hypothalamic Liraglutide Uptake and its Anti-Obesity Actions. Cell Metab. 2022 doi: 10.1016/j.cmet.2022.06.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gabery S, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5 doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rohrbach A, et al. Ablation of glucokinase-expressing tanycytes impacts energy balance and increases adiposity in mice. Mol Metab. 2021;53:101311. doi: 10.1016/j.molmet.2021.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoo S, et al. Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia. 2020;68:1987–2000. doi: 10.1002/glia.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35:159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muller-Fielitz H, et al. Tanycytes control the hormonal output of the hypothalamic-pituitary-thyroid axis. Nat Commun. 2017;8:484. doi: 10.1038/s41467-017-00604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farkas E, et al. A Glial-Neuronal Circuit in the Median Eminence Regulates Thyrotropin Releasing Hormone-Release via the Endocannabinoid System. iScience. 2020;23:100921. doi: 10.1016/j.isci.2020.100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goodman T, Hajihosseini MK. Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front Neurosci. 2015;9:387. doi: 10.3389/fnins.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yoo S, Blackshaw S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog Neurobiol. 2018;170:53–66. doi: 10.1016/j.pneurobio.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiss S, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robins SC, et al. alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- 118.Goodman T, et al. Fibroblast growth factor 10 is a negative regulator of postnatal neurogenesis in the mouse hypothalamus. Development. 2020;147 doi: 10.1242/dev.180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Recabal A, et al. The FGF2-induced tanycyte proliferation involves a connexin 43 hemichannel/purinergic-dependent pathway. J Neurochem. 2021;156:182–199. doi: 10.1111/jnc.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orellana JA, et al. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60:53–68. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee DA, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoo S, et al. Control of neurogenic competence in mammalian hypothalamic tanycytes. Sci Adv. 2021;7 doi: 10.1126/sciadv.abg3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mu W, et al. Hypothalamic Rax(+) tanycytes contribute to tissue repair and tumorigenesis upon oncogene activation in mice. Nat Commun. 2021;12:2288. doi: 10.1038/s41467-021-22640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Surbhi, Wittmann G, Low MJ, Lechan RM. Adult-born proopiomelanocortin neurons derived from Rax-expressing precursors mitigate the metabolic effects of congenital hypothalamic proopiomelanocortin deficiency. Mol Metab. 2021;53:101312. doi: 10.1016/j.molmet.2021.101312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Son JE, et al. Irx3 and Irx5 in Ins2-Cre(+) cells regulate hypothalamic postnatal neurogenesis and leptin response. Nat Metab. 2021;3:701–713. doi: 10.1038/s42255-021-00382-y. [DOI] [PubMed] [Google Scholar]
- 126.Dou Z, Son JE, Hui CC. Irx3 and Irx5 - Novel Regulatory Factors of Postnatal Hypothalamic Neurogenesis. Front Neurosci. 2021;15:763856. doi: 10.3389/fnins.2021.763856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mirzadeh Z, et al. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat Commun. 2017;8:13759. doi: 10.1038/ncomms13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Faubel R, Westendorf C, Bodenschatz E, Eichele G. Cilia-based flow network in the brain ventricles. Science. 2016;353:176–178. doi: 10.1126/science.aae0450. [DOI] [PubMed] [Google Scholar]
- 129.Genzen JR, Yang D, Ravid K, Bordey A. Activation of adenosine A2B receptors enhances ciliary beat frequency in mouse lateral ventricle ependymal cells. Cerebrospinal Fluid Res. 2009;6:15. doi: 10.1186/1743-8454-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Genzen JR, Platel JC, Rubio ME, Bordey A. Ependymal cells along the lateral ventricle express functional P2X(7) receptors. Purinergic Signal. 2009;5:299–307. doi: 10.1007/s11302-009-9143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Conductier G, et al. Control of ventricular ciliary beating by the melanin concentrating hormone-expressing neurons of the lateral hypothalamus: a functional imaging survey. Front Endocrinol (Lausanne) 2013;4:182. doi: 10.3389/fendo.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noble EE, et al. Control of Feeding Behavior by Cerebral Ventricular Volume Transmission of Melanin-Concentrating Hormone. Cell Metab. 2018;28:55–68.:e57. doi: 10.1016/j.cmet.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Massadi O, et al. Multifaceted actions of melanin-concentrating hormone on mammalian energy homeostasis. Nat Rev Endocrinol. 2021;17:745–755. doi: 10.1038/s41574-021-00559-1. [DOI] [PubMed] [Google Scholar]
- 134.Zilkha-Falb R, Kaushansky N, Ben-Nun A. The Median Eminence, A New Oligodendrogenic Niche in the Adult Mouse Brain. Stem Cell Reports. 2020;14:1076–1092. doi: 10.1016/j.stemcr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marsters CM, et al. Oligodendrocyte development in the embryonic tuberal hypothalamus and the influence of Ascl1. Neural Dev. 2016;11:20. doi: 10.1186/s13064-016-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kohnke S, et al. Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep. 2021;36:109362. doi: 10.1016/j.celrep.2021.109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ren Z, et al. Conditional knockout of leptin receptor in neural stem cells leads to obesity in mice and affects neuronal differentiation in the hypothalamus early after birth. Mol Brain. 2020;13:109. doi: 10.1186/s13041-020-00647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alonge KM, et al. Hypothalamic perineuronal net assembly is required for sustained diabetes remission induced by fibroblast growth factor 1 in rats. Nat Metab. 2020;2:1025–1033. doi: 10.1038/s42255-020-00275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saab AS, et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron. 2016;91:119–132. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ou Z, et al. A GPR17-cAMP-Lactate Signaling Axis in Oligodendrocytes Regulates Whole-Body Metabolism. Cell Rep. 2019;26:2984–2997.:e2984. doi: 10.1016/j.celrep.2019.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ren H, et al. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–1326. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ren H, Cook JR, Kon N, Accili D. Gpr17 in AgRP Neurons Regulates Feeding and Sensitivity to Insulin and Leptin. Diabetes. 2015;64:3670–3679. doi: 10.2337/db15-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Le Thuc O, et al. Hypothalamic Inflammation and Energy Balance Disruptions: Spotlight on Chemokines. Front Endocrinol (Lausanne) 2017;8:197. doi: 10.3389/fendo.2017.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wisse BE, et al. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology. 2007;148:5230–5237. doi: 10.1210/en.2007-0394. [DOI] [PubMed] [Google Scholar]
- 146.Jang PG, et al. NF-kappaB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia. J Biol Chem. 2010;285:9706–9715. doi: 10.1074/jbc.M109.070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jin S, et al. Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis. Sci Rep. 2016;6:29424. doi: 10.1038/srep29424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Le Thuc O, et al. Central CCL2 signaling onto MCH neurons mediates metabolic and behavioral adaptation to inflammation. EMBO Rep. 2016;17:1738–1752. doi: 10.15252/embr.201541499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan W, et al. Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des. 2011;17:3729–3740. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Banks WA. Anorectic effects of circulating cytokines: role of the vascular blood-brain barrier. Nutrition. 2001;17:434–437. doi: 10.1016/s0899-9007(01)00507-x. [DOI] [PubMed] [Google Scholar]
- 151.Valdearcos M, et al. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim F, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang G, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Heiss CN, et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021;35:109163. doi: 10.1016/j.celrep.2021.109163. [DOI] [PubMed] [Google Scholar]
- 155.Kim JD, Yoon NA, Jin S, Diano S. Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding. Cell Metab. 2019;30:952–962.:e955. doi: 10.1016/j.cmet.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dorfman MD, et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat Commun. 2017;8:14556. doi: 10.1038/ncomms14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cansell C, et al. Dietary fat exacerbates postprandial hypothalamic inflammation involving glial fibrillary acidic protein-positive cells and microglia in male mice. Glia. 2021;69:42–60. doi: 10.1002/glia.23882. [DOI] [PubMed] [Google Scholar]
- 158.Chowen JA, Argente-Arizon P, Freire-Regatillo A, Argente J. Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Front Neuroendocrinol. 2018;48:3–12. doi: 10.1016/j.yfrne.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 159.Binder AK, Winuthayanon Wipawee, Hewitt SylviaC, Couse JohnF, Korach KS. Steroid Receptors in the Uterus and Ovary. Academic Press; 2015. [Google Scholar]
- 160.Giacobini P, et al. Brain endothelial cells control fertility through ovarian-steroid-dependent release of semaphorin 3A. PLoS Biol. 2014;12:e1001808. doi: 10.1371/journal.pbio.1001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR. Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor beta1 release via prostaglandin E2 production and induces cell plasticity. J Neurosci. 2003;23:10622–10632. doi: 10.1523/JNEUROSCI.23-33-10622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Prevot V, et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pellegrino G, et al. GnRH neurons recruit astrocytes in infancy to facilitate network integration and sexual maturation. Nat Neurosci. 2021;24:1660–1672. doi: 10.1038/s41593-021-00960-z. [DOI] [PubMed] [Google Scholar]
- 165.Zeng F, Wang Y, Kloepfer LA, Wang S, Harris RC. ErbB4 deletion predisposes to development of metabolic syndrome in mice. Am J Physiol Endocrinol Metab. 2018;315:E583–E593. doi: 10.1152/ajpendo.00166.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Day FR, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464. doi: 10.1038/ncomms9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Prevot V, Sharif A. The polygamous GnRH neuron: Astrocytic and tanycytic communication with a neuroendocrine neuronal population. J Neuroendocrinol. 2022:e13104. doi: 10.1111/jne.13104. [DOI] [PubMed] [Google Scholar]
- 168.Swamydas M, Bessert D, Skoff R. Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J Neurosci Res. 2009;87:3306–3319. doi: 10.1002/jnr.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cerghet M, et al. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26:1439–1447. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yasuda K, et al. Sex-specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Res. 2020;46:101866. doi: 10.1016/j.scr.2020.101866. [DOI] [PubMed] [Google Scholar]
- 171.Marraudino M, et al. G Protein-Coupled Estrogen Receptor Immunoreactivity in the Rat Hypothalamus Is Widely Distributed in Neurons, Astrocytes, and Oligodendrocytes, Fluctuates during the Estrous Cycle, and Is Sexually Dimorphic. Neuroendocrinology. 2021;111:660–677. doi: 10.1159/000509583. [DOI] [PubMed] [Google Scholar]
- 172.Villa A, et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018;23:3501–3511. doi: 10.1016/j.celrep.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guneykaya D, et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018;24:2773–2783.:e2776. doi: 10.1016/j.celrep.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 174.Tramunt B, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ludwig MQ, et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat Metab. 2021;3:530–545. doi: 10.1038/s42255-021-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ludwig MQ, Todorov PV, Egerod KL, Olson DP, Pers TH. Single-Cell Mapping of GLP-1 and GIP Receptor Expression in the Dorsal Vagal Complex. Diabetes. 2021;70:1945–1955. doi: 10.2337/dbi21-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Filippi BM, et al. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes. 2014;63:892–899. doi: 10.2337/db13-1044. [DOI] [PubMed] [Google Scholar]
- 178.Hayes MR, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Williams EK, et al. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Muller TD, et al. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu Rev Nutr. 2014;34:237–260. doi: 10.1146/annurev-nutr-071812-161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reiner DJ, et al. Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. J Neurosci. 2016;36:3531–3540. doi: 10.1523/JNEUROSCI.3579-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yulyaningsih E, et al. Acute Lesioning and Rapid Repair of Hypothalamic Neurons outside the Blood-Brain Barrier. Cell Rep. 2017;19:2257–2271. doi: 10.1016/j.celrep.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Luo L, et al. Optimizing Nervous System-Specific Gene Targeting with Cre Driver Lines: Prevalence of Germline Recombination and Influencing Factors. Neuron. 2020;106:37–65.:e35. doi: 10.1016/j.neuron.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pak T, Yoo S, Miranda-Angulo AL, Wang H, Blackshaw S. Rax-CreERT2 knock-in mice: a tool for selective and conditional gene deletion in progenitor cells and radial glia of the retina and hypothalamus. PLoS One. 2014;9:e90381. doi: 10.1371/journal.pone.0090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou Y, et al. Author Correction: Temporal dynamic reorganization of 3D chromatin architecture in hormone-induced breast cancer and endocrine resistance. Nat Commun. 2020;11:1967. doi: 10.1038/s41467-020-15789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Magnani L, et al. Genome-wide reprogramming of the chromatin landscape underlies endocrine therapy resistance in breast cancer. Proc Natl Acad Sci U S A. 2013;110:E1490–1499. doi: 10.1073/pnas.1219992110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu Z, et al. Short-term tamoxifen treatment has long-term effects on metabolism in high-fat diet-fed mice with involvement of Nmnat2 in POMC neurons. FEBS Lett. 2018;592:3305–3316. doi: 10.1002/1873-3468.13240. [DOI] [PubMed] [Google Scholar]
- 190.O’Carroll SJ, Cook WH, Young D. AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front Mol Neurosci. 2020;13:618020. doi: 10.3389/fnmol.2020.618020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhu H, et al. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis. 2016;54:439–446. doi: 10.1002/dvg.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]